
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 20 June 2023
Sec. Alloimmunity and Transplantation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1211171
 Chengxin Luo1,2†
Chengxin Luo1,2† Xiangtao Huang1,2†
Xiangtao Huang1,2† Ling Wei1,2†
Ling Wei1,2† Guixian Wu1,2
Guixian Wu1,2 Yarui Huang1,2
Yarui Huang1,2 Yaqun Ding1,2
Yaqun Ding1,2 Zhen Huang1,2
Zhen Huang1,2 Jieping Chen1,2
Jieping Chen1,2 Xi Li3*
Xi Li3* Yunding Zou1,2*
Yunding Zou1,2* Shuangnian Xu1,2*
Shuangnian Xu1,2*Objective: Steroids-refractory (SR) acute graft-versus-host disease (aGVHD) is a life-threatening condition in patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT), but the optimal second-line therapy still has not been established. We aimed to perform a systematic review and meta-analysis of randomized controlled trials (RCTs) to compare the efficacy and safety of different second-line therapy regimens.
Methods: Literature search in MEDLINE, Embase, Cochrane Library and China Biology Medicine databases were performed to retrieve RCTs comparing the efficacy and safety of different therapy regimens for patients with SR aGVHD. Meta-analysis was conducted with Review Manager version 5.3. The primary outcome is the overall response rate (ORR) at day 28. Pooled relative risk (RR) and 95% confidence interval (CI) were calculated with the Mantel-Haenszel method.
Results: Eight eligible RCTs were included, involving 1127 patients with SR aGVHD and a broad range of second-line therapy regimens. Meta-analysis of 3 trials investigating the effects of adding mesenchymal stroma cells (MSCs) to other second-line therapy regimens suggested that the addition of MSCs is associated with significantly improvement in ORR at day 28 (RR = 1.15, 95% CI = 1.01–1.32, P = 0.04), especially in patients with severe (grade III–IV or grade C–D) aGVHD (RR = 1.26, 95% CI = 1.04–1.52, P = 0.02) and patients with multiorgan involved (RR = 1.27, 95% CI = 1.05–1.55, P = 0.01). No significant difference was observed betwwen the MSCs group and control group in consideration of overall survival and serious adverse events. Treatment outcomes of the other trials were comprehensively reviewed, ruxolitinib showed significantly higher ORR and complete response rate at day 28, higher durable overall response at day 56 and longer failure-free survival in comparison with other regimens; inolimomab shows similar 1-year therapy success rate but superior long-term overall survial in comparison with anti-thymocyte globulin, other comparisons did not show significant differences in efficacy.
Conclusions: Adding MSCs to other second-line therapy regimens is associated with significantly improved ORR, ruxolitinib showed significantly better efficacy outcomes in comparison with other regimens in patients with SR aGVHD. Further well-designed RCTs and integrated studies are required to determine the optimal treatment.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022342487.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a key treatment strategy for patients with high-risk hematological malignancies and severe non-malignant hematological disorders. The applications of allo-HSCT continuously increased over the past several decades (1). Despite substantial advancements in transplantation technologies, acute graft-versus-host disease (aGVHD) remains one of the most common complications and crucial contributing factor for transplant-related mortality (TRM) in patients undergoing allo-HSCT (2). Approximately 40%–60% of patients developed moderate to severe (grade II–IV) aGVHD after allo-HSCT despite standard prophylaxis (3, 4). Severe aGVHD is a life-threatening condition, associating with a dismal long-term overall survival (OS) lower than 30% for grade III disease and lower than 5% in patients with grade IV disease (5, 6). Above all, aGVHD is a major challenge in clinical practice limiting the application of allo-HSCT and compromising its benefits.
Steroids remains the first-line therapy for aGVHD, however, only 40%–60% of patients can achieve durable response with initial therapy (7–9). Patients with grade III-IV aGVHD, hyperacute GVHD, older age or multiple organs involvement are associated with high risk of treatment failure (10–12). The prognosis of patients with steroids-refractory aGVHD is extremely poor, with a GVHD-related mortality around 70% at 2 years (10, 13). Currently available second-line therapy for patients with steroids-refractory aGVHD mainly include mycophenolate mofetil (MMF), tacrolimus, anti-thymocyte globulin (ATG), Janus kinase (JAK) 1/2 inhibitor ruxolitinib, interleukin 2 receptor (IL-2R) antibodies (daclizumab, inolimomab, basiliximab), mammalian target of rapamycin (mTOR) inhibitors (sirolimus, everolimus), tumor necrosis factor-alpha (TNF-α) inhibitors (infliximab, etanercept), anti-CD52 antibody alemtuzumab, anti-α4β7 integrin antibody vedolizumab, extracorporal photopheresis (ECP) and mesenchymal stroma cells (MSCs) used alone or in combination (2, 9, 14). However, the optimal second-line therapy still has not been established (2, 15). In this study, we performed a systematic review and meta-analysis of randomized controlled trials (RCTs) comparing different second-line therapies in patients with steroids-refractory aGVHD following allo-HSCT, aiming to provide evidences for regimens selection in clinical practice and clues for future study design.
This study was reported according to preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (16). The protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) and available online (registration number CRD42022342487). We searched MEDLINE (by Ovid), Embase, Cochrane Library and China Biology Medicine (CBM) databases on April 6, 2022 with no date and language restriction, the search procedure was repeated on April 13, 2023. The main search terms are “graft versus host disease” or “GVHD” in combination with “refractory”, “resistant”, “persistent”, “second-line” or “salvage”. Reference lists of eligible trials and relevant reviews were manually checked for additional trials.
Two investigators (CXL and XTH) independently assessed eligibility of retrieved citations; disagreements were resolved by discussion with a third investigator (SNX). The inclusion criteria are: (і) included patients receiving allo-HSCT as therapy for hematological disorders, and developing steroids-refractory aGVHD of any grade following allo-HSCT; (ii) compared the efficacy and safety of two or more different second-line therapy regimens for steroids-refractory aGVHD; (iii) study design is randomized controlled trial (RCT). According to previously published agreements, steroids-refractory aGVHD is defined as: disease progression after at least 3 days of treatment with methylprednisolone ≥ 2 mg/kg/day or equivalent; lack of response after at least 7 days of treatment; or failure to taper the methylprednisolone dose to < 0.5 mg/kg/day or the prednisone dose to < 0.6 mg/kg/day (17, 18). Retrospective studies, single-arm studies, dose-escalating studies, case reports or case series, and post-hoc analysis were excluded.
Two investigators (CXL and LW) independently extracted data on trial characteristics (first author, publication year, study design), patients’ characteristics (age, gender, underlying disease, donor type, graft source, grade of aGVHD), type and dosage of second-line therapy regimens, median follow-up and treatment outcomes. The primary outcome is the overall response rate (ORR) at day 28, which is defined as the proportion of patients who achieved complete response (CR) or partial response (PR) at day 28. Secondary outcomes include CR rate, OS, and adverse events (AEs). For dichotomous data, number of patients with events and total number of patients analyzed were extracted. For time-to-event data, hazard ratios (HRs) and 95% confidence intervals (CIs) were extracted. The ln(HR) and standard error (SE) were calculated directly, or estimated from the log-rank P value and the number of events with the previously established methods when the HRs and 95% CIs were not reported (19). For trials with multiple publications, survival data were extracted from the report with the longest follow-up. Predesigned forms were used to extracted data and cross-checked to reach a consensus between the two investigators.
Methodological quality of included RCTs were assessed with the Cochrane risk of bias tool based on following aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias (20). Two investigators independently performed risk of bias assessment; any disagreements were resolved by consensus.
All statistical analyses were performed with Review Manager version 5.3 (Revman, The Cochrane Collaboration, 2014). For dichotomous data, the pooled risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using the Mantel-Haenszel method. For time-to-event data, the pooled HRs and 95% CIs were calculated using the generic inverse-variance method. Heterogeneity was assessed with Chi-square test and I2 statistic. Random-effects model was used when there is significant heterogeneity (P ≤ 0.1, I2 > 50%), otherwise fixed-effects model was used. Subgroup analyses were performed according to the patients’ age (< 18 years and ≥ 18 years), grade of aGVHD, involved organ (skin, gastrointestinal, and liver), and the number of involved organs (single organ and multiorgan). All P values are two-sided, and a P value of < 0.05 indicated statistical significance except that of heterogeneity test.
The databases search retrieved 5185 potentially relevant records. We removed 783 duplicates and excluded 4016 irrelevant records based on title and abstract, the remaining 386 records were included for further screening. Subsequently, we excluded 38 studies on GVHD prophylaxis, 23 studies on first-line therapy of aGVHD, 11 dose-finding studies, 6 protocol and 281 non-RCT studies. Ultimately, 27 records (19 conferences abstract and 8 full-text article) for 8 eligible RCTs were included for the systematic review (21–29). The flow chart of study selection is provided in Figure 1.
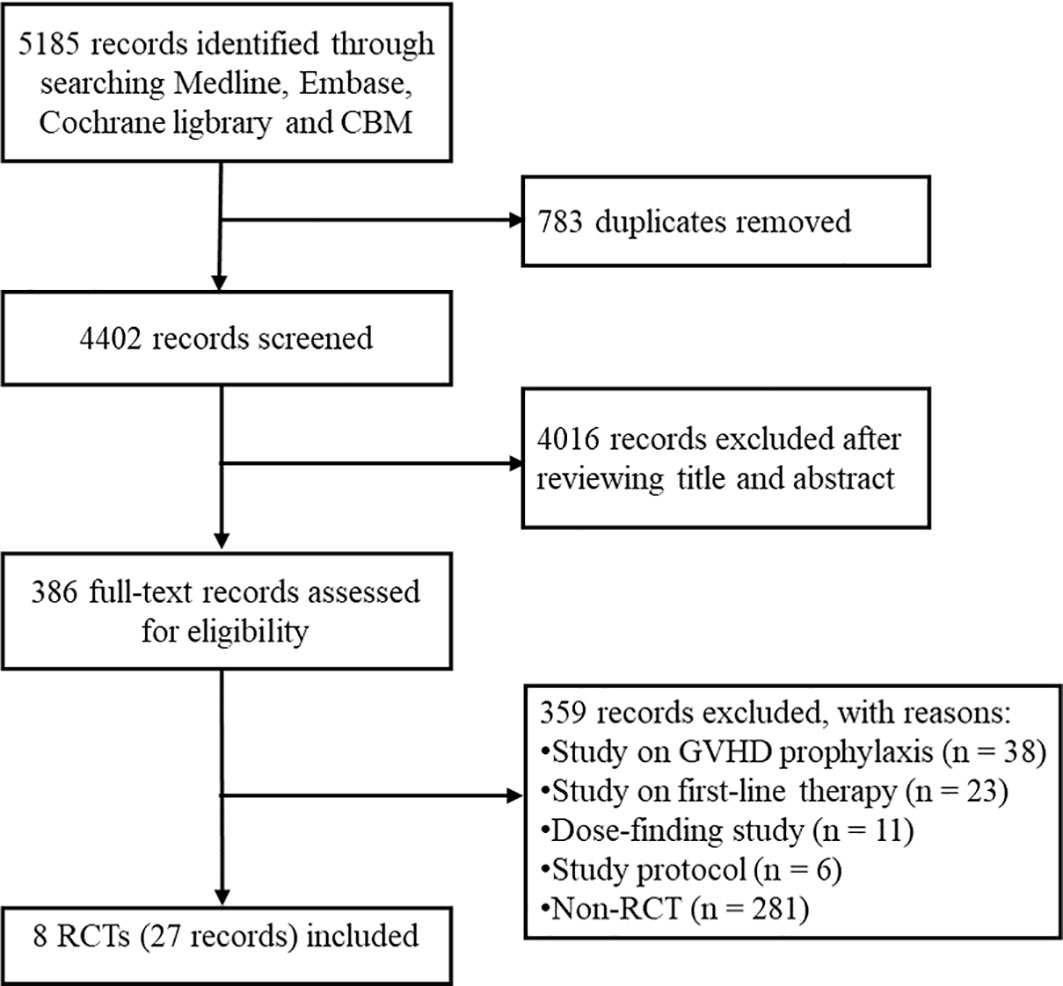
Figure 1 The flow chart of study selection. CBM, China Biology Medicine; GVHD, graft-versus-host disease; RCT, randomized controlled trial.
The characteristics of the 8 included RCTs are summarized in Table 1. The specific definition of steroids-refractory aGVHD in each included trail is summarized in Supplementary Table 1. A broad range of second-line therapy strategies for steroids-refractory aGVHD are investigated, the dosage of all regimens are provided in Supplementary Table 2. Three trials investigated the effects of adding MSCs to other second-line therapy regimens (including MMF, ATG, infliximab, etanercept, daclizumab, pentostatin, denileukin diftitox, ECP, alemtuzumab, and basiliximab plus cyclosporine or tacrolimus) (21–23). One trial (the REACH2 trial) compared the efficacy and safety of ruxolitinib with other best available care (investigator’s choice from ATG, ECP, MSCs, LD-MTX, MMF, everolimus, sirolimus, etanercept, or infliximab) (24). Other trials compared high-dose methylprednisolone (HD-MP) plus OKT3 (a murine anti-CD3 monoclonal antibody) or ATG with HD-MP alone, or compared ATG with inolimomab (a monoclonal antibody against IL-2R) or ABX-CBL (a murine monoclonal antibody against CD147) (25–29). The primary endpoints were largely different, including ORR (at day 28, 30 or 100), durable complete response, therapy success rate, and OS at day 180. The Median follow-up ranges from 180 days to 58.4 months (Table 1).
A total of 1127 patients were included and most of them were diagnosed with hematological malignancies (Table 1). Patient’s age, gender and grade of aGVHD were well matched between the experimental group and control group in 7 of the included trials that with enough information. As for donor type, 5 trials with relevant information included both related and unrelated donors. Graft sources were reported in 5 trials, which included both bone marrow (BM) and peripheral blood stem cells (PBSCs). Two trials included small proportions of patients (0.6%-12%) receiving cord blood (CB) transplantation (23, 24).
The results of risk of bias assessment suggest that random sequence generation is adequate in 3 trials, allocation sequence concealment is adequate in 2 trials, whereas the other trials did not provide sufficient information to evaluate selection bias (Supplementary Figure 1). As for blinding of participants and personnel, 2 trials are double-blind, 4 trials are open-label. Outcome assessments were performed in blinded manner in 3 trials. All of the included trials are free from attrition bias, reporting bias and any other bias except for one trial that only reported as a conference abstract and did not provide sufficient information to evaluate.
Treatment outcomes including overall response rate (ORR), complete respou rate and overall survival of all included trials are summarized in Table 2. The ORR at day 28 were reported in 4 trials, of which 3 trials investigated the effects of adding MSCs to other second-line therapy regimens (21–23). The ORR at day 28 of those second-line therapy regimens without MSCs ranges from 36.4% to 70.7%, addition of MSCs increased it to 47.6-82.8% (Table 2). The result of meta-analysis suggested that adding MSCs to other second-line therapy regimens is associated with significant increase of ORR at day 28 (RR = 1.15, 95% CI = 1.01–1.32, P = 0.04; Figure 2A). Subgroup analyses were performed based on patients’ age, grade of aGVHD, involved organ, and the number of involved organs, suggests that the addition of MSCs is associated with significant improve of ORR at day 28 in patients with severe (grade III–IV or grade C–D) aGVHD (RR = 1.26, 95% CI = 1.04–1.52, P = 0.02) and patients with multiorgan involved (RR = 1.27, 95% CI = 1.05–1.55, P = 0.01) although the subgroup differences were not statistically significant (Figures 3A, B). Subgroup analyses according to patients’ age and involved organ did not show any statistically significant results (Supplementary Figures 2, 3).
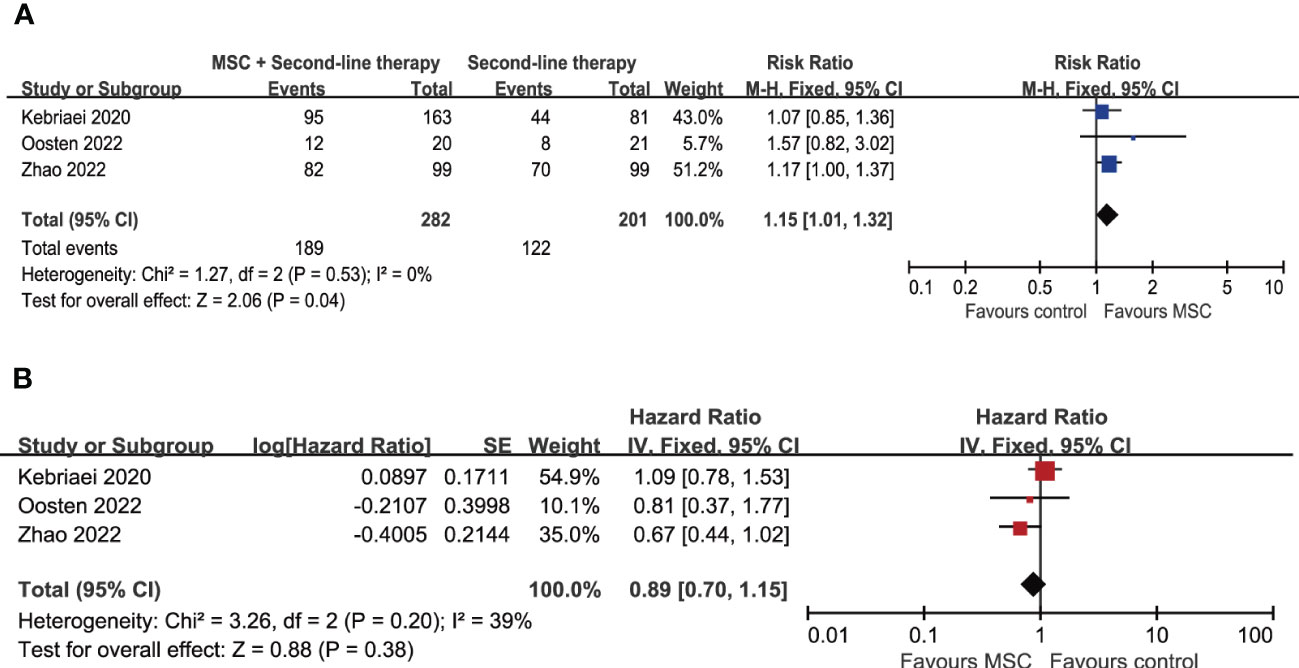
Figure 2 Forest plot for meta-analysis including MSC-related trials. (A) Overall response rate at day 28. (B) Overall survival. MSC, mesenchymal stem cells.
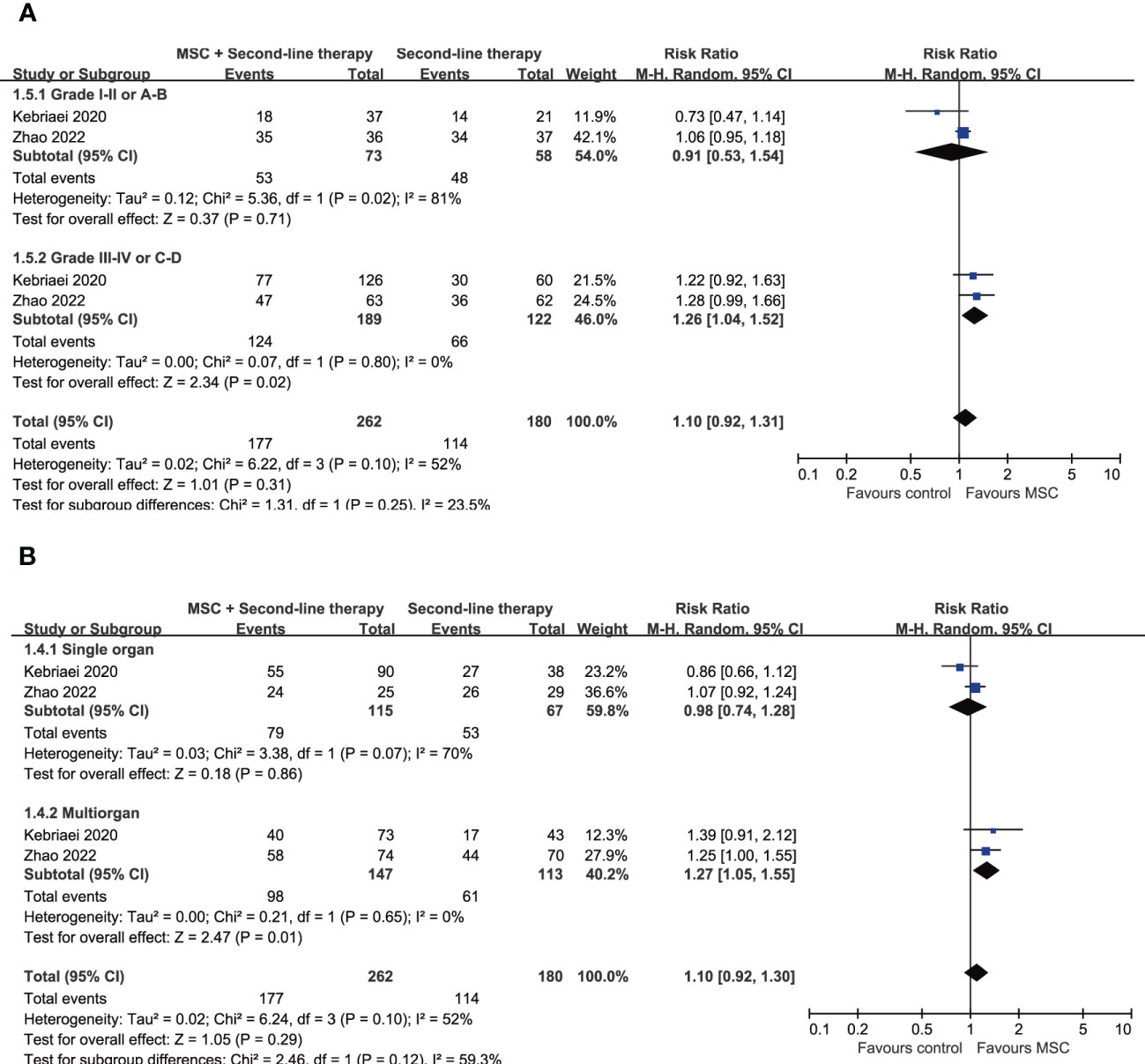
Figure 3 Forest plot for subgroup analysis on ORR at day 28 according to (A) the grade of aGVHD and (B) the number of involved organs. aGVHD, acute graft-versus-host disease; MSC, mesenchymal stem cells.
Another trial (the REACH2 trial) reporting ORR at day 28 as primary endpoint compared the efficacy of ruxolitinib with investigator’s choice from other second-line therapy regimens, including ATG, etanercept, ECP, infiliximab, MSCs and MMF (24). The ORR at day 28 of these regimens ranges from 30% to 60%, leading to a total ORR of 39% (Table 2). Ruxolitinib is associated with a significantly higher ORR at day 28 (62% versus 39%, P < 0.001) and durable overall response at day 56 (40% versus 22%, P < 0.001) in comprison with investigator’s choice.
The other 4 trials reported ORR at different time phase or 1-year therapy success rate (Table 2). Two trials investigated the efficacy of HD-MP plus ATG or anti-CD3 antibody OKT3, suggested that HD-MP plus ATG did not significantly improve ORR at day 30 over HD-MP alone (55% versus 48%; P = 0.3), and HD-MP plus OKT3 did not significantly improve ORR at day 100 over HD-MP alone (53% versus 33%; P = 0.06) (27, 29). One trial compared the efficacy of anti-CD147 antibody ABX-CBL with ATG, reported an ORR of 56% for the ABX-CBL group at a median of 22 days (range 7–72 days) and 57% for the ATG group at a median of 28 days (range 2–50 days), no significant difference was observed (28). The other trial compared inolimomab with ATG and reported 1-year therapy success rate as the primary endpoin (defined as the overall survival at 1 year without replacement of the baseline allocated treatment), and no significant differences was observed (28.5% versus 21.5%; P = 0.188) (25, 26).
The complete response (CR) rate were reported in 5 of the included trials (reviewed in Table 2). One of the 2 trials investigating the effects of adding MSCs with data regarding CR rate showed that the addition of MSCs led to a significantly improvement in CR rate at day 28 (56.6% versus 40.4%, P = 0.02), whereas another trial reported durable complete response (DCR, defined as CR for at least 28 consecutive days within the first 100 days after enrollment) as primary endpoint and no significant difference was observed (35% versus 30%, P = 0.42) (21, 23). The REACH2 trial reported that ruxolitinib led to significantly higher CR rate at day 28 in comparison with investigator’s choice from other second-line therapy (34% versus 19%, P = 0.004) (24). As for the trial comparing HD-MP plus ATG with HD-MP alone, CR rate at dat 30 was reported, and no significant difference was observed (33% versus 24%, P = 0.40) (29). For ABX-CBL versus ATG, The CR rates are 29% at a median of 77 days (range 14-77 days) in ABX-CBL group versus 32% at a meidan of 78 days (range 21-88 days) in ATG group (28).
After a median follow-up ranging from 180 days to 58.4 months, the 8 included trials reported overall survival (OS) at 180 days, 6 months, 1 year, 18-months, 3 years or the end of follow-up (summarized in Table 2). Meta-analysis of the 3 trials investigating the effects of adding MSCs to other second-line therapy regimens suggests that there is no significant difference in OS (HR = 0.89, 95% CI = 0.70–1.15, P = 0.38; Figure 2B). As for other comparisons, ruxolitinib did not lead to significant improvement in median OS (11.1 months versus 6.5 month; HR = 0.83, 95% CI = 0.60–1.15) but significantly prolonged median failure-free survival (5 months versus 1 month; HR = 0.46, 95% CI = 0.35–0.60); inolimomab showed more favorable OS over ATG (HR = 0.57, 95% CI = 0.35–0.95, P = 0.03) after long-term follow-up (up to 104 months, with a median 58.4 months) (25). The other trials did not report significant differences in OS between experimental group and control group (Table 2). The cumulative incidence of relapse of underlying disease (at 180 days, 1 year, 18-months, or 3 years) are provided in 4 trials, no significant difference is reported (Table 2).
The most common adverse events (AEs) reported in the 8 included trials are hematologic toxicities and infection (summarized in Table 2). One trial investigating the effects of adding MSCs to other second-line therapy regimens suggested that the MSCs group is associated with lower incidences of grade 3–4 hematologic toxicities (37.4% versus 53.5%, P = 0.022) and grade 3–4 infection (65.7% versus 78.8%, P = 0.039) (21). The REACH2 trial reported that ruxolitinib is associated with higher rates of thrombocytopenia (33% versus 18%, P = 0.003) compared with the investigator’s choice group (24). In comparison with ATG, inolimomab showed slightly lower incidences of viral infection (78% versus 92%, P = 0.05), and ABX-CBL showed lower incidences of pneumonia (33% versus 65%, P = 0.002). In comparison with HD-MP alone, HD-MP plus OKT3 is associated with lower risk of viral infection (35% versus 72%, P = 0.001), but caused cytokine release syndrome (CRS) at a rate around 60%. Other reported data in about AEs did not show any significant difference between experimental group and control group.
Meta-analyses results show that the MSCs plus other second-line therapy regimens is associated with similar risk of serious AEs (RR = 0.99, 95% CI = 0.88–1.12, P = 0.91), serious AEs with outcome of death (RR = 0.95, 95% CI = 0.56–1.59, P = 0.83), and infection-related death (RR = 1.17, 95% CI = 0.51–2.66, P = 0.71) in comparison with the control group (Supplementary Figure 4).
Steroid-refractory aGVHD is a life-threatening conditioning resulting in mortality rates higher than 90%. Treatment of steroid-refractory aGVHD remains a great challenge in clinical practice, the standard strategy still has not been established. This study performed a systematic review and meta-analysis of RCTs comparing the efficacy and safety of different second-line therapy regimens in patients with steroid-refractory aGVHD undergoing allo-HSCT. The reported treatment outcomes (including overall response rate, complete response rate, overall survival and main safety outcomes) of all second-line therapy in 8 eligible RCTs were comprehensively reviewed, and meta-analysis of 3 trials about MSCs was performed. We find that the addition of MSCs to other second-line therapy regimens are associated with significantly improved ORR at day 28, especially in patients with severe (grade III–IV or grade C–D) aGVHD and patients with multiorgan involved. The overall survival, risk of serious AE and serious AE-related death are similar between the MSCs and non-MSCs group. As for other comparisons, ruxolitinib showed significantly higher ORR and CR rate than other best available treatments; inolimomab was associated with superior long-term OS versus ATG. These evidences suggest that MSCs-contained second-line therapy regimens and ruxolitinib are asscociated with favorable efficacy outcomes in patients with steroid-refractory aGVHD.
The benefits from MSCs in the prophylaxis and treatment of immune-related disorders are widely investigated due to its multipotency and immunomodulatory properties (30). Various mechanisms are involved in the immunoregulating activities of MSCs, such as suppressing the proliferation and activation of CD4+ T cells, increasng the percentage of regulatory T cells, and modifying the cytokine secretion profile of different immune cells (30–32). In patients undergoing allo-HSCT, co-transplantation of MSCs can promotes engraftment and reduce the risk of severe aGVHD (33–35). For the treatment of GVHD, MSCs are often administrated in combination with other regimens, and the efficacy varies greatly due to the differences in MSCs source and dosage, disease characteristics and the combined regimens (35–38). Commercial MSCs product Remestemcel-L (Prochymal) was approved pediatric patients in Canada and New Zealand, and Temcell was approved in Japan, but the role of MSCs in the treatment of steroid-refractory aGVHD still require to be established (38). A meta-analysis of 13 non-randomised studies obtained an ORR of 72% and a 6-month survival of 63% for MSCs treatment in patients with steroid-refractory aGVHD (37). Afterwards, three RCTs were published and our meta-analyses results suggested that the addition of MSCs to other second-line therapy is associated with significant improvement in ORR, especially in patients with severe disease and multiorgan involvement (21–23). An ongoing phase 3 RCT (NCT04629833) comparing MSCs with best available therapy (BAT) in patients with steroid-refractory aGVHD will be helpful to further establish the benefits of MSCs (39). However, the source and dosage of MSCs, and the choice of combined regimens vary across these 3 published RCTs. Considering the combined regimens, the highest ORR (82.8%) and CR rate (56.6%) at day 28 were reported when MSCs are administrated in combination with basiliximab and calcineurin inhibitor (reviewed in Table 2), further studies are required to determine the optimal treatment strategy of MSCs in patients with different characteristics.
Ruxolitinib, and selective inhibitor of Janus kinase (JAK) 1 and JAK2, is the only approved drugs for the treatment of steroid-refractory aGVHD by US Food and Drug Administration (FDA). The JAK1/2 signaling plays an important role in the mechasims of GVHD through mediating the activation and proinflammatoy cytokines release of T cell, neutrophils and dendritic cells (DCs) (40, 41). Preclinical studies suggested that ruxolitinib can effectively inhibit JAK1/2 signaling, and ameliorate both acute and chronic GVHD while preserving graft-versus-tumor activity (42, 43). For patients with steroid-refractory aGVHD, ruxolitinib orally given at a dose of 5–10 mg achieved a ORR at day 28 of 55–82% with acceptable toxicity (24, 44, 45). The multicenter RCT (REACH2 trial) reported that compared with a control group incorporting a series of seond-line therapy regimens, ruxolitinib led to significantly higher ORR at day 28, higher durable overall response at day 56 and superior failure-free survival (24). A recently published meta-analysis including both prospective and retrospective studies reported an ORR of 77% and a 6-month survival of 63.9% for ruxolitinib in patients with steroid-refractory aGVHD, seem to be comparable to that of MSCs- or basiliximab-based second-line therapy regimens (46). RCT directly comparing the efficacy and safety of ruxolitinib with specific second-line regimens such as MSCs- and basiliximab-based regimen is unavailable, further trials and integrated studies are required to validate the advantages of ruxolitinib over other srategies.
Antagonists of IL-2R represent another attractive option for the therapy of steroid-refractory aGVHD. Blocking IL-2 signaling with IL-2R antagonists such as antibodies against IL-2R alpha chain (CD25) (basiliximab, daclizumab and inolimomab) and denileukin difititox (a recombinant fusion protein composed of IL-2 fragment and diphtheria toxin) show promising activity in steroid-refractory aGVHD (47–50). The effects of combining IL-2R antagonists with inhibitors of TNF-α signaling (etanercept and infliximab) were also investigated (51–54). According to the 2 RCTs included in our review, basiliximab achieved an ORR at day 28 of 70.7% and 82.8% with or without MSCs respectively, inolimomab achieved a 1-year therapy success rate of 28.5% and an advantage in long-term survival over ATG (21, 25). No RCT comparing the efficacy of these four IL-2R antagonists is available, a meta-analysis pooling data from both prospective and retrospective studies suggested that basiliximab-based therapy is associated the highest ORR of 81%, followed by 71% for daclizumab, 56% for denileukin difititox and 54% for inolimomab (55). Well-designed prospective studies will be helpful to validate the advantages of basiliximab over other IL-2R antagonists or other regimens in the therapy of steroid-refractory aGVHD.
There are several regimens of other mechanisms that were not involved in included trials, such as the anti-α4β7 integrins monoclonal antibody vedolizumab and the anti-interleukin-6 receptor (IL-6R) monoclonal antibody tocilizumab. A systematic review and meta-analysis have evaluated the efficacy of vedolizumab in the treatment of gastrointestinal aGVHD and obtained a pooled long-term ORR higher than 70% (56). As for steroid-refractory gastrointestinal aGVHD, several retrospective studies of small sample size were published and the reported ORR ranges from 45% to 79% (57–59). The efficacy of tocilizumab in steroid-refractory aGVHD was also only evaluated in a few retrospective studies with small sample size, reporting CR rate of 40%-60% (60, 61). Further studies are warranted to determine the efficacy and safety of these regimens with different mechanisms.
There are several limitations in this study. First of all, the number of published RCTs regarding therapy of steroid-refractory aGVHD is limited. Secondly, the comparative statistical analysis failed to include all investigated regimens since the regimens applied in the control groups varied largely across included tirals, including HD-MP, ATG and an incoporation of a series of best available choice, making it impractical to integrated evidences with traditional or network-meta-analysis. In future research, unification of control regimen will be helpful to increase the application of analyzed results for guiding clinical practice. Thirdly, the reported efficacy outcomes also varied across included tirals, making it impractical to integrate data from different trials. We choose ORR at day 28 as the primary outcome since it is proposed as the best endpoint for aGVHD therapeutic trials in predicting transplantation-related mortality (8). However, the ORR at day 28 are only reported in 4 included RCTs. Lastly, long-term follow-up data, which we think are very important for patients undergoing allo-HSCT, are absent in several included studies.
In conclusion, this systematic review and meta-analysis of RCTs suggests that MSCs-contained second-line therapy regimens and ruxolitinib are associated with favorable efficacy outcomes in patients with steroids-refractory aGVHD, but further well-designed RCTs and integrated studies are still required to determine the optimal treatment.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
SX, YZ, XL and JC contributed to the study conception and design, supervised the study and made critical revision in the manuscript. CL, XH and LW performed database searching, study selection, data extraction, statistical analyses and manuscript writing. GW, YH, YD, and ZH helped with data extraction, data checking and data interpretation. All authors contributed to the article and approved the submitted version.
This study was partly supported by the National Natural Science Foundation of China (NSFC82000169), Natural Science Foundation of Chongqing (cstc2020jcyj-msxmX1058), Project from Southwest Hospital (2022DZXDX004), Project from Third Military Medical University (2021XJS15), and the Logistics Project (AWS17J007). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1211171/full#supplementary-material
1. Chang YJ, Pei XY, Huang XJ. Haematopoietic stem-cell transplantation in China in the era of targeted therapies: current advances, challenges, and future directions. Lancet Haematol (2022) 9(12):e919–e29. doi: 10.1016/S2352-3026(22)00293-9
2. Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European society for blood and marrow transplantation. Lancet Haematol (2020) 7(2):e157–e67. doi: 10.1016/S2352-3026(19)30256-X
3. Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood (2012) 119(1):296–307. doi: 10.1182/blood-2011-06-364265
4. Martinez-Cibrian N, Zeiser R, Perez-Simon JA. Graft-versus-host disease prophylaxis: pathophysiology-based review on current approaches and future directions. Blood Rev (2021) 48:100792. doi: 10.1016/j.blre.2020.100792
5. Cahn JY, Klein JP, Lee SJ, Milpied N, Blaise D, Antin JH, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint société française de greffe de moëlle et thérapie cellulaire (SFGM-TC), Dana Farber cancer institute (DFCI), and international bone marrow transplant registry (IBMTR) prospective study. Blood (2005) 106(4):1495–500. doi: 10.1182/blood-2004-11-4557
6. Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet (London England). (2009) 373(9674):1550–61. doi: 10.1016/S0140-6736(09)60237-3
7. MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood marrow Transplant (2002) 8(7):387–94. doi: 10.1053/bbmt.2002.v8.pm12171485
8. de Masson A, Bouaziz JD, Peffault de Latour R, Wittnebel S, Ribaud P, Rubio MT, et al. Limited efficacy and tolerance of imatinib mesylate in steroid-refractory sclerodermatous chronic GVHD. Blood (2012) 120(25):5089–90. doi: 10.1182/blood-2012-09-453928
9. Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American society of blood and marrow transplantation. Biol Blood marrow Transplant (2012) 18(8):1150–63. doi: 10.1016/j.bbmt.2012.04.005
10. Westin JR, Saliba RM, De Lima M, Alousi A, Hosing C, Qazilbash MH, et al. Steroid-refractory acute GVHD: predictors and outcomes. Adv hematol (2011) 2011:601953. doi: 10.1155/2011/601953
11. MacMillan ML, Robin M, Harris AC, DeFor TE, Martin PJ, Alousi A, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood marrow Transplant (2015) 21(4):761–7. doi: 10.1016/j.bbmt.2015.01.001
12. El Jurdi N, Rayes A, MacMillan ML, Holtan SG, DeFor TE, Witte J, et al. Steroid-dependent acute GVHD after allogeneic hematopoietic cell transplantation: risk factors and clinical outcomes. Blood advances. (2021) 5(5):1352–9. doi: 10.1182/bloodadvances.2020003937
13. Rashidi A, DeFor TE, Holtan SG, Blazar BR, Weisdorf DJ, MacMillan ML. Outcomes and predictors of response in steroid-refractory acute graft-versus-Host disease. Biol Blood marrow Transplant (2019) 25(11):2297–302. doi: 10.1016/j.bbmt.2019.07.017
14. Kasikis S, Etra A, Levine JE. Current and emerging targeted therapies for acute graft-Versus-Host disease. Biodrugs (2021) 35(1):19–33. doi: 10.1007/s40259-020-00454-7
15. Zeiser R, Blazar BR. Acute graft-versus-Host disease - biologic process, prevention, and therapy. New Engl J Med (2017) 377(22):2167–79. doi: 10.1056/NEJMra1609337
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed) (2021) 372:n71. doi: 10.1136/bmj.n71
17. Mohty M, Holler E, Jagasia M, Jenq R, Malard F, Martin P, et al. Refractory acute graft-versus-host disease: a new working definition beyond corticosteroid refractoriness. Blood (2020) 136(17):1903–6. doi: 10.1182/blood.2020007336
18. von Bubnoff N, Ihorst G, Grishina O, Röthling N, Bertz H, Duyster J, et al. Ruxolitinib in GvHD (RIG) study: a multicenter, randomized phase 2 trial to determine the response rate of ruxolitinib and best available treatment (BAT) versus BAT in steroid-refractory acute graft-versus-host disease (aGvHD) (NCT02396628). BMC cancer. (2018) 18(1):1132. doi: 10.1186/s12885-018-5045-7
19. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
20. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. (Chichester (UK): John Wiley & Sons) (2019).
21. Zhao K, Lin R, Fan Z, Chen X, Wang Y, Huang F, et al. Mesenchymal stromal cells plus basiliximab, calcineurin inhibitor as treatment of steroid-resistant acute graft-versus-host disease: a multicenter, randomized, phase 3, open-label trial. J Hematol Oncol (2022) 15(1):22. doi: 10.1186/s13045-022-01240-4
22. Oosten L, Van Pel M, van der Holt B, Bach E, Cross M, Roelofs H, et al. Treatment of severe steroid-refractory acute GVHD with mesenchymal stromal cells: results of the phase III randomized double-blind multi-center HOVON-113 trial. Hemasphere (2022) 6:2384–5. doi: 10.1097/01.HS9.0000848304.76865.3a
23. Kebriaei P, Hayes J, Daly A, Uberti J, Marks DI, Soiffer R, et al. A phase 3 randomized study of remestemcel-l versus placebo added to second-line therapy in patients with steroid-refractory acute graft-versus-Host disease. Biol Blood Marrow Transplantation. (2020) 26(5):835–44. doi: 10.1016/j.bbmt.2019.08.029
24. Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-Host disease. New Engl J Med (2020) 382(19):1800–10. doi: 10.1056/NEJMoa1917635
25. Socie G, Milpied N, Yakoub-Agha I, Bay JO, Furst S, Bilger K, et al. Long-term follow-up of a phase 3 clinical trial of inolimomab for the treatment of primary steroid refractory aGVHD. Blood Advances. (2019) 3(2):184–6. doi: 10.1182/bloodadvances.2018028282
26. Socie G, Vigouroux S, Yakoub-Agha I, Bay JO, Furst S, Bilger K, et al. A phase 3 randomized trial comparing inolimomab vs usual care in steroid-resistant acute GVHD. Blood (2017) 129(5):643–9. doi: 10.1182/blood-2016-09-738625
27. Knop S, Hebart H, Gratwohl A, Kliem C, Faul C, Holler E, et al. Treatment of steroid-resistant acute GVHD with OKT3 and high-dose steroids results in better disease control and lower incidence of infectious complications when compared to high-dose steroids alone: a randomized multicenter trial by the EBMT chronic leukemia working party. Leukemia (2007) 21(8):1830–3. doi: 10.1038/sj.leu.2404731
28. Macmillan ML, Couriel D, Weisdorf DJ, Schwab G, Havrilla N, Fleming TR, et al. A phase 2/3 multicenter randomized clinical trial of ABX-CBL versus ATG as secondary therapy for steroid-resistant acute graft-versus-host disease. Blood (2007) 109(6):2657–62. doi: 10.1182/blood-2006-08-013995
29. Van Lint MT, Milone G, Leotta S, Uderzo C, Scime R, Dallorso S, et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood (2006) 107(10):4177–81. doi: 10.1182/blood-2005-12-4851
30. Markov A, Thangavelu L, Aravindhan S, Zekiy AO, Jarahian M, Chartrand MS, et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther (2021) 12(1):192. doi: 10.1186/s13287-021-02265-1
31. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood (2005) 105(4):1815–22. doi: 10.1182/blood-2004-04-1559
32. Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther (2013) 4(3):65. doi: 10.1186/scrt216
33. Lee SH, Lee MW, Yoo KH, Kim DS, Son MH, Sung KW, et al. Co-Transplantation of third-party umbilical cord blood-derived MSCs promotes engraftment in children undergoing unrelated umbilical cord blood transplantation. Bone marrow transplantation. (2013) 48(8):1040–5. doi: 10.1038/bmt.2013.7
34. Li T, Luo C, Zhang J, Wei L, Sun W, Xie Q, et al. Efficacy and safety of mesenchymal stem cells co-infusion in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. Stem Cell Res Ther (2021) 12(1):246. doi: 10.1186/s13287-020-02064-0
35. Morata-Tarifa C, Macías-Sánchez MDM, Gutiérrez-Pizarraya A, Sanchez-Pernaute R. Mesenchymal stromal cells for the prophylaxis and treatment of graft-versus-host disease-a meta-analysis. Stem Cell Res Ther (2020) 11(1):64. doi: 10.1186/s13287-020-01592-z
36. Chen X, Wang C, Yin J, Xu J, Wei J, Zhang Y. Efficacy of mesenchymal stem cell therapy for steroid-refractory acute graft-Versus-Host disease following allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. PloS One (2015) 10(8):e0136991. doi: 10.1371/journal.pone.0136991
37. Hashmi S, Ahmed M, Murad MH, Litzow MR, Adams RH, Ball LM, et al. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. Lancet Haematol (2016) 3(1):e45–52. doi: 10.1016/S2352-3026(15)00224-0
38. Murata M, Teshima T. Treatment of steroid-refractory acute graft-Versus-Host disease using commercial mesenchymal stem cell products. Front Immunol (2021) 12:724380. doi: 10.3389/fimmu.2021.724380
39. Zeiser R, Osswald E, Moeller AK, Bader P. A randomized, open label, multicentre, phase 3 trial of first line treatment with msc versus bat in patients with steroid refractory acute GVHD (idunn trial). Bone marrow transplantation. (2022) 57:217–8. doi: 10.1038/s41409-022-01798-0
40. Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood (2014) 123(24):3832–42. doi: 10.1182/blood-2013-12-543736
41. Schroeder MA, Choi J, Staser K, DiPersio JF. The role of janus kinase signaling in graft-Versus-Host disease and graft versus leukemia. Biol Blood marrow Transplant (2018) 24(6):1125–34. doi: 10.1016/j.bbmt.2017.12.797
42. Carniti C, Gimondi S, Vendramin A, Recordati C, Confalonieri D, Bermema A, et al. Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res (2015) 21(16):3740–9. doi: 10.1158/1078-0432.CCR-14-2758
43. Huarte E, Peel M, Juvekar A, Dubé P, Sarah S, Stephens L, et al. Ruxolitinib, a JAK1/JAK2 selective inhibitor, ameliorates acute and chronic steroid-refractory GvHD mouse models. Immunotherapy (2021) 13(12):977–87. doi: 10.2217/imt-2021-0013
44. Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia (2015) 29(10):2062–8. doi: 10.1038/leu.2015.212
45. Jagasia M, Perales MA, Schroeder MA, Ali H, Shah NN, Chen YB, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood (2020) 135(20):1739–49. doi: 10.1182/blood.2020004823
46. Fan S, Huo WX, Yang Y, Shen MZ, Mo XD. Efficacy and safety of ruxolitinib in steroid-refractory graft-versus-host disease: a meta-analysis. Front Immunol (2022) 13:954268. doi: 10.3389/fimmu.2022.954268
47. Tang FF, Cheng YF, Xu LP, Zhang XH, Yan CH, Han W, et al. Basiliximab as treatment for steroid-refractory acute graft-versus-Host disease in pediatric patients after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2020) 26(2):351–7. doi: 10.1016/j.bbmt.2019.10.031
48. Miano M, Cuzzubbo D, Terranova P, Giardino S, Lanino E, Morreale G, et al. Daclizumab as useful treatment in refractory acute GVHD: a paediatric experience. Bone marrow transplantation. (2009) 43(5):423–7. doi: 10.1038/bmt.2008.331
49. Piñana JL, Valcárcel D, Martino R, Moreno ME, Sureda A, Briones J, et al. Encouraging results with inolimomab (anti-IL-2 receptor) as treatment for refractory acute graft-versus-host disease. Biol Blood marrow Transplant (2006) 12(11):1135–41. doi: 10.1016/j.bbmt.2006.06.010
50. Ho VT, Zahrieh D, Hochberg E, Micale E, Levin J, Reynolds C, et al. Safety and efficacy of denileukin diftitox in patients with steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood (2004) 104(4):1224–6. doi: 10.1182/blood-2004-01-0028
51. Nadeau M, Perreault S, Seropian S, Foss F, Isufi I, Cooper DL. The use of basiliximab-infliximab combination for the treatment of severe gastrointestinal acute GvHD. Bone marrow transplantation. (2016) 51(2):273–6. doi: 10.1038/bmt.2015.247
52. Rager A, Frey N, Goldstein SC, Reshef R, Hexner EO, Loren A, et al. Inflammatory cytokine inhibition with combination daclizumab and infliximab for steroid-refractory acute GVHD. Bone marrow transplantation. (2011) 46(3):430–5. doi: 10.1038/bmt.2010.117
53. van Groningen LF, Liefferink AM, de Haan AF, Schaap NP, Donnelly JP, Blijlevens NM, et al. Combination therapy with inolimomab and etanercept for severe steroid-refractory acute graft-versus-Host disease. Biol Blood marrow Transplant (2016) 22(1):179–82. doi: 10.1016/j.bbmt.2015.08.039
54. Wolff D, Roessler V, Steiner B, Wilhelm S, Weirich V, Brenmoehl J, et al. Treatment of steroid-resistant acute graft-versus-host disease with daclizumab and etanercept. Bone marrow transplantation. (2005) 35(10):1003–10. doi: 10.1038/sj.bmt.1704929
55. Shen MZ, Li JX, Zhang XH, Xu LP, Wang Y, Liu KY, et al. Meta-analysis of interleukin-2 receptor antagonists as the treatment for steroid-refractory acute graft-Versus-Host disease. Front Immunol (2021) 12:749266. doi: 10.3389/fimmu.2021.749266
56. Li AC, Dong C, Tay ST, Ananthakrishnan A, Ma KS. Vedolizumab for acute gastrointestinal graft-versus-host disease: a systematic review and meta-analysis. Front Immunol (2022) 13:1025350. doi: 10.3389/fimmu.2022.1025350
57. Danylesko I, Bukauskas A, Paulson M, Peceliunas V, Gedde-Dahl DYT, Shimoni A, et al. Anti-α4β7 integrin monoclonal antibody (vedolizumab) for the treatment of steroid-resistant severe intestinal acute graft-versus-host disease. Bone marrow transplantation. (2019) 54(7):987–93. doi: 10.1038/s41409-018-0364-5
58. Fløisand Y, Lazarevic VL, Maertens J, Mattsson J, Shah NN, Zachée P, et al. Safety and effectiveness of vedolizumab in patients with steroid-refractory gastrointestinal acute graft-versus-Host disease: a retrospective record review. Biol Blood marrow Transplant (2019) 25(4):720–7. doi: 10.1016/j.bbmt.2018.11.013
59. Mehta RS, Saliba RM, Jan A, Shigle TL, Wang E, Nieto Y, et al. Vedolizumab for steroid refractory lower gastrointestinal tract graft-Versus-Host disease. Transplant Cell Ther (2021) 27(3):272.e1–.e5. doi: 10.1016/j.jtct.2020.12.011
60. Yucebay F, Matthews C, Puto M, Li J, William B, Jaglowski SM, et al. Tocilizumab as first-line therapy for steroid-refractory acute graft-versus-host-disease: analysis of a single-center experience. Leuk Lymphoma. (2019) 60(9):2223–9. doi: 10.1080/10428194.2019.1573996
Keywords: acute graft-versus-host disease, steroids-refractory, second-line therapy, mesenchymal stroma cells, ruxolitinib, meta-analysis, randomized controlled trials
Citation: Luo C, Huang X, Wei L, Wu G, Huang Y, Ding Y, Huang Z, Chen J, Li X, Zou Y and Xu S (2023) Second-line therapy for patients with steroid-refractory aGVHD: systematic review and meta-analysis of randomized controlled trials. Front. Immunol. 14:1211171. doi: 10.3389/fimmu.2023.1211171
Received: 25 April 2023; Accepted: 08 June 2023;
Published: 20 June 2023.
Edited by:
Giuseppe Milone, University Hospital Polyclinic Vittorio Emanuele, ItalyReviewed by:
Xiao-Dong Mo, Peking University People’s Hospital, ChinaCopyright © 2023 Luo, Huang, Wei, Wu, Huang, Ding, Huang, Chen, Li, Zou and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Li, bGl4aTE5ODh4bkAxNjMuY29t; Yunding Zou, em91eXVuZGluZ0AxNjMuY29t; Shuangnian Xu, eHVzaHVhbmduaWFuQHRtbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.