- 1Beijing Key Laboratory of New Techniques of Tuberculosis Diagnosis and Treatment, Senior Department of Tuberculosis, The 8th Medical Center of PLA General Hospital, Beijing, China
- 2Hebei North University, Zhangjiakou, Hebei, China
Introduction
Bacillus Calmette-Guérin (BCG) is the only licensed vaccine for tuberculosis (TB) prevention (1). A systematic review evaluated the effects on non-specific and all-cause mortality in children under 5 vaccinated with BCG, and the results showed that BCG vaccination was associated with reduced all-cause mortality, with an average relative risk of 0.70 in the five clinical trials and 0.47 in the nine high-bias observational studies included in the analysis (2). Another study conducted in northern Sweden found similar results that BCG vaccination was associated with a reduction in non-TB infectious deaths (RR = 0.75 (0.58-0.97), especially respiratory infections (3). Similarly, a case-control study in southeastern Brazil found that BCG reduced the risk of death from pneumonia in children under 1 year of age by 50% (4). These studies demonstrated that BCG vaccination can induce non-specific protective effects against non-mycobacterial infections. These non-specific protective effects may be related to three immunological mechanisms: trained immunity, heterologous immunity, and anti-inflammatory effect (5).
BCG-induced trained immunity has attracted the most interest of the three immune mechanisms described above. BCG-induced trained immunity involves the reprogramming of innate immune cells through epigenetic reprogramming (such as DNA modifications, noncoding RNAs, histone modifications, and chromatin remodeling) (6, 7), metabolic reprogramming (such as glycolysis, oxidative phosphorylation, and glutamine catabolism pathways) (8, 9), and long-term protection mediated by hematopoietic stem cells (10), resulting in enhanced responsiveness and protection against a range of infections (11). Therefore, BCG has been widely used in cancer therapy, infectious disease prevention, autoimmune disease treatment, and other diseases such as Alzheimer’s disease and Parkinson’s disease (11–13).
Based on the above facts, a hypothesis has been proposed in recent years: BCG-induced trained immunity can reduce the incidence, hospitalization, severe disease, and mortality of COVID-19 (14). Therefore, in the early stage of the COVID-19 pandemic, researchers conducted a large number of ecological, epidemiological, and statistical analyses on the potential of BCG to reduce COVID-19 morbidity and mortality, based on the incidence and mortality reported by the World Health Organization (WHO) and individual countries, supplemented by BCG vaccination rates in individual countries (5). However, the results of these observational studies are often highly heterogeneous due to many confounding factors. To address this challenge, researchers have set out to test this hypothesis in clinical trials with relatively high levels of evidence. Over 56 clinical trials were conducted in the Netherlands, India, Brazil, Denmark, Germany, the USA, and Greece to assess the effect of BCG vaccination on reducing the number of infections, days away from work, hospital admissions, morbidity, and mortality (15). To date, ten of these trials have published their results, including NCT04659941 (16), NCT04435379 (17), NCT04414267 (18), NCT04379336 (19), NCT04328441 (20, 21), RBR-4kjqtg (22), NCT04417335 (23), NCT04648800 (24), and CTRI/2020/07/026668 (25) (Table 1). However, the results of these clinical trials remain heterogeneous, making it impossible to draw any firm conclusions from them. Thus, we make the following recommendations for the design of clinical trials to evaluate the protective effect of BCG on COVID-19, which may help to address these heterogeneities.
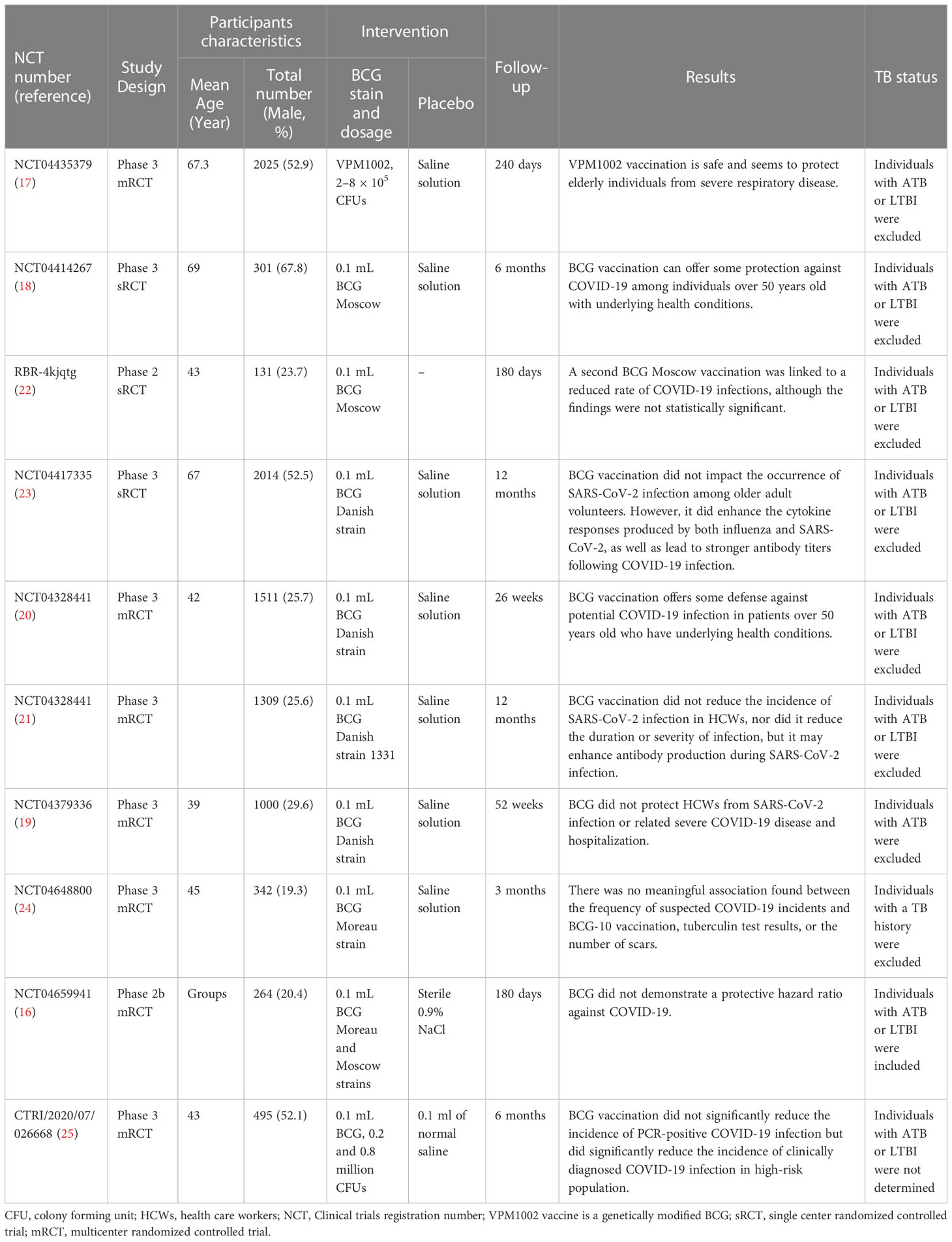
Table 1 Randomized controlled trials (RCTs) on evaluating BCG vaccination effectiveness against COVID-19.
Participants with ATB and LTBI should be excluded from the clinical trial
The BCG-induced trained immunity can be interfered by the existence of immune responses induced by the Mycobacterium tuberculosis, which should be indirectly detected by tuberculin skin test (TST) or M. tuberculosis antigen-specific interferon-gamma release assays (IGRAs) (18). Early in the COVID-19 pandemic, epidemiologic data from Europe suggested a lower prevalence of COVID-19 in individuals with a higher prevalence of latent tuberculosis infection (LTBI) [r(20): - 0.5511 ~ - 0.6338; p < 0.001] (26). Similarly, a retrospective study from Turkey investigated the association between TST induration diameter and the clinical course of COVID-19 and showed that TST positivity was higher in patients with milder COVID-19 (P<0.001) and a large induration diameter (P<0.001) (27). Another study evaluated the severity of disease in COVID-19 patients in relation to LTBI infection rates and its correlation with laboratory parameters and clinical outcomes and found that individuals with LTBI who developed COVID-19 had significantly less severe disease, accompanied by higher lymphocyte and monocyte counts (28). These findings are interesting and suggest a potential protective effect of LTBI against COVID-19. However, it is important to note that correlation does not necessarily equal causation and these findings need to be interpreted with caution. It is possible that other factors, such as age, comorbidities, and socioeconomic status, may confound these findings. Regardless, further research is needed to better understand the relationship between LTBI and COVID-19 outcomes. If a protective effect of LTBI can be confirmed, including participants with LTBI may confound the evaluation of BCG efficacy in protecting against COVID-19.
We investigated ten randomized controlled trials (RCTs) published in the last two years (Table 1), including six clinical trials indeed excluded participants with ATB or LTBI (17, 18, 20, 22, 23), two clinical trials excluded participants with ATB or a history of ATB (19, 24), one clinical trial included participants with ATB or LTBI (16), and one clinical trial didn’t report the status of ATB and LTBI (25). Interestingly, we found that four trials that excluded participants with ATB and LTBI supported a possible protective effect of BCG against COVID-19 (17, 18, 22, 23), and two studies showed that BCG vaccination did not reduce absenteeism, incidence, hospitalization, or associated severe COVID-19 disease among healthcare workers (HCWs) (20, 21). In contrast, two clinical trials that excluded only participants with ATB or a history of ATB but not LTBI suggested that BCG vaccination did not reduce the incidence, hospitalization, or associated severe COVID-19 disease in HCWs (19, 24). In a recent study, “The effect of BCG vaccination on infection and antibody levels against SARS-CoV-2-The results of ProBCG: a multicenter randomized clinical trial in Brazil”, published in the International Journal of Infectious Diseases, the authors found that BCG did not show a protective hazard ratio against COVID-19 (16), consistent with two clinical trials only excluded participants with ATB or an ATB history (19, 24). Furthermore, we found that the participants with ATB or LTBI were included in this study. The authors stated that the original inclusion and exclusion criteria for this study were HCWs 18 years of age or older who agreed to provide written informed consent, excluding pregnant women and those who had received a COVID-19-specific vaccine. Therefore, this clinical trial failed to exclude individuals with ATB and LTBI, making it impossible to distinguish the trained immunity induced by M. tuberculosis infection from those induced by BCG. In December 2022, Sanjeev Sinha and colleagues published a phase III multicenter RCT in Infectious Diseases and Therapy to evaluate the protective efficacy of BCG against COVID-19 in high-risk populations (25). The results showed that BCG vaccination could not significantly reduce the incidence of PCR-positive COVID-19 in high-risk populations but could reduce the incidence of clinically diagnosed COVID-19. A major limitation of the study was that it did not explicitly report the ATB and LTBI status of the enrolled participants.
Based on the expanding evidence supporting the association between LTBI and COVID-19 outcomes, we propose that randomized controlled trials (RCTs) aimed at assessing the protective efficacy of BCG vaccination against COVID-19 should exclude participants with ATB or LTBI, as determined by either the TST or IGRAs, during the trial design stage. This exclusion will enhance precision and accuracy of the trial results by controlling for potential confounders and ensuring that the observed association, if any, between BCG vaccination and COVID-19 efficacy is attributable to the vaccination alone. This recommendation should be further evaluated and implemented by the scientific and medical communities to optimize the design of upcoming RCTs.
One clinical trial would ideally use one BCG strain
Since its initial development in 1921, the Bacillus Calmette-Guérin (BCG) vaccine has undergone various modifications, resulting in several strains, including early strains such as BCG Russia, Japan, Moreau, and Sweden, and later strains such as BCG Denmark, BCG Tice, BCG Sweden, BCG Frappier, BCG Pasteur, BCG Prague, BCG Glaxo, and BCG Connaught (29). These various strains exhibit differences in immunogenicity, protective efficiency, and adverse effects. A comparative study utilizing the guinea pig model of pulmonary tuberculosis, evaluated the protection efficacy of six commonly used BCG strains, including BCG Japanese, BCG Danish, BCG Glaxo, BCG Connaught, BCG Pasteur, and BCG Tice, showing that BCG Tice had the best protection efficiency, while BCG Glaxo had relatively poor protection (30). Interestingly, an additional randomized trial conducted in Hong Kong involving 303,092 neonates showed that the incidence of TB after vaccination with BCG-Glaxo was 1.8 times higher than that following vaccination with BCG-Pasteur (31). A cohort study in Kazakhstan found that BCG Japan, BCG Serbia, and BCG Russia provided 69%, 43%, and 22% protection against clinically diagnosed TB and 92%, 82%, and 51% protection against culture-confirmed TB, respectively (32).
Furthermore, studies indicate that BCG strains differ not only in the prevention of TB, but also in the therapeutic effects of BCG on non-muscle-invasive bladder cancers (33, 34). A separate study involving Brazilian infants revealed differing cytokine expression profiles elicited by BCG Moro and BCG Russian strains, with BCG Moro inducing higher levels of cytokines such as IL-2, IL-4, and IL-10, compared to BCG Russian (35).
These findings highlight the importance of considering the type of BCG strains used in evaluating its protective efficacy against COVID-19 (36). Some BCG strains may act as a confounding factor, thus affecting the outcomes of clinical trials. Among the four BCG strains used in previously published clinical trials (Table 1), BCG Moscow, BCG Danish, BCG Moreau strains, and VPM1002 (a genetically modified BCG) strain were evaluated. Additionally, nine clinical trials with participant numbers ranging from 131 to 2025 assessed the efficacy of specific strains of BCG against COVID-19 (17–25). One study with a sample size of only 264 participants evaluated the protective efficacy of BCG Moreau and Moscow strains against COVID-19 (16). Although this study did not reach its originally calculated sample size of 376 individuals for each group, the findings provide valuable insights, even when considering the further subdivision of the BCG group into the BCG Moreau and BCG Moscow subgroups, which reduced the sample size in each group. Future clinical trials should consider these findings when designing trials to evaluate the protective efficacy of different BCG strains against COVID-19.
Conclusions
In summary, to ensure the accuracy of RCTs evaluating the protective effect of BCG against COVID-19, we recommend excluding individuals with a history of TB, including ATB and LTBI. Furthermore, the usage of two or more BCG strains in a clinical trial should be avoided unless the sample size of each subgroup is large enough to compensate for potential confounding factors. These guidelines can help prevent bias and enhance the validity and generalizability of clinical trial results, ultimately contributing to the development of effective strategies for COVID-19 prevention and control.
Author contributions
Conceptualization: WG; Data collection and data analysis: WG, YL, YX, and LZ; Funding acquisition: WG; Software: YL, YX, and LZ; Writing - original draft: WG; Writing - review and editing: WPG, YPL, YX, and LZ. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National key research and development program of China (grant number 2022YFA1303503) and the Eighth Medical Center of PLA General Hospital (grant number MS202211002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Calmette A. Preventive vaccination against tuberculosis with BCG. Proc R Soc Med (1931) 24:1481–90. doi: 10.1177/003591573102401109
2. Higgins JP, Soares-Weiser K, López-López JA, Kakourou A, Chaplin K, Christensen H, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. Bmj (2016) 355:i5170. doi: 10.1136/bmj.i5170
3. Schaltz-Buchholzer F, Kjær Sørensen M, Benn CS, Aaby P. The introduction of BCG vaccination to neonates in northern Sweden, 1927-31: re-analysis of historical data to understand the lower mortality among BCG-vaccinated children. Vaccine (2022) 40:1516–24. doi: 10.1016/j.vaccine.2021.06.006
4. Niobey FM, Duchiade MP, Vasconcelos AG, de Carvalho ML, Leal Mdo C, Valente JG. [Risk factors for death caused by pneumonia in children younger than 1 year old in a metropolitan region of southeastern brazil. a case- control study]. Rev Saude Publica (1992) 26:229–38. doi: 10.1590/s0034-89101992000400004
5. Gong W, Mao Y, Li Y, Qi Y. BCG Vaccination: a potential tool against COVID-19 and COVID-19-like black swan incidents. Int Immunopharmacol (2022) 108:108870. doi: 10.1016/j.intimp.2022.108870
6. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille calmette-guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U.S.A. (2012) 109:17537–42. doi: 10.1073/pnas.1202870109
7. Zhang Q, Cao X. Epigenetic regulation of the innate immune response to infection. Nat Rev Immunol (2019) 19:417–32. doi: 10.1038/s41577-019-0151-6
8. Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep (2016) 17:2562–71. doi: 10.1016/j.celrep.2016.11.011
9. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science (2014) 345:1250684. doi: 10.1126/science.1250684
10. Cirovic B, de Bree LCJ, Groh L, Blok BA, Chan J, van der Velden W, et al. BCG Vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe (2020) 28:322–334.e325. doi: 10.1016/j.chom.2020.05.014
11. Chen J, Gao L, Wu X, Fan Y, Liu M, Peng L, et al. BCG-Induced trained immunity: history, mechanisms and potential applications. J Transl Med (2023) 21:106. doi: 10.1186/s12967-023-03944-8
12. Klinger D, Hill BL, Barda N, Halperin E, Gofrit ON, Greenblatt CL, et al. Bladder cancer immunotherapy by BCG is associated with a significantly reduced risk of alzheimer’s disease and parkinson’s disease. Vaccines (Basel) (2021) 9(5):491. doi: 10.3390/vaccines9050491
13. Kühtreiber WM, Tran L, Kim T, Dybala M, Nguyen B, Plager S, et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines (2018) 3:23. doi: 10.1038/s41541-018-0062-8
14. Gong W, An H, Wang J, Cheng P, Qi Y. The natural effect of BCG vaccination on COVID-19: the debate continues. Front Immunol (2022) 13:953228. doi: 10.3389/fimmu.2022.953228
15. Wang J, Zhang Q, Wang H, Gong W. The potential roles of BCG vaccine in the prevention or treatment of COVID-19. Front Biosci (Landmark Ed) (2022) 27:157. doi: 10.31083/j.fbl2705157
16. Santos AP, Werneck GL, Dalvi APR, Dos Santos CC, Tierno P, Condelo HS, et al. The effect of BCG vaccination on infection and antibody levels against SARS-CoV-2-The results of ProBCG: a multicenter randomized clinical trial in Brazil. Int J Infect Dis (2023) 130:8–16. doi: 10.1016/j.ijid.2023.02.014
17. Blossey AM, Brückner S, May M, Parzmair GP, Sharma H, Shaligram U, et al. VPM1002 as prophylaxis against severe respiratory tract infections including coronavirus disease 2019 in the elderly: a phase 3 randomized, double-blind, placebo-controlled, multicenter clinical study. Clin Infect Dis (2023) 76:1304–10. doi: 10.1093/cid/ciac881
18. Tsilika M, Taks E, Dolianitis K, Kotsaki A, Leventogiannis K, Damoulari C, et al. ACTIVATE-2: a double-blind randomized trial of BCG vaccination against COVID-19 in individuals at risk. Front Immunol (2022) 13:873067. doi: 10.3389/fimmu.2022.873067
19. Upton CM, van Wijk RC, Mockeliunas L, Simonsson USH, McHarry K, van den Hoogen G, et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: a double-blind, randomised, controlled, phase 3 trial. eClinicalMedicine (2022) 48:101414. doi: 10.1016/j.eclinm.2022.101414
20. Ten Doesschate T, van der Vaart TW, Debisarun PA, Taks E, Moorlag S, Paternotte N, et al. Bacillus calmette-guérin vaccine to reduce healthcare worker absenteeism in COVID-19 pandemic, a randomized controlled trial. Clin Microbiol Infect (2022) 28:1278–85. doi: 10.1016/j.cmi.2022.04.009
21. Claus J, Ten Doesschate T, Gumbs C, van Werkhoven CH, van der Vaart TW, Janssen AB, et al. BCG Vaccination of health care workers does not reduce SARS-CoV-2 infections nor infection severity or duration: a randomized placebo-controlled trial. mBio (2023) 14:e0035623. doi: 10.1128/mbio.00356-23
22. dos Anjos LRB, da Costa AC, Cardoso A, Guimarães RA, Rodrigues RL, Ribeiro KM, et al. Efficacy and safety of BCG revaccination with M. bovis BCG moscow to prevent COVID-19 infection in health care workers: a randomized phase II clinical trial. Front Immunol (2022) 13:841868. doi: 10.3389/fimmu.2022.841868
23. Moorlag S, Taks E, Ten Doesschate T, van der Vaart TW, Janssen AB, Müller L, et al. Efficacy of BCG vaccination against respiratory tract infections in older adults during the coronavirus disease 2019 pandemic. Clin Infect Dis (2022) 75:e938–46. doi: 10.1093/cid/ciac182
24. Czajka H, Zapolnik P, Krzych Ł., Kmiecik W, Stopyra L, Nowakowska A, et al. A multi-center, randomised, double-blind, placebo-controlled phase iii clinical trial evaluating the impact of BCG re-vaccination on the incidence and severity of SARS-CoV-2 infections among symptomatic healthcare professionals during the COVID-19 pandemic in poland–first results. Vaccines (2022) 10:314. doi: 10.3390/vaccines10020314
25. Sinha S, Ajayababu A, Thukral H, Gupta S, Guha SK, Basu A, et al. Efficacy of bacillus calmette-guérin (BCG) vaccination in reducing the incidence and severity of COVID-19 in high-risk population (BRIC): a phase III, multi-centre, quadruple-blind randomised control trial. Infect Dis Ther (2022) 11:2205–17. doi: 10.1007/s40121-022-00703-y
26. Singh S, Maurya RP, Singh RK. “Trained immunity” from mycobacterium spp. exposure or BCG vaccination and COVID-19 outcomes. PloS Pathog (2020) 16:e1008969. doi: 10.1371/journal.ppat.1008969
27. Arslan Gulen T, Bayraktar M, Yaksi N, Kayabas U. Is the course of COVID-19 associated with tuberculin skin test diameter? a retrospective study. J Med Virol (2022) 94:1020–6. doi: 10.1002/jmv.27414
28. Madan M, Baldwa B, Raja A, Tyagi R, Dwivedi T, Mohan A, et al. Impact of latent tuberculosis on severity and outcomes in admitted COVID-19 patients. Cureus (2021) 13:e19882. doi: 10.7759/cureus.19882
29. Aspatwar A, Gong W, Wang S, Wu X, Parkkila S. Tuberculosis vaccine BCG: the magical effect of the old vaccine in the fight against the COVID-19 pandemic. Int Rev Immunol (2022) 41:283–96. doi: 10.1080/08830185.2021.1922685
30. Horwitz MA, Harth G, Dillon BJ, Maslesa-Galić S. Commonly administered BCG strains including an evolutionarily early strain and evolutionarily late strains of disparate genealogy induce comparable protective immunity against tuberculosis. Vaccine (2009) 27:441–5. doi: 10.1016/j.vaccine.2008.10.058
31. Jayaraman K, Adhisivam B, Nallasivan S, Krishnan RG, Kamalarathnam C, Bharathi M, et al. Two randomized trials of the effect of the Russian strain of bacillus calmette-guérin alone or with oral polio vaccine on neonatal mortality in infants weighing <2000 g in India. Pediatr Infect Dis J (2019) 38:198–202. doi: 10.1097/inf.0000000000002198
32. Favorov M, Ali M, Tursunbayeva A, Aitmagambetova I, Kilgore P, Ismailov S, et al. Comparative tuberculosis (TB) prevention effectiveness in children of bacillus calmette-guérin (BCG) vaccines from different sources, Kazakhstan. PloS One (2012) 7:e32567. doi: 10.1371/journal.pone.0032567
33. Chen YK, Huang EY, Chang YH, Kuo JY, Chung HJ, Wu HH, et al. The comparison of different BCG strains in the intravesical treatment of non-muscle invasive urothelial carcinoma of urinary bladder-a real-world practice. J Chin Med Assoc (2022) 85:928–34. doi: 10.1097/jcma.0000000000000768
34. Del Giudice F, Busetto GM, Gross MS, Maggi M, Sciarra A, Salciccia S, et al. Efficacy of three BCG strains (Connaught, TICE and RIVM) with or without secondary resection (re-TUR) for intermediate/high-risk non-muscle-invasive bladder cancers: results from a retrospective single-institution cohort analysis. J Cancer Res Clin Oncol (2021) 147:3073–80. doi: 10.1007/s00432-021-03571-0
35. Bitencourt J, Sarno A, Oliveira C, Souza RA, Lima CC, Takenami I, et al. Comparing cytokine production and clinical response following vaccination with BCG moreau and BCG Russia strains in a Brazilian infant population. Vaccine (2021) 39:3189–96. doi: 10.1016/j.vaccine.2021.04.028
Keywords: Bacillus Calmette-Guérin (BCG), tuberculosis (TB), COVID-19, clinical trial, latent tuberculosis infection (LTBI)
Citation: Gong W, Liu Y, Xue Y and Zhuang L (2023) Two issues should be noted when designing a clinical trial to evaluate BCG effects on COVID-19. Front. Immunol. 14:1207212. doi: 10.3389/fimmu.2023.1207212
Received: 17 April 2023; Accepted: 12 May 2023;
Published: 23 May 2023.
Edited by:
Bingdong Zhu, Lanzhou University, ChinaReviewed by:
Zhidong Hu, Fudan University, ChinaCopyright © 2023 Gong, Liu, Xue and Zhuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenping Gong, Z3dwODkxMDE1QHdodS5lZHUuY24=
 Wenping Gong
Wenping Gong Yinping Liu1
Yinping Liu1 Li Zhuang
Li Zhuang