- Department of Pathology, China-Japan Union Hospital, Jilin University, Changchun, Jilin, China
Bladder cancer is one of the common malignant urothelial tumors. Post-translational modification (PTMs), including ubiquitination, acetylation, methylation, and phosphorylation, have been revealed to participate in bladder cancer initiation and progression. Ubiquitination is the common PTM, which is conducted by E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzyme and E3 ubiquitin-protein ligase. E3 ubiquitin ligases play a key role in bladder oncogenesis and progression and drug resistance in bladder cancer. Therefore, in this review, we summarize current knowledge regarding the functions of E3 ubiquitin ligases in bladder cancer development. Moreover, we provide the evidence of E3 ubiquitin ligases in regulation of immunotherapy in bladder cancer. Furthermore, we mention the multiple compounds that target E3 ubiquitin ligases to improve the therapy efficacy of bladder cancer. We hope our review can stimulate researchers and clinicians to investigate whether and how targeting E3 ubiquitin ligases acts a novel strategy for bladder cancer therapy.
Introduction
Bladder cancer is one of the common malignant tumors worldwide (1). It was estimated that there are 82,290 new cases and 16,710 deaths in 2023 in the United States. In men, bladder cancer was the eight-leading cause of cancer-associated death in the United States (2). In the world, there were approximately 573,278 new cases with bladder cancer and 212,536 deaths due to this disease (3, 4). Tobacco smoking could be a reason for bladder cancer incidence. In addition, some risk factors, such as chemicals and aromatic amines, arsenic contamination and aluminum, could increase the bladder cancer development. The treatments of bladder cancer often include endoscopic resection, chemotherapy, radiation, intravesical immunotherapy and combination therapy (5–7). The gold standard therapy for MIBC was chemotherapy and radical cystectomy. Bladder-sparing trimodal therapy is also available for MIBC patients. Chemoimmunotherapy is the key strategy for bladder cancer with metastatic feature (8). The treatment of immunotherapy and immune checkpoint inhibitors has not shown the good efficacy in bladder cancer patients (9). However, bladder cancer exhibits immune evasion and poor outcomes, suggesting that novel therapies need to be developed for treating bladder cancer (10).
Several genes have been known to regulate development and aggressiveness in bladder cancer, including Wnt, STAT3, PI3K, AKT, mTOR and PTEN (11–15). For instance, monocarboxylate transporter isoform 1 (MCT1) has been found to govern aggressive and metabolic phenotypes in bladder cancer because higher expression of MCT1 was associated with lymph node, poor survival and distant metastasis (16, 17). Silencing of MCT1 blocked proliferation, invasion, migration and altered the expression of EMT-associated proteins (16). MCT1, MCT4 and CD147 displayed a prognostic implication and a potential role in bladder cancer metabolism (18). MCT1 and CD147 also participated in cisplatin resistance and tumor aggressiveness in bladder cancer (19). In addition, some proteins could be post-translationally modified, including phosphorylation, ubiquitination, acetylation, glycosylation, methylation and SUMOylation (20). Post-translational modification (PTM) has been known to govern tumorigenesis and progression in various cancer types, including bladder cancer (21, 22).
PTMs include ubiquitination, acetylation, phosphorylation, methylation, hydroxylation, lipidation, palmitoylation, and glycosylation (23–27). Autophagy-lysosome pathway and the ubiquitin-proteasomal system (UPS) are common PTMs to control protein stability (28, 29). Ubiquitination is an ATP-mediated process: an E1 ubiquitin-activating enzyme activates ubiquitin, E2 ubiquitin-conjugating enzyme links ubiquitin via a transesterification reaction, E3 ubiquitin-protein ligase makes the binding between E2 enzyme and substrate proteins, leading to ubiquitin transfer from E2 to the specific substrate (30, 31). E3 ubiquitin ligases are critically involved in oncogenesis and progression as well as drug resistance in bladder cancer (32, 33). Targeting E3 ubiquitin ligases has demonstrated to be a novel approach for bladder cancer therapy (34). In this review, we summarize current knowledge regarding the roles of E3 ubiquitin ligases in bladder oncogenesis. Furthermore, we discuss the insights of E3 ubiquitin ligases in regulation of immunotherapy in bladder cancer. Moreover, we highlight the efforts on targeting E3 ubiquitin ligases to improve the efficacy of bladder cancer treatments. We hope our review can encourage researchers to explore how can improve the benefit of bladder cancer therapy via targeting E3 ubiquitin ligases.
Deubiquitinases in bladder cancer
USP2a
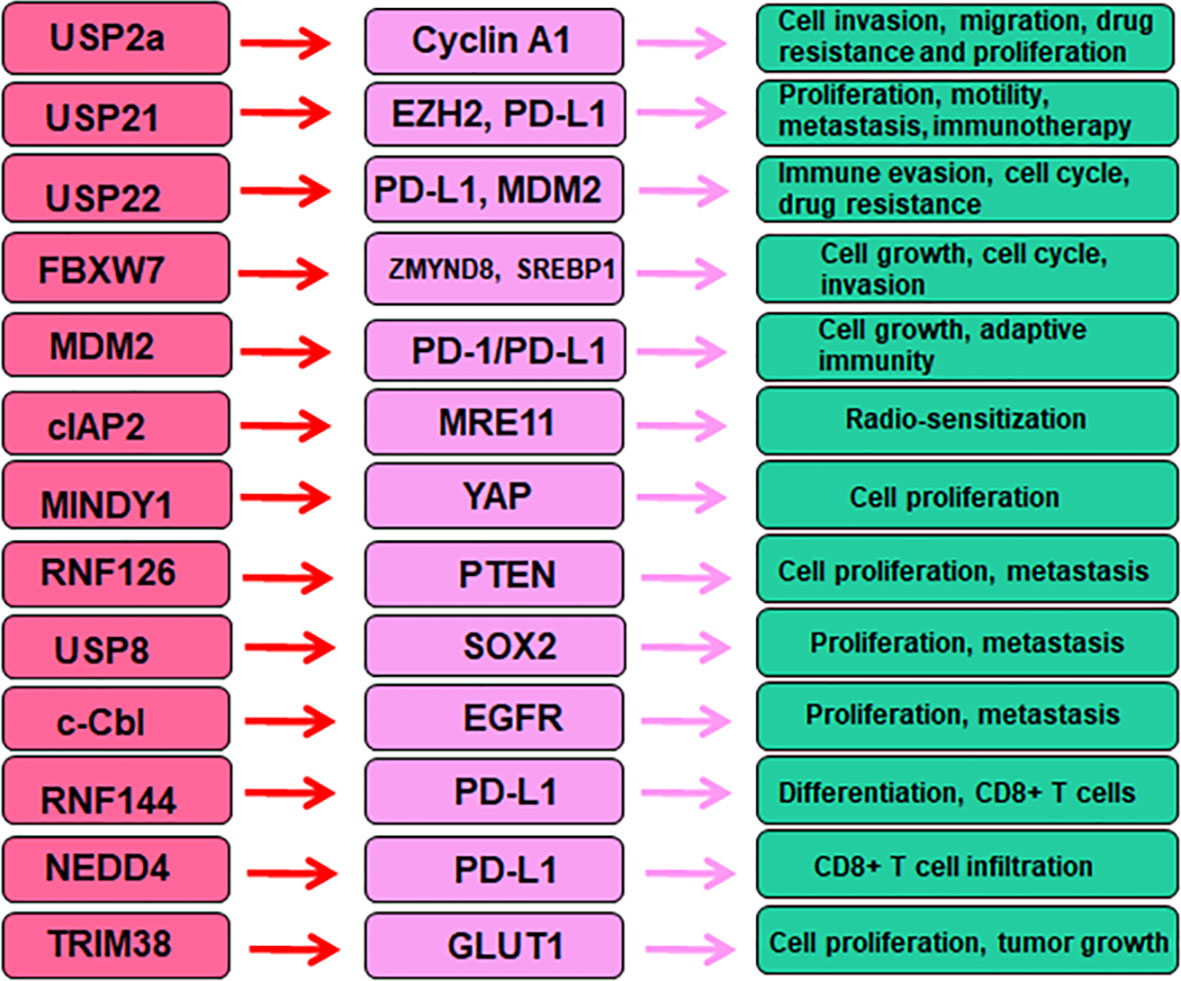
USP2a has been reported to regulate oncogenesis and progression in a variety of human cancers (35–37). USP2a mRNA expression was reduced in bladder cancer tissues compared with age-matched bladder tissues, and USP2a mRNA expression was decreased in higher stage of MIBC (38). Kim et al. found that USP2a increased tumor progression in part via regulation of cyclin A1 in bladder cancer (39). Specifically, overexpression of USP2a increased cell invasion, migration, chemotherapeutic drug resistance and proliferation. Downregulation of USP2a showed the opposite effects in bladder cancer. USP2a overexpression increased the Erk/MAPK phosphorylation after HB-EGF stimulation in T24 cells. Overexpression of USP2a in T24 cells caused more resistance to cisplatin-induced apoptosis due to inhibition of the cleaved form of PARP (c-PARP). USP2a interacted with cyclin A1 and blocked the ubiquitination of cyclin A1, contributing to cyclin A1 accumulation, which led to promotion of cell proliferation in bladder cancer (39). Frizzled 8-associated APF (antiproliferative factor) maintained the stability of p53 via modulation of USP2a and murine double minute 2 (MDM2) (40). APF decreased USP2a expression and caused MDM2 ubiquitination, leading to inhibition of the interaction between p53 and MDM2, thereby impairing p53 ubiquitination (40). Overexpression of USP2a increased cell growth through upregulation of cyclin D1 at the mRNA and protein levels, while depletion of USP2a reduced cell proliferation in part via increased cellular p53 levels in T24 cells (40).
USP21
USP21 has been gradually uncovered the essential role in carcinogenesis (41, 42). One integrative assay of 1q23.3 copy number gain in urothelial cancer patients with metastasis after platinum-based chemotherapy demonstrated that USP21, F11R, PPOX, DEDD, PFDN2 genes were closed linked to poor outcomes (43). Similarly, USP14 and USP21 were found to be associated with chemoresistance in bladder urothelial carcinoma with metastasis (44). Another study showed that USP21 expression was elevated in bladder cancer. High expression of USP21 was closely correlated with tumor metastasis and tumor size. Intriguingly, poorer survival rate was found in bladder cancer patients with higher levels of USP21 (45). In bladder cancer cells, increased expression of USP21 promoted cell proliferation, stimulated cell migration and invasion, enhanced tumor metastasis (45). Notably, overexpression of USP21 led to the development of EMT. Mechanistically, USP21 deubiquitinated EZH2 and stabilized its protein levels. USP21 could be a potential target for bladder cancer therapy (45). PD-L1 is observed in membrane of immune cells ad tumor cells. PD-L1 can bind to PD-1, leading to protection of tumor cells from an immune attack. The inhibitors of PD-1/PD-L1 can impair this binding and enhance the immune response against tumor cells (46, 47). USP21 has been identified to act as a deubiquitinase of PD-L1. Increased USP21 elevated PD-L1 abundance, whereas depletion of USP21 promoted PD-L1 degradation. Hence, targeting USP21 could be helpful to improve tumor immunotherapy (48).
USP22
USP22 has been known to involve in tumor cell proliferation, invasion, stemness, cell cycle arrest, metastasis, immune response and drug resistance in human cancer (49). In bladder cancer, silencing USP22 by siRNAs induced cell cycle arrest and attenuated cell proliferation (50). USP22 siRNA transfection increased the expression of p53 and p21, decreased cyclin E expression in bladder cancer cells. Silencing of USP22 promoted the degradation of MDM2 in bladder cancer cells. USP22 siRNA transfection induced cell cycle at G0/G1 phase via upregulation of p53, p21 and downregulation of cyclin E in bladder cancer cells (50). Depletion of USP22 expression retarded the tumor growth of implanted bladder cancer cells in mice (50). Another study also revealed that USP22 depletion reduced cell cycle progression and retarded tumor growth in animal models of bladder cancer, liver cancer, lung cancer, breast cancer and ovarian cancer (51). USP22 has been reported to regulate immune evasion and drug sensitivity in cancer (52). USP22 has been identified to work as a new regulator of PD-L1. USP22 interacted with PD-L1 and maintained PD-L1 stability via deubiquitination in A549, H1299 and H1792 NSCLC cells (53). USP22 also interacted with CSN5 and kept its stability via deubiquitination. Either CSN5 or USP22 enhanced the binding of PD-L1 with the other one. The K6, K11, K27, K29, K33 and K63-linked ubiquitin chains were removed by USP22 in PD-L1 and CSN5 in HEK293FT cells. Hence, USP22 governed the PD-L1 protein levels via CSN5/PD-L1 pathway in HEK293FT cells (53). Silencing of USP22 enhanced T cell cytotoxicity and blocked lung tumorigenesis. This study showed a critical role of USP22 in regulation of immune evasion via maintenance of PD-L1 protein levels (53). It is required to define the role of USP22 in bladder tumorigenesis.
E3 ubiquitin ligases in bladder cancer
FBXW7
FBXW7 belongs to F-box protein family and shows a tumor suppressive function in cancer development (54). F-box proteins target numerous substrates and regulate proliferation, metastasis, EMT, cancer stem cells, and drug resistance (55–57). FBXW7 exhibited single nucleotide variants or insertion or deletion in non-schistosomiasis related-squamous cell carcinoma of urinary bladder (NSR-SCCUB) patients (58). NSR-SCCUB is not common type in urothelial carcinoma, which could have genomic alterations (58). FBXW7 targeted an epigenetic regulator ZMYND8 for ubiquitination and degradation in bladder cancer (59). ZMYND8 increased cell viability and colony formation, migrative ability in bladder cancer. FBXW7 interacted with and degraded ZMYND8 in a polyubiquitin-dependent manner. By a gene set enrichment analysis, ZMYND8 was observed to be positively correlated to tumor stemness markers, including FOXM1, SOX2 and NANOG (59). One group revealed that overexpression of p65 increased cell migration via FBXW7-induced ubiquitination and degradation of RhoGDIα protein in bladder cancer (60). RhoGDIα protein was found to be a p65 downstream target and mediated p65-induced cell migration in bladder cancer. Mechanistically, p65 enhanced FBXW7 stability via attenuating the mRNA transcription of PTEN (60). Hence, p65 inhibited PTEN mRNA transcription and subsequently promoted FBXW7 stability, leading to degradation of RhoGDIα in bladder cancer cells (60). Liu et al. found that upregulation of FBXW7 reduced the invasion and growth of bladder cancer cells, caused cell cycle arrest at G0/G1 phase. Increased FBXW7 activated GSK-3β phosphorylation and inhibited the expression of SREBP1a in bladder cancer cells (61). SREBP1 is a transcription factor, including two isoforms, SREBP-1a and SREBP-1c, which regulates the expression of lipogenesis genes. Studies have shown that SREBP1 regulates the expression of stearoyl-CoA desaturase, fatty acid synthase, and acetyl-CoA carboxylase (62). FBXW7 can bind with SREBP1a by a co-immunoprecipitation assay. In vivo study further validated the role of FBXW7 in regulation of SREBP1a (61). The role of FBXW7 in bladder cancer indicated that targeting FBXW7 is a novel approach for bladder cancer therapy.
MDM2
MDM2 (mouse double minute 2 homologue) is involved in tumorigenesis mainly targeting p53 protein in different cancer types, including bladder cancer (63, 64). In 1994, upregulation of MDM2 and p53 expression was observed in bladder cancer patients (65). Moreover, p53 and MDM2 were found to be key factors in the progression of bladder cancer (66). There was an association between TP53 (codon 72, arginine> proline), MDM2 (SNP309, T>G) polymorphisms and patient’s survival in bladder cancer after chemoradiotherapy (CRT) (67). Patients with MDM2 T/G + G/G genotypes exhibited a good survival rate after CRT. TP53 and MDM2 with more than two of variant alleles exhibited an improved survival (67). For example, MDM2 SNP309 G-variant was revealed to be correlated with tumor cell invasive growth and the risk of bladder cancer (68, 69). Mao et al. found that OCT3/4 increased tumor immune escape via upregulation of TET1 and NRF2 expression, leading to enhancement of MDM2 expression, which contributed to acceleration of tumor immune evasion in bladder cancer (70). Small-molecule MDM2 inhibitors have been detected in clinical trials for improving the efficacy of cancer treatment (71, 72). MDM2 inhibitor APG-115 was reported to enhance the efficacy of PD-1 blockade via increasing anticancer immunity in the tumor microenvironment (73). One MDM2 inhibitor, AMG-232, sensitized tumor cells to T-cell-induced killing in tumors with high expression of MDM2 (74). The MDM2 ligand Nutlin-3 modulated the expression of PD-L1 and CD276 (75). Nutlin-3 induced the expression of PD-L1, while MDM2 did not bind PD-L1 (75). Suppression of MDM2 by HDM201 inhibitor facilitated anticancer responses via interaction with the stromal and immune microenvironment in tumor cells with p53 wild-type (76). MDM2 gene amplification could be a useful biomarker for prediction of a better response for targeted therapies in PD-L1 positive or negative urothelial bladder cancer (77).
TRIM38
TRIM38 functions as a SUMO ligase or an E3 ubiquitin ligase and targets several cellular signaling components (78). Glucose transporter type 1 (GLUT1) was upregulated in bladder cancer and correlated with poor survival rate and poor prognosis in patients with bladder cancer (79, 80). Moreover, GLUT1 was identified as an independent biomarker for prognosis in bladder cancer patients after radical cystectomy treatment (81). GLUT1 was also taken part in cisplatin resistance in bladder cancer, which can be regulated by miR-218 (82). According to TCGA bladder cancer database, TRIM38 expression was low in bladder cancer patients. Lower expression of TRIM38 was linked to shorter survival rate and worse prognosis in patients with bladder cancer (83). TRIM38 was further found to regulate proliferation, stemness and invasion of bladder cancer cells. Strikingly, TRIM38 had an interaction with GLUT1 and enhanced the ubiquitination and degradation of GLUT1 in bladder cancer cells. Accordingly, BAY-876, an inhibitor of GLUT1, inhibited proliferation and tumor growth in bladder cancer cells and mouse models (83).
Other deubiquitinases and E3 ubiquitin ligases
Accumulating evidence has shown that many E3 ubiquitin ligases are involved in bladder tumorigenesis. For instance, the E3 ubiquitin ligase cIAP2 (cellular inhibitor of apoptosis protein 2) was elevated after inhibition of histone deacetylase (HDAC) in bladder cancer. MRE11, which regulates DNA repair pathways and double-strand breaks, was also inhibited by HDAC inhibitors (84). The cIAP2 was found to bind with MRE11 and governed radio-sensitization after HDAC inhibitor treatment. cIAP2 modulated the ubiquitination of MRE11 and caused the downregulation of MRE11 in bladder cancer cells (84). Therefore, cIAP2 might be a promising target for improving chemoradiation strategy in bladder cancer. Suppression of GRIM19 expression impaired ubiquitination-mediated degradation of Bcl-xL in bladder cancer cells, conferring to promotion of cisplatin chemoresistance (85). Overexpression of GRIM19 potentiated cisplatin sensitivity and reduced the invasion and proliferation of bladder cancer cells, which was due to attenuation of Bcl-xL polyubiquitination and degradation (85).
Yes-associated protein (YAP) is one of key effectors in the Hippo tumor suppressor pathway, which regulates organ size and tissue growth and tumorigenesis (86, 87). Luo et al. reported that MINDY1, a DUB enzyme, interacted with YAP and acted as a deubiquitylase of YAP to stabilize YAP protein levels in bladder cancer (88). Consistently, silencing of MINDY1 reduced proliferation of bladder cancer cells. Overexpression of YAP abrogated the MINDY1 depletion-induced inhibition of cell proliferation in bladder cancer cells (88). Connective tissue growth factor (CTGF) controls differentiation, adhesion and proliferation, and involves in Hippo pathway, NF-κB and p53 pathways, leading to regulation of cancer, inflammation and fibrosis (89). Cysteine-rich protein 61 (CYR61) was reported to involve in the development of melanoma (90), glioma (91) and esophageal squamous cell carcinoma (92). Exosomal miR-217 mimic promoted migration and proliferation in 5637 and T24 cells via upregulation of YAP and its targets, such as CTGF, CYR61 and ANKRD1 (93). Downregulation of MINDY1 disrupted the YAP stabilization and inhibited the expression of YAP downstream genes, such as CTGF, CYR61 and ANKRD1 in bladder cancer (88). MINDY1 could be a possible biomarker and therapeutic target for bladder cancer (88). RNF126 (ring finger protein 126), acting as a E3 ubiquitin ligase, has been reported to be overexpressed in numerous cancer types and correlated with tumorigenesis (94). RNF126 expression was elevated in bladder cancer tissues via a TCGA database analysis. Depletion of RNF126 remarkably impaired proliferation and metastasis of bladder cancer cells via modulation of the EGFR/PI3K/AKT pathway. RNF126 silencing reduced EGFR expression and AKT phosphorylation, slightly inhibited PI3K expression, and remarkably increased the PTEN protein levels in UMUC3 and T24 cells. The mRNA levels of AKT and EGFR were reduced after RNF126 downregulation, but PTEN mRNA levels did not change in RNF126-silencing cells. Notably, PTEN was identified as a new substrate of RNF126 (95). RNF126 bound to PTEN and led to polyubiquitination and degradation of PTEN. Inhibition of RNF126 oncoprotein could be a novel approach for bladder cancer therapy (95). It has been known that c-Cbl is an E3 ubiquitin ligase that targets its substrates for degradation (96). C-Cbl was reported to target the EGFR for ubiquitination and degradation (97). Another study revealed that USP8 can regulate SOX2 ubiquitination and degradation in bladder cancer (98).
Deubiquitinases and E3 ubiquitin ligases regulate immunotherapy
The E3 ubiquitin ligases have been approved as important factors to govern the tumor microenvironment and affect immunotherapy in human cancers (99). Evidence has dissected that the E3 ubiquitin ligases control PD-1/PD-L1 protein levels and enhance tumor immunotherapy (100). For example, FBXO38, FBXW7 and C-Cbl target PD-1, whereas SPOP and FBXO22 target PD-L1. In addition, USP7, USP8 and USP22 target PD-L1 to maintain the PD-L1 protein levels (100, 101).
RNF144A regulates PD-L1
RNF144A is an E3 ubiquitin ligase for the degradation of DNA-PKcs (DNA-dependent protein kinase catalytic subunit), leading to promotion of apoptosis during DNA damage (102, 103). RNF144A governed PARP inhibitor sensitivity via targeting PARP1 in ubiquitin-dependent manner in breast cancer cells (104). In addition, RNF144A expression was decreased due to promoter hypermethylation in breast cancer cells (105). Moreover, RNF144A targeted the stability of HSPA2 via ubiquitin-dependent regulation in breast cancer (106). Furthermore, RNF144A degraded YY1 and inhibited the expression of GMFG as well as suppressed oncogenesis in breast cancer (107). RNF144A maintained the activation of EGFR signaling pathway to enhance EGF-involved cell proliferation (108). RNF144A controlled the stability of LIN28B via the uniquitin-proteasome manner and inhibited stem cell properties in ovarian cancer cells (109).
In bladder cancer cells, depletion of RNF144A elevated the stabilization of PD-L1 protein and enhanced carcinogen-mediated bladder oncogenesis (110). Mice with RNF144A deficiency were more prone to initiation of bladder cancer after carcinogen exposure. RNF144A knockout mice displayed the higher expression of PD-L1. RNF144A can bind with PD-L1 and enhanced ubiquitination and disruption of PD-L1 in the intracellular vesicles and plasma membrane (110). RNF144A depletion in mice caused a decrease of tumor infiltration CD8+ T-cells in the carcinogen-induced bladder cancer. Moreover, RNF144A depletion stimulated cellular differentiation, showing that a luminal subtype marker GATA3 was increased in RNF144A knockout tumors (110). This phenotype could be due to that RNF144A maintained EGFR expression. Hence, depletion of RNF144A increased the expression of PD-L1, DNA-PKcs and BMI1, resulting in the carcinogen-mediated the development of bladder cancer (110).
NEDD4 regulates PD-L1
An E3 ubiquitin ligase NEDD4 (also known as NEDD4-1) belongs to NEDD4 family, which has shown a critical function in carcinogenesis and progression (111, 112). NEDD4 performs its biological functions via targeting numerous substrates for ubiquitination and degradation (113, 114). NEDD4 has been revealed to regulate many functions, including growth, cell cycle, proliferation, differentiation, invasion, motility, apoptosis, necrosis, autophagy and metastasis (115). NEDD4 has been identified to take part in bladder cancer initiation and development. Inhibition of LAPTM5 blocked cell viability and growth and caused cell cycle arrest at G0/G1 phase via inhibition of p38 and ERK1/2 activation in bladder cancer (116). Depletion of NEDD4 suppressed the transportation of LAPTM5 from Golgi to lysosome, which could affect bladder tumorigenesis (116). Suppression of NEDD4 displayed antitumor activity in bladder cancer cells (117). Mao et al. found that NEDD4 can bind to KLF8 (Kruppel-like factor 8) and target the miR-132 and NRF2 (nuclear factor E2-related factor 2) axis in bladder cancer, contributing to acceleration of tumor growth, recurrence and lung metastasis (118). NEDD4 depletion reduced K63-linked polyubiquitination of KLF8 and inhibited the stability and transcriptional ability of KLF8 (118). NEDD4 promoted the interaction between KLF-8 and miR-132 promoter region, resulting in suppression of miR-132. Moreover, miR-132 inhibited the expression of NRF2 in bladder cancer cells, leading to repression of cell migration and viability (118).
Fibroblast growth factor receptor 3 (FGFR3) has been known to play a key role in bladder cancer development. FGFR3 rearrangements and missense mutations were reported in bladder cancer (119). One study showed that suppression of FGFR3 increased PD-L1 protein levels in FGFR3-expressing bladder cancer due to influencing its ubiquitination, leading to suppression of the anticancer activity of CD8+ T cells. FGFR3 expression was negatively associated with PD-L1 expression levels in bladder cancer tissues. FGFR3 activation can promote NEDD4 phosphorylation. NEDD4 catalyzed K48-linked polyubiquitination of PD-L1 via their interactions. CD8+ T-cell infiltration and anticancer ability were largely impaired because of upregulation of PD-L1 in bladder tumor cells in mice with NEDD4 knockout bladder cancer. Targeting FGFR3 and PD-L1 increased CD8+ T-cell-induced anticancer efficacy and exhibited effective tumor suppression in bladder cancer. This work provided a molecular clue among NEDD4, PD-L1 and FGFR3, suggesting that targeted therapy in combination with immune therapy could be much better for the treatment of bladder cancer. Therefore, NEDD4 targets PD-L1 for ubiquitination and destruction in FGFR3-overexpressing bladder cancer, indicating that NEDD4 is associated with immune surveillance via regulation of PD-L1 in bladder cancer (120). One group showed that a natural compound lycorine downregulated the expression of NEDD4 in bladder cancer, leading to suppression of cell growth and invasiveness (121). Hence, natural compounds targeting NEDD4 could be useful to improve immunotherapy in bladder cancer.
USP7 regulates PD-L1 expression
USP7 (ubiquitin-specific protease 7), also named as HAUSP (herpesvirus-associated protease), has been discovered to be associated with oncogenesis in some cancer types, including bladder cancer (122–125). USP7 has been revealed to control the anti-tumor immune responses. Inhibition of USP7 by its inhibitors impedes the activity of Treg cells, enhances polarization of tumor-related macrophages in tumor cells (126). It has been reported that USP7 modulated the expression levels of CCDC6 in bladder cancer. One USP7 inhibitor, P5091, regulated CCDC6 degradation and enhanced cell sensitivity to PARP inhibitors. Combined therapy with DNA damage inducer RRx-001 and P5091 promoted the tumor cell sensitivity to PARP inhibitors (127).
DNA methylation is regulated by DNMTs (DNA methyltransferases). SB216763, an inhibitor of GSK3 (glycogen synthase kinase-3), increased cell proliferation and upregulated the expression of pGSK3β, β-catenin and DNMT1 (128). The expression of USP7, DNMT1, UHRF1 and β-catenin was inhibited after re-expression of WIF-1 and treatment with DNMT1 inhibitor DAC (128). One study revealed that PD-L1 expression was positively associated with USP7 levels in gastric cancer patients. USP7 directly bound to PD-L1 and stabilize it (129). Abrogation of USP7 impaired the interaction between PD-1 and PD-L1, leading to sensitization of cancer cells to T cell killing in cancer cells and in mice. In addition, inhibition of USP7 by its inhibitor reduced cell proliferation due to p53 stabilization in gastric cancer cells (129). Hence, USP7 suppression by its inhibitors not only blocked gastric tumor cell proliferation but also inhibit the expression of PD-L1 to improve anti-cancer immune response in gastric cancer (129). It is required to explore whether USP7 inhibitors could enhance the immune response of bladder cancer. USP7 inhibitors have been developed to perform anticancer ability in various cancer types (130). It is necessary to determine whether these USP7 inhibitors can improve immunotherapy in bladder cancer.
Other E3 ubiquitin ligases regulate immunotherapy
One group used TCGA and GEO database to analyze ubiquitination-related molecular subtypes for bladder cancer (131). This group found a total of four ubiquitination-related molecular subtypes of bladder cancer. These four subgroups had various tumor microenvironment, prognosis, clinical characteristics and PD-L1 expression level. In addition, six ubiquitination-related genes (URGs), including HLA-A, UBE2D1, UBE2T, USP5, TMEM128 and UBE2N, could be useful for prognostic markers (131).
Compounds regulate E3 ligases in bladder cancer
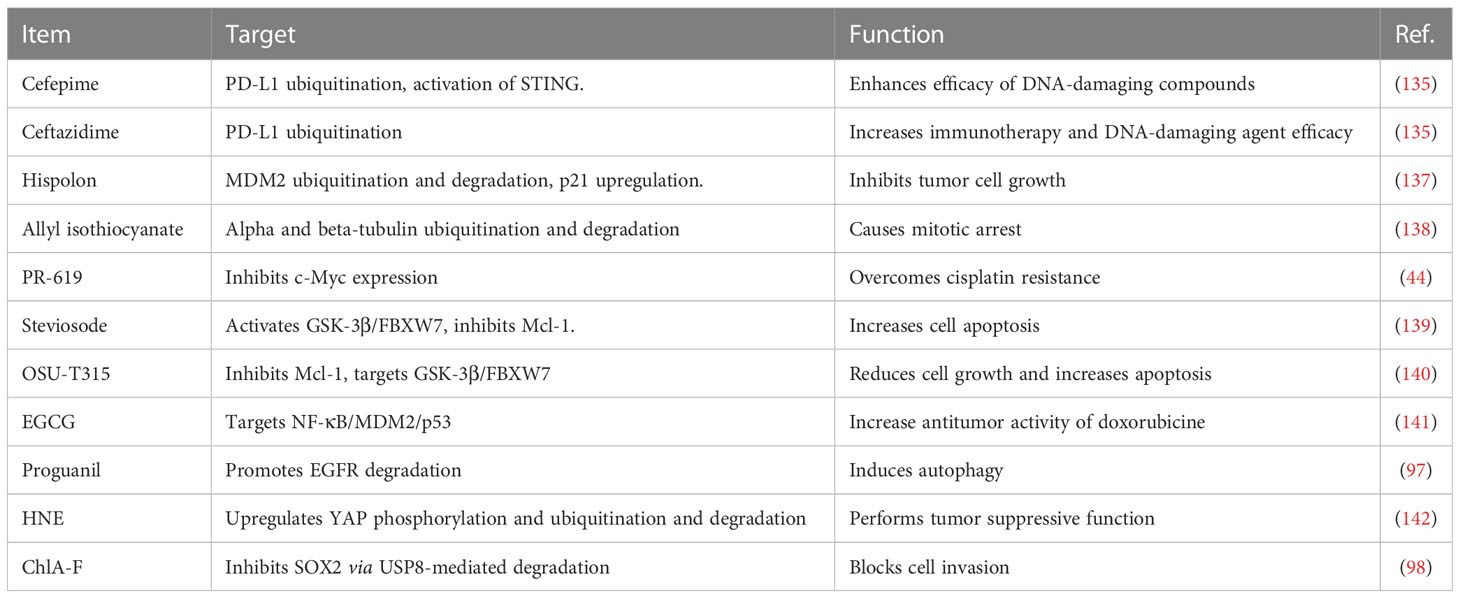
In recent years, some compounds have been uncovered to regulate the expression of E3 ubiquitin ligases in human malignancies, including bladder cancer (132–134). β-lactam cephalosporin antibiotic cefepime has been uncovered to deplete PD-L1 and promote tumor DNA damage and increase sensitivity of DNA-damaging compounds in multiple tumor cell lines, such as bladder cancer, melanoma, GBM (glioblastoma multiforme) and ovarian cancer (135). Cefepime inhibited tumor PD-L1 via regulation of its ubiquitination, enhanced efficacy of DNA-damaging compounds in mice, stimulated immunogenic tumor STING pathway. Ceftazidime exhibited the similar performance as cefepime in regulation of PD-L1 and DNA-damaging agent therapeutic efficacy. Taken together, cefepime and ceftazidime could improve immunotherapy and DNA-damaging agent efficacy in bladder cancer (135). Hispolon from Phellinus linteus is a natural polyphenol and conducted a function as a cancer killer via targeting several signaling pathways (136). Hisplon inhibited tumor cell growth via upregulation of p21 in bladder cancer cells (137). Hispolon promoted the ubiquitination and degradation of MDM2 in bladder cancer cells. ERK1/2 was activated and recruited to MDM2 and led to MDM2 ubiquitination. Inhibition of ERK1/2 by U0126 blocked hispolon-mediated caspase-7 cleavage. Hence, hispolon downregulated MDM2 via degradation in bladder cancer (137).
Allyl isothiocyanate was often obtained from cruciferous vegetables and caused mitotic arrest via upregulation of ubiquitination and degradation of alpha and beta-tubulin in bladder cancer cells (138). PR-619 was an inhibitor of deubiquitylating enzymes and overcame cisplatin resistance via the inhibition of c-Myc in bladder urothelial carcinoma cells (44). Stevioside was identified by high-throughput screening as a useful compound to increase cell apoptosis via activation of GSK-3β and induction of FBXW7, contributing to downregulation of MCL-1 in bladder cancer (139). Similarly, OSU-T315, an inhibitor of integrin-linked kinase, was observed to inhibit Mcl-1 expression levels via targeting the GSK-3β/FBXW7 axis in bladder cancer cells (140). Green tea polyphenol EGCG plays a tumor suppressive role in bladder cancer via inactivation of NF-kappa B. Moreover, EGCG promoted the anticancer activity of doxorubicine via modulation of NF-κB/MDM2/p53 pathway in bladder cancer (141). Proguanil, which is often used as an anti-malarial drug, inhibited the cell growth by promotion of EGFR degradation and induction of autophagy in bladder cancer (97). Proguanil enhanced the interaction between EGFR and Caveolin-1, leading to endocytosis and recruiting c-Cbl to elevate EGFR degradation via the lysosomal pathway (97). 4-hydroxynonenal (HNE), a pro-oxidant compound, conducted tumor suppressive function via altering several signaling pathways. HNE upregulated YAP phosphorylation and ubiquitination, caused promotion of YAP proteasomal degradation in bladder cancer cells (142). One compound ChlA-F blocked cell invasion via inhibition of SOX2 protein by USP8-mediated SOX2 degradation in bladder cancer (98). Therefore, compounds can regulate E3 ubiquitin ligases to enhance the ubiquitination and degradation of specific targets, which lead to antitumor activity in bladder cancer (Table 1).
Noncoding RNAs target E3 ligases
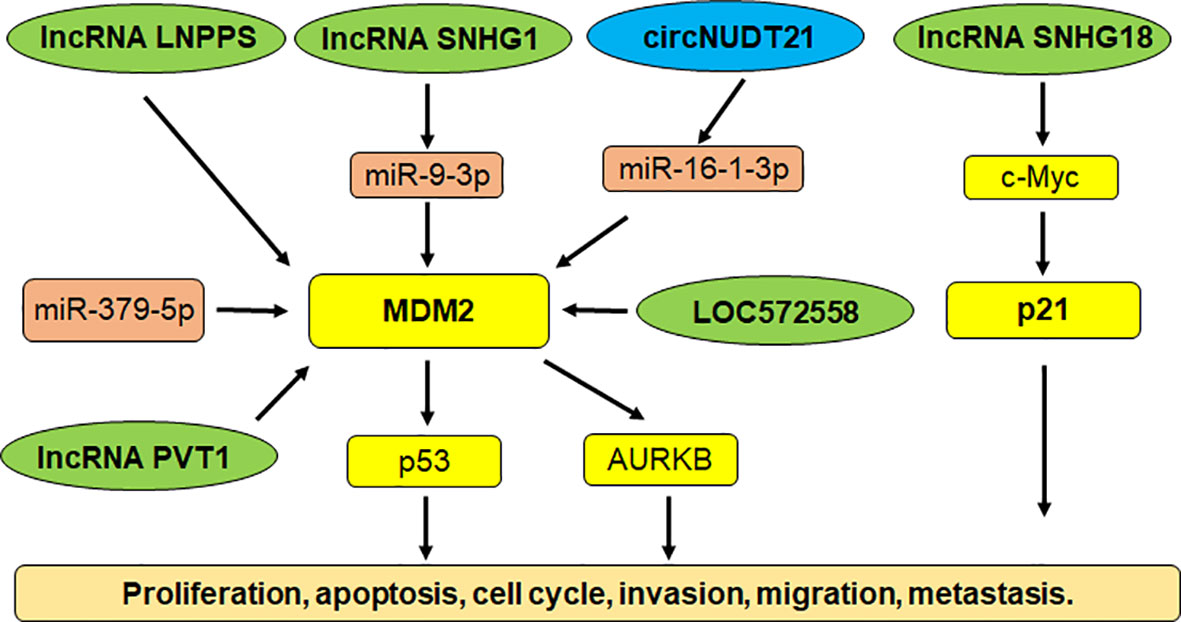
Multiple studies have shown that noncoding RNAs govern carcinogenesis in bladder cancer (143–146). Noncoding RNAs are transcribed from DNA, but not translated into proteins, including microRNAs (miRNAs), lncRNAs (log noncoding RNAs), siRNAs (small interfering RNAs), snRNAs (small nuclear RNAs) and piRNAs (147–149). Noncoding RNAs target E3 ubiquitin ligases to control bladder cancer initiation and progression. For example, miR-143 inhibited the expression of MDM2 and performed a tumor suppressive function via inhibition of cell growth and migration in bladder cancer (150). LncRNA SNHG1 sponged miR-9-3p expression and upregulated the expression of MDM2 in bladder cancer cells. MDM2 targeted PPARγ for ubiquitination and degradation, leading to facilitating the development of bladder cancer (151). LncRNA LNPPS displayed a tumor suppressive function via modulation of MDM2/p53 degradation in bladder cancer (152). LncRNA SNHG18 was downregulated in tumor specimens of bladder cancer patients. The bladder cancer patients with high expression of SNHG18 had a better survival rate. Upregulation of SNHG18 reduced proliferation of bladder cancer cells and decreased tumor sizes in mice (153). SNHG18 impaired the expression of c-Myc via targeting its ubiquitination and degradation, resulting in p21 upregulation in bladder cancer (153).
LncRNA PVT1 promoted the expression of MDM2 and accelerated the p53 ubiquitination and degradation, leading to promoting cell invasion and cell resistance to doxorubicin (154). AURKB (Aurora kinase B) was increased after MDM2 upregulation induced by lncRNA PVT1 in bladder cancer cells. AURKB further promoted the p53 ubiquitination that was induced by MDM2 (154). LncRNA LOC572558 overexpression was downregulated in tumor tissues of bladder cancer patients. In T24 and 5637 bladder tumor cells, upregulation of LOC572558 suppressed cell growth and invasion, induced apoptosis and caused cell cycle arrest, which was correlated with p53 phosphorylation, MDM2, AKT dephosphorylation (155). Chen et al. reported that a circRNA circNUDT21 altered the miR-16-1-3p/MDM2/p53 axis and accelerated tumor progression in bladder cancer (156). Hence, noncoding RNAs are pivotal to regulate E3 ubiquitin ligases in bladder tumorigenesis (Figure 1).
Conclusions and future perspectives
In conclusion, E3 ubiquitin ligases are critical in bladder cancer initiation and development via targeting specific substrates. E3 ubiquitin ligases alter tumor immunotherapy and drug resistance in bladder cancer (Figure 2). Targeting E3 ubiquitin ligases could be an effective strategy for bladder cancer therapy. It is necessary to mention several points regarding the roles of E3 ubiquitin ligases in bladder cancer. First, besides ubiquitination, there are many other types of PTMs to involve in bladder tumorigenesis. For example, activation of autophagy altered acetylation profile relevant for mechanotransduction in bladder tumor cells (157). PD-L1 methylation was found to be an independent biomarker for patient survival in bladder cancer (158). Histone demethylase JMJD1A promoted glycolysis via coactivation of HIF1α and led to promotion of urinary bladder cancer progression (159). SIRT1 (silent information regulator sirtulin 1), a NAD+ dependent deacetylase, elevated the expression of GLUT1 and stimulated tumor progression in bladder cancer via modulation of glucose uptake (160).
Second, in addition to E3 ubiquitin ligases, E2 enzyme has also been involved in bladder carcinogenesis. Ubiquitin-conjugating enzyme E2S (UBE2S) is a type of E2 enzyme in the ubiquitin system, which has displayed several activities in carcinogenesis (161). UBE2S has been suggested to promote the ovarian cancer development via targeting PI3K/AKT/mTOR pathway and modulating cell apoptosis and cell cycle (162). UBE2S reduced cell chemosensitivity via regulation of PTEN-AKT pathway in hepatocellular carcinoma (163). UBE2S expression was increased in urinary bladder cancer cells. Knockdown of UBE2S led to reduction of proliferation and induction of cell apoptosis, while upregulation of UBE2S resulted in an inverse phenotype in bladder cancer cells (164). Moreover, UBE2S performed the oncogenic functions via modulation of the mTORC1 pathway in bladder cancer cells. UBE2S targeted tuberous sclerosis 1 (TSC1) for ubiquitous degradation (164). Collectively, UBE2S promoted bladder cancer progression via degradation of TSC1 and activation of mTOR signaling pathway.
Third, noncoding RNAs have been identified as potential biomarkers for bladder cancer prognosis (165, 166). Besides lncRNAs, miRNAs and circRNAs, one study showed that PIWI-interacting RNAs (piRNAs) and snRNAs are important in bladder carcinogenesis (167, 168). In this work, it has been shown that 106 piRNAs were increased and 91 piRNAs were decreased in bladder tumor specimens. Upregulation of piRABC reduced proliferation, colony formation, but enhanced cell apoptosis in bladder cancer cells. Moreover, piRABC increased the expression of TNFSF4 protein in bladder cancer cells (167). Fourth, several F-box proteins have been described to target PD-1/PD-L1 in cancers; however, whether other F-box proteins can regulate immunotherapy is unclear. For example, FBXO45 has shown an essential role in tumorigenesis and malignant progression (169–171). FBXO22 targeted PD-L1 for degradation and sensitized tumor cells to DNA damage (172). FBXO1, FBXO20, FBXO22, FBXO28, FBXO32 and FBXO45 have been found to be associated with immune infiltration in pancreatic cancer (173). Hence, it is required to explore whether these F-box proteins are involved in immunotherapy in bladder cancer.
Fifth, it has been validated that PROTACs are novel tools for the enhancement of immunotherapy in human cancers (174). PROTACs have been designed to degrade a protein of interest (POI), resulting in a reduction of the expression of the POI (175, 176). One study has shown that one BET (bromodomain and extraterminal domain) inhibitor mivebresib synergized with a Bcl-xL PROTAC degrader PZ703b increased cell apoptosis through the mitochondrial pathway in bladder cancer (177). Another study showed that BRD4 PROTAC degrader QCA570 increased the degradation of BRD4 protein, leading to induction of cell apoptosis and cell cycle arrest, which caused antiproliferation ability in bladder cancer (178). All in a word, E3 ubiquitin ligases are essential for the initiation and progression of bladder cancer. Regulation of E3 ubiquitin ligases might be a potential therapeutic strategy for bladder cancer treatment.
Data availability statement
The data in this study are available from the corresponding author on reasonable request.
Author contributions
XMW and YZ wrote the manuscript. YW and HC made the tables and figures. XJW edited and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the Young Scientists Fund of the National Natural Science Foundation of China (grant No. 81700198), the Science and Technology Development Project of Jilin Province (grant No. 20190701064GH), Natural Science Foundation of Jilin Province of China (NO. 20210101328JC and YDZJ202201ZYTS243).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ANKRD1, ankyrin repeat domain 1; BET, bromodomain and extraterminal domain; CSN5, COP9 signalosome subunit 5; CTGF, connective tissue growth factor; CYR61, cysteine-rich angiogenic inducer 61; EGFR, epidermal growth factor receptor; ERK1/2, extracellular signal-regulated kinase 1/2; GRIM19, gene associated with retinoid-interferon-induced mortality-19; LAPTM5, lysosomal-associated multispanning membrane protein 5; mTORC1, mammalian target of rapamycin complex 1; MIBC, muscle invasive bladder cancer; MRE11, meiotic recombination 11 homolog; NSCLC, non-small cell lung cancer; NSR-SCCUB, non-schistosomiasis related-squamous cell carcinoma of urinary bladder; NEDD4, neuronally expressed developmentally downregulated 4; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand-1; PI3K, phosphoinositide 3-kinase; POI, protein of interest; PROTACs, proteolysis targeting chimeras; PTMs, post-translational modifications; qRT-PCR, quantitative real-time polymerase chain reaction; SIRT1, silent information regulator sirtulin 1; SOX2, Sex-determining region Y-box 2; SREBP1a, sterol regulatory element-binding protein 1a; TCGA, the Cancer Genome Atlas; TCR, T cell receptor; TILs, tumor-infiltration T cells; USP7, ubiquitin-specific processing protease 7.
References
1. van Hoogstraten LMC, Vrieling A, van der Heijden AG, Kogevinas M, Richters A, Kiemeney LA. Global trends in the epidemiology of bladder cancer: challenges for public health and clinical practice. Nat Rev Clin Oncol (2023) 20(5):287–304. doi: 10.1038/s41571-023-00744-3
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
3. Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA (2020) 324(19):1980–91. doi: 10.1001/jama.2020.17598
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
5. Yang Y, Wang C, Li Z, Lu Q, Li Y. Precise diagnosis and treatment of non-muscle invasive bladder cancer - a clinical perspective. Front Oncol (2023) 13:1042552. doi: 10.3389/fonc.2023.1042552
6. Shi Y, Mathis BJ, He Y, Yang X. The current progress and future options of multiple therapy and potential biomarkers for muscle-invasive bladder cancer. Biomedicines (2023) 11(2). doi: 10.3390/biomedicines11020539
7. Kombathula SH, Hoskin P. Pushing the envelope: the role of radiation therapy in non-muscle-Invasive bladder cancer. Semin Radiat Oncol (2023) 33(1):21–5. doi: 10.1016/j.semradonc.2022.10.003
8. Semeniuk-Wojtas A, Poddebniak-Strama K, Modzelewska M, Baryla M, Dziag-Dudek E, Syrylo T, et al. Tumour microenvironment as a predictive factor for immunotherapy in non-muscle-invasive bladder cancer. Cancer Immunol Immunother (2023). doi: 10.1007/s00262-023-03376-9
9. Saidian A, Dolendo I, Sharabi A, Stewart TF, Rose B, McKay RR, et al. The current and future promises of combination radiation and immunotherapy for genitourinary cancers. Cancers (Basel) (2022) 15(1). doi: 10.3390/cancers15010127
10. Crispen PL, Kusmartsev S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol Immunother (2020) 69(1):3–14. doi: 10.1007/s00262-019-02443-4
11. Golus M, Bugajski P, Chorbinska J, Krajewski W, Leminski A, Saczko J, et al. STAT3 and its pathways' dysregulation-underestimated role in urological tumors. Cells (2022) 11(19). doi: 10.3390/cells11193024
12. Huan J, Grivas P, Birch J, Hansel DE. Emerging roles for mammalian target of rapamycin (mTOR) complexes in bladder cancer progression and therapy. Cancers (Basel) (2022) 14(6). doi: 10.3390/cancers14061555
13. Wu G, Weng W, Xia P, Yan S, Zhong C, Xie L, et al. Wnt signalling pathway in bladder cancer. Cell Signal (2021) 79:109886. doi: 10.1016/j.cellsig.2020.109886
14. Ashrafizadeh M, Zarrabi A, Samarghandian S, Najafi M. PTEN: what we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur J Pharmacol (2020) 881:173226. doi: 10.1016/j.ejphar.2020.173226
15. Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol (2018) 1655:335–50. doi: 10.1007/978-1-4939-7234-0_23
16. Zhang G, Zhang Y, Dong D, Wang F, Ma X, Guan F, et al. MCT1 regulates aggressive and metabolic phenotypes in bladder cancer. J Cancer (2018) 9(14):2492–501. doi: 10.7150/jca.25257
17. Silva A, Cerqueira MC, Rosa B, Sobral C, Pinto-Ribeiro F, Costa MF, et al. Prognostic value of monocarboxylate transporter 1 overexpression in cancer: a systematic review. Int J Mol Sci (2023) 24(6). doi: 10.3390/ijms24065141
18. Choi JW, Kim Y, Lee JH, Kim YS. Prognostic significance of lactate/proton symporters MCT1, MCT4, and their chaperone CD147 expressions in urothelial carcinoma of the bladder. Urology (2014) 84(1):245.e9–15. doi: 10.1016/j.urology.2014.03.031
19. Afonso J, Santos LL, Miranda-Goncalves V, Morais A, Amaro T, Longatto-Filho A, et al. CD147 and MCT1-potential partners in bladder cancer aggressiveness and cisplatin resistance. Mol Carcinog (2015) 54(11):1451–66. doi: 10.1002/mc.22222
20. Chen T, Liu J, Li S, Wang P, Shang G. The role of protein arginine n-methyltransferases in inflammation. Semin Cell Dev Biol (2022). doi: 10.1016/j.semcdb.2022.08.005
21. Oo HZ, Seiler R, Black PC, Daugaard M. Post-translational modifications in bladder cancer: expanding the tumor target repertoire. Urol Oncol (2020) 38(12):858–66. doi: 10.1016/j.urolonc.2018.09.001
22. Wang W, Liu W, Chen Q, Yuan Y, Wang P. Targeting CSC-related transcription factors by E3 ubiquitin ligases for cancer therapy. Semin Cancer Biol (2022) 87:84–97. doi: 10.1016/j.semcancer.2022.11.002
23. Lee JM, Hammaren HM, Savitski MM, Baek SH. Control of protein stability by post-translational modifications. Nat Commun (2023) 14(1):201. doi: 10.1038/s41467-023-35795-8
24. Yang J, Song C, Zhan X. The role of protein acetylation in carcinogenesis and targeted drug discovery. Front Endocrinol (Lausanne) (2022) 13:972312. doi: 10.3389/fendo.2022.972312
25. Lopez-Banuelos L, Vega L. Inhibition of acetylation, is it enough to fight cancer? Crit Rev Oncol Hematol (2022) 176:103752. doi: 10.1016/j.critrevonc.2022.103752
26. Kim I, Park JW. Hypoxia-driven epigenetic regulation in cancer progression: a focus on histone methylation and its modifying enzymes. Cancer Lett (2020) 489:41–9. doi: 10.1016/j.canlet.2020.05.025
27. Zhou B, Hao Q, Liang Y, Kong E. Protein palmitoylation in cancer: molecular functions and therapeutic potential. Mol Oncol (2023) 17(1):3–26. doi: 10.1002/1878-0261.13308
28. Wang P, Dai X, Jiang W, Li Y, Wei W. RBR E3 ubiquitin ligases in tumorigenesis. Semin Cancer Biol (2020) 67(Pt 2):131–44. doi: 10.1016/j.semcancer.2020.05.002
29. Heo AJ, Ji CH, Kwon YT. The Cys/N-degron pathway in the ubiquitin-proteasome system and autophagy. Trends Cell Biol (2023) 33(3):247–59. doi: 10.1016/j.tcb.2022.07.005
30. Squair DR, Virdee S. A new dawn beyond lysine ubiquitination. Nat Chem Biol (2022) 18(8):802–11. doi: 10.1038/s41589-022-01088-2
31. Sun T, Liu Z, Yang Q. The role of ubiquitination and deubiquitination in cancer metabolism. Mol Cancer (2020) 19(1):146. doi: 10.1186/s12943-020-01262-x
32. Li H, Yang F, Chang K, Yu X, Guan F, Li X. The synergistic function of long and short forms of beta4GalT1 in p53-mediated drug resistance in bladder cancer cells. Biochim Biophys Acta Mol Cell Res (2023) 1870(2):119409. doi: 10.1016/j.bbamcr.2022.119409
33. Jia M, Su B, Mo L, Qiu W, Ying J, Lin P, et al. Circadian clock protein CRY1 prevents paclitaxel−induced senescence of bladder cancer cells by promoting p53 degradation. Oncol Rep (2021) 45(3):1033–43. doi: 10.3892/or.2020.7914
34. Chen C, Matesic LE. The Nedd4-like family of E3 ubiquitin ligases and cancer. Cancer Metastasis Rev (2007) 26(3–4):587–604. doi: 10.1007/s10555-007-9091-x
35. Kim J, Alavi Naini F, Sun Y, Ma L. Ubiquitin-specific peptidase 2a (USP2a) deubiquitinates and stabilizes beta-catenin. Am J Cancer Res (2018) 8(9):1823–36.
36. Zhao Y, Wang X, Wang Q, Deng Y, Li K, Zhang M, et al. USP2a supports metastasis by tuning TGF-beta signaling. Cell Rep (2018) 22(9):2442–54. doi: 10.1016/j.celrep.2018.02.007
37. Benassi B, Flavin R, Marchionni L, Zanata S, Pan Y, Chowdhury D, et al. MYC is activated by USP2a-mediated modulation of microRNAs in prostate cancer. Cancer Discov (2012) 2(3):236–47. doi: 10.1158/2159-8290.CD-11-0219
38. Jeong P, Ha YS, Yun SJ, Yoon HY, Freeman MR, Kim J, et al. Assess the expression of ubiquitin specific protease USP2a for bladder cancer diagnosis. BMC Urol (2015) 15:80. doi: 10.1186/s12894-015-0074-x
39. Kim J, Kim WJ, Liu Z, Loda M, Freeman MR. The ubiquitin-specific protease USP2a enhances tumor progression by targeting cyclin A1 in bladder cancer. Cell Cycle (2012) 11(6):1123–30. doi: 10.4161/cc.11.6.19550
40. Kim J, Keay SK, You S, Loda M, Freeman MR. A synthetic form of frizzled 8-associated antiproliferative factor enhances p53 stability through USP2a and MDM2. PLoS One (2012) 7(12):e50392. doi: 10.1371/journal.pone.0050392
41. An T, Lu Y, Yan X, Hou J. Insights into the properties, biological functions, and regulation of USP21. Front Pharmacol (2022) 13:944089. doi: 10.3389/fphar.2022.944089
42. Bonacci T, Emanuele MJ. Dissenting degradation: deubiquitinases in cell cycle and cancer. Semin Cancer Biol (2020) 67(Pt 2):145–58. doi: 10.1016/j.semcancer.2020.03.008
43. Riester M, Werner L, Bellmunt J, Selvarajah S, Guancial EA, Weir BA, et al. Integrative analysis of 1q23.3 copy-number gain in metastatic urothelial carcinoma. Clin Cancer Res (2014) 20(7):1873–83. doi: 10.1158/1078-0432.CCR-13-0759
44. Hsu FS, Lin WC, Kuo KL, Chiu YL, Hsu CH, Liao SM, et al. PR-619, a general inhibitor of deubiquitylating enzymes, diminishes cisplatin resistance in urothelial carcinoma cells through the suppression of c-myc: an in vitro and In Vivo study. Int J Mol Sci (2021) 22(21). doi: 10.3390/ijms222111706
45. Chen Y, Zhou B, Chen D. USP21 promotes cell proliferation and metastasis through suppressing EZH2 ubiquitination in bladder carcinoma. Onco Targets Ther (2017) 10:681–9. doi: 10.2147/OTT.S124795
46. Yin S, Chen Z, Chen D, Yan D. Strategies targeting PD-L1 expression and associated opportunities for cancer combination therapy. Theranostics (2023) 13(5):1520–44. doi: 10.7150/thno.80091
47. Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol (2021) 18(6):345–62. doi: 10.1038/s41571-021-00473-5
48. Yang S, Yan H, Wu Y, Shan B, Zhou D, Liu X, et al. Deubiquitination and stabilization of PD-L1 by USP21. Am J Transl Res (2021) 13(11):12763–74.
49. Feng T, Ling S, Xu C, Ying L, Su D, Xu X. Ubiquitin-specific peptidase 22 in cancer. Cancer Lett (2021) 514:30–7. doi: 10.1016/j.canlet.2021.05.004
50. Lv L, Xiao XY, Gu ZH, Zeng FQ, Huang LQ, Jiang GS. Silencing USP22 by asymmetric structure of interfering RNA inhibits proliferation and induces cell cycle arrest in bladder cancer cells. Mol Cell Biochem (2011) 346(1-2):11–21. doi: 10.1007/s11010-010-0585-4
51. Gennaro VJ, Stanek TJ, Peck AR, Sun Y, Wang F, Qie S, et al. Control of CCND1 ubiquitylation by the catalytic SAGA subunit USP22 is essential for cell cycle progression through G1 in cancer cells. Proc Natl Acad Sci U S A (2018) 115(40):E9298–307. doi: 10.1073/pnas.1807704115
52. Guo J, Zhao J, Fu W, Xu Q, Huang D. Immune evasion and drug resistance mediated by USP22 in cancer: novel targets and mechanisms. Front Immunol (2022) 13:918314. doi: 10.3389/fimmu.2022.918314
53. Wang Y, Sun Q, Mu N, Sun X, Wang Y, Fan S, et al. The deubiquitinase USP22 regulates PD-L1 degradation in human cancer cells. Cell Commun Signal (2020) 18(1):112. doi: 10.1186/s12964-020-00612-y
54. Wang L, Ye X, Liu Y, Wei W, Wang Z. Aberrant regulation of FBW7 in cancer. Oncotarget (2014) 5(8):2000–15. doi: 10.18632/oncotarget.1859
55. Yan L, Lin M, Pan S, Assaraf YG, Wang ZW, Zhu X. Emerging roles of f-box proteins in cancer drug resistance. Drug Resist Updat (2020) 49:100673. doi: 10.1016/j.drup.2019.100673
56. Wang Z, Liu P, Inuzuka H, Wei W. Roles of f-box proteins in cancer. Nat Rev Cancer (2014) 14(4):233–47. doi: 10.1038/nrc3700
57. Lin M, Xu Y, Gao Y, Pan C, Zhu X, Wang ZW. Regulation of f-box proteins by noncoding RNAs in human cancers. Cancer Lett (2019) 466:61–70. doi: 10.1016/j.canlet.2019.09.008
58. Al-Ezzi EM, Veitch ZW, Salah SH, van der Kwast TH, Stockley TL, Selvarajah S, et al. Genomic characterization of non-schistosomiasis-related squamous cell carcinoma of the urinary bladder: a retrospective exploratory study. PLoS One (2021) 16(12):e0259272. doi: 10.1371/journal.pone.0259272
59. Qiu F, Jin Y, Pu J, Huang Y, Hou J, Zhao X, et al. Aberrant FBXW7-mediated ubiquitination and degradation of ZMYND8 enhances tumor progression and stemness in bladder cancer. Exp Cell Res (2021) 407(2):112807. doi: 10.1016/j.yexcr.2021.112807
60. Zhu J, Li Y, Chen C, Ma J, Sun W, Tian Z, et al. NF-kappaB p65 overexpression promotes bladder cancer cell migration via FBW7-mediated degradation of RhoGDIalpha protein. Neoplasia (2017) 19(9):672–83. doi: 10.1016/j.neo.2017.06.002
61. Liu F, Liu X, Deng W, Yang X, Fu B. Overexpression of f-box and WD repeat domain containing 7 prevents tumor growth of bladder cancer cells through regulating SREBP1a. Transl Androl Urol (2022) 11(3):367–76. doi: 10.21037/tau-22-146
62. Zhao Q, Lin X, Wang G. Targeting SREBP-1-Mediated lipogenesis as potential strategies for cancer. Front Oncol (2022) 12:952371. doi: 10.3389/fonc.2022.952371
63. Klein AM, de Queiroz RM, Venkatesh D, Prives C. The roles and regulation of MDM2 and MDMX: it is not just about p53. Genes Dev (2021) 35(9-10):575–601. doi: 10.1101/gad.347872.120
64. Dobbelstein M, Levine AJ. Mdm2: open questions. Cancer Sci (2020) 111(7):2203–11. doi: 10.1111/cas.14433
65. Lianes P, Orlow I, Zhang ZF, Oliva MR, Sarkis AS, Reuter VE, et al. Altered patterns of MDM2 and TP53 expression in human bladder cancer. J Natl Cancer Inst (1994) 86(17):1325–30. doi: 10.1093/jnci/86.17.1325
66. Schmitz-Drager BJ, Kushima M, Goebell P, Jax TW, Gerharz CD, Bultel H, et al. p53 and MDM2 in the development and progression of bladder cancer. Eur Urol (1997) 32(4):487–93.
67. Shinohara A, Sakano S, Hinoda Y, Nishijima J, Kawai Y, Misumi T, et al. Association of TP53 and MDM2 polymorphisms with survival in bladder cancer patients treated with chemoradiotherapy. Cancer Sci (2009) 100(12):2376–82. doi: 10.1111/j.1349-7006.2009.01331.x
68. Hitzenbichler F, Stoehr CG, Rogenhofer M, Wieland WF, Ruemmele P, Hartmann A, et al. Mdm2 SNP309 G-variant is associated with invasive growth of human urinary bladder cancer. Pathobiology (2014) 81(2):53–9. doi: 10.1159/000355976
69. Xie L, Sun Y, Chen T, Tian D, Li Y, Zhang Y, et al. Association between MDM2 SNP309 T>G polymorphism and the risk of bladder cancer: new data in a Chinese population and an updated meta-analysis. Onco Targets Ther (2015) 8:3679–90. doi: 10.2147/OTT.S95353
70. Mao M, Yang L, Hu J, Liu B, Liu C, Zhang X, et al. OCT3/4 enhances tumor immune response by upregulating the TET1-dependent NRF2/MDM2 axis in bladder cancer. Genomics (2021) 113(4):2122–33. doi: 10.1016/j.ygeno.2021.04.033
71. Wang S, Chen FE. Small-molecule MDM2 inhibitors in clinical trials for cancer therapy. Eur J Med Chem (2022) 236:114334. doi: 10.1016/j.ejmech.2022.114334
72. Konopleva M, Martinelli G, Daver N, Papayannidis C, Wei A, Higgins B, et al. MDM2 inhibition: an important step forward in cancer therapy. Leukemia (2020) 34(11):2858–74. doi: 10.1038/s41375-020-0949-z
73. Fang DD, Tang Q, Kong Y, Wang Q, Gu J, Fang X, et al. MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment. J Immunother Cancer (2019) 7(1):327. doi: 10.1186/s40425-019-0750-6
74. Sahin I, Zhang S, Navaraj A, Zhou L, Dizon D, Safran H, et al. AMG-232 sensitizes high MDM2-expressing tumor cells to T-cell-mediated killing. Cell Death Discov (2020) 6:57. doi: 10.1038/s41420-020-0292-1
75. Li R, Zatloukalova P, Muller P, Gil-Mir M, Kote S, Wilkinson S, et al. The MDM2 ligand nutlin-3 differentially alters expression of the immune blockade receptors PD-L1 and CD276. Cell Mol Biol Lett (2020) 25:41. doi: 10.1186/s11658-020-00233-w
76. Wang HQ, Mulford IJ, Sharp F, Liang J, Kurtulus S, Trabucco G, et al. Inhibition of MDM2 promotes antitumor responses in p53 wild-type cancer cells through their interaction with the immune and stromal microenvironment. Cancer Res (2021) 81(11):3079–91. doi: 10.1158/0008-5472.CAN-20-0189
77. Brunelli M, Tafuri A, Cima L, Cerruto MA, Milella M, Zivi A, et al. MDM2 gene amplification as selection tool for innovative targeted approaches in PD-L1 positive or negative muscle-invasive urothelial bladder carcinoma. J Clin Pathol (2022) 75(1):39–44. doi: 10.1136/jclinpath-2020-207089
78. Hu MM, Shu HB. Multifaceted roles of TRIM38 in innate immune and inflammatory responses. Cell Mol Immunol (2017) 14(4):331–8. doi: 10.1038/cmi.2016.66
79. Palit V, Phillips RM, Puri R, Shah T, Bibby MC. Expression of HIF-1alpha and glut-1 in human bladder cancer. Oncol Rep (2005) 14(4):909–13. doi: 10.3892/or.14.4.909
80. Al-Maghrabi JA, Qureshi IA, Khabaz MN. Immunhistochemical expression of GLUT1 is associated with low grade and low stage of urinary bladder cancer. Int J Clin Exp Pathol (2019) 12(8):3049–57.
81. Bostrom PJ, Thoms J, Sykes J, Ahmed O, Evans A, van Rhijn BW, et al. Hypoxia marker GLUT-1 (Glucose transporter 1) is an independent prognostic factor for survival in bladder cancer patients treated with radical cystectomy. Bladder Cancer (2016) 2(1):101–9. doi: 10.3233/BLC-150033
82. Li P, Yang X, Cheng Y, Zhang X, Yang C, Deng X, et al. MicroRNA-218 increases the sensitivity of bladder cancer to cisplatin by targeting Glut1. Cell Physiol Biochem (2017) 41(3):921–32. doi: 10.1159/000460505
83. Wang X, He H, Rui W, Zhang N, Zhu Y, Xie X. TRIM38 triggers the uniquitination and degradation of glucose transporter type 1 (GLUT1) to restrict tumor progression in bladder cancer. J Transl Med (2021) 19(1):508. doi: 10.1186/s12967-021-03173-x
84. Nicholson J, Jevons SJ, Groselj B, Ellermann S, Konietzny R, Kerr M, et al. E3 ligase cIAP2 mediates downregulation of MRE11 and radiosensitization in response to HDAC inhibition in bladder cancer. Cancer Res (2017) 77(11):3027–39. doi: 10.1158/0008-5472.CAN-16-3232
85. Ni F, Yan CY, Zhou S, Hui PY, Du YH, Zheng L, et al. Repression of GRIM19 expression potentiates cisplatin chemoresistance in advanced bladder cancer cells via disrupting ubiquitination-mediated bcl-xL degradation. Cancer Chemother Pharmacol (2018) 82(4):593–605. doi: 10.1007/s00280-018-3651-3
86. Piccolo S, Panciera T, Contessotto P, Cordenonsi M. YAP/TAZ as master regulators in cancer: modulation, function and therapeutic approaches. Nat Cancer (2023) 4(1):9–26. doi: 10.1038/s43018-022-00473-z
87. Zanconato F, Cordenonsi M, Piccolo S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat Rev Cancer (2019) 19(8):454–64. doi: 10.1038/s41568-019-0168-y
88. Luo Y, Zhou J, Tang J, Zhou F, He Z, Liu T, et al. MINDY1 promotes bladder cancer progression by stabilizing YAP. Cancer Cell Int (2021) 21(1):395. doi: 10.1186/s12935-021-02095-4
89. Fu M, Peng D, Lan T, Wei Y, Wei X. Multifunctional regulatory protein connective tissue growth factor (CTGF): a potential therapeutic target for diverse diseases. Acta Pharm Sin B (2022) 12(4):1740–60. doi: 10.1016/j.apsb.2022.01.007
90. Chen J, Liu Y, Sun Q, Wang B, Li N, Chen X. CYR61 suppresses growth of human malignant melanoma. Oncol Rep (2016) 36(5):2697–704. doi: 10.3892/or.2016.5124
91. Zhang H, Geng D, Gao J, Qi Y, Shi Y, Wang Y, et al. Expression and significance of Hippo/YAP signaling in glioma progression. Tumour Biol (2016) 37:15665–76. doi: 10.1007/s13277-016-5318-1
92. Zhao Y, Schetter AJ, Yang GB, Nguyen G, Mathe EA, Li P, et al. microRNA and inflammatory gene expression as prognostic marker for overall survival in esophageal squamous cell carcinoma. Int J Cancer (2013) 132(12):2901–9. doi: 10.1002/ijc.27954
93. Huang ZM, Wang H, Ji ZG. Bladder mesenchymal stromal cell-derived exosomal miRNA-217 modulates bladder cancer cell survival through hippo-YAP pathway. Inflammation Res (2021) 70(9):959–69. doi: 10.1007/s00011-021-01494-7
94. Zhang R, Liu W, Sun J, Kong Y, Chen C. Roles of RNF126 and BCA2 E3 ubiquitin ligases in DNA damage repair signaling and targeted cancer therapy. Pharmacol Res (2020) 155:104748. doi: 10.1016/j.phrs.2020.104748
95. Xu H, Ju L, Xiong Y, Yu M, Zhou F, Qian K, et al. E3 ubiquitin ligase RNF126 affects bladder cancer progression through regulation of PTEN stability. Cell Death Dis (2021) 12(3):239. doi: 10.1038/s41419-021-03521-1
96. Noble M, Mayer-Proschel M, Li Z, Dong T, Cui W, Proschel C, et al. Redox biology in normal cells and cancer: restoring function of the redox/Fyn/c-cbl pathway in cancer cells offers new approaches to cancer treatment. Free Radic Biol Med (2015) 79:300–23. doi: 10.1016/j.freeradbiomed.2014.10.860
97. Xiao D, Hu X, Peng M, Deng J, Zhou S, Xu S, et al. Inhibitory role of proguanil on the growth of bladder cancer via enhancing EGFR degradation and inhibiting its downstream signaling pathway to induce autophagy. Cell Death Dis (2022) 13(5):499. doi: 10.1038/s41419-022-04937-z
98. Hua X, Huang M, Deng X, Xu J, Luo Y, Xie Q, et al. The inhibitory effect of compound ChlA-f on human bladder cancer cell invasion can be attributed to its blockage of SOX2 protein. Cell Death Differ (2020) 27(2):632–45. doi: 10.1038/s41418-019-0377-7
99. Liu J, Chen T, Li S, Liu W, Wang P, Shang G. Targeting matrix metalloproteinases by E3 ubiquitin ligases as a way to regulate the tumor microenvironment for cancer therapy. Semin Cancer Biol (2022) 86(Pt 2):259–68. doi: 10.1016/j.semcancer.2022.06.004
100. Hou B, Chen T, Zhang H, Li J, Wang P, Shang G. The E3 ubiquitin ligases regulate PD-1/PD-L1 protein levels in tumor microenvironment to improve immunotherapy. Front Immunol (2023) 14:1123244. doi: 10.3389/fimmu.2023.1123244
101. Hu X, Wang J, Chu M, Liu Y, Wang ZW, Zhu X. Emerging role of ubiquitination in the regulation of PD-1/PD-L1 in cancer immunotherapy. Mol Ther (2021) 29(3):908–19. doi: 10.1016/j.ymthe.2020.12.032
102. Ho SR, Mahanic CS, Lee YJ, Lin WC. RNF144A, an E3 ubiquitin ligase for DNA-PKcs, promotes apoptosis during DNA damage. Proc Natl Acad Sci U S A (2014) 111(26):E2646–55. doi: 10.1073/pnas.1323107111
103. Tsai CL, Yang PS, Hsu FM, Cheng AL, Yu WN, Cheng JC. Topoisomerase I inhibition radiosensitizing hepatocellular carcinoma by RNF144A-mediated DNA-PKcs ubiquitination and natural killer cell cytotoxicity. J Clin Transl Hepatol (2023) 11(3):614–25. doi: 10.14218/JCTH.2022.00271
104. Zhang Y, Liao XH, Xie HY, Shao ZM, Li DQ. RBR-type E3 ubiquitin ligase RNF144A targets PARP1 for ubiquitin-dependent degradation and regulates PARP inhibitor sensitivity in breast cancer cells. Oncotarget (2017) 8(55):94505–18. doi: 10.18632/oncotarget.21784
105. Zhang Y, Yang YL, Zhang FL, Liao XH, Shao ZM, Li DQ. Epigenetic silencing of RNF144A expression in breast cancer cells through promoter hypermethylation and MBD4. Cancer Med (2018) 7(4):1317–25. doi: 10.1002/cam4.1324
106. Yang YL, Zhang Y, Li DD, Zhang FL, Liu HY, Liao XH, et al. RNF144A functions as a tumor suppressor in breast cancer through ubiquitin ligase activity-dependent regulation of stability and oncogenic functions of HSPA2. Cell Death Differ (2020) 27(3):1105–18. doi: 10.1038/s41418-019-0400-z
107. Zhang YL, Cao JL, Zhang Y, Liao L, Deng L, Yang SY, et al. RNF144A exerts tumor suppressor function in breast cancer through targeting YY1 for proteasomal degradation to downregulate GMFG expression. Med Oncol (2022) 39(4):48. doi: 10.1007/s12032-021-01631-6
108. Ho SR, Lin WC. RNF144A sustains EGFR signaling to promote EGF-dependent cell proliferation. J Biol Chem (2018) 293(42):16307–23. doi: 10.1074/jbc.RA118.002887
109. Li Y, Wang J, Wang F, Chen W, Gao C, Wang J. RNF144A suppresses ovarian cancer stem cell properties and tumor progression through regulation of LIN28B degradation via the ubiquitin-proteasome pathway. Cell Biol Toxicol (2022) 38(5):809–24. doi: 10.1007/s10565-021-09609-w
110. Ho SR, Lee YC, Ittmann MM, Lin FT, Chan KS, Lin WC. RNF144A deficiency promotes PD-L1 protein stabilization and carcinogen-induced bladder tumorigenesis. Cancer Lett (2021) 520:344–60. doi: 10.1016/j.canlet.2021.08.008
111. Wang ZW, Hu X, Ye M, Lin M, Chu M, Shen X. NEDD4 E3 ligase: functions and mechanism in human cancer. Semin Cancer Biol (2020) 67(Pt 2):92–101. doi: 10.1016/j.semcancer.2020.03.006
112. Ye X, Wang L, Shang B, Wang Z, Wei W. NEDD4: a promising target for cancer therapy. Curr Cancer Drug Targets (2014) 14(6):549–56. doi: 10.2174/1568009614666140725092430
113. Jayaprakash S, Hegde M, BharathwajChetty B, Girisa S, Alqahtani MS, Abbas M, et al. Unraveling the potential role of NEDD4-like E3 ligases in cancer. Int J Mol Sci (2022) 23(20). doi: 10.3390/ijms232012380
114. Chen C, Matesic LE. The Nedd4-like family of E3 ubiquitin ligases and cancer. Cancer Metastasis Rev (2007) 26(3-4):587–604. doi: 10.1007/s10555-007-9091-x
115. Zhang R, Shi S. The role of NEDD4 related HECT-type E3 ubiquitin ligases in defective autophagy in cancer cells: molecular mechanisms and therapeutic perspectives. Mol Med (2023) 29(1):34. doi: 10.1186/s10020-023-00628-3
116. Chen L, Wang G, Luo Y, Wang Y, Xie C, Jiang W, et al. Downregulation of LAPTM5 suppresses cell proliferation and viability inducing cell cycle arrest at G0/G1 phase of bladder cancer cells. Int J Oncol (2017) 50(1):263–71. doi: 10.3892/ijo.2016.3788
117. Wen W, Li J, Wang L, Xing Y, Li X, Ruan H, et al. Inhibition of NEDD4 inhibits cell growth and invasion and induces cell apoptosis in bladder cancer cells. Cell Cycle (2017) 16(16):1509–14. doi: 10.1080/15384101.2017.1338220
118. Mao M, Yang L, Hu J, Liu B, Zhang X, Liu Y, et al. Oncogenic E3 ubiquitin ligase NEDD4 binds to KLF8 and regulates the microRNA-132/NRF2 axis in bladder cancer. Exp Mol Med (2022) 54(1):47–60. doi: 10.1038/s12276-021-00663-2
119. Ascione CM, Napolitano F, Esposito D, Servetto A, Belli S, Santaniello A, et al. Role of FGFR3 in bladder cancer: treatment landscape and future challenges. Cancer Treat Rev (2023) 115:102530. doi: 10.1016/j.ctrv.2023.102530
120. Jing W, Wang G, Cui Z, Xiong G, Jiang X, Li Y, et al. FGFR3 destabilizes PD-L1 via NEDD4 to control T-cell-Mediated bladder cancer immune surveillance. Cancer Res (2022) 82(1):114–29. doi: 10.1158/0008-5472.CAN-21-2362
121. Yang C, Xiang H, Fu K, Jin L, Yuan F, Xue B, et al. Lycorine suppresses cell growth and invasion via down-regulation of NEDD4 ligase in bladder cancer. Am J Cancer Res (2022) 12(10):4708–20.
122. Oliveira RI, Guedes RA, Salvador JAR. Highlights in USP7 inhibitors for cancer treatment. Front Chem (2022) 10:1005727. doi: 10.3389/fchem.2022.1005727
123. Zhou L, Ouyang T, Li M, Hong T, Mhs A, Meng W, et al. Ubiquitin-specific peptidase 7: a novel deubiquitinase that regulates protein homeostasis and cancers. Front Oncol (2021) 11:784672. doi: 10.3389/fonc.2021.784672
124. Lu J, Zhao H, Yu C, Kang Y, Yang X. Targeting ubiquitin-specific protease 7 (USP7) in cancer: a new insight to overcome drug resistance. Front Pharmacol (2021) 12:648491. doi: 10.3389/fphar.2021.648491
125. Nininahazwe L, Liu B, He C, Zhang H, Chen ZS. The emerging nature of ubiquitin-specific protease 7 (USP7): a new target in cancer therapy. Drug Discov Today (2021) 26(2):490–502. doi: 10.1016/j.drudis.2020.10.028
126. Korenev G, Yakukhnov S, Druk A, Golovina A, Chasov V, Mirgayazova R, et al. USP7 inhibitors in cancer immunotherapy: current status and perspective. Cancers (Basel) (2022) 14(22). doi: 10.3390/cancers14225539
127. Morra F, Merolla F, Criscuolo D, Insabato L, Giannella R, Ilardi G, et al. CCDC6 and USP7 expression levels suggest novel treatment options in high-grade urothelial bladder cancer. J Exp Clin Cancer Res (2019) 38(1):90. doi: 10.1186/s13046-019-1087-1
128. Varol N, Konac E, Bilen CY. Does wnt/beta-catenin pathway contribute to the stability of DNMT1 expression in urological cancer cell lines? Exp Biol Med (Maywood) (2015) 240(5):624–30. doi: 10.1177/1535370214556951
129. Wang Z, Kang W, Li O, Qi F, Wang J, You Y, et al. Abrogation of USP7 is an alternative strategy to downregulate PD-L1 and sensitize gastric cancer cells to T cells killing. Acta Pharm Sin B (2021) 11(3):694–707. doi: 10.1016/j.apsb.2020.11.005
130. Li P, Liu Y, Liu HM. A patent review of ubiquitin-specific protease 7 (USP7) inhibitors (2014-present). Expert Opin Ther Pat (2022) 32(7):753–67. doi: 10.1080/13543776.2022.2058873
131. Cai H, Chen H, Huang Q, Zhu JM, Ke ZB, Lin YZ, et al. Ubiquitination-related molecular subtypes and a novel prognostic index for bladder cancer patients. Pathol Oncol Res (2021) 27:1609941. doi: 10.3389/pore.2021.1609941
132. Sampson C, Wang Q, Otkur W, Zhao H, Lu Y, Liu X, et al. The roles of E3 ubiquitin ligases in cancer progression and targeted therapy. Clin Transl Med (2023) 13(3):e1204. doi: 10.1002/ctm2.1204
133. LaPlante G, Zhang W. Targeting the ubiquitin-proteasome system for cancer therapeutics by small-molecule inhibitors. Cancers (Basel) (2021) 13(12). doi: 10.3390/cancers13123079
134. Deng L, Meng T, Chen L, Wei W, Wang P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther (2020) 5(1):11. doi: 10.1038/s41392-020-0107-0
135. Murray C, Galvan E, Ontiveros C, Deng Y, Bai H, Padron AS, et al. Pharmacologic tumor PDL1 depletion with cefepime or ceftazidime promotes DNA damage and sensitivity to DNA-damaging agents. Int J Mol Sci (2022) 23(9). doi: 10.3390/ijms23095129
136. Sarfraz A, Rasul A, Sarfraz I, Shah MA, Hussain G, Shafiq N, et al. Hispolon: a natural polyphenol and emerging cancer killer by multiple cellular signaling pathways. Environ Res (2020) 190:110017. doi: 10.1016/j.envres.2020.110017
137. Lu TL, Huang GJ, Lu TJ, Wu JB, Wu CH, Yang TC, et al. Hispolon from phellinus linteus has antiproliferative effects via MDM2-recruited ERK1/2 activity in breast and bladder cancer cells. Food Chem Toxicol (2009) 47(8):2013–21. doi: 10.1016/j.fct.2009.05.023
138. Geng F, Tang L, Li Y, Yang L, Choi KS, Kazim AL, et al. Allyl isothiocyanate arrests cancer cells in mitosis, and mitotic arrest in turn leads to apoptosis via bcl-2 protein phosphorylation. J Biol Chem (2011) 286(37):32259–67. doi: 10.1074/jbc.M111.278127
139. Chen C, Na X, Wang L, Yu R. High-throughput screening identifies stevioside as a potent agent to induce apoptosis in bladder cancer cells. Biochem Pharmacol (2022) 203:115166. doi: 10.1016/j.bcp.2022.115166
140. Li Z, Ren D, Chen C, Sun L, Fang K. OSU-T315 and doxorubicin synergistically induce apoptosis via mitochondrial pathway in bladder cancer cells. Cell Biol Int (2022) 46(10):1672–81. doi: 10.1002/cbin.11855
141. Luo KW, Zhu XH, Zhao T, Zhong J, Gao HC, Luo XL, et al. EGCG enhanced the anti-tumor effect of doxorubicine in bladder cancer via NF-kappaB/MDM2/p53 pathway. Front Cell Dev Biol (2020) 8:606123. doi: 10.3389/fcell.2020.606123
142. Cucci MA, Compagnone A, Daga M, Grattarola M, Ullio C, Roetto A, et al. Post-translational inhibition of YAP oncogene expression by 4-hydroxynonenal in bladder cancer cells. Free Radic Biol Med (2019) 141:205–19. doi: 10.1016/j.freeradbiomed.2019.06.009
143. Jiang W, Xia J, Xie S, Zou R, Pan S, Wang ZW, et al. Long non-coding RNAs as a determinant of cancer drug resistance: towards the overcoming of chemoresistance via modulation of lncRNAs. Drug Resist Updat (2020) 50:100683. doi: 10.1016/j.drup.2020.100683
144. Lima APB, da Silva GN. Long non-coding RNA and chemoresistance in bladder cancer - a mini review. Cancer Invest (2023) 41(2):164–72. doi: 10.1080/07357907.2022.2146703
145. Zhang ZH, Wang Y, Zhang Y, Zheng SF, Feng T, Tian X, et al. The function and mechanisms of action of circular RNAs in urologic cancer. Mol Cancer (2023) 22(1):61. doi: 10.1186/s12943-023-01766-2
146. Wang H, Feng Y, Zheng X, Xu X. The diagnostic and therapeutic role of snoRNA and lincRNA in bladder cancer. Cancers (Basel) (2023) 15(4). doi: 10.3390/cancers15041007
147. Liu J, Shang G. The roles of noncoding RNAs in the development of osteosarcoma stem cells and potential therapeutic targets. Front Cell Dev Biol (2022) 10:773038. doi: 10.3389/fcell.2022.773038
148. Xie W, Chu M, Song G, Zuo Z, Han Z, Chen C, et al. Emerging roles of long noncoding RNAs in chemoresistance of pancreatic cancer. Semin Cancer Biol (2022) 83:303–18. doi: 10.1016/j.semcancer.2020.11.004
149. Beylerli O, Gareev I, Sufianov A, Ilyasova T, Guang Y. Long noncoding RNAs as promising biomarkers in cancer. Noncoding RNA Res (2022) 7(2):66–70. doi: 10.1016/j.ncrna.2022.02.004
150. Asghariazar V, Kadkhodayi M, Mansoori B, Mohammadi A, Baradaran B. Restoration of miR-143 reduces migration and proliferation of bladder cancer cells by regulating signaling pathways involved in EMT. Mol Cell Probes (2022) 61:101794. doi: 10.1016/j.mcp.2022.101794
151. Cai H, Xu H, Lu H, Xu W, Liu H, Wang X, et al. LncRNA SNHG1 facilitates tumor proliferation and represses apoptosis by regulating PPARgamma ubiquitination in bladder cancer. Cancers (Basel) (2022) 14(19). doi: 10.3390/cancers14194740
152. Li J, Wang Y, Zhang X, Yang X, Qi Q, Mi Q, et al. Characterisation of a novel transcript LNPPS acting as tumour suppressor in bladder cancer via PDCD5-mediated p53 degradation blockage. Clin Transl Med (2023) 13(1):e1149. doi: 10.1002/ctm2.1149
153. Ke M, Sun N, Lin Z, Zhang P, Hu Y, Wu S, et al. SNHG18 inhibits bladder cancer cell proliferation by increasing p21 transcription through destabilizing c-myc protein. Cancer Cell Int (2023) 23(1):48. doi: 10.1186/s12935-023-02887-w
154. Jiang X, Li H, Fang Y, Xu C. LncRNA PVT1 contributes to invasion and doxorubicin resistance of bladder cancer cells through promoting MDM2 expression and AURKB-mediated p53 ubiquitination. Environ Toxicol (2022) 37(6):1495–508. doi: 10.1002/tox.23501
155. Zhu Y, Dai B, Zhang H, Shi G, Shen Y, Ye D. Long non-coding RNA LOC572558 inhibits bladder cancer cell proliferation and tumor growth by regulating the AKT-MDM2-p53 signaling axis. Cancer Lett (2016) 380(2):369–74. doi: 10.1016/j.canlet.2016.04.030
156. Chen L, Li W, Li Z, Song Y, Zhao J, Chen Z, et al. circNUDT21 promotes bladder cancer progression by modulating the miR-16-1-3p/MDM2/p53 axis. Mol Ther Nucleic Acids (2021) 26:625–36. doi: 10.1016/j.omtn.2021.08.032
157. Jobst M, Kiss E, Gerner C, Marko D, Del Favero G. Activation of autophagy triggers mitochondrial loss and changes acetylation profile relevant for mechanotransduction in bladder cancer cells. Arch Toxicol (2023) 97(1):217–33. doi: 10.1007/s00204-022-03375-2
158. Xu J, Wei L, Liu H, Lei Y, Zhu Y, Liang C, et al. CD274 (PD-L1) methylation is an independent predictor for bladder cancer patients' survival. Cancer Invest (2022) 40(3):228–33. doi: 10.1080/07357907.2022.2028805
159. Wan W, Peng K, Li M, Qin L, Tong Z, Yan J, et al. Histone demethylase JMJD1A promotes urinary bladder cancer progression by enhancing glycolysis through coactivation of hypoxia inducible factor 1alpha. Oncogene (2017) 36(27):3868–77. doi: 10.1038/onc.2017.13
160. Chen J, Cao L, Li Z, Li Y. SIRT1 promotes GLUT1 expression and bladder cancer progression via regulation of glucose uptake. Hum Cell (2019) 32(2):193–201. doi: 10.1007/s13577-019-00237-5
161. Bao H, Luo Y, Ding G, Fu Z. A pan-cancer analysis of UBE2S in tumorigenesis, prognosis, pathway, immune infiltration and evasion, and therapy response from an immune-oncology perspective. J Oncol (2022) 2022:3982539. doi: 10.1155/2022/3982539
162. Zhang M, Liu Y, Yin Y, Sun Z, Wang Y, Zhang Z, et al. UBE2S promotes the development of ovarian cancer by promoting PI3K/AKT/mTOR signaling pathway to regulate cell cycle and apoptosis. Mol Med (2022) 28(1):62. doi: 10.1186/s10020-022-00489-2
163. Gui L, Zhang S, Xu Y, Zhang H, Zhu Y, Kong L. UBE2S promotes cell chemoresistance through PTEN-AKT signaling in hepatocellular carcinoma. Cell Death Discovery (2021) 7(1):357. doi: 10.1038/s41420-021-00750-3
164. Tang H, Fang T, Ji M, Wang JP, Song LL, Zhang QY, et al. UBE2S exerts oncogenic activities in urinary bladder cancer by ubiquitinating TSC1. Biochem Biophys Res Commun (2021) 578:7–14. doi: 10.1016/j.bbrc.2021.08.057
165. Jiang L, Sun G, Zou L, Guan Y, Hang Y, Liu Y, et al. Noncoding RNAs as a potential biomarker for the prognosis of bladder cancer: a systematic review and meta-analysis. Expert Rev Mol Diagn (2023) 23(4):325–34. doi: 10.1080/14737159.2023.2195554
166. Li K, Yao T, Wang Z. lncRNA-mediated ceRNA network in bladder cancer. Noncoding RNA Res (2023) 8(2):135–45. doi: 10.1016/j.ncrna.2022.12.002
167. Chu H, Hui G, Yuan L, Shi D, Wang Y, Du M, et al. Identification of novel piRNAs in bladder cancer. Cancer Lett (2015) 356(2 Pt B):561–7. doi: 10.1016/j.canlet.2014.10.004
168. Wang Z, Wang X, Wang Y, Tang S, Feng C, Pan L, et al. Transcriptomic analysis of gene networks regulated by U11 small nuclear RNA in bladder cancer. Front Genet (2021) 12:695597. doi: 10.3389/fgene.2021.695597
169. Wang Q, Wu L, Cao R, Gao J, Chai D, Qin Y, et al. Fbxo45 promotes the malignant development of esophageal squamous cell carcinoma by targeting GGNBP2 for ubiquitination and degradation. Oncogene (2022) 41(43):4795–807. doi: 10.1038/s41388-022-02468-7
170. Cao T, Cui Y, Wang Y, Wu L, Yu K, Chen K, et al. CACNA1C-AS2 inhibits cell proliferation and suppresses cell migration and invasion via targeting FBXO45 and PI3K/AKT/mTOR pathways in glioma. Apoptosis (2022) 27(11-12):979–91. doi: 10.1007/s10495-022-01764-7
171. Wu L, Yu K, Chen K, Zhu X, Yang Z, Wang Q, et al. Fbxo45 facilitates pancreatic carcinoma progression by targeting USP49 for ubiquitination and degradation. Cell Death Dis (2022) 13(3):231. doi: 10.1038/s41419-022-04675-2
172. De S, Holvey-Bates EG, Mahen K, Willard B, Stark GR. The ubiquitin E3 ligase FBXO22 degrades PD-L1 and sensitizes cancer cells to DNA damage. Proc Natl Acad Sci U S A (2021) 118(47):e2112674118. doi: 10.1073/pnas.2112674118
173. Zhang Y, Liu Q, Cui M, Wang M, Hua S, Gao J, et al. Comprehensive analysis of expression, prognostic value, and immune infiltration for ubiquitination-related FBXOs in pancreatic ductal adenocarcinoma. Front Immunol (2021) 12:774435. doi: 10.3389/fimmu.2021.774435
174. Li S, Chen T, Liu J, Zhang H, Li J, Wang Z, et al. PROTACs: novel tools for improving immunotherapy in cancer. Cancer Lett (2023) 560:216128. doi: 10.1016/j.canlet.2023.216128
175. Liu J, Ma J, Liu Y, Xia J, Li Y, Wang ZP, et al. PROTACs: a novel strategy for cancer therapy. Semin Cancer Biol (2020) 67(Pt 2):171–9. doi: 10.1016/j.semcancer.2020.02.006
176. Wang ZW, Liu Y, Zhu X. PhotoPROTACs: a novel biotechnology for cancer treatment. Trends Cell Biol (2020) 30(10):749–51. doi: 10.1016/j.tcb.2020.08.003
177. Xu Y, Lei Z, Zhu J, Wan L. Mivebresib synergized with PZ703b, a novel bcl-xl PROTAC degrader, induces apoptosis in bladder cancer cells via the mitochondrial pathway. Biochem Biophys Res Commun (2022) 623:120–6. doi: 10.1016/j.bbrc.2022.07.045
Keywords: E3 ligases, bladder cancer, immunotherapy, resistance, ubiquitination
Citation: Wang X, Zhang Y, Wu Y, Cheng H and Wang X (2023) The role of E3 ubiquitin ligases and deubiquitinases in bladder cancer development and immunotherapy. Front. Immunol. 14:1202633. doi: 10.3389/fimmu.2023.1202633
Received: 09 April 2023; Accepted: 21 April 2023;
Published: 05 May 2023.
Edited by:
Zichuan Liu, Tianjin University, ChinaReviewed by:
Xin Mu, Tianjin University, ChinaZhi-Wei Wang, Wenzhou Medical University, China
Naoe Taira Nihira, St. Marianna University School of Medicine, Japan
Copyright © 2023 Wang, Zhang, Wu, Cheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueju Wang, eHVlanVAamx1LmVkdS5jbg==
†These authors share first authorship
 Xuemei Wang†
Xuemei Wang† Xueju Wang
Xueju Wang