- Department of Hematology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China; Medical Cosmetic Center, Chengdu Second People's Hospital; Minhang Hospital, Fudan University, Shanghai, China
Neutrophils play a critical role in the immune response to infection and tissue injury. However, recent studies have shown that neutrophils are a heterogeneous population with distinct subtypes that differ in their functional properties. Moreover, aging can alter neutrophil function and exacerbate immune dysregulation. In this review, we discuss the concept of neutrophil heterogeneity and how it may be affected by aging. We then examine the implications of neutrophil heterogeneity and aging for COVID-19 pathogenesis and wound healing. Specifically, we summarize the evidence for neutrophil involvement in COVID-19 and the potential mechanisms underlying neutrophil recruitment and activation in this disease. We also review the literature on the role of neutrophils in the wound healing process and how aging and neutrophil heterogeneity may impact wound healing outcomes. Finally, we discuss the potential for neutrophil-targeted therapies to improve clinical outcomes in COVID-19 and wound healing.
1 Introduction
Neutrophils are critical immune cells that play a vital role in the body's response to infection and tissue injury (1). However, recent studies have identified that these cells are a heterogeneous population with distinct subtypes that exhibit unique functional properties (2, 3). Recent scRNA-seq research reveals significant heterogeneity among neutrophils, contradicting prior views of their homogeneity. By analyzing thousands of mouse neutrophils, eight distinct subsets were identified, each with unique functions and maturation processes under normal and infectious conditions. Bacterial infection primes these subsets for enhanced activity without disrupting their heterogeneity, facilitating deeper exploration of neutrophil-related diseases, biomarkers, and therapies at a single-cell resolution (4, 5). Recent research has revealed neutrophil heterogeneity, with CD66b(+) cells exhibiting neutrophil-like morphology in inflammation. These cells possess immunosuppressive or proinflammatory properties and are referred to as LDNs, LDGs, G-MDSCs, or immunosuppressive neutrophils. However, due to the absence of specific markers, their precise phenotype and function remain unclear. This article provides an overview of mature and immature neutrophil subsets with immunosuppressive or proinflammatory characteristics, addressing unresolved questions and gaps in our understanding of neutrophil heterogeneity (6, 7). Aging is known to have an impact on neutrophil function, leading to immune dysregulation, which may contribute to the severity of certain diseases (8). In this review, we aim to discuss the concept of neutrophil heterogeneity and its relationship with aging, specifically examining how it may impact COVID-19 pathogenesis and wound healing (9). We will provide an overview of the current understanding of neutrophil involvement in COVID-19, including the potential mechanisms underlying neutrophil activation and recruitment (10). Additionally, we will summarize the role of neutrophils in the wound healing process and discuss how aging and neutrophil heterogeneity may influence wound healing outcomes (11). Finally, we will review the potential of neutrophil-targeted therapies to improve clinical outcomes in COVID-19 and wound healing (12). Overall, a better understanding of neutrophil heterogeneity and its impact on immune function and disease pathogenesis may lead to the development of more targeted and effective therapies (13). Neutrophil heterogeneity is a relatively new concept that has emerged in recent years, and there is still much to be learned about the functional diversity of these cells (14). However, the potential clinical implications of this heterogeneity are significant (15). For example, recent studies have suggested that certain subtypes of neutrophils may be more effective at clearing infections, while others may contribute to tissue damage and inflammation (16). This raises the possibility of selectively targeting specific neutrophil subtypes for therapeutic purposes, which may be particularly relevant in the context of COVID-19, where an uncontrolled immune response can contribute to disease severity (17). Moreover, the dysregulation of neutrophil heterogeneity may contribute to the impaired wound healing seen in conditions such as diabetes, highlighting the need for novel approaches to modulate neutrophil function and heterogeneity (18). Overall, a better understanding of the complex biology of neutrophils and their heterogeneity will be crucial in developing new therapeutic strategies to improve clinical outcomes in a range of disease settings.
2 Neutrophil basics
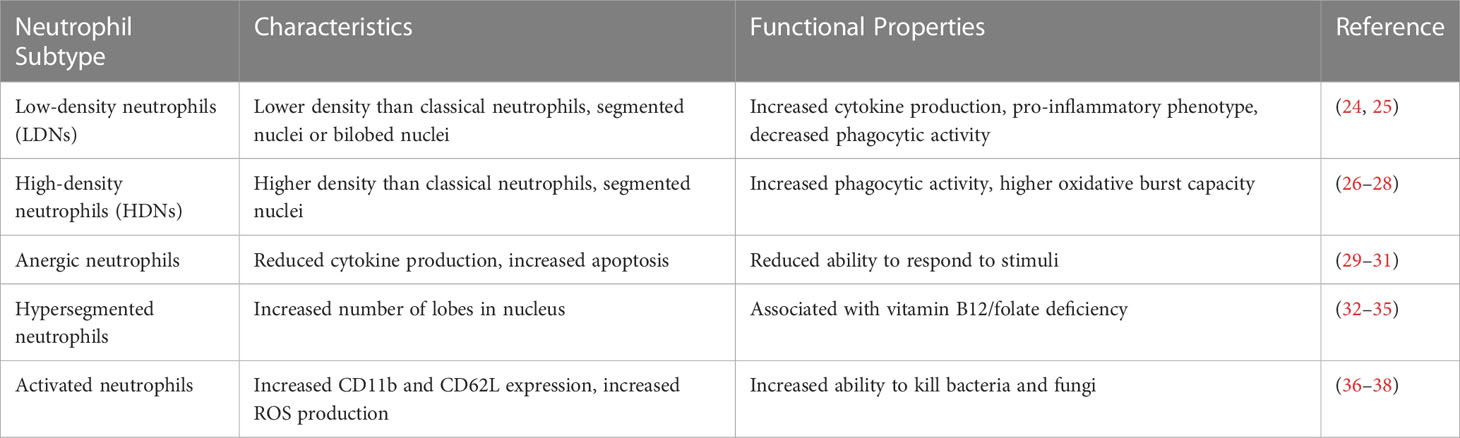
Neutrophils are a type of white blood cell that play a crucial role in the immune system's response to infection and inflammation (19). These cells are the first responders to an infection, as they are rapidly recruited to the site of infection or injury in large numbers (20). Once they arrive at the site of infection, neutrophils use a variety of mechanisms to eliminate pathogens, including phagocytosis, the release of antimicrobial agents, and the formation of neutrophil extracellular traps (NETs) (21). Neutrophils also play a critical role in modulating the inflammatory response, as they release cytokines and chemokines that recruit other immune cells to the site of infection (22). However, in some cases, excessive neutrophil activation can contribute to tissue damage and exacerbate inflammatory diseases. Thus, understanding the basic biology of neutrophils is essential for developing new therapies for infections and inflammatory disorders (23). Table 1 provides a comparison of the characteristics and functional properties of different neutrophil subtypes, highlighting the heterogeneity of these immune cells. In recent years, emerging research has shed light on the previously underappreciated ability of neutrophils to influence adaptive immunity (39). Neutrophils can interact with various immune cells, including dendritic cells, T cells, and B cells, and modulate their functions (40). This interaction suggests that neutrophils may play a more intricate role in shaping and regulating the adaptive immune response than previously believed (41). Recent studies have highlighted the capacity of neutrophils to induce the maturation of antigen-presenting cells (APCs) (42), particularly dendritic cells (DCs). Neutrophils can directly interact with DCs and promote their maturation by releasing pro-inflammatory cytokines, such as TNF-α and IL-12 (43). This activation of DCs leads to enhanced antigen presentation and subsequent activation of T cells, bridging the innate and adaptive immune responses (44). Notably, research has demonstrated that neutrophils can promote DC maturation in various infectious and inflammatory contexts (3, 45), providing valuable insights into the dynamic interplay between these immune cell populations. Recent progress in immunology has revealed an intriguing concept: neutrophils, traditionally considered as short-lived phagocytes, can also function as antigen-presenting cells (APCs). Neutrophils possess the ability to phagocytose pathogens, process the captured antigens (46), and subsequently present them on major histocompatibility complex class II (MHC-II) molecules, similar to classical APCs such as dendritic cells (42). This process involves the internalization of pathogens into neutrophil phagosomes, where the antigens are degraded and loaded onto MHC-II molecules. Consequently, neutrophils can engage with T cells, initiating adaptive immune responses and blurring the conventional boundaries between innate and adaptive immunity. These findings shed light on the versatile roles of neutrophils in orchestrating immune responses beyond their traditional phagocytic functions. The discovery that neutrophils can induce APC maturation and function as APCs themselves has significant implications for our understanding of immune responses and disease processes. This newfound role places neutrophils at the intersection of innate and adaptive immunity, highlighting their ability to bridge these two arms of the immune system. By directly influencing the maturation of APCs, neutrophils contribute to the initiation and regulation of adaptive immune responses. This finding opens up new avenues for exploring the dynamic interplay between neutrophils, APCs, and other immune cells, potentially leading to innovative therapeutic strategies for infectious diseases, autoimmunity, and cancer. Current research in the field of neutrophils functioning as APCs has provided valuable insights into the dynamic and complex nature of immune responses. However, there is still much to be explored and understood regarding the specific mechanisms and functional consequences of neutrophil-mediated APC maturation. Future studies could focus on elucidating the molecular pathways involved in this process, identifying the signals that trigger neutrophil transition into APCs, and investigating the impact of neutrophil-mediated APC maturation on various disease contexts. Continued research in this field holds immense potential to broaden our understanding of immune regulation and may pave the way for novel therapeutic interventions targeting immune-related disorders.
2.1 Explain the basic characteristics of neutrophils and their life cycle
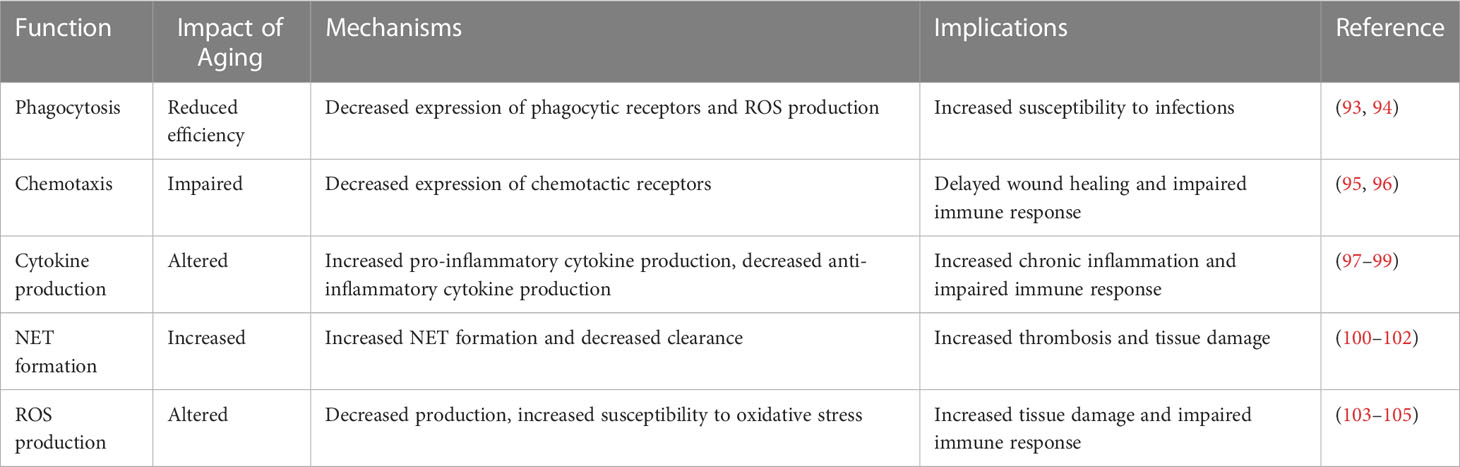
Neutrophils, a vital constituent of granulocytes or white blood cells, are indispensable to the innate immune response against infection and inflammation (47). They are recognized by their multilobed nuclei and cytoplasmic granules (48), that house an array of enzymes and antimicrobial agents (49). Known for their brief lifespan, ranging from a few hours to a few days (50), neutrophils are perpetually synthesized in the bone marrow and then dispatched into circulation. However, it's crucial to mention that neutrophils do not solely depend on this circulation for their immunological function. Current research underscores the significant role of marginated and tissue-resident neutrophils as first responders during infections and tissue injuries (51–53). These neutrophils swiftly counteract the spread of pathogens, thereby organizing the subsequent immune response. Once activated by chemotactic factors such as cytokines and chemokines, neutrophils navigate their way to the infection or inflammation site to eliminate pathogens employing a multitude of mechanisms (54). Their life cycle concludes with programmed cell death, or apoptosis, post which phagocytic cells, for instance, macrophages, clear them (55). This clearance of apoptotic neutrophils is paramount for inflammation resolution and prevention of tissue damage (56). Recent evidence indicates that aging significantly impacts neutrophil functionality, potentially triggering immune dysregulation. Aging is seemingly linked to a decline in neutrophil efficiency, as observed in the impairment of phagocytosis and oxidative burst, diminished cytokine production, and altered chemotaxis (57). Such alterations heighten the susceptibility to infections and inflammatory diseases in the elderly (58–60). Additionally, aging can reconfigure the gene expression profile of neutrophils, impacting their response to stimuli. Several factors, including changes in the bone marrow microenvironment, fluctuating levels of circulating hormones and cytokines, and cellular damage accumulation, are hypothesized to drive these age-related alterations in neutrophil function (61, 62). Therefore, it is crucial to consider the effect of aging on neutrophil functionality while conceptualizing new therapeutic interventions for infections and inflammatory conditions (63). Further investigations are warranted to unravel the mechanisms propelling age-induced changes in neutrophil function, thereby enabling the development of strategies to modulate neutrophil functionality in older adults. Table 2 compares the impact of aging on various neutrophil functions, including phagocytosis, cytokine production, and ROS production, emphasizing the importance of considering the impact of aging on immune function.
3 Neutrophil heterogeneity
Discuss the concept of neutrophil heterogeneity and its implications
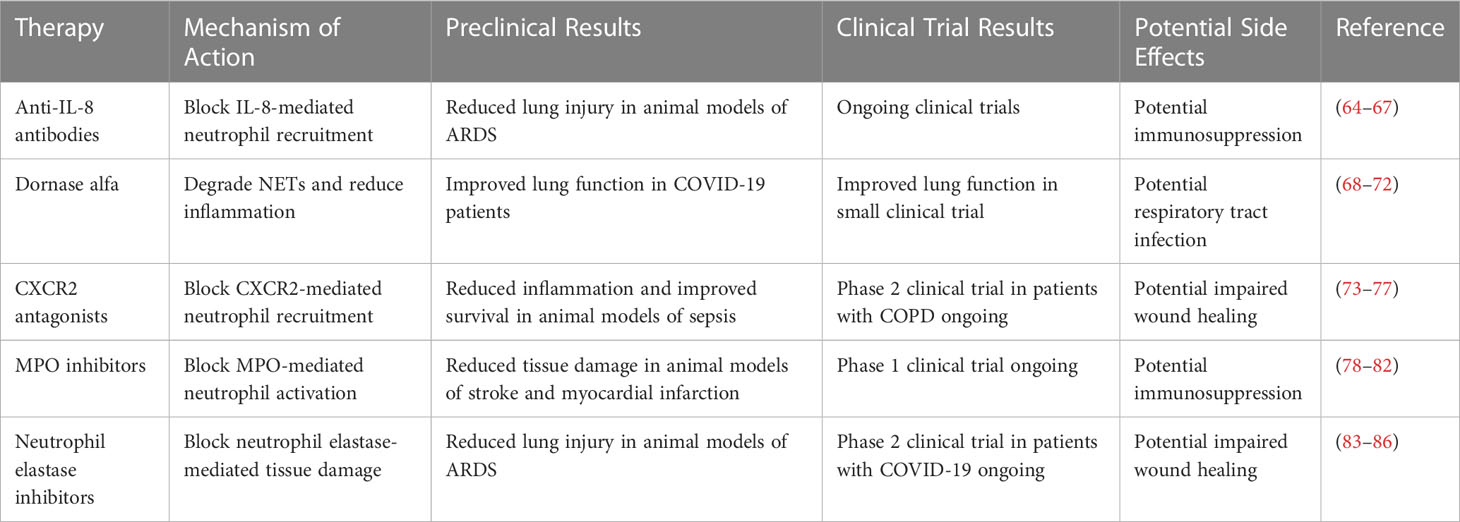
Recent studies have revealed that neutrophils are a heterogeneous population of cells with distinct subtypes that differ in their morphology, gene expression profile, and functional properties (87). The concept of neutrophil heterogeneity has important implications for our understanding of the immune response and the pathogenesis of various diseases (88). For example, different subtypes of neutrophils may have different functions in the immune response, with some subtypes being more effective at phagocytosis and others being more efficient at producing reactive oxygen species (89). Moreover, neutrophil heterogeneity may contribute to the development of chronic inflammation and autoimmunity, as some subtypes of neutrophils have been shown to be involved in the pathogenesis of these conditions (90). The identification of neutrophil subtypes has been facilitated by advances in single-cell genomics and proteomics techniques, which allow for the characterization of individual cells at the molecular level (91). The discovery of neutrophil heterogeneity has opened up new avenues for research into the immune response and the development of new therapies for inflammatory and autoimmune diseases (92). The potential of neutrophil-targeted therapies in various diseases is summarized in Table 3, which lists the mechanism of action, preclinical and clinical trial results, and potential side effects of different therapies. Recent advances in single-cell genomics and proteomics techniques have enabled the identification and characterization of different subtypes of neutrophils (106). These subtypes are distinguished by differences in their gene expression profiles, cell surface markers, and functional properties (107). It is notable that low-density neutrophils (LDNs) and high-density neutrophils (HDNs) were first identified back in 1986 (108). More recent studies have expanded our understanding of these subtypes, revealing that LDNs are typically more abundant in patients with autoimmune and inflammatory diseases and are associated with increased production of pro-inflammatory cytokines (109). In contrast, HDNs are more efficient at phagocytosis and have a higher oxidative burst capacity (110). Other subtypes of neutrophils have also been identified based on differences in their response to cytokines and chemokines (111). For example, neutrophils primed with interferon-gamma (IFN-γ) have been shown to be more efficient at phagocytosis and killing of bacteria (112). The functional differences between these subtypes of neutrophils have important implications for our understanding of the immune response and the development of new therapies for inflammatory and autoimmune diseases (113). Recent advances in the field of neutrophil biology have clearly underlined the heterogeneous nature of these critical immune cells (52). Neutrophils are no longer considered a homogenous population, but rather a highly versatile group with subsets that differ in their phenotypes and functions (114), crucial in maintaining homeostasis as well as responding to disease states. The heterogeneity of neutrophils can be influenced by several factors including the nature of the stimulus, the microenvironment, and their maturity (115). For example, research has highlighted the existence of low-density neutrophils (LDNs) and high-density neutrophils (HDNs) (116), which can be distinguished based on their buoyant density, morphological, and functional characteristics (117). LDNs are generally associated with chronic inflammation and are known to possess pro-inflammatory characteristics (117), while HDNs are usually found in healthy individuals, carrying out regular neutrophil functions such as phagocytosis and degranulation (46). Furthermore, the process of aging also significantly impacts neutrophil heterogeneity, introducing another level of complexity (118). The aforementioned heterogeneity plays a critical role during wound healing; different subsets of neutrophils have been found to be involved at various stages of wound healing (119), from the early inflammatory phase, where they act as the first line of defense against potential pathogens, to later stages, where they aid in tissue repair and regeneration (57, 120, 121). Therefore, a comprehensive understanding of neutrophil heterogeneity, along with the factors influencing it, is crucial for the elucidation of the complex role these cells play in health and disease. Recent studies have suggested that aging can impact neutrophil heterogeneity and contribute to immune dysregulation (120). For example, aging has been associated with changes in the gene expression profile of neutrophils, which may alter their functional properties and contribute to immune dysfunction (121). Moreover, aging can lead to the accumulation of cellular damage, which may affect the ability of neutrophils to respond to stimuli (57). These changes may also affect the balance between different subtypes of neutrophils, leading to an altered immune response (122). For example, aging has been associated with a shift towards pro-inflammatory neutrophil subtypes, which may contribute to chronic inflammation and increased susceptibility to infections (123). Additionally, aging can affect the bone marrow microenvironment, which may impact the production and differentiation of neutrophils (124). These findings highlight the importance of considering the impact of aging on neutrophil heterogeneity when developing new therapies for infectious and inflammatory diseases in older adults (125). Further research is needed to better understand the mechanisms underlying the impact of aging on neutrophil heterogeneity and immune function. Recent progress in research has highlighted the potential effects of aging and neutrophil heterogeneity on COVID-19, offering valuable insights into disease pathogenesis and clinical outcomes. Aging is associated with immunosenescence, a gradual decline in immune system function, which can impair the body's ability to effectively combat viral infections such as COVID-19. This age-related decline in immune response is of particular concern as it may contribute to increased disease severity and poorer outcomes in older individuals. Additionally, studies have revealed that neutrophils, a type of white blood cell crucial for immune defense, exhibit significant heterogeneity in their response to viral infections. This heterogeneity can impact the immune response to COVID-19, leading to variations in disease progression and patient outcomes. Understanding the underlying mechanisms and functional differences in neutrophil subsets holds promise for developing targeted therapeutic interventions and personalized treatment strategies for COVID-19 patients. Aging is a significant risk factor for severe outcomes in many infectious diseases, including COVID-19. The major contributor to this increased risk is immunosenescence, a state of gradual immune system deterioration that arises naturally with age. Immunosenescence impacts both the innate and adaptive arms of the immune response, including neutrophil function.
4 Neutrophils and COVID-19
4.1 Summarize the current understanding of neutrophil involvement in COVID-19 pathogenesis
COVID-19 is a respiratory illness caused by the SARS-CoV-2 virus that has rapidly spread across the globe (126), leading to a global pandemic. Recent studies have suggested that neutrophils may play a crucial role in the pathogenesis of COVID-19 (127). Neutrophils are rapidly recruited to the lungs of COVID-19 patients and have been shown to release large amounts of reactive oxygen species (ROS) and pro-inflammatory cytokines, which contribute to the development of acute respiratory distress syndrome (ARDS) and multi-organ failure (128). Moreover, neutrophil extracellular traps (NETs) have been identified in the lungs of COVID-19 patients, which can contribute to thrombosis and tissue damage (129). The dysregulated neutrophil response in COVID-19 may be driven by a combination of factors, including virus-induced immune dysregulation, cytokine storm, and bacterial co-infections (130). These findings suggest that targeting neutrophil activation and recruitment may be a promising strategy for the treatment of COVID-19 (131). Several ongoing clinical trials are currently investigating the efficacy of neutrophil-targeted therapies in COVID-19 treatment. As we delve deeper into the interplay of aging, neutrophil heterogeneity, and COVID-19 severity, we can observe distinct patterns. These patterns, summarized in Table 1, clearly show that the immunosenescence and the nature of the neutrophil response vary significantly across age groups. These variations can influence the severity of COVID-19 symptoms, which generally tend to be milder in younger adults and more severe in older individuals. This highlights the importance of age and immune function, specifically neutrophil behavior, in determining the clinical outcomes of COVID-19 (Supplementary Table 1). Neutrophils are the most abundant type of white blood cell and form an essential first line of defense against infections (132, 133). They are traditionally considered short-lived, reactive cells that rapidly respond to infection signals (134). However, recent studies have begun to reveal the complex and varied nature of neutrophil biology (132). Far from being a homogenous population, neutrophils can be differentiated into various subpopulations based on their phenotype, function, and the context of the immune response (46, 135). In the context of COVID-19, neutrophil heterogeneity appears to play a critical role (136). The study underlines the crucial role of neutrophils in COVID-19, identifying a link between increased immature neutrophil populations and disease severity, while suggesting potential therapies targeting neutrophil-induced tissue damage (136). Different neutrophil subsets can be found in patients with varying severity of the disease (137, 138). Some subsets are associated with a heightened inflammatory response, often linked to severe disease and negative outcomes (24, 139, 140), while other subsets appear to be more regulatory, promoting resolution of inflammation and tissue repair (19). These observations have led to the emerging concept of "neutrophil plasticity" – the ability of neutrophils to dynamically adapt their functions in response to changes in the environment (124, 141). The age-related decline in immune function can exacerbate the dysregulation of the neutrophil response, leading to an overactive, damaging immune reaction – a situation often seen in severe COVID-19 cases (8). This study probes the association between severe COVID-19 and the aging immune system, particularly focusing on the exacerbated dysregulation of neutrophil response in the elderly. It posits that interventions targeting age-associated pathways could fortify immunity across diverse age cohorts, hence potentially reducing fatalities and enduring disabilities triggered by the pandemic (8). The characteristic 'cytokine storm' in severe COVID-19, driven by a hyperactive immune response, can cause extensive tissue damage and organ failure, leading to critical illness or death (142, 143). Older individuals, due to their compromised immune system, are more susceptible to this dysregulated immune response, explaining, at least in part, their increased vulnerability to severe disease (144, 145). Understanding the relationship between aging, neutrophil heterogeneity, and COVID-19 progression is essential for developing targeted therapies (146). By identifying key mechanisms driving neutrophil behavior in the context of age and COVID-19, researchers may develop strategies to modulate the immune response, mitigating the harmful effects while promoting protective immunity (147). Such an approach could involve the use of pharmaceutical agents to modify neutrophil function or the design of personalized treatment strategies based on a patient's specific neutrophil subset profile (148, 149). In conclusion, the complexities of the aging immune system and the multifaceted nature of neutrophil biology hold both challenges and opportunities for tackling COVID-19. Further research in these areas has the potential to yield significant improvements in the clinical management of the disease, particularly for vulnerable older populations.
4.2 Discuss the potential mechanisms underlying neutrophil activation and recruitment in COVID-19
The mechanisms underlying neutrophil activation and recruitment in COVID-19 are complex and multifactorial (150). The SARS-CoV-2 virus is known to directly infect and activate immune cells, including neutrophils, through binding to the angiotensin-converting enzyme 2 (ACE2) receptor (151). This activation can lead to the release of cytokines and chemokines, which further recruit and activate neutrophils to the site of infection (152). Moreover, COVID-19 is associated with a cytokine storm, which is characterized by the overproduction of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) (153). These cytokines can activate neutrophils and promote their recruitment to the lungs, where they contribute to the development of ARDS and tissue damage (154). Additionally, bacterial co-infections are common in COVID-19 patients, and the presence of bacterial products, such as lipopolysaccharides (LPS), can further activate and recruit neutrophils (154). The dysregulated neutrophil response in COVID-19 may be a result of a combination of these factors, and understanding the underlying mechanisms may lead to the development of new therapeutic approaches to target neutrophil activation and recruitment in COVID-19 (155).
4.3 Evaluate the evidence for neutrophil-targeted therapies in COVID-19 treatment
There is growing interest in the potential of neutrophil-targeted therapies as a strategy for the treatment of COVID-19 (156). Several preclinical studies have suggested that blocking neutrophil recruitment and activation may improve clinical outcomes in COVID-19. For example, the use of anti-IL-8 antibodies (10), which block the recruitment of neutrophils, has been shown to reduce lung injury in animal models of COVID-19 (157). Other approaches, such as the use of NET inhibitors, have also shown promise in preclinical studies (158). Moreover, several ongoing clinical trials are investigating the efficacy of neutrophil-targeted therapies in COVID-19 treatment (159). One example is the use of dornase alfa, an FDA-approved medication used in the treatment of cystic fibrosis, which has been shown to degrade NETs and improve lung function in COVID-19 patients (160). However, there are also concerns that targeting neutrophils may impair the immune response to the virus, and the long-term effects of these therapies are not yet fully understood. Therefore, further research is needed to evaluate the safety and efficacy of neutrophil-targeted therapies in COVID-19 treatment (159).
5 Neutrophils and wound healing
Neutrophils play a critical role in the early stages of the wound healing process (161). Once a tissue injury occurs, neutrophils are rapidly recruited to the site of the wound, where they remove debris and bacteria through phagocytosis and the release of reactive oxygen species (ROS) (162). Neutrophils also release cytokines and chemokines, which recruit other immune cells to the site of the wound and promote angiogenesis and tissue remodeling (163). However, the excessive accumulation of neutrophils can also contribute to tissue damage and impair the wound healing process (164). Therefore, the balance between the pro-inflammatory and anti-inflammatory functions of neutrophils is critical for optimal wound healing (165). Recent studies have also suggested that different subtypes of neutrophils may have distinct functions in the wound healing process (164), and that the dysregulation of neutrophil heterogeneity may contribute to impaired wound healing in some conditions, such as diabetes (18). These findings suggest that targeting neutrophil function and heterogeneity may have therapeutic potential for improving wound healing outcomes. Recent studies have suggested that aging and neutrophil heterogeneity can impact the wound healing process (46). Aging is associated with a decline in neutrophil function, including impaired phagocytosis and oxidative burst, reduced cytokine production, and altered chemotaxis (166). These changes may impair the ability of neutrophils to effectively remove debris and bacteria from the site of the wound, leading to delayed wound healing and increased susceptibility to infections (167). Moreover, aging can also alter the gene expression profile of neutrophils and affect their response to stimuli, which may contribute to impaired wound healing (168). In addition, recent studies have identified different subtypes of neutrophils that have distinct functions in the wound healing process, such as those that are more efficient at phagocytosis or cytokine production (169). The dysregulation of neutrophil heterogeneity may contribute to impaired wound healing in some conditions, such as diabetes, where an imbalance between different neutrophil subtypes has been reported (170). These findings highlight the importance of considering the impact of aging and neutrophil heterogeneity on the wound healing process when developing new therapies to improve wound healing outcomes (171). Further research is needed to better understand the mechanisms underlying these effects and develop strategies to modulate neutrophil function and heterogeneity in older adults and those with impaired wound healing (172). The development of neutrophil-targeted therapies may hold promise for improving wound healing outcomes (12), particularly in conditions where impaired neutrophil function or dysregulated neutrophil activation is implicated in delayed healing (173). Recent studies have investigated various approaches for targeting neutrophils in the context of wound healing, including the use of anti-inflammatory agents (174), such as corticosteroids, and the inhibition of neutrophil-derived ROS and proteases (175). For example, the use of the ROS scavenger, N-acetylcysteine (NAC), has been shown to promote wound healing in diabetic mice by reducing oxidative stress and promoting angiogenesis (176). In addition, the use of protease inhibitors, such as serpinB1, has been shown to improve wound healing outcomes by reducing the activity of neutrophil-derived proteases (177), which can impair tissue regeneration. Moreover, recent studies have also suggested that modulating the balance between different subtypes of neutrophils may have therapeutic potential for improving wound healing outcomes (178). However, the safety and efficacy of these therapies in humans is not yet fully understood, and further research is needed to optimize these approaches and evaluate their potential clinical utility (179).
5.1 Impacts of COVID-19 on wound healing: nutritional status, skin manifestations, and immunosuppression
In the wake of recent advancements, a better understanding of how severe COVID-19 illness can detrimentally affect a patient's nutritional status has been developed (180–182). Nutrition is an integral component of wound healing, providing the necessary elements for tissue repair and immune function (183–185). Patients suffering from severe COVID-19, however, may experience drastic changes in their nutritional status due to various factors such as decreased appetite (186), increased metabolic demand due to the infection, or digestive complications associated with the disease (187, 188). This state of malnutrition may subsequently impede the wound healing process (189). Insufficient intake of protein, for example, can hinder tissue synthesis, while deficiencies in vitamins and minerals can disrupt collagen formation and immune response (190, 191), both crucial for wound recovery. Hence, malnutrition not only delays wound healing but also escalates the risk of complications, such as infection or wound dehiscence (192, 193). This understanding underscores the need for thorough nutritional assessment and appropriate dietary interventions in managing wound care for COVID-19 patient (194, 195). In light of recent studies, it has been observed that some patients with severe COVID-19 exhibit skin manifestations (196, 197), such as rashes or pseudo-chilblain lesions, colloquially known as "COVID toes." These dermatological symptoms likely arise from the virus's interaction with cells in the skin or as part of the body's immune response to the virus (198, 199). However, the precise correlation between these skin changes and wound healing remains elusive (200, 201). Some theories suggest that the increased inflammatory response associated with these skin conditions could potentially affect the phases of wound healing, which are inflammation, proliferation, and remodeling (202, 203). For instance, an exaggerated inflammatory response might lead to prolonged or chronic inflammation, delaying the progression to the subsequent phases of wound healing (204–206). Furthermore, if COVID-19 affects the blood vessels in the skin, as has been suggested by the presentation of pseudo-chilblain lesions (206, 207), this could impair the delivery of oxygen and nutrients essential for wound healing (208, 209). Ongoing research aims to elucidate the mechanisms underlying these observations, which will be crucial in tailoring wound care strategies for patients with severe COVID-19. Progressing research has begun to understand the intersection of COVID-19 and the immune system's responses, particularly regarding wound healing (210). Current research explores how COVID-19, immune responses, and wound healing are interlinked. Chronic conditions like MetS and T2DM often cause inflammation (211). COVID-19, due to an atypical immune response, triggers a unique cytokine storm, exacerbating inflammation. The prolonged inflammatory responses by SARS-CoV-2 can result in chronic inflammation and potential damage, such as fibrosis and pancreatic islet apoptosis (212). One of the significant discoveries is that COVID-19 infection often results in a reduced number of lymphocytes - a type of white blood cell that is vital in the immune response (213, 214). This is critical because lymphocytes, including T-cells and B-cells, play a pivotal role in the wound healing process, which includes phases of inflammation, proliferation, and remodeling (215, 216). They help orchestrate other cells' activities, release cytokines, and aid in combating potential infections at the wound site (184, 217). Therefore, a reduction in lymphocyte count, or immunosuppression, due to COVID-19 can potentially delay or impair the wound healing process (218, 219). This has significant implications for patient care, particularly for those who may require surgery or those with pre-existing wounds. Further research is needed to fully understand this process and develop strategies to support wound healing in the context of COVID-19 (220, 221). Considering recent progress in understanding the effects of severe COVID-19 on wound healing, a multi-faceted approach is necessary for future therapy development and improvement of mechanisms. Enhanced nutritional support should be a focus area, considering the role of malnutrition in impeding wound healing (222). Novel strategies to manage appetite loss and digestion complications should be explored along with high-protein diets or supplements and adequate micronutrient intake. Simultaneously, dermatological treatments should be considered for patients exhibiting skin manifestations (199, 223, 224). Unraveling the links between skin changes and wound healing could lead to targeted topical treatments that manage inflammation and support the skin's natural healing process. Furthermore, immunotherapy might be crucial given COVID-19's impact on lymphocyte counts. Therapies to restore lymphocyte function or count, such as immunomodulatory drugs or cytokine therapies, may offer new avenues for supporting wound healing (225). Personalized medicine, combining nutritional support, skin care, and immunotherapy, could maximize patient outcomes, necessitating further research into individual variations. Comprehensive and ongoing research into these mechanisms will be pivotal to develop robust therapeutic strategies and improve patient recovery and quality of life in the context of COVID-19.
6 Conclusion
In this review, we discussed recent progress in understanding the role of neutrophils in various physiological and pathological processes, including immune response, aging, COVID-19, and wound healing. We highlighted the importance of considering the heterogeneity of neutrophils and its impact on immune function and disease pathogenesis. We also reviewed the potential of neutrophil-targeted therapies in various diseases, including COVID-19, and discussed the potential effects of aging and neutrophil heterogeneity on wound healing outcomes. The research in this field has identified novel therapeutic targets and provided insights into the underlying mechanisms of disease pathogenesis. The implications of this research for future studies and clinical practice are significant, as it may lead to the development of more targeted and effective therapies for infectious and inflammatory diseases and improved wound healing outcomes. Moreover, the identification of neutrophil subtypes and the characterization of their functions may facilitate the development of personalized medicine approaches. However, further research is needed to fully understand the complexity of neutrophil biology and its impact on various disease processes.
Author contributions
WW: writing, editing, reviewing, and conceptualization. CX: writing, editing, reviewing, and conceptualization. LY: writing, editing, reviewing, and conceptualization. CL: reviewing and editing. CC: reviewing, editing, and supervision. ZQ: writing and revision of the manuscript. YL: writing and conceptualization. YZ: revision and managing the project. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Sichuan Provincial People's Hospital Program (2020LY08) and Sichuan Science and Technology Program (2020YFS0434).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1201651/full#supplementary-material
References
1. Liu L, Sun B. Neutrophil pyroptosis: new perspectives on sepsis. Cell Mol Life Sci CMLS (2019) 76(11):2031–42. doi: 10.1007/s00018-019-03060-1
2. Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res (2018) 371(3):531–9. doi: 10.1007/s00441-017-2785-7
3. Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev (2016) 273(1):329–43. doi: 10.1111/imr.12459
4. Wigerblad G, Cao Q, Brooks S, Naz F, Gadkari M, Jiang K, et al. Single-cell analysis reveals the range of transcriptional states of circulating human neutrophils. J Immunol (2022) 209(4):772–82. doi: 10.4049/jimmunol.2200154
5. Xie X, Shi Q, Wu P, Zhang X, Kambara H, Su J, et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat Immunol (2020) 21(9):1119–33. doi: 10.1038/s41590-020-0736-z
6. Deniset JF, Kubes P. Neutrophil heterogeneity: Bona fide subsets or polarization states? J leukocyte Biol (2018) 103(5):829–38. doi: 10.1002/JLB.3RI0917-361R
7. Scapini P, Marini O, Tecchio C, Cassatella MA. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev (2016) 273(1):48–60. doi: 10.1111/imr.12448
8. Bartleson JM, Radenkovic D, Covarrubias AJ, Furman D, Winer DA, Verdin E. SARS-coV-2, COVID-19 and the ageing immune system. Nat Aging (2021) 1(9):769–82. doi: 10.1038/s43587-021-00114-7
9. Lynch SM, Guo G, Gibson DS, Bjourson AJ, Rai TS. Role of senescence and aging in SARS-coV-2 infection and COVID-19 disease. Cells (2021) 10(12):3367. doi: 10.3390/cells10123367
10. Caillon A, Trimaille A, Favre J, Jesel L, Morel O, Kauffenstein G. Role of neutrophils, platelets, and extracellular vesicles and their interactions in COVID-19-associated thrombopathy. J Thromb haemostasis JTH (2022) 20(1):17–31. doi: 10.1111/jth.15566
11. Nolan E, Malanchi I. Connecting the dots: Neutrophils at the interface of tissue regeneration and cancer. Semin Immunology Elsevier (2022) p:101598. doi: 10.1016/j.smim.2022.101598
12. Van Bruggen S, Martinod K. The coming of age of neutrophil extracellular traps in thrombosis: Where are we now and where are we headed? Immunol Rev (2023) 314(1):376–98. doi: 10.1111/imr.13179
13. Mincham KT, Bruno N, Singanayagam A, Snelgrove RJ. Our evolving view of neutrophils in defining the pathology of chronic lung disease. Immunology (2021) 164(4):701–21. doi: 10.1111/imm.13419
14. Siwicki M, Pittet MJ. Versatile neutrophil functions in cancer. In: Seminars in Immunology. Amsterdam, Netherlands: Elsevier (2021). p. 101538.
15. Ramón YCS, Sesé M, Capdevila C, Aasen T, De Mattos-Arruda L, Diaz-Cano SJ, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med (Berlin Germany) (2020) 98(2):161–77. doi: 10.1007/s00109-020-01874-2
16. Singhal A, Kumar S. Neutrophil and remnant clearance in immunity and inflammation. Immunology (2022) 165(1):22–43. doi: 10.1111/imm.13423
17. Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal transduction targeted Ther (2020) 5(1):84. doi: 10.1038/s41392-020-0191-1
18. Barman PK, Koh TJ. Macrophage dysregulation and impaired skin wound healing in diabetes. Front Cell Dev Biol (2020) 8:528. doi: 10.3389/fcell.2020.00528
19. Patel AA, Ginhoux F, Yona S. Monocytes, macrophages, dendritic cells and neutrophils: an update on lifespan kinetics in health and disease. Immunology (2021) 163(3):250–61. doi: 10.1111/imm.13320
20. Alam A, Thelin EP, Tajsic T, Khan DZ, Khellaf A, Patani R, et al. Cellular infiltration in traumatic brain injury. J Neuroinflamm (2020) 17(1):328. doi: 10.1186/s12974-020-02005-x
21. Block H, Zarbock A. A fragile balance: does neutrophil extracellular trap formation drive pulmonary disease progression? Cells (2021) 10(8):1932. doi: 10.3390/cells10081932
22. Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb haemostasis JTH (2014) 12(11):1764–75. doi: 10.1111/jth.12730
23. Hajishengallis G. New developments in neutrophil biology and periodontitis. Periodontology (2000) 82(1):78–92. doi: 10.1111/prd.12313
24. Morrissey SM, Geller AE, Hu X, Tieri D, Ding C, Klaes CK, et al. A specific low-density neutrophil population correlates with hypercoagulation and disease severity in hospitalized COVID-19 patients. JCI Insight (2021) 6(9). doi: 10.1172/jci.insight.148435
25. Sas AR, Carbajal KS, Jerome AD, Menon R, Yoon C, Kalinski AL, et al. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat Immunol (2020) 21(12):1496–505. doi: 10.1038/s41590-020-00813-0
26. Tavares IF, Dos Santos JB, Pacheco FDS, Gandini M, Mariante RM, Rodrigues TF, et al. Mycobacterium leprae induces neutrophilic degranulation and low-density neutrophil generation during erythema nodosum leprosum. Front Med (2021) 8:711623. doi: 10.3389/fmed.2021.711623
27. Cho Y, Bukong TN, Tornai D, Babuta M, Vlachos IS, Kanata E, et al. Neutrophil extracellular traps contribute to liver damage and increase defective low-density neutrophils in alcohol-associated hepatitis. J Hepatol (2023) 78(1):28–44. doi: 10.1016/j.jhep.2022.08.029
28. Cerecedo D, Martínez-Vieyra I, López-Villegas EO, Hernández-Cruz A, Loza-Huerta ADC. Heterogeneity of neutrophils in arterial hypertension. Exp Cell Res (2021) 402(2):112577. doi: 10.1016/j.yexcr.2021.112577
29. Chen H, Zhang X, Su H, Zeng J, Chan H, Li Q, et al. Immune dysregulation and RNA N6-methyladenosine modification in sepsis, Wiley interdisciplinary reviews. RNA (2023) 14(3):e1764. doi: 10.1002/wrna.1764
30. Albillos A, Martin-Mateos R, van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol (2022) 19(2):112–34. doi: 10.1038/s41575-021-00520-7
31. Ganesh K, Joshi MB. Neutrophil sub-types in maintaining immune homeostasis during steady state, infections and sterile inflammation. Inflammation Res Off J Eur Histamine Res Soc (2023) 72(6):1175–92. doi: 10.1007/s00011-023-01737-9
32. Domellöf M, Berglund SK. Nutritional anemia in infants and children. In: Karakochuk CD, Zimmermann MB, Moretti D, Kraemer K, editors. Nutritional anemia. Cham: Springer International Publishing (2022). p. 77–90.
33. Kraus RF, Gruber MA. Neutrophils-from bone marrow to first-line defense of the innate immune system. Front Immunol (2021) 12:767175. doi: 10.3389/fimmu.2021.767175
34. Tsui M, Min W, Ng S, Dobbs K, Notarangelo LD, Dror Y, et al. The use of induced pluripotent stem cells to study the effects of adenosine deaminase deficiency on human neutrophil development. Front Immunol (2021) 12:748519. doi: 10.3389/fimmu.2021.748519
35. Dutta A, Bhagat S, Paul S, Katz JP, Sengupta D, Bhargava D. Neutrophils in cancer and potential therapeutic strategies using neutrophil-derived exosomes, vaccines. (2023) 11(6):. doi: 10.3390/vaccines11061028
36. Arve-Butler S, Schmidt T, Mossberg A, Berthold E, Gullstrand B, Bengtsson AA, et al. Synovial fluid neutrophils in oligoarticular juvenile idiopathic arthritis have an altered phenotype and impaired effector functions. Arthritis Res Ther (2021) 23(1):109. doi: 10.1186/s13075-021-02483-1
37. Lauszus JS, Eriksen PL, Hansen MM, Eriksen LL, Shawcross DL, Vilstrup H, et al. Activation and functional priming of blood neutrophils in non-alcoholic fatty liver disease increases in non-alcoholic steatohepatitis. Clin Exp Gastroenterol (2021) 14:441–9. doi: 10.2147/CEG.S329424
38. Sengupta S, Caldwell CC, Nomellini V. Distinct neutrophil populations in the spleen during PICS. Front Immunol (2020) 11:804. doi: 10.3389/fimmu.2020.00804
39. Kvedaraite E. Neutrophil-T cell crosstalk in inflammatory bowel disease. Immunology (2021) 164(4):657–64. doi: 10.1111/imm.13391
40. Costa S, Bevilacqua D, Cassatella MA, Scapini P. Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology (2019) 156(1):23–32. doi: 10.1111/imm.13005
41. Li Y, Wang W, Yang F, Xu Y, Feng C, Zhao Y. The regulatory roles of neutrophils in adaptive immunity. Cell communication Signaling CCS (2019) 17(1):147. doi: 10.1186/s12964-019-0471-y
42. Meinderts SM, Baker G, van Wijk S, Beuger BM, Geissler J, Jansen MH, et al. Neutrophils acquire antigen-presenting cell features after phagocytosis of IgG-opsonized erythrocytes. Blood Adv (2019) 3(11):1761–73. doi: 10.1182/bloodadvances.2018028753
43. Bhattacharya P, Ismail N, Saxena A, Gannavaram S, Dey R, Oljuskin T, et al. Neutrophil-dendritic cell interaction plays an important role in live attenuated Leishmania vaccine induced immunity. PloS Negl Trop Dis (2022) 16(2):e0010224. doi: 10.1371/journal.pntd.0010224
44. Del Prete A, Salvi V, Soriani A, Laffranchi M, Sozio F, Bosisio D, et al. Dendritic cell subsets in cancer immunity and tumor antigen sensing. In: Cellular & Molecular Immunology. Germany, Berlin: Springer Nature (2023).
45. Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil. Immunity (2021) 54(7):1377–91. doi: 10.1016/j.immuni.2021.06.006
46. Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P. Neutrophil diversity in health and disease. Trends Immunol (2019) 40(7):565–83. doi: 10.1016/j.it.2019.04.012
47. Nedeva C, Menassa J, Duan M, Liu C, Doerflinger M, Kueh AJ, et al. TREML4 receptor regulates inflammation and innate immune cell death during polymicrobial sepsis. Nat Immunol (2020) 21(12):1585–96. doi: 10.1038/s41590-020-0789-z
48. Al-Marzooqi W, Al-Aufi SK, Ali H, El-Zaiat HM, Al-Kharousi K, Al-Adwani SR, et al. Comparative study of ultrastructural observations on blood cells of local Omani and cobb 500 broiler chickens: ultrastructural observations on blood cells of two strains of chickens. J Agric Mar Sci [JAMS] (2023) 28(1):62–9. doi: 10.53541/jams.vol28iss1pp62-69
49. Gupta S, Undale VR, Lakhadive K. Novel targets for antimicrobials. Turkish J Pharm Sci (2020) 17(5):565–75. doi: 10.4274/tjps.galenos.2020.90197
50. Ballesteros I, Rubio-Ponce A, Genua M, Lusito E, Kwok I, Fernández-Calvo G, et al. Co-option of neutrophil fates by tissue environments. Cell (2020) 183(5):1282–1297.e18. doi: 10.1016/j.cell.2020.10.003
51. Ng LG, Liu Z, Kwok I, Ginhoux F. Origin and heterogeneity of tissue myeloid cells: A focus on GMP-derived monocytes and neutrophils. Annu Rev Immunol (2023) 41:375–404. doi: 10.1146/annurev-immunol-081022-113627
52. Siwicki M, Kubes P. Neutrophils in host defense, healing, and hypersensitivity: Dynamic cells within a dynamic host. J Allergy Clin Immunol (2023) 151(3):634–55. doi: 10.1016/j.jaci.2022.12.004
53. Turgeon ML. Immunology & Serology in laboratory medicine-E-book. Amsterdam, Netherlands: Elsevier Health Sciences (2020).
54. de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol (2016) 16(6):378–91. doi: 10.1038/nri.2016.49
55. Yamamoto M, Aizawa R. Maintaining a protective state for human periodontal tissue. Periodontology (2000) 86(1):142–56. doi: 10.1111/prd.12367
56. Lee M, Lee SY, Bae YS. Emerging roles of neutrophils in immune homeostasis. BMB Rep (2022) 55(10):473–80. doi: 10.5483/BMBRep.2022.55.10.115
57. Clark D, Kotronia E, Ramsay SE. Frailty, aging, and periodontal disease: Basic biologic considerations. Periodontology (2000) 87(1):143–56. doi: 10.1111/prd.12380
58. Finger CE, Moreno-Gonzalez I, Gutierrez A, Moruno-Manchon JF, McCullough LD. Age-related immune alterations and cerebrovascular inflammation. Mol Psychiatry (2022) 27(2):803–18. doi: 10.1038/s41380-021-01361-1
59. Barkaway A, Rolas L, Joulia R, Bodkin J, Lenn T, Owen-Woods C, et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity (2021) 54(7):1494–1510.e7. doi: 10.1016/j.immuni.2021.04.025
60. Simmons SR, Bhalla M, Herring SE, Tchalla EYI, Bou Ghanem EN. Older but Not Wiser: the Age-Driven Changes in Neutrophil Responses during Pulmonary Infections. Infection Immun (2021) 89(4). doi: 10.1128/IAI.00653-20
61. Xie X, Shi Q, Wu P, Zhang X, Kambara H, Su J, et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat Immunol (2020) 21(9):1119–33. doi: 10.1038/s41590-020-0736-z
62. Trtica Majnarić L, Guljaš S, Bosnić Z, Šerić V, Wittlinger T. Neutrophil-to-lymphocyte ratio as a cardiovascular risk marker may be less efficient in women than in men. biomolecules (2021) 11(4):528. doi: 10.3390/biom11040528
63. Rodrigues LP, Teixeira VR, Alencar-Silva T, Simonassi-Paiva B, Pereira RW, Pogue R, et al. Hallmarks of aging and immunosenescence: Connecting the dots. Cytokine Growth Factor Rev (2021) 59:9–21. doi: 10.1016/j.cytogfr.2021.01.006
64. Hammond ME, Lapointe GR, Feucht PH, Hilt S, Gallegos CA, Gordon CA, et al. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J Immunol (1995) 155(3):1428–33. doi: 10.4049/jimmunol.155.3.1428
65. Jorens PG, Richman-Eisenstat JB, Housset BP, Massion PP, Ueki I, Nadel JA. Pseudomonas-induced neutrophil recruitment in the dog airway in vivo is mediated in part by IL-8 and inhibited by a leumedin. Eur Respir J (1994) 7(11):1925–31. doi: 10.1183/09031936.94.07111925
66. Zhu X, Xiao L, Huo R, Zhang J, Lin J, Xie J, et al. Cyr61 is involved in neutrophil infiltration in joints by inducing IL-8 production by fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther (2013) 15(6):R187. doi: 10.1186/ar4377
67. Bilusic M, Heery CR, Collins JM, Donahue RN, Palena C, Madan RA, et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Immunother Cancer (2019) 7(1):240. doi: 10.1186/s40425-019-0706-x
68. Holliday ZM, Earhart AP, Alnijoumi MM, Krvavac A, Allen LH, Schrum AG. Non-randomized trial of dornase alfa for acute respiratory distress syndrome secondary to covid-19. Front Immunol (2021) 12:714833. doi: 10.3389/fimmu.2021.714833
69. Earhart AP, Holliday ZM, Hofmann HV, Schrum AG. Consideration of dornase alfa for the treatment of severe COVID-19 acute respiratory distress syndrome. New Microbes New infections (2020) 35:100689. doi: 10.1016/j.nmni.2020.100689
70. Weber AG, Chau AS, Egeblad M, Barnes BJ, Janowitz T. Nebulized in-line endotracheal dornase alfa and albuterol administered to mechanically ventilated COVID-19 patients: a case series. Mol Med (Cambridge Mass.) (2020) 26(1):91. doi: 10.1186/s10020-020-00215-w
71. Fisher J, Mohanty T, Karlsson CAQ, Khademi SMH, Malmström E, Frigyesi A, et al. Proteome profiling of recombinant DNase therapy in reducing NETs and aiding recovery in COVID-19 patients. Mol Cell Proteomics MCP (2021) 20:100113. doi: 10.1016/j.mcpro.2021.100113
72. Englert H, Rangaswamy C, Deppermann C, Sperhake JP, Krisp C, Schreier D, et al. Defective NET clearance contributes to sustained FXII activation in COVID-19-associated pulmonary thrombo-inflammation. EBioMedicine (2021) 67:103382. doi: 10.1016/j.ebiom.2021.103382
73. Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest (2012) 122(9):3127–44. doi: 10.1172/JCI61067
74. Stadtmann A, Zarbock A. CXCR2: from bench to bedside. Front Immunol (2012) 3:263. doi: 10.3389/fimmu.2012.00263
75. Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, et al. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infection Immun (2001) 69(4):2017–24. doi: 10.1128/IAI.69.4.2017-2024.2001
76. Ashar HK, Pulavendran S, Rudd JM, Maram P, Achanta M, Chow VTK, et al. Administration of a CXC chemokine receptor 2 (CXCR2) antagonist, SCH527123, together with oseltamivir suppresses NETosis and protects mice from lethal influenza and piglets from swine-influenza infection. Am J Pathol (2021) 191(4):669–85. doi: 10.1016/j.ajpath.2020.12.013
77. Lee SK, Kim SD, Kook M, Lee HY, Ghim J, Choi Y, et al. Phospholipase D2 drives mortality in sepsis by inhibiting neutrophil extracellular trap formation and down-regulating CXCR2. J Exp Med (2015) 212(9):1381–90. doi: 10.1084/jem.20141813
78. Breckwoldt MO, Chen JW, Stangenberg L, Aikawa E, Rodriguez E, Qiu S, et al. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci United States America (2008) 105(47):18584–9. doi: 10.1073/pnas.0803945105
79. El Kebir D, József L, Pan W, Filep JG. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ Res (2008) 103(4):352–9. doi: 10.1161/01.RES.0000326772.76822.7a
80. Davies MJ, Hawkins CL. The role of myeloperoxidase in biomolecule modification. Chronic Inflammation Disease Antioxidants Redox Signaling (2020) 32(13):957–81. doi: 10.1089/ars.2020.8030
81. Forghani R, Kim HJ, Wojtkiewicz GR, Bure L, Wu Y, Hayase M, et al. Myeloperoxidase propagates damage and is a potential therapeutic target for subacute stroke. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab (2015) 35(3):485–93. doi: 10.1038/jcbfm.2014.222
82. Ali M, Pulli B, Courties G, Tricot B, Sebas M, Iwamoto Y, et al. Myeloperoxidase inhibition improves ventricular function and remodeling after experimental myocardial infarction, JACC. Basic to Trans Sci (2016) 1(7):633–43. doi: 10.1007/s00540-017-2311-9
83. Aikawa N, Kawasaki Y. Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther Clin Risk Manage (2014) 10:621–9. doi: 10.2147/TCRM.S65066
84. Lee JM, Yeo CD, Lee HY, Rhee CK, Kim IK, Lee DG, et al. Inhibition of neutrophil elastase contributes to attenuation of lipopolysaccharide-induced acute lung injury during neutropenia recovery in mice. J Anesth (2017) 31(3):397–404. doi: 10.1007/s00540-017-2311-9
85. Tsai YF, Hwang TL. Neutrophil elastase inhibitors: a patent review and potential applications for inflammatory lung diseases (2010 - 2014). Expert Opin Ther patents (2015) 25(10):1145–58. doi: 10.1517/13543776.2015.1061998
86. Sahebnasagh A, Saghafi F, Safdari M, Khataminia M, Sadremomtaz A, Talaei Z, et al. Neutrophil elastase inhibitor (sivelestat) may be a promising therapeutic option for management of acute lung injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19. J Clin Pharm Ther (2020) 45(6):1515–9. doi: 10.1111/jcpt.13251
87. Buonfiglioli A, Hambardzumyan D. Macrophages and microglia: the cerberus of glioblastoma. Acta Neuropathologica Commun (2021) 9(1):54. doi: 10.1186/s40478-021-01156-z
88. Gause WC, Rothlin C, Loke PN. Heterogeneity in the initiation, development and function of type 2 immunity. Nat Rev Immunol (2020) 20(10):603–14 doi: 10.1038/s41577-020-0301-x
89. Zhao C, Deng H, Chen X. Harnessing immune response using reactive oxygen Species-Generating/Eliminating inorganic biomaterials for disease treatment. Advanced Drug Delivery Rev (2022) 188:114456. doi: 10.1016/j.addr.2022.114456
90. Wigerblad G, Kaplan MJ. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol (2023) 23(5):274–88. doi: 10.1038/s41577-022-00787-0
91. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol (2020) 17(8):807–21. doi: 10.1038/s41423-020-0488-6
92. Caetano AJ, Redhead Y, Karim F, Dhami P, Kannambath S, Nuamah R, et al. Spatially resolved transcriptomics reveals pro-inflammatory fibroblast involved in lymphocyte recruitment through CXCL8 and CXCL10. eLife (2023) 12:e81525. doi: 10.7554/eLife.81525.sa2
93. Moniot A, Braux J, Siboni R, Guillaume C, Audonnet S, Allart-Simon I, et al. Inhibition of recruitment and activation of neutrophils by pyridazinone-scaffold-based compounds. Int J Mol Sci (2022) 23(13):7226. doi: 10.3390/ijms23137226
94. Uribe-Querol E, Rosales C. Phagocytosis: our current understanding of a universal biological process. Front Immunol (2020) 11:1066. doi: 10.3389/fimmu.2020.01066
95. Metzemaekers M, Gouwy M, Proost P. Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol Immunol (2020) 17. doi: 10.1038/s41423-020-0412-0
96. Guimarães-Bastos D, Frony AC, Barja-Fidalgo C, Moraes JA. Melanoma-derived extracellular vesicles skew neutrophils into a pro-tumor phenotype. J leukocyte Biol (2022) 111(3):585–96. doi: 10.1002/JLB.3A0120-050RR
97. Abaricia JO, Shah AH, Musselman RM, Olivares-Navarrete R. Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis. Biomaterials Sci (2020) 8(8):2289–99. doi: 10.1039/C9BM01474H
98. Chen J, Hall S, Vitetta L. Altered gut microbial metabolites could mediate the effects of risk factors in Covid-19. Rev Med Virol (2021) 31(5):1–13. doi: 10.1002/rmv.2211
99. Darif D, Hammi I, Kihel A, El Idrissi Saik I, Guessous F, Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microbial pathogenesis (2021) 153:104799. doi: 10.1016/j.micpath.2021.104799
100. Keir HR, Chalmers JD. Neutrophil extracellular traps in chronic lung disease: implications for pathogenesis and therapy. Eur Respir Rev an Off J Eur Respir Soc (2022) 31(163). doi: 10.1183/16000617.0241-2021
101. Alflen A, Lopez PA, Hartmann AK, Maxeiner J, Bosmann M, Sharma A, et al. Neutrophil extracellular traps impair fungal clearance in a mouse model of invasive pulmonary aspergillosis. Immunobiology (2020) 225(1):151867. doi: 10.1016/j.imbio.2019.11.002
102. Yoshida Y, Takeshita S, Kawamura Y, Kanai T, Tsujita Y, Nonoyama S. Enhanced formation of neutrophil extracellular traps in Kawasaki disease. Pediatr Res (2020) 87(6):998–1004. doi: 10.1038/s41390-019-0710-3
103. Sharma P, Jha A. D. S, oxidative stress and antioxidative defense systems in plants growing under abiotic stresses. In: Handbook of plant and crop stress, 3rd Edition. Florida: CRC Press, Taylor & Francis Publishing Company (2010). p. 2010.
104. Burgos-Morón E, Abad-Jiménez Z, Marañón AM, Iannantuoni F, Escribano-López I, López-Domènech S, et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J Clin Med (2019) 8(9). doi: 10.3390/jcm8091385
105. Ruegsegger GN, Vanderboom PM, Dasari S, Klaus KA, Kabiraj P, McCarthy CB, et al. Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI Insight (2019) 4(18). doi: 10.1172/jci.insight.130681
106. Ratnasiri K, Wilk AJ, Lee MJ, Khatri P, Blish CA. Single-cell RNA-seq methods to interrogate virus-host interactions. In: Seminars in Immunopathology. Amsterdam, Netherlands: Springer (2023). p. 71–89.
107. Poulin J-F, Gaertner Z, Moreno-Ramos OA, Awatramani R. Classification of midbrain dopamine neurons using single-cell gene expression profiling approaches. Trends Neurosci (2020) 43(3):155–69. doi: 10.1016/j.tins.2020.01.004
108. Valadez-Cosmes P, Maitz K, Kindler O, Raftopoulou S, Kienzl M, Santiso A, et al. Identification of novel low-density neutrophil markers through unbiased high-dimensional flow cytometry screening in non-small cell lung cancer patients. Front Immunol (2021) 12:703846. doi: 10.3389/fimmu.2021.703846
109. Mes L, Steffen U, Chen HJ, Veth J, Hoepel W, Griffith GR, et al. IgA2 immune complexes selectively promote inflammation by human CD103(+) dendritic cells. Front Immunol (2023) 14:1116435. doi: 10.3389/fimmu.2023.1116435
110. Aridgides DS, Mellinger DL, Armstrong DA, Hazlett HF, Dessaint JA, Hampton TH, et al. Functional and metabolic impairment in cigarette smoke-exposed macrophages is tied to oxidative stress. Sci Rep (2019) 9(1):9624. doi: 10.1038/s41598-019-46045-7
111. Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol (2019) 16(10):601–20. doi: 10.1038/s41571-019-0222-4
112. Tran AC, Diogo GR, Paul MJ, Copland A, Hart P, Mehta N, et al. Mucosal therapy of multi-drug resistant tuberculosis with igA and interferon-γ. Front Immunol (2020) 11:582833. doi: 10.3389/fimmu.2020.582833
113. Fu X, Liu H, Huang G, Dai SS. The emerging role of neutrophils in autoimmune-associated disorders: effector, predictor, and therapeutic targets. MedComm (2021) 2(3):402–13. doi: 10.1002/mco2.69
114. Tecchio C, Cassatella MA. Uncovering the multifaceted roles played by neutrophils in allogeneic hematopoietic stem cell transplantation. Cell Mol Immunol (2021) 18(4):905–18. doi: 10.1038/s41423-020-00581-9
115. Rawat K, Syeda S, Shrivastava A. Neutrophil-derived granule cargoes: paving the way for tumor growth and progression. Cancer metastasis Rev (2021) 40(1):221–44. doi: 10.1007/s10555-020-09951-1
116. Cohen TS, Takahashi V, Bonnell J, Tovchigrechko A, Chaerkady R, Yu W, et al. Staphylococcus aureus drives expansion of low-density neutrophils in diabetic mice. J Clin Invest (2019) 129(5):2133–44. doi: 10.1172/JCI126938
117. Cesta MC, Zippoli M, Marsiglia C, Gavioli EM, Cremonesi G, Khan A, et al. Neutrophil activation and neutrophil extracellular traps (NETs) in COVID-19 ARDS and immunothrombosis. Eur J Immunol (2023) 53(1):e2250010. doi: 10.1002/eji.202250010
118. Wigerblad G, Kaplan MJ. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol (2023) 23(5):274–88. doi: 10.1038/s41577-022-00787-0
119. Liu Y, Zhang M, Liao Y, Chen H, Su D, Tao Y, et al. Human umbilical cord mesenchymal stem cell-derived exosomes promote murine skin wound healing by neutrophil and macrophage modulations revealed by single-cell RNA sequencing. Front Immunol (2023) 14:1142088. doi: 10.3389/fimmu.2023.1142088
120. Murao A, Aziz M, Wang P. Neutrophil heterogeneity in sepsis: the role of damage-associated molecular patterns. Shock (2023) 59(2):239–46. doi: 10.1097/SHK.0000000000002019
121. Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol (2019) 19(4):255–65. doi: 10.1038/s41577-019-0141-8
122. Jobin K, Müller DN, Jantsch J, Kurts C. Sodium and its manifold impact on our immune system. Trends Immunol (2021) 42(6):469–79. doi: 10.1016/j.it.2021.04.002
123. Mogilenko DA, Shchukina I, Artyomov MN. Immune ageing at single-cell resolution. Nat Rev Immunol (2022) 22(8):484–98. doi: 10.1038/s41577-021-00646-4
124. Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol (2022) 22(3):173–87. doi: 10.1038/s41577-021-00571-6
125. Bonaventura A, Montecucco F, Dallegri F, Carbone F, Lüscher TF, Camici GG, et al. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc Res (2019) 115(8):1266–85. doi: 10.1093/cvr/cvz084
126. Yashavantha Rao HC, Jayabaskaran C. The emergence of a novel coronavirus (SARS-CoV-2) disease and their neuroinvasive propensity may affect in COVID-19 patients. J Med Virol (2020) 92(7):786–90. doi: 10.1002/jmv.25918
127. Thierry AR, Roch B. Neutrophil Extracellular Traps and By-Products Play a Key Role in COVID-19: Pathogenesis, Risk Factors, and Therapy. J Clin Med (2020) 9(9). doi: 10.3390/jcm9092942
128. Arcanjo A, Logullo J, Menezes CCB, Giangiarulo TCdeSC, Reis MCD, Castro GMMde, et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci Rep (2020) 10(1):19630. doi: 10.1038/s41598-020-76781-0
129. Liana P, Liberty IA, Murti K, Hafy Z, Salim EM, Zulkarnain M, et al. A systematic review on neutrophil extracellular traps and its prognostication role in COVID-19 patients. Immunologic Res (2022) 70(4):449–60. doi: 10.1007/s12026-022-09293-w
130. Mellett L, Khader SA. S100A8/A9 in COVID-19 pathogenesis: Impact on clinical outcomes. Cytokine Growth Factor Rev (2022) 63:90–7. doi: 10.1016/j.cytogfr.2021.10.004
131. Meng QF, Tian R, Long H, Wu X, Lai J, Zharkova O, et al. Capturing cytokines with advanced materials: A potential strategy to tackle COVID-19 cytokine storm. Adv Mater (2021) 33(20):e2100012. doi: 10.1002/adma.202100012
132. Németh T, Sperandio M, Mócsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discovery (2020) 19(4):253–75. doi: 10.1038/s41573-019-0054-z
133. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol (2012) 30:459–89. doi: 10.1146/annurev-immunol-020711-074942
134. Ulfig A, Leichert LI. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell Mol Life Sci CMLS (2021) 78(2):385–414. doi: 10.1007/s00018-020-03591-y
135. Tay SH, Celhar T, Fairhurst AM. Low-density neutrophils in systemic lupus erythematosus. Arthritis Rheumatol (2020) 72(10):1587–95. doi: 10.1002/art.41395
136. Reusch N, De Domenico E, Bonaguro L, Schulte-Schrepping J, Baßler K, Schultze JL, et al. Neutrophils in COVID-19. Front Immunol (2021) 12:652470. doi: 10.3389/fimmu.2021.652470
137. deKay JT, Emery IF, Rud J, Eldridge A, Lord C, Gagnon DJ, et al. DEspR(high) neutrophils are associated with critical illness in COVID-19. Sci Rep (2021) 11(1):22463. doi: 10.1038/s41598-021-01943-7
138. McLeish KR, Shrestha R, Vashishta A, Rane MJ, Barati MT, Brier ME, et al. Differential functional responses of neutrophil subsets in severe COVID-19 patients. Front Immunol (2022) 13:879686. doi: 10.3389/fimmu.2022.879686
139. Gutiérrez-Bautista JF, Rodriguez-Nicolas A, Rosales-Castillo A, Jiménez P, Garrido F, Anderson P, et al. Negative clinical evolution in COVID-19 patients is frequently accompanied with an increased proportion of undifferentiated th cells and a strong underrepresentation of the th1 subset. Front Immunol (2020) 11:596553. doi: 10.3389/fimmu.2020.596553
140. Nasrollahi H, Talepoor AG, Saleh Z, Vakili ME, Heydarinezhad P, Karami N, et al. Immune responses in mildly versus critically ill COVID-19 patients. Front Immunol (2023) 14:1077236. doi: 10.3389/fimmu.2023.1077236
141. Hidalgo A, Casanova-Acebes M. Dimensions of neutrophil life and fate. Semin Immunol (2021) 57:101506. doi: 10.1016/j.smim.2021.101506
142. Vanderbeke L, Van Mol P, Van Herck Y, De Smet F, Humblet-Baron S, Martinod K, et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun (2021) 12(1):4117. doi: 10.1038/s41467-021-24360-w
143. Tang L, Yin Z, Hu Y, Mei H. Controlling cytokine storm is vital in COVID-19. Front Immunol (2020) 11:570993. doi: 10.3389/fimmu.2020.570993
144. Cunha LL, Perazzio SF, Azzi J, Cravedi P, Riella LV. Remodeling of the immune response with aging: immunosenescence and its potential impact on COVID-19 immune response. Front Immunol (2020) 11:1748. doi: 10.3389/fimmu.2020.01748
145. Bajaj V, Gadi N, Spihlman AP, Wu SC, Choi CH, Moulton VR. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front Physiol (2020) 11:571416. doi: 10.3389/fphys.2020.571416
146. Qin G, Liu S, Yang L, Yu W, Zhang Y. Myeloid cells in COVID-19 microenvironment. Signal transduction targeted Ther (2021) 6(1):372. doi: 10.1038/s41392-021-00792-0
147. Ho JQ, Sepand MR, Bigdelou B, Shekarian T, Esfandyarpour R, Chauhan P, et al. The immune response to COVID-19: Does sex matter? Immunol (2022) 166(4):429–43. doi: 10.1111/imm.13487
148. Haschka D, Tymoszuk P, Bsteh G, Petzer V, Berek K, Theurl I, et al. Expansion of neutrophils and classical and nonclassical monocytes as a hallmark in relapsing-remitting multiple sclerosis. Front Immunol (2020) 11:594. doi: 10.3389/fimmu.2020.00594
149. Engelen SE, Robinson AJB, Zurke YX, Monaco C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat Rev Cardiol (2022) 19(8):522–42. doi: 10.1038/s41569-021-00668-4
150. Loo J, Spittle DA, Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax (2021) 76(4):412–20. doi: 10.1136/thoraxjnl-2020-216243
151. Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol (2020) 251(3):228–48. doi: 10.1002/path.5471
152. Ma Y, Zhang Y, Zhu L. Role of neutrophils in acute viral infection. Immun Inflamm Dis (2021) 9(4):1186–96. doi: 10.1002/iid3.500
153. Roshanravan N, Seif F, Ostadrahimi A, Pouraghaei M, Ghaffari S. Targeting cytokine storm to manage patients with COVID-19: A mini-review. Arch Med Res (2020) 51(7):608–12. doi: 10.1016/j.arcmed.2020.06.012
154. Johansson C, Kirsebom FCM. Neutrophils in respiratory viral infections. Mucosal Immunol (2021) 14(4):815–27. doi: 10.1038/s41385-021-00397-4
155. Hazeldine J, Lord JM. Neutrophils and COVID-19: active participants and rational therapeutic targets. Front Immunol (2021) 12:680134. doi: 10.3389/fimmu.2021.680134
156. Dennison D, Al Khabori M, Al Mamari S, Aurelio A, Al Hinai H, Al Maamari K, et al. Circulating activated neutrophils in COVID-19: An independent predictor for mechanical ventilation and death. Int J Infect Dis IJID Off Publ Int Soc Infect Dis (2021) 106:155–9. doi: 10.1016/j.ijid.2021.03.066
157. Lartey NL, Valle-Reyes S, Vargas-Robles H, Jiménez-Camacho KE, Guerrero-Fonseca IM, Castellanos-Martínez R, et al. ADAM17/MMP inhibition prevents neutrophilia and lung injury in a mouse model of COVID-19. J leukocyte Biol (2022) 111(6):1147–58. doi: 10.1002/JLB.3COVA0421-195RR
158. Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discovery (2021) 20(9):689–709. doi: 10.1038/s41573-021-00233-1
159. Pollock J, Chalmers JD. The immunomodulatory effects of macrolide antibiotics in respiratory disease. Pulmonary Pharmacol Ther (2021) 71:102095. doi: 10.1016/j.pupt.2021.102095
160. Okur HK, Yalcin K, Tastan C, Demir S, Yurtsever B, Karakus GS, et al. Preliminary report of in vitro and in vivo effectiveness of dornase alfa on SARS-CoV-2 infection. New Microbes New infections (2020) 37:100756. doi: 10.1016/j.nmni.2020.100756
161. Arenas Gómez CM, Sabin KZ, Echeverri K. Wound healing across the animal kingdom: Crosstalk between the immune system and the extracellular matrix. Dev dynamics an Off Publ Am Assoc Anatomists (2020) 249(7):834–46. doi: 10.1002/dvdy.178
162. Ma Y. Role of neutrophils in cardiac injury and repair following myocardial infarction. Cells (2021) 10(7):1676. doi: 10.3390/cells10071676
163. Mahmud Z, Rahman A, Mishu ID, Kabir Y. Mechanistic insights into the interplays between neutrophils and other immune cells in cancer development and progression. Cancer metastasis Rev (2022) 41(2):405–32. doi: 10.1007/s10555-022-10024-8
164. Phillipson M, Kubes P. The healing power of neutrophils. Trends Immunol (2019) 40(7):635–47. doi: 10.1016/j.it.2019.05.001
165. Chalise U, Becirovic-Agic M, Lindsey ML. The cardiac wound healing response to myocardial infarction. WIREs Mech Dis (2023) 15(1):e1584. doi: 10.1002/wsbm.1584
166. Gluba-Brzózka A, Franczyk B, Rysz-Górzyńska M, Rokicki R, Koziarska-Rościszewska M, Rysz J. Pathomechanisms of immunological disturbances in β-thalassemia. Int J Mol Sci (2021) 22(18). doi: 10.3390/ijms22189677
167. Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol (2020) 10(9):200223. doi: 10.1098/rsob.200223
168. Andersson CK, Iwasaki J, Cook J, Robinson P, Nagakumar P, Mogren S, et al. Impaired airway epithelial cell wound-healing capacity is associated with airway remodelling following RSV infection in severe preschool wheeze. Allergy (2020) 75(12):3195–207. doi: 10.1111/all.14466
169. Wilgus TA, Roy S, McDaniel JC. Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care (2013) 2(7):379–88. doi: 10.1089/wound.2012.0383
170. Ganesh GV, Ramkumar KM. Macrophage mediation in normal and diabetic wound healing responses. Inflammation Res Off J Eur Histamine Res Soc (2020) 69(4):347–63. doi: 10.1007/s00011-020-01328-y
171. Riedl J, Popp C, Eide C, Ebens C, Tolar J. Mesenchymal stromal cells in wound healing applications: role of the secretome, targeted delivery and impact on recessive dystrophic epidermolysis bullosa treatment. Cytotherapy (2021) 23(11):961–73. doi: 10.1016/j.jcyt.2021.06.004
172. Pastar I, Marjanovic J, Stone RC, Chen V, Burgess JL, Mervis JS, et al. Epigenetic regulation of cellular functions in wound healing. Exp Dermatol (2021) 30(8):1073–89. doi: 10.1111/exd.14325
173. Serini S, Calviello G. New insights on the effects of dietary omega-3 fatty acids on impaired skin healing in diabetes and chronic venous leg ulcers, foods (Basel, Switzerland). (2021) 10(10):. doi: 10.3390/foods10102306
174. Shofler D, Rai V, Mansager S, Cramer K, Agrawal DK. Impact of resolvin mediators in the immunopathology of diabetes and wound healing. Expert Rev Clin Immunol (2021) 17(6):681–90. doi: 10.1080/1744666X.2021.1912598
175. Aloe C, Wang H, Vlahos R, Irving L, Steinfort D, Bozinovski S. Emerging and multifaceted role of neutrophils in lung cancer. Trans Lung Cancer Res (2021) 10(6):2806–18. doi: 10.21037/tlcr-20-760
176. Wang G, Yang F, Zhou W, Xiao N, Luo M, Tang Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomedicine Pharmacotherapy (2023) 157:114004. doi: 10.1016/j.biopha.2022.114004
177. Burster T, Mustafa Z, Myrzakhmetova D, Zhanapiya A, Zimecki M. Hindrance of the proteolytic activity of neutrophil-derived serine proteases by serine protease inhibitors as a management of cardiovascular diseases and chronic inflammation. Front Chem (2021) 9:784003. doi: 10.3389/fchem.2021.784003
178. Dias C, Nylandsted J. Plasma membrane integrity in health and disease: significance and therapeutic potential. Cell Discovery (2021) 7(1):4. doi: 10.1038/s41421-020-00233-2
179. Torous J, Bucci S, Bell IH, Kessing LV, Faurholt-Jepsen M, Whelan P, et al. The growing field of digital psychiatry: current evidence and the future of apps, social media, chatbots, and virtual reality. World Psychiatry Off J World Psychiatr Assoc (WPA) (2021) 20(3):318–35. doi: 10.1002/wps.20883
180. Cardinali DP, Brown GM, Reiter RJ, Pandi-Perumal SR. Elderly as a High-risk Group during COVID-19 Pandemic: Effect of Circadian Misalignment, Sleep Dysregulation and Melatonin Administration. Sleep Vigil (2020) 4(2):81–7. doi: 10.1007/s41782-020-00111-7
181. Fernández-Quintela A, Milton-Laskibar I, Trepiana J, Gómez-Zorita S, Kajarabille N, Léniz A, et al. Key Aspects in Nutritional Management of COVID-19 Patients. J Clin Med (2020) 9(8):2589. doi: 10.3390/jcm9082589
182. Im JH, Je YS, Baek J, Chung MH, Kwon HY, Lee JS. Nutritional status of patients with COVID-19. Int J Infect Dis IJID Off Publ Int Soc Infect Dis (2020) 100:390–3. doi: 10.1016/j.ijid.2020.08.018
183. Livingstone C. Zinc: physiology, deficiency, and parenteral nutrition. Nutr Clin Pract Off Publ Am Soc Parenteral Enteral Nutr (2015) 30(3):371–82. doi: 10.1177/0884533615570376
184. Kharaziha M, Baidya A, Annabi N. Rational design of immunomodulatory hydrogels for chronic wound healing. Advanced Materials (2021) 33(39):2100176. doi: 10.1002/adma.202100176
185. Li R, Liu K, Huang X, Li D, Ding J, Liu B, et al. Bioactive materials promote wound healing through modulation of cell behaviors. Advanced Sci (Weinheim Baden-Wurttemberg Germany) (2022) 9(10):e2105152. doi: 10.1002/advs.202105152
186. Antwi J, Appiah B, Oluwakuse B, Abu BAZ. The nutrition-COVID-19 interplay: a review. Curr Nutr Rep (2021) 10(4):364–74. doi: 10.1007/s13668-021-00380-2
187. Gavriatopoulou M, Korompoki E, Fotiou D, Ntanasis-Stathopoulos I, Psaltopoulou T, Kastritis E, et al. Organ-specific manifestations of COVID-19 infection. Clin Exp Med (2020) 20(4):493–506. doi: 10.1007/s10238-020-00648-x
188. Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes/metabolism Res Rev (2021) 37(2):e3377. doi: 10.1002/dmrr.3377
189. Palmieri B, Vadalà M, Laurino C. Nutrition in wound healing: investigation of the molecular mechanisms. narrative review J Wound Care (2019) 28(10):683–93. doi: 10.12968/jowc.2019.28.10.683
190. Sussman G. Nutrition and diabetes, understanding diabetic foot: A comprehensive guide for general practitioners. World Sci (2023) 215–25.
191. Munoz N, Posthauer ME. Nutrition strategies for pressure injury management: Implementing the 2019 International Clinical Practice Guideline. Nutr Clin Pract Off Publ Am Soc Parenteral Enteral Nutr (2022) 37(3):567–82. doi: 10.1002/ncp.10762
192. Almeida RP, Mokete L, Sikhauli N, Sekeitto AR, Pietrzak J. The draining surgical wound post total hip and knee arthroplasty: what are my options? A narrative review EFORT Open Rev (2021) 6(10):872–80. doi: 10.1302/2058-5241.6.200054
193. Lender O, Göbölös L, Bajwa G, Bhatnagar G. Sternal wound infections after sternotomy: risk factors, prevention and management. J Wound Care (2022) 31(Sup6):S22–s30. doi: 10.12968/jowc.2022.31.Sup6.S22
194. Chadha SA, Stout MA, Goyal PK, Nguyen CV, Lu KQ. Assessing unmet needs in patients with hidradenitis suppurativa. Arch Dermatol Res (2023), 1–6. doi: 10.1007/s00403-023-02645-y
195. Wang W, Yan Y, Guo Z, Hou H, Garcia M, Tan X, et al. All around suboptimal health - a joint position paper of the Suboptimal Health Study Consortium and European Association for Predictive. Prev Personalised Medicine EPMA J (2021) 12(4):403–33. doi: 10.1007/s13167-021-00253-2
196. Kanitakis J, Lesort C, Danset M, Jullien D. Chilblain-like acral lesions during the COVID-19 pandemic ("COVID toes"): Histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol (2020) 83(3):870–5. doi: 10.1016/j.jaad.2020.05.145
197. Carrascosa JM, Morillas V, Bielsa I, Munera-Campos M. Cutaneous manifestations in the context of SARS-coV-2 infection (COVID-19). Actas dermo-sifiliograficas (2020) 111(9):734–42. doi: 10.1016/j.ad.2020.08.002
198. Criado PR, Pagliari C, Carneiro FRO, Quaresma JAS. Lessons from dermatology about inflammatory responses in Covid-19. Rev Med Virol (2020) 30(5):e2130. doi: 10.1002/rmv.2130
199. Fernández-Lázaro D, Garrosa M. Identification, mechanism, and treatment of skin lesions in COVID-19: A review. Viruses (2021) 13(10):1916. doi: 10.3390/v13101916
200. Foster DS, Januszyk M, Yost KE, Chinta MS, Gulati GS, Nguyen AT, et al. Integrated spatial multiomics reveals fibroblast fate during tissue repair. Proc Natl Acad Sci United States America (2021) 118(41). doi: 10.1073/pnas.2110025118
201. Lambert AW, Weinberg RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. (2021) 21(5):325–38. doi: 10.1038/s41568-021-00332-6
202. Sun Q, Hu S, Lou Z, Gao J. The macrophage polarization in inflammatory dermatosis and its potential drug candidates. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie (2023) 161:114469. doi: 10.1016/j.biopha.2023.114469
203. Dong J, Chen L, Zhang Y, Jayaswal N, Mezghani I, Zhang W, et al. Mast cells in diabetes and diabetic wound healing. Adv Ther (2020) 37(11):4519–37. doi: 10.1007/s12325-020-01499-4
204. Yoo HHB, Marin FL. Treating inflammation associated with pulmonary hypertension: an overview of the literature. Int J Gen Med (2022) 15:1075–83. doi: 10.2147/IJGM.S295463
205. Cooper PO, Haas MR, NooNepalle SKR, Shook BA. Dermal drivers of injury-induced inflammation: contribution of adipocytes and fibroblasts. Int J Mol Sci (2021) 22(4):352–9. doi: 10.3390/ijms22041933
206. Holzer-Geissler JCJ, Schwingenschuh S, Zacharias M, Einsiedler J, Kainz S, Reisenegger P, et al. The Impact of Prolonged Inflammation on Wound Healing. Biomedicines (2022) 10(4):957–981. doi: 10.3390/biomedicines10040856
207. Sadeghzadeh-Bazargan A, Rezai M, Nobari NN, Mozafarpoor S, Goodarzi A. Skin manifestations as potential symptoms of diffuse vascular injury in critical COVID-19 patients. J cutaneous Pathol (2021) 48(10):1266–76. doi: 10.1111/cup.14059
208. Dalisson B, Barralet J. Bioinorganics and wound healing. Advanced Healthcare Materials (2019) 8(18):1900764. doi: 10.1002/adhm.201900764
209. Gushiken LFS, Beserra FP, Bastos JK, Jackson CJ, Pellizzon CH. Cutaneous wound healing: an update from physiopathology to current therapies, life (Basel, Switzerland). (2021) 11(7):. doi: 10.3390/life11070665
210. Mentella MC, Scaldaferri F, Gasbarrini A, Miggiano GAD. The role of nutrition in the COVID-19 pandemic. Nutrients (2021) 13(4). doi: 10.3390/nu13041093
211. Hayden MR. An immediate and long-term complication of COVID-19 may be type 2 diabetes mellitus: the central role of β-cell dysfunction Apoptosis and Exploration of Possible Mechanisms. Cells (2020) 9(11). doi: 10.3390/cells9112475
212. Hayden MR. An immediate and long-term complication of COVID-19 may be type 2 diabetes mellitus: the central role of β-cell dysfunction, apoptosis and exploration of possible mechanisms. Cells (2020). doi: 10.3390/cells9112475
213. Aghbash PS, Eslami N, Shirvaliloo M, Baghi HB. Viral coinfections in COVID-19. J Med Virol (2021) 93(9):5310–22. doi: 10.1002/jmv.27102
214. Vafadar Moradi E, Teimouri A, Rezaee R, Morovatdar N, Foroughian M, Layegh P, et al. Increased age, neutrophil-to-lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID-19 mortality. Am J Emergency Med (2021) 40:11–4. doi: 10.1016/j.ajem.2020.12.003
215. Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of acute and chronic wound healing. biomolecules (2021) 11(5). doi: 10.3390/biom11050700
216. Mu R, Souza SCde, Liao Z, Dong L, Wang C. Reprograming the immune niche for skin tissue regeneration - From cellular mechanisms to biomaterials applications. Adv Drug Delivery Rev (2022) 185:114298. doi: 10.1016/j.addr.2022.114298
217. Zhang B, Su Y, Zhou J, Zheng Y, Zhu D. Toward a better regeneration through implant-mediated immunomodulation: harnessing the immune responses. Advanced Sci (Weinheim Baden-Wurttemberg Germany) (2021) 8(16):e2100446. doi: 10.1002/advs.202100446
218. Patidar GK, Land KJ, Vrielink H, Rahimi-Levene N, Dann EJ, Al-Humaidan H, et al. Understanding the role of therapeutic plasma exchange in COVID-19: preliminary guidance and practices. Vox sanguinis (2021) 116(7):798–807. doi: 10.1111/vox.13067
219. Nhean S, Varela ME, Nguyen YN, Juarez A, Huynh T, Udeh D, et al. COVID-19: A review of potential treatments (Corticosteroids, remdesivir, tocilizumab, bamlanivimab/etesevimab, and casirivimab/imdevimab) and pharmacological considerations. J Pharm Pract (2023) 36(2):407–17. doi: 10.1177/08971900211048139
220. Barchitta M, Maugeri A, Favara G, Magnano San Lio R, Evola G, Agodi A, et al. Nutrition and wound healing: an overview focusing on the beneficial effects of curcumin. Int J Mol Sci (2019) 20(5). doi: 10.3390/ijms20051119
221. Scalise A, Falcone M, Avruscio G, Brocco E, Ciacco E, Parodi A, et al. What COVID-19 taught us: New opportunities and pathways from telemedicine and novel antiseptics in wound healing. Int Wound J (2022) 19(5):987–95. doi: 10.1111/iwj.13695
222. Meehan A, Partridge J, Jonnalagadda SS. Clinical and economic value of nutrition in healthcare: A nurse's perspective. Nutr Clin Pract Off Publ Am Soc Parenteral Enteral Nutr (2019) 34(6):832–8. doi: 10.1002/ncp.10405
223. Seirafianpour F, Sodagar S, Pour Mohammad A, Panahi P, Mozafarpoor S, Almasi S, et al. Cutaneous manifestations and considerations in COVID-19 pandemic: A systematic review. Dermatologic Ther (2020) 33(6):e13986. doi: 10.1111/dth.13986
224. Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allergy Asthma Rep (2020) 20(12):75. doi: 10.1007/s11882-020-00974-w
Keywords: neutrophil, aging, heterogeneity, COVID-19, wound healing, immune response
Citation: Liu Y, Xiang C, Que Z, Li C, Wang W, Yin L, Chu C and Zhou Y (2023) Neutrophil heterogeneity and aging: implications for COVID-19 and wound healing. Front. Immunol. 14:1201651. doi: 10.3389/fimmu.2023.1201651
Received: 06 April 2023; Accepted: 02 August 2023;
Published: 28 November 2023.
Edited by:
Joseph T. Y. Lau, University at Buffalo, United StatesCopyright © 2023 Liu, Xiang, Que, Li, Wang, Yin, Chu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Yin, MzA0MDg5NzY3QHFxLmNvbQ==; Wen Wang, MTU1Mzc2MDQ0QHFxLmNvbQ==; Chenyu Chu, dG4wMDk5Mjc4NkBxcS5jb20=; Yin Zhou, MzA3MTI0NDU5QHFxLmNvbQ==
†These authors have contributed equally to this work
 Yi Liu
Yi Liu Changlan Xiang†
Changlan Xiang†