- 1Department of Respiratory Medicine, The Third People’s Hospital of Honghe Prefecture, Gejiu, China
- 2Department of Thoracic Surgery , The Third People’s Hospital of Honghe Prefecture, Gejiu, China
- 3Department of Thoracic Surgery, Yunnan Cancer Center, The Third Affiliated Hospital of Kunming Medical University, Kunming, China
- 4Department of Oncology, Gejiu City People’s Hospital, Diannan Central Hospital of Honghe Prefecture, The Fifth Affiliated Hospital of Kunming Medical University, Gejiu, China
Immune checkpoint inhibitors (ICIs) target the negative regulatory pathway of T cells and effectively reactive the anti-tumor immune function of T cells by blocking the key pathway of the immune escape mechanism of the tumor—PD-1/PD-L1, and fundamentally changing the prospect of immunotherapy for non-small cell lung cancer patients. However, such promising immunotherapy is overshadowed by Hyperprogressive Disease, a response pattern associated with unwanted accelerated tumor growth and characterized by poor prognosis in a fraction of treated patients. This review comprehensively provides an overview of Hyperprogressive Disease in immune checkpoint inhibitor-based immunotherapy for non-small cell lung cancer including its definition, biomarkers, mechanisms, and treatment. A better understanding of the black side of immune checkpoint inhibitors therapy will provide a more profound insight into the pros and cons of immunotherapy.
Introduction
Lung cancer is a serious life-threatening disease, and non-small cell lung cancer (NSCLC) is one of its most prevalent subtypes (1, 2). Immune checkpoint inhibitors (ICIs), as PD-1/PD-L1 inhibitors based immunotherapy has made revolutionized effects and become a milestone in the treatment history of NSCLC (3). However, PD-1/PD-L1 blockade can lead to an unsatisfactory response pattern characterized by accelerated tumor growth and associated with poor prognosis——Hyperprogressive Disease (HPD) (4). Detrimental patterns such as HPD and early death (ED) have been respectively observed in a proportion of NSCLC patients treated with ICIs (5). Overall survival (OS) is significantly reduced in NSCLC patients who develop HPD after PD-1/PD-L1 inhibitors blockade (4). For instance, although the PD-1 antibody Nivolumab is quite effective in clinical practice, HPD is not rare in patients with advanced NSCLC treated with Nivolumab and paralleled with a poor prognosis (6, 7). There are also several case reports about HPD events after treatment with another PD-1 inhibitor Pembrolizumab and the PD-L1 inhibitor - Durvalumab (8, 9). However, the definition and predictive biomarkers of HPD in NSCLC remain controversial, and the associated clinicopathological features or biological mechanisms are not yet determined. This significantly restricts the utilization of ICIs in patients with NSCLC (Figure 1).
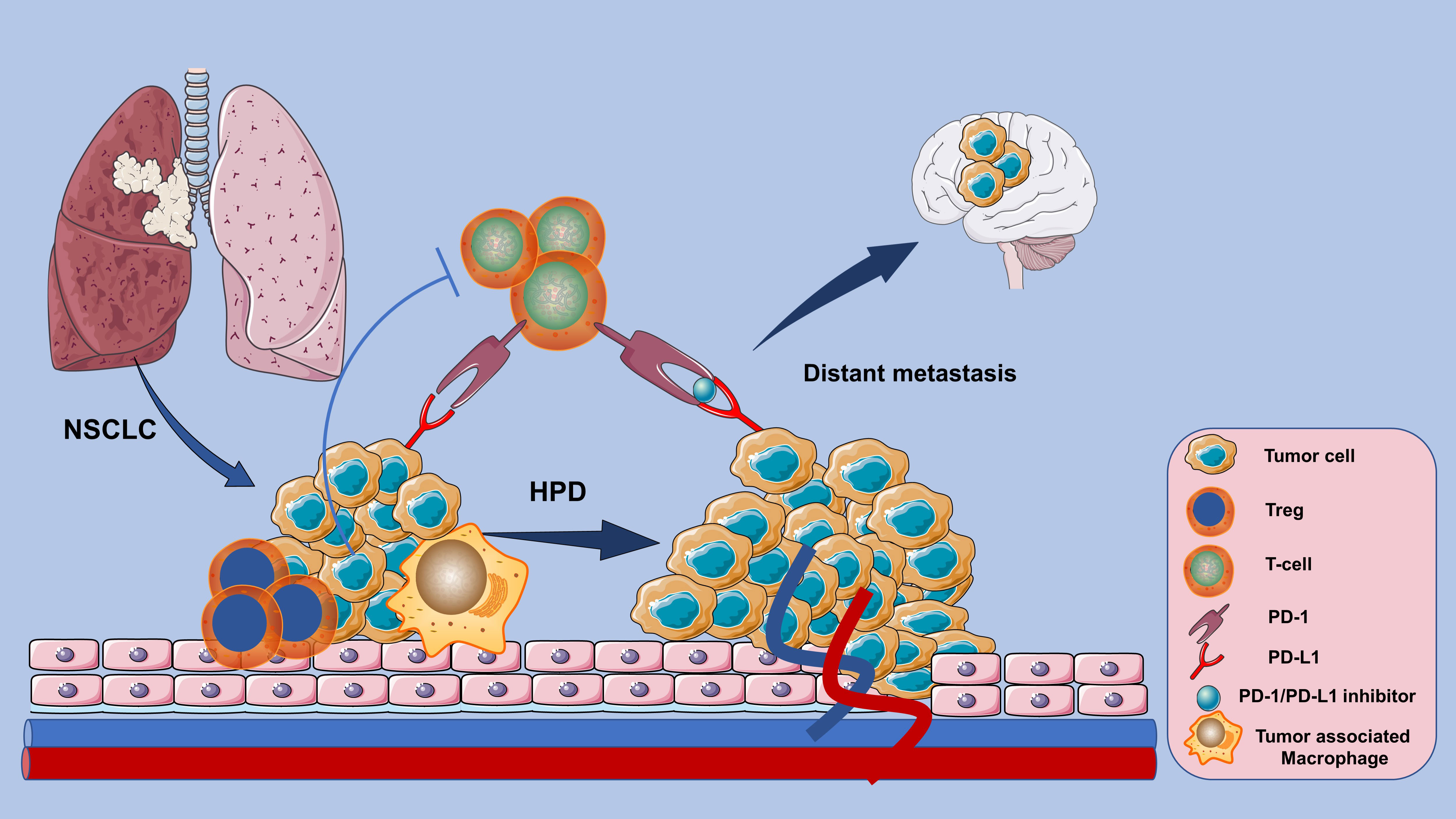
Figure 1 HPD response patterns associated with poor prognosis in NSCLC patients whose tumors instead accelerated in growth after PD-1/PD-L1 inhibitor-based immunotherapy.
Definition of HPD in NSCLC immunotherapy
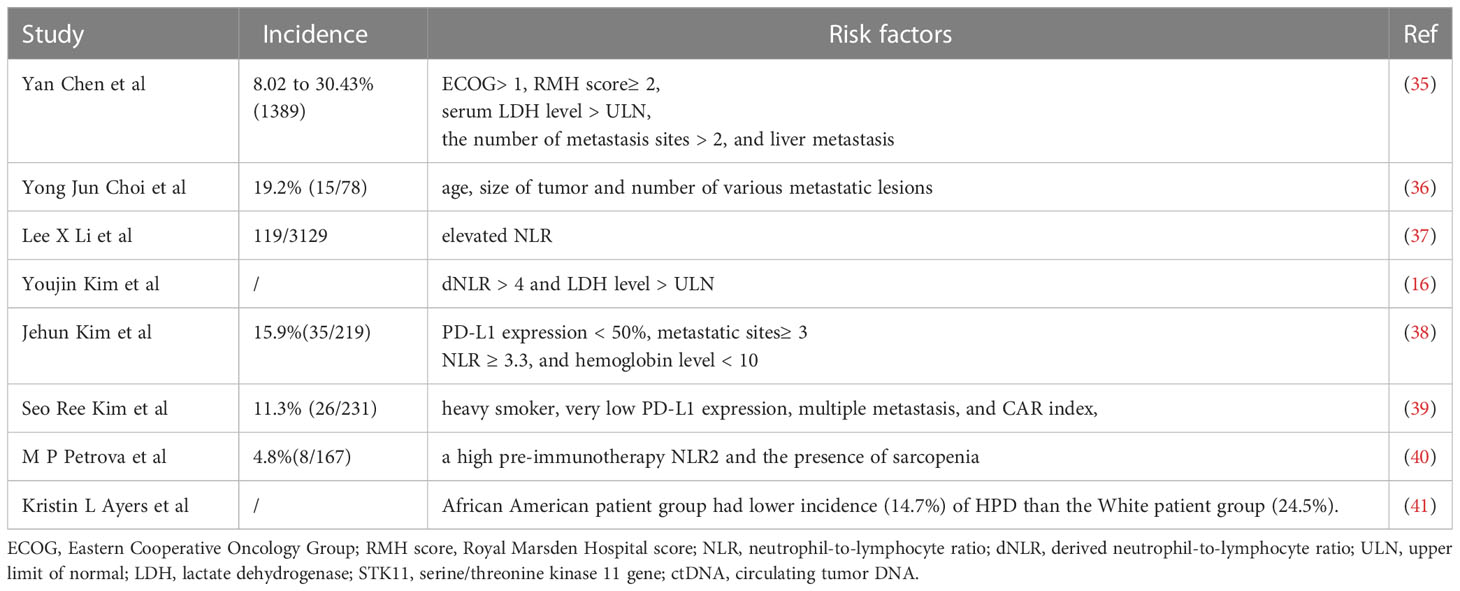
The accelerate growth in tumor size and volume measured by computed tomography(CT) during ICIs blockade are the most objective characteristics of HPD by using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria (10). However, the main limitation of conventional response assessment criteria RECIST 1.1 remains due to the inadequate ability to capture the response to immunotherapies and the inapplicability to patients without pre-baseline imaging or progression on unmeasurable lesions (11, 12). Therefore, novel criteria like iRECIST is clinically used as a response evaluation tool in patients undergoing immunotherapy (13). Despite the improvement, the definition of HPD has not been standardized and the prevalence of it varies based on different criteria (14) (Table 1). The assessment criteria should address the relevance of the clinical presentation, poor prognosis, and biological behavior of the NSCLC (21). The standard definition of HPD should be continuously optimized to guide better PD-1/PD-L1 inhibitors immunotherapy.
Differentiating HPD from pseudoprogression
However, definitions based on radiological assessment alone have substantial technical limitations. The possibility of pseudoprogression (PsPD) exists when progressive deterioration of pulmonary infiltrative shadows is observed within 4 weeks among advanced NSCLC patients after the initial administration of anti-PD-1 antibody (22). Current clinical and radiological assessment strategies are inadequate to distinguish PsPD with HPD. PsPD has a similar response pattern of tumor increase or appearance of new lesions monitored by imaging at the beginning of treatment with ICIs, but shrinks later, whereas HPD is a rapid and poor prognosis progression pattern (23, 24). Consequently, repeat biopsies should be considered even if radiographic tumor progression is detected during immunotherapy (25–27). Besides, immune system-related response criteria such as NLR and ctDNA also have the potential to differentiate HPD from PsPD (28).
Clinical characteristics and biomarkers for HPD
Conventional imaging methods are restricted to determine HPD. Image-based radiomic markers extracted from baseline CT of advanced NSCLC treated with PD-1/PD-L1 inhibitors including the features of peritumoral texture and nodule vessel-related tortuosity may have prospective value for identifying the HPD. Meanwhile, using radiomics features at the lesion-level analysis has the same effect. The novel radiomic models have translational implications to distinguish vulnerable NSCLC patients at risk of HPD (29–31).
In addition to imaging indices, sensitive predictive markers of positive and negative responses to immunotherapy and clinical factors that identify high-risk NSCLC populations that potentially progress to HPD after treatment with ICIs should be continuously developed (32, 33). There are lots of clinicopathological features associated with HPD in NSCLC patients treated with ICIs, as HPD was found associated with higher age (>65 years old) rather than higher tumor burden or specific tumor type (34) (Table 2). Subsequently, the related risk-predicting model based on clinical features is under exploring. Lung immune prognostic index (LIPI) based on dNLR > 3 and LDH > ULN is a promising tool for selecting patients who may not benefit from ICIs therapy (42).
Genomic profiles is another key component of risk prediction models for HPD after immunotherapy. A case report illustrates that patients carrying EGFR exon 20 insertion and MYC amplification have the risk of developing to HPD after Nivolumab blockade (43). Besides, the coexistence of STK11 gene mutations and KRAS mutations can be used as potential biomarkers for HPD (16). Simultaneously, MDM2 family amplification or EGFR aberrations are closely linked with increasing TGR after PD-1/PD-L1 inhibitors monotherapy (44). Furthermore, long non-coding RNA (lncRNA) plays a critical role in the immune regulation of LUAD and the immune-related lncRNAs (IRLs) manifest a promising prediction value of ICIs efficacy in LUAD. Patients with low risk might gain benefits from ICIs whereas some have a risk of HPD (45). Additionally, Liquid biopsy could be assisted to identify patients at high risk of HPD, and ctDNA may be a novel prognostic biomarker of PD-1 blockade (46, 47).
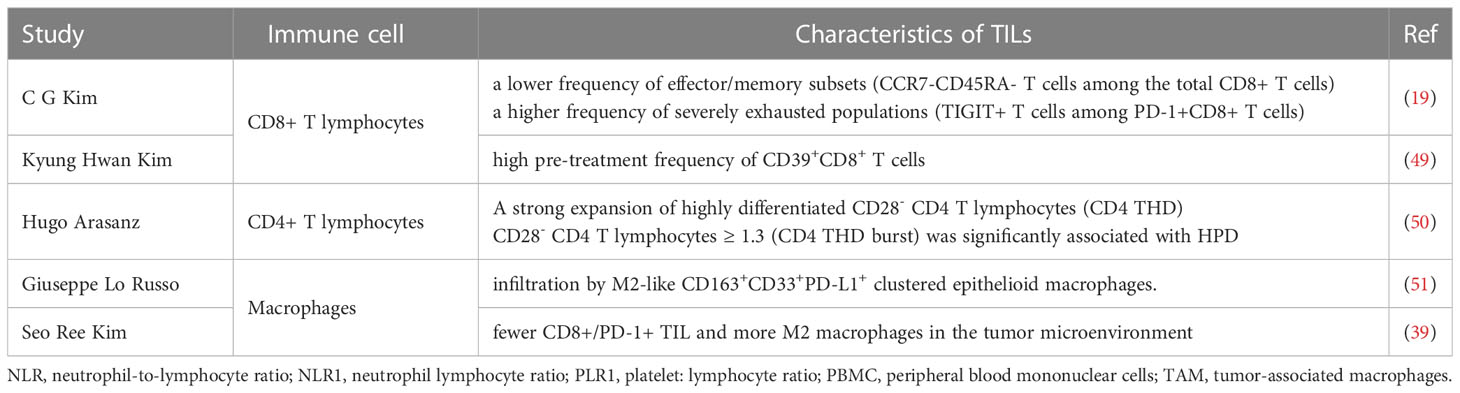
There are more and more studies evaluating the predictive and prognostic value of the various immune cells in pretreatment tissue samples and identifying determinants associated with response in patients with NSCLC treated with ICIs. Levels of tumor-infiltrating lymphocytes (TILs) were strongly and independently associated with response to ICIs therapy (48). These studies illustrate that the different predictive and prognostic values for infiltrating immune cells in tumor tissue may help in selecting patients for ICIs. More importantly, the patient’s TILs assessment is relatively easy to incorporate into the pathology laboratory workflow, easy to perform and inexpensive. Besides, the analyzing of immune cell of PBMC gradually draws more and more attention (Table 3).
Mechanism of HPD in NSCLC
Exploring the mechanisms of HPD in NSCLC is critical for understanding immunotherapy represented by ICIs. The tumour microenvironment (TME) is involved in influencing the response to immunotherapy as it plays a predominant role in the multiple interactions between tumor cells and the immune system (52). The biological basis and mechanisms of HPD are being elucidated and some studies have proposed immune checkpoint antibody-Fc/FcR interactions on macrophages as a mechanism of HPD after PD-1/PD-L1 blockade. Reprogramming of tumor associated macrophage (TAM) with the involvement of the Fc receptor of ICIs contribut to the induction of HPD (51). While, a study revealed that HPD was significantly linked with intratumoral B-cell density but not T-cell or macrophage (53). An animal model of a regulatory T cell (Treg)-dominated TME formed by selective depletion of CD8+ T cells by targeting CD8β antigen with near-infrared photoimmunotherapy (NIR-PIT) has shown that HPD after PD-1 blockade can be partly responsible for an imbalance between effector T cells and Tregs in the TME (54). Intrestingly, the interaction between the redox and immune system may lead to the local immunosuppression in the TME which accelerate tumor growth. Such as the administration of IgG4 and glutathione could promote tumor growth in the mouse lung cancer model (55).
Notably, analysis of the pathological features of patients who developed HPD during Pembrolizumab treatment for NSCLC suggests that the pathological type conversion of adenocarcinoma to small cell carcinoma may be the cause of HPD during ICIs treatment (56). Furthermore, changes in PD-L1 expression in tumor tissues may also be associated with HPD (8). It has been demonstrated that HPD can be prevented in preclinical models by targeting the IFNγ-PKM2-β-catenin axis. Tandem through the immunogenic, metabolic, and oncogenic pathway of the IFNγ-PKM2-β-catenin cascade is the primary mechanism of ICIs-associated HPD (57). There is an urgent need for further expansion of the scope of research and invasive research tools, and in-depth exploration of the underlying molecular mechanisms is of paramount importance.
The management of HPD in NSCLC
A comprehensive and thorough study of the mechanisms involved not only provides a plausible explanation for HPD, but also offers new opportunities to manipulate this mechanism to improve cancer immunotherapy. PD-1 blockade may promote the proliferation of highly suppressive PD-1+ eTreg cells, leading to suppression of antitumor immunity and HPD. Therefore targeting depletion of eTreg cells in tumor tissue would be an effective strategy for the treatment and prevention of HPD (58). More importantly, salvage treatment after the onset of HPD in NSCLC is also under active investigation in clinical practice. Alternative therapies, like high-dose corticosteroids, antibiotics and drainage, can be effective in treating the symptoms of HPD caused by Nivolumab (59). Besides, termination of immunotherapy should be discussed after the onset of HPD is monitored and an early switch to cytotoxic therapy is essential to avoid further disease progression (60). For instance, a comparative study retrospectively screened patients with pathologically confirmed advanced or recurrent NSCLC demontrated that the HPD rate was significantly lower in the combination therapy (cytotoxic chemotherapy plus PD-1/PD-L1 inhibitor) group than in the PD-1/PD-L1 inhibitor monotherapy group (61). Chemotherapy has the value to increase a tumor’s response to immunotherapy and overcome the associated resistance (62). The combination therapy warrant further study to reduce the incidence of HPD. Moreover, informing patients of the risk of HPD is an indispensable component before the administration of ICIs. Health auhorities and trial sponsors are under obligation to monitor tumor progression in trials to help oncologists properly inform patients of the expected incidence of HPD.
Discussion
Immunotherapy based on immune checkpoint inhibitors has brought revolutionary clinical benefits to patients with NSCLC, however, immunotherapy is also a double-edged sword that may bring about serious response patterns such as HPD, which deviates from the original intent of immunotherapy’s excellent clinical efficacy and high safety profile. The lack of consensus on the definitional criteria and biological basis of HPD necessitates larger studies and multicenter collaborations to standardize the criteria. How to maximize the efficacy and minimize the HPD caused by ICIS while consolidating existing therapeutic gains to benefit more NSCLC patients remains an open question. The importance of positive predictive markers for screening NSCLC patients who may benefit from immunotherapy with ICIs and the role of developing negative response predictive markers to screen out subgroups of NSCLC that do not benefit or may even develop HPD cannot be underestimated, therefor identifying potential molecular mechanisms and developing predictive biomarkers for HPD is an important direction.
Author contributions
Conception and design: LZ, YL. Administrative support: TC, TN, JH. Provision of study materials: TC, XT. Collection and assembly of data: LZ, YH. Data analysis and interpretation: YL. Manuscript writing: All authors. Final approval of manuscript: All authors. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Li Z, Feiyue Z, Gaofeng L. Traditional Chinese medicine and lung cancer–from theory to practice. BioMed Pharmacother (2021) 137. doi: 10.1016/j.biopha.2021.111381
3. Li Z, Feiyue Z, Gaofeng L, Haifeng L. Lung cancer and oncolytic virotherapy–enemy's enemy. Transl Oncol (2023) 27. doi: 10.1016/j.tranon.2022.101563
4. Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol (2018) 4(11):1543–52. doi: 10.1001/jamaoncol.2018.3676
5. Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Comparison of fast-progression, hyperprogressive disease, and early deaths in advanced non-Small-Cell lung cancer treated with PD-1/PD-L1 inhibitors or chemotherapy. JCO Precis Oncol (2020) 4:829–40. doi: 10.1200/PO.20.00021
6. Costantini A, Fallet V, Corny J, Friard S, Chouaid C, Duchemann B, et al. Nivolumab-refractory patients with advanced non-small-cell lung cancer. Lung Cancer (Amsterdam Netherlands) (2019) 130:128–34. doi: 10.1016/j.lungcan.2019.01.015
7. Kim SH, Choi CM, Lee DH, Kim SW, Yoon S, Kim WS, et al. Clinical outcomes of nivolumab in patients with advanced non-small cell lung cancer in real-world practice, with an emphasis on hyper-progressive disease. J Cancer Res Clin Oncol (2020) 146(11):3025–36. doi: 10.1007/s00432-020-03293-9
8. Tanaka Y, Matsubara O, Asada K, Muramatsu A, Suzuki M, Shirai T, et al. Hyperprogressive disease after treatment with pembrolizumab in lung adenocarcinoma: an autopsy case study. Respir Med Case Rep (2019) 28:100885. doi: 10.1016/j.rmcr.2019.100885
9. Khreis TJ, Azar IH, Patel R, Mehdi SA. Durvalumab-induced hyperprogressive disease in nonmetastatic lung cancer. J Oncol Pract (2019) 15(4):217–9. doi: 10.1200/JOP.18.00739
10. Lo Russo G, Facchinetti F, Tiseo M, Garassino MC, Ferrara R. Hyperprogressive disease upon immune checkpoint blockade: focus on non-small cell lung cancer. Curr Oncol Rep (2020) 22(5):41. doi: 10.1007/s11912-020-00908-9
11. Léger MA, Routy B, Juneau D. FDG PET/CT for evaluation of immunotherapy response in lung cancer patients. Semin Nucl Med (2022) 52(6):707–19. doi: 10.1053/j.semnuclmed.2022.04.010
12. Champiat S, Ferrara R, Massard C, Besse B, Marabelle A, Soria JC, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol (2018) 15(12):748–62. doi: 10.1038/s41571-018-0111-2
13. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol (2017) 18(3):e143–e52. doi: 10.1016/S1470-2045(17)30074-8
14. Park HJ, Kim KW, Won SE, Yoon S, Chae YK, Tirumani SH, et al. Definition, incidence, and challenges for assessment of hyperprogressive disease during cancer treatment with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Netw Open (2021) 4(3):e211136. doi: 10.1001/jamanetworkopen.2021.1136
15. Matos I, Martin-Liberal J, García-Ruiz A, Hierro C, Ochoa de Olza M, Viaplana C, et al. Capturing hyperprogressive disease with immune-checkpoint inhibitors using RECIST 1.1 criteria. Clin Cancer Res (2020) 26(8):1846–55. doi: 10.1158/1078-0432.CCR-19-2226
16. Kim Y, Kim CH, Lee HY, Lee SH, Kim HS, Lee S, et al. Comprehensive clinical and genetic characterization of hyperprogression based on volumetry in advanced non-small cell lung cancer treated with immune checkpoint inhibitor. J Thorac Oncol (2019) 14(9):1608–18. doi: 10.1016/j.jtho.2019.05.033
17. Ten Berge D, Hurkmans DP, den Besten I, Kloover JS, Mathijssen RHJ, Debets R, et al. Tumour growth rate as a tool for response evaluation during PD-1 treatment for non-small cell lung cancer: a retrospective analysis. ERJ Open Res (2019) 5(4):00179–2019. doi: 10.1183/23120541.00179-2019
18. Kas B, Talbot H, Ferrara R, Richard C, Lamarque JP, Pitre-Champagnat S, et al. Clarification of definitions of hyperprogressive disease during immunotherapy for non-small cell lung cancer. JAMA Oncol (2020) 6(7):1039–46. doi: 10.1001/jamaoncol.2020.1634
19. Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH, Ahn BC, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol (2019) 30(7):1104–13. doi: 10.1093/annonc/mdz123
20. Abbar B, De Castelbajac V, Gougis P, Assoun S, Pluvy J, Tesmoingt C, et al. Definitions, outcomes, and management of hyperprogression in patients with non-small-cell lung cancer treated with immune checkpoint inhibitors. Lung Cancer (2021) 152:109–18. doi: 10.1016/j.lungcan.2020.12.026
21. Wang M, Huang H, Xu Z, Li Z, Shen L, Yu Y, et al. Proposal for multiple new lesions as complement of hyperprogressive disease in NSCLC patients treated with PD-1/PD-L1 immunotherapy. Lung Cancer (2022) 173:28–34. doi: 10.1016/j.lungcan.2022.09.001
22. Hashimoto K, Kaira K, Kawasaki T, Yamaguchi O, Kagamu H. Psuedoprogression mimicking hyperprogressive disease after pembrolizumab treatment in a patient with lung cancer. Lung Cancer (2020) 139:221–3. doi: 10.1016/j.lungcan.2019.11.019
23. Rashid AS, Venigalla S, Dzeda M, Masters GA. A case report of the clinico-radiologic challenges of assessing treatment response after stereotactic radiation of oligometastases preceded by immunotherapy: pseudoprogression, mixed response patterns, and opportunities for precision radiation. Cureus (2019) 11(3):e4264. doi: 10.7759/cureus.4264
24. Guaitoli G, Baldessari C, Bertolini F, Tomasello C, Cascinu S, Barbieri F. Are we ready to describe response or progression to immunotherapy in lung cancer? Crit Rev Oncol Hematol (2019) 138:112–9. doi: 10.1016/j.critrevonc.2019.04.002
25. Shionoya Y, Hirohashi Y, Takahashi H, Hashimoto M, Nishiyama K, Takakuwa Y, et al. Possible pseudo-progression of non-small cell lung carcinoma in a patient with clinical hyper-progression associated with trousseau syndrome who was treated with pembrolizumab: a case report. Anticancer Res (2021) 41(7):3699–706. doi: 10.21873/anticanres.15161
26. Ando C, Ichihara E, Kano H, Iwamoto Y, Hirabae A, Nakasuka T, et al. Granulation tissue-induced pseudo-relapse during nivolumab treatment in advanced non-small cell lung cancer. In Vivo (2019) 33(6):2113–5. doi: 10.21873/invivo.11711
27. Kurman JS, Murgu SD. Hyperprogressive disease in patients with non-small cell lung cancer on immunotherapy. J Thorac Dis (2018) 10(2):1124–8. doi: 10.21037/jtd.2018.01.79
28. Zhou L, Zhang M, Li R, Xue J, Lu Y. Pseudoprogression and hyperprogression in lung cancer: a comprehensive review of literature. J Cancer Res Clin Oncol (2020) 146(12):3269–79. doi: 10.1007/s00432-020-03360-1
29. Vaidya P, Bera K, Patil PD, Gupta A, Jain P, Alilou M, et al. Novel, non-invasive imaging approach to identify patients with advanced non-small cell lung cancer at risk of hyperprogressive disease with immune checkpoint blockade. J Immunother Cancer (2020) 8(2):e001343. doi: 10.1136/jitc-2020-001343
30. Song C, Park H, Lee HY, Lee S, Ahn JH, Lee SH. Evaluation of response to immune checkpoint inhibitors using a radiomics, lesion-level approach. Cancers (Basel) (2021) 13(23):6050. doi: 10.3390/cancers13236050
31. Tunali I, Gray JE, Qi J, Abdalah M, Jeong DK, Guvenis A, et al. Novel clinical and radiomic predictors of rapid disease progression phenotypes among lung cancer patients treated with immunotherapy: an early report. Lung Cancer (2019) 129:75–9. doi: 10.1016/j.lungcan.2019.01.010
32. Castello A, Rossi S, Mazziotti E, Toschi L, Lopci E. Hyperprogressive disease in patients with non-small cell lung cancer treated with checkpoint inhibitors: the role of (18)F-FDG PET/CT. J Nucl Med (2020) 61(6):821–6. doi: 10.2967/jnumed.119.237768
33. Wang X, Wang F, Zhong M, Yarden Y, Fu L. The biomarkers of hyperprogressive disease in PD-1/PD-L1 blockage therapy. Mol Cancer (2020) 19(1):81. doi: 10.1186/s12943-020-01200-x
34. Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res (2017) 23(8):1920–8. doi: 10.1158/1078-0432.CCR-16-1741
35. Chen Y, Hu J, Bu F, Zhang H, Fei K, Zhang P. Clinical characteristics of hyperprogressive disease in NSCLC after treatment with immune checkpoint inhibitor: a systematic review and meta-analysis. BMC Cancer (2020) 20(1):707. doi: 10.1186/s12885-020-07206-4
36. Choi YJ, Kim T, Kim EY, Lee SH, Kwon DS, Chang YS. Prediction model for hyperprogressive disease in non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer (2020) 11(10):2793–803. doi: 10.1111/1759-7714.13594
37. Li LX, Cappuzzo F, Matos I, Socinski MA, Hopkins AM, Sorich MJ. Low risk of hyperprogression with first-line chemoimmunotherapy for advanced non-small cell lung cancer: pooled analysis of 7 clinical trials. Oncologist (2023) 28(4):e205–e211. doi: 10.1093/oncolo/oyad043
38. Kim J, Kim T, Jang TW, Kang H, Kim MH, Yoon SH, et al. Clinical outcomes of hyperprogression based on volumetry in non-small cell lung cancer after immune checkpoint inhibitor treatment. Thorac Cancer (2022) 13(15):2170–9. doi: 10.1111/1759-7714.14539
39. Kim SR, Chun SH, Kim JR, Kim SY, Seo JY, Jung CK, et al. The implications of clinical risk factors, CAR index, and compositional changes of immune cells on hyperprogressive disease in non-small cell lung cancer patients receiving immunotherapy. BMC Cancer (2021) 21(1):19. doi: 10.1186/s12885-020-07727-y
40. Petrova MP, Donev IS, Radanova MA, Eneva MI, Dimitrova EG, Valchev GN, et al. Sarcopenia and high NLR are associated with the development of hyperprogressive disease after second-line pembrolizumab in patients with non-small-cell lung cancer. Clin Exp Immunol (2020) 202(3):353–62. doi: 10.1111/cei.13505
41. Ayers KL, Mullaney T, Zhou X, Liu JJ, Lee K, Ma M, et al. Analysis of real-world data to investigate the impact of race and ethnicity on response to programmed cell death-1 and programmed cell death-ligand 1 inhibitors in advanced non-small cell lung cancers. Oncologist (2021) 26(7):e1226–e39. doi: 10.1002/onco.13780
42. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol (2018) 4(3):351–7. doi: 10.1001/jamaoncol.2017.4771
43. Huang X, Xia L, Lan F, Shao YW, Li W, Xia Y. Treatment of nivolumab results in hyperprogressive disease in a patient harboring EGFR exon 20 insertion and MYC amplification. J Thorac Oncol (2019) 14(9):e189–e91. doi: 10.1016/j.jtho.2019.04.009
44. Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res (2017) 23(15):4242–50. doi: 10.1158/1078-0432.CCR-16-3133
45. Zeng Z, Liang Y, Shi J, Xiao L, Tang L, Guo Y, et al. Identification and application of a novel immune-related lncRNA signature on the prognosis and immunotherapy for lung adenocarcinoma. Diagnostics (Basel) (2022) 12(11):2891. doi: 10.3390/diagnostics12112891
46. Zulato E, Del Bianco P, Nardo G, Attili I, Pavan A, Boscolo Bragadin A, et al. Longitudinal liquid biopsy anticipates hyperprogression and early death in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors. Br J Cancer (2022) 127(11):2034–42. doi: 10.1038/s41416-022-01978-1
47. Chen Y, Li X, Liu G, Chen S, Xu M, Song L, et al. ctDNA concentration, MIKI67 mutations and hyper-progressive disease related gene mutations are prognostic markers for camrelizumab and apatinib combined multiline treatment in advanced NSCLC. Front Oncol (2020) 10:1706. doi: 10.3389/fonc.2020.01706
48. Rakaee M, Adib E, Ricciuti B, Sholl LM, Shi W, Alessi JV, et al. Association of machine learning-based assessment of tumor-infiltrating lymphocytes on standard histologic images with outcomes of immunotherapy in patients with NSCLC. JAMA Oncol (2023) 9(1):51–60. doi: 10.1001/jamaoncol.2022.4933
49. Kim KH, Hur JY, Koh J, Cho J, Ku BM, Koh JY, et al. Immunological characteristics of hyperprogressive disease in patients with non-small cell lung cancer treated with anti-PD-1/PD-L1 abs. Immune Netw (2020) 20(6):e48. doi: 10.4110/in.2020.20.e4
50. Arasanz H, Zuazo M, Bocanegra A, Gato M, Martínez-Aguillo M, Morilla I, et al. Early detection of hyperprogressive disease in non-small cell lung cancer by monitoring of systemic T cell dynamics. Cancers (Basel) (2020) 12(2):344. doi: 10.3390/cancers12020344
51. Lo Russo G, Moro M, Sommariva M, Cancila V, Boeri M, Centonze G, et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res (2019) 25(3):989–99. doi: 10.1158/1078-0432.CCR-18-1390
52. Genova C, Dellepiane C, Carrega P, Sommariva S, Ferlazzo G, Pronzato P, et al. Therapeutic implications of tumor microenvironment in lung cancer: focus on immune checkpoint blockade. Front Immunol (2021) 12:799455. doi: 10.3389/fimmu.2021.799455
53. Ku BM, Kim Y, Lee KY, Kim SY, Sun JM, Lee SH, et al. Tumor infiltrated immune cell types support distinct immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. Eur J Immunol (2021) 51(4):956–64. doi: 10.1002/eji.202048966
54. Wakiyama H, Kato T, Furusawa A, Okada R, Inagaki F, Furumoto H, et al. Treg-dominant tumor microenvironment is responsible for hyperprogressive disease after PD-1 blockade therapy. Cancer Immunol Res (2022) 10(11):1386–97. doi: 10.1158/2326-6066.CIR-22-0041
55. Zhang W, Quan Y, Ma X, Zeng L, Li J, Chen S, et al. Synergistic effect of glutathione and IgG4 in immune evasion and the implication for cancer immunotherapy. Redox Biol (2023) 60:102608. doi: 10.1016/j.redox.2023.102608
56. Okeya K, Kawagishi Y, Muranaka E, Izumida T, Tsuji H, Takeda S. Hyperprogressive disease in lung cancer with transformation of adenocarcinoma to small-cell carcinoma during pembrolizumab therapy. Intern Med (2019) 58(22):3295–8. doi: 10.2169/internalmedicine.2892-19
57. Li G, Choi JE, Kryczek I, Sun Y, Liao P, Li S, et al. Intersection of immune and oncometabolic pathways drives cancer hyperprogression during immunotherapy [J]. Cancer Cell (2023) 41(2):304–22.e7. doi: 10.1016/j.ccell.2022.12.008
58. Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci USA (2019) 116(20):9999–10008. doi: 10.1073/pnas.1822001116
59. Kanazu M, Edahiro R, Krebe H, Nishida K, Ishijima M, Uenami T, et al. Hyperprogressive disease in patients with non-small cell lung cancer treated with nivolumab: a case series. Thorac Cancer (2018) 9(12):1782–7. doi: 10.1111/1759-7714.12894
60. Grecea M, Marabelle A, Ammari S, Massard C, Champiat S. Managing hyperprogressive disease in the era of programmed cell death protein 1/Programmed death-ligand 1 blockade: a case discussion and review of the literature. Oncologist (2020) 25(5):369–74. doi: 10.1634/theoncologist.2019-0671
61. Matsuo N, Azuma K, Kojima T, Ishii H, Tokito T, Yamada K, et al. Comparative incidence of immune-related adverse events and hyperprogressive disease in patients with non-small cell lung cancer receiving immune checkpoint inhibitors with and without chemotherapy. Invest New Drugs (2021) 39(4):1150–8. doi: 10.1007/s10637-021-01069-7
Keywords: non-small cell lung cancer, PD-1/PD-L1, response pattern, hyperprogressive disease, immunotherapy
Citation: Li Y, Chen T, Nie TY, Han J, He Y, Tang X and Zhang L (2023) Hyperprogressive disease in non-small cell lung cancer after PD-1/PD-L1 inhibitors immunotherapy: underlying killer. Front. Immunol. 14:1200875. doi: 10.3389/fimmu.2023.1200875
Received: 05 April 2023; Accepted: 24 April 2023;
Published: 22 May 2023.
Edited by:
Vera Rebmann, University of Duisburg-Essen, GermanyReviewed by:
Jianjun Zhang, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2023 Li, Chen, Nie, Han, He, Tang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanping Li, MzkyNDI3MjhAMTYzLmNvbQ==; Li Zhang, RHJlYW1tYWtlci1saUBvdXRsb29rLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yanping Li1*†
Yanping Li1*† Li Zhang
Li Zhang