- 1Department of Spine Surgery, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of High Technology for Plant Protection, Plant Protection Research Institute, Guangdong Academy of Agricultural Science, Guangzhou, Guangdong, China
1 Introduction
Stinging by social Hymenoptera species such as honeybees, vespids, and ants is a major causes of anaphylaxis (1). Hymenopteran stings only cause minor local inflammation and reactions in most people. However, patients with venom allergy are at risk for systemic allergic reactions, which are a leading cause of anaphylaxis fatalities (2). Fire ants (Solenopsis) are aggressive species and named for the burning pain they inflict. Indeed, stinging ant venom allergy has become a significant public health concern in parts of the world where the fire ants are endemic (3).
Red imported fire ant (RIFA), Solenopsis invicta Buren, is a major notorious invasive ant species that appears in the list of 100 of the world’s worst invasive alien species (4). It can inflict serious economic and ecological damage on households, electricity service, communications, wildlife, agriculture, recreation areas, and bring a huge threat to human health and life (5, 6). Given the widespread distribution of RIFA in human-inhabited areas, reports of fire ant attacks and stings are common. For instance, more than 30% of people in fire ant-infested areas have suffered stings each year in the southeast region of the United States and China (6, 7).
RIFA is of major medical importance, and its toxicity mechanism is fairly unique. RIFA venom mainly including water-insoluble alkaloid differs from the venoms of honeybees, vespids, which are composed largely of protein-containing aqueous solutions (2, 8). Undoubtedly, it is the venom that makes RIFA such a significant health hazard to humans. However, RIFA venom also has a positive side which can significantly inhibit some key symptoms of psoriasis and malaria (9). Therefore, exploring the biological role of RIFA venom and making them beneficial will provide a new scheme for the development and application of RIFA. Here, we summarize the current research on RIFA venoms and put forward the idea that RIFA venoms have potential anti-tumor effects.
2 Components of red imported fire ant venom and its physiological activity
After being stung by RIFA, human body will feel burning pain. A few people will have allergic reactions to toxic proteins, and even allergic shock in severe cases (1, 10, 11). The powerful virulence of S. invicta is closely related to its venom gland secretions, which are produced in the poison gland and mainly composed of insoluble alkaloids and trace protein (2, 8, 12, 13). Four protein antigens (SoliI-IV, allergic proteins) were identified in water-soluble protein peptides (small molecules and enzymes) of the fire ant venom, which can cause allergic reactions (14–17). The insoluble alkaloids are mainly composed of 2-methyl-6-alkyl or alkenyl piperidines and piperideines, which can promote mast cells to release histamine and vasoactive amines, causing cell necrosis, pain and abscess (18, 19). Alkaloids with many kinds of activities were identified from the RIFA venom. These small nitrogen heterocyclic compounds have a variety of pharmacological activities. 2-methyl-6-undecylpiperidine (solenopsin A) is a powerful poison hemolysin and skin necrosis of alkaloids that make the cells release histamine (19, 20). cis- and trans-2-methyl-6-undecylpiperidine (isosolenopsin A and solenopsin A) interferes with the coupling between ion channels and the recognition sites of vertebrate nicotinic acetylcholine receptors (21). After intravenous injection of the alkaloids into mice, it was found that the alkaloids can seriously damage the central nervous system and cardiovascular system of mice, indicating that the alkaloids can penetrate the blood-brain barrier and cause dizziness, seizures, cardiopulmonary complications, death and other consequences when the injection dose ranges from 3-30 mg/kg (21). Solenopsin A and analogs can ceramide and help to restore the barrier function of the skin (9, 22). Experiments have proved that some key symptoms of psoriasis can be significantly inhibited. When the local area of the lesion treated with solenopsin A was studied by immunohistochemistry, it was found that the number of CD4+ T cells, CD8+ T cells and CD11c+ dendritic cells decreased significantly. RIFA venom may become a new therapeutic method for the development of psoriasis treatment (22, 23). In addition, solenopsins can inhibit the formation of Pseudomonas fluorescens and other biofilms, and reduce bacterial adhesion significantly (24).
Collectively, RIFA venom has a variety of active ingredients, and plays a variety of biological functions, and can even regulate human physiological functions. Therefore, RIFA venom has the potential to be converted into clinical therapeutic drugs.
3 Biological venom and tumor treatment
Malignant tumor is one of the most serious diseases threatening human health. Surprisingly, a variety of biological venoms, such as bee venom, snake venom, toad venom and scorpion venom, have been found to have therapeutic effects on tumor (25–32). These venoms promote apoptosis, autophagy and lysis of tumor cells by regulating gene expression of tumor cells and cytotoxicity. Inhibition of tumor cell proliferation, adhesion, migration and invasion, inhibition of tumor angiogenesis and other effects to play an anti-tumor role (25–32). These biotoxins are mainly composed of peptides, proteins and alkaloids. It is worth noting that bee venom, snake venom, toad venom and scorpion venom can promote tumor cell apoptosis by inhibiting the activation of phosphatidylinoinosiol 3-kinase (PI3K) and phospho-Akt (p-Akt). And inhibit tumor angiogenesis to shrink tumor volume (25–32). Interestingly, solenopsin A in RIFA venom inhibited the angiogenesis of zebrafish by delaying the formation of angiogenesis precursor or bud in vivo (33). In vitro and cellular experiments, solenopsin A showed relatively selective inhibition of Akt activation in a competitive manner with ATP. In addition, in cellular experiments, solenopsin A also regulated the downstream pathway by inhibiting PI3K activation (33). The PI3K/Akt pathway is involved in the regulation of various cellular functions in vivo, including proliferation, cytoskeletal organization, survival, and carcinogenesis (34–38). Akt is an important drug target for cancer and inflammatory diseases. Akt inhibitors are divided into ATP competitive Akt inhibitors and allosteric Akt inhibitors. At present, the effectiveness and specificity of Akt inhibitors are not satisfactory, and have a variety of adverse reactions (39). Therefore, the development of tumor therapeutics targeting Akt has a broad application prospect. It is worth noting that the active components in the above biological venom, such as bee venom, toad venom, snake venom and scorpion venom, can not only regulate PI3K/Akt pathway, but also inhibit angiogenesis through Akt/VEGF pathway and mTOR/VEGF pathway, inhibit tumor growth by regulating the expression levels of cyclin, p21, p27, p38 and HIF- 1 α pathway and promote tumor cell lysis and apoptosis by up-regulating the expression of RIP1, RIP3, PARP-1 and ERK signaling pathway (29, 40–42). Therefore, the anti-tumor effect of RIFA venom through other pathways remains to be further explored. In addition, as mentioned earlier, RIFA venom plays a role in regulating the number of immune cells in psoriasis, in which CD4+T cells and CD8+T cells play an important role in anti-tumor immunity. Therefore, RIFA venom may play an anti-tumor effect by regulating the immune system (22, 43, 44).
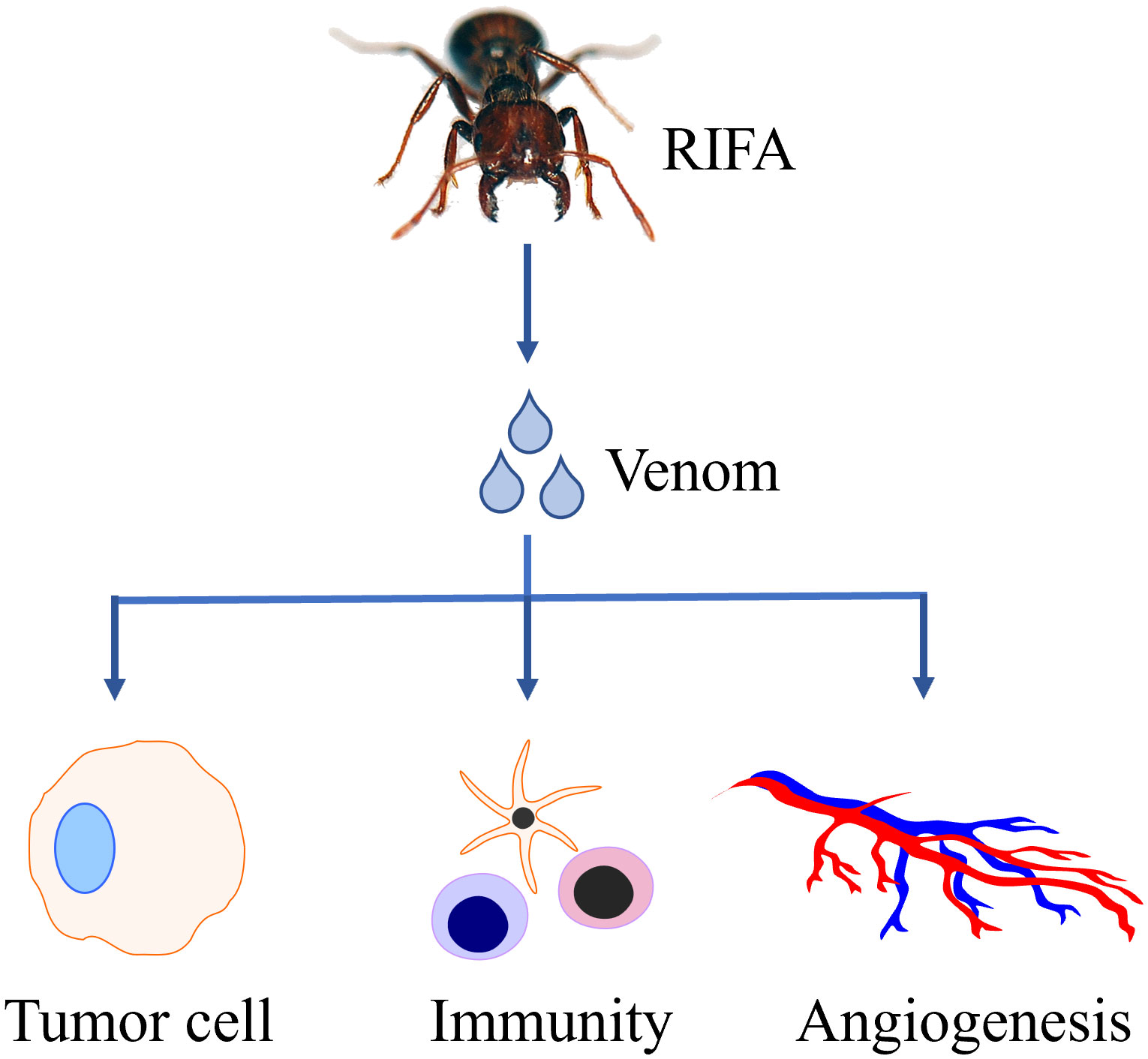
Collectively, RIFA venom is likely to exert its anti-tumor effect through PI3K pathway, regulation of angiogenesis, regulation of immune system and other unknown pathways (Figure 1).
4 Discussion
The invasion of RIFA has caused serious economic losses and ecological disasters (45, 46). After being stung of the fire ant, people will have pain, allergic reactions, and even allergic shock (1, 10, 11). RIFA venom is mainly composed of allergic proteins (SoliI-IV) and alkaloids that can cause allergic reactions (14, 15). To explore the biological effects of RIFA venom will be helpful for the potential clinical therapeutic agent for tumor treatment. Many physiological effects of alkaloid components in RIFA venom have been explored, such as promoting mast cells to release histamines and vasoactive amines, causing cell necrosis, causing pain and pustular reaction at the sting site (18, 19), promoting hemolysis and skin necrosis (19), interfering with the coupling between ion channels and the recognition sites of vertebrate nicotinic acetylcholine receptors. It can cause damage to the central nervous system and cardiovascular system (21), promote the recovery of skin barrier function (9, 22), regulates the number of local immune cells in the lesion (23), etc. It is worth noting that solenopsin can inhibit Akt and inhibit angiogenesis in vitro through PI3K signaling pathway (33). These findings are consistent with the fact that biotoxins such as bee venom, snake venom, toad venom, and scorpion venom can promote tumor cell apoptosis and reduce tumor size by inhibiting tumor angiogenesis by inhibiting the activation of phosphatidylinostat 3-kinase (PI3K) and phospho-Akt (p-Akt) (25–32). Bee venom, snake venom, toad venom and scorpion venom have been found to play an anti-tumor role by regulating gene expression of tumor cells, cytotoxicity, promoting apoptosis, autophagy and lysis of tumor cells, inhibiting tumor cell proliferation, adhesion, migration and invasion, and inhibiting tumor angiogenesis. However, there are few studies on RIFA venom, and only the PI3K/Akt pathway has been found to play its anti-tumor function, the PI3K/Akt pathway is closely related to malignant tumors, and Akt is an important drug target for cancer and inflammatory diseases. Therefore, the development of Akt targeted tumor drugs has a wide application prospect. In addition, RIFA venom can also regulate the number of immune cells, and its anti-tumor effect may be exerted by regulating the immune system, either. Finally, many components of RIFA venom need to be further explored and their valuable functions and applications explored. Unfortunately, the evolutionary reason for anti-tumor function of ant venom is currently unknown based on limited evidence. We speculate that interactions might exist between ants and mammals, shaping the physiology for both animals. The technology of separation and synthesis of active components in RIFA venom has been maturing, which makes it possible for us to study and utilize specific venom (14, 47).
Collectively, RIFA venom has a variety of effects and has the potential to be used as a clinical therapeutic agent. Its inhibitory effect on P13K-Akt pathway is similar to that of bee venom, snake venom, toad venom and scorpion venom, suggesting that RIFA venom has potential anti-tumor ability.
Author contributions
Conceptualization, data curation, writing-original draft preparation, writing-review and editing: all authors. Supervision and funding acquisition: KC, GQ. All authors have read and agreed to the final version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2021YFD1000500 to GQ), and National Natural Science Foundation of China (82072513 to KC), Science and Technology Program of Guangzhou (202102080182 to KC).
Acknowledgments
We wish to thank the National Key R & D Program of China, the National Natural Science Foundation of China, and the Science and Technology Program of Guangzhou for their generous support. We also wish to thank Zhiguang Xu for his guidance on this article and the relevant authors in the citations.
Conflict of interest
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wanandy T, Mulcahy E, Lau WY, Brown SGA, Wiese MD. Global view on ant venom allergy: from allergenic components to clinical management. Clin Rev Allergy Immunol (2022) 62(1):123–44. doi: 10.1007/s12016-021-08858-1
2. Fitzgerald KT, Flood AA. Hymenoptera stings. Clin Tech Small Anim Pract (2006) 21(4):194–204. doi: 10.1053/j.ctsap.2006.10.002
3. Arif F, Williams M. Hymenoptera stings. In: StatPearls. Treasure Island (FL: StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC (2023).
4. Lowe S. BM, Boudjelas S, De Poorter M. 100 of the world’s worst invasive alien species. In: A selection from the global invasive species database. The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN) (2000), 12. Available at: www.issg.org/booklet.pdf.
5. Vinson SB. Invasion of the red imported fire ant (Hymenoptera: Formicidae): spread, biology and impact. Am Entomol (1997) 43(1)23–39.
6. Wang L, Lu Y, Li R, Zeng L, Du J, Huang X, et al. Mental health effects caused by red imported fire ant attacks (Solenopsis invicta). Plos One (2018) 13(6):e0199424.
7. Kumari J, Sah RK, Mohaideen S NM, Ahmad S, Pati S. Studying the rationale of fire ant sting therapy usage by the tribal natives of bastar revealed ant venom-derived peptides with promising anti-malarial activity. Toxins (2022) 14(11):789.
8. Lockey RF. The imported fire ant: immunopathologic significance. Hosp Pract (Off Ed) (1990) 25(3):109–12, 115-24. doi: 10.1080/21548331.1990.11703926
9. Karlsson I, Zhou X, Thomas R, Smith AT, Bonner MY, Bakshi P, et al. Solenopsin a and analogs exhibit ceramide-like biological activity. Vasc Cell (2015) 7:5. doi: 10.1186/s13221-015-0030-2
10. Kruse B, Anderson J, Simon LV. Fire ant bites. In: StatPearls. Treasure Island (FL: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC (2022).
11. Potiwat R, Sitcharungsi R. Ant allergens and hypersensitivity reactions in response to ant stings. Asian Pac J Allergy Immunol (2015) 33(4):267–75.
12. Chen J, Zhao Y, Li XC, Zhao JH. Pyridine alkaloids in the venom of imported fire ants. J Agric Food Chem (2019) 67(41):11388–95. doi: 10.1021/acs.jafc.9b03631
13. dos Santos Pinto JR, Fox EG, Saidemberg DM, Santos LD, da Silva Menegasso AR, Costa-Manso E, et al. Proteomic view of the venom from the fire ant Solenopsis invicta buren. J Proteome Res (2012) 11(9):4643–53. doi: 10.1021/pr300451g
14. Hoffman DR, Dove DE, Jacobson RS. Allergens in hymenoptera venom. XX. isolation of four allergens from imported fire ant (Solenopsis invicta) venom. J Allergy Clin Immunol (1988) 82(5 Pt 1):818–27. doi: 10.1016/0091-6749(88)90084-x
15. Hoffman DR. Allergens in hymenoptera venom. XVII. allergenic components of Solenopsis invicta (imported fire ant) venom. J Allergy Clin Immunol (1987) 80(3 Pt 1):300–6. doi: 10.1159/000234394
16. Lockwood SA, Haghipour-Peasley J, Hoffman DR, Deslippe RJ. Identification, expression, and immuno-reactivity of sol i 2 & sol i 4 venom proteins of queen red imported fire ants, Solenopsis invicta buren (Hymenoptera: formicidae). Toxicon (2012) 60(5):752–9. doi: 10.1016/j.toxicon.2012.05.011
17. Hoffman DR, Sakell RH, Schmidt M. Sol i 1, the phospholipase allergen of imported fire ant venom. J Allergy Clin Immunol (2005) 115(3):611–6. doi: 10.1016/j.jaci.2004.11.020
18. Chen J, Cantrell CL, Shang HW, Rojas MG. Piperideine alkaloids from the poison gland of the red imported fire ant (Hymenoptera: formicidae). J Agric Food Chem (2009) 57(8):3128–33. doi: 10.1021/jf803561y
19. Lind NK. Mechanism of action of fire ant (Solenopsis) venoms. i. lytic release of histamine from mast cells. Toxicon (1982) 20(5):831–40. doi: 10.1016/0041-0101(82)90070-8
20. Macconnell JG, Blum MS, Fales HM. Alkaloid from fire ant venom: identification and synthesis. Science (1970) 168(3933):840–1. doi: 10.1126/science.168.3933.840
21. Howell G, Butler J, Deshazo RD, Farley JM, Liu HL, Nanayakkara NP, et al. Cardiodepressant and neurologic actions of Solenopsis invicta (imported fire ant) venom alkaloids. Ann Allergy Asthma Immunol (2005) 94(3):380–6. doi: 10.1016/S1081-1206(10)60991-X
22. Arbiser JL, Nowak R, Michaels K, Skabytska Y, Biedermann T, Lewis MJ, et al. Evidence for biochemical barrier restoration: topical solenopsin analogs improve inflammation and acanthosis in the KC-Tie2 mouse model of psoriasis. Sci Rep (2017) 7(1):11198. doi: 10.1038/s41598-017-10580-y
23. Arbiser JL, Bonner MY, Ward N, Elsey N, Rao S. Selenium unmasks protective iron armor: a possible defense against cutaneous inflammation and cancer. Biochim Biophys Acta Gen Subj (2018) 1862(11):2518–27. doi: 10.1016/j.bbagen.2018.05.018
24. Carvalho DB, Fox EGP, Santos DGD, Sousa JS, Freire DMG, Nogueira FCS, et al. Fire ant venom alkaloids inhibit biofilm formation. Toxins (Basel) (2019) 11(7):420. doi: 10.3390/toxins11070420
25. Oršolić N. Bee venom in cancer therapy. Cancer Metastasis Rev (2012) 31(1-2):173–94. doi: 10.1007/s10555-011-9339-3
26. Kwon NY, Sung SH, Sung HK, Park JK. Anticancer activity of bee venom components against breast cancer. Toxins (Basel) (2022) 14(7):460. doi: 10.3390/toxins14070460
27. Dueñas-Cuellar RA, Santana CJC, Magalhães ACM, Pires OR Jr, Fontes W, Castro MS. Scorpion toxins and ion channels: potential applications in cancer therapy. Toxins (Basel) (2020) 12(5):326. doi: 10.3390/toxins12050326
28. Urra FA, Araya-Maturana R. Targeting metastasis with snake toxins: molecular mechanisms. Toxins (Basel) (2017) 9(12):390. doi: 10.3390/toxins9120390
29. Li FJ, Hu JH, Ren X, Zhou CM, Liu Q, Zhang YQ. Toad venom: a comprehensive review of chemical constituents, anticancer activities, and mechanisms. Arch Pharm (Weinheim) (2021) 354(7):e2100060. doi: 10.1002/ardp.202100060
30. Díaz-García A, Varela D. Voltage-gated K(+)/Na(+) channels and scorpion venom toxins in cancer. Front Pharmacol (2020) 11:913. doi: 10.3389/fphar.2020.00913
31. Moga MA, Dimienescu OG, Arvatescu CA, Ifteni P, Ples L. Anticancer activity of toxins from bee and snake venom-an overview on ovarian cancer. Molecules (2018) 23(3):692. doi: 10.3390/molecules23030692
32. Rady I, Siddiqui IA, Rady M, Mukhtar H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett (2017) 402:16–31. doi: 10.1016/j.canlet.2017.05.010
33. Arbiser JL, Kau T, Konar M, Narra K, Ramchandran R, Summers SA, et al. Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis. Blood (2007) 109(2):560–5. doi: 10.1182/blood-2006-06-029934
34. Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK. The akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci USA (1998) 95(25):14950–5. doi: 10.1073/pnas.95.25.14950
35. Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science (1991) 254(5029):274–7. doi: 10.1126/science.254.5029.274
36. Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, et al. Suppression of c-myc-induced apoptosis by ras signalling through PI(3)K and PKB. Nature (1997) 385(6616):544–8. doi: 10.1038/385544a0
37. Watton SJ, Downward J. Akt/PKB localisation and 3’ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr Biol (1999) 9(8):433–6. doi: 10.1016/S0960-9822(99)80192-4
38. Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev (2004) 30(2):193–204. doi: 10.1016/j.ctrv.2003.07.007
39. Uko NE, Güner OF, Matesic DF, Bowen JP. Akt pathway inhibitors. Curr Top Med Chem (2020) 20(10):883–900. doi: 10.2174/1568026620666200224101808
40. Shi P, Xie S, Yang J, Zhang Y, Han S, Su S, et al. Pharmacological effects and mechanisms of bee venom and its main components: recent progress and perspective. Front Pharmacol (2022) 13:1001553. doi: 10.3389/fphar.2022.1001553
41. Bialves TS, Bastos Junior CLQ, Cordeiro MF, Boyle RT. Snake venom, a potential treatment for melanoma. a systematic review. Int J Biol Macromol (2023) 231:123367. doi: 10.1016/j.ijbiomac.2023.123367
42. Mikaelian AG, Traboulay E, Zhang XM, Yeritsyan E, Pedersen PL, Ko YH, et al. Pleiotropic anticancer properties of scorpion venom peptides: rhopalurus princeps venom as an anticancer agent. Drug Des Devel Ther (2020) 14:881–93. doi: 10.2147/DDDT.S231008
43. Speiser DE, Chijioke O, Schaeuble K, Münz C. CD4(+) T cells in cancer. Nat Cancer (2023) 4(3):317–29. doi: 10.1038/s43018-023-00521-2
44. St Paul M, Ohashi PS. The roles of CD8(+) T cell subsets in antitumor immunity. Trends Cell Biol (2020) 30(9):695–704. doi: 10.1016/j.tcb.2020.06.003
45. Kemp SF, deShazo RD, Moffitt JE, Williams DF, Buhner WA 2nd. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol (2000) 105(4):683–91. doi: 10.1067/mai.2000.105707
46. Helms JA 4th, Godfrey AP, Ames T, Bridge ES. Are invasive fire ants kept in check by native aerial insectivores? Biol Lett (2016) 12(5):20160059. doi: 10.1098/rsbl.2016.0059
Keywords: Solenopsis invicta Buren, venom, alkaloid, tumor, antitumor activity, Solenopsin A
Citation: Mo Y, Shi Q, Qi G and Chen K (2023) Potential anti-tumor effects of Solenopsis invicta venom. Front. Immunol. 14:1200659. doi: 10.3389/fimmu.2023.1200659
Received: 05 April 2023; Accepted: 08 May 2023;
Published: 22 May 2023.
Edited by:
Yifei Wang, Southern Medical University, ChinaCopyright © 2023 Mo, Shi, Qi and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kebing Chen, Y2hrYmluZ0BtYWlsLnN5c3UuZWR1LmNu; Guojun Qi, cWlnakBnZHBwcmkuY29t
 Yizhang Mo
Yizhang Mo Qingxing Shi2
Qingxing Shi2 Guojun Qi
Guojun Qi Kebing Chen
Kebing Chen