
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 07 July 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1198562
 Jian Huang1†
Jian Huang1† Zhen-Guang Wang1†
Zhen-Guang Wang1† Qi-Fei Tao1†
Qi-Fei Tao1† Yun Yang1
Yun Yang1 Sheng-Xian Yuan1
Sheng-Xian Yuan1 Fang-Ming Gu1
Fang-Ming Gu1 Hui Liu1
Hui Liu1 Ze-Ya Pan1*
Ze-Ya Pan1* Bei-Ge Jiang1*
Bei-Ge Jiang1* Wan Yee Lau1,2*
Wan Yee Lau1,2* Wei-Ping Zhou1*
Wei-Ping Zhou1*Background: Reports on Lenvatinib-based therapies show promising treatment outcomes for patients with unresectable hepatocellular carcinoma (uHCC). However, the effect and safety of Lenvatinib-based therapies still need to be further studies.
Methods: This was a retrospective, single-center study on the safety and treatment efficacy of Lenvatinib-based combination therapies for uHCC Patients. The primary endpoints were progression-free survival (PFS) and overall survival (OS). The secondary endpoints were progressive disease (PD), stable disease (SD), partial response (PR), and complete response (CR).
Results: Of 91 patients, there were 16 females and 75 males with uHCC who received systemic therapies based on Lenvatinib in our center. Forty-six patients (50.5%) received Lenvatinib combined with PD-1 antibody treatment. All these patients also received local therapy with the exception of 2 patients. The remaining 36 patinets received Lenvatinib combined with transcatheter arterial chemoembolization (TACE), 1 patient treated Lenvatinib combined with radiotherapy, 8 patients received Lenvatinib alone. At a median treatment time of 8 months, the objective response rate (ORR) of the entire cohort was 58.2% (53 patients), including 7 patients with CR and 46 patients with PR. 21 patients (23.1%) had SD. The disease control rate (DCR) of all patients was 81.3% (74 patients). However, 17 patients (18.7%) developed PD. The 1- and 2-year cumulative OS rates for the entire cohort were 66.8% and 39.3%, while the corresponding PFS rates were 38.0% and 17.1%, respectively. Univariate and multivariate Cox regression analysis revealed multiple tumor sites to be an independent OS risk factor for uHCC patients (HR=2.204, 95% CI=1.104-4.399, P=0.025). The most frequently reported adverse events in all patients were AST elevation (51.6%), followed by hypertension (33.0%), ALT elevation (26.4%), and decreased appetite (25.3%). After a combination treatment of Lenvatinib-based therapies, 15 patients met the criteria for salvage liver resection and underwent down-staging hepatectomy with a curative intent. The combination of PD-1 treatment was not very effective in improving the prognosis of uHCC patients treated with Lenvatinib combined with TACE.
Conclusion: Our study demonstrated that a proportive of patients benefited from Lenvatinib-based combination therapies with manageable safety profiles, allowing these patients to undergo downstaging surgery with curative intent.
Hepatocellular carcinoma (HCC) accounts for 75-85% of all liver cancers and is the fourth leading cause of cancer-related mortality worldwide (1). The vast majority of cases of HCC are associated with chronic liver diseases such as chronic viral hepatitis and nonalcoholic fatty liver disease (2). Patients with unresectable HCC (uHCC) have an abysmal prognosis, and the late diagnosis is exacerbated by the absence of sysptoms and the paucity of effective screening programs for early diagnosis (3). Constraints for surgical resection as a common tool used for “care” come from factors such as chronic cirrhosis, large tumor size, and extrahepatic metastases. Systemic therapy is commonly used in patients with uHCC to prolong survival, enhance quality of life, and provide improved treatment outcomes.
Tyrosine kinase inhibitors (TKIs) are often used to treat uHCC. Lenvatinib, functioning as a multiple kinase inhibitor against the FGFR 1-4, VEGFR 1-3, RET, PDGF receptor α, and KIT, has been approved as the first-line systemic therapy drug for uHCC (1). The overall survival (OS) for advanced HCC patients treated with Lenvatinib has been shown to be non-inferior to sorafenib in the phase 3 clinical trial ‘REFLECT’ (2). The overall response rate (ORR) in the study on Lenvatinib in treating uHCC using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria was 24.1%. However, 99% of patients with uHCC in this study developed treatment-emergent adverse events (TEAEs). Previous studies have also showed that combination treatments comprising of vascular endothelial growth factor (VEGF)-mediated immune suppression and PD-1 inhibitors, such as pembrolizumab, are promising options in achieving better prognosis for HCC patients, especially in HBV-related HCC (3, 4). The synergistic effect of the combinations lies in decreased number of tumor-associated macrophages and a high proportion of CD8+ T cells, both of which can increase the antitumor activity of PD-1 inhibitors (5). For patients with HCC in the intermediate and advanced stages, transcatheter arterial chemoembolization (TACE) has become the gold standard of treatment (6). It has been shown that TACE has a synergistic effect with systemic therapies because it upregulates the expressions of fibroblast growth factor (FGF) and VEGF (7), and stimulates tumor-associated antigens release and immunogenic death of cancer cells (8, 9). Other local treatments such as radiotherapy, radiofrequency ablation, and hepatic artery infusion chemotherapy (HAIC), also showed definite therapeutic effects on advanced HCC (10). uHCC with a large tumor size and rich blood supply can limit the treatment outcome of local treatments, making the need for them to be combined with other therapies to obtain satisfactory long-term survival results.
Whether or not combined therapies can be used to enhance treatment efficacy but without increasing adverse effects are the major concerns for clinicians. However, the benefits and drawbacks of using Lenvatinib-based combination treatments in HCC are still unclear. This retrospective study evaluated treatment efficacy and adverse effects of Lenvatinib-based combination therapies on uHCC patients treated in our center.
Patients who wre included in this retrospective study came from the Eastern Hepatobiliary Surgery Hospital (EHBH) between December 2018 to October 2020. Data on clinical outcomes and histopathological findings were collected prospectively and examined retrospectively. The Declaration of Helsinki was followed, and the EHBH Hospital’s Ethics Committee approved this research. All participants provided written informed consent for the treatment and for their data to be used for clinical research.
All eligible patients were independently diagnosed and evaluated by two experienced pathologists using the Barcelona Clinic Liver Cancer (BCLC) staging (11) and the Child-Pugh classification. Based on the Liver Cancer Study Group of Japan, uHCC was defined as bi-lobar liver involvement due to multiple or large solitary tumors, insufficient future liver remnant, extrahepatic metastasis or major vessels invasion, including inferior vena cava and portal vein (12).
The inclusion criteria were patients with: (1) uHCC confirmed by clinical features or histopathological biopsy; (2) Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 or 1; (3) Child-Pugh class A or B; (4) at least one measurable target lesion by mRECIST. The exclusion criteria were patients with: (1) ECOG-PS score >1; (2) Child-Pugh class C; (3) a history of other cancers; (4) incomplete data. Patients with uHCC who simultaneously received other forms of therapy such as TACE, radiotherapy, radiofrequency ablation, or chemotherapy were also included in this study. Surgeons based on the relevant guidelines combined with patient’s general condition, tumor burden status, laboratory tests, imaging examination and other indicators to determine which treatments to receive.
Clinical characteristics of patients including sex, age, liver cirrhosis, albumin-bilirubin (ALBI) grading, liver function, hepatitis virus status and tumor biomarkers were collected before treatment. Tumor status, including extrahepatic metastases, macrovascular invasion, BCLC staging and CNLC staging (13) were determined based on the results of imaging examinations. We used the following formula to mathematically derive ALBI: (log 10 bilirubin (umol/L) x 0.66) + (albumin (g/L) x -0.085) (14). The threshold level of HBV-DNA was used as we have previously described (15).
Lenvatinib (Eisai Inc., Woodcliff Lake, NJ, USA) was administered to all patients at a daily dose of 8 mg for individuals weighing <60 kg and 12 mg for those weighing ≥ 60 kg as administered in the REFLECT trial. All dose interruptions and reductions were carried out strictly using the REFLECT trial’s protocol. In most patients, Lenvatinib therapy was given for 8 months (range 5-11 months). Labeled patients were given 200 mg of pembrolizumab (Merck, New York, NJ) intravenously once every three weeks. Severe adverse events which warranted to discontinue treatment was adopted. The Seldinger technique was used to carry out TACE by catheterization of the hepatic artery through the percutaneous femoral artery to selectively or superselectively intubate the artery that supplies blood to the tumor. Proper amount of lipiodol and gelatin sponge, as well as the chemotherapeutic drugs of fluorouracil, epirubicin, and platinum were administered through the catheter. The chemotherapy medication doses were based on the patient’s tumor stage, body area, and physical condition.
CT or MRI was conducted to evaluate the treated tumor(s) once every 6-8 weeks, or when there were signs or symptoms indicating tumor progression. The RECIST v1.1 was used to grade tumor responses. Grading of AEs complied with CTCAE v5.0. Radiotherapy and radiofrequency ablation were performed based on the patient’s condition to achieve the best treatment effect.
The primary endpoints were progression free survival (PFS) and OS. PFS was defined as the period between the first day of Lenvatinib treatment and the date of disease progression was measured by RECIST v1.1 or death. OS was measured from the first day of Lenvatinib treatment to the date the patient died. The secondary endpoints were tumor response rates, including CR, PR, SD, and PD. The ORR was calculated as the sum of PR and CR, while the DCR was the sum of PR, CR, and SD. The treatment effect of each patient was evaluated by mRECIST based on contrast-enhanced CT or MRI. The AEs were graded using the NCI-CTCAE v5.0.
Every patient was followed-up once every 6-8 weeks for monitoring and evaluation. Regular blood and urine tests, liver and kidney liver functions, thyroid function, myocardial enzymes, HBV-DNA, and tumor markers including Prothrombin caused by Vitamin K Absence or Antagonist-II (PIVKA-II) and alpha-fetoprotein (AFP) and CT or MRI to assess tumor response were performed at each follow-up visit. Additionally, routine chest X-rays were done. With patient’s informed consent, radical resection with postoperative adjuvant treatment were carried out after any patient who met the tumor-downstaging criteria for salvage resection. Lenvatinib was discontinued for 1 week, and PD-1 was discontinued for 1 month before and after surgery. The remaining patients were followed-up until they developed PD, with symptomatic progression, developed severe toxicity to drug treatment, or withdrew their consents.
SPSS 26.0 (IBM, New York, United States) and Graph Pad Prism 9.0 (San Diego, CA, USA) were used for data processing and analysis. Data were presented as mean ± SD for continuous variables and as percentiles for categorical ones. Univariate and multivariate analysis were carried out using the Cox proportional hazards model. Fisher’s exact or Chi-squared tests were used for differences between categorical variables. Continuous variables were compared by the Student’s t-test or Mann-Whitney U test. The Kaplan-Meier technique was used for survival analysis and compared using the log-rank test. A P<0.05 was deemed statistically significant.
This study included 91 patients with uHCC. There were 16 females and 75 males who received Lenvatinib-based systemic therapies between December 2018 and October 2020. The median age was 53 years (interquartile range, 46.0-58.0), and 78 patients (85.7%) were HBsAg-positive, 52 patients (57.1%) had liver cirrhosis, nearly all patients were in Child-Pugh class A, and only three patients (3.3%) in Child-Pugh class B. Tumor markers showed 64 patients (70.3%) to have AFP levels above 20μ g/L, and 76 patients (83.5%) had elevated PIVKA-II levels. The median tumor diameter was 6.0 cm (interquartile range, 4.0-9.0cm), and 58 patients (63.7%) had multiple tumors. Ten patients (11.0%) had lymph node metastasis, 21 patients (23.1%) had extrahepatic metastasis, and 29 patients (31.9%) had macrovascular invasion, which included portal vein and hepatic vein invasion. Tumor staging, showed 57.1% and 56.0% of patients were categorized as BCLC stage C, and China Live Cancer (CNLC) stage IIIa-IIIb, respectively. Specifically, among the BCLC stage A patients, three patients with tumor recurrence after surgery were reluctant to undergo further surgery, five patients had unresectable HCC due to large tumor sizes, and one patient received targeted therapy for multiple tumors including multiple liver resections. Notably, 15 patients who received the systemic treatment were down-staged to salvage liver resection aiming at clinical cure. Table 1 provides the summary of the baseline characteristics of the patients.
Table 2 is a summary of the treatment regimens used in this study. Most patients were treated with systemic and local therapy, and only seven patients (7.6%) were treated with Lenvatinib alone. The majority of local therapy consisted of TACE, radiotherapy, radiofrequency ablation, and hepatic artery infusion chemotherapy (HAIC). TACE was administered to 78 patients (85.7%), of whom 44 patients (48.4%) were treated once, and 9 patients (9.8%) were treated for more than four times. Based on the patient’s condition,46 patients (50.5%) received Lenvatinib combined with PD-1 antibody therapy, with only 2 patients who received this combination treatment alone while the remainding patients (n=44) received this combination treatment together with local therapy. Eighty-two patients (90.1%) received 8 mg/day of Lenvatinib, while 9 (9.9%) received 12 mg/day.
The ORR of the entire cohort was 58.2% (n=53) after a median treatment of 8 months, there were 7 patients with CR and 46 patients with PR. In addition 21 patients (23.1%) achieved SD. The DCR for all the patients was 81.3% (n=74). The remaining 17 patients (18.7%) had PD. Table 3 summarizes the response rates as stratified by the BCLC staging.
The patients were observed for a duration ranging from 4-37 months (median 21 months). The 1- and 2-year cumulative OS rates for the entire cohort were 66.8% and 39.3%, while the corresponding PFS rates were 38.0% and 17.7%, respectively. The median PFS and OS were 10 and 17 months, respectively (Figure 1). On stratification using the BCLC staging, the median OS were 17 months for both the BCLC stages B and C. (Figure 2A). The median PFS for the BCLC stages A, B, and C were 23.5, 11, and 10 months, respectively (Figure 2B). Univariate and multivariate Cox regression analyses on the entire patient cohort for prognosis revealed that multiple tumor sites to be an independent risk factor for OS in uHCC patients (HR=2.204, 95% CI=1.104-4.399, P=0.025) (Table 4). Table 5 shows 11 patients received alternative therapies following progression of the tumors.
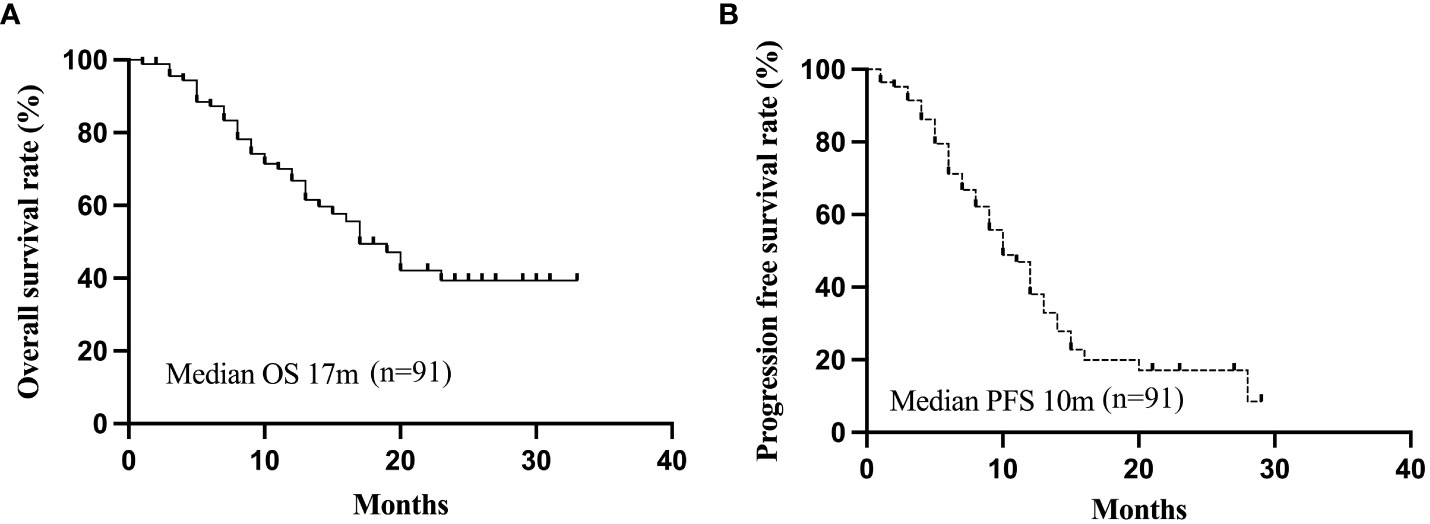
Figure 1 The all-patients Kaplan-Meier curves for OS (A) and PFS (B). OS, overall survival; PFS, progression-free survival.
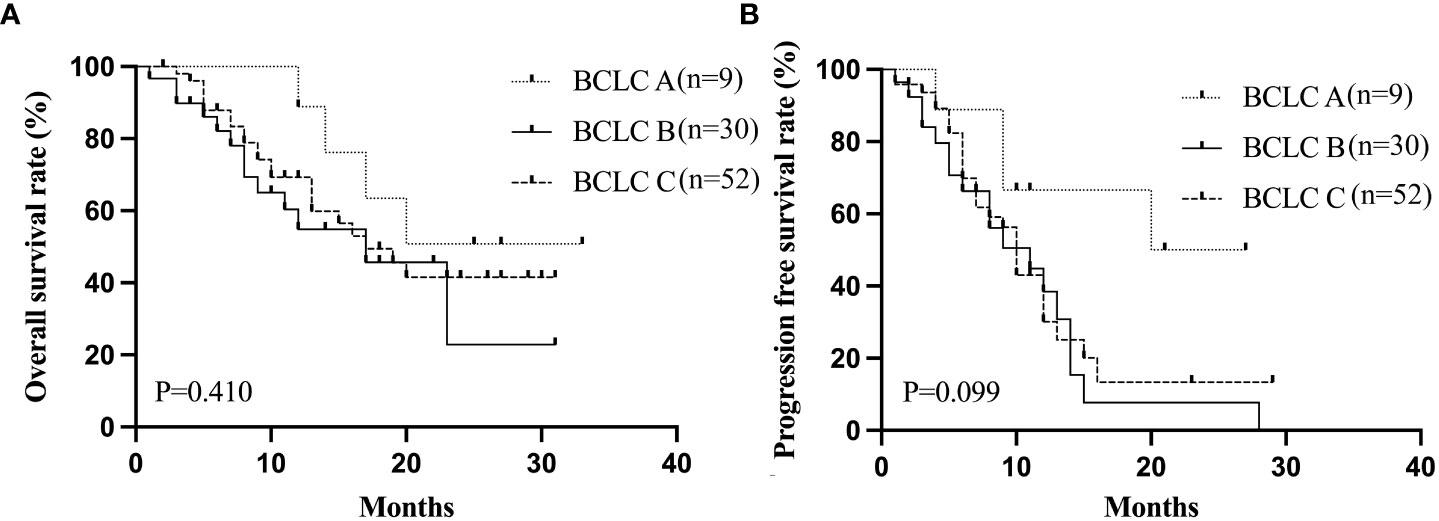
Figure 2 The Kaplan-Meier curves for OS (A) and PFS (B) stratified by BCLC staging. OS, overall survival; PFS, progression-free survival.
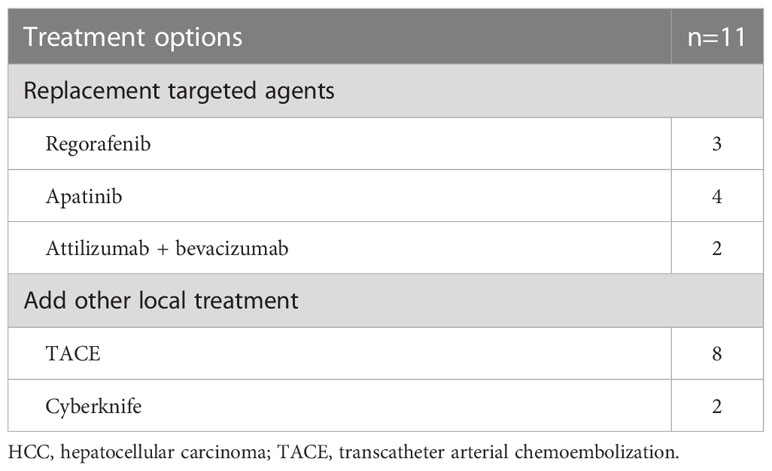
Table 5 Alternative treatment options for patients with advanced unresectable HCC after tumor progression.
In this study, all AEs were assessed and found to be tolerable and mild, and there were no toxicity-related deaths. The AEs in patients consisted of elevated AST levels (51.6%), followed by hypertension (33%), elevated ALT levels (26.4%), and decreased appetite (25.3%). AEs of grade 3 or higher were experienced in 24.2% of all patients to include AST elevation (6.6%), hypertension (4.4%), diarrhea (4.4%), increased blood bilirubin (4.4%), decreased appetite (3.3%), and fatigue (1.1%) (Table 6).
Following Lenvatinib-based combination therapies, the tumors in 15 patients were down-staged to become resectable using salvage liver resection with a curative intent. Seven (23.3%) of these 15 patients who received down-staging resection initially had BCLC stage B disease, while the remaining 8 patients (15.4%) initially had BCLC stage C disease. (Table 7). The baseline clinicopathologic characteristics of uHCC patients who achieved ORR after treatment as stratified by surgery are shown in Table 8, with no significantly differences in these characteristics which existed between the surgical and non-surgical groups. The 1- year cumulative OS and PFS rates for the surgical group were 73.3% and 27.7%, compared with the non-surgical group of 62.6% and 66.5%, respectively. The 2- year cumulative OS and PFS for the surgical group was not reached, while the non-surgical group was 34.4% and 36.4%.The median PFS of the surgical group was 13 months, compared with the non-surgical group of 16 months. The median OS of the surgical group was not reached, while the non-surgical group was 17 months. The two groups had no significant differences in OS (P=0.237) and PFS (P=0.262). Figure 3 shows the Kaplan-Meier curves of OS and PFS for patients who achieved ORR as stratified by surgery.
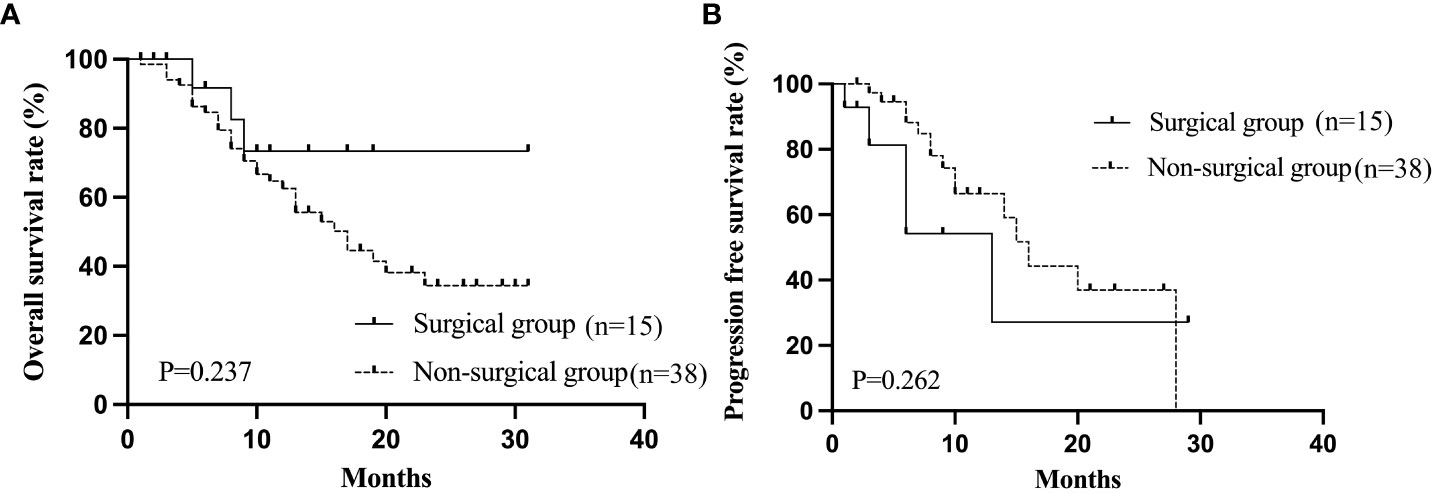
Figure 3 The Kaplan-Meier curves of OS (A) and PFS (B) stratified by surgery in patients who achieved ORR. OS, overall survival; PFS, progression-free survival; ORR, objective response rate.
The baseline clinical characteristics of uHCC patients who achieved ORR following treatment with Lenvatinib and TACE are shown in Table 9. Patients (n=42) were divided into two groups based on the PD-1 treatment. The proportion of patients with macrovascular invasion was greater in the Lenvatinib + TACE + PD-1 group (n=20) than in the Lenvatinib + TACE group (n=22, P=0.034). The median OS and PFS were 16 months and 11 months in the Lenvatinib + TACE + PD-1 group and 17 months and 10 months in the Lenvatinib + TACE group, respectively. Figure 4 depicts the Kaplan-Meier curves for OS (P=0.202) and PFS (P=0.566) for these patients. As the proportions of macrovascular invasion were significantly different between the Lenvatinib + TACE + PD-1 group and the Lenvatinib + TACE group, the effect of radiotherapy on patients with macrovascular invasion was also evaluated. Figure 5 depicts the Kaplan-Meier curves for OS (P=0.883) and PFS (P=0.613) in patients with macrovascular invasion, with or without radiotherapy. The median OS and PFS of the macrovascular invasion group were 13 months and 10 months in the radiotherapy group compared with 16 months and 12 months in the group without radiotherapy, respectively.
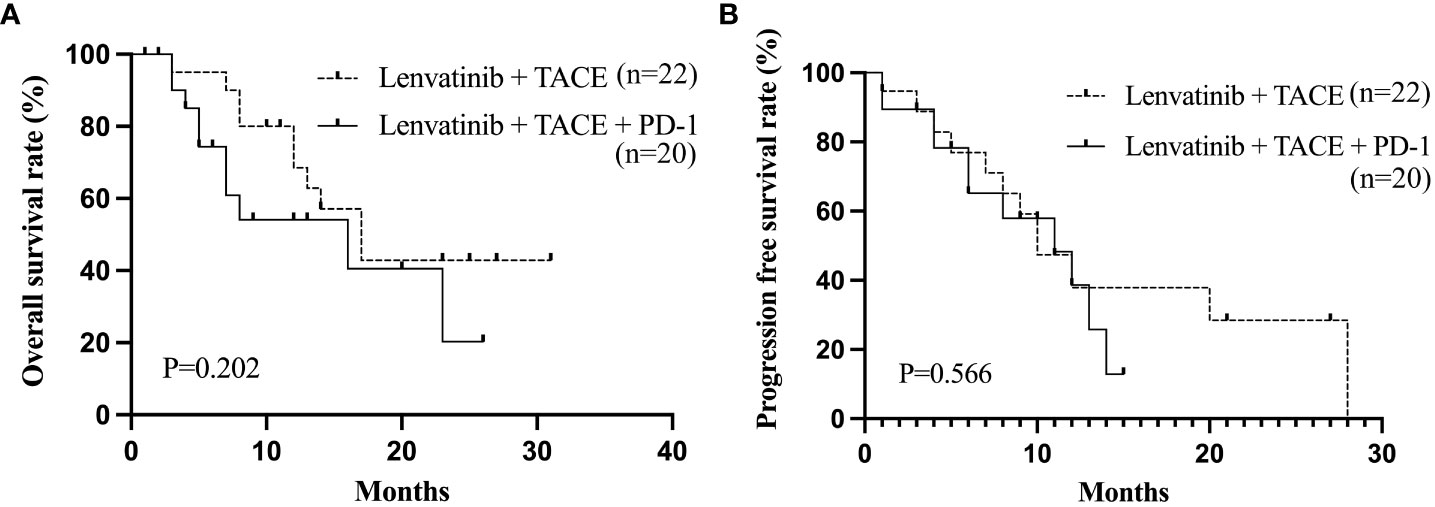
Figure 4 The Kaplan-Meier curves of OS (A) and PFS (B) of uHCC patients who achieved ORR after Lenvatinib and TACE treatment. OS, overall survival; PFS, progression-free survival; ORR, objective response rate; uHCC, unresectable hepatocellular carcinoma.

Figure 5 The Kaplan-Meier curves of OS (A) and PFS (B) of uHCC patients with macrovascular invasion with or without radiotherapy. OS, overall survival; PFS, progression-free survival; uHCC, unresectable hepatocellular carcinoma.
Our study supports that Lenvatinib-based combination therapies for patients with uHCC to be efficacious and safe, with the potential of tumor down-staging followed by salvage liver resection in a proportion of patients with initially incurable diseases to become curable, the whole cohort of patients had a median OS of 17 months and PFS of 10 months. The results of our study were in general consistant with the results reported by Finn et al. in a phase 1b single arm study (16) with median OS for patients with uHCC treated with Lenvatinib + pembrolizumab being 22 months, and median PFS being 9.3 months as assessed by mRECIST. The ORR was 46%, and the CR reached 11%. All these results show Lenvatinib-based combination therapies to be a promising treatment for uHCC patients.
In a mouse model, Lenvatinib which targets FGFR 1-4, VEGFR 1-3, PDGF α receptor, RET, and KIT, was shown to reduce the number of monocytes and macrophages while increasing the number of CD8+ T cells. Such effects can enhance the immunomodulatory activity when combined with PD-1 antibody (3, 16). A recent study by Torrens and colleagues found that in addition to inhibiting angiogenesis in tumor tissues, combined Lenvatinib with pembrolizumab exerted distinct immunomodulatory effects by stimulating immunological pathways, decreasing Treg cell infiltration, and blocking TGF-β signaling (17). In a systematic review, combined Lenvatinib with pembrolizumab demonstrated the highest absolute ORR when compared with any first-line treatment for uHCC (18). The combination of Lenvatinib with immune checkpoint inhibitors (ICIs) is promising in treating advanced HCC patients, as recently shown by an analysis of safety and efficacy study conducted by Huang et al. (19). However, in our study, PD-1 when combined with Lenvatinib and TACE did not significantly improve the prognosis of uHCC patients. This suggests that there is no definite correlation among the different types of combination therapy with the therapeutic benefit for patients, and the therapeutic effects may not be improved with more combinations of treatments. In our study, when 22 patients who received Lenvatinib and TACE alone were compared with 20 patients who received in addition PD-1 therapy, and these two groups of patient had similar baseline characteristics with the exception for macrovascular invasion, there was no significant difference in survival outcomes after treatment. Further subgroup analysis of macrovascular invasion was performed. When radiotherapy was added, the median OS of the radiotherapy subgroup on uHCC patients with macrovascular invasion was 13 months, a finding which is similar to the median survival of 12.3 months after radiotherapy reported by Tang et al. (20). All these findings suggest that treatment responses to combined therapy can be related to treatment tolerance by patients, limitations imposed by treatment adverse effects, and/or drug interactions in various combined treatment regimens. Therefore, studies to find out the appropriate combination therapies are important in treating HCC patients with advanced stages of diseases.
Using univariate and multivariate Cox regression analysis, multiple tumor sites was identified as an independent risk factor for post-treatment survival in uHCC patients. To our knowledge, our study is the first to identify multiple tumor sites to be an independent prognostic risk factor in patients with uHCC treated with Lenvatinib-based combination treatments. Yang et al. studied combined TACE with ICIs and TKIs for treatment of uHCC. They found 19 of 31 patients with multiple lesions had a favorable tumor response (21), suggesting that the combined therapy to be a treatment option for patients with uHCC with multiple tumor sites. More researches should be done to find better treatment options for patients with multiple tumor sites which is known to have a poor prognosis.
TACE is the therapy which is commonly used for patients with BCLC intermediate stage HCC, with a high tumor response and a tolerable safety profile (22). Previous studies show that the local anticancer effect of TACE is attributed to its abilities to exacerbate hypoxia in cancer cells (23, 24). Hypoxia in cancer cells triggers production of hypoxia-related factors, which increases VEGF and fibroblast growth factor levels and promotes tumor angiogenesis. In the combined therapy regimen of TACE with Lenvatinib, the better drug can inhibit the kinase activities of VEGF receptors to counteract the adverse effects of TACE, thus resulting in improved anticancer effects. Prior clinical studies indicated that Lenvatinib plus TACE provided survival benefits for patients with uHCC (25, 26). Chen et al. showed that the pembrolizumab-Lenvatinib-TACE sequential therapy prolonged the median PFS and OS in patients with uHCC (27). All these findings suggest that therapy using TACE and Lenvatinib plus pembrolizumab can provide clinical benefits in treating advanced uHCC patients. In our study, some patients treated with TACE and Lenvatinib plus pembrolizumab achieved a prolonged, although not statistically significant, median survival.
The most common adverse events in our study were AST elevation, hypertension, ALT elevation, and decreased appetite, which were similar as reported by the previous phase Ib Study (28). The incidence of grade 3-4 adverse events in our cohort was 24.2%, a figure which is high enough to draw to the attention of clinicians using Lenvatinib-based combination therapies. In general, the incidence of adverse events with combination therapy is higher than with monotherapy. Whether it is local or systemic therapy, the more therapies used, the more are the adverse events, which can have significantly increased impact on the patient’s liver and kidney functions, and can adversely affect the therapeutic effect (29). Therefore, the challenge to use combination therapies in treating uHCC is to find out how to obtain the optional treatment dosages to improve treatment effectiveness with minimal advise effects.
After Lenvatinib-based combination therapies, 15 patients were tumor-downstaged to undergo salvage liver resection with curative intent, making this study to be the first report with the largest sample size of patients with uHCC who underwent down-staging surgery after Lenvatinib-based treatments. While there was no discernible improvement in survival time for those who had surgery compared to those who did not, this provides a novel therapeutic avenue for uHCC patients who were having incurable disease to become potentically curable. The underlying reason why liver resection still needs to be carried out after tumor downstaging is that in a significant proportion of patients, small clusters of cancer cells were left behind which could later lead to HCC recurrence. Excision of the remaining lesions is expected to extend the durations of tumor-free and overall survivals. Qiao et al. performed liver transplantation after treatment with a PD-1 inhibitor and Lenvatinib in 7 patients with HCC. The results suggested promising efficacy with tolerable mortality in the surgically treated patients with uHCC (30). Currently, there are no guidelines or consensus on how and when to carry out salvage surgery for patients after tumor down-staging in patients after treatment with the combination of Lenvatinib and pembrolizumab.
This study has several limitations. First, it is a retrospective study with a small sample size and with a short follow-up. Second, there is a potential risk of selection bias. Third, this study covered a wide range of BCLC stages of uHCC, to include stages A, B, and C. The effectiveness of Lenvatinib is known to vary among the different BCLC stages of HCC. Exclusion of the impact of tumor staging is required in future studies. Fourth, the uHCC patients included in this study were treated with Lenvatinib-based therapy in combination with different other treatments including TACE, PD-1, radiotherapy and so on. These different combinations can result in biases on treatment outcomes, and our conclusions should be interpreted with caution. Fifth, the lack of feedback from biological data in our study warrants further investigation. Finally, only 15 patients in this study had tumor-downstaging after therapy who could fulfill the criteria for salvage surgical resection. The sample size was small, although salvage surgery had a certain impact on prognosis of such patients. A large sample, multicenter, randomized controlled study is needed for further verification.
Among all the uHCC patients in this study, 58.2% had an objective response rate. For uHCC patients who had an initially poor prognoses, combining anti-angiogenic therapy, immunotherapy, and local treatments have opened up new treatment options. Tumor down-staging followed by salvage liver resection can offer a potential for clinical cure for patients with uHCC who initially is assessed to have incurable diseases.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Eastern Hepatobiliary Surgery Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
(I) Conception and design: All authors. (II) Administrative support: Z-YP, WL,W-PZ and B-GJ. (III) Provision of study materials or patients: YY, S-XY, F-MG and HL. (IV) Collection and assembly of data: JH, Z-GW and Q-FT. (V) Data analysis and interpretation: All authors. (VI) Manuscript writing: All authors. (VII) Final approval of manuscript: All authors. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (81672721), the Program of Meng Chao Talent Training (Z-YP, B-GJ) and the Open Project Program of Key Laboratory of Minimally Invasive Techniques & Rapid Rehabilitation of Digestive System Tumor of Zhejiang Province (21SZDSYS05).
We would like to thank all the staff members for their contributions to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs (2019) 79(6):665–74. doi: 10.1007/s40265-019-01116-x
2. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet (2018) 391(10126):1163–73. doi: 10.1016/s0140-6736(18)30207-1
3. Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci (2018) 109(12):3993–4002. doi: 10.1111/cas.13806
4. Zhu AX, Finn RS, Ikeda M, Sung MW, Baron AD, Kudo M, et al. A phase ib study of lenvatinib (Len) plus pembrolizumab (Pembro) in unresectable hepatocellular carcinoma (Uhcc). J Clin Oncol (2020) 38(15_suppl):4519. doi: 10.1200/JCO.2020.38.15_suppl.4519
5. Kato Y, Bao X, Macgrath S, Tabata K, Hori Y, Tachino S, et al. Lenvatinib mesilate (Len) enhanced antitumor activity of a pd-1 blockade agent by potentiating Th1 immune response. Ann Oncol (2016) 27:vi1. doi: 10.1093/annonc/mdw362.02
6. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology (2018) 67(1):358–80. doi: 10.1002/hep.29086
7. Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter arterial chemoembolization (Tace) in hepatocellular carcinoma (Hcc): the role of angiogenesis and invasiveness. Am J Gastroenterol (2008) 103(4):914–21. doi: 10.1111/j.1572-0241.2007.01712.x
8. Kohles N, Nagel D, Jungst D, Stieber P, Holdenrieder S. Predictive value of immunogenic cell death biomarkers Hmgb1, srage, and dnase in liver cancer patients receiving transarterial chemoembolization therapy. Tumour Biol (2012) 33(6):2401–9. doi: 10.1007/s13277-012-0504-2
9. Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, et al. Unmasking of alpha-Fetoprotein-Specific Cd4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol (2007) 178(3):1914–22. doi: 10.4049/jimmunol.178.3.1914
10. Inchingolo R, Posa A, Mariappan M, Spiliopoulos S. Locoregional treatments for hepatocellular carcinoma: current evidence and future directions. World J Gastroenterol (2019) 25(32):4614–28. doi: 10.3748/wjg.v25.i32.4614
11. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (2018) 391(10127):1301–14. doi: 10.1016/S0140-6736(18)30010-2
12. Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan society of hepatology (Jsh) 2010 updated version. Dig Dis (2011) 29(3):339–64. doi: 10.1159/000327577
13. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese Clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr (2020) 9(4):452–63. doi: 10.21037/hbsn-20-480
14. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the albi grade. J Clin Oncol (2015) 33(6):550–8. doi: 10.1200/JCO.2014.57.9151
15. Huang J, Liu FC, Li L, Zhou WP, Jiang BG, Pan ZY. Nomograms to predict the long-time prognosis in patients with alpha-fetoprotein negative hepatocellular carcinoma following radical resection. Cancer Med (2020) 9(8):2791–802. doi: 10.1002/cam4.2944
16. Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-Pd-1 antibody combination treatment activates Cd8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PloS One (2019) 14(2):e0212513. doi: 10.1371/journal.pone.0212513
17. Torrens L, Montironi C, Puigvehí M, Mesropian A, Leslie J, Haber PK, et al. Immunomodulatory effects of lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatology (2021) 74(5):2652–69. doi: 10.1002/hep.32023
18. Shek D, Read SA, Nagrial A, Carlino MS, Gao B, George J, et al. Immune-checkpoint inhibitors for advanced hepatocellular carcinoma: a synopsis of response rates. Oncologist (2021) 26(7):e1216–e25. doi: 10.1002/onco.13776
19. Huang X, Xu L, Ma T, Yin X, Huang Z, Ran Y, et al. Lenvatinib plus immune checkpoint inhibitors improve survival in advanced hepatocellular carcinoma: a retrospective study. Front Oncol (2021) 11:751159. doi: 10.3389/fonc.2021.751159
20. Tang QH, Li AJ, Yang GM, Lai EC, Zhou WP, Jiang ZH, et al. Surgical resection versus conformal radiotherapy combined with tace for resectable hepatocellular carcinoma with portal vein tumor thrombus: a comparative study. World J Surg (2013) 37(6):1362–70. doi: 10.1007/s00268-013-1969-x
21. Yang F, Yang J, Xiang W, Zhong BY, Li WC, Shen J, et al. Safety and efficacy of transarterial chemoembolization combined with immune checkpoint inhibitors and tyrosine kinase inhibitors for hepatocellular carcinoma. Front Oncol (2021) 11:657512. doi: 10.3389/fonc.2021.657512
22. Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, et al. Lenvatinib combined with anti-Pd-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma (2021) 8:1233–40. doi: 10.2147/JHC.S332420
23. Han G, Berhane S, Toyoda H, Bettinger D, Elshaarawy O, Chan AWH, et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: a response-based approach. Hepatology (2020) 72(1):198–212. doi: 10.1002/hep.31022
24. Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant (2009) 9(8):1920–8. doi: 10.1111/j.1600-6143.2009.02695.x
25. Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, Tominaga L, et al. Lenvatinib-transarterial chemoembolization sequential therapy as an effective treatment at progression during lenvatinib therapy for advanced hepatocellular carcinoma. Liver Cancer (2020) 9(6):756–70. doi: 10.1159/000510299
26. Shimose S, Kawaguchi T, Tanaka M, Iwamoto H, Miyazaki K, Moriyama E, et al. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: a multicenter cohort study using data mining analysis. Oncol Lett (2020) 20(3):2257–65. doi: 10.3892/ol.2020.11758
27. Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, et al. Lenvatinib plus tace with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring pd-L1 expression: a retrospective study. J Cancer Res Clin Oncol (2022) 148(8):2115-2125. doi: 10.1007/s00432-021-03767-4
28. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/jco.20.00808
29. Wang Y, Jiang M, Zhu J, Qu J, Qin K, Zhao D, et al. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. BioMed Pharmacother (2020) 132:110797. doi: 10.1016/j.biopha.2020.110797
Keywords: hepatocellular carcinoma, Lenvatinib, combination therapy, PD-1, tumor-downstaging
Citation: Huang J, Wang Z-G, Tao Q-F, Yang Y, Yuan S-X, Gu F-M, Liu H, Pan Z-Y, Jiang B-G, Lau WY and Zhou W-P (2023) Efficacy and safety of Lenvatinib-based combination therapies for patients with unresectable hepatocellular carcinoma: a single center retrospective study. Front. Immunol. 14:1198562. doi: 10.3389/fimmu.2023.1198562
Received: 01 April 2023; Accepted: 23 June 2023;
Published: 07 July 2023.
Edited by:
Tahseen H. Nasti, Emory University, United StatesReviewed by:
Mingyue Cai, The Second Affiliated Hospital of Guangzhou Medical University, ChinaCopyright © 2023 Huang, Wang, Tao, Yang, Yuan, Gu, Liu, Pan, Jiang, Lau and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Ping Zhou, ZWhwaHdwQDEyNi5jb20=; Wan Yee Lau, am9zZXBobGF1QGN1aGsuZWR1Lmhr; Bei-Ge Jiang, amlhbmdfYmVpZ2VAYWxpeXVuLmNvbQ==; Ze-Ya Pan, aGpsdWZmeUAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.