
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 19 June 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1196793
Introduction: Immune checkpoint inhibitor (ICI) combination therapy has changed the treatment landscape for metastatic renal cell carcinoma (mRCC). However, little evidence exists on the treatment-related severe adverse events (SAEs) and fatal adverse events (FAEs) of ICI combination therapy in mRCC.
Method: We searched PubMed, Embase, and Cochrane Library databases to evaluate randomized controlled trials (RCTs) of ICI combination therapy versus conventional tyrosine kinase inhibitor (TKI)-targeted therapy in mRCC. Data on SAEs and FAEs were analyzed using revman5.4 software.
Results: Eight RCTs (n=5380) were identified. The analysis showed no differences in SAEs (60.5% vs. 64.5%) and FAEs (1.2% vs. 0.8%) between the ICI and TKI groups (odds ratio [OR], 0.83; 95%CI 0.58−1.19, p=0.300 and OR, 1.54; 95%CI 0.89−2.69, p=0.120, respectively). ICI-combination therapy was associated with less risk of hematotoxicities, including anemia (OR, 0.24, 95%CI 0.15–0.38, p<0.001), neutropenia (OR, 0.07, 95%CI 0.03–0.14, p<0.001), and thrombocytopenia (OR, 0.05, 95%CI 0.02−0.12, p<0.001), but with increased risks of hepatotoxicities (ALT increase [OR, 3.39, 95%CI 2.39–4.81, p<0.001] and AST increase [OR, 2.71, 95%CI 1.81−4.07, p<0.001]), gastrointestinal toxicities (amylase level increase [OR, 2.32, 95%CI 1.33–4.05, p=0.003] and decreased appetite [OR, 1.77, 95%CI 1.08–2.92, p=0.020]), endocrine toxicity (adrenal insufficiency [OR, 11.27, 95%CI 1.55–81.87, p=0.020]) and nephrotoxicity of proteinuria (OR, 2.21, 95%CI 1.06−4.61, p=0.030).
Conclusions: Compared with TKI, ICI combination therapy has less hematotoxicity in mRCC but more specific hepatotoxicity, gastrointestinal toxicity, endocrine toxicity, and nephrotoxicity, with a similar severe toxicity profile.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023412669.
Kidney cancer is one of the most common and dangerous cancer killers worldwide, and its incidence increases annually (1). Clear Cell renal cell carcinoma (RCC) accounts for approximately 90% of all kidney cancer pathologies (2). For decades, highly effective treatments for metastatic RCC (mRCC) have been lacking, and patients’ prognoses are very poor (3, 4). Vascular endothelial growth factor (VEGF)-tyrosine kinase inhibitor (TKI)-targeted therapies, such as sunitinib and cabozantinib, have certain efficacy and are recommended by international guidelines as the first-line treatment for mRCC; however, their overall treatment effect is not ideal, with a 5-year overall survival rate of less than 10% (5, 6). Moreover, VEGF-TKI–targeted agents have very common and unique adverse drug reactions that add pain to patients, including hypertension, proteinuria, hemorrhagic disease, and hand and foot syndrome (7).
In recent years, researchers have found that tumor cells can inhibit the immune function of T cells by promoting the activation of immune checkpoint molecules, thus avoiding being killed by the human immune system (8). With the development of immunotherapies and the approved application of immune checkpoint inhibitors (ICIs), ICI-based therapies have become promising treatment strategies for mRCC (9). Because of the widespread use of ICIs, immune-related adverse events have attracted considerable clinical attention. ICI immunotherapy is reported to cause multiple organ toxicities, including skin toxicity, gastrointestinal toxicity, liver toxicity, cardiovascular toxicity, and hematological toxicity. Some serious adverse events (SAEs) have a low incidence, including immune-associated pneumonia, myocarditis, and hepatitis; however, once an SAE occurs, it can be fatal (10, 11).
Combination therapies with ICIs have been widely used to treat mRCC. However, whether this combination strategy increases drug-related toxicity, particularly severe and fatal adverse events (FAEs), remains unclear. To explore the safety profile of ICI combination therapy and provide guidance for clinical treatment with ICIs, we conducted this meta-analysis comparing the SAEs and FAEs of ICI combination therapy with those of standard-of-care TKI-targeted therapy in patients with mRCC.
We searched the online electronic databases PubMed, Embase, and the Cochrane Library from inception until January 2023. The search keywords were kidney cancer, renal cell carcinoma, renal cancer, immunotherapy, immune checkpoint inhibitors, PD-1, PD-L1, CTLA-4, nivolumab, pembrolizumab, camrelizumab, sintilimab, toripalimab, atezolizumab, avelumab, durvalumab, tremelimumab, and ipilimumab. Only published randomized controlled trials (RCTs) were included; the languages were restricted to English.
We included only the studies that met the following inclusion criteria (1): Participants: patients with metastatic, pathological, or histologically confirmed RCC (2); Interventions: Combination therapy with ICIs (e.g., ICI in combination with chemotherapy, targeted therapy, or endocrine therapy) (3); Comparison: TKI-targeted therapy (4); Outcome: at least one safety outcome for SAEs or FAEs was reported. The exclusion criteria included non-randomized controlled studies, retrospective studies, preclinical studies, animal studies, conference abstracts, reviews, case reports, letters, expert consensus literature, comments, articles with fewer than 10 patients, and incomplete data.
The literature was screened independently by two researchers (FYN and XL), and relevant data were extracted and recorded using a standardized data collection form. Disagreements were resolved by a third independent researcher (MDC), and the final data were reviewed. Data were extracted from each study, including the study name, author information, year of publication, trial design, stage, number of patients, sex, age, treatment regimen, drug dose, and a detailed record of treatment-related SAEs and FAEs information. The SAEs were defined as Grade 3 or greater adverse events. FAEs were defined as Grade 5 adverse events (treatment-related deaths).
Two independent researchers (HJF and LPH) assessed bias in the selected studies using the Cochrane systematic risk of bias instrument (12), including random sequence generation, allocation concealment, blinding, blindness of outcome assessment, completeness of outcome data, reporting of selective outcomes, and other deviations. Any disagreements during the evaluation were resolved through discussion and consultation with an additional researcher (LXJ).
The meta-analysis was performed using revman5.4 software. Differences were considered statistically significant at p<0.05. We used the odds ratio (OR) and 95% confidence interval (CI) for the risk assessment of SAEs and FAEs. Interstudy heterogeneity was determined using the Cochran Q and I2 statistical tests. Heterogeneity was assessed as high when I2 was >50% and low when I2 was <50%. A fixed-effects model was used when the statistical homogeneity was achieved (I2<50%). When statistical heterogeneity occurred between studies (I2>50%), a random-effects model was used for analysis.
After searching the databases, 2766 articles were retrieved. After screening and removing duplicates carefully, 8 RCTs comprising 5380 total patients with previously untreated mRCC were included in the analysis (Figure 1). Among the comparisons with TKI were 3 PD-1 plus VEGF inhibitors (pembrolizumab plus lenvatinib [13], nivolumab plus cabozantinib [14], and pembrolizumab plus axitinib [15]), 3 PD-L1 plus VEGF inhibitors (2 atezolizumab plus bevacizumab [16,18] and 1 avelumab plus axitinib [17]), and 2 PD-L1 plus CTLA-4 inhibitors (both nivolumab plus ipilimumab [19,20]). Among the 8 included trials, 6 were Phase 3 clinical studies and 2 were Phase 2. The selected studies were published between 2018 and 2022. The detailed characteristics of the included studies are shown in Table 1.
In the eight included studies, the incidence of SAEs was 60.5% in the ICI combination group (n=2735) and 64.9% in the TKI group (n=2645) (Table 2). As shown in Figure 2, the meta-analysis found no statistical difference in the risk of SAEs between the two treatment groups (OR, 0.83, 95%CI 0.58−1.19, p=0.300). Subgroup analysis showed that, compared with TKI alone, PD-1 plus CTLA-4 inhibitors had a lower risk of SAEs (OR, 0.49, 95%CI 0.39−0.61, p<0.001), while PD-1 plus VEGF inhibitors had a greater risk of SAEs (OR, 1.39, 95% CI 1.09−1.79, p=0.008). In the PD-L1 plus VEGF inhibitors subgroup, no difference in SAEs was found between the two treatment groups (OR, 0.68, 95%CI 0.45−1.03, p=0.070). The most common SAEs in the ICI combination group were hypertension (18.6%), increased lipase level (8.9%), increased ALT level (6.6%), diarrhea (6.1%), and increased amylase level (5.6%); the incidence of these corresponding SAEs was 17.2%, 6.6%, 2.0%, 4.8%, and 2.6% in the TKI group, respectively (Table 2; Figure 3). The meta-analysis showed that ICI-combination therapy had significantly less risk of hematotoxicities, including anemia (OR, 0.24, 95%CI 0.15–0.38, p<0.001), neutropenia (OR, 0.07, 95%CI 0.03–0.14, p<0.001), and thrombocytopenia (OR, 0.05, 95%CI 0.02−0.12, p<0.001) than TKI. However, for digestive toxicity and nephrotoxicity, ICI-combination therapy was associated with significantly greater risks of an ALT increase (OR, 3.39, 95%CI 2.39–4.81, p<0.001), AST increase (OR, 2.71, 95%CI 1.81−4.07, p<0.001), amylase level increase (OR, 2.32, 95%CI 1.33–4.05, p=0.003), decreased appetite (OR, 1.77, 95%CI 1.08–2.92, p=0.020), and proteinuria (OR, 2.21, 95% CI 1.06−4.61, p=0.030). In addition, greater risks of rash (OR, 5.01; 95%CI 2.11–11.9, p=0.003) and adrenal insufficiency (OR, 11.27; 95%CI 1.55–81.87, p=0.020) and less risk of mucosal inflammation (OR, 0.37; 95%CI 0.19–0.69, p=0.002) were observed with ICI combination therapy (Table 2).
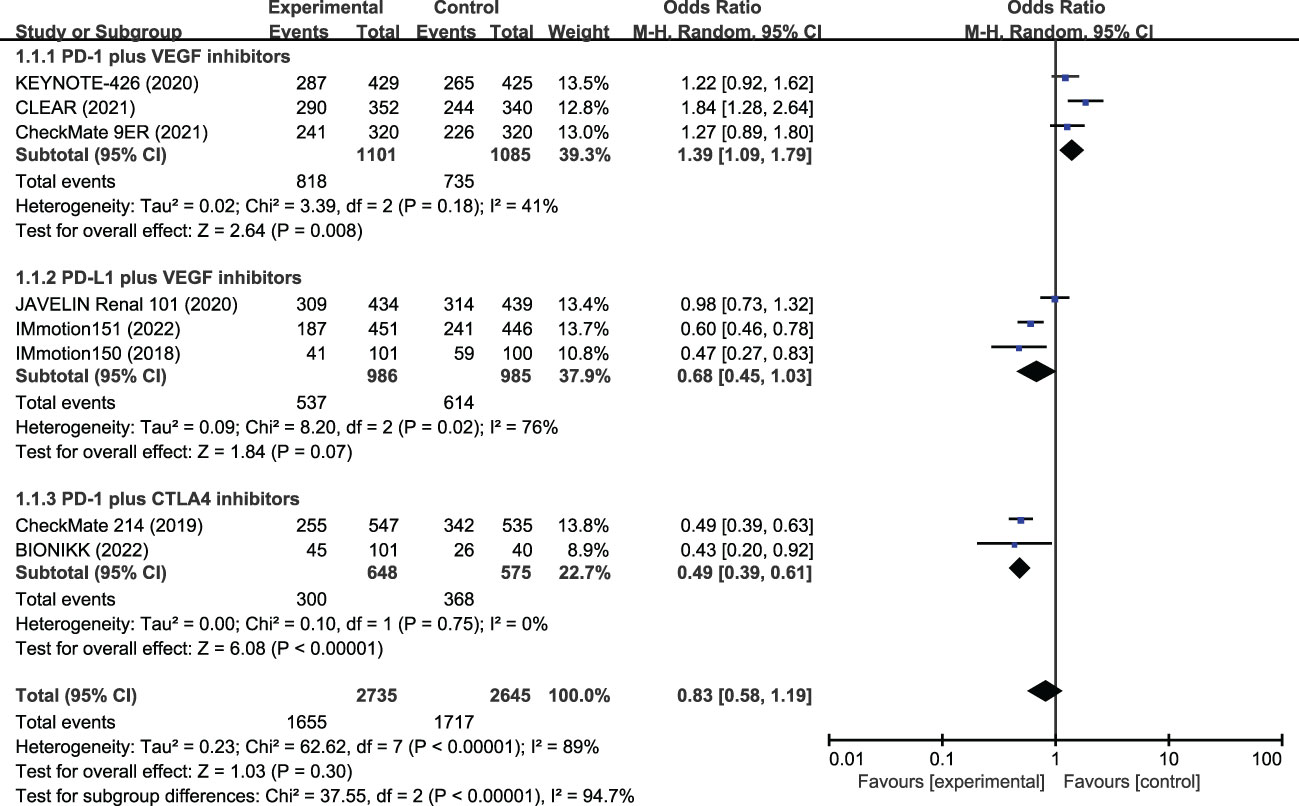
Figure 2 Meta-analysis of severe adverse events of immune checkpoint inhibitor combination therapy versus tyrosine kinase inhibitors in metastatic renal cell carcinoma. PD-1, programmed cell death protein-1; PD-L1, programmed cell death 1 ligand 1; VEGF, vascular endothelial growth factor; CTLA-4, cytotoxic T lymphocyte antigen 4.
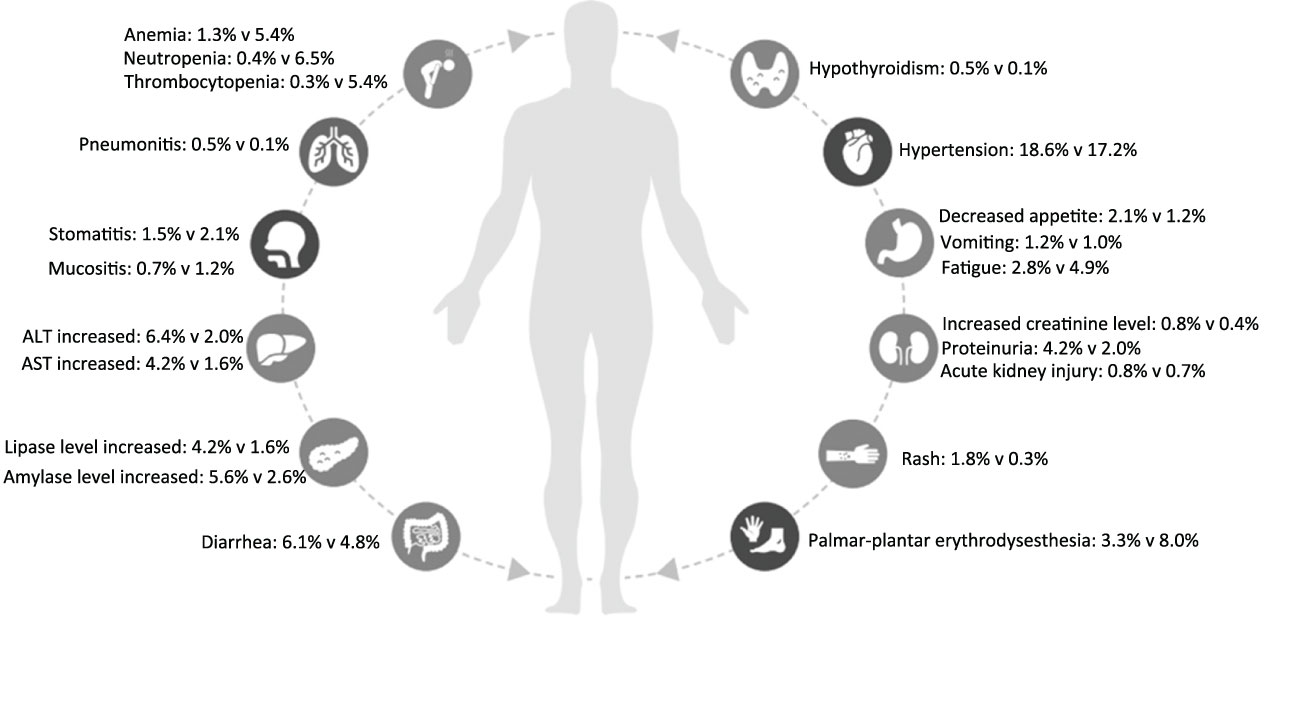
Figure 3 Severe adverse events of immune checkpoint inhibitor combination therapy versus tyrosine kinase inhibitors in each organ system for patients with metastatic renal cell carcinoma.
Data on FAEs were reported in all included studies. The most common FAEs in the ICI-combination group (n=2735) were pneumonitis (4 patients), hepatitis (3 patients), hemorrhagic events (3 patients), sepsis (3 patients), myocarditis (2 patients), myasthenic syndrome (2 patients), and sudden death (2 patients). In the TKI group (n=2645), they were heart failure (2 patients), pneumonia (2 patients), gastrointestinal hemorrhage (2 patients), and cardiac arrest (2 patients). The incidences of FAEs were 1.2% and 0.8% in the ICI and TKI combination groups, respectively (Table 3). The meta-analysis suggested the incidence of FAEs had no statistical difference (OR, 1.54, 95%CI 0.89−2.69, p=0.830) between the two treatment groups (Figure 4). Subgroup analysis showed that a similar risk of FAEs was found for PD-1 plus VEGR inhibitors (OR, 1.58, 95%CI 0.71−3.49, p=0.260), PD-L1 plus VGFR inhibitors (OR, 2.00, 95%CI 0.60−6.67, p=0.260), and PD-L1 with CTLA-4 inhibitors (OR, 1.23, 95%CI 0.45−3.40, p=0.690).
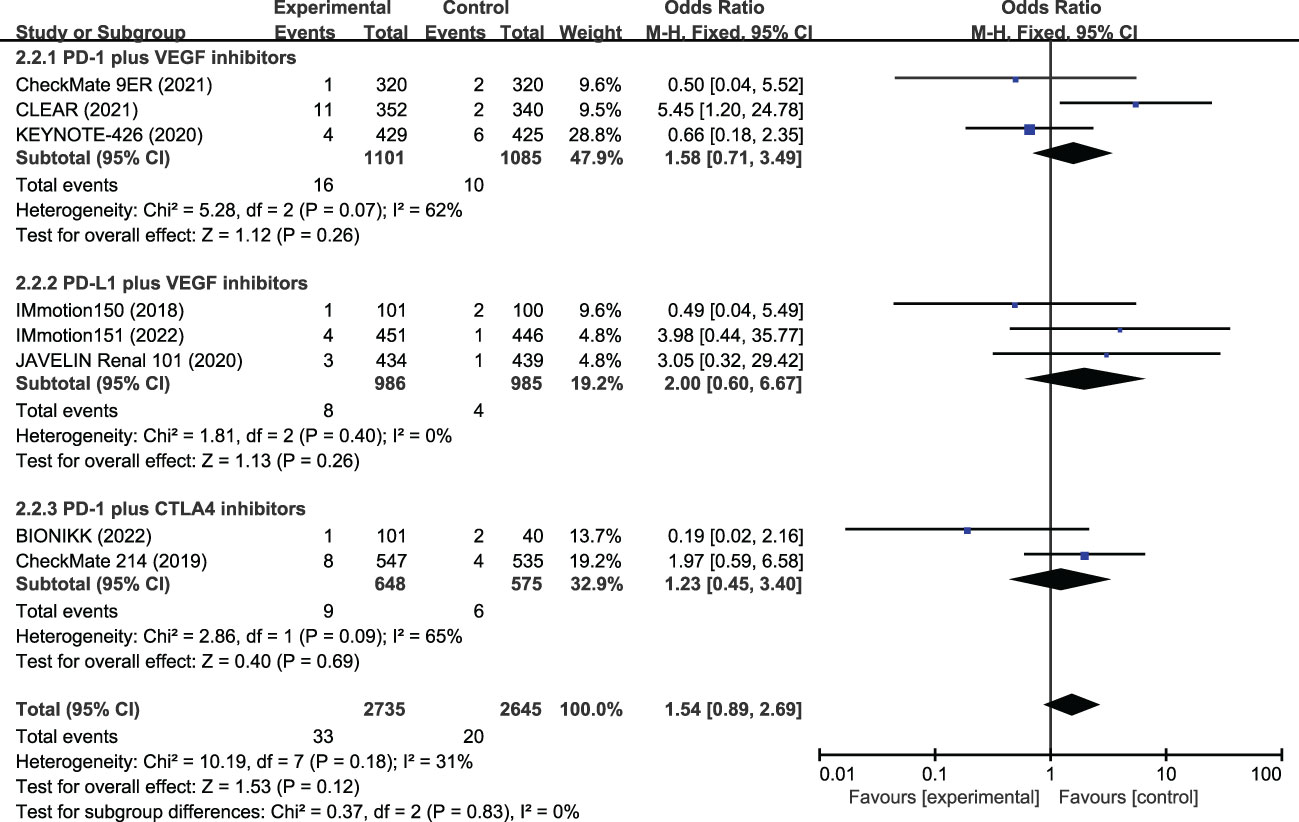
Figure 4 Meta-analysis of fatal adverse events of immune checkpoint inhibitor combination therapy versus tyrosine kinase inhibitor in metastatic renal cell carcinoma. PD-1, programmed cell death protein-1; PD-L1, programmed cell death 1 ligand 1; VEGF, vascular endothelial growth factor; CTLA-4, cytotoxic T lymphocyte antigen 4.
All eight included RCTs were assessed as high quality using the Cochrane Collaboration tool. The details are shown in Figure 5.
For over a decade, immunotherapy with ICIs has been important in cancer treatment. ICI combination therapy has demonstrated broad therapeutic prospects for several solid cancers, including RCC (21–25). With the widespread use of ICI-based immunotherapy for RCC, the safety of ICI combination therapy is receiving increasing attention from clinicians.
To the best of our knowledge, this study is the first to perform a comprehensive evaluation of the severe and fatal toxicities of ICI combination therapy compared with standard VEGF-TKI–targeted therapy in patients with mRCC. The analysis showed that ICI combination therapy did not cause more SAEs or FAEs than TKI. Furthermore, the incidence of FAEs with ICI combination therapy was very low (1.2%). The most common FAEs were pneumonitis, hepatitis, hemorrhagic events, and myocarditis, which deserve attention in clinical practice. Wang et al. (26) evaluated the fatal toxic effects associated with ICIs in cancer, measuring the toxicity-related fatality rates for anti–PD-1, anti–PD-L1, anti–CTLA-4, and anti–PD-1/PD-L1 plus anti–CTLA-4 treatments as 0.36%, 0.38%, 1.08%, and 1.23%, respectively. Their findings supported the low incidence of FAEs in cancer patients treated with dual immunotherapy (PD-1/PD-L1 plus CTLA-4). Except for hypertension (18.6%), the incidence of SAEs with ICI combination therapy was low (<10%). These results suggest that ICI combination therapy has a good safety profile for the treatment of mRCC.
Considering the possible effect of different ICI combinations on SAEs, we conducted a subgroup analysis according to different therapeutic regimens and found that PD-L1 plus VEGF inhibitors had similar SAEs, and PD-1 plus CTLA-4 inhibitors had fewer SAEs than TKI. However, PD-1 plus VEGF inhibitors were associated with more SAEs than TKI alone. Similar results have been reported. For example, in the IMbrave150 trial, Finn et al. (27) reported that patients receiving atenizumab (anti–PD-L1) and bevacizumab had a similar risk of Grade 3 or 4 adverse events (56.5% vs. 55.1%, respectively) as those receiving sorafenib as the first-line treatment for unresectable hepatocellular carcinoma (HCC). In addition, Ren et al. (28) reported that sintilimab (anti–PD-1) plus bevacizumab biosimilar showed more SAEs (32% vs. 19%) than sorafenib in patients with unresectable HCC. This finding suggests that, despite ICI combination therapy having a good overall safety profile, some differences in toxicities among different ICI combinations may need to be treated differently.
In addition to severe toxicity overall, we also focused on the SAEs in each organ system. We found that ICI combination therapy had significantly less hematotoxicity, including anemia, neutropenia, and thrombocytopenia, than TKI. This result may indicate that ICIs have a protective effect on the blood system, thus reducing the damage to bone marrow cells caused by TKI-targeted agents. Kramer et al. (29) reported that hematological immune-related adverse events could affect all hematopoietic blood cell lineages and may persist or even be fatal; therefore, clinicians should monitor blood counts closely, if necessary. Although hematotoxicities are rare, we found that patients receiving ICI combination therapy showed greater hepatotoxicity (ALT and AST increases) than those receiving TKI; some died of immune-related hepatitis. Wang et al. (30) reported that using ICIs was associated with greater hepatotoxicity than all other systematic treatments and that ICI combination therapy had a greater risk of hepatotoxicity (including hepatic failure) than ICI monotherapy. Because of the significant association between ICI-based therapy and hepatotoxicity, clinicians should focus more attention on this safety signal. For nephrotoxicity, ICI combination therapy did not increase the risk of creatinine and renal failure but significantly increased the risk of proteinuria. Wu et al. (31) reported that adding ICI to VEGF inhibitors might cause hypertension and proteinuria. Ning et al. (32) reported that, after long-term targeted therapy, the use of combination therapy further aggravated proteinuria in patients with mRCC. In addition, ICI combination therapy showed a higher frequency of decreased appetite, increased lipase levels, and adrenal insufficiency than TKI; therefore, gastrointestinal toxicity and endocrine toxicity cannot be easily ignored when selecting ICI combination therapy for patients with mRCC. Therefore, although the overall safety was good, the toxicity of ICI combination therapy was found to have high organ specificity, which deserves attention in clinical practice. Another noteworthy aspect is that in this analysis, most included RCTs used sunitinib as a control drug. However, in current clinical practice, cabozantinib has become a preferred choice of monotherapy for patients with mRCC. So, whether our results are applicable to other TKI drugs, more detailed research is required.
This study had some limitations. First, only a few combined modes of ICI therapy are currently used to treat mRCC, including PD-1/PD-L1 plus VGFR inhibitors and PD-1 plus CTLA-4 inhibitors. No combination of ICI with chemotherapy was studied; therefore, toxicity results must be analyzed and generalized to other tumors carefully. Second, we found the results of each ICI-combination subgroup were different when we analyzed the SAEs of the patients. We point this out because different types of ICI, including inhibitors of PD-1/PD-L1 and CTLA-4, have different antitumor mechanisms, so each combination needs to be treated differently. Third, because clear cell RCC was the main inclusion criteria in all presented clinical trials, our results may not be extrapolated to another histology type of RCC. Finally, because of limited data, we could not conduct a subgroup analysis according to the different ICI combinations for each SAE, which may have affected the results of these toxicities.
The current meta-analysis demonstrates that, compared with TKI, ICI combination therapy has a safe profile in general and does not increase SAEs and FAEs in patients with mRCC. Moreover, ICI combination therapy showed significantly less hematotoxicity than TKI. However, higher frequencies of SAEs were also observed, including hepatotoxicity, gastrointestinal toxicity, endocrine toxicity, and nephrotoxicity, warranting closer attention in clinical and future investigations.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Y-NF, G-YX, and LX designed the study. D-CM and J-FH screened the articles and extracted the relevant data. P-HL, G-YX, and X-JL contributed to the statistical analysis. Y-NF and LX wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SC declared a shared affiliation with the authors to the handling editor at time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
RCC, renal cell carcinoma; ICI, immune checkpoint inhibitor; TKI, tyrosine kinase inhibitor; OR, odds ratio; PD-1, programmed cell death protein 1; PD-L1, programmed cell death 1 ligand 1; CTLA-4, cytotoxic T lymphocyte antigen 4; RCT, randomized controlled trial.
1. Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam JA, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol (2022) 82(5):529–42. doi: 10.1016/j.eururo.2022.08.019
2. Capitanio U, Montorsi F. Renal cancer. Lancet (2016) 387(10021):894–906. doi: 10.1016/S0140-6736(15)00046-X
3. Vento JA, Rini BI. Treatment of refractory metastatic renal cell carcinoma. Cancers (Basel) (2022) 14(20):5005. doi: 10.3390/cancers14205005
4. Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Electronic address:Y2xpbmljYWxndWlkZWxpbmVzQGVzbW8ub3Jn. renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol (2019) 30(5):706–20. doi: 10.1093/annonc/mdz056
5. Rathmell WK, Rumble RB, Van Veldhuizen PJ, Al-Ahmadie H, Emamekhoo H, Hauke RJ, et al. Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol (2022) 40(25):2957–95. doi: 10.1200/JCO.22.00868
6. Cooley LS, Rudewicz J, Souleyreau W, Emanuelli A, Alvarez-Arenas A, Clarke K, et al. Experimental and computational modeling for signature and biomarker discovery of renal cell carcinoma progression. Mol Cancer (2021) 20(1):136. doi: 10.1186/s12943-021-01416-5
7. Hofmann F, Hwang EC, Lam TB, Bex A, Yuan Y, Marconi LS, et al. Targeted therapy for metastatic renal cell carcinoma. Cochrane Database Syst Rev (2020) 10(10):CD012796. doi: 10.1002/14651858.CD012796.pub2
8. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev (2010) 236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x
9. Díaz-Montero CM, Rini BI, Finke JH. The immunology of renal cell carcinoma. Nat Rev Nephrol (2020) 16(12):721–35. doi: 10.1038/s41581-020-0316-3
10. Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev (2016) 44:51–60. doi: 10.1016/j.ctrv.2016.02.001
11. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol (2017) 28(10):2377–85. doi: 10.1093/annonc/mdx286
12. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane statistical methods group. the cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
13. Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med (2021) 384(14):1289–300. doi: 10.1056/NEJMoa2035716
14. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med (2021) 384(9):829–41. doi: 10.1056/NEJMoa2026982
15. Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol (2020) 21(12):1563–73. doi: 10.1016/S1470-2045(20)30436-8
16. Motzer RJ, Powles T, Atkins MB, Escudier B, McDermott DF, Alekseev BY, et al. Final overall survival and molecular analysis in IMmotion151, a phase 3 trial comparing atezolizumab plus bevacizumab vs sunitinib in patients with previously untreated metastatic renal cell carcinoma. JAMA Oncol (2022) 8(2):275–80. doi: 10.1001/jamaoncol.2021.5981
17. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med (2019) 380(12):1103–15. doi: 10.1056/NEJMoa1816047
18. McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med (2018) 24(6):749–57. doi: 10.1038/s41591-018-0053-3
19. Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol (2019) 20(10):1370–85. doi: 10.1016/S1470-2045(19)30413-9
20. Vano YA, Elaidi R, Bennamoun M, Chevreau C, Borchiellini D, Pannier D, et al. Nivolumab, nivolumab-ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): a biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol (2022) 23(5):612–24. doi: 10.1016/S1470-2045(22)00128-0
21. Ferrara R, Imbimbo M, Malouf R, Paget-Bailly S, Calais F, Marchal C, et al. Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer. Cochrane Database Syst Rev (2020) 12(12):CD013257. doi: 10.1002/14651858.CD013257
22. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2022) 23(8):995–1008. doi: 10.1016/S1470-2045(22)00326-6
23. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
24. Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2020) 21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8
25. Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature (2022) 603(7903):942–8. doi: 10.1038/s41586-022-04508-4
26. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol (2018) 4(12):1721–8. doi: 10.1001/jamaoncol.2018.3923
27. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
28. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol (2021) 22(7):977–90. doi: 10.1016/S1470-2045(21)00252-7
29. Kramer R, Zaremba A, Moreira A, Ugurel S, Johnson DB, Hassel JC, et al. Hematological immune related adverse events after treatment with immune checkpoint inhibitors. Eur J Cancer (2021) 147:170–81. doi: 10.1016/j.ejca.2021.01.013
30. Wang H, Yang H, Zhou X, Zhang X. Hepatotoxicity associated with immune checkpoint inhibitors in clinical practice: a study leveraging data from the US food and drug administration's adverse event reporting system. Clin Ther (2023) 45(2):151–9. doi: 10.1016/j.clinthera.2023.01.001
31. Wu Z, Chen Q, Qu L, Li M, Wang L, Mir MC, et al. Adverse events of immune checkpoint inhibitors therapy for urologic cancer patients in clinical trials: a collaborative systematic review and meta-analysis. Eur Urol (2022) 81(4):414–25. doi: 10.1016/j.eururo.2022.01.028
Keywords: renal cell carcinoma, tyrosine kinase inhibitor, immune checkpoint inhibitor, serious adverse events, fatal adverse events
Citation: Feng Y-N, Xie G-Y, Xiao L, Mo D-C, Huang J-F, Luo P-H and Liang X-J (2023) Severe and fatal adverse events of immune checkpoint inhibitor combination therapy in patients with metastatic renal cell carcinoma: a systematic review and meta-analysis. Front. Immunol. 14:1196793. doi: 10.3389/fimmu.2023.1196793
Received: 30 March 2023; Accepted: 05 June 2023;
Published: 19 June 2023.
Edited by:
Anand Rotte, Arcellx Inc., United StatesReviewed by:
Shaohua Chen, Guangxi Medical University Cancer Hospital, ChinaCopyright © 2023 Feng, Xie, Xiao, Mo, Huang, Luo and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao-Ning Feng, MzE3ZG9kb0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.