- 1Department of Pediatrics, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Republic of Korea
- 2Department of Pediatrics, School of Medicine, Inha University, Incheon, Republic of Korea
- 3Department of Pediatrics, School of Medicine, Kyungpook National University, Daegu, Republic of Korea
- 4Department of Radiology, School of Medicine, Kyungpook National University, Daegu, Republic of Korea
Introduction: It is well known that infliximab (IFX) trough levels (TLs) are associated with endoscopic healing (EH) in Crohn’s disease (CD). We investigated whether IFX TLs are associated with transmural healing (TH) in pediatric patients with CD following 1-year treatment.
Methods: Pediatric patients with CD treated with IFX were included in this single-center prospective study. IFX TL tests, magnetic resonance enterography (MRE), and colonoscopies were simultaneously conducted after 1-year IFX treatment. TH was defined as a wall thickness of ≤3 mm without inflammatory signs evaluated using MRE. EH was defined as a Simple Endoscopic Score for Crohn’s disease of <3 points on colonoscopy.
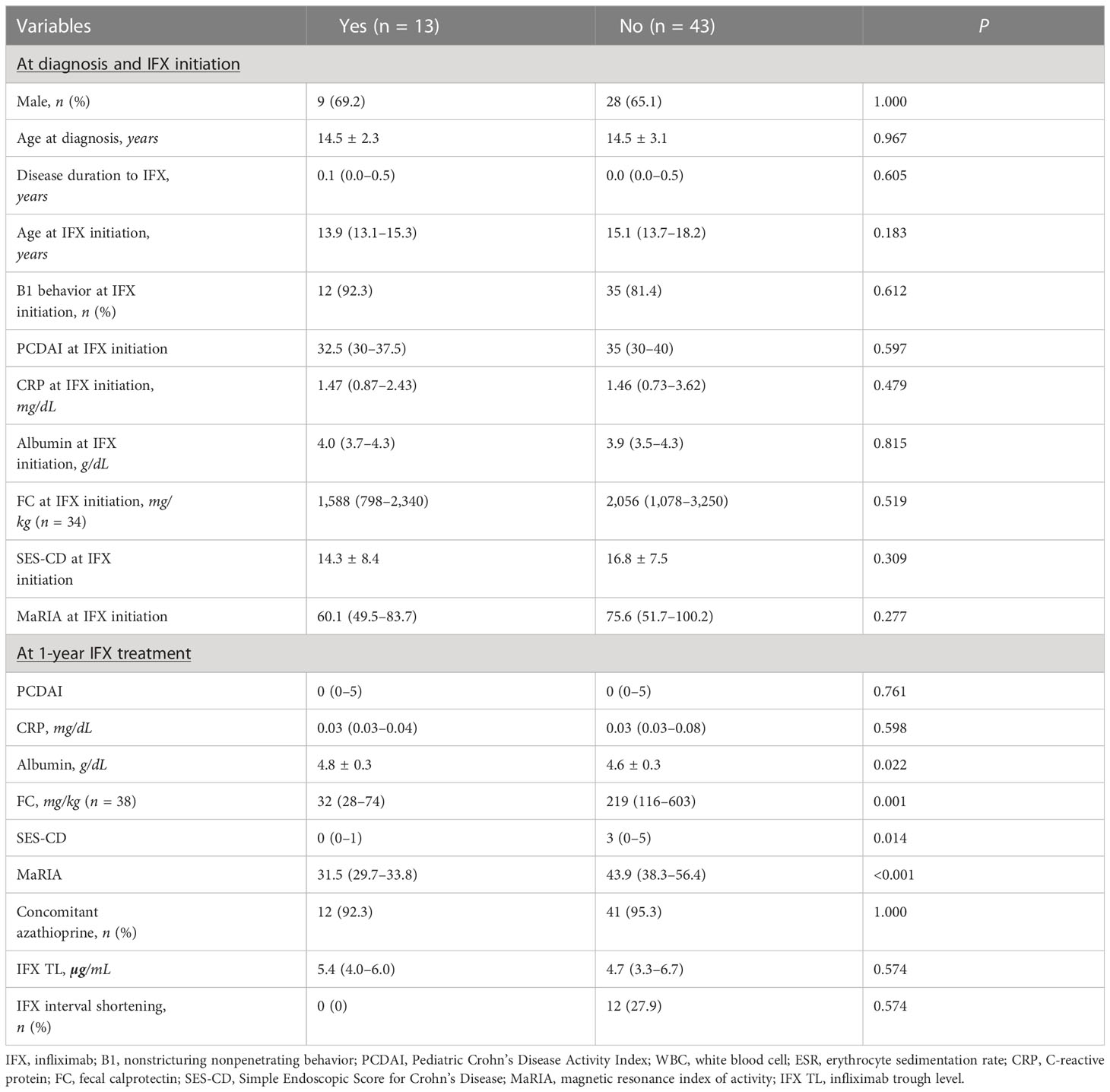
Results: Fifty-six patients were included. EH and TH were observed in 60.7% (34/56) and 23.2% (13/56) of patients, respectively. IFX TLs were higher in patients with EH (median, 5.6 vs. 3.4 µg/mL, P = 0.002), whereas IFX TLs showed no significant difference in patients with and without TH (median, 5.4 vs. 4.7 µg/mL, P = 0.574). No significant difference was observed in EH and TH between patients whose intervals were shortened or not. Multivariate logistic regression analysis showed that IFX TLs and disease duration to IFX initiation were associated with EH (odds ratio [OR] = 1.82, P = 0.001, and OR = 0.43, P = 0.02, respectively).
Discussion: In pediatric patients with CD, IFX TLs were associated with EH but not with TH. Further studies investigating long-term TH and proactive dosing based on therapeutic drug monitoring may clarify whether an association between IFX TLs and TH exists.
1 Introduction
Crohn’s disease (CD) is an inflammatory bowel disease that is characterized by the development of chronic lesions throughout the gastrointestinal tract due to a systemic inflammatory reaction. If inappropriately treated, strictures and fistulas can cause irreversible intestinal damage and may even require surgery, such as bowel resection and stoma formation (1, 2). However, the advent of anti-tumor necrosis factor (anti-TNF) agents, including infliximab (IFX) and adalimumab, as treatments of CD has allowed the rapid achievement and maintenance of remission in patients of all ages, including pediatric patients (3–6). This treatment has thus improved the quality of life of patients with CD. Moreover, current treatment strategies have evolved to include the administration of biologics, including anti-TNF alpha, in the early inflammatory phase of the disease before complications develop (7–9).
Beyond simple clinical remission, the treatment of CD aims to achieve deep remission. Deep remission is an evolving concept that lacks a widely mutual definition; however, it is generally considered a state of clinical remission (symptom control) with evidence of endoscopic healing (EH) (10). More recently, the concept of transmural healing (TH) is emerging. TH is evaluated by measuring bowel wall thickness and signal intensity using a noninvasive measurement tool, ultrasonography, or magnetic resonance enterography (MRE). Recently, some studies have compared EH and TH, with all suggesting that TH could be a potential treatment endpoint in CD (11–14).
IFX is an anti-TNF alpha drug used for CD treatment, for which the therapeutic trough level (TL) range (≥3 µg/mL) has been discussed in several studies (15–17). Recently, attempts to proactively evaluate TLs are undertaken to guide clinical decision-making regarding dose intensification (18, 19). Moreover, clinicians have demonstrated a relationship between IFX TL and EH, suggesting that EH occurs at concentrations of 5 µg/mL or higher in pediatric patients with CD (20). A previous study in adult CD recommended maintaining an IFX concentration of 6–10 µg/mL to reach EH (21). In the aforementioned studies, the association between EH and IFX TL has been demonstrated; however, data regarding the association between IFX TL and TH are limited. Therefore, we aimed to investigate whether IFX TLs were associated with TH in pediatric patients with CD at 1-year IFX treatment.
2 Methods
2.1 Patients and study design
This was a single-center prospective study conducted at Kyungpook National University Children’s Hospital. Participants were pediatric patients under the age of 19 and diagnosed with moderate-to-severe luminal CD requiring IFX treatment between January 2020 and December 2021. All patients were followed up for more than 1 year. CD was diagnosed following the revised Porto criteria of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (22). Patients were classified with moderate-to-severe CD based on a Pediatric Crohn’s Disease Activity Index (PCDAI) score of 30 points or more (23).
Indications for IFX treatment included patients with relapse during maintenance treatment with immunomodulators, or top-down treatment with IFX owing to the severity of CD at the time of diagnosis. Patients who lost their response to IFX underwent interval shortening of IFX doses to 4–6 weeks. Secondary loss of response (LOR) was defined as symptomatic inflammatory relapse (PCDAI ≥10 points with elevated C-reactive protein [CRP] or calprotectin levels) and/or endoscopic or radiologically confirmed relapse following successful primary response (24). All patients underwent colonoscopy, which was inserted up to the terminal ileum, and MRE at 1 year following treatment with IFX. Patients who were 19 years or older at the initiation of treatment with IFX, used IFX for perianal CD treatment, were not available for extra blood sampling to measure IFX TLs, and failed MRE at 1 year were excluded from this study. Routine tests of IFX TLs were checked only during the 1-year study period; however, tests were also performed in those when LOR was suspected.
The primary endpoints in this study were endoscopic and transmural remission rates following IFX treatment. The secondary endpoint was evaluation of the association of IFX TLs with EH or TH. The tertiary endpoint was evaluation of factors that affect EH and TH. This study was approved by the Institutional Review Board of Kyungpook National University Chilgok Hospital and conducted in accordance with the Declaration of Helsinki.
2.2 Data collection
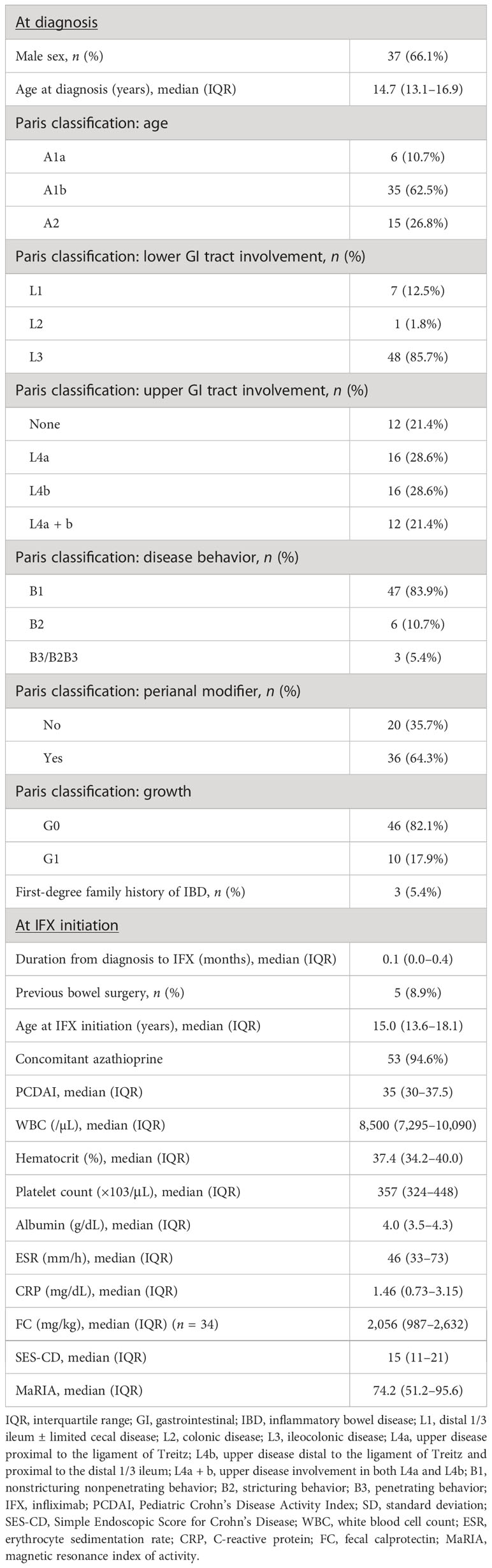
Baseline demographic data, including age at diagnosis, sex, body mass index, growth status, family history of inflammatory bowel disease, previous history of surgery associated with CD, and use of concomitant medications, were collected. Disease phenotype at diagnosis was assessed according to the Paris classification (25). Laboratory evaluation, including inflammatory markers such as erythrocyte sedimentation rate (ESR) and CRP levels, were also collected at the time of diagnosis and at 1 year following treatment. Serum IFX TLs were measured at 1 year in each sample using a commercial enzyme-linked immunosorbent assay kit (Matriks Biotechnology Co., Ltd., Ankara, Turkey).
Colonoscopy and MRE were conducted before IFX intiation and at 1 year following treatment. The severity of mucosal involvement was assessed using the Simple Endoscopic Score for Crohn’s disease (SES-CD). All MRE images were separately interpreted by two board-certified gastrointestinal radiologists. The radiologists were blinded to the clinical information and endoscopic scores of the enrolled patients. To evaluate the degree of TH, the magnetic resonance index of activity (MaRIA) score was assessed (14, 25–28).
2.3 Definitions
Clinical remission was defined as a PCDAI score of <10 points. Biochemical remission was defined as a CRP level of <0.3 mg/dL. SES-CD was calculated as the sum of endoscopic parameters (ulcer size, ulcerated surface, affected surface, and luminal narrowing) of the terminal ileum and each segment of the colon. EH was defined as an SES-CD of <3 points. TH was defined as a wall thickness of ≤3 mm, with the absence of ulcers, edema, enhancement, and complications on all ileocolonic segments evaluated using MRE. Complex perianal fistulas were defined as those involving the upper part of the sphincter complex (e.g., high intersphincteric, high transsphincteric, suprasphincteric, or extrasphincteric origin of the fistula tract); have multiple external openings (tracts); are associated with pain or fluctuation suggesting a perianal abscess; and/or are associated with a rectovaginal fistula or anorectal stricture (29).
2.4 Statistical analysis
Comparative data for continuous variables were described as median values with interquartile ranges or mean values with standard deviations. Student’s t-test, Wilcoxon’s rank-sum test, or Bonferroni t-test were used to compare continuous variables in the groups. Chi-squared test or Fisher’s exact test was used to compare categorical variables in the groups. To examine the association between EH or TH and other variables, univariate and multivariate logistic regression analyses were performed. To investigate the crude odds ratio (OR), univariate logistic regression analysis was performed. Factors with a p-value of <0.1 in the univariate analysis were included in the multivariate analysis. Results were expressed as adjusted ORs with 95% confidence intervals (CIs). A p-value of ≤0.05 was considered statistically significant. All statistical analyses were performed using R version 3.2.3.
3 Results
3.1 Baseline characteristics at diagnosis and IFX initiation
A total of 56 patients were included in this study. Investigation of the baseline characteristics at diagnosis showed a male predominance and a median diagnosis age of 14.7 years, with most patients showing L3 involvement, and approximately 80% with L4 involvement. Overall, 47 (84%) and 36 (64%) of patients were classified as having B1 behavior and perianal disease modifiers, respectively. Growth failure was observed in approximately one-fifth of patients (Table 1). Baseline characteristics at IFX initiation showed a median disease duration to IFX of 0.1 years, with 53 (95%) receiving concomitant azathioprine. Five patients (8.9%) had a history of prior bowel surgery before starting IFX. Three patients received ileocecectomy, and two received small bowel resection and anastomosis. The median PCDAI score was 35 points, median ESR was 46 mm/h, median CRP level was 1.5 mg/dL, median SES-CD was 15 points, and median MaRIA score was 74.2 points (Table 1).
3.2 Remission rates at 1-year treatment with IFX
Remission rates at 1-year treatment with IFX showed that 92.9% (52/56) of patients were in clinical remission, and 87.5% (49/56) had a CRP level of <0.3 mg/dL. Furthermore, EH and TH were observed in 60.7% (34/56) and 23.2% (13/56) of patients, respectively (Figure 1).
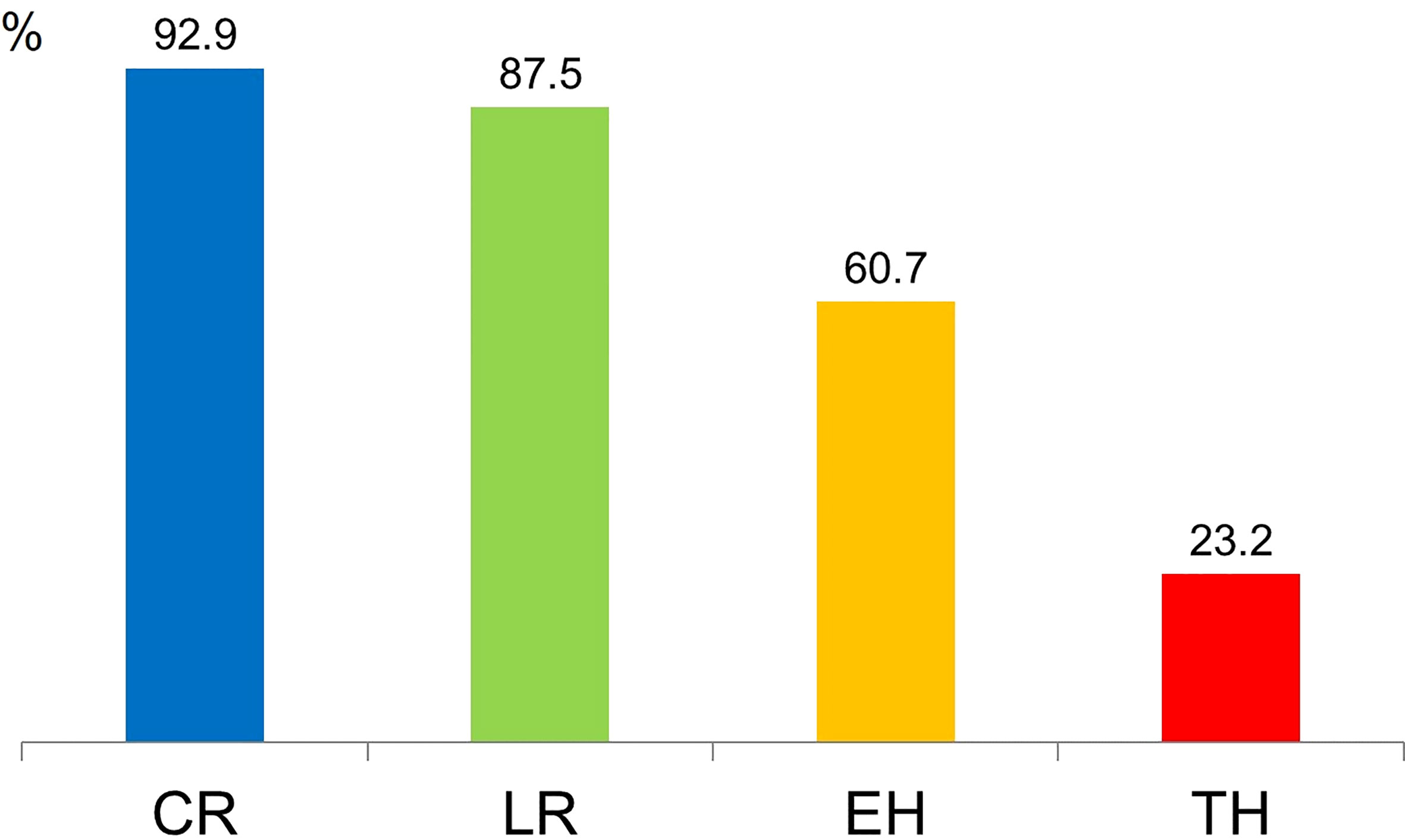
Figure 1 Remission rates following 1-year treatment with infliximab. CR, clinical remission; LR, laboratory remission; EH, endoscopic healing; TH, transmural healing.
3.3 Comparison between patients with and without TH
Comparison between patients with and without TH at 1-year IFX treatment showed no significant differences at baseline (Table 2). However, at 1-year IFX treatment, the albumin level was significantly higher in patients with TH (4.8 ± 0.3 vs. 4.6 ± 0.3 g/dL, P = 0.022), whereas SES-CD (median, 0 vs. 3 points, P = 0.014) and MaRIA (median, 31.5 [29.7–33.8] vs. 43.9 [38.3–56.4] points, P < 0.001) scores were significantly lower. Furthermore, all 13 patients who achieved TH had EH. Since the definition of EH was an SES-CD of 0–2 points, the SES-CD of 13 patients who achieved TH was further analyzed. Of the 13 patients, 10, 2, and 1 scored 0, 1, and 2 points, respectively, showing that 76.9% of the patients who reached TH had an SES-CD of 0 points.
3.4 IFX TLs according to remission status and dosing intervals
The comparison of IFX TLs according to remission status showed that patients with EH had significantly higher IFX TLs than those without EH (median, 5.6 vs. 3.4 μg/mL, P = 0.002), whereas no statistically significant difference was observed between those with and without TH (median, 5.4 vs. 4.7 μg/mL, P = 0.574) (Figure 2). The quartiles of IFX TL and proportion of patients with EH and TH are described in Figure 3. When IFX TLs were compared between the EH + TH, EH + no TH, and no EH + no TH groups, the median values of IFX TLs between the EH + TH and EH + no TH groups were 5.7 and 5.4 μg/mL, respectively, with no significant difference. However, in the case of the no EH + no TH group, IFX TLs were statistically significantly lower with median TLs of 3.4 μg/mL (P = 0.002) than the other two groups (Figure 4A). As all patients who had reached TH also had achieved EH, there were no patients who had only TH without EH (TH + no EH). The dosing intervals of IFX were shortened in 12 patients during maintenance. The comparison of IFX TLs according to interval shortening showed significantly higher levels in patients whose intervals were shortened (median, 7.8 vs. 4.7 μg/mL, P = 0.029) (Figure 4B). Antibodies to IFX were detected in 2 of 12 patients with shortened dosing intervals.
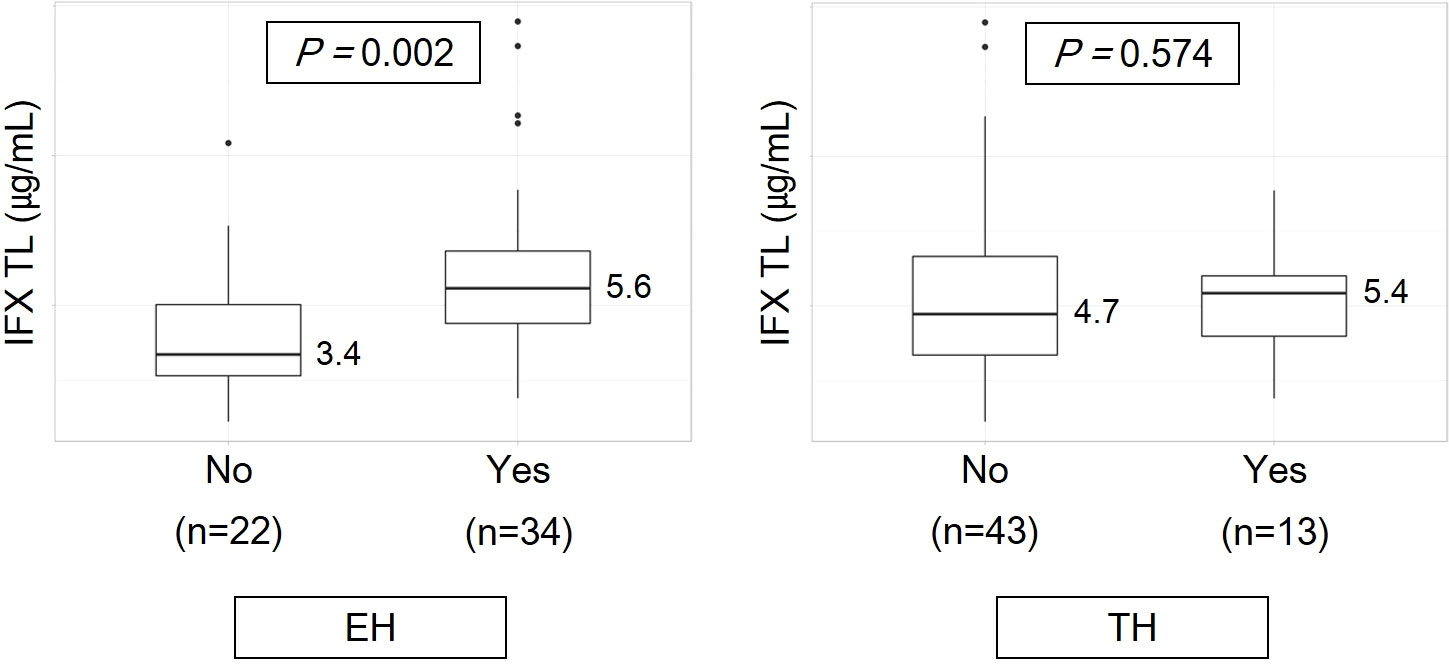
Figure 2 Infliximab trough levels according to remission status. EH, endoscopic healing; IFX TL, infliximab trough level; TH, transmural healing.
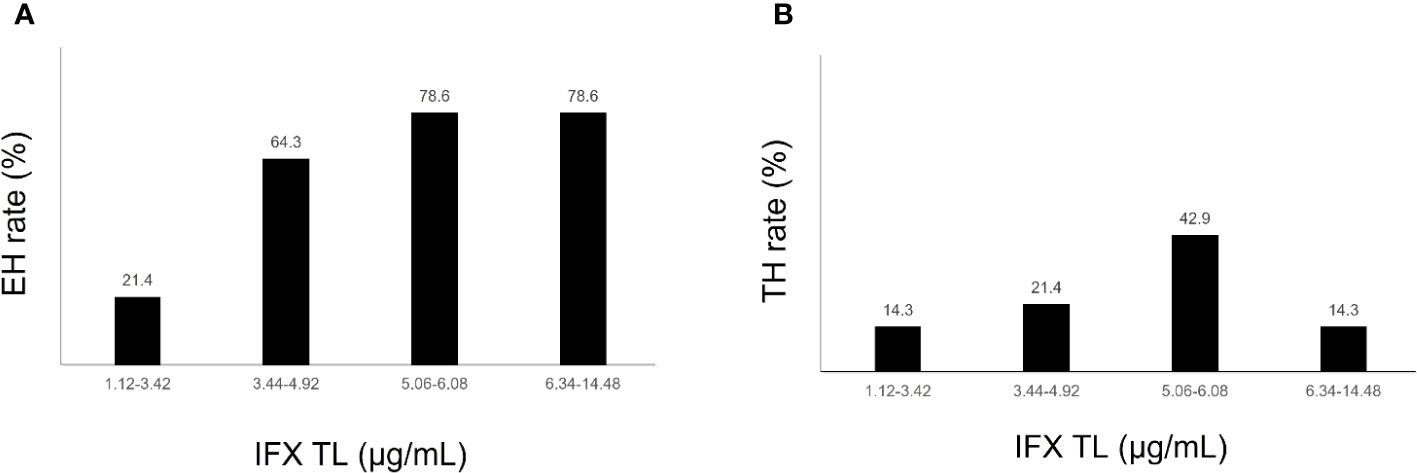
Figure 3 (A) Endoscopic healing rates according to quartiles of infliximab trough levels. (B) Transmural healing rates according to quartiles of infliximab trough levels. EH, endoscopic healing; IFX TL, infliximab trough level; TH, transmural healing.

Figure 4 (A) Comparison of infliximab trough levels between the EH + TH, EH + no TH, and no EH + no TH groups. (B) Infliximab trough levels according to dosing intervals. EH, endoscopic healing; IFX TL, infliximab trough level; TH, transmural healing.
3.5 Remission rates according to dosing intervals
Remission rates according to dosing intervals showed that the rates of EH were similar between patients whose intervals were and were not shortened. Meanwhile, those whose intervals were shortened had a relatively lower proportion of patients with TH than those receiving IFX with the standard dosing interval of 8 weeks, although statistical significance was not observed (Figure 5).
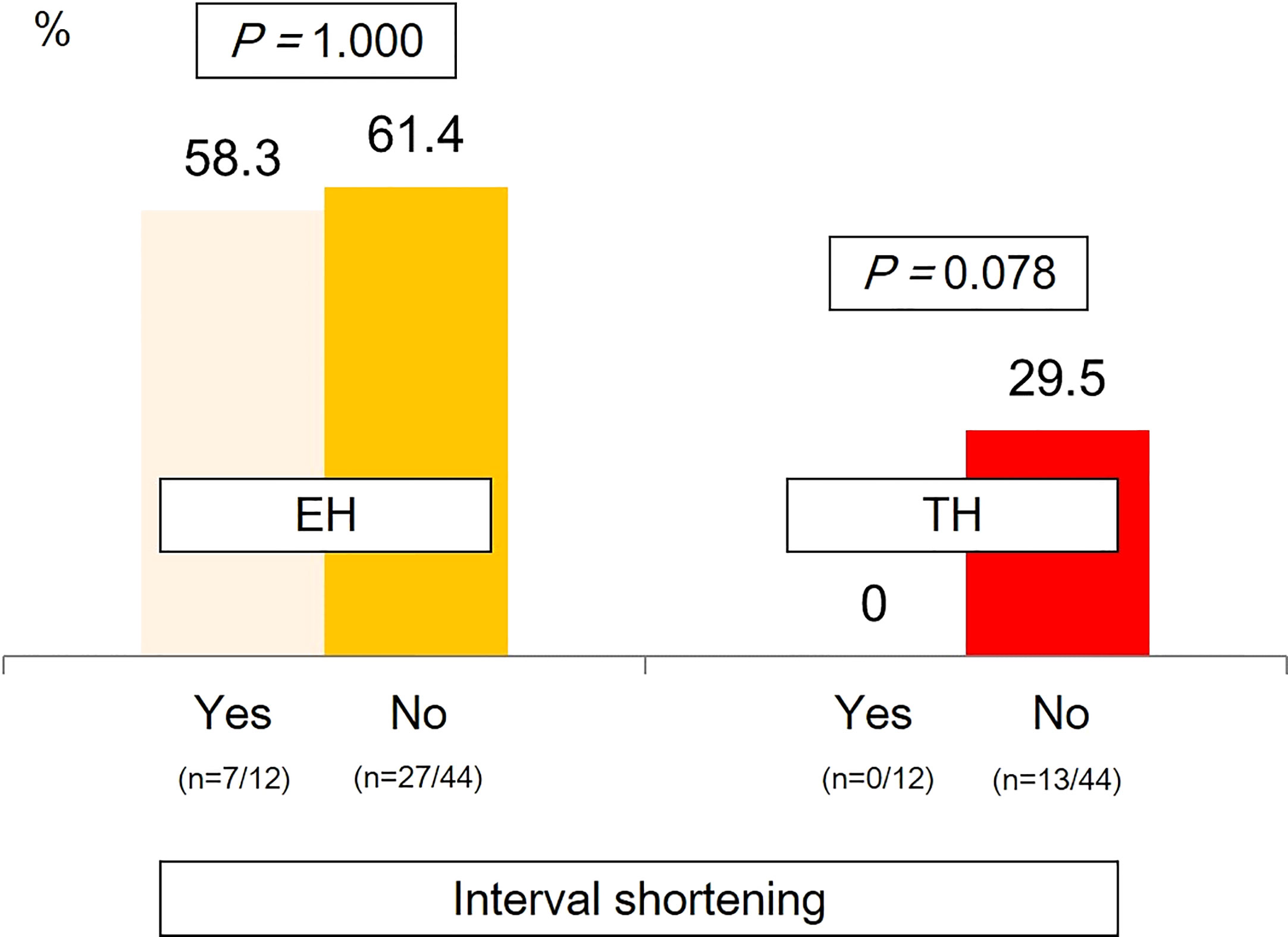
Figure 5 Remission rates according to dosing intervals. EH, endoscopic healing; TH, transmural healing.
3.6 Factors associated with TH and EH at 1-year IFX treatment
In univariate analysis, albumin level (OR, 10.16; 95% CI, 1.37–92.62; P = 0.028) and SES-CD (OR, 0.60; 95% CI, 0.32–0.89; P = 0.046) at 1-year IFX treatment were significantly associated with TH. However, these associations were not significant in multivariate analysis (Table 3). Meanwhile, disease duration to IFX (OR, 0.43; 95% CI, 0.18–0.80; P = 0.023), MaRIA score at IFX initiation (OR, 0.97; 95% CI, 0.94–1.00; P = 0.046), and IFX TLs (OR, 1.76; 95% CI, 1.30–2.68; P = 0.002) were associated with EH at 1-year IFX treatment (Table 4).
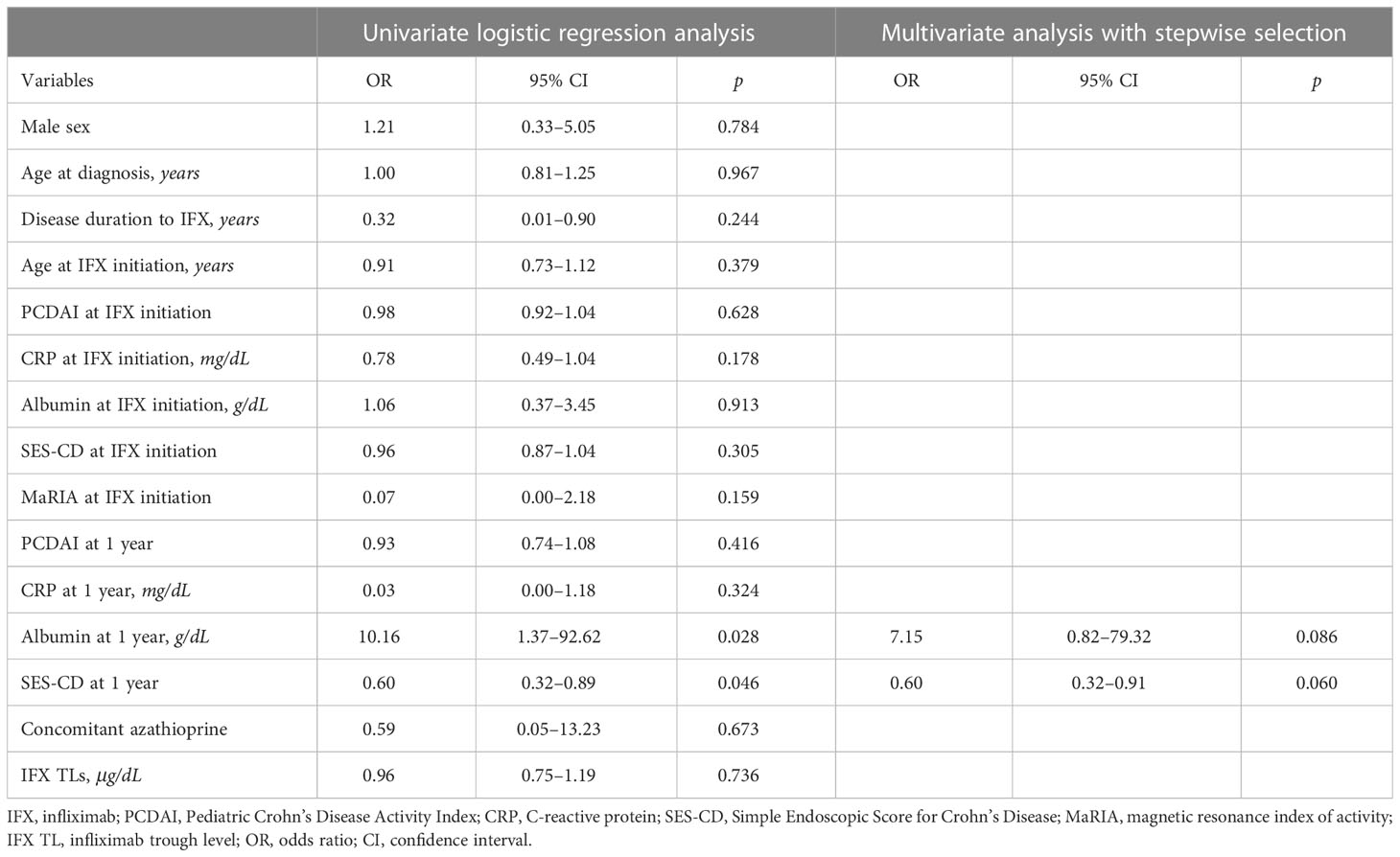
Table 3 Logistic regression analyses of factors associated with transmural healing at 1-year IFX treatment.
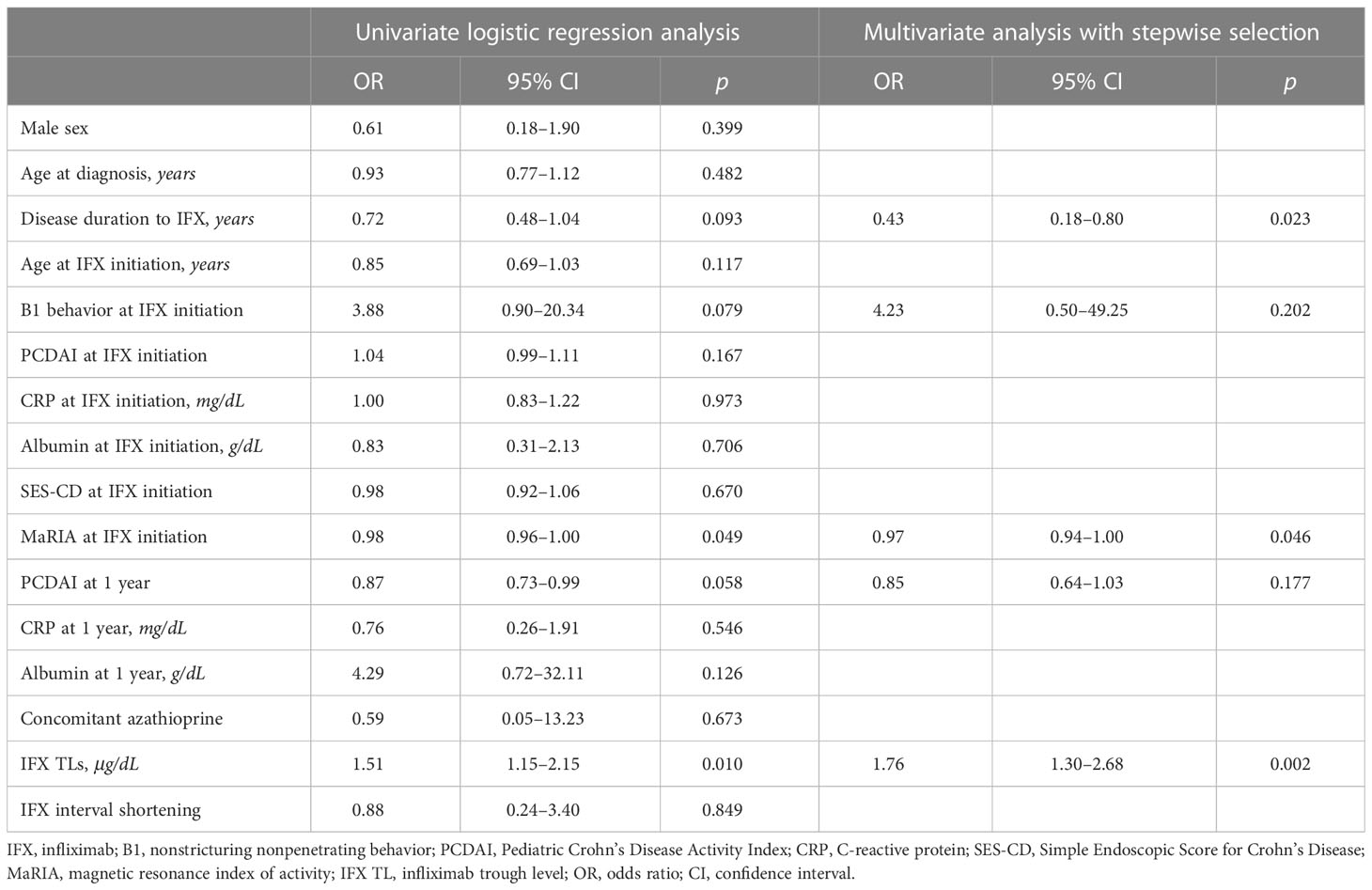
Table 4 Logistic regression analyses of factors associated with mucosal healing at 1-year IFX treatment.
4 Discussion
This study prospectively evaluated whether IFX TLs were associated with TH in pediatric patients with CD. As several recent studies have argued that TH is a promising new treatment target in CD, evaluating the relationship between IFX TLs and TH is necessary to develop new therapeutic approaches.
Consistent with previous studies, we observed a significant difference in IFX TLs between the two groups of patients with and without EH (20, 21, 30, 31); however, no difference in IFX TLs was observed between the two groups of patients with and without TH (Figure 2). Consequently, the authors considered TH as a deeper target for remission than EH, as patients with TH may be a subgroup of patients with EH. Unlike ulcerative colitis (UC), patients with CD exhibit deep ulcers rather than superficial ulcers; therefore, although the surface of the ulcer is healed, some inflammatory responses inside the mucosa or bowel wall (e.g., increase in blood flow and recruitment of immune cells) may yet remain.
IFX, an anti-TNF alpha agent, blocks the cytokine downstream of the inflammation process of CD; however, it does not inhibit a series of reactions that increase blood flow, recruit inflammatory cells (monocytes and macrophages), and differentiate T helper cells, which are upstream of the inflammatory process (32–34). Considering this mechanism, IFX treatment alone may prevent direct mucosal injury but not all inflammatory signaling pathways. This presents the possibility that EH can be achieved but TH cannot be achieved. In this regard, treatments other than IFX may be required to reach TH.
An additional point to consider is whether an IFX TL above the known therapeutic range is needed to obtain an association between TH and IFX TLs. This is because past studies have reported that higher IFX TL concentrations are required to treat fistulizing perianal lesions, and recent studies have evaluated the need for high-dose IFX treatment before LOR develops (35–37). However, evidence for higher IFX concentrations and doses to achieve TH remain insufficient to date. To verify this, additional studies are needed.
The third possible reason that IFX TLs were not associated with TH is that the 1-year treatment period set in this study may be relatively short to achieve TH. In 2013, Van Assche et al. reported the results of MRE up to 6 months following IFX treatment, and only 13% of patients showed normalization of MRE findings, leading them to conclude that the 6-month treatment period was insufficient for observing the treatment effect (38). However, according to the study of Castiglione et al. in 2016, the TH rate evaluated at 2 years following IFX treatment increased to 23% (39).
The fourth reason is minor but may nevertheless affect the outcome. There is no standardized definition of TH to date. In 2021, Geyl et al. compiled 17 papers that evaluated TH in a systematic review (40); the method for defining TH in each study was summarized, showing that all studies used bowel wall thickening, MaRIA score, or SES-CD. Similar to our study, most studies defined TH using bowel wall thickness; however, the standard for thickness varied, with some studies setting the upper limit as 3 mm and others as 7 mm (12, 41–51). Compared with other studies, our study’s TH definition was relatively strict. These stringent criteria may have resulted in an underestimation of the number of patients who reached TH, which may have prevented us from obtaining statistically significant results.
The results of the logistic regression analysis (Tables 3, 4) support the aforementioned results. As mucosal defects result in a loss of albumin due to leakage, a high albumin level can be used as a predictor of TH (Table 3). Furthermore, as the SES-CD represents EH, and EH can be observed as a preceding remission of TH, patients who reach EH may have a higher probability of reaching TH. Therefore, the SES-CD can be used as a predictor of TH. As shown in the results of the correlation between EH and IFX TLs, a high IFX TL could predict EH in the logistic regression analysis (Table 4). Moreover, logistic regression analysis showed that achieving EH is difficult since the period from the time of diagnosis to the use of IFX increases. Consistently, previous studies have reported that rapid application of biologics results in a high remission rate (52–54).
The strength of this study is that it is the first to evaluate the relationship between IFX TLs and TH in pediatric patients with CD. Even when expanding this scope to include studies in adults, only one study has evaluated the association between IFX TLs and TH to date. This paper was published very recently, in 2022 (55), and evaluated TH using ultrasonography, not MRE, and suggested that lower maintenance IFX TLs are associated with sonographic parameters of inflammation in UC and CD. Similar to our study, this paper also states that further studies are required to determine whether targeting higher IFX TLs can increase sonographic TH. The primary limitation of this study is that only 13 patients reached TH, which was too low to reach statistical significance. However, the total number of patients, 56, was relatively large for a prospective study with pediatric patients. Furthermore, this study is the first to evaluate the relationship between IFX TLs and TH in pediatric patients.
5 Conclusion
The results of our analysis showed that higher IFX TLs were associated with EH but not with TH in pediatric patients with CD treated with IFX. TH should be considered a concept of deeper remission than EH, and higher TLs than conventional therapeutic IFX TLs or long-term treatment may be required to reach TH. The results of this study may serve as a basis for further studies investigating long-term TH and proactive dosing based on therapeutic drug monitoring, which may clarify whether an association between IFX TLs and TH exists.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Institutional Review Board of Kyungpook National University Chilgok Hospital. Written informed consent to participate in this study was provided by the participant’ legal guardian/next of kin.
Author contributions
SYC contributed in the analysis and interpretation of data, drafting of the initial manuscript, and critical revision for important intellectual content. YK contributed in the analysis and interpretation of data, and drafting of the initial manuscript. SJC contributed in acquisition of data, and drafting of the initial manuscript. SL contributed in in the analysis and interpretation of data, and critical revision for important intellectual content. B-HC contributed in acquisition, analysis and interpretation of data, and critical revision for important intellectual content. BK contributed in the conception and design of the study, acquisition, analysis and interpretation of data, statistical analysis, and critical revision for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2021R1A2C1011004 and 2022R1G1A1010896).
Acknowledgments
There are no additional acknowledgments associated with this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet (2007) 369:1641–57. doi: 10.1016/S0140-6736(07)60751-X
2. Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of crohn’s disease in a population-based cohort. Gastroenterol (2010) 139:1147–55. doi: 10.1053/j.gastro.2010.06.070
3. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for crohn’s disease: the ACCENT I randomised trial. Lancet (2002) 359:1541–9. doi: 10.1016/S0140-6736(02)08512-4
4. Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, et al. Adalimumab for maintenance treatment of crohn’s disease: results of the CLASSIC II trial. Gut (2007) 56:1232–9. doi: 10.1136/gut.2006.106781
5. Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe crohn’s disease in children. Gastroenterol (2007) 132:863–73. doi: 10.1053/j.gastro.2006.12.003
6. Hyams JS, Griffiths A, Markowitz J, Baldassano RN, Faubion WA Jr., Colletti RB, et al. Safety and efficacy of adalimumab for moderate to severe crohn’s disease in children. Gastroenterol (2012) 143:365–74.e2. doi: 10.1053/j.gastro.2012.04.046
7. Danese S, Fiorino G, Fernandes C, Peyrin-Biroulet L. Catching the therapeutic window of opportunity in early crohn’s disease. Curr Drug targets (2014) 15:1056–63. doi: 10.2174/1389450115666140908125738
8. Kang B, Choi SY, Kim HS, Kim K, Lee YM, Choe YH. Mucosal healing in paediatric patients with moderate-to-severe luminal crohn’s disease under combined immunosuppression: escalation versus early treatment. J Crohn’s Colitis (2016) 10:1279–86. doi: 10.1093/ecco-jcc/jjw086
9. Kang B, Choe YH. Early biologic treatment in pediatric crohn’s disease: catching the therapeutic window of opportunity in early disease by treat-to-target. Pediatr gastroenterol Hepatol Nutr (2018) 21:1–11. doi: 10.5223/pghn.2018.21.1.1
10. Colombel J-F, Louis E, Peyrin-Biroulet L, Sandborn WJ, Panaccione R. Deep remission: a new concept? Digestive Diseases (2012) 30:107–11. doi: 10.1159/000342732
11. Ma L, Li W, Zhuang N, Yang H, Liu W, Zhou W, et al. Comparison of transmural healing and mucosal healing as predictors of positive long-term outcomes in crohn’s disease. Ther Adv gastroenterol (2021) 14:17562848211016259. doi: 10.1177/17562848211016259
12. Lafeuille P, Hordonneau C, Vignette J, Blayac L, Dapoigny M, Reymond M, et al. Transmural healing and MRI healing are associated with lower risk of bowel damage progression than endoscopic mucosal healing in crohn’s disease. Alimentary Pharmacol Ther (2021) 53:577–86. doi: 10.1111/apt.16232
13. Buisson A, Hordonneau C, Deepak P. Transmural healing and MRI remission: new promising therapeutic targets in crohn’s disease. Inflammatory bowel diseases (2017) 23:E44–E5. doi: 10.1097/MIB.0000000000001219
14. Choi SY, Kim ES, Jeon TY, Lee YM, Lee SM, Choe BH, et al. Transmural healing evaluated by magnetic resonance enterography in paediatric patients with crohn’s disease receiving maintenance treatment with biologics. Alimentary Pharmacol Ther (2022) 56:1146–56. doi: 10.1111/apt.17161
15. Bortlik M, Duricova D, Malickova K, Machkova N, Bouzkova E, Hrdlicka L, et al. Infliximab trough levels may predict sustained response to infliximab in patients with crohn’s disease. J Crohn’s Colitis (2013) 7:736–43. doi: 10.1016/j.crohns.2012.10.019
16. Marits P, Landucci L, Sundin U, Davidsdottir L, Nilsson J, Befrits R, et al. Trough s-infliximab and antibodies towards infliximab in a cohort of 79 IBD patients with maintenance infliximab treatment. J Crohn’s Colitis (2014) 8:881–9. doi: 10.1016/j.crohns.2014.01.009
17. Merras-Salmio L, Kolho K-L. Clinical use of infliximab trough levels and antibodies to infliximab in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr (2017) 64:272–8. doi: 10.1097/MPG.0000000000001258
18. Vaughn BP, Martinez-Vazquez M, Patwardhan VR, Moss AC, Sandborn WJ, Cheifetz AS. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflammatory bowel diseases (2014) 20:1996–2003. doi: 10.1097/MIB.0000000000000156
19. Fernandes SR, Bernardo S, Simões C, Gonçalves AR, Valente A, Baldaia C, et al. Proactive infliximab drug monitoring is superior to conventional management in inflammatory bowel disease. Inflammatory bowel diseases (2020) 26:263–70. doi: 10.1093/ibd/izz131
20. Kang B, Choi SY, Choi YO, Lee S-Y, Baek S-Y, Sohn I, et al. Infliximab trough levels are associated with mucosal healing during maintenance treatment with infliximab in paediatric crohn’s disease. J Crohn’s Colitis (2019) 13:189–97. doi: 10.1093/ecco-jcc/jjy155
21. Ungar B, Levy I, Yavne Y, Yavzori M, Picard O, Fudim E, et al. Optimizing anti-TNF-α therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol (2016) 14:550–7.e2. doi: 10.1016/j.cgh.2015.10.025
22. Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr (2014) 58:795–806. doi: 10.1097/mpg.0000000000000239
23. Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric crohn’s disease activity index. J Pediatr Gastroenterol Nutr (1991) 12:439–47. doi: 10.1097/00005176-199105000-00005
24. Grover Z, Biron R, Carman N, Lewindon P. Predictors of response to infliximab in children with luminal crohn’s disease. J Crohn’s Colitis (2014) 8:739–46. doi: 10.1016/j.crohns.2013.12.017
25. Rimola J, Rodríguez S, García-Bosch O, Ordás I, Ayala E, Aceituno M, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic crohn’s disease. Gut (2009) 58:1113–20. doi: 10.1136/gut.2008.167957
26. Rimola J, Ordás I, Rodriguez S, García-Bosch O, Aceituno M, Llach J, et al. Magnetic resonance imaging for evaluation of crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflammatory bowel diseases (2011) 17:1759–68. doi: 10.1002/ibd.21551
27. Ordás I, Rimola J, Rodríguez S, Paredes JM, Martínez-Pérez MJ, Blanc E, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with crohn’s disease. Gastroenterol (2014) 146:374–82. doi: 10.1053/j.gastro.2013.10.055
28. Kang B, Choi SY, Chi S, Lim Y, Jeon TY, Choe YH. Baseline wall thickness is lower in mucosa-healed segments 1 year after infliximab in pediatric crohn disease patients. J Pediatr Gastroenterol Nutr (2017) 64:279–85. doi: 10.1097/MPG.0000000000001222
29. American Gastroenterological Association Clinical Practice Committee. American Gastroenterological association medical position statement: perianal crohn’s disease. Gastroenterol (2003) 125:1503–7. doi: 10.1016/j.gastro.2003.08.024
30. Paul S, Del Tedesco E, Marotte H, Rinaudo-Gaujous M, Moreau A, Phelip J-M, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflammatory Bowel Diseases (2013) 19:2568–76. doi: 10.1097/MIB.0b013e3182a77b41
31. Barnes EL, Allegretti JR. Are anti-tumor necrosis factor trough levels predictive of mucosal healing in patients with inflammatory bowel disease? J Clin gastroenterol (2016) 50:733–41. doi: 10.1097/MCG.0000000000000441
32. Chang JT. Pathophysiology of inflammatory bowel diseases. New Engl J Med (2020) 383:2652–64. doi: 10.1056/NEJMra2002697
33. Lee SH, eun Kwon J, Cho M-L. Immunological pathogenesis of inflammatory bowel disease. Intestinal Res (2018) 16:26. doi: 10.5217/ir.2018.16.1.26
34. Li N, Shi R-H. Updated review on immune factors in pathogenesis of crohn’s disease. World J Gastroenterol (2018) 24:15. doi: 10.3748/wjg.v24.i1.15
35. El-Matary W, Walters TD, Huynh HQ, deBruyn J, Mack DR, Jacobson K, et al. Higher postinduction infliximab serum trough levels are associated with healing of fistulizing perianal crohn’s disease in children. Inflammatory Bowel Diseases (2019) 25:150–5. doi: 10.1093/ibd/izy217
36. Kwon Y, Kim E-S, Kim Y-Z, Choe Y-H, Kim M-J. Cytokine profile at diagnosis affecting trough concentration of infliximab in pediatric crohn’s disease. Biomedicines (2022) 10:2372. doi: 10.3390/biomedicines10102372
37. Hendler SA, Cohen BL, Colombel J-F, Sands BE, Mayer L, Agarwal S. High-dose infliximab therapy in crohn’s disease: clinical experience, safety, and efficacy. J Crohn’s Colitis (2015) 9:266–75. doi: 10.1093/ecco-jcc/jju026
38. Van Assche G, Herrmann KA, Louis E, Everett SM, Colombel J-F, Rahier J-F, et al. Effects of infliximab therapy on transmural lesions as assessed by magnetic resonance enteroclysis in patients with ileal crohn’s disease. J Crohn’s Colitis (2013) 7:950–7. doi: 10.1016/j.crohns.2013.01.011
39. Castiglione F, Mainenti P, Testa A, Imperatore N, De Palma GD, Maurea S, et al. Cross-sectional evaluation of transmural healing in patients with crohn’s disease on maintenance treatment with anti-TNF alpha agents. Digestive Liver Disease (2017) 49:484–9. doi: 10.1016/j.dld.2017.02.014
40. Geyl S, Guillo L, Laurent V, D’Amico F, Danese S, Peyrin-Biroulet L. Transmural healing as a therapeutic goal in crohn’s disease: a systematic review. Lancet Gastroenterol Hepatol (2021) 6:659–67. doi: 10.1016/S2468-1253(21)00096-0
41. Civitelli F, Nuti F, Oliva S, Messina L, La Torre G, Viola F, et al. Looking beyond mucosal healing: effect of biologic therapy on transmural healing in pediatric crohn’s disease. Inflammatory bowel diseases (2016) 22:2418–24. doi: 10.1097/MIB.0000000000000897
42. Eder P, Łykowska-Szuber L, Katulska K, Stawczyk-Eder K, Krela-Kaźmierczak I, Klimczak K, et al. Intestinal healing after anti-TNF induction therapy predicts long-term response to one-year treatment in patients with ileocolonic crohn’s disease naive to anti-TNF agents. Gastroenterol Review/Przegląd Gastroenterologiczny (2016) 11:187–93. doi: 10.5114/pg.2015.55185
43. Sauer CG, Middleton JP, McCracken C, Loewen J, Braithwaite K, Alazraki A, et al. Magnetic resonance enterography healing and magnetic resonance enterography remission predicts improved outcome in pediatric crohn disease. J Pediatr Gastroenterol Nutr (2016) 62:378–83. doi: 10.1097/MPG.0000000000000976
44. Weinstein-Nakar I, Focht G, Church P, Walters TD, Abitbol G, Anupindi S, et al. Associations among mucosal and transmural healing and fecal level of calprotectin in children with crohn’s disease. Clin Gastroenterol Hepatol (2018) 16:1089–97. doi: 10.1016/j.cgh.2018.01.024
45. Laterza L, Piscaglia AC, Minordi LM, Scoleri I, Larosa L, Poscia A, et al. Multiparametric evaluation predicts different mid-term outcomes in crohn’s disease. Digestive Diseases (2018) 36:184–93. doi: 10.1159/000487589
46. Fernandes SR, Rodrigues RV, Bernardo S, Cortez-Pinto J, Rosa I, da Silva JP, et al. Transmural healing is associated with improved long-term outcomes of patients with crohn’s disease. Inflammatory bowel diseases (2017) 23:1403–9. doi: 10.1097/MIB.0000000000001143
47. Deepak P, Fletcher JG, Fidler JL, Barlow JM, Sheedy SP, Kolbe AB, et al. Radiological response is associated with better long-term outcomes and is a potential treatment target in patients with small bowel crohn’s disease. Off J Am Coll Gastroenterology| ACG (2016) 111:997–1006. doi: 10.1038/ajg.2016.177
48. Hallé E, Azahaf M, Duveau N, Lambin T, Nachury M, Branche J, et al. Radiological response is associated with better outcomes and should be considered a therapeutic target in crohn’s disease. Digestive Dis Sci (2020) 65:2664–74. doi: 10.1007/s10620-019-05979-8
49. Ripollés T, Paredes JM, Martínez-Pérez MJ, Rimola J, Jauregui-Amezaga A, Bouzas R, et al. Ultrasonographic changes at 12 weeks of anti-TNF drugs predict 1-year sonographic response and clinical outcome in crohn’s disease: a multicenter study. Inflammatory bowel diseases (2016) 22:2465–73. doi: 10.1097/MIB.0000000000000882
50. Noh SM, Oh EH, Park SH, Lee JB, Kim JY, Park JC, et al. Association of faecal calprotectin level and combined endoscopic and radiological healing in patients with crohn’s disease receiving anti-tumour necrosis factor therapy. J Crohn’s Colitis (2020) 14:1231–40. doi: 10.1093/ecco-jcc/jjaa042
51. Buisson A, Hordonneau C, Goutorbe F, Allimant C, Goutte M, Reymond M, et al. Bowel wall healing assessed using magnetic resonance imaging predicts sustained clinical remission and decreased risk of surgery in crohn’s disease. J Gastroenterol (2019) 54:312–20. doi: 10.1007/s00535-018-1505-8
52. Lee YM, Kang B, Lee Y, Kim MJ, Choe YH. Infliximab “top-down” strategy is superior to “step-up” in maintaining long-term remission in the treatment of pediatric crohn disease. J Pediatr Gastroenterol Nutr (2015) 60:737–43. doi: 10.1097/MPG.0000000000000711
53. Lee JS, Lee JH, Lee JH, Lee HJ, Kim MJ, Lee HJ, et al. Efficacy of early treatment with infliximab in pediatric crohn’s disease. World J gastroenterol: WJG (2010) 16:1776. doi: 10.3748/wjg.v16.i14.1776
54. Berg DR, Colombel J-F, Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflammatory bowel diseases (2019) 25:1896–905. doi: 10.1093/ibd/izz059
Keywords: Crohn’s disease, infliximab, magnetic resonance enterography, transmural healing, trough levels
Citation: Choi SY, Kwon Y, Choi S, Lee SM, Choe B-H and Kang B (2023) Infliximab trough levels are associated with endoscopic healing but not with transmural healing at one year treatment with infliximab in pediatric patients with Crohn’s disease. Front. Immunol. 14:1192827. doi: 10.3389/fimmu.2023.1192827
Received: 23 March 2023; Accepted: 12 June 2023;
Published: 23 June 2023.
Edited by:
Ciro Romano, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Philip Bufler, Charité University Medicine Berlin, GermanyWay Seah Lee, University of Malaya, Malaysia
Zubin Grover, Dalhousie University, Canada
Copyright © 2023 Choi, Kwon, Choi, Lee, Choe and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ben Kang, YmVua2FuZ0BrbnUuYWMua3I=
†These authors have contributed equally to this work and share first authorship
 So Yoon Choi
So Yoon Choi Yiyoung Kwon
Yiyoung Kwon Sujin Choi3
Sujin Choi3 So Mi Lee
So Mi Lee Ben Kang
Ben Kang