- 1Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
- 2Department of Laboratory Medicine, The People’s Hospital of Dongtai City, Dongtai, China
- 3Hangzhou Institute of Cardiovascular Diseases, Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou, China
- 4Department of Clinical Laboratory, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Gusu School, Nanjing Medical University, Suzhou, Jiangsu, China
Due to its widespread occurrence and high mortality rate, hepatocellular carcinoma (HCC) is an abhorrent kind of cancer. Immunotherapy is a hot spot in the field of cancer treatment, represented by immune checkpoint inhibitors (ICIs), which aim to improve the immune system’s ability to recognize, target and eliminate cancer cells. The composition of the HCC immune microenvironment is the result of the interaction of immunosuppressive cells, immune effector cells, cytokine environment, and tumor cell intrinsic signaling pathway, and immunotherapy with strong anti-tumor immunity has received more and more research attention due to the limited responsiveness of HCC to ICI monotherapy. There is evidence of an organic combination of radiotherapy, chemotherapy, anti-angiogenic agents and ICI catering to the unmet medical needs of HCC. Moreover, immunotherapies such as adoptive cellular therapy (ACT), cancer vaccines and cytokines also show encouraging efficacy. It can significantly improve the ability of the immune system to eradicate tumor cells. This article reviews the role of immunotherapy in HCC, hoping to improve the effect of immunotherapy and develop personalized treatment regimens.
1 Introduction
The liver is the sixth most common site of primary cancer in humans, and as one of the most common malignant tumors, liver cancer has the fourth most lethal rate in the world (1). It is estimated that by 2025, more than 1 million people will develop liver cancer each year (2). Among them, the incidence of hepatocellular carcinoma (HCC) accounts for 80–90% of primary liver cancer (3), with a dismal prognosis and a relative five-year survival rate of 18% (4), placing a huge load on healthcare. HCC occurs predominantly in men between the ages of 60 and 70 years (5, 6), and person with hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infection, excessive alcohol use, nonalcoholic steatohepatitis (NAFLD), and a family history of liver cancer is at high risk for HCC (7–10). Although anti-HBV and anti-HCV therapy can significantly reduce the risk of HCC, it still cannot completely avoid the occurrence of HCC (11, 12). Serum alpha-fetoprotein (AFP) is a commonly used and important indicator for the diagnosis of early HCC and the monitoring of curative effect (13). However, HCC is typically detected at a level that is intermediate to advanced (14). The overall survival (OS) rate after liver resection for patients with advanced HCC has been unsatisfactory on the basis of large data (15).
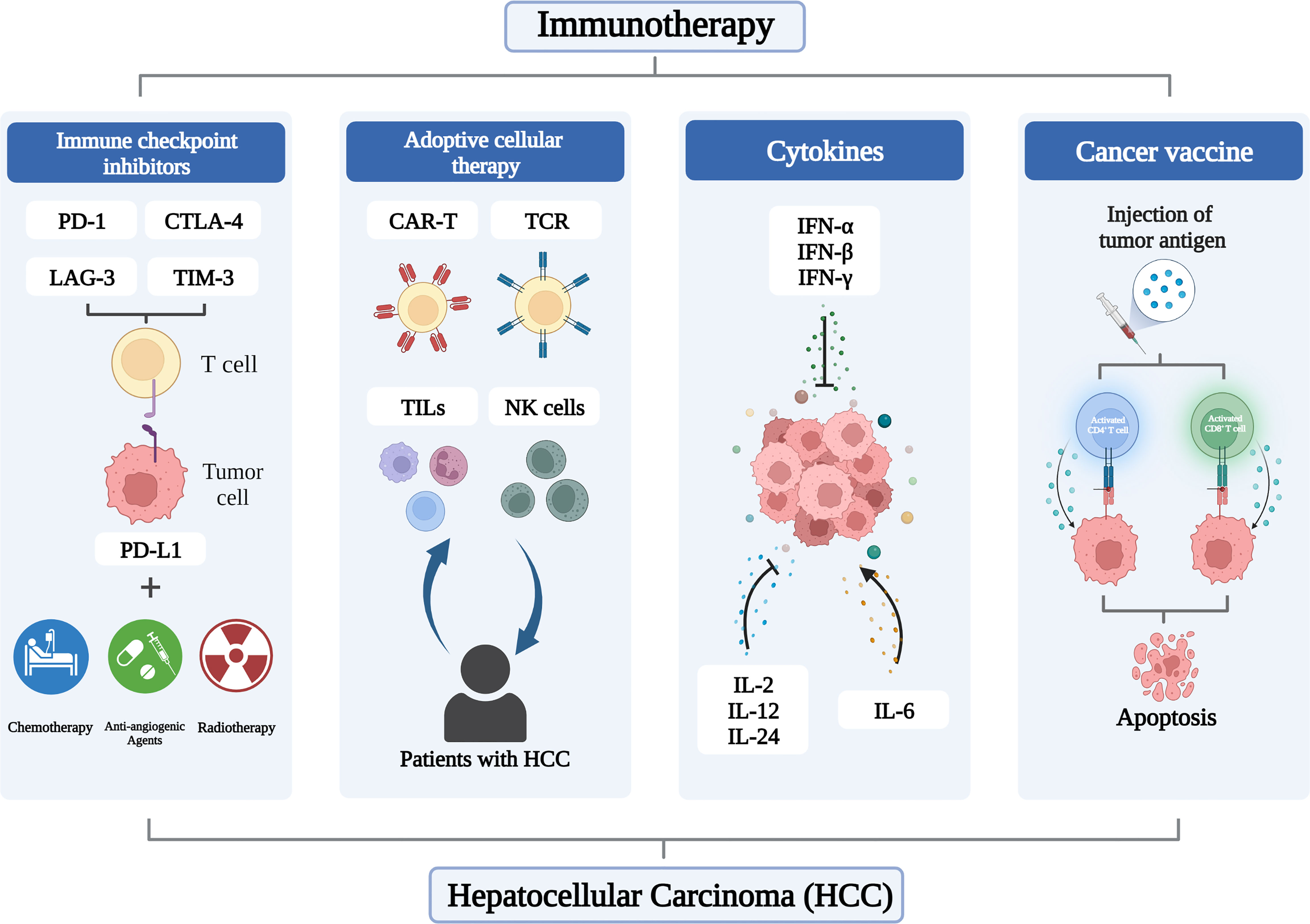
Antitumor therapies combining liver transplantation, percutaneous ablation, transarterial chemoembolization (TACE), and radiation embolization have made great strides, but the choice is largely dependent on tumor load, location, and comorbidities (16). Indeed, systemic molecular therapies have been a mainstay of treatment for advanced HCC for more than a decade, with first-line agents, including the oral multityrosine kinase inhibitors (TKIs) sorafenib, lenvatinib, and donafinib, and second-line agents, including the antiangiogenic agents regorafenib and apatinib (17). The clinical prognosis of cancer patients will be impacted by immune infiltration in the tumor microenvironment (TME), which has been proven to have a profound influence in tumor formation. An effective immune response can eradicate malignant cells or impair their phenotype and function. However, cancer cells have evolved mechanisms such as defective antigen presentation and recruitment of immunosuppressive cell populations to evade immune surveillance (18), and antitumor immune responses are suppressed. In recent years, immunotherapy, including immune checkpoint inhibitors (ICIs), adoptive cell therapy (ACT), cancer vaccines and cytokines, has shown exciting efficacy in melanoma and non-small cell lung cancer, and is revolutionizing the treatment of HCC (Figure 1).
This review emphasizes challenges and options to effectively treat HCC patients by describing the fundamental processes of immunotherapy and its therapeutic benefits in HCC. In addition, we suggested innovations and techniques to improve the performance of immunotherapy based on the characteristics of HCC.
2 Immune checkpoint inhibitors
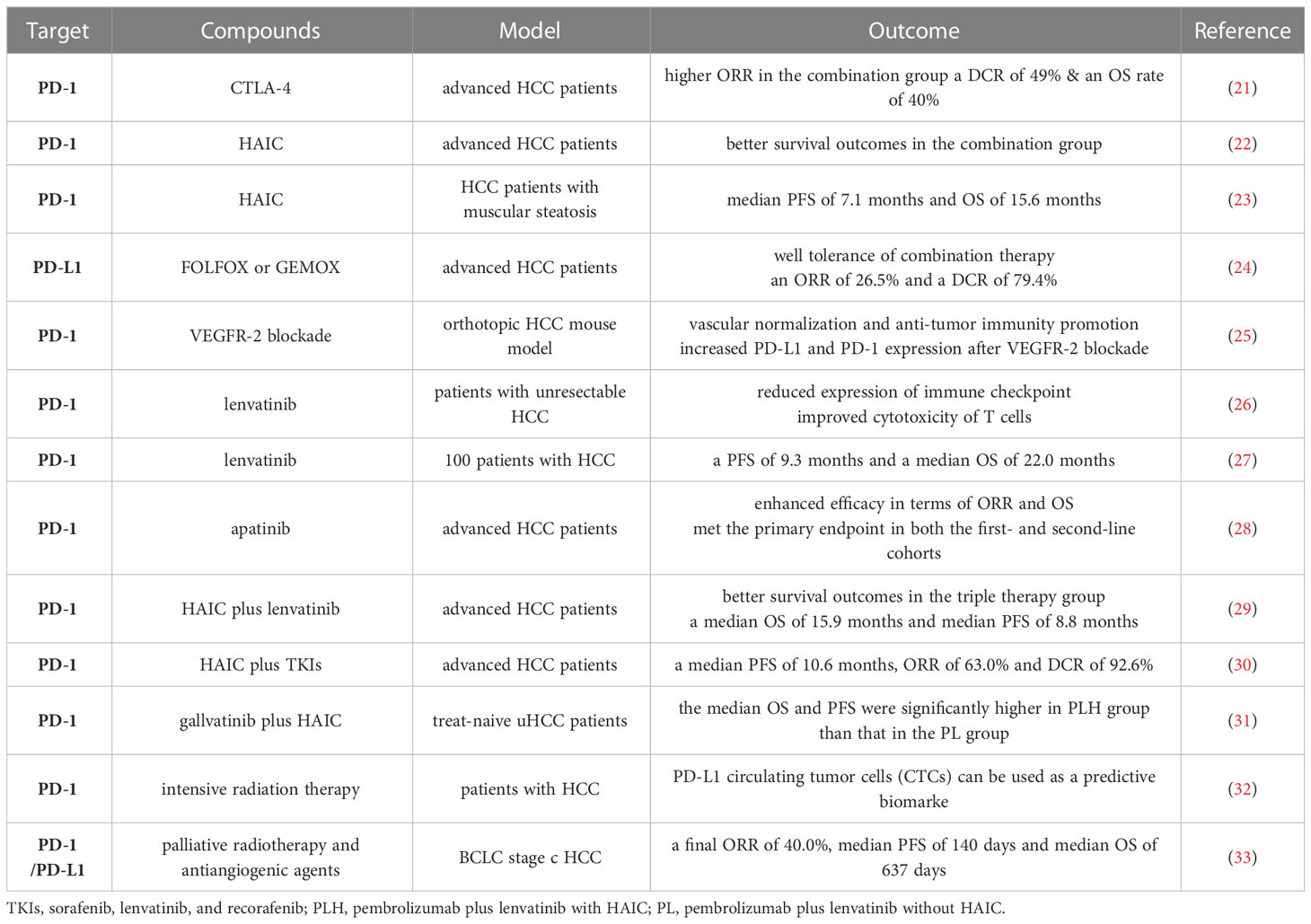
Elimination of co-inhibitory signals is an effective way to regulate autoimmune responses. Immune checkpoint molecules are inhibitory regulatory molecules of the immune system that are critical for maintaining self-tolerance, preventing autoimmune responses, and minimizing tissue damage by controlling the timing and intensity of the immune response (19, 20). ICIs with PD-1, PD-L1, CTLA-4, TIM-3 and LAG-3 as representative targets have shown surprising safety and efficacy in the treatment of HCC (Table 1).
Inhibitors of PD-1, PDL-1, and CTLA-4 are the backbone of clinical practice or of systemic therapies in development for hepatocellular carcinoma. The CTLA-4 blocker tremelimumab’s anti-tumor and antiviral effects in HCC patients were first investigated by Bruno et al. (34). Finally, patients showed a median time to progression (TTP) of 6.48 months and a disease control rate (DCR) of 76%, revealing for the first time the transformative function of ICIs in the treatment of HCC. A phase I/II study published 4 years later by Anthony et al. first evaluated the efficacy of anti-PD-1 monoclonal antibody nabuliumab in patients with advanced HCC (35), showing an objective response rate (ORR) of 20%, a DCR of 64%, a median OS of 13.2 months as well as a median duration of response of 9.9 months, which further confirmed the potential of ICIs for HCC. In 2018, another single-arm phase II trial conducted by Colombo et al. tested the clinical efficacy and safety of pembrolizumab in 104 patients with advanced hepatocellular carcinoma who had been previously treated with sorafenib (36). The results showed an ORR rate of 17% and 44% of patients had stable disease. 77% of patients had a sustained response for ≥ 9 months, median PFS of 4.9 months, and median OS of 12.9 months, indicating that the safety and toxicity of pembrolizumab in HCC patients are manageable.
In addition to the above three types of immune checkpoints, TIM-3 and LAG-3 can also target and stimulate anti-tumor immune responses. Liu et al. (37) studied the expression, function, and regulation of the Tim-3/galectin-9 pathway in patients with HBV-related HCC, founding that the interaction of Tim-3 and galectin-9 impaired T cell effector function in HCC, and Tim-3 expression was negatively correlated with clinical outcome in patients with HBV-related HCC. LAG3 binds MHC class II molecules with high affinity, is upregulated upon T cell activation, and provides a negative signal to T cells (38). Guo et al. (39) found that serum LAG-3 levels significantly increased in HCC patients compared with healthy controls, and patients with higher LAG-3 levels had a poor prognosis after TACE. These preclinical studies demonstrated the potential of TIM-3 with LAG-3 and provided support for the subsequent combination therapy of multiple ICIs.
2.1 Combination of two ICIs
Single ICI may have limited efficacy in the treatment of HCC, but combinations of ICIs, such as anti-PD-1, anti-PD-L1, and anti-CTLA-4, have shown excellent efficacy in tumors such as non-small-cell lung cancer (40), melanoma (41), and colorectal cancer (42). Yau et al. (21) tested the safety of combination therapy with nivolumab (NIVO) and ipilimumab (IPI) in patients with advanced HCC and found that the ORR in the combination group was twice as high as that in the NIVO monotherapy group, with a DCR of 49% and 24-mo OS rate of 40%. This encouraging result led to accelerated FDA approval of this combination for the treatment of HCC patients after sorafenib. Single-agent pembrolizumab and nivolumab plus ipilimumab are now approved as second-line treatments for patients with disease progression from first-line TKIs (43). Liu et al. (37) found that the up-regulation of TIM-3 and/or PD-1 expression on tumor infiltrating lymphocytes (TILs) weakened their functions and was closely related to the disease progression of HBV-related HCC. The anti-tumor effect of TILs was restored after blocking TIM-3 and PD-1, which provided new ideas for the combination of immune checkpoint inhibitors. Currently, dual blockade of LAG-3 with anti-PD-1 treatment is being tested in a Phase I trial (NCT01968109) (44).
2.2 Combination with chemotherapy
Hepatic arterial infusion chemotherapy (HAIC) is an emerging therapy that has attracted much attention due to its high response rate and favorable survival for advanced liver cancer. In the Asian region, HAIC has been used as a sorafenib replacement therapy for patients with advanced HCC (45, 46). As a locoregional interventional therapy, HAIC not only maximizes tumor cell killing but also reduces systemic toxicity of chemotherapy agents through first-pass effects in the liver (47). Despite the promising efficacy of HAIC against unresectable tumors, its ability to inhibit extrahepatic metastasis remains unsatisfactory.
Mei et al. (22) investigated the effect of HAIC combined with anti-PD-1 immunotherapy (HAICAP) in advanced HCC and showed that patients in the HAICAP group had significantly better survival outcomes than those in the HAIC group. Muscular steatosis refers to the abnormal distribution of adipose tissue between and within muscle cells, which leads to excessive fat deposition in muscle, resulting in the decline of muscle mass, limb function and physical fitness. Yi et al. (23) evaluated the effects of anti-PD-1 immunotherapy and HAIC in HCC patients with varying degrees of muscular steatosis. The median PFS was 7.1 months and OS was 15.6 months. It was also found that patients with muscular steatosis had accelerated disease progression, increased levels of complications, prolonged hospital stay and poor prognosis after receiving treatment. A phase II study (24) focused on the clinical benefit of combining the anti-PD-L1 antibody camrelizumab with oxaliplatin-based chemotherapy (FOLFOX or GEMOX) in HCC patients and found an ORR of 26.5%, a DCR of 79.4%, a median TTR of 2.0 months, and a median PFS of 5.5 months. Camrelizumab plus FOLFOX or GEMOX chemotherapy is well tolerated and may provide a new option for patients with advanced HCC.
2.3 Combination with anti-angiogenic agents
HCC is a highly vascularized tumor (48), and clinical trials have successfully shown the critical role of targeting VEGF-driven angiogenesis in HCC (49). Anti-angiogenic agents inhibits tumor growth by normalizing tumor vasculature and disrupting the hypoxic tumor microenvironment (50). In 2020, atezolizumab-bevacizumab combination became the standard first-line systemic treatment for advanced HCC for its significant efficacy (43) and other combinations are being explored. Shigeta et al. (25) used orthotopic transplantation or induced mouse models of HCC to examine the effect of anti-PD-1/VEGFR-2 combination therapy on survival. VEGFR-2/PD-1 dual blockade can promote the normalization of blood vessels, reprogram the immune microenvironment, and promote the anti-tumor immunity of HCC. A nonrandomized, open-label, phase II trial by Xu et al. (28) evaluated the efficacy of camrelizumab plus apatinib in patients with advanced HCC who were naive or refractory/intolerant to first-line targeted therapy. The results showed that the combination of camrelizumab and apatinib achieved effective efficacy in terms of ORR and OS and met the primary endpoint in both the first- and second-line cohorts.
Deng et al. (26) explored the optimal combination of anti-PD-1 antibody and TKIs in patients with unresectable HCC and the possible mechanism of combined therapy. They found that lenvatinib can reduce the expression of PD-1, CTLA-4 and Tim-3 on T cells promoted by VEGFA and basic fibroblast growth factor, and improve the cytotoxicity of T cells. The combination of anti-PD-1 antibody and lenvatinib has a more effective anti-tumor effect than sorafenib or BGJ398. An Ib multicenter open-label study of 100 patients was designed to assess the tolerability, safety, and efficacy of lenvatinib plus pembrolizumab in the treatment of unresectable HCC (27). In this study, lenvatinib plus pembrolizumab produced a definitive response rate, with a PFS of 9.3 months and a median OS of 22.0 months. This suggests that lenvatinib plus PD-1 inhibition and pembrolizumab have promising antitumor effects.
2.4 Triple therapy
Anti-angiogenic agents combined with PD-1 or PD-L1 inhibitors have shown promising survival results in the treatment of unresectable HCC tumors. HAIC has also attracted attention for its high response rate and favorable survival in patients with advanced HCC. The combination of these three factors provides a new option for personalized treatment of different HCC patients.
A retrospective study was designed to compare survival among patients with advanced HCC who received HAIC plus lenvatinib plus a PD-1 inhibitor (HPL) with survival among patients who received lenvatinib plus a PD-1 inhibitor (PL) (29). The results showed that the survival outcome of patients in the HPL group was significantly better than that in the PL group, with a median OS of 15.9 months and a median PFS of 8.8 months, which were almost twice of PL group. All adverse events were assessed as mild and manageable, and no toxicity-related deaths occurred during follow-up. Liu et al. (30) investigated the efficacy and safety of HAIC combined with anti-PD-1 immunotherapy versus TKIs. Finally, the median PFS was 10.6 months, ORR was 63.0%, and DCR was 92.6% in the patients who received triple therapy, which was satisfying. Song et al. (31) evaluated the efficacy and safety of pembrolizumab, lenvatinib plus HAIC (PLH) versus pembrolizumab and lenvatinib (PL) in selected treat-naive uHCC patients. At the final follow-up, the median OS was 17.7 months in PLH group and 12.6 months in PL group. And the median PFS in the PLH group (10.9 months) was also significantly higher than that in the PL group (6.8 months), confirming that the combination of PD-L1 inhibitor, HAIC and lenvatinib can also improve survival rate.
2.5 Combination of radiotherapy
Recent studies have found that the use of local radiotherapy may stimulate antitumor immune responses by increasing apoptosis and necrosis of tumor cells and subsequently increasing the expression of antigen presentation and immunomodulatory genes (51). This may improve the response to immunotherapy, increase efficiency and reduce adverse effects. The strategy of combining immunotherapy with radiotherapy has shown promising results in clinical and basic studies. PD-L1 circulating tumor cells (CTCs) can be used as a predictive biomarker in patients with HCC who receive PD-1 inhibitors in combination with intensive radiation therapy (IMRT) and antiangiogenic therapy (32). Zhong et al. (33) explored the safety and clinical efficacy of PD-1/PD-L1 inhibitors combined with palliative radiotherapy and antiangiogenic agents in the treatment of BCLC stage C HCC. The final ORR was 40.0%, the median PFS was 140 days, and the median OS was 637 days, which proved that PD-1/PD-L1 inhibitors combined with palliative radiotherapy and anti-angiogenesis therapy were reliable without unexpected adverse events. Additional studies exploring clinical benefit are needed.
3 Adoptive cellular therapy
ACT is also an emerging type of immunotherapy that involves harvesting human T cells, growing them in vitro, increasing their number or targeted killing, and then injecting them back into the patient to kill cancer cells in the blood or tissue (52). Compared with traditional methods, ACT has the characteristics of high specificity, short onset and less interference from internal factors. Four classes of ACT that have made progress include chimeric antigen receptor T-cell (CAR-T), genetically engineered T-cell receptor (TCR), tumor-infiltrating lymphocytes (TILs) and natural killer (NK) cells. ACT has shown remarkable results in hematologic tumors, such as B-cell leukemia (53) and multiple myeloma (54).
3.1 Tumor-infiltrating lymphocytes and natural killer cells
TILs are lymphocytes that leave the blood and enter the tumor, and have a wide range of antigen recognition roles in tumor cells. Among them, T cell populations such as CD8 and CD4 play a key role in tumor control through mechanisms such as production of proinflammatory cytokines and promotion of plasma cell production (55, 56).
Ding et al. (57) conducted a meta-analysis of 7905 patients from 46 studies to evaluate the prognostic impact of TILs in HCC patients. The results showed that for TILs subsets, the density of CD8+, FOXP3+, CD3+ and granzyme B+ lymphocytes was significantly associated with improved survival, and the density of FOXP3+ TILs in the intratumoral (IT) was the most important prognostic marker. Higher CD8+ TIL and granzyme B+ T lymphocyte infiltration rate in the IT of patients are associated with better OS, and high CD3+ density predicts worse OS. This indicates that some TIL subsets can be used as prognostic biomarkers for HCC. Huang et al. (58) evaluated the expression of FoxP3 regulatory T cells (Tregs), CD4, CD8, and CD34 in tumor and surrounding tissues of 54 HCC patients by immunohistochemistry. It was found that the density of Tregs within the tumor was significantly elevated, whereas the density of CD8+T cells was lower. Tumor-infiltrating Tregs may promote HCC progression by promoting angiogenesis and decreasing CD8 + T cells, and are considered to be poor prognostic indicators for HCC. Whether ILs can exert normal anti-tumor activity depends on the expression level of inhibitory receptors on their surface. Pfister et al. (59) reported the progressive accumulation of depleted CD8+ PD-1 T cells in NASH-affected livers, and elimination of enriched CD8+ PD-1 T cells reduced liver injury and HCC incidence. Overall, TIL has been shown to exhibit complex anti-tumor and pro-tumor properties in HCC. As a therapeutic strategy, TILs expand immune cells from the immunosuppressive tumor microenvironment and infuse them back into the patient. However, clinical studies on the therapeutic effect of TILs in HCC are still limited.
NK cells are unique cytotoxic lymphocytes that play a crucial role in fighting tumors and infections. The importance of NK cells and their activated receptor-ligand axis in the immune surveillance of HCC has been extensively studied. Major histocompatibility complex class I chain-related protein A (MICA) is the human ligand for the NKG2D receptor on NK cells, and binding of MICA triggers NK cells and enhances antigen-specific tumor immunity (60). Reduced MICA expression in HCC tissues is associated with lower PFS and OS in patients 53 (61).
A in vitro study conducted by Kim et al. (62) showed that HI CD56bright NK cells could produce significant killing effects on the human HCC cell line SNU398, a result that supports the next step in the investigation of the immunotherapy potential of NK cells. The clinical efficacy of immunotherapy with irreversible electroporation (IRE) in combination with allogeneic NK cells for stage IV HCC was evaluated in 40 patients (63). The results showed a synergistic effect of IRE and NK cell therapy, which not only enhanced the immune function, but also reduced the expression of AFP, showing good clinical efficacy. Weng et al. (64) randomized 85 patients with HCC after TACE and radiofrequency ablation to immunotherapy or no adjuvant therapy. Autologous cytokine-induced killer (CIK) cells were infused through the hepatic artery. CD3+, CD4+, CD56+ cells and CD4+/CD8+ ratio were increased in the CIK treatment group, while the recurrence rates at 1 year and 18 months were significantly decreased, indicating the important role of CIK in the treatment of HCC.
3.2 Chimeric antigen receptor T-cell and genetically engineered T-cell receptor
The major advantage of CAR-T cells, which are genetically modified lymphocytes, is the ability to recognize extracellular antigens that are presented independently of HLA without the need for antigen presentation to the surface by MHC, making more cancer cells vulnerable to attack (65). The application of CAR-T in HCC has also received extensive attention in recent years.
Secretion of IL-7 and CCL19 (7×19) improves the infiltration and survival of mouse CAR-T cells in vivo (66). Pang et al. (67) co-transduced T cells with CAR vector and 7×19 lentivirus to further verify the anti-HCC efficacy of 7× 19-secreting CAR-T cells. It was found that the liver tumor lesions significantly shrank on day 10 after receiving CAR-T cell injection and completely disappeared after one month. However, the number of patients enrolled in this study is small, and larger studies are still needed to prove the safety and efficacy of CAR-T cells. While HCC tumor organoids and CD39 HBV-CAR-T cells were co-cultured, Zou et al. (68) discovered that the anti-tumor effects of the CD39 HBV-CAR-T cells were enhanced when the PD-1, TIM-3, and LAG-3 checkpoints were down-regulated. Glypican-3 (GPC3) is a cell membrane cancer fetoprotein that is highly expressed in various solid tumors (69). A large body of evidence has shown that CAR-T cells targeting GPC3 can inhibit the growth of HCC cells. In a preclinical study, CAR-T cells were generated on the basis of humanized YP7 (hYP7) and HN3 antibodies (70). They found that CAR (hYP7) T cells suppressed GPC3-positive HCC in a mouse model of HCC, possibly by inducing perforin and granzyme mediated apoptosis or reducing Wnt signaling in tumor cells. Expanded and modified peripheral blood V1 T cells were employed by KAkkouk et al. (71) to produce GPC3-specific CAR and sIL-15. The generated CAR/sIL-15 V1 T cells demonstrated improved antitumor activity and successfully slowed the development of the HCC tumor. Patients with HCC may benefit from treatment with CAR-T cells that target GPC3.
The α and β chains of the TCR are key components that determine T-cell antigen specificity, and the TCR recognizes antigens through enzymatically cleaved peptides that are presented on the cell surface by MHC molecules (72). In TCR-T therapy, T cells are edited to express the TCRα and β chains, giving them tumor-targeting specificity (73).
Zhu et al. (74) identified AFP-specific TCR genes in mice and transduced human T cells with mouse TCR genes to bind HLA-A2/AFP158 tetamers. TCR-T cells specifically killed HLA-A2AFP HepG2 HCC tumor cells but had no obvious toxicity to normal primary hepatocytes in vitro. Meng et al. (75) studied the safety of HBV-TCR-T cell immunotherapy in eight patients with advanced HBV-HCC who did not qualify for liver transplantation. None of the patients showed acute adverse effects during or immediately after the injection, and they were subsequently well tolerated. The final median OS was 33.1 months, and the median TTP was 6.18 months. HBV-TCR T cells have the potential to treat HCC recurrence after liver transplantation, but their efficacy may be hindered by the immunosuppressive therapy required to prevent graft rejection. Hafezi et al. (76) engineered TCR-T cells molecularly to preserve their versatility in these patients while minimizing the risk associated with organ rejection. The results showed that patients with HBV-HCC after liver transplantation who received different immunosuppressive drugs showed different degrees of peripheral blood monocytes activation after HBV-TCR T cell infusion, and the disease progression was controlled.
4 Cancer vaccines
One of the key factors that needs to be addressed to achieve clinical benefit in HCC is to trigger an immune response, and cancer vaccines are an ideal immunotherapy strategy. As a key part of tumor vaccine design, tumor antigens can be traditionally divided into tumor-associated antigen (TAA) and tumor-specific antigen (TSA). There is yet little research on TSA. A preclinical experiment has demonstrated that lentinan-induced tumor-specific antigens have a significant impact on the anti-tumor immune response and immune system activation (77). Currently, the rationale for most therapeutic cancer vaccines is based on TAA to elicit an antitumor immune response to eliminate tumor cells that express these antigens (78).
A study by Mizukoshi et al. have identified some TAA in HCC and observed the activation of directed T cell responses, including Cyclophilin B, SART2, SART3, p53, MRP3, AFP and hTERT, among others (79). HepaVac-101 is a first single-arm human phase I/II multicenter cancer vaccine trial against HCC (80). 22 HCC patients were injected with the peptide antigen IMA970A and the TLR7/8/RIG I agonist CV8102, and the results showed that vaccination had a good safety profile and elicited TAA-specific immune responses in the general population of patients. Chen et al. (81) linked the XCL1 chemokine to GPC3, which is overexpressed in HCC, to construct the XCL3-GPC3 fusion molecule as a liver cancer vaccine. dendritic cells (DCS) targeted by the vaccine enhance the infiltration of antigen-specific CD8 T cells and NK cells and inhibit tumor formation and growth, with antitumor effects further enhanced by the administration of anti-PD-1. The effectiveness of cancer vaccines is limited by immunosuppressive TME, indicating the need for improvements. The effectiveness of a novel combinatorial approach based on metronomic chemotherapy and vaccination is examined in a mouse model (82). Comparing the combinatorial treatment to the vaccination alone results in a more specific T cell response, which correlates to a lower prevalence of Tregs. Such results are very encouraging and may open the door to useful advancements in immunotherapeutic approaches for HCC.
New tumor-selective vectors, key components that enhance antigen-specific immune response, can improve anticancer efficacy and circumvent systemic toxicity (83). Huang et al. (84) designed tumor-targeting lipid dendrimeric calcium phosphate (TT-LDCP) nanoparticles (NPs) using thymitine-functionalized dendrimeric polymers to efficiently deliver siRNA and pDNA into HCC cells, increase tumor invasion and CD8 T cell activation, and enhance the efficacy of cancer vaccines. And inhibit the progression of HCC. The Oxford 40 ligand (OX40L), a tumor necrosis factor receptor, is also a promising target for mRNA cancer vaccines (85). One study evaluated the anti-HCC effect and immune activation mechanism of a lipid nanoparticle-encapsulated OX40L mRNA cancer vaccine in vitro and in vivo. The results showed that the OX40L mRNA vaccine effectively induced T cell activation in vivo and inhibited tumor progression (86).
5 Cytokines
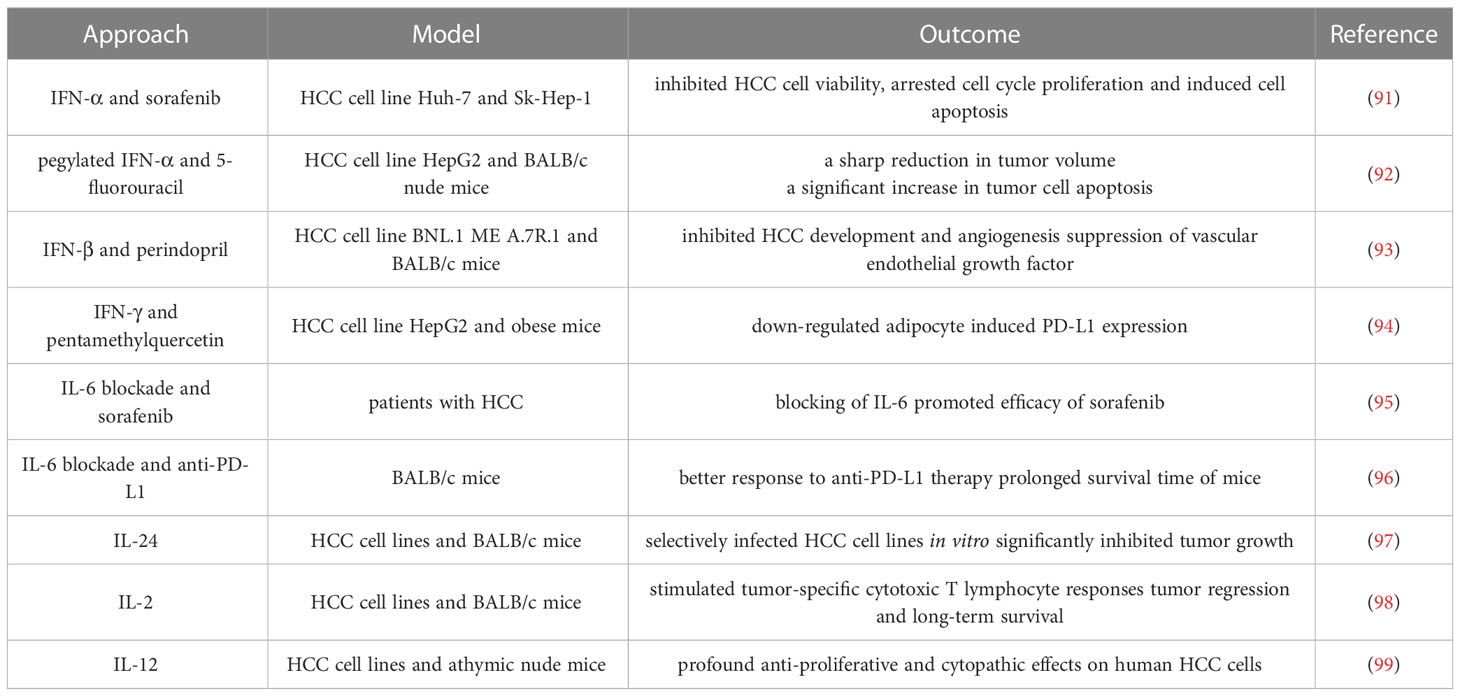
Cytokines are a class of small molecule proteins with a wide range of biological activities that are synthesized and secreted by immune cells and some non-immune cells in response to stimulation (87, 88). Cytokines mediate cell-to-cell communication and have diverse functions, including regulation of innate and adaptive immunity, cell growth, and repair of injured tissues (89). With the comprehensive development of tumor immunotherapy, cytokine therapy has entered a new era, with cytokines such as interferon (IFN) and interleukin (IL) becoming an important circuit in tumor immunotherapy (88, 90). Since HCC is characterized by a low degree of immune infiltration, the use of cytokines to expand the proliferation of immune cells and induce the recruitment of immune cells is a feasible method to improve anti-tumor immunity (Table 2).
5.1 Interferons
The discovery of IFN provided the greatest impetus for research in all cytokine studies (100). IFN can be divided into three classes: I, II and III. IFN-I is mainly expressed by innate immune cells (101), IFN-II is produced mainly by T cells and NK cells (102), whereas IFN-III expression varies according to tissue (103).
IFN-I, represented by IFN-α and IFN-β, not only affects tumor cell development through cytotoxic, cytosuppressive and antiangiogenic effects (104), but also enhances TAA expression by up-regulating MHC class I proteins (105). A frequent hallmark of noninvasive cancers is loss of IFN-I signaling. Thus, the application of IFN-I is being widely explored. Wang et al. (91) investigated the combined effects of IFN-α and sorafenib on HCC and found that the combination therapy synergistically inhibited HCC cell viability, arrested cell cycle proliferation and induced apoptosis of HCC cells by regulating the expression levels of cyclin A and cyclin B as well as pro-survival Bcl-2 family proteins. Hagiwara et al. (92) investigated the anti-HCC effect of combination therapy with pegylated interferon (PEG-IFN)-α and 5-fluorouracil (5-FU), and found that the combination group had a reduction in tumor volume and a significant increase in tumor cell apoptosis compared with the monotherapy group. This is related to the increased expression of p53 protein and mRNA induced by PEG-IFN. Noguchi et al. (93) elucidated the combined effects of the clinically used angiotensin I converting enzyme (ACE) inhibitor perindopril (PE) and IFN-β on HCC development and angiogenesis in mice. It was found that both PE and IFN-β significantly inhibited HCC occurrence and tumor neovascularization, but the effect of low-dose IFN was weaker than that of PE. Additional preclinical and clinical research is required to examine more potent combo therapies.
IFN-γ, the only member of IFN-II, also plays an important role in tumor immune regulation. Patients with HCC have been found to have fewer mucosa-associated invariant T (MAIT) cells in the peripheral blood and liver than healthy controls, and these cells produce a corresponding reduction in IFN-γ (106). Evidence suggests that interferon signaling plays a key role in regulating the efficacy and sensitivity of ICIs against a variety of tumor types. Wu et al. (107) used Kaplan-Meier survival analysis based on HCC database and found that among the nine interferon regulatory factors (IRFs) that regulate interferon signaling, decreased expression of IRF8 was associated with poor prognosis in HCC patients. However, IFN-γ and PD-1 signaling pathways were significantly inhibited in HCC patients with low IRF8, which indirectly revealed the relationship between IFN-γ and HCC prognosis. Li et al. (94) found that the natural flavonoid pentamethylquercetin (PMQ) could partially inhibit HCC progression in obese mice by down-regulating adipocyte induced PD-L1 expression through IFN-γ signaling. However, some studies have shown that IFN-γ shows a pro-tumor effect in HCC mice model. The complex role of IFN-γ in TME needs to be investigated urgently to exert its antitumor effect.
5.2 Interleukins
ILs are key elements for orchestrating the TME and controlling tumor-immune-cell crosstalk, enabling both a conducive environment for cancer growth and critical for an effective tumor-directed immune response (90). Therefore, these properties of ILs can be exploited to improve immunotherapy to increase effectiveness and limit side effects.
IL-6 is a multifunctional inflammatory cytokine with very low expression in normal human cells and increased concentrations in the serum of patients with hepatitis and HCC (108). The mechanism of action of IL-6 in HCC has been extensively studied. The IL-6/STAT3 signaling pathway is involved in various physiological processes, including cell growth, differentiation, and immune regulation. Aberrant activation of the IL-6/STAT3 signaling pathway has been detected in HCC (109), with an impact on the proliferation, invasion, metastasis, immune escape, and drug resistance of HCC cells (110). IL-6/STAT3 signaling upregulates HNRNPC expression in HCC cells, and knockdown of HNRNPC significantly inhibits IL-6/STAT3-enhanced HCC metastasis (111). Yang et al. (95) explored the effect of IL-6 on the sensitivity of HCC cells treated with sorafenib and its mechanism, and found that siIL-6 further promoted sorafenib to impede proliferation and induce apoptosis, suggesting that blocking IL-6 could be used as a potential therapeutic approach for sorafenib sensitivity of HCC cells. Liu et al. (96) observed that IL-6 impaired anti-tumor immunity by suppressing TNF- and IFN-expression on tumor-infiltrating CD6 cells after the co-treatment of HCC with IL-6 inhibition and anti-PD-L1 checkpoint inhibitors. Anti-PD-L1 medication had a stronger effect on tumors when paired with anti-IL-6, and mice survived noticeably longer as a result.
The possible treatment of other types of ILs for HCC has also been discussed. Deng et al. (97) constructed a recombinant oncolytic virus vaccinia based on a vaccinia virus carrying the IL-24 gene (VG9-IL-24). Evaluation showed that VG9-IL-24 effectively infected HCC cell lines but not normal hepatocytes in vitro. In vivo, tumor growth was significantly inhibited and VG9-IL-24-treated mice lived longer. Interleukin-2 (IL-2) is an anticancer cytokine that triggers human innate and adaptive immunity by stimulating T cell proliferation and lymphocyte infiltration into tumor sites. Sun et al. (98) investigated the ability of recombinant adenovirus injection expressing IL-2 (rAd-IL-2) to inhibit tumor cell growth in HCC in HCC tumor model. The results showed that rAd-IL-2 significantly stimulated tumor-specific cytotoxic T lymphocyte responses by inducing the recruitment of CD4+ and CD8+ T cells to tumors, leading to tumor regression and long-term survival of mice during the 120-day treatment period. The study by El-Shemi et al. (99) examined the therapeutic efficacy of two armed oncolytic adenoviruses encoding the human TRAIL gene (Ad-ΔB/TRAIL) and the IL-12 gene (Ad-ΔB/IL-12) in a preclinical model of human HCC. It was found that the combination therapy exhibited profound anti-proliferative and cytopathic effects on human HCC cells and exerted potent tumor killing effect in vivo. More clinical trials are still in urgent need to apply ILs to broader usage.
6 Conclusions
Immunotherapy is expected to achieve a new breakthrough in the radical treatment of tumors and become the mainstream method of tumor treatment because of its specificity and high efficiency, which can free the body from harmful treatment. However, due to the low degree of immune infiltration in HCC, the response to immunotherapy is limited. Although the efficacy of ICIs alone has been confirmed in dozens of clinical trials, multiple ICIs or ICIs combined with chemotherapy, anti-angiogenesis drugs, and radiotherapy are still better choices to improve ORR. In addition, immunotherapy such as ACT, tumor vaccines and cytokines are gradually being studied. However, most research has focused on the preclinical phase, and there is an urgent need to find additional therapies that are safe enough to proceed to clinical trials or to combine several classes of immunotherapies to develop personalized treatment regimens. With all the research activity in the HCC field advancing, we will undoubtedly continue to see exciting advances in immunotherapy for HCC.
Author contributions
JL, SX and PD had the idea and drafted the work. ZX, CG and ML performed the literature search. JW and LH and critically revised the work. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82272396) and Suzhou Medical and Health Science and Technology Innovation Project (No. SKY2022057).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xiang Z, Wu J, Li J, Zheng S, Wei X, Xu X. Gut microbiota modulation: a viable strategy to address medical needs in hepatocellular carcinoma and liver transplantation. Engineering (2023). doi: 10.1016/j.eng.2022.12.012
2. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
3. Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol (2018) 19(3):222–32. doi: 10.1038/s41590-018-0044-z
4. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
5. Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, et al. International trends in hepatocellular carcinoma incidence, 1978–2012. Int J Cancer (2020) 147(2):317–30. doi: 10.1002/ijc.32723
6. Wolfe W, Xiang Z, Yu X, Li P, Chen H, Yao M, et al. The challenge of applications of probiotics in gastrointestinal diseases. Advanced Gut Microbiome Res (2023) 2023. doi: 10.1155/2023/1984200
7. Villanueva A. Hepatocellular carcinoma. N Engl J Med (2019) 380(15):1450–62. doi: 10.1056/NEJMra1713263
8. Wu J, Guo N, Zhang X, Xiong C, Liu J, Xu Y, et al. Hev-lfs: a novel scoring model for patients with hepatitis e virus-related liver failure. J Viral Hepat (2019) 26(11):1334–43. doi: 10.1111/jvh.13174
9. Wu J, Huang F, Ling Z, Liu S, Liu J, Fan J, et al. Altered faecal microbiota on the expression of Th cells responses in the exacerbation of patients with hepatitis e infection. J Viral Hepat (2020) 27(11):1243–52. doi: 10.1111/jvh.13344
10. Xiang Z, Li J, Lu D, Wei X, Xu X. Advances in multi-omics research on viral hepatitis. Front Microbiol (2022) 3365. doi: 10.3389/fmicb.2022.987324
11. Hou JL, Zhao W, Lee C, Hann HW, Peng CY, Tanwandee T, et al. Outcomes of long-term treatment of chronic hbv infection with entecavir or other agents from a randomized trial in 24 countries. Clin Gastroenterol Hepatol (2020) 18(2):457–67.e21. doi: 10.1016/j.cgh.2019.07.010
12. Zhao T, Yang Z, Yu J, Lu J, Li L, Xu X, et al. Comparing long-term outcomes of entecavir and tenofovir disoproxil fumarate in liver transplant patients. Advanced Gut Microbiome Res (2022) 2022. doi: 10.1155/2022/4779960
13. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol (2004) 130(7):417–22. doi: 10.1007/s00432-004-0552-0
14. Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (Hcc): epidemiology, etiology and molecular classification. Adv Cancer Res (2021) 149:1–61. doi: 10.1016/bs.acr.2020.10.001
15. Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg (2014) 260(2):329–40. doi: 10.1097/sla.0000000000000236
16. Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. Easl clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol (2018) 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
17. Commission GOoNH. Standard for diagnosis and treatment of primary liver cancer (2022 edition). Zhonghua Gan Zang Bing Za Zhi (2022) 38(2):288–303. doi: 10.3969/j.issn.1001-5256.2022.02.009
18. Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol (2007) 25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609
19. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
20. Xiong C-q, Zhou H-c, Wu J, Guo N-Z. The protective effects and the involved mechanisms of tanshinone iia on sepsis-induced brain damage in mice. Inflammation (2019) 42:354–64. doi: 10.1007/s10753-018-0899-z
21. Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B, et al. Nivolumab (Nivo) + ipilimumab (Ipi) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (Ahcc): results from checkmate 040. J Clin Oncol (2019) 37(15_suppl):4012–. doi: 10.1200/JCO.2019.37.15_suppl.4012
22. Mei J, Li SH, Li QJ, Sun XQ, Lu LH, Lin WP, et al. Anti-Pd-1 immunotherapy improves the efficacy of hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma. J Hepatocell Carcinoma (2021) 8:167–76. doi: 10.2147/jhc.S298538
23. Yi X, Fu Y, Long Q, Zhao Y, Li S, Zhou C, et al. Myosteatosis can predict unfavorable outcomes in advanced hepatocellular carcinoma patients treated with hepatic artery infusion chemotherapy and anti-Pd-1 immunotherapy. Front Oncol (2022) 12:892192. doi: 10.3389/fonc.2022.892192
24. Qin S, Chen Z, Liu Y, Xiong J, Ren Z, Meng Z, et al. A phase ii study of anti–Pd-1 antibody camrelizumab plus Folfox4 or gemox systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J Clin Oncol (2019) 37(15_suppl):4074–. doi: 10.1200/JCO.2019.37.15_suppl.4074
25. Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology (2020) 71(4):1247–61. doi: 10.1002/hep.30889
26. Deng H, Kan A, Lyu N, Mu L, Han Y, Liu L, et al. Dual vascular endothelial growth factor receptor and fibroblast growth factor receptor inhibition elicits antitumor immunity and enhances programmed cell death-1 checkpoint blockade in hepatocellular carcinoma. Liver Cancer (2020) 9(3):338–57. doi: 10.1159/000505695
27. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/jco.20.00808
28. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (Rescue): a nonrandomized, open-label, phase ii trial. Clin Cancer Res (2021) 27(4):1003–11. doi: 10.1158/1078-0432.Ccr-20-2571
29. Mei J, Tang YH, Wei W, Shi M, Zheng L, Li SH, et al. Hepatic arterial infusion chemotherapy combined with pd-1 inhibitors plus lenvatinib versus pd-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol (2021) 11:618206. doi: 10.3389/fonc.2021.618206
30. Liu B-J, Gao S, Zhu X, Guo J-H, Kou F-X, Liu S-X, et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-Pd-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy (2021) 13(17):1395–405. doi: 10.2217/imt-2021-0192
31. Owonikoko TK, Park K, Govindan R, Ready N, Reck M, Peters S, et al. Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: checkmate 451. J Clin Oncol (2021) 39(12):1349–59. doi: 10.1200/JCO.20.02212
32. Su K, Guo L, He K, Rao M, Zhang J, Yang X, et al. Pd-L1 expression on circulating tumor cells can be a predictive biomarker to pd-1 inhibitors combined with radiotherapy and antiangiogenic therapy in advanced hepatocellular carcinoma. Front Oncol (2022) 12:873830. doi: 10.3389/fonc.2022.873830
33. Zhong L, Wu D, Peng W, Sheng H, Xiao Y, Zhang X, et al. Safety of pd-1/Pd-L1 inhibitors combined with palliative radiotherapy and anti-angiogenic therapy in advanced hepatocellular carcinoma. Front Oncol (2021) 11:686621. doi: 10.3389/fonc.2021.686621
34. Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of ctla-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis c. J Hepatol (2013) 59(1):81–8. doi: 10.1016/j.jhep.2013.02.022
35. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (Checkmate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2
36. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (Keynote-224): a non-randomised, open-label phase 2 trial. Lancet Oncol (2018) 19(7):940–52. doi: 10.1016/S1470-2045(18)30351-6
37. Liu F, Zeng G, Zhou S, He X, Sun N, Zhu X, et al. Blocking Tim-3 or/and pd-1 reverses dysfunction of tumor-infiltrating lymphocytes in hbv-related hepatocellular carcinoma. Bull du Cancer (2018) 105(5):493–501. doi: 10.1016/j.bulcan.2018.01.018
38. Andrews LP, Marciscano AE, Drake CG, Vignali DAA. Lag3 (Cd223) as a cancer immunotherapy target. Immunol Rev (2017) 276(1):80–96. doi: 10.1111/imr.12519
39. Guo M, Qi F, Rao Q, Sun J, Du X, Qi Z, et al. Serum lag-3 predicts outcome and treatment response in hepatocellular carcinoma patients with transarterial chemoembolization. Front Immunol (2021) 12:754961. doi: 10.3389/fimmu.2021.754961
40. Paz-Ares LG, Ramalingam SS, Ciuleanu TE, Lee JS, Urban L, Caro RB, et al. First-line nivolumab plus ipilimumab in advanced nsclc: 4-year outcomes from the randomized, open-label, phase 3 checkmate 227 part 1 trial. J Thorac Oncol (2022) 17(2):289–308. doi: 10.1016/j.jtho.2021.09.010
41. Meerveld-Eggink A, Rozeman EA, Lalezari F, van Thienen JV, Haanen J, Blank CU. Short-term ctla-4 blockade directly followed by pd-1 blockade in advanced melanoma patients: a single-center experience. Ann Oncol (2017) 28(4):862–7. doi: 10.1093/annonc/mdw692
42. Majidpoor J, Mortezaee K. The efficacy of pd-1/Pd-L1 blockade in cold cancers and future perspectives. Clin Immunol (2021) 226:108707. doi: 10.1016/j.clim.2021.108707
43. Sperandio RC, Pestana RC, Miyamura BV, Kaseb AO. Hepatocellular carcinoma immunotherapy. Annu Rev Med (2022) 73(1):267–78. doi: 10.1146/annurev-med-042220-021121
44. Hato T, Zhu AX, Duda DG. Rationally combining anti-vegf therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy (2016) 8(3):299–313. doi: 10.2217/imt.15.126
45. Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, et al. Jsh consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer (2014) 3(3-4):458–68. doi: 10.1159/000343875
46. Liu M, Shi J, Mou T, Wang Y, Wu Z, Shen A. Systematic review of hepatic arterial infusion chemotherapy versus sorafenib in patients with hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol Hepatol (2020) 35(8):1277–87. doi: 10.1111/jgh.15010
47. Tsai W-L, Lai K-H, Liang H-L, Hsu P-I, Chan H-H, Chen W-C, et al. Hepatic arterial infusion chemotherapy for patients with huge unresectable hepatocellular carcinoma. PloS One (2014) 9(5):e92784. doi: 10.1371/journal.pone.0092784
48. Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res (2019) 25(3):912–20. doi: 10.1158/1078-0432.Ccr-18-1254
49. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med (2008) 359(4):378–90. doi: 10.1056/NEJMoa0708857
50. Finn RS, Bentley G, Britten CD, Amado R, Busuttil RW. Targeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse model. Liver Int (2009) 29(2):284–90. doi: 10.1111/j.1478-3231.2008.01762.x
51. Liao Y-P, Wang C-C, Butterfield LH, Economou JS, Ribas A, Meng WS, et al. Ionizing radiation affects human mart-1 melanoma antigen processing and presentation by dendritic Cells1. J Immunol (2004) 173(4):2462–9. doi: 10.4049/jimmunol.173.4.2462
52. Kirtane K, Elmariah H, Chung CH, Abate-Daga D. Adoptive cellular therapy in solid tumor malignancies: review of the literature and challenges ahead. J Immunother Cancer (2021) 9(7). doi: 10.1136/jitc-2021-002723
53. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel car T-cell therapy in refractory Large b-cell lymphoma. N Engl J Med (2017) 377(26):2531–44. doi: 10.1056/NEJMoa1707447
54. Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z car T cell therapy in b cell acute lymphoblastic leukemia. Sci Transl Med (2014) 6(224):224ra25. doi: 10.1126/scitranslmed.3008226
55. Ruterbusch M, Pruner KB, Shehata L, Pepper M. In vivo Cd4(+) T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev Immunol (2020) 38:705–25. doi: 10.1146/annurev-immunol-103019-085803
56. Zander R, Schauder D, Xin G, Nguyen C, Wu X, Zajac A, et al. Cd4(+) T cell help is required for the formation of a cytolytic Cd8(+) T cell subset that protects against chronic infection and cancer. Immunity (2019) 51(6):1028–42.e4. doi: 10.1016/j.immuni.2019.10.009
57. Ding W, Xu X, Qian Y, Xue W, Wang Y, Du J, et al. Prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: a meta-analysis. Med (Baltimore) (2018) 97(50):e13301. doi: 10.1097/md.0000000000013301
58. Huang Y, Wang FM, Wang T, Wang YJ, Zhu ZY, Gao YT, et al. Tumor-infiltrating Foxp3+ tregs and Cd8+ T cells affect the prognosis of hepatocellular carcinoma patients. Digestion (2012) 86(4):329–37. doi: 10.1159/000342801
59. Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. Nash Limits anti-tumour surveillance in immunotherapy-treated hcc. Nature (2021) 592(7854):450–6. doi: 10.1038/s41586-021-03362-0
60. Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of nk cells and T cells by Nkg2d, a receptor for stress-inducible mica. Science (1999) 285(5428):727–9. doi: 10.1126/science.285.5428.727
61. Zhang J, Xu Z, Zhou X, Zhang H, Yang N, Wu Y, et al. Loss of expression of mhc class I-related chain a (Mica) is a frequent event and predicts poor survival in patients with hepatocellular carcinoma. Int J Clin Exp Pathol (2014) 7(6):3123–31.
62. Kim N, Park PJ, Jung MK, Song G-W, Jung D-H, Lee S-G, et al. Differential function of natural killer cells in the liver graft perfusate of Korean population. In: Transplantation proceedings. Elsevier.
63. Alnaggar M, Lin M, Mesmar A, Liang S, Qaid A, Xu K, et al. Allogenic natural killer cell immunotherapy combined with irreversible electroporation for stage iv hepatocellular carcinoma: survival outcome. Cell Physiol Biochem (2018) 48(5):1882–93. doi: 10.1159/000492509
64. Weng D-S, Zhou J, Zhou Q-M, Zhao M, Wang Q-J, Huang L-X, et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunotherapy (2008) 31(1):63–71. doi: 10.1097/CJI.0b013e31815a121b
65. Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of car T cells. Nat Rev Cancer (2021) 21(3):145–61. doi: 10.1038/s41568-020-00323-z
66. Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. Il-7 and Ccl19 expression in car-T cells improves immune cell infiltration and car-T cell survival in the tumor. Nat Biotechnol (2018) 36(4):346–51. doi: 10.1038/nbt.4086
67. Pang N, Shi J, Qin L, Chen A, Tang Y, Yang H, et al. Il-7 and Ccl19-secreting car-T cell therapy for tumors with positive glypican-3 or mesothelin. J Hematol Oncol (2021) 14(1):118. doi: 10.1186/s13045-021-01128-9
68. Zou F, Tan J, Liu T, Liu B, Tang Y, Zhang H, et al. The Cd39(+) hbv surface protein-targeted car-T and personalized tumor-reactive Cd8(+) T cells exhibit potent anti-hcc activity. Mol Ther (2021) 29(5):1794–807. doi: 10.1016/j.ymthe.2021.01.021
69. Shimizu Y, Suzuki T, Yoshikawa T, Endo I, Nakatsura T. Next-generation cancer immunotherapy targeting glypican-3. Front Oncol (2019) 9:248. doi: 10.3389/fonc.2019.00248
70. Popat S, Curioni-Fontecedro A, Dafni U, Shah R, O'Brien M, Pope A, et al. A multicentre randomised phase iii trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European thoracic oncology platform (Etop 9-15) promise-meso trial. Ann Oncol (2020) 31(12):1734–45. doi: 10.1016/j.annonc.2020.09.009
71. Makkouk A, Yang XC, Barca T, Lucas A, Turkoz M, Wong JTS, et al. Off-the-Shelf Vδ1 gamma delta T cells engineered with glypican-3 (Gpc-3)-Specific chimeric antigen receptor (Car) and soluble il-15 display robust antitumor efficacy against hepatocellular carcinoma. J Immunother Cancer (2021) 9(12). doi: 10.1136/jitc-2021-003441
72. DembiĆ Z, Haas W, Weiss S, McCubrey J, Kiefer H, von Boehmer H, et al. Transfer of specificity by murine α and β T-cell receptor genes. Nature (1986) 320(6059):232–8. doi: 10.1038/320232a0
73. Shafer P, Kelly LM, Hoyos V. Cancer therapy with tcr-engineered T cells: current strategies, challenges, and prospects. Front Immunol (2022) 13:835762. doi: 10.3389/fimmu.2022.835762
74. Zhu W, Peng Y, Wang L, Hong Y, Jiang X, Li Q, et al. Identification of α-Fetoprotein-Specific T-cell receptors for hepatocellular carcinoma immunotherapy. Hepatology (2018) 68(2):574–89. doi: 10.1002/hep.29844
75. Meng F, Zhao J, Tan AT, Hu W, Wang SY, Jin J, et al. Immunotherapy of hbv-related advanced hepatocellular carcinoma with short-term hbv-specific tcr expressed T cells: results of dose escalation, phase I trial. Hepatol Int (2021) 15(6):1402–12. doi: 10.1007/s12072-021-10250-2
76. Hafezi M, Lin M, Chia A, Chua A, Ho ZZ, Fam R, et al. Immunosuppressive drug-resistant armored T-cell receptor T cells for immune therapy of hcc in liver transplant patients. Hepatology (2021) 74(1):200–13. doi: 10.1002/hep.31662
77. Wang Y, Han X, Li YD, Zhao SY, Zhang DJ, Zhao ZH, et al. Effects of tumor-specific antigen induced by lentinan on murine H22 hepatocellular carcinoma immunoprophylaxis. Eur Rev Med Pharmacol Sci (2015) 19(23):4516–24.
78. Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol (2022) 15(1):28. doi: 10.1186/s13045-022-01247-x
79. Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, et al. Comparative analysis of various tumor-associated antigen-specific T-cell responses in patients with hepatocellular carcinoma. Hepatology (2011) 53(4):1206–16. doi: 10.1002/hep.24149
80. Löffler MW, Gori S, Izzo F, Mayer-Mokler A, Ascierto PA, Königsrainer A, et al. Phase I/Ii multicenter trial of a novel therapeutic cancer vaccine, hepavac-101, for hepatocellular carcinoma. Clin Cancer Res (2022) 28(12):2555–66. doi: 10.1158/1078-0432.Ccr-21-4424
81. Chen K, Wu Z, Zhao H, Wang Y, Ge Y, Wang D, et al. Xcl1/Glypican-3 fusion gene immunization generates potent antitumor cellular immunity and enhances anti-Pd-1 efficacy. Cancer Immunol Res (2020) 8(1):81–93. doi: 10.1158/2326-6066.Cir-19-0210
82. Tagliamonte M, Petrizzo A, Napolitano M, Luciano A, Arra C, Maiolino P, et al. Novel metronomic chemotherapy and cancer vaccine combinatorial strategy for hepatocellular carcinoma in a mouse model. Cancer Immunol Immunother (2015) 64(10):1305–14. doi: 10.1007/s00262-015-1698-0
83. Galluzzi L, Chan TA, Kroemer G, Wolchok JD, López-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Trans Med (2018) 10(459):eaat7807. doi: 10.1126/scitranslmed.aat7807
84. Huang K-W, Hsu F-F, Qiu JT, Chern G-J, Lee Y-A, Chang C-C, et al. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci Adv (2020) 6(3):eaax5032. doi: 10.1126/sciadv.aax5032
85. Webb GJ, Hirschfield GM, Lane PJL. Ox40, Ox40l and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol (2016) 50(3):312–32. doi: 10.1007/s12016-015-8498-3
86. Deng Z, Yang H, Tian Y, Liu Z, Sun F, Yang P. An Ox40l mrna vaccine inhibits the growth of hepatocellular carcinoma. Front Oncol (2022) 12:975408. doi: 10.3389/fonc.2022.975408
87. Rose-John S. Interleukin-6 family cytokines. Cold Spring Harbor Perspect Biol (2018) 10(2):a028415. doi: 10.1101/cshperspect.a028415
88. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer (2009) 9(5):361–71. doi: 10.1038/nrc2628
89. Zheng X, Wu Y, Bi J, Huang Y, Cheng Y, Li Y, et al. The use of supercytokines, immunocytokines, engager cytokines, and other synthetic cytokines in immunotherapy. Cell Mol Immunol (2022) 19(2):192–209. doi: 10.1038/s41423-021-00786-6
90. Briukhovetska D, Dörr J, Endres S, Libby P, Dinarello CA, Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer (2021) 21(8):481–99. doi: 10.1038/s41568-021-00363-z
91. Wang L, Jia D, Duan F, Sun Z, Liu X, Zhou L, et al. Combined anti-tumor effects of ifn-α and sorafenib on hepatocellular carcinoma in vitro and in vivo. Biochem Biophys Res Commun (2012) 422(4):687–92. doi: 10.1016/j.bbrc.2012.05.056
92. Hagiwara S, Kudo M, Nakatani T, Sakaguchi Y, Nagashima M, Fukuta N, et al. Combination therapy with peg-Ifn-α and 5-fu inhibits Hepg2 tumour cell growth in nude mice by apoptosis of P53. Br J Cancer (2007) 97(11):1532–7. doi: 10.1038/sj.bjc.6604058
93. Noguchi R, Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Yanase K, et al. Combination of interferon-beta and the angiotensin-converting enzyme inhibitor, perindopril, attenuates murine hepatocellular carcinoma development and angiogenesis. Clin Cancer Res (2003) 9(16 Pt 1):6038–45.
94. Li Z, Gao WQ, Wang P, Wang TQ, Xu WC, Zhu XY, et al. Pentamethylquercetin inhibits hepatocellular carcinoma progression and adipocytes-induced pd-L1 expression Via ifn-Γ signaling. Curr Cancer Drug Targets (2020) 20(11):868–74. doi: 10.2174/1568009620999200730184514
95. Yang J, Wang J, Luo J. Decreased il-6 induces sensitivity of hepatocellular carcinoma cells to sorafenib. Pathol - Res Pract (2019) 215(10):152565. doi: 10.1016/j.prp.2019.152565
96. Liu H, Shen J, Lu K. Il-6 and pd-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun (2017) 486(2):239–44. doi: 10.1016/j.bbrc.2017.02.128
97. Deng L, Yang X, Ding Y, Fan J, Peng Y, Xu D, et al. Oncolytic therapy with vaccinia virus carrying il-24 for hepatocellular carcinoma. Virol J (2022) 19(1):44. doi: 10.1186/s12985-022-01779-1
98. Sun Y, Wu H, Chen G, Huang X, Shan Y, Shi H, et al. Genetically engineered recombinant adenovirus expressing Interleukin−2 for hepatocellular carcinoma therapy. Mol Med Rep (2018) 17(1):300–6. doi: 10.3892/mmr.2017.7922
99. El-Shemi AG, Ashshi AM, Na Y, Li Y, Basalamah M, Al-Allaf FA, et al. Combined therapy with oncolytic adenoviruses encoding trail and il-12 genes markedly suppressed human hepatocellular carcinoma both in vitro and in an orthotopic transplanted mouse model. J Exp Clin Cancer Res (2016) 35:74. doi: 10.1186/s13046-016-0353-8
100. Negishi H, Taniguchi T, Yanai H. The interferon (Ifn) class of cytokines and the ifn regulatory factor (Irf) transcription factor family. Cold Spring Harb Perspect Biol (2018) 10(11). doi: 10.1101/cshperspect.a028423
101. Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res (2011) 17(9):2619–27. doi: 10.1158/1078-0432.Ccr-10-1114
102. Wheelock EF. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science (1965) 149(3681):310–1. doi: 10.1126/science.149.3681.310
103. Eslam M, George J. Targeting ifn-Λ: therapeutic implications. Expert Opin Ther Targets (2016) 20(12):1425–32. doi: 10.1080/14728222.2016.1241242
104. Zhou L, Zhang Y, Wang Y, Zhang M, Sun W, Dai T, et al. A dual role of type I interferons in antitumor immunity. Advanced Biosyst (2020) 4(11):1900237. doi: 10.1002/adbi.201900237
105. Chen J, Cao Y, Markelc B, Kaeppler J, Vermeer JA, Muschel RJ. Type I ifn protects cancer cells from Cd8+ T cell-mediated cytotoxicity after radiation. J Clin Invest (2019) 129(10):4224–38. doi: 10.1172/jci127458
106. Huang W, Ye D, He W, He X, Shi X, Gao Y. Activated but impaired ifn-Γ production of mucosal-associated invariant T cells in patients with hepatocellular carcinoma. J Immunother Cancer (2021) 9(11). doi: 10.1136/jitc-2021-003685
107. Wu H, Li Y, Shi G, Du S, Wang X, Ye W, et al. Hepatic interferon regulatory factor 8 expression suppresses hepatocellular carcinoma progression and enhances the response to anti–programmed cell death protein-1 therapy. Hepatology (2022) 76(6):1602–16. doi: 10.1002/hep.32316
108. Rincon M, Irvin CG. Role of il-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci (2012) 8(9):1281–90. doi: 10.7150/ijbs.4874
109. Lee C, Cheung ST. Stat3: an emerging therapeutic target for hepatocellular carcinoma. Cancers (Basel) (2019) 11(11). doi: 10.3390/cancers11111646
110. Xu J, Lin H, Wu G, Zhu M, Li M. Il-6/Stat3 is a promising therapeutic target for hepatocellular carcinoma. Front Oncol (2021) 11:760971. doi: 10.3389/fonc.2021.760971
Keywords: hepatocellular carcinoma (HCC), immunotherapy, immune checkpoint inhibitors, adoptive cellular therapy, cancer vaccines, cytokines
Citation: Li J, Xuan S, Dong P, Xiang Z, Gao C, Li M, Huang L and Wu J (2023) Immunotherapy of hepatocellular carcinoma: recent progress and new strategy. Front. Immunol. 14:1192506. doi: 10.3389/fimmu.2023.1192506
Received: 23 March 2023; Accepted: 28 April 2023;
Published: 10 May 2023.
Edited by:
Min Yao, Nantong University, ChinaReviewed by:
Yuzhu Dai, The 903rd Hosipital of the PLA, ChinaZhijie Xu, Central South University, China
Zhenjian Zhuo, Guangzhou Medical University, China
Copyright © 2023 Li, Xuan, Dong, Xiang, Gao, Li, Huang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wu, wujianglinxing@163.com; Lan Huang, 446009533@qq.com
†These authors have contributed equally to this work
 Jiarui Li
Jiarui Li