
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 26 June 2023
Sec. Primary Immunodeficiencies
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1187959
This article is part of the Research TopicWomen in Primary ImmunodeficienciesView all 8 articles
 Elena Sophia Fratini1,2†
Elena Sophia Fratini1,2† Maddalena Migliavacca1,3†
Maddalena Migliavacca1,3† Federica Barzaghi1,3
Federica Barzaghi1,3 Claudia Fossati3
Claudia Fossati3 Stefania Giannelli3
Stefania Giannelli3 Ilaria Monti3
Ilaria Monti3 Miriam Casiraghi3
Miriam Casiraghi3 Francesca Ferrua1,3
Francesca Ferrua1,3 Salvatore Recupero1,3
Salvatore Recupero1,3 Giulia Consiglieri1,3
Giulia Consiglieri1,3 Valeria Calbi1,3
Valeria Calbi1,3 Francesca Tucci1,3
Francesca Tucci1,3 Vera Gallo1,3
Vera Gallo1,3 Maria Ester Bernardo1,2,3
Maria Ester Bernardo1,2,3 Sabina Cenciarelli1,2
Sabina Cenciarelli1,2 Monica Palmoni1,2
Monica Palmoni1,2 Margherita Moni1,2
Margherita Moni1,2 Luca Galimberti1,2
Luca Galimberti1,2 Marzia Duse4
Marzia Duse4 Lucia Leonardi4
Lucia Leonardi4 Elena Sieni5
Elena Sieni5 Elena Soncini6
Elena Soncini6 Fulvio Porta6
Fulvio Porta6 Lucia Dora Notarangelo7
Lucia Dora Notarangelo7 Raffaella De Santis8
Raffaella De Santis8 Saverio Ladogana8
Saverio Ladogana8 Alessandro Aiuti1,2,3†
Alessandro Aiuti1,2,3† Maria Pia Cicalese1,2,3*†
Maria Pia Cicalese1,2,3*†Hemophagocytic inflammatory syndrome (HIS) is a rare form of secondary hemophagocytic lymphohistiocytosis caused by an impaired equilibrium between natural killer and cytotoxic T-cell activity, evolving in hypercytokinemia and multiorgan failure. In the context of inborn errors of immunity, HIS occurrence has been reported in severe combined immunodeficiency (SCID) patients, including two cases of adenosine deaminase deficient-SCID (ADA-SCID). Here we describe two additional pediatric cases of ADA-SCID patients who developed HIS. In the first case, HIS was triggered by infectious complications while the patient was on enzyme replacement therapy; the patient was treated with high-dose corticosteroids and intravenous immunoglobulins with HIS remission. However, the patient required HLA-identical sibling donor hematopoietic stem cell transplantation (HSCT) for a definitive cure of ADA-SCID, without HIS relapse up to 13 years after HSCT. The second patient presented HIS 2 years after hematopoietic stem cell gene therapy (GT), secondarily to Varicella-Zoster vaccination and despite CD4+ and CD8+ lymphocytes’ reconstitution in line with other ADA SCID patients treated with GT. The child responded to trilinear immunosuppressive therapy (corticosteroids, Cyclosporine A, Anakinra). We observed the persistence of gene-corrected cells up to 5 years post-GT, without HIS relapse. These new cases of children with HIS, together with those reported in the literature, support the hypothesis that a major dysregulation in the immune system can occur in ADA-SCID patients. Our cases show that early identification of the disease is imperative and that a variable degree of immunosuppression could be an effective treatment while allogeneic HSCT is required only in cases of refractoriness. A deeper knowledge of immunologic patterns contributing to HIS pathogenesis in ADA-SCID patients is desirable, to identify new targeted treatments and ensure patients’ long-term recovery.
Hemophagocytic lymphohistiocytosis (HLH) is a major dysregulation of the immune system. Consequent hypercytokinemia and auto-inflammatory syndrome can evolve, if untreated, into multiorgan failure and death. Diagnosis usually comes delayed, with an impact on patient survival and long-term complications, so early recognition of the disease is crucial. For this purpose, the Histiocyte Society established new diagnostic guidelines in 2004, where three additional criteria were introduced; low/absent NK cell activity, hyperferritinemia, and high-soluble interleukin-2-receptor levels (IL2r) (1). Current treatment regimens for HLH include immune suppressants, immunomodulators, and definitive cure by allogeneic hematopoietic stem cell transplantation (HSCT) in selected patients.
Familial HLH (FHL) usually affects infants that carry a spectrum of inherited defects in cytotoxic lymphocyte function, often triggered by underlying uncontrolled infections. HLH may also arise as a secondary condition resulting from persistent antigen stimulation due to autoimmune disease or malignancy (2). More widely, any defect in T cells or NK/phagocytic function or major constitutional immunological abnormality may contribute to HLH pathogenesis. In this view, studying HLH has contributed to shedding light on the immune system functioning and nowadays gives new leads to advances in the understanding of the pattern of perpetuation of the inflammation, uncovering possible targets in the pathway for autoinflammatory diseases.
A secondary form of HLH, Hemophagocytic inflammatory syndrome (HIS), has been described in selected Inborn Errors of Immunity (IEI) as in defects of MAGT1, GATA2 WAS, and ADA2 genes, but also in patients with chronic granulomatous disease (CGD) and severe combined immunodeficiencies (SCID) (3, 4), as a deficit of ILRG2, RAG1, IL7RA, CD3E, and Adenosine Deaminase-SCID (ADA-SCID) (5, 6).
ADA‐SCID is a life‐threatening immunodeficiency, characterized by lymphopenia in B/T/NK subpopulations, failure to thrive, severe infections, and autoimmunity (7). Allo-HSCT and Strimvelis an ex-vivo retroviral hematopoietic stem cell gene therapy (HSC-GT) approved in the EU, are standard-of-care treatments for ADA-SCID (8, 9).
Patients with HIS in the context of IEIs present major diagnostic and therapeutic implications, related to the branch of the immune system involved in the HLH genesis and to the treatment choice, spanning from immunosuppressive agents, with related increased infection risk, to allo-HSCT in refractory cases.
In our study, we described two HIS cases emerging in ADA-SCID patients. Moreover, we provide a report of two cases described in the literature.
We evaluated retrospectively HLH-2004 criteria in two ADA-SCID children followed in San Raffaele Hospital between 2005 and 2020. Both patients had a previous history of enzyme replacement therapy (ERT) with Polyethylene glycol-modified adenosine deaminase (PEG-ADA) before HIS onset and survived without recurrence. Therapeutic strategies were discussed with the families and treatments did not lead to adverse or unanticipated events. Family adherence to physicians’ indications was complete. No persistent organ damage or cognitive/mental health issues related to the hospitalization or to the medications were documented.
Case 1. A 2-year-old Italian child affected by ADA-SCID was referred to our center to undergo experimental HSC-GT (10). The patient was on oral steroids for chronic autoimmune hemolytic anemia (AHA). A central venous line catheter was inserted under sedation and the procedure was complicated by right pneumothorax, promptly drained. Five days after surgery the child presented with fever and a diffuse maculopapular rash on the trunk, eyelids, hands, and feet. Contextually, high inflammatory indexes were observed (CRP>500 mg/L, nv <0.5), with mild hepatomegaly confirmed by ultrasound. The fever continued unremittingly in the following days. The blood cultures were positive for Staphylococcus epidermidis and hominis, therefore intravenous antibiotic therapy (vancomycin, rifampin, and imipenem), and empirical intravenous immunoglobulins (IVIg) and antifungal treatment were started. Heart US showed possible vegetation on the central venous line tip in the right atrium, therefore, suspected infectious endocarditis, Port-a-cath was removed. After surgery, left pleural hematic effusion was evident, with consumptive coagulopathy (INR 2.1, nv 0.8-1-2, D dimer 13 µg/mL nv 0.27-0.77, mild thrombocytopenia). Three weeks after surgery, the patient’s clinical conditions worsened, with spiking high fever and dyspnea. A total body CT scan was performed documenting recrudescence of left pleural effusion with subsequent drainage of bloody fluid. The child, upon family request, was transferred to the referring hospital. Despite an improvement in general condition, the patient continued to have fever, diffuse skin rash, and hepatomegaly. Laboratory exams (Table 1) further showed increased neutrophil counts, lymphopenia, hypertriglyceridemia and hyperfibrinogemia, D Dimer 114.9 (nv <0.5), and CRP 160 mg/l (nv <0.5). HIS was empirically diagnosed despite HLH criteria not being fully evaluated due to the urgent need for patient’s management, therefore steroid therapy with Methylprednisolone 2 mg/kg/day was started, further increased up to 5 mg/kg/day with prompt remission of symptoms, despite the persistence of systemic signs of inflammation. The prominent hypercoagulative state was complicated by endocaval thrombi along the course of the femoral central venous line, requiring antithrombotic therapy with good response.
ERT was stopped for two months and IVIg was administered once a week for one month. After two months of high-dose corticosteroid therapy, progressive tapering was started, with suspension 5 months after HIS onset, contextual complete resolution of the clinical signs, and laboratory tests normalization. After complete resolution of the disease (9 months after the onset), the patient was treated with HSC-GT in our center receiving 10.7 * 106 CD34+/kg with a vector copy number (VCN) equal to 0.12 cp/cell (11). Unfortunately, graft failure of gene-corrected cells occurred (10), thus the child reintroduced ERT and subsequently received an HLA identical sibling HSCT (unavailable at the time of GT) without conditioning at the referring Centre. HSCT resulted in successful engraftment without recurrence of HIS, and 13 years after HSCT.
Case 2. A 4-year-old male child affected by ADA-SCID was started on PEG-ADA at 3 months of life and treated in 2017 with Strimvelis (12) at 1 year of age. Medicinal Product dose and VCN were in line with the subjects treated in the clinical development program (13).
Clinical history following infusion of Strimvelis was unremarkable, without autoinflammatory/autoimmune issues, until 2 years of follow-up. The patient’s lymphocyte counts and subpopulations were progressively improving, although mild lymphopenia for their age was still present (Figure 1).
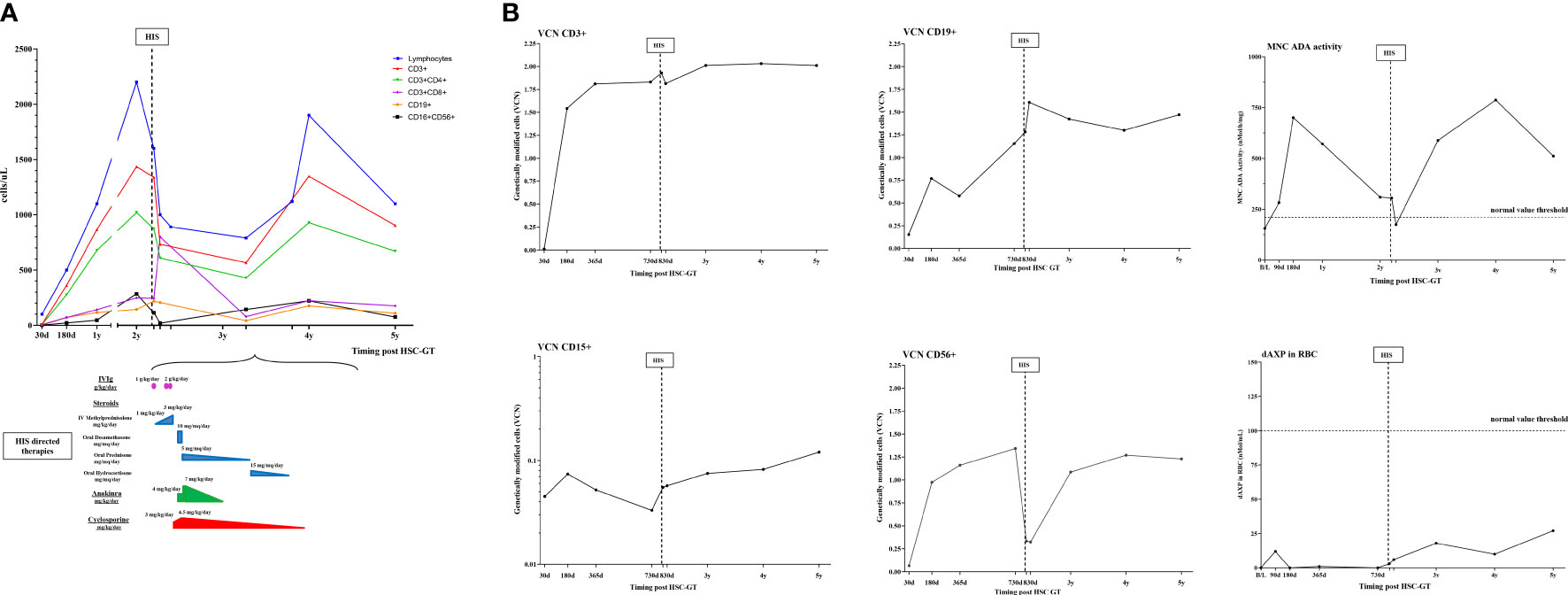
Figure 1 Schematic representation of immunological, molecular, and biochemical parameters and HIS-directed therapy in case 2. (A) Kinetics of lymphocytes subpopulations (CD3+,CD3+CD4+,CD3+CD8+,CD19+,CD16+CD156+) post HSC-GT over time (upper panel) and HIS-directed therapies (numbers indicate starting dose and maximum dose used) (lower panel); (B) Vector copy number in lymphocytes subpopulations (CD3+,CD19+,CD56+) and granulocytes (CD15+) and ADA-SCID biochemical analysis (ADA activity on mononuclear cells and purine metabolites in red blood cells). MNC, mononucleated cells; dAXP, Deoxyadenosine nucleotide percentage; RBC, red blood cells; d, days; B/L, baseline.
Molecular exams showed transduced cells in all hematopoietic lineages, good enzyme activity, and satisfactory detoxification. CD3+CD4+ and CD16+CD56+ were within normal limits, with a good naive/memory ratio. CD3+CD8+ and B cells were lower than normal for age in particular CD3+ count was more than 0.5 × 109/l, and CD3+CD8+ count was more than 0.2 × 109/l with adequate proliferative response to mitogens. (Figure 1). Ig supplementation was stopped 8 months post-GT and the child received inactivated vaccines (hexavalent, pneumococcus, and meningococcus ACWY and B) resulting in a robust antibody response.
As part of the local standard vaccination schedule, anti-Varicella zoster (VZV) immunization was performed 2 years after GT. One week after vaccination the child presented a low-grade intermittent fever lasting two weeks. Because of this fever, left ankle monoarticular arthritis, and a diffuse salmon maculopapular rash, the child was admitted to a local hospital.
Blood exams revealed hyperferritinemia (Table 1), therefore Methylprednisolone 1 mg/kg/day was started, along with intravenous broad-spectrum antibiotics and antiviral therapy.
Prompt amelioration of the child’s conditions was observed, however, after a week the fever pattern worsened, with a persistent rise in inflammatory indexes. Increased doses of Methylprednisolone at 2mg/kg/day and IVIg at 1g/kg were administered. Bone marrow biopsy did not show signs of hemophagocytosis.
The child was transferred for additional immunologic investigations to our unit and he was started on oral dexamethasone 10 mg/m2/d. At admission, laboratory exams, reported in Table 1, showed: elevated inflammatory indexes with CRP 25 mg/L (nv <0.5), ESR 57 mm/1h (nv 1-15), mild anemia, and thrombocytopenia. On immunophenotype analysis reduction in lymphocyte counts, in particular of CD56+ was observed, with a decreased VCN in CD56+ subpopulation (Figure 1). VZV DNA on plasma was negative (Table 1). Coagulopathy was observed with APTT < 23 sec, and factor VIII (300%, nv 65-140) without evidence of thrombosis. In the following months, after the progressive tapering of corticosteroid therapy, relapse of fever and a diffuse maculopapular rash was observed. Heart US showed normal coronary arteries echostructure. Diagnosis of HIS triggered by Varicella zoster vaccination was formulated, fulfilling 5 out of 8 HLH-2004 criteria. Immunosuppressive therapy was optimized by increasing the dose of corticosteroids, starting Cyclosporin A (CyA) and an anti-IL1 receptor antagonist (Anakinra) (Figure 1).
Improvement of clinical conditions and normalization of inflammatory and liver function indexes were observed in the following months. Anakinra and CyA were stopped after 12 and 18 months from HIS onset, respectively. At the latest follow-up, following discontinuation of immunosuppression, lymphocytes were below normal for age, within the lower range of ADA-SCID patients treated with HSC-GT, with normal response to mitogens. Furthermore, persistence of gene-corrected cells and recovery of transduced CD56+ cells was observed.
Details of case 3 and case 4, identified in the literature, are provided in Tables 1 and 2.
In ADA-SCID, HIS occurred as a complication of various immunologic triggers such as vaccination, infections, and post-HSCT, as in case 3 (6), but also as a first finding of IEI with a still unknown diagnosis, as in case 4 (5). Misdiagnosis with a septic status complicated the children’s treatments. In fact, the diagnosis was delayed in all cases because immunologic dysfunction was suspected only when the patients’ clinical conditions deteriorated.
The characteristics of our two patients were in line with the other two cases reported in the literature (Cases 3 and 4) (Table 2). In all cases, increased liver function enzymes, LDH, ferritinemia, and trygliceridemia with a procoagulant state were observed. IL2r, when tested, was dramatically elevated in all the patients (Table 1). Unremittent fever, skin rash, and hepatomegaly were common to all children, while additional symptoms were arthritis, disseminated intravascular coagulation, and acute kidney failure. Cytopenia (Bi/Trilinear) was a constant finding, whether associated with clear hemophagocytosis signs or not, as observed in case 3. Pancytopenia was present in case 3, while mild anemia and thrombocytopenia were present in cases 1 and 4.
Some of the most updated tests specific to HLH were performed in cases 2 and 3 (i.e. perforin analysis, while NK activity was not feasible due to lymphopenia in case 2). The patient in case 1 did not undergo testing because of the lack of published HLH guidelines at that time (1). Genetic analysis for familiar HLH was performed and found negative in case 4.
Autoimmunity is known to be associated with ADA deficiency (14) and case 1 displayed overt autoimmune manifestations before the onset of HIS (AHA), while case 2 presented a positive family history (father with intestinal bowel disease diagnosis).
In our cases, different therapeutic approaches were successful in managing HIS, and an escalation strategy to more aggressive approaches was chosen, to guarantee safety. Eventually, haploidentical HSCT was performed as a rescue strategy in case 3 (6).
In case 1 high-dose corticosteroids, IVIg, and a short washout from ERT were attempted and were sufficient to silence HIS. Low-dose Busulfan conditioning performed prior to HSC-GT could have had a role in maintaining remission. However, due to GT failure, the patient received a subsequent HLA-identical HSCT without conditioning after 2 years from HSC-GT, with successful outcome and with no HIS recurrence.
Post HSC-GT, rescue HSCT was performed in case 3 due to a refractory HIS. Haplo-HSCT was primed with emapalumab and conditioning with etoposide, cyclophosphamide, anti-thyroglobulin, and CyA, due to multiple episodes of graft rejection (7). The patient is currently 4 years post-HSCT in good clinical conditions, without HIS relapse or cGvHD.
Interestingly, the cause of failure of HSC-GT engraftment in cases 1 and 3 could have been multifactorial; ongoing infections as well as the immunodysregulated background of both patients could have negatively influenced the quality of the HSC and BM microenvironment, possibly contributing to HIS pathogenesis.
Our data in case 2 provide also useful insights into the kinetics of gene-corrected cells during the occurrence of HIS. Despite good immunological reconstitution, HIS was accompanied by a reduction in NK absolute count in the context of generalized lymphopenia, with a relative increase of untransduced NK gene-corrected cells (lower VCN) (Figure 1). We speculate that mostly residual uncorrected NK cells could have contributed to sustaining HIS priming and maintenance, in the presence of a powerful immunologic trigger such as the VZV immunization. In this case, HIS was controlled by immunosuppressive drugs (i.e. corticosteroids, Anakinra, CyA), suggesting that despite the initial immune dysregulation, gene-corrected cells were sufficient to restore normal immune functions.
The occurrence of HIS manifestations in ADA-SCID confirms that these patients carry a significant burden of immune dysregulation. Autoimmunity/autoinflammation manifested in literature both in patients on ERT and in those who have undergone BMT or GT, having achieved variable degrees of immune reconstitution (14).
It remains unclear which mechanisms engage in HIS development, but it is highly reasonable that both T lymphocytes and NK cells could play a role, failing to control excessive inflammatory responses and leading to a cytokine storm.
In conclusion, our series indicates that the occurrence of unexplained fever with marked hyperferritinemia, sudden cytopenias, or coagulopathy in a patient with ADA-SCID should be urgently investigated for HIS. HIS may be more common in ADA-SCID and IEIs than recognized, as clinical and laboratory features in these patients may camouflage behind the occurrence of severe infectious complications associated with the underlying genetic condition.
We report two rare cases of HIS associated with ADA-SCID and review the existing literature. Both HIS diagnosis and treatment resulted challenging in ADA-SCID patients. Characterization of the multifactorial innate and adaptive cellular immunity dysregulation processes will be important to better understand the pathogenetic mechanism of HIS in ADA-SCID and other IEIs. HIS treatment remains challenging, spanning from immunosuppressive/immunomodulatory drugs to a complete immune-hematological system replacement by allo-HSCT, which becomes imperative in case of poor response or refractoriness. Ultimately, once again congenital immunodeficiencies show to have an unbreakable bond with HIS.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
ESF and MMi provided clinical care for the patient, participated to data collection and analysis and wrote the manuscript. BF,MC, FF, RS, GC, VC, FT, VG, MB, SC, LL, MD provided clinical care for the patient. ESo, LN, FP, DR, SL provided clinical care for the patient and participated to data collection. MP, MMo, LG contributed to manuscript drafting and contributed to data collection. FC contributed to data collection. SG, IM and ESi performed molecular and immunological studies on patient’s samples. AA is the ADASCID gene therapy clinical trial principal investigator, contributed to data interpretation and manuscript writing. MC is the ADASCID gene therapy clinical trial investigator, coordinated data collection, analysis and interpretation, provided clinical care for the patient, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
The work was partially supported by Fondazione Telethon and grants from the European Commission (ERARE-3-JTC 2015 EUROCID, AA), Ministero della Salute, Ricerca Finalizzata NET-2011-02350069 (to AA, CC). AA is the recipient of Else Kröner-Fresenius-Stiftung (EKFS) prize.
We acknowledge the support of Dr. F Carlucci for metabolic analyses of ADA-SCID patients treated in our center.
The San Raffaele Telethon Institute for Gene Therapy SR-TIGET is a joint venture between the Telethon Foundation and Ospedale San Raffaele OSR. Gene therapy for ADA-SCID was developed at SR-TIGET and licensed to GlaxoSmithKline GSK in 2010. Strimvelis Marketing Authorization in Europe occurred in 2016 under GSK holding and the product is currently licensed in Iceland, Norway, Liechtenstein, and UK. Orchard Therapeutics Netherlands B.V. is the Marketing Authorization Holder in the EU and Orchard Therapeutics Europe Limited is the Marketing Authorization Holder in the UK. AA was the PI of pilot and pivotal SR-TIGET clinical trial of gene therapy for ADA SCID. MC the PI of the Strimvelis Registry.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer (2007) 48(2):124–31. doi: 10.1002/pbc.21039
2. Allen CE, McClain KL. Pathophysiology and epidemiology of hemophagocytic lymphohistiocytosis. Hematol Am Soc Hematol Educ Program (2015) 2015:177–82. doi: 10.1182/asheducation-2015.1.177
3. Bousfiha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol (2020) 40(1):66–81. doi: 10.1007/s10875-020-00758-x
4. Bode SF, Ammann S, Al-Herz W, Bataneant M, Dvorak CC, Gehring S, et al. Inborn errors working party of the EBMT. the syndrome of hemophagocytic lymphohistiocytosis in primary immunodeficiencies: implications for differential diagnosis and pathogenesis. Haematologica (2015) 100(7):978–88. doi: 10.3324/haematol.2014.121608
5. Chidambaram AC, Maulik K, Ramamoorthy JG, Parameswaran N. A novel mutation of adenosine deaminase causing SCID presenting as hemophagocytic lymphohistiocytosis with acute kidney injury. Br J Haematol (2020) 191(3):509–12. doi: 10.1111/bjh.17058
6. Tucci F, Gallo V, Barzaghi F, Ferrua F, Migliavacca M, Calbi V, et al. Emapalumab treatment in an ADA-SCID patient with refractory hemophagocytic lymphohistiocytosis-related graft failure and disseminated bacillus calmette-guérin infection. Haematologica (2021) 106(2):641–6. doi: 10.3324/haematol.2020.255620
7. Gaspar HB, Aiuti A, Porta F, Candotti F, Hershfield MS, Notarangelo LD. How I treat ADA deficiency. Blood (2009) 114(17):3524–32. doi: 10.1182/blood-2009-06-189209
8. Lankester AC, Albert MH, Booth C, Gennery AR, Güngör T, Hönig M, et al. Inborn errors working party of the European society for blood and marrow transplantation and the European society for immune deficiencies, and European reference network on rare primary immunodeficiency autoinflammatory autoimmune diseases (RITA). EBMT/ESID inborn errors working party guidelines for hematopoietic stem cell transplantation for inborn errors of immunity. Bone Marrow Transplant (2021) 56(9):2052–62doi: 10.1038/s41409-021-01378-8
9. Grunebaum E, Booth C, Cuvelier GDE, Loves R, Aiuti A, Kohn DB. Updated management guidelines for adenosine deaminase deficiency. J Allergy Clin Immunol Pract (2023) 11(6):1665–1675. doi: 10.1016/j.jaip.2023.01.032
10. Cicalese MP, Ferrua F, Castagnaro L, Pajno R, Barzaghi F, Giannelli S, et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood (2016) 128(1):45–54. doi: 10.1182/blood-2016-01-688226
11. Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med (2009) 360(5):447–58. doi: 10.1056/NEJMoa0805817
12. Cicalese MP, et al. poster number PD41. (2022). Availavle at: https://esidmeeting.org/e-posters/
13. Cicalese MP, Ferrua F, Castagnaro L, Rolfe K, De Boever E, Reinhardt RR, et al. Gene therapy for adenosine deaminase deficiency: a comprehensive evaluation of short- and medium-term safety. Mol Ther (2018). 26(3):917–931. doi: 10.1016/j.ymthe.2017.12.022.
Keywords: hemophagocytic inflammatory syndrome, hemophagocytic lymphohistiocytosis (HLH), SCID, ADA-SCID, primary immunodeficiency
Citation: Fratini ES, Migliavacca M, Barzaghi F, Fossati C, Giannelli S, Monti I, Casiraghi M, Ferrua F, Recupero S, Consiglieri G, Calbi V, Tucci F, Gallo V, Bernardo ME, Cenciarelli S, Palmoni M, Moni M, Galimberti L, Duse M, Leonardi L, Sieni E, Soncini E, Porta F, Notarangelo LD, De Santis R, Ladogana S, Aiuti A and Cicalese MP (2023) Hemophagocytic inflammatory syndrome in ADA-SCID: report of two cases and literature review. Front. Immunol. 14:1187959. doi: 10.3389/fimmu.2023.1187959
Received: 16 March 2023; Accepted: 05 June 2023;
Published: 26 June 2023.
Edited by:
Sara Sebnem Kilic, Bursa Uludağ University, TürkiyeReviewed by:
Nufar Marcus, Schneider Children’s Medical Center, IsraelCopyright © 2023 Fratini, Migliavacca, Barzaghi, Fossati, Giannelli, Monti, Casiraghi, Ferrua, Recupero, Consiglieri, Calbi, Tucci, Gallo, Bernardo, Cenciarelli, Palmoni, Moni, Galimberti, Duse, Leonardi, Sieni, Soncini, Porta, Notarangelo, De Santis, Ladogana, Aiuti and Cicalese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Pia Cicalese, Y2ljYWxlc2UubWFyaWFwaWFAaHNyLml0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.