- 1Public Health and Scientific Research, Social and Scientific Systems, Inc., a DLH Holdings Corp. Company, Durham, NC, United States
- 2Department of Oral Biology, University of Florida, Gainesville, FL, United States
- 3Department of Laboratory Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 4Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, United States
- 5Biostatistics and Computational Biology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, United States
- 6Clinical Research Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, United States
Objective: Recent studies report high-titer anti-dense fine speckled 70 (DFS70) autoantibodies in persons with inflammatory conditions, but the clinical significance remains unclear. Our goals were to estimate anti-DFS70 autoantibody prevalence, identify correlates, and assess time trends.
Methods: Serum antinuclear antibodies (ANA) were measured by indirect immunofluorescence assay on HEp-2 cells in 13,519 participants ≥12 years old from three time periods (1988–1991, 1999–2004, 2011–2012) of the National Health and Nutrition Examination Survey. ANA-positive participants with dense fine speckled staining were evaluated for anti-DFS70 antibodies by enzyme-linked immunosorbent assay. We used logistic models adjusted for survey-design variables to estimate period-specific anti-DFS70 antibody prevalence in the US, and we further adjusted for sex, age, and race/ethnicity to identify correlates and assess time trends.
Results: Women were more likely than men (odds ratio (OR)=2.97), black persons were less likely than white persons (OR=0.60), and active smokers were less likely than nonsmokers (OR=0.28) to have anti-DFS70 antibodies. The prevalence of anti-DFS70 antibodies increased from 1.6% in 1988-1991 to 2.5% in 1999-2004 to 4.0% in 2011-2012, which corresponds to 3.2 million, 5.8 million, and 10.4 million seropositive individuals, respectively. This increasing time trend in the US population (P<0.0001) was modified in some subgroups and was not explained by concurrent changes in tobacco smoke exposure. Some, but not all, anti-DFS70 antibody correlates and time trends resembled those reported for total ANA.
Conclusion: More research is needed to elucidate anti-DFS70 antibody triggers, their pathologic or potentially protective influences on disease, and their possible clinical implications.
Introduction
Autoimmune diseases are a diverse group of disorders characterized by damaging immune responses to self-antigens, and which, outside of some environmental and iatrogenic exposures, are of unknown etiology (1, 2). They impact 5% or more of the population, with increasing rates observed recently (3–8), but it remains unclear whether these positive time trends are due to changes in recognition and diagnosis, or if they represent true temporal increases in incidence, possibly related to environmental exposures (9).
Antinuclear antibodies (ANA) are a common biomarker of autoimmunity, observed in patients with various autoimmune diseases. However, ANA are also observed in the general population and are associated with sociodemographic factors such as older age and female sex (10, 11), genetic factors (12), and environmental exposures, including infections, medications, and organic and inorganic chemicals and toxins (4, 13, 14). Parallel to increases in autoimmune diseases, ANA prevalence has also increased (15).
There are many types and target specificities of ANA, and some are more clinically relevant than others (16, 17). The dense fine speckled (DFS) staining pattern observed by indirect immunofluorescence assay (IFA) on HEp-2 cells is often, but not always, indicative of autoantibodies to a specific 70 kDa protein known as DFS70 (18, 19). The first reports on anti-DFS70 antibodies suggested associations with atopic dermatitis and other conditions (20). More recent studies suggest their association with younger age groups (21–23) and indicate they are the second most common IFA staining pattern in apparently healthy individuals (16). The detection of high-titer monospecific anti-DFS70 antibodies can help rule out the diagnosis of systemic autoimmune rheumatic diseases (SARD), as they are rarely observed in persons with these conditions (16, 17, 24–27).
Our primarily exploratory and descriptive study focused on anti-DFS70 antibodies and used US National Health and Nutrition Examination Survey (NHANES) data from 1988 through 2012 to estimate prevalence, identify correlates, and assess time trends (15). In addition to evaluating anti-DFS70 antibody associations with standard sociodemographic factors such as sex, age, race/ethnicity, education, and economic status, we also evaluated several health-related factors, including body mass index, tobacco smoke exposure, alcohol consumption, C-reactive protein level, general health status, poor health preventing work, and allergens. As a secondary goal, we investigated whether time trends in factor-based subgroups differed from the overall time trend, which might suggest trend modification. Additionally, we investigated whether anti-DFS70 antibody correlates and time trends resembled those observed previously for the broader category of total ANA (10, 15), or alternatively might have either driven or attenuated them.
Subjects and methods
Study population
We measured ANA in 13,519 NHANES participants ≥12 years old, sampled from three time periods: 1988-1991 (4,727 persons), 1999-2004 (4,527 persons), and 2011-2012 (4,265 persons) as detailed previously (15). The NHANES sampled the civilian noninstitutionalized US population and provided sampling weights to adjust for nonresponse and the probability of selection into each ANA subsample (28), which allows for nationally representative estimates. The NHANES protocol was approved by the Human Subjects Institutional Review Board of the US Centers for Disease Control and Prevention (CDC). Written informed consent was obtained from all participants.
Antibody assessment
Surplus NHANES serum samples from the three cohorts were collected at the same point in time and ANA were assessed by IFA on HEp-2 cells with a NOVA View system (INOVA Diagnostics, San Diego, CA) and graded 0-4 as described earlier (10, 15). Nuclear, cytoplasmic, and mitotic patterns of ANA were identified according to international consensus criteria (17). Independent readings were initially made by two experienced evaluators, blinded with respect to sample characteristics and time period. The two raters agreed on >95% of the ANA intensities and patterns; differences were resolved by consensus or adjudicated by a third blinded rater (EKLC). Repeat testing of random samples showed >98% concordance (10). All ANA-positive participants with the DFS (AC-2) IFA staining pattern were evaluated by an enzyme-linked immunosorbent assay (ELISA) for anti-DFS70 antibodies (Medical & Biological Laboratories, 7808, Japan) and classified as positive when the average value in two duplicate wells was >15 unit/mL, per manufacturer instructions. All sera were analyzed using the same methods, same reagents, and same equipment by the same evaluators in the same laboratory. Twenty-three randomly selected anti-DFS70-positive sera and all sera with a value near 15 were repeated and validated using the same assay methodology.
Endpoints
Our statistical analysis focused on binary indicators of total ANA and anti-DFS70 antibody positivity/negativity. Participants with ANA grades 1-4 were treated as positive, while those with a grade of 0 were treated as negative (15). Among ANA-positive participants with DFS (AC-2) IFA staining, those with anti-DFS70 antibodies detected by ELISA were treated as positive, while all others were treated as negative, including those not tested with ELISA because they were ANA-negative. Our primary goal was to estimate prevalence, identify correlates, and assess time trends for anti-DFS70 antibodies, but for completeness we did the same for total ANA. We investigated the degree to which anti-DFS70 antibodies affected the total ANA results with a sensitivity analysis of “other” ANA, which excluded persons positive for anti-DFS70 antibodies and treated the remaining ANA-positives as positive for other ANA.
Explanatory variables
Sex, age, and race/ethnicity are known correlates of ANA and modifiers of ANA time trends (15), so we included these variables in our anti-DFS70 antibody analyses. Age was treated as a continuous variable when used as an adjustment covariate but categorized into three groups when used for stratification: adolescents (12-19 years), younger adults (20-49 years), or older adults (≥50 years). Race/ethnicity was categorized as white persons, black persons, Mexican Americans, or others. We also examined body mass index (BMI), poverty income ratio (PIR), current smoking exposure, alcohol consumption, education, and C-reactive protein, which were included and defined in our previous ANA studies (10). For exploratory purposes, we investigated health-related variables related to general health status, poor health preventing work, and allergens. Self-reported general health status was categorized as excellent, very good, good, or fair/poor. An indicator that poor health prevented work was based on whether participants reported that physical, mental, or emotional problems kept them from working. An allergen indicator was based on whether a positive skin test, administered by NHANES staff at enrollment, showed a participant was allergic to Alternaria alternata, Bermuda grass, cats, German cockroaches, dust mites, peanuts, short ragweed, Russian thistle, rye grass, or white oak. Supplemental Table S1 lists all participant characteristics studied.
Data availability
There were 13,519 NHANES participants in our study. Data on sex, age, and race/ethnicity were available for everyone. However, for some participants, data on the other variables were not collected, were purposely excluded, or were missing. For example, general health status was not collected by NHANES for any participants in Period 1; was only collected for adults in some survey cycles, so we excluded all adolescents (ages <20 years) for consistency in the analysis; and was missing for some participants in Periods 2 and 3. For each variable and every period (and all periods combined), Supplemental Table S2 lists the number and percentage of participants who were analyzed, were not surveyed, were excluded from the analysis, and were missing data; several NHANES restrictions based on age and period (or survey cycle) are described in the footnotes.
Statistical analysis
We estimated the prevalence (population proportion) of total ANA, anti-DFS70 antibodies, and other ANA, both overall and in factor-based subgroups. Prevalence estimates and 95% confidence intervals (CIs) were derived from logistic regression models for antibody positivity, adjusted for the survey design variables (sampling strata, clusters, and weights), the factor defining the subgroup, and in some cases time period. The number of antibody-positive persons in each period was estimated by multiplying our period-specific prevalence estimate by the period-specific CDC estimate of the civilian noninstitutionalized US population ≥12 years old (https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx#population-totals). We evaluated the association between antibody status and an individual factor category, relative to a reference category, with a prevalence odds ratio (OR) and a 95% CI from a logistic model for antibody positivity, adjusted for period, sex, age, and race/ethnicity. The association between antibody status and a factor in its entirety was assessed by an F-test from a statistical contrast. We investigated time trends overall and in subgroups (to identify trend modifiers) by fitting two logistic models for antibody positivity; both adjusted for sex, age, and race/ethnicity. One model added a categorical covariate for period, from which ORs and CIs were calculated to assess how antibody positivity differed in each period relative to the first. The other model added a quantitative covariate for the time between period midpoints (0, 12, or 22 years), and a time trend was assessed with a χ2-test to judge whether the period covariate’s coefficient was zero. We assessed whether the time trends in all subgroups defined by a given factor truly differed or simply reflected the overall trend (e.g., were male and female trends distinct, such that sex was a trend modifier) by adding a time-by-factor interaction to the model and testing whether its coefficient(s) equaled zero. Because smoking habits changed over these same time periods, we also explored whether time trends were attenuated after further adjustment for smoking exposure (i.e., could trends be explained by those behavioral changes).
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and all accounted for the survey design variables (sampling strata, clusters, and weights). The sampling weights allowed for population-representative estimates by adjusting for survey nonresponse and selection probabilities (28). The covariate-adjusted analyses incorporated a restricted cubic spline (29) in age, categorical variables for sex and race/ethnicity, and either a categorical or quantitative variable for period. We used the SurveyLogistic procedure to perform the logistic regression analyses, with domain statements to properly handle the sampling weights in subgroup analyses. Variance estimates, used to construct the 95% CIs, were obtained by the Taylor series method. All P-values were 2-sided and unadjusted for multiple comparisons.
Results
Participant characteristics
The sample distributions of all participant characteristics considered in our analyses are shown in Supplemental Table S1, for each time period separately and for all periods combined. The proportions of some characteristics were relatively consistent across periods, while others varied. Sample distributions can be useful, but these unadjusted proportions are not nationally representative estimates because they do not account for the survey-sampling weights.
Antibody positivity
Of the 13,519 participants, 1,857 (13.7%) were positive for total ANA and 312 (2.3%) were positive for anti-DFS70 antibodies. These counts and percentages are broken down into subgroups defined by categories of selected factors in Table 1 (and additional factors in Supplemental Table S3). Though not shown, 484 (3.6%) of the 13,519 participants had the DFS (AC-2) staining pattern, of which 312 (64.5%) were positive for anti-DFS70 antibodies. Note that these unadjusted antibody positivity proportions are not nationally representative prevalence estimates.
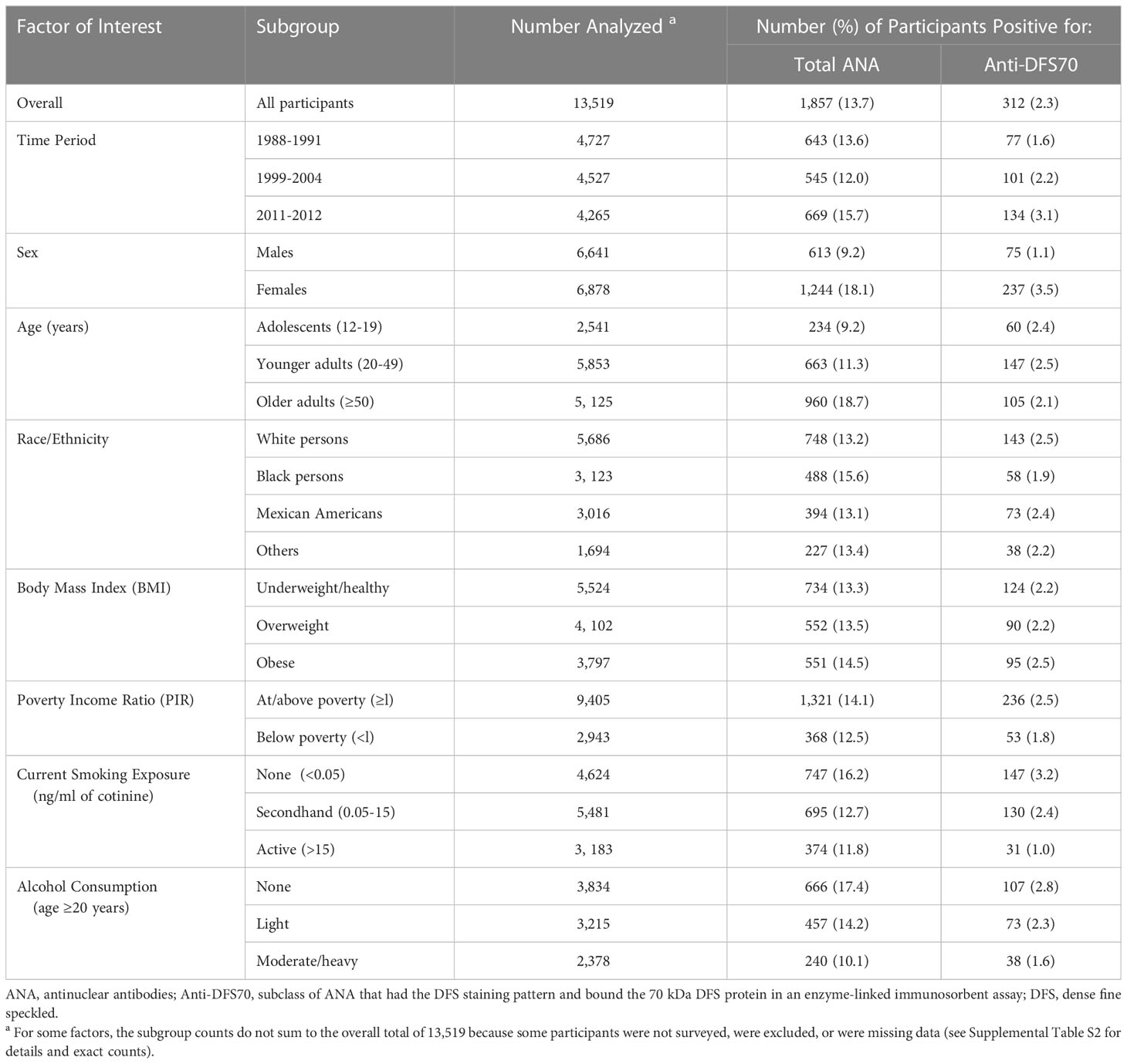
Table 1 Sample distributions of total ANA and anti-DFS70 antibody positivity overall and in selected subgroups.
Prevalence estimates
Adjusted only for the survey-design variables, the period-specific population-representative estimates of anti-DFS70 antibody prevalence are 1.57% (95% CI=1.20-2.04%) in 1988-1991, 2.48% (95% CI=1.85-3.33%) in 1999-2004, and 4.04% (95% CI=3.12-5.23%) in 2011-2012 (Table 2). The corresponding estimated numbers of individuals in the US who were positive for anti-DFS70 antibodies are 3.2 million (95% CI=2.4-4.1), 5.8 million (95% CI=4.3-7.8), and 10.4 million (95% CI=8.0-13.5). For comparison, the respective period-specific prevalence estimates for total ANA are 11.0% (95% CI=9.7-12.5%), 11.4% (95% CI=10.2-12.8%), and 16.1% (95% CI=14.5-17.9%), and the estimated numbers of ANA-positive individuals are 22.4 million (95% CI=19.6-25.4), 26.6 million (95% CI=23.8-29.7), and 41.6 million (95% CI=37.4-46.1). Prevalence estimates for total ANA and anti-DFS70 antibodies are reported for the full population and selected subgroups in Table 2 and for additional subgroups in Supplemental Table S4; the subgroup estimates were essentially averaged over time periods.
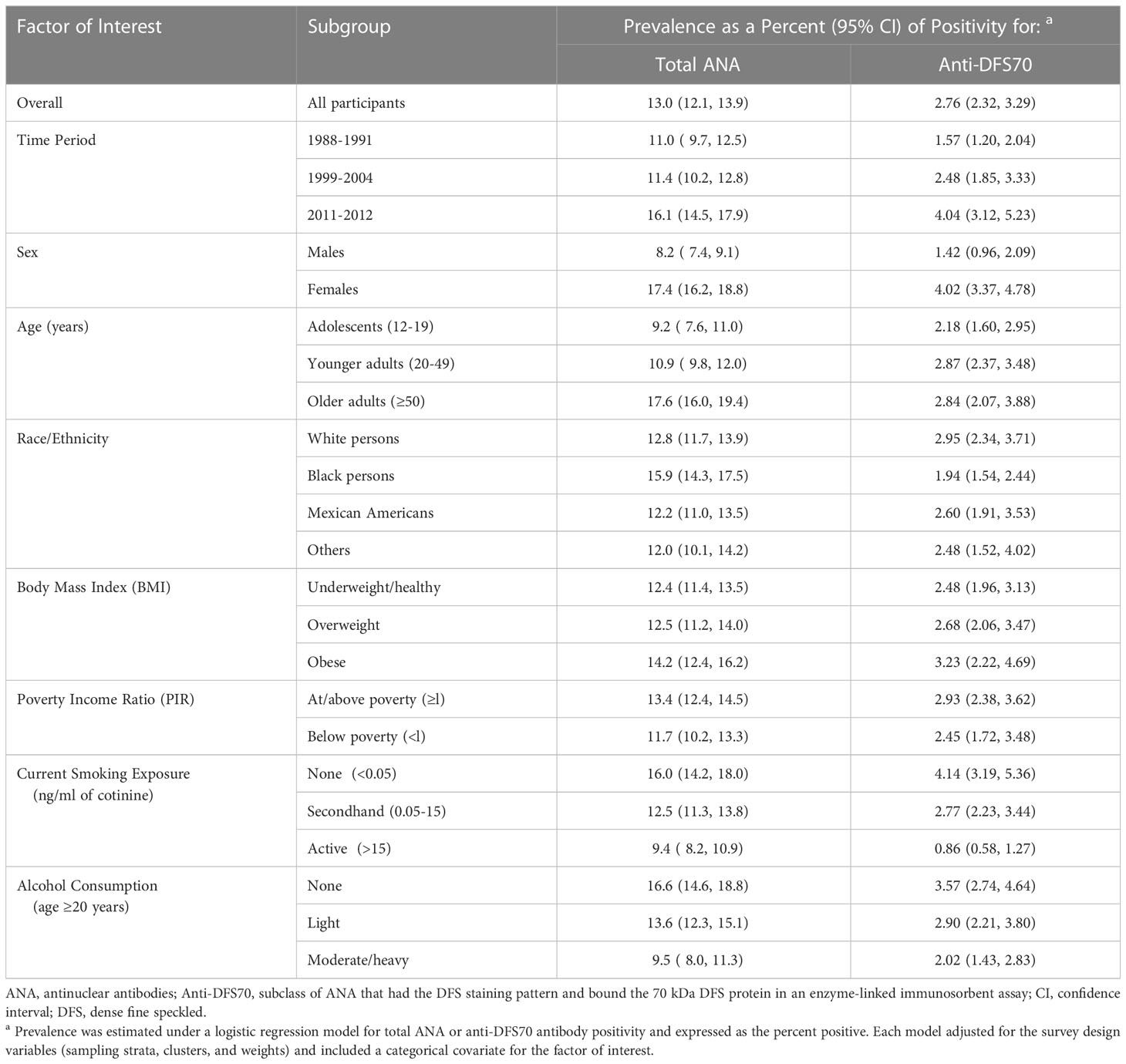
Table 2 Prevalence estimates for total ANA and anti-DFS70 antibody positivity overall and in selected subgroups.
In contrast, period-specific prevalence estimates are shown for the full population and selected subgroups in Figure 1 (and additional subgroups in Supplemental Figure S1). The full population estimates in Figure 1 are visual representations of the numerical values given for time period in Table 2, but the subgroup estimates in Figure 1 and Supplemental Figure S1 are period-specific, while those in Table 2 and Supplemental Table S4 are not. These plots clearly illustrate previously noted increases in total ANA positivity over time (15) and several known total ANA associations, including higher prevalence in females, older adults, and black persons (10, 15). Some, but not all, of these relationships are also seen for anti-DFS70 antibodies. For example, as with total ANA, the estimated prevalence of anti-DFS70 antibodies increased over time and was higher in females, but unlike total ANA, it was not higher in black persons and appeared unrelated to age.
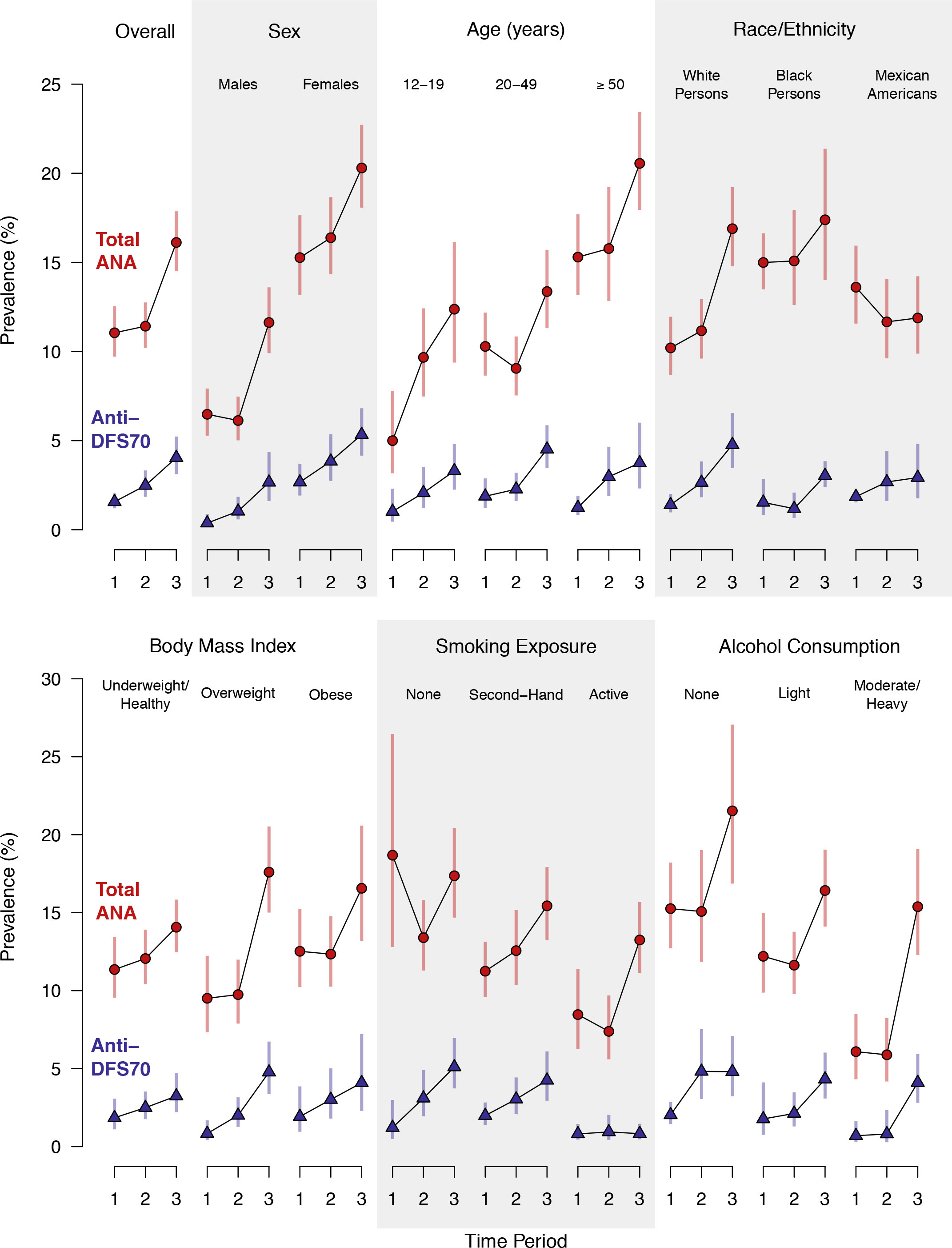
Figure 1 Prevalence of antinuclear antibodies (ANA) and anti-dense fine speckled 70 (DFS70) antibodies in the US population ≥12 years old and in selected subgroups. Separate estimates are plotted for Period 1 (1988–1991), Period 2 (1999–2004), and Period 3 (2011–2012). The prevalence estimates and 95% confidence intervals (CIs) for total ANA are represented by red circles and red vertical lines, respectively, while the anti-DFS70 antibody prevalence estimates and 95% CIs are represented by blue triangles and blue vertical lines. Period-specific prevalence estimates are connected by black lines to visualize time trends. The estimates were derived from a logistic regression model for total ANA or anti-DFS70 antibody positivity, which stratified by the factor defining the subgroup and adjusted for the survey-design variables (sampling strata, clusters, and weights) and a categorical covariate for time period. Participants with missing data for the factor defining the subgroup were excluded only from that subgroup analysis.
Correlates
Covariate-adjusted analyses replicated previously observed (10, 15) ANA associations with time period (P<0.0001), sex (P<0.0001), age (P<0.0001), and race/ethnicity (P=0.0008) (Table 3), with higher total ANA prevalence in the last period, females, older adults, and black persons. Period and sex were also associated with anti-DFS70 antibodies (P<0.0001), but age was not (P=0.168), and the evidence for race/ethnicity was marginal (P=0.015) and suggested lower rather than higher prevalence in black persons. However, there was strong evidence that current smoking exposure was inversely associated with anti-DFS70 antibody prevalence (P<0.0001), in contrast to the weak evidence for total ANA (P=0.046). No other factor was clearly associated with total ANA or anti-DFS70 antibodies (Table 3 and Supplemental Table S5).
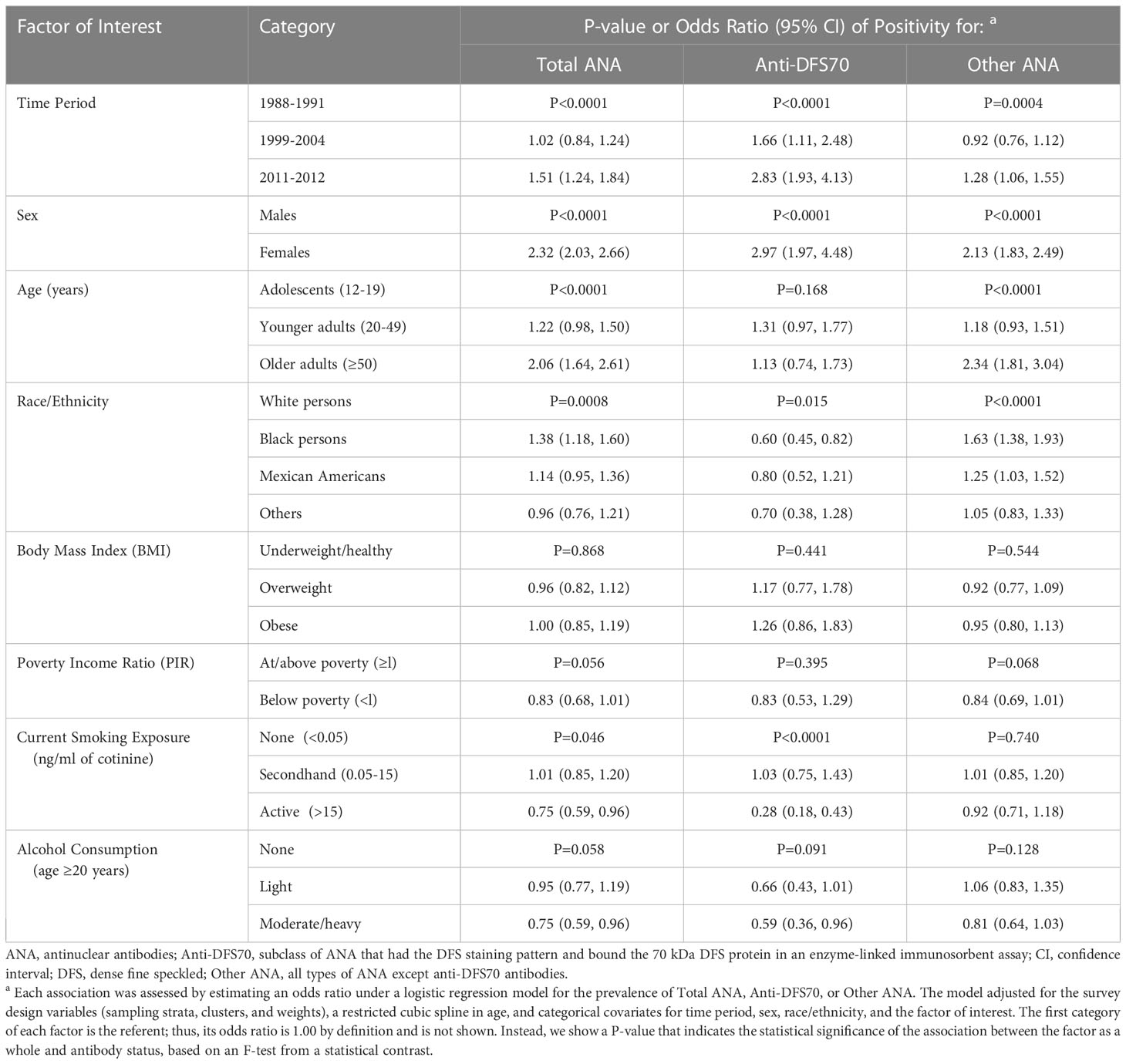
Table 3 Covariate-adjusted odds ratios for assessing associations between selected factors and total ANA, anti-DFS70 antibodies, and other ANA.
Total ANA prevalence increased in the last time period relative to the first (OR=1.51; 95% CI=1.24-1.84), but the positive time trend for anti-DFS70 antibody prevalence was even steeper, with OR=1.66 (95% CI=1.11-2.48) in 1999-2004 and OR=2.83 (95% CI=1.93-4.13) in 2011-2012. Also, while the odds of having ANA in general were over two times higher in females than males (OR=2.32; 95% CI=2.03-2.66), the odds of specifically having anti-DFS70 antibodies were roughly three times higher in females (OR=2.97; 95% CI=1.97-4.48). The prevalence of total ANA increased with age, with OR=1.22 (95% CI=0.98-1.50) for younger adults relative to adolescents and OR=2.06 (95% CI=1.64-2.61) for older adults, but there was little evidence of an age association with anti-DFS70 antibodies. Black persons had a higher prevalence of total ANA than white persons (OR=1.38; 95% CI=1.18-1.60), but a lower prevalence of anti-DFS70 antibodies (OR=0.60; 95% CI=0.45-0.82). Three-fourths as many active smokers had ANA in general relative to nonsmokers (OR=0.75; 95% CI=0.59-0.96), but that proportion was close to one-fourth for anti-DFS70 antibodies specifically (OR=0.28; 95% CI=0.18-0.43). All association results are shown in Table 3 and Supplemental Table S5.
Time trends
There was strong evidence (P<0.0001) that the overall prevalence of both total ANA and anti-DFS70 antibodies increased over time (Table 4). Adjusted for covariates, the estimated ORs for total ANA in the second and third periods relative to the first were 1.02 (95% CI=0.84-1.24) and 1.51 (95% CI=1.24-1.84), respectively, and the corresponding OR estimates for anti-DFS70 antibodies were 1.66 (95% CI=1.11-2.48) and 2.83 (95% CI=1.93-4.13).
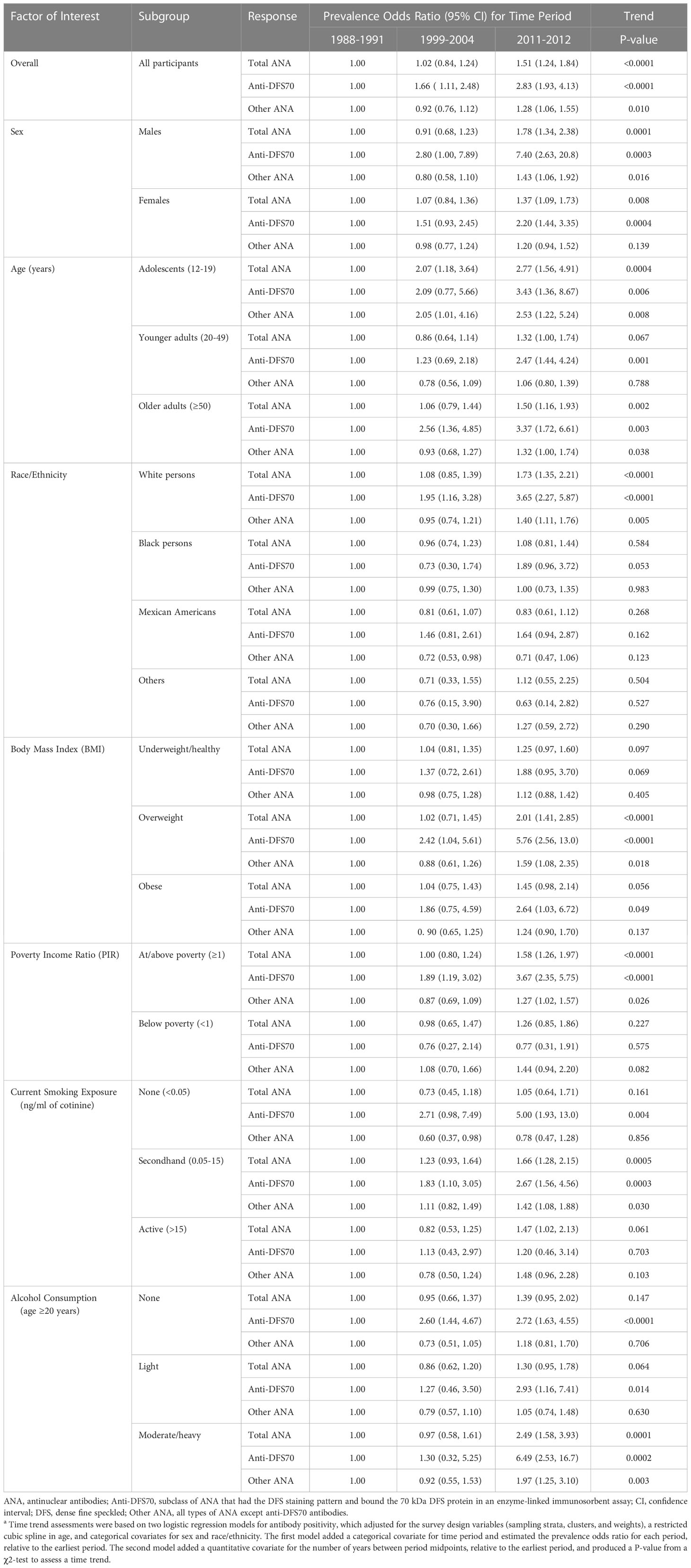
Table 4 Covariate-adjusted assessments of time trends for total ANA, anti-DFS70 antibodies, and other ANA in selected subgroupsa.
In subgroup analyses, the increasing time trend for total ANA was seen in both males (P=0.0001) and females (P=0.008), as well as in adolescents (P=0.0004) and adults ≥50 years old (P=0.002), but not adults 20-49 years old (Table 4). The positive time trend for total ANA was also observed in white persons (P<0.0001), overweight individuals (P<0.0001), persons living at or above the poverty level (P<0.0001), persons exposed to secondhand tobacco smoke (P=0.0005), and moderate to heavy drinkers (P=0.0001) (Table 4), as well as in persons for whom poor health did (P=0.004) or did not (P=0.001) prevent them from working (Supplemental Table S6). These time trends for total ANA are depicted in Figure 1 and Supplemental Figure S1.
Increases in anti-DFS70 antibodies over time were seen in most of the same subgroups: males (P=0.0003), females (P=0.0004), adolescents (P=0.006), older adults (P=0.003), white persons (P<0.0001), overweight individuals (P<0.0001), individuals living at or above the poverty level (P<0.0001), persons exposed to secondhand tobacco smoke (P=0.0003), and moderate to heavy drinkers (P=0.0002). Unlike total ANA, though, the positive anti-DFS70 antibody time trend was also seen in younger adults (P=0.001), nonsmokers (P=0.004), nondrinkers (P<0.0001), and persons with very high C-reactive protein levels (P<0.0001) (Table 4; Supplemental Table S6; Figure 1; Supplemental Figure S1).
We assessed whether the time trends in subgroups defined by a given factor’s categories were different or statistically consistent with the overall time trend. We found marginal evidence (0.001<P<0.05) that the ANA time trends for subgroups defined by age (P=0.036), race/ethnicity (P=0.002), and alcohol consumption (P=0.014) differed, as did the anti-DFS70 antibody time trends for subgroups defined by sex (P=0.025), PIR (P=0.005), current smoking exposure (P=0.034), and C-reactive protein (P=0.002) (Supplemental Table S7).
Additional analyses
We investigated whether anti-DFS70 antibody correlates and time trends systematically differed from those for total ANA by analyzing “other” ANA (i.e., any type of ANA except anti-DFS70 antibodies). There was weak evidence that current smoking exposure was inversely associated with total ANA (P=0.046), strong evidence for anti-DFS70 antibodies (P<0.0001), and no evidence for other ANA (P=0.740), suggesting that anti-DFS70 antibodies were driving the inverse association between total ANA and smoking (Table 3). As for increases in prevalence across periods, the overall positive time trend for other ANA was smaller than that for total ANA and anti-DFS70 antibodies, suggesting that the anti-DFS70 antibody time trend contributed to the trend for total ANA (Table 4). Similar evidence of this relationship was seen in both sexes, older adults, white persons, overweight individuals, individuals at or above the poverty level, persons exposed to secondhand tobacco smoke, and moderate to heavy drinkers (Table 4), as well as in persons with more than a high school education (Supplemental Table S6).
Smoking exposure decreased over the 25-year span of our study and was inversely associated with anti-DFS70 antibodies, which could confound the observed anti-DFS70 antibody time trends. Thus, we repeated our covariate-adjusted analyses after adding a covariate for smoking exposure and found that, relative to Period 1, anti-DFS70 antibody prevalence increased in Period 2 (OR=1.65; 95% CI=1.08-2.52) and again in Period 3 (OR=2.69; 95% CI=1.80-4.02). These odds ratios are similar to those from our original analysis, which adjusted for sex, age, and race/ethnicity, but not smoking exposure (Table 3). In both analyses, the statistical evidence of a positive time trend was strong (P<0.0001), suggesting that changes in smoking over this 25-year span cannot explain the increase in anti-DFS70 antibody prevalence over time.
Discussion
Our main goal was to estimate the prevalence of anti-DFS70 antibodies, identify factors associated with them, and assess changes over time. A secondary goal was to evaluate whether subgroup time trends were heterogeneous, as a way of discovering possible drivers or protective factors that could steepen or flatten the general trend. A tertiary goal was to compare our results for anti-DFS70 antibodies with established results for total ANA. In view of these goals, we summarize our findings below, after a brief review of the relevance of anti-DFS70 antibodies. We also discuss strengths and limitations of our study and several other points of interest.
The standard HEp-2 IFA for ANA detects a heterogeneous group of autoantibodies and is a common screening tool when a clinical presentation suggests a possible autoimmune disease (17). However, relatively little is known about the natural history of ANA in the absence of an autoimmune disease. Staining patterns are important for understanding the relevance of ANA in pre-clinical, symptomatic, and healthy populations. Recently, thirty ANA patterns identified by alphanumeric codes (AC-0 to AC-29) were described and classified by the committee for the International Consensus on ANA Patterns (ICAP) (18, 30), and the clinical relevance was summarized online (www.ANApatterns.org) (17). The DFS (AC-2) staining pattern is associated with anti-DFS70 antibodies, which are more common in healthy individuals than in those with SARD (16, 31). The autoantigen DFS70 is a transcription co-activator regulating the expression of oxidative-stress, inflammatory, and antioxidant genes (32). The consensus is that individuals with high-titer monospecific anti-DFS70 antibodies (i.e., no other well-known autoantibodies that are markers for autoimmune diseases) seldom have SARD (16, 17, 24–27).
The selection of the anti-DFS70 ELISA used in this study was based on its commercial availability at the time and the overall laboratory experience with alternatives for anti-DFS70 testing (33). A natural question is why some samples were positive in the DFS/AC-2 IFA but negative in the anti-DFS70 ELISA. Further study focused primarily on the few higher-titered DFS/AC-2 positive samples. We attempted to identify the target antigen(s) in these sera using Western blot and immunoprecipitation-mass spectrometry, as described in the characterization of other autoantibody reference materials (33–35). No target antigen was consistently identified, though the data clearly showed that these sera did not recognize DFS70 in either Western blot or immunoprecipitation-mass spectrometry. These sera may be similar to some that the community refers to as showing a “pseudo DFS” pattern. Further work is needed to characterize these sera as “pseudo DFS” in the absence of a well-defined autoantigen target or clinical relevance. Our observations are consistent with a recent publication that reports sera with a DFS-like or pseudo-DFS pattern did not react with DFS70, which suggests they may recognize diverse proteins or conformational epitopes (19). The percentage of sera negative for anti-DFS70 in the DFS/AC-2 pool is expected to vary substantially in different laboratories, based on many factors. Harmonization will need to consider variations in experience reading IFA, selection of a validated anti-DFS70 assay, and proper use of available anti-DFS70 reference material (33).
The large NHANES databases and serum repositories allowed us to explore anti-DSF70 antibody prevalence, correlates, and time trends in nationally representative samples of the civilian noninstitutionalized US population ≥12 years old. Anti-DFS70 antibody prevalence increased from 1.6% in 1988-1991 to 2.5% in 1999-2004 to 4.0% in 2011-2012, which correspond to 3.2 million, 5.8 million, and 10.4 million seropositive individuals, respectively. Thus, over this 25-year span, we observed a 2.5-fold increase in the prevalence for anti-DFS70 antibodies (1.6% to 4.0%) compared with a 1.5-fold increase for total ANA (11.0% to 16.1%). Analyses accounting for sex, age, and race/ethnicity showed this positive trend was statistically significant (P<0.0001), with anti-DFS70 antibody prevalence being 66% higher in 1999-2004 (1.66-fold increase) and 183% higher in 2011-2012 (2.83-fold increase), relative to 1988-1991. Covariate-adjusted analyses also revealed anti-DFS70 antibody associations with sex and smoking (both P<0.0001), where the odds of having anti-DFS70 antibodies were three times higher in females than in males and one-fourth as high in active smokers as in nonsmokers. There was also some evidence of a race/ethnicity association (P=0.015), with black persons only 60% as likely as white persons to have anti-DFS70 antibodies. We did not find clear evidence of anti-DFS70 antibody associations with other factors considered.
Both total ANA and anti-DFS70 antibodies increased significantly over time and both were associated with sex and race/ethnicity, but the clear age association with total ANA was not apparent for anti-DFS70 antibodies, and the obvious smoking association with anti-DFS70 antibodies was only weakly supported for total ANA. Our analysis of other ANA suggested anti-DFS70 antibodies may have driven the inverse association between total ANA and smoking, but played little to no role in the more obvious ANA associations with sex, age, and race/ethnicity.
We also investigated whether the overall anti-DFS70 antibody time trend was seen in subgroups. Anti-DFS70 antibody prevalence increased over time in both sexes, all three age groups, white persons, overweight individuals, persons at or above the poverty level, nonsmokers, persons exposed to secondhand smoke, alcohol abstainers, moderate to heavy alcohol drinkers, persons with more than a high school education, and persons with very high C-reactive protein levels. In many of these subgroups, similar time trends were observed for the broader category of total ANA. However, there was little evidence of a time trend for total ANA in younger adults, nonsmokers, alcohol abstainers, or persons with very high C-reactive protein levels. It is not clear why some association patterns for anti-DSF70 antibodies differ from those for total ANA, but distinct mechanisms could be at play, as has been hypothesized to explain why smoking is a risk factor for some diseases but protective for others (36).
Our study had several strengths. Total ANA and anti-DFS70 antibody cohorts were large, spanned 25 years, and were weighted to be representative of the civilian noninstitutionalized US population ≥12 years old. All assays were performed in the same laboratory and used the same evaluators, methods, and equipment. Our analyses accounted for many sociodemographic factors and health conditions as potential correlates or modifiers. We focused on persons confirmed positive for anti-DFS70 antibodies by ELISA among those screened positive for DFS staining by IFA; this is consistent with the standard protocol for a typical clinical immunology laboratory, which usually only screens for anti-DFS70 antibodies in sera with positive DFS staining.
Our findings are also subject to limitations: 1) the NHANES excluded institutionalized persons, such as the elderly in residential care; 2) statistical analyses were based on cross-sectional data rather than longitudinal data with repeated measures; 3) neither total ANA nor anti-DFS70 antibodies were assessed in children <12 years old; 4) some information was self-reported; 5) not all participants were tested for anti-DFS70 antibodies (i.e., only those positive for ANA with the DFS staining pattern); and 6) there were inadequate clinical data available to allow for disease association studies.
Some individuals may have had anti-DFS70 antibodies but did not test positive for DFS (AC-2) staining if there were other autoantibody signals that hindered the detection of the DFS pattern. For example, if someone had both anti-DFS70 and anti-DNA antibodies, the anti-DNA antibodies could have produced a strong homogeneous staining pattern that obscured the DFS pattern; however, there were few participants with strong homogeneous staining in the NHANES cohorts. We did not perform the ELISA test for anti-DFS70 antibodies among non-DFS-positive participants due to cost considerations and because most clinical laboratories do not test for anti-DFS70 antibodies without prior HEp-2 IFA pre-screening. However, when a clear DFS pattern was observed, it was likely from high-titer anti-DFS70 antibodies (37). Thus, our findings relate mostly to high-titer or monospecific anti-DFS70 antibodies, which have been reported mainly in non-SARD or healthy individuals (38), and therefore our analyses probably underestimated the true prevalence of anti-DFS70 antibodies.
As discussed above and reviewed by others (32, 39), the search for clinical associations between anti-DFS70 antibodies and well-defined disease conditions has not yielded clear results. There is general agreement that high-titer monospecific anti-DFS70 antibodies tend to be associated with non-SARD conditions, including certain gynecologic syndromes, atopic disorders, skin disorders (psoriasis and alopecia areata), fibromyalgia/chronic pain syndrome/chronic fatigue syndrome, Hashimoto’s thyroiditis, and prostate cancer (38, 40–42). However, recent studies have also shown anti-DFS70 antibodies in persons with SARD, including systemic lupus erythematosus, rheumatoid arthritis, undifferentiated connective tissue disease, and Sjögren’s Syndrome (38, 40). Our data revealed a substantial prevalence of anti-DFS70 antibodies in the NHANES cohorts, and increasingly so in the more recent cohorts. Perhaps anti-DFS70 antibodies are not as strongly linked to a particular disease as they are to specific, but yet to be fully defined, exposures in our rapidly changing environment (8). One possible explanation for the weak association between anti-DFS70 antibodies and particular diseases may be the increasingly high relative prevalence of anti-DFS70 antibodies in the general population, as shown in the present study. In a future study, we plan to investigate possible associations between anti-DFS70 antibodies and additional environmental exposures and health conditions.
In conclusion, anti-DFS70 antibodies in the US were associated with sex and exposure to tobacco smoke, as well as the period of evaluation. Specifically, their prevalence was higher in females, lower in active smokers, and increased substantially over the 25-year span of our study. This positive time trend was observed in both the general population and many subgroups. We also found some evidence of an association between race/ethnicity and anti-DFS70 antibodies, with black persons having a lower prevalence than white persons. The known age association with total ANA, however, was not obvious for anti-DFS70 antibodies. More research is needed to understand the risk factors, pathologic or potentially protective influences on disease (43), and clinical implications of anti-DFS70 antibodies.
Data availability statement
All of the NHANES data that we analyzed in this study are freely and publicly available from the CDC web sites.
Ethics statement
The NHANES protocol was approved by the Human Subjects Institutional Review Board of the US Centers for Disease Control and Prevention (CDC). The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors were involved in drafting the paper or revising it critically for important intellectual content. EC had full access to the data and takes responsibility for its integrity and the accuracy of the data analysis. Study conception and design: GD, BZ, CP, CW, FM, and EC. Acquisition of data: GD, BZ, CC, FM, and EC. Analysis and interpretation of data: GD, BZ, CC, CP, CW, FM, and EC. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences under project Z01 ES101074 and under contracts HHSN273201600011C GS-00F-173CA/75N96022F00055 to Social & Scientific Systems, a DLH Holdings Corp. Company.
Acknowledgments
We thank Drs. Marvin Fritzler and Luis Andrade for their very helpful comments, Justin Nicholas and Rodrigo Mora for technical laboratory assistance, and Dr. Geraldine McQuillan for administrative and regulatory assistance. We also thank the members of the NHANES Autoimmunity Study Group (including Drs. Linda Birnbaum, Richard Cohn, Dori Germolec, Minoru Satoh, Nigel Walker, Irene Whitt, and Darryl Zeldin) for initiating the studies that motivated much of this research. Finally, we thank Dr. Michael Mahler (Inova Diagnostics/Werfen) for providing the use of the NOVA View automated fluorescence microscope system for data collection.
Conflict of interest
Authors GD and CC were employed by the company Social and Scientific Systems, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The interpretations and conclusions contained herein are those of the authors and do not necessarily represent positions of the authors’ affiliations.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1186439/full#supplementary-material
Supplementary Figure 1 | Prevalence of antinuclear antibodies (ANA) and anti-dense fine speckled 70 (DFS70) antibodies in additional subgroups. Separate estimates are plotted for Period 1 (1988–1991), Period 2 (1999–2004), and Period 3 (2011–2012). The prevalence estimates and 95% confidence intervals (CIs) for total ANA are represented by red circles and red vertical lines, respectively, while the anti-DFS70 antibody prevalence estimates and 95% CIs are represented by blue triangles and blue vertical lines. Period-specific prevalence estimates are connected by black lines to visualize time trends. The estimates were derived from a logistic regression model for total ANA or anti-DFS70 antibody positivity, which stratified by the factor defining the subgroup and adjusted for the survey-design variables (sampling strata, clusters, and weights) and a categorical covariate for time period. Participants with missing data for the factor defining the subgroup were excluded only from that subgroup analysis.
References
1. Davidson A, Diamond B. Autoimmune diseases. N Engl J Med (2001) 345(5):340–50. doi: 10.1056/nejm200108023450506
2. Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med (2015) 278(4):369–95. doi: 10.1111/joim.12395
3. Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med (2002) 347(12):911–20. doi: 10.1056/NEJMra020100
4. Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences expert panel workshop. J Autoimmun (2012) 39(4):259–71. doi: 10.1016/j.jaut.2012.05.002
5. Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis (2015) 3:151–5. doi: 10.12691/ijcd-3-4-8
6. Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med (2017) 376(15):1419–29. doi: 10.1056/NEJMoa1610187
7. Fatoye F, Gebrye T, Svenson LW. Real-world incidence and prevalence of systemic lupus erythematosus in Alberta, Canada. Rheumatol Int (2018) 38(9):1721–6. doi: 10.1007/s00296-018-4091-4
8. Miller FW. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr Opin Immunol (2023) 80:102266. doi: 10.1016/j.coi.2022.102266
9. Schmidt CW. Questions persist: environmental factors in autoimmune disease. Environ Health Perspect (2011) 119(6):A249–53. doi: 10.1289/ehp.119-a248
10. Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum (2012) 64(7):2319–27. doi: 10.1002/art.34380
11. Parks CG, Miller FW, Satoh M, Chan EK, Andrushchenko Z, Birnbaum LS, et al. Reproductive and hormonal risk factors for antinuclear antibodies (ANA) in a representative sample of U.S. women. Cancer Epidemiol Biomarkers Prev (2014) 23(11):2492–502. doi: 10.1158/1055-9965.Epi-14-0429
12. Liao KP, Kurreeman F, Li G, Duclos G, Murphy S, Guzman R, et al. Associations of autoantibodies, autoimmune risk alleles, and clinical diagnoses from the electronic medical records in rheumatoid arthritis cases and non-rheumatoid arthritis controls. Arthritis Rheum (2013) 65(3):571–81. doi: 10.1002/art.37801
13. Chang C, Gershwin ME. Drugs and autoimmunity–a contemporary review and mechanistic approach. J Autoimmun (2010) 34(3):J266–75. doi: 10.1016/j.jaut.2009.11.012
14. Dinse GE, Co CA, Parks CG, Weinberg CR, Xie G, Chan EKL, et al. Expanded assessment of xenobiotic associations with antinuclear antibodies in the United States, 1988-2012. Environ Int (2022) 166:107376. doi: 10.1016/j.envint.2022.107376
15. Dinse GE, Parks CG, Weinberg CR, Co CA, Wilkerson J, Zeldin DC, et al. Increasing prevalence of antinuclear antibodies in the United States. Arthritis Rheumatol (2022) 74(12):2032–41. doi: 10.1002/art.42330
16. Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, Andrade LE. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum (2011) 63(1):191–200. doi: 10.1002/art.30084
17. Damoiseaux J, Andrade LEC, Carballo OG, Conrad K, Francescantonio PLC, Fritzler MJ, et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the international consensus on ANA patterns (ICAP) perspective. Ann Rheum Dis (2019) 78(7):879–89. doi: 10.1136/annrheumdis-2018-214436
18. Chan EK, Damoiseaux J, Carballo OG, Conrad K, de Melo Cruvinel W, Francescantonio PL, et al. Report of the first international consensus on standardized nomenclature of antinuclear antibody HEp-2 cell patterns 2014-2015. Front Immunol (2015) 6:412. doi: 10.3389/fimmu.2015.00412
19. Sanchez-Hernandez ES, Ortiz-Hernandez GL, Ochoa PT, Reeves M, Bizzaro N, Andrade LEC, et al. The nuclear dense fine speckled (DFS) immunofluorescence pattern: not all roads lead to DFS70/Ledgfp75. Diagnostics (Basel) (2023) 13(2). doi: 10.3390/diagnostics13020222
20. Ochs RL, Muro Y, Si Y, Ge H, Chan EK, Tan EM. Autoantibodies to DFS 70 Kd/Transcription coactivator P75 in atopic dermatitis and other conditions. J Allergy Clin Immunol (2000) 105(6 Pt 1):1211–20. doi: 10.1067/mai.2000.107039
21. Watanabe A, Kodera M, Sugiura K, Usuda T, Tan EM, Takasaki Y, et al. Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum (2004) 50(3):892–900. doi: 10.1002/art.20096
22. Röber N, Höpner U, Aringer M, Sebastian S, Gräßler A, Lüthke K, et al. Anti-DFS70 antibodies without disease specific antibodies help to rule out ANA associated rheumatic diseases. In: Conrad K, Chan EKL, Andrade LEC, Steiner G, Pruijn GJM, Shoenfeld Y, editors. Autoantibody research to standardized diagnostic assays in the management of human diseases. Lengerich: Pabst Science Publishers (2015). p. 173–4.
23. Conrad K, Röber N, Andrade LE, Mahler M. The clinical relevance of anti-DFS70 autoantibodies. Clin Rev Allergy Immunol (2017) 52(2):202–16. doi: 10.1007/s12016-016-8564-5
24. Mahler M, Hanly JG, Fritzler MJ. Importance of the dense fine speckled pattern on HEp-2 cells and anti-DFS70 antibodies for the diagnosis of systemic autoimmune diseases. Autoimmun Rev (2012) 11(9):642–5. doi: 10.1016/j.autrev.2011.11.005
25. Shovman O, Gilburd B, Chayat C, Amital H, Langevitz P, Watad A, et al. Prevalence of anti-DFS70 antibodies in patients with and without systemic autoimmune rheumatic diseases. Clin Exp Rheumatol (2018) 36(1):121–6.
26. Deng C, Qu X, Cheng S, Zeng X, Li Y, Fei Y. Decision-making value of nuclear dense fine speckled pattern in systemic autoimmune rheumatic disease: trick or treat? Ann Rheum Dis (2020) 79(8):e92. doi: 10.1136/annrheumdis-2019-215587
27. Mockenhaupt LM, Dolscheid-Pommerich R, Stoffel-Wagner B, Behning C, Brossart P, Schäfer VS. Autoantibodies to dense-Fine-Speckled 70 (DFS70) do not necessarily rule out connective tissue diseases. Semin Arthritis Rheum (2022) 52:151936. doi: 10.1016/j.semarthrit.2021.12.006
28. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999-2010. Vital Health Stat 2 (2013) 161):1–24.
29. Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: NY: Springer-Verlag (2001).
30. Andrade LEC, Klotz W, Herold M, Conrad K, Rönnelid J, Fritzler MJ, et al. International consensus on antinuclear antibody patterns: definition of the AC-29 pattern associated with antibodies to DNA topoisomerase I. Clin Chem Lab Med (2018) 56(10):1783–8. doi: 10.1515/cclm-2018-0188
31. Mahler M, Andrade LE, Casiano CA, Malyavantham K, Fritzler MJ. Implications for redefining the dense fine speckled and related indirect immunofluorescence patterns. Expert Rev Clin Immunol (2019) 15(5):447–8. doi: 10.1080/1744666x.2019.1596802
32. Ochs RL, Mahler M, Basu A, Rios-Colon L, Sanchez TW, Andrade LE, et al. The significance of autoantibodies to DFS70/Ledgfp75 in health and disease: integrating basic science with clinical understanding. Clin Exp Med (2016) 16(3):273–93. doi: 10.1007/s10238-015-0367-0
33. Dellavance A, Baldo DC, Zheng B, Mora RA, Fritzler MJ, Hiepe F, et al. Establishment of an international autoantibody reference standard for human anti-DFS70 antibodies: proof-of-Concept study for a novel megapool strategy by pooling individual specific sera. Clin Chem Lab Med (2019) 57(11):1754–63. doi: 10.1515/cclm-2019-0087
34. Calise SJ, Zheng B, Hasegawa T, Satoh M, Isailovic N, Ceribelli A, et al. Reference standards for the detection of anti-mitochondrial and anti-Rods/Rings autoantibodies. Clin Chem Lab Med (2018) 56(10):1789–98. doi: 10.1515/cclm-2017-1152
35. Zheng B, Mora RA, Fritzler MJ, Satoh M, Bloch DB, Garcia-De La Torre I, et al. Establishment of international autoantibody reference standards for the detection of autoantibodies directed against PML bodies, GW bodies, and NuMa protein. Clin Chem Lab Med (2020) 59(1):197–207. doi: 10.1515/cclm-2020-0981
36. Perricone C, Versini M, Ben-Ami D, Gertel S, Watad A, Segel MJ, et al. Smoke and autoimmunity: the fire behind the disease. Autoimmun Rev (2016) 15(4):354–74. doi: 10.1016/j.autrev.2016.01.001
37. La Jeon Y, Kang SY, Lee WI, Kim MH. Clinical aspects of the dense fine speckled pattern in indirect immunofluorescence-antinuclear antibody screening and its association with DFS70 autoantibodies. Ann Clin Lab Sci (2019) 49(4):496–502.
38. Infantino M, Pregnolato F, Bentow C, Mahler M, Benucci M, Li Gobbi F, et al. Only monospecific anti-DFS70 antibodies aid in the exclusion of antinuclear antibody associated rheumatic diseases: an Italian experience. Clin Chem Lab Med (2019) 57(11):1764–9. doi: 10.1515/cclm-2019-0454
39. Ortiz-Hernandez GL, Sanchez-Hernandez ES, Casiano CA. Twenty years of research on the DFS70/Ledgf autoantibody-autoantigen system: many lessons learned but still many questions. Auto Immun Highlights (2020) 11(1):3. doi: 10.1186/s13317-020-0126-4
40. Zheng B, Wang Z, Mora RA, Liu A, Li C, Liu D, et al. Anti-DFS70 antibodies among patient and healthy population cohorts in China: results from a multicenter training program showing spontaneous abortion and pediatric systemic autoimmune rheumatic diseases are common in anti-DFS70 positive patients. Front Immunol (2020) 11:562138. doi: 10.3389/fimmu.2020.562138
41. Lundgren MC, Sapkota S, Peterson DJ, Crosson JT. The antinuclear antibody dense fine speckled pattern and possible clinical associations: an indication of a proinflammatory microenvironment. J Immunol Methods (2021) 488:112904. doi: 10.1016/j.jim.2020.112904
42. Rincón-Riaño D, Fernández-Ávila DG, Acero-Molina D, Bello Gualtero JM, Romero-Sánchez C. Frequency of ANA/DFS70 autoantibodies in Colombian patients with undifferentiated connective tissue disease. Reumatismo (2022) 74(2). doi: 10.4081/reumatismo.2022.1420
Keywords: antinuclear antibodies (ANA), autoantibody, autoimmune disease, dense fine speckled (DFS), dense fine speckled 70 (DFS70), National Health and Nutrition Examination Survey (NHANES), population study
Citation: Dinse GE, Zheng B, Co CA, Parks CG, Weinberg CR, Miller FW and Chan EKL (2023) Anti-dense fine speckled 70 (DFS70) autoantibodies: correlates and increasing prevalence in the United States. Front. Immunol. 14:1186439. doi: 10.3389/fimmu.2023.1186439
Received: 14 March 2023; Accepted: 22 May 2023;
Published: 23 June 2023.
Edited by:
Allen Jay Rosenspire, Wayne State University, United StatesReviewed by:
Carlos A. Casiano, Loma Linda University, United StatesLieve Van Hoovels, OLV Hospital, Belgium
Copyright © 2023 Dinse, Zheng, Co, Parks, Weinberg, Miller and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edward K. L. Chan, ZWNoYW5AdWZsLmVkdQ==
 Gregg E. Dinse
Gregg E. Dinse Bing Zheng
Bing Zheng Caroll A. Co1
Caroll A. Co1 Christine G. Parks
Christine G. Parks Clarice R. Weinberg
Clarice R. Weinberg Frederick W. Miller
Frederick W. Miller Edward K. L. Chan
Edward K. L. Chan