- 1Mayo Clinic Graduate School of Biomedical Sciences, Department of Immunology, Mayo Clinic, Rochester, MN, United States
- 2Division of Clinical Microbiology, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, United States
- 3Division of Public Health, Infectious Diseases, and Occupational Medicine, Department of Medicine, Mayo Clinic, Rochester, MN, United States
Background: Periprosthetic joint infection (PJI) is a devastating complication of total joint arthroplasty surgery. Increased densities of activated mast cells have been predicted to be present in PJI compared to non-infectious arthroplasty failure based on analysis of transcriptomic data, but their presence in PJI-associated periprosthetic tissues has not been visually confirmed.
Objective: This preliminary study investigated the presence and activation status of mast cells in periprosthetic tissues associated with PJI.
Methods: Periprosthetic tissues from five PJI cases and three arthroplasty failures due to instability and one due to stiffness were immunohistochemically stained using tryptase and microscopically evaluated to enumerate mast cells and evaluate overall activation status within tissue samples. Mast cell activation was evidenced by the release of tryptase into the extracellular space surrounding mast cells.
Results: Mast cells were found in all samples, with average cellular densities of 22 and 26 cells/mm2 tissue in PJI and uninfected samples, respectively (p, 0.6610). Apparent mast cell activation and degranulation was readily observed throughout each of the five PJI samples studied, but not in any of the uninfected samples studied.
Conclusion: While preliminary, these findings provide evidence for a role of mast cells in the immune response in PJI. Additional investigation of the role of mast cells during arthroplasty failure is warranted, providing a better understanding of underlying biology and informing potential diagnostic and treatment targets.
1 Introduction
Total joint arthroplasty (TJA) is a common surgical procedure to treat joint pain and restore mobility. Arthroplasty failure is a major complication of TJA, caused by periprosthetic joint infection (PJI) and several non-infectious etiologies, including instability, stiffness, and aseptic loosening (1, 2). PJI is associated with biofilm formation by bacteria growing on implanted devices. Arthroplasty failure may necessitate surgical intervention, with intensive antimicrobial treatment also needed for PJI.
Recently, our group used an RNA-sequencing-based cellular deconvolution method, CIBERSORTx, to predict immune cellularity based on interrogation of bulk transcriptomic data from PJI and non-infectious arthroplasty failure (NIAF) sonicate fluid (derived from sonicating resected implants) (3). This evaluation suggested that activated mast cells may be elevated in PJI compared to NIAF, based on an elevation of a predicted activated mast cell transcriptomic signature informed by a combination of IL1A, IL1B, CCL4, and CCL20 expression. While these results gave evidence of a potential role of mast cells in the immune response to PJI, they were limited by the analytical method, as cellular populations were based solely on predictive deconvolution algorithms and not on actual cellular analyses.
Connective tissue resident mast cells are known to act as “sentinel cells” upon stimulation by bacteria during infection, detecting pathogen-associated molecular patterns, and initiating downstream antimicrobial inflammation through secretion of extracellular tryptase and chymase proteases, recruitment of phagocytic neutrophils, and direct antigen presentation to the adaptive immune response (4–7). The presence and inflammatory roles of synovial mast cells in rheumatoid arthritis, osteoarthritis, and arthrofibrosis has been described (8–11). Additionally, the presence and activation of mast cells in tissues surrounding implanted hips have previously been shown during aseptic loosening, although their role in other causes of NIAF is unexplored (12, 13). While infiltration and inflammatory impact of other cell types, such as neutrophils and macrophages, is well-described in PJI, the potential presence and role of mast cells during PJI were a novel finding.
This preliminary study aimed to determine whether activated mast cells can be visualized in peri-prosthetic tissues during PJI, confirming results of a prediction made based on transcriptomic analysis of sonicate fluid.
2 Materials and methods
2.1 Patient sample cohort
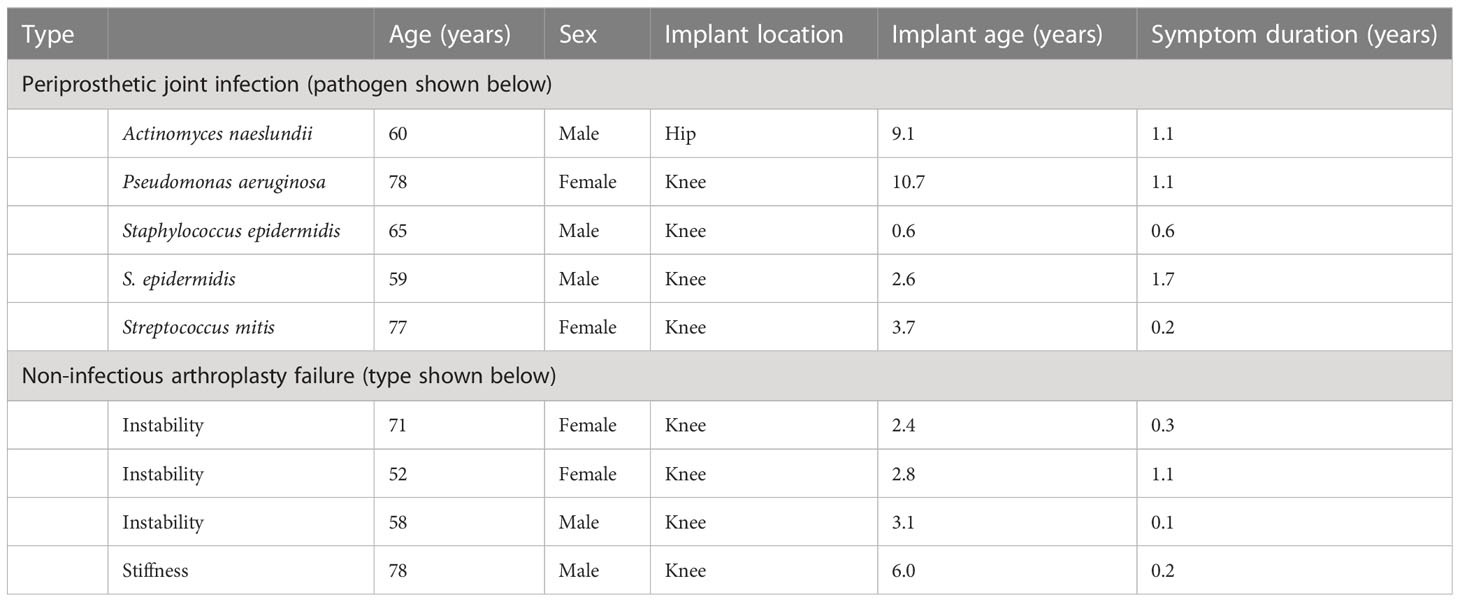
Periprosthetic tissue samples were taken during knee and hip arthroplasty revision surgery, as approved by the Mayo Clinic Institutional Review Board (09-000808). Paired tissues from NIAF sonicate fluids (n=4) with the lowest predicted activated mast cell populations and from PJI sonicate fluids (n=5) with the highest predicted activated mast cell populations by cellular deconvolution (3) were examined and are described in Table 1. The average age of the subjects studied was 66.4 years and ranged from 52 to 78 years. Five of the nine (56%) were from male subjects. NIAF samples included those associated with instability (n=3) and stiffness (n=1). PJI was determined by Musculoskeletal Infection Society (MSIS) diagnostic criteria (14) and was associated with Staphylococcus epidermidis (n=2), Actinomyces naeslundii (n=1), Pseudomonas aeruginosa (n=1), and Streptococcus mitis (n=1). Three of the five PJI subjects had had symptoms lasting ≥1 year, with two having had symptoms lasting <1 year (15). One PJI sample was from a total hip arthroplasty revision surgery, with the remaining samples, including four with PJI and four with NIAF, from total knee arthroplasty revision surgeries. The average age of the implant for each sample was 4.6 years, ranging from 0.6 to 10.7 years.
2.2 Immunohistochemical staining, microscopy, and image analysis
Formalin-fixed paraffin-embedded (FFPE) tissue blocks were sectioned and stained using a clinically validated immunohistochemistry (IHC) staining protocol with anti-mast cell tryptase antibody AA1 (Agilent Dako, Santa Clara, CA, USA) at a 1/2,000 dilution (16–19). Protease 2 was used as a pretreatment step with OptiView DAB IHC Detection. IHC-stained slides were imaged using a KEYENCE BZ-X800 All-in-One Fluorescence Microscope (KEYENCE, Osaka, Japan). The microscopist was blinded as to whether samples were from PJI or NIAF. For imaging, 31 × 21 total fields at 10×, accounting for an approximately 32 mm × 16 mm slide area, were stitched together using the BZ-X800 Analyzer (KEYENCE). The numbers of mast cells and total tissue surface areas within stitched images were determined using ImageJ v1.53a (20). Mast cell activation was evaluated under 40× and 100× magnification.
2.3 Statistical analysis and graphical representation
Mast cells per mm2 tissue sample were calculated and plotted using GraphPad Prism 9 v9.5.1 (San Diego, CA, USA). Statistical significance was determined via Welch’s two-sample t-test.
3 Results
Tryptase IHC-stained slides were histopathologically evaluated to determine mast cell densities in periprosthetic tissues. Average mast cell densities in PJI and NIAF samples were 22 and 26 cells/mm2 tissue, respectively (Figure 1; Welch’s two-sample t-test p-value of 0.6610).
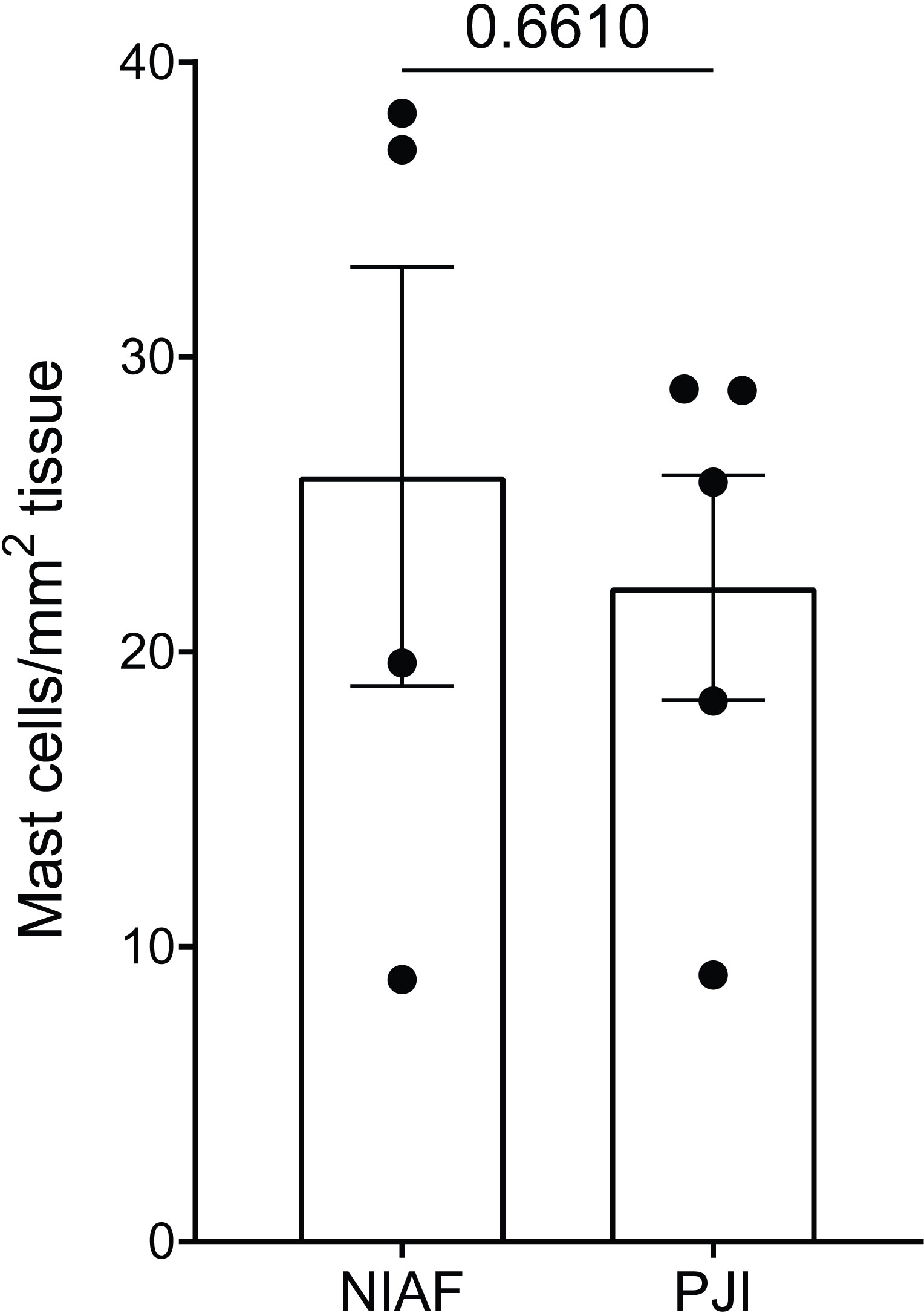
Figure 1 Density of mast cells in periprosthetic tissue from non-infectious arthroplasty failure [NIAF; instability (n=3) and stiffness (n=1)] and periprosthetic joint infection (PJI, n=5) samples. Total mast cells count per mm2 tissue in NIAF and PJI samples were enumerated by tryptase immunohistochemical staining. Statistical significance was determined via Welch’s two-sample t-test.
Apparent mast cell activation and degranulation were evaluated microscopically in each of the samples. A release of tryptase from mast cells into the extracellular space was observed throughout each of the PJI samples, whereas tryptase in uninfected tissues remained largely punctate, isolated within cellular membranes, and not released into the surrounding tissue (Figure 2).
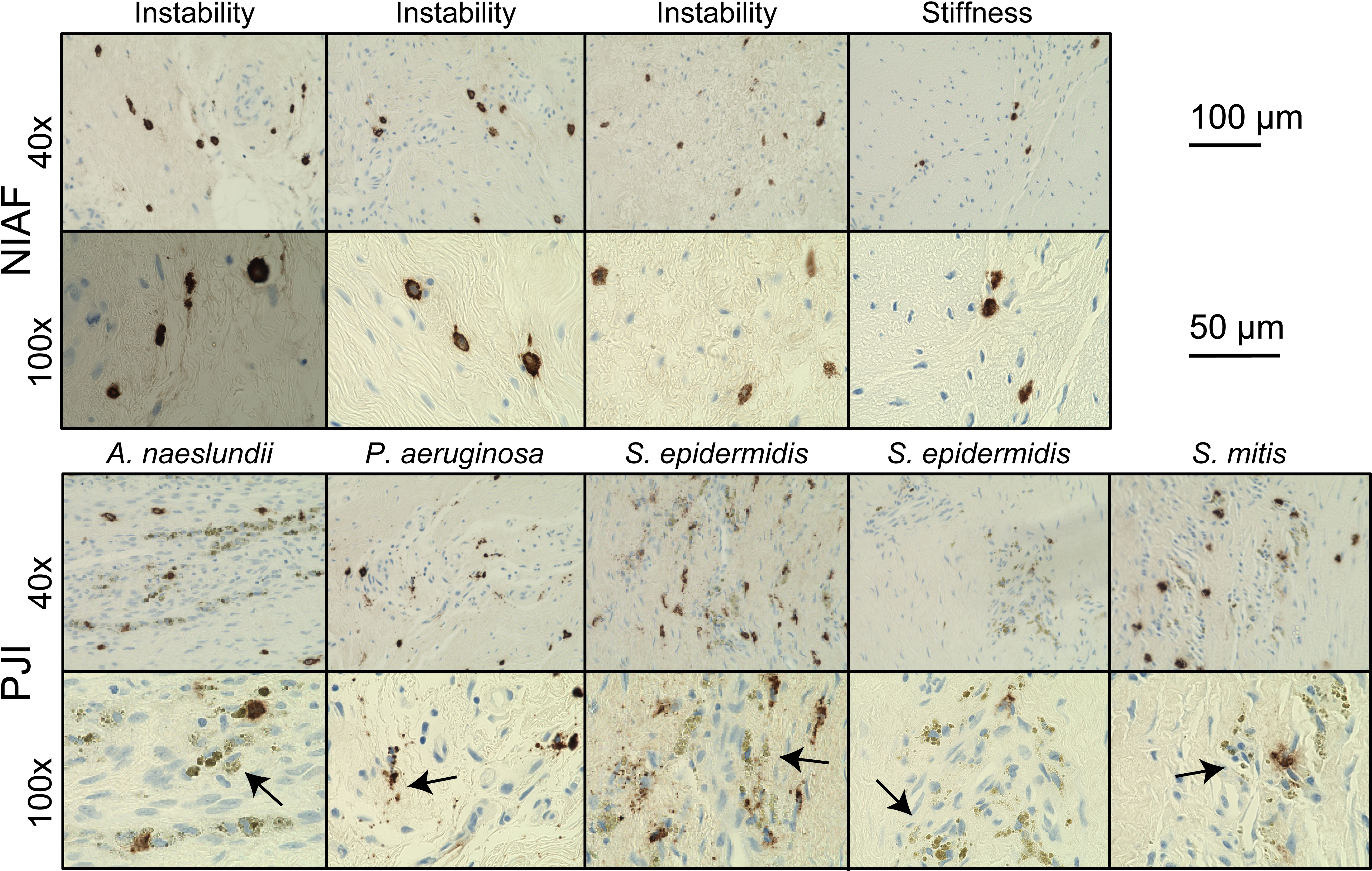
Figure 2 Mast cell activation in periprosthetic tissue from arthroplasty failure due to instability, arthroplasty failure due to stiffness, and periprosthetic joint infection (PJI). Representative images of mast cells in instability or stiffness (top) and PJI (bottom) samples at 40× and 100× magnification, showing apparent degranulation of activated mast cells (arrows) by tryptase immunohistochemical staining in PJI. Scale bars indicate 200 µm at 40× and 100 µm at 100×. Images depicted are from two causes of non-infectious of arthroplasty failure (NIAF), instability (n=3) and stiffness (n=1), and five PJIs, including Actinomyces naeslundii (n=1), Pseudomonas aeruginosa (n=1), Staphylococcus epidermidis (n=2), and Streptococcus mitis (n=1) PJI.
4 Discussion
In this preliminary study, periprosthetic tissues from five PJI and four uninfected arthroplasty failure cases were stained for tryptase, microscopically imaged, and analyzed to determine and compare overall mast cell density and activation status. Results, although preliminary, show for the first time the presence of mast cells in PJI-associated periprosthetic tissues, supporting a potential role for mast cell activation in the immune response in PJI.
Limberg et al. recently described enumerated mast cell densities in periprosthetic tissues from patients undergoing total knee arthroplasty for arthrofibrosis, a subtype of NIAF (10). Mast cell densities found here not only recapitulate those previously reported in non-infected tissues but also show similar numbers of mast cells in PJI-associated tissues. Overall, there was no statistically significant difference in mast cell numbers between PJI and NIAF samples. This is not unexpected as mast cells are considered long-lived tissue-resident cells initially seeded from fetal origins and, therefore, not expected to infiltrate into the tissues during infection (7). Of particular interest, however, was that mast cells appeared to be activated in PJI samples, with apparent increased release of extracellular tryptase, compared to the limited number and types of NIAF samples evaluated. These results support those of previously reported RNA-based predicted activated mast cell populations in sonicate fluid samples from PJI (3).
As known contributors to the chemoattraction and activation of innate immune cells during other types of bacterial infection (4–7), the results found here provide evidence for a potential role of activated mast cells in the infiltration of macrophages and neutrophils into the synovium during PJI (2, 21). Local therapeutic targeting to activate mast cells has recently been shown to enhance clearance of bacterial skin infections in mice and limit reinfection (22). A similar approach may be relevant to the treatment of PJI, paired with traditional antimicrobial treatment.
In this study, there was a lack of presumed degranulation in instability- and stiffness-associated NIAF samples. This is in contrast with previous studies, where degranulation of mast cells was observed in hip tissues from aseptic loosening (12, 13). This may be because the non-infected tissues samples analyzed here were chosen from paired sonicate fluids having low predicted activated mast cell populations, from results of transcriptomic-based cellular deconvolution. There may be differences in mast cell activation related to the underlying pathology causing NIAF. Alterations in activation status between different etiologies of NIAF are of particular interest, as they may be a potential differentiative biomarker. As such, further investigation into mast cell activation in different types of NIAF is warranted.
This preliminary study has several limitations. First, the sample cohort examined was small and not necessarily fully representative of the disease states studied; tissues were from only four NIAF and five PJI subjects and were specifically picked based on previously predicted activated mast cell quantification by cellular deconvolution in associated sonicate fluids (3). In addition, only two etiologies of NIAF, instability and stiffness, were evaluated (12, 13). While limited, this cohort does provide initial confirmatory characterization of mast cells within periprosthetic tissues associated with PJI. Next, this study lacks inclusion of native joint tissues. Whether mast cell densities and activation status differ between tissues from joints with or without implanted devices is unknown. Lastly, while this staining method is clinically validated to determine mast cell quantification, only a single mast cell stain, tryptase IHC, was utilized to determine both cell densities and activation status. Confirmatory investigations using additional cellular quantification and activation status assessment methods are needed.
The findings presented support further investigation and characterization of the role of mast cells in PJI.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Mayo Clinic Institutional Review Board (09-000808 - “Detection of Biofilms on Explanted Orthopedic Devices”). The ethics committee waived the requirement of written informed consent for participation.
Author contributions
CF contributed to study design, conducted laboratory experiments and data analysis, and drafted the manuscript. RP contributed to study conception, study design, and drafting of the manuscript. Both authors read and approved the final manuscript.
Funding
The research reported here was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number NIH R01 AR056647. CF was supported by the Mayo Clinic Graduate School of Biomedical Sciences and the Ph.D. Training Grant in Basic Immunology (NIH R25 GM055252 24).
Conflict of interest
Author RP reports grants from the companies ContraFect, TenNor Therapeutics Limited, and BIOFIRE. Author RP is a consultant to PhAST, Torus Biosystems, Day Zero Diagnostics, Mammoth Biosciences, and HealthTrackRx; monies are paid to Mayo Clinic. Mayo Clinic and RP have a relationship with Pathogenomix. RP has research supported by Adaptive Phage Therapeutics. Mayo Clinic has a royalty-bearing know-how agreement and equity in Adaptive Phage Therapeutics. RP is also a consultant to Netflix, Abbott Laboratories, Oxford Nanopore Technologies, and CARB-X. In addition, RP has a patent on Bordetella pertussis/parapertussis PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued. RP receives honoraria from the NBME, Up-to-Date and the Infectious Diseases Board Review Course.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev (2014) 27(2):302–45. doi: 10.1128/CMR.00111-13
2. Patel R. Periprosthetic joint infection. N Engl J Med (2023) 388(3):251–62. doi: 10.1056/NEJMra2203477
3. Fisher CR, Krull JE, Bhagwate A, Masters T, Greenwood-Quaintance KE, Abdel MP, et al. Sonicate fluid cellularity predicted by transcriptomic deconvolution differentiates infectious from non-infectious arthroplasty failure. J Bone Joint Surg Am (2023) 105(1):63–73. doi: 10.2106/JBJS.22.00605
4. Johnzon CF, Ronnberg E, Pejler G. The role of mast cells in bacterial infection. Am J Pathol (2016) 186(1):4–14. doi: 10.1016/j.ajpath.2015.06.024
5. Piliponsky AM, Acharya M, Shubin NJ. Mast cells in viral, bacterial, and fungal infection immunity. Int J Mol Sci (2019) 20(12):2851. doi: 10.3390/ijms20122851
6. Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: A multi-functional master cell. Front Immunol (2015) 6:620. doi: 10.3389/fimmu.2015.00620
7. St John AL, Rathore APS, Ginhoux F. New perspectives on the origins and heterogeneity of mast cells. Nat Rev Immunol (2023) 23(1):55–68. doi: 10.1038/s41577-022-00731-2
8. Nigrovic PA, Binstadt BA, Monach PA, Johnsen A, Gurish M, Iwakura Y, et al. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc Natl Acad Sci U S A (2007) 104(7):2325–30. doi: 10.1073/pnas.0610852103
9. Nigrovic PA, Lee DM. Synovial mast cells: Role in acute and chronic arthritis. Immunol Rev (2007) 217(1):19–37. doi: 10.1111/j.1600-065X.2007.00506.x
10. Limberg AK, Salib CG, Tibbo ME, Vargas-Hernandez JS, Bettencourt JW, Bayram B, et al. Immune cell populations differ in patients undergoing revision total knee arthroplasty for arthrofibrosis. Sci Rep (2022) 12(1):22627. doi: 10.1038/s41598-022-22175-3
11. Chen Z, Ma Y, Li X, Deng Z, Zheng M, Zheng Q. The immune cell landscape in different anatomical structures of knee in osteoarthritis: A gene expression-based study. BioMed Res Int (2020) 2020:9647072. doi: 10.1155/2020/9647072
12. Qiu J, Beckman MJ, Qian J, JIranek W. Simultaneous labeling of mast cell proteases and protease mRNAs at the bone-implant interface of aseptically loosened hip implants. J Orthop Res (2005) 23(4):942–8. doi: 10.1016/j.orthres.2005.04.008
13. Solovieva SA, Ceponis A, Konttinen YT, Takagi M, Suda A, Eklund KK, et al. Mast cells in loosening of totally replaced hips. Clin Orthop Relat Res (1996) 1996(322):158–65. doi: 10.1097/00003086-199601000-00020
14. Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: An evidence-based and validated criteria. J Arthroplasty (2018) 33(5):1309–14.e2. doi: 10.1016/j.arth.2018.02.078
15. ROmano CL, ROmano D, Logoluso N, Drago L. Bone and joint infections in adults: A comprehensive classification proposal. Eur Orthop Traumatol (2011) 1(6):207–17. doi: 10.1007/s12570-011-0056-8
16. Horny HP, Sotlar K, Stellmacher F, Krokowski M, Agis H, Schwartz LB, et al. The tryptase positive compact round cell infiltrate of the bone marrow (TROCI-BM): A novel histopathological finding requiring the application of lineage specific markers. J Clin Pathol (2006) 59(3):298–302. doi: 10.1136/jcp.2005.028738
17. Irani AM, Bradford TR, Kepley CL, Schechter NM, Schwartz LB. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem (1989) 37(10):1509–15. doi: 10.1177/37.10.2674273
18. Li CY. Diagnosis of mastocytosis: Value of cytochemistry and immunohistochemistry. Leuk Res (2001) 25(7):537–41. doi: 10.1016/S0145-2126(01)00029-7
19. Li WV, Kapadia SB, Sonmez-Alpan E, Swerdlow SH. Immunohistochemical characterization of mast cell disease in paraffin sections using tryptase, CD68, myeloperoxidase, lysozyme, and CD20 antibodies. Mod Pathol (1996) 9(10):982–8.
20. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods (2012) 9(7):671–5. doi: 10.1038/nmeth.2089
21. Fisher CR, Patel R. Profiling the immune response to periprosthetic joint infection and non-infectious arthroplasty failure. Antibiotics (2023) 12(2). doi: 10.3390/antibiotics12020296
Keywords: mast cells, periprosthetic joint infection, non-infectious arthroplasty failure, periprosthetic tissues, tryptase
Citation: Fisher CR and Patel R (2023) Activated mast cells in periprosthetic joint infection-associated tissue. Front. Immunol. 14:1183977. doi: 10.3389/fimmu.2023.1183977
Received: 10 March 2023; Accepted: 31 July 2023;
Published: 16 August 2023.
Edited by:
Beate E. Kehrel, University Hospital Münster, GermanyReviewed by:
Stefan Frischbutter, Charité University Medicine Berlin, GermanyJochen Salber, Ruhr-University Bochum, Germany
Copyright © 2023 Fisher and Patel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robin Patel, patel.robin@mayo.edu
 Cody R. Fisher
Cody R. Fisher Robin Patel
Robin Patel