- The Second Clinical Medical College, Xuzhou Medical University, Xuzhou, China
Background: Neuropathic pain is caused by a neurological injury or disease and can have a significant impact on people’s daily lives. Studies have shown that neuropathic pain is commonly associated with neurodegenerative diseases. In recent years, there has been a lot of literature on the relationship between neuropathic pain and neurodegenerative diseases. However, bibliometrics is rarely used in analyzing the general aspects of studies on neuropathic pain in neurodegenerative diseases.
Methods: The bibliometric analysis software CiteSpace and VOSviewer were used to analyze the knowledge graph of 387 studies in the Science Citation Index Expanded of the Web of Science Core Collection Database.
Results: We obtained 2,036 documents through the search, leaving 387 documents after culling. 387 documents were used for the data analysis. The data analysis showed that 330 papers related to neuropathic pain in neurodegenerative diseases were published from 2007–2022, accounting for 85.27% of all published literature. In terms of contributions to the scientific study of neuropathic pain, the United States is in the top tier, with the highest number of publications, citations, and H-indexes.
Conclusion: The findings in our study may provide researchers with useful information about research trends, frontiers, and cooperative institutions. Multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease are the three most studied neurodegenerative diseases. Among the pathological basis of neurodegenerative diseases, microglia-regulated neuroinflammation is a hot research topic. Deep brain stimulation and gamma knife radiosurgery are two popular treatments.
1 Introduction
Neurodegenerative diseases are characterized by neurodegenerative changes or apoptosis which can worsen over time and lead to functional impairment (1). Common neurodegenerative diseases include multiple sclerosis (MS), Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease and Amyotrophic lateral sclerosis. Studies have shown that patients with neurodegenerative diseases often present with pain and that the pain described by some patients with neurodegenerative diseases may be related to neuropathic pain (NP) (2).
NP is a chronic condition caused by dysfunction of the central or peripheral nervous system and its symptoms include spontaneous pain, nociceptive hyperalgesia and tactile allodynia (3). As a common form of pain in neurodegenerative diseases, NP causes chronic physical and psychological suffering and places a tremendous burden on patients, physicians and the health care system (4). However, due to the specific nature of neurodegenerative diseases (e.g. cognitive dysfunction), patients are less able to recognize and self-report pain (5), which increases negative emotions in patients with neurodegenerative diseases and not only further aggravates their cognitive dysfunction, but also seriously affects their quality of life and expected medical outcomes. It is therefore necessary to focus on pain in patients with neurodegenerative diseases. Studies have shown that abnormal activation of central microglia (6), neuroinflammation (7, 8), demyelinating lesions (9), altered cell signaling (10, 11), and acquired senescence/cell death (12) are mechanisms that contribute to the pathogenesis of neurodegenerative diseases and that some of these mechanisms are also mechanisms that induce NP, suggesting a close link between the two (13).
Bibliometric analysis is an emerging tool to obtain more quantitative information on cooperation between various organizations, the influence of publications, and developing trends (14, 15). At present, there are no bibliometric analysis papers focusing on the role of NP in neurodegenerative diseases. Therefore, we conducted a detailed search of the Web of Science Core Collection Database and analyzed the results through Microsoft Excel (version 2019), CiteSpace (version 6.1.R6 and version 5.6.R5) and other software to summarize previous research results and provide references for subsequent studies.
2 Materials and methods
2.1 Search strategy
The data of this study came from the Science Citation Index-Expanded of the Web of Science Core Collection Database. We conducted a literature search on January 2, 2023, and 2,036 pieces of literature were retrieved. The retrieval strategy was as follows: TS= (“Neuropathic Pain” OR “Neuralgia”) AND TS = (“multiple sclerosis” OR “amyotrophic lateral sclerosis” OR “Parkinson’s” OR “Parkinson disease” OR “Alzheimer’s” OR “Alzheimer disease” OR “Huntington’s” OR “Huntington disease” OR Neurodegenerative). The papers obtained were from 1994–2022 and the type of literature was an article or a review. The search strategy identified papers that mention these terms in the title, abstract, author keywords, or keywords plus. After that, we checked the titles, abstracts, author keywords, and keywords plus of all the documents, and eliminated those that were not relevant. Finally, 387 documents remained after elimination, and all of them were identified by CiteSpace.
2.2 Analytical tool
We extracted the literature downloaded from the Science Citation Index-Expanded of the Web of Science Core Collection Database using Excel, and the extracted data included full records and the references cited. CiteSpace (16) is a visual analysis software developed by Dr. Chao-Mei Chen based on the theory of citation analysis. We used the software to transform quantitative literature data into visual maps and networks that provide key information including research hotspots, frontiers, and country cooperation between countries/regions/institutions. There are different nodes and links in various CiteSpace visual graphs. The nodes can represent different keywords, countries, institutions, journals, etc. The larger the node, the more emergence or citations in this area. The links between nodes represent their relationships. When countries/regions/institutions represented by node appear in the same document, there is a connecting line between the two. Different colors represent different years of issue. The newer the date, the warmer the color of the node/link, and the earlier the date, the cooler the color of the node/link. The centrality indicates the importance of this node in the network, and in CiteSpace, nodes with purple rings are considered to be centrally located with high centrality. In addition, we used VOSviewer (version 1.6.18) to assist in analysis and mapping.
3 Results
3.1 Analysis of publication outputs
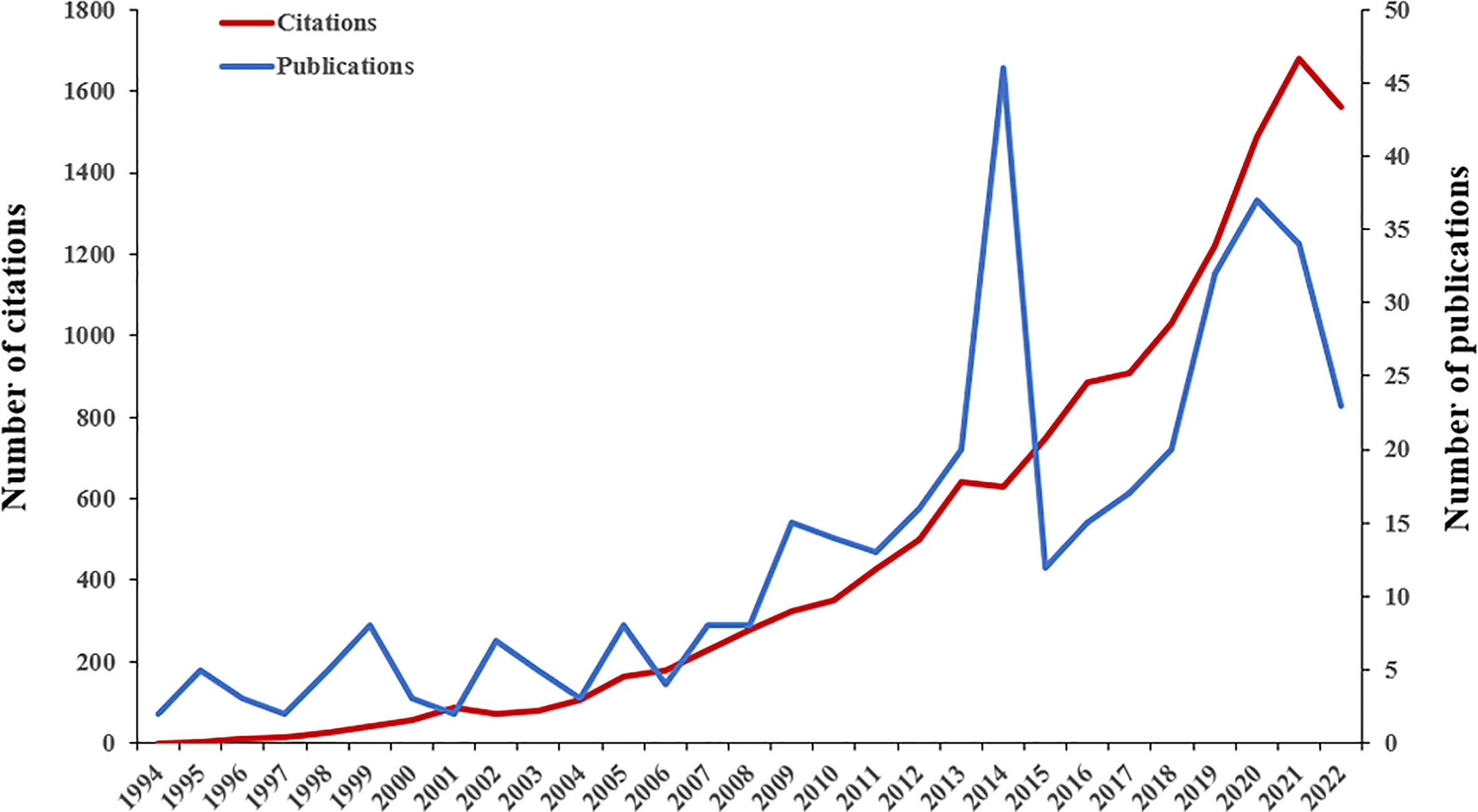
A total of 387 papers from 1994 to 2022 were eventually used for data analysis. According to the time trend analysis (Figure 1), the publications increased from 2 in 1994 to 23 in 2022. The number of publications fluctuated. The growth of publications in the literature could be roughly divided into three stages (1994–2006, 2007–2014, and 2015–2022). The number of publications fluctuated at a lower level from 1994 to 2006. Next, the number of publications increased fluctuating from 2007 to 2014 and peaked in 2014. In the third stage, there was a largely year-on-year trend of increasing numbers in 2015–2020, but in 2022 there was a large drop in the number of publications. Although the second and third stages covered only 8 years, the number of articles published reached 140 and 190 respectively, far exceeding the first stage. In total, the papers published in the third stage accounted for 49.10% of all published literature. Figure 1 also shows a gradual increase in the number of citations to the literature. 387 papers were cited 13,731 times and the average number of citations per item was 35.48.
3.2 Analysis of countries and institution
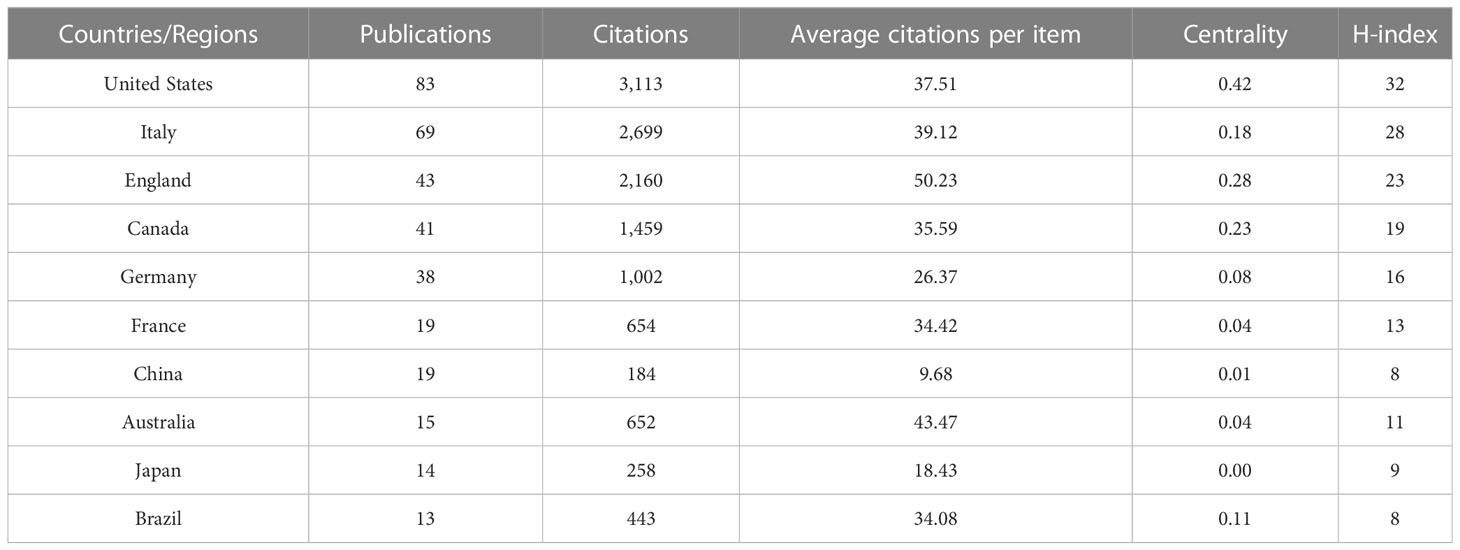
The 387 papers were contributed by 51 different countries/regions. The top ten countries/regions by number of publications are shown in Table 1. According to the number of publications, the United States (83) was the most prolific country, followed by Italy (69), England (43), and Canada (41). The United States also had the highest number of citations (3,113) and H-index (32). In order of centrality, the top three countries were the United States (0.42), Italy (0.18), and England (0.28). However, some prolific countries such as Germany, France, and China had a centrality of less than 0.1. Figure 2 shows the cooperation links between each country/region.
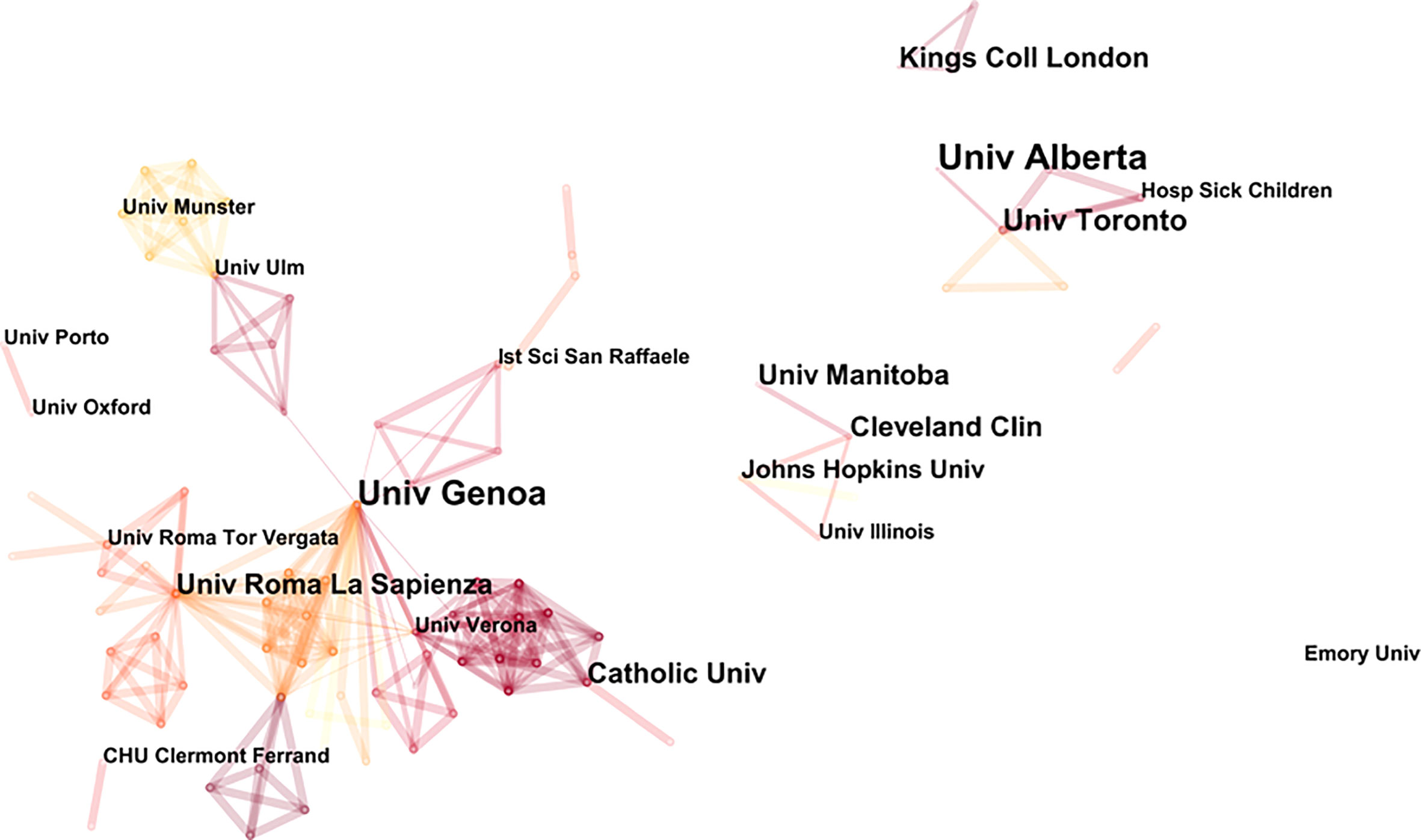
Prolific institutions in publications on NP in neurodegenerative diseases are presented in Table 2. The most prolific institution was the University of Alberta (9 publications), followed by the University of Genoa (8 publications) and the University of Toronto (6 publications). Most of the top ten most prolific institutions were from Canada and the United States. Figure 3 illustrates the partnership between the different institutions. The University of Genoa and the Sapienza University of Rome were two important nodes in this network.
3.3 Analysis of journals
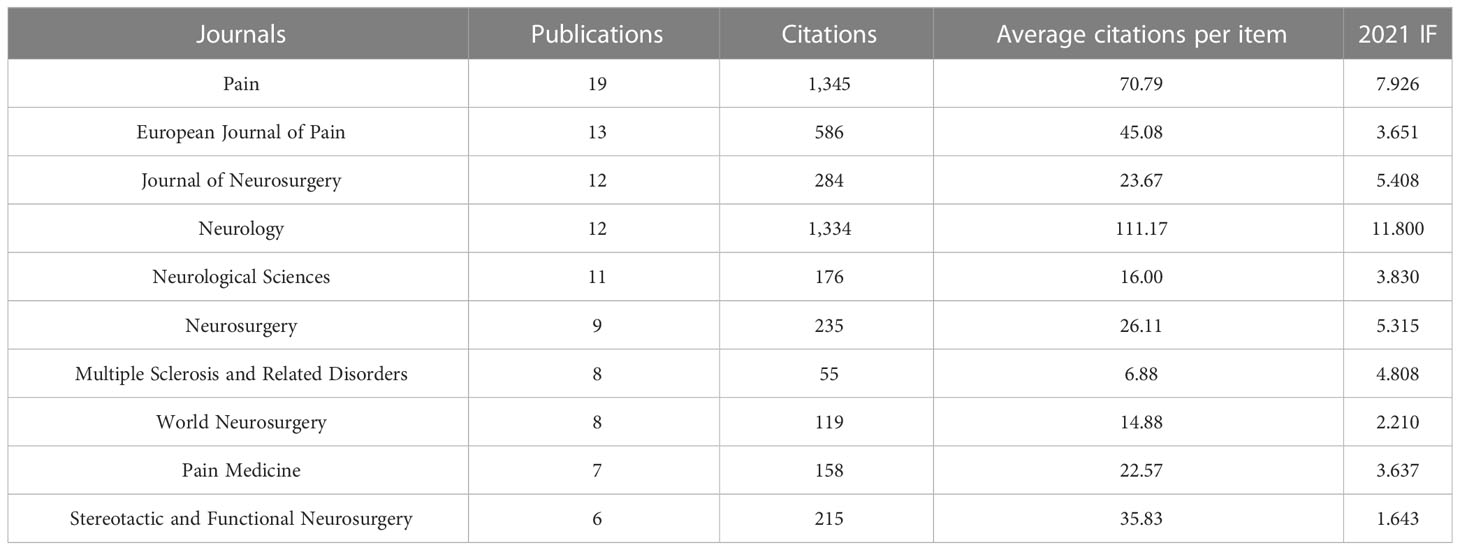
The 387 papers were published in 197 different journals. The top ten productive journals in publishing articles or reviews on NP in neurodegenerative diseases are shown in Table 3. They accounted for 27.13% (105 publications) of the total publication outputs. Through the results of journals, we found that Pain published the highest number of literatures (19, 4.910%), followed by European Journal of Pain (13, 3.359%), Journal of Neurosurgery (12, 3.101%), and Neurology (12, 3.101%). Among the top 10 journals, Pain was cited the most and Neurology was cited the second most, with these two journals being cited far more often than others. Neurology also had the highest average citations per item (111.17; IF2021, 11.8).
3.4 Analysis of authors
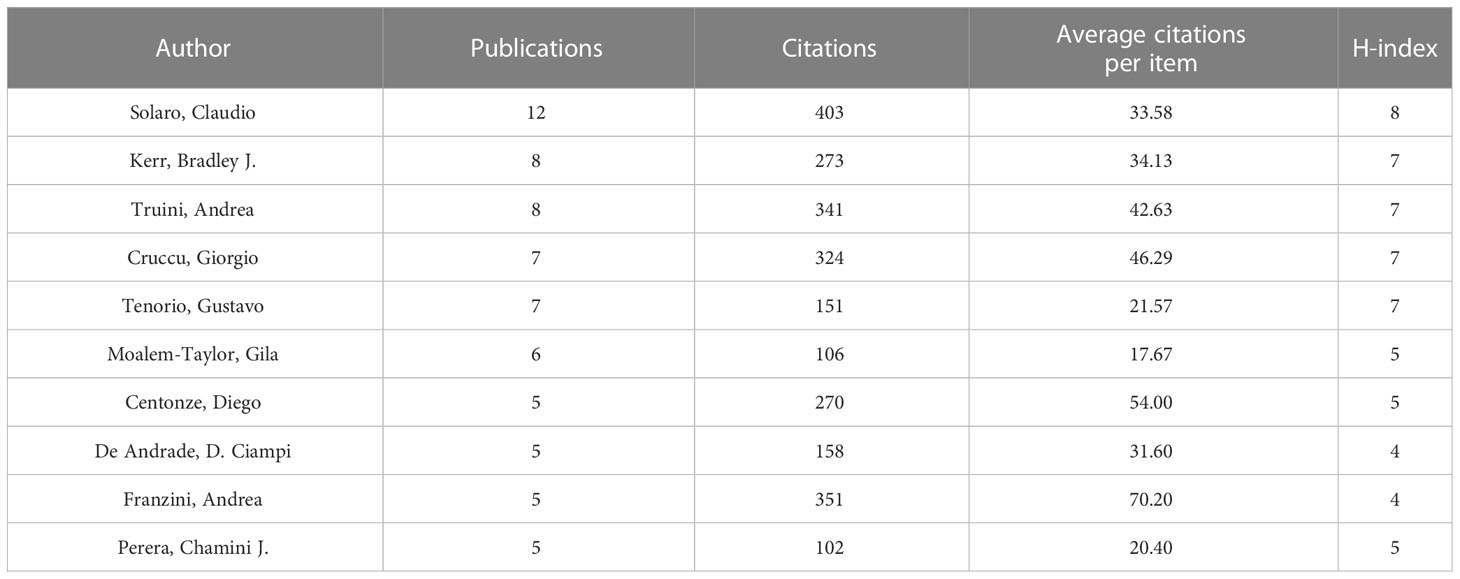
The 387 papers on NP in neurodegenerative diseases were contributed by 1,871 authors. Table 4 shows the top ten authors by publication. Among the most prolific authors, Solaro, Claudio ranked the first with 12 publications, followed by Kerr, Bradley J. (8 publications), Truini, Andrea (8 publications) and Cruccu, Giorgio (7 publications). Solaro, Claudio had the most citations (403), followed by Franzini, Andrea (351). Franzini, Andrea had the highest number of average citations per item, far more than anyone else.
3.5 Analysis of the 10 most-cited papers
The number of citations of a study’s published literatures can be used to demonstrate its academic impact on the field of study. Table 5 lists the ten most-cited papers and the average top ten cited papers per year on NP in neurodegenerative diseases. The ten most-cited papers ranged from 205 to 447, and the combined citations of the ten most-cited papers accounting for 22.23% of the total citations of 387 publications. The most-cited paper was “Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis” by Rog, David J, published in Neurology in 2005.
Among the 10 most-cited papers, only one was from the last five years. “Cannabinoids and the expanded endocannabinoid system in neurological disorders” by Cristino, Luigia, published in Nature reviews neurology is the most recent and highly cited literature in the field, as well as the most cited on average per year. These highly cited papers focus mainly on the prevalence of neurodegenerative diseases, the characteristics of the disease such as NP, and the treatment of NP.
3.6 Analysis of keywords
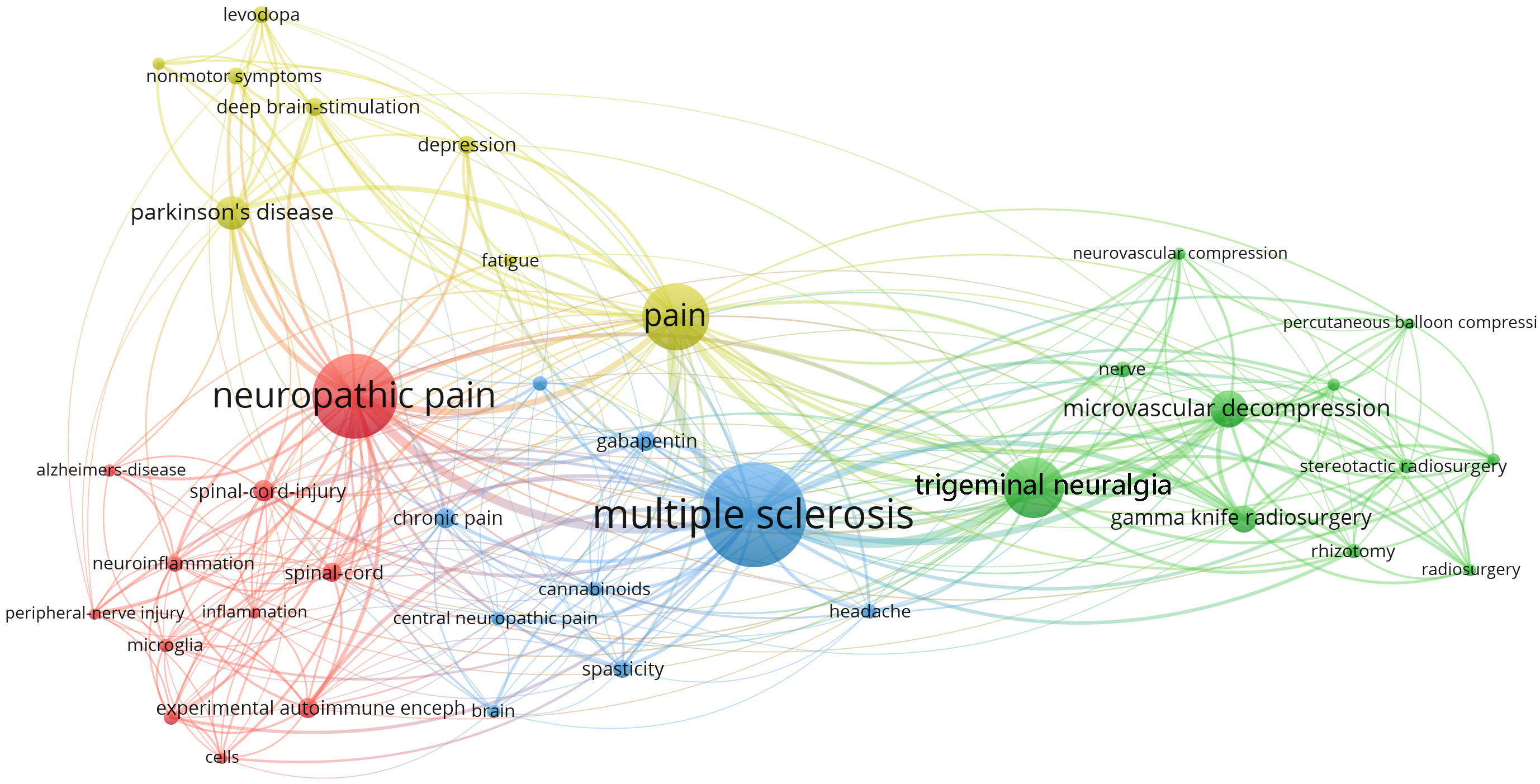
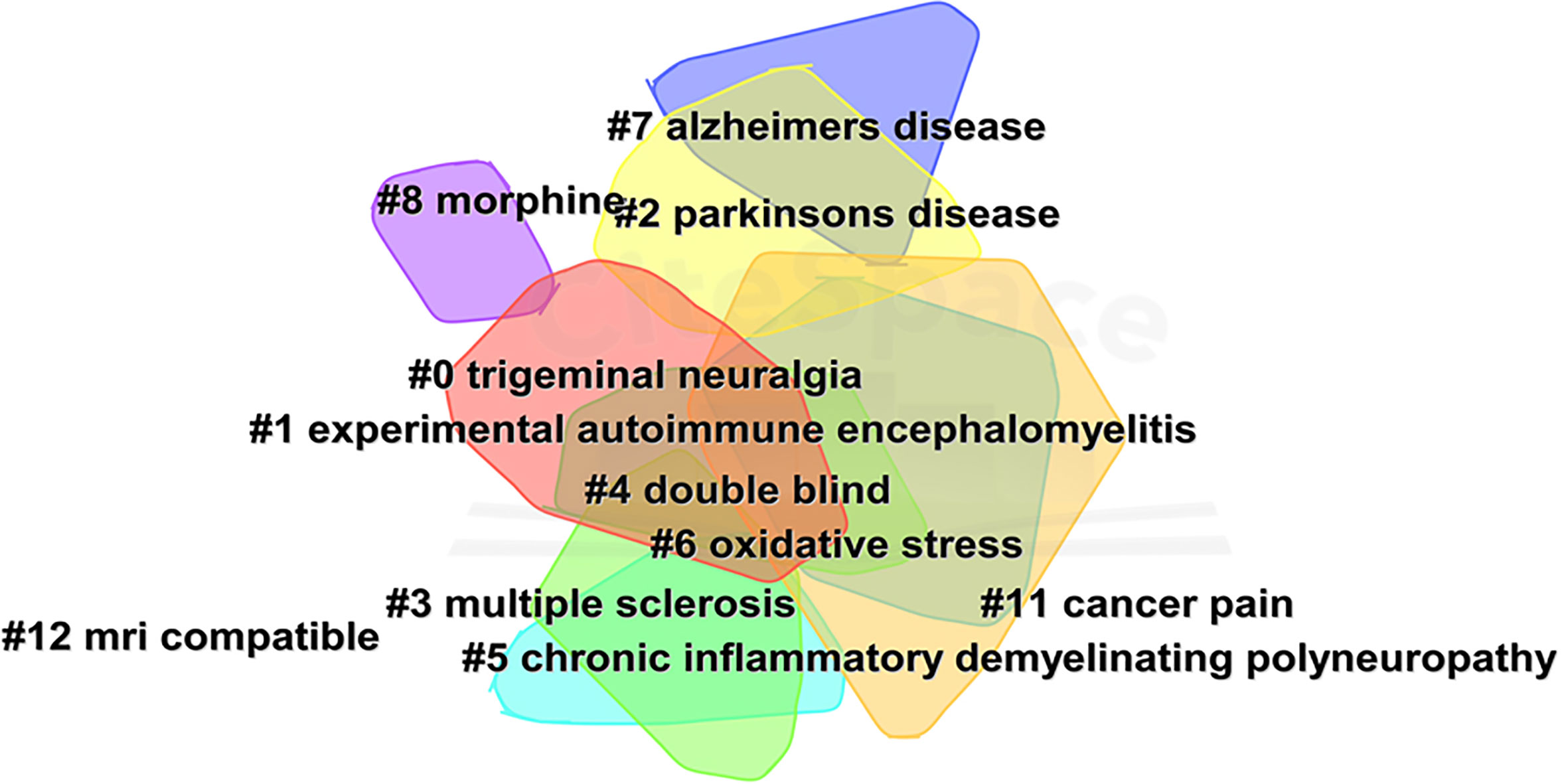
Figure 4 shows the network of keyword co-occurrence. Nodes represent different keywords, and lines represent relationships between keywords. Red, yellow, blue, and green represent the four categories centered on “neuropathic pain,” “pain,” “multiple sclerosis,” and “trigeminal neuralgia,” respectively. “Multiple sclerosis,” “neuropathic pain,” “pain,” and “trigeminal neuralgia” were the four most important keywords in this network. Current research keywords could be classified into eleven clusters, as shown in Figure 5 (Unreasonable clusters such as #9 and #10 have been eliminated). The larger the number of cluster labels, the more documents are included under that cluster. Figure 6 shows the top 25 keywords with the strongest citation bursts since 1994. Keywords with citation bursts can reflect the development of a knowledge field. Burst terms can reflect the research frontiers in the field. From Figure 6, the time of the beginning and end of the research frontier can be seen, and the length of the red bar shows the lasting time of the research frontier. The strength of the outbreak represents the intensity of the mutation. “Parkinson’s disease,” “central pain,” and “nonmotor symptom” were the top three keywords with the strongest citation. The newest research frontiers were “nonmotor symptom,” “system,” “prevalence,” and “mice”.

Figure 6 The keywords with the strongest citation bursts of publications on NP in neurodegenerative disease research.
4 Discussion
4.1 Global research trends on NP
This study presents a bibliometric analysis of NP in neurodegenerative diseases between 1994 and 2022. The number of publications has shown a slow but erratic growth trend over time. It grew rapidly from 2013 to 2014, peaking in 2014. 2015 saw an abrupt decrease, and the number of publications per year increased from 2016 to 2020 and then decreased in 2021. The citation frequency of the literature has been increased year by year, showing an upward trend, with the largest increase in 2017–2021. The top three countries/regions in terms of the number of publications and citations were the United States, Italy, and England. The three countries/regions with the highest number of average citations per item were England, Australia, and Italy. The United States had the largest number of publications, citations, centrality, and H-index, indicating its significant academic influence in the field. The fact that nearly hundreds of countries around the world contribute to the field indicates that research in this area has a broad global reach. The University of Genoa and Sapienza University of Rome had academic collaborations with many other institutions and were two important nodes in the network of institutional co-occurrence (Figure 3). Except the University of Genoa and the Sapienza University of Rome, the other institutions had less centrality and future research needs to strengthen collaboration between core institutions in different countries. Among the top ten active journals, Pain was the most cited (1,345 citations) and Neurology had the highest number of average citations per item (111.17 citations; IF2021, 11.8), Pain also had the most publications (19).
4.2 Research hotspots and frontiers on NP
4.2.1 Relationship between MS, PD, AD and NP
Among the top ten papers cited (Table 5), the paper titled, “Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases”, was published by Wang, H et al. in Neuroscience in 2002. This study proposed that NP and neurodegenerative diseases shared common pathophysiological characteristics. In recent years, studies have shown that microglia-mediated neuroinflammation, which is an important pathological basis for diseases including neurodegenerative diseases and NP, is a hot topic of interest for researchers. Interestingly, microglia and neuroinflammation were also found to connect NP to MS, PD, and AD in our keyword co-occurrence map (Figure 4), suggesting a potential link between neurodegenerative diseases and NP (Table 6).

Table 6 The common pathophysiological characteristics between neuropathic pain and Multiple sclerosis, Alzheimer’s disease, Parkinson’s disease and Huntington’s disease.
Microglia-mediated neuroinflammation is an important pathological basis for the development of neurodegenerative diseases and is also closely associated with disease progression. Neuroinflammation is an immune response activated by microglia and astrocytes in the central nervous system (brain and spinal cord) and usually associated with direct damage to the nervous system. Neuroinflammation is present in MS, PD, AD and many other neurodegenerative diseases (17). Microglia are a major component of the intrinsic immune system of the brain and spinal cord and can respond rapidly when the body is exposed to injury or infection. Studies have shown that microglia could regulate neuroinflammation associated with neurodegenerative pathologies (18). Neurodegenerative diseases may involve neuronal apoptosis, which stimulates microglia activation and promotes inflammation. Persistent inflammation generates excess free radicals, which further exacerbate oxidative stress thereby generating excess reactive oxygen species and causing irreversible cellular damage. Cellular damage leads to cytokine necrosis, which causes apoptosis and further aggravates neuroinflammation. This forms a recurrent cycle (Figure 7) that eventually induces central and peripheral sensitization, leading to NP (19, 20). According to the keyword analysis, MS, PD, and AD were the three categories of neurodegenerative diseases with the highest attention in this field.
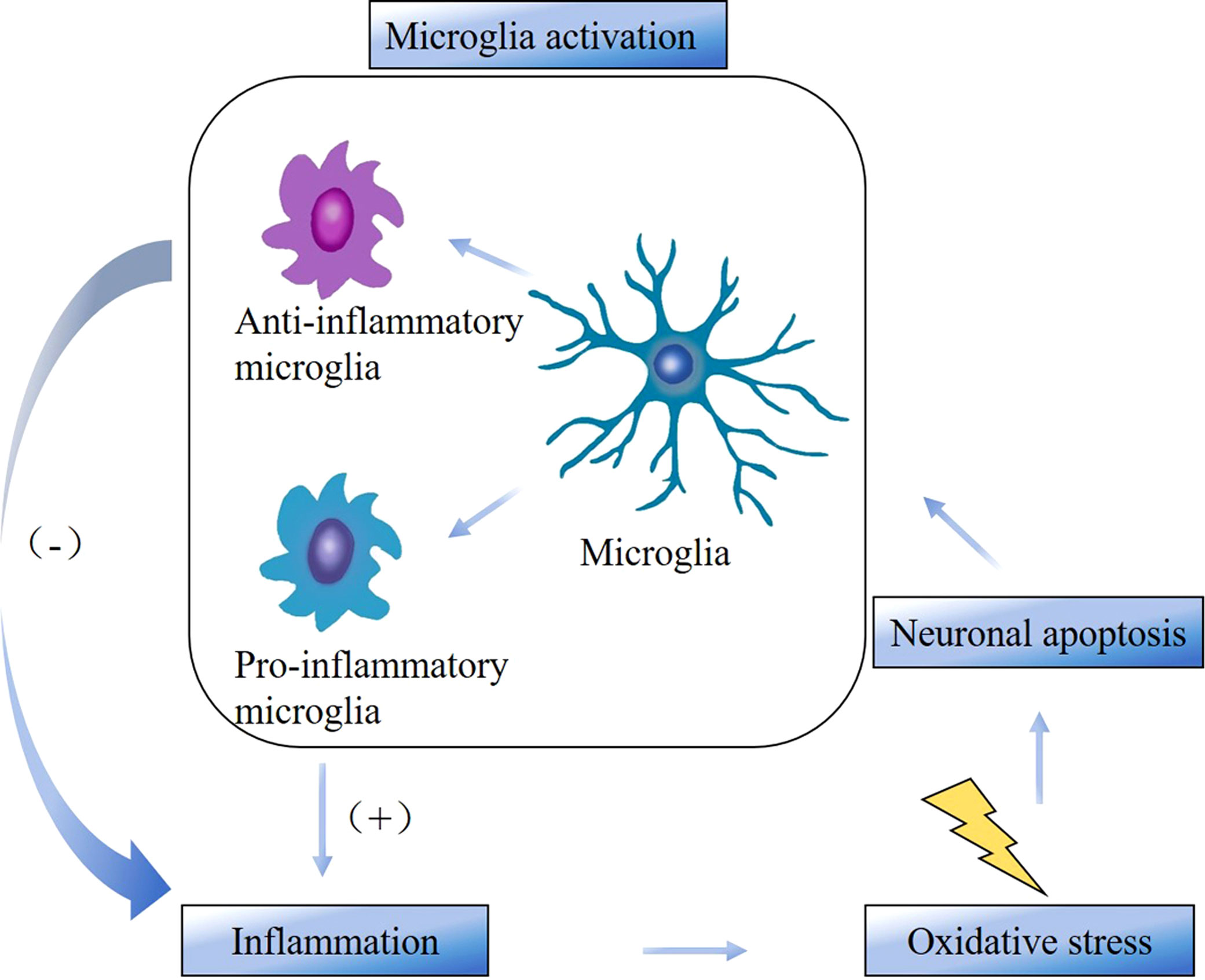
Figure 7 The cycle between microglia activation, inflammation, oxidative stress, and neuronal apoptosis.
MS is a chronic immune-mediated inflammatory demyelinating disease of the central nervous system and is one of the common neurodegenerative diseases (21). The main symptom of the disease is loss of motor and sensory function, and it is now becoming recognized that chronic pain is also a major problem that affects 50% to 80% of patients with MS. A highly cited systematic review by O’Conner et al. found that the point prevalence of pain in MS patients approached 50%, with approximately 75% of patients reporting pain within one month of assessment (22). Pain is a common complication of MS (23), and NP is a major mechanism of increased risk of pain in patients with MS (24). A systematic review showed an overall prevalence of chronic pain of 52.1% in 274 patients, with the lower extremities being most frequently involved (36.9%). Of these patients, 89 had NP, with an overall prevalence of 23.7%, and was associated with involvement of the sensory function system. Pain intensity was significantly higher in patients with NP than in patients without NP (25). Pain adversely affects family life, ability to work, etc., and pain in MS patients is affected by age, duration of the disease, degree of functional impairment, spasticity, depression, and fatigue (26, 27). Pain in MS patients includes extremity pain, trigeminal neuralgia (TN), Lhermitte’s sign, painful tonic spasms, back pain and headache (26, 28). Pain in MS is central in more than half of patients and is associated with mechanical or thermal nociceptive sensitization (29). In the co-occurrence map of keywords (Figure 4), TN was one of the most important nodes linked to MS. Meanwhile, TN was linked to “neurovascular compression” and “microvascular decompression”. TN is the most characteristic NP in MS. Possible causes of MS-related TN include the destruction of main afferent nerves by pontine plaques, neurovascular compression, and demyelinating lesions affecting the nerves. Other sensory disturbances, including persistent pain and sensory deficits, can be caused by damage to secondary neurons in the spinal trigeminal complex (30–32). Figure 6 shows that “Experimental autoimmune encephalomyelitis” was a research frontier of interest for many researchers in 2018–2019. Because it shares many clinical and neuropathological characteristics with MS, it is widely used to study MS. Olechowski et al. found that inflammation and reactive glial cell proliferation could be key mediators of abnormal pain in female C57BL/6 mice immunized with myelin oligodendrocyte glycoprotein MOG (35–55), and that model could serve as a tool to study NP in MS (33, 34). Other clinical and preclinical model studies also confirmed that demyelination and inflammation played an important role in the pathogenesis of NP in MS, and the progression of MS was associated with abnormal activation of microglia and astrocytes (29).
PD is a multi-system, progressive, incurable neurodegenerative disorder (35), associated with the loss of dopaminergic neurons in the substantia nigra of the brain. As the disease progresses, motor impairment and nonmotor symptoms are a considerable burden of disease. The outbreak graph for keywords (Figure 6) shows that “nonmotor symptom” was one of the latest frontiers of research. Pain has been shown to begin with clinical episodes of PD or follow thereafter as a nonmotor feature of PD (36). Beiske et al. published the study titled, “Pain in Parkinson’s disease: Prevalence and characteristics” in Pain in 2009 (37). They conducted questionnaires, neurological examinations, and structured interviews with 176 family-living Parkinson’s patients, and found that pain was significantly more common in Parkinson’s patients compared to the general population. The prevalence of pain in the PD population is 40% to 85% (38). In patients with PD it is generally described as dystonic pain and non-dystonic pain. Of the non-dystonic sources of pain, there has been less research around central pain (39). Schestatsky et al. found that primary central pain in patients with PD was due to abnormal control of the effects of pain input on autonomic centers and that dysfunction may occur in dopamine-dependent centers that regulated autonomic function and inhibitory regulation of pain input (40, 41). Neuroinflammation is one of the hot spots of NP research in recent years and one of the important immune modulations in PD. It has been found that postmortem PD patients had a significant increase in reactive microglia in the substantia nigra (42) and significant activation of microglia had been observed in various animal models (43).This suggests that abnormal activation of microglia might lead to elevated pro-inflammatory factors and degeneration of dopaminergic neurons in the midbrain region, resulting in pain (44).
AD is also a common neurodegenerative disorder characterized by persistent neuroinflammation leading to overall cognitive decline and progressive loss of memory and reasoning skills (45). The amyloid cascade hypothesis proposes that the misfolded and aggregated β-amyloid protein (Aβ) triggers the pathological characteristics of AD, such as neuronal cell death (46, 47), which requires clearance to maintain normal neuronal function. Many patients with AD suffer from NP, and studies have shown that pain can cause cognitive dysfunction (48, 49), patients with pain in multiple parts of the body have a higher risk of dementia (50). Studies have shown that microglia-regulated neuroinflammation plays a key role in the pathogenesis of AD (51). Activated microglia have a dual role in the clearance of Aβ. Anti-inflammatory microglia activation contributes to Aβ clearance which reduces Aβ accumulation (17, 52), whereas pro-inflammatory microglia activation can induce dysregulated neuroinflammatory responses and leads to synapse loss, neuronal distress, and Aβ production (53, 54). The use of targeting microglia in the central nervous system can provide a new reference for diagnosis and intervention in AD.
4.2.2 Treatment of NP
The treatment of NP in neurodegenerative diseases is a great challenge due to the heterogeneity of etiology, symptoms, and underlying mechanisms. Treatment of NP can be divided into pharmacological and non-pharmacological treatments. Non-pharmacological treatments can continue to be divided into invasive and non-invasive treatments.
The main drugs currently used to treat NP are nonsteroidal anti-inflammatory drugs, opioids, antidepressants, and anticonvulsants. Anticonvulsants and antidepressants are the recommended first-line treatment drugs. “Gabapentin” and “cannabinoids” were the two drugs that appear on the map of keyword co-occurrence (Figure 4), and “gabapentin” was also a frontier of research during 2001-2012. Among the top ten cited papers (Table 5), there were 4 papers related to “gabapentin” and “cannabinoids”. These four papers are also among the top ten averages citations per year.
Gabapentin is an anticonvulsant. Studies have shown that gabapentin was particularly effective in relieving allergic reactions and nociceptive sensitization in animal models (55). The drug had been clearly shown to be effective in the treatment of NP in diabetic neuropathy (56) and postherpetic neuralgia (57). Its more favorable side effects and lack of drug-drug interactions in different patients (including the elderly) made it an attractive drug (58). Therefore, gabapentin was once considered an important drug for the treatment of NP syndromes (58, 59). Available studies have shown that short-term use (three months) could lead to adverse cardiovascular disease (60) while long-term use of gabapentin could damage musculoskeletal conditions (61–63). Knowing the potential complications can have a considerable impact on the treatment plan given by the clinician. When gabapentin is limited by adverse effects, the cannabinoid is emerging as a promising drug for the treatment of NP. The analgesic effects of exogenous cannabinoids have been extensively demonstrated in experimental models of NP, and there is growing evidence that cannabinoids can provide analgesia in situations that are difficult to treat with other treatments (64–66). However, the duration of treatment and the route of administration that provides better tolerability and safety are still lacking in-depth studies (67). Most guidelines recommend the use of monotherapy as the first and second treatment options (68, 69). Many years ago, researchers have suggested that drug combination therapy is also justified because NP results from multiple mechanisms, but few studies of combination therapy have been documented (70). Combining different medications can sometimes improve analgesia and/or tolerability (71), but the increased use of combination medications also poses some safety concerns. In 2022, Hahn et al. (72) found that opioid-gabapentin combination therapy, despite reducing gastrointestinal adverse effects, may also lead to an increased risk of central nervous system depression and death. A recent meta-analysis (73) compared the efficacy of combinations of two or more drugs with placebo or at least one monotherapy in adults with NP. Their findings failed to demonstrate that the combination of opioid-antidepressants, opioid-gabapentin, and gabapentin-antidepressants was superior to the two monotherapies. Thus, in cases where monotherapy has limited efficacy, combination therapy may be used as a complementary treatment but also requires close monitoring of individual doses to ensure safety.
In the map of keyword co-occurrence (Figure 4), “microvascular decompression,” “gamma knife radiosurgery” were associated with MS and TN, and “deep brain stimulation” was associated with PD, suggesting that microvascular decompression, gamma knife radiosurgery, and deep brain stimulation were common non-pharmacological treatments for NP in neurodegenerative diseases such as MS and PD.
Among invasive treatments, microvascular decompression is considered a safe and effective vascular compression treatment for MS-related TN. It can also be recommended as the preferred procedure in some cases of MS (74). In addition, gamma knife radiosurgery is also an effective surgical treatment for MS-related TN (75–78).
In recent years, a number of non-invasive treatments have become popular with patients. Deep brain stimulation of the subthalamic nucleus has been shown to reduce the incidence of pain and improve the quality of life in patients with idiopathic PD with refractory motor fluctuations (79). In addition, studies have shown that anodal transcranial direct current stimulation reduced pain scores in patients with central chronic pain in MS, and this effect lasted beyond the stimulation period, resulting in long-term clinical effects (80, 81). Other studies have found that transcranial magnetic stimulation could improve motor and other symptoms associated with neurodegenerative processes such as NP, amyotrophic lateral sclerosis, MS, AD, PD, or Huntington’s disease (82).
Besides, exercise has been gaining popularity in recent years as a convenient and low-cost non-invasive treatment with multiple health benefits. In animal models of NP, several studies have found that exercise could reduce mechanical and thermal nociceptive sensitization (83, 84). Human studies have shown that moderate aerobic exercise, combining aerobic and resistance exercise, or high-intensity interval training, could reduce some NP. However, some indicators of NP such as thermal pain thresholds could not be improved (85). Exercise shows beneficial effects on NP in PD. Exercise acts on the muscle activity response, and myokines released during muscle contraction can modulate its activity by interacting with receptors in microglia. On the one hand, it can inhibit the pro-inflammatory mediators induced by microglia activation in PD, thus reducing the level of pro-inflammatory cytokines. On the other hand, it upregulates anti-inflammatory cytokines, which increases the anti-inflammatory level in the central nervous system and exerts neuroprotective activity (86). Exercise prevents cell death of dopaminergic neurons and modulates pain through dopaminergic and non-dopaminergic pain inhibitory pathways. However, it is not clear whether exercise exacerbates pain and impedes neuroplastic changes thereby affecting pain regulation in the central nervous system. In exercise training, there is still a need to avoid acute exacerbation of pain after exercise, while allowing for the establishment of physical activity tolerance (87). The pattern, intensity, frequency, and duration of exercise doses required for NP need to be systematically investigated (88).
The current treatment of NP in neurodegenerative diseases is mainly symptomatic, while mechanism-based treatments are lacking. Highly effective drugs are still lacking in pharmacological treatments.
5 Benefits and limitations
This study is the first bibliometric analysis of the literature related to NP in neurodegenerative diseases from 1994 to 2022. The data for the bibliometric analysis were obtained from the Science Citation Index-Expanded of the Web of Science Core Collection Database. 2,036 papers were retrieved from the database with a specific search strategy. By checking the titles, abstracts, author keywords, and keywords plus of all the documents, we eliminated the irrelevant papers and finally left 387 papers. A total of 197 different journals were included in the study, which is quite rich in data. In addition, the study covered a wide range of fields including neuroscience, surgery, pharmacology, immunology, and biochemistry. This study analyzed annual postings, prolific journals, articles with high citations, collaborations between different countries/institutions, research hotspots, and frontiers in the field. The findings in this study may provide researchers with useful information about research trends, frontiers, and cooperative institutions. Furthermore, CiteSpace and VOSviewer were used to transform quantitative literature data into visual maps and networks, making the information easy to understand. However, it was impossible to avoid the limitations of the study. Due to the format requirements of CiteSpace, all data in this study were only retrieved from the Science Citation Index-Expanded of the Web of Science Core Collection Database so the relevant publications may not be comprehensive. In addition, articles published after the search date could not be included in our analysis, leading to a lack of timeliness in the study.
6 Conclusion
This study demonstrates from a historical perspective that the field of research on NP in neurodegenerative diseases is well developed and has broad prospects, helping us realize the main research countries and institutions, core journals, overall development trend, hotspots, and research frontiers. Some prolific institutions with low centrality still need to strengthen further academic associations to expand their influence in this field. MS, PD, and AD are the three most studied neurodegenerative diseases. Among the pathological basis of neurodegenerative diseases, microglia-regulated neuroinflammation is a hot research topic. Due to the heterogeneity of NP in neurodegenerative diseases in terms of mechanism, etiology, symptoms, and treatment, its treatment remains challenging. Deep brain stimulation and gamma knife radiation therapy are two popular methods. Most of the current treatment revolves around the disease, and a mechanism-based approach to treatment is still lacking.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
YF was in charge of literature collation, analysis, and writing. CG and CZ was in charge of literature collation and article proofreading. WZ was in charge of proofreading. JG and BC was in charge of concept design, article proofreading and project guidance. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Youth Fund of Jiangsu Basic Research Program in 2021 (BK20210907), Medical Research Project of Jiangsu Commission of Health (Z2022004) and Xuzhou Medical University Outstanding Talents Start-up Fund (RC20552057).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hou LL, Hong T. Stem cells and neurodegenerative diseases. Sci China Ser C-Life Sci (2008) 51(4):287–94. doi: 10.1007/s11427-008-0049-1
2. Hendriksen E, van Bergeijk D, Oosting RS, Redegeld FA. Mast cells in neuroinflammation and brain disorders. Neurosci Biobehav Rev (2017) 79:119–33. doi: 10.1016/j.neubiorev.2017.05.001
3. Inoue K. Purinergic signaling in microglia in the pathogenesis of neuropathic pain. Proc Japan Acad Ser B-Physical Biol Sci (2017) 93(4):174–82. doi: 10.2183/pjab.93.011
4. Smith BH, Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep (2012) 16(3):191–8. doi: 10.1007/s11916-012-0256-0
5. Achterberg WP, Pieper MJC, van Dalen-Kok AH, de Waal MWM, Husebo BS, Lautenbacher S, et al. Pain management in patients with dementia. Clin Interventions Aging (2013) 8:1471–82. doi: 10.2147/cia.S36739
6. Guo SR, Wang H, Yin YF. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front Aging Neurosci (2022) 14:815347. doi: 10.3389/fnagi.2022.815347
7. Jiang H, Zhang Y, Yue J, Shi YC, Xiao B, Xiao WB, et al. Non-coding rnas: the neuroinflammatory regulators in neurodegenerative diseases. Front Neurol (2022) 13:929290. doi: 10.3389/fneur.2022.929290
8. Giallongo S, Longhitano L, Denaro S, D'Aprile S, Torrisi F, La Spina E, et al. The role of epigenetics in neuroinflammatory-driven diseases. Int J Mol Sci (2022) 23(23):15218. doi: 10.3390/ijms232315218
9. Huang YY, Dreyfus CF. The role of growth factors as a therapeutic approach to demyelinating disease. Exp Neurol (2016) 283:531–40. doi: 10.1016/j.expneurol.2016.02.023
10. Liedhegner EAS, Gao XH, Mieyal JJ. Mechanisms of altered redox regulation in neurodegenerative diseases-focus on s-glutathionylation. Antioxidants Redox Signaling (2012) 16(6):543–66. doi: 10.1089/ars.2011.4119
11. Varela L, Garcia-Rendueles MER. Oncogenic pathways in neurodegenerative diseases. Int J Mol Sci (2022) 23(6). doi: 10.3390/ijms23063223
12. Chi H, Chang HY, Sang TK. Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci (2018) 19(10):3082. doi: 10.3390/ijms19103082
13. Wang H, Sun H, Della Penna K, Benz RJ, Xu J, Gerhold DL, et al. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience (2002) 114(3):529–46. doi: 10.1016/s0306-4522(02)00341-x
14. Ellegaard O, Wallin JA. The bibliometric analysis of scholarly production: how great is the impact? Scientometrics (2015) 105(3):1809–31. doi: 10.1007/s11192-015-1645-z
15. Albuquerque PC, Castro MJC, Santos-Gandelman J, Oliveira AC, Peralta JM, Rodrigues ML. Bibliometric indicators of the zika outbreak. PloS Negl Trop Dis (2017) 11(1):6. doi: 10.1371/journal.pntd.0005132
16. Chen CM. Citespace ii: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol (2006) 57(3):359–77. doi: 10.1002/asi.20317
17. Edison P, Brooks DJ. Role of neuroinflammation in the trajectory of alzheimer's disease and in vivo quantification using pet. J Alzheimers Dis (2018) 64:S339–S51. doi: 10.3233/jad-179929
18. Kwon HS, Koh S-H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Trans Neurodegeneration (2020) 9(1):1–12. doi: 10.1186/s40035-020-00221-2
19. Zhang Z-J, Jiang B-C, Gao Y-J. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci (2017) 74(18):3275–91. doi: 10.1007/s00018-017-2513-1
20. Cai Y, Xu J, Cheng Q. Proto-oncogene tyrosine-protein kinase src (Src) inhibition in microglia relieves neuroinflammation in neuropathic pain mouse models. Bioengineered (2021) 12(2):11390–8. doi: 10.1080/21655979.2021.2008694
21. Koudriavtseva T, Mainero C. Neuroinflammation, neurodegeneration and regeneration in multiple sclerosis: intercorrelated manifestations of the immune response. Neural Regeneration Res (2016) 11(11):1727–30. doi: 10.4103/1673-5374.194804
22. O'Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain (2008) 137(1):96–111. doi: 10.1016/j.pain.2007.08.024
23. Solaro C, Cella M, Signori A, Martinelli V, Radaelli M, Centonze D, et al. Identifying neuropathic pain in patients with multiple sclerosis: a cross-sectional multicenter study using highly specific criteria. J Neurol (2018) 265(4):828–35. doi: 10.1007/s00415-018-8758-2
24. Burkill S, Montgomery S, Kockum I, Piehl F, Strid P, Hillert J, et al. The association between multiple sclerosis and pain medications. Pain (2019) 160(2):424–32. doi: 10.1097/j.pain.0000000000001429
25. Ferraro D, Plantone D, Morselli F, Dallari G, Simone AM, Vitetta F, et al. Systematic assessment and characterization of chronic pain in multiple sclerosis patients. Neurological Sci (2018) 39(3):445–53. doi: 10.1007/s10072-017-3217-x
26. Kahraman T, Ozdogar AT, Ertekin O, Ozakbas S. Frequency, type, distribution of pain and related factors in persons with multiple sclerosis'. Multiple Sclerosis Related Disord (2019) 28:221–5. doi: 10.1016/j.msard.2019.01.002
27. Heitmann H, Biberacher V, Tiemann L, Buck D, Loleit V, Selter RC, et al. Prevalence of neuropathic pain in early multiple sclerosis. Multiple Sclerosis J (2016) 22(9):1224–30. doi: 10.1177/1352458515613643
28. Brola W, Mitosek-Szewczyk K, Opara J. Symptomatology and pathogenesis of different types of pain in multiple sclerosis. Neurologia I Neurochirurgia Polska (2014) 48(4):272–9. doi: 10.1016/j.pjnns.2014.07.009
29. Robinson RR, Dietz AK, Maroof AM, Asmis R, Forsthuber TG. The role of glial-neuronal metabolic cooperation in modulating progression of multiple sclerosis and neuropathic pain. Immunotherapy (2019) 11(2):129–47. doi: 10.2217/imt-2018-0153
30. Gass A, Kitchen N, MacManus DG, Moseley IF, Hennerici MG, Miller DH. Trigeminal neuralgia in patients with multiple sclerosis: lesion localization with magnetic resonance imaging. Neurology (1997) 49(4):1142–4. doi: 10.1212/wnl.49.4.1142
31. Meaney JFM, Watt JWG, Eldridge PR, Whitehouse GH, Wells JCD, Miles JB. Association between trigeminal neuralgia and multiple-sclerosis - role of magnetic-Resonance-Imaging. J Neurol Neurosurg Psychiatry (1995) 59(3):253–9. doi: 10.1136/jnnp.59.3.253
32. Cruccu G, Biasiotta A, Di Rezze S, Fiorelli M, Galeotti F, Innocenti P, et al. Trigeminal neuralgia and pain related to multiple sclerosis. Pain (2009) 143(3):186–91. doi: 10.1016/j.pain.2008.12.026
33. Olechowski CJ, Truong JJ, Kerr BJ. Neuropathic pain behaviours in a chronic-relapsing model of experimental autoimmune encephalomyelitis (Eae). Pain (2009) 141(1-2):156–64. doi: 10.1016/j.pain.2008.11.002
34. Duffy SS, Lees JG, Perera CJ, Moalem-Taylor G. Managing neuropathic pain in multiple sclerosis: pharmacological interventions. Medicinal Chem (2018) 14(2):106–19. doi: 10.2174/1573406413666170906122508
35. Zhou ZX, Ye PH, Li XH, Zhang YX, Li MH, Chen QY, et al. Synaptic potentiation of anterior cingulate cortex contributes to chronic pain of parkinson's disease. Mol Brain (2021) 14(1):1–17. doi: 10.1186/s13041-021-00870-y
36. Defazio G, Berardelli A, Fabbrini G, Martino D, Fincati E, Fiaschi A, et al. Pain as a nonmotor symptom of Parkinson disease - evidence from a case-control study. Arch Neurol (2008) 65(9):1191–4. doi: 10.1001/archneurol.2008.2
37. Beiske AG, Loge JH, Ronningen A, Svensson E. Pain in parkinson's disease: prevalence and characteristics. Pain (2009) 141(1-2):173–7. doi: 10.1016/j.pain.2008.12.004
38. Rana AQ, Qureshi D, Sabeh W, Mosabbir A, Rahman E, Sarfraz Z, et al. Pharmacological therapies for pain in parkinson's disease - a review paper. Expert Rev Neurother (2017) 17(12):1209–19. doi: 10.1080/14737175.2017.1385393
39. Moreno CB, Hernandez-Beltran N, Munevar D, Gutierrez-Alvarez AM. Central neuropathic pain in parkinson's disease. Neurologia (2012) 27(8):500–3. doi: 10.1016/j.nrl.2011.08.001
40. Blanchet PJ, Brefel-Courbon C. Chronic pain and pain processing in parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry (2018) 87:200–6. doi: 10.1016/j.pnpbp.2017.10.010
41. Schestatsky P, Kumru H, Valls-Sole J, Valldeoriola F, Marti MJ, Tolosa E, et al. Neurophysiologic study of central pain in patients with Parkinson disease. Neurology (2007) 69(23):2162–9. doi: 10.1212/01.wnl.0000295669.12443.d3
42. Roca V, Casabona JC, Radice P, Murta V, Pitossi FJ. The degenerating substantia nigra as a susceptible region for gene transfer-mediated inflammation. Parkinsons Dis (2011) 2011:931572. doi: 10.4061/2011/931572
43. Stefanova N. Microglia in parkinson's disease. J Parkinsons Dis (2022) 12:S105–S12. doi: 10.3233/jpd-223237
44. Ho MS. Microglia in parkinson's disease. Adv Exp Med Biol (2019) 1175:335–53. doi: https://doi.org/10.1007/978-981-13-9913-8_13
45. Marttinen M, Takalo M, Natunen T, Wittrahm R, Gabbouj S, Kemppainen S, et al. Molecular mechanisms of synaptotoxicity and neuroinflammation in alzheimer's disease. Front Neurosci (2018) 12:963. doi: 10.3389/fnins.2018.00963
46. Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of alzheimer's disease. Trends Pharmacol Sci (1991) 12(10):383–8. doi: 10.1016/0165-6147(91)90609-v
47. Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Sci (New York NY) (1992) 256(5054):184–5. doi: 10.1126/science.1566067
48. Xu H, Yue CJ, Chen L. Post-transcriptional regulation of soluble guanylate cyclase that governs neuropathic pain in alzheimer's disease. J Alzheimers Dis (2019) 71(4):1331–8. doi: 10.3233/jad-190743
49. Wang RG, Man YY, Zhou MY, Zhu YZ, Wang LW, Yang JP. Neuropathic pain-induced cognitive dysfunction and down-regulation of neuronal pentraxin 2 in the cortex and hippocampus. Neuroreport (2021) 32(3):274–83. doi: 10.1097/wnr.0000000000001584
50. Zhao W, Zhao L, Chang X, Lu X, Tu Y. Elevated dementia risk, cognitive decline, and hippocampal atrophy in multisite chronic pain. Proc Natl Acad Sci United States America (2023) 120(9):e2215192120–e. doi: 10.1073/pnas.2215192120
51. Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol (2021) 17(3):157–72. doi: 10.1038/s41582-020-00435-y
52. Ziegler-Waldkirch S, d'Errico P, Sauer J-F, Erny D, Savanthrapadian S, Loreth D, et al. Seed-induced a beta deposition is modulated by microglia under environmental enrichment in a mouse model of alzheimer's disease. EMBO J (2018) 37(2):167–82. doi: 10.15252/embj.201797021
53. Jin J, Guo J, Cai HB, Zhao CC, Wang H, Liu ZY, et al. M2-like microglia polarization attenuates neuropathic pain associated with alzheimer's disease. J Alzheimers Dis (2020) 76(4):1255–65. doi: 10.3233/jad-200099
54. Si ZZ, Zou CJ, Mei X, Li XF, Luo H, Shen Y, et al. Targeting neuroinflammation in alzheimer's disease: from mechanisms to clinical applications. Neural Regeneration Res (2023) 18(4):708–15. doi: 10.4103/1673-5374.353484
55. Klazas M, Naamneh MS, Zheng W, Lazarovici P. Gabapentin increases intra-epidermal and peptidergic nerve fibers density and alleviates allodynia and thermal hyperalgesia in a mouse model of acute taxol-induced peripheral neuropathy. Biomedicines (2022) 10(12):3190. doi: 10.3390/biomedicines10123190
56. D'Souza RS, Barman R, Joseph A, Abd-Elsayed A. Evidence-based treatment of painful diabetic neuropathy: a systematic review. Curr Pain Headache Rep (2022) 26(8):583–94. doi: 10.1007/s11916-022-01061-7
57. Cao X, Shen Z, Wang X, Zhao J, Liu W, Jiang G. A meta-analysis of randomized controlled trials comparing the efficacy and safety of pregabalin and gabapentin in the treatment of postherpetic neuralgia. Pain Ther (2023) 12(1):1–18. doi: 10.1007/s40122-022-00451-4
58. Rose MA, Kam PCA. Gabapentin: pharmacology and its use in pain management. Anaesthesia (2002) 57(5):451–62. doi: 10.1046/j.0003-2409.2001.02399.x
59. Khan OA. Gabapentin relieves trigeminal neuralgia in multiple sclerosis patients. Neurology (1998) 51(2):611–4. doi: 10.1212/wnl.51.2.611
60. Pan YH, Davis PB, Kaebler DC, Blankfield RP, Xu R. Cardiovascular risk of gabapentin and pregabalin in patients with diabetic neuropathy. Cardiovasc Diabetol (2022) 21(1):170. doi: 10.1186/s12933-022-01610-9
61. Fernandez PCR, Wright CS, Warden SJ, Hum J, Farach-Carson MC, Thompson WR. Effects of gabapentin and pregabalin on calcium homeostasis: implications for physical rehabilitation of musculoskeletal tissues. Curr Osteoporosis Rep (2022) 20(6):365–78. doi: 10.1007/s11914-022-00750-x
62. Russo M, Graham B, Santarelli DM. Gabapentin-friend or foe? Pain Pract (2023) 23(1):63–9. doi: 10.1111/papr.13165
63. Jorgensen EB, Overgaard LK, Folkestad L, Damkier P, Hallas J, Henriksen DP. The risk of fragility fractures following initiation of gabapentin and pregabalin-a Danish, nationwide, high-dimensional propensity score-matched cohort study. Basic Clin Pharmacol Toxicology (2023) 132(5):384–391. doi: 10.1111/bcpt.13825
64. Fine PG, Rosenfeld MJ. Cannabinoids for neuropathic pain. Curr Pain Headache Rep (2014) 18(10):1–9. doi: 10.1007/s11916-014-0451-2
65. Boychuk DG, Goddard G, Mauro G, Orellana MF. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache (2015) 29(1):7–14. doi: 10.11607/ofph.1274
66. Hossain MZ, Ando H, Unno S, Kitagawa J. Targeting peripherally restricted cannabinoid receptor 1, cannabinoid receptor 2, and endocannabinoid-degrading enzymes for the treatment of neuropathic pain including neuropathic orofacial pain. Int J Mol Sci (2020) 21(4):1423. doi: 10.3390/ijms21041423
67. Quintero JM, Pulido G, Giraldo LF, Leon MX, Diaz LE, Bustos RH. A systematic review on cannabinoids for neuropathic pain administered by routes other than oral or inhalation. Plants-Basel (2022) 11(10):1357. doi: 10.3390/plants11101357
68. Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, et al. Efns guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol (2006) 13(11):1153–69. doi: 10.1111/j.1468-1331.2006.01511.x
69. Moulin DE, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian pain society. Pain Res Manage (2014) 19(6):328–35. doi: 10.1155/2014/754693
70. Eisenberg E, Suzan E. Drug combinations in the treatment of neuropathic pain. Curr Pain Headache Rep (2014) 18(12):1–8. doi: 10.1007/s11916-014-0463-y
71. Jesus CHA, Ferreira MV, Gasparin AT, Rosa ES, Genaro K, Crippa JAD, et al. Cannabidiol enhances the antinociceptive effects of morphine and attenuates opioid-induced tolerance in the chronic constriction injury model. Behav Brain Res (2022) 435:114076. doi: 10.1016/j.bbr.2022.114076
72. Hahn J, Jo Y, Yoo SH, Shin J, Yu YM, Ah YM. Risk of major adverse events associated with gabapentinoid and opioid combination therapy: a systematic review and meta-analysis. Front Pharmacol (2022) 13:1009950. doi: 10.3389/fphar.2022.1009950
73. Balanaser M, Carley M, Baron R, Finnerup NB, Moore RA, Rowbotham MC, et al. Combination pharmacotherapy for the treatment of neuropathic pain in adults: systematic review and meta-analysis. Pain (2023) 164(2):230–51. doi: 10.1097/j.pain.0000000000002688
74. Broggi G, Ferroli P, Franzini A, Servello D, Dones I. Microvascular decompression for trigeminal neuralgia: comments on a series of 250 cases, including 10 patients with multiple sclerosis. J Neurol Neurosurg Psychiatry (2000) 68(1):59–64. doi: 10.1136/jnnp.68.1.59
75. Rogers CL, Shetter AG, Ponce FA, Fiedler JA, Smith KA, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia associated with multiple sclerosis. J Neurosurg (2002) 97:529–32. doi: 10.3171/jns.2002.97.supplement_5.0529
76. Lee AT, Raygor KP, Elefant F, Ward MM, Wang DD, Barbaro NM, et al. Comparison of stereotactic radiosurgery and radiofrequency ablation for trigeminal neuralgia in multiple sclerosis patients. Stereotactic Funct Neurosurg (2020) 98(6):378–85. doi: 10.1159/000509315
77. Verheul JB, Hanssens PEJ, Lie ST, Leenstra S, Piersma H, Beute GN. Gamma knife surgery for trigeminal neuralgia: a review of 450 consecutive cases. J Neurosurg (2010) 113:160–7. doi: 10.3171/2010.7.Gks10978
78. Mathieu D, Effendi K, Blanchard J, Seguin M. Comparative study of gamma knife surgery and percutaneous retrogasserian glycerol rhizotomy for trigeminal neuralgia in patients with multiple sclerosis clinical article. J Neurosurg (2012) 117:175–80. doi: 10.3171/2012.6.Gks12987
79. Cury RG, Galhardoni R, Fonoff ET, Ghilardi MGD, Fonoff F, Arnaut D, et al. Effects of deep brain stimulation on pain and other nonmotor symptoms in Parkinson disease. Neurology (2014) 83(16):1403–9. doi: 10.1212/wnl.0000000000000887
80. Mori F, Codeca C, Kusayanagi H, Monteleone F, Buttari F, Fiore S, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain (2010) 11(5):436–42. doi: 10.1016/j.jpain.2009.08.011
81. Young J, Zoghi M, Khan F, Galea MP. The effect of transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis: randomized controlled trial. Pain Med (2020) 21(12):3451–7. doi: 10.1093/pm/pnaa128
82. Medina FJ, Tunez I. Huntington's disease: the value of transcranial meganetic stimulation. Curr Medicinal Chem (2010) 17(23):2482–91. doi: 10.2174/092986710791556078
83. Yamaoka S, Oshima Y, Horiuchi H, Morino T, Hino M, Miura H, et al. Altered gene expression of Rnf34 and pacap possibly involved in mechanism of exercise-induced analgesia for neuropathic pain in rats. Int J Mol Sci (2017) 18(9):1962. doi: 10.3390/ijms18091962
84. Shen J, Fox LE, Cheng J. Swim therapy reduces mechanical allodynia and thermal hyperalgesia induced by chronic constriction nerve injury in rats. Pain Med (2013) 14(4):516–25. doi: 10.1111/pme.12057
85. Leitzelar BN, Koltyn KF. Exercise and neuropathic pain: a general overview of preclinical and clinical research. Sports Medicine-Open (2021) 7(1):1–16. doi: 10.1186/s40798-021-00307-9
86. Rieger de Almeida EJ, Ibrahim HJ, Chitolina Schetinger MR, de Andrade CM, Cardoso AM. Modulation of inflammatory mediators and microglial activation through physical exercise in alzheimer's and parkinson's diseases. Neurochemical Res (2022) 47(11):3221–40. doi: 10.1007/s11064-022-03713-x
87. Allen NE, Moloney N, van Vliet V, Canning CG. The rationale for exercise in the management of pain in parkinson's disease. J Parkinsons Dis (2015) 5(2):229–39. doi: 10.3233/jpd-140508
Keywords: bibliometric, neuroinflammation, microglia, neuropathic pain (NP), neurodegenerative diseases
Citation: Fu Y, Gong C, Zhu C, Zhong W, Guo J and Chen B (2023) Research trends and hotspots of neuropathic pain in neurodegenerative diseases: a bibliometric analysis. Front. Immunol. 14:1182411. doi: 10.3389/fimmu.2023.1182411
Received: 08 March 2023; Accepted: 23 June 2023;
Published: 12 July 2023.
Edited by:
Daniela Adamo, University of Naples Federico II, ItalyCopyright © 2023 Fu, Gong, Zhu, Zhong, Guo and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binglin Chen, MTE4Nzg3ODU3MkBxcS5jb20=; Jiabao Guo, Z2xvcmlhMDkxM2dqYkAxNjMuY29t
 Yujie Fu
Yujie Fu Chan Gong
Chan Gong Chenchen Zhu
Chenchen Zhu Weiquan Zhong
Weiquan Zhong Jiabao Guo
Jiabao Guo Binglin Chen
Binglin Chen