
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Immunol. , 22 June 2023
Sec. Alloimmunity and Transplantation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1182251
This article is part of the Research Topic Novel Mechanism and Strategies to Overcome Relapse after Allogeneic Stem Cell Transplantation View all 6 articles
 Yimei Feng1†
Yimei Feng1† Ting Chen1†
Ting Chen1† Yun Zhang1†
Yun Zhang1† Han Yao1
Han Yao1 Ping Wang1
Ping Wang1 Lu Wang1
Lu Wang1 Kaniel Cassady2
Kaniel Cassady2 Zhongmin Zou3
Zhongmin Zou3 Yuqing Liu1
Yuqing Liu1 Lu Zhao1
Lu Zhao1 Lei Gao1
Lei Gao1 Xi Zhang1*
Xi Zhang1* Peiyan Kong1*
Peiyan Kong1*Introduction: While allogeneic hematopoietic stem cell transplantation (allo-HSCT) can be a curative regimen for acute myeloid leukemia (AML), relapse of AML remains a serious risk post-transplantation. Once relapsed, salvage options are limited and management of AML is difficult. Here we designed a prospective study to examine the efficacy and tolerability of maintenance therapy with azacytidine (AZA) plus low-dose lenalidomide (LEN) to prevent relapse after allo-HSCT for AML patients (ChiCTR2200061803).
Methods: AML patients post-allo-HSCT were treated with AZA (75 mg/m2 for 7 days), followed by LEN (5 mg/m2, day 10-28), and a 4-week resting interval, which was defined as one treatment cycle. A total of 8 cycles was recommended.
Results: 37 patients were enrolled, 25 patients received at least 5 cycles, and 16 patients finished all 8 cycles. With a median follow-up time of 608 (43-1440) days, the estimated 1-year disease free survival (DFS) was 82%, cumulative incidence of relapse (CIR) was 18%, and overall survival (OS) was 100%. Three patients (8%) had grade 1-2 neutropenia without fever; one patient developed grade 3-4 thrombocytopenia and minor subdural hematoma; 4/37 patients (11%) developed chronic GVHD with a score of 1-2, without requiring systemic treatment; No patient developed acute GVHD. After AZA/LEN prophylaxis, increasing numbers of CD56+NK and CD8+ T, and decreasing of CD19+ B cells were observed.
Discussion: Azacitidine combined with low-dose lenalidomide was observed to be an effective relapse prophylaxis option after allo-HSCT in AML patients, and can be administered safely without significantly increasing the risk of GVHD, infection and other AEs.
Clinical Trial Registration: www.chictr.org, identifier ChiCTR2200061803.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) plays an important role in the treatment of intermediate and high-risk acute myeloid leukemia (AML). However, 30-80% of patients are destined to relapse following allo-HSCT (1). Relapse is now the major cause of treatment failure in AML patients after HSCT. Following relapse, either a second transplantation or donor lymphocyte infusion (DLI) can be used to treat relapsed disease, but the efficacy is unsatisfactory at present (2, 3). Early intervention during AML relapse may improve patient outcomes (4), while the holy grail post-allo-HSCT remains the prevention of relapse. With the advent of new drugs, there is an increased focus on the optimization of prophylaxis regimens after transplantation to improve the long-term survival of AML patients.
Recently, two drugs azacitidine (AZA, hypomethylating agent/HMA) and lenalidomide (LEN, immunomodulator/IMiD), have each been shown to possess significant anti-leukemic activity against AML (5, 6). In relapsed patients post-allo-HSCT, 15-20% of patients achieved a complete remission (CR) when receiving AZA treatment, but median time to CR was 108 days (7), highlighting a potential opportunity to optimize AZA therapy post-allo-HSCT. Craddock et al (8). conducted a dose-finding study of LEN administered in combination with AZA in relapsed AML patients after transplantation. The maximum tolerated dose of LEN was 25 mg. In the entire patient set, the median OS of the responders was better than 10 months’ median OS observed in non-responders significantly (9). However, because LEN can activate NK cells and T cells, it has been reported that the use of LEN may cause severe graft-versus-host disease (GVHD) in patients after allo-SCT (10, 11). On the other hand, AZA can accelerate reconstitution of T regulatory cells after transplantation, induce immune tolerance, and reduce the risk of GVHD (12, 13). Therefore, we hypothesized that combination of AZA and low-dose LEN could simultaneously provide anti-leukemic activity after transplantation without increasing, overall, the risk of severe GVHD. Here we describe a prospective study to examine the efficacy and tolerability of maintenance therapy with AZA plus low-dose LEN to prevent relapse after allo-HSCT for AML patients.
This clinical trial (ChiCTR2200061803 at the Chinese Clinical Trial Registry, www.chictr.org) was initiated by our medical center (Xinqiao Hospital) as a prospective, open-label, single-arm trial design of LEN in combination with AZA in AML patients who received allo-HSCT. The enrolled patients received AZA treatment (75 mg/m2 for 7 days) on day 1, approximately 100 days post-HSCT, followed by oral administration of lenalidomide from the day 10 (5 mg/m2/day) to day 28, followed by a one-month rest interval, which was defined as one cycle. In total, 8 cycles were recommended as a complete treatment course. Patient enrollment flow chart and a diagram of the prophylaxis regimen is summarized in Figure 1.
Patient inclusion criteria: (1) Relapsed and refractory (R/R) AML patients who received allo-HSCT (for criteria of R/R AML refer to Chinese guidelines for the diagnosis and treatment of relapsed/refractory acute myelogenous leukemia (14)); (2) Successful hematopoietic reconstitution after transplantation and without GVHD (3). The acute GVHD (aGVHD) below grade 2, and/or chronic GVHD (cGVHD) below score of 2, who don’t need systemic treatment for GVHD; (3) Patients without severe infection or organ failure after transplantation.
Patient exclusion criteria: (1) Patients who have already relapsed (including molecular and cytological relapses) at the beginning of this protocol after transplantation; (2) Patients with grade 2 or above acute GVHD or chronic GVHD with a score of more than 2; (3) Those who were allergic to the study drugs; (4) Those who researchers assessed as unfit.
Leukemia relapse monitoring was performed by assessing bone marrow once a month in the first 6 months post-HSCT and thereafter every 2+ months, depending on the patient’s condition. The frequency of BM examination could be increased if necessary. Flow cytometry (FCM) and real-time quantitative polymerase chain reaction (qRT-PCR) were used to monitor minimal residual disease (MRD). The MRD monitoring interval was the same as that for the bone marrow biopsy. MRD positivity was defined as >0.01% of cells with leukemia-associated aberrant immune phenotypes in the bone marrow (15), or transcript level ≥0.001% for leukemia-related genes, including AML1/ETO, FLT3-ITD, DNMT3A, MLLAF9, MLL/AF4, etc. Patients were scored as MRD positive if they had 2 consecutive positive results using FCM or PCR or both FCM and PCR were positive in a single sample. Regimen-related hematological toxicity was monitored once a week in the outpatient clinic, including routine blood, liver, and kidney function. Analysis of lymphocyte subsets was also performed for the enrolled patients, including T, B, and NK cell analysis. The Adverse Events assessment is according to the National Cancer Institute Common Terminology Criteria 4.03.
The follow-up time point of the article ended in May 15, 2023. The primary endpoint was incidence of relapse; secondary endpoints included overall survival (OS), disease-free survival (DFS), and safety of the medication regimen. OS was measured from the time of prophylaxis intervention to death from any cause. DFS was defined as the time from prophylaxis intervention to relapse (including molecular and cytological relapse), progression, or death. Cumulative incidence of relapse (CIR) was defined as the time from prophylaxis to disease relapse or progression. Non-relapse mortality (NRM) was measured from the time of transplantation to death from any cause other than disease relapse or disease progression. The one-way ANOVA test was used to analyze the proportional difference of lymphocyte subsets among different timepoints. The Kaplan–Meier estimator was used to estimate the survival curves. P < 0.05 was assigned as statistical significance. Graphpad prism (8.0) was used to carry out all the above statistical analyses and the drawing of survival curve.
A total of 37 AML patients were enrolled in the study, including 21 males (57%) and 16 females (43%), with a median age of 31 years (4-58years). Disease status before transplantation for all patients was either CR (complete remission) or PR (partial remission), CR with MRD negative accounted for 81% (30/37), CR morphologically but MRD positive accounted for 14% (5/37). Two patients (5%) achieved PR before transplantation. The MRD of all patients was evaluated by flow cytometry, and 8 patients were fusion gene positive at first diagnosis as detected by PCR. The risk stratification of the disease was as 9 intermediate risk patients (24%) and 28 high risk patients (76%). Donors were classified as 7 HLA-identical sibling donors (19%), 13 Haplo-identical donors (35%), and 17 unrelated donors (46%). The median chemotherapy cycle before transplantation was 4 cycles (3-8 cycles). The median CD34+ cells numbers for transplantation was 7×106/kg (2.86-11.57). The median time to neutrophil engraftment (≥0.5×109/L) after transplantation was 15 (10-23) days, while that of platelet engraftment (≥20×109/L) was 16 (11-27) days (Table 1).
Sequential AZA and LEN prophylaxis therapy was safe and well tolerated. The median number of prophylaxis cycles was 7 (range, 1-8 cycles), while 25 patients received at least 5 cycles, and 16 patients finished all 8 cycles. The adverse events included a total of 3/37 patients (8%) who displayed grade 1-2 neutropenia while none had agranulocytosis with fever; one patient with grade 3-4 thrombocytopenia developed minor subdural hematoma, and recovered safely after symptom-oriented treatment; 5/37 patients (14%) exhibited mild rash with pruritus; 4/37 patients (11%) developed cGVHD following treatment with AZA/LEN with a score of 1-2 not requiring systemic treatment cGVHD; No patient developed symptoms consistent with aGVHD. The NRM was zero.
With a median follow-up time of 608 (43-1440) days, Five patients (14%) had molecular relapse but three patients became MRD negative again after continued treatment with the AZA/LEN regimen, one attaining MRD negativity after DLI and venetoclax treatment, and one patient was lost to follow-up. There were 4 patients who developed cytological relapse, received other subsequent treatment and were still alive with disease (Figure 2). Calculating results demonstrated that the estimated 1-year CIR was 18%,1-year DFS was 82%, and OS was 100% (Figure 3).
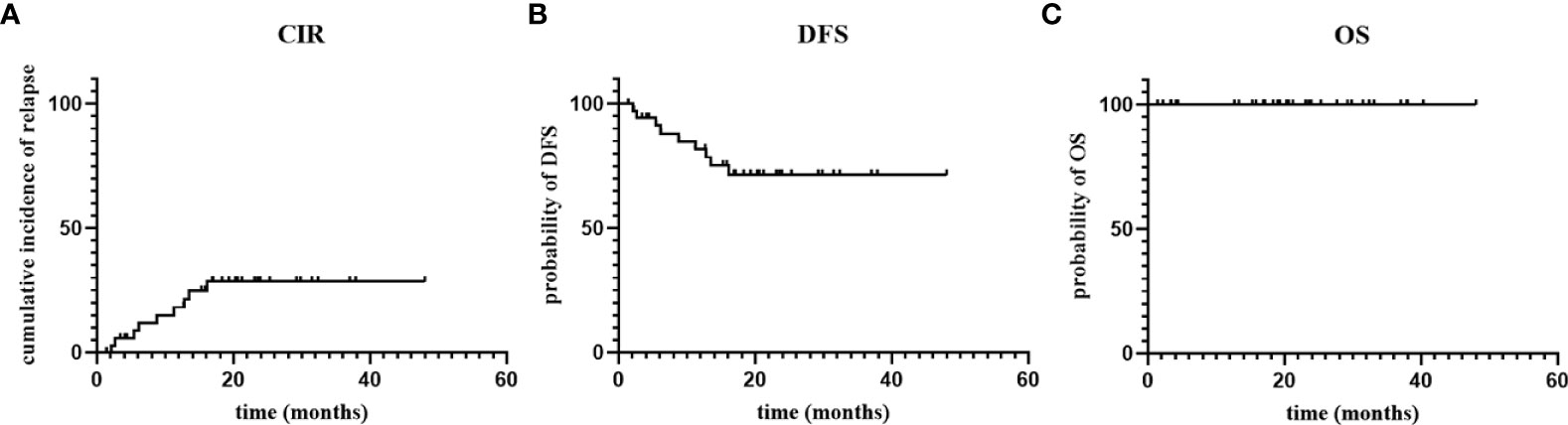
Figure 3 The cumulative incidence of relapse (CIR), disease-free survival (DFS) and overall survival (OS).
Flow cytometry analysis was performed to measure the lymphocyte subsets at four different timepoints: AML patients post-allo-HSCT prior to AZA/LEN prophylaxis (baseline), following the first month after receiving AZA/LEN prophylaxis (1M), the second month after receiving AZA/LEN prophylaxis (2M), and the third month after receiving AZA/LEN prophylaxis (3M). We measured immune cell subsets in some of the patients and observed a significant change in the proportions of CD4+ T, CD8+ T, CD19+ B and CD56+ NK cells subsets after prophylaxis. While the percentage of CD3+ T cells did not change following prophylaxis with AZA/LEN, both CD8+T and CD56+NK cell percentages in these patients were increased in the periphery after AZA and LEN intervention. In contrast, CD19+ B cells decreased dramatically following AZA/LEN administration (Figure 4).
Leukemia relapse after allo-HSCT is the major cause of treatment failure for AML patients. Once relapsed, salvage treatments are limited. Therefore, a prophylaxis regimen that can prevent AML relapse post-HSCT is needed. Currently, the most common prophylaxis regimens for preventing relapse after allo-HSCT include tyrosine kinase inhibitors (TKI), HMAs, immune checkpoint inhibitors (ICI), donor lymphocyte infusions (DLI), and immunotherapy. In this study, azacitidine combined with lenalidomide (AZA/LEN), as a novel relapse prophylaxis option, acquired satisfactory CIR, DFS and OS rate, and importantly evoked less adverse events. This regimen provided an effective and safe relapse-preventive therapy for AML patients after allo-HSCT.
The impact of AZA in the post transplantation setting is controversial (16). Several studies have previously reported AZA as an effective prophylactic treatment after allo-HSCT (17–19). In contrast, other studies failed to demonstrate a beneficial impact of post-transplantation prophylactic AZA on patient outcomes (20, 21). Moreover, AZA may lead to severe cytopenia, especially in early post-transplant period in frail patients (22). Lenalidomide, an immunomodulatory drug, is another candidate for potential maintenance therapy after allo-HSCT (23). It has broad effects on cytokines, immune cells, and angiogenesis, such as increasing the activity of NK cells and cytotoxic T cells and playing a specific role in the GVL (graft versus leukemia) effect. However, it may also enhance aGVHD even at modest doses of 5 to 10 mg10 (24). A striking observation in this study was the tolerability of LEN with acceptable rates of GVHD in patients after allo-HSCT. One possible explanation is the timing of administration, as we administered LEN 100 days post-allo-HSCT, outside the window of aGVHD. It could also be related to the combination with AZA, which may augment T-regulatory cell expansion and decrease the risk of GVHD (9, 25). Additionally, HMA application after transplantation can reactivate tumor suppressor genes and re-expression HLA-DR in tumor cells (26), and is associated with an increase in the proportion of WT1 positive cytotoxic T lymphocytes (WT1+CTL) (27). Of note, combined AZA/LEN therapy has been used as successful salvage therapy in patients who had relapsed post-transplantation. In one example, 7 of 15 (47%) patients who received at least three cycles of LEN/AZA salvage achieved a major clinical response as 3 CR, 3 CR with incomplete blood count recovery, and 1 PR patient) (8).
Our strategy in this paper was to move AZA/LEN administration earlier post-allo-HSCT to assess impact on preventing relapse after transplantation in AML patients, rather than as a salvage treatment after relapse has occurred. Our study enrolled 37 patients, with a median follow-up of 608 days. Under the intervention of AZA/LEN, only 9 cases relapsed, and the remaining 28 cases survived with disease-free in the observation period. The results showed 82% of 1-year DFS, 18% of CIR, 100% of OS, 0% aGVHD and 11% of cGVHD, which was superior to DLI based regimen with 1-year DFS 58-62.5%%, 74.5-78.8% of OS and aGVHD 7-8.7% (28–30). Notable, we defined the starting time of OS, DFS, and CIR from the implementation of prophylaxis, with the aim of better reflecting the clinical efficacy of this prophylactic measure, because there are too many interference factors such as chemotherapy and transplantation which cannot simply reflect the effectiveness of prophylaxis, if started from diagnosis or transplantation. Anyway, we re-analyzed the outcomes from time of transplantation, and found that the estimated 1-year CIR was 12%, and 1-year DFS was 88% (Supplementary File), that are superior than statistical data of which from the beginning of prophylaxis. It is speculated that benefited from the dual effects of transplantation conditioning regimen and prophylaxis regimen. Meanwhile, the state of MRD before transplantation is an important factor determining relapse after transplantation, and we found that for patients with MRD positive before transplantation, maintenance therapy earlier after transplantation can help better reduce relapse, so we are currently conducting a phase 2 study, using the AZA+LEN regimen in advance within 100 days, when hematopoietic reconstruction after transplantation. Although this paper is limited to a single arm and small size study, we demonstrated that AZA can be safely administered post-SCT in combination with LEN for prophylaxis, which seems to be associated with a higher anti-leukemic activity.
The detailed mechanism of anti-leukemic activity of AZA+LEN remains to be explored. We assessed lymphocyte subsets, and observed a significant increase in the proportions of CD8+ T, and CD56+ NK cells subsets after prophylaxis. It is speculated that cytotoxic CD8+ T cells and NK cells activation can augment GVL effect to restrain relapse. AZA has previously been shown to up-regulate tumor antigen expression on AML blasts and can also induce a CD8+ T-cell response post-allograft (19, 31), whereas post-allograft lenalidomide induces strong NK cell-mediated anti-tumor activity (23). Most reports mentioned that LEN may cause GVHD, but there were also opposite reports recognizing LEN as a useful prophylactic agent for aGVHD-induced mortality through the inhibition on lymphocyte migration to the gastrointestinal tract in mice model (32). In our study, the number of CD19+ B cells ratio decreased dramatically after prophylaxis. However, the impact of this reduction on relapse and GVHD pathogenesis, such as reduction in autoantibodies, must be further explored. Additionally, detection of other immune subsets (such as Tregs, Bregs, MDSC, etc.) and other biomarkers such as cytokines (chemokines, inflammatory factors, etc.) will be further explored in future studies.
In conclusion, the results of the current study suggest that maintenance treatment with AZA and low-dose LEN combination introduced around 100 days after allo-HSCT is surprisingly efficacious, with an acceptable toxicity profile and impressive long-term disease control. In addition, this treatment did not have much impact on cGVHD, and its impact on GVL requires further clarification with more indicators. However, this is only a small sample, single-arm, one center clinical study, and definitely is worthy of further investigation in a larger cohort with longer follow-up period.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the ethics committees of Xinqiao Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
YF and PK contributed to the design and conceptualization of the research, design of data analyses, interpretation of data, collection of data, and writing of the manuscript. TC, LW, HY, YL, LZ and LG managed patients and reviewed this paper. YZ and PW collected FCM data and draw the figures. KC, ZZ, and XZ edited this review. XZ and TC funded the work. All authors contributed to the article and approved the submitted version.
This work was supported by General Project of Chongqing Natural Science (cstc2020jcyj-msxmX0448 and cstc2020jcyj-msxmX1086), National Natural Science Foundation of China (No. 82020108004, 82200243), National Center for Clinical Medicine Research on Blood System Diseases 2020 Open Project (Key Project 2020ZKZC02).
Author KC was employed by Regeneron Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1182251/full#supplementary-material
1. Craddock C, Versluis J, Labopin M, Socie G, Huynh A, Deconinck E, et al. Distinct factors determine the kinetics of disease relapse in adults transplanted for acute myeloid leukaemia. J Internal Med (2018) 283(4):371–79. doi: 10.1111/joim.12720
2. Christopeit M, Kuss O, Finke J, Bacher U, Beelen DW, Bornhauser M, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol (2013) 31(26):3259–71. doi: 10.1200/JCO.2012.44.7961
3. Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT acute leukemia working party. J Clin Oncol (2007) 25(31):4938–45. doi: 10.1200/JCO.2007.11.6053
4. Gao L, Zhang Y, Wang S, Kong P, Su Y, Hu J, et al. Effect of rhG-CSF combined with decitabine prophylaxis on relapse of patients with high-risk MRD-negative AML after HSCT: an open-label, multicenter, randomized controlled trial. J Clin Oncol (2020) 38(36):4249–59. doi: 10.1200/JCO.19.03277
5. Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood (2015) 126(3):291–9. doi: 10.1182/blood-2015-01-621664
6. Fehniger TA, Uy GL, Trinkaus K, Nelson AD, Demland J, Abboud CN, et al. A phase 2 study of high-dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood (2011) 117(6):1828–33. doi: 10.1182/blood-2010-07-297143
7. Craddock C, Labopin M, Robin M, Finke J, Chevallier P, Yakoub-Agha I, et al. Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica (2016) 101(7):879–83. doi: 10.3324/haematol.2015.140996
8. Craddock C, Slade D, De Santo C, Wheat R, Ferguson P, Hodgkinson A, et al. Combination lenalidomide and azacitidine: a novel salvage therapy in patients who relapse after allogeneic stem-cell transplantation for acute myeloid leukemia. J Clin Oncol (2019) 37(7):580–88. doi: 10.1200/JCO.18.00889
9. Ciotti G, Marconi G, Martinelli G. Hypomethylating agent-based combination therapies to treat post-hematopoietic stem cell transplant relapse of acute myeloid leukemia. Front Oncol (2021) 11(810387). doi: 10.3389/fonc.2021.810387
10. Sockel K, Bornhaeuser M, Mischak-Weissinger E, Trenschel R, Wermke M, Unzicker C, et al. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial. Haematologica (2012) 97(9):e34–5. doi: 10.3324/haematol.2012.067629
11. Kneppers E, van der Holt B, Kersten MJ, Zweegman S, Meijer E, Huls G, et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 trial. Blood (2011) 118(9):2413–9. doi: 10.1182/blood-2011-04-348292
12. Kungwankiattichai S, Ponvilawan B, Roy C, Tunsing P, Kuchenbauer F, Owattanapanich W. Maintenance with hypomethylating agents after allogeneic stem cell transplantation in acute myeloid leukemia and myelodysplastic syndrome: a systematic review and meta-analysis. Front Med (Lausanne) (2022) 9(801632). doi: 10.3389/fmed.2022.801632
13. Choi J, Ritchey J, Prior JL, Holt M, Shannon WD, Deych E, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood (2010) 116(1):129–39. doi: 10.1182/blood-2009-12-257253
14. Leukemia, Lymphoma Group CSoHCMA. Chinese Guidelines for the diagnosis and treatment of relapsed/refractory acute myelogenous leukemia (2021). Zhonghua Xue Ye Xue Za Zhi (2021) 42(8):624–27. doi: 10.3760/cma.j.issn.0253-2727.2021.08.002
15. Zhang Y, Wang P, Cassady K, Zou Z, Li Y, Deng X, et al. Pretransplantation minimal residual disease monitoring by multiparameter flow cytometry predicts outcomes of AML patients receiving allogeneic hematopoietic stem cell transplantation. Transpl Immunol (2022) 72(101596). doi: 10.1016/j.trim.2022.101596
16. Yang G, Wang X, Huang S, Huang R, Wei J, Wang X, et al. Generalist in allogeneic hematopoietic stem cell transplantation for MDS or AML: epigenetic therapy. Front Immunol (2022) 13(1034438). doi: 10.3389/fimmu.2022.1034438
17. Tamura A, Ishida T, Saito A, Yamamoto N, Yokoi T, Uemura S, et al. Low-dose azacitidine maintenance therapy after allogeneic stem cell transplantation for high-risk pediatric acute myeloid leukemia. Pediatr Blood Cancer (2018) 65(10):e27284. doi: 10.1002/pbc.27284
18. de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer (2010) 116(23):5420–31. doi: 10.1002/cncr.25500
19. Craddock C, Jilani N, Siddique S, Yap C, Khan J, Nagra S, et al. Tolerability and clinical activity of post-transplantation azacitidine in patients allografted for acute myeloid leukemia treated on the RICAZA trial. Biol Blood Marrow Transplant (2016) 22(2):385–90. doi: 10.1016/j.bbmt.2015.09.004
20. Maples KT, Sabo RT, McCarty JM, Toor AA, Hawks KG. Maintenance azacitidine after myeloablative allogeneic hematopoietic cell transplantation for myeloid malignancies. Leuk Lymphoma (2018) 59(12):2836–41. doi: 10.1080/10428194.2018.1443334
21. Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Popat U, et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv (2020) 4(21):5580–88. doi: 10.1182/bloodadvances.2020002544
22. Silverman LR, Fenaux P, Mufti GJ, Santini V, Hellstrom-Lindberg E, Gattermann N, et al. Continued azacitidine therapy beyond time of first response improves quality of response in patients with higher-risk myelodysplastic syndromes. Cancer (2011) 117(12):2697–702. doi: 10.1002/cncr.25774
23. Wolschke C, Stubig T, Hegenbart U, Schonland S, Heinzelmann M, Hildebrandt Y, et al. Postallograft lenalidomide induces strong NK cell-mediated antimyeloma activity and risk for T cell-mediated GvHD: results from a phase I/II dose-finding study. Exp Hematol (2013) 41(2):134–42 e3. doi: 10.1016/j.exphem.2012.10.004
24. Wei A, Tan P, Perruzza S, Govindaraj C, Fleming S, McManus J, et al. Maintenance lenalidomide in combination with 5-azacitidine as post-remission therapy for acute myeloid leukaemia. Br J Haematol (2015) 169(2):199–210. doi: 10.1111/bjh.13281
25. Landman S, Cruijsen M, Urbano PCM, Huls G, van Erp PEJ, van Rijssen E, et al. DNA Methyltransferase inhibition promotes Th1 polarization in human CD4(+)CD25(high) FOXP3(+) regulatory T cells but does not affect their suppressive capacity. J Immunol Res (2018) 4973964. doi: 10.1155/2018/4973964
26. Mora-Garcia Mde L, Duenas-Gonzalez A, Hernandez-Montes J, de la Cruz-Hernandez E, Perez-Cardenas E, Weiss-Steider B, et al. Up-regulation of HLA class-I antigen expression and antigen-specific CTL response in cervical cancer cells by the demethylating agent hydralazine and the histone deacetylase inhibitor valproic acid. J Transl Med (2006) 4(55). doi: 10.1186/1479-5876-4-55
27. Wong KK, Hassan R, Yaacob NS. Hypomethylating agents and immunotherapy: therapeutic synergism in acute myeloid leukemia and myelodysplastic syndromes. Front Oncol (2021) 11(624742). doi: 10.3389/fonc.2021.624742
28. Zhu CY, Chen GF, Zhou W, Hou C, Wang XK, Wang FY, et al. Outcome and prognostic factors of high-risk acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Ann Transplant (2019) 24:328–40. doi: 10.12659/AOT.915381
29. Xiao H, Li L, Pang Y, Wu Y, Jiang Z, Liu Z, et al. Sequential treatment combining cladribine-based re-induction, myeloablative allogeneic HSCT, and prophylactic donor lymphocyte infusion: a promising treatment for refractory acute myeloid leukemia. Ann Hematol (2018) 97(12):2479–90. doi: 10.1007/s00277-018-3453-z
30. Pan WY, Li KX, Wu HY, He YZ, Du JW, Zheng YL, et al. Efficacy and safety of cladribine-based intensified conditioning regimen in hematopoietic stem cell transplantation in patients with high-risk acute myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi (2022) 30(1):65–71. doi: 10.19746/j.cnki.issn.1009-2137.2022.01.011
31. Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood (2012) 119(14):3361–9. doi: 10.1182/blood-2011-09-377044
32. Tsubokura Y, Yoshimura H, Satake A, Nasa Y, Tsuji R, Ito T, et al. Early administration of lenalidomide after allogeneic hematopoietic stem cell transplantation suppresses graft-versus-host disease by inhibiting T-cell migration to the gastrointestinal tract. Immun Inflamm Dis (2022) 10(9):e688. doi: 10.1002/iid3.688
Keywords: allo-SCT, azacitidine, lenalidomide, maintenance therapy, AML
Citation: Feng Y, Chen T, Zhang Y, Yao H, Wang P, Wang L, Cassady K, Zou Z, Liu Y, Zhao L, Gao L, Zhang X and Kong P (2023) Azacitidine and lenalidomide combination: a novel relapse prophylaxis regimen after allogeneic hematopoietic stem-cell transplantation in patients with acute myeloid leukemia. Front. Immunol. 14:1182251. doi: 10.3389/fimmu.2023.1182251
Received: 08 March 2023; Accepted: 07 June 2023;
Published: 22 June 2023.
Edited by:
Liangding Hu, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Sravanti Rangaraju, University of Alabama at Birmingham, United StatesCopyright © 2023 Feng, Chen, Zhang, Yao, Wang, Wang, Cassady, Zou, Liu, Zhao, Gao, Zhang and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Zhang, emhhbmd4eGlAc2luYS5jb20=; Peiyan Kong, cGVpeWFua29uZ0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.