
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 14 April 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1178682
This article is part of the Research TopicImmunotherapy and Small Molecule Inhibitors as Combinational Cancer TherapeuticsView all 19 articles
Anaplastic thyroid carcinoma, BRAF non-V600, NRAS, combination immunotherapy and targeted therapy, case report. Anaplastic thyroid carcinoma (ATC) is a rare type of thyroid cancer with a mortality rate near 100%. BRAF V600 and NRAS mutations are the most common drivers of ATC. While patients with BRAF V600-mutated ATC can be treated with BRAF-targeted therapy, there is no effective treatment for ATC driven by NRAS or non-V600 BRAF mutations. For patients with untargetable driver mutations, immunotherapy provides an alternative treatment option. Here, we present a metastatic ATC patient with PD-L1 positive (tumor proportion score of 60%) tumor and NRAS Q61R/BRAF D594N mutations, who progressed on PD-1 antibody sintilimab plus angiogenesis inhibitor anlotinib. The class 3 BRAF mutant D594N is sensitive to the inhibition of MEK inhibitor trametinib, and its oncogenic activity also depends on CRAF, which can be inhibited by BRAF inhibitor dabrafenib. For these reasons, the patient received a salvage treatment regime of dabrafenib, trametinib, and sintilimab, which resulted in a complete pathological response. To our best knowledge, this is the first report of successful treatment of ATC patients with concurrent NRAS/BRAF non-V600 mutations with the combination of immunotherapy and targeted therapy. Further investigation is required to decipher the mechanism by which the combination of dabrafenib/trametinib with PD-1 antibody overcomes initial immunotherapy resistance likely mediated by concurrent BRAF and NRAS mutations.
Anaplastic thyroid carcinoma (ATC) is one of the most aggressive solid tumors with a disease-specific mortality rate near 100% (1). Genomic profiling studies revealed that the major drivers of ATC are TP53, TERT, BRAF, and NRAS mutations (2). While ATC patients with BRAF V600 mutation are eligible for BRAF-targeted therapy, there is no effective treatment for NRAS-mutated ATC patients (1).
In the past decade, cancer immunotherapy has shifted the paradigm of cancer treatment. Based on the results of the KEYNOTE-158 trial, FDA approved PD-1 antibody pembrolizumab as a treatment option for patients with TMB-H (≥ 10 mut/Mb) solid tumors, which was also endorsed by the NCCN thyroid cancer guideline for ATC treatment (1). Of note, the KETNOTE-158 trial only included two patients with thyroid cancer. Recently, an investigational PD-1 antibody spartalizumab showed promising efficacy in a phase 2 trial of ATC, in which spartalizumab achieved an overall response rate (ORR) of 29% and 35% in the PD-L1 positive and high PD-L1 (≥ 50%) subgroups, respectively (3). Responses were seen in BRAF wild-type and BRAF-mutant patients. These results indicated that PD-1 antibody might be a treatment option for ATC patients with high PD-L1 expression, irrespective of their BRAF mutation status.
Both immune checkpoint blockade (ICB) and BRAF-targeted therapies have been approved for the treatment of BRAF V600-mutant melanoma (4). Of note, abnormal activation of the MAPK signaling can result in tumor-intrinsic resistance to ICB through the modulation of tumor microenvironment (TME) (5). The triplet combination of BRAF inhibitor (BRAFi), MEK inhibitor (MEKi), and PD-1/L1 antibodies have been tested in clinical trials of BRAF V600-mutant melanoma (6–8). Although the triplet regimes did not improve the response rate compared with the doublets, they improved the duration of response. Furthermore, two triplets improved progression-free survival (PFS) in the IMspire150 and KEYNOTE-022 trials (6, 7). Therefore, the NCCN guideline for cutaneous melanoma recommended two triplets regimes (vemurafenib/cobimetinib/atezolizumab and dabrafenib/trametinib/pembrolizumab) as first-line therapy options for unresectable or metastatic melanoma patients with BRAF V600 mutation (4).
According to the mechanisms of activation, BRAF mutants can be classified into three groups (9). Class 1 and 2 mutants function as RAS-independent monomers and dimers, respectively (9). Class 3 mutants have impaired kinase activity, and their oncogenic activity depends on RAS and CRAF (9). Because coexisting BRAF mutations synergize with RAS mutations in the amplification of downstream MAPK signaling, the treatment of solid tumors with coexisting BRAF/RAS mutations is more challenging than those with BRAF or RAS mutations alone. Here, we presented a PD-L1-positive metastatic ATC patient with coexisting NRAS and class 3 BRAF mutations. After progression on PD-1 antibody sintilimab plus an angiogenesis inhibitor anlotinib, she achieved a pathological complete response with the triplet regime of BRAFi dabrafenib, MEKi trametinib, and a domestic PD-1 antibody sintilimab (Figure 1A).
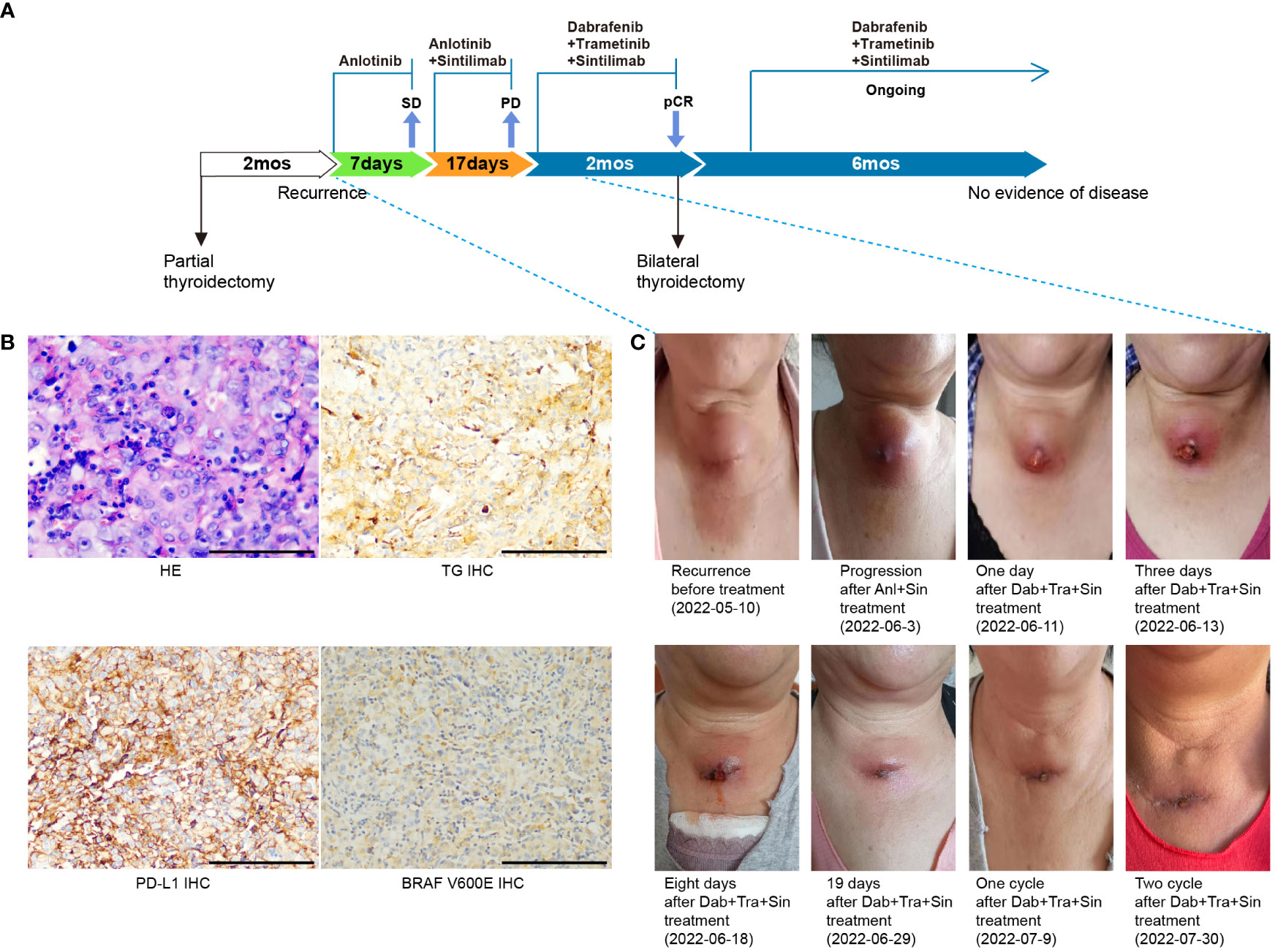
Figure 1 Case summary. (A) Summary of disease course and treatment procedure. pCR, pathological complete response; SD, stable disease; PD, progressive disease; mo, months. (B) H&E, TG, BRAF V600E and PD-L1 staining of the primary tumor. Scale bars: 50 µm. H&E: hematoxylin and eosin. (C) Representative images showing disease recurrence/progression on sintilimab plus anlotonib, and the patient’s response to the triplet regime of sintilimab, dabrafenib, and trametinib.
A 61-year-old female presented with a rapidly enlarging neck mass in November 2021. Contrast-enhanced computed tomography (CT) revealed a 1.9×1.8 cm nodule and a 3.2×2.6 cm nodule in the left and right thyroid lobes, respectively. She underwent surgical resection of partial thyroid right lobe and thyroid left lobe mass on Jan 25th, 2022. Postoperative histopathological examination confirmed multifocal anaplastic thyroid carcinoma with areas of necrosis and calcifications in the right lobe, and nodular goiter in the left. Immunohistochemical (IHC) staining was positive for Ki-67 (80%), Vimentin, TTF-1, P53, Cyclin D1, Pax-8, TG (weak, 1+), BRAF V600E (weak, 1+), and negative for AE1/AE3, CD56, Calcitonin, LCA, and HMB45 (Figure 1B). Two months later, a progressively enlarging mass appeared in her right neck, indicating disease progression (Figure 1A). PET-CT imaging revealed multiple hypoechoic nodules in cervical region VI, with the largest located in the center of 4.0×2.8 cm (Figures 1C, 2A). The patient was then referred to our hospital. She had a poor Eastern Cooperative Oncology Group (ECOG) performance status of 2, difficulty in breathing, and choking with deglutition.
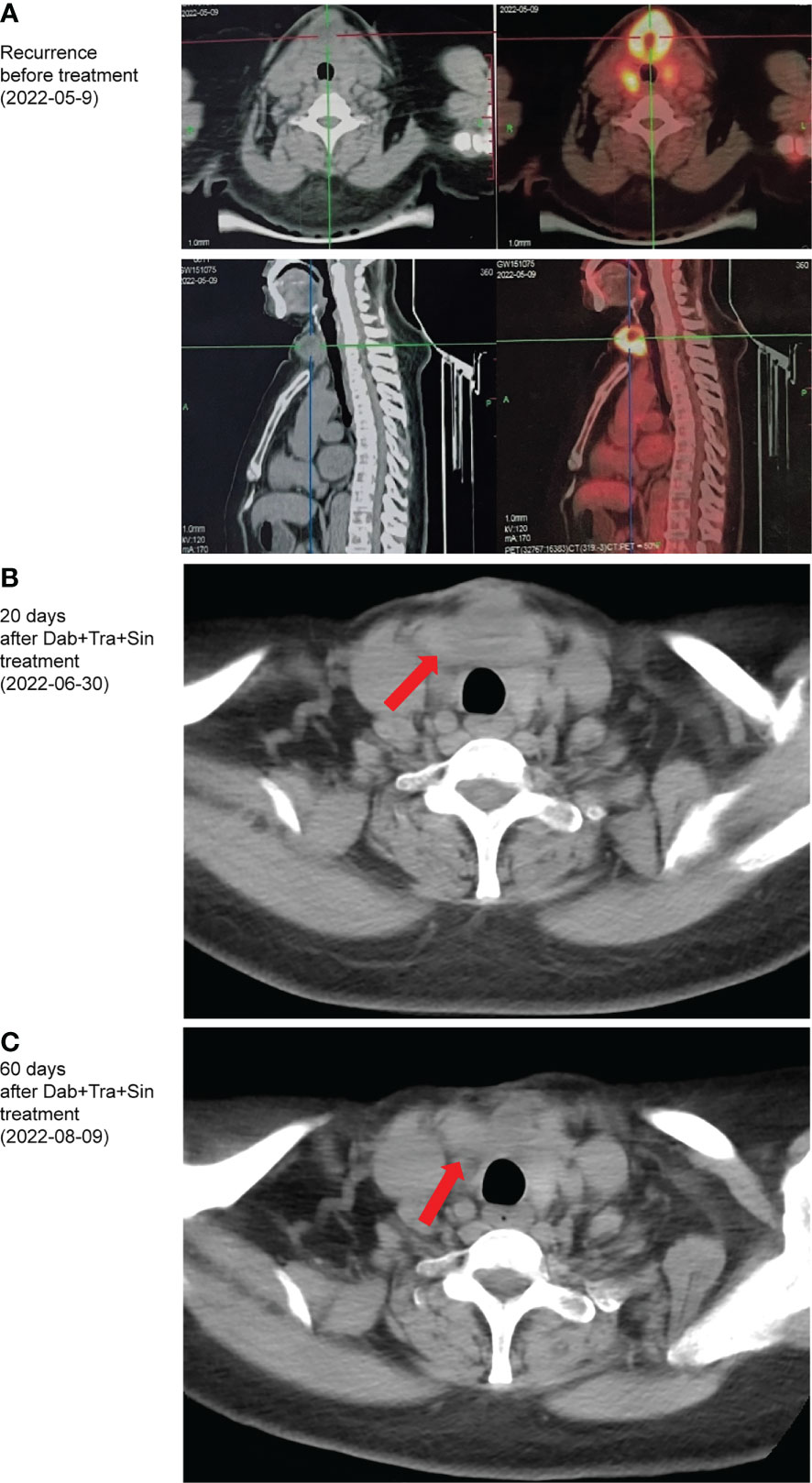
Figure 2 Computed tomography images showing the patient’ recurrence (A), as well as response to the triplet regime of sintilimab, dabrafenib, and trametinib after 20 (B), 60 (C) days of treatment.
Because radical surgery was not suitable, the patient was treated with anlotinib (10mg, QD, 2 weeks on/1 week off), a multi-targeted tyrosine kinase inhibitor targeting tumor angiogenesis and proliferation from May 13th. The lesion soon shrank by about 20%, and the patient’s skin color returned to normal. Genetic testing of the resected tumor tissue with a multi-gene next-generation sequencing (NGS) panel (Onco Panscan™, Genetron Health) revealed a tumor mutational burden of 1.41 mutations per megabase (mut/Mb), microsatellite status stable (MSS), TERT promoter mutation, an NRAS Q61R mutation with a variant allele fraction (VAF) of 10.6%, and a BRAF D594N mutation with a VAF of 12.6% (Table 1). PD-L1 IHC assay (PD-L1 IHC 22C3 pharmDx assay, Agilent Technologies, Carpinteria, CA, USA) showed a TPS of 60% and a CPS of 80 (Figure 1B). We previously reported the successful treatment of a PD-L1-positive, NRAS Q61R-mutated metastatic ATC patient with sintilimab plus an angiogenesis inhibitor anlotinib (10). Given the similarity of these two ATC patients, sintilimab (200 mg, Q3W) was added on May 21st, 2022. Unfortunately, disease progression occurred, including neck mass enlargement to 4.4×3.0 cm, the reappearance of breathing difficulty, and choking with deglutition. And this doublet regime was left off after one cycle (Figures 1A, C).
MAPK signaling amplification driven by class 3 BRAF mutant D594N is sensitive to the inhibition of trametinib (9). Furthermore, the oncogenic activity of class 3 BRAF mutants depends on CRAF (11), which can be inhibited with dabrafenib (12). Of note, the efficacy of dabrafenib plus trametinib has not been established in NRAS-driven solid tumors. Given the coexistence of NRAS Q61R and BRAF D594N mutations, the patient was then treated with a salvage regime consisting of dabrafenib (75 mg, BID), trametinib (2 mg, QD), and sintilimab (200mg, Q3W) from Jun 10th, 2022. After nine days, regression of all metastases in the bilateral neck was noted, and breathing/swallowing returned to normal (Figures 1A, C). After one cycle, the neck mass continued to shrink, and the ulcerated area was healed (Figures 1C, 2B). Fever (39.5°C) was the only adverse event observed during this triplet regime treatment. Her temperature returned to normal within 24 hours of taking off dabrafenib. The triplet regime was resumed after her temperature remained normal for 24 hours, and no subsequent fever occurred. After two cycles (six weeks) of treatment, radical surgery was evaluated as feasible (Figures 1C, 2C). The patient discontinued dabrafenib and trametinib on Aug 11th, 2022. And bilateral residual thyroidectomy plus neck lymph node dissection were performed the next day. Postoperative pathology indicated that she had a pathological complete response (pCR). Given the high risk of ATC patients with concomitant BRAF/RAS and TERT mutations (13), she continued on the triplet regime from Sep 20th and remained in remission with an excellent quality of life until the last follow-up in March 2023 (Figure 1A).
The three major histological types of thyroid cancers are differentiated thyroid carcinoma (DTC), medullary thyroid carcinoma (MTC), and anaplastic thyroid carcinoma (ATC) (1). DTC represents more than 95% of thyroid cancer cases and has a very good prognosis. In contrast, ATC patients have a poor prognosis with a historical median overall survival (OS) of four months (14). ATC responds poorly to conventional thyroid cancer treatment options including surgery, radiation therapy, chemotherapy, and radioactive iodine (RAI) therapy (1). The NCCN guideline for thyroid carcinoma recommended that ATC patients with locally resectable disease can be treated with multimodal therapy, and those with actionable mutations (BRAF, NTRK, ALK, RET, MSI, dMMR, TMB-H) can be treated with targeted therapy or immunotherapy (1).
Recent genomic profiling studies revealed that activating BRAF and RAS mutations are major drivers of thyroid cancer. The TCGA study characterized the landscape of papillary thyroid carcinoma (PTC), the most common type of thyroid cancer (15). Among 496 PTC patients, 60% carried BRAF mutations, and 13% had RAS mutations. Compared to PTC, genomic studies of large ATC cohorts are rare. In one study of 126 ATC patients, 45% had BRAF alterations (all V600E except for 1 deletion), and 24% had RAS mutations (13). In another ATC cohort (n = 196), 41% had BRAF mutations and 27% had RAS mutations (2). Interestingly, the latter study also observed non-V600 BRAF mutations in PTC, one precursor of ATC. These rare BRAF alterations include BRAF fusions, K601E, G469A, V600_K601delinsE, V600_K601>D, V600_W604>R, and V600_S605>D. Of note, BRAF V600E and RAS mutations are mutually exclusive in these thyroid cancer cohorts.
Abnormal activation of the RAF-RAS-MAPK signaling pathway is identified in more than 30% of human cancers (16). BRAF is an established therapeutic target in colorectal cancer (CRC), non-small cell lung cancer (NSCLC), melanoma, and ATC (17). Recently, FDA granted accelerated approval of dabrafenib in combination with trametinib for the treatment of almost all BRAF V600-mutated solid tumors. In contrast, there is no effective therapy for NRAS-mutated solid tumors. Furthermore, coexisting NRAS mutations can result in resistance to dabrafenib/trametinib (18, 19). Therefore, the treatment of solid tumors driven by NRAS mutation alone or in combination with BRAF mutations represents a clinical challenge.
Recent pieces of evidence indicated that immunotherapy could be an effective treatment option for ATC. In a single-center study, PD-1 antibody pembrolizumab or nivolumab achieved an overall response rate (ORR) of 16% in 13 advanced or metastatic ATC patients, including seven patients with BRAF V600E mutations (20). And responses were ongoing in four individuals. Similarly, in a phase 2 trial of advanced/metastatic ATC (n = 42), an investigational PD-1 antibody spartalizumab achieved an overall ORR of 19%, including three complete responses (CRs) and five partial responses (PRs) (3).
Given the poor prognosis and limited therapeutic options of ATC, several multitargeted tyrosine kinase inhibitors (TKIs) including sorafenib, sunitinib, imatinib, and pazopanib have been evaluated in clinical trials with unsatisfactory results. Anlotinib is a novel multitarget TKI which has been approved in China for the treatment of medullary thyroid cancer (MTC), (NSCLC), small cell lung cancer (SCLC), soft tissue sarcoma (STS) and radioactive iodine-refractory differentiated thyroid cancer (RAIR-DTC) (21, 22). In a single-arm phase 2 trial, neoadjuvant anlotinib therapy achieved an ORR of 76.9% in patients with advanced thyroid cancer (23). As most patients treated with TKI will develop resistance, the combination of PD-1 antibodies with TKIs has been proposed as a strategy to overcome TKI resistance in cancer patients. In a retrospective study at the MD Anderson Cancer Center, the addition of pembrolizumab to TKIs resulted in a best overall response (BOR) of 42% in ATC patients who progressed on TKIs (24). Sintilimab is a PD-1 antibody approved in China for the treatment of NSCLC, Hodgin’s lymphoma, hepatocellular carcinoma, and esophageal squamous cell carcinoma (ESCC) (25). Recently, the combination of sintilimab and anlotinib has shown promising antitumor activity and tolerable safety profiles in cervical cancer, endometrial cancer, hepatocellular carcinoma, and biliary tract cancer (26–29).
Previously, we reported that sintilimab plus anlotinib achieved a remarkable response in a metastatic NRAS-mutated ATC patient with high PD-L1 expression (10). In this study, our patient briefly responded to anlotinib but quickly developed resistance to sintilimab plus anlotinib. We suspected that the distinct responses to sintilimab plus anlotinib in these two PD-L1-positive, NRAS-mutated ATC patients could be mediated by the class 3 BRAF mutation D594N, which synergizes with RAS mutations in the amplification of downstream MAPK signaling (9). The oncogenic activity of class 3 BRAF mutants depends on the kinase activity of CRAF (9), which can be inhibited by BRAFi dabrafenib. Furthermore, class 3 BRAF mutants are sensitive to the inhibition of MEKi trametinib (9). Although the antitumor activity of MAPK-targeted therapy has not been established in NRAS-mutated solid tumors, this approach could counteract the synergistic effect of coexisting NRAS/BRAF mutations and render the tumor sensitive to immunotherapy.
Activation of the MAPK pathway plays a key role in tumor-intrinsic resistance to immune checkpoint blockade (30). In patients with triple-negative breast cancer, activation of the RAS-MAPK pathway was associated with reduced tumor-infiltrating lymphocytes (TILs) (31). However, trametinib treatment upregulated the expression of PD-L1 and MHC-I/II in mouse mammary tumor-derived cell lines in vitro and in vivo. Similarly, in BRAF V600-mutant melanoma, hyperactivated MAPK signaling inhibits T cell infiltration through the production of VEGF, which was reversed by the administration of a BRAFi (32). Other studies showed that the addition of dabrafenib/trametinib to immune checkpoint blockade (ICB) resulted in higher antitumor activity than ICB alone (33, 34). These preclinical and clinical studies led to the hypothesis that the combination of immunotherapy and MAPK-targeted therapy might provide better clinical benefits than each alone. The efficacy of BRAFi/MEKi plus PD-1/PD-L1 antibody triplets in BRAF-mutated solid tumors has been tested in three pivotal trials of melanoma (IMspire150, KEYNOTE-022, COMBI-i). Based on the results of the IMspire150 trial, FDA approved the combination of BRAFi vemurafenib, MEKi cobimetinib, and PD-L1 antibody atezolizumab for the treatment of BRAF V600-mutated melanoma. Because class 3 BRAF mutants were resistant to vemurafenib but sensitive to trametinib, we selected the dabrafenib/trametinib doublet to combine with sintilimab as the salvage therapy for our patient, which achieved a complete pathological response.
In addition to triplet therapy, optimal sequencing of immunotherapy and targeted therapy represents another strategy to improve outcomes for patients with BRAF-mutated solid tumors. In murine models of BRAF/NRAS-driven melanoma, anti-PD-1/L1 antibody lead-in before MAPK inhibitor combination optimized antitumor activity by promoting T cell clonal expansion and macrophage polarization (35). Consistently, results of two trials (DREAMseq and SECOMBIT) showed that nivolumab/ipilimumab followed by BRAFi/MEKi doublets led to superior overall survival in patients with advanced BRAF-mutant melanoma when compared with the opposite treatment sequence (36, 37). Similarly, data from the phase 3 NEMO trial revealed that previous immunotherapy led to better clinical benefits of MEKi binimetinib in patients with NRAS-mutant melanoma (38). While these results were obtained in patients with BRAF/NRAS-mutant melanoma, we can not rule out the possibility that a brief preceding immunotherapy of our patient may also contribute to her response to the BRAFi/MEKi/PD-1 antibody triplet regime.
Given this patient’s remarkable response, further investigation is required to explore the potential of the BRAFi/MEKi/PD-1 antibody triplet in patients with NRAS- or BRAF-mutated solid tumors refractory to immunotherapy and BRAF-targeted therapy. Furthermore, clinical trials of sequential immunotherapy and targeted therapy in ATC should be pursued.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Concept and design: LG, SL, YZ, XH. Acquisition, analysis, and interpretation of data: LG, XL, YC. Drafting of the manuscript: YC, XL. Critical revision of the manuscript for important intellectual content: TM, XH, SL. Technical and material support: LG, XH, YZ. Study supervision: SL. All authors contributed to the article and approved the submitted version.
The authors are grateful to the patient for her kind cooperation.
XL and TM are employees of Genetron Health Beijing Technology, Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw (2022) 20:925–51. doi: 10.6004/jnccn.2022.0040
2. Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res (2018) 24:3059–68. doi: 10.1158/1078-0432.CCR-18-0373
3. Capdevila J, Wirth LJ, Ernst T, Ponce Aix S, Lin CC, Ramlau R, et al. PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol (2020) 38:2620–7. doi: 10.1200/JCO.19.02727
4. Swetter SM, Thompson JA, Albertini MR, Barker CA, Baumgartner J, Boland G, et al. NCCN guidelines® insights: Melanoma: Cutaneous, version 2.2021. J Natl Compr Cancer Network (2021) 19:364–76. doi: 10.6004/jnccn.2021.0018
5. Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S. Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ book. Am Soc Clin Oncol Annu Meeting (2019) 39:147–64. doi: 10.1200/EDBK_240837
6. Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London England) (2020) 395:1835–44. doi: 10.1016/S0140-6736(20)30934-X
7. Ferrucci PF, Di Giacomo AM, Del Vecchio M, Atkinson V, Schmidt H, Schachter J, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer (2020) 8(2):e001806. doi: 10.1136/jitc-2020-001806
8. Dummer R, Long GV, Robert C, Tawbi HA, Flaherty KT, Ascierto PA, et al. Randomized phase III trial evaluating spartalizumab plus dabrafenib and trametinib for BRAF V600-mutant unresectable or metastatic melanoma. J Clin Oncol (2022) 40:1428–38. doi: 10.1200/JCO.21.01601
9. Yao Z, Yaeger R, Rodrik-Outmezguine VS, Tao A, Torres NM, Chang MT, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature (2017) 548:234–8. doi: 10.1038/nature23291
10. Gui L, Liu S, Zhang Y, Shi Y. A remarkable and durable response to sintilimab and anlotinib in the first-line treatment of an anaplastic thyroid carcinoma without targetable genomic alterations: A case report. OncoTargets Ther (2021) 14:2741–6. doi: 10.2147/OTT.S305196
11. Trojaniello C, Vitale MG, Ascierto PA. Triplet combination of BRAF, MEK and PD-1/PD-L1 blockade in melanoma: the more the better? Curr Opin Oncol (2021) 33:133–8. doi: 10.1097/CCO.0000000000000709
12. King AJ, Arnone MR, Bleam MR, Moss KG, Yang J, Fedorowicz KE, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PloS One (2013) 8:e67583. doi: 10.1371/journal.pone.0067583
13. Xu B, Fuchs T, Dogan S, Landa I, Katabi N, Fagin JA, et al. Dissecting anaplastic thyroid carcinoma: A comprehensive clinical, histologic, immunophenotypic, and molecular study of 360 cases. Thyroid (2020) 30:1505–17. doi: 10.1089/thy.2020.0086
14. Lin B, Ma H, Ma M, Zhang Z, Sun Z, Hsieh IY, et al. The incidence and survival analysis for anaplastic thyroid cancer: a SEER database analysis. Am J Trans Res (2019) 11:5888–96.
15. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell (2014) 159:676–90. doi: 10.1016/j.cell.2014.09.050
16. Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discovery (2014) 13:928–42. doi: 10.1038/nrd4281
17. Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, et al. Comprehensive characterization of cancer driver genes and mutations. Cell (2018) 173:371–385.e18. doi: 10.1016/j.cell.2018.02.060
18. Chapman PB. Mechanisms of resistance to RAF inhibition in melanomas harboring a BRAF mutation. Am Soc Clin Oncol Educ book. Am Soc Clin Oncol Annu Meeting (2013) 33:e80–e82. doi: 10.1200/EdBook_AM.2013.33.80
19. Cabanillas ME, Dadu R, Iyer P, Wanland KB, Busaidy NL, Ying A, et al. Acquired secondary RAS mutation in BRAF(V600E)-mutated thyroid cancer patients treated with BRAF inhibitors. Thyroid (2020) 30:1288–96. doi: 10.1089/thy.2019.0514
20. Hatashima A, Archambeau B, Armbruster H, Xu M, Shah M, Konda B, et al. An evaluation of clinical efficacy of immune checkpoint inhibitors for patients with anaplastic thyroid carcinoma. Thyroid (2022) 32:926–36. doi: 10.1089/thy.2022.0073
21. Li S. Anlotinib: A novel targeted drug for bone and soft tissue sarcoma. Front Oncol (2021) 11:664853. doi: 10.3389/fonc.2021.664853
22. Lin Y, Qin S, Li Z, Yang H, Fu W, Li S, et al. Apatinib vs placebo in patients with locally advanced or metastatic, radioactive iodine-refractory differentiated thyroid cancer: The REALITY randomized clinical trial. JAMA Oncol (2022) 8:242–50. doi: 10.1001/jamaoncol.2021.6268
23. Huang NS, Wei WJ, Xiang J, Chen JY, Guan Q, Lu ZW, et al. The efficacy and safety of anlotinib in neoadjuvant treatment of locally advanced thyroid cancer: A single-arm phase II clinical trial. Thyroid (2021) 31:1808–13. doi: 10.1089/thy.2021.0307
24. Iyer PC, Dadu R, Gule-Monroe M, Busaidy NL, Ferrarotto R, Habra MA, et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J immunotherapy Cancer (2018) 6:68. doi: 10.1186/s40425-018-0378-y
25. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ (Clinical Res ed.) (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
26. Xu Q, Wang J, Sun Y, Lin Y, Liu J, Zhuo Y, et al. Efficacy and safety of sintilimab plus anlotinib for PD-L1-Positive recurrent or metastatic cervical cancer: A multicenter, single-arm, prospective phase II trial. J Clin Oncol (2022) 40:1795–805. doi: 10.1200/JCO.21.02091
27. Wei W, Ban X, Yang F, Li J, Cheng X, Zhang R, et al. Phase II trial of efficacy, safety and biomarker analysis of sintilimab plus anlotinib for patients with recurrent or advanced endometrial cancer. J Immunother Cancer (2022) 10(5):e004338. doi: 10.1136/jitc-2021-004338
28. Chen X, Li W, Wu X, Zhao F, Wang D, Wu H, et al. Safety and efficacy of sintilimab and anlotinib as first line treatment for advanced hepatocellular carcinoma (KEEP-G04): A single-arm phase 2 study. Front Oncol (2022) 12:909035. doi: 10.3389/fonc.2022.909035
29. Jin S, Zhao R, Zhou C, Zhong Q, Shi J, Su C, et al. Feasibility and tolerability of sintilimab plus anlotinib as the second-line therapy for patients with advanced biliary tract cancers: An open-label, single-arm, phase II clinical trial. Int J Cancer (2022) 152(8):1648–58. doi: 10.1002/ijc.34372
30. Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol (2020) 20:25–39. doi: 10.1038/s41577-019-0218-4
31. Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: Therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res (2016) 22:1499–509. doi: 10.1158/1078-0432.CCR-15-1125
32. Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res (2013) 19:393–403. doi: 10.1158/1078-0432.CCR-12-1626
33. Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T, et al. The BRAF and MEK inhibitors dabrafenib and trametinib: Effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res (2015) 21:1639–51. doi: 10.1158/1078-0432.CCR-14-2339
34. Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Trans Med (2015) 7:279ra41. doi: 10.1126/scitranslmed.aaa4691
35. Wang Y, Liu S, Yang Z, Algazi AP, Lomeli SH, Wang Y, et al. Anti-PD-1/L1 lead-in before MAPK inhibitor combination maximizes antitumor immunity and efficacy. Cancer Cell (2021) 39:1375–1387.e6. doi: 10.1016/j.ccell.2021.07.023
36. Atkins MB, Lee SJ, Chmielowski B, Tarhini AA, Cohen GI, Truong TG, et al. Combination dabrafenib and trametinib versus combination nivolumab and ipilimumab for patients with advanced BRAF-mutant melanoma: The DREAMseq trial-ECOG-ACRIN EA6134. J Clin Oncol (2023) 41:186–97. doi: 10.1200/JCO.22.01763
37. Ascierto PA, Mandalà M, Ferrucci PF, Guidoboni M, Rutkowski P, Ferraresi V, et al. Sequencing of ipilimumab plus nivolumab and encorafenib plus binimetinib for untreated BRAF-mutated metastatic melanoma (SECOMBIT): A randomized, three-arm, open-label phase II trial. J Clin Oncol (2023) 41:212–21. doi: 10.1200/JCO.21.02961
Keywords: anaplastic thyroid carcinoma (ATC), BRAF non-V600E, NRAS, combination immunotherapy and targeted therapy, case report
Citation: Gui L, Zhu Y, Li X, He X, Ma T, Cai Y and Liu S (2023) Case report: Complete response of an anaplastic thyroid carcinoma patient with NRAS Q61R/BRAF D594N mutations to the triplet of dabrafenib, trametinib and PD-1 antibody. Front. Immunol. 14:1178682. doi: 10.3389/fimmu.2023.1178682
Received: 03 March 2023; Accepted: 30 March 2023;
Published: 14 April 2023.
Edited by:
Shariq Qayyum, Brigham and Women’s Hospital and Harvard Medical School, United StatesReviewed by:
Laura Deborah Locati, University of Pavia, ItalyCopyright © 2023 Gui, Zhu, Li, He, Ma, Cai and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoyan Liu, c2hhb3lhbmxpdS5iakAyNjMubmV0; Yi Cai, eWljYWkxMDhAZ21haWwuY29t
†These authors contributed equally as co-first authors to this article.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.