- 1Department of Gastroenterology, Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Shaanxi Province Key Laboratory of Gastrointestinal Motility Disorders, Laboratory of Digestive Diseases of the Second Affiliated Hospital of Xi'an Jiaotong University, Xi’an, China
CCL13/MCP-4 belongs to the CC chemokine family, which induces chemotaxis in many immune cells. Despite extensive research into its function in numerous disorders, a thorough analysis of CCL13 is not yet accessible. The role of CCL13 in human disorders and existing CCL13-focused therapies are outlined in this study. The function of CCL13 in rheumatic diseases, skin conditions, and cancer is comparatively well-established, and some studies also suggest that it may be involved in ocular disorders, orthopedic conditions, nasal polyps, and obesity. We also give an overview of research that found very little evidence of CCL13 in HIV, nephritis, and multiple sclerosis. Even though CCL13-mediated inflammation is frequently linked to disease pathogenesis, it’s fascinating to note that in some conditions, like primary biliary cholangitis (PBC) and suicide, it might even act as a preventative measure.
1 Introduction
Chemotactic cytokines are divided into four subfamilies based on how their amino-terminal (N-terminal) cysteines are arranged: CXC, CC, XC, and CX3C; their main function is to induce directional cell migration or the migration of cells drawn to chemotactic factors towards the source of the chemotactic factor along the signal of increased chemotactic concentration. CC chemotactic factor family member CCL13, also known as MCP-4 (monocyte chemoattractant protein 4) (1). CCL13 can bind to CCR1, CCR2, CCR3, CCR5, and CCR11, causing eosinophils, monocytes, T cells, and immature dendritic cells to migrate (2) (Figure 1). In addition to its chemotactic activity, CCL13 has been shown to induce eosinophil degranulation, basophil histamine release, adhesion molecule expression, and secretion of pro-inflammatory cytokines in epithelial, endothelial, and muscle cells. Besides which, research has revealed that CCL13 and its derived peptides have antibacterial activity against Gram-negative bacteria (3, 4). The antimicrobial activity of cytokines may be one of the body’s defenses; therefore, elevated CCL13 in certain diseases may be associated with anti-infective properties.
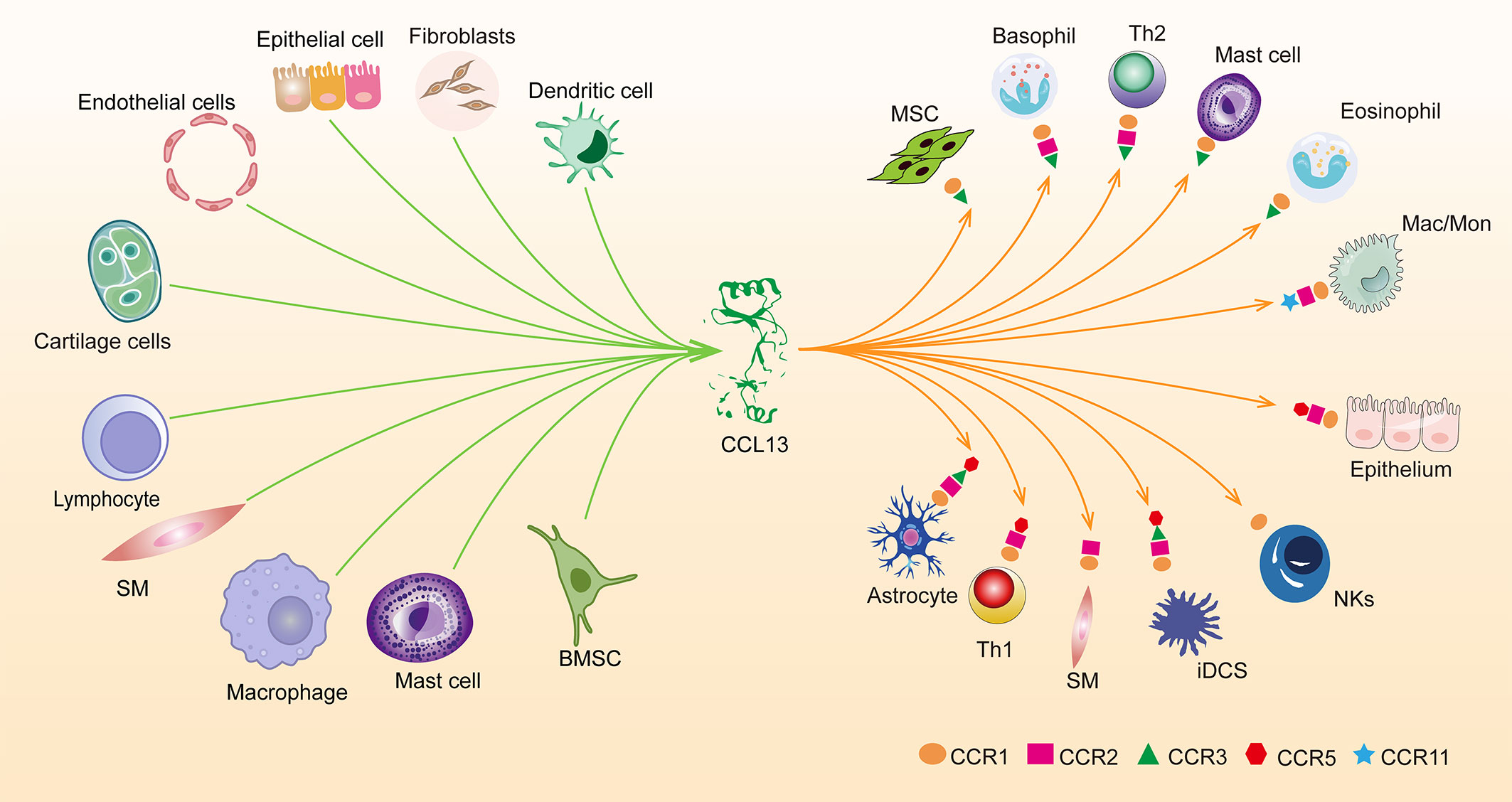
Figure 1 Overview of cells releasing CCL13 at the protein level and the expression of five receptors for CCL13 in cells. smooth muscle cells (SM), Macrophages (Mac), Monocytes (Mon), immature dendritic cells (iDCs), Natural killer (NKs), Helper T Lymphocyte (Th), Bone Marrow Stromal Cells (BMSC).
Many organs, including the small intestine, thymus, colon, lung, trachea, stomach, and lymph nodes, express CCL13 at the transcription level. At the protein level, CCL13 has been reported to be present in knee chondrocytes, human proximal renal tubular epithelial cells, etc. (Table 1). mRNA expression of CCL13 is upregulated in various diseases, but protein-level expression has rarely been validated. Studies have demonstrated that the proteomics of CCL13 exhibits inconsistency with mRNA expression. Therefore, CCL13 mRNA-based studies require validation by protein analysis to establish convincing conclusions (5).
This article seeks to present a thorough analysis of CCL13, a summary of the function that CCL13 plays in disease, and a discussion of its probable activation pathways (Figure 2). Additionally, we will discuss intervention strategies that can prevent CCL13 from functioning (Table 2).
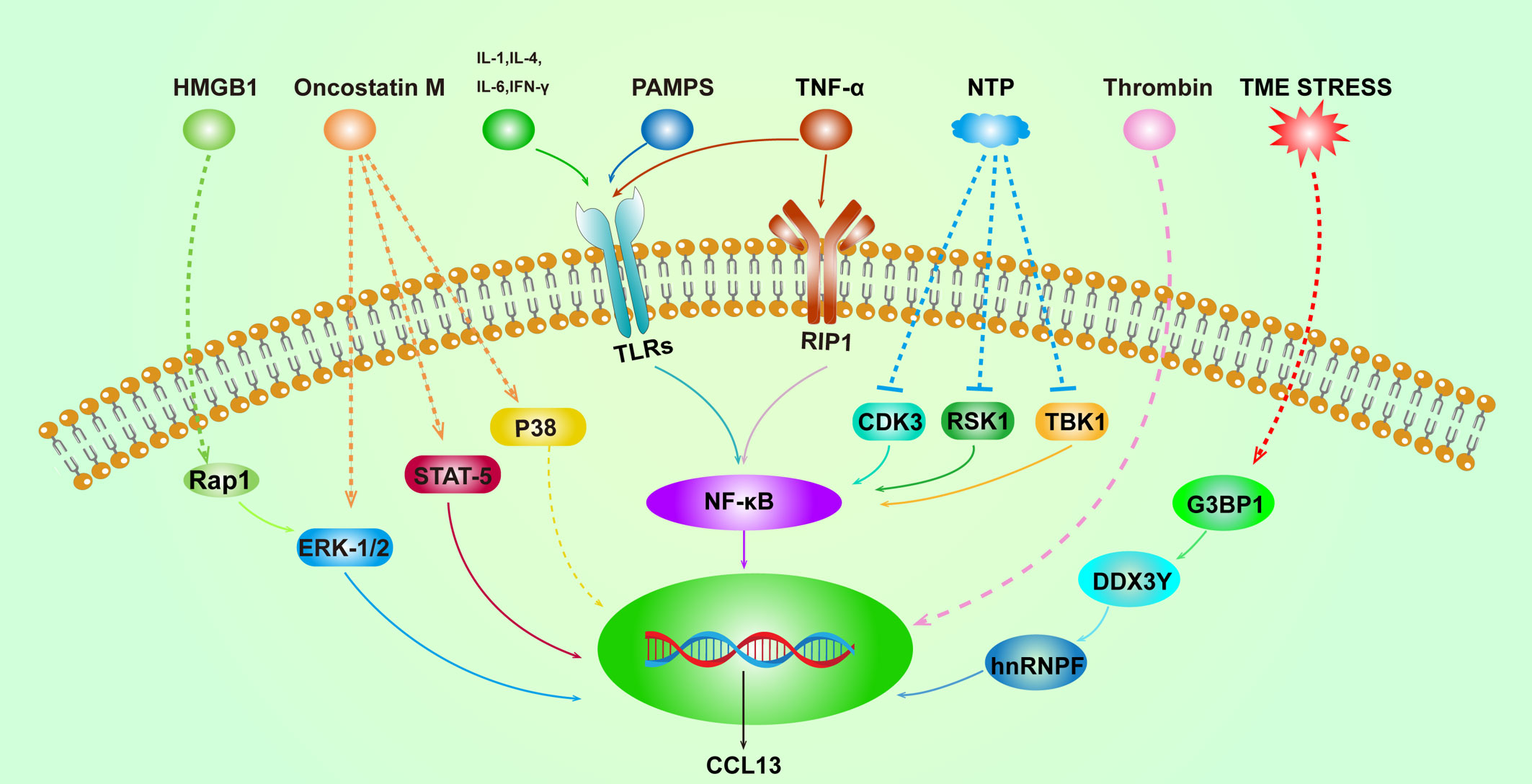
Figure 2 Overview of the activation pathways of CCL13. Solid lines represent well-defined relationships, and dashed lines represent intermediate molecular mechanisms unknown. High mobility group box 1 (HMGB1), Ras-Associated Protein 1 (Rap1), the tumor micro-environment (TME), and non-thermal plasma (NTP).
2 CCL13 and respiratory diseases
CCL13 and other chemokines within its family have been extensively investigated in diseases such as asthma, COPD, allergic pneumonia, and upper and lower respiratory tract infections. These diseases are characterized by inflammatory cell infiltration, which is mediated by multiple chemokines. Inflammatory cell infiltration can be triggered by pathogens or non-pathogenic factors.
2.1 CCL13 and asthma
Asthma is a heterogeneous lung disease with different phenotypes and unique potential mechanisms. In the past decade, people have conducted extensive research on the cellular and molecular mechanisms of asthma. The aggregation of eosinophils, type 2 helper T cells (Th2), and monocytes in the airway leads to changes in lung structure, which then leads to the decline of respiratory function. The most important pathological process of asthma is abnormal Th2 inflammation, which is mediated by Th2 cytokines, such as IL-4. About 50% of mild and moderate asthma and most severe asthma cases are induced by Th2-dependent inflammation. However, non-Th2 cytokines, including TNF-α, can also mediate Th2-low asthma (23). Airway epithelial cells can be activated by cytokines (IL-1β, IFN-γ,TNF-α, etc.) or PAMPS (Pathogen-related molecular patterns) via the TLRs-NF-κb pathway and releases CCL13, which recruits eosinophils and promotes the polarization of M2 macrophages to mediate the progression of asthma (2, 24, 25). When compared to patients with asthma who had their condition under control with glucocorticoids, people with uncontrolled asthma had greater CCL13 levels (26). Monocytes and eosinophils in sputum from children with asthma exhibited CCL13 and CCR3, but lymphocytes solely expressed CCL13; CCL13 is negatively correlated with peak expiratory flow and is downregulated in asthma remission (27). Elevated blood CCL13 concentrations have been observed in children with severe asthma, and it has been suggested that blood CCL13 levels can help in characterizing the severity of asthma in children (28). Mechanistically, CCL5, CCL7, CCL13, as well as CCL11 and CCL24, act synergistically to recruit eosinophils into the airways (29). Furthermore, Toll-like receptor 7 and 8 (TLR7/8) mediates the antiviral immune response by recognizing mainly viral RNA, and the increased response of the CCL13 gene to TLR7/8 agonists in the nasal mucosa of asthma patients may reflect the role of the virus in asthma progression (30). Interestingly, CDIP-2, which is a peptide derived from CCL13, has been shown to reduce chemokine-mediated function, decrease leukocyte recruitment, and reduce cytokine production by interacting with CCR1, CCR2, and CCR3 (31, 32). These results imply that CDIP-2 has the potential to alleviate airway inflammation and could be a promising therapeutic target for asthma. IL-13 is known to play a key role in the pathogenesis of asthma, and its upregulation has been associated with increased levels of CCL13 in the serum of asthma patients; Using Lebrikizumab, a monoclonal antibody that blocks the IL-13Rα1/IL-4Rα receptors, has had good results in lowering the serum concentrations of CCL17, CCL13, and total IgE in asthma patients (16). Yet it should be highlighted that, as shown in another trial, a single blockage of IL-13 was found to be insufficient to improve lung function in asthma patients who were not getting inhaled steroids (33). Bcl6 appears to be a promising inflammatory substance that inhibits histone acetylation of the chromatin of the gene cluster in lung epithelial cells by binding to a specific site of the CCL13 gene, leading to the downregulation of CCL13 (34).
The role of the CCL13-Th2 axis in promoting the entry of M2 macrophages and eosinophils into the airways and triggering airway inflammation in asthma is well established. While targeting type 2 cytokines to downregulate CCL13 has shown effectiveness, blocking upstream mediators of CCL13 alone has not been proven to be sufficient in achieving favorable outcomes.
2.2 CCL13 and COPD
Chronic Obstructive Pulmonary Disease (COPD) is a serious chronic respiratory condition which is currently the third greatest cause of death worldwide (35). Airway inflammation and damaging emphysema characterize COPD. In terms of pathology, it entails infiltration of inflammatory cells, modification of the airways, and irreversible damage of the lungs, among these, chronic inflammation is essential for the emergence of COPD (36). CCL13 has been linked to the development of COPD and has been shown to significantly increase when human lung tissue is stimulated by respirable smoke extracts from cooking (37). Contrarily, analysis of macrophages from bronchoalveolar lavage fluid (BALF) of COPD patients revealed primarily non-polarized macrophages with reduced gene expression of CD163, CD40, and CCL13, which are involved in pathogen recognition and processing; non polarized macrophages are primarily to blame for the decreased phagocytic capacity and the reduced ability to recognize and handle pathogens in lung macrophages (38). Moreover, a study by Ghebre et al. (39) revealed that the microbiome spectrum in sputum from patients with asthma and COPD seems to reflect different lineages of inflammatory mediators, and the upregulation of CCL13 and other type 2 mediators suggests the possibility of the infection of the bacterial phylum Bacteroidetes; type 1 mediators such as CXCL10 are more closely related to the phyla Actinobacteria and Firmicutes; pro-inflammatory mediators such as IL-1β are increased following the bacterial phylum Proteobacteria infection; these inflammatory lineages imply heterogeneity in bacterial related exacerbations of asthma and COPD, and suggest that endotype may be more important than the diagnosis of the disease itself. However, Proteobacteria, Actinobacteria, and Firmicutes are associated with other types of media. At last, bronchoalveolar lavage and biopsy samples’ content of CCL13 and other MCP family members may not be specific in identifying asthma, tuberculosis, nodular disease, and chronic bronchitis (40).
The different stages of the illness may play a role in the contradictory findings of CCL13 in the progression of COPD. CCL13 may add to chronic inflammation and tissue damage in the early phases of COPD. The detection and removal of pathogens by macrophages, as well as tissue healing and regeneration, may be promoted by CCL13 in later phases, when the lung tissue is already damaged. It’s also conceivable that CCL13’s dual function is context-dependent and affected by a variety of elements, including the pathogens involved, the host immune system, and additional environmental variables. Clarification of CCL13’s function in the development of COPD and its possible medicinal uses requires more study.
2.3 Acute eosinophilic pneumonia and hypersensitivity pneumonitis
Acute eosinophilic pneumonia (AEP) is a condition characterized by a significant infiltration of eosinophils in the lungs. Interestingly, eosinophil migration in endothelial cells is only induced by AEP-BALF, but not BALF from patients with hypersensitivity pneumonitis. The accumulation of eosinophils in AEP is mediated by CCR3 ligands, such as CCL13 and, CCL24 (41). The CCR3 antagonist YM-355179 has been shown to have potential in the treatment of eosinophil-associated allergic inflammatory disorders by blocking chemokine-mediated intracellular Ca2+ influx; this mechanism prevents the accumulation of eosinophils in affected tissues, thereby reducing the severity of the associated inflammation (42).
Although CCR1 and CCR3 are receptors for CCL13-induced eosinophil-triggered allergies, it is crucial to remember that CCR1 also has a function in CCL13-induced allergic pneumonia. Further investigation into the role of CCR1 in this mechanism may shed light on the etiology of allergy illnesses linked to eosinophils and possibly reveal novel targets for therapy.
2.4 Acute upper and lower respiratory tract infections
Acute lower respiratory infection (ALRI) is a prevalent infectious disease that affects individuals of all ages. ALRI in young children are known to be most frequently brought on by the respiratory syncytial virus (RSV) (43). According to a study, levels of CCL13 were found to be higher in patients with ALRI who were hospitalized from the start to day 6 after admission, but it wasn’t correlated with the severity of their symptoms; in fact, it was even downregulated in severe cases of patients with a CURB-65 score of >3 (suggestive of severe pneumonia) (44). Based on the available evidence, the relationship between CCL13 and ALRI remains unclear. While some studies suggest a potential role for CCL13 in ALRI, there is currently no definitive evidence linking the expression level of CCL13 to the severity of ALRI symptoms, further research is needed to better understand the potential role of CCL13 in the pathogenesis and clinical course of ALRI. Finally, In upper respiratory tract infections, CCL13 and CCL7 have been suggested to play a role in the recruitment of macrophages in children with confirmed viral infections of the upper respiratory tract (45).
3 CCL13 and rheumatism
3.1 CCL13 and RA
Rheumatoid arthritis (RA) is a chronic inflammatory disease that is one of the most prevalent autoimmune disorders. The disease primarily affects the joints, resulting in inflammation of the synovial membrane, which can cause joint swelling, stiffness, and pain (46). Serum CCL13 levels and expression in synovial and cartilage tissues are elevated in RA patients. The joint is the main site of inflammation in RA, and the cells known to produce CCL13 in the joint are synovial fibroblasts and chondrocytes (6, 47). Synovial fibroblasts promote chondrocyte catabolism and the development of synovial bone fragmentation, and they also have an aggressive inflammatory, stromal regulatory, and invasive character. These elements work together to cause joint destruction (48). Oncostatin M (OSM) is necessary for IL-6 and TNF- to positively regulate CCL13 expression in synovial fibroblasts. STAT-5, ERK-1/2, and p38 are involved in the production of CCL13 in response to OSM, and the increased CCL13 inhibits H2O2-induced apoptosis in synovial fibroblasts, enhances intra-synovial macrophage infiltration and angiogenesis, and aids in the course of the disease (47, 49). Studies have also demonstrated that the MAPK pathway mediates CCL13-induced proliferation of synovial fibroblasts and that these effects can be completely inhibited by PD98059 (17). In rheumatoid arthritis, estrogen has been found to decrease synovial fibroblast death and increase CCL13 expression, and this process may explain why women are more likely than males to get RA (50). In cartilage tissue, CCL13 is substantially expressed at both the mRNA and protein levels. CCL13, which is released by chondrocytes and aids in the deterioration of joints in RA, encourages the formation of rheumatoid synovial cells (6). Hintzen et al. (47, 49) reported that whereas all types of examined fibroblasts showed evidence of efficient OSM-induced signaling, only synovial fibroblasts were found to release CCL13. Although synovial fibroblasts are the primary source of CCL13 in the context of RA, several studies have demonstrated that CCL13 can be produced in response to different stimuli by other types of fibroblasts, such as dermal fibroblasts, colonic subepithelial myofibroblasts, and nasal polyp fibroblasts (51–53).
3.2 CCL13 and OA
Inflammation is one of the key mechanisms in the complicated joint condition known as osteoarthritis(OA), and imaging is a crucial way to diagnose OA (54). Inflammatory molecules known as the danger-associated molecular pattern (DAMP) contribute to the inflammatory process in OA joints. Alkaline calcium phosphate crystals, which are OA-associated DAMPs, can downregulate the expression of CCL13 and MRC1 (M2 markers) in macrophages (55); however, clinical studies have shown that CCL13 levels in serum and synovial fluid (SF) correlate with the severity of OA as determined by imaging (56). Patients with KL4 knee OA have significantly higher levels of CCL13 in serum and SF than those with KL2 and KL3, and patients with KL3 knee OA have significantly higher levels of CCL13 in SF than those with KL2 (The KL grading system is the most commonly used to assess the severity of joint disease in patients with OA, with grade 0 being the normal knee and grade 4 being the most severely affected) (56). It is evident that CCL13 exhibits distinct expression patterns in OA, and this pattern of expression in macrophages merits exploratory research.
3.3 CCL13 and other rheumatic diseases
Sjogren’s syndrome (SS) is an autoimmune disease, antibodies associated with SS can upregulate the expression of CC chemokines (CCL2, CCL13, and CCL20) in human salivary gland epithelial cells via the TACE/TNF-α/NF-κB signaling pathway (57). Nevertheless, in a cross-sectional study comparing several illnesses, serum CCL13 levels were measured in patients with systemic sclerosis (SSc), dermatomyositis, systemic lupus erythematosus (SLE), and healthy individuals, and it was discovered that only SSc patients had elevated levels of serum CCL13 compared to the control group (58, 59). In conclusion, while CCL13 cannot be used to diagnose rheumatism, it may be coupled with other disease-specific markers to increase the accuracy of the diagnosis.
4 CCL13 and skin diseases
Atopic dermatitis (AD) and alopecia areata are the two primary dermatological disorders linked to CCL13 that have been described in the literature, with the former being more clearly linked.
4.1 CCL13 and AD
Atopic dermatitis (AD) is a prevalent chronic inflammatory skin disease. The development of AD is influenced by various factors, including genetic predisposition, skin barrier dysfunction, and innate and adaptive immune dysregulation (60). CCL13 has been extensively studied as a Th2-associated marker in various tissues, including human PBMCs, dendritic cells, macrophages, and lesional skin tissue from patients with AD. However, the findings have not always been consistent. In lesional skin tissue from AD patients of all ages, CCL13 mRNA expression is significantly increased and has been shown to contribute to IgE synthesis (61–63). Furthermore, in older AD patients, CCL13 is considered a risk factor for atherosclerosis, indicating that these people might gain from screening for and treatment of cardiovascular disease (64). Proteomic analysis of blister fluid in AD patients has shown that CCL13 is one of the most upregulated proteins (7). However, the basal mRNA expression of CCL13 is downregulated in the PBMCs of AD patients, and CCL13 secretion into PBMCs is significantly increased upon stimulation with TLR2 ligands (65). It has been suggested that age-specific therapies may be beneficial for AD because CCL13 levels decrease significantly in diseased tissue and blood With increasing age (64). Targeting CCL13 for AD treatment is in its infancy, and an in vitro experiment (8) has pointed out that TNF-α promotes CCL13 gene expression in both IκB dependent and non-dependent pathways by binding to RIP1(receptor interacting protein 1); Non-thermal plasma (NTP) inhibited NF-κB pathway in a non-IκB-dependent manner and downregulated CCL13 expression in AD mice, and the combination of NTP and 1% hydrocortisone cream was found to be more effective. In 2018, the FDA authorized the oral medication ASN002, a dual JAK/SYK inhibitor, for the treatment of moderate to severe atopic dermatitis; ASN002 significantly reverses the transcriptome of lesional skin to a nonlesional phenotype and inhibits key inflammatory pathways involved in the pathogenesis of AD, including CCL13-mediated Th2-associated inflammation (18).
Indeed, age is a significant factor in determining the function of CCL13 in AD, and more investigation is required to understand why CCL13 expression in AD-lesioned tissues and PBMC differs.
4.2 CCL13 and alopecia areata
Alopecia areata is a common autoimmune disorder characterized by the immune-mediated destruction of hair follicles. One of the main events in the etiology of alopecia areata is the loss of hair follicle immune privilege, although the underlying processes are complicated and may involve both local and systemic immune dysregulation (66). Alopecia areata has traditionally been thought to be associated with Th1 activation, but recently there is ample evidence that Th2 may also mediate the development of alopecia areata. GWAS studies suggest a role for Th2-associated genes in the pathogenesis of alopecia areata (67). Elevated Th2 markers in the blood and skin of patients with alopecia areata may be involved in systemic inflammation and could be a potential indicator of the severity of the disease (9, 68). Upilumab, an IL-4R inhibitor, blocks the Th2 axis in patients with alopecia areata, and these patients showed downregulation of Th2-related markers (CCL13, CCL18, CCL26, CCL24) starting at week 12 of treatment with dupilumab, significant upregulation of the hair keratin gene-set at week 24, and clinical improvement in patients with alopecia areata at week 48 (69). This demonstrates how Th2-related cytokines can suppress the expression of hair keratins and be harmful in alopecia areata. To ascertain the function of particular Th2 inhibition in the treatment of alopecia areata, larger size and longer clinical trials are still necessary before the Th2 axis may be targeted for the treatment of alopecia areata.
5 CCL13 and digestive system disease
5.1 CCL13 and hepatic disease
According to Townsend et al.’s research, CCL13 expression is reduced in hepatitis D patients; this change in chemokine levels may contribute to the faster disease development in hepatitis D patients (70). On the other hand, secondary bacterial infections in cirrhotic individuals may be linked to the increase of CCL13 transcript levels in their serum and duodenal mucosa (71). Although little is known about CCL13’s role in the development of cirrhosis, recent research suggests that it may slow the advancement of the disease through type 2 immunity. However, viruses can counteract this effect in a number of ways. Elevated CCL13 may be a protective factor and needs to be further investigated, given that Gram-negative bacteria are more frequently seen in bacterial infections associated with cirrhosis.
Primary biliary cholangitis (PBC), is a chronic autoimmune liver disease characterized by cholestasis and the presence of anti-mitochondrial antibodies in the bloodstream (72). It was shown that Th2 chemokines were downregulated in early PBC, where serum CCL13 was elevated in early and decreased in late of PBC, and negatively correlated with PBC staging (r = -0.373), preventing early disease progression (73). Given the marked eosinophil infiltration in the portal vein in patients with early PBC, the increased CCL13 in the serum of patients with early PBC might be associated with this, and the downregulation of CCL13 in the late stages seems to indicate that it is not involved in the subsequent progression of PBC.
5.2 CCL13 and IBD
Inflammatory bowel disease (IBD) is an idiopathic inflammatory disease of the intestinal tract that primarily includes ulcerative colitis (UC) and Crohn’s disease (CD). Although its pathogenesis is unclear, chronic lesions of CD show an excessive Th1 response and a Th2 pattern is present in the mucosa of lesions in early CD and UC (74). Studies have shown that the effects of pharmacological interventions alone on chemokines in patients with IBD appear to exhibit targeting. Only CXCL10 was significantly downregulated in the blood of patients with CD treated with atorvastatin, while the other eight chemokines, including CCL13, were not significantly changed (75). Interestingly, in a clinical study that included UC and CD, after treatment with vedolizumab, although serum CCL13 levels increased in the treatment group as a whole; when patients were subdivided into groups with and without responders, CCL28 was down-regulated in responders, whereas CCL13 was up-regulated in non-responders, and they concluded that CCL13 levels at the initiation of treatment may predict the potential prognostic value of vedolizumab (76). The mechanism of CCL13 in IBD may be related to its induction of adhesion molecule expression in epithelial cells, and increased expression of adhesion molecules allows more cells of the innate immune system (such as monocytes or neutrophils) to enter the site of inflammation. The greater CCL13 levels in non-responders following therapy may be partially explained by this.
6 CCL13 and renal diseases
Studies on individuals who experienced acute renal allograft rejection and vasculitis glomerulonephritis revealed that CCL13 was primarily expressed in peritubular, periglomerular, and perivascular sites and was linked to the infiltration of CD3+ lymphocytes and CD68+ monocytes/macrophages. Proximal tubular epithelial cells also displayed low levels of CCL13 protein expression, which could be seen by protein blotting. The upregulation of CCL13 expression is a result of pro-inflammatory cytokines in response and plays an important role in monocyte/macrophage recruitment and retention in renal inflammation (10). In addition, kidney donor quality significantly affects renal transplantation outcomes, such as recipients of kidney donors from the elderly who exhibit low post-transplant renal function and short graft lifespan; a single-center, retrospective, observational study showed that compared to standard criteria donors and living donors, elderly and expanded criteria donors have upregulated CCL13 at the transcriptional level and may be involved in post-transplant renal inflammation and renal function impairment (77).
7 CCL13 and ocular disorders
Ocular uveitis can be a symptom of a variety of connective tissue disorders, including seronegative spondyloarthritis (SpA), nodular disease, pseudoarthrosis, recurrent polychondritis, and granulomatous polyangiitis. Yet uveitis that manifests as SpA has its own distinctive features, notably with a strong link to the presence of HLA-B27 (78). For this reason, uveitis presenting with spondyloarthritis is collectively referred to as B27-associated uveitis, which typically develops in young adults but can also affect children and adolescents. Studies have shown that people with HLA-B27-associated uveitis have 255-fold higher levels of CCL13 in their aqueous humor than healthy individuals. Additionally, compared to granulomatous uveitis, non-granulomatous uveitis exhibits a considerably higher level of CCL13 (79). This suggests that CCL13 is involved in the pathogenesis of non-granulomatous uveitis and that the associated immune responses may be more effective in this type of uveitis, particularly in HLA-B27-associated uveitis.
Patients with ischemic retinal vein occlusion (RVO) had modestly elevated levels of vitreous CCL13, which correlated with vitreous hemorrhage and may have indicated how severe the retinal inflammation was (80). In patients with primary rheumatogenic retinal detachment (RD), CCL13 levels are higher in vitreous fluid (VF) than in macular holes (MH), and several cytokines seem to be involved in the immune initiation and profibrotic processes after RD, and these cytokines may be related to the increased intraocular fluid volume and higher mobility of the fluid around the refractive apparatus of the anterior chamber (81). Furthermore, in vitro experiments have demonstrated that treatment of corneal stromal fibroblasts with thrombin resulted in a significant increase in CCL13 mRNA expression (up 2-fold) and protein production (from 0 to 14 pg/ml) and that CCL13 played a crucial role in mediating the thrombin-triggered immune response (11).
8 CCL13 and nasal polyps and rhinitis
Nasal polyposis (NP) is an inflammatory disease with Th2 skewing, and it is widely believed that certain chemotactic agents and Th2 factors play a crucial role in its pathogenesis. One study showed that fibroblasts may be a major source of Th2 chemokines and that TLR2, 3, 4, and 5 ligands can synergistically induce the production of CCL13 in nasal polyp fibroblasts when combined with IL-4, while TLR7/8 or 9 ligands do not induce its production (53). The expression of Th2 markers, including CCL13, was not found to be altered in nasal polyposis (NP) patients receiving glucocorticoid (GC) therapy; this lack of change in expression levels may suggest that T-cell-driven NP inflammatory mediators are resistant to the effects of GC treatment (82).
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a condition characterized by a type 2 immune response, with overproduction of IgE and infiltration of eosinophils (83). Much of the research on the immunopathology of CRSwNP has relied on transcriptomics. However, Workman et al. (5) noted that proteomic analysis can reveal inconsistencies with mRNA expression, with tissue proteomic and transcriptomic analyses of CRSwNP polyp tissue found significantly elevated CCL13 mRNA levels, but no change in protein expression, and this implies that research based on mRNA of CCL13 should be supported by protein.
Allergic rhinitis is also an inflammatory disease characterized by excessive production of local type 2 cytokines and increased eosinophils in tissues. In patients with seasonal allergic rhinitis, under nasal allergen stimulation, the level of CCL13 in nasal secretions increases 3.7-fold, while IL-10 and IL-4 significantly decrease; CCL13 may worsen, while IL-10 may alleviate nasal mucosa allergy (12). In addition, similar to asthma, increased responsiveness of CCL13 to TLR7/8 agonists has also been observed in allergic rhinitis. The response to nasal administration of Resiquimod (a specific TLR7/8 agonist) can be used to simply assess nasal mucosal responsiveness (30).
9 CCL13 and obesity and its complications
In recent years, much interest has been generated in the fundamental mechanisms causing the global rise in obesity. Many health issues related to obesity, including coronary atherosclerosis, have been linked in studies to the emergence of persistent low-grade inflammation in the body (84). A positive energy balance and an increased anabolic state, especially in adipocytes, may be the initial stimulus in the case of obesity. This results in the release of chemokines that activate an adaptive inflammatory response, which encourages the healthy expansion of adipocytes while lowering energy stores (85).
It has been demonstrated that adipocytes from obese individuals exhibit higher expression of CCL13 compared to those from lean individuals (86), and elevated levels of CCL13 are positively associated with BMI (87, 88). According to in vitro studies, CCL13 expression in preadipocytes is low at baseline but steadily rises following differentiation (89).
Integrated analysis of miRNA and genome-wide data from epicardial adipose tissue (EAT) in patients with coronary artery disease (CAD) suggests that altered metabolic and inflammatory regulation is a hallmark of EAT in CAD. Furthermore, miR-103-3p/CCL13 appears to be a novel candidate that plays a role in EAT function and CAD (90). Additionally, CCL13/CCR2 has been shown to promote the formation of carotid plaques and may act as a link between the activation of platelets and monocytes (91). Coincidentally, data from mouse models confirm that when fed a high-fat diet, mice lacking CCR2 eat less and are less likely to become obese, and CCR2 antagonist therapy lowers inflammatory aspects of obesity, such as macrophage infiltration in adipose tissue (92). Earlier on, a study of adipokines in obesity and related comorbidities suggests that CCL13, a brand-new biomarker for extreme obesity, may exacerbate subclinical atherosclerosis in persons with obesity by affecting circulating levels of major atherosclerotic markers. After bariatric surgery, CCL13 was significantly reduced, indicating that reduced levels of CCL13 may be one of the mechanisms leading to cardiovascular risk reduction in obese patients following bariatric surgery (93).
Together with CAD, obesity has become a significant risk factor for periodontitis. Notably, levels of CCL13 and high-sensitivity C-reactive protein (hs-CRP) were found to be significantly elevated in both gingival crevicular fluid and serum, and a positive correlation between CCL13 and periodontal parameters (94). When the relationship between obesity, chronic periodontitis, and serum CCL13 concentrations was examined, Pradeep et al. (95) discovered that obese patients with chronic periodontitis had significantly higher serum CCL13 concentrations than the non-obese group. All these discoveries implies that CCL13 and hs-CRP may be markers of chronic inflammation in obesity and periodontal disease.
10 CCL13 and tissue repair
Wound healing is a complex process that occurs in response to skin damage, and involves a series of reparative events that aim to restore the protective skin barrier (96). When the wound is inflamed, the probability of forming a hypertrophic scar will greatly increase (97, 98). CCL13 is barely expressed in normal scar formation but is expressed for a longer period in hyperplastic scar formation (99). An animal study showed that Imiquimod significantly inhibited the expression of CCL13 mRNA and upregulated Th1-associated chemokines in rabbits at 21 days to 63 days after surgery to reduce collagen deposition and the extent of fibrosis and thus inhibit scar hyperplasia in a manner that regulated the Th1 and Th2 axes (19).
The process of bone healing is largely affected by the stability of the fixation (biomechanics) as well as the blood supply to the healing site. This repair process is composed of several phases, including inflammation, repair, and remodeling (100). High mobility group box 1 (HMGB1), a ubiquitous inflammatory factor in fractures, promotes the secretion of CCL4 and CCL13 from mesenchymal stem cells (MSCs) by activating the Ras-associated protein-1 (Rap1) signaling pathway, and these chemokines mediate the migration of MSCs, which in turn promotes fracture healing (13, 101, 102).
11 CCL13 and neurological disorders
Multiple sclerosis (MS) is an immune-related central nervous system disease (103). In MS, upregulation of CCL13 levels in the brain tissue and cerebrospinal fluid can induce monocyte chemotaxis and the secretion of inflammatory cytokines in lymphocytes, ultimately, causing oligodendrocyte activation and myelin destruction (20, 104). The downregulation of CCL13 protein expression in the plasma of MS patients in the third trimester of pregnancy and its increase after delivery may be related to the immune tolerance established during pregnancy, this expression pattern of CCL13 may reflect the disease activity of MS during pregnancy (14). Indeed, the haplotype gene in CCL13 is associated with susceptibility to MS in both rat and human genomes (104). In addition, the administration of intravenous immunoglobulin to patients with MS downregulates CCL13 expression in peripheral T cells, which in turn may inhibit T cell proliferation (20, 104).
12 CCL13 and mental illness
Mental illness refers to a range of clinical manifestations arising from brain dysfunction that is influenced by various biological, psychological, and social environmental factors, resulting in varying degrees of impairment in cognitive, emotional, volitional, and behavioral mental activities (105). In recent years, despite significant advances in molecular mechanisms in neuroscience, few biomarkers have made their way into clinical psychiatric practice (106). CCL13 has been shown to drive chemotaxis of pro-inflammatory cells to the inflamed or injured central nervous system (CNS), therefore, chemokines could potentially serve as novel diagnostic and therapeutic targets in psychiatric disorders (107).
In individuals with post-traumatic stress disorder (PTSD), there is an increase in serum levels of CCL13, CCL20, and CXCL6, which may indicate a higher risk for developing PTSD. In contrast, CX3CL1 may serve as a marker for recovery; more than any of these, CCL13 shows a positive correlation with scores on the PTSD Checklist (PCL), suggesting that CCL13 levels may be associated with the severity of PTSD symptoms (108). Another study revealed that plasma levels of CCL13/CCL2 ratios were approximately twofold higher in individuals with PTSD, without significant changes observed in cerebrospinal fluid levels, these ratios remained constant over circadian time, regardless of gender, body mass index, or age at the time of trauma, and may be potential circadian biomarkers for chronic PTSD (109). All of these suggest that the diagnosis of PTSD based on a single marker is difficult, and a combination of multiple substances may be more promising. However, unlike in PTSD, brain tissue levels of CCL13 were significantly lower in the dorsolateral prefrontal cortex of individuals who completed suicide, revealing that chemokine alterations may be suicide-specific immunological mechanisms (110). Interestingly, in a 12-year follow-up study, individuals who attempted suicide had lower levels of CCL13, CCL11, CCL4, CCL2, and CCL17 in their cerebrospinal fluid and plasma compared to those with psychosis who had never experienced suicidal thoughts (111). This long-term study strongly demonstrates that chemokines such as CCL13 are of great research value, as they are likely to play a role in the maintenance of immune homeostasis in the organism, which is critical for mood stabilization and regulation.
13 CCL13 and AIDS
The pathogenesis of Acquired Immune Deficiency Syndrome(AIDS) is primarily a result of cellular immunodeficiency caused by the direct and indirect actions of HIV (112). The primary targets of HIV have activated CD4+ T lymphocytes (113); and in untreated HIV-infected patients, the macrophage phenotype has been reported to shift from M1 in the early stages of infection to M2 in the later stages and can inhibit viral replication (114).
According to a multicenter AIDS cohort study, CCL13 detectability was found to be greater than 80%, and elevated plasma levels of CCL13 may be associated with more rapid disease progression in HIV-infected individuals (115, 116). In a multicenter AIDS cohort study (MACS) conducted from 1984 to 2009, CCL13 was found to be higher in the group of SUP (exposed to HARRT with HIV RNA suppressed to less than 50 copies/ml plasma) compared to the HAART-naive (NAI) group, on the timeline, CCL13 increased significantly in the first year of viral suppression, followed by a uniformly flat trajectory, HIV suppression appeared to increase the levels of the M2-associated chemokines (117). In addition, the growth hormone-releasing hormone (GHRH) analog tesamorelin significantly reduced CCL13 expression in HIV populations with metabolic dysregulation, and systemic and end-organ inflammation, suggesting that enhancement of the GH axis may improve immune activation in this population (21).
14 CCL13 and cancer
Cancer growth and response to therapy are both significantly influenced by inflammation, with chronic inflammation increasing tumor progression and therapeutic resistance. Acute inflammatory responses, on the other hand, frequently promote dendritic cell (DC) maturation and antigen presentation, which can drive anti-tumor immune responses. Multiple chemokines, such as those in the CCL and CXCL families, have been identified as key regulators of inflammation initiation and regression (118). The majority of tumor-associated macrophages (TAMs) in most solid tumors are M2-type TAMs (M2 TAMs), which are essential for controlling the immunosuppressive tumor microenvironment, encouraging tumor angiogenesis, and promoting tumor spread (15). CCL13 is a crucial chemokine for M2 TAMs and may be involved in its function of it (Table 3).
High blood CCL13 levels were independently a marker for predicting distant metastasis in colorectal cancer, according to logistic regression analysis, and they were substantially linked with advanced age, advanced T-stage, distant metastasis, and UICC stage in colorectal cancer (119). In cases of gastric cancer, the levels of CCL13 reflect the distinct response patterns of fibroblasts in specific tumor sites toward cancer cell invasion. Elevated levels of CCL13 can be indicative of submucosal invasion by tumor cells (120). In M2 TAMs, stress granule (SG) formation was stimulated by tumor micro-environment (TME) stress, and SG increased DDX3Y/hnRNPF mediated mRNA stability of CCL13, which in turn enhanced CCL13 expression and promoted the metastasis of oral squamous cell carcinoma metastasis, however, the above molecular expression and phenotype are reversed upon knockdown of the G3BP1 (15). Intriguingly, tissues from individuals with metastatic salivary adenoid cystic carcinoma(SACC) showed higher expression of CCL13 in the absence of tumor recurrence or perineural invasion than in the presence of tumor recurrence (121). Moreover, CCL13 is linked to 2-microglobulin (2-MG) levels in multiple myeloma, poor prognosis in prostate adenocarcinoma, and cell proliferation in breast cancer (122–124). Likewise, hepatocellular carcinoma (HCC) tumor tissues contain considerably more CCL13 mRNA than normal tissue, however this is unrelated to the clinical prognosis (125). In ovarian cancer, CCL13 triggers epithelial-mesenchymal transition via the p38 MAPK pathway (128). Chronic hypoxia does not seem to affect the expression of CCL13, according to in vitro experiments using breast cancer, hepatocellular carcinoma, and lung adenocarcinoma cells (129).
Further to that, it has been discovered that CCL13 protein expression is augmented in the plasma of pediatric Hodgkin’s lymphoma patients and is related to a sluggish early response; and for Hodgkin’s lymphoma patients, biologically-based risk stratification algorithms that incorporate CCL13 could be taken into consideration to enhance treatment results and reduce toxicity (126, 127). In cutaneous T-cell lymphoma (CTCL), CCL13+ monocytes/macrophages play a role in mediating immunosuppression by interacting with malignant T cells, and blocking the S100A9-TLR4 interaction with tasquinimod has been shown to inactivate the NF-κB pathway, leading to inhibition of CTCL tumor cell growth and induction of apoptosis (22).
15 Discussion
In summary, the main pathogenic mechanism of inflammation involves the recruitment and activation of immune cells, specifically Th2 and M2 macrophages, to inflamed tissues through the chemokine CCL13. Despite the potential of blocking the chemokine system as an important area of anti-inflammatory drug development, only a few drugs, such as ASN002 (a dual-action inhibitor of JAK/SYK), lebrikizumab (IL-13Rα1/IL-4Rα receptor blockade), maraviroc (a CCR5 antagonist), and plerixafor (a CXCR4 antagonist), have received FDA approval (16, 18, 33, 130, 131). However, a single blockade of CCL13 upstream or downstream does not appear to be effective due to high structural overlap and functional crossover between chemokines (33). Additionally, many other immune agents that can modulate chemokines are still not well studied and the molecular mechanisms are still unclear. For another, it is worth noting that CCL13 has been reported to have a positive effect on human diseases such as PBC and suicide, and further exploration of its mechanism is warranted. It should be noted that elevated CCL13 may be linked to antimicrobial resistance, and it ought to take this into account given the potential of CO infections like COPD and cirrhosis (39, 71).
It is crucial to keep in mind that while examining the connection between CCL13 and illness, age is a component that cannot be disregarded. Much research on CCL13 on both youngsters and the elderly has yielded conflicting findings (61, 64). Likewise, as some research suggests that the two may exhibit divergent expression patterns, simultaneous monitoring of lesion locations and peripheral blood chemokines is required (37, 38, 65). Furthermore, insufficient research has been done on the locations where the CCL13 protein is expressed. Several research (5, 132)indicate that CCL13 is not consistently expressed at the levels of transcription and translation, and rather than focusing exclusively on identifying changes in expression at the transcriptional level, future research should validate results through protein analysis.
Author contributions
LL wrote the main manuscript text and prepared the figures and tables. LW made suggestions on the framework of the review. YS reviewed and revised the first draft. LM gave instructions on the drawing. YR and FY helped search literature. FD designed the study, revised and gave final approval of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (NO. 81770540), and the Key Research and Development Project of Shaanxi Province (NO. 2020SF235).
Acknowledgments
The authors thank Dr. Yikai Wang of the Second Affiliated Hospital of Xi’an Jiaotong University for providing support for our article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev (1999) 10(1):61–86. doi: 10.1016/s1359-6101(99)00005-2
2. Mendez-Enriquez E, Garcia-Zepeda EA. The multiple faces of CCL13 in immunity and inflammation. Inflammopharmacology (2013) 21(6):397–406. doi: 10.1007/s10787-013-0177-5
3. GarciaZepeda EA, Combadiere C, Rothenberg ME, Sarafi MN, Lavigne F, Hamid Q, et al. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J Immunol (1996) 157(12):5613–26. doi: 10.1016/S0022-1759(96)00181-0
4. Luster AD, Rothenberg ME. Role of the monocyte chemoattractant protein and eotaxin subfamily of chemokines in allergic inflammation. J Leukoc Biol (1997) 62(5):620–33. doi: 10.1002/jlb.62.5.620
5. Workman AD, Nocera AL, Mueller SK, Otu HH, Libermann TA, Bleier BS. Translating transcription: proteomics in chronic rhinosinusitis with nasal polyps reveals significant discordance with messenger RNA expression. Int Forum Allergy Rhinol (2019) 9(7):776–86. doi: 10.1002/alr.22315
6. Iwamoto T, Okamoto H, Iikuni N, Takeuchi M, Toyama Y, Tomatsu T, et al. Monocyte chemoattractant protein-4 (MCP-4)/CCL13 is highly expressed in cartilage from patients with rheumatoid arthritis. Rheumatology (2006) 45(4):421–4. doi: 10.1093/rheumatology/kei209
7. Rojahn TB, Vorstandlechner V, Krausgruber T, Bauer WM, Alkon N, Bangert C, et al. Single-cell transcriptomics combined with interstitial fluid proteomics defines cell type-specific immune regulation in atopic dermatitis. J Allergy Clin Immunol (2020) 146(5):1056–69. doi: 10.1016/j.jaci.2020.03.041
8. Choi JH, Song YS, Lee HJ, Hong JW, Kim GC. Inhibition of inflammatory reactions in 2,4-dinitrochlorobenzene induced Nc/Nga atopic dermatitis mice by non-thermal plasma. Sci Rep (2016) 6:27376. doi: 10.1038/srep27376
9. Glickman JW, Dubin C, Renert-Yuval Y, Dahabreh D, Kimmel GW, Auyeung K, et al. Cross-sectional study of blood biomarkers of patients with moderate to severe alopecia areata reveals systemic immune and cardiovascular biomarker dysregulation. J Am Acad Dermatol (2021) 84(2):370–80. doi: 10.1016/j.jaad.2020.04.138
10. Chakravorty SJ, Howie AJ, Girdlestone J, Gentle D, Savage COS. Potential role for monocyte chemotactic protein-4 (MCP-4) in monocyte/macrophage recruitment in acute renal inflammation. J Pathol (2001) 194(2):239–46. doi: 10.1002/path.877
11. Twining SS, Warejcka DJ. Thrombin increases human corneal fibroblast expression of the chemokines, CXCL1, CCL2, IL-6, IL-8, CXCL10 and CCL13. Arvo Meeting Abstracts (2012) 53(6).
12. Baumann R, Rabaszowski M, Stenin I, Tilgner L, Scheckenbach K, Wiltfang J, et al. Comparison of the nasal release of IL-4, IL-10, IL-17, CCL13/MCP-4, and CCL26/eotaxin-3 in allergic rhinitis during season and after allergen challenge. Am J Rhinol Allergy (2013) 27(4):266–72. doi: 10.2500/ajra.2013.27.3913
13. Lin F, Xue DT, Xie T, Pan ZJ. HMGB1 promotes cellular chemokine synthesis and potentiates mesenchymal stromal cell migration via Rap1 activation. Mol Med Rep (2016) 14(2):1283–9. doi: 10.3892/mmr.2016.5398
14. Lingehed GP, Hellberg S, Huang JS, Khademi M, Kockum I, Carlsson H, et al. Plasma protein profiling reveals dynamic immunomodulatory changes in multiple sclerosis patients during pregnancy. Front Immunol (2022) 13:930947. doi: 10.3389/fimmu.2022.930947
15. Liu ZX, Rui T, Lin ZY, Xie SL, Zhou B, Fu M, et al. Tumor-associated macrophages promote metastasis of oral squamous cell carcinoma via CCL13 regulated by stress granule. Cancers (2022) 14(20):5081. doi: 10.3390/cancers14205081
16. Agrawal S, Townley RG. Role of periostin, FENO, IL-13, lebrikzumab, other IL-13 antagonist and dual IL-4/IL-13 antagonist in asthma. Expert Opin Biol Ther (2014) 14(2):165–81. doi: 10.1517/14712598.2014.859673
17. Iwamoto T, Okamoto H, Kobayashi S, Ikari K, Toyama Y, Tomatsu T, et al. A role of monocyte chemoattractant protein-4 (MCP-4)/CCL13 from chondrocytes in rheumatoid arthritis. FEBS J (2007) 274(18):4904–12. doi: 10.1111/j.1742-4658.2007.06013.x
18. Pavel AB, Song T, Kim HJ, Del Duca E, Krueger JG, Dubin C, et al. Oral janus kinase/SYK inhibition (ASN002) suppresses inflammation and improves epidermal barrier markers in patients with atopic dermatitis. J Allergy Clin Immunol (2019) 144(4):1011–24. doi: 10.1016/j.jaci.2019.07.013
19. Chen BG, Li HC, Xia W. Imiquimod regulating Th1 and Th2 cell-related chemokines to inhibit scar hyperplasia. Int Wound J (2019) 16(6):1281–8. doi: 10.1111/iwj.13183
20. Pigard N, Elovaara I, Kuusisto H, Paalavuo R, Dastidar P, Zimmermann K, et al. Therapeutic activities of intravenous immunoglobulins in multiple sclerosis involve modulation of chemokine expression. J Neuroimmunol (2009) 209(1-2):114–20. doi: 10.1016/j.jneuroim.2009.01.014
21. Stanley TL, Fourman LT, Wong LP, Sadreyev R, Billingsley JM, Feldpausch MN, et al. Growth hormone releasing hormone reduces circulating markers of immune activation in parallel with effects on hepatic immune pathways in individuals with HIV-infection and nonalcoholic fatty liver disease. Clin Infect Dis (2021) 73(4):621–30. doi: 10.1093/cid/ciab019
22. Du YX, Cai Y, Lv Y, Zhang LS, Yang H, Liu QZ, et al. Single-cell RNA sequencing unveils the communications between malignant T and myeloid cells contributing to tumor growth and immunosuppression in cutaneous T-cell lymphoma. Cancer Lett (2022) 551:215972. doi: 10.1016/j.canlet.2022.215972
23. Habib N, Pasha MA, Tang DD. Current understanding of asthma pathogenesis and biomarkers. Cells (2022) 11(17):2764. doi: 10.3390/cells11172764
24. Winkler C, Witte L, Moraw N, Faulenbach C, Muller M, Holz O, et al. Impact of endobronchial allergen provocation on macrophage phenotype in asthmatics. BMC Immunol (2014) 15(1):12. doi: 10.1186/1471-2172-15-12
25. Lamkhioued B, Garcia-Zepeda EA, Abi-Younes S, Nakamura H, Jedrzkiewicz S, Wagner L, et al. Monocyte chemoattractant protein (MCP)-4 expression in the airways of patients with asthma. induction in epithelial cells and mononuclear cells by proinflammatory cytokines. Am J Respir Crit Care Med (2000) 162(2 Pt 1):723–32. doi: 10.1164/ajrccm.162.2.9901080
26. Delgado-Dolset MI, Obeso D, Rodriguez-Coira J, Tarin C, Tan G, Cumplido JA, et al. Understanding uncontrolled severe allergic asthma by integration of omic and clinical data. Allergy (2022) 77(6):1772–85. doi: 10.1111/all.15192
27. El-Gamal YM, Heshmat NM, Awwad KS, Ezzat MHM, Al-Aziz MMA, El-Gabbas ZM. Monocyte chemotactic protein-4 (MCP-4; CCL-13) and the CC chemokine receptor 3 (CCR3) in sputum of asthmatic children. J Allergy Clin Immunol (2007) 119(1):S72–2. doi: 10.1016/j.jaci.2006.11.304
28. Adel-Patient K, Grauso M, Abou-Taam R, Guillon B, Dietrich C, Machavoine F, et al. A comprehensive analysis of immune constituents in blood and bronchoalveolar lavage allows identification of an immune signature of severe asthma in children. Front Immunol (2021) 12:700521. doi: 10.3389/fimmu.2021.700521
29. Rojas-Ramos E, Avalos AF, Perez-Fernandez L, Cuevas-Schacht F, Valencia-Maqueda E, Teran LM. Role of the chemokines RANTES, monocyte chemotactic proteins-3 and-4, and eotaxins-1 and-2 in childhood asthma. Eur Respir J (2003) 22(2):310–6. doi: 10.1183/09031936.03.00084802
30. Jha A, Thwaites R, Tunstall T, Kon OM, Shattock R, Openshaw P, et al. Enhanced in vivo vivo mucosal interferon and chemokine responses to a single stranded RNA analogue (R848) in participants with asthma. Erj Open Res (2019) 5. doi: 10.1183/23120541.lungscienceconference-2019.OP01
31. Mendez-Enriquez E, Medina-Tamayo J, Soldevila G, Fortoul TI, Anton B, Flores-Romo L, et al. A CCL chemokine-derived peptide (CDIP-2) exerts anti-inflammatory activity via CCR1, CCR2 and CCR3 chemokine receptors: implications as a potential therapeutic treatment of asthma. Int Immunopharmacol (2014) 20(1):1–11. doi: 10.1016/j.intimp.2014.01.032
32. Mendez-Enriquez E, Melendez Y, Martinez F, Baay G, Huerta-Yepez S, Gonzalez-Bonilla C, et al. CDIP-2, a synthetic peptide derived from chemokine (C-c motif) ligand 13 (CCL13), ameliorates allergic airway inflammation. Clin Exp Immunol (2008) 152(2):354–63. doi: 10.1111/j.1365-2249.2008.03619.x
33. Noonan M, Korenblat P, Mosesova S, Scheerens H, Arron JR, Zheng Y, et al. Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids. J Allergy Clin Immunol (2013) 132(3):567. doi: 10.1016/j.jaci.2013.03.051
34. Seto T, Yoshitake M, Ogasawara T, Ikari J, Sakamoto A, Hatano M, et al. Bcl6 in pulmonary epithelium coordinately controls the expression of the CC-type chemokine genes and attenuates allergic airway inflammation. Clin Exp Allergy (2011) 41(11):1568–78. doi: 10.1111/j.1365-2222.2011.03836.x
35. Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [CPH] study): a national cross-sectional study. Lancet (2018) 391(10131):1706–17. doi: 10.1016/S0140-6736(18)30841-9
36. Higham A, Quinn AM, Cancado JED, Singh D. The pathology of small airways disease in COPD: historical aspects and future directions. Respir Res (2019) 20(1):49. doi: 10.1186/s12931-019-1017-y
37. Binaya KC, Mahapatra PS, Thakker D, Henry AP, Billington CK, Sayers I, et al. Proinflammatory effects in ex vivo human lung tissue of respirable smoke extracts from indoor cooking in Nepal. Ann Am Thorac Soc (2020) 17(6):688–98. doi: 10.1513/AnnalsATS.201911-827OC
38. Akata K, Yamasaki K, Leitao FS, Yang CX, Takiguchi H, Sahin B, et al. Abundance of non-polarized lung macrophages with poor phagocytic function in chronic obstructive pulmonary disease (COPD). Biomedicines (2020) 8(10):398. doi: 10.3390/biomedicines8100398
39. Ghebre MA, Pang PH, Diver S, Desai D, Bafadhel M, Haldar K, et al. Biological exacerbation clusters demonstrate asthma and chronic obstructive pulmonary disease overlap with distinct mediator and microbiome profiles. J Allergy Clin Immunol (2018) 141(6):2027–36. doi: 10.1016/j.jaci.2018.04.013
40. Miotto D, Christodoulopoulos P, Olivenstein R, Taha R, Cameron L, Tsicopoulos A, et al. Expression of IFN-gamma-inducible protein; monocyte chemotactic proteins 1, 3, and 4; and eotaxin in T(H)1-and T(H)2-mediated lung diseases. J Allergy Clin Immunol (2001) 107(4):664–70. doi: 10.1067/mai.2001.113524
41. Nakagome K, Shoda H, Shirai T, Nishihara F, Soma T, Uchida Y, et al. Eosinophil transendothelial migration induced by the bronchoalveolar lavage fluid of acute eosinophilic pneumonia. Respirology (2017) 22(5):913–21. doi: 10.1111/resp.12982
42. Morokata T, Suzuki K, Masunaga Y, Taguchi K, Morihira K, Sato I, et al. A novel, selective, and orally available antagonist for CC chemokine receptor 3. J Pharmacol Exp Ther (2006) 317(1):244–50. doi: 10.1124/jpet.105.097048
43. Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet (London England) (2022) 399(10340):2047–64. doi: 10.1016/s0140-6736(22)00478-0
44. Relster MM, Holm A, Pedersen C. Plasma cytokines eotaxin, MIP-1, MCP-4, and vascular endothelial growth factor in acute lower respiratory tract infection. Apmis (2017) 125(2):148–56. doi: 10.1111/apm.12636
45. Santiago J, Hernandez-Cruz JL, Manjarrez-Zavala ME, Montes-Vizuet R, Rosete-Olvera DP, Tapia-Diaz AM, et al. Role of monocyte chemotactic protein-3 and-4 in children with virus exacerbation of asthma. Eur Respir J (2008) 32(5):1243–9. doi: 10.1183/09031936.00085107
46. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet (2016) 388(10055):2023–38. doi: 10.1016/s0140-6736(16)30173-8
47. Hintzen C, Quaiser S, Pap T, Heinrich PC, Hermanns HM. Induction of CCL13 expression in synovial fibroblasts highlights a significant role of oncostatin m in rheumatoid arthritis. Arthritis Rheumatism (2009) 60(7):1932–43. doi: 10.1002/art.24602
48. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. New Engl J Med (2011) 365(23):2205–19. doi: 10.1056/NEJMra1004965
49. Yamaguchi A, Nozawa K, Fujishiro M, Kawasaki M, Suzuki F, Takamori K, et al. CC motif chemokine ligand 13 is associated with rheumatoid arthritis pathogenesis. Modern Rheumatol (2013) 23(5):856–63. doi: 10.3109/s10165-012-0752-4
50. Yamaguchi A, Nozawa K, Fujishiro M, Kawasaki M, Takamori K, Ogawa H, et al. Estrogen inhibits apoptosis and promotes CC motif chemokine ligand 13 expression on synovial fibroblasts in rheumatoid arthritis. Immunopharmacol Immunotoxicol (2012) 34(5):852–7. doi: 10.3109/08923973.2012.664149
51. Hein H, Schluter C, Kulke R, Christophers E, Schroder JM, Bartels J. Genomic organization, sequence analysis and transcriptional regulation of the human MCP-4 chemokine gene (SCYA13) in dermal fibroblasts: a comparison to other eosinophilic beta-chemokines. Biochem Biophys Res Commun (1999) 255(2):470–6. doi: 10.1006/bbrc.1999.0216
52. Yagi Y, Andoh A, Nishida A, Shioya M, Nishimura T, Hashimoto T, et al. Interleukin-31 stimulates production of inflammatory mediators from human colonic subepithelial myofibroblasts. Int J Mol Med (2007) 19(6):941–6. doi: 10.3892/ijmm.19.6.941
53. Nonaka M, Fukumoto A, Ogihara N, Pawankar R, Sakanushi A, Yagi T. Expression of MCP-4 by TLR ligand-stimulated nasal polyp fibroblasts. Acta Oto-Laryngologica (2007) 127(12):1304–9. doi: 10.1080/00016480701242444
54. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet (2019) 393(10182):1745–59. doi: 10.1016/s0140-6736(19)30417-9
55. Mahon OR, Kelly DJ, McCarthy GM, Dunne A. Osteoarthritis-associated basic calcium phosphate crystals alter immune cell metabolism and promote M1 macrophage polarization. Osteoarthritis Cartilage (2020) 28(5):603–12. doi: 10.1016/j.joca.2019.10.010
56. Gao F, Tian J, Pan HL, Gao J, Yao M. Association of CCL13 levels in serum and synovial fluid with the radiographic severity of knee osteoarthritis. J Invest Med (2015) 63(3):545–7. doi: 10.1097/jim.0000000000000150
57. Lisi S, Sisto M, Lofrumento DD, D’Amore M. Sjogren’s syndrome autoantibodies provoke changes in gene expression profiles of inflammatory cytokines triggering a pathway involving TACE/NF-kappa b. Lab Invest (2012) 92(4):615–24. doi: 10.1038/labinvest.2011.190
58. Yanaba K, Yoshizaki A, Muroi E, Hara T, Ogawa F, Shimizu K, et al. CCL13 is a promising diagnostic marker for systemic sclerosis. Br J Dermatol (2010) 162(2):332–6. doi: 10.1111/j.1365-2133.2009.09507.x
59. Gambichler T, Yilmaz E, Hoxtermann S, Kolios A, Moritz R, Bechara FG, et al. Serum CCL13 levels in patients with systemic sclerosis and controls. Br J Dermatol (2011) 165(1):216–8. doi: 10.1111/j.1365-2133.2011.10330.x
60. Lipozencic J, Pastar Z, Kulisic SM, Pavic I. Immunologic aspects of atopic dermatitis. Acta Dermatovenerol Croatica (2009) 17(3):226–34.
61. Brunner PM, He H, Pavel AB, Czarnowicki T, Lefferdink R, Erickson T, et al. The blood proteomic signature of early-onset pediatric atopic dermatitis shows systemic inflammation and is distinct from adult long-standing disease. J Am Acad Dermatol (2019) 81(2):510–9. doi: 10.1016/j.jaad.2019.04.036
62. Lang CCV, Renert-Yuval Y, Del Duca E, Pavel AB, Wu JN, Zhang N, et al. Immune and barrier characterization of atopic dermatitis skin phenotype in Tanzanian patients. Ann Allergy Asthma Immunol (2021) 127(3):334–41. doi: 10.1016/j.anai.2021.04.023
63. Plager DA, Leontovich AA, Henke SA, Davis MDP, McEvoy MT, Sciallis GF, et al. Early cutaneous gene transcription changes in adult atopic dermatitis and potential clinical implications. Exp Dermatol (2007) 16(1):28–36. doi: 10.1111/j.1600-0625.2006.00504.x
64. Zhou LS, Leonard A, Pavel AB, Malik K, Raja A, Glickman J, et al. Age-specific changes in the molecular phenotype of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol (2019) 144(1):144–56. doi: 10.1016/j.jaci.2019.01.015
65. Yu YY, Lin DX, Cai XQ, Cui DN, Fang R, Zhang W, et al. Enhancement of chemokine mRNA expression by toll-like receptor 2 stimulation in human peripheral blood mononuclear cells of patients with atopic dermatitis. BioMed Res Int (2020) 2020:1497175. doi: 10.1155/2020/1497175
66. Han G. The changing landscape of alopecia areata: an introduction. Adv Ther (2017) 34(7):1584–5. doi: 10.1007/s12325-017-0544-5
67. Petukhova L, Christiano AM. Functional interpretation of genome-wide association study evidence in alopecia areata. J Invest Dermatol (2016) 136(1):314–7. doi: 10.1038/jid.2015.402
68. Wang D, Xu XM, Li XZ, Shi J, Tong XL, Chen J, et al. ccl13 is upregulated in alopecia areata lesions and is correlated with disease severity. Exp Dermatol (2021) 30(5):723–32. doi: 10.1111/exd.14293
69. Renert-Yuval Y, Pavel AB, Del Duca E, Facheris P, Pagan AD, Bose S, et al. Scalp biomarkers during dupilumab treatment support Th2 pathway pathogenicity in alopecia areata. Allergy (2022) 00:1–13. doi: 10.1111/all.15561
70. Townsend EC, Zhang GY, Ali R, Firke M, Moon MS, Han MAT, et al. The balance of type 1 and type 2 immune responses in the contexts of hepatitis b infection and hepatitis d infection. J Gastroenterol Hepatol (2019) 34(4):764–75. doi: 10.1111/jgh.14617
71. Du Plessis J, Vanheel H, Janssen CEI, Roos L, Slavik T, Stivaktas PI, et al. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol (2013) 58(6):1125–32. doi: 10.1016/j.jhep.2013.01.038
72. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet (2013) 382(9904):1587–99. doi: 10.1016/s0140-6736(13)60096-3
73. Mu N, Lin F, Jiang ZG, Liang Y, Yang ZX. Characteristics of serum chemokine profile in primary biliary cholangitis. Cytokine (2020) 136:155291. doi: 10.1016/j.cyto.2020.155291
74. Jugde F, Alizadeh M, Boissier C, Chantry D, Siproudhis L, Corbinais S, et al. Quantitation of chemokines (MDC, TARC) expression in mucosa from crohn’s disease and ulcerative colitis. Eur Cytokine Netw (2001) 12(3):468–77.
75. Grip O, Janciauskiene S. Atorvastatin reduces plasma levels of chemokine (CXCL10) in patients with crohn’s disease. PloS One (2009) 4(5):e5263. doi: 10.1371/journal.pone.0005263
76. Zwicker S, Lira-Junior R, Hoog C, Almer S, Bostrom EA. Systemic chemokine levels with “Gut-specific” vedolizumab in patients with inflammatory bowel DiseaseA pilot study. Int J Mol Sci (2017) 18(8):1827. doi: 10.3390/ijms18081827
77. Hruba P, Krejcik Z, Dostalova Merkerova M, Klema J, Stranecky V, Slatinska J, et al. Molecular fingerprints of borderline changes in kidney allografts are influenced by donor category. Front Immunol (2020) 11:423. doi: 10.3389/fimmu.2020.00423
78. Pathanapitoon K, Dodds EM, Cunningham ET, Rothova A. Clinical spectrum of HLA-B27-associated ocular inflammation. Ocular Immunol Inflammation (2017) 25(4):569–76. doi: 10.1080/09273948.2016.1185527
79. Abu El-Asrar AM, Berghmans N, Al-Obeidan SA, Gikandi PW, Opdenakker G, Van Damme J, et al. The CC chemokines CCL8, CCL13 and CCL20 are local inflammatory biomarkers of HLA-B27-associated uveitis. Acta Ophthalmol (2019) 97(1):E122–8. doi: 10.1111/aos.13835
80. Zeng YK, Cao D, Yu HH, Zhuang XN, Yang DW, Hu YY, et al. Comprehensive analysis of vitreous chemokines involved in ischemic retinal vein occlusion. Mol Vision (2019) 25:756–65.
81. Garweg JG, Zandi S, Pfister I, Rieben R, Skowronska M, Tappeiner C. Cytokine profiles of phakic and pseudophakic eyes with primary retinal detachment. Acta Ophthalmol (2019) 97(4):E580–8. doi: 10.1111/aos.13998
82. Li CW, Zhang KK, Li TY, Lin ZB, Li YY, de Lafaille MAC, et al. Expression profiles of regulatory and helper T-cell-associated genes in nasal polyposis. Allergy (2012) 67(6):732–40. doi: 10.1111/j.1398-9995.2012.02811.x
83. Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Journal (2017) 12(Issue):331–57. doi: 10.1146/annurev-pathol-052016-100401
84. Hotamisligil GS. Inflammation and metabolic disorders. Nature (2006) 444(7121):860–7. doi: 10.1038/nature05485
85. Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell (2015) 160(5):816–27. doi: 10.1016/j.cell.2015.02.010
86. Ignacio RMC, Gibbs CR, Lee ES, Son DS. Differential chemokine signature between human preadipocytes and adipocytes. Immune Network (2016) 16(3):189–94. doi: 10.4110/in.2016.16.3.189
87. Hashimoto I, Wada J, Hida A, Baba M, Miyatake N, Eguchi J, et al. Elevated serum monocyte chemoattractant protein-4 and chronic inflammation in overweight subjects. Obesity (2006) 14(5):799–811. doi: 10.1038/oby.2006.93
88. Kitahara CM, Trabert B, Katki HA, Chaturvedi AK, Kemp TJ, Pinto LA, et al. Body mass index, physical activity, and serum markers of inflammation, immunity, and insulin resistance. Cancer Epidemiol Biomarkers Prev (2014) 23(12):2840–9. doi: 10.1158/1055-9965.Epi-14-0699-t
89. Alomar SY, Gentili A, Zaibi MS, Kepczynska MA, Trayhurn P. IL-1 beta (interleukin-1 beta) stimulates the production and release of multiple cytokines and chemokines by human preadipocytes. Arch Physiol Biochem (2016) 122(3):117–22. doi: 10.3109/13813455.2016.1156706
90. Vacca M, Di Eusanio M, Cariello M, Graziano G, D’Amore S, Petridis FD, et al. Integrative miRNA and whole-genome analyses of epicardial adipose tissue in patients with coronary atherosclerosis. Cardiovasc Res (2016) 109(2):228–39. doi: 10.1093/cvr/cvv266
91. Breland UM, Michelsen AE, Skjelland M, Folkersen L, Krohg-Sorensen K, Russell D, et al. Raised MCP-4 levels in symptomatic carotid atherosclerosis: an inflammatory link between platelet and monocyte activation. Cardiovasc Res (2010) 86(2):265–73. doi: 10.1093/cvr/cvq044
92. Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci (2003) 100(12):7265–70. doi: 10.1073/pnas.1133870100
93. Gentili A, Zaibi MS, Alomar SY, De Vuono S, Ricci MA, Alaeddin A, et al. Circulating levels of the adipokines monocyte chemotactic protein-4 (MCP-4), macrophage inflammatory protein-1 beta (MIP-1 beta), and eotaxin-3 in severe obesity and following bariatric surgery. Hormone Metab Res (2016) 48(12):847–53. doi: 10.1055/s-0042-108731
94. Kumari M, Pradeep AR, Priyanka N, Kalra N, Naik SB. Crevicular and serum levels of monocyte chemoattractant protein-4 and high-sensitivity c-reactive protein in periodontal health and disease. Arch Oral Biol (2014) 59(6):645–53. doi: 10.1016/j.archoralbio.2014.03.012
95. Pradeep AR, Kumari M, Kalra N, Priyanka N. Correlation of MCP-4 and high-sensitivity c-reactive protein as a marker of inflammation in obesity and chronic periodontitis. Cytokine (2013) 61(3):772–7. doi: 10.1016/j.cyto.2012.12.022
96. Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol (2012) 26(7):812–20. doi: 10.1111/j.1468-3083.2011.04415.x
97. Jie L, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clinics Dermatol (2007) 25(1):9–18. doi: 10.1016/j.clindermatol.2006.09.007
98. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Trans Med (2014) 6(265):265sr266. doi: 10.1126/scitranslmed.3009337
99. Chen BG, Li HC, Xia W. The role of Th1/Th2 cell chemokine expression in hypertrophic scar. Int Wound J (2020) 17(1):197–205. doi: 10.1111/iwj.13257
100. Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol (2012) 8(3):133–43. doi: 10.1038/nrrheum.2012.1
101. Lejmi E, Perriraz N, Clément S, Morel P, Baertschiger R, Christofilopoulos P, et al. Inflammatory chemokines MIP-1δ and MIP-3α are involved in the migration of multipotent mesenchymal stromal cells induced by hepatoma cells. Stem Cells Dev (2015) 24(10):1223–35. doi: 10.1089/scd.2014.0176
102. Zhang F, Wang C, Wang H, Lu M, Li Y, Feng H, et al. Ox-LDL promotes migration and adhesion of bone marrow-derived mesenchymal stem cells via regulation of MCP-1 expression. Mediators Inflammation (2013) 2013:691023. doi: 10.1155/2013/691023
103. Sand IK. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr Opin Neurol (2015) 28(3):193–205. doi: 10.1097/wco.0000000000000206
104. Ockinger J, Stridh P, Beyeen AD, Lundmark F, Seddighzadeh M, Oturai A, et al. Genetic variants of CC chemokine genes in experimental autoimmune encephalomyelitis, multiple sclerosis and rheumatoid arthritis. Genes Immun (2010) 11(2):142–54. doi: 10.1038/gene.2009.82
105. Too LS, Spittal MJ, Bugeja L, Reifels L, Butterworth P, Pirkis J. The association between mental disorders and suicide: a systematic review and meta-analysis of record linkage studies. J Affect Disord (2019) 259:302–13. doi: 10.1016/j.jad.2019.08.054
106. Herron JW, Nerurkar L, Cavanagh J. Neuroimmune biomarkers in mental illness. Journal (2018) 40(Issue):45–78. doi: 10.1007/7854_2018_45
107. Stuart MJ, Singhal G, Baune BT. Systematic review of the neurobiological relevance of chemokines to psychiatric disorders. Front Cell Neurosci (2015) 9:357. doi: 10.3389/fncel.2015.00357
108. Zhang L, Hu XZ, Li XX, Chen Z, Benedek DM, Fullerton CS, et al. Potential chemokine biomarkers associated with PTSD onset, risk and resilience as well as stress responses in US military service members. Trans Psychiatry (2020) 10(1):31. doi: 10.1038/s41398-020-0693-1
109. Dalgard C, Eidelman O, Jozwik C, Olsen CH, Srivastava M, Biswas R, et al. The MCP-4/MCP-1 ratio in plasma is a candidate circadian biomarker for chronic post-traumatic stress disorder. Trans Psychiatry (2017) 7(2):e1025–5. doi: 10.1038/tp.2016.285
110. Shinko Y, Otsuka I, Okazaki S, Horai T, Boku S, Takahashi M, et al. Chemokine alterations in the postmortem brains of suicide completers. J Psychiatr Res (2020) 120:29–33. doi: 10.1016/j.jpsychires.2019.10.008
111. Janelidze S, Ventorp F, Erhardt S, Hansson O, Minthon L, Flax J, et al. Altered chemokine levels in the cerebrospinal fluid and plasma of suicide attempters. Psychoneuroendocrinology (2013) 38(6):853–62. doi: 10.1016/j.psyneuen.2012.09.010
112. Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, Viall AH, et al. Human immunodeficiency virus transmission at each step of the care continuum in the united states. JAMA Internal Med (2015) 175(4):588–96. doi: 10.1001/jamainternmed.2014.8180
113. Maartens G, Celum C, Lewin SR. HIV Infection: epidemiology, pathogenesis, treatment, and prevention. Lancet (2014) 384(9939):258–71. doi: 10.1016/s0140-6736(14)60164-1
114. Cassol E, Cassetta L, Rizzi C, Alfano M, Poli G. M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J Immunol (2009) 182(10):6237–46. doi: 10.4049/jimmunol.0803447
115. Kapaata A, Balinda SN, Hare J, Leonova O, Kikaire B, Egesa M, et al. Infection with HIV-1 subtype d among acutely infected ugandans is associated with higher median concentration of cytokines compared to subtype a. IJID Regions (Online) (2022) 3:89–95. doi: 10.1016/j.ijregi.2022.03.007
116. McKay HS, Margolick JB, Martinez-Maza O, Lopez J, Phair J, Rappocciolo G, et al. Multiplex assay reliability and long-term intra-individual variation of serologic inflammatory biomarkers. Cytokine (2017) 90:185–92. doi: 10.1016/j.cyto.2016.09.018
117. Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. Aids (2015) 29(4):463–71. doi: 10.1097/qad.0000000000000545
118. Zhao HK, Wu L, Yan GF, Chen Y, Zhou MY, Wu YZ, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduction Targeted Ther (2021) 6(1):263. doi: 10.1038/s41392-021-00658-5
119. Okugawa Y, Toiyama Y, Mohri Y, Tanaka K, Kawamura M, Hiro J, et al. Elevated serum concentration of monocyte chemotactic protein 4 (MCP-4) as a novel non-invasive prognostic and predictive biomarker for detection of metastasis in colorectal cancer. J Surg Oncol (2016) 114(4):483–9. doi: 10.1002/jso.24335
120. Abe A, Nagatsuma AK, Higuchi Y, Nakamura Y, Yanagihara K, Ochiai A. Site-specific fibroblasts regulate site-specific inflammatory niche formation in gastric cancer. Gastric Cancer (2017) 20(1):92–103. doi: 10.1007/s10120-015-0584-y
121. Mays AC, Feng X, Browne JD, Sullivan CA. Chemokine and chemokine receptor profiles in metastatic salivary adenoid cystic carcinoma. Anticancer Res (2016) 36(8):4013–8.
122. Huang Y, Huang J, Zhang P, Luo J, Cheng P, Miao L, et al. Integrated analysis of hub gene expression in multiple myeloma. J BUON (2021) 26(5):2040–52.
123. Zhao X, Hu DX, Li J, Zhao GZ, Tang W, Cheng HL. Database mining of genes of prognostic value for the prostate adenocarcinoma microenvironment using the cancer gene atlas. BioMed Res Int (2020) 2020:5019793. doi: 10.1155/2020/5019793
124. Franzen B, Alexeyenko A, Kamali-Moghaddam M, Hatschek T, Kanter L, Ramqvist T, et al. Protein profiling of fine-needle aspirates reveals subtype-associated immune signatures and involvement of chemokines in breast cancer. Mol Oncol (2019) 13(2):376–91. doi: 10.1002/1878-0261.12410
125. She SP, Ren LY, Chen P, Wang MY, Chen DB, Wang Y, et al. Functional roles of chemokine receptor CCR2 and its ligands in liver disease. Front Immunol (2022) 13:812431. doi: 10.3389/fimmu.2022.812431
126. Maggio EM, Van den Berg A, Visser L, Diepstra A, Kluiver J, Emmens R, et al. Common and differential chemokine expression patterns in RS cells of NLP, EBV positive and negative classical Hodgkin lymphomas. Int J Cancer (2002) 99(5):665–72. doi: 10.1002/ijc.10399
127. Agrusa JE, Scull BP, Abhyankar HA, Lin H, Ozuah NW, Chakraborty R, et al. Defining the inflammatory plasma proteome in pediatric Hodgkin lymphoma. Cancers (2020) 12(12):3603. doi: 10.3390/cancers12123603
128. Li ST, Hu YX, Liu OX, Li X, Lin B. Prognostic biomarker MCP-4 triggers epithelial-mesenchymal transition via the p38 MAPK pathway in ovarian cancer. Front Oncol (2022) 12:1034737. doi: 10.3389/fonc.2022.1034737
129. Korbecki J, Kojder K, Barczak K, Siminska D, Gutowska I, Chlubek D, et al. Hypoxia alters the expression of CC chemokines and CC chemokine receptors in a tumor-a literature review. Int J Mol Sci (2020) 21(16):5647. doi: 10.3390/ijms21165647
130. Tan QX, Zhu Y, Li J, Chen ZX, Han GW, Kufareva I, et al. Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science (2013) 341(6152):1387–90. doi: 10.1126/science.1241475
131. Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, et al. International union of pharmacology. LXXXIX. update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev (2014) 66(1):1–79. doi: 10.1124/pr.113.007724
Keywords: CCL13, MCP-4, cytokines, human diseases, Th2, NF-κB, type 2 immunity
Citation: Li L, Dai F, Wang L, Sun Y, Mei L, Ran Y and Ye F (2023) CCL13 and human diseases. Front. Immunol. 14:1176639. doi: 10.3389/fimmu.2023.1176639
Received: 28 February 2023; Accepted: 05 April 2023;
Published: 19 April 2023.
Edited by:
Vadim Gaponenko, University of Illinois at Chicago, United StatesReviewed by:
Kanami Orihara, Tokyo Institute of Technology, JapanHung-Jen Chen, Amsterdam University Medical Center, Netherlands
Copyright © 2023 Li, Dai, Wang, Sun, Mei, Ran and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Dai, ZGFpZmVpNjhAeGp0dS5lZHUuY24=
 Laifu Li
Laifu Li Fei Dai
Fei Dai Lianli Wang1,2
Lianli Wang1,2 Lin Mei
Lin Mei