
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Immunol. , 13 June 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1174006
This article is part of the Research Topic A Roadmap Towards Immunotherapy in Pediatric Tumors View all 7 articles
Bacille Calmette-Guérin (BCG) vaccination supposedly imparts and augments “trained immunity” that cross-protects against multiple unrelated pathogens and enhances general immune surveillance. Gradual reductions in tuberculosis burden over the last 3–5 decades have resulted in the withdrawal of BCG vaccination mandates from developed industrialized countries while reducing to a single neonatal shot in the rest. Concurrently, a steady increase in early childhood Brain and CNS (BCNS) tumors has occurred. Though immunological causes of pediatric BCNS cancer are suspected, the identification of a causal protective variable with intervention potential has remained elusive. An examination of the countries with contrasting vaccination policies indicates significantly lower BCNS cancer incidence in 0–4-year-olds (per hundredthousand) of countries following neonatal BCG inoculations (n=146) vs. non-BCG countries (n=33) [Mean: 1.26 vs. 2.64; Median: 0.985 vs. 2.8; IQR: 0.31–2.0 vs. 2.4–3.2; P=<0.0001 (two-tailed)]. Remarkably, natural Mycobacterium spp. reexposure likelihood is negatively correlated with BCNS cancer incidence in 0-4-year-olds of all affected countries [r(154): −0.6085, P=<0.0001]. Seemingly, neonatal BCG vaccination and natural “boosting” are associated with a 15–20-fold lower BCNS cancer incidence. In this opinion article, we attempt to synthesize existing evidence implying the immunological basis of early childhood BCNS cancer incidence and briefly indicate possible causes that could have precluded objective analysis of the existing data in the past. We draw the attention of the stakeholders to consider the comprehensive evaluation of immune training as a potential protective variable through well-designed controlled clinical trials or registry-based studies as feasible for its potential applications in reducing childhood BCNS cancer incidence.
Bacille Calmette-Guérin (BCG), a derivative of Mycobacterium bovis (a member of M. tuberculosis complex), is the most widely used early childhood vaccine that is in use for over 100 years for protecting against tuberculosis (TB) (1). It offers the greatest protection against miliary TB and tuberculous meningitis and to a lesser extent against pulmonary TB (2). However, the non-specific protection offered by BCG against other common pathogens, sepsis, and unrelated conditions has been associated with up to a 50% reduction in early childhood mortality rate in different studies (3–7). BCG is supposed to provide this protection through the induction of granulopoiesis, the activation of heterologous T-cell immunity, and enhanced non-specific innate immunity committed through epigenetic and metabolic reprogramming (8–15). Mechanistically, the inoculation is supposed to bring about functional reprogramming of the cells of innate immune response (e.g., Monocytes, macrophages, NK cells, etc.) that leads to better immune surveillance and response (9–16). In the case of children growing up in ‘hygienic’ conditions, it has been hypothesized to provide the necessary immune stimulus required for “normal” immune system development that may reduce the incidence of common childhood cancers and immune conditions, including autoimmune diseases (17–21). The ability of BCG to potentiate cell-mediated response, which is believed to be important for cancer, has led to its evaluation for preventive and therapeutic potential in many observational studies and clinical trials (15, 18–20, 22–28). However, except in the case of bladder cancer, its preventive and therapeutic potential remains debated and largely uncertain due to the lack of comprehensive and consistent data along with a theoretical framework that could at least attempt to explain different conflicting observations (15, 19, 20, 22). The evidence generated so far seems to be overwhelmingly negating the potential of BCG in protecting against cancers. We envision this apparent uncertainty about the potential benefits of BCG vaccination and conflicting outcomes in different studies to be resulting from the inappropriate generalization of the potency of different BCG vaccines in activating the innate immune system (29, 30), omissions about the requirement of boosters (31, 32), longevity of conferred non-specific “trained immunity” that seldom lasts a few years in the absence of a booster (31–33), treating all cancers as a homogeneous lot disregarding their origin (e.g., embryonal, mutational: sporadic, germline, primary, secondary, etc.) and associated inherent differences, non-consideration of the immune status of subjects and their exposure to a specific intervention and risk factors, etc. in different studies reported in (10, 15, 17–20, 22).
Early childhood cancers are hypothesized to result from aberrant embryonic remnants that could have been otherwise eliminated during ‘normal’ development had the appropriate immune stimulus and training been available in the form of common pathogens and microbes to which we have been exposed during evolution (17–20, 22, 28). Based on the observation of children brought up in supposedly more hygienic conditions, a critical role for natural exposure to pathogens for immune reprogramming and maturation has been proposed (17–20, 34–36). ‘Hygienic’ conditions have been associated with increased allergy and atopic conditions, susceptibility to life-threatening infections, and sometimes even with the differential incidence rates of cancers (9, 11, 17, 19–22). However, for the lack of consistent evidence pinpointing a preventive/protective agent or intervention that may have a direct causal relationship, ambivalence about their actual potential in cancer incidence prevention has remained (15, 17, 19, 22, 28).
Brain and other central nervous system (BCNS) cancer remains the second most frequently occurring childhood cancer and the most frequent cause of cancer mortality (37). The updated estimates for all cancers and ages periodically produced by the International Agency for Research on Cancer, WHO as a part of the GLOBOCAN project (https://gco.iarc.fr/today/home) are a comprehensive source of cancer incidence estimates and related information. Incidentally, the industrialized western world with high living standards and hygiene has higher incidence rates of both early childhood and later-age BCNS cancer (37). Most of the countries for which estimates are available have been steadily registering an annual increase in childhood BCNS cancer incidence for the last 3-5 decades (37). The improvement in TB prevalence and incidence resulting from improved hygiene and living standards globally had also seen a concomitant reversal of BCG mandates, and changes in BCG booster schedules, or altogether scrapping in several countries (38, 39). For the majority of countries/territories with data, the estimated annual % change in total BCNS cancer incidence has been positive for recent decades (Supplementary Figure 1) (37). The childhood BCNS cancer incidence is highest in 0-4-Year-olds (0-4Y-Old or younger than 5 years) and decreases in older 5-14Y-old children. GLOBOCAN study estimates BCNS cancer incidence and associated mortality among 0-4Y-olds during the year 2020 to be 9252 and 4256, respectively, which accounted for about 16% of incidence and 20% of associated deaths from all cancer types combined (37). Despite, advances in diagnostics and therapeutics, the preventive vaccines and causative risk factors for the majority of early childhood BCNS cancers remain unidentified and uncharacterized (40–43). Though previously the occurrence of malignant and embryonic origin BCNS tumors in children <5 years has been variously associated (positively as well as negatively) with risk factors like socioeconomic position (41, 42, 44), allergy (42, 45, 46), infections or exposure to pathogens early in life (47, 48), human developmental index (HDI), etc. (41–48), the identification of potential protective risk factors with an intervention potential (i.e., employable or actionable) to reduce the incidence of BCNS cancers has so far remained an unfulfilled aspiration.
Compared to BCNS cancers arising in any other age group, the early childhood incidences are predominantly of embryonal origin and malignant, and their incidence rates decrease with age (40–43). The scientific community may be aware of the fact that although globally BCNS cancer incidence rates pick up again around 20 years of age and keep going up with age (37, 40, 41), they are overwhelmingly not of the same origin (up to 10-fold) (42, 43). This offers an opportunity to evaluate any potential role of immune modulation and maturation, more specifically that of BCG vaccination, the most widely given vaccine, on the incidence of childhood BCNS cancer, if any. We surmise that if early childhood BCG vaccination-induced/primed non-specific trained immunity could play any role in cancer incidence arising from abnormal immune system development and maturation, their effect would be more discernible in early childhood cancers, which allegedly arise from embryonic remnants that could not be eliminated during early development for the lack of appropriate and timely immune system stimulation (priming and maturation). The policy on BCG vaccination varies by country (Table 1) (39). Currently, the majority of middle- to low-income countries follow the BCG vaccination policy for newborns, while non-BCG mandating countries (No-BCG) are mostly affluent high-income countries (51) with very high (vh-) to high (h-) HDI – a composite measure of “a long and healthy life, access to knowledge and a decent standard of living” (49). The existence of disparities in BCG vaccination policies (39) and TB incidence rates (i.e., M. tuberculosis complex exposure possibility) across countries (50) provides a unique opportunity to test the hypothesis that BCG vaccination and/or “boosting” may be potentially protective (alternatively non-protective) in early childhood BCNS cancer.

Table 1 Early Childhood Brain and other Central Nervous System (BCNS) cancer incidence in different HDI group countries with varying tuberculosis (TB) incidence rates (potential exposure) and correlation analysis.
Globally, the distribution of updated age-standardized incidence rates (ASR) of BCNS cancer for the recent year 2020 (37) varies widely from 0 to 5.4 per 100,000 (100k) in 0-4Y-olds (Table 1). Nevertheless, the widely mandated BCG vaccination seems associated with reduced childhood BCNS cancer incidence in 0-4Y-olds (Figure 1A). The countries of Europe and North America with no neonatal BCG vaccination policy in place (‘No-BCG’ or ‘NB’) are some of the worst affected countries, while incidence rates in countries with BCG vaccination in place (BCG countries) do have some similar high incidence rate countries, but the majority seem to cluster towards lower incidence rates. The incidence in No-BCG and BCG countries significantly differ (Mean: 2.64 vs 1.26; Median: 2.8 vs 0.985; IQR; 2.4–3.2 vs 0.31–2.0; P = <0.0001 (two-tailed)). The high to low incidence rates of BCNS cancer among countries also display an interesting association with countries’ income brackets (51). The higher income brackets have higher average incidence rates than the lower income brackets (Figure 1A: right side Y axis). The previous observations made in small-scale studies performed in different countries that identified the socioeconomic position (SEP) of families as a potential risk factor positively associated with childhood BCNS cancer incidence (52, 53), seem to be applicable globally. However, the occurrence of high incidence rates (ASR) in certain BCG vaccinating countries of Europe [notably, Montenegro (5.4), Estonia (4.3), Croatia (4.3), North Macedonia (3.6), Republic of Moldova (3.5), Lithuania (3.4), Serbia, Poland (3.3)] along with wide variation in incidence rates among BCG vaccinating countries 0 – 5.4, casts doubt on the ability of single BCG shots alone to protect young children from BCNS cancers, as could have been the case in some of the past studies reported in (15, 19, 20, 22) that failed to find an association between BCG vaccination and cancer incidence.
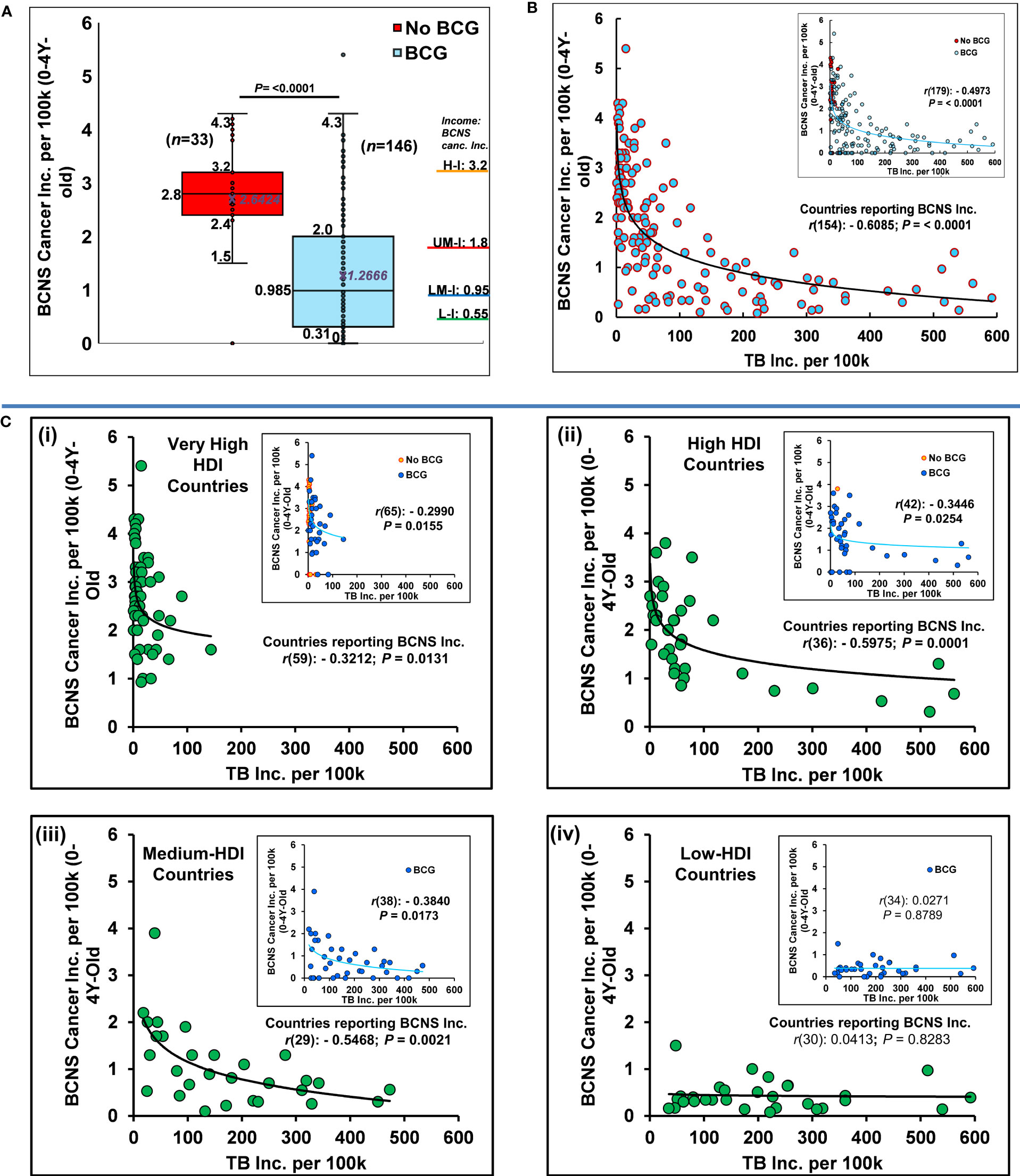
Figure 1 BCG vaccination and M. tuberculosis complex exposure negatively associated with BCNS cancer incidence in 0-4Y-old children. (A) Globally, the distribution of age-standardized BCNS cancer incidence rates (ASR) among countries (n=179) are shown as Box and whisker plot. BCG-vaccinated children had significantly reduced incidence (t-test: two-tailed, equal/unequal variance) than the non-vaccinated (No BCG). The average incidence rate seems directly proportional to income levels [H-I: High ($13,205 or more); UM-I: Upper Middle (between $4,256 and $13,205); LM-I: Lower Middle ($1,086 and $4,255); L-I: Low income (≤$1,086) as defined by World Bank, 2023 (51) (For CI refer to (37)]. (B) The probability of natural boosting (exposure to the M. tuberculosis complex) is strongly associated with lower BCNS cancer incidence in the affected countries/territories. (C) BCNS cancer incidence in countries belonging to different HDI subgroups: The distribution of BCNS cancer incidence with TB incidence rates among very high HDI [panel C(i)], high HDI [panel C(ii)], medium HDI [panel C(iii)], and low HDI [panel C(iv)] countries indicates a significant negative correlation between BCNS cancer incidence and the supposed probability of M. tuberculosis complex exposure among countries except in the group of low-HDI countries (also refer to Table 1 for subgroup analysis). Inset has all countries color-coded for BCG and No BCG groups. The trend lines are drawn to guide the eye. Note the presence of outliers in the No-BCG and BCG groups. Most of the high BCNS incidence-displaying countries of the BCG group, including the outlier, are clustered in low background M. tuberculosis complex exposure regions with TB incidence rates of < 100 per 100k (B, C) for the year 2020.
A missing explanatory variable for the observed reduced BCNS cancer incidence in BCG countries could be the ‘boosting’ stimulus arising from natural exposure to boosting events (also priming for No-BCG countries) in the form of M. tuberculosis complex exposure specific to populations. It may be pertinent here to reiterate for the scientists from other backgrounds that M. tuberculosis complex exposure does not invariably cause TB but rather only sustained long-term exposure of susceptible individuals, which constitutes a small fraction of the population – variously estimated to be <1-5%, is supposed to result in clinical TB (33, 54). Assuming the BCG vaccination could be playing a role in the BCNS cancer incidence in young children, the higher incidence in some countries could be envisioned to result from the inability of a single BCG inoculation to sufficiently activate the immune system in the absence of proper, timely boosting. Revaccination has been indicated to enhance the non-specific protective effects of BCG in early childhood (3, 55, 56). The neonates in countries with higher TB prevalence or incidence rates (50) could be more frequently exposed to natural boosting events than those born in low TB incidence countries.
Surprisingly, when we look at the covariation of BCNS cancer incidence rates (37) with countries’ TB incidence rates (50) they are found to be significantly negatively correlated even when completely disregarding their BCG vaccination policy (Table 1). This correlation further improves on consideration of the countries reporting childhood BCNS cancer incidence [Table 1, Affected countries: r(154): –0.6085, P = <0.0001; All countries (affected + non-affected) r(179): –0.4973; P = <0.0001]. The consideration of only BCG countries slightly decreases the correlation that could be potentially indicative of the important role of exposure to the M. tuberculosis complex in industrialized No-BCG countries with overall higher “hygiene” as well. This view gets support from the existence of negative correlation in No-BCG countries belonging to very high HDI (See the correlation analysis for countries of very high HDI subgroup in Table 1). Looking at the distribution of BCNS cancer incidence, it seems to be decreasing exponentially with the increase in TB incidence rates of the countries (i.e., increased probability of natural exposure: priming and boosting). The seeming outlier of the BCG vaccination group that reported BCNS cancer incidence of 5.4 (i.e., Montenegro) as well as other high incidence reporting countries happened to be some of the least TB incidence (M. tuberculosis complex exposure) countries of the No-BCG and BCG group of countries (Figure 1B). The increase in TB incidence rates up to 100 per 100,000 (100k), is seemingly associated with a four-fold reduction in BCNS cancer incidence among 0-4Y-olds, which further goes down by two-fold on an increase in TB incidence to 200 per 100k. The same trend follows till the highest TB incidence rate of about 600 per 100k, potentially indicating TB incidence rates in populations as a risk factor inversely correlated with early childhood BCNS cancer incidence rates.
The reporting of childhood BCNS cancer incidence across countries has not been uniform. The projected estimates could be more skewed in low HDI countries because of the possible lack of education, medical infrastructure, diagnostics, affordability, and reporting. Historical estimates for many countries display fluctuations in 3–8-year periods with extreme variations on sex-specific incidence rates, potentially indicative of more imprecise estimates, and underline a need for better data reporting and collation than current (37). Thus, consideration of the latest BCNS cancer incidence estimates in very high HDI (vh-HDI) and high HDI (h-HDI) countries and to a lesser extent medium HDI (m-HDI) may be expected to more reliably illustrate the association, if any. The low HDI countries alone may not reliably show any significant association due to the supposed high M. tuberculosis complex exposure (supposedly not a limiting factor any more) and extremely low BCNS incidence rates combined with diagnostic and reporting limitations, including the TB incidence rates. Remarkably, the BCNS cancer incidence among 0-4Y-olds in vh-HDI, h-HDI and m-HDI countries during 2020 was found to be significantly but negatively correlated with their TB incidence rates, except in the low-HDI countries as expected [Figure 1C (i-iv); vh-HDI: r(65): −0.2990, P = 0.0155; h-HDI: r(42): −0.3446; P = 0.0254; m-HDI: r(38): −0.3840, P = 0.0173; l-HDI: r(34): 0.0271, P = 0.8789; See Table 1]. For the associations in various subgroups, refer to Table 1. The negative association between childhood BCNS cancer incidence and exposure to the M. tuberculosis complex significantly improved on consideration of only those countries that were reporting incidence of childhood BCNS cancer (affected countries) both as a group of countries (ALL) as well as HDI subgroups [Total: r(154): −0.6085; P = <0.0001; vh-HDI: r(59): −0.3212, P = 0.0131; h-HDI: r(36): −0.5975, P = 0.0001; m-HDI: r(29): −0.5468, P = 0.0021]. The negative association remained prominent for relevant subgroups of countries that had significant M. tuberculosis complex exposure and BCG vaccination policy in place [BCG countries: ALL affected r(125): −0.5595; P = <0.0001; h-HDI: r(34): −0.6072; P = 0.0001; m-HDI: r(29): −0.5467; P = 0.0021; Table 1 subgroups]. Together, they suggest that natural boosting combined with BCG vaccination may be protectively related to early childhood BCNS cancer incidence.
The early childhood BCNS cancer incidence in countries appears to be negatively associated with the prevailing possibility of exposure to the M. tuberculosis complex. The observations presented could be construed as indicative of non-exposure to M. tuberculosis complex or natural “boosting” as an unidentified risk factor for childhood BCNS cancer, possibly functionally related to the “Hygiene Hypothesis” and associated trained immunity. The boosting event(s) could be necessary for supposed priming or activation (immunomodulation), and reprogramming of the metabolism for desired outcomes. We suspect the current regimen of single neonatal BCG inoculation may not be sufficient to cause desired immune activations in environments lacking boosting opportunities. Possibly, multiple shot regimens, as used to be given earlier in many countries (39) or suggested for enhancing nonspecific effects (55, 56) may be necessary for providing the required stimulus and boosting to stem the steady annual rise of early childhood BCNS cancer incidence globally (37). However, since a large majority of early childhood BCNS cancers are supposedly embryonic developmental remnants, the likelihood of the mother’s immune system getting modulated by exposure to BCG and/or M. tuberculosis complex itself and in effect modulating and moderating that of the embryo (in utero, including endocrine) for ‘normal’ development remains a possibility (20, 28). We would be inclined to suggest the pouring and pooling of resources for a thorough evaluation of BCG’s potential in reducing childhood BCNS cancer incidence. With BCNS cancers in children on the rise, carefully planned, appropriately controlled, multicentric clinical trials, preferably coordinated and managed by WHO, should be performed in high-incidence countries, both No-BCG and BCG countries, to reliably evaluate the qualitative and quantitative contributions of neonatal BCG inoculation(s), boosting, and the mother’s immune system modulation. The countries with zero incidence rates could provide additional insights in terms of etiological agents as well as help identify unknown risk factors specific to countries that are responsible for huge differences in the BCNS cancer incidence rate even among socially similar vh-HDI countries (e.g., Iceland, Luxembourg vs. Germany, Ireland, Greece, Montenegro, etc.). If immunomodulation has a role in the increased incidence of BCNS cancers in childhood, the recent change in schedule and policy from multiple to single to no BCG inoculations could be costing us >10-fold higher incidence and mortality.
The inherent limitations of the cancer incidence dataset arising from data availability and the methodology employed (57), especially in lower HDI countries, should also be considered when planning for exploratory evaluation studies. As BCNS cancer incidence reporting globally remains fragmentary (37, 57), the studies, whether prospective or registry-based retrospective, should wherever possible be performed in high incidence but low TB prevalence countries, carefully controlling for age, trained immunity status, mothers’ immunization, etc. to arrive at meaningful conclusions about its preventive interventional potential. Consideration of existing trends of incidence in previous relevant years (37) as a baseline could provide additional pointers to help reliably estimate the impact of specific interventions in clinical trials.
BCG vaccinations coupled with timely natural “boosting” could be causally associated with a reduction in childhood BCNS cancer incidence. Future research may be directed at evaluating the same in carefully controlled trials and identifying country-specific differences and modifiers. It is needed to improve our understanding of the role of BCG vaccination and the mechanistic details of the potential protective immune augmentation process. It may help devise ways to stem the steady rise of early childhood cancer incidence with similar components. Additionally, the findings could indicate inoculation regimens that are better suited for lowering early childhood cancer incidence. The revised multiple BCG vaccinations, as they used to be, may have to be reintroduced globally to reduce childhood cancer incidence. The countries with higher incidence rates (ASR per 100k), e.g., Montenegro (5.4), Estonia (4.3), USA (4.3), Greece (4.2), Canada (4.1), Israel (4), Slovakia (3.9), Slovenia (3.9), etc., are going to gain the most in terms of morbidity and mortality reduction. However, a lot needs to be done to reliably ascertain the potential benefits of BCG vaccination in reducing cancer incidence, or the lack thereof, before this could be again reintroduced or written off.
SS conceived the idea, designed the research, analyzed the data, and wrote the paper. RS and AD wrote the paper. All authors contributed to the article and approved the submitted version.
The laboratory of SS is supported by a seed grant from IoE, Banaras Hindu University (No. R/Dev/D/IoE/Seed Grant/2020-21/Dr. Samer Singh). The work presented is unfunded.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1174006/full#supplementary-material
Supplementary Figure 1 | Estimated Annual Percentage Change of Brain and Central Nervous System Cancer Incidence ASR per 100,000 in 0-4Y-olds. Majority of No-BCG and BCG countries/territories (n=44) display positive annual percentage change in combined incidence for sexes in 0-4Y-olds (last 15 years data). Bars show CI (Available at https://gco.iarc.fr/overtime/en/dataviz/eapc?populations=38000_3600_7600_10000_11200_12400_15200_15600_17000_19100_18800_20300_20800_21800_25000_27600_23300_35200_35600_37200_37600_39200_41000_41400_42800_44000_47000_52800_55400_57800_61600_70300_70500_72400_75200_75600_76400_79200_80000_80400_82630_82610_84000_82620&sexes=1_2&multiple_populations=1&years=2018&cancers=23&types=0&key=asr&age_end=0&group_cancers=0&multiple_cancers=0&mode=population&age_start=0&group_years=0&eapc_span=15&ul=1). The historical estimates remain weak. The observed sex-specific incidence variation in the past for specific years or over time could be indicative of reporting issues. (Available at https://gco.iarc.fr/overtime/en/dataviz/trends?populations=38000&sexes=1_2&multiple_populations=1&years=1943_2018&cancers=23&types=0&key=asr&age_end=0&group_cancers=0&multiple_cancers=0&mode=population&age_start=0&group_years=0).
1. UNICEF. Bacillus calmette-guérin (BCG) supply and demand update (2019). Available at: https://www.unicef.org/supply/reports/bacillus-calmette-gu%C3%A9rin-bcg-supply-and-demand-update.
2. Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet (2006) 367(9517):1173–80. doi: 10.1016/S0140-6736(06)68507-3
3. Trunk G, Davidović M, Bohlius J. Non-specific effects of bacillus calmette-guérin: a systematic review and meta-analysis of randomized controlled trials. Vaccines (Basel) (2023) 11(1):121. doi: 10.3390/vaccines11010121
4. Biering-Sørensen S, Aaby P, Lund N, Monteiro I, Jensen KJ, Eriksen HB, et al. Early BCG-Denmark and neonatal mortality among infants weighing <2500 g: a randomised controlled trial. Clin Inf Dis (2017) 65:1183–90. doi: 10.1093/cid/cix525
5. Higgins JPT, Soares-Weiser K, Lopez-Lopez JA, Kakourou A, Chaplin K, Christensen H, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ (2016) 355:i5170. doi: 10.1136/bmj.i5170
6. Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis (2011) 204(2):245–52. doi: 10.1093/infdis/jir240
7. Nankabirwa V, Tumwine JK, Mugaba PM, Tylleskär T, Sommerfelt H, PROMISE-EBF Study Group. Child survival and BCG vaccination: a community based prospective cohort study in Uganda. BMC Public Health (2015) 15:175. doi: 10.1186/s12889-015-1497-8
8. Brook B, Harbeson DJ, Shannon CP, Cai B, He D, Ben-Othman R, et al. BCG Vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci Transl Med (2020) 12(542):eaax4517. doi: 10.1126/scitranslmed.aax4517
9. Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science (2016) 352(6284):aaf1098. doi: 10.1126/science.aaf1098
10. Cvián C, Fernández-Fierro A, Retamal-Díaz A, Díaz FE, Vasquez AE, Lay MK, et al. BCG-Induced cross-protection and development of trained immunity: implication for vaccine design. Front Immunol (2019) 10:2806. doi: 10.3389/fimmu.2019.02806
11. Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol (2013) 34(9):431–9. doi: 10.1016/j.it.2013.04.004
12. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille calmette-guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A (2012) 109(43):17537–42. doi: 10.1073/pnas.1202870109
13. Arts RJ, Joosten LA, Netea MG. Immunometabolic circuits in trained immunity. Semin Immunol (2016) 28(5):425–30. doi: 10.1016/j.smim.2016.09.002
14. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science (2014) 345(6204):1250684. doi: 10.1126/science.1250684
15. Singh AK, Netea MG, Bishai WR. BCG Turns 100: its nontraditional uses against viruses, cancer, and immunologic diseases. J Clin Invest (2021) 131(11):e148291. doi: 10.1172/JCI148291
16. Saeed S, Quintin J, Kerstens HHD, Rao NA, Aghajanirefah A, Matarese F, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science (2014) 345:1251086. doi: 10.1126/science.1251086
17. Greaves M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat Rev Cancer (2018) 18(8):471–84. doi: 10.1038/s41568-018-0015-6
18. Rosenthal SR. Cancer precursors and their control by BCG. Dev Biol Stand (1986) 58(Pt A):401–16.
19. Grange JM, Stanford JL, vaccination BCG. And cancer. Tubercle (1990) 71(1):61–4. doi: 10.1016/0041-3879(90)90063-e
20. Grange JM, Stanford JL, Stanford CA, Kölmel KF. Vaccination strategies to reduce the risk of leukaemia and melanoma. J R Soc Med (2003) 96(8):389–92. doi: 10.1258/jrsm.96.8.389
21. Angelidou A, Pittet LF, Faustman D, Curtis N, Levy O. BCG Vaccine’s off-target effects on allergic, inflammatory, and autoimmune diseases: worth another shot? J Allergy Clin Immunol (2022) 149(1):51–4. doi: 10.1016/j.jaci.2021.09.034
22. Marron M, Brackmann LK, Kuhse P, Christianson L, Langner I, Haug U, et al. Vaccination and the risk of childhood cancer-a systematic review and meta-analysis. Front Oncol (2021) 10:610843. doi: 10.3389/fonc.2020.610843
23. Davignon L, Robillard P, Lemonde P, Frappier ABCG. Vaccination and leukemia mortality. Lancet (1970) 2(7674):638. doi: 10.1016/s0140-6736(70)91402-9
24. Rosenthal SR, Crispen RG, Thorne MG, Piekarski N, Raisys N, Rettig PG, et al. And leukemia mortality. JAMA (1972) 222(12):1543–4. doi: 10.1001/jama.1972.03210120041010
25. Snider DE, Comstock GW, Martinez I, Caras GJ. Efficacy of BCG vaccination in prevention of cancer: an update. J Natl Cancer Inst (1978) 60(4):785–8. doi: 10.1093/jnci/60.4.785
26. Nathanson L. Use of BCG in the treatment of human neoplasms: a review. Semin Oncol (1974) 1(4):337–50.
27. Bast RC Jr, Zbar B, Borsos T, Rapp HJ. BCG And cancer. N Engl J Med (1974) 290(26):1458–69. doi: 10.1056/NEJM197406272902605
28. Hauer J, Fischer U, Borkhardt A. Toward prevention of childhood ALL by early-life immune training. Blood (2021) 138(16):1412–28. doi: 10.1182/blood.2020009895
29. Angelidou A, Conti MG, Diray-Arce J, Benn CS, Shann F, Netea MG, et al. Licensed bacille calmette-guérin (BCG) formulations differ markedly in bacterial viability, RNA content and innate immune activation. Vaccine (2020) 38(9):2229–40. doi: 10.1016/j.vaccine.2019.11.060
30. Miyasaka M. Is BCG vaccination causally related to reduced COVID-19 mortality? EMBO Mol Med (2020) 12(6):e12661. doi: 10.15252/emmm.202012661
31. Rakshit S, Ahmed A, Adiga V, Sundararaj BK, Sahoo PN, Kenneth J, et al. BCG Revaccination boosts adaptive polyfunctional Th1/Th17 and innate effectors in IGRA+ and IGRA–Indian adults. JCI Insight (2019) 4(24):e130540. doi: 10.1172/jci.insight.130540
32. Menzies D. Interpretation of repeated tuberculin tests. boosting, conversion, and reversion. Am J Respir Crit Care Med (1999) 159(1):15–21. doi: 10.1164/ajrccm.159.1.9801120
33. Singh S, Maurya RP, Singh RK. “Trained immunity” from mycobacterium spp. exposure or BCG vaccination and COVID-19 outcomes. PloS Pathog (2020) 16(10):e1008969. doi: 10.1371/journal.ppat.1008969
34. Strachan DP. Hay fever, hygiene, and household size. BMJ (Clinical Res ed) (1989) 299(6710):1259–60. doi: 10.1136/bmj.299.6710.1259
35. Stanford JL, Stanford CA, Grange JM. Environmental echoes. Sci Progr (2001) 84(Pt 2):105–24. doi: 10.3184/003685001783239014
36. Grange JM, Stanford JL, Stanford CA. Campbell De morgan’s ‘Observations on cancer’, and their relevance today. J R Soc Med (2002) 95:296–9. doi: 10.1177/014107680209500609
37. GCO. WHO GLOBOCAN 2020 (2023). The International Agency for Research on Cancer’ (IARC), WHO. Available at: https://gco.iarc.fr/today/home (Accessed 05 February 2023).
38. Dara M, Acosta CD, Rusovich V, Zellweger JP, Centis R, Migliori GB, et al. Bacille calmette-guérin vaccination: the current situation in Europe. Eur Respir J (2014) 43(1):24–35. doi: 10.1183/09031936.00113413
39. Zwerling A, Behr M, Verma A, Brewer T, Menzies D, Pai M. World atlas of BCG policies and practices (2020). Available at: http://www.bcgatlas.org/index.php.
40. Adel Fahmideh M, Scheurer ME. Pediatric brain tumors: descriptive epidemiology, risk factors, and future directions. Cancer Epidemiol Biomarkers Prev (2021) 30(5):813–21. doi: 10.1158/1055-9965.EPI-20-1443
41. Ostrom QT, Adel Fahmideh M, Cote DJ, Muskens IS, Schraw JM, Scheurer ME, et al. Risk factors for childhood and adult primary brain tumors. Neuro Oncol (2019) 21(11):1357–75. doi: 10.1093/neuonc/noz123
42. Ostrom QT, Francis SS, Barnholtz-Sloan JS. Epidemiology of brain and other CNS tumors. Curr Neurol Neurosci Rep (2021) 21(12):68. doi: 10.1007/s11910-021-01152-9
43. Nabors LB, Ammirati M, Bierman PJ, Brem H, Butowski N, Chamberlain MC, et al. Central nervous system cancers. J Natl Compr Canc Netw (2013) 11(9):1114–51. doi: 10.6004/jnccn.2013.0132
44. Porter AB, Lachance DH, Johnson DR. Socioeconomic status and glioblastoma risk: a population-based analysis. Cancer Causes Control (2015) 26(2):179–85. doi: 10.1007/s10552-014-0496-x
45. Turner MC. Epidemiology: allergy history, IgE, and cancer. Cancer Immunol Immunother (2012) 61(9):1493–510. doi: 10.1007/s00262-011-1180-6
46. Cui Y, Hill AW. Atopy and specific cancer sites: a review of epidemiological studies. Clin Rev Allergy Immunol (2016) 51(3):338–52. doi: 10.1007/s12016-016-8559-2
47. Johnson KJ, Cullen J, Barnholtz-Sloan JS, Ostrom QT, Langer CE, Turner MC, et al. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol Biomarkers Prev (2014) 23(12):2716–36. doi: 10.1158/1055-9965.EPI-14-0207
48. Lupatsch JE, Bailey HD, Lacour B, Dufour C, Bertozzi AI, Leblond P, et al. Childhood brain tumours, early infections and immune stimulation: a pooled analysis of the ESCALE and ESTELLE case-control studies (SFCE, France). Cancer Epidemiol (2018) 52:1–9. doi: 10.1016/j.canep.2017.10.015
49. UNDP. HUMAN DEVELOPMENT REPORT 2021/2022. United Nations Development Programme. UN Plaza, New York, NY 10017 USA: United Nations (2022). Available at: https://hdr.undp.org/system/files/documents/global-report-document/hdr2021-22pdf_1.pdf.
50. WHO. Global tuberculosis programme: WHO TB burden estimates. Available at: https://www.who.int/teams/global-tuberculosis-programme/data (Accessed 05 Feb 2023).
51. World Bank. World bank country and lending groups (2023). The World Bank Group. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (Accessed 05 Feb 2023).
52. Erdmann F, Hvidtfeldt UA, Sørensen M, Raaschou-Nielsen O. Socioeconomic differences in the risk of childhood central nervous system tumors in Denmark: a nationwide registerbased case-control study. Cancer causes control: CCC (2020) 31(10):915–29. doi: 10.1007/s10552-020-01332-x
53. Francis SS, Wang R, Enders C, Prado I, Wiemels JL, Ma X, et al. Socioeconomic status and childhood central nervous system tumors in California. Cancer causes control: CCC (2021) 32(1):27–39. doi: 10.1007/s10552-020-01348-3
54. WHO. Latent tuberculosis in fection: updated and consolidated guidelines for programmatic management (2018). Available at: https://apps.who.int/iris/bitstream/handle/10665/260233/9789241550239-eng.pdf;jsessionid=24D3F1097E3884502A9C1A.
55. Roth A, Benn CB, Ravn H, Rodrigues A, Lisse IM, Yazdanbakhsh M, et al. Effect of revaccination with BCG in early childhood on mortality: randomised trial in Guinea-Bissau. BMJ (2010) 340:c671. doi: 10.1136/bmj.c671
56. Benn CS, Fisker AB, Whittle HC, Aaby P. Revaccination with live attenuated vaccines confer additional beneficial nonspecific effects on overall survival: a review. EBioMedicine (2016) 10:312–7. doi: 10.1016/j.ebiom.2016.07.016
Keywords: cancer discovery, pediatric cancer, brain and CNS cancer, childhood vaccinations, BCG vaccine, trained immunity, cancer prevention, Mycobacterium spp.
Citation: Singh S, Diwakar A and Singh RK (2023) BCG vaccination policy, natural boosting and pediatric brain and CNS tumor incidences. Front. Immunol. 14:1174006. doi: 10.3389/fimmu.2023.1174006
Received: 25 February 2023; Accepted: 25 May 2023;
Published: 13 June 2023.
Edited by:
Aqeel Ahmad, University of Florida, United StatesReviewed by:
Wafaa M. Rashed, Ahram Canadian University, EgyptCopyright © 2023 Singh, Diwakar and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samer Singh, c2FtZXIuc2luZ2gxMEBiaHUuYWMuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.