
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 30 June 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1172369
This article is part of the Research TopicAutoimmune Diseases in ChildhoodView all 24 articles
 Giorgia Paldino1
Giorgia Paldino1 Maria Felicia Faienza2
Maria Felicia Faienza2 Marco Cappa3
Marco Cappa3 Andrea Pietrobattista4
Andrea Pietrobattista4 Donatella Capalbo5
Donatella Capalbo5 Mariella Valenzise6
Mariella Valenzise6 Vito Lampasona7
Vito Lampasona7 Annamaria Cudini1
Annamaria Cudini1 Elena Carbone1
Elena Carbone1 Olivia Pagliarosi1
Olivia Pagliarosi1 Giuseppe Maggiore4
Giuseppe Maggiore4 Mariacarolina Salerno8
Mariacarolina Salerno8 Corrado Betterle9
Corrado Betterle9 Alessandra Fierabracci1*
Alessandra Fierabracci1*Introduction: Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) syndrome is a rare monogenic disease determined by biallelic mutations in AIRE gene, which encodes a transcription factor essential for central immune tolerance. Classic diagnosis is determined by the presence of two of the main APECED clinical diseases: chronic mucocutaneous candidiasis, chronic hypoparathyroidism, and Addison’s disease. Non-endocrine autoimmunity, involving the liver, intestine, eyes, and kidneys, is generally reported in a minority of European patients, while American APECED patients have a higher tendency of developing organ-specific non-endocrine manifestations early in life. This observation led to the revision of the diagnostic criteria to permit earlier diagnosis based on the appearance of one classic triad symptom or one non-classical manifestation at a young age in the presence of IFNωAbs or AIRE mutations (Ferre-Lionakis criteria).
Patients and methods: We analyzed the clinical, genetic, and autoantibody (Ab) profiles in a series of 14 pediatric Italian APECED patients with gastrointestinal manifestations (seven male and seven female patients). Ten patients presented hepatitis (APECED-associated hepatitis (APAH)), while seven were affected by constipation, diarrhea, and malabsorption. Four patients had developed APAH before classic triad symptoms.
Results: Based on the age of appearance of non-endocrine manifestations including APAH and gastro-enteropathy, the Ferre-Lionakis criteria would have allowed an expedited diagnosis in 11/14 patients. Abs to tryptophan hydroxylase (TPHAb) and hepatic aromatic l-amino acid decarboxylase (AADC) were significantly associated with APECED patients of the present series. Abs to cP4501A2 were detectable in the serum of 4/8 patients with APAH, and Abs to cP4502A6 were detectable in 3/8 patients. AADC Abs tested positive in 5/7 patients, which is indicative of gastrointestinal dysfunction in APECED and TPHAb in 5/7 patients with gastrointestinal dysfunction. IFNAb was significantly associated with the syndrome.
Conclusion: Although Ferre-Lionakis expanded criteria applied to the American cohorts of APECED patients would require validation in independent large cohorts of European patients, the results of this study emphasize the importance to evaluate the presence and the age of appearance of APAH and autoimmune enteropathy even in European cohorts for an earlier APECED diagnosis. An earlier APECED diagnosis would also allow the prevention of episodes of life-threatening hypocalcemic seizures and adrenal crisis, which are the main manifestations of undiagnosed APECED.
Autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED, OMIM240300) syndrome is a rare monogenic disease due to biallelic mutations in autoimmune regulator (AIRE) gene (1). Classic diagnostic criteria are the presence of two of the three main APECED symptoms: chronic mucocutaneous candidiasis (CMC), chronic hypoparathyroidism (CH), and Addison’s disease (AD). Non-endocrine autoimmunity involving the liver, stomach, intestine, eyes, and kidneys is generally reported in a minority of patients (1).
Although it depends on the series analyzed, approximately 25% of APECED patients in different populations are affected by gastro-enteropathy (GE) manifestations (2) including celiac disease, cystic fibrosis, pancreatic exocrine insufficiency (PEI), autoimmune intestinal disease (2) and loss of enterochromaffin cells (3, 4), intestinal infections, gastritis due to an autoimmune attack against gastric parietal cells leading to gastric atrophy, and pernicious anemia (5). Circulating autoantibody (Abs) against parietal cells (anti-gastric parietal cells (PCAs)), i.e., anti-sodium potassium channel molecule and anti-intrinsic factor (IFA), are detectable. Candida esophagitis is characterized by pseudomembranotic lesions, erosion, and ulceration with retrosternal pain while swallowing and dysphagia (6, 7). Intestinal candidiasis may also be responsible for watery diarrhea and malabsorption (7). Autoimmune enteropathy is characterized by small intestinal villous atrophy, protracted diarrhea, no response to exclusion diets, and no severe immunodeficiency (2, 3, 8). Abs to tryptophan hydroxylase (TPHAb), an intestinal autoantigen expressed in serotonin-producing cells in the central nervous system and the intestine, were detected in APECED (8). Lymphangiectasia of the small intestine may also be responsible for steatorrhea in APECED patients (9).
Among the other non-endocrine manifestations, APECED-associated hepatitis (APAH) has been described to occur at different prevalence in large series of APECED patients among different ethnicities (reviewed (rev) in 10). Further, APAH is the first manifestation in less than 2% of APECED patients (rev in 11). Abs are detected against hepatic aromatic l-amino acid decarboxylase (AADC), cytochrome P450 family 1 subfamily A member 2 (CYP1A2), histidine decarboxylase (HDC), bactericidal permeability-increasing fold-containing B1, TPH, and p450 21-hydroxylase (21-OH, CYP21A2). No liver-specific autoantigen has been so far identified in all patients with APAH, and it has not been possible to specifically distinguish APAH from classical autoimmune hepatitis (AIH) (10). The homozygous c.967_979del13 AIRE mutation is the most frequently associated genotype to APAH development (10).
American APECED patients showed enrichment of organ-specific non-endocrine manifestations early in life when compared to European cohorts (11). Between 40% and 80% of the patients had non-major disorders at onset, while the occurrence of classical manifestations was delayed (11). This observation led to the revision of the diagnostic clinical criteria (11), which would permit earlier diagnosis based on the appearance of one classic triad (CMC, AD, and CH) (occurring below 20 years of age) and one non-classical manifestation (enamel hypoplasia, periodic fever with rash, non-infectious keratitis, or autoimmune hepatitis) at a young age in presence of IFNωAb or AIRE mutations (11, 12). In light of the foregoing, in this manuscript, we report and discuss the clinical, genetic, and Ab profile in a series of 14 pediatric Italian APECED patients with APAH or gastrointestinal manifestations highlighting common features and evaluating whether their early enrichment would allow an expedited clinical diagnosis of APECED similar to the American cohort.
The overall population consists of 14 cases (seven male and seven female patients): 10 unrelated patients (Table 1, Nos. 1, 2, and 7–14) and two couples of siblings (Nos. 3–4 and 5–6). All patients were recruited from continental Italy except for patient No. 8, who was recruited from Sicily. Although all the patients were referred and diagnosed in childhood, eight patients (Nos. 4–8 and 11–13) are currently adults.
The mean actual age was 16.5 years (range, 6.5–34.2). The mean age at referral was 4.8 years (range, 1–19.7). Ages at the presentation of the three major components of APECED, clinical manifestations, molecular genetics, and Ab profile of patients are analytically reported in Table 1. In Table 1, we have also summarized the minor APECED manifestations that have been detected so far in the whole series, as well as the results of both genetic analyses and Ab screening. Patient Nos. 2, 9, and 10 included in Table 1 are described for the first time in the present report, whereas the remaining cases have already been reported elsewhere (13–19). The patients were recruited from the University Department of Pediatrics (DPUO), the Endocrinology Unit, and the Hepatology, Gastroenterology and Nutrition Unit of Children’s Hospital Bambino Gesù (OPBG) IRCCS, in Rome (patient Nos. 1–4 and 10–14 in Table 1); from the Unit of Endocrinology and Rare Endocrine Diseases of Bari University (patient Nos. 9 and 10 in Table 1); from the Pediatric Endocrinology Units of the Department of Mother and Child; from the Department of Translational Medical Sciences of the University Federico II of Naples (patient Nos. 5–7 in Table 1); and from the Department of Human Pathology of Adulthood and Childhood of the University of Messina (patient No. 8 in Table 1).
The clinical history of the 14 patients, from the first cardinal manifestation to the APECED diagnosis, was retrospectively reconstructed based on questionnaires addressed to the families by the authors, whereas their subsequent clinical evolution, from the time of diagnosis onward, was assessed based on clinical records of the patients at our centers. For the diagnosis of APECED, we used the criteria proposed by Husebye et al. (12). Informed consent was obtained from all those who took part in the present study in accordance with the Declaration of Helsinki. The investigation was approved by the local Institutional Review Board (IRB) of OPBG, which regulates using of human samples for experimental studies (Study Protocol No. 1385_OPBG_2017).
Ab screening was performed in all cases at the time of diagnosis and periodically repeated during follow-up. The patients’ sera were assayed for insulin-dependent diabetes mellitus (type 1 diabetes (T1D))-related Abs, i.e., islet cell antibodies (ICAs) by immunofluorescence (IFL), anti-glutamic acid decarboxylase isoform 65 (GADAb) (First anti-GAD ELISA RSR, Cardiff, UK), anti-tyrosine phosphatase-related islet antigen 2 (IA2Ab) (First anti-IA2 ELISA RSR), anti-insulin (IAA) (Medzyme Corporation, Montreal, QC, Canada), and anti-zinc transporter 8 Abs (ZnT8Ab) (anti-ZnT8 RSR) by enzyme-linked immunosorbent assay (ELISA); and for thyroid-related Abs, i.e., anti-thyrotropin (TSH)-receptor (TRAb) immunoassay, Immulite TSI (Siemens Healthcare, Tarrytown, NY, USA), thyroglobulin (TgAb), and thyroperoxidase (TPOAb) via electrochemiluminescence immunoassay (ECLIA) (Siemens, Erlangen, Germany). Celiac-disease-related Abs were screened by chemiluminescence (CLIA, Quanta-Flash-Werfen, Monza, Milan, Italy), i.e., anti-transglutaminase (TRGAb) (CLIA, Quanta-Flash-Werfen, Monza, Milan, Italy) and by fluorimetric enzyme-linked immunoassay (FEIA), i.e., extractable nuclear antigen (ENA, SSA/RoAb) (ELiA, Thermo Fisher, Waltham, MA, USA) and by IFL endomysial Ab (EMA) (Werfen, Barcelona, Spain). Specific hepatic Abs, i.e., anti-liver–kidney microsomal (LKMAb) and anti-liver cytosol type 1 (LC1Ab), anti-ribosomal (RAb), and PCA were measured by indirect IFL (KSL-Werfen), while anti-soluble liver antigen IgG (SLA-IgG) (Euroimmun Italia s.r.l., Padua, Italy) via ELISA and IFA (ELiA, Thermo Fisher) were assayed via FEIA. Abs against autoantigen cytochrome P450 21-hydroxylase (21OHAb), hepatic autoantigens cytochrome P4501A2 (cP4501A2Ab and CYP1A2Ab) and P4502D6 (cP4502D6Ab), anti-villin (VillinAb), anti-75kDa (HarmoninAb and AIE-75Ab), and anti-interferonα4 (IFNα4Ab) were tested by luciferase immunoprecipitation system (LIPS, S. Raffaele Hospital, Milan, Italy) (22). Abs to autoantigen adrenal cortex (ACA) cells by IFL (Medical Systems, Milan, Italy), Abs to adrenal and gonadal autoantigens, cytochrome P450 side chain cleavage enzyme (SCCAb), adrenal autoantigen cP450c17α-hydroxylase (17αOHAb), hepatic aromatic l-amino acid decarboxylase (AADC), and TPHAb were tested by specific methods as previously reported (23–25) (S. Raffaele Hospital, Milan, Italy). IFNωAb was assayed by RIA in collaboration with FIRS Laboratories RSR Ltd (Cardiff, UK).
Non-organ specific Abs anti-nuclear (ANA), anti-neutrophil cytoplasmic (ANCA) (Elettrochimica s.r.l., Mainate, Varese), anti-double-stranded DNA (anti-dsDNA) (bioMérieux Italia S.p.A, Bagno a Ripoli, Florence, Italy), anti-reticulin (ARA), anti-mitochondrial (AMA), and anti-smooth muscle cell (SMA) were also tested by IFL (KSL-Werfen). Anti-cardiolipin Ab (CAb) was tested by FEIA (ELIA Thermo Fisher), and anti-glycoprotein 210 Ab (gp210Ab), which is indicative of primary biliary cirrhosis, was tested by Dot Blot assay (Alifax, Polverara, Padua, Italy).
All 14 exons and flanking exon–intron boundaries of the AIRE gene were sequenced in the DNA of recruited patients (26) according to already described protocols (Genetic Analyzer 3500 Applied Biosystems HITACHI System, Thermo Fisher Scientific, Rodano, Italy). Next-generation sequencing (NGS) was applied to the analysis of DNA samples of patient Nos. 1 and 2, and data were analyzed with the DRAGHEN Germline v3 algorithm (Illumina, San Diego, CA, USA).
In the present series, all APECED patients presented APAH or autoimmune enteropathies (AIEs) (Tables 1, 2, Figure 1, Supplementary Figure 1) (13–19). A description of unpublished case reports is reported in the Supplementary Text, Supplementary Figure 1 and Table 1.
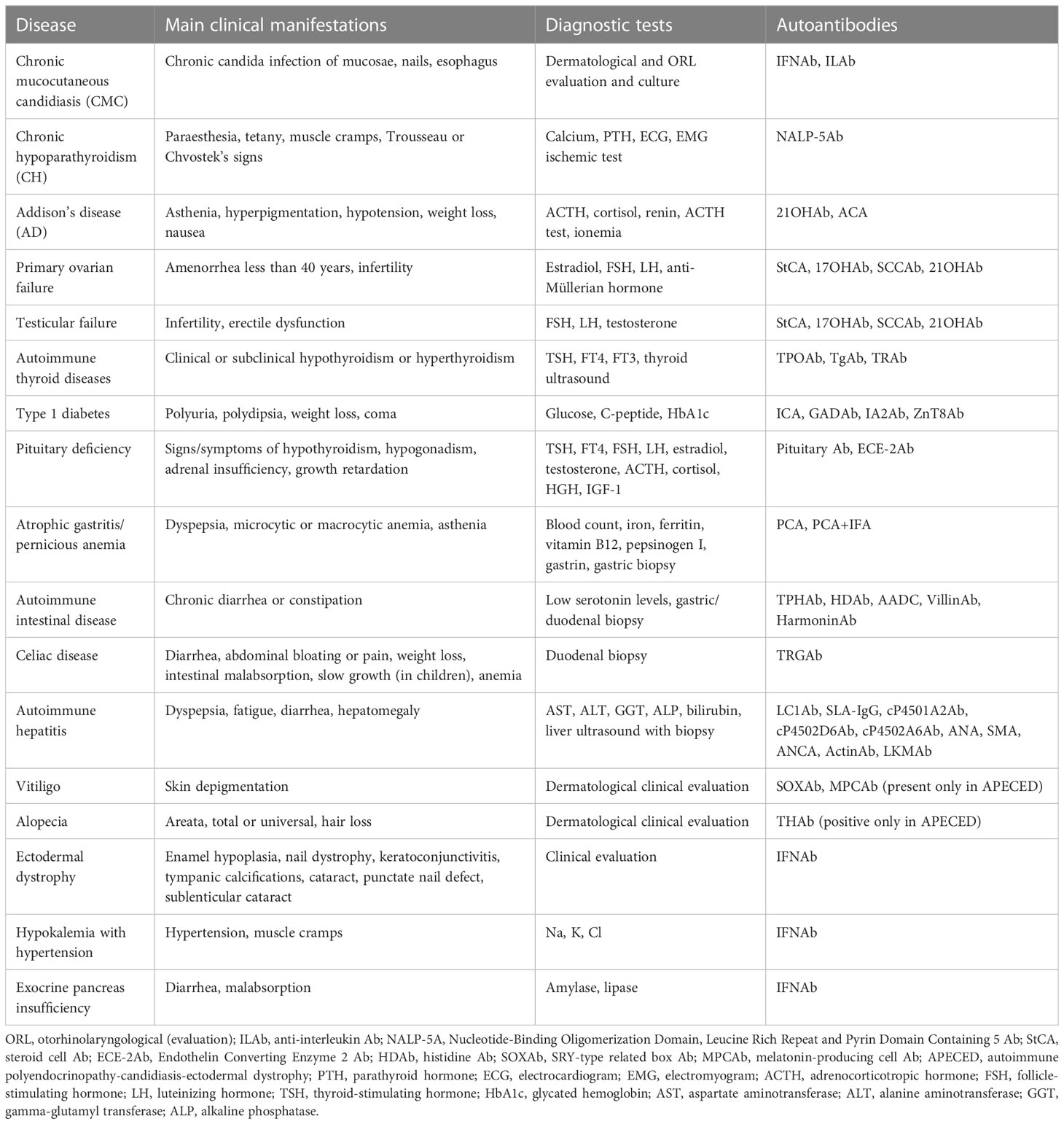
Table 2 Clinical manifestations, diagnostic criteria, and autoantibody tests for each disorder of the 14 APECED patients.
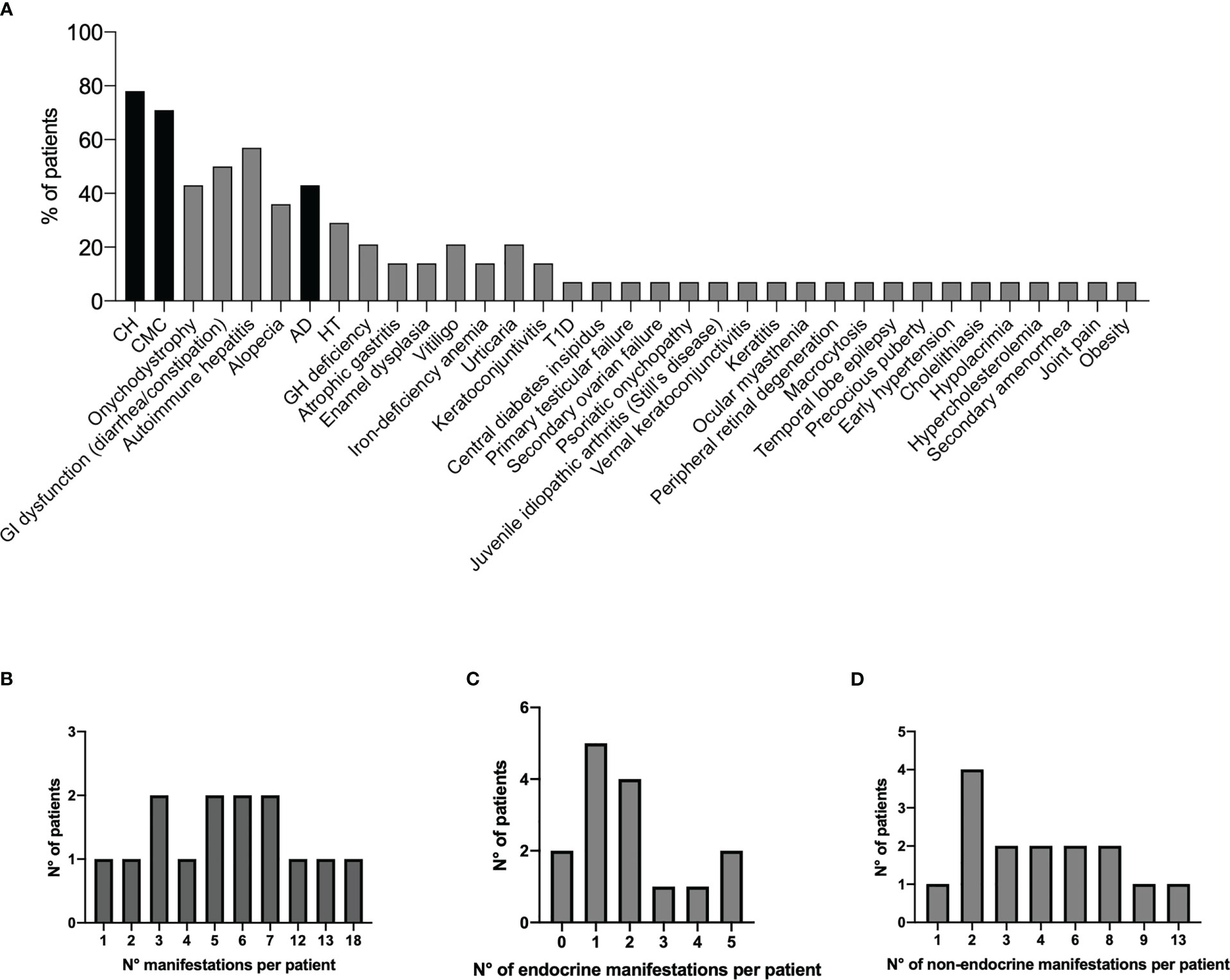
Figure 1 Prevalence of all disease manifestations in the 14 Italian APECED patients (A). The black bars represent the classical diagnostic triad. Distribution of total clinical manifestations (B), endocrinopathies (C), and non-endocrine manifestations (D) per patient. APECED, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy.
Nine patients (Nos. 1–5, 8, and 12–14) presented APAH (Table 1). The mean age of APAH diagnosis was 8.1 years (range, 1.5–13). In three patients (Nos. 1, 3, and 5), APAH occurrence was the first disease manifestation, while three patients (Nos. 2, 4, and 14) developed it following CH, two (Nos. 8 and 12) following CMC, and one (No. 13) developed APAH following CH and simultaneously with CMC. APAH-related specificities LKMAb, which target cytochrome P4502D6 (cP4502D6 and CYP2D6) autoantigen (27), tested positive in 3/9 patients (Nos. 4, 12, and 13) and TPH in 4/8 (Nos. 2, 5, 8, and 12); AADC was enriched in 6/7 (Nos. 1–3, 5, 8, and 12), cP450c21Ab in 4/6 (Nos. 1–4), and cP4501A2Ab in one patient (No. 12). Regarding other APAH-related Abs, ANA was present in 3/10 (Nos. 2, 3, and 14), SMA was present in 3/10 patients (Nos. 1, 3, and 12), ANCA and LC1Ab tested negative in all those tested (Nos. 1–5, 13, and 14 and Nos. 1–4, 13, and 14, respectively), and lastly, SLA-IgG was absent in all those tested (Nos. 1–4, 13, and 14) (Table 1). The most frequent AIRE mutation was c.967_979del13 (4/9 patients, Nos. 1, 3, 4, and 14) (10).
Seven patients (Nos. 5–11) were affected by signs of constipation, diarrhea, and/or malabsorption (Table 1). The mean age of intestinal dysfunction diagnosis was 6.1 years (range, 3.9–9). Two patients (Nos. 8 and 11) were also affected by iron-deficiency anemia and two (Nos. 5 and 10) by atrophic gastritis, and of them was affected with pernicious anemia (No. 10). The sera of five patients (Nos. 5, 7, 8, 10, and 11) tested positive for TPHAb and five (Nos. 5, 6, 8, 10, and 11) for AADC Abs known to be associated with AIE in APECED. AIE-75 and VillinAb tested negative in Nos. 5–10. cP450c21Ab tested positive in 3/6 patients (Nos. 6, 7, and 9) with gastrointestinal dysfunction. PCA was positive in 1/7 (No. 5), while IFA was present in 1/5 (No. 10) patients (Table 1). Six of six patients’ sera (Nos. 5–10) tested positive for IFNωAb and IFN-α4Ab (2). None of the patients presented celiac-related Abs. No specific AIRE genotype was prevalent.
Eleven of 12 (Nos. 1–8 and 10–12) of the whole series were positive for IFNω and 11/12 (Nos. 1–11) for IFNα4Ab.
Two patients (Nos. 5 and 8) with AIE also presented clinical laboratory parameters indicative of autoimmune hepatitis (vide supra).
In this study, we have characterized the clinical presentation and autoantibody profile of APAH and gastrointestinal manifestations in a series of 14 Italian APECED patients. Of these, nine patients (Nos. 1, 3–8, 11, and 12) were previously reported by our group (13–19), while five patients were newly described in the present study (Nos. 2, 9, 10, 13, and 14) and were added to the published series of 158 APECED patients recruited from across Italy (14).
In the analysis conducted by Garelli et al. (14) on 568 patients from different ethnicities, APAH occurs with an overall prevalence of between 4% and 43% of patients. In particular, in the Italian APECED series, APAH was diagnosed in 21.5% of patients by the end of the follow-up (14). Autoimmune intestinal diseases were diagnosed between 5% and 54% of APECED patients and autoimmune gastritis/pernicious anemia in 4% to 45% of patients. In the Italian APECED series, 29.7% of patients developed autoimmune intestinal dysfunction; autoimmune gastritis was diagnosed in 25.9% of APECED patients, and pernicious anemia was diagnosed in 21/41 of patients.
The analysis of the present Italian APECED series showed a significant expansion of disease manifestations with 35 clinical entities in varying frequencies (from 7% to 78%, Figure 1A, Table 1) with a median of 5.5 manifestations per patient (mean 6.6; range, 1–18; Figure 1B). The observed spectrum and frequency of endocrine and non-endocrine manifestations (median of 1.5 endocrine manifestations per patient; mean 2; range, 0–5 (Figure 1C); median of 3.5 non-endocrine manifestations; mean 4.6; range, 1–13 (Figure 1D), respectively) were similar to those of published cohorts (rev in 12). Further, the putative association between eight manifestations and APECED/AIRE is highly debatable; these include IgA deficit, septic arthritis, periodic rash-associated fever, kidney cysts, headaches, polyps, posterior reversible encephalopathy syndrome (PRES), and abscesses.
Of note, organ-specific non-endocrine manifestations were enriched in the present series with autoimmune hepatitis and gastrointestinal dysfunction seen in 57% (814) and 50% (7/14) of patients, respectively (Supplementary Figure 1). Remarkably, in published cohorts, circulating autoantibodies were found in approximately 80% of non-APECED patients affected by AIH (28). Two clinically distinct subtypes of AIH can be distinguished based on Ab patterns: AIH-1 and AIH-2 (29). AIH-1 patients had the presence of circulating ANA and/or SMA. Antibodies directed against SLA/liver-pancreas antigen (LP) (30, 31) specifically occurred in AIH patients. Abs against neutrophilic antigens can be at high prevalence (32). A little overlap was found between AIH-1 and AIH-2 patients (28, 29). Patients affected by AIH-2 were characterized by LKM-1Ab. Often associated with AIH-2 was LC1 and less frequently Abs against UDP-glucuronosyltransferases (LKM3Ab). The prevalence and diagnostic value of autoantibodies associated with idiopathic AIH remain to be determined for patients with APAH. In APECED, molecular targets of different hepatic autoantigens have been identified: P4501A2 (33, 34), cytochrome P4502A6 (CYP2A6) (35/36), and AADC (35) (vide supra). Remarkably, AADCs were detected in 92% of Finnish patients with APAH, but they were also frequent in those with no other signs of APAH (35).
Overall autoantibodies against TPHAb and AADC were significantly associated in APECED patients of the present series (Table 1). The last AADC specificity was also significantly associated with autoimmune biopsy-proven hepatopathy. CYP1A2 and AADC are known hepatic autoantigens in APECED (34). CYP1A2Ab was detected in the serum of 4/8 patients with APAH and CYP2A6Ab in the serum of 3/8 patients. Overall, these data confirm the association with APAH discovered in the previous series (27). As regards CYP2A6 and CYP1A2, they are two of six cytochromes P450 that are highly expressed in human liver microsomes (36). CYP1A2 seems to be not expressed in extrahepatic tissues, while CYP2A6 is also expressed in human kidneys (37). CYP2D6Ab was detected in the serum of two patients with APAH (Nos. 3 and 12); in one case, it was associated with CYP1A2Ab. As regards CYP2D6, it is the major autoantigen of LKM-1Ab widely used as a diagnostic marker of AIH-2, which was detectable in 95%–100% of patients (28).
Clinical enteropathy was diagnosed in seven patients; AADC Abs tested positive in 5/7 patients, indicating gastrointestinal dysfunction in APECED. Consistent with published literature (38), the presence of TPHAb was significantly associated with APECED being present in the serum of 5/7 patients. In contrast, VillinAb and HarmoninAb tested negative, as they were reported to be detectable only in a few APECED patients, while these are specific markers of IPEX (39).
Among other cP450 disease-related specificities, adrenal and steroid cell antibodies are detectable in APECED patients and directed against cP450ssc and cP450c17 (17αOH) (40–42). In the present study, 5/12 patients had cP450sscAb and 2/12 had cP450c17Ab. In the literature, there is some disagreement on whether cP450c21 is an autoantigen in APECED. As regards this matter, Uibo et al. (1994) reported that this specificity is present in 15/50 APECED patients (42), while Winqvist (1995) (43, 44) and Clemente in the Sardinian cohort failed to detect it (33). In our series, we confirmed the presence of cP450c21Ab in 9/12 APECED patients.
Almost all patients with APECED usually develop high levels of neutralizing Ab anti-type 1 interferons, which are often detectable before the appearance of clinical symptoms of autoimmunity (40). Indeed, 11/14 patients of the present series (except for patient Nos. 9) showed circulating IFNωAb, and 11/14 patients showed circulating IFNα4Ab; in two patients, IFNAb was not investigated.
On a general ground, a genotype/phenotype correlation has been rarely observed in the APECED series (rev in 10). In the present small series of APECED patients, the most frequent AIRE mutation was c.967_979del13, which was detected in the DNA of 4/9 patients with APAH as previously reported (10). No specific AIRE genotype was prevalent in APECED patients with gastrointestinal manifestations: this does not allow to establish a clear genotype/phenotype correlation.
Although the application of Ferre-Lionakis expanded criteria (11, 12) would require validation in independent large cohorts of patients, the early enrichment of organ-specific non-endocrine manifestations, similar to the American cohort investigated, has allowed an expedited clinical diagnosis in 11/14 patients (Nos. 1–5, 8–12, and 14) of the present series. On a general ground based on Ferre-Lionakis criteria, the presence of APAH or gastrointestinal manifestations in association with one symptom of the classical triad could lead to a clinical diagnosis that should be further confirmed by AIRE gene sequencing and detection of IFNωAb (11). This emphasizes the importance in the clinical practice of the definition of APAH and gastrointestinal dysfunction for an earlier diagnosis of APECED even in European cohorts. An earlier diagnosis would also allow the prevention of episodes of life-threatening hypocalcemic seizures and adrenal crisis, which are the main manifestations of undiagnosed APECED.
The datasets presented in this article are not readily available because of ethical consent for patients. All reported mutations of the AIRE gene are already described in the literature. Requests to access the datasets should be directed to corresponding authors and clinicians Marco Cappa, Maria Felicia Faienza, Giuseppe Maggiore, Mariacarolina Salerno.
The studies involving human participants were reviewed and approved by Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
GP, OP, AC, and EC analyzed data and conducted genetic screening. MF, MC, AP, DC, MV, GM, and MS provided patients with clinical data and contributed to writing the manuscript. VL conducted hepatopathy and enteropathy-related Ab determinations. CB contributed to writing the manuscript and provided critical revision. AF designed the study; analyzed, interpreted, and discussed the clinical and laboratory data; and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Italian Ministry of Health with “current research funds”.
We acknowledge the technical support of Anna Lo Russo and Alessia Palma for their contribution to data analysis, collection of biological samples, and AIRE gene screening.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1172369/full#supplementary-material
AADC, hepatic aromatic l-amino acid decarboxylase; Abs, autoantibodies; ACA, autoantigen adrenal cortex; AD, Addison’s disease; AGA, anti-gliadin; AIH, autoimmune hepatitis; ANA, Abs anti-nuclear; ANCA, anti-neutrophil cytoplasmic; ARA, anti-reticulin; AMA, anti-mitochondrial; AIRE, autoimmune regulator; APAH, APECED-associated hepatitis; APECED, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy; CMC, chronic mucocutaneous candidiasis; cP450, cytochrome P450; ENA, extractable nuclear antigen; EGDS, Esophagogastroduodenoscopy; EMA, endomysial Ab; GADAb, anti-glutamic acid decarboxylase isoform 65; GE, gastro-enteropathy; CH, chronic hypoparathyroidism; HT, Hashimoto thyroiditis; IAA, anti-insulin Ab; IA2Ab, anti-tyrosine phosphatase-related islet antigen 2; ICAs, islet cell antibodies; IFA, anti-intrinsic factor; IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome); IFN, anti-interferon; LC1Ab, anti-liver cytosol type 1; LKMAb, anti-liver–kidney microsomal; PCA, anti-gastric parietal cell; Pt, patient; RAb, anti-ribosomal; SCCAb, cytochrome P450 side chain cleavage enzyme; SLA-IgG, anti-soluble liver antigen IgG; SMA, anti-smooth muscle cell; T1D, type 1 diabetes; TgAb, thyroglobulin; TPHAb, tryptophan hydroxylase; TRAb, TSH-receptor antibody; TSH, anti-thyrotropin; TPOAb, thyroperoxidase; TRGAb, anti-transglutaminase; VillinAb, anti-villin; HarmoninAb, anti-75kDa; ZnT8Ab, anti-zinc transporter 8.
1. Fierabracci A. Recent insights into the role and molecular mechanisms of the autoimmune regulator (AIRE) gene in autoimmunity. Autoimmun Rev (2011) 10:137–43. doi: 10.1016/j.autrev.2010.08.019
2. Kluger N, Jokinen M, Krohn K, Ranki A. Gastrointestinal manifestations in APECED syndrome. J Clin Gastroenterol (2013) 47:112–20. doi: 10.1097/MCG.0b013e31827356e1
3. Posovszky C, Lahr G, von Schnurbein J, Buderus S, Findeisen A, Schröder, et al. Loss of enteroendocrine cells in autoimmune-polyendocrine-candidiasis-ectodermal-dystrophy (APECED) syndrome with gastrointestinal dysfunction. J Clin Endocrinol Metab (2012) 97:E292–300. doi: 10.1210/jc.2011-2044
4. Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab (2006) 91:2843–50. doi: 10.1210/jc.2005-2611
5. Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med (1997) 337:1441–8. doi: 10.1056/NEJM199711133372007
6. Kisand K, Wolff ASB, Podkrajsek KT, Tserel L, Link M, Kisand KV, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med (2010) 207:299–308. doi: 10.1084/jem.20091669
7. Eyerich K, Eyerich S, Hiller J, Behrendt H, Traidl-Hoffmann C. Chronic mucocutaneous candidiasis, from bench to bedside. Eur J Dermatol (2010) 20:260–5. doi: 10.1684/ejd.2010.0910
8. Ekwall O, Hedstrand H, Grimelius L, Haavik J, Perheentupa J, Gustafsson J, et al. Identification of tryptophan hydroxylase as an intestinal autoantigen. Lancet (1998) 352:279–83. doi: 10.1016/S0140-6736(97)11050-9
9. Bereket A, Lowenheim M, Blethen SL, Kane P, Wilson TA. Intestinal lymphangiectasia in a patient with autoimmune polyglandular disease type I and steatorrhea. J Clin Endocrinol Metab (1995) 80:933–5. doi: 10.1210/jcem.80.3.7883852
10. Chascsa DM, Ferré EMN, Hadjiyannis Y, Alao H, Natarajan M, Quinones M, et al. APECED-associated hepatitis: clinical, biochemical, histological and treatment data from a Large, predominantly American cohort. Hepatology (2021) 73:1088–104. doi: 10.1002/hep.31421
11. Ferre EMN, Rose SR, Rosenzweig SD, Burbelo PD, Romito KR, Niemela JE, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight (2016) 1:e88782. doi: 10.1172/jci.insight.88782
12. Husebye ES, Anderson MS, Kämpe O. Autoimmune polyendocrine syndromes. N Engl J Med (2018) 378:1132–41. doi: 10.1056/NEJMra1713301
13. Pietrobattista A, Della Corte C, Francalanci P, Lepri FR, Maggiore G. AIRE mutation triggering acute liver failure: between genetic testing and treatment options. Pediatr Transplant (2021) 25:e14118. doi: 10.1111/petr.14118
14. Garelli S, Dalla Costa M, Sabbadin C, Barollo S, Rubin B, Scarpa R, et al. Autoimmune polyendocrine syndrome type 1: an Italian survey on 158 patients. J Endocrinol Invest (2021) 44:2493–510. doi: 10.1007/s40618-021-01585-6
15. Valenzise M, Fierabracci A, Cappa M, Porcelli P, Barcellona R, De Luca F, et al. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy: report of seven additional Sicilian patients and overview of the overall series from Sicily. Horm Res Paediatr (2014) 82:127–32. doi: 10.1159/000363537
16. Cervato S, Mariniello B, Lazzarotto F, Morlin L, Zanchetta R, Radetti G, et al. Evaluation of the autoimmune regulator (AIRE) gene mutations in a cohort of Italian patients with autoimmune-polyendocrinopathy-candidiasis-ectodermal-dystrophy (APECED) and in their relatives. Clin Endocrinol (Oxf) (2009) 70:421–8. doi: 10.1111/j.1365-2265.2008.03318.x
17. Lintas C, Cappa M, Comparcola D, Nobili V, Fierabracci A. An 8-year-old boy with autoimmune hepatitis and candida onychosis as the first symptoms of autoimmune polyglandular syndrome (APS1): identification of a new homozygous mutation in the autoimmune regulator gene (AIRE). Eur J Pediatr (2008) 167:949–53. doi: 10.1007/s00431-007-0599-4
18. Capalbo D, Mazza C, Giordano R, Improda N, Arvat E, Cervato S, et al. Molecular background and genotype-phenotype correlation in autoimmune-polyendocrinopathy-candidiasis-ectodermal-distrophy patients from campania and in their relatives. J Endocrinol Invest (2012) 35:169–73. doi: 10.3275/7677
19. Improda N, Capalbo D, Cirillo E, Cerbone M, Esposito A, Pignata C, et al. Cutaneous vasculitis in patients with autoimmune polyendocrine syndrome type 1: report of a case and brief review of the literature. BMC Pediatr (2014) 14:272. doi: 10.1186/1471-2431-14-272
20. Capalbo D, Elefante A, Spagnuolo MI, Mazza C, Betterle C, Pignata C, et al. Posterior reversible encephalopathy syndrome in a child during an accelerated phase of a severe APECED phenotype due to an uncommon mutation of AIRE. Clin Endocrinol (Oxf) (2008) 69:511–3. doi: 10.1111/j.1365-2265.2008.03206.x
21. Capalbo D, Fusco A, Aloj G, Improda N, Vitiello L, Dianzani U, et al. High intrafamilial variability in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy: a case study. J Endocrinol Invest (2012) 35:77–81. doi: 10.3275/8055
22. Lampasona V, Passerini L, Barzaghi F, Lombardoni C, Bazzigaluppi E, Brigatti C, et al. Autoantibodies to harmonin and villin are diagnostic markers in children with IPEX syndrome. PloS One (2013) 8:e78664. doi: 10.1371/journal.pone.0078664
23. Chen S, Sawicka J, Betterle C, Powell M, Prentice L, Volpato M, et al. Autoantibodies to steroidogenic enzymes in autoimmune polyglandular syndrome, addison's disease, and premature ovarian failure. J Clin Endocrinol Metab (1996) 81:1871–6. doi: 10.1210/jcem.81.5.8626850
24. Dal Pra C, Chen S, Betterle, Zanchetta R, McGrath V, Furmaniak J, et al. Autoantibodies to human tryptophan hydroxylase and aromatic l-amino acid decarboxylase. Eur J Endocrinol (2004) 150:313–21. doi: 10.1530/eje.0.1500313
25. Tanaka H, Perez SM, Powell M, Sanders JF, Sawicka J, Chen S, et al. Steroid 21-hydroxylase autoantibodies: measurements with a new immunoprecipitation assay. J Clin Endocrinol Metab (1997) 82:1440–6. doi: 10.1210/jcem.82.5.3929
26. Palma A, Gianchecchi E, Palombi M, Luciano R, Di Carlo P, Crinò A, et al. Analysis of the autoimmune regulator gene in patients with autoimmune non-APECED polyendocrinopathies. Genomics (2013) 102:163–8. doi: 10.1016/j.ygeno.2013.04.016
27. Clemente MG, Meloni A, Obermayer-Straub P, Frau F, Manns MP, De Virgiliis S. Two cytochromes P450 are major hepatocellular autoantigens in autoimmune polyglandular syndrome type 1. Gastroenterology (1998) 114:324–8. doi: 10.1016/s0016-5085(98)70484-6
28. Obermayer-Straub P, Strassburg CP, Manns MP. Autoimmune hepatitis. J Hepatol (2000) 32:181–97. doi: 10.1016/s0168-8278(00)80425-0
29. Czaja AJ, Manns MP. The validity and importance of subtypes in autoimmune hepatitis: a point of view. Am J Gastroenterol (1995) 90:1206–11.
30. Manns M, Gerken G, Kyriatsoulis Ahimada T, Yamazaki H, Mimura M, Staritz M, et al. Characterisation of a new subgroup of autoimmune chronic active hepatitis by autoantibodies against a soluble liver antigen. Lancet (1987) 1:292–4. doi: 10.1016/s0140-6736(87)92024-1
31. Stechemesser E, Klein R, Berg PA. Characterization and clinical relevance of liver-pancreas antibodies in autoimmune hepatitis. Hepatology (1993) 18:1–9. doi: 10.1002/hep.1840180102
32. Targan SR, Landers C, Vidrich A, Czaja AJ. High-titer antineutrophil cytoplasmic antibodies in type-1 autoimmune hepatitis. Gastroenterology (1995) 108:1159–66. doi: 10.1016/0016-5085(95)90215-5
33. Clemente MG, Obermayer-Straub P, Meloni A, Strassburg CP, Arangino V, Tukey RH, et al. Cytochrome P450 1A2 is a hepatic autoantigen in autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab (1997) 82:1353–61. doi: 10.1210/jcem.82.5.3913
34. Gebre-Medhin G, Husebye ES, Gustafsson J, Winqvist O, Goksøyr A, Rorsman F, et al. Cytochrome P450IA2 and aromatic l-amino acid decarboxylase are hepatic autoantigens in autoimmune polyendocrine syndrome type I. FEBS Lett (1997) 412:439–45. doi: 10.1016/s0014-5793(97)00797-7
35. Husebye ES, Gebre-Medhin G, Tuomi T, Perheentupa J, Landin-Olsson M, Gustafsson J, et al. Autoantibodies against aromatic l-amino acid decarboxylase in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab (1997) 82:147–50. doi: 10.1210/jcem.82.1.3647
36. Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 caucasians. J Pharmacol Exp Ther (1994) 270:414–23.
37. Kharasch ED, Hankins DC, Thummel KE. Human kidney methoxyflurane and sevoflurane metabolism: intrarenal fluoride production as a possible mechanism of methoxyflurane nephrotoxicity. Anethesiology (1995) 82:689–99. doi: 10.1097/00000542-199503000-00011
38. Chida N, Kobayashi I, Takezaki S, Ueki M, Yamazaki Y, Garelli S, et al. Disease specificity of anti-tryptophan hydroxylase-1 and anti-AIE-75 autoantibodies in APECED and IPEX syndrome. Clin Immunol (2015) 15:36–42. doi: 10.1016/j.clim.2014.10.010
39. Kluger N, Jokinen M, Lintulahti A, Krohn K, Ranki A. Gastrointestinal immunity against tryptophan hydroxylase-1, aromatic l-amino-acid decarboxylase, AIE-75, villin and paneth cells in APECED. Clin Immunol (2015) 158:212–20. doi: 10.1016/j.clim.2015.03.012
40. Krohn K, Uibo R, Aavik E, Peterson P, Savilahti K. Identification by molecular cloning of an autoantigen associated with addison's disease as steroid 17 alpha-hydroxylase. Lancet (1992) 339:770–3. doi: 10.1016/0140-6736(92)91894-e
41. Uibo R, Aavik E, Peterson P, Perheentupa J, Aranko S, Pelkonen R, et al. Autoantibodies to cytochrome P450 enzymes P450scc, P450c17, and P450c21 in autoimmune polyglandular disease types I and II and in isolated addison's disease. J Clin Endocrinol Metab (1994) 78:323–8. doi: 10.1210/jcem.78.2.8106620
42. Winqvist O, Gustafsson J, Rorsman F, Karlsson FA, Kämpe O. Two different cytochrome P450 enzymes are the adrenal antigens in autoimmune polyendocrine syndrome type I and addison's disease. J Clin Invest (1993) 92:2377–85. doi: 10.1172/JCI116843
43. Winqvist O, Gebre-Medhin G, Gustafsson J, Ritzén EM, Lundkvist O, Karlsson FA, et al. Identification of main gonadal autoantigens in patients with adrenal insufficiency and associated ovarian failure. J Clin Endocrinol Metab (1995) 80:1717–23. doi: 10.1210/jcem.80.5.7745025
Keywords: APECED, autoimmune hepatitis, autoimmune gastro-enteropathy, AIRE, diagnostic criteria, autoimmune gastroenteropathy
Citation: Paldino G, Faienza MF, Cappa M, Pietrobattista A, Capalbo D, Valenzise M, Lampasona V, Cudini A, Carbone E, Pagliarosi O, Maggiore G, Salerno M, Betterle C and Fierabracci A (2023) Analysis of a series of Italian APECED patients with autoimmune hepatitis and gastro-enteropathies. Front. Immunol. 14:1172369. doi: 10.3389/fimmu.2023.1172369
Received: 23 February 2023; Accepted: 05 June 2023;
Published: 30 June 2023.
Edited by:
Magda Carneiro-Sampaio, University of São Paulo, BrazilReviewed by:
Antonio Carlos Pastorino, University of São Paulo, BrazilCopyright © 2023 Paldino, Faienza, Cappa, Pietrobattista, Capalbo, Valenzise, Lampasona, Cudini, Carbone, Pagliarosi, Maggiore, Salerno, Betterle and Fierabracci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandra Fierabracci, YWxlc3NhbmRyYS5maWVyYWJyYWNjaUBvcGJnLm5ldA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.