- 1School of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 2Department of Pharmacy, Chung Shan Medical University Hospital, Taichung, Taiwan
- 3Department of Pharmacology, Chung Shan Medical University, Taichung, Taiwan
- 4Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 5Department of Obstetrics and Gynecology, E-Da Hospital, I-Shou University, Kaohsiung, Taiwan
- 6Department of Health Services Administration, China Medical University, Taichung, Taiwan
Background: In previous studies, it was reported that non-alcoholic fatty liver disease (NAFLD) incidence and prevalence increased in children with atopic dermatitis. Nevertheless, the actual association between the two diseases has not been fully proven in large-scale studies, and real-world evidence is missing. The objective of this nationwide, longitudinal cohort study was to evaluate the association between NAFLD and atopic dermatitis.
Methods: The National Health Insurance Research Database in Taiwan was utilized in this study. Patients with records of NAFLD diagnosis were recruited as the experimental group, and patients having less than three outpatient visits or one inpatient visiting record due to NAFLD were excluded from the study design. Non-NAFLD controls were matched based on a 1:4 propensity score matching. Potential confounders including age, gender, comorbidity, and medical utilization status were considered as covariates. The risk of future atopic dermatitis would be evaluated based on multivariate Cox proportional hazard regression.
Results: Compared with people without NAFLD, a decreased risk of atopic dermatitis in NALFD patients had been observed (aHR = 0.93, 95% CI 0.87–0.98). The trend was especially presented in young NAFLD patients. In patients younger than 40 years old, a 20% decreased risk of atopic dermatitis was reported (aHR = 0.80, 95% CI 0.70–0.92).
Conclusion: People with NAFLD were not associated with an increased risk of atopic dermatitis. Conversely, a 0.93-fold risk was noted in NAFLD patients, compared with NAFLD-free controls. Future studies are warranted to evaluate further the mechanism regarding the interplay between the inflammatory mechanisms of NAFLD and atopic dermatitis.
Introduction
With increasing prevalence, non-alcoholic fatty liver disease (NAFLD) causes a gradually increasing health burden worldwide (1). The prevalence of NAFLD varies in different regions. In a recent meta-analysis, the global prevalence was reported to be approximately 25% (2). NAFLD was strongly associated with an increased risk of liver cirrhosis and hepatocellular carcinoma, leading to worsened prognosis and increased mortality rate (2, 3). The influence of NAFLD on health status was not limited to hepatic dysfunction. Multisystem comorbidities, including cardiovascular, renal, and endocrinological dysfunctions, were reported to be presented in NAFLD patients (4).
Patients with atopic dermatitis present pruritus and skin lesions at various sites (5). Environmental factors such as climate, diet, and chemical exposure could contribute to the onset of atopic dermatitis (6, 7). Moreover, factors including antibiotic use and comorbidity status could also serve as potential risk factors of atopic dermatitis (6, 8). In previous studies, it was reported that NAFLD incidence and prevalence increased in children with atopic dermatitis (9, 10). Nevertheless, the actual association has not been fully proven in large-scale studies.
Evaluating the risk of atopic dermatitis in NAFLD patients could be potentially useful serving as references for clinicians caring for NAFLD patients. However, studies evaluating the risk of atopic dermatitis in NAFLD patients were not available, and real-world evidence is missing. Therefore, we conducted a nationwide, longitudinal cohort study to evaluate the association between NAFLD and atopic dermatitis.
Material and methods
Data source
The data source of this retrospective cohort study was based on the datasets from the National Health Insurance Research Database (NHIRD) in Taiwan. Data in NHIRD were recorded based on medical claims and related information retrieved from the mandatory, single-payer National Health Insurance (NHI) program. The NHI covered most of the citizens (>99%) in Taiwan, which could be representative of the population. The NHIRD has been widely utilized as the data source in previous real-world observational studies (11, 12). Deidentified information including inpatient/outpatient visits and diagnosis was recorded in the NHIRD. The International Classification of Disease, 9th and 10th revision, Clinical Modification (ICD-9-CM, ICD-10-CM) codes were applied for disease recording. Since information that could identify any specific individual has been deidentified, informed consent for the current study was exempted.
Study population, exposure, and outcomes
Data from 2000 to 2018 in NHIRD have been extracted for analysis. Patients with NAFLD were recruited as the NAFLD group. In the NHIRD, diagnosis was made after evaluations from specialists. However, several additional criteria were set to ensure validity and quality for disease definition: 1) Only patients who were diagnosed with NAFLD between 2004 and 2013 with the index date matching the control group were included in the cohort. In this case, we could ensure that each analyzed patient could have at least a 5-year term for follow-up. 2) To ensure the validity of participant recruitment, patients having less than three outpatient visits or one inpatient visiting record due to NAFLD were excluded from the study design (13). 3) Patients younger than 18 years old were excluded from the study. 4) People who withdrew from the NHIRD or were deceased before the endpoint were censored and were not included for further analysis. 5) To ensure that the outcome measurement could represent new-onset atopic dermatitis, patients with a previous record of atopic dermatitis were excluded from the study. 6) To avoid the presence of outcomes being influenced by liver diseases other than NAFLD, patients with a previous record of chronic liver diseases (including liver fibrosis, cirrhosis, and hepatic cancer) before the index date were excluded from the study. Healthy control groups without NAFLD were randomly selected. Propensity score matching (PSM) in age, sex, income level, urbanization, comorbidity burden, and enrolled year was performed to address the differences in baseline characteristics. After PSM, each patient in the NAFLD group was randomly matched with four NAFLD-free healthy people in the control group. To address possible confounding bias, multivariate Cox regression analysis was performed to adjust variables including related comorbidities such as hypertension, diabetes, hyperlipidemia, myocardial infarction, coronary artery disease, chronic kidney disease, obesity, alcoholism, major depressive disorder, inflammatory bowel disease, asthma, psoriasis, allergic rhinitis, conjunctivitis, and urticaria. The study endpoint was when the study participants were diagnosed with atopic dermatitis. To improve the validity of atopic dermatitis diagnosis, only events consisting of more than three outpatient visits or one inpatient record due to atopic dermatitis were defined as outcome events (14). Each person in the study was followed up from the index date to the date of diagnosis of atopic dermatitis (outcome event), quitting registration from the NHI or on 31 December 2018 (the endpoint of the current study).
Statistical analysis
p-values smaller than 0.05 are considered to be significant differences between the data for comparison. Standardized mean difference (SMD) has been utilized to evaluate the difference between the NAFLD group and the healthy control group regarding baseline characteristics. SMD values smaller than 0.1 represent a negligible difference. To evaluate the risk of incident outcome events, the hazard ratio (HR) was calculated. Each HR was presented with a 95% confidence interval (CI). All statistical analyses were performed by SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
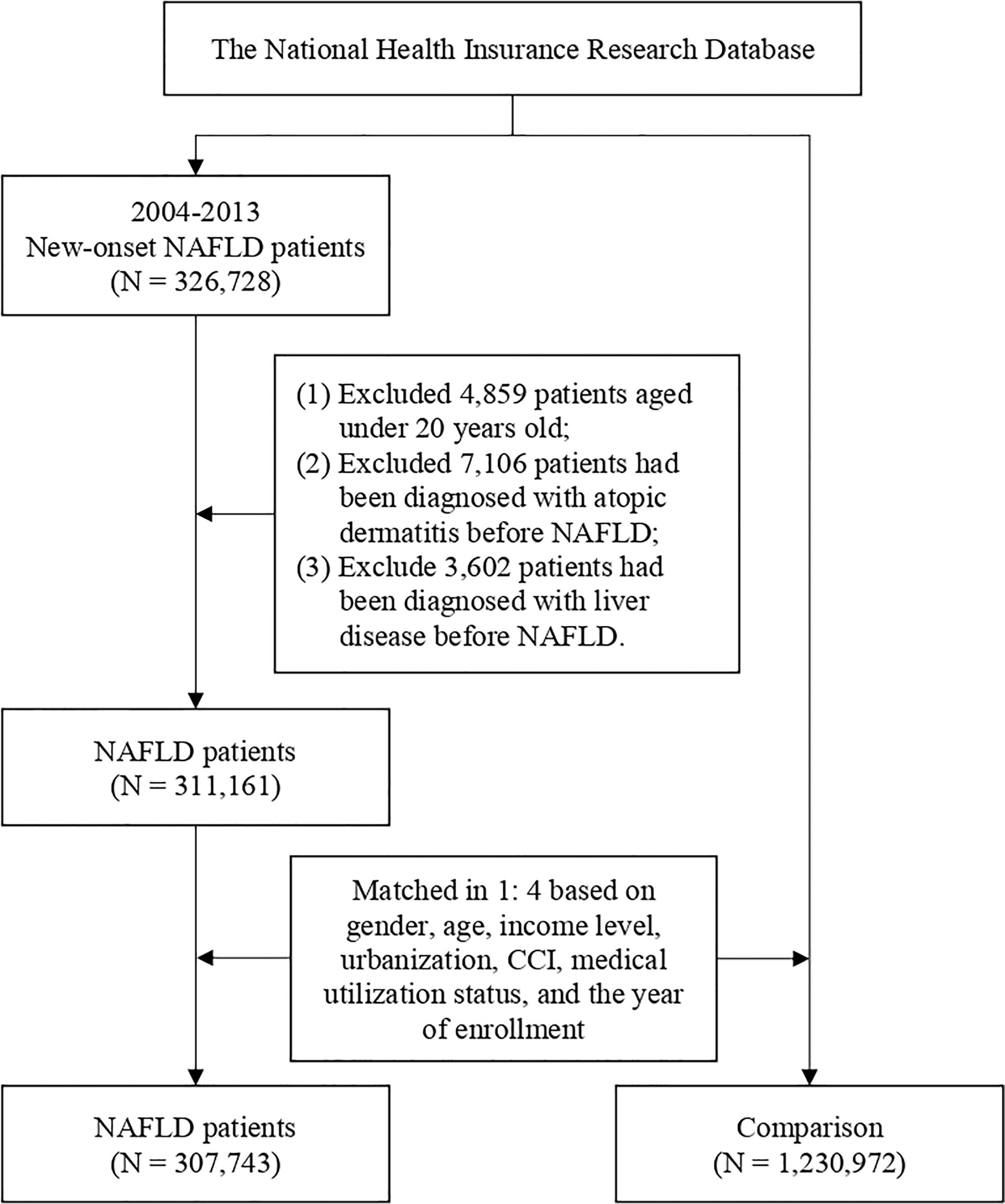
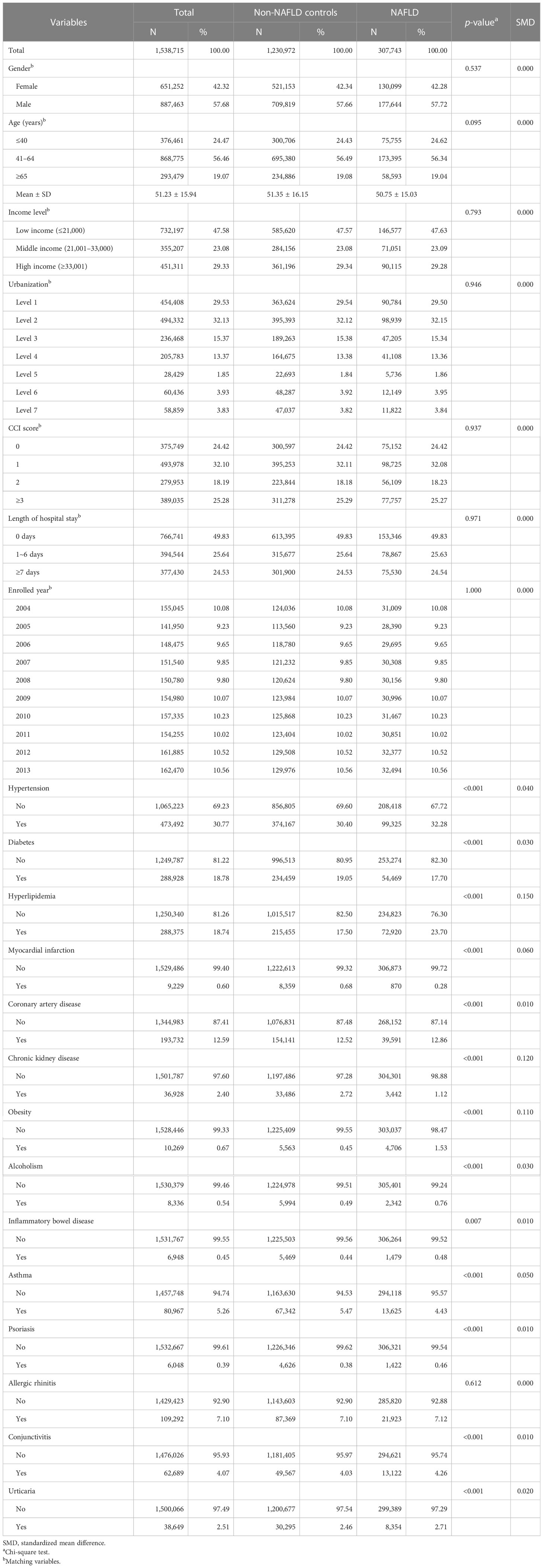
In total, 307,743 patients with NAFLD and 1,230,972 healthy controls were enrolled in the current study (Figure 1). In the NAFLD group, 42% of the patients were men and 58% of the patients were women. The mean age of NAFLD patients was 51.2 years old. Comorbidity burden has been evaluated via the Charlson comorbidity index (CCI), a validated assessment tool used to evaluate the burden of comorbidities and to predict patients’ prognostic factors, which has been utilized in previous real-world studies (13, 15). After propensity score matching, differences between related variates become statistically insignificant (Table 1).
Risk of NAFLD patients developing a topic dermatitis
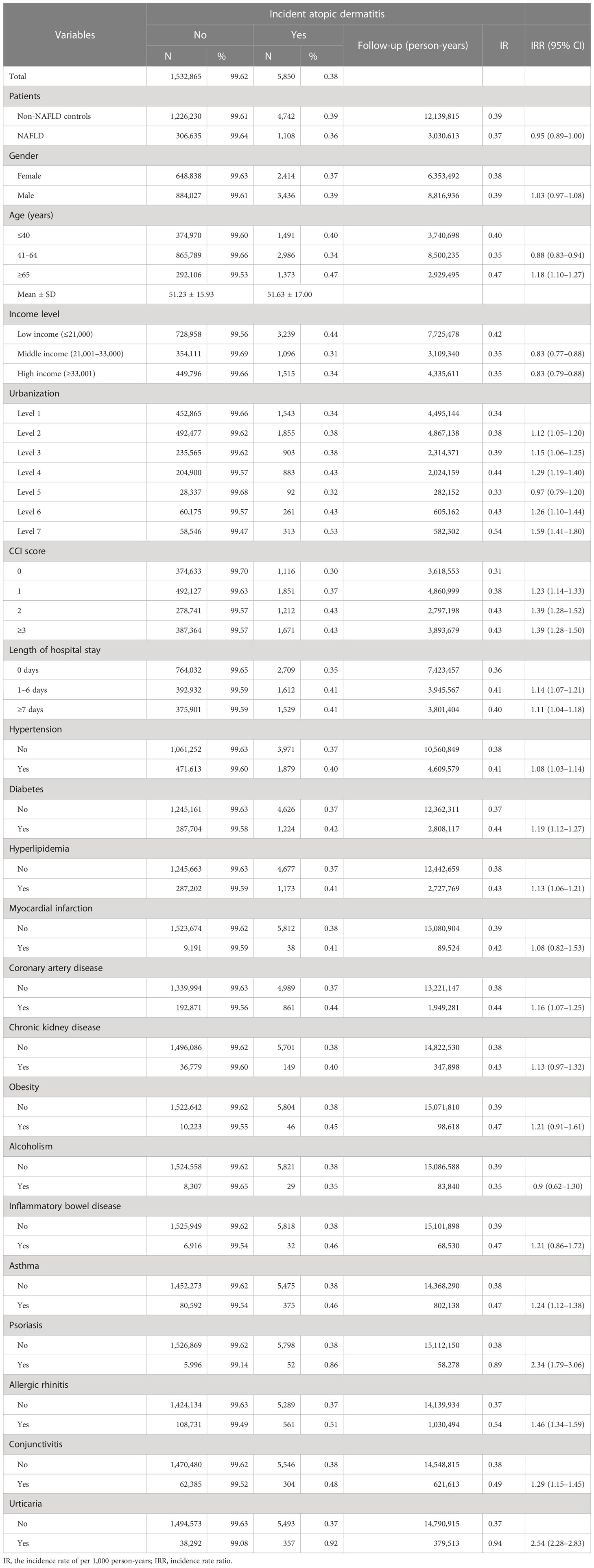
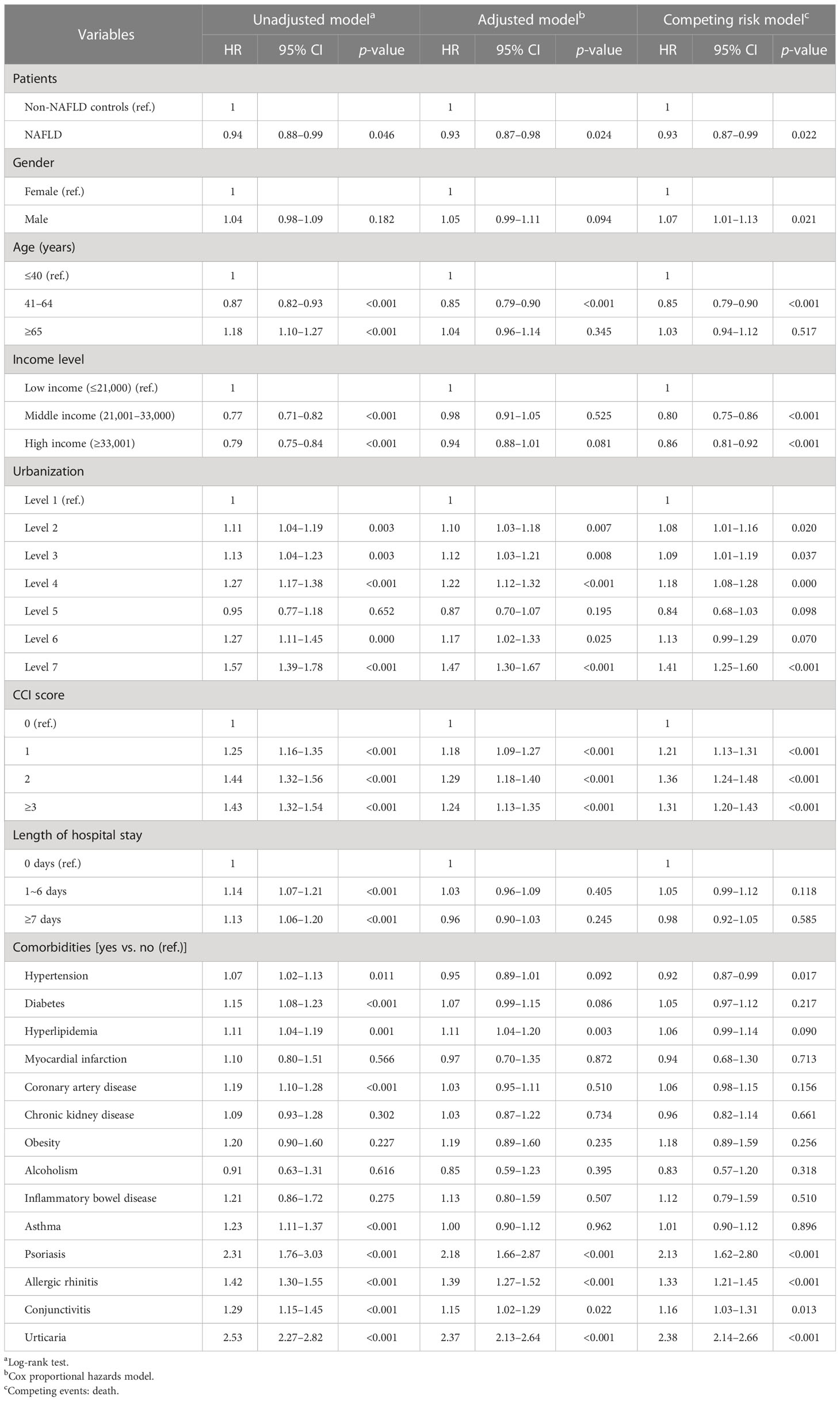
For patients with NAFLD, the incidence rate of new-onset atopic dermatitis was 0.37 per 1,000 person-years, whereas for healthy controls without NAFLD, the incidence rate of atopic dermatitis was 0.39 per 1,000 person-years (Table 2). In the Cox regression model, compared with healthy controls without NAFLD, people with NALFD had a statistically significant lower risk of developing atopic dermatitis (HR = 0.94, 95% CI 0.88–0.99). The significance remained after adjusting related covariates (aHR = 0.93, 95% CI 0.87–0.98). For patients with inflammatory or allergic comorbidities, including psoriasis, allergic rhinitis, and urticaria, the risk of atopic dermatitis was significantly higher than in controls (Table 3).
Stratification analysis
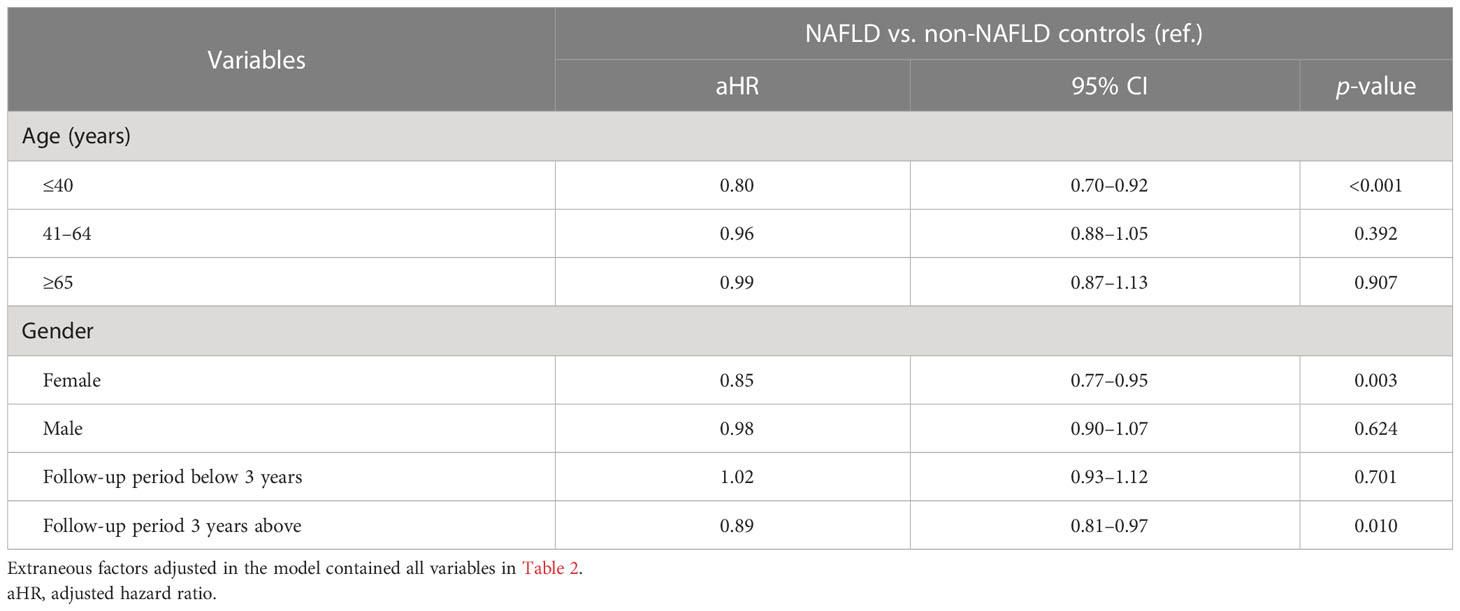
In the age-stratified subgroup analyses, a decreasing risk was enhanced in patients younger than 40 years old, with a 20% decreased risk compared with non-NAFLD controls (aHR = 0.80, 95% CI 0.70–0.92) (Table 4). As for patients older than 40 years old, the significance of the NAFLD–atopic dermatitis association was not observed. For female NAFLD patients, the risk of atopic dermatitis was lower than in controls (aHR = 0.85, 95% CI 0.77–0.95). Moreover, a decreasing risk of atopic dermatitis was only observed in patients who were followed up for more than 3 years (aHR = 0.89, 95% CI 0.81–0.97). For those having less than 3 years of follow-up, the trend was statistically insignificant.
Discussion
In the current retrospective cohort study, we utilized a nationwide database to evaluate the risk of atopic dermatitis in people with NAFLD. Compared with people without NAFLD, a decreased risk of atopic dermatitis in NALFD patients had been observed (aHR = 0.93, 95% CI 0.87–0.98). The trend was especially presented in young NAFLD patients. In patients younger than 40 years old, a 20% decreased risk of atopic dermatitis was reported (aHR = 0.80, 95% CI 0.70–0.92).
NAFLD could influence multiple organ systems. Due to the influence of metabolic factors including obesity or diabetes, steatosis of hepatocytes was noted in NAFLD patients (16). In the pathogenesis of NAFLD, secretion of proinflammatory cytokines would lead to chronic inflammation, causing the potential association between NAFLD and various comorbidities. The common comorbidities of NAFLD include diabetes, cardiovascular diseases, and renal diseases (17). As for dermatological involvements, previous studies identified the association between NAFLD and psoriasis (13, 18). Previous articles suggested that elevated secretion of non-esterified fatty acids would be presented in liver tissue due to the risk factors of NAFLD, such as insulin resistance (19). Under this circumstance, oxidative stress could be possibly increased, leading to a subsequent inflammatory response (19, 20). A population-based study from South Korea hypothesized that through the excess accumulation of free fatty acids, chronic inflammation status could potentially lead to airway impairment and remodeling, attributed to the higher incidence of asthma in NAFLD patients (21).
The association between atopic dermatitis and NAFLD has been discussed in various previous studies. Fatty liver diseases have been regarded as one of the possible comorbidities of atopic dermatitis based on the available evidence to date (22). However, most studies were limited to a small sample size, a cross-sectional design, or animal models. In an animal study based on a mouse model, it was reported that in atopic dermatitis lesions, lipid metabolism would be influenced and the accumulation of cholesterol in hepatic tissues was noted (23). Another single-center study in Japan reported that the prevalence of fatty liver diseases was observed to be increased in pediatric populations (9). To the best of our knowledge, though an increased prevalence of atopic dermatitis was noted in previous literature, the actual mechanism regarding the interplay between NAFLD and atopic dermatitis is still unconfirmed. Moreover, previous studies did not focus on the longitudinal influence of NAFLD and the subsequent atopic dermatitis incidence. The current study provided real-world evidence regarding the knowledge gap of this issue and suggested that NAFLD patients were associated with a lower risk of atopic dermatitis. The results of our current study revealed that though the prevalence of NAFLD might be high in people with atopic dermatitis, the reversed association was not observed in longitudinal settings. To the best of our knowledge, the current mechanism hypotheses in the pathogenesis of NAFLD might not be sufficient to properly attribute the lower incidence of atopic dermatitis in the NAFLD group. However, we hypothesize that after NAFLD diagnosis, changes in the patients’ lifestyle could occur and therefore influence the incidence of atopic dermatitis. Given that we were not able to access lifestyle information and the exposure to allergens in patients, we believe that further large-scale studies might be necessary to evaluate the molecular mechanism in NAFLD patients and evaluate the potential role of lifestyle in the observed association. In the Cox regression analysis, it was presented that patients with coronary artery disease were associated with atopic dermatitis in the unadjusted model. However, after adjustment of covariates potentially influencing the incidence of atopic dermatitis, including age, sex, urbanization status, socioeconomic status, medical utilization status, and other comorbidities, the association became insignificant. The difference indicates that confounding biases could influence the interpretation of the results and should be prudently considered. A recent Mendelian randomization study also reported a non-significant causal association between atopic dermatitis and coronary heart diseases, which also corresponded to the observed trend in the adjusted model in the current study (24).
The limitations of this study should be stated. First, given that this study was based on a real-world and retrospective study setting, residual confounders could still exist even though we have tried our best to perform adjustments on potential confounders. The association observed in retrospective cohort studies should be carefully interpreted and should not be regarded as causation. Second, in the current study, the definition of diseases and other covariates was based on the ICD-9/10 codes, which could lead to potential misclassification bias. Previous studies had intended to validate the positive predictive values of ICD-9/10 of NAFLD and atopic dermatitis in claim-based datasets (25, 26). However, in our current study, misclassification bias could still exist due to the limitation of administrative codes. To the best of our knowledge, validation studies evaluating the positive predictive value of ICD codes of atopic dermatitis in Taiwanese population were not available. Although we have adopted in the current study the ICD coding algorithms utilized in a previous NHIRD study to define atopic dermatitis, some of the atopic dermatitis cases could still be potentially misclassified. Third, since environmental, lifestyle, and genetic information was not available in the NHIRD, we were not able to set these factors as covariates to evaluate the outcomes. Fourth, since the information on the widely used NAFLD severity indicators such as NAFLD fibrosis score or steatosis, activity, and fibrosis (SAF) score was not available in the NHIRD, we were not able to precisely define the severity of NAFLD (27, 28). Therefore, stratification analysis based on NAFLD severity was not available in the current study. Fifth, datasets from the NHIRD of Taiwan might not be generalizable to other populations with cultural, genetic, or environmental differences. The aforementioned limitations should be noted by the authors, and the results of the current study should be prudently interpreted.
As a conclusion, people with NAFLD were not associated with an increased risk of atopic dermatitis. Conversely, a 0.93-fold risk was noted in NAFLD patients, compared with NAFLD-free controls. Future studies are warranted to evaluate further the mechanism regarding the interplay between the inflammatory mechanisms of NAFLD and atopic dermatitis.
Funding
This research was funded by the Chung Shan Medical University Hospital, Taiwan (CSH-2021-A-007), and China Medical University, Taiwan (CMU111-MF-111).
Acknowledgments
We are grateful to the Health Data Science Center, China Medical University Hospital, for providing administrative, technical, and funding support, which has contributed to the completion of this study. This study is based, in part, on data released by the Health and Welfare Data Science Center, Ministry of Health and Welfare.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The interpretation and conclusions contained herein do not represent those of the Ministry of Health and Welfare.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1171804/full#supplementary-material
Abbreviations
NAFLD, non-alcoholic fatty liver disease; aHR, adjusted hazard ratio; NHIRD, National Health Insurance Research Database; ICD-9-CM, International Classification of Disease, Ninth Revision, Clinical Modification; 95% CI, 95% confidence interval.
References
1. Neuschwander-Tetri BA. Non-alcoholic fatty liver disease. BMC Med (2017) 15(1):45. doi: 10.1186/s12916-017-0806-8
2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (2016) 64(1):73–84. doi: 10.1002/hep.28431
3. Johnston MP, Patel J, Byrne CD. Causes of mortality in non-alcoholic fatty liver disease (NAFLD) and alcohol related fatty liver disease (AFLD). Curr Pharm Des (2020) 26(10):1079–92. doi: 10.2174/1381612826666200128094231
4. Glass LM, Hunt CM, Fuchs M, Su GL. Comorbidities and nonalcoholic fatty liver disease: the chicken, the egg, or both? Fed Pract (2019) 36(2):64–71.
6. Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab (2015) 66(Suppl 1):8–16. doi: 10.1159/000370220
7. Yong SB, Gau SY, Guo YC, Wei JC. Allergy from perspective of environmental pollution effects: from an aspect of atopic dermatitis, immune system, and atmospheric hazards-a narrative review of current evidences. Environ Sci pollut Res Int (2022) 29(38):57091–57101. doi: 10.1007/s11356-022-21582-3
8. Dai YX, Tai YH, Chang YT, Chen TJ, Chen MH. Bidirectional association between psoriasis and atopic dermatitis: A nationwide population-based cohort study. Dermatology (2021) 237(4):521–7. doi: 10.1159/000514581
9. Kimata H. Prevalence of fatty liver in non-obese Japanese children with atopic dermatitis. Indian Pediatr (2005) 42(6):587–93.
10. Kimata H. Increased incidence of fatty liver in non-obese Japanese children under 1 year of age with or without atopic dermatitis. Public Health (2006) 120(2):176–8. doi: 10.1016/j.puhe.2005.02.006
11. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan's National Health Insurance Research Database: past and future. Clin Epidemiol (2019) 11:349–58. doi: 10.2147/CLEP.S196293
12. Lin LY, Warren-Gash C, Smeeth L, Chen PC. Data resource profile: the National Health Insurance Research Database (NHIRD). Epidemiol Health (2018) 40:e2018062. doi: 10.4178/epih.e2018062
13. Gau SY, Huang KH, Lee CH, Kuan YH, Tsai TH, Lee CY. Bidirectional association between psoriasis and nonalcoholic fatty liver disease: real-world evidence from two longitudinal cohort studies. Front Immunol (2022) 13:840106. doi: 10.3389/fimmu.2022.840106
14. Lin TL, Wu CY, Yen JJ, Juan CK, Chang YL, Ho HJ, et al. Fracture risks in patients with atopic dermatitis: A nationwide matched cohort study. Ann Allergy Asthma Immunol (2021) 127(6):667–673.e662. doi: 10.1016/j.anai.2021.09.004
15. Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson comorbidity index: A critical review of clinimetric properties. Psychother Psychosom (2022) 91(1):8–35. doi: 10.1159/000521288
16. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet (2021) 397(10290):2212–24. doi: 10.1016/S0140-6736(20)32511-3
17. Rosato V, Masarone M, Dallio M, Federico A, Aglitti A, Persico M. NAFLD and extra-hepatic comorbidities: current evidence on a multi-organ metabolic syndrome. Int J Environ Res Public Health (2019) 16(18). doi: 10.3390/ijerph16183415
18. Ruan Z, Lu T, Chen Y, Yuan M, Yu H, Liu R, et al. Association between psoriasis and nonalcoholic fatty liver disease among outpatient US adults. JAMA Dermatol (2022) 158(7):745–53. doi: 10.1001/jamadermatol.2022.1609
19. Mantovani A, Gisondi P, Lonardo A, Targher G. Relationship between non-alcoholic fatty liver disease and psoriasis: A novel hepato-dermal axis? Int J Mol Sci (2016) 17(2):217. doi: 10.3390/ijms17020217
20. Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol (2015) 16(5):448–57. doi: 10.1038/ni.3153
21. Roh JH, Lee H, Yun-Jeong B, Park CS, Kim HJ, Yoon SY. A nationwide survey of the association between nonalcoholic fatty liver disease and the incidence of asthma in Korean adults. PloS One (2022) 17(1):e0262715. doi: 10.1371/journal.pone.0262715
22. Silverberg JI, Silverberg NB. Atopic dermatitis: update on pathogenesis and comorbidities. Curr Dermatol Rep (2012) 1(4):168–78. doi: 10.1007/s13671-012-0021-y
23. Seino S, Tanaka Y, Honma T, Yanaka M, Sato K, Shinohara N, et al. et al: Atopic dermatitis causes lipid accumulation in the liver of NC/Nga mouse. J Clin Biochem Nutr (2012) 50(2):152–7. doi: 10.3164/jcbn.11-29
24. Huang J, Gui Y, Wu J, Xie Y. Investigating the association of atopic dermatitis with ischemic stroke and coronary heart disease: A mendelian randomization study. Front Genet (2022) 13:956850. doi: 10.3389/fgene.2022.956850
25. Corey KE, Kartoun U, Zheng H, Shaw SY. Development and validation of an algorithm to identify nonalcoholic fatty liver disease in the electronic medical record. Dig Dis Sci (2016) 61(3):913–9. doi: 10.1007/s10620-015-3952-x
26. Hsu DY, Dalal P, Sable KA, Voruganti N, Nardone B, West DP, et al. Validation of International Classification of Disease Ninth Revision codes for atopic dermatitis. Allergy (2017) 72(7):1091–5. doi: 10.1111/all.13113
27. Nascimbeni F, Bedossa P, Fedchuk L, Pais R, Charlotte F, Lebray P, et al. Clinical validation of the FLIP algorithm and the SAF score in patients with non-alcoholic fatty liver disease. J Hepatol (2020) 72(5):828–38. doi: 10.1016/j.jhep.2019.12.008
Keywords: non-alcoholic fatty liver disease, atopic dermatitis, NHIRD, cohort study, epidemiology
Citation: Gau S-Y, Huang C-H, Yang Y, Tsai T-H, Huang K-H and Lee C-Y (2023) The association between non-alcoholic fatty liver disease and atopic dermatitis: a population-based cohort study. Front. Immunol. 14:1171804. doi: 10.3389/fimmu.2023.1171804
Received: 22 February 2023; Accepted: 24 July 2023;
Published: 18 August 2023.
Edited by:
Javier Crespo, Marqués de Valdecilla Health Research Institute (IDIVAL), SpainReviewed by:
Paula Iruzubieta, Marqués de Valdecilla Health Research Institute (IDIVAL), SpainMaría Teresa Arias Loste, Marqués de Valdecilla Health Research Institute (IDIVAL), Spain
Copyright © 2023 Gau, Huang, Yang, Tsai, Huang and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chien-Ying Lee, Y3NoZDAxNUBjc211LmVkdS50dw==; Kuang-Hua Huang, a2hodWFuZ0BtYWlsLmNtdS5lZHUudHc=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Shuo-Yan Gau
Shuo-Yan Gau Ching-Hua Huang2,3,4†
Ching-Hua Huang2,3,4† Chien-Ying Lee
Chien-Ying Lee