
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Immunol., 31 May 2023
Sec. Inflammation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1167301
This article is part of the Research TopicEmerging Talents in Inflammation: 2022View all 6 articles
A leading thinker on inflammation wrote that “inflammation is associated with almost every major human disease” (1). Inflammation is a defense process of the body against stimuli and a biological response of the immune system to harmful stimuli (2). Inflammation is usually beneficial, but once the inflammatory reaction is out of balance, it will be harmful to the body (3). Inflammation has a great impact on human health, which has been paid more and more attention by researchers. As far as 2021 is concerned, 13905 articles identified “inflammation” as the keyword, and 1284 articles included the word “inflammation” in the title (4). This not only reflects the importance of studying inflammation, but also emphasizes the urgency of inflammation research.
Dysregulated inflammatory reaction can lead to infectious, autoimmune, neurological, cardiovascular, renal and tumor diseases (5–8). Although researchers have put a lot of efforts into biological understanding and drug development, and some interventions have been successful in clinical trial (4), the prevention and monitoring of some inflammation is still a problem. Early monitoring of the prevention and recovery process can effectively reduce the impact of inflammation on people’s health. Although traditional drugs can effectively prevent inflammation, it is always difficult for people to take drugs frequently. Emerging triboelectric nanogenerators (TENG) provides a new prevention and monitoring scheme to address this challenge (9–11). The latest research progress in soft electronics has proposed flexible and stretchable functional sensors (12–14). TENG combines flexible materials and wearable characteristics, and has been successfully applied in the health and medical fields as implantable medical sensors (15–17), biological sensors (18–21), monitoring sensors (22–24), etc. TENG is lightweight, highly flexible and elasticity (25, 26), and can directly contact the skin or organ surface for inflammation prevention and monitoring.
In this paper, the application of the emerging TENG in the prevention and monitoring of inflammation such as cervical spondylitis, lumbar spondylosis, rheumatoid arthritis has been fully discussed. Flexible medical sensors, biosensors and monitoring sensors made of materials with different characteristics have been successfully applied to the prevention and monitoring of some inflammation and diseases. Additionally, this paper also discusses the advantages of TENG combined with artificial intelligence (AI) in disease prevention and monitoring, and the development of TENG in the medical field promoted by AI. At the end of the article, the challenges of the development of TENG and AI in the field of inflammation are analyzed, and its development prospects are prospected.
Dysregulation of inflammation can seriously affect health. There are many reasons for its formation, including biological factors, physical factors, chemical factors (27), etc. TENG is a powerful technology to convert mechanical energy into electrical energy, which plays a vital role in physiological signal monitoring (28–30), disease prevention and treatment (31), microbial control (32), etc. For example, Wang et al. reported a TENG-based portable spirometer that effectively monitors lung activity (33). The prevention and monitoring of some inflammations (such as cervical spondylitis, lumbar spondylitis, asthma, etc.) through TENG-based equipment contributes to innovation and development in the field of inflammation. Cheng et al. proposed the application of innovative machine learning in inflammation (34), which is of great significance in the application of TENG and AI in inflammation.
With the improvement of people’s living standards, more and more attention has been paid to the health problems caused by inflammation, and various inflammation prevention, monitoring and treatment technologies have been developed. The development of TENG broadens the boundary for the study of inflammation. Li et al. developed a reel shaped tensile sensing device to reduce the risk of spinal disease (35). The device is a high-precision stretchable sensor that combines retractable badge reel and grating-structured (and the Kapton surface is etched with ions to improve the output performance) (Figure 1A). The device is installed at the cervical, lumbar or other joints of the body, and the sensor generates different electrical signals through different stretching or contracting conditions. We can obtain real-time detection signals through the monitoring terminal to judge the state of the spine, which will help reduce the risk of cervical spondylosis, lumbar spondylosis and other spinal diseases caused by abnormal posture.
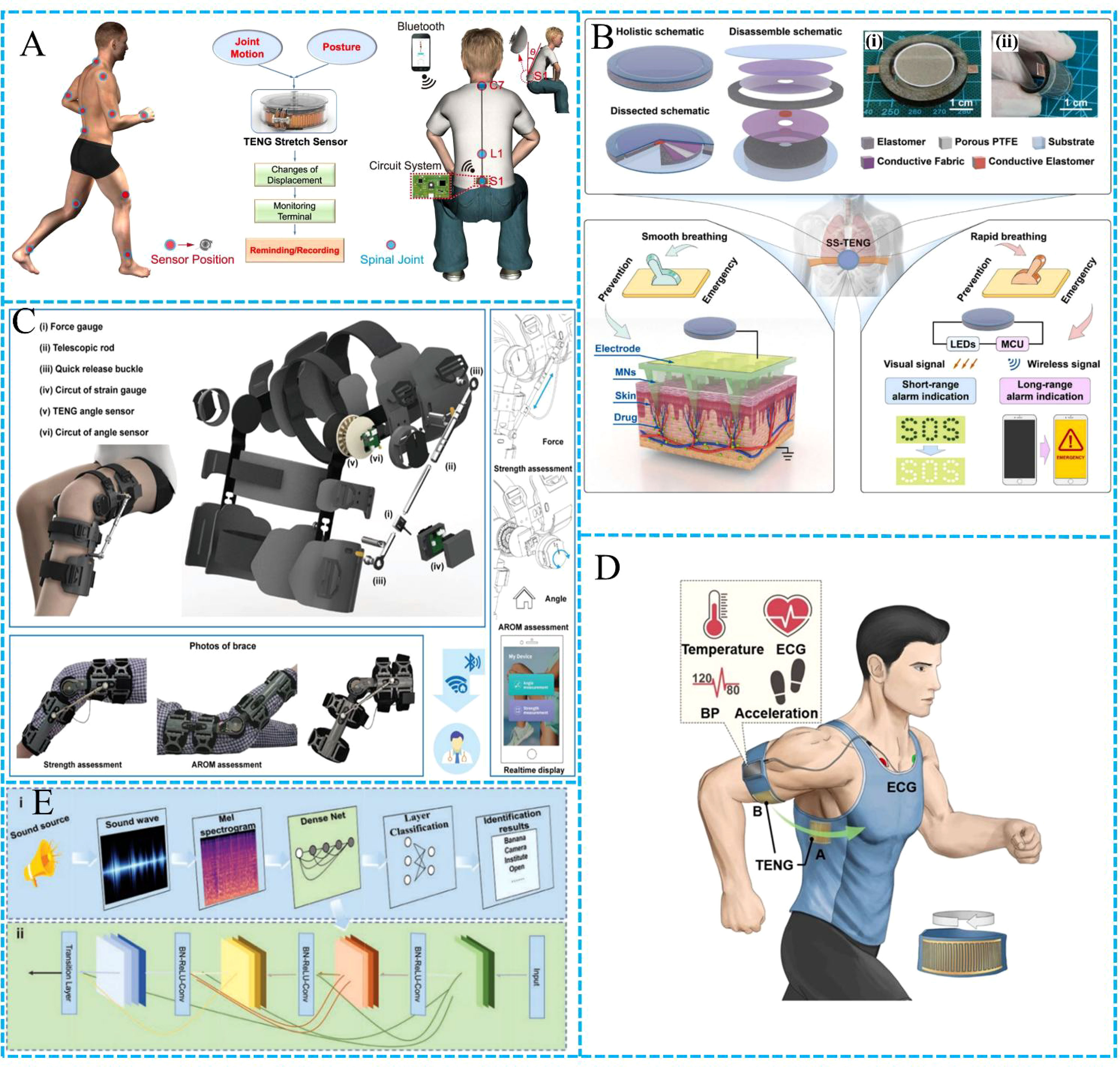
Figure 1 Application of TENG. (A) Illustration of the thin, lightweight, and wearable stretch sensor placed around the full body to monitor the joint and spinal motions (35). (B) Basic architecture and operation for respiration-mediated self-switched TENG (36). (C) Schematic illustration of the rehabilitation brace system (37). (D) Schematic diagram of self-powered wireless physiological monitoring system (38). (E) The application diagram of ETAS in voice-text conversion (39).
Yu et al. have developed a self-switched TENG mediated by respiratory motions to provide prevention and emergency functions for asthma (36). The system ingeniously designs an asthma service system with different characteristics of materials such as polyethylene terephthalate (PET) and conductive fabric, which has both prevention and emergency functions and can drive drugs into the body (Figure 1B). In the emergency state, the alarm module is driven and send emergency information to the monitoring terminal, so as to effectively prevent and monitor the potential safety hazards caused by asthma.
It is worth noting that these prevention technologies are self-powered, which greatly simplifies the external equipment of traditional prevention technologies and makes prevention more efficient. Therefore, the prevention technology based on TENG has promising application prospects in the field of inflammation.
Inflammation monitoring is critical. Osteoarthritis is a common inflammation in the elderly, particularly knee osteoarthritis, which not only brings pain and impaired mobility to patients (40, 41), but also may cause social psychological anxiety and depression (42, 43). Luo et al. developed a portable wearable total knee arthroplasty (TKA) patient rehabilitation monitoring and evaluation system based on TENG (37). The system is mainly composed of two sensing modules: force sensor for measuring isometric muscle strength and active angle sensor for detecting joint range of motion (Figure 1C). The system visualizes the relationship between torque and force, as well as the range of motion of the patient’s joints, and then transmits the measurement information to the mobile phone for analysis, giving rehabilitation indicators (isometric muscle test score (IMTS)), and quantitatively evaluating TKA rehabilitation.
Yan et al. developed a set of self-powered wireless physiological monitoring system (Figure 1D). The TENG and flexible thin-film solar cell are integrated, without external power supply and with high output power (38). The system can effectively monitor inflammation, collect blood pressure, body temperature, movement and other parameters, and transmit the data to the terminal, realizing real-time continuous monitoring of the physical state of the subjects.
The asthma prevention and emergency system based on TENG developed by Yu et al. can not only reduce the incidence rate of asthma patients, but also monitor the status of asthma patients, push drugs for them, and send dangerous signals to the control terminal (36). Lin et al. synthesized 3D dendritic TiO2 nanostructures to prepare ultraviolet radiation (UVR) photodetectors, and this structure can be used for TENG (44) to monitor the intensity of ultraviolet radiation and protect health.
As TENG develops in the field of inflammation, its contribution to inflammation monitoring will increase. The development of TENG can improve our quality of life and even open up new space for telemedicine.
Inflammation is linked to several diseases, some of which can damage the eardrum and affect hearing. Jiang et al. reported an ultra-thin eardrum-like triboelectric acoustic sensor (ETAS) that is a boon for people with hearing loss (39). The equipment is assembled by electrospinning technology after silver plating on the nanofiber film, and it is thin and highly sensitive. ETAS is combined with AI algorithm to achieve real-time voice conversion, with a high recognition rate (92.64%), as shown in Figure 1E. The system combines the TENG and AI algorithm, so that hearing impaired people have a clear opportunity to listen to the world, and also provides new ideas for inflammation research. Liu et al. also reported a self-powered artificial auditory pathway based on TENG (45), which is composed of a TENG and a field effect synaptic transistor. TENG converts the collected voice signal into electrical signal, and the transistor performs signal conversion and neural morphology operation to simulate the biological acoustic function. The system uses k-nearest neighbors (KNN) algorithm to effectively implement sound detection.
As we all know, the current social development is closely related to AI technology, and AI technology also plays an important role in medical treatment. The deep learning model provides great help for disease prediction, diagnosis and even treatment (46, 47). TENG-based sensors can collect a large amount of data and provide the data basis for AI algorithm. The combination of the two has played a crucial role in medical treatment (48). Therefore, AI-TENG has great research value and application prospect in the field of inflammation.
Although TENG and AI can be helpful for inflammation prevention and monitoring, there are still some limitations in the application of AI technology in TENG. For example, Many AI technologies still lack universal mechanisms, fail to reveal the underlying relationships between structures and materials, and require further exploration between features and predictions in AI models (49–51). Further, for actual prevention and monitoring, there is an urgent need to address specific AI models corresponding to different types of inflammation, and the method to collect and analyze real-time data.
TENG has many advantages, including low cost, high efficiency, softness, wearable, self-powered, and combined with AI algorithm, it has great application prospects in the prevention and monitoring of inflammation, even in the treatment. However, the development of new things always faces many challenges. Here, we divide the challenge into two parts, one is the challenge of AI in TENG: Firstly, most AI technologies cannot reveal the potential relationship between materials, structures and outputs. Secondly, there is a lack of generalized mechanism or understanding of the triboelectric effect. Finally, the over fitting related to AI algorithm, which heavily depends on the quality of input data, is also a challenge. The second is the challenge of AI-TENG in the field of inflammation. First of all, how to analyze the collected data is a problem. Secondly, how to design appropriate algorithms for inflammation research is also a great challenge. Finally, how to evaluate the inflammatory state according to real-time data and AI algorithm. Today, with the rapid development of 5G networks and cloud computing, we have enough opportunities to meet these challenges.
The application of TENG and AI technology to the field of inflammation provides a new direction for inflammation research and new hope for some difficult to solve inflammation. On the one hand, TENG has strong sensing and data collection capabilities, and its structure is variable, so it can design different TENG for different inflammation. On the other hand, AI technology involves many algorithms, such as artificial neural network (ANN), decision tree, linear classifier, etc. We can choose different algorithms for different inflammation to achieve accurate prevention, monitoring and treatment. Additionally, the combination of AI and TENG can not only design and optimize the TENG according to the actual demand, but also optimize the AI algorithm subject to the actual situation. The combination of TENG and AI technology has great application prospects in the field of inflammation. They will make the medical service system more real-time, accurate and diverse.
EZ and ZZ: conceptualization,writing and revise. ZZ and CH: supervision and funding acquisition. All authors contributed to the article and approved the submitted version.
This paper is supported by Guangxi Key Laboratory of Automatic Detecting Technology and Instruments (No.YQ23211).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Medzhitov R. The spectrum of inflammatory responses. Science (2021) 374(6571):1070–5. doi: 10.1126/science.abi5200
2. Kiss AL. Inflammation in focus: the beginning and the end. Pathol Oncol Res (2022) 27:169. doi: 10.3389/pore.2021.1610136
3. Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol (2023) 159–173. doi: 10.1038/s41577-022-00760-x
4. Nathan C. Nonresolving inflammation redux. Immunity (2022) 55(4):592–605. doi: 10.1016/j.immuni.2022.03.016
5. Roda G, Ng SC, Kotze PG, Argollo M, Panaccione R, Spinelli A, et al. Crohn’s disease. Nat Rev Dis Primers (2020) 6(1):22. doi: 10.1038/s41572-020-0183-z
6. Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol (2022) 22(11):657–73. doi: 10.1038/s41577-022-00684-6
7. Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol (2021) 18(9):666–82. doi: 10.1038/s41569-021-00552-1
8. Basso PJ, Andrade-Oliveira V, Camara NOS. Targeting immune cell metabolism in kidney diseases. Nat Rev Nephrol (2021) 17(7):465–80. doi: 10.1038/s41581-021-00413-7
9. Wang ZL. On maxwell’s displacement current for energy and sensors: the origin of nanogenerators. Mater Today (2017) 20(2):74–82. doi: 10.1016/j.mattod.2016.12.001
10. Fan FR, Tian ZQ, Wang ZL. Flexible triboelectric generator! Nano Energy (2012) 1(2):328–34. doi: 10.1016/j.nanoen.2012.01.004
11. Gao HQ, Hu MA, Ding JF, Xia BL, Yuan GL, Sun HS, et al. Investigation of contact electrification between 2d mxenes and Mos2 through density functional theory and triboelectric probes. Adv Funct Mater (2023) 10:2213410. doi: 10.1002/adfm.202213410
12. Yamada T, Hayamizu Y, Yamamoto Y, Yomogida Y, Izadi-Najafabadi A, Futaba DN, et al. A stretchable carbon nanotube strain sensor for human-motion detection. Nat Nanotechnol (2011) 6(5):296–301. doi: 10.1038/nnano.2011.36
13. Son D, Kang J, Vardoulis O, Kim Y, Matsuhisa N, Oh JY, et al. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat Nanotechnol (2018) 13(11):1057. doi: 10.1038/s41565-018-0244-6
14. Ray TR, Choi J, Bandodkar AJ, Krishnan S, Gutruf P, Tian LM, et al. Bio-integrated wearable systems: a comprehensive review. Chem Rev (2019) 119(8):5461–533. doi: 10.1021/acs.chemrev.8b00573
15. Zhang WLH, Zhang LL, Gao HL, Yang WY, Wang S, Xing LL, et al. Self-powered implantable skin-like glucometer for real-time detection of blood glucose level in vivo. Nano-Micro Lett (2018) 10(2):1–11. doi: 10.1007/s40820-017-0185-x
16. Ouyang H, Liu Z, Li N, Shi BJ, Zou Y, Xie F, et al. Symbiotic cardiac pacemaker. Nat Commun (2019) 10:1821. doi: 10.1038/s41467-019-09851-1
17. Zhao CC, Feng HQ, Zhang LJ, Li Z, Zou Y, Tan PC, et al. Highly efficient in vivo cancer therapy by an implantable magnet triboelectric nanogenerator. Adv Funct Mater (2019) 29(41):1808640. doi: 10.1002/adfm.201808640
18. Meng KY, Chen J, Li XS, Wu YF, Fan WJ, Zhou ZH, et al. Flexible weaving constructed self-powered pressure sensor enabling continuous diagnosis of cardiovascular disease and measurement of cuffless blood pressure. Adv Funct Mater (2019) 29(5):1806388. doi: 10.1002/adfm.201806388
19. Jiang W, Li H, Liu Z, Li Z, Tian JJ, Shi BJ, et al. Fully bioabsorbable natural-Materials-Based triboelectric nanogenerators. Adv Mater (2018) 30(32):1801895. doi: 10.1002/adma.201801895
20. Zhao LM, Li H, Meng JP, Wang AC, Tan PC, Zou Y, et al. Reversible conversion between Schottky and ohmic contacts for highly sensitive, multifunctional biosensors. Adv Funct Mater (2020) 30(5):1907999. doi: 10.1002/adfm.201907999
21. Shan YZ, Feng HQ, Li Z. Electrical stimulation for nervous system injury: research progress and prospects. Acta Physico-Chimica Sin (2020) 36(12). doi: 10.3866/pku.Whxb202005038
22. Wang ZR, Hao Z, Yu SF, De Moraes CG, Suh LH, Zhao XZ, et al. An ultraflexible and stretchable aptameric graphene nanosensor for biomarker detection and monitoring. Adv Funct Mater (2019) 29(44):1905202. doi: 10.1002/adfm.201905202
23. Zhang Q, Liang QJ, Zhang Z, Kang Z, Liao QL, Ding Y, et al. Electromagnetic shielding hybrid nanogenerator for health monitoring and protection. Adv Funct Mater (2018) 28(1):1703801. doi: 10.1002/adfm.201703801
24. Cheng Y, Lu X, Chan KH, Wang RR, Cao ZR, Sun J, et al. A stretchable fiber nanogenerator for versatile mechanical energy harvesting and self-powered full-range personal healthcare monitoring. Nano Energy (2017) 41:511–8. doi: 10.1016/j.nanoen.2017.10.010
25. Qu XC, Liu Y, Liu Z, Li Z. Assistive devices for the people with disabilities enabled by triboelectric nanogenerators. J Physics-Materials (2021) 4(3):034015. doi: 10.1088/2515-7639/ac0092
26. Zhang JH, Xu QH, Li H, Zhang SY, Jiang YW, Hu N, et al. Self-powered electrodeposition system for Sub-10-Nm silver nanoparticles with high-efficiency antibacterial activity. J Phys Chem Lett (2022) 13(29):6721–30. doi: 10.1021/acs.jpclett.2c01737
27. Tsoupras A, Lordan R, Zabetakis I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients (2018) 10(5):604. doi: 10.3390/nu10050604
28. Yao G, Kang L, Li CC, Chen SH, Wang Q, Yang JZ, et al. A self-powered implantable and bioresorbable electrostimulation device for biofeedback bone fracture healing. Proc Natl Acad Sci USA (2021) 118(28):e2100772118. doi: 10.1073/pnas.2100772118
29. Pullano SA, Critello DC, Fiorillo AS. Triboelectric-induced pseudo-icg for cardiovascular risk assessment on flexible electronics. Nano Energy (2020) 67:104278. doi: 10.1016/j.nanoen.2019.104278
30. Liu BH, Libanori A, Zhou YH, Xiao X, Xie GZ, Zhao X, et al. Simultaneous biomechanical and biochemical monitoring for self-powered breath analysis. ACS Appl Mater Interfaces (2022) 14(5):7301–10. doi: 10.1021/acsami.1c22457
31. Wang H, Wang JH, He TYY, Li Z, Lee C. Direct muscle stimulation using diode-amplified triboelectric nanogenerators (Tengs). Nano Energy (2019) 63:103844. doi: 10.1016/j.nanoen.2019.06.040
32. Shi R, Zhang JS, Tian JJ, Zhao CC, Li Z, Zhang YZ, et al. An effective self-powered strategy to endow titanium implant surface with associated activity of anti-biofilm and osteogenesis. Nano Energy (2020) 77:105201. doi: 10.1016/j.nanoen.2020.105201
33. Xu QH, Fang YS, Jing BQS, Hu N, Lin K, Pan YF, et al. A portable triboelectric spirometer for wireless pulmonary function monitoring. Biosens Bioelectron (2021) 187:8. doi: 10.1016/j.bios.2021.113329
34. Zhao SY, Zhang L, Ji W, Shi YC, Lai GC, Chi H, et al. Machine learning-based characterization of cuprotosis-related biomarkers and immune infiltration in parkinson’s disease. Front Genet (2022) 13:1010361. doi: 10.3389/fgene.2022.1010361
35. Li CY, Liu D, Xu CQ, Wang ZM, Shu S, Sun ZR, et al. Sensing of joint and spinal bending or stretching via a retractable and wearable badge reel. Nat Commun (2021) 12(1):2950. doi: 10.1038/s41467-021-23207-8
36. Yu B, Zhou L, Zhang X, Hu G, Min H, Qiu Y, et al. Respiration-mediated self-switched triboelectric nanogenerator for wearable point-of-Care prevention and alarm of asthma. Nano Energy (2023) 106:108058. doi: 10.1016/j.nanoen.2022.108058
37. Luo JZ, Li YS, He M, Wang ZM, Li CY, Liu D, et al. Rehabilitation of total knee arthroplasty by integrating conjoint isometric myodynamia and real-time rotation sensing system. Adv Sci (2022) 9(8):2105219. doi: 10.1002/advs.202105219
38. Yan W, Ma C, Cai X, Sun Y, Zhang G, Song W. Self-powered and wireless physiological monitoring system with integrated power supply and sensors. Nano Energy (2023) 108:108203. doi: 10.1016/j.nanoen.2023.108203
39. Jiang Y, Zhang YF, Ning C, Ji QQ, Peng X, Dong K, et al. Ultrathin eardrum-inspired self-powered acoustic sensor for vocal synchronization recognition with the assistance of machine learning. Small (2022) 18(13):2106960. doi: 10.1002/smll.202106960
40. Roos EM, Herzog W, Block JA, Bennell KL. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat Rev Rheumatol (2011) 7(1):57–63. doi: 10.1038/nrrheum.2010.195
41. Becker R, Berth A, Nehring M, Awiszus F. Neuromuscular quadriceps dysfunction prior to osteoarthritis of the knee. J Orthopaedic Res (2004) 22(4):768–73. doi: 10.1016/j.orthres.2003.11.004
42. Creamer P, Lethbridge-Cejku M, Hochberg MC. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology (2000) 39(5):490–6. doi: 10.1093/rheumatology/39.5.490
43. Sherman AM. Social relations and depressive symptoms in older adults with knee osteoarthritis. Soc Sci Med (2003) 56(2):247–57. doi: 10.1016/s0277-9536(02)00023-0
44. Lin ZH, Cheng G, Yang Y, Zhou YS, Lee S, Wang ZL. Triboelectric nanogenerator as an active uv photodetector. Adv Funct Mater (2014) 24(19):2810–6. doi: 10.1002/adfm.201302838
45. Liu YQ, Li EL, Wang XM, Chen QZ, Zhou YL, Hu YY, et al. Self-powered artificial auditory pathway for intelligent neuromorphic computing and sound detection. Nano Energy (2020) 78:105403. doi: 10.1016/j.nanoen.2020.105403
46. Lu MY, Chen TY, Williamson DFK, Zhao M, Shady M, Lipkova J, et al. Ai-based pathology predicts origins for cancers of unknown primary. Nature (2021) 594(7861):106. doi: 10.1038/s41586-021-03512-4
47. Kather JN, Pearson AT, Halama N, Jager D, Krause J, Loosen SH, et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat Med (2019) 25(7):1054. doi: 10.1038/s41591-019-0462-y
48. Chen MH, Zhou YK, Lang JY, Li LJ, Zhang Y. Triboelectric nanogenerator and artificial intelligence to promote precision medicine for cancer. Nano Energy (2022) 92:106783. doi: 10.1016/j.nanoen.2021.106783
49. Jiao PC. Emerging artificial intelligence in piezoelectric and triboelectric nanogenerators. Nano Energy (2021) 88:21. doi: 10.1016/j.nanoen.2021.106227
50. Jiao PC, Alavi AH. Evolutionary computation for design and characterization of nanoscale metastructures. Appl Mater Today (2020) 21:9. doi: 10.1016/j.apmt.2020.100816
51. Vasudevan RK, Choudhary K, Mehta A, Smith R, Kusne G, Tavazza F, et al. Materials science in the artificial intelligence age: high-throughput library generation, machine learning, and a pathway from correlations to the underpinning physics. MRS Commun (2019) 9(3):821–38. doi: 10.1557/mrc.2019.95
Keywords: inflammation, triboelectric nanogenerators, artificial intelligence, prevention, monitoring
Citation: Zhao E, Hu C and Zhu Z (2023) Emerging triboelectric nanogenerators for the prevention and monitoring of inflammation. Front. Immunol. 14:1167301. doi: 10.3389/fimmu.2023.1167301
Received: 16 February 2023; Accepted: 15 May 2023;
Published: 31 May 2023.
Edited by:
Fabrice Cognasse, INSERM U1059 SAnté INgéniérie BIOlogie, FranceReviewed by:
Songyun Zhao, Wuxi People’s Hospital Affiliated to Nanjing Medical University, ChinaCopyright © 2023 Zhao, Hu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyuan Zhu, enl1YW56aHVAc3d1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.