
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 24 March 2023
Sec. NK and Innate Lymphoid Cell Biology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1166451
One would expect maternal immune cells to attack the invading trophoblast as the placenta is semi-allogenic. However, they appear to cooperate with the trophoblast in disrupting the arterial wall which has been determined in several studies. uNK cells are a particular type of immune cell that appears to play a role in pregnancy. As in pregnancy, the key contributors to trophoblast invasion appear to be a unique combination of genes, which appear to regulate multiple components of the interactions between placental and maternal cells, called HLA class 1b genes. The HLA class 1b genes have few alleles, which makes them unlikely to be recognized as foreign by the maternal cells. The low polymorphic properties of these particular HLAs may aid trophoblasts in actively avoiding immune attacks. This review gives a complete description of the mechanisms of interaction between HLAs and maternal uNK cells in humans.
A major component of the mother’s leukocytes population is composed of uterine natural killer (uNK) cells, which are approximately 70% of the total decidual leukocyte population in the first trimester of pregnancy (1–3). uNK cells are lymphocytes that survey the body for abnormal cells. uNK cells are present in low numbers even before pregnancy, during the menstrual cycle’s proliferative and early secretory phase. The number of uNK cells in the endometrium increases through the late secretory phase of the menstrual cycle and continues to rise in early pregnancy. The uNK cells accumulate in the decidua until week 20 of gestation, with the highest numbers present in early pregnancy.
uNK cells play a unique role in decidualization and trophoblast implantation, and uNK cells have been suggested to be remarkably different from other subsets of CD56bright NK cells in peripheral blood (4–10). The origin of uNK cells remains unclear, but uNK cells (CD56brightCD16dim) have been detected in both non-pregnant and pregnant uterine tissue (9, 11). uNK cells have been found to secrete different types of cytokines compared to peripheral NK (pNK) cells and are poorly cytotoxin in normal pregnancies (12). uNK cells do appear to produce low proportion of cytotoxic proteins, but this is not thought sufficient to kill the invading trophoblasts (13). Similar to cytotoxin secretion, secretion of cytokine is also influenced by the activating and inhibitory receptors on the surface of uNK cells.
NK cell function is determined by a balance of activation and inhibition signalling induced by trans-membrane receptors (14, 15). Integration of these numerous inputs results in cytotoxin and/or cytokine secretion. This process involves the engagement of ligands with the receptors, as well as the action of pro-inflammatory cytokines such as IL-1, IL-2, IL-12, IL-15, IL-18, IL-21 and IFNα,β (8, 16, 17) released by antigen-presenting cells (APCs) (18). NK cells do not have a dominant activation receptor, so several receptors or coactivations are required to secrete cytotoxins. If activation signalling dominates, NK cells become activated. Following activation, an NK cell can secrete cytotoxins and various types of cytokines. Cytotoxin secretion is suppressed by the recognition of ligands that the NK cell receptors detect on ‘target’ cells (19). Upon contact with other cells recognised as undesirable, activated NK cells are able to mediate cell killing via two mechanisms, exocytosis of perforin/granzyme granules and signalling via TNF receptors (8, 19).
The interaction between NK cells and target cells requires docking between NK cell receptors and target cell ligands (Figure 1). There are two hypotheses regarding how NK cells interact with pathogens or foreign cells. The ‘missing-self’ hypothesis is one of the simple recognition strategies for NK cells. This hypothesis suggests that NK cells attack target cells that lack ‘self-molecules’, which usually exist in a healthy or normal cell (20). The action of NK cells can be described as ‘activation upon recognition of the unexpected and inactivation upon recognition of the expected’. Another recognition strategy is called the ‘induced self’ hypothesis, where NK cell activation happens because of the expression of ligands for NK cell-activating receptors. The activation of NK cells in this situation is induced under cellular stress conditions, like viral infection (21). Both recognition methods may work simultaneously for NK cells to maximally discriminate between normal cells and infected target cells (22).
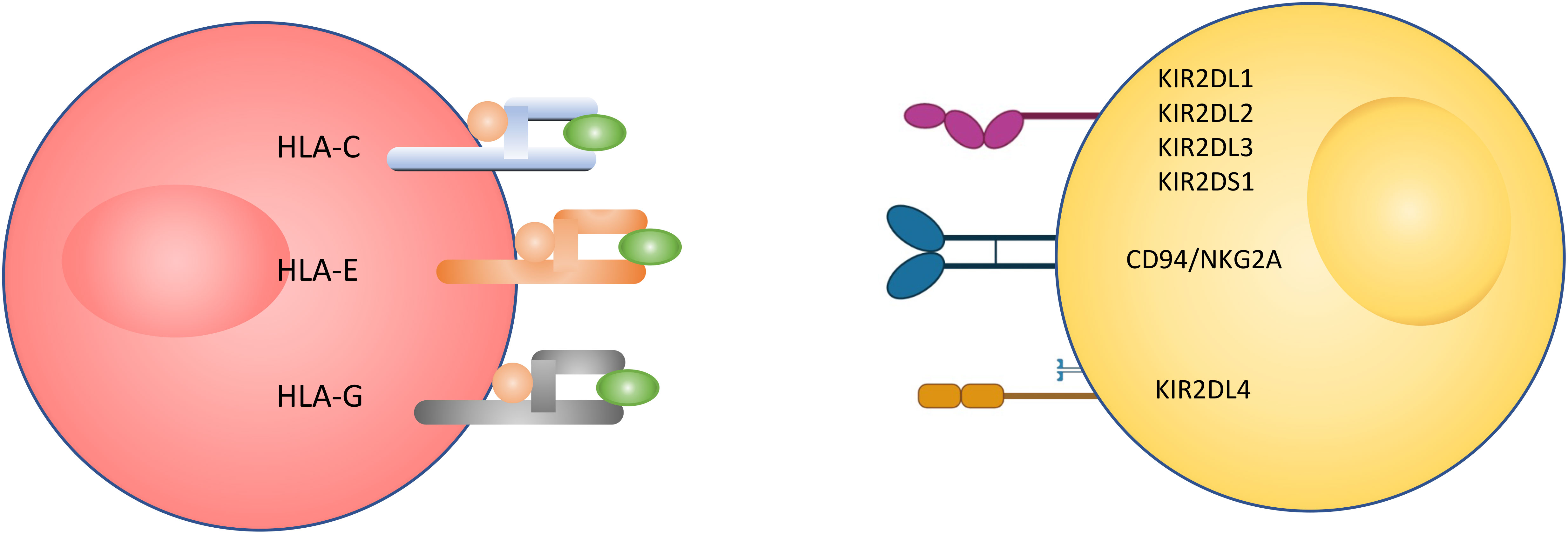
Figure 1 Illustration of NK cell interaction with a target cell. The interaction between the two cells happens through receptors expressed on the NK cell surface and ligand expressed on the target cell surface.
NK cells can be divided into two distinct populations: CD56dim and CD56bright, which differ in their distribution of homing properties. CD56dim makes up 90% of human peripheral blood, while CD56bright represents the main lymphocyte population in human decidua (10). Normally CD56dim cells express high levels of CD16 (23), the strongest inducer of cytotoxicity by IL-2 activated NK cells (17). While CD56bright cells express no, or low levels, of CD16. So, CD56dim and CD56bright cells secrete different types or levels of cytotoxins and cytokines (6, 16). As there is a distinction between the NK cells based on both CD56and CD16, the population of NK cells is also recognized as being split into CD56dimCD16+ and CD56brightCD16-cohorts. Another difference between cells in the two populations of CD56is perforin expression. It is expressed in high levels in CD56dim, while CD56bright cells express 10-fold lower perforin (16, 23).
A large number of NK cell receptors have been studied and the number is still growing (Figure 2) (6, 29–32). There are three major families of natural killer receptors (NKRs). The first family is the killer cell Ig-like receptor (KIR). This group of receptors recognizes human leukocyte antigen (HLA) -A, -B and -C on target cells. The expression of KIR isoforms on NK cells is regulated by the methylation of KIR gene loci (21). A second group is C-type lectins, such as CD94 and NKG2, which recognize HLA-E and MICA (ligands on tumour, infected and/or stressed cells) on target cells. A third group is the natural cytotoxicity receptors (NCRs) which comprise activation receptors to signal cells’ lysis. Among them are NKp44, Nkp46, NKp80 and NKp30 (21), which interact with viral hemagglutinin, nuclear factor HLA-B-associated transcript 3, heparin sulphate proteoglycans, C-type lectin and B7-H6 (21). There is a small family of receptors in humans, called leukocyte immunglobulin-like transcript (ILT) receptors, which are inhibitory receptors that bind to the HLA class I ligand.

Figure 2 Receptors of interest in human natural killer cell. Activating (highlighted in yellow) and inhibitory (highlighted in blue) receptors expressed on (A) pNK and (B) uNK surfaces, and ligands that bind to them (24–28).
The KIR receptor group is of particular interest in this study because of its capacity to bind with HLA-G and HLA-C, which have both been found to play important roles in trophoblast invasion. Commonly, inhibitory KIRs contain ITIM (immunoreceptor tyrosine-based inhibition motifs) regions in their cytoplasmic tail, and activation KIRs contain ITAM (immunoreceptor tyrosine-based activation motif) regions in their cytoplasmic tail. ITIM is defined by a consensus of amino acid sequence (I/L/V/S) xYxx (L/V), where the x represents any amino acid. Once an inhibition receptor engages with a target cell ligand, the tyrosine residue on the ITIM is phosphorylated and activates inhibition signalling downstream. In contrast, the ITAM consensus sequence is defined by (D/E)xxYxx(L/I)x(6-12)Yxx(L/I), where x(6-12) represents 6-12 possible amino acids (some say x(6-8)) (30, 31, 33). Upon engagement with the ligand on the target cell, the tyrosine residue is phosphorylated by Src family protein tyrosine kinase (SFK). This initiates the activation signalling pathway of an NK cell (30, 34).
MHCs or HLAs are normally highly variable between individuals, as they are encoded by numerous highly polymorphic genes, meaning that it is extremely unlikely that two people will possess cells with the same set of HLA molecules. There is strict regulation of HLA genes, and production of their proteins, by invading trophoblasts (35). While there are multiple mechanisms that may contribute to immune tolerance in pregnancy, a particular class of non-classical HLA-E and -G, and classical HLA-C appear to play a key role in maternal immune response. Another important mechanism by which the fetus avoids the maternal immunologic response is the failure of trophoblasts to express classical HLA class Ia, comprising HLA-A and -B. Without expression of HLA-A and -B molecules, trophoblasts are unlikely to be killed by NKs through secretion of cytotoxic. In contrast to classical HLA (HLA-A, -B and -C), there is limited variability between individuals in HLA-E and -G (35, 36). HLA-E and -G have low numbers of alleles that differ at the protein level, HLA-E has 2 alleles and HLA-G has five alleles that do not alter the amino acid sequence (35). It is suggested that because of the limited variability in HLA-E and -G that uNK cells are “poor killers of the usual NK cell targets” (35). HLA-E, HLA-C and more so HLA-G, are thought to be highly expressed in placental cells to 1) provide recognition for uNK cells that is not highly variable between individuals, and 2) to inhibit the toxicity of uNKs. However, the true nature of the interaction between invading placental cells and uNK cells is not well understood. In this section we will address HLA-G, -C and -E and their potential roles in pregnancy.
Most of the natural killer cell receptors that have potential ligands expressed by extravillous trophoblasts (EVTs) have been described. HLA-G binds to members of the LILR family, including LILRB1, LILRB2 and KIR2DL4. HLA-E binds to NKG2C and its inhibitory counterpart NKG2A. Classical HLA-C allotypes that are polymorphic bind to members of the KIR2DL/S family (24). While NKG2C and KIR2DS1 are activating, LILRB1/2, KIR2DL1/2/3 and NKG2A are inhibitory. In contrast, KIR2DL4 can be either activating or inhibitory depending on the amino acid residues in the domain. During the first 8-10 weeks of gestation, KIR2DL1/2/3 and KIR2DS1 expression on uNK cells is very high compared to pNK cells from the same women at the same time, and NKG2A is expressed on almost all uNK and LILRB1 is expressed on 30-40% of uNK cells. This suggests a majority of uNK cells may bind to HLA-C expressed by EVTs (24, 37, 38).
HLA-G is the MHC complex that is most abundant during term pregnancy. HLA-G stimulates uNK cells’ secretion of cytokines and further induces immune tolerance, controlling EVT invasion, and contributing to vascular remodelling of the spiral arteries (39). HLA-G is a homodimer expressed almost exclusively by EVTs (40). HLA-G has eight exons and a specific feature of HLA-G is the seven alternative splicings that permit the formation of various isoforms of HLA-G; four membrane-bound and three soluble proteins. The highly expressed HLA-G isoforms in trophoblasts during early pregnancy are membrane-bound HLA-G1 and soluble HLA-G5. While mRNAs for HLA-G4 and G7 are not abundant in placentas (35), HLA-G is readily detected in the EVTs that invade the maternal endometrium (14, 41). Soluble HLA-G has been detected in the peripheral blood and other biological fluids of pregnant women (42). Additional studies reported that soluble HLA-G was detected in the culture medium from human IVF-derived embryos. The presence of HLA was significantly associated with subsequent pregnancy following transfer to recipients (43–46).
In the uterus, HLA-G appears to have important interactions with uNK cell surface receptors, in particular the killer cell immunoglobulin-like receptor (KIR2DL4) and plays an important role in placental development. Rajagopalan & Long (47) demonstrated that KIR2DL4 interacts with cells expressing HLA-G but not with cells expressing HLA class I molecules. Unlike other KIR members, KIR2DL4 is expressed by all NK cells. KIR2DL4 is a unique KIR member since it has both activation and inhibition features (29, 48). KIR2DL4 has an ITIM region and also a positively charged arginine amino acid residue in the transmembrane region that is a feature of activation receptors. Therefore, many describe KIR2DL4 as an activating receptor with inhibition potential (22, 32, 47–49). KIR2DL4 and HLA-G ligation and treatment of uNK cells produce a proinflammatory cytokine, interferon- (IFN).
Another important MHC molecule in pregnancy that is expressed by EVTs is the classical class Ia HLA-C molecule (7). Although HLA-C is moderately polymorphic, both paternal and maternal alleles of HLA-C are expressed by trophoblasts, which could reserve the semi-allogenic characteristics of HLA-C (7). In addition, the allelic disparity at the HLA-C locus is improbable to cause failed pregnancy (35). Hiby et al. (50) suggested an imbalance in stimulation of uNK immunoglobulin-like receptors (KIR) by HLA-C in pre-eclampsia in human pregnancy (50). KIR specificity for HLA-C is expressed in high density on uNK cells compared to pNK cells in pregnant women, indicating the high density of HLA-C expressed by uNK cells.
HLA-C is the only classical HLA known to be expressed in trophoblasts. Because both KIR and HLA-C are polymorphic, maternal KIR and fetal HLA-C genetic combination can vary between pregnancies (50). KIR exists in two groups, A and B, which are specified as inhibiting and activating uNK, respectively. The KIR haplotype A is a simpler and mainly inhibitory KIR, while the B KIR haplotype is complicated and mainly activating. Women with homozygous AA have a higher risk of pre-eclampsia than those who are BB and heterozygous AB (50). HLA-C allotypes exist in two groups; HLA-C1 and HLA-C2, according to the particular amino acid residue at position 80 (51). HLA-C1 has asparagine (Asn) and acts as a ligand for inhibitory KIR2DL2 and KIR2DL3. HLA-C2 has a lysine (Lys) at residue 80 and is a ligand for the inhibitory KIR2DL1 receptor and the activating KIR2DS1 receptor. HLA-C2 is a stronger ligand than HLA-C1 upon interaction with the receptors (50).
A combination of fetal HLA-C2 and a maternal AA KIR genotype will most probably lead to pre-eclampsia and poor placentation (29, 50, 52). As an example, HLA-C2 and KIR2DL1 binding is expected to cause pre-eclampsia. Overly inhibited uNK cells may cause EVTs to prematurely stop the remodelling of spiral arteries and this inadequate remodelling thereby increases the risk of pre-eclampsia (52). Therefore, Hiby et al. (50) suggested that high inhibition signalling in uNK cells favours pre-eclampsia. However, the strong uNK cells inhibition could be balanced by the interaction of HLA-C2 with activation KIR AB/BB genotypes receptors. Parham (52) suggested that activating KIR lowers the likelihood of pre-eclampsia and the absence of activating KIR favours pre-eclampsia.
A recent study indicated that human birth weight is regulated by the interaction between maternal KIR and paternal HLA-C2 (50). This suggests that the frequent inhibitory signals in maternal KIRs produce small babies but frequent activating KIR signals result in big babies, compared to normal growth. Thus, the balance between activation and inhibition contributes to successful pregnancy by maintaining the fetus birth weight between the two extremes (53). Investigations have shown that KIR2DS1 is the gene with the most significant effect on human birth weight. Although KIR2DS5 has also been shown to increase birth weight, the impact is not significant (50).
There is a high expression of inhibitory receptor CD94/NKG2A on uNK cells, which suggests the HLA-E ligand exists in maternal decidua (7). The interaction between CD94/NKG2A and HLA-E is believed to prevent the lysis of both maternal and paternal tissue cells in the vicinity of uNK cells. HLA-G complexes with HLA-E and binds CD94/NKG2C, giving enough affinity to trigger an NK cell response. This means that HLA-G needs HLA-E to influence the maternal immune response. This mechanism will still occur even if the fetus is homozygous for the HLA-G null allele because the leader peptide will continue to be translated (51, 54). HLA-E could be the major inhibitory regulator for uNK cells, rather than HLA-G (1). Knowing that HLA-E is the only ligand for the CD94/NKG2A heterodimer, this suggestion is supported by the large amount of CD94/NKG2A found in the maternal decidua (35, 55).
Inhibitory receptors regulate the activities of various activating receptors and have diverse signalling pathways downstream. NKs are inhibited by inhibition receptors that recognize MHC class I. Inhibitory receptor engagement reduces tyrosine phosphorylation. For example, the CD94/NKG2A inhibition receptor complex recognizes HLA-E, which results in phosphorylation of HLA-E and CD94/NKG2A complex by SFKs. This further leads to the recruitment of phosphatase SHP to the NKG2A complex (at the ITIM region). A common feature of inhibition receptors involved in these protective functions is the presence of ITIM in the cytoplasmic tail. The functional consequence of SHP recruitment by ITIM-containing receptors in NK cells is well established. Vav is a direct target of SHP when SHP is recruited to the ITIM region. Signals from inhibitory receptors dephosphorylate Vav through SHP. However, coaggregation between activating and inhibitory receptors is required for inhibition to occur.
The known differences in receptor-ligand interactions between pNKs and uNK cells are illustrated in Figure 2. As described earlier, the important ligands expressed on trophoblasts are HLA-C, -E and -G. These are the ligands that therefore are assumed determine the response of uNKs to trophoblasts. As reported in the literature, we hypothesised that the level of cytokine secretion is increased in uNK cells compared to pNK cells.
Papuchova et al. (56) analysis of the HLA-C, -E, and -G differential expression shows that HLA-C and -E expression during term pregnancy (>37 weeks) was significantly lower compared to the first trimester (6-12 weeks). Meanwhile, the first trimester EVT has the highest levels of HLA-C and term pregnancy EVT has the highest levels of HLA-G. These differences suggest that EVT has a distinct ability for antigen presentation and interaction with uNK at different stages of pregnancy (57).
Generally, IFNγ and TNFα are the cytokines secreted in the highest levels by NK cells (25, 30). NK cells also secrete GM-CSF (23, 58). For particular NK receptors, there is evidence that KIR2DL4 receptor activation by HLA-G induces the production of IFNγ and TNFα (48, 58, 59). The production of IFNγ by KIR2DL4 is blocked by inhibition of MAPK pathway. Experimental work by Sharkey (2008) showed that KIR activation includes KIR2DS1 activation by HLA-C and results in increased secretion of IFNγ (40). HLA-E and HLA-G also stimulate the secretion of IFNγ, TNFα and GM-CSF in large granular lymphocytes (LGL) (56).
Immune cells have been found aggregated around spiral arteries in early pregnancy, near the invading trophoblast (60–62). Smith et al. (62) and Rana et al. (63) suggested that spiral artery remodelling is initiated by uNK cells. A reduced number of uNK cells have been detected in pregnant mothers with pre-eclampsia and intrauterine growth restriction (IUGR), which is believed to cause poor spiral artery remodelling (2). The key contributors to trophoblast invasion appear to be a unique combination of HLA genes. The low polymorphic properties of these particular HLAs may aid trophoblasts in actively avoiding immune attacks (64). We concluded that the uNK lack of the ligand-receptor pairs that are reported in pNK which are ICAM, MICA, HLA-A and HLA-B ligands and their respective receptors ITG, NKG2D, KIR2DL1 and KIR2DL3.
The author confirms being the sole contributor of this work and has approved it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol (2002) 2(9):656–63. doi: 10.1038/nri886
2. Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol (2017) 17(8):469–82. doi: 10.1038/nri.2017.64
3. Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol (2009) 82(1):24–31. doi: 10.1016/j.jri.2009.08.001
4. Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: A unique innate immunoregulatory role for the CD56bright subset. Blood J Am Soc Hematol (2001) 97(10):3146–51. doi: 10.1182/blood.V97.10.3146
5. Wendt K, Wilk E, Buyny S, Buer J, Schmidt RE, Jacobs R. Gene and protein characteristics reflect functional diversity of CD56dim and CD56bright NK cells. J leukocyte Biol (2006) 80(6):1529–41. doi: 10.1189/jlb.0306191
6. Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol (2001) 19(1):197–223. doi: 10.1146/annurev.immunol.19.1.197
7. King A, Hiby SE, Gardner L, Joseph S, Bowen JM, Verma S, et al. Recognition of trophoblast HLA class I molecules by decidual NK cell receptors - A review. Placenta (2000) 21:S81–5. doi: 10.1053/plac.1999.0520
8. Sojka DK, Yang L, Yokoyama WM. Uterine natural killer cells. Front Immunol (2019) 10:960. doi: 10.3389/fimmu.2019.00960
9. Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol (2013) 31:387–411. doi: 10.1146/annurev-immunol-032712-100003
10. Yamada H, Shimada S, Kato EH, Morikawa M, Iwabuchi K, Kishi R, et al. Decrease in a specific killer cell immunoglobulin-like receptor on peripheral natural killer cells in women with recurrent spontaneous abortion of unexplained etiology. Am J Reprod Immunol (2004) 51(3):241–7. doi: 10.1111/j.1600-0897.2004.00139.x
11. Lash GE, Robson SC, Bulmer JN. Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta (2010) 31:S87–92. doi: 10.1016/j.placenta.2009.12.022
12. Gaynor LM, Colucci F. Uterine natural killer cells: Functional distinctions and influence on pregnancy in humans and mice. Front Immunol (2017) 8:467. doi: 10.3389/fimmu.2017.00467
13. Bouteiller PL. HLA-G in the human placenta: Expression and potential functions. Biochem Soc Trans (2000) 28(2):208–12. doi: 10.1042/bst0280208
14. Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science (2004) 306(5701):1517–9. doi: 10.1126/science.1103478
15. Long EO, Sik Kim H, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu Rev Immunol (2013) 31:227–58. doi: 10.1146/annurev-immunol-020711-075005
16. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol (2001) 22(11):633–40. doi: 10.1016/S1471-4906(01)02060-9
17. Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev (2006) 214(1):73–91. doi: 10.1111/j.1600-065X.2006.00457.x
18. Caligiuri MA. Human natural killer cells. Blood J Am Soc Hematol (2008) 112(3):461–9. doi: 10.1182/blood-2007-09-077438
19. Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, et al. Activation of NK cell cytotoxicity. Mol Immunol (2005) 42(4):501–10. doi: 10.1016/j.molimm.2004.07.034
20. Kärre K. Natural killer cell recognition of missing self. Nat Immunol (2008) 9(5):477–80. doi: 10.1038/ni0508-477
21. Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol (2011) 89(2):216–24. doi: 10.1038/icb.2010.78
22. Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol (2006) 6(7):520–31. doi: 10.1038/nri1863
23. Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood J Am Soc Hematol (2010) 115(11):2167–76. doi: 10.1182/blood-2009-08-238469
24. Moffett A, Colucci F. Co-Evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol Rev (2015) 267(1):283–97. doi: 10.1111/imr.12323
25. Maghazachi AA. Insights into seven and single transmembrane-spanning domain receptors and their signaling pathways in human natural killer cells. Pharmacol Rev (2005) 57(3):339–57. doi: 10.1124/pr.57.3.5
26. Dybkaer K, Iqbal J, Zhou G, Geng H, Xiao L, Schmitz A, et al. Genome wide transcriptional analysis of resting and IL2 activated human natural killer cells: Gene expression signatures indicative of novel molecular signaling pathways. BMC Genomics (2007) 8(1):1–17. doi: 10.1186/1471-2164-8-230
27. McIntire RH, Hunt JS. Antigen presenting cells and HLA-G–a review. Placenta (2005) 26:S104–9. doi: 10.1016/j.placenta.2005.01.006
28. Brown D, Trowsdale J, Allen R. The LILR family: Modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens (2004) 64(3):215–25. doi: 10.1111/j.0001-2815.2004.00290.x
29. Middleton D, Gonzelez F. The extensive polymorphism of KIR genes. Immunology (2010) 129(1):8–19. doi: 10.1111/j.1365-2567.2009.03208.x
30. Barrow AD, Trowsdale J. You say ITAM and I say ITIM, let's call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immunol (2006) 36(7):1646–53. doi: 10.1002/eji.200636195
31. Billadeau DD, Leibson PJ. ITAMs versus ITIMs: Striking a balance during cell regulation. J Clin Invest (2002) 109(2):161–8. doi: 10.1172/JCI0214843
32. Rajagopalan S, Long EO. KIR2DL4 (CD158d): an activation receptor for HLA-G. Front Immunol (2012) 3:258. doi: 10.3389/fimmu.2012.00258
33. Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol (2008) 9(5):495–502. doi: 10.1038/ni1581
34. Humphrey MB, Lanier LL, Nakamura MC. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev (2005) 208(1):50–65. doi: 10.1111/j.0105-2896.2005.00325.x
35. Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J (2005) 19(7):681–93. doi: 10.1096/fj.04-2078rev
36. Golos TG, Bondarenko GI, Dambaeva SV, Breburda EE, Durning M. On the role of placental major histocompatibility complex and decidual leukocytes in implantation and pregnancy success using non-human primate models. Int J Dev Biol (2010) 54(2-3):431. doi: 10.1387/ijdb.082797tg
37. Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, et al. Maternal uterine NK cell–activating receptor KIR2DS1 enhances placentation. J Clin Invest (2013) 123(10):4264–72. doi: 10.1172/JCI68991
38. Sharkey AM, Gardner L, Hiby S, Farrell L, Apps R, Masters L, et al. Killer ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-c and alters with gestational age. J Immunol (2008) 181(1):39–46. doi: 10.4049/jimmunol.181.1.39
39. Li C, Houser BL, Nicotra ML, Strominger JL. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci (2009) 106(14):5767–72. doi: 10.1073/pnas.0901173106
40. Dendrou CA, Petersen J, Rossjohn J, Fugger L. HLA variation and disease. Nat Rev Immunol (2018) 18(5):325–39. doi: 10.1038/nri.2017.143
41. Hunt JS, Langat DK, McIntire RH, Morales PJ. The role of HLA-G in human pregnancy. Reprod Biol Endocrinol (2006) 4(1):1–8. doi: 10.1186/1477-7827-4-S1-S10
42. Hunt JS, Petroff MG, Morales P, Sedlmayr P, Geraghty DE, Ober C. HLA-G in reproduction: studies on the maternal–fetal interface. Hum Immunol (2000) 61(11):1113–7. doi: 10.1016/S0198-8859(00)00195-6
43. Fuzzi B, Rizzo R, Criscuoli L, Noci I, Melchiorri L, Scarselli B, et al. HLA-G expression in early embryos is a fundamental prerequisite for the obtainment of pregnancy. Eur J Immunol (2002) 32(2):311–5. doi: 10.1002/1521-4141(200202)32:2<311::AID-IMMU311>3.0.CO;2-8
44. Yie SM, Balakier H, Motamedi G, Librach CL. Secretion of human leukocyte antigen-G by human embryos is associated with a higher in vitro fertilization pregnancy rate. Fertil Steril (2005) 83(1):30–6. doi: 10.1016/j.fertnstert.2004.06.059
45. Sher G, Keskintepe L, Fisch JD, Acacio BA, Ahlering P, Batzofin J, et al. Soluble human leukocyte antigen G expression in phase I culture media at 46 hours after fertilization predicts pregnancy and implantation from day 3 embryo transfer. Fertil Steril (2005) 83(5):1410–3. doi: 10.1016/j.fertnstert.2004.11.061
46. Desai N, Filipovits J, Goldfarb J. Secretion of soluble HLA-G by day 3 human embryos associated with higher pregnancy and implantation rates: Assay of culture media using a new ELISA kit. Reprod BioMedicine Online (2006) 13(2):272–7. doi: 10.1016/S1472-6483(10)60626-8
47. Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G–specific receptor expressed on all natural killer cells. J Exp Med (1999) 189(7):1093–100. doi: 10.1084/jem.189.7.1093
48. Rajagopalan S, Fu J, Long EO. Cutting edge: induction of IFN-γ production but not cytotoxicity by the killer cell ig-like receptor KIR2DL4 (CD158d) in resting NK cells. J Immunol (2001) 167(4):1877–81. doi: 10.4049/jimmunol.167.4.1877
49. Faure M, Long EO. KIR2DL4 (CD158d), an NK cell-activating receptor with inhibitory potential. J Immunol (2002) 168(12):6208–14. doi: 10.4049/jimmunol.168.12.6208
50. Hiby SE, Walker JJ, O'shaughnessy KM, Redman CW, Carrington M, Trowsdale J, et al. Combinations of maternal KIR and fetal HLA-c genes influence the risk of preeclampsia and reproductive success. J Exp Med (2004) 200(8):957–65. doi: 10.1084/jem.20041214
51. Hviid TVF. HLA-G in human reproduction: aspects of genetics, function and pregnancy complications. Hum Reprod Update (2006) 12(3):209–32. doi: 10.1093/humupd/dmi048
52. Parham P. NK cells and trophoblasts: Partners in pregnancy. J Exp Med (2004) 200(8):951–5. doi: 10.1084/jem.20041783
53. Hiby SE, Apps R, Chazara O, Farrell LE, Magnus P, Trogstad L, et al. Maternal KIR in combination with paternal HLA-C2 regulate human birth weight. J Immunol (2014) 192(11):5069–73. doi: 10.4049/jimmunol.1400577
54. Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, et al. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-e, f, and G in maternal-placental immune recognition. J Immunol (2003) 171(3):1376–84. doi: 10.4049/jimmunol.171.3.1376
55. Díaz-Hernández I, Alecsandru D, García-Velasco JA, Domínguez F. Uterine natural killer cells: from foe to friend in reproduction. Hum Reprod Update (2021) 27(4):720–46. doi: 10.1093/humupd/dmaa062
56. Papuchova H, Kshirsagar S, Xu L, Bougleux Gomes HA, Li Q, Iyer V, et al. Three types of HLA-g+ extravillous trophoblasts that have distinct immune regulatory properties. Proc Natl Acad Sci (2020) 117(27):15772–7. doi: 10.1073/pnas.2000484117
57. Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, Meer AVD, Joosten I, et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PloS Biol (2006) 4(1):e9. doi: 10.1371/journal.pbio.0040009
58. van der Meer A, Lukassen HGM, van Lierop MJC, Wijnands F, Mosselman S, Braat DD, et al. Membrane-bound HLA-G activates proliferation and interferon-γ production by uterine natural killer cells. Mol Hum Reprod (2004) 10(3):189–95. doi: 10.1093/molehr/gah032
59. Rieger L, Hofmeister V, Probe C, Dietl J, Weiss EH, Steck T, et al. Th1-and Th2-like cytokine production by first trimester decidual large granular lymphocytes is influenced by HLA-G and HLA-e. MHR: Basic Sci Reprod Med (2002) 8(3):255–61. doi: 10.1093/molehr/8.3.255
60. Harris LK. Trophoblast-vascular cell interactions in early pregnancy: How to remodel a vessel. Placenta (2010) 31:S93–8. doi: 10.1016/j.placenta.2009.12.012
61. Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol (2013) 13(2):133–44. doi: 10.1038/nri3370
62. Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol (2009) 174(5):1959–71. doi: 10.2353/ajpath.2009.080995
63. Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res (2019) 124(7):1094–112. doi: 10.1161/CIRCRESAHA.118.313276
Keywords: uterine natural killer (uNK) cells, NK receptor, natural killer cell (NK cell), immune cell, peripheral natural killer (pNK) cell
Citation: Ismail NI (2023) Relative expression of receptors in uterine natural killer cells compared to peripheral blood natural killer cells. Front. Immunol. 14:1166451. doi: 10.3389/fimmu.2023.1166451
Received: 15 February 2023; Accepted: 13 March 2023;
Published: 24 March 2023.
Edited by:
Laurent Brossay, Brown University, United StatesReviewed by:
Alice Gilman-Sachs, Rosalind Franklin University of Medicine and Science, United StatesCopyright © 2023 Ismail. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nurul Izza Ismail, bnVydWwuaXNtYWlsQHVzbS5teQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.