- 1Department of Neurosurgery, Zhejiang Rongjun Hospital, Jiaxing, China
- 2Department of Internal Medicine, Sunto Women & Children’s Hospital, Jiaxing, China
- 3Department of Neurology, Zhejiang Rongjun Hospital, Jiaxing, China
Although estrogen is predominantly related to the maintenance of reproductive functioning in females, it mediates various physiological effects in nearly all tissues, especially the central nervous system. Clinical trials have revealed that estrogen, especially 17β-estradiol, can attenuate cerebral damage caused by an ischemic stroke. One mechanism underlying this effect of 17β-estradiol is by modulating the responses of immune cells, indicating its utility as a novel therapeutic strategy for ischemic stroke. The present review summarizes the effect of sex on ischemic stroke progression, the role of estrogen as an immunomodulator in immune reactions, and the potential clinical value of estrogen replacement therapy. The data presented here will help better understand the immunomodulatory function of estrogen and may provide a basis for its novel therapeutic use in ischemic stroke.
1 Introduction
Stroke is the leading cause of morbidity and mortality after cardiac diseases and cancers, affecting 15 million individuals worldwide annually (1). As the risk of stroke increases with age, increasing life expectancy and rising aging population will significantly burden the economy (2). Reportedly, the economic burden of stroke has been estimated to reach $184.1 billion by 2030 (3). Moreover, ineffective treatments of stroke along with the aging of the population will increase the risk of mortality and long-term disability in the future (4). Currently, the primary barrier to establishing effective therapeutic strategies is the limited understanding of mechanisms underlying secondary neuronal damage after stroke-induced central nervous system (CNS) injuries (5). In addition, numerous preclinical studies have failed to translate into clinical use owing to the enrollment of only male animals; this emphasized the consideration of sex differences in stroke etiology. Currently, the Stroke Therapy Academic Industry Roundtable guidelines recommend considering age and sex differences when translating preclinical results in clinical trials (6, 7). Indeed, accumulative evidence has revealed that sex is an important factor in the etiology of stroke-induced CNS injuries (8). Therefore, a better understanding of the potential effect of sex can improve clinical prognosis and help establish novel therapeutic strategies. This study provides a comprehensive review of the mechanisms underlying sex-related differences observed in post-stroke CNS injuries, including genetic alteration, estrogen-mediated immune regulation, and downstream signaling pathways. Moreover, the review summarizes the anti-inflammatory effects of estrogen on the brain and emphasizes the clinical potential of estrogen-based therapeutic strategy in patients with stroke.
2 Incidence and severity of ischemic stroke based on sex
According to epidemiological data, stroke is a sexually dimorphic disease (9, 10). Although the incidence of stroke is much higher in men, women have been reported to have severe CNS injuries, higher morbidity and mortality, and greater post-stroke neurobehavioral deficits. However, according to the Greater Cincinnati/Northern Kentucky Stroke Study and data from the National Institutes of Neurological Disorders and Stroke, approximately 55,000 more women suffer from stroke annually than men, owing to the longer life expectancy in women (11). Although the incidence of stroke is higher for men, women with stroke have a higher mortality rate (58%), poorer recovery, and greater long-term disability (12). Currently, the incidence rate of stroke has decreased from 19.5% to 14.5% in men, but it has only modestly decreased from 18.0% to 16.1% in women (13). Moreover, women in the age groups of 19–30 and 45–54 years have a higher risk of stroke than men in the same age groups. This may be attributed to the alterations in estrogen status during this age. Particularly, the rapid decreases and alterations in estrogen levels lead to several disorders, which eventually increase the risk of stroke (14).
Although estrogen is a sex hormone in females that regulates reproductive function, it also plays a role in several pathological processes, especially in the CNS (14, 15). Accumulative evidence has demonstrated that estrogen, especially 17β-estradiol, has a protective role in brain injuries after ischemic stroke (16). This protective effect of 17β-estradiol is mediated by several mechanisms, including the regulation of local and systemic immune responses after stroke onset (17).
3 Effect of sex on post-stroke outcomes
It has been well documented that the sex chromosome complement is responsible for sexual dimorphism (XX for female and XY for male) (18). Accordingly, a previous study has assessed sex-related differences in the expression of X chromosome genes in patients with ischemic stroke (19). The study found that in females, X chromosome-related genes were enriched in TNFR1, interleukin (IL) 17, and natural killer cell signaling pathways, whereas in males, the X chromosome-related genes were highly expressed in pathways involved in cell development, cellular migration, and pro-inflammatory reactions (20, 21). Moreover, it has been demonstrated that genes associated with the X chromosome are responsible for cellular processes occurring after a stroke, including the activity of NF-κB activating protein, tissue inhibitor of metalloproteinase 1, and IL-1R-associated kinase (22). Specifically, the X chromosome contains several estrogen-response element (ERE) sequences. A previous study has identified 12,515 ERE sites in the human genome and 11,810 in the mouse genome. Yang et al. assessed 300 mice and reported more than 10,000 genes exhibiting sex-dependent expression in somatic tissues (23). Reportedly, approximately 13% of genes in the brain contribute to sexual dimorphism, including 355 in females and 257 in males (24).
To date, several studies have focused on the effects of sex hormones on post-stroke outcomes. Studies have additionally revealed the role of genetic sex differences in post-stroke outcomes. Reportedly, the sex hormone estrogen plays a critical role in sexual dimorphism after vascular attacks; however, this effect of estrogen is not fully accounted for in post-stroke outcomes between males and females. Based on experimental studies, several cell death-associated signaling pathways function independently of sex hormones but have been found to function differently in both sexes. In males, ischemia-induced cell death is predominantly triggered via the activation of poly ADP-ribose polymerase (PARP-1), an NAD-dependent DNA repair enzyme. The activation of PARP-1 induces further DNA damage, which results in the release of mitochondrial apoptosis-inducing factor and its subsequent translocation into the nucleus (25). Under the condition of ischemic stroke, PARP-1 activation can be observed in both sexes; however, the neuroprotective effects of this pathway were abrogated in males (26). Moreover, the inhibition or genetic deletion of PARP-1 induced these neuroprotective effects only in males, suggesting a distinct mechanism of the PARP-1 pathway in this sex (27). In addition, the caspase-mediated apoptotic pathway is the predominant mechanism underlying cell death under the conditions of ischemic stroke-induced brain injury.
Cytosolic cytochrome C (Cyto C) is an essential component of the intrinsic caspase pathway. After a stroke, neurons exhibit greater resistance to nitrosative stress and higher Cyto C levels in females than males (28). The main subjects in the study were postmenopausal or old women or ovariectomized (OVX) female models. The lack of estrogen leads to a significant increase in Cyto C (5). Moreover, neuronal responses differ according to sex. A previous study has revealed that after stroke, the caspase expression pattern in females was different from that in males. In addition, pan-caspase inhibitors preferentially protected females, but no such significant effect was observed in males. On the contrary, post-stroke males exposed to oxygen-glucose deprivation were found to be preferentially protected by neuronal NOS (nNOS) inhibitors, whereas females were not (29). Of note, these sexually dimorphic protective effects of caspase inhibitors can be observed in intact females as well as in ovariectomized (OVX) females and females who receive estrogen replacement therapy (ERT), indicating that these neuroprotective effects are independent of sex hormones (30).
4 Downstream signaling pathways of estrogen
Estrogen is a lipophilic steroid hormone that can easily diffuse across cellular membranes, especially the blood–brain barrier (31). Naturally, estrogen is synthesized from cholesterol in the ovaries and occurs in three forms: estrone, estradiol, and estriol (32). Estrone is also known as E1, estradiol as E2, and estriol as E3. Particularly, estradiol exists as 17α and 17β-estradiol, of which 17β-estradiol is the most prevalent and most potent female gonadal hormone, followed by estrone and estriol (33). Recently, it has been revealed that the role of estrogen is not limited to reproductive function. Via the bloodstream, estrogen is distributed to various tissues, such as the cardiovascular system, immune system, and CNS, where it exerts distinct effects (34, 35).
Estrogen exerts its functions by binding to estrogen receptors (ERs), which are present in several tissues, including the brain parenchyma (36). To date, three types of ERs have been identified: ERα, ERβ, and G-protein coupled receptors (37–39). Reportedly, estrogen mediates its effects via two signaling pathways (40). The first is the genomic pathway, which involves ERα and ERβ. After the binding of estrogen to ER, the activated receptor forms a homodimer and is delivered into the cell nucleus. Here, estrogen further binds to the ERE in the promoter site of various genes and serves as a transcription factor (41). The second way by which estrogen elicits its effects is through a non-genomic mechanism. In this mechanism, the ligand–ER dimer can locate itself in the cytoplasm or at the membrane, eventually activating the downstream protein kinases and phosphatases (42).
5 Regulatory role of 17β-estradiol in neuroinflammation
Ischemic stroke damages the ischemic core in the brain owing to the sudden deprivation of blood flow, oxygen, and nutrients (43, 44). A variety of ischemic cascade reactions can be observed within a few minutes after stroke onset, including increased oxidative stress and mitochondrial dysfunction, which eventually lead to cell apoptosis. These cascade reactions also include cell death-associated damage-associated molecular patterns (DAMP) and subsequent inflammatory responses (45). DAMPs promote the local activation of microglia and induce the recruitment of leukocytes (46). In this microenvironment, immune cells secrete pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, which induce local or systemic inflammatory responses (46, 47). Accumulative evidence has revealed that such immune responses can aggravate cerebral ischemic injuries as well as promote nerve regeneration, attenuate inflammation, and improve tissue repair after stroke (48, 49). In addition, the such inflammatory responses in brain can be observed in several conditions, such as infection, and are often associated with an increased risk of ischemic stroke. In line with this, a study has revealed that approximately 30% patients developed stroke during infection (50).
While the protective effects of 17β-estradiol after stroke are partially mediated by the neuroprotective pathways in the brain and immune regulation, brain injuries and tissue repairs are dependent on the immune responses after stroke onset. Specifically, the administration of 17β-estradiol after stroke can protect neural function and promote recovery through immune regulation (51). Reportedly, 17β-estradiol regulates inflammation via the activation of macrophages and release of anti-inflammatory cytokines, including IL-10 and TGF-β (52) These inflammatory responses observed in response to 17β-estradiol differ with sex throughout the lifespan and may contribute to the sexually dimorphic responses observed after stroke. However, there is still limited understanding regarding the relationship between sex hormones and immune regulation.
5.1 Estrogen and innate immunity
Stroke is associated with a significant increase in estrogen levels, which suggests an immediate physiological reaction following brain injury. The rapid, local production of estrogen has been reported to be associated with the activation of innate immunity (53). Reportedly, 17β-estradiol can inhibit the production and release of pro-inflammatory cytokines, including IL-1β, IL-6, and TNFα. This explains the role of 17β-estradiol in modulating immune responses, implicating its use as a therapeutic strategy (54).
5.1.1 Estrogen and microglia
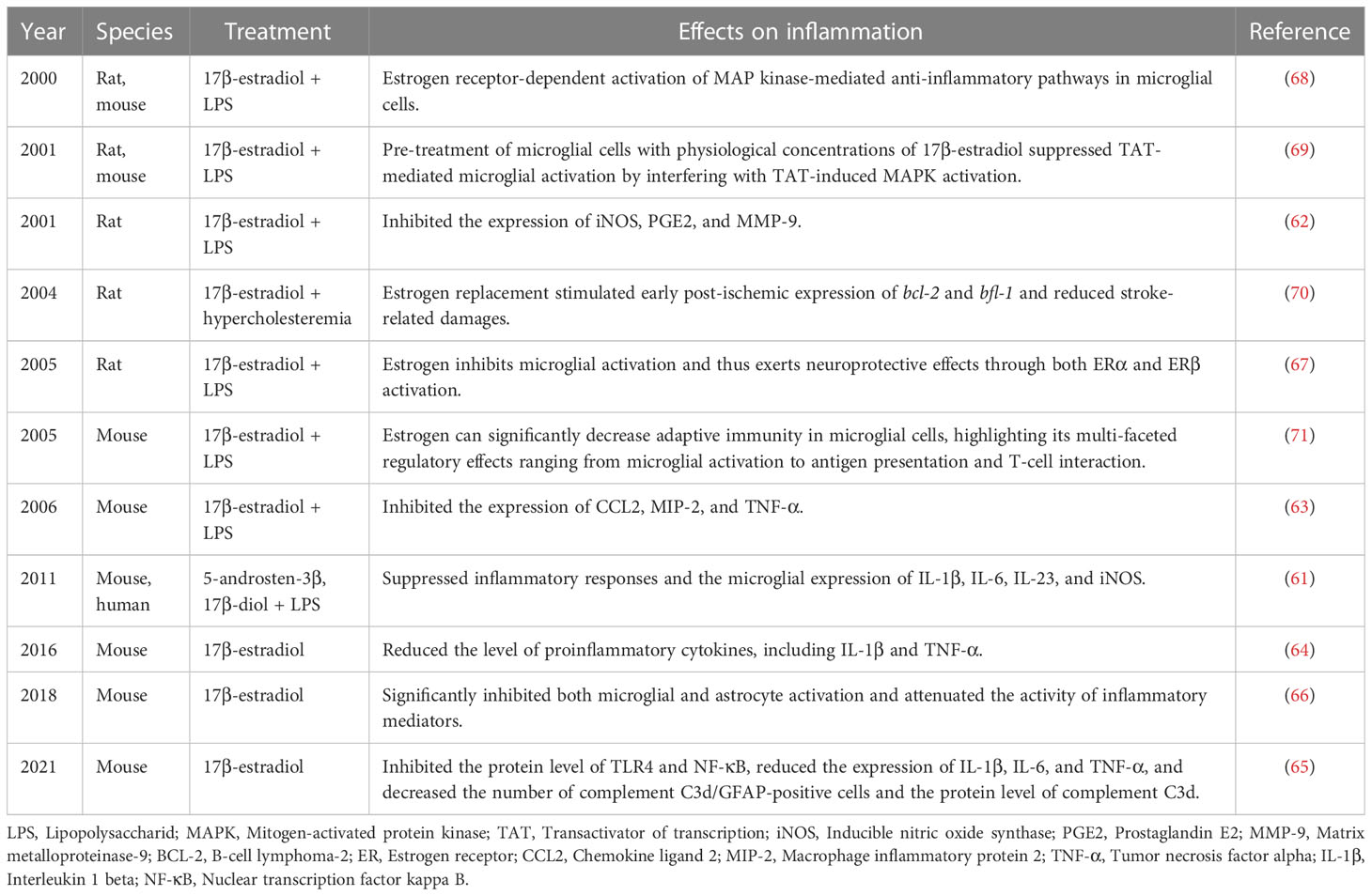
Microglia are derived from the primary myeloid precursors and are the resident immune cells in CNS (55). Accordingly, the critical role of microglia in brain injuries has been extensively explored. It has been reported that following brain injury, the number of microglia and the intensity of immune responses differ based on sex (56). A preclinical study has demonstrated that female mice have more microglia in the hippocampus than male mice. In addition, old female mice were found to have more microglia than younger ones, which accounts for the production and release of sex hormones (57). Moreover, according to an experimental study, the administration of estrogen can suppress microglial proliferation (58). In OVX female mice, ERT was found to reduce the number of microglial cells in the aged group. Furthermore, ovariectomy was found to increase the number of microglia and abrogate the restraining effects on microglial activation (59). Reportedly, estrogen induces the maintenance of resting phenotype with ramified morphology and downregulates MHCII expression (60). Furthermore, the ERs preset on microglia allow for estrogen to mediate its effects. In mouse models of middle cerebral artery occlusion, ERα knockout was found to activate the microglia and further induce larger infarcts. These additionally provide evidence for an ADIOL/ERβ/CtBP-transcription pathway that regulates inflammatory responses in microglia and can be targeted by selective ERβ modulators (61). Moreover, estrogen can inhibit microglial activation and reduce the level of proinflammatory cytokines, thus mediating neuroprotective effects through both ERα and ERβ activation (62–64).
In animal models of traumatic brain injury, estrogen was found to inhibit TLR4 and NF-κB protein expression; reduce the expression of the proinflammatory factors IL-1β, IL-6, and TNF-α; and decrease the number of complement C3d/GFAP-positive cells and complement C3d protein expression (65). Similarly, in animal models of spinal cord injury, microglial and astrocyte activation were both significantly inhibited, along with attenuation of the secretion of inflammatory mediators (66). Specifically, the location of ERβ in the microglial cytoplasm suggests the involvement of the nonclassical effects of estrogen on microglia (67). To this extent, a study has demonstrated that in microglial cells, estrogen-mediated anti-inflammatory pathways are mediated by the activation of the mitogen-activated protein kinase (MAPK) signaling pathway (68, 69). Notably, ERT has been found to stimulate early postischemic expression of bcl-2 and bfl-1 and reduce brain injury (70). In addition, estrogen can significantly downregulate factors mediating adaptive immunity in microglial cells; this highlights the multi-faceted regulatory effects of estrogen on microglial parameters related to antigen presentation and T-cell interaction (71) (Table 1).
5.1.2 Estrogen and monocytes
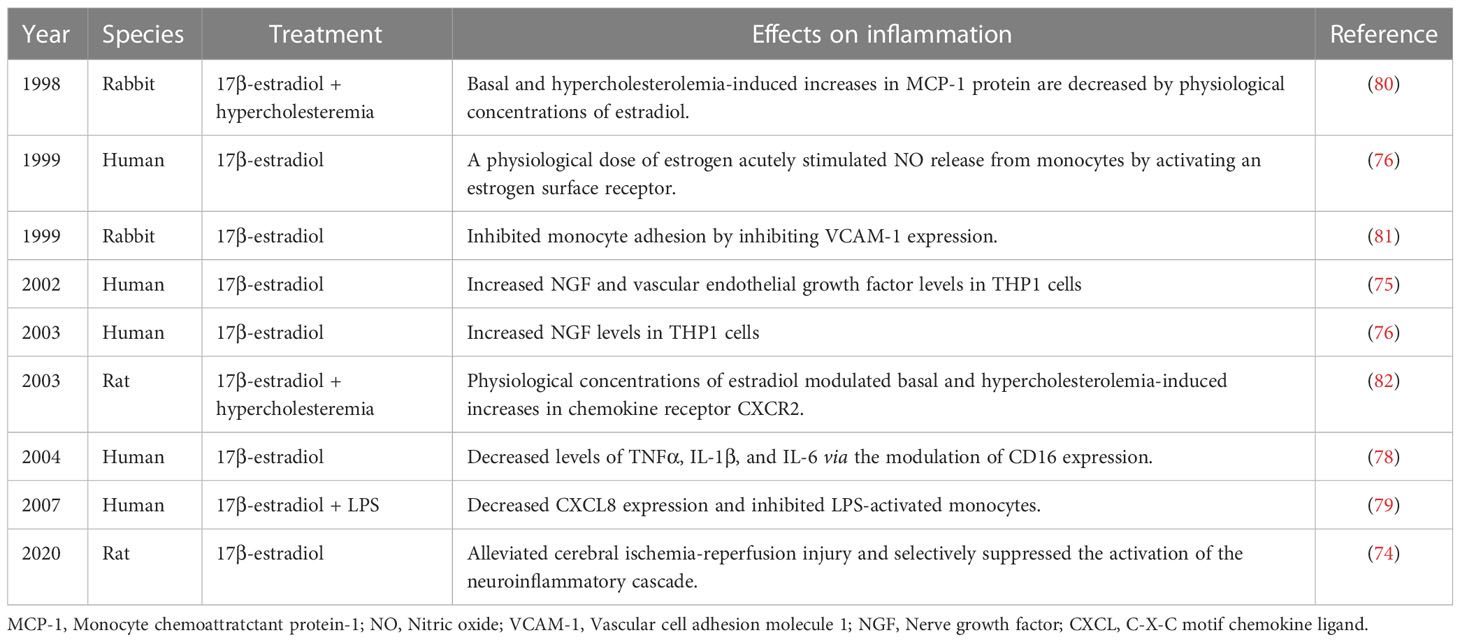
Monocytes and macrophages play an important role in the resolution of inflammation by scavenging apoptotic neutrophils. In response to inflammatory signals from injury sites, monocytes are rapidly mobilized. Although it has been reported that monocyte infiltration in the injured brain mediates both beneficial and detrimental effects on immune regulation after stroke, the number of monocytes is positively associated with the risk of post-stroke infection (72). Reportedly, estrogen affects the functioning of monocytes by significantly altering myelopoiesis and monocyte migration. Specifically, during ovulation and gestation in females, the count of circulating monocytes is much higher than during other stages of the reproductive cycle. Estrogen also affects monocyte adhesion (73) by reducing the migratory and adhesive capacity of monocytes. This way estrogen limits stroke-induced inflammatory reactions, further alleviating the cerebral ischemia-reperfusion injury and selectively suppressing the activation of the neuroinflammatory cascade (74). In addition, several studies have demonstrated the anti-inflammatory effects of estrogen on monocytes. It has been reported that 17β-estradiol can induce the formation of vascular endothelial growth factor, while dihydrotestosterone can antagonize the effect of 17β-estradiol by regulating adenylate cyclase in THP-1 cells, which is mediated by GPR130 (75, 76). Another study demonstrated that a physiological dose of estrogen acutely stimulates nitric oxide release from human monocytes by activating estrogen surface receptors that are coupled to increases in intracellular calcium (77). Moreover, estrogen was found to inhibit proinflammatory cytokine release from activated monocytes partly by modulating CD16 expression (78, 79). In addition, at physiological concentrations, estradiol mediates monocyte adhesion as well as basal and hypercholesterolemia-induced increases in CXCR2 and MCP-1 expression (80–82) (Table 2). These data highlight that estrogen plays a role in post-stroke brain injury by significantly affecting monocytes and their functioning.
5.1.3 Estrogen and macrophages
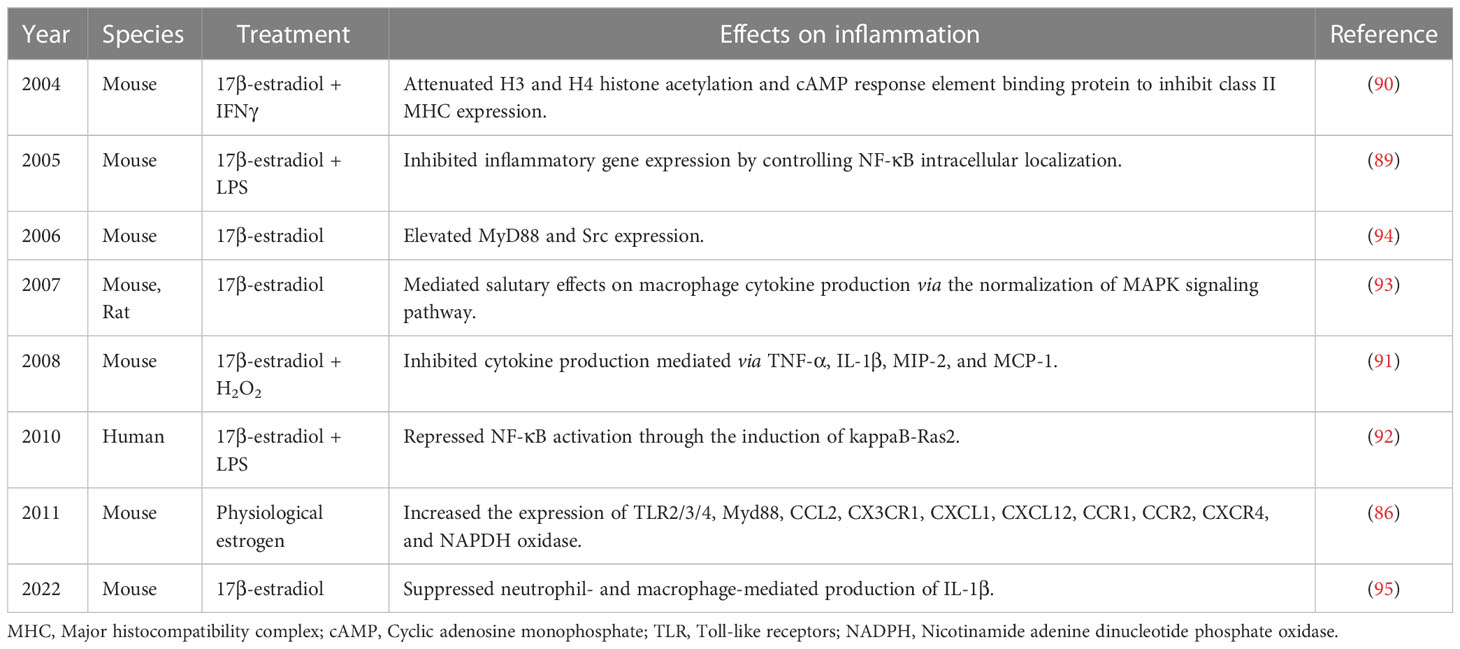
Hematopoietic stem cell-derived macrophages are another type of resident immune cells, apart from microglia, in CNS (83). These resident macrophages and microglia share overlapping features and have similar biomarkers, which complicate the assessment of their unique roles in estrogen-mediated neuroprotective effects. In addition, resident macrophages can continually renew from the bone marrow, unlike microglia (84, 85). So far, the potential role of resident macrophages is limited and based on the study of peripheral macrophages and cell lines. Therefore, accurate discrimination between the roles of microglia and macrophages after stroke requires further study. Reportedly, the proliferation and function of resident macrophages are associated with sex. A recent study focused on the changes in cell composition and immune function with sex and revealed higher resident leukocytes in the pleural and peritoneal cavities in females (86). In addition, resident macrophages had higher Toll-like receptor (TLR) expression and greater phagocytic and NADPH oxidase activities in females than in males (87). Reportedly, OVX can abrogate such effects caused by sex differences as well as regulate chemokine function, and macrophage count in females to the level observed in males. Interestingly, it has been revealed that OVX does not significantly alter T lymphocyte counts, suggesting no association of these lymphocytes with gonadal steroids (88). So far, although the role of sex differences in T lymphocyte populations has been explored in several autoimmune diseases, their effects in patients with stroke have not been fully investigated. Moreover, 17β-estradiol has been shown to inhibit the expression of inflammatory genes (TNF-α, IL-1β, MIP-2, and MCP-1) by controlling NF-κB or MAPK signaling pathway and attenuating H3 and H4 histone acetylation as well as cAMP response element binding protein-binding protein in macrophages (89–95) (Table 3). The above mentioned results of studies demonstrated the pivotal role of macrophages in inflammatory response post-ischemic stroke.
5.1.4 Estrogen and neutrophils
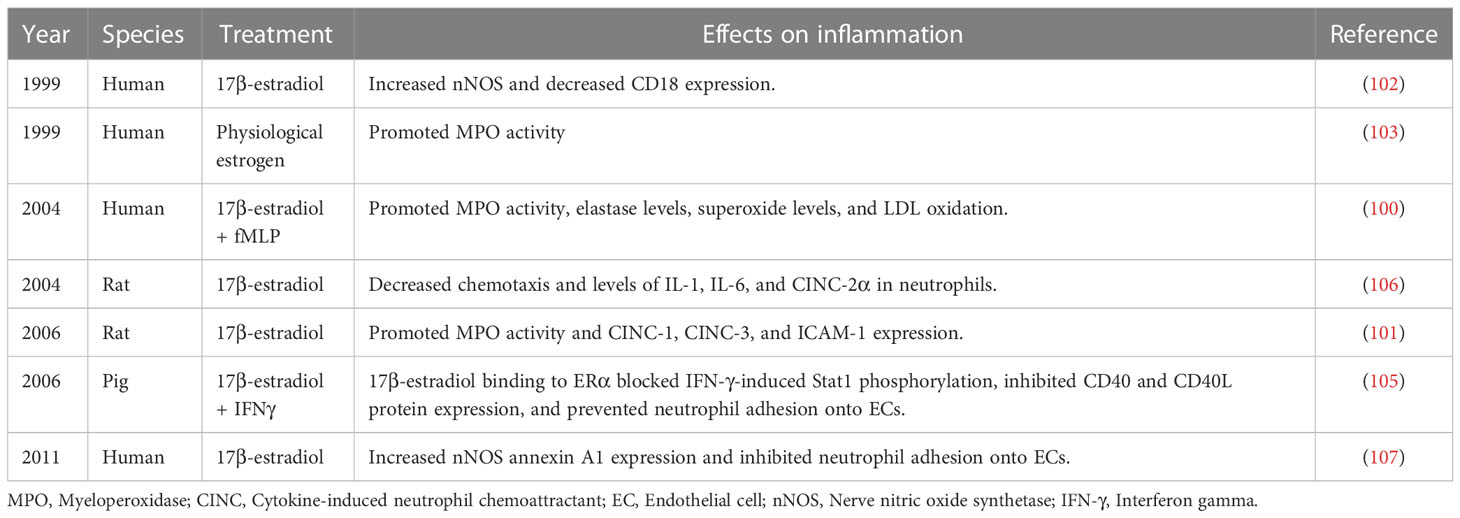
Stroke is associated with the disruption of blood–brain barrier, following which neutrophils immediately migrate into the injured sites (96). At the early stage, neutrophil infiltration can activate monocyte-derived macrophages to scavenge debris. Moreover, at sites of inflammation, neutrophils release pro-inflammatory cytokines that recruit more leukocytes. However, excessive neutrophil infiltration exacerbates brain injury (97). According to clinical studies, neutrophil accumulation is related to poor prognosis in patients with brain injuries (98). Several studies have revealed a positive correlation between estrogen levels and neutrophil counts (95, 99). To this extent, a study has reported that in females, myeloperoxidase activity in neutrophils is higher than that in males. Moreover, estrogen has been reported to promote the degranulation and release of myeloperoxidase, elastase, cytokine-induced neutrophil chemo-attractant-1 (CINC)-1, CINC-3, and intercellular adhesion molecule-1 as well as increase nNOS expression and superoxide levels in neutrophils (100–103). The dynamics of neutrophil infiltration after stroke into the brain parenchyma and subsequent neutrophil-mediated responses have yet to be completely determined (104). Available studies suggest that 17β-estradiol can inhibit the synthesis of neutrophil chemoattractants at ischemic sites and regulate excessive neutrophil infiltration. Moreover, 17β-estradiol can inhibit neutrophil adhesion to endothelial cells; mediate the clearance of neutrophils; and inhibit the expression of chemotaxis, IL-1, IL-6, and CINC-2α to neutrophils by binding to Erα. It can additionally inhibit IFN-γ-induced Stat1 phosphorylation as well as downregulate CD40 and CD40L protein expression (105–107) (Table 4).
5.1.5 Estrogen and dendritic cells
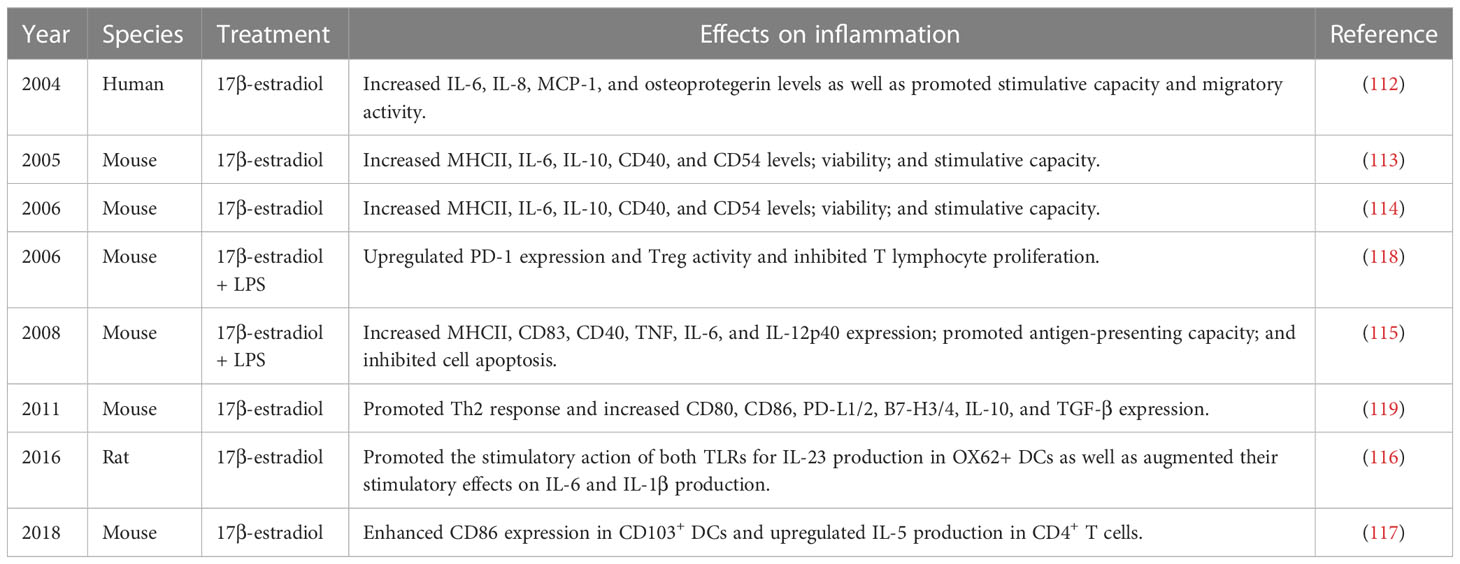
DCs are considered the only antigen-presenting cells that are involved in post-stroke injury and have a remarkable ability to activate memory and naïve T lymphocytes (108). DCs are a member of innate immunity and play a critical role in the phagocytosis and release of inflammatory factors. They additionally play a unique role in bridging innate and adaptive immunity (109), owing to which these cells have been correlated in a wide range of diseases, including atherosclerosis, various cancers, and more recently, stroke (110). Accumulative evidence has revealed increased recruitment of DCs and their potential role in brain injury following stroke onset. Moreover, an animal study has revealed the presence of DCs with high MHCII and CD80 expression at 72 hours after reperfusion and has demonstrated their association with lymphocyte migration in a time-dependent manner (111). In general, increased DC infiltration in the brain parenchyma after stroke may be positively correlated to the degree of injury. Estrogen administration in experimental autoimmune encephalomyelitis (EAE) mice has demonstrated significant improvement in their neurobehavior and outcomes as well as a marked reduction in the DC count. In addition, 17β-estradiol was found to upregulate the expression of MHCII, IL-10, CD40, CD83, CD54, IL-6, IL-8, MCP-1, and osteoprotegerin; upregulate stimulative capacity, migratory activity, and antigen-presenting capacity; and inhibit cell apoptosis (112–115). DCs are considered the link between innate and adaptive immunity. Moreover, DCs harbor antigens specific to T lymphocytes, which on activation initiate adaptive immune responses. Correspondingly, estrogen can induce DC differentiation and MHC expression, which facilitate T lymphocyte-mediated immune responses in the brain. Specifically, 17β-estradiol treatment is of great clinical value in regulating DCs. A study has reported that 17β-estradiol administration induces both the TLRs for IL-23 production in OX62+ DCs, thus stimulating IL-6 and IL-1β production (116). In addition, 17β-estradiol induces CD86 expression in CD103+ DCs after allergen-mediated upregulation of IL-5 in CD4+ T cells (117) (Table 5).
5.2 Estrogen and adaptive immunity
Evidence shows that 17β-estradiol can modulate the immune microenvironment and control the infiltration of T lymphocytes in the brain. The infiltration of cytotoxic T lymphocytes (CD8+) exacerbates brain injury, while T regulatory cells (Tregs) mediate anti-inflammatory effects and are responsible for reducing lesion volume and improving prognosis after stroke (120). 17β-estradiol administration can promote Treg responses through the inhibition of Th1- and Th17-derived cytokines or by directly promoting the proliferation of Tregs, upregulating PD-1 expression, and downregulating T lymphocyte proliferation (118, 121). Additionally, 17β-estradiol can promote Th2 response, thus upregulating the expression of CD80, CD86, PD-L1/2, B7-H3/4, IL-10, and TGF-β (119). Estrogen also plays a critical role in the regulation of B lymphocytes. Previous studies have shown that estradiol treatment protected purified B cells from apoptosis. Similarly, estradiol was shown to protect mice splenic B cells of from serum-deficiency-induced apoptosis; however, no effect was observed on the proliferation of B cells (122).
6 ERT: estrogen and immune regulation
It is well known that ERα is widely localized in the brain, including the forebrain, hypothalamus, and hippocampus (123). The binding of estrogen to ERα in the brain is responsible for several effects in both the physiological and pathological states. Reportedly, several genes are associated with neuronal survival and are regulated by an ERE-containing promoter. In particular, 17β-estradiol can promote the transcription of such genes and exert neuroprotective roles. This has been demonstrated in a study that found that 17β-estradiol could promote the transcription of a wide range of genes (124). After brain injury, 17β-estradiol administration can promote the upregulation of cell survival proteins, including phosphoinositide 3-kinase, cyclic-AMP response element-binding protein (CREB), Bcl-2, Bcl-x, c-fos, and c-jun. Moreover, 17β-estradiol inhibits the expression of apoptosis-related proteins, including Fas, FADD, and Bax, thus subsequently downregulating Cyto C release (125). Accumulative evidence has revealed the effects of downstream signaling of 17β-estradiol. Administration of 17β-estradiol can activate the MAPK pathway and promote the phosphorylation of CREB (126). In addition, it can inhibit the activation of caspase-3/8 and thus suppress ischemia-induced acetylation of p53 (127).
In the context of stroke, the neuroprotective effects of 17β-estradiol have been demonstrated in OVX animal models of ischemic stroke. OVX animals are the widely accepted model for post-menopausal women, as the removal of ovaries in female animals effectively mimics the diminished estrogen levels observed in post-menopausal women (128). Based on an extensive review of experimental studies, estrogen was found to reduce lesion volume after transient or permanent cerebral ischemic stroke in a dose-dependent manner. Therefore, pre-treatment with estrogen is considered as a prevention strategy for stroke onset, while post-treatment with estrogen is a potential therapeutic strategy (129).
Currently, the positive effects of estrogen in patients with stroke have been well investigated. However, according to the results of the Women’s Estrogen for Stroke Trial and the Women’s Health Initiative trial, estrogen may increase the incident risk of stroke, which limits the establishment of a therapeutic strategy for stroke based on estrogen (130). Of note, the therapeutic time window and dose are the critical factors in the treatment of patients with stroke. A previous clinical trial has reported that early administration of ERT was associated with a lower risk of stroke onset than later administration in the post-menopause phase (131). Moreover, lower doses of estrogen exert stronger neuroprotective effects against stroke.
Long-term administration of high-dose, micronized estradiol in healthy women remarkedly increases the level of C-reactive protein (CRP), a bio-marker used to assess vascular risk (132). Similarly, the levels of endogenous estradiol are negatively associated with CRP levels in young women, suggesting the anti-inflammatory effects of estrogen (133). It has been suggested that ERT should be started immediately at menopause to achieve maximal beneficial effect.
7 Potential therapeutic effects of estrogen in patients with stroke
According to previous studies, 17β-estradiol can effectively attenuate brain injuries, reduce infarct area, and promote recovery in animal stroke models. However, these positive effects have not been well supported by clinical data (134). Furthermore, a variety of clinical trials have reported that ERT does not induce protective effects against primary or secondary brain injuries and instead increases the risk of stroke onset (130, 135).
The difference in the effectiveness of estrogen observed in experimental and clinical studies may be attributable to differences in conditions being treated. Experimental studies assessed the treatment of ischemic cerebral injury, whereas the clinical trials focused on the prevention of stroke, which has not been thoroughly explored in experimental studies. In clinical trials, time, duration, and dosage are the important factors that mediate the negative effects of ERT (136). Based on clinical data, ERT can increase the risk of venous thrombosis, whereas a percutaneous approach to estrogen administration can reduce this risk (137, 138). Therefore, low dosage, short-term treatment, and a percutaneous approach will avoid increasing the risk of stroke onset and may formulate a safe alternative for the prevention and treatment of stroke. In a nested case-control study, short-term percutaneous treatment with estrogen was considered the safest choice against ischemic stroke (139). Compared with the oral approach, the percutaneous approach avoids the first-pass metabolism of estrogen in the liver. This prevents interaction with coagulation factors, inflammatory cytokines, and sex hormone binding protein, which reduces the risk of venous thromboembolism, which is often observed with oral estrogen. According to a clinical study, estrogen levels increase after stroke. Moreover, inflammatory control and the addition of exogenous hormones are likely to improve neural function in elderly male patients with stroke (140).
8 Conclusion
Generally, the host response to sterile inflammation is considered as a beneficial reaction; however, a hyper-immune response or altered signaling can lead to homeostatic imbalance and induce further chronic inflammation. Evidence has revealed that prolonged neuroinflammation is detrimental to clinical prognosis of stroke. Moreover, stroke-induced immunosuppression can increase susceptibility to infections, which complicates treatment. Because of the delayed diagnosis and subsequent delay in treatment, it is essential to establish new treatments with wider treatment windows. Neuro-inflammation is an attractive target for treatment of stroke owing to its wide therapeutic window. Therefore, several studies have attempted to identify novel neuroprotective strategies to target immune reactions in patients with stroke.
Previous experimental studies have demonstrated that estrogen is a potent immunomodulator and is considered a neuro-protective molecule in ischemic stroke (34, 35). Specifically, after stroke onset, the release of pro-inflammatory cytokines can aggravate brain damage. Although the use of estrogen or its analogs to regulate immune responses requires further exploration, ERT could be a potential treatment that targets immune responses in patients with stroke. Irrespective, experimental and clinical studies have not reached a consensus regarding the role of estrogen in alleviating post-stroke brain injury. This may be attributed to the conditions being targeted, as experimental research is mainly aimed at the treatment of acute ischemic stroke, while clinical trials are aimed at the prevention of primary or secondary stroke (141, 142). Furthermore, 17β-estradiol can regulate the immune system by inhibiting the release of pro-inflammatory cytokines and attenuating inflammatory reactions (143, 144). Despite the limited pre-clinical data regarding the effects of 17β-estradiol on immune regulation, the positive effects of 17β-estradiol on attenuating inflammatory reactions have been proven. Understanding the effects of 17β-estradiol provides the opportunity to explore novel therapeutic strategies while avoiding the controversial off-target effects of estrogen. Irrespective, it is necessary to establish more animal models to replicate the clinical conditions to provide a basis for future clinical trials.
Author contributions
All authors contributed to the general design of the study. LX conceptualized the review. XZ drafted the review. YL prepared the tables. YS, YL and LX revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ding Q, Liu S, Yao Y, Liu H, Cai T, Han L. Global, regional, and national burden of ischemic stroke, 1990-2019. Neurology (2022) 98(3):e279–e90. doi: 10.1212/wnl.0000000000013115
2. Jiang S, Li T, Ji T, Yi W, Yang Z, Wang S, et al. Ampk: potential therapeutic target for ischemic stroke. Theranostics (2018) 8(16):4535–51. doi: 10.7150/thno.25674
3. Wang YJ, Li ZX, Gu HQ, Zhai Y, Jiang Y, Zhao XQ, et al. China Stroke statistics 2019: a report from the national center for healthcare quality management in neurological diseases, China national clinical research center for neurological diseases, the Chinese stroke association, national center for chronic and non-communicable disease control and prevention, Chinese center for disease control and prevention and institute for global neuroscience and stroke collaborations. Stroke Vasc Neurol (2020) 5(3):211–39. doi: 10.1136/svn-2020-000457
4. Hankey GJ. Stroke. Lancet (London England) (2017) 389(10069):641–54. doi: 10.1016/s0140-6736(16)30962-x
5. Hurford R, Sekhar A, Hughes TAT, Muir KW. Diagnosis and management of acute ischaemic stroke. Pract Neurol (2020) 20(4):304–16. doi: 10.1136/practneurol-2020-002557
6. Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke (2009) 40(6):2244–50. doi: 10.1161/strokeaha.108.541128
7. Saver JL, Chaisinanunkul N, Campbell BCV, Grotta JC, Hill MD, Khatri P, et al. Standardized nomenclature for modified rankin scale global disability outcomes: consensus recommendations from stroke therapy academic industry roundtable xi. Stroke (2021) 52(9):3054–62. doi: 10.1161/strokeaha.121.034480
8. Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol (2008) 7(10):915–26. doi: 10.1016/s1474-4422(08)70193-5
9. Zhou H, Chen W, Pan Y, Suo Y, Meng X, Li H, et al. Effect of sex differences on prognosis of intravenous thrombolysis: data from the thrombolysis implementation and monitor of acute ischemic stroke in China (Tims-China). Stroke Vasc Neurol (2021) 6(1):10–5. doi: 10.1136/svn-2020-000351
10. Etherton MR, Wu O, Cougo P, Lorenzano S, Li H, Cloonan L, et al. Sex-specific differences in white matter microvascular integrity after ischaemic stroke. Stroke Vasc Neurol (2019) 4(4):198–205. doi: 10.1136/svn-2019-000268
11. Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the greater Cincinnati/Northern Kentucky stroke study. Stroke (2010) 41(7):1326–31. doi: 10.1161/strokeaha.109.575043
12. Xu J, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2021. NCHS Data Brief (2022) 456):1–8.
13. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics–2011 update: a report from the American heart association. Circulation (2011) 123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701
14. Zhang J, Li H, Xu Z, Lu J, Cao C, Shen H, et al. Oestrogen ameliorates blood-brain barrier damage after experimental subarachnoid haemorrhage via the shh pathway in Male rats. Stroke Vasc Neurol (2022). doi: 10.1136/svn-2022-001907
15. Rexrode KM, Madsen TE, Yu AYX, Carcel C, Lichtman JH, Miller EC. The impact of sex and gender on stroke. Circ Res (2022) 130(4):512–28. doi: 10.1161/circresaha.121.319915
16. Petrone AB, Simpkins JW, Barr TL. 17β-estradiol and inflammation: implications for ischemic stroke. Aging Dis (2014) 5(5):340–5. doi: 10.14336/ad.2014.0500340
17. Patkar S, Uwanogho D, Modo M, Tate RJ, Plevin R, Carswell HVO. Targeting 17β-estradiol biosynthesis in neural stem cells improves stroke outcome. Front Cell Neurosci (2022) 16:917181. doi: 10.3389/fncel.2022.917181
18. Turtzo LC, Siegel C, McCullough LD. X Chromosome dosage and the response to cerebral ischemia. J Neurosci (2011) 31(37):13255–9. doi: 10.1523/jneurosci.0621-11.2011
19. Cabrera Zapata LE, Garcia-Segura LM, Cambiasso MJ, Arevalo MA. Genetics and epigenetics of the X and y chromosomes in the sexual differentiation of the brain. Int J Mol Sci (2022) 23(20). doi: 10.3390/ijms232012288
20. Yang X, Rutkovsky AC, Zhou J, Zhong Y, Reese J, Schnell T, et al. Characterization of altered gene expression and histone methylation in peripheral blood mononuclear cells regulating inflammation in covid-19 patients. J Immunol (2022) 208(8):1968–77. doi: 10.4049/jimmunol.2101099
21. Park SJ, Wu CH, Gordon JD, Zhong X, Emami A, Safa AR. Taxol induces caspase-10-Dependent apoptosis. J Biol Chem (2004) 279(49):51057–67. doi: 10.1074/jbc.M406543200
22. Carrel L. Willard HF. X-Inactivation Profile Reveals Extensive Variability X-Linked Gene Expression Females. Nat (2005) 434(7031):400–4. doi: 10.1038/nature03479
23. Bourdeau V, Deschênes J, Métivier R, Nagai Y, Nguyen D, Bretschneider N, et al. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol (2004) 18(6):1411–27. doi: 10.1210/me.2003-0441
24. Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci (2004) 5(9):701–8. doi: 10.1038/nrn1494
25. Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Trans Med (2008) 6:33. doi: 10.1186/1479-5876-6-33
26. Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, et al. Apoptosis-inducing factor mediates Poly(Adp-ribose) (Par) polymer-induced cell death. Proc Natl Acad Sci USA (2006) 103(48):18314–9. doi: 10.1073/pnas.0606528103
27. Vagnerova K, Liu K, Ardeshiri A, Cheng J, Murphy SJ, Hurn PD, et al. Poly (Adp-ribose) polymerase-1 initiated neuronal cell death pathway–do androgens matter? Neuroscience (2010) 166(2):476–81. doi: 10.1016/j.neuroscience.2009.12.041
28. Kim TH, Vemuganti R. Effect of sex and age interactions on functional outcome after stroke. CNS Neurosci Ther (2015) 21(4):327–36. doi: 10.1111/cns.12346
29. Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, et al. Specific caspase inhibitor q-Vd-Oph prevents neonatal stroke in P7 rat: a role for gender. J Neurochem (2007) 100(4):1062–71. doi: 10.1111/j.1471-4159.2006.04269.x
30. Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke (2009) 40(5):1842–8. doi: 10.1161/strokeaha.108.538686
31. Hao D, Li J, Wang J, Meng Y, Zhao Z, Zhang C, et al. Non-classical estrogen signaling in ovarian cancer improves chemo-sensitivity and patients outcome. Theranostics (2019) 9(13):3952–65. doi: 10.7150/thno.30814
32. Shen G, Ren H, Shang Q, Zhang Z, Zhao W, Yu X, et al. Mir-128 plays a critical role in murine osteoclastogenesis and estrogen deficiency-induced bone loss. Theranostics (2020) 10(10):4334–48. doi: 10.7150/thno.42982
33. Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav (2014) 66(4):602–18. doi: 10.1016/j.yhbeh.2014.08.011
34. Tang X, Fang M, Cheng R, Zhang Z, Wang Y, Shen C, et al. Iron-deficiency and estrogen are associated with ischemic stroke by up-regulating transferrin to induce hypercoagulability. Circ Res (2020) 127(5):651–63. doi: 10.1161/circresaha.119.316453
35. Carwile E, Wagner AK, Crago E, Alexander SA. Estrogen and stroke: a review of the current literature. J Neurosci Nurs (2009) 41(1):18–25. doi: 10.1097/JNN.0b013e31819345f8
36. Yang M, Liu Q, Huang T, Tan W, Qu L, Chen T, et al. Dysfunction of estrogen-related receptor alpha-dependent hepatic vldl secretion contributes to sex disparity in Nafld/Nash development. Theranostics (2020) 10(24):10874–91. doi: 10.7150/thno.47037
37. Hong-Goka BC, Chang FL. Estrogen receptors alpha and beta in choroid plexus epithelial cells in alzheimer’s disease. Neurosci Lett (2004) 360(3):113–6. doi: 10.1016/j.neulet.2004.01.075
38. Wang Z, Xie R, Yang X, Yin H, Li X, Liu T, et al. Female mice lacking erβ display Excitatory/Inhibitory synaptic imbalance to drive the pathogenesis of temporal lobe epilepsy. Theranostics (2021) 11(12):6074–89. doi: 10.7150/thno.56331
39. Wang XW, Yuan LJ, Yang Y, Zhang M, Chen WF. Igf-1 inhibits Mptp/Mpp(+)-induced autophagy on dopaminergic neurons through the igf-1r/Pi3k-Akt-Mtor pathway and gper. Am J Physiol Endocrinol Metab (2020) 319(4):E734–e43. doi: 10.1152/ajpendo.00071.2020
40. Ma Y, Niu E, Xie F, Liu M, Sun M, Peng Y, et al. Electroacupuncture reactivates estrogen receptors to restore the neuroprotective effect of estrogen against cerebral ischemic stroke in long-term ovariectomized rats. Brain Behav (2021) 11(10):e2316. doi: 10.1002/brb3.2316
41. Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol (2019) 116:135–70. doi: 10.1016/bs.apcsb.2019.01.001
42. Khan MZI, Uzair M, Nazli A, Chen JZ. An overview on estrogen receptors signaling and its ligands in breast cancer. Eur J Med Chem (2022) 241:114658. doi: 10.1016/j.ejmech.2022.114658
43. Zong X, Li Y, Liu C, Qi W, Han D, Tucker L, et al. Theta-burst transcranial magnetic stimulation promotes stroke recovery by vascular protection and neovascularization. Theranostics (2020) 10(26):12090–110. doi: 10.7150/thno.51573
44. Hollist M, Morgan L, Cabatbat R, Au K, Kirmani MF, Kirmani BF. Acute stroke management: overview and recent updates. Aging Dis (2021) 12(4):1000–9. doi: 10.14336/ad.2021.0311
45. Maehara N, Taniguchi K, Okuno A, Ando H, Hirota A, Li Z, et al. Aim/Cd5l attenuates damps in the injured brain and thereby ameliorates ischemic stroke. Cell Rep (2021) 36(11):1096–93. doi: 10.1016/j.celrep.2021.109693
46. Liu DL, Hong Z, Li JY, Yang YX, Chen C, Du JR. Phthalide derivative Cd21 attenuates tissue plasminogen activator-induced hemorrhagic transformation in ischemic stroke by enhancing macrophage scavenger receptor 1-mediated damp (Peroxiredoxin 1) clearance. J Neuroinflamm (2021) 18(1):143. doi: 10.1186/s12974-021-02170-7
47. Zhu H, Hu S, Li Y, Sun Y, Xiong X, Hu X, et al. Interleukins and ischemic stroke. Front Immunol (2022) 13:828447. doi: 10.3389/fimmu.2022.828447
48. Li Y, Zhu ZY, Huang TT, Zhou YX, Wang X, Yang LQ, et al. The peripheral immune response after stroke-a double edge sword for blood-brain barrier integrity. CNS Neurosci Ther (2018) 24(12):1115–28. doi: 10.1111/cns.13081
49. Becker KJ. Strain-related differences in the immune response: relevance to human stroke. Transl Stroke Res (2016) 7(4):303–12. doi: 10.1007/s12975-016-0455-9
50. Shi K, Wood K, Shi FD, Wang X, Liu Q. Stroke-induced immunosuppression and poststroke infection. Stroke Vasc Neurol (2018) 3(1):34–41. doi: 10.1136/svn-2017-000123
51. Acosta-Martínez M. Shaping microglial phenotypes through estrogen receptors: relevance to sex-specific neuroinflammatory responses to brain injury and disease. J Pharmacol Exp Ther (2020) 375(1):223–36. doi: 10.1124/jpet.119.264598
52. Akabori H, Moeinpour F, Bland KI, Chaudry IH. Mechanism of the anti-inflammatory effect of 17beta-estradiol on brain following trauma-hemorrhage. Shock (Augusta Ga) (2010) 33(1):43–8. doi: 10.1097/SHK.0b013e3181b0ebcb
53. Cho YE, Lee H, Bae HR, Kim H, Yun S, Vorn R, et al. Circulating immune cell landscape in patients who had mild ischaemic stroke. Stroke Vasc Neurol (2022) 7(4):319–27. doi: 10.1136/svn-2021-001224
54. Ritzel RM, Capozzi LA, McCullough LD. Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke. Horm Behav (2013) 63(2):238–53. doi: 10.1016/j.yhbeh.2012.04.007
55. Zhang A, Liu Y, Xu H, Zhang Z, Wang X, Yuan L, et al. Ccl17 exerts neuroprotection through activation of Ccr4/Mtorc2 axis in microglia after subarachnoid haemorrhage in rats. Stroke Vasc Neurol (2022) 8(1):4–16. doi: 10.1136/svn-2022-001659
56. Zhang S. Microglial activation after ischaemic stroke. Stroke Vasc Neurol (2019) 4(2):71–4. doi: 10.1136/svn-2018-000196
57. Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, et al. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res (2002) 956(1):30–5. doi: 10.1016/s0006-8993(02)03475-3
58. Peng R, Dai W, Li D, Li Y. Gonadal hormone trigger the dynamic microglial alterations through Traf6/Tak1 axis that correlate with depressive behaviors. J Psychiatr Res (2022) 152:128–38. doi: 10.1016/j.jpsychires.2022.06.026
59. Gyenes A, Hoyk Z, Csakvari E, Siklos L, Parducz A. 17β-estradiol attenuates injury-induced microglia activation in the oculomotor nucleus. Neuroscience (2010) 171(3):677–82. doi: 10.1016/j.neuroscience.2010.09.033
60. Prat A, Behrendt M, Marcinkiewicz E, Boridy S, Sairam RM, Seidah NG, et al. A novel mouse model of alzheimer’s disease with chronic estrogen deficiency leads to glial cell activation and hypertrophy. J Aging Res (2011) 2011:251517. doi: 10.4061/2011/251517
61. Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An adiol-Erβ-Ctbp transrepression pathway negatively regulates microglia-mediated inflammation. Cell (2011) 145(4):584–95. doi: 10.1016/j.cell.2011.03.050
62. Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, et al. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci (2001) 21(6):1809–18. doi: 10.1523/jneurosci.21-06-01809.2001
63. Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology (2006) 147(5):2263–72. doi: 10.1210/en.2005-1330
64. Zhao TZ, Ding Q, Hu J, He SM, Shi F, Ma LT. Gper expressed on microglia mediates the anti-inflammatory effect of estradiol in ischemic stroke. Brain Behav (2016) 6(4):e00449. doi: 10.1002/brb3.449
65. Wang J, Hou Y, Zhang L, Liu M, Zhao J, Zhang Z, et al. Estrogen attenuates traumatic brain injury by inhibiting the activation of microglia and astrocyte-mediated neuroinflammatory responses. Mol Neurobiol (2021) 58(3):1052–61. doi: 10.1007/s12035-020-02171-2
66. Lee JY, Choi HY, Ju BG, Yune TY. Estrogen alleviates neuropathic pain induced after spinal cord injury by inhibiting microglia and astrocyte activation. Biochim Biophys Acta Mol Basis Dis (2018) 1864(7):2472–80. doi: 10.1016/j.bbadis.2018.04.006
67. Liu X, Fan XL, Zhao Y, Luo GR, Li XP, Li R, et al. Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-alpha and estrogen receptor-beta in microglia. J Neurosci Res (2005) 81(5):653–65. doi: 10.1002/jnr.20583
68. Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology (2000) 141(10):3646–56. doi: 10.1210/endo.141.10.7693
69. Bruce-Keller AJ, Barger SW, Moss NI, Pham JT, Keller JN, Nath A. Pro-inflammatory and pro-oxidant properties of the hiv protein tat in a microglial cell line: attenuation by 17 beta-estradiol. J Neurochem (2001) 78(6):1315–24. doi: 10.1046/j.1471-4159.2001.00511.x
70. Zhang L, Nair A, Krady K, Corpe C, Bonneau RH, Simpson IA, et al. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. J Clin Invest (2004) 113(1):85–95. doi: 10.1172/jci18336
71. Dimayuga FO, Reed JL, Carnero GA, Wang C, Dimayuga ER, Dimayuga VM, et al. Estrogen and brain inflammation: effects on microglial expression of mhc, costimulatory molecules and cytokines. J Neuroimmunol (2005) 161(1-2):123–36. doi: 10.1016/j.jneuroim.2004.12.016
72. Li S, Liu K, Zhang R, Gao Y, Fang H, Liu X, et al. Lower lymphocyte to monocyte ratio is a potential predictor of poor outcome in patients with cerebral venous sinus thrombosis. Stroke Vasc Neurol (2019) 4(3):148–53. doi: 10.1136/svn-2018-000180
73. Maoz H, Kaiser N, Halimi M, Barak V, Haimovitz A, Weinstein D, et al. The effect of estradiol on human myelomonocytic cells. 1. Enhancement Colony Formation. J Reprod Immunol (1985) 7(4):325–35. doi: 10.1016/0165-0378(85)90027-0
74. Scheld M, Heymann F, Zhao W, Tohidnezhad M, Clarner T, Beyer C, et al. Modulatory effect of 17β-estradiol on myeloid cell infiltration into the Male rat brain after ischemic stroke. J Steroid Biochem Mol Biol (2020) 202:105667. doi: 10.1016/j.jsbmb.2020.105667
75. Kanda N, Watanabe S. 17beta-estradiol enhances vascular endothelial growth factor production and dihydrotestosterone antagonizes the enhancement Via the regulation of adenylate cyclase in differentiated thp-1 cells. J Invest Dermatol (2002) 118(3):519–29. doi: 10.1046/j.0022-202x.2002.01672.x
76. Kanda N, Watanabe S. 17beta-estradiol enhances the production of nerve growth factor in thp-1-Derived macrophages or peripheral blood monocyte-derived macrophages. J Invest Dermatol (2003) 121(4):771–80. doi: 10.1046/j.1523-1747.2003.12487.x
77. Stefano GB, Prevot V, Beauvillain JC, Fimiani C, Welters I, Cadet P, et al. Estradiol coupling to human monocyte nitric oxide release is dependent on intracellular calcium transients: evidence for an estrogen surface receptor. J Immunol (1999) 163(7):3758–63.
78. Kramer PR, Kramer SF, Guan G. 17 beta-estradiol regulates cytokine release through modulation of Cd16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheum (2004) 50(6):1967–75. doi: 10.1002/art.20309
79. Pioli PA, Jensen AL, Weaver LK, Amiel E, Shen Z, Shen L, et al. Estradiol attenuates lipopolysaccharide-induced cxc chemokine ligand 8 production by human peripheral blood monocytes. J Immunol (2007) 179(9):6284–90. doi: 10.4049/jimmunol.179.9.6284
80. Pervin S, Singh R, Rosenfeld ME, Navab M, Chaudhuri G, Nathan L. Estradiol suppresses mcp-1 expression in vivo: implications for atherosclerosis. Arterioscler Thromb Vasc Biol (1998) 18(10):1575–82. doi: 10.1161/01.atv.18.10.1575
81. Nathan L, Pervin S, Singh R, Rosenfeld M, Chaudhuri G. Estradiol inhibits leukocyte adhesion and transendothelial migration in rabbits in vivo: possible mechanisms for gender differences in atherosclerosis. Circ Res (1999) 85(4):377–85. doi: 10.1161/01.res.85.4.377
82. Lei ZB, Fu XJ, Lu ZT, Wang BC, Liu XL, You NZ. Effect of estradiol on chemokine receptor Cxcr2 expression in rats: implications for atherosclerosis. Acta Pharmacol Sin (2003) 24(7):670–4.
83. Zhang A, Xu Y, Xu H, Ren J, Meng T, Ni Y, et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting Ccl17. Theranostics (2021) 11(8):3839–52. doi: 10.7150/thno.53749
84. Amo-Aparicio J, Garcia-Garcia J, Francos-Quijorna I, Urpi A, Esteve-Codina A, Gut M, et al. Interleukin-4 and interleukin-13 induce different metabolic profiles in microglia and macrophages that relate with divergent outcomes after spinal cord injury. Theranostics (2021) 11(20):9805–20. doi: 10.7150/thno.65203
85. Kerkhofs D, van Hagen BT, Milanova IV, Schell KJ, van Essen H, Wijnands E, et al. Pharmacological depletion of microglia and perivascular macrophages prevents vascular cognitive impairment in ang ii-induced hypertension. Theranostics (2020) 10(21):9512–27. doi: 10.7150/thno.44394
86. Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood (2011) 118(22):5918–27. doi: 10.1182/blood-2011-03-340281
87. Di Florio DN, Sin J, Coronado MJ, Atwal PS, Fairweather D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol (2020) 31:101482. doi: 10.1016/j.redox.2020.101482
88. Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Trans Med (2009) 7:97. doi: 10.1186/1479-5876-7-97
89. Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling nf-kappab intracellular localization. Mol Cell Biol (2005) 25(8):2957–68. doi: 10.1128/mcb.25.8.2957-2968.2005
90. Adamski J, Ma Z, Nozell S, Benveniste EN. 17beta-estradiol inhibits class ii major histocompatibility complex (Mhc) expression: influence on histone modifications and cbp recruitment to the class ii mhc promoter. Mol Endocrinol (2004) 18(8):1963–74. doi: 10.1210/me.2004-0098
91. Huang H, He J, Yuan Y, Aoyagi E, Takenaka H, Itagaki T, et al. Opposing effects of estradiol and progesterone on the oxidative stress-induced production of chemokine and proinflammatory cytokines in murine peritoneal macrophages. J Med Invest (2008) 55(1-2):133–41. doi: 10.2152/jmi.55.133
92. Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses nf-kappa b activation through coordinated regulation of let-7a and mir-125b in primary human macrophages. J Immunol (2010) 184(9):5029–37. doi: 10.4049/jimmunol.0903463
93. Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, et al. Estrogen receptor-alpha predominantly mediates the salutary effects of 17beta-estradiol on splenic macrophages following trauma-hemorrhage. Am J Physiol Cell Physiol (2007) 293(3):C978–84. doi: 10.1152/ajpcell.00092.2007
94. Zheng R, Pan G, Thobe BM, Choudhry MA, Matsutani T, Samy TS, et al. Myd88 and src are differentially regulated in kupffer cells of males and proestrus females following hypoxia. Mol Med (2006) 12(4-6):65–73. doi: 10.2119/2006-00030.Zheng
95. Adachi A, Honda T, Egawa G, Kanameishi S, Takimoto R, Miyake T, et al. Estradiol suppresses psoriatic inflammation in mice by regulating neutrophil and macrophage functions. J Allergy Clin Immunol (2022) 150(4):909–19.e8. doi: 10.1016/j.jaci.2022.03.028
96. Zhang C, Ling CL, Pang L, Wang Q, Liu JX, Wang BS, et al. Direct macromolecular drug delivery to cerebral ischemia area using neutrophil-mediated nanoparticles. Theranostics (2017) 7(13):3260–75. doi: 10.7150/thno.19979
97. Li G, Ma Q, Wang R, Fan Z, Tao Z, Liu P, et al. Diagnostic and immunosuppressive potential of elevated mir-424 levels in circulating immune cells of ischemic stroke patients. Aging Dis (2018) 9(2):172–81. doi: 10.14336/ad.2017.0602
98. Nguyen VA, Crewther SG, Howells DW, Wijeratne T, Ma H, Hankey GJ, et al. Acute routine leukocyte and neutrophil counts are predictive of poststroke recovery at 3 and 12 months poststroke: an exploratory study. Neurorehabil Neural Repair (2020) 34(9):844–55. doi: 10.1177/1545968320948607
99. Lim CL, Or YZ, Ong Z, Chung HH, Hayashi H, Shrestha S, et al. Estrogen exacerbates mammary involution through neutrophil-dependent and -independent mechanism. Elife (2020) 9. doi: 10.7554/eLife.57274
100. Chiang K, Parthasarathy S, Santanam N. Estrogen, neutrophils and oxidation. Life Sci (2004) 75(20):2425–38. doi: 10.1016/j.lfs.2004.04.035
101. Yu HP, Shimizu T, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, et al. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. J Leukoc Biol (2006) 79(5):963–70. doi: 10.1189/jlb.1005596
102. Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience (1999) 89(2):567–78. doi: 10.1016/s0306-4522(98)00340-6
103. Kabutomori O, Yanagihara T, Iwatani Y, Kawarazaki A, Kabutomori M. Sex difference in myeloperoxidase activity of neutrophils. Am J Hematol (1999) 60(4):312–3. doi: 10.1002/(sici)1096-8652(199904)60:4<312::aid-ajh13>3.0.co;2-k
104. Tran TK, MacFarlane GR, Kong RY, O’Connor WA, Yu RM. Potential mechanisms underlying estrogen-induced expression of the molluscan estrogen receptor (Er) gene. Aquat Toxicol (2016) 179:82–94. doi: 10.1016/j.aquatox.2016.08.015
105. Geraldes P, Gagnon S, Hadjadj S, Merhi Y, Sirois MG, Cloutier I, et al. Estradiol blocks the induction of Cd40 and Cd40l expression on endothelial cells and prevents neutrophil adhesion: an eralpha-mediated pathway. Cardiovasc Res (2006) 71(3):566–73. doi: 10.1016/j.cardiores.2006.05.015
106. Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, et al. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation (2004) 110(12):1664–9. doi: 10.1161/01.Cir.0000142050.19488.C7
107. Nadkarni S, Cooper D, Brancaleone V, Bena S, Perretti M. Activation of the annexin A1 pathway underlies the protective effects exerted by estrogen in polymorphonuclear leukocytes. Arterioscler Thromb Vasc Biol (2011) 31(11):2749–59. doi: 10.1161/atvbaha.111.235176
108. Yao RQ, Li ZX, Wang LX, Li YX, Zheng LY, Dong N, et al. Single-cell transcriptome profiling of the immune space-time landscape reveals dendritic cell regulatory program in polymicrobial sepsis. Theranostics (2022) 12(10):4606–28. doi: 10.7150/thno.72760
109. Hoffman RA, Huang S, Chalasani G, Vallejo AN. Disparate recruitment and retention of plasmacytoid dendritic cells to the small intestinal mucosa between young and aged mice. Aging Dis (2021) 12(5):1183–96. doi: 10.14336/ad.2021.0119
110. Lee HT, Liu SP, Lin CH, Lee SW, Hsu CY, Sytwu HK, et al. A crucial role of Cxcl14 for promoting regulatory T cells activation in stroke. Theranostics (2017) 7(4):855–75. doi: 10.7150/thno.17558
111. Newman TA, Galea I, van Rooijen N, Perry VH. Blood-derived dendritic cells in an acute brain injury. J Neuroimmunol (2005) 166(1-2):167–72. doi: 10.1016/j.jneuroim.2005.04.026
112. Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood (2004) 104(5):1404–10. doi: 10.1182/blood-2003-10-3380
113. Yang L, Liang J, Yao G, Chen P, Hou Y. 17beta-estradiol regulates the numbers, endocytosis, stimulative capacity and il-10 secretion of mouse spleen dendritic cells. Toxicol Lett (2005) 155(2):239–46. doi: 10.1016/j.toxlet.2004.09.016
114. Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res (2006) 16(8):995–1004. doi: 10.1101/gr.5217506
115. Kawasaki T, Choudhry MA, Suzuki T, Schwacha MG, Bland KI, Chaudry IH. 17beta-estradiol’s salutary effects on splenic dendritic cell functions following trauma-hemorrhage are mediated Via estrogen receptor-alpha. Mol Immunol (2008) 45(2):376–85. doi: 10.1016/j.molimm.2007.06.148
116. Stojić-Vukanić Z, Bufan B, Pilipović I, Vujnović I, Nacka-Aleksić M, Petrović R, et al. Estradiol enhances capacity of tlr-matured splenic dendritic cells to polarize Cd4+ lymphocytes into il-17/Gm-Csf-Producing cells in vitro. Int Immunopharmacol (2016) 40:244–53. doi: 10.1016/j.intimp.2016.09.001
117. Masuda C, Miyasaka T, Kawakami K, Inokuchi J, Kawano T, Dobashi-Okuyama K, et al. Sex-based differences in Cd103(+) dendritic cells promote female-predominant Th2 cytokine production during allergic asthma. Clin Exp Allergy (2018) 48(4):379–93. doi: 10.1111/cea.13081
118. Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of treg cells, and enhanced expression of the pd-1 costimulatory pathway. J Neurosci Res (2006) 84(2):370–8. doi: 10.1002/jnr.20881
119. Papenfuss TL, Powell ND, McClain MA, Bedarf A, Singh A, Gienapp IE, et al. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J Immunol (2011) 186(6):3346–55. doi: 10.4049/jimmunol.1001322
120. Nadkarni S, McArthur S. Oestrogen and immunomodulation: new mechanisms that impact on peripheral and central immunity. Curr Opin Pharmacol (2013) 13(4):576–81. doi: 10.1016/j.coph.2013.05.007
121. Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory mechanisms in ischemic stroke: focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci (2020) 21(18). doi: 10.3390/ijms21186454
122. Fu Y, Li L, Liu X, Ma C, Zhang J, Jiao Y, et al. Estrogen promotes b cell activation in vitro through down-regulating Cd80 molecule expression. Gynecol Endocrinol (2011) 27(8):593–6. doi: 10.3109/09513590.2010.507281
123. Saito K, Cui H. Emerging roles of estrogen-related receptors in the brain: potential interactions with estrogen signaling. Int J Mol Sci (2018) 19(4). doi: 10.3390/ijms19041091
124. Lu Y, Sareddy GR, Wang J, Wang R, Li Y, Dong Y, et al. Neuron-derived estrogen regulates synaptic plasticity and memory. J Neurosci (2019) 39(15):2792–809. doi: 10.1523/jneurosci.1970-18.2019
125. Wang J, Sareddy GR, Lu Y, Pratap UP, Tang F, Greene KM, et al. Astrocyte-derived estrogen regulates reactive astrogliosis and is neuroprotective following ischemic brain injury. J Neurosci (2020) 40(50):9751–71. doi: 10.1523/jneurosci.0888-20.2020
126. Jiang H, Xiao L, Jin K, Shao B. Estrogen administration attenuates post-stroke depression by enhancing Creb/Bdnf/Trkb signaling in the rat hippocampus. Exp Ther Med (2021) 21(5):433. doi: 10.3892/etm.2021.9850
127. Saeed K, Jo MH, Park JS, Alam SI, Khan I, Ahmad R, et al. 17β-estradiol abrogates oxidative stress and neuroinflammation after cortical stab wound injury. Antioxidants (Basel) (2021) 10(11). doi: 10.3390/antiox10111682
128. Quispe Calla NE, Vicetti Miguel RD, Aceves KM, Huang H, Howitt B, Cherpes TL. Ovariectomized mice and postmenopausal women exhibit analogous loss of genital epithelial integrity. Tissue Barriers (2021) 9(2):1865760. doi: 10.1080/21688370.2020.1865760
129. Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Transl Stroke Res (2013) 4(4):390–401. doi: 10.1007/s12975-012-0230-5
130. Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the women’s health initiative: a randomized trial. Jama (2003) 289(20):2673–84. doi: 10.1001/jama.289.20.2673
131. Lisabeth L, Bushnell C. Stroke risk in women: the role of menopause and hormone therapy. Lancet Neurol (2012) 11(1):82–91. doi: 10.1016/s1474-4422(11)70269-1
132. Prestwood KM, Unson C, Kulldorff M, Cushman M. The effect of different doses of micronized 17beta-estradiol on c-reactive protein, interleukin-6, and lipids in older women. J Gerontol A Biol Sci Med Sci (2004) 59(8):827–32. doi: 10.1093/gerona/59.8.m827
133. Gaskins AJ, Wilchesky M, Mumford SL, Whitcomb BW, Browne RW, Wactawski-Wende J, et al. Endogenous reproductive hormones and c-reactive protein across the menstrual cycle: the biocycle study. Am J Epidemiol (2012) 175(5):423–31. doi: 10.1093/aje/kwr343
134. Leon RL, Li X, Huber JD, Rosen CL. Worsened outcome from middle cerebral artery occlusion in aged rats receiving 17β-estradiol. Endocrinology (2012) 153(7):3386–93. doi: 10.1210/en.2011-1859
135. Tannen RL, Weiner MG, Xie D, Barnhart K. A simulation using data from a primary care practice database closely replicated the women’s health initiative trial. J Clin Epidemiol (2007) 60(7):686–95. doi: 10.1016/j.jclinepi.2006.10.012
136. Shoupe D. Individualizing hormone therapy to minimize risk: accurate assessment of risks and benefits. Womens Health (Lond) (2011) 7(4):475–85. doi: 10.2217/whe.11.42
137. Olié V, Canonico M, Scarabin PY. Risk of venous thrombosis with oral versus transdermal estrogen therapy among postmenopausal women. Curr Opin Hematol (2010) 17(5):457–63. doi: 10.1097/MOH.0b013e32833c07bc
138. Speroff L. Transdermal hormone therapy and the risk of stroke and venous thrombosis. Climacteric (2010) 13(5):429–32. doi: 10.3109/13697137.2010.507111
139. Canonico M, Carcaillon L, Plu-Bureau G, Oger E, Singh-Manoux A, Tubert-Bitter P, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke (2016) 47(7):1734–41. doi: 10.1161/strokeaha.116.013052
140. Goodman MP. Are all estrogens created equal? a review of oral vs. transdermal therapy. J Womens Health (Larchmt) (2012) 21(2):161–9. doi: 10.1089/jwh.2011.2839
141. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. Jama (2002) 288(3):321–33. doi: 10.1001/jama.288.3.321
142. Kim T, Chelluboina B, Chokkalla AK, Vemuganti R. Age and sex differences in the pathophysiology of acute cns injury. Neurochem Int (2019) 127:22–8. doi: 10.1016/j.neuint.2019.01.012
143. Shen HH, Zhang T, Yang HL, Lai ZZ, Zhou WJ, Mei J, et al. Ovarian hormones-Autophagy-Immunity axis in menstruation and endometriosis. Theranostics (2021) 11(7):3512–26. doi: 10.7150/thno.55241
Keywords: ischemic stroke, estrogen, 17β-estradiol, neuroinflammation, neuroprotection, immunomodulation, therapy
Citation: Zhong X, Sun Y, Lu Y and Xu L (2023) Immunomodulatory role of estrogen in ischemic stroke: neuroinflammation and effect of sex. Front. Immunol. 14:1164258. doi: 10.3389/fimmu.2023.1164258
Received: 12 February 2023; Accepted: 12 April 2023;
Published: 25 April 2023.
Edited by:
Xiangsheng Zhang, Capital Medical University, ChinaReviewed by:
Yingfeng Wan, University of Michigan, United StatesAnke Zhang, Shanghai Jiao Tong University, China
Xuemei Liu, Dongfang Hospital, China
Copyright © 2023 Zhong, Sun, Lu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Xu, eHVsZWkyMDIwMTJAMTYzLmNvbQ==
 Xiaojun Zhong1
Xiaojun Zhong1 Lei Xu
Lei Xu