- 1Department of Experimental Neuroendocrinology, Maj Institute of Pharmacology Polish Academy of Sciences, Krakow, Poland
- 2Department of Cell Biology and Histology, School of Medicine and Nursing, Tumor Microenvironment Group, Basque Country University, Leioa, Spain
- 3Department of Microbiology, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, Krakow, Poland
- 4Malopolska Centre of Biotechnology, Jagiellonian University, Krakow, Poland
- 5Department of Psychiatry, Faculty of Medicine, King Chulalongkorn Memorial Hospital, Bangkok, Thailand
- 6Department of Psychiatry, Medical University of Plovdiv, Plovdiv, Bulgaria
- 7IMPACT Strategic Research Centre, Deakin University, Geelong, VIC, Australia
- 8Kyung Hee University, Seoul, Republic of Korea
Due to the high prevalence of depression among cancer patients, antidepressant medications are frequently administered as adjuvant treatment. However, the safety of such medications in the development of metastasis is unclear. In this study, we investigated the effects of fluoxetine, desipramine, and mirtazapine on the liver metastasis of murine C26 colon carcinoma (cc). Balb/c male mice were administered these antidepressants intraperitoneally (i.p.) for 14 days following intrasplenic injections of C26 colon carcinoma cells. Desipramine and fluoxetine, but not mirtazapine, significantly increased the number of tumor foci and total volume of the tumor in liver tissue. This effect was associated with a decrease in the ability of splenocytes to produce interleukin (IL)-1β and interferon (IFN)-γ and an increase in their ability to produce interleukin (IL)-10. Similar changes were observed in plasma IL-1β, IFN-γ, and IL-10 levels. The current study demonstrates that the stimulatory effect of desipramine and fluoxetine, but not mirtazapine, on experimental colon cancer liver metastasis is associated with a suppression of immune defenses against the tumor.
1 Introduction
Effective drug therapy for depression is important for patients with cancer, considering that the quality of life is often destroyed because of the stress connected with the fear of illness progression, loss of independence and dignity, the side effects of surgery, radio- and/or chemotherapy, and fear of death. The recently published study shows that antidepressants such as selective serotonin reuptake inhibitors (SSRIs) and mirtazapine, were effective and well tolerated in children and adolescents with cancer and psychiatric comorbidities (1).
Some reports have suggested that antidepressants may be linked with the formation and growth of tumors, raising concern about prescribing such medication on a regular basis (2–5). On the contrary, some preclinical studies showed no carcinogenesis in mice receiving a human-equivalent dose of antidepressant drugs for 24 months (4), as well as some clinical systematic rereviews and dose-response meta-analyses found that antidepressant use did not increase the incidence risk of cancers and even decreased the incidence risk of breast cancer, along with a non-linear or linear dose-response relationship (6). Therefore, the effects of these antidepressant drugs on tumors remain unclear.
There is also evidence that antidepressants have negative immunoregulatory and anti-inflammatory effects (7–10). Thus, in human depression and animal models of depression antidepressants, such as tricyclics and SSRIs, suppress the production of interferon (IFN)-γ and interleukin (IL)-1β and increase the production of IL-10, a negative immunoregulatory cytokine. These immunoregulatory effects may play a role in tumor progression or metastasis processes.
Fluoxetine hydrochloride, an SSRI, blocks the reuptake of serotonin into the presynaptic nerve terminals, resulting in enhanced synaptic serotonin levels. It is one of the most commonly used antidepressants, due to its efficacy, safety, and tolerability. The SSRIs citalopram, escitalopram, sertraline, and fluoxetine are generally the first-line choice for the treatment of depression, bulimia nervosa, and obsessive-compulsive disorder. Desipramine displays an antidepressant property similar to that of other tricyclic antidepressants (TCAs). It is the active “in vivo” metabolite of imipramine and as such, shares many of imipramine’s pharmacologic effects. It is not considered a first-line treatment since the introduction of SSRIs. Desipramine inhibits the reuptake of noradrenaline much stronger than serotonin. Mirtazapine is an effective new-generation antidepressant, which enhances noradrenergic neurotransmission via an antagonistic action at α2-adrenergic auto- and heteroreceptors. Mirtazapine has been suggested as a first choice during cancer immunotherapy because of its potent anti-nausea effects, which stimulate appetite in cancer patients (11, 12).
The aim of this work was to evaluate the effect of chronic treatment with three antidepressants representing various pharmacological groups: desipramine, fluoxetine, and mirtazapine on the metastatic potential of murine C26 colon carcinoma cells to the liver. Colorectal cancer is one of the most aggressive cancers in Western countries. Patients suffering from this cancer die from metastases, mainly to the liver, rather than from the primary tumor. Hence, we examined the effect of repeated administration of antidepressants on the development of metastatic foci in the liver. Furthermore, to better understand the association between antidepressant effects on tumor pathology and immune system activity, the T-cell proliferative response to concanavalin A (Con A), and the production of anti- and pro-tumoral cytokines: IL-1β, IL-10, and IFN-γ were also studied.
2 Materials and methods
2.1 Animals
The experiment was performed in the Basque Country University School of Medicine & Nursing on 75 animals (6–8 weeks old male Balb/c mice) obtained from Charles River, Barcelona, Spain. Animals were housed in up to five per cage, at 22°C and 40% humidity under a 12-hour light-dark cycle, with free access to water and standard food. All the proceedings were approved by the Basque Country University Ethical Committee (CEID) and by institutional, national, and international guidelines regarding the protection and care of animals used for scientific purposes.
2.2 Cell culture
Murine C26 colon carcinoma cell line is syngeneic to Balb/c mice (13). C26 cc cell line was obtained from ATCC (Manassas, VA, USA) and was cultured in culture flasks filled with RPMI-1640 supplemented with 10% Fetal Calf Serum, penicillin, and streptomycin. The cell cultures were maintained at 37°C in 5% CO2. Cells in the exponential growth phase were harvested at 80% confluence, the cell suspension was centrifuged, washed once with phosphate-buffered saline (PBS), and resuspended in PBS at a concentration of 2 × 106 cells per mL. Cell viability was determined by trypan blue exclusion. Only single-cell suspensions with 95% viability were used for inoculation.
2.3 In vivo experimental development of hepatic metastases
Animals were divided into five experimental groups consisting in five experimental units each. The experiment was repeated three times given a total of N=75, n=15, and of N=25, n=5 per repetition. To induce hepatic metastases in vivo, C26 cc cells were suspended at a concentration of 1.5 × 105 in 0.1 ml of Hanks’ Balanced salt solution (HBBS) and were intrasplenically (i.s.) injected into anesthetized mice (Nembutal, 50 mg/kg) as previously described (14). Mice were anesthetized for portal blood collection, and then sacrificed by cervical dislocation on the 15th day after the injection of cancer cells and livers were removed. Livers were fixed by immersion in Zinc solution for 12 hours at room temperature, and then, paraffin-embedded. A minimum of six 5-μm thick tissue sections of the liver (three groups, separated 1 mm) were stained with hematoxylin and eosin (H-E). An integrated image analysis system (Olympus Microimage 4.0 capture kit) connected to an Olympus BX51TF microscope was used to quantify the number, average diameter, and position coordinates of metastases. The percentage of the liver volume occupied by metastatic tissue and metastases density (foci number/100 mm3) were also determined (15). In the case of outliers, animals dying before the established endpoint, with ascites and/or inflammation, and/or little or no sign of growth in the spleen and/or liver will not be considered for metastatic quantification.
2.4 Antidepressant drugs treatment
Balb/c mice were subdivided into 5 groups of 5 animals each and received daily intraperitoneal (i.p.) injections of either desipramine (10 mg/kg, Research Biochemicals International, USA), fluoxetine (10 mg/kg, The Lilly Laboratories, USA), or mirtazapine (20 mg/kg, Organon, The Netherlands) for 14 days, respectively (16, 17). Desipramine and fluoxetine were dissolved in 0.9% saline, whereas mirtazapine was suspended in a 1% aqueous solution of Tween 80. The Control group for desipramine and fluoxetine groups received i.p. injection of saline (NaCl group), whereas the control group for mirtazapine studies received 1% aqueous solution of Tween 80 (Tween group). The first drug injection was given 2 h after injection of tumor cells, and the last drug injection 24 hours before sacrificing the animals. The experiments were repeated three times.
2.5 Proliferative response of splenocytes to mitogen stimulation in vitro
Spleens from tumor-bearing mice were aseptically removed, minced in PBS-EDTA (1% w/v) using a pair of scissors, and passed through fine steel (70 μm diameter) mesh to obtain a homogeneous cell suspension. After centrifugation (380 × g at 4°C for 10 min), the pelleted cells were washed three times in PBS and resuspended in RPMI 1640 complete medium. Splenocytes were cultured for 72 h at a concentration of 4 × 106 cells/ml in the presence or absence of 2.5 μg/ml Con A. Proliferation was quantified utilizing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) assay and the absorbance was evaluated after 4 hours in an ELISA reader (Bio-Rad, USA) at 570 nm (18).
2.6 Determination of cytokines
Previously collected blood from tumor-bearing mice was centrifuged and serum was collected and stored at -80°C for analysis of cytokines later. Splenocyte suspensions were seeded at a concentration of 4 × 106 cells/ml in 24-well Corning tissue culture plates and were then stimulated with Con A solution (2.5 µg/ml) and LPS solution (5 µg/ml) simultaneously or remained unstimulated. Cell-free supernatants were collected 48 h later and stored at -20°C until use. IL-1β, IL-10, and IFN-γ levels were estimated in serum and supernatants. All the enzyme-linked immunosorbent assays (ELISA) were based on monoclonal-monoclonal antibody pairs and were performed using DuoSet ELISA Development Kits (R&D Systems, Abingdon, UK) following manufacturer instructions.
2.7 Statistical analysis
For statistical analysis of the results, Statistica 9.0 software (Statsoft, Tulsa, USA), run on a PC-compatible computer, was used. Intraexperimental statistical significance was evaluated by a two-tailed unpaired t-Test and the differences between groups were considered statistically significant when p<0.05. Then, differences between independent experiments were evaluated using a one-way analysis of variance (ANOVA) followed by Dunnett's test. The normality of variable distributions and homogeneity of variances were verified by the Shapiro-Wilk and Levene’s tests, respectively. The values that exceeded 2-fold the standard deviation from the average were discarded from the analyses. The p-values lower than 0.05 were regarded as statistically significant.
3 Results
3.1 The effect of antidepressant drugs on C26 colon carcinoma liver metastasis development
In order to analyze the effect of antidepressant drugs on the metastatic potential of C26 cc cells to the liver, tumor cells were injected i.s. as described in the “Materials and methods” section. The animals were treated with a daily dose of either antidepressant or vehicle until the day before sacrifice 15 days after tumor cell injection. Figure 1 shows that both desipramine and fluoxetine significantly increased the number of C26 cc cell foci and the overall metastatic volume estimated as the percentage of liver tissue occupied by the tumor when compared to that tissue from vehicle-treated control mice. On the contrary, mirtazapine did not modulate the number and volume of metastases in comparison to vehicle-treated control mice.
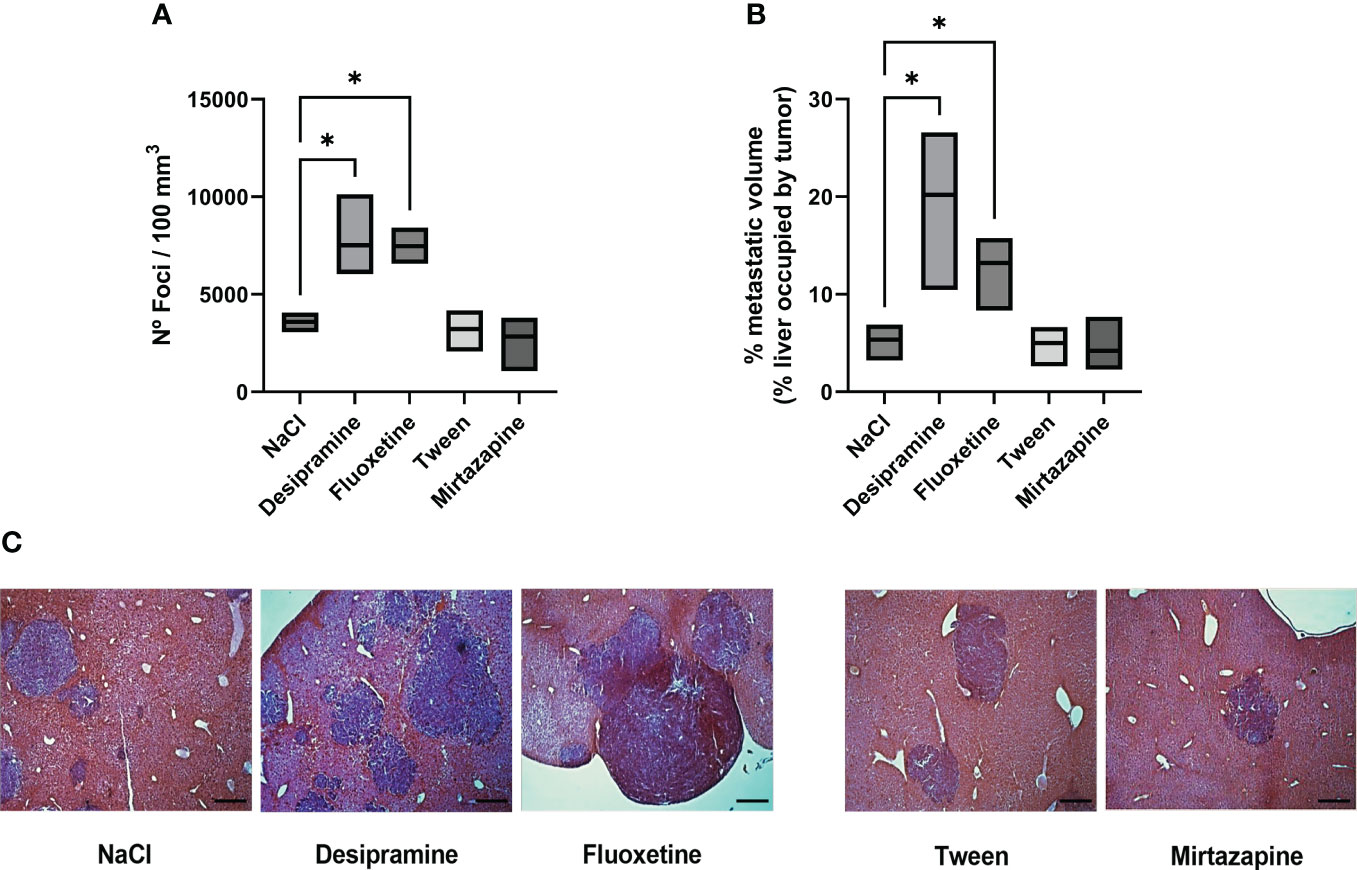
Figure 1 Effect of antidepressants on C26 colon carcinoma (cc) metastatic colonization to the liver. Balb/c mice were injected intrasplenically (i.s.) with 1.5 × 105 C26 colon carcinoma cells. Animals were treated intraperitoneally (i.p.) with a daily dose of desipramine (10 mg/kg), fluoxetine (10 mg/kg), mirtazapine (20 mg/kg), or proper vehicle (0.9% NaCl or 1% Tween) for 14 days. The first drug injection was given 2 h after intrasplenic injection of tumor cells. After 15 days mice were sacrificed and livers were processed for histology. A series of three experiments (5 mice per treatment) was carried out to determine metastatic growth in the liver; the number of foci per 100 mm3 of liver tissue (A), metastatic volume (overall tumor tissue volume of liver occupied by metastasis) (B), microphotography of H-E staining of livers collected from tumor-bearing mice after antidepressant treatment, bar = 250 µm (C). Data represent the minimum, maximum and mean values of three micro experiments, the effects of the different treatments on the rate of metastatic development were compared by one-way analysis of variance (ANOVA), *p<0.05.
3.2 Production of cytokines
Next, the levels of serum inflammatory cytokines involved in the immune response developed during metastatic development were analyzed. Figures 2A, B, 3A, B show that the levels of both serum and spleen IFN-γ and IL-1β were decreased after desipramine- and fluoxetine- but not those of mirtazapine in C26 cc cells-bearing mice after 14 days of treatment. Particularly drastic was the decrease in spleen IFN-γ levels in desipramine-treated tumor-bearing mice (up to 96%, Figure 3A). Desipramine significantly increased (214%) serum IL-10 levels (Figure 2C), whilst desipramine and fluoxetine increased the levels of this cytokine produced by the splenocytes collected from tumor-bearing mice (Figure 3C).
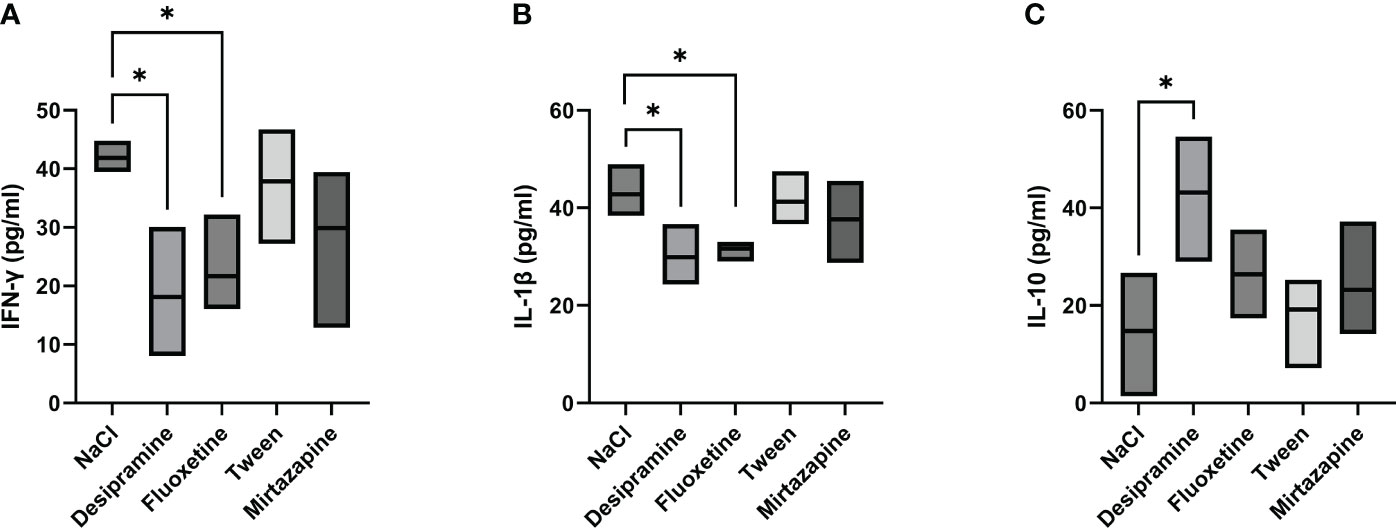
Figure 2 Effect of antidepressants on cytokine levels in serum from C26 colon carcinoma-bearing mice. Balb/c mice were injected i.s. with 1.5 × 105 C26 colon carcinoma cells. Animals were treated i.p. with a daily dose of desipramine (10 mg/kg), fluoxetine (10 mg/kg), mirtazapine (20 mg/kg), or proper vehicle (0.9% NaCl or 1% Tween). After 15 days mice were sacrificed and serum was collected for cytokine analysis. A series of three experiments (5 mice per treatment) was carried out to determine levels of IFN-γ (A), IL-1 β (B), and IL-10 (C). Data represent the minimum, maximum and mean of three micro experiments. The effects of the different treatments on cytokine levels were compared by one-way analysis of variance (ANOVA), *p<0.05.
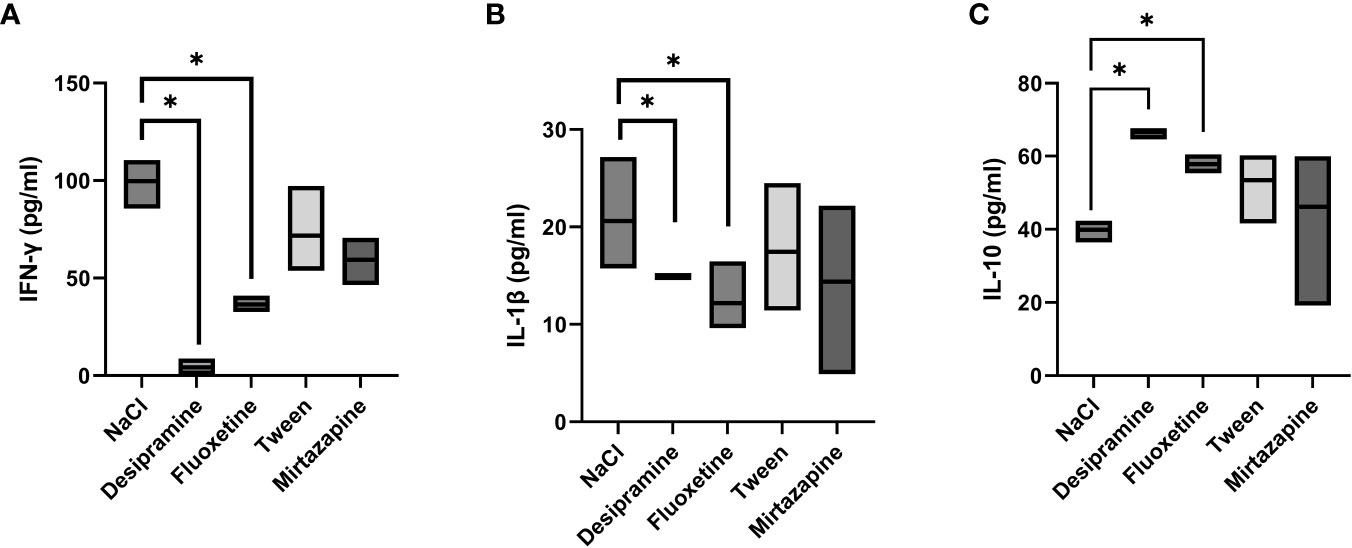
Figure 3 Effect of antidepressants on cytokine levels produced by spleen lymphocytes from C26 colon carcinoma-bearing mice. Balb/c mice were injected i.s. with 1.5 × 105 C26 colon carcinoma cells. Animals were treated i.p. with a daily dose of desipramine (10 mg/kg), fluoxetine (10 mg/kg), mirtazapine (20 mg/kg), or proper vehicle (0.9% NaCl or 1% Tween). After 15 days mice were sacrificed and spleens were collected for cytokine analysis. After isolation, spleen lymphocytes were cultured for 48 h in presence of Con A and LPS simultaneously stimulation. A series of three experiments (5 mice per treatment) was carried out to determine levels of IFN-γ (A), IL-1β (B), and IL-10 (C). Data represent the minimum, maximum and mean of three micro experiments. The effects of the different treatments on cytokine levels were compared by one-way analysis of variance (ANOVA), *p<0.05.
3.3 Proliferative activity of splenocytes of tumors bearing mice
The proliferation of splenocytes is a key factor in antitumor functions. Thus, we analyzed the proliferative activity of splenocytes in tumor-bearing mice treated with an antidepressant. Desipramine and fluoxetine significantly inhibited the proliferative activity of splenocytes isolated from C26 cc cell-bearing mice by 36% and 28% respectively. Conversely, the blastogenic response of splenocytes to Con A was not changed in mirtazapine-treated Balb/c mice with C26 cc metastasis in the liver (Figure 4).
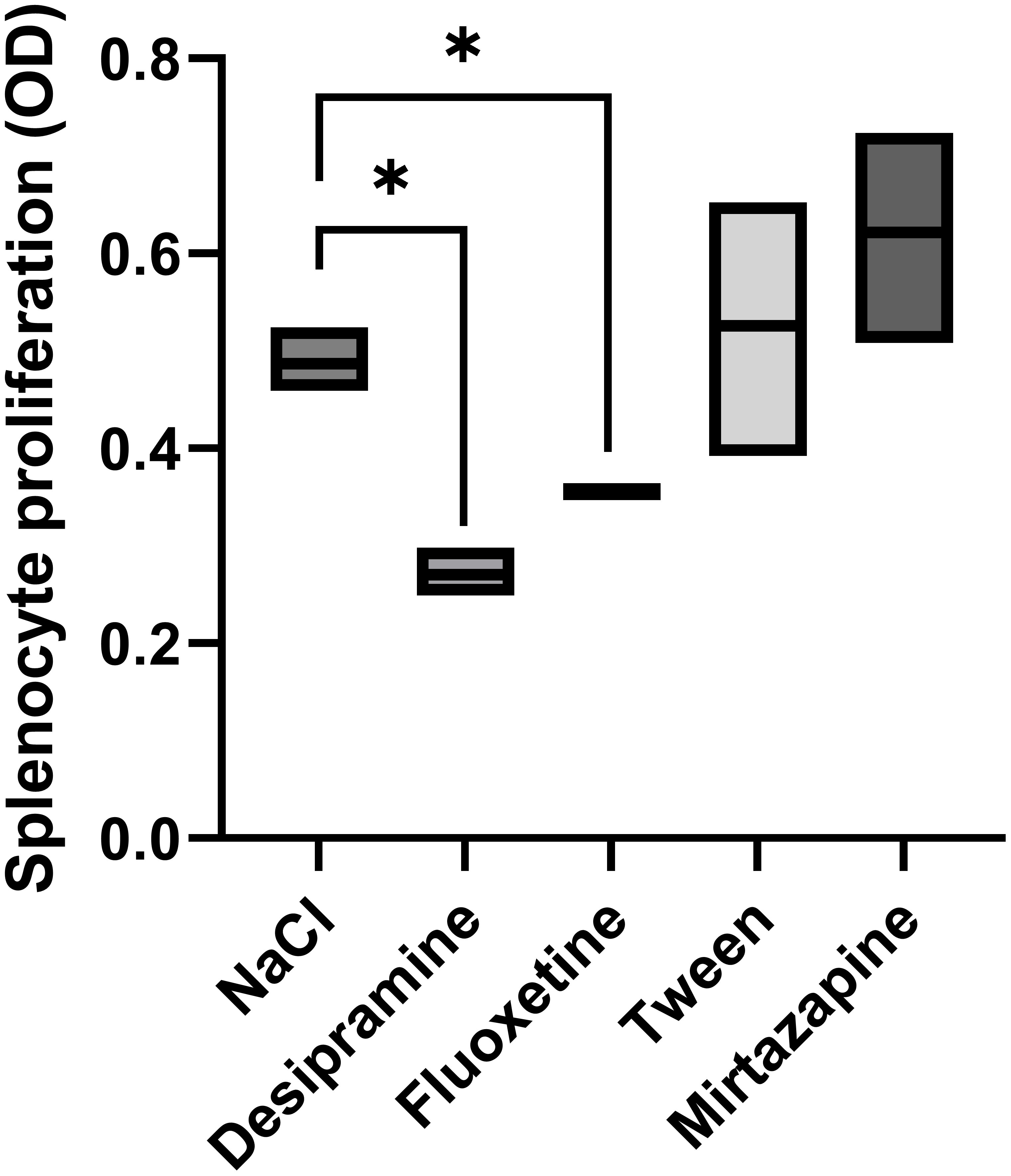
Figure 4 Effect of antidepressants on spleen lymphocyte proliferation. Animals were treated i.p. with a daily dose of desipramine (10 mg/kg), fluoxetine (10 mg/kg), mirtazapine (20 mg/kg), or proper vehicle (0.9% NaCl or 1% Tween). Spleen lymphocytes were isolated from spleens collected from C26 colon carcinoma-bearing mice 15 days after tumor cell injection. After isolation, spleen lymphocytes were cultured for 72 h in the presence of Con A, and the proliferation of lymphocytes was measured by MTT assay. Data represent min, max and mean of three micro experiments. The effects of the different treatments on splenocyte proliferation levels were compared by one-way analysis of variance (ANOVA), *p<0.05.
4 Discussion
Antidepressants are used in cancer patients, but the effects of antidepressant drugs on the progression of cancer metastasis are not fully elucidated. In the present study, we aimed to put some light by analyzing the effects of several antidepressants in the metastatic progression of murine C26 colon carcinoma to the liver. The main findings of this study are that treatment of C26 colon carcinoma cells-bearing mice with desipramine or fluoxetine: 1) increases the metastatic potential of C26 cc cells to the liver; 2) inhibits the proliferative activity of splenocytes; and 3) decreases the production of pro-inflammatory cytokines (IFN-γ, IL-1β) whilst increasing the production of the anti-inflammatory cytokine IL-10. Mirtazapine, on the other hand, had no effect on colon carcinoma growth in the liver as well as on immune system activity.
To the best of our knowledge, the effect of antidepressant drugs on C26 cc tumor progress in a unique model of liver metastasis formation after intra-splenic tumor cells injection has not been studied yet. The intra-splenic injection of tumor cells offers advantages over other murine models, such as consistent metastatic development to the liver at a uniform time, peripheral blood immune analysis, and high reproducibility. Also, tumor cells are allowed to reach the liver through their natural way, that is, through the portal vein (19). Previous studies showed that antidepressant drugs given after injection of tumor cells inhibit jejunal and colonic tumors in rats and xenografts of human colorectal and prostate cancers in athymic nude mice (20) or enhance tumor growth. Brandes et al. (4) found that fluoxetine stimulated the development of chemically-induced tumors in rodents but the doses were much higher than those used in our study. Fluoxetine in a dose of 20-80 mg/kg per day decreases the latency of C-3 fibrosarcoma and aggregates the weight of melanoma and the rate and frequency of development of mammary tumors in rats fed with dimethylbenzanthracene. The prometastatic effect of fluoxetine has been used in invasive and metastatic models in human HepG2 cells (21). The levels of serotonin, which are increased by SSRIs, may promote tumor growth in human hepatocellular cancer (22).
Moreover, fluoxetine (below 25 µM) stimulates the accumulation of fat in primary hepatocytes, causing the liver to become fatty, favoring a profibrotic environment which may, in turn, contribute to the development of liver sclerosis and liver cancer (23). Moreover, fluoxetine treatment may increase the number of breast cancer metastases to the brain in a mouse model (24). Contrary to the above-mentioned data several reports revealed a beneficial, anti-tumor effect of fluoxetine. Treatment of fluoxetine significantly decreased the progression of hepatocellular carcinoma Hep3B and non-small cell lung cancer CL1-5-F4 tumors (25). Similar anti-tumor and anti-metastatic properties have been reported for imipramine, related to the dose-dependent inhibition of Fascin-1, the overexpression of which is correlated with the progression of colorectal tumors (26). Furthermore, a recent retrospective cohort study revealed that individuals prescribed fluoxetine and other SSRIs had a reduced risk of bladder cancer (27). The differences observed in tumor development might be explained by differences in the tumor microenvironment affected by the progression of cancer. In fact, the phenotypic and genotypic characteristics of tumor cells from a primary tumor might differ greatly from those observed in their metastatic counterparts. Under such conditions, the immune microenvironment may also adapt to the new scenario. In addition, the present model focuses on the later steps of the metastatic process, namely, adhesion to secondary organ endothelium, transmigration to the organ parenchyma, and final colonization, including the avascular and vascular phases. Further studies should put some light on the effects of these anti-depressants in earlier metastatic steps, such as primary tumor detachment, intravasation, and pre-metastatic niche formation.
In our previous studies, we observed that chronic desipramine and fluoxetine treatment, administered before the injection of tumor cells, induced gastrointestinal tract, peritoneal cavity, skin, and spleen metastasis formation by B16F10 melanoma cells in C57BL/6J male and female mice (7, 28). The effect was age and “temperament”-dependent. Desipramine pretreatment dramatically promoted metastasis formation and increased mortality rate in young high-active males whereas fluoxetine pretreatment induced such effect in young high-active females (28). In our former studies in the lung metastasis cancer model induced by i.v. injection of MADB 106 cells to Wistar rats subjected to stress procedure according to the chronic mild stress (CMS) model of depression, pro-metastatic effects caused by desipramine treatment were observed. These studies showed a very disadvantageous effect of desipramine on tumor progression in the lungs of both stress highly-sensitive and stress non-reactive (stress resistant) animals (29). Moreover, desipramine injection decreased the number of T CD8+ cells and cytotoxicity of natural killer (NK) cells as well as skew macrophage activity towards M2 functional phenotype (30).
The development of cancer metastases is particularly affected by the inhibition of immune activity associated with immune checkpoints (ICP), especially the PD1 (programmed death receptor 1) present, among others, on CD8+ T cells, natural killer cells and macrophages, and its ligand PD-L1 (programmed death receptor ligand 1) presented on cancer cells (31, 32). Our primary results show a reduction in PD-L1 expression in the brain tissue of two different species of animals in two animal models of depression (Curzytek K, Malicki S, Kubera MW, Maes M, et al., paper in preparation). This observation confirms our hypothesis that depressive disorders may correlate with ICPs dysregulation, both in the nervous and immune systems. It suggested a significant impact of antidepressants on the expression of PD-1 and/or PDL-1 on cells of the immune system and cancer cells (increased expression of PD-1 on cells eliminating cancer cells and, at the same time, increased expression of PD-L1 on cancer cells, induced by some antidepressants, may be an important cause of the progression of the neoplastic process).
The third drug evaluated in this study – mirtazapine – did not show any effect on either metastases formation or the tested immune parameters. Mirtazapine appears to be a promising drug in the alleviation of cancer-related depression symptoms, although more randomized controlled trials are needed to define its effectiveness and safety in the cancer population (33).
The promotion of metastasis formation in animals treated with fluoxetine and desipramine is probably connected with the inhibition of anti-cancer cell-mediated immunity. In the present study, we have shown that fluoxetine and desipramine decrease Con A-induced splenocyte proliferation. Moreover, this was accompanied by a decrease in the ability of splenocytes to produce IL-1β and IFN-γ, and by an increase in IL-10 production and similar changes in plasma cytokine levels. However, our former studies were shown that simvastatin (used to decrease the high level of blood cholesterol) although exhibits pleiotropic effects on carcinogenesis, inhibits the release of IL-8 and IL-6 from colorectal cell lines and decreases serum IL-6 level in patients with advanced colorectal cancer (34).
In the present study, we observed a reduction in IL-1β levels after desipramine and fluoxetine treatment which correlates with an increase in the metastatic burden observed in the liver. This is in contrast with other several studies showing that IL-1β produced by tumor-associated macrophages supports colon cancer cell growth (35) and stimulates the adhesion of colon carcinoma cells to mesothelial monolayers (36) and lung microvascular endothelium (37). Moreover, it was shown that sialyl Lewis X-expressing antigens on colon cancer cells act as ligands for selectins, induced by IL-1β, on human liver endothelial cells (38). On the other hand IL-18, a cytokine with strong antitumor activity and an IFN-γ-inducing factor in experimental models requires processing by an IL-1 converting enzyme (39). Therefore, we may speculate that a decrease of IL-18 may also occur in antidepressant-treated C26 cc metastasis-bearing Balb/c mice, avoiding the mounting of an efficient immune response in the liver. In addition, a decrease of IL-1β, via a reduction in the production of IL-2 in T spleen cells, may be responsible for the decreased proliferative activity of splenocytes. Also, the involvement of both pro-inflammatory cytokines in the development of an adequate angiogenic vascular might suggests that the effect of antidepressants examined in this study is independent of this pathway, and relates in a unique way to the local immune response developed in the liver during metastatic progression of colorectal cancer.
Bevers et al. (2022) showed a significant role in the activation of dendritic cells, tumor-specific T CD8+ cells, and macrophages in the spleen and an increase in the production of Type I interferons by splenocytes in the formation of an anti-tumor response in C57BL/6J mice (40).
Maybe in our study desipramine and fluoxetine inhibits the spleen and liver formation of long-lasting tumor-specific T CD8+ cells, which showed a high degree of multifunctionality and provoked high antitumor efficiency.
IFN-γ is a multifunctional cytokine that plays important role in activating innate and adaptive immune response via enhancing NK cells, T cells, and natural killer T (NKT) cells (41). The decrease of IFN-γ production predisposes to the development of primary and transplanted tumors, whereas increases promote host response to tumors, although the mechanisms explaining these effects of cytokines have remained elusive (42). It was shown that IFN-γ has antiproliferative effects on pancreatic cancer cells and inhibits pancreatic tumor growth in Balb/c nu/nu mice (43) and experimentally induced skin carcinogenesis (44).
Conversely, IL-10 levels play an important role in the modulation of tumor progression: high levels of IL-10 in a tumor microenvironment block the activity of antigen-presenting cells (APC), cytotoxic T cells, and NK cells and stimulate tumor growth. We observed that the higher metastatic growth in C26 cc cell-bearing mice after desipramine and fluoxetine treatment may be associated with an increased ability of splenocytes to produce IL-10. This mechanism may decrease anti-tumor immunity and enhance metastasis by promoting the ability to circulate C26 cc cells to survive in the host liver. This is in accordance with the increase in IL-10 levels in soluble Intercellular Adhesion Molecule 1 (sICAM-1)-induced hepatic metastasis formation (14). IL-10 may also enhance weakly metastatic human colorectal carcinoma metastasis into nude mouse liver via inhibition of the production of nitric oxide (NO), reactive oxygen species (ROS), and upregulation of expression of inducible nitric oxide synthetase (iNOS) in the host liver (29, 45).
A bioinformatics approach identified antitumor effects of tricyclic antidepressants in small cell lung cancer (SCLC) and other high-grade neuroendocrine carcinomas (grade 3 neuroendocrine carcinomas, G3NEC) that were subsequently validated in preclinical models (46). Unfortunately, our research shows that desipramine increases the formation of metastases to the liver and in this situation, we cannot recommend repositioning desipramine in clinical trials in colorectal cancer.
In conclusion, the present study showed prometastatic effects of desipramine and fluoxetine, but not mirtazapine, by modulating secondary liver colonization during colon carcinoma progression, and that these effects are associated with the effects of antidepressants on cytokines, i.e., enhanced IL-10 and reduced IL-1 β and IFN-γ levels.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Basque Country University Ethical Committee (CEID).
Author contributions
Conceptualization, MK and BA; methodology, BA and MK; formal analysis, BA, BG, and KC; investigation, BA; resources BA and MK; writing – original draft preparation, MK and BA; writing – review and editing, BA, BG, KC, MK, SM, and MM; supervision, MK; project administration, MK; funding acquisition, MK. All authors contributed to the article and approved the submitted version.
Funding
This work has been supported by the statutory funds of the Laboratory of Immunoendocrinology, Department of Experimental Neuroendocrinology, Maj Institute of Pharmacology Polish Academy of Sciences, 31-343 Kraków, Smętna 12 street, Poland.
Acknowledgments
The authors are entirely responsible for the scientific content of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lavan O, Peled O, Avishai-Neumann M, Weizman A, Yahel A, Apter A, et al. Psychopharmacology in the pediatric oncology and bone marrow transplant units: antidepressant treatment. J Child Adolesc Psychopharmacol (2022) 32:153–61. doi: 10.1089/cap.2021.0083
2. Steingart AB, Cotterchio M. Do antidepressants cause, promote, or inhibit cancers? J Clin Epidemiol (1995) 48:1407–12. doi: 10.1016/0895-4356(95)00545-5
3. Sternbach H. Are antidepressants carcinogenic? a review of preclinical and clinical studies. J Clin Psychiatry (2003) 64:1153–62. doi: 10.4088/JCP.v64n1003
4. Brandes LJ, Arron RJ, Bogdonavic RP, Tong J, Zaborniak CL, Hogg GR, et al. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res (1992) 52:3796–800.
5. Amerio A, Gálvez JF, Odone A, Dalley SA, Ghaemi SN. Carcinogenicity of psychotropic drugs: a systematic review of US food and drug administration-required preclinical in vivo studies. Aust N Z J Psychiatry (2015) 49:686–96. doi: 10.1177/0004867415582231
6. Zhuang Y, Pang X, Qi Y, Zhang T, Cao G, Xue H, et al. The incidence risk of breast and gynecological cancer by antidepressant use: a systematic review and dose-response meta-analysis of epidemiological studies involving 160,727 patients. Front Oncol (2022) 12:939636. doi: 10.3389/fonc.2022.939636
7. Kubera M, Grygier B, Arteta B, Urbańska K, Basta-Kaim A, Budziszewska B, et al. Age-dependent stimulatory effect of desipramine and fluoxetine pretreatment on metastasis formation by B16F10 melanoma in male C57BL/6 mice. Pharmacol Rep (2009) 61:1113–26. doi: 10.1016/s1734-1140(09)70174-4
8. Kubera M, Lin AH, Kenis G, Bosmans E, Bockstaele v, Maes M. Anti-inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol (2001) 21:199–206. doi: 10.1097/00004714-200104000-00012
9. Kubera M, Simbirtsev A, Mathison R, Maes M. Effects of repeated fluoxetine and citalopram administration on cytokine release in C57BL/6 mice. Psychiatry Res (2000) 96:255–66. doi: 10.1016/s0165-1781(00)00184-0
10. Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De Jongh R, et al. Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology (1999) 20:370–9. doi: 10.1016/S0893-133X(98)00088-8
11. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev (2001) 7:249–64. doi: 10.1111/j.1527-3458.2001.tb00198.x
12. Kast RE. Mirtazapine may be useful in treating nausea and insomnia of cancer chemotherapy. Support Care Cancer (2001) 9:469–70. doi: 10.1007/s005200000215
13. Sato N, Michaelides MC, Wallack MK. Transplantation immunity and cross-protection of two cultured murine colon lines. J Natl Cancer Inst (1983) 70:261–5.
14. Arteta B, Lasuen N, Lopategi A, Sveinbjörnsson B, Smedsrød B, Vidal-Vanaclocha F. Colon carcinoma cell interaction with liver sinusoidal endothelium inhibits organ-specific antitumor immunity through interleukin-1-induced mannose receptor in mice. Hepatology (2010) 51:2172–82. doi: 10.1002/hep.23590
15. Valcárcel M, Arteta B, Jaureguibeitia A, Lopategi A, Martínez I, Mendoza L, et al. Three-dimensional growth as multicellular spheroid activates the proangiogenic phenotype of colorectal carcinoma cells via LFA-1-dependent VEGF: implications on hepatic micrometastasis. J Transl Med (2008) 6:57–69. doi: 10.1186/1479-5876-6-57
16. Grygier B, Arteta B, Kubera M, Basta-Kaim A, Budziszewska B, Leśkiewicz M, et al. Inhibitory effect of antidepressants on B16F10 melanoma tumor growth. Pharmacol Rep (2013) 65(3):672–81. doi: 10.1016/s1734-1140(13)71045-4
17. Rénéric JP, Bouvard M, Stinus L. In the rat forced swimming test, chronic but not subacute administration of dual 5-HT/NA antidepressant treatments may produce greater effects than selective drugs. Behav Brain Res (2002) 136(2):521–32. doi: 10.1016/s0166-4328(02)00203-6
18. Budziszewska B, Leśkiewicz M, Kubera M, Jaworska-Feil L, Kajta M, Lasoń W. Estrone, but not 17 beta-estradiol, attenuates kainate-induced seizures and toxicity in male mice. Exp Clin Endocrinol Diabetes. (2001) 109:168–73. doi: 10.1055/s-2001-14841
19. Soares KC, Foley K, Olino K, Leubner A, Mayo SC, Jain A, et al. A preclinical murine model of hepatic metastases. J Vis Exp (2014) 91):51677. doi: 10.3791/51677
20. Abdul M, Logothetis CJ, Hoosein NM. Growth-inhibitory effects of serotonin uptake inhibitors on human prostate carcinoma cell lines. J Urology. (1995) 154:247–50. doi: 10.1016/S0022-5347(01)67288-4
21. Tian YS, Chen KC, Zulkefli ND, Maner RS, Hsieh CL. Evaluation of the inhibitory effects of genipin on the fluoxetine-induced invasive and metastatic model in human HepG2 cells. Molecules (2018) 23(12):3327. doi: 10.3390/molecules23123327
22. Soll C, Jang JH, Riener MO, Moritz W, Wild PJ, Graf R, et al. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology (2010) 51:1244–54. doi: 10.1002/hep.23441
23. Feng XM, Xiong J, Qin H, Liu W, Chen RN, Shang W, et al. Fluoxetine induces hepatic lipid accumulation via both promotion of the SREBP1c-related lipogenesis and reduction of lipolysis in primary mouse hepatocytes. CNS Neurosci Ther (2012) 18:974–80. doi: 10.1111/cns.12014
24. Shapovalov Y, Zettel M, Spielman SC, Amico-Ruvio SA, Kelly EA, Sipe GO, et al. Fluoxetine modulates breast cancer metastasis to the brain in a murine model. BMC Cancer. (2014) 14:598. doi: 10.1186/1471-2407-14-598
25. Hsu LC, Tu HF, Hsu FT, Yueh PF, Chiang IT. Beneficial effect of fluoxetine on anti-tumor progression on hepatocellular carcinoma and non-small cell lung cancer bearing animal model. BioMed Pharmacother. (2020) 126:110054. doi: 10.1016/j.biopha.2020.110054
26. Alburquerque-González B, Bernabé-García M, Montoro-García S, Bernabé-García Á, Rodrigues PC, Ruiz Sanz J, et al. New role of the antidepressant imipramine as a Fascin1 inhibitor in colorectal cancer cells. Exp Mol Med (2020) 52(2):281–92. doi: 10.1038/s12276-020-0389-x
27. Liu YC, Chen VC, Lu ML, Lee MJ, McIntyre RS, Majeed A, et al. The association between selective serotonin reuptake inhibitors (SSRIs) use and the risk of bladder cancer: a nationwide population-based cohort study. Cancers (Basel). (2020) 12(5):1184. doi: 10.3390/cancers12051184
28. Kubera M, Grygier B, Wrona D, Rogóż Z, Roman A, Basta-Kaim A, et al. Stimulatory effect of antidepressant drug pretreatment on progression of B16F10 melanoma in high-active male and female C57BL/6J mice. J Neuroimmunol. (2011) 240-241:34–44. doi: 10.1016/j.jneuroim.2011.09.006
29. Grygier B, Kubera M, Wrona D, Roman A, Basta-Kaim A, Gruca P, et al. Stimulatory effect of desipramine on lung metastases of adenocarcinoma MADB 106 in stress highly-sensitive and stress non-reactive rats. Prog Neuropsychopharmacol Biol Psychiatry (2018) 80:279–90. doi: 10.1016/j.pnpbp.2017.04.024
30. Ko JH, Jung BG, Park YS, Lee BJ. Inhibitory effects of interferon-gamma plasmid DNA on DMBA-TPA induced mouse skin carcinogenesis. Cancer Gene Ther (2011) 18:646–54. doi: 10.1038/cgt.2011.36
31. Muscari I, Fierabracci A, Adorisio S, Moretti M, Cannarile L, Thi Minh Hong V, et al. Glucocorticoids and natural killer cells: a suppressive relationship. Biochem Pharmacol (2022) 198:114930. doi: 10.1016/j.bcp.2022.114930
32. Soldi LR, Silva VLC, Rabelo DH, Uehara IA, Silva MJB. Reactivation of natural killer cells with monoclonal antibodies in the microenvironment of malignant neoplasms. J Cancer Res Clin Oncol (2023). doi: 10.1007/s00432-023-04575-8
33. Economos G, Lovell N, Johnston A, Higginson IJ. What is the evidence for mirtazapine in treating cancer-related symptomatology? a systematic review. Support Care Cancer. (2020) 28(4):1597–606. doi: 10.1007/s00520-019-05229-7
34. Malicki S, Winiarski M, Matlok M, Kostarczyk W, Guzdek A, Konturek PC. IL-6 and IL-8 responses of colorectal cancer in vivo and in vitro cancer cells subjected to simvastatin. J Physiol Pharmacol (2009) 60:141–6.
35. Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene (2009) 28:3892–902. doi: 10.1038/onc.2009.247
36. van Grevenstein WM, Hofland LJ, van Rossen MEE, van Koetsveld PM, Jeekel J, van Eijck CHJ. Inflammatory cytokines stimulate the adhesion of colon carcinoma cells to mesothelial monolayers. Dig Dis Sci (2007) 52:2775–83. doi: 10.1007/s10620-007-9778-4
37. ten Kate M, Hofland LJ, van Grevenstein WM, van Koetsveld PV, Jeekel J, van Eijck CH. Influence of proinflammatory cytokines on the adhesion of human colon carcinoma cells to lung microvascular endothelium. Int J Cancer. (2004) 112:943–50. doi: 10.1002/ijc.20506
38. Matsushita Y, Kitajima S, Goto M, Tezuka Y, Sagara M, Imamura H, et al. Selectins induced by interleukin-1beta on the human liver endothelial cells act as ligands for sialyl Lewis X-expressing human colon cancer cell metastasis. Cancer Lett (1998) 133:151–60. doi: 10.1016/s0304-3835(98)00220-1
39. Nagai H, Hara I, Horikawa T, Oka M, Kamidono S, Ichihashi M. Gene transfer of secreted-type modified interleukin-18 gene to B16F10 melanoma cells suppresses in vivo tumor growth through inhibition of tumor vessel formation. J Invest Dermatol (2002) 119:541–8. doi: 10.1046/j.1523-1747.2002.01866.x
40. Bevers S, Kooijmans SAA, Van de Velde E, Evers MJW, Seghers S, Gitz-Francois JJJM, et al. mRNA-LNP vaccines tuned for systemic immunization induce strong antitumor immunity by engaging splenic immune cells. Mol Ther (2022) 30:3078–94. doi: 10.1016/j.ymthe.2022.07.007○
41. Saito TI, Li HW, Sykes M. Invariant NKT cells are required for antitumor responses induced by host-versus-graft responses. J Immunol (2010) 185:2099–105. doi: 10.4049/jimmunol.0901985
42. Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev (2002) 13:95–109. doi: 10.1016/s1359-6101(01)00038-7
43. Lange F, Rateitschak K, Fitzner B, Pöhland R, Wolkenhauer O, Jaster R. Studies on mechanisms of interferon-gamma action in pancreatic cancer using a data-driven and model-based approach. Mol Cancer. (2011) 10:1–13. doi: 10.1186/1476-4598-10-13
44. Jessup JM, Laguinge L, Lin S, Samara R, Aufman K, Battle P, et al. Carcinoembryonic antigen induction of IL-10 and IL-6 inhibits hepatic ischemic/reperfusion injury to colorectal carcinoma cells. Int J Cancer. (2004) 111:332–7. doi: 10.1002/ijc.20264
45. Jessup JM, Samara R, Battle P, Laguinge LM. Carcinoembryonic antigen promotes tumor cell survival in liver through an IL-10-dependent pathway. Clin Exp Metastasis. (2004) 21:709–17. doi: 10.1007/s10585-004-7705-z
Keywords: antidepressants, desipramine, fluoxetine, mirtazapine, C26 colon carcinoma, cytokines
Citation: Kubera M, Arteta B, Grygier B, Curzytek K, Malicki S and Maes M (2023) Stimulatory effect of fluoxetine and desipramine, but not mirtazapine on C26 colon carcinoma hepatic metastases formation: association with cytokines. Front. Immunol. 14:1160977. doi: 10.3389/fimmu.2023.1160977
Received: 07 February 2023; Accepted: 30 May 2023;
Published: 20 June 2023.
Edited by:
Pietro Ghezzi, University of Urbino Carlo Bo, ItalyReviewed by:
Toshiyuki Murai, Osaka University, JapanDavid Martín Hernández, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Spain
Copyright © 2023 Kubera, Arteta, Grygier, Curzytek, Malicki and Maes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta Kubera, a3ViZXJhQGlmLXBhbi5rcmFrb3cucGw=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Marta Kubera, orcid.org/0000-0003-4401-5055
Beatriz Arteta, orcid.org/0000-0003-3253-3162
Beata Grygier, orcid.org/0000-0002-7236-7720
Katarzyna Curzytek, orcid.org/0000-0002-6354-1563
Stanisław Malicki, orcid.org/0000-0001-8778-9259
Michael Maes, orcid.org/0000-0002-2012-871X
 Marta Kubera
Marta Kubera Beatriz Arteta
Beatriz Arteta Beata Grygier
Beata Grygier Katarzyna Curzytek
Katarzyna Curzytek Stanisław Malicki
Stanisław Malicki Michael Maes
Michael Maes