- 1Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS and PUMC), Beijing, China
- 2Department of Breast Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS and PUMC), Beijing, China
Hepatocellular carcinoma (HCC) is often diagnosed at an unresectable stage without opportunities for curative therapy. Future liver remnant (FLR) insufficiency limits the range of patients who can undergo radical resection. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) can ultimately achieve short-term hypertrophy of the FLR in patients with viral hepatitis-related fibrosis/cirrhosis and R0 resection. However, the influence of immune checkpoint inhibitors (ICIs) on liver regeneration remains unknown. We report two patients diagnosed with Barcelona Clinic Liver Cancer (BCLC)-B stage hepatitis B virus (HBV)-related HCC who underwent pioneering ALPPS after immunotherapy without posthepatectomy liver failure (PHLF). ALPPS has been shown to be safe and feasible in patients with HCC who underwent immunotherapy previously for the first time and might provide an alternative salvage option for future conversion therapy of HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world, ranking sixth in morbidity and fourth in cancer-related deaths (1). Majority of patients are diagnosed at an advanced stage without the opportunity to receive curative therapies including ablation and surgery (2). The volume of the future liver remnant (FLR) acts as an important limiting factor in the determination of tumour resectability (3). Approximately 80% of patients with HCC have a background of chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection and liver cirrhosis, and severity of these conditions is negatively correlated with the rate of hypertrophy of the FLR (3). Posthepatectomy liver failure (PHLF) caused by FLR insufficiency eliminates of the hope of radical resection (4).
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) can achieve rapid FLR growth in two weeks with a low risk of tumour progression during therapy (5). ALPPS has been shown to be safe and feasible in selected patients with viral hepatitis-related HCC (6).
Immune checkpoint inhibitors (ICIs) can restore the antitumor function of effector T cells through the inhibition of immune checkpoint molecules such as programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), which are revolutionizing the management of HCC (7–9). ICIs also affect other PD-1-expressed cells such as B cells, macrophages, and natural killer T (NKT) cells, and inflammatory processes in tumor microenvironment (10–12). It’s worth noting that liver regeneration and hepatocarcinogenesis involve multiple common events and processes (13). However, the exact association between ICIs and liver hypertrophy after hepatectomy remains unclear.
In the era of immunotherapy for solid tumors, the application of ALPPS in HCC patients with fibrosis/cirrhosis receiving ICIs has not been reported previously. Here, we report two cases of Barcelona Clinic Liver Cancer (BCLC)-B stage HCC patients who received combination therapy followed by pioneering ALPPS and completed conversion therapy successfully without serious perioperative complications.
Case presentation
Case 1
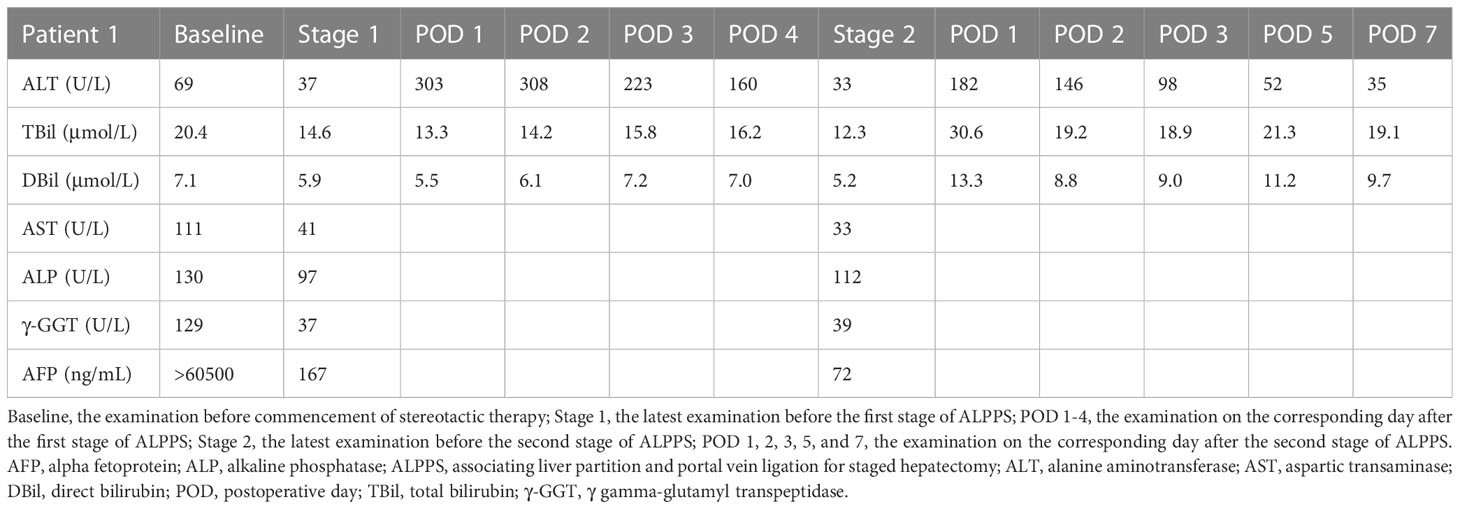
A 54-year-old male presented to our hospital because of upper abdominal pain. The patient weighed 65 kg, his height was 167 cm, and the standard liver volume (SLV) was 1231 mL, which was calculated based on the Urata formula (14). Laboratory examination (Table 1) showed alpha fetoprotein (AFP) >60500 ng/mL, total bilirubin (TBil) 20.4 μmol/L, direct bilirubin (DBil) 7.1 μmol/L, alanine transaminase (ALT) 69 U/L, and hepatitis B surface antigen (HBsAg) (+). Abdominal enhanced computed tomography (CT) indicated liver cirrhosis, a 14.9 cm × 11.8 cm mass located in the right liver and a 1.5 cm × 1.0 cm mass located in the left liver both with heterogeneous rapid enhancement of the mass in the arterial phase (Figure 1A). The patient was diagnosed with chronic hepatitis B from childhood and had regular antiviral therapy while finding the masses in the liver. The clinical diagnosis was HCC with BCLC-B stage. According to the BCLC strategy, transarterial chemoembolization (TACE) and a combination of the targeted drug (lenvatinib, 8 mg once a day) and ICIs (camrelizumab, 200 mg every three weeks) were performed (Figure 1F). After two courses of TACE and five cycles of immunotherapy, abdominal enhanced CT showed that the range of enhancement was significantly smaller than before. Although the diameter of the lesion was slightly smaller, the lesion reached partial response (PR; i.e., >30% decrease in the sum of diameters of viable (enhancing) target lesions) according to modified Response Evaluation Criteria Solid Tumours (mRECIST) (15). Laboratory examination before the surgery showed AFP 167.0 ng/mL, TBil 14.6 μmol/L, DBil 5.9 μmol/L, aspartic transaminase (AST) 41 U/L, and ALT 37 U/L. The volume of FLR was 475 mL, as calculated by abdominal CT (Figure 1B). An insufficient FLR could be linked to a high risk of postoperative liver failure after first-stage resection. After having obtained the consent of the patient and his family, the first stage of ALPPS and resection of the left lesion by laparoscopy was performed three months after the first course of TACE. A postoperative liver function test (LFT) indicated TBil 13.3 μmol/L, DBil 5.5 μmol/L, and ALT 303 U/L. Liver function improved after liver protection therapy. Twenty-one days after the first-stage surgery, abdominal CT indicated that the FLR increased to 576 mL (Figure 1C), and the rate of FLR/SLV was 46.8%, which could meet the needs of the body. The second stage of ALPPS was completed, and the TBil, DBil and ALT were 30.6 μmol/L, 13.3 μmol/L and 182 U/L, respectively, on the first day after surgery. Changes in liver function are shown in Figure 1E. The FLR was 708 mL (Figure 1D), and the patient was discharged 7 days after second-stage ALPPS. A pathology report demonstrated a mass in the left liver with moderately differentiated HCC and a mass in the right liver with extensive haemorrhage and necrosis and no clear residual tumour cells, which reached pathological complete response (pCR). The systemic therapy was restored two months after ALPPS with the same regimen as before in the follow-up clinic. The follow-up examination showed no recurrence on imaging and sufficient FLR on that day.
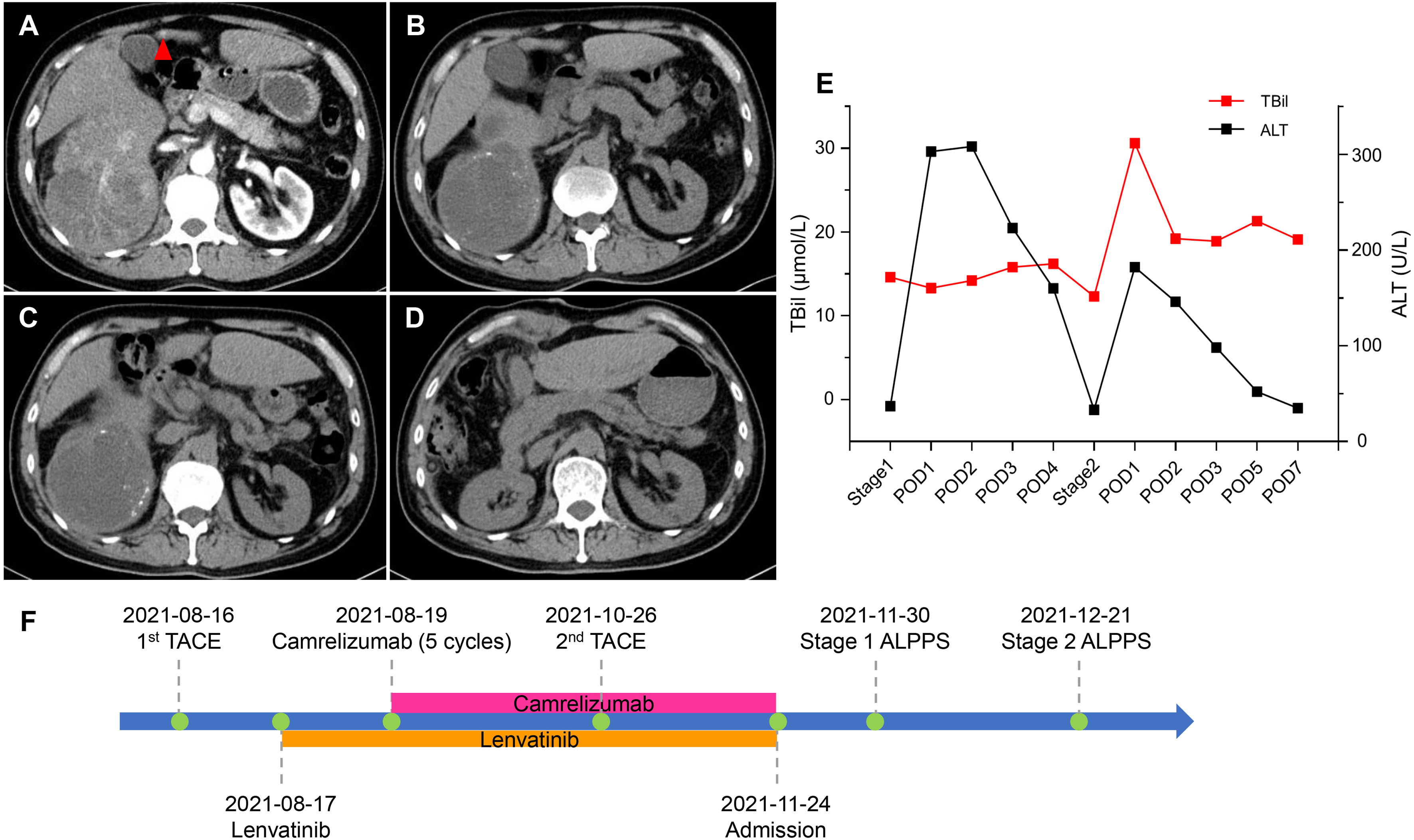
Figure 1 CT scans and changes in liver function of 1st patient. (A) Arterial phase of CT before stereotactic therapy. A lesion (red triangle) measuring 1.5 cm × 1.0 cm in liver segment 4b. (B) Plain scan before stage 1 ALPPS. (C) Plain scan before stage 2 ALPPS. (D) Plain scan 7 days after stage 2 ALPPS. (E) Changes in liver function during ALPPS. After stage 1, TBil remained a stable level, whereas ALT transiently rose but quickly declined. After stage 2, TBil showed no inordinate increases and quickly returned to normal, and ALT showed no significant upturns. (F) Timeline of treatment process. ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; ALT, alanine aminotransferase; CT, computer tomography; POD, postoperative day; TACE, transarterial chemoembolization; TBil, total bilirubin.
Case 2
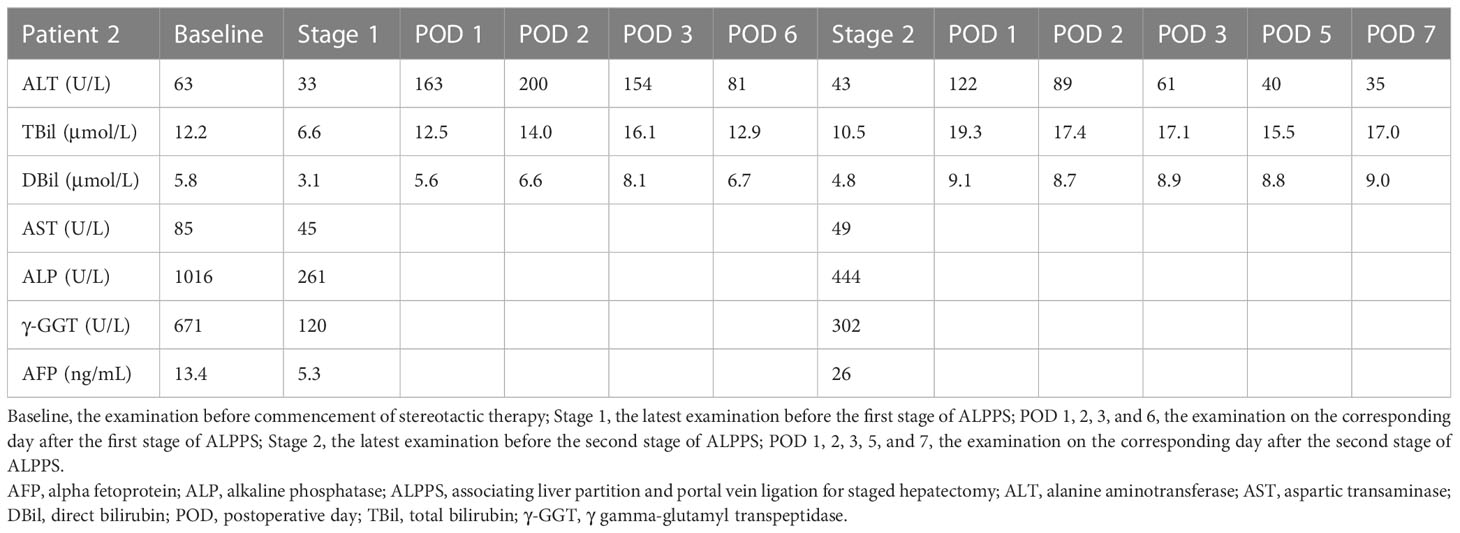
A 48-year-old male was admitted to our hospital due to a palpable mass in the right upper abdomen. The patient weighed 60 kg with a height of 178 cm, and the SLV was 1217 mL. Laboratory examination (Table 2) revealed TBil 12.2 μmol/L, DBil 5.8 μmol/L, alkaline phosphatase (ALP) 1016 U/L, γ gamma-glutamyl transpeptidase (γ-GGT) 671 U/L, HBV-DNA 735780 IU/L, and HBsAg (+). AFP was at the normal level. Abdominal enhanced CT showed a 16.2 cm × 11.2 cm mass situated in the right liver with inhomogeneous enhancement of the mass in the arterial phase and multiple intrahepatic metastatic lesions (Figure 2A). In addition, the patient was administered interferon therapy when diagnosed with HBV. According to the BCLC-B stage, stereotactic therapy for HCC was performed (i.e., TACE combined with 8 mg lenvatinib once a day and 200 mg camrelizumab every three weeks, Figure 2F). Abdominal CT showed that the diameter of the lesion was reduced from 16.2 cm to 9.7 cm (Figure 2B) after three courses of TACE and ten cycles of camrelizumab. LFT was almost normal, except ALP 261 U/L and γ-GGT 120 U/L. The first stage of ALPPS was performed with laparoscopy after obtaining the consent of the patient and his family. LFT suggested TBil 12.5 μmol/L, DBil 5.6 μmol/L, and ALT 163 U/L on the first day after surgery. One month after the first-stage surgery, the volume of FLR was 473 mL, which was more hypertrophic than the baseline volume of FLR (356 mL) (Figure 2C). The rate of FLR/SLV was 38.9%, and the patient received right hepatectomy plus right caudate-leaf resection by laparotomy. Changes in liver function are shown in Figure 2E. The pathology report suggested moderately differentiated hepatocellular carcinoma with satellite nodules. Systemic therapy was restored one week after second-stage ALPPS. Re-examination of CT one month after second-stage ALPPS indicated sufficient FLR (Figure 2D).
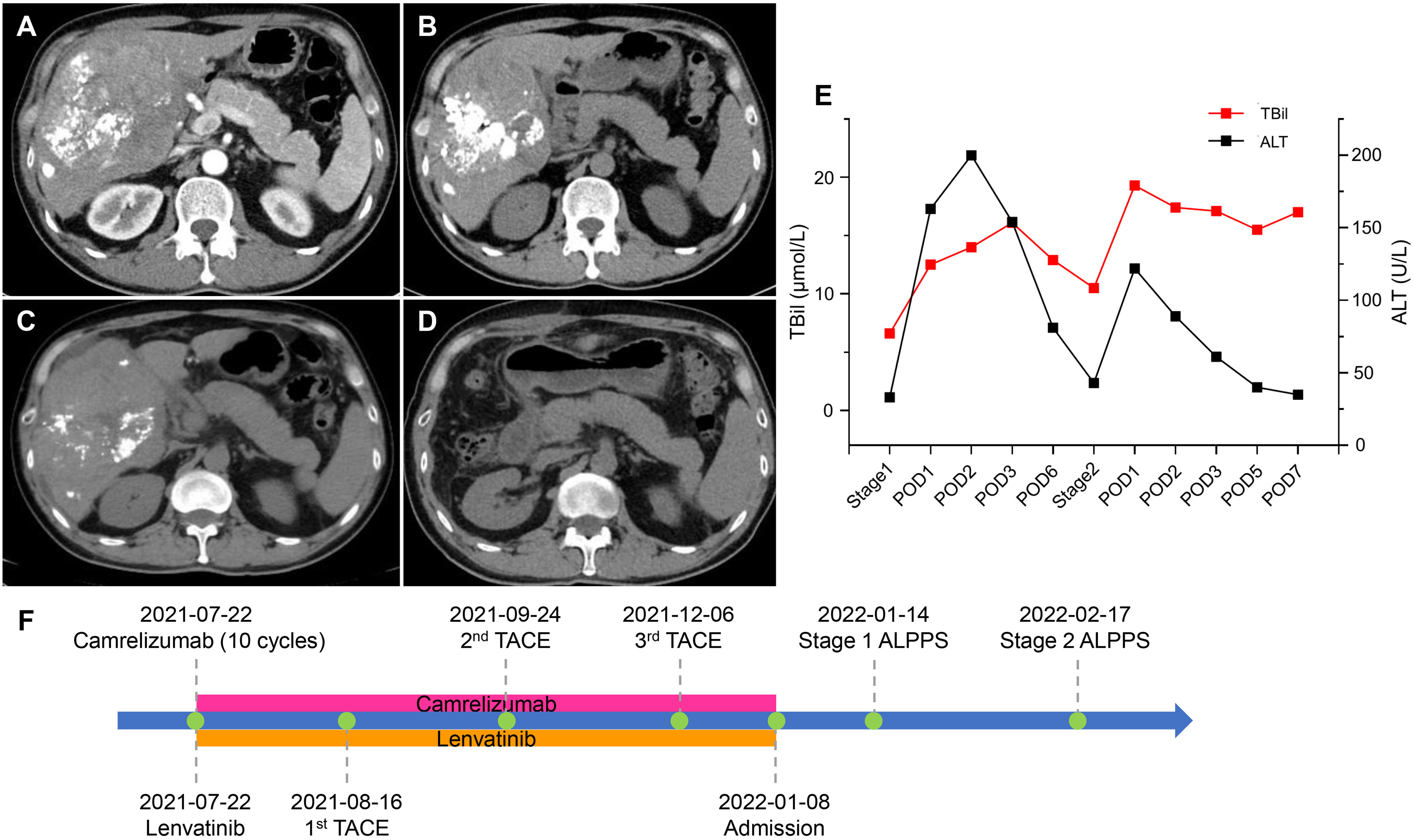
Figure 2 CT scans and changes in liver function of 2nd patient. (A) Arterial phase of CT after two courses of TACE. (B) Plain scan before stage 1 ALPPS. (C) Plain scan before stage 2 ALPPS. (D) Plain scan one month after stage 2 ALPPS. (E) Changes in liver function during ALPPS. After stage 1, TBil escalated within the normal level, whereas ALT transiently rose but quickly declined. After stage 2, TBil showed no inordinate increases, and ALT showed no significant upturns. (F) Timeline of treatment process. ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; ALT, alanine aminotransferase; CT, computer tomography; POD, postoperative day; TACE, transarterial chemoembolization; TBil, total bilirubin.
Discussion
ALPPS is a surgical strategy that can achieve radical resection of surgically unresectable HBV-related HCC by short-term hypertrophy of the FLR (3). However, the survival benefit of ALPPS may be limited at the beginning of the confirmed diagnosis in patients with BCLC-B stage HCC involving both left and right livers or large masses with satellite lesions (16). Currently, most guidelines recommend TACE and/or systemic therapy as the first choice for intermediate and advanced staged HCC (16). Our previous study suggested that stereotactic therapy (i.e., a combination of systemic and locoregional therapy) could safely achieve tumour downstaging and R0 resection in HCC patients with extrahepatic metastasis (17). The overall 5-year survival rate for patients with intermediate or advanced stage HCC after downstaging and conversion followed by resection is 50%-60%, which is close to the survival rate after resection with early-stage HCC (18). Aggressive stereotactic therapy achieved simultaneous radiographic and serological responses, resulting in tumour downstaging in the present patients. Achievement of oncological unresectable conversion indicated a survival benefit after further radical resection.
Previous studies suggested that ALPPS was superior to TACE combined with portal vein embolization in the overall conversion of insufficient FLR (5). After a sufficient evaluation, we performed a pioneering ALPPS in the present patients. Although the rates of FLR hypertrophy (i.e., 4.8 mL and 3.9 mL per day) after the first stage were lower than those in patients who had not received systemic therapy (5), the second stage was finally completed within a month. This result suggested that the hypertrophic speed might be influenced by systemic therapy in addition to viral hepatitis-related fibrosis/cirrhosis (5) and intrahepatic tumour burden (19). Inflammation and cytokine release from overactivation of immune cells after treatment with ICIs may cause liver injury (20). Previous studies demonstrated that the overproduction of IFN-γ by NKT cells negatively regulated liver regeneration in HBV transgenic mice (21). Interestingly, the production of IFN-γ was decreased in NKT cells of HCC patients, and anti-PD-1 drugs could improve function of NKT cells including enhanced IFN-γ level (22). These findings may provide a potential explanation on impaired liver regeneration in HBV-related HCC patients after treatment with ICIs. However, this study had some limitations such as a lack of pathological examination on FLR and control cases. Larger samples are needed to elucidate the mechanisms for the liver regeneration influenced by ICIs.
Furthermore, it is necessary to have a useful method to predict adequate FLR hypertrophy before ALPPS (23) which was guaranteed to perform the surgery with the greatest possible safety (24). The absence of PHLF indicated a sufficient FLR for these patients. These cases demonstrate that patients with a satisfactory objective response rate according to not only the RECIST 1.1 (Case 2) but also the mRECIST guidelines (Case 1) could receive ALPPS. These patients completed conversion therapy and achieved the goal of cure. In addition, we performed first-stage ALPPS by laparoscopy rather than a conventional procedure. The use of this procedure could reduce the high rate of occurrence of procedure-related mortality and morbidity, such as bile leakage and sepsis, by diminishing invasiveness (25).
It is worth noting that in Case 1, considering the insufficiency of FLR and the toxicity of chemotherapy to the liver, the right liver lesion was selected accurately during TACE. The left liver lesion without locoregional therapy was resected in first-stage ALPPS. Interestingly, postoperative pathology revealed pCR in the large lesion of the right liver, while residual tumour cells were observed in the small lesion of the left liver. The stereotactic therapy of enhanced immunotherapy by locoregional therapy was validated in a patient with a concurrent course of disease.
FLR hypertrophy might be influenced by immunotherapy, and further researches are warranted. Nevertheless, ALPPS is feasible and safe for the treatment of selected patients with HBV-related HCC who underwent prior therapy with ICIs. ALPPS might be an alternative strategy to salvage insufficient FLR during conversion therapy.
Ethics statement
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
CN and GL collected clinical data and drafted the manuscript. JZ participated in the management of the patient. XY, YX, and HZ designed treatment plan and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
3. Wang Z, Peng Y, Hu J, Wang X, Sun H, Sun J, et al. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis b virus-related hepatocellular carcinoma: a single center study of 45 patients. Ann Surg (2020) 271:534–41. doi: 10.1097/sla.0000000000002942
4. Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel M, et al. Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the international ALPPS registry. Ann Surg (2015) 262:780–785; discussion 785-786. doi: 10.1097/sla.0000000000001450
5. Li PP, Huang G, Jia NY, Pan ZY, Liu H, Yang Y, et al. Associating liver partition and portal vein ligation for staged hepatectomy versus sequential transarterial chemoembolization and portal vein embolization in staged hepatectomy for HBV-related hepatocellular carcinoma: a randomized comparative study. Hepatobil Surg Nutr (2022) 11:38–51. doi: 10.21037/hbsn-20-264
6. Chan ACY, Chok K, Dai JWC, Lo CM. Impact of split completeness on future liver remnant hypertrophy in associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in hepatocellular carcinoma: complete-ALPPS versus partial-ALPPS. Surgery (2017) 161:357–64. doi: 10.1016/j.surg.2016.07.029
7. Ahn E, Araki K, Hashimoto M, Li W, Riley JL, Cheung J, et al. Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci USA (2018) 115:4749–54. doi: 10.1073/pnas.1718217115
8. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov (2018) 8:1069–86. doi: 10.1158/2159-8290.cd-18-0367
9. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol (2022) 19:151–72. doi: 10.1038/s41571-021-00573-2
10. Wang DR, Wu XL, Sun YL. Therapeutic targets and biomarkers of tumor immunotherapy: response versus non-response. Signal Transduct Target Ther (2022) 7:331. doi: 10.1038/s41392-022-01136-2
11. Siwicki M, Gort-Freitas NA, Messemaker M, Bill R, Gungabeesoon J, Engblom C, et al. Resident kupffer cells and neutrophils drive liver toxicity in cancer immunotherapy. Sci Immunol (2021) 6:eabi7083. doi: 10.1126/sciimmunol.abi7083
12. Llewellyn HP, Arat S, Gao J, Wen J, Xia S, Kalabat D, et al. T Cells and monocyte-derived myeloid cells mediate immunotherapy-related hepatitis in a mouse model. J Hepatol (2021) 75:1083–95. doi: 10.1016/j.jhep.2021.06.037
13. Riddiough GE, Jalal Q, Perini MV, Majeed AW. Liver regeneration and liver metastasis. Semin Cancer Biol (2021) 71:86–97. doi: 10.1016/j.semcancer.2020.05.012
14. Urata K, Hashikura Y, Ikegami T, Terada M, Kawasaki S. Standard liver volume in adults. Transplant Proc (2000) 32:2093–4. doi: 10.1016/s0041-1345(00)01583-9
15. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol (2020) 72:288–306. doi: 10.1016/j.jhep.2019.09.026
16. Ke L, Shen R, Fan W, Hu W, Shen S, Li S, et al. The role of associating liver partition and portal vein ligation for staged hepatectomy in unresectable hepatitis b virus-related hepatocellular carcinoma. Ann Transl Med (2020) 8:1402. doi: 10.21037/atm-20-2420
17. Yang X, Xu H, Zuo B, Yang X, Bian J, Long J, et al. Downstaging and resection of hepatocellular carcinoma in patients with extrahepatic metastases after stereotactic therapy. Hepatobil Surg Nutr (2021) 10:434–42. doi: 10.21037/hbsn-21-188
18. Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg (2004) 240:299–305. doi: 10.1097/01.sla.0000133123.11932.19
19. Takahashi EA, Fleming CJ, Andrews JC. Future liver remnant hypertrophy after portal vein embolization is inversely correlated with intrahepatic tumor burden. J Vasc Interv Radiol (2019) 30:435–9. doi: 10.1016/j.jvir.2018.10.014
20. König D, Läubli H. Mechanisms of immune-related complications in cancer patients treated with immune checkpoint inhibitors. Pharmacology (2021) 106:123–36. doi: 10.1159/000509081
21. Dong Z, Zhang J, Sun R, Wei H, Tian Z. Impairment of liver regeneration correlates with activated hepatic NKT cells in HBV transgenic mice. Hepatology (2007) 45:1400–12. doi: 10.1002/hep.21597
22. Tao L, Wang S, Kang G, Jiang S, Yin W, Zong L, et al. PD-1 blockade improves the anti-tumor potency of exhausted CD3(+)CD56(+) NKT-like cells in patients with primary hepatocellular carcinoma. Oncoimmunology (2021) 10:2002068. doi: 10.1080/2162402x.2021.2002068
23. Chan A, Zhang WY, Chok K, Dai J, Ji R, Kwan C, et al. ALPPS versus portal vein embolization for hepatitis-related hepatocellular carcinoma: a changing paradigm in modulation of future liver remnant before major hepatectomy. Ann Surg (2021) 273:957–65. doi: 10.1097/sla.0000000000003433
24. Lopez-Lopez V, Robles-Campos R, Brusadin R, Lopez-Conesa A, de la Peña J, Caballero A, et al. ALPPS for hepatocarcinoma under cirrhosis: a feasible alternative to portal vein embolization. Ann Transl Med (2019) 7:691. doi: 10.21037/atm.2019.10.57
Keywords: unresectable hepatocellular carcinoma, immunotherapy, associating liver partition and portal vein ligation for staged hepatectomy, future liver remnant, immune checkpoint inhibitors
Citation: Ning C, Liu G, Zhang J, Yang X, Xu Y and Zhao H (2023) Case Report: The application of associating liver partition and portal vein ligation for staged hepatectomy in patients with hepatitis b virus-related hepatocellular carcinoma after undergoing treatment with an immune checkpoint inhibitor: a report of two cases. Front. Immunol. 14:1159885. doi: 10.3389/fimmu.2023.1159885
Received: 06 February 2023; Accepted: 19 April 2023;
Published: 09 May 2023.
Edited by:
Fanping Meng, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Marc Micó-Carnero, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), SpainDelavar Shahbazzadeh, Pasteur Institute of Iran (PII), Iran
Copyright © 2023 Ning, Liu, Zhang, Yang, Xu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobo Yang, eWFuZ3hpYW9ibzY3QHB1bWNoLmNu; Yiyao Xu, eHV5aXlhb0Bob3RtYWlsLmNvbQ==; Haitao Zhao, emhhb2h0QHB1bWNoLmNu
†These authors have contributed equally to this work and share first authorship
 Cong Ning1†
Cong Ning1† Junwei Zhang
Junwei Zhang Haitao Zhao
Haitao Zhao