- 1Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 2Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
Ulcerative colitis (UC), a type of inflammatory bowel disease characterized by recurring and incurable symptoms, causes immense suffering and economic burden for patients due to the limited treatment options available. Therefore, it is imperative to develop novel and promising strategies, as well as safe and effective drugs, for the clinical management of UC. Macrophages play a critical role as the initial line of defense in maintaining intestinal immune homeostasis, and their phenotypic transformation significantly influences the progression of UC. Scientific studies have demonstrated that directing macrophage polarization toward the M2 phenotype is an effective strategy for the prevention and treatment of UC. Phytochemicals derived from botanical sources have garnered the interest of the scientific community owing to their distinct bioactivity and nutritional value, which have been shown to confer beneficial protective effects against colonic inflammation. In this review, we explicated the influence of macrophage polarization on the development of UC and collated data on the significant potential of natural substances that can target the macrophage phenotype and elucidate the possible mechanism of action for its treatment. These findings may provide novel directions and references for the clinical management of UC.
1 Introduction
Ulcerative colitis (UC) is a chronic, idiopathic inflammatory disorder that affects the mucosa of the colon and rectum consecutively. It is listed by the World Health Organization as a modern refractory disease, with typical clinical symptoms including recurrent abdominal pain, diarrhea, and hematochezia. Over recent decades, the incidence rate of UC has surged globally, ranging from 0.5 to 31.5 per 100,000 individuals annually across various populations, according to relevant statistics. An escalating body of evidence has indicated that UC typically manifests in individuals aged 30 to 40 years, with no sex predominance (1–3). Despite significant advancements made in the past decades, the precise etiology of UC largely remains an enigma. Pathogenic factors, including genetic predisposition, environmental factors, intestinal dysbiosis, and immune dysregulation, are widely acknowledged as being involved in the development of UC (3, 4).
Lifelong treatment is almost imperative for patients diagnosed with UC due to the dearth of known preventive or fundamentally curative interventions. The current therapeutic goal is persistent remission, mucosal healing, and mitigation of the risk of colorectal neoplasia. As the major treatment option for UC, typical drugs used in the clinic mainly include aminosalicylates, corticosteroids, immunosuppressants, biological agents, and microecologics (5, 6), which have not achieved satisfactory results on account of the various adverse effects, drug tolerance, and the high rate of recrudescence, among others (7, 8). Hence, there is an urgent need to explore and develop novel safe and effective medications for UC.
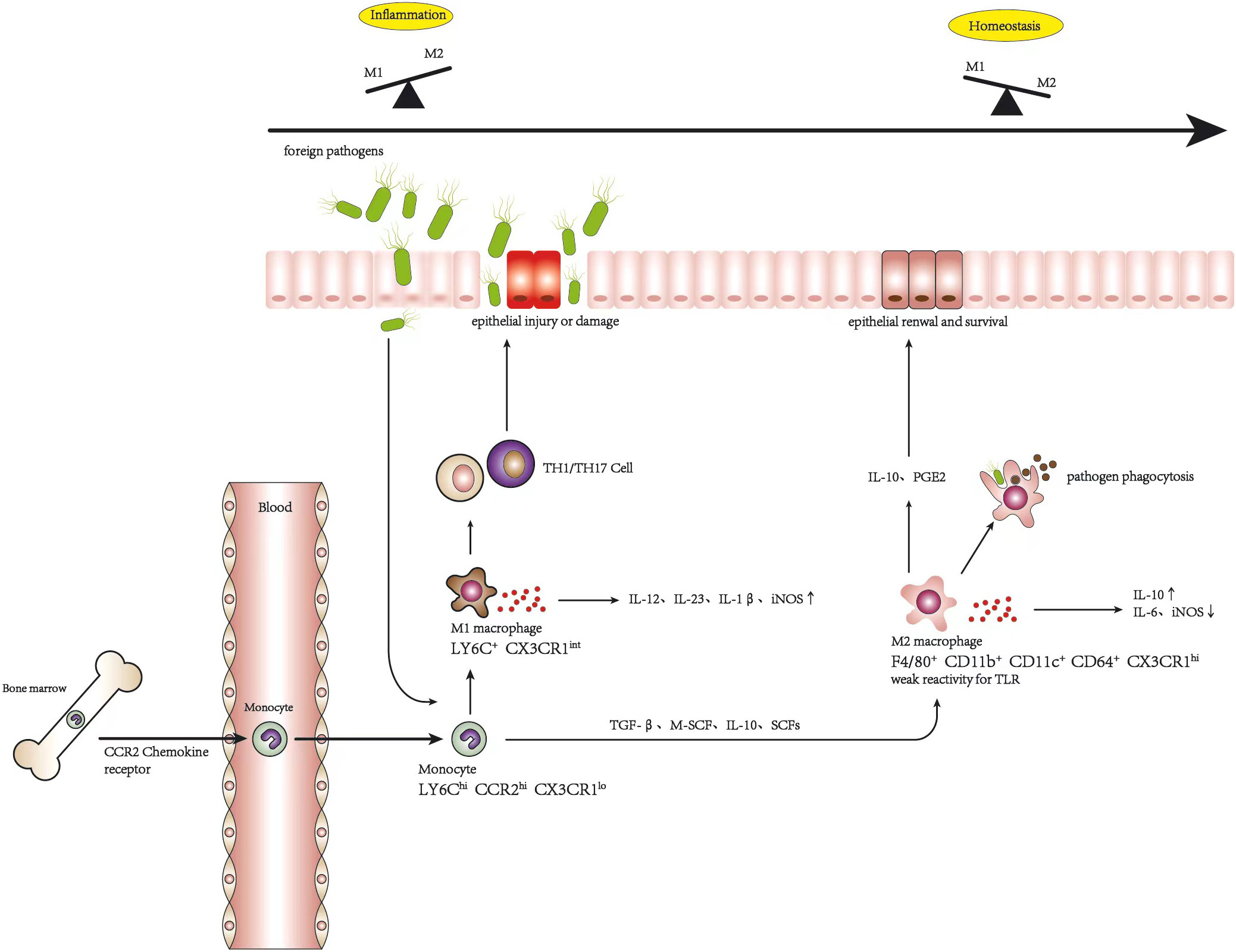
Macrophages, as the sentinels of intestinal immune homeostasis, can manifest diverse functional phenotypes in response to various environmental cues and stimuli, thereby either promoting or resolving intestinal inflammation, which play an essential role in the development of UC. Based on the in vitro model of monocyte-derived macrophages, the macrophage population can be divided into the classically activated M1 macrophages with pro-inflammatory activity and the alternatively activated M2 macrophages with anti-inflammatory characteristics. In intestinal homeostasis, resident macrophages usually present an M2 phenotype with low reactivity to Toll-like receptor (TLR) ligands to maintain tolerance to the different antigens from food and symbiotic microflora (9). On the contrary, in the colon of patients with UC, M1 macrophages play a dominant role with the excessive accumulation of pro-inflammatory factors, leading to the damage of the intestinal epithelial barrier and the imbalance of immune homeostasis (10, 11). Furthermore, there is compelling evidence that macrophage polarization tends to be a severe imbalanced condition in the intestinal tissues of patients with UC who are non-responsive to conventional treatments (12, 13). Therefore, targeting macrophage polarization is often an attractive aspect for UC therapy.
2 Macrophage
Evidence indicates that intestinal resident cells are derived from embryonic precursors that undergo continuous in situ proliferation during the neonatal period and are subsequently replaced by macrophages originating from peripheral blood with advancing age (14, 15). Due to their constant exposure to gut pathogens and their high energy consumption, lamina propria (LP) macrophages have a short life span and necessitate continuous replenishment by bone marrow-derived peripheral blood monocytes, which subsequently differentiate into mature macrophages in the gut (16). The most abundant macrophage population in the body is distributed in the gastrointestinal mucosa, especially in the LP near the epithelium, while a small proportion exists in the smooth muscle layer of the intestinal wall (16). Maintaining gut immune homeostasis is a cooperative and an elaborately dynamic process that requires moderate tolerance for the beneficial commensal microorganisms colonizing the gastrointestinal tract and the innocuous antigens from food substances. At the same time, it is vital to respond promptly to invading pathogens for host protection (17). As a crucial component of innate immunity, intestinal macrophages are polarized to different phenotypes by environmental cues, with the heterogeneous functions of identifying pathogens, phagocytosing microorganisms and debris, remodeling impaired tissues, supporting regulatory T cells, and regulating inflammation, which are considered as the main factors contributing to and maintaining intestinal homeostasis (18–20). Throughout the construction of monocyte-derived macrophage models in vitro, the macrophage population can be classified into two categories with opposing functions: the classically activated macrophages (M1 macrophages) that represent pro-inflammatory conditions and the alternatively activated macrophages (M2 macrophages) that represent anti-inflammatory conditions (21–25). Once the balance of macrophage polarization is broken, dysfunction will occur, impairing the ability to maintain homeostasis and sense signals of tissue damage in the body, which may lead to inflammatory diseases (26). It is worth noting that, while this taxonomic approach substantially contributes to the comprehension of the metabolic programming of the different macrophage functions, it may not fully reflect the in vivo condition of macrophages, which are influenced by complex environmental cues and may display features of both phenotypes (9, 16). Research in this area has shown that when this occurs to certain cytokines or complexes such as transforming growth factor beta (TGF-β), glucocorticoids, or the immune complex, macrophages become a continuum of activation forms alongside the M1/M2 axis, with similar but different transcription and functions (9, 27). However, categorization into either M1 or M2 brings benefits in macrophage polarization to understand their heterogeneous functions and their transformation.
2.1 M1 macrophages
Generally, M1 macrophages could be activated by tumor necrosis factor alpha (TNF-α) and TLR ligands such as lipopolysaccharides (LPS) or interferon gamma (IFN-γ); overexpress CD64, CD86, and CD16/32; and secrete high levels of the pro-inflammatory cytokines TNF-α, IL-1α, IL-1β, IL-6, IL-12, and IL-23, among others. In terms of function, the M1 phenotype possesses antigen presentation, pathogen elimination, and antitumor abilities. These macrophages synthesize nitric oxide (NO), which can mediate protection against infection and reactive oxygen species (ROS)-induced tissue damage, as well as impair tissue regeneration and wound healing. Moreover, a large amount of inducible nitric oxide synthase (iNOS) is excreted by M1 macrophages, which is regarded as an antimicrobial cytokine (28, 29).
2.2 M2 macrophages
Representing anti-inflammatory activity, M2 macrophages are identified by distinct markers including IL-10, CD206, and CD163 (30) and are polarized by stimulating the Th2 cytokines IL-4 and IL-13 via the activation of STAT6 through IL-4 receptor alpha (IL-4Rα). In addition, IL-10 can also induce the M2 phenotype by activating STAT3 via the IL-10 receptor (IL-10R). Functionally, M2 macrophages have the potent capacity of phagocytosis, obliterate the debris of apoptotic cells, accelerate tissue repair and wound healing, and possess pro-angiogenic and pro-fibrotic properties. In addition, these macrophages produce higher levels of IL-10 and arginase 1 (Arg-1), an effector enzyme in urea metabolism that inhibits immune responses (29, 31).
3 The origination of intestinal macrophages
Intestinal resident macrophages are mainly supplemented by circulating monocytes recruited to the mucosa, which express lymphocyte antigen 6C-high (LY6Chi), CC-chemokine receptor 2-high (CCR2hi), and CX3C-chemokine receptor 1-low (CX3CR1low) in a mouse model. Subsequently, after entering the intestinal mucosa and encountering special intestinal signals such as TGF-β, macrophage colony-stimulating factor 1 (CSF-1 or M-CSF), and IL-10, as well as some environmental cues including short-chain fatty acids (SCFAs) produced by the gut microbiota (32), LY6Chi monocytes begin to differentiate locally to mature resident macrophages. During this process, the monocyte population first occurs in major histocompatibility complex class II (MHCII) before increasing F4/80 with a decline in LY6C throughout a series of short-lived CX3CR1int intermediates (33). Mature intestinal macrophages can be distinguished by F4/80+, CD11b+, CD11c+, and CD64+ with a high expression of CX3CR1 (34). Previous research has shown that, in intestinal homeostasis, the majority of resident macrophages exhibit weak reactivity for the stimulation of TLR, increased production of anti-inflammatory cytokines such as IL-10, and suppression of pro-inflammatory cytokines such as iNOS and IL-6 (32), which express CD163 and CD206 (35), characterized more like an M2 macrophage. These macrophages play a pivotal role in the regulation of gut homeostasis via the clearance of harmful bacteria and adventive substances, IL-10, and the excretion of prostaglandin E2 to stimulate epithelial stem cell renewal and survival to promote the integrity of the epithelial barrier (36).
Circulating monocyte differentiation in the context of inflammation occurrence has been changed and disrupted compared with the above description, which is switched to polarize into a pro-inflammatory condition, M1 macrophage. During this period, the terminal differentiation process of LY6Chi monocytes into mature intestinal macrophages is disrupted, leading to the accumulation of LY6Chi monocytes, LY6Cint population, MHCII-positive and CX3CR1int immature macrophages. LY6CintCX3CR1int cells retain their pro-inflammatory capacity through the secretion of inflammatory cytokines, including IL-12, IL-23, and IL-1β, thereby promoting type 1 T helper (Th1) and Th17 immune responses and aggravating tissue damage (32). There is evidence that the proportion and the number of CX3CR1int macrophages obviously increase in mice after management with dextran sulfate sodium (DSS) (35).
Consequently, to some degree, Ly6Clo monocytes could be regarded as the macrophages of the circulatory system. Monocytes gradually undergo differentiation into resident macrophages in intestinal homeostasis. When the colon is inflamed, resident macrophages are still replenished from monocytes in blood circulation. However, the phenotype changes from tolerant to sensitive to ambient and pro-inflammatory, with high TLR expression, and the balance of macrophage polarization is deflected to M1 macrophages. More interestingly, research has revealed that Ly6Chi monocytes can also be transformed into Ly6Clo monocytes and subsequently returned to the bone marrow to replenish the local macrophage population (9, 37). Nevertheless, the reasons underlying the dysregulation of macrophage polarization are not yet fully understood and may be related to the exceptional accumulation of monocytes and their response to local alterations or repolarization between M1 and M2 macrophages. Of course, it cannot be ruled out that both scenarios may act in concert (9, 35).
4 Macrophage polarization affects the development of UC
The origination of macrophages posits that M1 and M2 macrophages are distinct subsets polarized from a common precursor, displaying diversity in phenotype and function, which can be polarized into various phenotypes in response to multiple environmental cues, consequently acquiring different abilities and transforming into each other under specific conditions (19, 35). CCR2+L6Cyhi monocytes can replenish macrophages in all phenotypes during intestinal homeostasis and inflammation, which differentiate as a continuum of CX3CR1int pool. UC is a chronic inflammatory disease driven initially by the disruption of the epithelial barrier, which is composed of a single layer of intestinal cells that connect with adjacent cells to form a continuous physical barrier, controlling the permeability of the luminal content (38). When foreign pathogens invade intestinal by crossing the injured epithelial layer, the balance of macrophages is deflected as M1 macrophages presenting a pro-inflammatory condition to engulf foreign materials and secrete pro-inflammatory cytokines such as TNF-α, IL-6, IL12, and IL-23, which promote immune responses mediated via Th1 and Th17 cells to protect the host from invasion (26). Under ideal conditions, the protective inflammatory response is self-limited and would completely resolve after the pathogens were eliminated, without causing tissue damage or impairment of wound healing due to excessive immune response. Generally, it is an active process controlled by the recruitment of M1 macrophages and the accumulation of M2 macrophages (32). However, the balance of macrophage polarization is destroyed gradually by the infiltration of more and more M1 macrophages, inflammatory cytokines are overexpressed, and a high level of iNOS is induced, which directly or indirectly affect intestinal epithelial cells, leading to their injury or necrosis, which elevates the occurrence and development of UC (9). The alteration of macrophage polarization in inflammation and homeostasis is shown in Figure 1. Furthermore, Lissner et al. revealed that M1 macrophages invaded intestinal deregulated tight junction proteins and induced epithelial cell apoptosis to disrupt the epithelial barrier directly (39). Correspondingly, there is evidence that the polarization of macrophages gives priority to the M1 phenotype in the intestinal mucosa of patients with inflammatory bowel disease (IBD) and in experimental colitis mice (11, 39, 40). It should be noted that, although colonic M1 macrophages predominate during colitis, M2-like resident macrophages are also present to combat inflammation and to facilitate wound healing, which helps resolve the inflammation (36). Indeed, promoting the phenotype of anti-inflammatory M2 macrophages has been considered a promising treatment for IBD. M2 macrophages play an important role in the alleviation of colitis.
5 Targeting macrophage polarization as an effective strategy for UC treatment
Given the significant impact of macrophage polarization on the development of UC, targeting the skewed axis represents a promising strategy for its prevention and treatment and has spurred extensive research. In recent years, studies have revealed that M1 macrophage enrichment in the colon biopsies of patients with IBD was positively correlated with disease severity (41). In addition, there is evidence that vedolizumab-induced reduction in the ratio of M1/M2 macrophages does contribute to the resolution of intestinal inflammation (42). It has been demonstrated that intraperitoneal or intravenous injection of exogenous bone marrow-derived M2 macrophages could effectively reduce the severity of colitis in mice caused by dinitrobenzene sulfonic acid (DNBS) (43, 44). Furthermore, it was evidenced that mice with deficient M2 macrophage polarization were more vulnerable to colitis induced by DSS (45). Caprioli et al. demonstrated that the downregulation of M1 macrophage pathway genes was connected to the mucosal healing of patients with IBD through treatment with infliximab, and M1 macrophages were significantly decreased, which was associated with the increased rate of macrophage apoptosis, representing a key mechanism for the therapeutic success of antitumor necrosis factor antibodies (46). Parallel results exist showing a prominent increase of the proportion of M2 macrophages in patients with IBD responding to infliximab therapy, but not in non-responders (30, 47). Eissa et al. have provided proof that the intestinal mucosa of patients diagnosed with UC is characterized by abundant infiltration of pro-inflammatory macrophages, producing a significant number of inflammatory mediators (e.g., TNF-α, IL-1β, and IL-6) through the activation of the nuclear factor kappa B (NF-κB) signaling pathway, which is negatively correlated with chromofungin (CHR), a short peptide with antimicrobial effects encoded from chromogranin A exon IV that is downregulated in UC. Moreover, exogenous CHR administration significantly mitigates colitis associated with a reduction of M1 macrophage markers (48). In addition, it has been revealed that intracolonic administration of CHR can increase M2 macrophage polarization, which decrease colonic collagen deposition and sustain the homeostasis of intestinal epithelial cells, thus protecting against colitis induced by DSS (49). Follistatin-like protein 1 (FSTL1), a pleiotropic cytokine that participates in a comprehensive spectrum of physiological and pathogenic processes, exhibits a highly expressional acitivity in human and mouse UC. It facilitates pro-inflammatory M1 phenotype macrophages and inhibits the M2 anti-inflammatory phenotype, leading to the excessive production of various inflammatory cytokines in vitro and in vivo. Li et al. found that the inhibition of FSTL1 could lead to UC remission, and the phenomenon disappears with the depletion of macrophages (50). Park et al. showed that adipose tissue-derived mesenchymal stem cells (ASCs) reduced the large amount of macrophages and the M1 macrophage population to mitigate UC in a model of DSS-induced mice. In the cell culture experiment, it was indicated that ASCs take effect by promoting the phenotype transition from M1 to M2, leading to anti-inflammatory cytokine proliferation (51). Cao et al. also reported that extracellular vesicles (EVs) secreted by bone marrow mesenchymal stem cells (BMSCs) could reinforce M2 macrophage polarization, supported by increased level of CD163 as the M2 marker, to effectively lessen the severity of UC. This result appeared to be associated with the JAK1/STAT1/STAT6 signaling pathway (52). These discoveries presume that the polarization of macrophages may be connected to mucosal healing in patients with IBD and could be an effective therapy for this disease.
6 Multiple phytochemicals show therapeutic prospects in UC treatment by targeting macrophage polarization
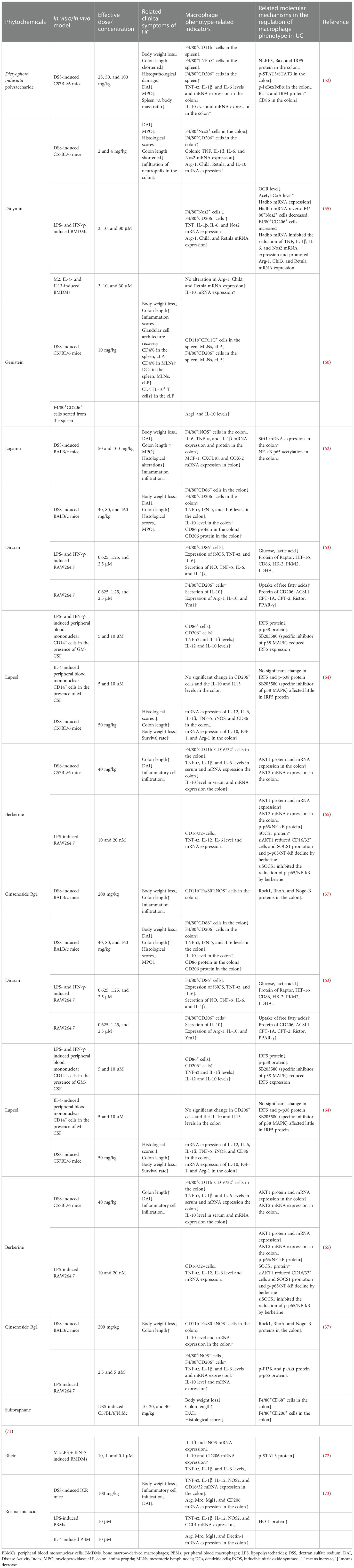
The first-line therapy currently used in the clinic for mild to moderate UC is mainly 5-aminosalicylic acid (5-ASA) drugs, which can be administered as suppositories, enemas, or oral preparations (53). Patients who do not respond to or do not achieve remission with 5-ASA drugs can be treated with corticosteroids (54), but glucocorticoids should not be used to maintain remission because of their lack of long-term efficacy and the risk of side effects (55). Thiopurines or biologic drugs, or both, should be used in patients with moderate to severe colitis, but long-term use must be carefully monitored for associated adverse effects, such as lymphoproliferative disorders (56, 57). Surgical treatment is usually indicated for uncontrollable massive bleeding, perforation, or endoscopically unresectable UC-associated adverse lesions (58). Phytochemicals, which are extracted from nature and with widely available sources, have been confirmed to possess abundant biological activities, as well as relatively low toxicity and high efficacy, which are particularly prominent in antitumor applications (59–61). The utilization of natural products in the management and prevention of various ailments can be traced back to ancient times, owing to their remarkable and indisputable effectiveness. The wealth of active compounds and diverse agent functions in natural products has always been an appealing prospect for researchers to explore and investigate novel phytochemical entity drugs with fewer adverse effects, thereby leading to more effective clinical application. In recent decades, a wealth of data has emerged indicating that numerous active ingredients sourced from plants and natural products hold immense potential in the management of IBD. This article aimed to present recent research focused on the treatment of UC using natural product-derived drugs that have been experimentally confirmed to be beneficial in vitro or in vivo based on online search protocols including PubMed, Web of Science, and Elsevier SD. The following key search terms were used: ‘ulcerative colitis,’ ‘Inflammatory bowel disease,’ ‘colitis,’ ‘Intestinal inflammation,’ ‘macrophage,’ ‘polarization,’ and ‘natural products,’ ‘compound,’ ‘phytochemical.’ The phytochemicals targeting macrophage polarization in UC treatment are listed in Table 1.
Dictyophora indusiata polysaccharide (DIP)
Dictyophora indusiata polysaccharide (DIP) isolated from dictyophora indusiate one of the most popular edible mushrooms due to its daintiness and multi-nutrition, was reported to possess potent antioxidant and anti-inflammatory activities in vitro (74, 75). Recent evidence has shown that DIP could conspicuously alleviate the severity of colitis in DSS-induced mice, and this mitigation is associated with the restoration of gut microbiota function and gut epithelial integrity, improvement of oxidative stress, and regulation of macrophage polarization balance (62, 76, 77). Wang et al. found that treatment with DIP significantly reduced M1 macrophage polarization and promoted the M2 phenotype in the spleen of mice orally administered DSS, and the macrophages marked by CD86 in the colon were also inhibited, which were consistent with the deregulation of the expression of TNF-α, IL-6, and IL-1β and the high secretion of IL-10 after DIP administration. In addition, DIP downregulated the activation of the NF-κB, STAT3, and NLRP3 signaling pathways in the colon of mice treated with DSS, which may be associated with the mechanism of macrophage polarization balance (62). Significantly, the biological activity of polysaccharides is highly correlated to their conformation of space, which means that their efficacy would greatly weaken or even vanish once they are degraded into monosaccharides or oligosaccharides.
Didymin
Didymin, a dietary glycoside widely distributed in citrus fruits such as mandarin, bergamot, orange, Origanum, and Vulgare Duanxueliu, has attracted attention due to its antioxidant capacity (63). Recently, Lv et al. have found that didymin can effectively reduce colitis in mice by targeting macrophage polarization to the M2 phenotype. Their experiment results showed that didymin decreased the proportion of M1 and increased M2 in the colon of mice induced by DSS, and mice injected with exogenous M1 macrophages after being administered with clodronate liposomes to deplete autologous macrophages exhibited more sensitivity to DSS; however, the severity of colitis was declined by didymin management before exogenous M1 macrophages injection. Interestingly, didymin resisted M1 macrophage polarization, but there was no alteration on M2 macrophages and on the expression of Arg-1, Chil3, and Retnla, indicating that the effect of didymin on ameliorating colitis is dependent on the transformation of M1 macrophages toward M2. A further study suggested that the macrophage phenotype modulation of didymin is presented through the improvement of fatty acid oxidation (FAO) by fortifying the expression of Hadhb (60).
Genistein
Genistein, an isoflavonoid compound widely distributed in soy-based products (78), also called phytoestrogen owing to a pattern resembling estradiol, shows high anti-inflammatory, anticancer, antioxidant, and antidiabetic properties and has attracted interest in medical research (64, 79, 80). Recently, it has been revealed that genistein could conspicuously mitigate experimental colitis, which is associated with targeting macrophage polarization. The results of the experiment indicated that the administration of genistein resulted in the decline of M1 and the elevation of M2 macrophages in the spleen, mesenteric lymph nodes (MLNs), and colon lamina propria (cLP) of DSS-induced mice. Apart from this, the M2 macrophages sorted from colitis mice induced by genistein highly expressed Arg-1 and IL-10 compared with those managed using phosphate-buffered saline (PBS). However, the detailed mechanism of how genistein shifts M1 macrophages toward the M2 phenotype still remains unclear (81).
Loganin
Loganin, a type of bioactive iridoid glycoside extracted from traditional Chinese medicine, commonly called Cornus officinalis, was established to have potent anti-depression, neuropathic protection, and anti-inflammation effects (82–84). Yuan et al. reported that loganin could prominently alleviate the pathologic alterations of DSS-induced colitis, increase the tight junction proteins to protect the intestinal epithelial barrier, and inhibit the expression of colonic pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α (85). Another study demonstrated the high expression of Sirt1, inhibition of the acetylation of NF-κB p65, and the suppression of loganin in the M1 macrophages of colitis mice, which was counteracted after using the Sirt1 inhibitor Ex527. These findings suggest that the therapeutic potential may have involved the inhibition of M1 macrophages regulated by the Sirt1/NF-κB pathway (65).
Dioscin
Dioscin is a steroid saponin isolated from Dioscorea nipponica (86), which has been reported to be a potential therapeutic component for colitis. It showed high availability in suppressing glycolysis and promoting FAO to predispose macrophage polarization from M1 toward M2. Interestingly, the agonist of the mTORC1 signal could reverse the effects of dioscin on the downregulation of glycolysis and the counteraction of the HIF-1α protein expression, which is indispensable for the transcription of the inflammatory cytokines and metabolic genes associated with glycolysis, resulting in the abortion of M1 macrophage decline. In addition, after administration of the mammalian target of rapamycin complex 2 (mTORC2) inhibitor, the enhancement of dioscin on the peroxisome proliferator-activated receptor gamma (PPAR-γ) protein and FAO-related enzymes was prominently impaired, and the promotion of M2 macrophages was counteracted as well. These findings demonstrated that dioscin modulated the polarization and metabolism of macrophages by regulating the mTORC1/HIF-1α and mTORC2/PPAR-γ signaling pathways to mitigate the severity of colitis, which was further confirmed in experimental colitis mice (87). Similarly, Shi et al. showed that dioscin catalyzed the expression of miR-125a-5p to shift macrophages toward the M2 phenotype, thereby restoring the intestinal epithelial barrier function and facilitating experimental colitis (88).
Lupeol
Lupeol is a triterpenoid compound with exclusive bioactivity found in numerous natural plants including Albizia lebbeck and Alnus glutinosa (89). It has been reported that lupeol exhibited protective effects against colitis in experimental animals, and this involved blocking the NF-κB signaling of intestinal epithelial cells and modulating macrophages leaning toward the M2 phenotype to relieve inflammatory responses (66, 90). Zhu et al. observed that IRF5, a key transcription factor associated with M1 macrophages, was remarkably reduced after lupeol incubation of M1 macrophages induced by LPS and IFN-γ with exposure to granulocyte-macrophage colony-stimulating factor (GM-CSF), but the same results were not detected in M2 macrophages, which were inferred to be bound with the modulation of a specific signaling pathway. This hypothesis was subsequently confirmed as studies showed that the p38 mitogen-activated protein kinase (MAPK) phosphorylation of M1 macrophages was reduced by lupeol, which was counteracted by the use of the p38 MAPK inhibitor (90). Therefore, lupeol possibly inhibits IRF5 through a specific receptor and downstream signaling pathway, such as p38 MAPK, to switch M1 macrophages toward M2.
Berberine
Berberine, a plant isoquinoline alkaloid largely found in the root of Coptis chinensis (91), has been proven to be beneficial in colitis treatment through various mechanisms, such as inhibiting the IFN-γ and JAK2/STAT3 signaling pathways to attenuate inflammatory responses (67, 92), regulating the intestinal mucosal immune homeostasis through the Wnt/β-catenin pathway (93), and reducing the activation of the MAPK and NF-κB signaling pathways to decrease pro-inflammatory cytokine production (68). Recently, Yunxin et al. have reported that berberine could correct macrophage polarization imbalance by inhibiting differentiation of the M1 phenotype to prevent colitis development directly by upregulating the AKT1 pathway and the protein expression of SOCS1, one of the target genes of AKT1, and decreasing the level of NF-κB phosphorylation. In addition, it was observed that knocking out the AKT1 gene reversed the effect of berberine on the modulation of SOCS1 and NF-κB phosphorylation protein expression. The downregulation of berberine on M1 macrophage polarization and related pro-inflammatory cytokines, such as IL-6 and TNF-α, was also neutralized on account of AKT1 small interfering RNA (siRNA) transfection, suggesting that the inhibitory activity of berberine on M1 polarization is dependent on the AKT1/SOCS1/NF-κB signaling pathway (94).
Ginsenoside Rg1
Ginsenoside Rg1 is a major active constituent of Panax ginseng and has been reported to be an anti-inflammatory treatment for various diseases (69). Recent evidence has shown that ginsenoside Rg1 could conspicuously ameliorate the severity of symptoms and reduce the inflammatory response by downregulating the expression of TNF-α, IL-33, IL-6, and CCL-2 in a DSS-induced colitis mouse model (40, 95). Ginsenoside Rg1 has been reported to be a good regulator of macrophage polarization, which increased the M2 phenotype and inhibited the M1 phenotype, similar to Y27632, a specific inhibitor of Rock1. Furthermore, it is worth noting that the increase in the expression of the Rock1, RhoA, and Nogo-B proteins in the colonic tissues of colitis mice was attenuated by ginsenoside Rg1 and Y27632, demonstrating that the trends of Nogo signaling in the regulation of the macrophage phenotype in colitis mice were largely consistent with ginsenoside Rg1. These results may imply that regulation by ginsenoside Rg1 of the phenotype of macrophages in colitis mice may be associated with the Nogo-B signaling pathway (40).
Baicalin
Baicalin, one of the active ingredients of Scutellaria baicalensis Georgi, was proven to be therapeutic in IBD (70, 96). Zhu et al. investigated the anti-inflammatory effect of baicalin against LPS-induced mouse peritoneal macrophages and found that it could effectively inhibit the LPS-induced promotion of the inflammatory macrophage subset of M1, reducing the ratio of M1/M2. Consistently, they found that baicalin treatment obviously mitigated the severity of DSS-induced colitis in mice (97). The related mechanism may involve the regulation of the IRF4/IRF5 protein expression of baicalin, as the results showed that baicalin could directly facilitate the protein expression of IRF4 and block that of IRF5, and the decline of the M1/M2 of baicalin was reversed after IRF4 siRNA transfection (97).
Toosendanin (TSN)
Toosendanin (TSN) is a triterpenoid distributed in the bark or fruits of a type of commonly used Chinese herbal medicine, known as Melia toosendan Sieb et Zucc (59). Fan et al. determined that TSN could alleviate the symptoms of DSS-induced mice by reducing the inflammatory responses and macrophage polarization, as the experiment results showed that TSN could downregulate the percentage of the M1 phenotype and the expression of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, but promoted M2 macrophages. A further study revealed that the activation of NLRP3 induced by DSS in the colonic macrophage of colitis mice was reversed by TSN, which influenced the composition of IL-1β. Interestingly, TSN acted as an activator of the NFE-related factor 2 (Nrf2) signaling pathway, a key transcription factor facilitating the antioxidant response via the synthesis of heme oxygenase-1 (HO-1), which modulated IL-10 production to affect the macrophage phenotype (71). The results showed that the decline of the colonic expression of Nrf2 and HO-1 in mice induced by DSS was counteracted by TSN management (98). This evidence implied that TSN regulation of macrophage alteration attenuating DSS-induced colitis is associated with the NLRP3 and Nrf2/HO-1 pathways, but the specificity of the relationship needs further validation.
Artemisinin
Artemisinin is the main active compound isolated from Artemisia annua L, which was initially popular for its strong antimalarial properties, but which has also been revealed in recent years to exert various activities such as antivirus, anti-parasite, tumor suppression, and inflammation prevention (72). Previous evidence showed the protective function of artemisinin against DSS-induced colitis in mice, which involved the induction of CYP3A expression through the activation of the pregnane X receptor (PXR) (99). A study by Huai et al. provided proof that the inflammatory colonic tissues of patients with Crohn’s disease (CD) presented significantly increased M2 macrophages marked by CD11b+CD206+ and reduction of the pro-inflammatory cytokine expression after the administration of artemisinin in vitro. In addition, it has been suggested that artemisinin could mitigate the symptoms of colitis via upregulating the macrophages of murine colitis tissues polarized to the M2 phenotype, which may be associated with inhibiting the MYD88 and ERK signaling pathways owing to evidence showing that artemisinin significantly suppressed MyD88 activation and ERK phosphorylation in the colon tissue of a DSS-induced mouse model (100). However, the specific mechanism of artemisinin on the MYD88 and ERK signaling pathways affecting the regulation of macrophage phenotype remains to be further studied.
Tiliroside
Tiliroside, a natural flavonoid derived from several medicinal and dietary plants, such as linden, rosehip, and strawberry, was revealed to exhibit anti-inflammatory, antioxidant, anticarcinogenic, and hepatoprotective activities (73). Zhuang et al. reported that the protective function of tiliroside in UC was related to the blocking of M1 macrophage polarization, and this effect was mainly achieved by accelerating the proteasomal degradation of HIF-1α, consequently attenuating glycolysis. This was validated in the experiments showing that tiliroside could significantly decrease the extraction of 2-NBDG, a fluorescent deoxyglucose analog widely used in detecting cellular glucose uptake; the gene expression of glycolytic enzymes such as glucose transporter 1 (Glut1), enolase 1 (Eno1), and pyruvate kinase M (Pkm); and the production of lactate in bone marrow-derived macrophages (BMDMs) induced by LPS and IFN-γ. Another evidence exhibited tiliroside prominently downregulating the protein level of HIF-1α, but had no effect on the mRNA expression. In addition, tiliroside ceased to be effective after using clodronate liposomes, which can significantly deplete macrophages in vivo, suggesting that tiliroside inhibited colitis through a macrophage-dependent mechanism (101). In a word, the findings above showed the potential of tiliroside as a therapeutic strategy for UC through targeting the HIF-1a/glycolysis pathway to mediate M1 macrophage reduction.
Platycodin D (PLD)
Platycodin D (PLD) is a triterpenoid saponin extracted from the root of the Platycodon grandiflorum plant (102). Guo et al. studied the anti-inflammatory effects of PLD on DSS-induced colitis in mice, as well as on LPS-induced RAW264.7, and found that PLD was effective in mitigating colitis through shifting macrophage polarization to deflect the M2 phenotype. Further examination showed that the property of PLD on the regulation of macrophage polarization involved the activation of the PI3K/Akt pathway and the inhibition of the NF-κB pathway, as data revealed the upregulation of p-PI3K and p-Akt proteins with a decline of the nuclear translocation of the p65 subunit after PLD administration in LPS-stimulated RAW264.7 cells, which was further confirmed to be or at least partly dependent on adenosine 5′-monophosphate-activated protein kinase (AMPK) due to the effect of PLD on PI3K/Akt and NF-κB pathway modulation being reduced after the knockdown of AMPK. It is noteworthy that a higher dose of PLD exerted a lower anti-inflammatory effect on the macrophages managed by LPS compared to a lower dose, which may be associated with the modest suppression of cell activity (103).
Sulforaphane
Sulforaphane is a dietary isothiocyanate widely distributed in cruciferous vegetables such as broccoli, cabbage, and Brussels sprouts. It possesses great antioxidant and anti-inflammatory activities (104). Studies on DSS-induced colitis in mice found that management with sulforaphane could conspicuously improve the clinical symptoms of colitis and the damaged epithelial integrity (105). Sun et al. reported that sulforaphane elevated the IL-10 production of LPS- and IFN-γ-induced BMDMs and switched the macrophages from the M1 to the M2 phenotype with the activation of STAT3. Moreover, after the neutralization of IL-10, the effect of sulforaphane on the M2 phenotype priority was suppressed, as well as the level of STAT3 phosphorylation, implying the modulation of sulforaphane on macrophage polarization mediating the phenotype switch from M1 to M2 in murine colitis caused by DSS, and this effect was closely related to the activation of the IL-10/STAT3 signaling pathways (106).
Rhein
Rhein is a natural flavonoid compound derived from rhubarb that is widely used as a traditional Chinese medicine to treat edema, constipation, and inflammation (107). It has been reported that rhein has potential in alleviating DSS-induced colitis through regulating macrophage polarization toward the M2 phenotype, i.e., toward the anti-inflammatory condition. Experimental data from RAW264.7 cells showed that the relative expression of M1 markers and pro-inflammation mediators were significantly inhibited after the administration of rhein, but the results for M2 were totally reversed. In addition, researchers found that rhein could prevent the activation of the Nox2 redox-mediated NLRP3 inflammasome and modulate the Nrf2-dependent redox balance to block the maturation and secretion of IL-1β in macrophages, one of the key pro-inflammatory cytokines (108).
Rosmarinic acid (RA)
Rosmarinic acid (RA) is a natural compound extracted from plants of the Lamiaceae family, including rosemary, lemon balm, and mint (109). Some studies have investigated the protective effects of RA against colitis in mice induced by DSS and found that it is a potential anti-inflammatory candidate for UC treatment (110, 111). Mai et al. reported that RA could inhibit M1 macrophages with the promotion of M2 in both the colonic tissues of DSS-induced mice and in peripheral blood macrophages cultured in vitro and upregulate the protein level of HO-1. In addition, the inhibition of RA of the LPS-mediated NF-κB p65 translocation into the nucleus was shortened by interdicting HO-1; moreover, the administration of the NF-κB inhibitor BAY11-7082 had no significant effect on the modulation of macrophage differentiation by RA. These results indicated that RA dampened M1 macrophage polarization via promoting HO-1 to impede the NF-κB pathway in ameliorating experimental colitis (111).
7 Conclusion and future perspectives
As a modern refractory disease, UC has negatively impacted the quality of life of patients due to its recurrence and obstinacy, with intolerable symptoms such as frequent hematochezia and abdominal pain (58). A series of studies have demonstrated the great importance of the imbalance of macrophage polarization in the development of UC; therefore, targeting macrophage polarization tendency to the anti-inflammatory phenotype, i.e., of M2, is a potential therapeutic option for UC (21, 30, 36, 112). As the hotspot of new drug development, natural products exhibit abundant bioactivities and nutritional value. In this paper, we summarized more than a dozen investigated phytochemicals extracted from diverse plants, including didymin, genistein, loganin, etc., which could ameliorate experimental colitis by modulating macrophage polarization (60, 65, 81). Their chemical structures include flavonoid, polyphenol, alkaloid, and terpenoid derivatives, and the related modulatory mechanisms involved regulating Hadhb-mediated FAO, the Sirt1/NF-κB signaling pathway, and mTORC2/PPAR-γ signaling, among others (60, 65, 87). The aforementioned findings demonstrated that phytochemicals have promising prospects in mitigating the symptoms of UC by modulating macrophage polarization. However, investigations pertaining to their therapeutic efficacy in patients with UC are yet to be conducted, as all current research has been limited to experimental animal models. Furthermore, the underlying regulatory mechanisms and the potential toxicity of these phytochemicals, which act as regulators of macrophage polarization, require further elucidation. Consequently, further research should concentrate on the toxicity and safety of phytochemicals with effects on the regulation of macrophage polarization, as well as on particular mechanisms that are needed to promote natural regulators of macrophage polarization as UC therapy.
Author contributions
RS, JL, and TM provided direction and guidance for this manuscript. KW wrote the whole manuscript. XL, MW, YY, LS, ZJ, and HJ were responsible for the collation of the paper. RS and TM made significant revisions to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81874386), Chinese Medicine Inheritance and Innovation “OneHundred Million” Talent Project Qihuang Scholar (to JL) and Capital’s Funds for Health Improvement and Research (shoufa 2022-4-4205).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. da Silva BC, Lyra AC, Rocha R, Santana GO. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J Gastroenterol (2014) 20(28):9458–67. doi: 10.3748/wjg.v20.i28.9458
2. Torres J, Halfvarson J, Rodriguez-Lago I, Hedin CRH, Jess T, Dubinsky M, et al. Results of the seventh scientific workshop of ECCO: precision medicine in IBD-prediction and prevention of inflammatory bowel disease. J Crohns Colitis (2021) 15(9):1443–54. doi: 10.1093/ecco-jcc/jjab048
3. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J-F. Ulcerative colitis. Lancet (2017) 389(10080):1756–70. doi: 10.1016/S0140-6736(16)32126-2
4. Younis N, Zarif R, Mahfouz R. Inflammatory bowel disease: between genetics and microbiota. Mol Biol Rep (2020) 47(4):3053–63. doi: 10.1007/s11033-020-05318-5
5. Bhattacharya S, Cross RK. Medical treatment of ulcerative colitis. Semin Colon Rectal Surg (2022) 33. doi: 10.1016/j.scrs.2022.100863
6. Kayal M, Shah S. Ulcerative colitis: current and emerging treatment strategies. J Clin Med (2019) 9(1):94. doi: 10.3390/jcm9010094
7. Li C, Wang J, Ma R, Li L, Wu W, Cai D, et al. Natural-derived alkaloids exhibit great potential in the treatment of ulcerative colitis. Pharmacol Res (2022) 175:105972. doi: 10.1016/j.phrs.2021.105972
8. Weisshof R, El Jurdi K, Zmeter N, Rubin DT. Emerging therapies for inflammatory bowel disease. Adv Ther (2018) 35(11):1746–62. doi: 10.1007/s12325-018-0795-9
9. Han X, Ding S, Jiang H, Liu G. Roles of macrophages in the development and treatment of gut inflammation. Front Cell Dev Biol (2021) 9:625423. doi: 10.3389/fcell.2021.625423
10. Li M, Xue Q, Yang X, Aung LHH, Yang Y, Yu T. The pathophysiological role of macrophages in colitis and their treatment. Recent Adv Microb Diversity (2022), 277–97. doi: 10.1016/B978-0-12-822368-0.00013-X
11. Grimm MC, Pullman WE, Bennett GM, Sullivan PJ, Pavli P, Doe WF. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J Gastroenterol Hepatol (1995) 10(4):387–95. doi: 10.1111/j.1440-1746.1995.tb01589.x
12. Koelink PJ, Bloemendaal FM, Li B, Westera L, Vogels EWM, van Roest M, et al. Anti-TNF therapy in IBD exerts its therapeutic effect through macrophage IL-10 signalling. Gut (2020) 69(6):1053–63. doi: 10.1136/gutjnl-2019-318264
13. Vos ACW, Wildenberg ME, Arijs I, Duijvestein M, Verhaar AP, de Hertogh G, et al. Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflammatory Bowel Dis (2012) 18(3):401–8. doi: 10.1002/ibd.21818
14. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
15. Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol (2014) 15(10):929–37. doi: 10.1038/ni.2967
16. Delfini M, Stakenborg N, Viola MF, Boeckxstaens G. Macrophages in the gut: masters in multitasking. Immunity (2022) 55(9):1530–48. doi: 10.1016/j.immuni.2022.08.005
17. Moreira Lopes TC, Mosser DM, Gonçalves R. Macrophage polarization in intestinal inflammation and gut homeostasis. Inflamm Res (2020) 69(12):1163–72. doi: 10.1007/s00011-020-01398-y
18. Wang S, Ye Q, Zeng X, Qiao S. Functions of macrophages in the maintenance of intestinal homeostasis. J Immunol Res (2019) 2019:1512969. doi: 10.1155/2019/1512969
19. Kuhl AA, Erben U, Kredel LI, Siegmund B. Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol (2015) 6:613. doi: 10.3389/fimmu.2015.00613
20. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol (2013) 229(2):176–85. doi: 10.1002/path.4133
21. Seyedizade SS, Afshari K, Bayat S, Rahmani F, Momtaz S, Rezaei N, et al. Current status of M1 and M2 macrophages pathway as drug targets for inflammatory bowel disease. Arch Immunol Ther Exp (Warsz) (2020) 68(2):10. doi: 10.1007/s00005-020-00576-4
22. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol (2008) 8(12):958–69. doi: 10.1038/nri2448
23. Mackaness GB. Cellular resistance to infection. J Exp Med (1962) 116(3):381–406. doi: 10.1084/jem.116.3.381
24. Gordon S. Alternative activation of macrophages. Nat Rev Immunol (2003) 3(1):23–35. doi: 10.1038/nri978
25. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol (2000) 164(12):6166–73. doi: 10.4049/jimmunol.164.12.6166
26. Yang Z, Lin S, Feng W, Liu Y, Song Z, Pan G, et al. A potential therapeutic target in traditional Chinese medicine for ulcerative colitis: macrophage polarization. Front Pharmacol (2022) 13:999179. doi: 10.3389/fphar.2022.999179
27. Murray PJ. Macrophage polarization. Annu Rev Physiol (2017) 79:541–66. doi: 10.1146/annurev-physiol-022516-034339
28. Zhang J, Zhou X, Hao H. Macrophage phenotype-switching in cancer. Eur J Pharmacol (2022) 931:175229. doi: 10.1016/j.ejphar.2022.175229
29. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol (2018) 233(9):6425–40. doi: 10.1002/jcp.26429
30. Du Y, Rong L, Cong Y, Shen L, Zhang N, Wang B. Macrophage polarization: an effective approach to targeted therapy of inflammatory bowel disease. Expert Opin Ther Targets (2021) 25(3):191–209. doi: 10.1080/14728222.2021.1901079
31. Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol (2020) 877:173090. doi: 10.1016/j.ejphar.2020.173090
32. Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol (2019) 16(9):531–43. doi: 10.1038/s41575-019-0172-4
33. Bain CC, Mowat AM. The monocyte-macrophage axis in the intestine. Cell Immunol (2014) 291(1-2):41–8. doi: 10.1016/j.cellimm.2014.03.012
34. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol (2013) 14(10):986–95. doi: 10.1038/ni.2705
35. Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol (2013) 6(3):498–510. doi: 10.1038/mi.2012.89
36. Pan X, Zhu Q, Pan LL, Sun J. Macrophage immunometabolism in inflammatory bowel diseases: from pathogenesis to therapy. Pharmacol Ther (2022) 238:108176. doi: 10.1016/j.pharmthera.2022.108176
37. Gren ST, Grip O. Role of monocytes and intestinal macrophages in crohn’s disease and ulcerative colitis. Inflamm Bowel Dis (2016) 22(8):1992–8. doi: 10.1097/MIB.0000000000000824
38. Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med (2017) 49(5):e338. doi: 10.1038/emm.2017.20
39. Lissner D, Schumann M, Batra A, Kredel LI, Kuhl AA, Erben U, et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis (2015) 21(6):1297–305. doi: 10.1097/MIB.0000000000000384
40. Long J, Liu XK, Kang ZP, Wang MX, Zhao HM, Huang JQ, et al. Ginsenoside Rg1 ameliorated experimental colitis by regulating the balance of M1/M2 macrophage polarization and the homeostasis of intestinal flora. Eur J Pharmacol (2022) 917:174742. doi: 10.1016/j.ejphar.2022.174742
41. Liu H, Dasgupta S, Fu Y, Bailey B, Roy C, Lightcap E, et al. Subsets of mononuclear phagocytes are enriched in the inflamed colons of patients with IBD. BMC Immunol (2019) 20(1):42. doi: 10.1186/s12865-019-0322-z
42. Zeissig S, Rosati E, Dowds CM, Aden K, Bethge J, Schulte B, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut (2019) 68(1):25–39. doi: 10.1136/gutjnl-2018-316023
43. Ackermann M, Mucci A, McCabe A, Frei S, Wright K, Snapper SB, et al. Restored macrophage function ameliorates disease pathophysiology in a mouse model for IL10 receptor-deficient very early onset inflammatory bowel disease. J Crohns Colitis (2021) 15(9):1588–95. doi: 10.1093/ecco-jcc/jjab031
44. Hunter MM, Wang A, Parhar KS, Johnston MJ, Van Rooijen N, Beck PL, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology (2010) 138(4):1395–405. doi: 10.1053/j.gastro.2009.12.041
45. Takada Y, Hisamatsu T, Kamada N, Kitazume MT, Honda H, Oshima Y, et al. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J Immunol (2010) 184(5):2671–6. doi: 10.4049/jimmunol.0804012
46. Caprioli F, Bose F, Rossi RL, Petti L, Vigano C, Ciafardini C, et al. Reduction of CD68+ macrophages and decreased IL-17 expression in intestinal mucosa of patients with inflammatory bowel disease strongly correlate with endoscopic response and mucosal healing following infliximab therapy. Inflamm Bowel Dis (2013) 19(4):729–39. doi: 10.1097/MIB.0b013e318280292b
47. Vos AC, Wildenberg ME, Duijvestein M, Verhaar AP, van den Brink GR, Hommes DW. Anti-tumor necrosis factor-alpha antibodies induce regulatory macrophages in an fc region-dependent manner. Gastroenterology (2011) 140(1):221–30. doi: 10.1053/j.gastro.2010.10.008
48. Eissa N, Hussein H, Kermarrec L, Elgazzar O, Metz-Boutigue MH, Bernstein CN, et al. Chromofungin (CHR: CHGA47-66) is downregulated in persons with active ulcerative colitis and suppresses pro-inflammatory macrophage function through the inhibition of NF-kappaB signaling. Biochem Pharmacol (2017) 145:102–13. doi: 10.1016/j.bcp.2017.08.013
49. Eissa N, Hussein H, Kermarrec L, Grover J, Metz-Boutigue ME, Bernstein CN, et al. Chromofungin ameliorates the progression of colitis by regulating alternatively activated macrophages. Front Immunol (2017) 8:1131. doi: 10.3389/fimmu.2017.01131
50. Li G, Ren H, Wu X, Hu Q, Hong Z, Wang G, et al. Follistatin like protein-1 modulates macrophage polarization and aggravates dextran sodium sulfate-induced colitis. Int Immunopharmacol (2020) 83:106456. doi: 10.1016/j.intimp.2020.106456
51. Park HJ, Kim J, Saima FT, Rhee KJ, Hwang S, Kim MY, et al. Adipose-derived stem cells ameliorate colitis by suppression of inflammasome formation and regulation of M1-macrophage population through prostaglandin E2. Biochem Biophys Res Commun (2018) 498(4):988–95. doi: 10.1016/j.bbrc.2018.03.096
52. Cao L, Xu H, Wang G, Liu M, Tian D, Yuan Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int Immunopharmacol (2019) 72:264–74. doi: 10.1016/j.intimp.2019.04.020
53. Feagan BG, Chande N, MacDonald JK. Are there any differences in the efficacy and safety of different formulations of oral 5-ASA used for induction and maintenance of remission in ulcerative colitis? evidence from cochrane reviews. Inflamm Bowel Dis (2013) 19(9):2031–40. doi: 10.1097/MIB.0b013e3182920108
54. Kornbluth A, Sachar DB. Practice parameters committee of the American college of g. ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol (2010) 105(3):501–23. doi: 10.1038/ajg.2009.727
55. Chotiyarnwong P, McCloskey EV. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol (2020) 16(8):437–47. doi: 10.1038/s41574-020-0341-0
56. Beigel F, Steinborn A, Schnitzler F, Tillack C, Breiteneicher S, John JM, et al. Risk of malignancies in patients with inflammatory bowel disease treated with thiopurines or anti-TNF alpha antibodies. Pharmacoepidemiol Drug Saf (2014) 23(7):735–44. doi: 10.1002/pds.3621
57. Fukata N, Okazaki K, Omiya M, Matsushita M, Watanabe M, Members of the Ministry of H, et al. Hematologic malignancies in the Japanese patients with inflammatory bowel disease. J Gastroenterol (2014) 49(9):1299–306. doi: 10.1007/s00535-013-0873-3
58. Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Primers (2020) 6(1):74. doi: 10.1038/s41572-020-0205-x
59. He X, Wang N, Zhang Y, Huang X, Wang Y. The therapeutic potential of natural products for treating pancreatic cancer. Front Pharmacol (2022) 13:1051952. doi: 10.3389/fphar.2022.1051952
60. Lv Q, Xing Y, Liu Y, Chen Q, Xu J, Hu L, et al. Didymin switches M1-like toward M2-like macrophage to ameliorate ulcerative colitis via fatty acid oxidation. Pharmacol Res (2021) 169:105613. doi: 10.1016/j.phrs.2021.105613
61. Slezakova S, Ruda-Kucerova J. Anticancer activity of artemisinin and its derivatives. Anticancer Res (2017) 37(11):5995–6003. doi: 10.21873/anticanres.12046
62. Wang Y, Ji X, Yan M, Chen X, Kang M, Teng L, et al. Protective effect and mechanism of polysaccharide from dictyophora indusiata on dextran sodium sulfate-induced colitis in C57BL/6 mice. Int J Biol Macromol (2019) 140:973–84.
63. Yao Q, Lin MT, Zhu YD, Xu HL, Zhao YZ. Recent trends in potential therapeutic applications of the dietary flavonoid didymin. Molecules (2018) 23(10):2547. doi: 10.3390/molecules23102547
64. Khan FB, Singh P, Jamous YF, Ali SA, Abdullah, Uddin S, et al. Multifaceted pharmacological potentials of curcumin, genistein, and tanshinone IIA through proteomic approaches: an in-depth review. Cancers (Basel) (2022) 15(1):249. doi: 10.3390/cancers15010249
65. Liu S, Shen H, Li J, Gong Y, Bao H, Zhang J, et al. Loganin inhibits macrophage M1 polarization and modulates sirt1/NF-kappaB signaling pathway to attenuate ulcerative colitis. Bioengineered (2020) 11(1):628–39. doi: 10.1080/21655979.2020.1774992
66. Lee C, Lee JW, Seo JY, Hwang SW, Im JP, Kim JS. Lupeol inhibits LPS-induced NF-kappa b signaling in intestinal epithelial cells and macrophages, and attenuates acute and chronic murine colitis. Life Sci (2016) 146:100–8. doi: 10.1016/j.lfs.2016.01.001
67. Li X, Xu S, Zhang Y, Li K, Gao XJ, Guo MY. Berberine depresses inflammation and adjusts smooth muscle to ameliorate ulcerative colitis of cats by regulating gut microbiota. Microbiol Spectr (2022) 10(6):e0320722. doi: 10.1128/spectrum.03207-22
68. Yan F, Wang L, Shi Y, Cao H, Liu L, Washington MK, et al. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. Am J Physiol Gastrointest Liver Physiol (2012) 302(5):G504–14. doi: 10.1152/ajpgi.00312.2011
69. Jin J, Zhong Y, Long J, Wu T, Jiang Q, Wang H, et al. Ginsenoside Rg1 relieves experimental colitis by regulating balanced differentiation of Tfh/Treg cells. Int Immunopharmacol (2021) 100:108133. doi: 10.1016/j.intimp.2021.108133
70. Wang X, Xie L, Long J, Liu K, Lu J, Liang Y, et al. Therapeutic effect of baicalin on inflammatory bowel disease: a review. J Ethnopharmacol (2022) 283:114749. doi: 10.1016/j.jep.2021.114749
71. Song S, An J, Li Y, Liu S. Electroacupuncture at ST-36 ameliorates DSS-induced acute colitis via regulating macrophage polarization induced by suppressing NLRP3/IL-1beta and promoting Nrf2/HO-1. Mol Immunol (2019) 106:143–52. doi: 10.1016/j.molimm.2018.12.023
72. Ho WE, Peh HY, Chan TK, Wong WS. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther (2014) 142(1):126–39. doi: 10.1016/j.pharmthera.2013.12.001
73. Velagapudi R, Aderogba M, Olajide OA. Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-kappaB/p38-mediated neuroinflammation in activated BV2 microglia. Biochim Biophys Acta (2014) 1840(12):3311–9. doi: 10.1016/j.bbagen.2014.08.008
74. Deng C, Hu Z, Fu H, Hu M, Xu X, Chen J. Chemical analysis and antioxidant activity in vitro of a beta-d-glucan isolated from dictyophora indusiata. Int J Biol Macromol (2012) 51(1-2):70–5. doi: 10.1016/j.ijbiomac.2012.05.001
75. Wang Y, Lai L, Teng L, Li Y, Cheng J, Chen J, et al. Mechanism of the anti-inflammatory activity by a polysaccharide from dictyophora indusiata in lipopolysaccharide-stimulated macrophages. Int J Biol Macromol (2019) 126:1158–66. doi: 10.1016/j.ijbiomac.2019.01.022
76. Kanwal S, Joseph TP, Owusu L, Xiaomeng R, Meiqi L, Yi X. A polysaccharide isolated from dictyophora indusiata promotes recovery from antibiotic-driven intestinal dysbiosis and improves gut epithelial barrier function in a mouse model. Nutrients (2018) 10(8):1003. doi: 10.3390/nu10081003
77. Kanwal S, Joseph TP, Aliya S, Song S, Saleem MZ, Nisar MA, et al. Attenuation of DSS induced colitis by dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J Funct Foods (2020) 64:103641. doi: 10.1016/j.jff.2019.103641
78. Shete VS, Telange DR, Mahajan NM, Pethe AM, Mahapatra DK. Development of phospholipon(R)90H complex nanocarrier with enhanced oral bioavailability and anti-inflammatory potential of genistein. Drug Deliv (2023) 30(1):2162158. doi: 10.1080/10717544.2022.2162158
79. Geng Y, Chen S, Yang Y, Miao H, Li X, Li G, et al. Long-term exposure to genistein inhibits the proliferation of gallbladder cancer by downregulating the MCM complex. Sci Bull (Beijing) (2022) 67(8):813–24. doi: 10.1016/j.scib.2022.01.011
80. Zhang N, Zhang W, Guo X, Liu J, Li S, Zhang H, et al. Genistein protects against hyperglycemia and fatty liver disease in diet-induced prediabetes mice via activating hepatic insulin signaling pathway. Front Nutr (2022) 9:1072044. doi: 10.3389/fnut.2022.1072044
81. Abron JD, Singh NP, Price RL, Nagarkatti M, Nagarkatti PS, Singh UP. Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PloS One (2018) 13(7):e0199631. doi: 10.1371/journal.pone.0199631
82. Kou Y, Li Z, Yang T, Shen X, Wang X, Li H, et al. Therapeutic potential of plant iridoids in depression: a review. Pharm Biol (2022) 60(1):2167–81. doi: 10.1080/13880209.2022.2136206
83. Jang JH, Yang G, Seok JK, Kang HC, Cho YY, Lee HS, et al. Loganin prevents hepatic steatosis by blocking NLRP3 inflammasome activation. Biomol Ther (Seoul) (2023) 31(1):40–7. doi: 10.4062/biomolther.2022.077
84. Cheng KI, Chang YC, Chu LW, Hsieh SL, An LM, Dai ZK, et al. The iridoid glycoside loganin modulates autophagic flux following chronic constriction injury-induced neuropathic pain. Int J Mol Sci (2022) 23(24). doi: 10.3390/ijms232415873
85. Yuan J, Cheng W, Zhang G, Ma Q, Li X, Zhang B, et al. Protective effects of iridoid glycosides on acute colitis via inhibition of the inflammatory response mediated by the STAT3/NF-small ka, CyrillicB pathway. Int Immunopharmacol (2020) 81:106240. doi: 10.1016/j.intimp.2020.106240
86. Kou Y, Sun Q, Zhu R, Lin Z, Li Z, Xu H, et al. Dioscin induces M1 macrophage polarization through connexin-43 channels in tumor-associated-macrophages-mediated melanoma metastasis. Phytomedicine (2023) 109:154559. doi: 10.1016/j.phymed.2022.154559
87. Wu MM, Wang QM, Huang BY, Mai CT, Wang CL, Wang TT, et al. Dioscin ameliorates murine ulcerative colitis by regulating macrophage polarization. Pharmacol Res (2021) 172:105796. doi: 10.1016/j.phrs.2021.105796
88. Shi L, Zhang P, Jin R, Chen X, Dong L, Chen W. Dioscin ameliorates inflammatory bowel disease by up-regulating miR-125a-5p to regulate macrophage polarization. J Clin Lab Anal (2022) 36(6):e24455. doi: 10.1002/jcla.24455
89. Sohag AAM, Hossain MT, Rahaman MA, Rahman P, Hasan MS, Das RC, et al. Molecular pharmacology and therapeutic advances of the pentacyclic triterpene lupeol. Phytomedicine (2022) 99:154012. doi: 10.1016/j.phymed.2022.154012
90. Zhu Y, Li X, Chen J, Chen T, Shi Z, Lei M, et al. The pentacyclic triterpene lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. Int Immunopharmacol (2016) 30:74–84. doi: 10.1016/j.intimp.2015.11.031
91. Li YH, Xiao HT, Hu DD, Fatima S, Lin CY, Mu HX, et al. Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in C57BL/6 mice by suppressing Th17 responses. Pharmacol Res (2016) 110:227–39. doi: 10.1016/j.phrs.2016.02.010
92. Yang T, Ma X, Wang R, Liu H, Wei S, Jing M, et al. Berberine inhibits IFN-gamma signaling pathway in DSS-induced ulcerative colitis. Saudi Pharm J (2022) 30(6):764–78. doi: 10.1016/j.jsps.2022.03.015
93. Dong Y, Fan H, Zhang Z, Jiang F, Li M, Zhou H, et al. Berberine ameliorates DSS-induced intestinal mucosal barrier dysfunction through microbiota-dependence and wnt/beta-catenin pathway. Int J Biol Sci (2022) 18(4):1381–97. doi: 10.7150/ijbs.65476
94. Liu Y, Liu X, Hua W, Wei Q, Fang X, Zhao Z, et al. Berberine inhibits macrophage M1 polarization via AKT1/SOCS1/NF-kappaB signaling pathway to protect against DSS-induced colitis. Int Immunopharmacol (2018) 57:121–31. doi: 10.1016/j.intimp.2018.01.049
95. Zhu G, Wang H, Wang T, Shi F. Ginsenoside Rg1 attenuates the inflammatory response in DSS-induced mice colitis. Int Immunopharmacol (2017) 50:1–5. doi: 10.1016/j.intimp.2017.06.002
96. Shen J, Cheng J, Zhu S, Zhao J, Ye Q, Xu Y, et al. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int Immunopharmacol (2019) 73:193–200. doi: 10.1016/j.intimp.2019.04.052
97. Zhu W, Jin Z, Yu J, Liang J, Yang Q, Li F, et al. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int Immunopharmacol (2016) 35:119–26. doi: 10.1016/j.intimp.2016.03.030
98. Fan H, Chen W, Zhu J, Zhang J, Peng S. Toosendanin alleviates dextran sulfate sodium-induced colitis by inhibiting M1 macrophage polarization and regulating NLRP3 inflammasome and Nrf2/HO-1 signaling. Int Immunopharmacol (2019) 76:105909. doi: 10.1016/j.intimp.2019.105909
99. Hu D, Wang Y, Chen Z, Ma Z, You Q, Zhang X, et al. Artemisinin protects against dextran sulfate-sodium-induced inflammatory bowel disease, which is associated with activation of the pregnane X receptor. Eur J Pharmacol (2014) 738:273–84. doi: 10.1016/j.ejphar.2014.04.050
100. Huai M, Zeng J, Ge W. Artemisinin ameliorates intestinal inflammation by skewing macrophages to the M2 phenotype and inhibiting epithelial-mesenchymal transition. Int Immunopharmacol (2021) 91:107284. doi: 10.1016/j.intimp.2020.107284
101. Zhuang H, Lv Q, Zhong C, Cui Y, He L, Zhang C, et al. Tiliroside ameliorates ulcerative colitis by restoring the M1/M2 macrophage balance via the HIF-1alpha/glycolysis pathway. Front Immunol (2021) 12:649463. doi: 10.3389/fimmu.2021.649463
102. Wu Y, Huang D, Wang X, Pei C, Xiao W, Wang F, et al. Suppression of NLRP3 inflammasome by platycodin d via the TLR4/MyD88/NF-kappaB pathway contributes to attenuation of lipopolysaccharide induced acute lung injury in rats. Int Immunopharmacol (2021) 96:107621. doi: 10.1016/j.intimp.2021.107621
103. Guo R, Meng Q, Wang B, Li F. Anti-inflammatory effects of platycodin d on dextran sulfate sodium (DSS) induced colitis and e. coli lipopolysaccharide (LPS) induced inflammation. Int Immunopharmacol (2021) 94:107474. doi: 10.1016/j.intimp.2021.107474
104. Williams EJ, Guilleminault L, Berthon BS, Eslick S, Wright T, Karihaloo C, et al. Sulforaphane reduces pro-inflammatory response to palmitic acid in monocytes and adipose tissue macrophages. J Nutr Biochem (2022) 104:108978. doi: 10.1016/j.jnutbio.2022.108978
105. Zhang Y, Tan L, Li C, Wu H, Ran D, Zhang Z. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS. AMB Express (2020) 10(1):119. doi: 10.1186/s13568-020-0948-5
106. Sun Y, Tang J, Li C, Liu J, Liu H. Sulforaphane attenuates dextran sodium sulphate induced intestinal inflammation via IL-10/STAT3 signaling mediated macrophage phenotype switching. Food Sci Hum Wellness (2022) 11(1):129–42. doi: 10.1016/j.fshw.2021.07.014
107. Zhuang S, Zhong J, Zhou Q, Zhong Y, Liu P, Liu Z. Rhein protects against barrier disruption and inhibits inflammation in intestinal epithelial cells. Int Immunopharmacol (2019) 71:321–7. doi: 10.1016/j.intimp.2019.03.030
108. Zhou Y, Gao C, Vong CT, Tao H, Li H, Wang S, et al. Rhein regulates redox-mediated activation of NLRP3 inflammasomes in intestinal inflammation through macrophage-activated crosstalk. Br J Pharmacol (2022) 179(9):1978–97. doi: 10.1111/bph.15773
109. Hassanzadeh-Taheri M, Ahmadi-Zohan A, Mohammadifard M, Hosseini M. Rosmarinic acid attenuates lipopolysaccharide-induced neuroinflammation and cognitive impairment in rats. J Chem Neuroanat (2021) 117:102008. doi: 10.1016/j.jchemneu.2021.102008
110. Jin BR, Chung KS, Hwang S, Hwang SN, Rhee KJ, Lee M, et al. Rosmarinic acid represses colitis-associated colon cancer: a pivotal involvement of the TLR4-mediated NF-kappaB-STAT3 axis. Neoplasia (2021) 23(6):561–73. doi: 10.1016/j.neo.2021.05.002
111. Mai P, Chen C, Xiao X, Ma X, Shi Y, Miao G, et al. Rosmarinic acid protects against ulcerative colitis by regulating macrophage polarization depending on heme oxygenase-1 in mice. Eur J Inflamm (2020) 18. doi: 10.1177/2058739220959916
Keywords: macrophage, phenotype, polarization, phytochemicals, therapeutic effects, ulcerative colitis
Citation: Wang K, Mao T, Lu X, Wang M, Yun Y, Jia Z, Shi L, Jiang H, Li J and Shi R (2023) A potential therapeutic approach for ulcerative colitis: targeted regulation of macrophage polarization through phytochemicals. Front. Immunol. 14:1155077. doi: 10.3389/fimmu.2023.1155077
Received: 31 January 2023; Accepted: 20 March 2023;
Published: 01 May 2023.
Edited by:
Yanan Ma, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Lili Qu, UCHC, United StatesJingkai Zhou, City of Hope National Medical Center, United States
Guolong Zuo, University of California, San Francisco, United States
Xinhe Shan, Penn Medicine, United States
Copyright © 2023 Wang, Mao, Lu, Wang, Yun, Jia, Shi, Jiang, Li and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junxiang Li, bGlqdW54aWFuZzEyMjZAMTYzLmNvbQ==; Rui Shi, c2hpYWk1ODhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Ke Wang1,2†
Ke Wang1,2† Tangyou Mao
Tangyou Mao Junxiang Li
Junxiang Li Rui Shi
Rui Shi