
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 06 April 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1149931
This article is part of the Research TopicCancer Cell-intrinsic and -extrinsic Factors Affecting Tumor Immune EvasionView all 10 articles
Hepatocellular carcinoma (HCC) is the most common primary liver cancer, and the third leading cause of cancer-related deaths worldwide. HCC is characterized by insidious onset, and most patients are diagnosed at an advanced stage with a poor prognosis. Identification of biomarkers for HCC onset and progression is imperative to development of effective diagnostic and therapeutic strategies. CD147 is a glycoprotein that is involved in tumor cell invasion, metastasis and angiogenesis through multiple mechanisms. In this review, we describe the molecular structure of CD147 and its role in regulating HCC invasion, metastasis and angiogenesis. We highlight its potential as a diagnostic and therapeutic target for HCC.
Liver cancer, one of the most common malignant tumors in humans, is mainly divided into primary and secondary subgroups. Primary liver cancer is ranked seventh and second with regards to incidence and mortalities, respectively worldwide (1). Hepatocellular carcinoma (HCC), the leading type of primary liver cancer, is the third leading cause of cancer-related deaths worldwide, killing 745,500 patients each year (2, 3). In Asia, chronic hepatitis B virus (HBV) infection is the leading cause of HCC (4), whereas chronic hepatitis C virus (HCV), alcoholic cirrhosis and steatohepatitis are the main causes across Western countries (5). Other risk factors for HCC include heavy alcohol consumption, aflatoxin ingestion, obesity, type 2 diabetes and smoking (6, 7). Despite advances in diagnosis and treatment strategies, prognosis of HCC patients remains unsatisfactory, with a 5-year survival rate of only 15-20%, a rate that has changed little in the past 30 years (8, 9). These minute changes have been attributed to lack of reliable early biomarkers and the high economic challenge of effective treatment in countries with high risk factors (10). Current treatment modalities for HCC mainly involve surgical interventions, such as ablation, resection and organ transplantation (11, 12). However, these treatment approaches are often limited by late diagnosis, coupled with lack of transplantable organs or disease that progress beyond the Milan criteria (13). Therefore, urgent identification of novel molecular mechanisms and diagnostic markers is imperative to development of strategies for effective treatment of HCC.
Cluster of differentiation 147 (CD147), a glycoprotein originally known as a regulator of Matrix metalloproteinase (MMP), serves as a potential target for cancer therapy through cell-matrix and cell-cell interactions (14, 15). Studies have shown that CD147 is not only overexpressed in cancer cells, but also regulates cell proliferation, drug resistance and cell stromal adhesion properties (16–18). Earlier reports indicated that apart from regulating MMP, CD147 also plays a role in several other functions, and can also bind different molecular partners to regulate multiple signaling pathways (19, 20). In addition, CD147 is involved in angiogenesis by regulating production of vascular endothelial growth factor (VEGF) in tumor and stromal cells (21). In addition, CD147 acts on cancer-associated fibroblasts to promote tumorigenesis and development. It was found that CD147 is expressed on melanoma cells and induces tumor cell invasion by stimulating fibroblast production of matrix metalloproteinases (22). Xu et al. (23)found that CD147 transformed breast cancer static fibroblasts into cancer-associated fibroblasts.
Prospecting for novel mechanisms regulating HCC development, coupled with early HCC detection, can greatly improve chances of effective treatment. Studies have described the role of new diagnostic biomarkers in clinical and therapeutic management of HCC (24). Notably, numerous evidence indicates that CD147 is a promising diagnostic and therapeutic biomarker for HCC (25).
CD147 which plays numerous functions, was given different names by different researchers during early days, including gp42, BSG, and EMMPRIN, among others (26–28). The Human Genome Project uses the name BSG, while its corresponding gene and protein name is basigin (Ok blood group) (20). Apart from detection in all vertebrates. This gene is also homologous in Drosophila melanogaster and Schistosoma (29). The gene encoding CD147 is located on chromosome 19, p13.3, and comprises 10 exons on a ~12 kb fragment (30). A 30 bp element, located at the -142 to -112bp 5’ end of the gene, contains binding sites for specificity protein 1 (Sp1), activator protein 1 (AP1), transcription factor II (TFII) and early growth response factor-2, which are important for CD147 transcription (31, 32). The 3’ flanking region also has two hypoxia-inducible factor (HIF) binding sites (33, 34). Human Protein Database shows that four variants of CD147 has been encoded through an alternative promoter and splicing (35, 36) (Figure 1). The Ig-like structural domain is divided into four types, namely type V, C1, C2, and I. Notably, the latter type lies between types V and C. Moreover, CD147-1 has three Ig-like structural domains and is a retina-specific type (37, 38). CD147-2, a common classical isoform that is widely distributed, has two Ig-like structural domains and three asparagine-glycosylated aspartate sequence sites (39, 40). Structurally, one monomer of CD147 is composed of two domains, D1 corresponding to N-terminal domain and a C-terminal domain called D2 (Figure 1). On the other hand, CD147-3 and CD147-4 are less common and contain only 1 extracellular structural domain (Ig I). Studies have shown that CD147-3 can act as an endogenous inhibitor of CD147-2 by forming a heterodimer with it (35). Notably, the transmembrane region of BSG proteins comprises 23 amino acids that are highly conserved in the CD147 family as well as across species (41, 42). A fully conserved Glu in the middle of the transmembrane region is of particular interest, owing to the fact that it may not only mediate CD147 interactions with other adjacent proteins in the membrane but also regulate important CD147 functions. Moreover, the transmembrane region contains a typical leucine zipper structural domain that is involved in both membrane-protein interactions and multiple intracellular signaling pathways (41, 43).
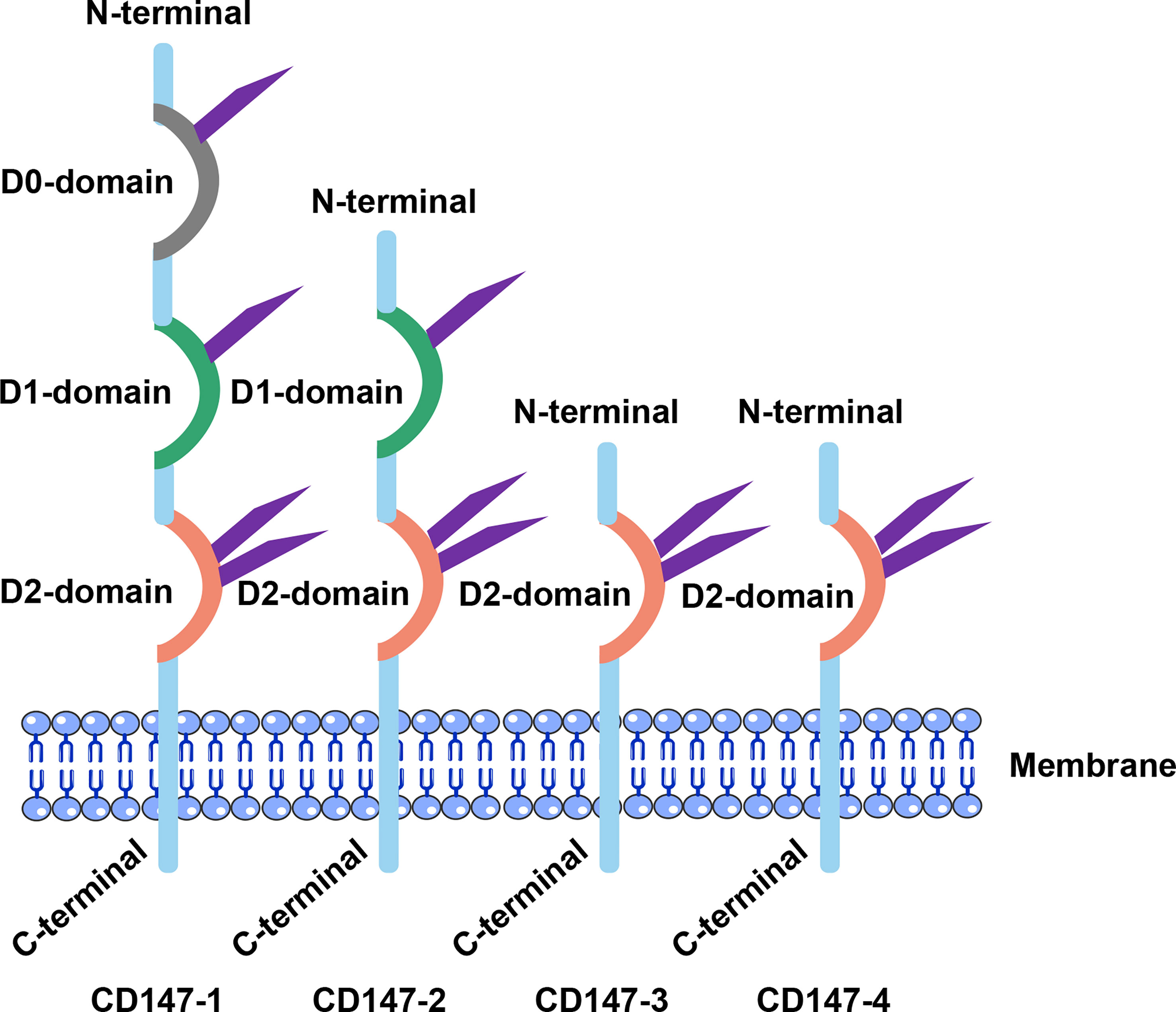
Figure 1 Structural characteristics of CD147. CD147’s extracellular segments differed significantly. D0: retina-specific structural domain; D1: Ig C2 structural domain; D2: Ig I structural domain.
Studies have shown that CD147 interacts with numerous partners, such as caveolin-1 (44), monocarboxylate transporter (45), CD98 and β1 integrin (46), to promote various processed including cell metabolism, proliferation, migration and invasion (47). In addition, the soluble form of CD147 was found to internalize and promote cell proliferation and migration through surface CD147 binding (48, 49). Another study demonstrated that CD147 overexpression mimics VEGF production through the PI3K/AKT signaling pathway, thereby directly promoting tumor angiogenesis (50). Moreover, Chen et al. (51) reported that CD147 was overexpressed in human umbilical vein endothelial cells, while its upregulation by specific siRNAs markedly suppressed angiogenesis in multiple ways, including proliferation, migration, secretion of MMPs, and activation of the PI3K/Akt pathway. It has been shown that CD147 is associated with the development of various solid tumors such as esophageal cancer, head and neck squamous cell carcinoma, oral squamous cell carcinoma, gastric cancer, colorectal cancer, and breast cancer (52–72). The biological roles of CD147 in different cancers are shown in Table 1.
Tumor invasion relies on a complex mechanism that includes cell adhesion, migration, and stromal degradation. CD147 enrichment on the surface of tumor cells is an important regulator of tumor mesenchymal interactions, as it stimulates the neighboring mesenchyme to promote synthesis of several MMPs (mainly MMP-2,9). These enzymes degrade the extracellular matrix composed of collagen, elastin, adhesion proteins and proteoglycans, thereby providing conducive conditions for cell metastatic movement (73–75). Previous studies have shown that CD147’s extracellular N-terminal region is critical for MMP induction (73, 76). The epithelial-mesenchymal transition (EMT) is a key developmental program that is often activated during cancer
invasion and metastasis. Ru et al. (77) demonstrated that CD147 plays an important role in invasion by promoting EMT of hepatocytes through the TGF-β signaling pathway (Figure 2).
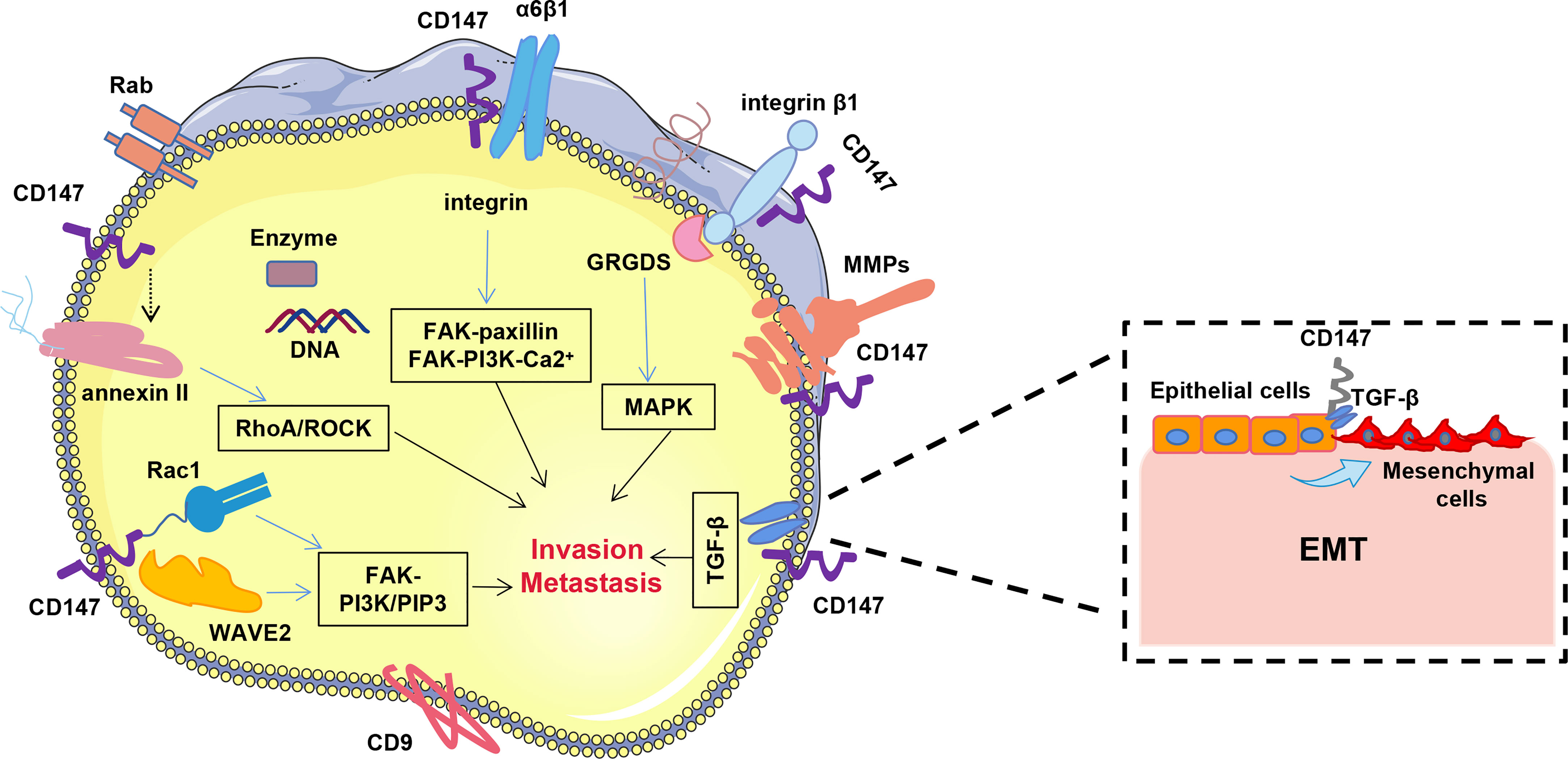
Figure 2 CD147 promotes hepatocellular carcinoma invasion and metastasis. CD147 promotes HCC invasion and metastasis mainly through FAK-PI3K-Ca2+, RhoA/ROCK, FAK-PI3K/PIP3,TGF-β and MAPK signaling pathways.
Cell motility plays a crucial role in tumor invasion and metastasis. Notably, CD147 promotes HCC invasion and metastasis through several different pathways, including integrin-mediated FAK-paxillin, FAK-PI3K-Ca2+, RhoA/ROCK, WAVE2, Rac1 and MAPK signaling pathways (Figure 2). Studies have shown that CD147 co-localizes with integrin α3β1 and α6β1 in hepatocellular carcinoma cells and mediates FAK-paxillin as well as FAK-PI3K-Ca2+ signaling pathways through their interaction to promote both invasive and metastatic potential of HCC cells (75, 78). Binding of CD147 to the integrin β1 subunit competitively prevents its binding to the GRGDS peptide, leading to cytoskeletal rearrangement (47). Conversely, CD147 inhibits the RhoA/ROCK signaling pathway and amoeboid motility in HCC cells by attenuating annexin II phosphorylation. Moreover, it also promotes localization of the Verprolin homolog 2 (WAVE2) membrane and activation of Rac1 in HCC cells via the integrin-FAK-PI3K/PIP3 signaling pathway, thereby contributing to formation of amoeboid and mesenchymal motility (79). Cui et al. (80) demonstrated that CD147 dimerization is essential for the induction of hepatocellular carcinoma MMPs and cell invasion via the MAPK pathway. Additional evidence has shown that membrane-linked protein II promotes HCC invasion and metastasis in vitro by interacting with CD147 (81).
Studies have shown that CD147 is also involved in tumor angiogenesis, a key component of the tumor microenvironment. CD147-induced MMP expression in tumor and stromal compartments subsequently mediates release of biologically active angiogenic growth factors from stromal binding complexes (82)(Figure 3). Tang et al. (83) demonstrated that CD147 stimulated tumor angiogenesis by upregulating VEGF and MMP expression in tumor and mesenchymal compartments. Results from both in vitro and in vivo tumor models indicated that tumor CD147 promoted production of endothelial VEGF soluble isoforms (especially the most angiogenic ones) and their major receptor VEGFR-2 via transcription factor HIF-2α (84). In addition, CD147 promotes capillary-like formation via VEGFR-2 and its ligand VEGF (21). A disintegrin and metallo-proteinases (ADAM) family of protein hydrolases is anchored to the cell membrane, is broadly expressed, evolutionarily conserved, and is the main enzyme involved in the extramembrane cleavage of molecules (85). It has been reported that ADAM12 cleaved the extracellular segment of CD147 and fully bound the free CD147 to the receptor cells, thus regulating tumor angiogenesis (86). Wu et al. (87)found that ADAM10 decomposes CD147 to produce cytoplasmic fragments and promotes HCC development by promoting autophagy.

Figure 3 CD147 promotes hepatocellular carcinoma angiogenesis. CD147 enhances production of MMPs and VEGF thereby promoting HCC angiogenesis.
Remodeling of the tumor microenvironmental matrix by VEGF and MMPs is essential for angiogenesis. Studies have shown that CD147 expression is positively correlated with VEGF, MMP-2, MMP-9 and microvessel density CD34 (MVD-CD34) expression in HCC tissues (88, 89). Results from in vitro and in vivo experiments showed that interfering with CD147 expression in mouse hepatocellular carcinoma cells not only significantly downregulated MMP-11 and VEGF-A expression at both mRNA and protein levels, but also suppressed invasiveness, adhesion and metastasis to lymph nodes (90). Another study demonstrated that Kaposi’s sarcoma-associated herpes virus (KSHV) promotes invasiveness of fibroblasts and endothelial cells by upregulating CD147 (91), while enhanced invasiveness of KSHV-infected endothelial cells was attributed to activation of VEGF through CD147-dependent PI3K/AKT and MAPK (92). Collectively, these data indicate that CD147 promotes angiogenesis by directly regulating secretion of MMP and VEGF on the one hand, and inducing HCC invasion by activating VEGF through the CD147-dependent PI3K/AKT and MAPK signaling pathways on the other.
Numerous studies have highlighted the significance of CD147 in tumor progression, thus affirming its role in tumor diagnosis. CD147 is overexpressed in a variety of cancers, such as lung, breast, prostate, stomach, and genitourinary cancers (93–97). Another study suggested that CD147 may be an important independent predictor of poor survival in HCC patients, owing to its role in tumor growth, invasion and angiogenesis (98). The results showed that the expression of CD147 was positively correlated with metalloproteinase-2, vascular endothelial growth factor and microvascular density CD34 in hepatocellular carcinoma patients. Patients with high CD147 expression had poor survival (98). Given the important role played by CD147 in tumor cell growth, survival and invasive metastasis, coupled with its widespread expression in human malignancies, researchers have employed proteomics techniques to analyze differential expression of proteins in liver cancer plasma/serum. Results indicate that CD147 antigen is specifically highly expressed in the plasma of liver cancer patients. Zhu et al. (99) suggested that the higher the expression of CD147 or the better the degree of tumor differentiation, the longer the survival of patients with liver cancer, thus an effective therapeutic target for interfering with or reversing HCC progression. On the other hand, Wu et al. (100) found that serum soluble CD147 levels were not only significantly higher in HCC patients than healthy subjects, but were also associated with tumor size and Child-Pugh classification. that the authors concluded that detection of soluble CD 147 has some value in HCC diagnosis. In addition, CD147 expression is strongly correlated with HCC prognosis. oliver et al. (101) demonstrated that HCC patients with low CD147 expression had longer survival. However, a meta-analysis by Peng et al. showed no correlation between HCC survival and CD147 expression (102).Taken together, these studies indicate that CD147 plays a crucial role in HCC progression, and affirm its potential as a diagnostic biomarker.
The role of CD147 in tumorigenesis has made it a new target for development of tumor therapies. The basic approach for targeted therapy entails down-regulating expression of CD147 protein via RNAi technology (103), small molecule compounds (104), anti-CD147 monoclonal antibodies (105, 106) or polyclonal antibodies (107) with the aim of blocking CD147 function. Multiple antigenic peptide vaccines have also been employed (108). To target CD147, the therapeutic agent Licartin (generic name (I131) metuximab injection) was developed as an anti-CD147 monoclonal antibody HAb18 coupled to the radioisotope I131. Results of a Phase I/II trials demonstrated that Licartin is safe, thus it was officially approved for clinical use by the Chinese State Food and Drug Administration (SFDA, registration number S20050039) (109). Results from a randomized trial showed that Licartin prevents HCC recurrence after liver transplantation (110). Despite Licartin’s efficacy in HCC, its clinical application has been limited by radioactive I131 component. Wang et al. (111) experimentally tested four anti-CD147 antibodies in HCC, and found that while 1B 3 and 3B 3 effectively inhibited MMP-2 secretion and cell invasion, HAb 18 Gedomab 1 and HAb18 Gedomab 2 exhibited opposite effects. Wang et al. developed an anti-CD147 antibody- HAb 18. The chimeric antibody cHAb 18 contains variable heavy and light chains of HAb 18 antibody and a constant region of human IgG 1 γ1. The authors found that cHAb 18 treatment not only effectively suppressed liver tumor metastasis but also prolonged survival in an in situ HCC mouse model (112). Apart from the anti-CD 147 antibody strategy, researchers have demonstrated that small molecule (AC-73) inhibitors of CD147 dimerization can suppress MMP-2 production in hepatocellular carcinoma via the CD147-ERK-STAT 3-MMP-2 signaling pathway (104). Tseng et al (113). used chimeric antigen receptor therapy with CAR-transduced T and NK cells that recognize the surface marker CD147 to effectively kill various malignant HCC cell lines in vitro, as well as HCC tumors in xenograft and patient-derived xenograft mouse models. These findings support the therapeutic potential of CD147-CAR-modified immune cells for HCC patients.
CD147 is a cell adhesion molecule involved in intercellular and extracellular matrix interactions. Functionally, it stimulates secretion of MMP without affecting production of tissue inhibitors of metalloproteinases (physiological inhibitors of MMP), thereby altering the collagenolytic balance to activate MMP (114). Members of the CD147 family widely differ in molecular weights, depending on the species, tissues and cells (115–117). Studies have shown that CD147 is not only highly expressed in HCC, but is also closely associated with its development (100). Notably, CD147 promotes HCC invasion and metastasis through integrin-mediated FAK-paxillin, FAK-PI3K-Ca2+, RhoA/ROCK, WAVE2 and Rac1 signaling pathways. In addition, CD147 can induce VEGF and MMPs formation to promote HCC angiogenesis. In this review, we have described the CD147 structure and its underlying molecular mechanism in HCC invasion, metastasis and angiogenesis. In addition, we have highlighted its potential as a diagnostic marker for HCC. HCC develops due to accumulation of multiple factors and interaction of multiple mechanisms. In addition, CD147 plays a role in the immune infiltration or immune escape of the tumor microenvironment. Chen et al (118). showed that CD147 regulates anti-tumor CD8 T cell responses to promote tumor immune escape. In recent years, extracellular vesicles (EVs) have been extensively studied. Study finds CD14-positive EVs are a novel biomarker of HCC and cholangiocarcinoma liquid biopsy that permit a non-invasive assessment of the presence and possible extent of these cancers in patients with advanced liver diseases (119). Yang et al (120). noted that immune cells- derived EVs containing integrin αMβ2 or CD147 may facilitate HCC metastasis.
Currently, there is no effective therapy available to treat HCC. Sorafenib is a widely used first-line standard agent for the treatment of advanced HCC, but has been shown to have low efficacy and severe side effects (121). Opdivo, a PD-1 blocker, has been approved by the U.S. Food and Drug Administration as a second-line treatment strategy for HCC patients previously treated with Sorafenib (122). CD147 is being investigated as a new target for HCC treatment. Anti-CD147 monoclonal antibody-targeted therapy for HCC is a promising strategy. Cost of use and security is a major challenge. Overcoming these problems will make CD147 prominent in the treatment of HCC. Notably, prognosis of HCC patients has seen little improvement in the last two decades, possibly due to limited information on the molecular mechanisms underlying its progression. Urgent elucidation of these mechanisms is imperative to future development of novel and effective therapeutic strategies and reliable diagnostic biomarkers for HCC patients.
DH, DR, and QJ searched for literature and wrote the first draft of this article. ML and JZ edited the manuscript. ZL edited figures. HS and TZ strictly reviewed the manuscript and polished the grammar. All authors contributed to the article and approved the submitted version.
This work was supported by funds from the National Natural Science Foundation of China (Grant No. 82260422), and Key R&D Planning Project of Jiangxi Science and Technology Commission, China (No. 20203BBGL73126).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
3. Oliveri RS, Wetterslev J, Gluud C. Hepatocellular carcinoma. Lancet (London England) (2012) 380(9840):470. doi: 10.1016/S0140-6736(12)61285-9
4. Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis b virus infection. Lancet (London England) (2018) 392(10161):2313–24. doi: 10.1016/S0140-6736(18)31865-8
5. Medavaram S, Zhang Y. Emerging therapies in advanced hepatocellular carcinoma. Exp Hematol Oncol (2018) 7:17. doi: 10.1186/s40164-018-0109-6
6. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin (2007) 57(1):43–66. doi: 10.3322/canjclin.57.1.43
7. Dimitroulis D, Damaskos C, Valsami S, Davakis S, Garmpis N, Spartalis E, et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J Gastroenterol (2017) 23(29):5282–94. doi: 10.3748/wjg.v23.i29.5282
8. Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist (2010) 15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05
9. Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol (2014) 28(5):753–70. doi: 10.1016/j.bpg.2014.08.007
10. El-Serag HB. Hepatocellular carcinoma. N Engl J Med (2011) 365(12):1118–27. doi: 10.1056/NEJMra1001683
11. Seshadri RM, Besur S, Niemeyer DJ, Templin M, McKillop IH, Swan RZ, et al. Survival analysis of patients with stage I and II hepatocellular carcinoma after a liver transplantation or liver resection. HPB (Oxford) (2014) 16(12):1102–9. doi: 10.1111/hpb.12300
12. Niemeyer DJ, Simo KA, Iannitti DA, McKillop IH. Ablation therapy for hepatocellular carcinoma: past, present and future perspectives. Hepat Oncol (2014) 1(1):67–79. doi: 10.2217/hep.13.8
13. Earl TM, Chapman WC. Hepatocellular carcinoma: Resection versus transplantation. Semin Liver Dis (2013) 33(3):282–92. doi: 10.1055/s-0033-1351783
14. Gabison EE, Huet E, Baudouin C, Menashi S. Direct epithelial-stromal interaction in corneal wound healing: Role of EMMPRIN/CD147 in MMPs induction and beyond. Prog Retin Eye Res (2009) 28(1):19–33. doi: 10.1016/j.preteyeres.2008.11.001
15. Gao C, Lu CH, Chen J. Biological characteristics of cluster of differentiation 147 and its relationship with tumour. Zhongguo Yi Xue Ke Xue Yuan Xue Bao (2016) 38(5):589–93. doi: 10.3881/j.issn.1000-503X.2016.05.018
16. Grass GD, Toole BP. How, with whom and when: an overview of CD147-mediated regulatory networks influencing matrix metalloproteinase activity. Biosci Rep (2015) 36(1):e00283. doi: 10.1042/BSR20150256
17. Chen X, Lin J, Kanekura T, Su J, Lin W, Xie H, et al. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res (2006) 66(23):11323–30. doi: 10.1158/0008-5472.CAN-06-1536
18. Yang JM, Xu Z, Wu H, Zhu H, Wu X, Hait WN. Overexpression of extracellular matrix metalloproteinase inducer in multidrug resistant cancer cells. Mol Cancer Res (2003) 1(6):420–7.
19. Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J Biochem (2016) 159(5):481–90. doi: 10.1093/jb/mvv127
20. Pennings GJ, Kritharides L. CD147 in cardiovascular disease and thrombosis. Semin Thromb Hemost (2014) 40(7):747–55. doi: 10.1182/blood-2009-04-217380
21. Bougatef F, Quemener C, Kellouche S, Naimi B, Podgorniak MP, Millot G, et al. EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2alpha-mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood (2009) 114(27):5547–56. doi: 10.1182/blood-2009-04-217380
22. Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer (2002) 99(4):520–8. doi: 10.1002/ijc.10390
23. Xu J, Lu Y, Qiu S, Chen ZN, Fan Z. A novel role of EMMPRIN/CD147 in transformation of quiescent fibroblasts to cancer-associated fibroblasts by breast cancer cells. Cancer Lett (2013) 335(2):380–6. doi: 10.1016/j.canlet.2013.02.054
24. Basil CF, Zhao Y, Zavaglia K, Jin P, Panelli MC, Voiculescu S, et al. Common cancer biomarkers. Cancer Res (2006) 66(6):2953–61. doi: 10.1158/0008-5472.CAN-05-3433
25. Fan H, Yi W, Wang C, Wang J. The clinicopathological significance and prognostic value of EMMPRIN overexpression in cancers: Evidence from 39 cohort studies. Oncotarget (2017) 8(47):82643–60. doi: 10.18632/oncotarget.19740
26. Miyauchi T, Masuzawa Y, Muramatsu T. The basigin group of the immunoglobulin superfamily: Complete conservation of a segment in and around transmembrane domains of human and mouse basigin and chicken HT7 antigen. J Biochem (1991) 110(5):770–4. doi: 10.1093/oxfordjournals.jbchem.a123657
27. Jiang JL, Zhou Q, Yu MK, Ho LS, Chen ZN, Chan HC. The involvement of HAb18G/CD147 in regulation of store-operated calcium entry and metastasis of human hepatoma cells. J Biol Chem (2001) 276(50):46870–7. doi: 10.1074/jbc.M108291200
28. Fadool JM, Linser PJ. 5A11 antigen is a cell recognition molecule which is involved in neuronal-glial interactions in avian neural retina. Dev Dyn (1993) 196(4):252–62. doi: 10.1002/aja.1001960406
29. Kadomatsu K, Muramatsu T. [Role of basigin, a glycoprotein belonging to the immunoglobulin superfamily, in the nervous system]. Tanpakushitsu Kakusan Koso (2004) 49(15 Suppl):2417–24.
30. Kaname T, Miyauchi T, Kuwano A, Matsuda Y, Muramatsu T, Kajii T. Mapping basigin (BSG), a member of the immunoglobulin superfamily, to 19p13.3. Cytogenet Cell Genet (1993) 64(3-4):195–7. doi: 10.1159/000133573
31. Liang L, Major T, Bocan T. Characterization of the promoter of human extracellular matrix metalloproteinase inducer (EMMPRIN). Gene (2002) 282(1-2):75–86. doi: 10.1016/S0378-1119(01)00847-2
32. Hahn JN, Kaushik DK, Yong VW. The role of EMMPRIN in T cell biology and immunological diseases. J Leukoc Biol (2015) 98(1):33–48. doi: 10.1189/jlb.3RU0215-045R
33. Yang HW, Tsai RY, Chen JP, Ju SP, Liao JF, Wei KC, et al. Fabrication of a nanogold-dot array for rapid and sensitive detection of vascular endothelial growth factor in human serum. ACS Appl Mater Interfaces (2016) 8(45):30845–52. doi: 10.1021/acsami.6b13329
34. Zhu X, Song Z, Zhang S, Nanda A, Li G. CD147: A novel modulator of inflammatory and immune disorders. Curr Med Chem (2014) 21(19):2138–45. doi: 10.2174/0929867321666131227163352
35. Liao CG, Kong LM, Song F, Xing JL, Wang LX, Sun ZJ, et al. Characterization of basigin isoforms and the inhibitory function of basigin-3 in human hepatocellular carcinoma proliferation and invasion. Mol Cell Biol (2011) 31(13):2591–604. doi: 10.1128/MCB.05160-11
36. Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, et al. Human protein reference database–2009 update. Nucleic Acids Res (2009) 37(Database issue):D767–72. doi: 10.1093/nar/gkn892
37. Redzic JS, Armstrong GS, Isern NG, Jones DN, Kieft JS, Eisenmesser EZ. The retinal specific CD147 Ig0 domain: from molecular structure to biological activity. J Mol Biol (2011) 411(1):68–82. doi: 10.1016/j.jmb.2011.04.060
38. Findinier J, Laurent S, Duchene T, Roussel X, Lancelon-Pin C, Cuine S, et al. Deletion of BSG1 in chlamydomonas reinhardtii leads to abnormal starch granule size and morphology. Sci Rep (2019) 9(1):1990. doi: 10.1038/s41598-019-39506-6
39. Yu XL, Hu T, Du JM, Ding JP, Yang XM, Zhang J, et al. Crystal structure of HAb18G/CD147: Implications for immunoglobulin superfamily homophilic adhesion. J Biol Chem (2008) 283(26):18056–65. doi: 10.1074/jbc.M802694200
40. Shih YT, Wang W, Hasenbeck A, Stone D, Zhao Y. Investigation of physicochemical, nutritional, and sensory qualities of muffins incorporated with dried brewer's spent grain flours as a source of dietary fiber and protein. J Food Sci (2020) 85(11):3943–53. doi: 10.1111/1750-3841.15483
41. Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost (2005) 93(2):199–204. doi: 10.1160/TH04-08-0536
44. Tang W, Hemler ME. Caveolin-1 regulates matrix metalloproteinases-1 induction and CD147/EMMPRIN cell surface clustering. J Biol Chem (2004) 279(12):11112–8. doi: 10.1074/jbc.M312947200
45. Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J (2000) 19(15):3896–904. doi: 10.1093/emboj/19.15.3896
46. Cho JY, Fox DA, Horejsi V, Sagawa K, Skubitz KM, Katz DR, et al. The functional interactions between CD98, beta1-integrins, and CD147 in the induction of U937 homotypic aggregation. Blood (2001) 98(2):374–82. doi: 10.1182/blood.V98.2.374
47. Li Y, Wu J, Song F, Tang J, Wang SJ, Yu XL, et al. Extracellular membrane-proximal domain of HAb18G/CD147 binds to metal ion-dependent adhesion site (MIDAS) motif of integrin beta1 to modulate malignant properties of hepatoma cells. J Biol Chem (2012) 287(7):4759–72. doi: 10.1074/jbc.M111.277699
48. Storry JR. The ok blood group system: An update. Immunohematology (2021) 37(1):18–9. doi: 10.21307/immunohematology-2021-004
49. Knutti N, Kuepper M, Friedrich K. Soluble extracellular matrix metalloproteinase inducer (EMMPRIN, EMN) regulates cancer-related cellular functions by homotypic interactions with surface CD147. FEBS J (2015) 282(21):4187–200. doi: 10.1111/febs.13414
50. Tang Y, Nakada MT, Rafferty P, Laraio J, McCabe FL, Millar H, et al. Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K-akt signaling pathway. Mol Cancer Res (2006) 4(6):371–7. doi: 10.1158/1541-7786.MCR-06-0042
51. Chen Y, Zhang H, Gou X, Horikawa Y, Xing J, Chen Z. Upregulation of HAb18G/CD147 in activated human umbilical vein endothelial cells enhances the angiogenesis. Cancer Lett (2009) 278(1):113–21. doi: 10.1016/j.canlet.2009.01.004
52. Yin Z, Cai H, Wang Z, Jiang Y, Proliferation PABI. Invasion, and angiogenesis in esophageal squamous cell carcinoma through regulating CD147. Drug Des Devel Ther (2020) 14:4561–73. doi: 10.2147/DDDT.S269915
53. Wan Y, Wu XY. [Expression and clinical significance of DAPK1 and CD147 in esophageal squamous cell carcinoma]. Zhonghua Zhong Liu Za Zhi (2012) 34(1):44–8.
54. Zhu S, Li Y, Mi L, Zhang Y, Zhang L, Gong L, et al. Clinical impact of HAb18G/CD147 expression in esophageal squamous cell carcinoma. Dig Dis Sci (2011) 56(12):3569–76. doi: 10.1007/s10620-011-1812-x
55. Huang L, Xu AM, Peng Q. CD147 and MMP-9 expressions in type II/III adenocarcinoma of esophagogastric junction and their clinicopathological significances. Int J Clin Exp Pathol (2015) 8(2):1929–37.
56. Sweeny L, Liu Z, Bush BD, Hartman Y, Zhou T, Rosenthal EL. CD147 and AGR2 expression promote cellular proliferation and metastasis of head and neck squamous cell carcinoma. Exp Cell Res (2012) 318(14):1788–98. doi: 10.1016/j.yexcr.2012.04.022
57. Yu B, Zhang Y, Wu K, Wang L, Jiang Y, Chen W, et al. CD147 promotes progression of head and neck squamous cell carcinoma via NF-kappa b signaling. J Cell Mol Med (2019) 23(2):954–66. doi: 10.1111/jcmm.13996
58. Cao Z, Xiang J, Li C. Expression of extracellular matrix metalloproteinase inducer and enhancement of the production of matrix metalloproteinase-1 in tongue squamous cell carcinoma. Int J Oral Maxillofac Surg (2009) 38(8):880–5. doi: 10.1016/j.ijom.2009.03.004
59. Haque A, Moriyama M, Kubota K, Ishiguro N, Sakamoto M, Chinju A, et al. CD206(+) tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci Rep (2019) 9(1):14611. doi: 10.1038/s41598-019-51149-1
60. Huang C, Sun Z, Sun Y, Chen X, Zhu X, Fan C, et al. Association of increased ligand cyclophilin a and receptor CD147 with hypoxia, angiogenesis, metastasis and prognosis of tongue squamous cell carcinoma. Histopathology (2012) 60(5):793–803. doi: 10.1111/j.1365-2559.2011.04130.x
61. Liu H, Xu XF, Zhao Y, Tang MC, Zhou YQ, Gao FH. NS-398 promotes pancreatic cancer cell invasion by CD147 and MMP-2 via the activation of P38. Mol Med Rep (2016) 13(3):2208–14. doi: 10.3892/mmr.2016.4783
62. Li M, Zhai Q, Bharadwaj U, Wang H, Li F, Fisher WE, et al. Cyclophilin a is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer (2006) 106(10):2284–94. doi: 10.1002/cncr.21862
63. Zhou Y, Zheng M, Liu Z, Yang H, Zhu P, Jiang JL, et al. CD147 promotes DNA damage response and gemcitabine resistance via targeting ATM/ATR/p53 and affects prognosis in pancreatic cancer. Biochem Biophys Res Commun (2020) 528(1):62–70. doi: 10.1016/j.bbrc.2020.05.005
64. Chu D, Zhu S, Li J, Ji G, Wang W, Wu G, et al. CD147 expression in human gastric cancer is associated with tumor recurrence and prognosis. PloS One (2014) 9(6):e101027. doi: 10.1371/journal.pone.0101027
65. Zheng HC, Gong BC. CD147 expression was positively linked to aggressiveness and worse prognosis of gastric cancer: A meta and bioinformatics analysis. Oncotarget (2017) 8(52):90358–70. doi: 10.18632/oncotarget.20089
66. Yang GL, Tao HR, Wang HW, Sun Y, Zhang LD, Zhang C, et al. Ara-c increases gastric cancer cell invasion by upregulating CD-147-MMP-2/MMP−9 via the ERK signaling pathway. Oncol Rep (2015) 33(4):2045–51. doi: 10.3892/or.2015.3748
67. Jiang Z, Zhang H, Liu C, Yin J, Tong S, Lv J, et al. beta3GnT8 promotes colorectal cancer cells invasion via CD147/MMP2/Galectin3 axis. Front Physiol (2018) 9:588. doi: 10.3389/fphys.2018.00588
68. Xu T, Zhou M, Peng L, Kong S, Miao R, Shi Y, et al. Upregulation of CD147 promotes cell invasion, epithelial-to-mesenchymal transition and activates MAPK/ERK signaling pathway in colorectal cancer. Int J Clin Exp Pathol (2014) 7(11):7432–41.
69. Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun (2014) 5:3591. doi: 10.1038/ncomms4591
70. Zhang Z, Lin M, Wang J, Yang F, Yang P, Liu Y, et al. Calycosin inhibits breast cancer cell migration and invasion by suppressing EMT. via BATF/TGF-beta1. Aging (Albany NY) (2021) 13(12):16009–23. doi: 10.18632/aging.203093
71. Li F, Zhang J, Guo J, Jia Y, Han Y, Wang Z. RNA Interference targeting CD147 inhibits metastasis and invasion of human breast cancer MCF-7 cells by downregulating MMP-9/VEGF expression. Acta Biochim Biophys Sin (Shanghai) (2018) 50(7):676–84. doi: 10.1093/abbs/gmy062
72. Liu Y, Xin T, Jiang QY, Huang DY, Shen WX, Li L, et al. CD147, MMP9 expression and clinical significance of basal-like breast cancer. Med Oncol (2013) 30(1):366. doi: 10.1007/s12032-012-0366-x
73. Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, et al. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res (1995) 55(2):434–9.
74. Guo H, Zucker S, Gordon MK, Toole BP, Biswas C. Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem (1997) 272(1):24–7. doi: 10.1074/jbc.272.1.24
75. Xu J, Xu HY, Zhang Q, Song F, Jiang JL, Yang XM, et al. HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol Cancer Res (2007) 5(6):605–14. doi: 10.1158/1541-7786.MCR-06-0286
76. Guo H, Li R, Zucker S, Toole BP. EMMPRIN (CD147), an inducer of matrix metalloproteinase synthesis, also binds interstitial collagenase to the tumor cell surface. Cancer Res (2000) 60(4):888–91.
77. Ru NY, Wu J, Chen ZN, Bian H. HAb18G/CD147 is involved in TGF-beta-induced epithelial-mesenchymal transition and hepatocellular carcinoma invasion. Cell Biol Int (2015) 39(1):44–51. doi: 10.1002/cbin.10341
78. Tang J, Wu YM, Zhao P, Yang XM, Jiang JL, Chen ZN. Overexpression of HAb18G/CD147 promotes invasion and metastasis via alpha3beta1 integrin mediated FAK-paxillin and FAK-PI3K-Ca2+ pathways. Cell Mol Life Sci (2008) 65(18):2933–42. doi: 10.1007/s00018-008-8315-8
79. Zhao P, Zhang W, Wang SJ, Yu XL, Tang J, Huang W, et al. HAb18G/CD147 promotes cell motility by regulating annexin II-activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology (2011) 54(6):2012–24. doi: 10.1002/hep.24592
80. Cui HY, Guo T, Wang SJ, Zhao P, Dong ZS, Zhang Y, et al. Dimerization is essential for HAb18G/CD147 promoting tumor invasion via MAPK pathway. Biochem Biophys Res Commun (2012) 419(3):517–22. doi: 10.1016/j.bbrc.2012.02.049
81. Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li Y, et al. Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer Sci (2010) 101(2):387–95. doi: 10.1111/j.1349-7006.2009.01420.x
82. Lafleur MA, Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in angiogenesis. Expert Rev Mol Med (2003) 5(23):1–39. doi: 10.1017/S1462399403006628
83. Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res (2005) 65(8):3193–9. doi: 10.1158/0008-5472.CAN-04-3605
84. Wang CH, Yao H, Chen LN, Jia JF, Wang L, Dai JY, et al. CD147 induces angiogenesis through a vascular endothelial growth factor and hypoxia-inducible transcription factor 1alpha-mediated pathway in rheumatoid arthritis. Arthritis Rheum (2012) 64(6):1818–27. doi: 10.1002/art.34341
85. Reiss K, Saftig P. The "a disintegrin and metalloprotease" (ADAM) family of sheddases: physiological and cellular functions. Semin Cell Dev Biol (2009) 20(2):126–37. doi: 10.1016/j.semcdb.2008.11.002
86. Albrechtsen R, Wewer Albrechtsen NJ, Gnosa S, Schwarz J, Dyrskjot L, Kveiborg M. Identification of ADAM12 as a novel basigin sheddase. Int J Mol Sci (2019) 20(8):1957. doi: 10.3390/ijms20081957
87. Wu B, Cui J, Yang XM, Liu ZY, Song F, Li L, et al. Cytoplasmic fragment of CD147 generated by regulated intramembrane proteolysis contributes to HCC by promoting autophagy. Cell Death Dis (2017) 8(7):e2925. doi: 10.1038/cddis.2017.251
88. Jia L, Wang H, Qu S, Miao X, Zhang J. CD147 regulates vascular endothelial growth factor-a expression, tumorigenicity, and chemosensitivity to curcumin in hepatocellular carcinoma. IUBMB Life (2008) 60(1):57–63. doi: 10.1002/iub.11
89. Zhang Q, Chen X, Zhou J, Zhang L, Zhao Q, Chen G, et al. CD147, MMP-2, MMP-9 and MVD-CD34 are significant predictors of recurrence after liver transplantation in hepatocellular carcinoma patients. Cancer Biol Ther (2006) 5(7):808–14. doi: 10.4161/cbt.5.7.2754
90. Jia L, Cao J, Wei W, Wang S, Zuo Y, Zhang J. CD147 depletion down-regulates matrix metalloproteinase-11, vascular endothelial growth factor-a expression and the lymphatic metastasis potential of murine hepatocarcinoma hca-f cells. Int J Biochem Cell Biol (2007) 39(11):2135–42. doi: 10.1016/j.biocel.2007.06.007
91. Qin Z, Dai L, Slomiany MG, Toole BP, Parsons C. Direct activation of emmprin and associated pathogenesis by an oncogenic herpesvirus. Cancer Res (2010) 70(10):3884–9. doi: 10.1158/0008-5472.CAN-09-4663
92. Dai L, Bratoeva M, Toole BP, Qin Z, Parsons C. KSHV activation of VEGF secretion and invasion for endothelial cells is mediated through viral upregulation of emmprin-induced signal transduction. Int J Cancer (2012) 131(4):834–43. doi: 10.1002/ijc.26428
93. Zhong X, Li M, Nie B, Wu F, Zhang L, Wang E, et al. Overexpressions of RACK1 and CD147 associated with poor prognosis in stage T1 pulmonary adenocarcinoma. Ann Surg Oncol (2013) 20(3):1044–52. doi: 10.1245/s10434-012-2377-4
94. Zhao S, Ma W, Zhang M, Tang D, Shi Q, Xu S, et al. High expression of CD147 and MMP-9 is correlated with poor prognosis of triple-negative breast cancer (TNBC) patients. Med Oncol (2013) 30(1):335. doi: 10.1007/s12032-012-0335-4
95. Li Y, Xu J, Chen L, Zhong WD, Zhang Z, Mi L, et al. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology (2009) 54(6):677–87. doi: 10.1111/j.1365-2559.2009.03280.x
96. Han ZD, Bi XC, Qin WJ, He HC, Dai QS, Zou J, et al. CD147 expression indicates unfavourable prognosis in prostate cancer. Pathol Oncol Res (2009) 15(3):369–74. doi: 10.1007/s12253-008-9131-z
97. Han ZD, He HC, Bi XC, Qin WJ, Dai QS, Zou J, et al. Expression and clinical significance of CD147 in genitourinary carcinomas. J Surg Res (2010) 160(2):260–7. doi: 10.1016/j.jss.2008.11.838
98. Zhang Q, Zhou J, Ku XM, Chen XG, Zhang L, Xu J, et al. Expression of CD147 as a significantly unfavorable prognostic factor in hepatocellular carcinoma. Eur J Cancer Prev (2007) 16(3):196–202. doi: 10.1097/01.cej.0000236245.40619.c3
99. Zhu S, Li Y, Zhang Y, Wang X, Gong L, Han X, et al. Expression and clinical implications of HAb18G/CD147 in hepatocellular carcinoma. Hepatol Res (2015) 45(1):97–106. doi: 10.1111/hepr.12320
100. Wu J, Hao ZW, Zhao YX, Yang XM, Tang H, Zhang X, et al. Full-length soluble CD147 promotes MMP-2 expression and is a potential serological marker in detection of hepatocellular carcinoma. J Transl Med (2014) 12:190. doi: 10.1186/1479-5876-12-190
101. Waidmann O, Koberle V, Bettinger D, Trojan J, Zeuzem S, Schultheiss M, et al. Diagnostic and prognostic significance of cell death and macrophage activation markers in patients with hepatocellular carcinoma. J Hepatol (2013) 59(4):769–79. doi: 10.1016/j.jhep.2013.06.008
102. Peng F, Li H, You Q, Li H, Wu D, Jiang C, et al. CD147 as a novel prognostic biomarker for hepatocellular carcinoma: A meta-analysis. BioMed Res Int (2017) 2017:5019367. doi: 10.1155/2017/5019367
103. Hatanaka M, Higashi Y, Kawai K, Su J, Zeng W, Chen X, et al. CD147-targeted siRNA in A375 malignant melanoma cells induces the phosphorylation of EGFR and downregulates cdc25C and MEK phosphorylation. Oncol Lett (2016) 11(4):2424–8. doi: 10.3892/ol.2016.4267
104. Fu ZG, Wang L, Cui HY, Peng JL, Wang SJ, Geng JJ, et al. A novel small-molecule compound targeting CD147 inhibits the motility and invasion of hepatocellular carcinoma cells. Oncotarget (2016) 7(8):9429–47. doi: 10.18632/oncotarget.6990
105. Agrawal SM, Silva C, Wang J, Tong JP, Yong VW. A novel anti-EMMPRIN function-blocking antibody reduces T cell proliferation and neurotoxicity: Relevance to multiple sclerosis. J Neuroinflamm (2012) 9:64. doi: 10.1186/1742-2094-9-64
106. Baba M, Inoue M, Itoh K, Nishizawa Y. Blocking CD147 induces cell death in cancer cells through impairment of glycolytic energy metabolism. Biochem Biophys Res Commun (2008) 374(1):111–6. doi: 10.1016/j.bbrc.2008.06.122
107. Walter M, Simanovich E, Brod V, Lahat N, Bitterman H, Rahat MA. An epitope-specific novel anti-EMMPRIN polyclonal antibody inhibits tumor progression. Oncoimmunology (2016) 5(2):e1078056. doi: 10.1080/2162402X.2015.1078056
108. Simanovich E, Brod V, Rahat MM, Drazdov E, Walter M, Shakya J, et al. Inhibition of tumor growth and metastasis by EMMPRIN multiple antigenic peptide (MAP) vaccination is mediated by immune modulation. Oncoimmunology (2017) 6(1):e1261778. doi: 10.1080/2162402X.2016.1261778
109. Chen ZN, Mi L, Xu J, Song F, Zhang Q, Zhang Z, et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: clinical phase I/II trials. Int J Radiat Oncol Biol Phys (2006) 65(2):435–44. doi: 10.1016/j.ijrobp.2005.12.034
110. Xu J, Shen ZY, Chen XG, Zhang Q, Bian HJ, Zhu P, et al. A randomized controlled trial of licartin for preventing hepatoma recurrence after liver transplantation. Hepatology (2007) 45(2):269–76. doi: 10.1002/hep.21465
111. Wang L, Ku XM, Li Y, Bian HJ, Zhang SH, Ye H, et al. Regulation of matrix metalloproteinase production and tumor cell invasion by four monoclonal antibodies against different epitopes of HAb18G/CD147 extracellular domain. Hybridoma (Larchmt) (2006) 25(2):60–7. doi: 10.1089/hyb.2006.25.60
112. Wang Y, Yuan L, Yang XM, Wei D, Wang B, Sun XX, et al. A chimeric antibody targeting CD147 inhibits hepatocellular carcinoma cell motility via FAK-PI3K-Akt-Girdin signaling pathway. Clin Exp Metastasis (2015) 32(1):39–53. doi: 10.1007/s10585-014-9689-7
113. Tseng HC, Xiong W, Badeti S, Yang Y, Ma M, Liu T, et al. Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nat Commun (2020) 11(1):4810. doi: 10.1038/s41467-020-18444-2
114. Gabison EE, Hoang-Xuan T, Mauviel A, Menashi S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie (2005) 87(3-4):361–8. doi: 10.1016/j.biochi.2004.09.023
115. Kanekura T, Miyauchi T, Tashiro M, Muramatsu T. Basigin, a new member of the immunoglobulin superfamily: Genes in different mammalian species, glycosylation changes in the molecule from adult organs and possible variation in the n-terminal sequences. Cell Struct Funct (1991) 16(1):23–30. doi: 10.1247/csf.16.23
116. Nehme CL, Fayos BE, Bartles JR. Distribution of the integral plasma membrane glycoprotein CE9 (MRC OX-47) among rat tissues and its induction by diverse stimuli of metabolic activation. Biochem J (1995) 310(Pt 2):693–8. doi: 10.1042/bj3100693
117. Li R, Huang L, Guo H, Toole BP. Basigin (murine EMMPRIN) stimulates matrix metalloproteinase production by fibroblasts. J Cell Physiol (2001) 186(3):371–9. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1042>3.0.CO;2-8
118. Chen Y, Xu J, Wu X, Yao H, Yan Z, Guo T, et al. CD147 regulates antitumor CD8(+) T-cell responses to facilitate tumor-immune escape. Cell Mol Immunol (2021) 18(8):1995–2009. doi: 10.1038/s41423-020-00570-y
119. Julich-Haertel H, Urban SK, Krawczyk M, Willms A, Jankowski K, Patkowski W, et al. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J Hepatol (2017) 67(2):282–92. doi: 10.1016/j.jhep.2017.02.024
120. Yang N, Li S, Li G, Zhang S, Tang X, Ni S, et al. The role of extracellular vesicles in mediating progression, metastasis and potential treatment of hepatocellular carcinoma. Oncotarget (2017) 8(2):3683–95. doi: 10.18632/oncotarget.12465
121. Ben Mousa A. Sorafenib in the treatment of advanced hepatocellular carcinoma. Saudi J Gastroenterol (2008) 14(1):40–2. doi: 10.4103/1319-3767.37808
122. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (London England) (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2
Keywords: hepatocellular carcinoma, CD147, metastasis, angiogenesis, diagnosis
Citation: Huang D, Rao D, Jin Q, Lai M, Zhang J, Lai Z, Shen H and Zhong T (2023) Role of CD147 in the development and diagnosis of hepatocellular carcinoma. Front. Immunol. 14:1149931. doi: 10.3389/fimmu.2023.1149931
Received: 23 January 2023; Accepted: 28 March 2023;
Published: 06 April 2023.
Edited by:
Xiangliang Yuan, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Manoj Kumar Kashyap, Amity University Gurgaon, IndiaCopyright © 2023 Huang, Rao, Jin, Lai, Zhang, Lai, Shen and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haibin Shen, ODIxOTk0NjAzQHFxLmNvbQ==; Tianyu Zhong, emhvbmd0aWFueXVAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.