
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 08 August 2023
Sec. B Cell Biology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1148632
This article is part of the Research Topic Next Generation B cell Targeting Therapies in Autoimmune Diseases View all 5 articles
 Daidi Zhao1†
Daidi Zhao1† Kaixi Ren1†
Kaixi Ren1† Jiarui Lu1†
Jiarui Lu1† Zhiqin Liu2
Zhiqin Liu2 Zunbo Li3
Zunbo Li3 Jun Wu4
Jun Wu4 Zhihao Xu5
Zhihao Xu5 Songdi Wu6
Songdi Wu6 Tao Lei7
Tao Lei7 Chao Ma8
Chao Ma8 Sijia Zhao1
Sijia Zhao1 Miao Bai1
Miao Bai1 Hongzeng Li1*
Hongzeng Li1* Jun Guo1*
Jun Guo1*Objective: To address a novel lower-dose rituximab (RTX) therapy strategy based on our clinical experience and assess its efficacy and safety in neuromyelitis optica spectrum disorder (NMOSD).
Methods: A multicenter, open-label, self-controlled, prospective follow-up study. Totally, 108 NMOSD patients were enrolled and a lower-dose RTX strategy was applied including 100 mg weekly for 3 weeks and then reinfusions every 6 months. Annualized relapse rate (ARR), the expanded disability status scale (EDSS) score and length of spinal cord lesions were included to evaluate the efficacy. Side effects were recorded to assess the safety profile.
Results: Of 108 patients, 80 (74.1%) initiated low-dose RTX therapy immediately after acute attack treatment and 33 (30.6%) initiated it after the first attack. During a median treatment period of 35.5 (22.0–48.8) months, significant decreases were observed in median ARR (1.1 [0.8–2.0] versus 0 [0–0.2], p < 0.001), EDSS score (3.5 [2.5–4.0] versus 2.0 [1.0–3.0], p < 0.001) and spinal cord lesion segments (5.0 [4.0–8.0] versus 3.0 [1.0–6.0], p < 0.001). The cumulative risk of relapses significantly decreased during the post- versus pre-RTX period (HR 0.238, 95%CI 0.160–0.356, p < 0.001) and on early therapy initiated within 24 months after disease onset versus delayed therapy (HR 0.506, 95%CI 0.258–0.994, p = 0.041). No serious side effects were recorded and all the subjects did not discontinue treatment due to RTX-related side effects.
Conclusion: Our research provided evidence supporting the lower-dose RTX strategy in treating NMOSD and reopened the issues of optimal dosage and therapy initiation timing.
Neuromyelitis optica spectrum disorder (NMOSD) is a chronic autoimmune inflammatory disease of the central nervous system mainly characterized by optic neuritis and longitudinal extensive transverse myelitis. Additionally, it may present with syndromes of area postrema, brainstem, diencephalon, and cerebrum (1). Since the discovery of pathogenic antibodies against water channel protein aquaporin-4 (AQP4) (2), it is considered a separate disease entity distinct from multiple sclerosis and has a higher prevalence in East Asian and African populations than in Caucasian population (3, 4). NMOSD is a rare but clinically aggressive disease, where cumulative damages from frequent clinical relapses would result in permanent disabilities and even mortality. Therefore, seeking effective and safe immunosuppressive or immunomodulatory drugs would be necessary to prevent relapses and reduce disability.
Nowadays, only three recombinant monoclonal antibodies have been approved to treat AQP4-IgG seropositive NMOSD given a good efficacy and safety profile confirmed in phase 3 trials, including IL-6R-targeting satralizumab (5, 6), CD19-targeting inebilizumab (7) and complement 5-targeting eculizumab (8). However, the economic costs and treatment compliance need to be taken into consideration when a long-term therapy would be applied for NMOSD. Moreover, no access or difficult access to these drugs in China limits their wide use and also promotes the attempts for alternative treatments. In the past two decades, off-label use of conventional immunosuppressive drugs such as mycophenolate mofetil (MMF) or azathioprine (AZA) has been extensively accepted (9, 10), but long-term daily medication affects treatment compliance to some extent. Instead, some biologic agents such as rituximab (RTX) have been increasingly applied to treat NMOSD (11–13) and are more effective than AZA or MMF in the prevention of subsequent clinical relapses and the improvement of severity of disability (14–16).
RTX is a monoclonal antibody against CD20 on the surface of B cells that was approved initially to treat non-Hodgkin lymphoma (17). Given the pathogenesis of AQP4-IgG mediated humoral immunity in NMOSD, B cell depletion by RTX off-label use has been considered as a possible treatment approach and shows good efficacy, and thus has been highly recommended as first-line therapy (18–20). However, there are no consensus on the protocols of RTX induction therapy and re-treatment, including optimal RTX dosage and timing of therapy initiation. In the majority of previous studies, patients received intravenous infusion of 1 g RTX twice 2 weeks apart or 375 mg/m2 once weekly for 4 weeks as induction therapy, and maintenance reinfusions were administered mainly based on circulating B-cell subset percentages or counts or at a pre-specified fixed interval (19). However, the timing for initiating RTX therapy remains undetermined. Additionally, long-term high-dose RTX therapy may increase the risk of adverse effects of immunosuppression especially fatal consequences (11, 19, 21). The associated costs and treatment compliance of long-term therapy should also be taken into consideration in the majority of NMOSD patients. Thus, as alternatives of conventional high-dose RTX therapy, any effective treatment strategy that minimizes unnecessary exposure to the drug and allows significant cost savings and safety would be beneficial. Up till now, lower doses have been suggested, up to 100 mg RTX per infusion, to find the minimal effective dose in NMOSD and other autoimmune diseases (13, 22, 23). Herein, we addressed a novel lower-dose RTX (LD-RTX) strategy deriving from our real-world clinical practice and assessed its effectiveness and safety in treating NMOSD in a multicenter, open-label, self-controlled, prospective follow-up study conducted in the northwest of China.
This is a multicenter, open-label, self-controlled, prospective follow-up study registered at ClinicalTrials.gov (No.: NCT04256252). A total of eligible 108 patients with NMOSD were enrolled from January 2014 to May 2019. Inclusion criteria included: (1) age between 16 and 75 years old, (2) fulfilling 2006 diagnostic criteria for NMO (24) or 2015 diagnostic criteria for NMOSD (25), (3) at least two relapses in past 2 years and/or at least one attack or relapse in past one years, (4) willingness to enrollment of this study and disease-related assessments, and (5) negative pregnancy tests for female subjects prior to inclusion, and effective contraception for all subjects during the study period. The following exclusion criteria were applied: (1) use of other immunosuppressive agents or discontinuation for less than three months, (2) white blood cell < 3×109/L, neutrophil < 1.5×109/L, hemoglobin < 85 g/L, or platelet < 80×109/L, (3) coexisting active liver disease or persistent transaminases elevation, (4) presence of serious blood, kidney, cardiovascular, and endocrine diseases, serious infection, or history of malignancies, (5) other chronic active immune diseases or stable conditions but requiring immunosuppressivr agents or glucocorticoids, (6) pregnant or lactating subjects and those with family planning, and (7) allergy to rituximab and its other components. The present study was approved by the Ethics Committee of Tangdu Hospital, Air Force Medical University (approval No. 2014120), and informed consent was obtained from all subjects or their legal representatives before LD-RTX therapy initiation.
The LD-RTX therapy protocol consisted of induction therapy and maintenance therapy. RTX was administered at a dose of 100 mg once weekly for 3 weeks as induction therapy. Maintenance therapy was reinfusions of RTX at 100 mg once every 6 months according to the percentages of circulating B cell subsets and patient’s preference. Rescue therapy for relapses was intravenous methylprednisolone 500-1000 mg for 5 consecutive days, and then oral prednisone was initiated at 0.6-1.0 mg/kg and decreased gradually over approximately 3 months. An additional cycle of RTX induction therapy was administered following intravenous methylprednisolone therapy for relapses. Before each RTX infusion, the patient received 0.3 g of oral Ibuprofen and 10 mg of intravenous dexamethasone to prevent possible flu-like symptoms and infusion-related allergic reactions. The percentages of circulating B cell subsets in peripheral blood mononuclear cells (PBMCs) were assessed by flow cytometry before and after LD-RTX induction therapy, and then every 6 months. B-cell depletion was defined as a percentage of CD19+ B cells lower than 1% and CD19+CD27+ memory B cells lower than 0.05%. Once B cell repopulation (percentages of CD19+ B cells > 1% and/or memory B cells > 0.05%) occurred at the pre-specified time points, a maintenance reinfusion of LD-RTX would be recommended.
All the subjects were treated with LD-RTX for at least 12 months. The primary outcome measure for efficacy assessments was annualized relapse rate (ARR) before and after LD-RTX therapy. Clinical relapses were defined as the occurrence of new neurological symptoms or the worsening of previous symptoms maintaining more than 24 hours, with an increase of DESS score by at least 0.5 points. The change of EDSS scores prior to and after LD-RTX therapy was used as the secondary outcome measure, and the lesions on spinal cord MRI were evaluated before and after LD-RTX therapy. In addition, the safety profile was assessed by recording all adverse events related to RTX use. During this study period, EDSS scores were evaluated by two qualified neurologists together.
Categorical data are shown as number with percentage and continuous data as median with interquartile range (IQR). The chi-square test or Fisher’s exact test was used for categorical data to compare the inter-group differences, and Wilcoxon rank sum test or Student’s t test was used for inter-group comparisons of continuous data with skew or normal distribution. The changes of ARR, EDSS and spinal cord lesions before and after LD-RTX therapy were analyzed by the Wilcoxon matched-pairs signed rank test. As previously suggested (14), pre-RTX ARR could be calculated only when disease duration prior to LD-RTX therapy initiation lasted for at least 6 months to avoid the potential overestimation. For ARR comparison, only patients with ARR values of both pre- and post-RTX were included. The Kaplan-Meier survival curve was adopted to analyze the cumulative risk of relapses during pre- versus post-RTX periods and in early versus delayed RTX therapy groups, and inter-group differences were compared by the Mantel-Cox log-rank test. All statistical analyses were performed using the SPSS 23.0 software, Graphpad Prism 7 and R software version 4.2.2 for Windows. Statistical significance was set at the level of p < 0.05.
A total of 108 eligible patients diagnosed with NMOSD were eventually enrolled in the present study, with the enrollment flowchart shown in Figure 1. Of them, 11 patients enrolled between January 2014 and November 2015 according to 2006 NMO diagnostic criteria initially also fulfilled 2015 NMOSD diagnostic criteria. Table 1 illustrates the baseline characteristics of all the patients, including 96 females and 12 males. AQP4-IgG was detected positive in sera from 92 patients, and the other 15 cases had negative results and one case with the result not available, with the comparison of clinical characteristics were revealed in Table 1. Overall, the median age at onset was 39.5 (IQR, 28.3–51.0) years and the median interval from disease onset to LD-RTX therapy initiation was 15.0 (2.0–46.8) months. Myelitis was the most common phenotype of the first attack with an incidence rate of 51.9%, followed by optic neuritis (31.5%). The clinical characteristics of patients who had experienced one attack or at least 2 attacks prior to LD-RTX therapy initiation were compared and shown in Table 2. LD-RTX therapy was initiated in 80 (74.1%) patients immediately after intravenous methylprednisolone rescue therapy for acute attacks, whereas other 28 (25.9%) initiated at the remission stage. All the patients had received immunotherapy prior to RTX use. Of them, 107 (99.1%) has ever treated with steroids, 13 (12.0%) with intravenous immunoglobulin, and 21 (19.4%) with other immunosuppressants including mycophenolate mofetil (MMF), azathioprine (AZA), ciclosporin A (CYC), and cyclophosphamide (CTX). Of note, these immunosuppressive agents had been discontinued for at least 3 months prior to LD-RTX therapy initiation. B-cell depletion was achieved in all subjects after LD-RTX induction therapy. The detailed data on relapses and LD-RTX therapy throughout disease course of all the 108 patients was shown in Figure 2. In total, 35 (32.4%) patients experienced at least one relapse during the post-RTX period, and the occurrence of each relapse and its time relevance to the last reinfusion of RTX was depicted in Supplemental Figure 1. Moreover, the comparison of clinical characteristics of patients with relapses or not during the post-RTX period was revealed in Supplemental Table 1. Of the 35 patients who experienced post-RTX relapses, 4 had switched from RTX to AZA or MMF till the last follow-up. In addition, three patients had been enrolled in other clinical trials but not due to dissatisfaction with the efficacy of LD-RTX therapy. One patient had died of depression not related to NMOSD.

Table 2 Comparison of clinical characteristics of patients according to the number of attacks prior to LD-RTX therapy initiation.
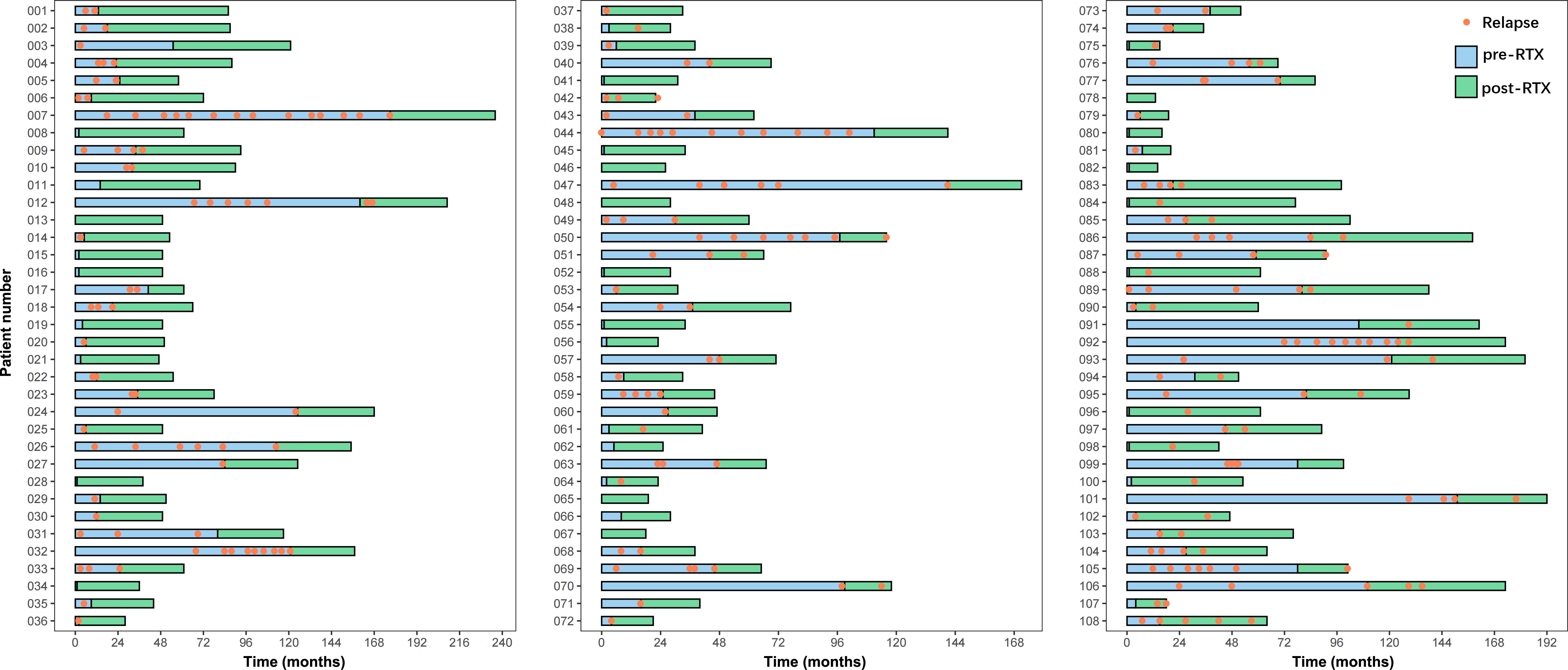
Figure 2 The detailed information on relapses and LD-RTX therapy throughout the disease course of all 108 patients. “0” on the x-axis represents the disease onset.
The efficacy of LD-RTX therapy was evaluated mainly by the changes in ARR and EDSS score during a median post-RTX follow-up period of 35.5 (22.0–48.8) months. Overall, a dramatic decrease of median ARR was achieved at the end of this study compared with that prior to RTX therapy (0 [0–0.2] versus 1.1 [0.8–2.0], Z -7.196, p < 0.001). It was of note that the overwhelming majority of the subjects (97.1%, 68/70) had a reduction in their ARRs (Figure 3A) and 73 (67.6%) were relapse-free. Similar to ARR, LD-RTX therapy also led to a significant decrease of EDSS score, with the median score from 3.5 (2.5–4.0) before RTX therapy to 2.0 (1.0–3.0) after therapy (Z -8.320, p < 0.001). Specifically, the EDSS score decreased in 93 (86.1%) cases and remained unchanged in 12 (11.1%) cases (Figure 3B).
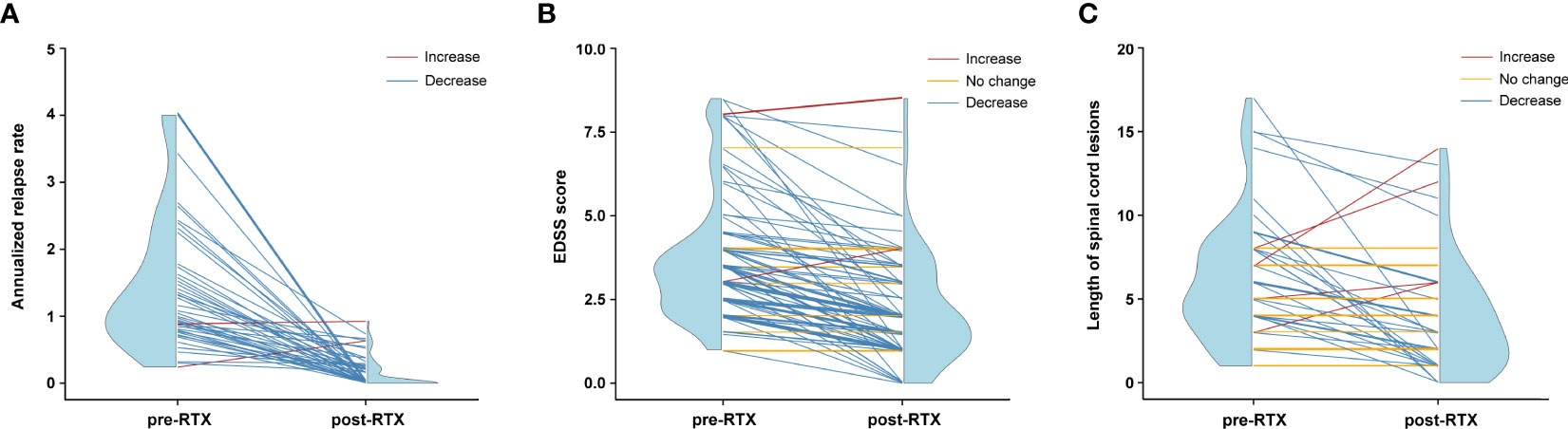
Figure 3 Efficacy assessments of LD-RTX therapy in each NMOSD patient. (A) Changes in annualized relapse rate (ARR) after therapy compared to before therapy (n = 70). Thirty-eight patients were not included due to a disease duration of less than 6 months prior to the first RTX infusion. Pre-RTX indicates the period from disease onset to the initiation of LD-RTX therapy, and post-RTX indicates the period from the initiation of LD-RTX therapy to the last follow-up. (B) Changes in EDSS score after therapy compared to before therapy (n = 108). (C) Changes in length of spinal cord lesions after therapy compared to before therapy (n = 53). The red line indicates an increased tendency, the yellow indicates no change and the blue indicates an decreased tendency. Blue half violin shows the distribution of efficacy measures at each time point. For (B, C), pre-RTX indicates the time point of disease onset and post-RTX indicates the time point of the last follow-up.
The median time to first relapse was 11.0 (4.8–24.0) months for the patients who experienced at least one relapse during the pre-RTX period, while the value was 12.0 (7.8–19.2) months for those during the post-RTX period (inter-group comparison, p = 0.501). Moreover, the median time to the first relapse was 14.0 (10.0–27.0) months for patients who experienced at least one relapse on early therapy initiated within 24 months after disease onset and 13.0 (4.0–18.0) months for those on delayed therapy (inter-group comparison, p = 0.316). We used the Kaplan-Meier survival curves to analyze the cumulative risk of developing relapses in all patients. The cumulative risk during the post-RTX period was significantly lower than that during the pre-RTX period (hazard ratio [HR] 0.238, 95%CI 0.160–0.356, p < 0.001; Figure 4A). As illustrated in Figure 4B, early RTX therapy was associated with a decreased cumulative risk compared to delayed therapy (HR 0.506, 95%CI 0.258–0.994, p = 0.041).
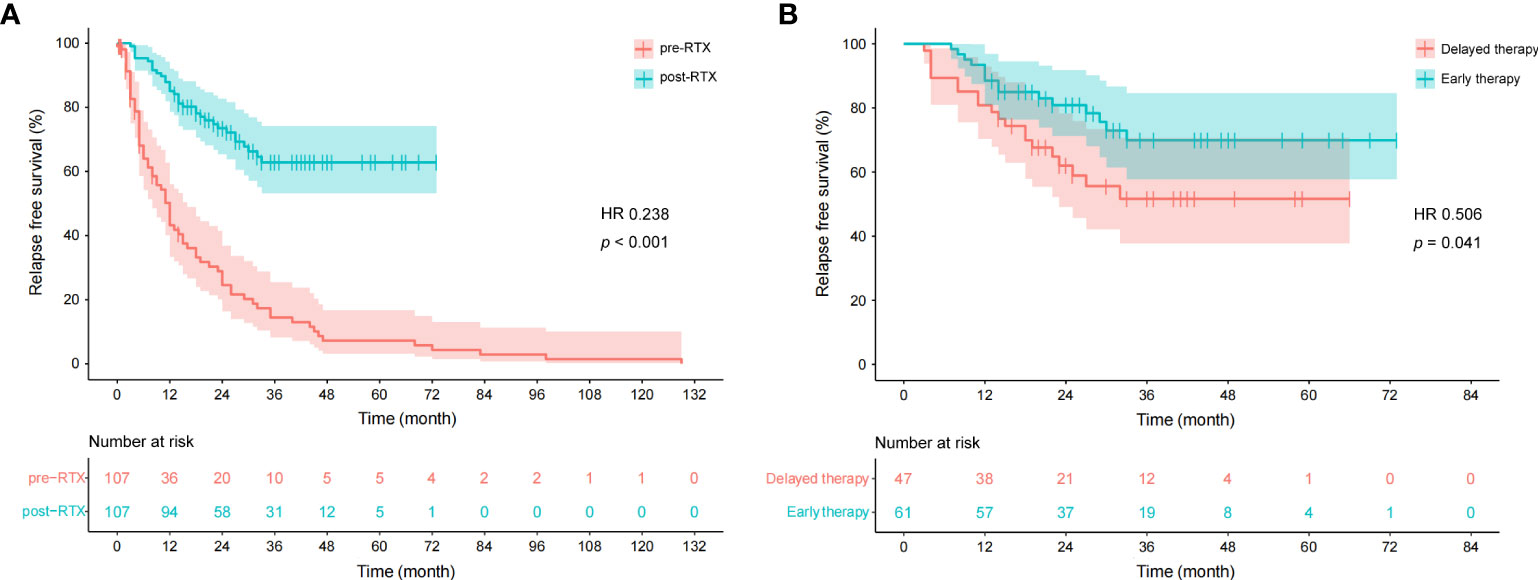
Figure 4 Cumulative survivals without relapse over time (months) as depicted by the Kaplan-Meier curve. (A) Comparison between pre- and post-RTX periods. One patient was not included due to the time from disease onset to the first relapse not available. “0” on the x-axis represents the disease onset in the pre-RTX group and the initiation of LD-RTX therapy in the post-RTX group. (B) Comparison between early therapy and delayed RTX therapy. “0” on the x-axis represents the initiation of LD-RTX therapy. Patients who experienced the first relapse from the pre-specified time point were censored, as presented by narrow vertical lines.
As an additional index of therapeutic efficacy, the changes in spinal cord lesions were compared in 53 patients who had available pre- and post-RTX MRI results. The median length of the lesion segments was 5.0 (4.0–8.0) before LD-RTX therapy and dropped to 3.0 (1.0–6.0) after therapy (Z -3.984, p < 0.001). As shown in Figure 3C, the decrease in the length of spinal cord lesions occurred in 31 (58.5%) cases and lesions disappeared completely in 6 (11.3%) cases. Although no changes in spinal cord segments were observed after RTX therapy in the remaining 16 (30.2%) cases, an obvious reduction of lesion volume was confirmed in these patients (data not shown).
Flow cytometry was performed to monitor the dynamics of circulating CD19+ B cell and CD19+CD27+ memory B cell subsets throughout LD-RTX therapy period. LD-RTX induction therapy led to B-cell depletion in all patients and this effect still remained at 3 months after induction. At 6 months after induction and thereafter, the median percentages of these two cell subsets in total PBMCs always maintained above the cut-off value of B-cell depletion (Figure 5), and similar percentages were detected when post-RTX clinical relapses occurred.
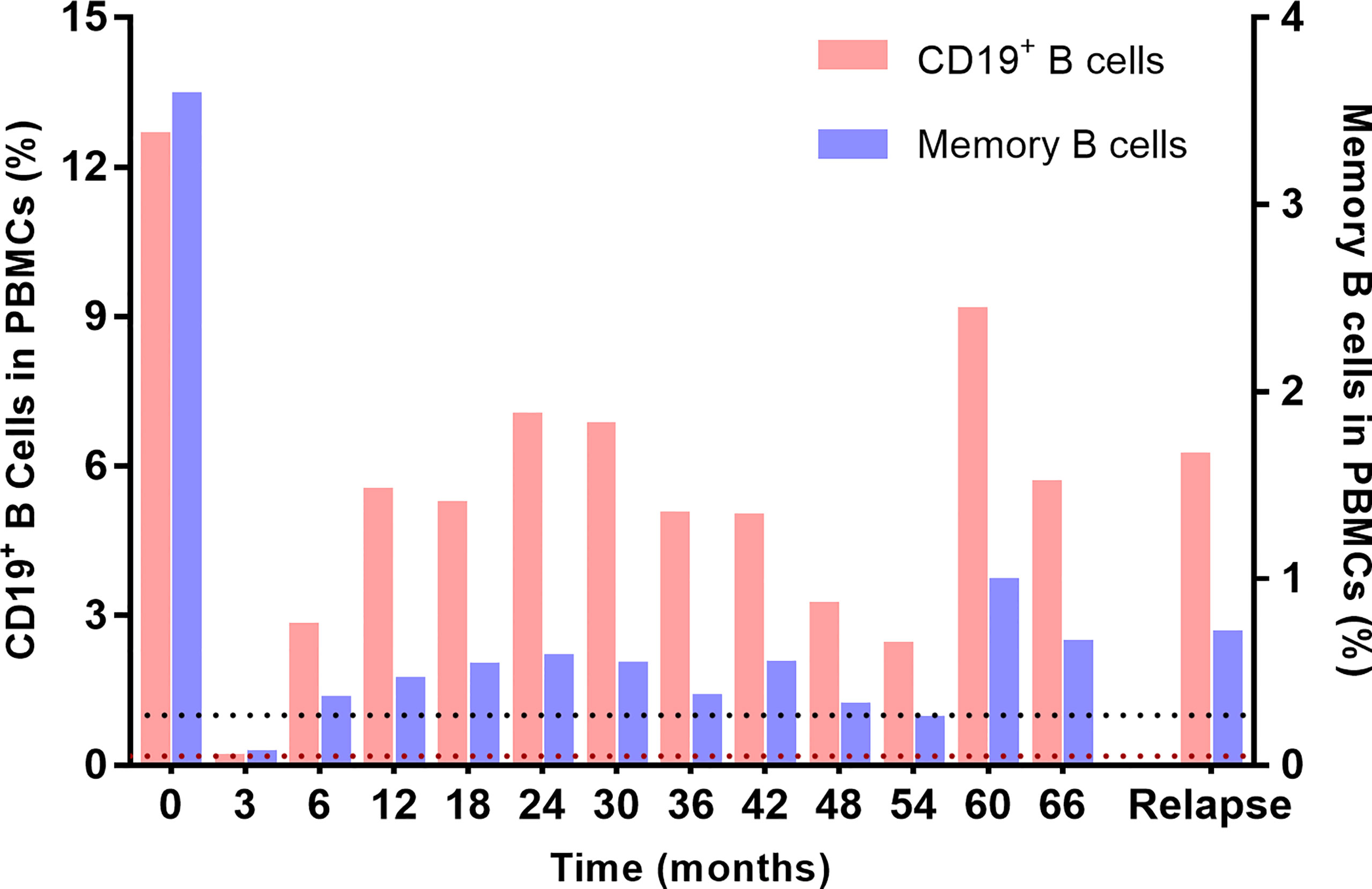
Figure 5 Dynamic monitoring of circulating CD19+ B cells and CD19+CD27+ memory B cells in PBMCs. The black dotted line represents the cut-off percentage of CD19+ B cells of 1%, and the brown dotted line represents the cut-off percentage of memory cells of 0.05%.
During the study period, 22 (20.4%) of 108 patients experienced RTX-related side effects, including infusion-related reactions and other adverse events. As shown in Table 3, skin rash was the most common infusion-related reaction occurring in 7 (31.8%) of the 22 patients, followed by flu-like symptoms (27.3%, 6/22), skin pruritus (18.2%, 4/22), sweating (13.6%, 3/22), palpitation (4.5%, 1/22) and laryngeal edema (4.5%, 1/22). All the side effects were mild and transient and could be rapidly relieved by lowering the infusion rate or by anti-allergic therapy. Other adverse events included alopecia (36.4%, 8/22), fatigue (9.1%, 2/22), muscle aches (9.1%, 2/22), urinary infection (4.5%, 1/22) and hemoglobin drop (4.5%, 1/22). No serious side effects such as tumors, progressive multifocal leukoencephalopathy, and serious infections were observed during the study period.
Rituximab (RTX) has been well recommended as first-line therapy to prevent relapses of NMOSD. However, there is no consensus on RTX dosage regimen due to the fact of off-label use and the lack of large-scale prospective randomized controlled trials. This study was led by Tangdu Hospital of Air Force Medical University, where a dose of 100 mg RTX once weekly for 3 weeks as induction therapy and reinfusions of 100 mg RTX every 6 months as maintenance therapy are the typical treatment approach for NMOSD patients. Based on the pilot satisfactory outcome of this strategy in our single center, we conducted this multicenter, open-label, self-controlled, prospective follow-up study in the northwest of China to clarify the effectiveness and safety of this LD-RTX strategy in treating NMOSD. The key findings include the following: (1) our LD-RTX strategy is demonstrated to be effective based on the significant decrease of ARR, EDSS score and length of spinal cord lesions after RTX therapy; (2) the satisfactory safety profile of our regimen is also confirmed based on a low risk of developing RTX-related side effects and a low switch rate to other immunosuppressants; and (3) the dramatic decrease of cumulative risk of relapses during post- versus pre-RTX therapy periods and on early versus delayed therapy reinforces the necessity and importance of initiating LD-RTX therapy as early as possible.
The efficacy of RTX in treating NMOSD has been widely demonstrated since the first report by Cree et al. in 2005 (12). However, the majority of prior studies conducted in Caucasian population have adopted the high dose of RTX induction regimen similar to that used in B cell lymphoma, i.e., 375 mg/m2 once weekly for 4 consecutive weeks or 1000 mg twice 2 weeks apart. The maintenance reinfusions (500-1000 mg/cycle) were administered every 6 to 9 months according to clinical functional status and patient’s choice (26) or depending on circulating B cell repopulation. Besides the economic costs of this high-dose regimen, the prescription should be given with caution due to the potentially serious adverse effects including death (19, 21). In such a perspective, low-dose strategies would be of great promise in the context of ensuring efficacy and safety. During the last decade, several groups from China have attempted various modified dosages of RTX strategies for NMOSD and achieved a good efficacy and safety profile, exhibiting a better efficacy than other immunosuppressive agents such as AZA or MMF (13, 27, 28). In these retrospective studies of small sample sizes, the RTX induction regimen included 100 mg once weekly for 3-4 consecutive weeks, and then the percentages of circulating CD19+ B cells and/or CD19+CD27+ memory B cells were monitored every 4-12 weeks. The maintenance infusion was restarted whenever B cells were repopulated. In contrast, a lower dose of RTX strategy was applied in this study than those in any prior studies, including 300 mg of induction dose and then repeated maintenance reinfusions of 100 mg at a fixed 6-month interval. Encouragingly, our LD-RTX strategy maintained good efficacy over a median of 35.5 months, as manifested by the marked reduction of median ARR by 100%, median EDSS score by 43%, and median length of spinal cord lesions by 40%. More notably, 67.6% of patients were relapse-free, 86.1% had improved EDSS scores and 58.5% had reduced or disappeared spinal cord lesion segments, which is similar to those of other studies assessing the effectiveness of low- or standard-dose RTX strategies in treating NMOSD (29–31). In our opinion, the lower dose of RTX strategy applied in this study would be a promising option for treating NMOSD. Additionally, our data provide insights into optimal RTX dosing exploration in the future.
To date, the timing of initiating RTX therapy in NMOSD patients remains undetermined. In a recent practical guideline of the NOMADMUS group from France, an expert consensus was obtained for recommending no window between the end of rescue therapy for acute attacks (i.e., high doses of steroids and/or plasma exchanges) and RTX therapy initiation (32). The view is well followed in this study where 80 (74.1%) patients started LD-RTX therapy immediately after acute attack treatment, which simulates the real-world setting in clinical practice. Another retrospective study provides a novel clue for early RTX use based on the finding that RTX therapy initiated within 24 months of NMO onset resulted in a more dramatic reduction of ARR, although the same dosages of RTX initiated 24 months later also achieved a significant ARR drop compared with before treatment (20). In this study, 61 patients received early LD-RTX therapy and more than half (54.1%, 33/61) of the cases were first-onset. The concept of starting RTX therapy earlier is further reinforced since the cumulative risk of relapses in the patients receiving early LD-RTX therapy significantly decreased compared to those receiving delayed therapy. Although the median time to the first relapse was not markedly prolonged during post- versus pre-RTX therapy periods and on early versus delayed therapy, a dramatic decrease of cumulative risk of relapses gives sufficient support to the use of RTX as early as possible and as sequential therapy following acute attack treatment.
Regarding the timing of maintenance RTX reinfusion, there is an absence of a standardized protocol. The majority of previous studies monitored the count and/or percentage of circulating CD19+ B cells, especially CD19+CD27+ memory cells at varying time intervals. A widely accepted cut-off of B-cell depletion are the percentage of CD19+ B cells lower than 1% (22), or that of memory B cells lower than 0.05% in the initial two years and 0.1% thereafter (18). RTX reinfusions were administered whenever B cells were repopulated, even though the dosage of RTX varied from one to another (13, 18, 20, 33, 34). Greenberg et al. (22) compared the time to B-cell repopulation (defined as a CD19 percentage of 2% or greater) after different RTX doses and found that 1000 mg per dose yielded more prolonged B-cell depletion than 100 mg per dose (184 ± 72 days versus 99 ± 36 days), which may provide support to more frequent reinfusion when low RTX dosing strategies were applied (13, 27, 28, 35, 36). Although the association of clinical relapses with B-cell repopulation has been well determined to date, it is of note that we have observed that many patients with B-cell repopulation maintained a long-term relapse-free condition in real-world clinical practice. Similar findings come from a recent study in which 2 NMOSD patients receiving low-dose RTX therapy maintained a relapse-free condition at the majority of time points when B-cell repopulated but not receiving reinfusions, raising the question of whether the low threshold of B cells is an obligatory monitoring indicator of repeated RTX reinfusions (37). In this study, a fixed 6-month interval of RTX reinfusion was determined based on our clinical experience and it was longer than those of other low-dose RTX strategies (13, 27, 28, 35, 36). Encouragingly, our strategy with a longer time interval of reinfusion is sufficient to maintain a clinical remission status, as demonstrated by 67.6% relapse-free patients at the end of the study. Thus, our results reopen the issue of the optimal cut-off level of B cells for repeated RTX reinfusions which required future investigation.
The safety and tolerability of our LD-RTX strategy are well established. The frequency of side effects in our study was lower than the conventional dose strategies (18, 34, 38) but similar to the low dose strategies (28, 36, 39). Consistent with these prior studies, the most common were infusion-related reactions. It is of note that no serious adverse events were recorded and no patients discontinued RTX treatment because of side effects related to RTX use. Meanwhile, no increment of the incidence rate of side effects were determined with the extension of therapy duration. Considering that long-term high-dose RTX may be accompanied by the increased risk of adverse effects of immunosuppression especially fatal outcomes (11, 19, 21), our strategy with a lower cumulative dose of RTX might become a better treatment option guaranteeing both efficacy and safety.
Our research has several limitations. Firstly, it was not designed in a randomized, double-blind manner. Thus, the control group was absent and it would inevitably limit the level of evidence from our data. Secondly, a high proportion (35.2%, 38/108) of patients have a disease duration of less than 6 months prior to the first RTX infusion. As a result, these patients were not included when comparing the ARR before and after RTX therapy. It was sufficient to avoid the potential overestimation, but future investigation with valid data for all enrolled subjects is warranted to achieve reliable conclusions. Thirdly, we notice that 74.1% of patients had no therapeutic window between the end of rescue therapy for acute attack and LD-RTX therapy initiation. Although this reflects real-world clinical situations, RTX therapy following glucocorticoids might have affected the post-RTX evaluations of EDSS score and length of spinal cord lesions. Thus, these two are only taken as the secondary outcome measures in the present study. Future prospective, well-designed investigations are required to validate the conclusions derived from our study.
In conclusion, a novel lower-dose RTX therapy strategy is addressed and new attempts in terms of RTX initiation and reinfusion timing are made in this study. Our LD-RTX therapy significantly reduces clinical relapses and disability and would be of great promise in the context of ensuring both efficacy and safety.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Tangdu Hospital, Air Force Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
HL and JG conceived and designed the protocol. ZQL, ZBL, JW, ZX, TL, CM, SZ and MB collected the data. DZ, KR and JL wrote the manuscript; DZ and KR analyzed the data. HL and JG critically revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 82171339), Key Research and Development Project of Shaanxi Province (Nos. 2022ZDLSF02-04; 2022SF-167), Excellent Personnel Foundation of Tangdu Hospital (to JG), Social Talent Fund of Tangdu Hospital (No. 2021SHRC003), and National Natural Science Foundation Promoting Project of Tangdu Hospital (No. 2021ZTXM010).
The authors highly appreciate the excellent work of Ms. Yao Wang in statistical analysis. We would like to thank Mr. Yu Han for his assistance in interpreting spinal cord MRI results. The authors gratefully acknowledge Kindstar Global and Shaanxi MYBiotech Co., Ltd. for professional assistance in detecting AQP4-IgG.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1148632/full#supplementary-material
1. Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers (2020) 6(1):85. doi: 10.1038/s41572-020-0214-9
2. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet (2004) 364(9451):2106–12. doi: 10.1016/S0140-6736(04)17551-X
3. Bizzoco E, Lolli F, Repice AM, Hakiki B, Falcini M, Barilaro A, et al. Prevalence of neuromyelitis optica spectrum disorder and phenotype distribution. J Neurol (2009) 256(11):1891–8. doi: 10.1007/s00415-009-5171-x
4. Sherman E, Han MH. Acute and chronic management of neuromyelitis optica spectrum disorder. Curr Treat Options Neurol (2015) 17(11):48. doi: 10.1007/s11940-015-0378-x
5. Yamamura T, Kleiter I, Fujihara K, Palace J, Greenberg B, Zakrzewska-Pniewska B, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med (2019) 381(22):2114–24. doi: 10.1056/NEJMoa1901747
6. Traboulsee A, Greenberg BM, Bennett JL, Szczechowski L, Fox E, Shkrobot S, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol (2020) 19(5):402–12. doi: 10.1016/S1474-4422(20)30078-8
7. Cree BAC, Bennett JL, Kim HJ, Weinshenker BG, Pittock SJ, Wingerchuk DM, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet (2019) 394(10206):1352–63. doi: 10.1016/S0140-6736(19)31817-3
8. Pittock SJ, Berthele A, Fujihara K, Kim HJ, Levy M, Palace J, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med (2019) 381(7):614–25. doi: 10.1056/NEJMoa1900866
9. Espiritu AI, Pasco PMD. Efficacy and tolerability of azathioprine for neuromyelitis optica spectrum disorder: A systematic review and meta-analysis. Mult Scler Relat Disord (2019) 33:22–32. doi: 10.1016/j.msard.2019.05.011
10. Songwisit S, Kosiyakul P, Jitprapaikulsan J, Prayoonwiwat N, Ungprasert P, Siritho S. Efficacy and safety of mycophenolate mofetil therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. Sci Rep (2020) 10(1):16727. doi: 10.1038/s41598-020-73882-8
11. Jacob A, Weinshenker BG, Violich I, McLinskey N, Krupp L, Fox RJ, et al. Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Arch Neurol (2008) 65(11):1443–8. doi: 10.1001/archneur.65.11.noc80069
12. Cree BA, Lamb S, Morgan K, Chen A, Waubant E, Genain C. An open label study of the effects of rituximab in neuromyelitis optica. Neurology (2005) 64(7):1270–2. doi: 10.1212/01.WNL.0000159399.81861.D5
13. Yang CS, Yang L, Li T, Zhang DQ, Jin WN, Li MS, et al. Responsiveness to reduced dosage of rituximab in Chinese patients with neuromyelitis optica. Neurology (2013) 81(8):710–3. doi: 10.1212/WNL.0b013e3182a1aac7
14. Mealy MA, Wingerchuk DM, Palace J, Greenberg BM, Levy M. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol (2014) 71(3):324–30. doi: 10.1001/jamaneurol.2013.5699
15. Torres J, Pruitt A, Balcer L, Galetta S, Markowitz C, Dahodwala N. Analysis of the treatment of neuromyelitis optica. J Neurol Sci (2015) 351(1-2):31–5. doi: 10.1016/j.jns.2015.02.012
16. Jeong IH, Park B, Kim SH, Hyun JW, Joo J, Kim HJ. Comparative analysis of treatment outcomes in patients with neuromyelitis optica spectrum disorder using multifaceted endpoints. Mult Scler (2016) 22(3):329–39. doi: 10.1177/1352458515587752
17. Grillo-López AJ. Rituximab: an insider’s historical perspective. Semin Oncol (2000) 27(6 Suppl 12):9–16.
18. Kim SH, Kim W, Li XF, Jung IJ, Kim HJ. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol (2011) 68(11):1412–20. doi: 10.1001/archneurol.2011.154
19. Damato V, Evoli A, Iorio R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: A systematic review and meta-analysis. JAMA Neurol (2016) 73(11):1342–8. doi: 10.1001/jamaneurol.2016.1637
20. Zéphir H, Bernard-Valnet R, Lebrun C, Outteryck O, Audoin B, Bourre B, et al. Rituximab as first-line therapy in neuromyelitis optica: efficiency and tolerability. J Neurol (2015) 262(10):2329–35. doi: 10.1007/s00415-015-7852-y
21. Etemadifar M, Salari M, Mirmosayyeb O, Serati M, Nikkhah R, Askari M, et al. Efficacy and safety of rituximab in neuromyelitis optica: Review of evidence. J Res Med Sci (2017) 22:18. doi: 10.4103/1735-1995.200275
22. Greenberg BM, Graves D, Remington G, Hardeman P, Mann M, Karandikar N, et al. Rituximab dosing and monitoring strategies in neuromyelitis optica patients: creating strategies for therapeutic success. Mult Scler (2012) 18(7):1022–6. doi: 10.1177/1352458511432896
23. Zaja F, Vianelli N, Volpetti S, Battista ML, Defina M, Palmieri S, et al. Low-dose rituximab in adult patients with primary immune thrombocytopenia. Eur J Haematol (2010) 85(4):329–34. doi: 10.1111/j.1600-0609.2010.01486.x
24. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology (2006) 66(10):1485–9. doi: 10.1212/01.wnl.0000216139.44259.74
25. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology (2015) 85(2):177–89. doi: 10.1212/WNL.0000000000001729
26. Ip VH, Lau AY, Au LW, Fan FS, Chan AY, Mok VC, et al. Rituximab reduces attacks in Chinese patients with neuromyelitis optica spectrum disorders. J Neurol Sci (2013) 324(1-2):38–9. doi: 10.1016/j.jns.2012.09.024
27. Yang Y, Wang CJ, Wang BJ, Zeng ZL, Guo SG. Comparison of efficacy and tolerability of azathioprine, mycophenolate mofetil, and lower dosages of rituximab among patients with neuromyelitis optica spectrum disorder. J Neurol Sci (2018) 385:192–7. doi: 10.1016/j.jns.2017.12.034
28. Zhang M, Zhang C, Bai P, Xue H, Wang G. Effectiveness of low dose of rituximab compared with azathioprine in Chinese patients with neuromyelitis optica: an over 2-year follow-up study. Acta Neurol Belg (2017) 117(3):695–702. doi: 10.1007/s13760-017-0795-6
29. Jade JD, Bansi S, Singhal B. Rituximab in neuromyelitis optica spectrum disorders: our experience. Ann Indian Acad Neurol (2017) 20(3):229–32. doi: 10.4103/aian.AIAN_499_16
30. Kim W, Kim SH, Kim HJ. New insights into neuromyelitis optica. J Clin Neurol (2011) 7(3):115–27. doi: 10.3988/jcn.2011.7.3.115
31. Kim SH, Jeong IH, Hyun JW, Joung A, Jo HJ, Hwang SH, et al. Treatment outcomes with rituximab in 100 patients with neuromyelitis optica: influence of FCGR3A polymorphisms on the therapeutic response to rituximab. JAMA Neurol (2015) 72(9):989–95. doi: 10.1001/jamaneurol.2015.1276
32. Ciron J, Audoin B, Bourre B, Brassat D, Durand-Dubief F, Laplaud D, et al. Recommendations for the use of Rituximab in neuromyelitis optica spectrum disorders. Rev Neurol (Paris) (2018) 174(4):255–64. doi: 10.1016/j.neurol.2017.11.005
33. Kim SH, Kim Y, Kim G, Park NY, Jang HM, Shin HJ, et al. Less frequent rituximab retreatment maintains remission of neuromyelitis optica spectrum disorder, following long-term rituximab treatment. J Neurol Neurosurg Psychiatry (2019) 90(4):486–7. doi: 10.1136/jnnp-2018-318465
34. Kim SH, Huh SY, Lee SJ, Joung A, Kim HJ. A 5-year follow-up of rituximab treatment in patients with neuromyelitis optica spectrum disorder. JAMA Neurol (2013) 70(9):1110–7. doi: 10.1001/jamaneurol.2013.3071
35. Li T, Zhang LJ, Zhang QX, Yang CS, Zhang C, Li YJ, et al. Anti-Rituximab antibody in patients with NMOSDs treated with low dose Rituximab. J Neuroimmunol (2018) 316:107–11. doi: 10.1016/j.jneuroim.2017.12.021
36. Zhao S, Zhou H, Xu Q, Dai H, Wei S. Efficacy of low-dose rituximab on neuromyelitis optica-associated optic neuritis. Front Neurol (2021) 12:637932. doi: 10.3389/fneur.2021.637932
37. Jing L, Yu H, Li J, Li Y, Liu X, Li H, et al. Is low threshold of B cells an obligatory monitoring indicator of repeated RTX infusion in the treatment of Neuromyelitis Optica spectrum disorder? - A report of two cases. Neuroimmunol Rep (2021) 1:100023. doi: 10.1016/j.nerep.2021.100023
38. Bedi GS, Brown AD, Delgado SR, Usmani N, Lam BL, Sheremata WA. Impact of rituximab on relapse rate and disability in neuromyelitis optica. Mult Scler (2011) 17(10):1225–30. doi: 10.1177/1352458511404586
Keywords: neuromyelitis optica spectrum disorder, rituximab, low-dose, efficacy, safety
Citation: Zhao D, Ren K, Lu J, Liu Z, Li Z, Wu J, Xu Z, Wu S, Lei T, Ma C, Zhao S, Bai M, Li H and Guo J (2023) Rituximab at lower dose for neuromyelitis optica spectrum disorder: a multicenter, open-label, self-controlled, prospective follow-up study. Front. Immunol. 14:1148632. doi: 10.3389/fimmu.2023.1148632
Received: 20 January 2023; Accepted: 14 July 2023;
Published: 08 August 2023.
Edited by:
Rui Li, University of Pennsylvania, United StatesReviewed by:
Abdorreza Naser Moghadasi, Tehran University of Medical Sciences, IranCopyright © 2023 Zhao, Ren, Lu, Liu, Li, Wu, Xu, Wu, Lei, Ma, Zhao, Bai, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Guo, Z3VvanVuXzgxQDE2My5jb20=; Hongzeng Li, bGxpaG9uZ3plbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.