- 1The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2Cancer Center, Department of Medical Oncology, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, Zhejiang, China
Immune checkpoint inhibitors (ICIs) are an integral antitumor therapy for many malignancies. Most patients show very good tolerability to ICIs; however, serious immune-related adverse events (irAEs) with ICIs have been well documented and prevent some patients from continuing ICIs or even become the direct cause of patient death. Cytopenia is a rare irAE but can be life-threatening. Here, we present the case of a 66-year-old male patient with metastatic lung adenocarcinoma who received two doses of chemotherapy + PD-1 antibody tislelizumab and developed pancytopenia after each dose. Although the first episode of pancytopenia resolved with a treatment regimen of granulocyte colony-stimulating factor (G-CSF), thrombopoietin (TPO), and red blood cell and platelet transfusion, the second episode showed extreme resistance to these treatments and improved only after the administration of steroids. His second pancytopenia episode resolved after a long course of treatment with methylprednisolone, G-CSF, TPO, hetrombopag and multiple red blood cell and platelet transfusions. However, he suffered a cerebral infarction when his platelet count was in the normal range and gradually recovered 1 week later. This case highlights the importance of the early recognition and management of hematological irAEs.
Introduction
Hematological immune-related adverse events (Hema-irAEs) with ICIs are very rare irAEs that manifest as thrombocytopenia, anemia, neutropenia, hypereosinophilia, bi-cytopenia, or even pancytopenia (1, 2). Recently, a French report (3) from the review of cases of ICI-related grade ≥2 cytopenia within the French pharmacovigilance database showed that 68 patients experienced 75 episodes of ICI-related cytopenia which consisted of thrombocytopenia (50.7%), autoimmune hemolytic anemia (25.3%), neutropenia (13.3%), pure red cell aplasia (8%), and aplastic anemia (2.7%). Nearly half of the cytopenia cases were grade ≥4, and 4.4% of patients died from cytopenia-related complications. The incidence for ICI-induced grade 4 neutropenia was reported to be 0.14% in a German melanoma center (4). The median onset and duration of ICI-related neutropenia was 10.5 weeks after the first ICI administration and 13 days, respectively (5). The treatment regimen for ICI-related neutropenia mainly included granulocyte colony-stimulating factor (G-CSF) and intravenous corticosteroids (4). However, patients with ICI-related thrombocytopenia were reported to be associated with worse overall survival compared those without thrombocytopenia or with thrombocytopenia unrelated to ICIs (6).
Bi-cytopenia was reported in 10.3% of ICI-related cytopenia (3). Nevertheless, cases of ICI-induced pancytopenia are extremely rare in the literature, and only scattered case reports are available (7–13).
Here, we report a case of pancytopenia induced by the PD-1 antibody tislelizumab, which was refractory to G-CSF, thrombopoietin, and thrombopoietin receptor agonists but responded well to steroids and was followed by a cerebral infarction after the resolution of cytopenia.
Case presentation
A 66-year-old male was diagnosed in 2022 with stage IVB (cT2aN3M1c) non-squamous non-small-cell lung cancer (NSCLC). He presented with a supraclavicular mass and pain in the right upper limb on his first visit. Computed tomography (CT) of the chest showed multiple masses in the superior lobe of the right lung, supraclavicular region, and right hilar and mediastinal lymph nodes (Figure 1A). Subsequent sodium fluoride (18F-NaF) positron emission tomography/computed tomography (PET/CT) confirmed the lesions found on CT and suggested additional metastases involving right axillary lymph nodes, bilateral adrenal glands and multiple bones (cervical vertebras, lumbar vertebras, left iliac crest, left side 4th rib) (Figure 1B). Core biopsy of the right supraclavicular mass confirmed the diagnosis of poorly differentiated adenocarcinoma. A panel of 10 genes (EGFR, KRAS, NRAS, BRAF, PIK3CA, ALK, ROS1, RET, MET, HER2) tested for driver mutation(s) by amplification refractory mutation system-polymerase chain reaction (ARMS-PCR) was negative for actionable molecular biomarkers. Tested with Dako 22C3 on Dako Autostainer Link 48, the expression level of PD-L1 on tumor cells was 5%.
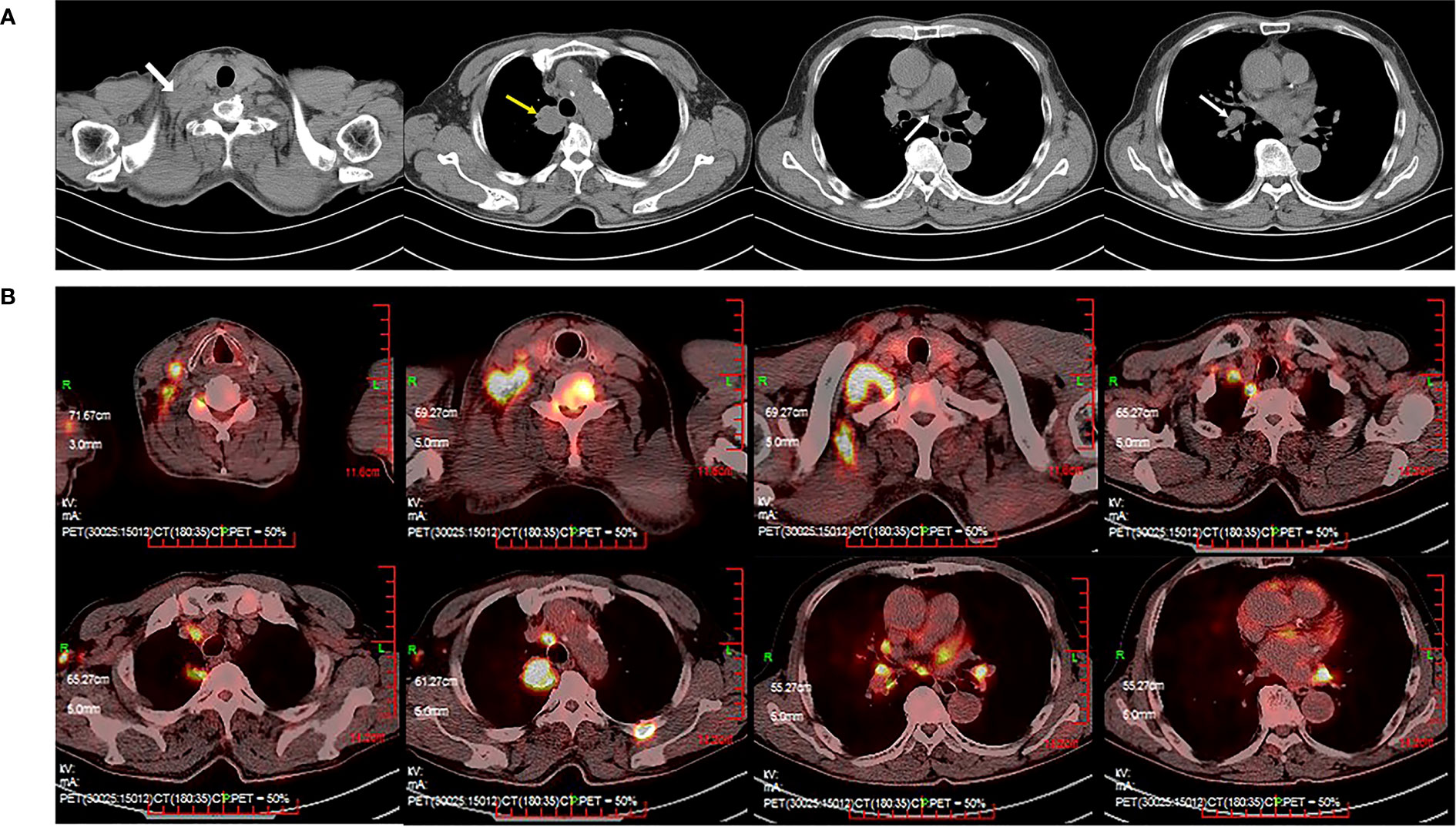
Figure 1 Computed tomography (CT) of the thorax with contrast and positron emission tomography/computed tomography (PET/CT) at the time of diagnosis. (A) Thoracic CT revealed multiple masses (the superior lobe of the right lung, supraclavicular region, right hilar and mediastinal lymph nodes). (B) PET/CT revealed suspicious primary lung cancer and metastasis.
The patient was given one cycle of carboplatin (AUC=4, Day 1) plus pemetrexed (500 mg/m2, Day 1) before the gene mutation test. Unfortunately, grade IV rash developed 9 days after chemotherapy. Twenty-one days after the first cycle of chemotherapy, carboplatin was then replaced by cisplatin for the second cycle of therapy, which consisted of cisplatin (75 mg/m2, Day 1) and pemetrexed (500 mg/m2, Day 1) to avoid possible carboplatin-induced rash. The patient went through the second chemotherapy course fairly well without any serious adverse events. The patient responded to chemotherapy well (partial remission) and reached stable disease during the following two cycles of chemotherapy plus immunotherapy (Supplemental Figure 1). Chest CT with intravenous contrast before the third cycle showed the shrinkage of the lung nodules (Supplemental Figure 1A). The PD-1 antibody tislelizumab (200 mg, Day 1) was therefore administered to the patient together with pemetrexed and cisplatin (TPC) for the third cycle of therapy. Ten days after the completion of the third cycle regimen, the patient presented with febrile neutropenia with an absolute neutrophil count (ANC) of 120/μL, anemia with a hemoglobin (Hb) level of 75 g/L and thrombocytopenia with a platelet count of 26,000/μL. Two days later, his myelosuppression kept worsening with Hb of 59 g/L and platelet count of 12,000/μL. According to CTCAE5.0, his anemia was graded as III and both neutropenia and thrombocytopenia were grade IV. He was admitted and diagnosed with chemotherapy-induced pancytopenia that resolved after the administration of 9 days of G-CSF and thrombopoietin (TPO) injection, intravenous (IV) antibiotics and platelet and packed red blood cell transfusion. Chest CT with intravenous contrast before the fourth cycle (4thC) showed slight further shrinkage of the lung nodules (stable disease) (Supplemental Figure 1B). In view of the efficacy and myelosuppression that was supposed to be related to chemotherapeutic agents from TPC regimen, chemotherapy dose reduction was carried out for the 4thC therapy that consisted of cisplatin (33 mg/m2, Day 1), pemetrexed (280 mg/m2, Day 1) and tislelizumab (200 mg, Day 1) administered 30 days after the third cycle. Nine days after the 4thC, the patient developed febrile neutropenia (ANC 500/μL) and anemia (Hb 74 g/L). The platelet count was 102,000/μL. Despite timely administration of G-CSF and IV antibiotics for 2 days, his ANC, Hb and platelet count continued to decrease to 410/μL, 66 g/L and 49,000/μL (11 days after the 4thC), respectively. He continued G-CSF and IV antibiotics, started TPO and hetrombopag, and received two transfusions of packed red blood cells (4.5 U) and platelets (34 U) during the following 9 days. Eighteen days after the 4thC, his ANC, Hb and platelet count were 220/μL, 74 g/L and 7,000/μL, respectively, which prompted us to suspect Hema-irAEs. After a multidisciplinary treatment (MDT) panel discussion, he continued G-CSF, TPO, hetrombopag, and IV antibiotics and started IV steroids with methylprednisolone (1.2 mg/kg) at 21 days after the 4thC. Two and 4 more transfusions of packed red blood cells (3 U) and platelets (51 U), respectively, were administered thereafter. His ANC and platelet count reached nadirs of 150/μL (20 days after the 4thC) and 3,000/μL (29 days after the 4thC), respectively. According to CTCAE5.0, his anemia was graded as III, but both neutropenia and thrombocytopenia were grade IV and life-threatening. His ANC and platelet count increased to 660/μL (3 days after the initiation of methylprednisolone) and 31,000/μL (9 days after the initiation of methylprednisolone), respectively. A peripheral blood smear was then performed 5 days after the initiation of methylprednisolone for abnormalities and showed a decrease of nucleated cells that consisted of predominantly neutrophils (81%), however, negative for malignant cells and parasites (Supplemental Figures 2A, B). Methylprednisolone was then tapered slowly from the seventh day of initiation over 6 weeks. His neutropenia and thrombocytopenia resolved on Days 8 (3,870/μL) and 20 (184,000/μL) of methylprednisolone treatment, respectively. His anemia also improved gradually, and he was discharged 40 days after the 4thC (Day 20 of methylprednisolone initiation) but returned to the emergency room with symptomatic epilepsy at night on the same day of discharge. Emergency CT of the brain revealed a large area of low density in the left occipital and temporal lobes suggestive of a cerebral infarction (Figure 2). Magnetic resonance angiography suggested no significant cerebrovascular stenosis or dilation. Chest CT suggested stable disease (Supplemental Figure 1C). He was hospitalized for 8 days and gradually recovered after discharge in terms of the cerebral infarction, leaving his lung cancer unaddressed.
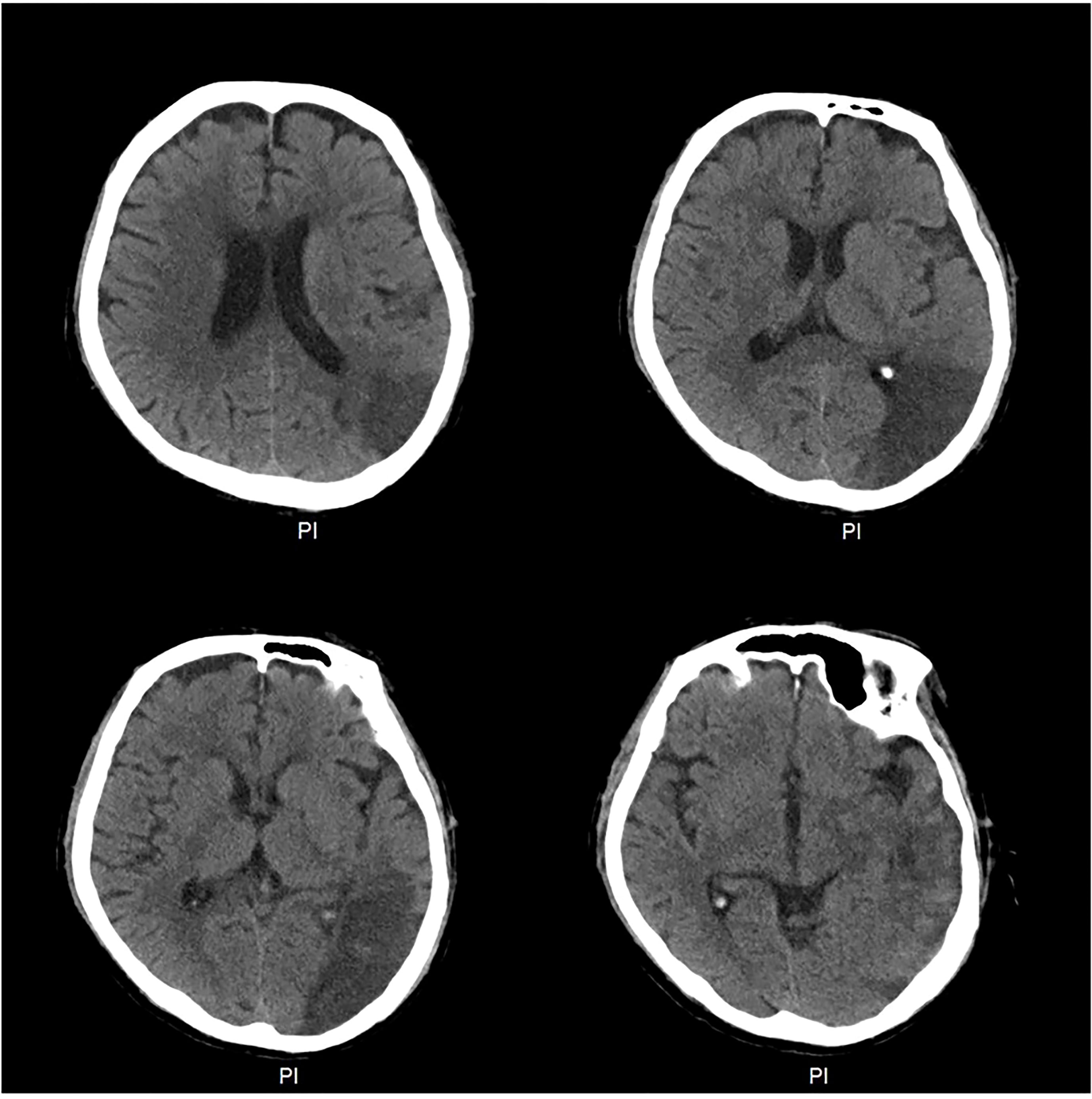
Figure 2 Computed tomography (CT) of the brain at the time of diagnosis of the cerebral infarction. CT of the brain revealed a large area of low density in the left occipital and temporal lobes.
Eighty-five days after the 4thC, bone marrow aspiration was pursued to investigate the possible invasion of bone marrow by cancer cells or parasites, which revealed a nonmalignant and parasite-free aspirate marrow specimen (Supplemental Figure 2).
Considering the progression of the lesions (Supplemental Figure 3A), he finally agreed to have a core rebiopsy of the right supraclavicular mass 4 months after the 4thC, and next-generation sequencing (NGS) was performed to screen for gene mutations, which revealed a mesenchymal epithelial transition (MET) exon 14 skipping mutation (METΔex14). He started savolitinib 400 mg once daily, and significant shrinkage of the right supraclavicular mass was observed 2 days after the initiation of savolitinib. Two weeks after the initiation of savolitinib, the right supraclavicular lesion became flat and invisible from the outside (Figure 3). Chest CT showed remarkable remission of all the lesions, and this remission continued, as revealed by chest CT (Supplemental Figure 3B) with intravenous contrast 66 days after the initiation of savolitinib. At the time of the preparation of this manuscript, he was on savolitinib and had returned to normal life activities.
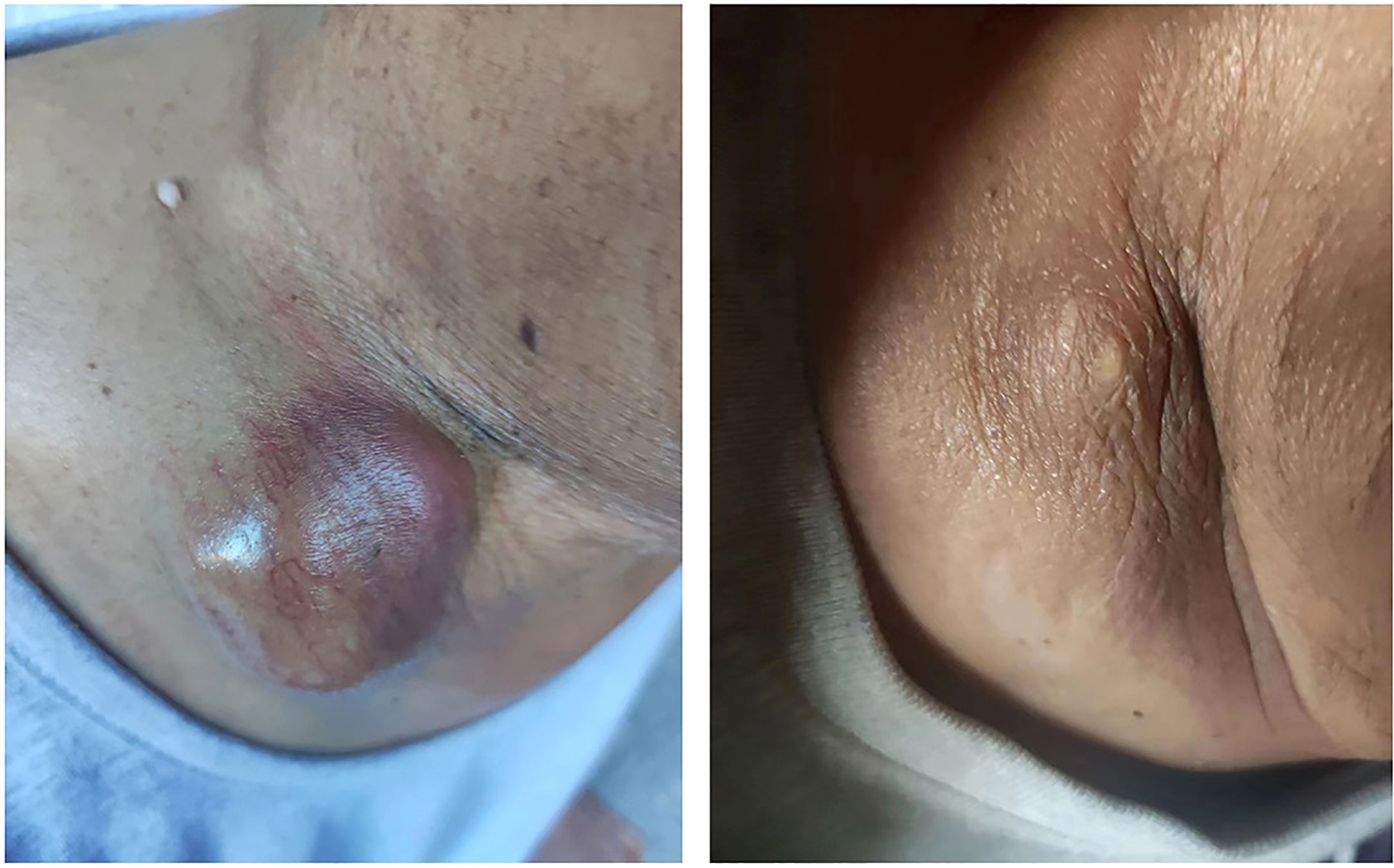
Figure 3 Supraclavicular mass before and after savolitinib treatment. A bulging mass was shown on the right supraclavicular area before treatment with savolitinib (left). The supraclavicular mass shrank dramatically after two weeks of treatment with savolitinib (400 mg/day) (right).
Patient perspective
Our patient reported the following about his experience: I did not expect to recover from the serious episodes of pancytopenia. I felt it important to strictly follow the physician’s suggestions. After taking savolitinib, I soon recovered from the episodes. I appreciated what my physicians had done during the course of my diagnosis and treatment.
Discussion
Myelosuppression is a common and rare AE of chemotherapy and ICIs, respectively (14). It was reported that the incidence of ICI-induced cytopenia is less than 0.5% in patients administered ICIs (15). Pancytopenia has rarely been reported in patients treated with ICIs (7–13). However, it is very difficult to distinguish chemotherapy-induced myelosuppression from Hema-irAEs when ICIs are administered concurrently with chemotherapy.
Taking a comprehensive review of the case, we thought the following supports the diagnosis of Hema-irAEs with tislelizumab but not chemotherapy or other causes induced myelosuppression. (i) The occurrence of his pancytopenia was closely associated with the administration of chemotherapy + tislelizumab, but not with chemotherapy alone (Figure 4). (ii) The first two cycles consisting of standard dose platinum-doublet did not induce pancytopenia, while the third cycle that contains standard dose platinum-doublet and tislelizumab triggered serious pancytopenia, and the fourth cycle, despite of sharp dose reduction of platinum-doublet (around 50% of previous dose) + full dose tislelizumab, induced even worse and life-threatening pancytopenia. (iii) The pancytopenia was refractory to G-CSF and thrombopoietin/hetrombopag but responded to 1.2 mg/kg methylprednisolone very well. (iv) Blood smear and bone marrow aspiration did not suggest malignant or parasitic blood or bone marrow environment. (v) The patient did not have any history of myelosuppression or immune disorders.
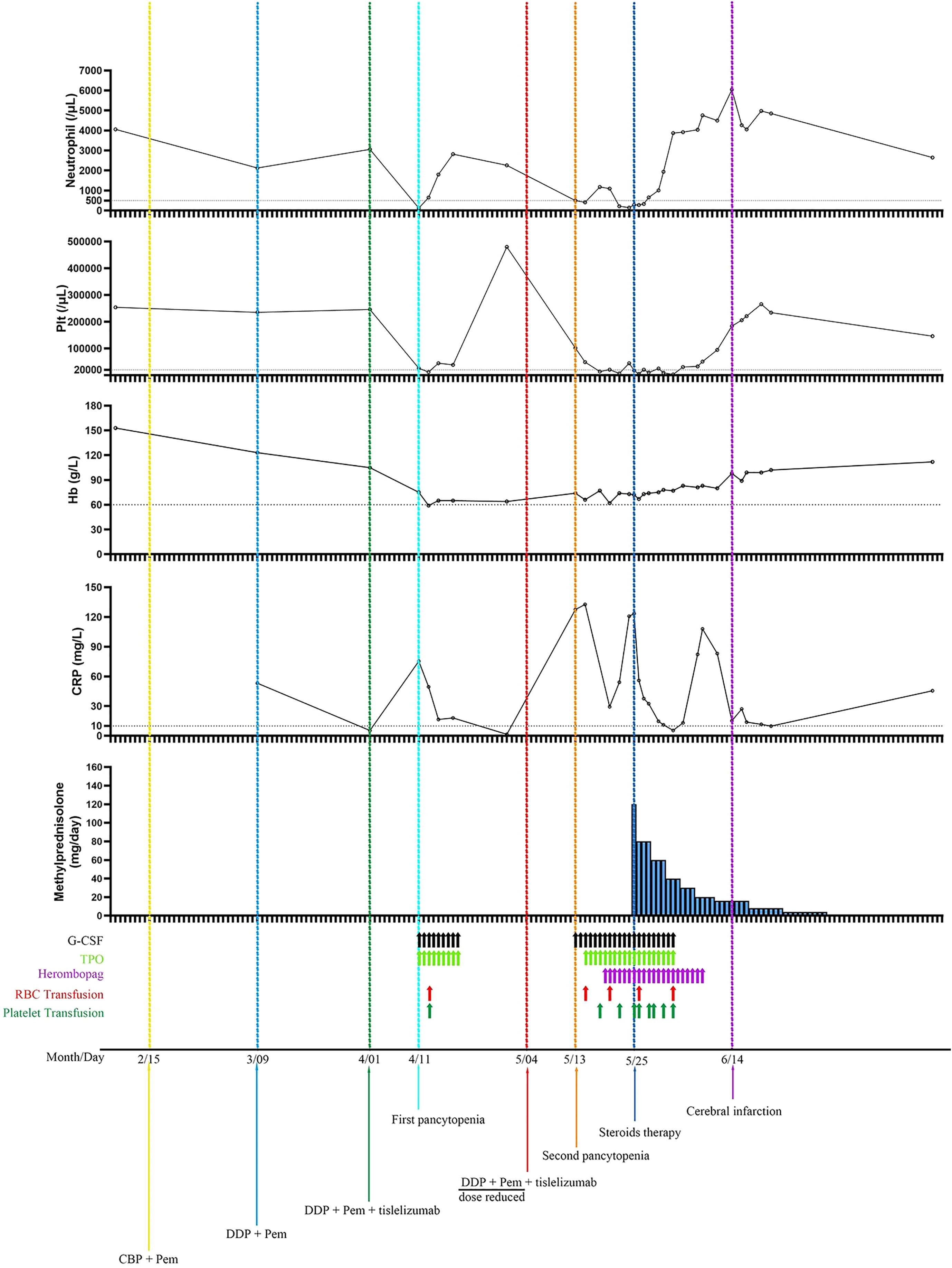
Figure 4 Neutrophil and platelet counts, hemoglobin and CRP concentrations as a function of time and treatments. The first pancytopenia was diagnosed on 10 days after the administration of 3rdC (chemotherapy plus tislelizumab) and resolved after the administration of 9 days of G-CSF and TPO injection, and one platelet and packed red blood cell transfusion. The second pancytopenia was diagnosed on 9 days after the 4thC (dose reduced chemotherapy plus full dose tislelizumab) and showed resistant to G-CSF, TPO, and transfusions of platelet and packed red blood cells, but responded well to steroids. Plt, platelet; Hb, hemoglobin; CRP, C reactive protein; G-CSF, granulocyte colony-stimulating factor; TPO, thrombopoietin; RBC, red blood cell; CBP, carboplatin; Pem, pemetrexed; DDP, cisplatin.
The cause of ICI-associated cytopenia possibly involves the cytotoxicity of ICI-activated T-cell- and/or B-cell-produced autoantibodies (16). Although there are some publications briefly describing Hema-irAEs, the criteria for the diagnosis and management of Hema-irAEs are not well-established (17–22). In this case, the patient does not have the history of autoimmune diseases or hematological disorders and he received two cycles of chemotherapy after the diagnosis of NSCLC and complained of oral ulcers after chemotherapy without outpatient visits or blood tests. Therefore, it was unclear whether the first two cycles of chemotherapy caused cytopenia. Ten days after the third cycle regimen containing the PD-1 antibody tislelizumab and the previous chemotherapy regimen, the patient developed grade III anemia and grade IV neutropenia and thrombocytopenia that necessitated the transfusion of red blood cells and platelets and injection of G-CSF and TPO. The pancytopenia resolved 9 days later without immunosuppression and remained in remission thereafter, which made it extremely difficult to determine the cause of the pancytopenia. Despite the 12-day delay and significant chemotherapy dose reduction in the fourth cycle regimen, the patient experienced a more severe and longer course of pancytopenia that was resistant to the above management for the first episode of pancytopenia. Despite 11 days of intensive care, the pancytopenia remained unimproved. However, only 3 days after the initiation of methylprednisolone, the patient’s ANC and Hb levels reversed from myelosuppression to 660/μL and 73 g/L, respectively, and his platelet count recovered to 31,000/μL after another 6 days of treatment with methylprednisolone. Finally, his neutropenia and thrombocytopenia resolved on Days 8 and 20 of methylprednisolone treatment, respectively. Therefore, despite the quick resolution of the first pancytopenia episode without immunosuppression, the second pancytopenia episode was extremely resistant to management without steroids but responded very well to subsequent methylprednisolone, strongly suggesting a likely Hema-irAE with tislelizumab. This case implied that higher grade and more refractory Hema-irAEs can occur in patients who resume ICIs after the resolution of previous Hema-irAEs, underlining the importance of the early recognition of Hema-irAEs. The median time to occurrence of ICI-related cytopenia ranges from 6 to 10.1 weeks (3, 15, 17, 20, 23). The patient whose case is described here developed pancytopenia only 10 days after the first dose of tislelizumab. A similar pattern of Hema-irAEs was also reported recently (24). The patient rejected a bone marrow test upon the resolution of neutropenia and thrombocytopenia at 40 days after the 4thC. Eighty-five days after the 4thC, bone marrow aspiration was performed to investigate the possible invasion of the bone marrow by cancer cells or parasites, and the findings further supported that the patient’s episodes of pancytopenia were Hema-irAEs.
Based on this case and a literature review, to make a timely and accurate diagnosis of ICI-related cytopenia, an MDT panel discussion is strongly suggested, and the following should be covered: the association of cytopenia with ICI administration, the effect of ICI withholding and/or immunosuppression on cytopenia reversal, other reasons that could be causes of the cytopenia, and the presence of autoantibodies reported to be associated with cytopenia.
The patient stopped TPO and hetrombopag when his platelet count reached 50,000/μL. His platelet count never went beyond the upper limit of normal range. However, he suffered a cerebral infarction when his platelet count was within the normal range (184,000/μL). Paradoxically, thrombocytopenia is accompanied by ischemic events (25–29), which indicates risk evaluation for cerebral vascular accident is warranted in cancer patients who experience thrombocytopenia.
Although the specimen for the first molecular testing by PCR was obtained by core biopsy, no known actionable mutation was found, and the METΔex14 revealed by a second testing with NGS strongly argued the importance of dynamic and repeated biopsy and molecular testing, especially by NGS.
Conclusions
Hema-irAEs are rare with ICIs but can be life-threatening. Early recognition of Hema-irAEs is vital to reduce the potential risks in these patients. An MDT panel discussion can help in the diagnosis and management of Hema-irAEs. Dynamic and repeated biopsy and molecular testing, especially with NGS, should be considered for patients with recurrent and metastatic NSCLC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethical Committee of Zhejiang Provincial People’s Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
H-YG and J-WZ took care of the patient during his hospitalization and prepared the data for this manuscript. Y-SW, Z-NM, X-MZ, and F-WW were members of the diagnosis and treatment group and were involved in the care of the patient. A-HZ and G-QW designed the outline of this case report and guided the preparation of this manuscript. G-QW also led the MDT panel discussion of this case. All authors are aware of and approve this submission. All authors contributed to the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1148425/full#supplementary-material
Supplementary Figure 1 | Computed tomography (CT) of the thorax during the course of anticancer therapy and management of pancytopenia (A) Two cycles of chemotherapy led to partial remission of lung cancer. (B) One cycle of dose-reduced chemotherapy+tislelizumab led to further shrinkage of multiple nodules. (C) Forty days after the second dose of tislelizumab, CT of the thorax indicated stable disease.
Supplementary Figure 2 | Blood smear and bone marrow examination (A,B) Blood smear and (C, D) bone marrow aspiration revealed non-malignant and parasite-free environment.
Supplementary Figure 3 | Computed tomography (CT) of the thorax before and after the administration of savolitinib (A) Four months after the second dose of tislelizumab, computed tomography (CT) of the thorax indicated progressive disease. (B) Chest CT showed partial remission of the lesions after two weeks of savolitinib treatment.
References
1. Liu X, Liang X, Liang J, Li Y, Wang J. Immune thrombocytopenia induced by immune checkpoint inhibitors in solid cancer: case report and literature review. Front Oncol (2020) 10:530478. doi: 10.3389/fonc.2020.530478
2. Ye X, Hu F, Zhai Y, Qin Y, Xu J, Guo X, et al. Hematological toxicities in immune checkpoint inhibitors: A pharmacovigilance study from 2014 to 2019. Hematol Oncol (2020) 38(4):565–75. doi: 10.1002/hon.2743
3. Martin M, Nguyen HM, Beuvon C, Bene J, Palassin P, Atzenhoffer M, et al. Immune checkpoint inhibitor-related cytopenias: about 68 cases from the french pharmacovigilance database. Cancers (2022) 14(20). doi: 10.3390/cancers14205030
4. Zaremba A, Kramer R, De Temple V, Bertram S, Salzmann M, Gesierich A, et al. Grade 4 neutropenia secondary to immune checkpoint inhibition - A descriptive observational retrospective multicenter analysis. Front Oncol (2021) 11:765608. doi: 10.3389/fonc.2021.765608
5. Boegeholz J, Brueggen CS, Pauli C, Dimitriou F, Haralambieva E, Dummer R, et al. Challenges in diagnosis and management of neutropenia upon exposure to immune-checkpoint inhibitors: meta-analysis of a rare immune-related adverse side effect. BMC Cancer (2020) 20(1):300. doi: 10.1186/s12885-020-06763-y
6. Haddad TC, Zhao S, Li M, Patel SH, Johns A, Grogan M, et al. Immune checkpoint inhibitor-related thrombocytopenia: incidence, risk factors and effect on survival. Cancer Immunol Immunother CII (2022) 71(5):1157–65. doi: 10.1007/s00262-021-03068-2
7. Atwal D, Joshi KP, Ravilla R, Mahmoud F. Pembrolizumab-induced pancytopenia: A case report. Permanente J (2017) 21:17–004. doi: 10.7812/TPP/17-004
8. Ni D, AlZahrani F, Smylie M. AIHA and pancytopenia as complications of pembrolizumab therapy for metastatic melanoma: A case report. Case Rep Oncol (2019) 12(2):456–65. doi: 10.1159/000500856
9. Cybulska-Stopa B, Gruchała A, Niemiec M. Immune-related pancytopenia induced by anti-PD-1 therapy - interrupt or continue treatment - the role of immunohistochemical examination. Case Rep Oncol (2019) 12(3):820–8. doi: 10.1159/000504130
10. Ueki Y, Suzuki M, Horikawa Y, Watanabe H, Yamaguchi Y, Morita C, et al. Pembrolizumab-induced pancytopenia in a patient with squamous cell lung cancer. Thorac Cancer (2020) 11(9):2731–5. doi: 10.1111/1759-7714.13582
11. Michot JM, Vargaftig J, Leduc C, Quere G, Burroni B, Lazarovici J, et al. Immune-related bone marrow failure following anti-PD1 therapy. Eur J Cancer (Oxford Engl 1990) (2017) 80:1–4. doi: 10.1016/j.ejca.2017.04.004
12. Tokumo K, Masuda T, Miyama T, Miura S, Yamaguchi K, Sakamoto S, et al. Nivolumab-induced severe pancytopenia in a patient with lung adenocarcinoma. Lung Cancer (2018) 119:21–4. doi: 10.1016/j.lungcan.2018.02.018
13. Uehara J, Yoshino K, Sugiyama E, Ohkuma K, Oaku S, Yamashita C, et al. Immune-related pancytopenia caused by nivolumab and ipilimumab combination therapy for unresectable melanoma of unknown primary. J Dermatol (2020) 47(6):e237–e9. doi: 10.1111/1346-8138.15341
14. Calvo R. Hematological side effects of immune checkpoint inhibitors: the example of immune-related thrombocytopenia. Front Pharmacol (2019) 10:454. doi: 10.3389/fphar.2019.00454
15. Delanoy N, Michot JM, Comont T, Kramkimel N, Lazarovici J, Dupont R, et al. Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet Haematol (2019) 6(1):e48–57. doi: 10.1016/S2352-3026(18)30175-3
16. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers (2020) 6(1):38. doi: 10.1038/s41572-020-0160-6
17. Kramer R, Zaremba A, Moreira A, Ugurel S, Johnson DB, Hassel JC, et al. Hematological immune related adverse events after treatment with immune checkpoint inhibitors. Eur J Cancer (Oxford Engl 1990) (2021) 147:170–81. doi: 10.1016/j.ejca.2021.01.013
18. Kroll M, Rojas-Hernandez C, Yee C. Hematologic complications of immune checkpoint inhibitors. Blood (2022) 139(25):3594–604. doi: 10.1182/blood.2020009016
19. Le Burel S, Champiat S, Mateus C, Marabelle A, Michot JM, Robert C, et al. Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur J Cancer (Oxford Engl 1990) (2017) 82:34–44. doi: 10.1016/j.ejca.2017.05.032
20. Tanios G, Doley P, Munker R. Autoimmune hemolytic anemia associated with the use of immune checkpoint inhibitors for cancer: 68 cases from the Food and Drug Administration database and review. Eur J Haematol (2019) 102(2):157–62. doi: 10.1111/ejh.13187
21. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol (2022) 33(12):1217–38. doi: 10.1016/j.annonc.2022.10.001
22. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer (2021) 9(6). doi: 10.1136/jitc-2021-002435
23. Kong BY, Micklethwaite KP, Swaminathan S, Kefford RF, Carlino MS. Autoimmune hemolytic anemia induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Res (2016) 26(2):202–4. doi: 10.1097/CMR.0000000000000232
24. Baek DW, Chae YS. Pembrolizumab-related autoimmune hemolytic anemia in a patient with metastatic lung adenocarcinoma: a case report. Yeungnam Univ J Med (2021) 38(4):366–70. doi: 10.12701/yujm.2021.00899
25. Hasegawa T, Ozaki Y, Inoue T, Watanabe Y, Fukuhara M, Yamaura T, et al. Nivolumab-related severe thrombocytopenia in a patient with relapsed lung adenocarcinoma: a case report and review of the literature. J Med Case Rep (2019) 13:1–6. doi: 10.1186/s13256-019-2245-y
26. Li Y, Xu Q, Guo X. Thromboembolic events secondary to tirofiban-induced thrombocytopenia being treated with thrombopoietin: A case report. Exp Ther Med (2016) 12(2):1177–80. doi: 10.3892/etm.2016.3439
27. Sasaki T, Yasuda T, Abe D, Miyano R, Kainaga M, Tomura N, et al. A case of multiple cerebral infarction preceding acute exacerbation of idiopathic thrombocytopenic purpura. J stroke Cerebrovasc Dis Off J Natl Stroke Assoc (2019) 28(3):789–91. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.026
28. Suzuki H, Tsunematsu T, Takahashi H, Yasuda S, Gyotoku D, Miyajima K, et al. A case of heparin-induced thrombocytopenia with subacute stent thrombosis, multiple cerebral infarction, and acute limb ischemia. J Cardiol Cases (2017) 15(5):145–9. doi: 10.1016/j.jccase.2016.12.013
Keywords: tislelizumab, pancytopenia, hematological irAEs, cerebral infarction, lung adenocarcinoma
Citation: Gu H-Y, Zhao J-W, Wang Y-S, Meng Z-N, Zhu X-M, Wang F-W, Zheng A-H and Wu G-Q (2023) Case Report: Life-threatening pancytopenia with tislelizumab followed by cerebral infarction in a patient with lung adenocarcinoma. Front. Immunol. 14:1148425. doi: 10.3389/fimmu.2023.1148425
Received: 20 January 2023; Accepted: 10 July 2023;
Published: 25 July 2023.
Edited by:
Xuelei Ma, Sichuan University, ChinaReviewed by:
Alexis Talbot, University of California, San Francisco, United StatesKarthik Gnanapandithan, Mayo Clinic Florida, United States
Copyright © 2023 Gu, Zhao, Wang, Meng, Zhu, Wang, Zheng and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ai-Hong Zheng, emFoMDU4MTg4OThAMTYzLmNvbQ==; Guo-Qing Wu, Z3F3dXpzdUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Hang-Yu Gu1†
Hang-Yu Gu1† Guo-Qing Wu
Guo-Qing Wu