- 1Institute of Human Genetics, Polish Academy of Sciences Poznan, Poznan, Poland
- 2Department of Gastroenterology, Dietetics, and Internal Diseases, Poznan University of Medical Sciences, Poznan, Poland
- 3Department of Environmental Medicine/Department of Gastroenterology, Human Nutrition and Internal Medicine, Poznan University of Medical Sciences, Poznan, Poland
- 4Department of Biochemistry and Biotechnology, Poznań University of Life Sciences, Poznań, Poland
- 5Department of Pediatric Gastroenterology and Metabolic Diseases, Poznan University of Medical Sciences, Poznan, Poland
- 6Department of Biotechnology, Institute of Natural Fibres and Medicinal Plants National Research Institute, Poznan, Poland
- 7Institute of Genetics and Animal Biotechnology, Magdalenka, Poland
- 8Institute of Neurobiology, Bulgarian Academy of Sciences, Sofia, Bulgaria
- 9Department of Pharmacognosy, University of Vienna, Vienna, Austria
- 10UCIBIO – Applied Molecular Biosciences Unit, MEDTECH, Laboratory of Pharmaceutical Technology, Department of Drug Sciences, Faculty of Pharmacy, University of Porto, Porto, Portugal
- 11Associate Laboratory i4HB - Institute for Health and Bioeconomy, Faculty of Pharmacy, University of Porto, Porto, Portugal
Commonly used clinical strategies against coronavirus disease 19 (COVID-19), including the potential role of monoclonal antibodies for site-specific targeted drug delivery, are discussed here. Solid lipid nanoparticles (SLN) tailored with tocilizumab (TCZ) and loading cannabidiol (CBD) are proposed for the treatment of COVID-19 by oral route. TCZ, as a humanized IgG1 monoclonal antibody and an interleukin-6 (IL-6) receptor agonist, can attenuate cytokine storm in patients infected with SARS-CoV-2. CBD (an anti-inflammatory cannabinoid and TCZ agonist) alleviates anxiety, schizophrenia, and depression. CBD, obtained from Cannabis sativa L., is known to modulate gene expression and inflammation and also shows anti-cancer and anti-inflammatory properties. It has also been recognized to modulate angiotensin-converting enzyme II (ACE2) expression in SARS-CoV-2 target tissues. It has already been proven that immunosuppressive drugs targeting the IL-6 receptor may ameliorate lethal inflammatory responses in COVID-19 patients. TCZ, as an immunosuppressive drug, is mainly used to treat rheumatoid arthritis, although several attempts have been made to use it in the active hyperinflammatory phase of COVID-19, with promising outcomes. TCZ is currently administered intravenously. It this review, we discuss the potential advances on the use of SLN for oral administration of TCZ-tailored CBD-loaded SLN, as an innovative platform for managing SARS-CoV-2 and related infections.
1 Highlights
● Tocilizumab (TCZ) attenuates cytokine storm in SARS-CoV-2-infected patients;
● Cannabidiol (CBD) promotes alleviation of anxiety, schizophrenia and depression;
● High levels of CBD from Cannabis sativa L. may be used to modulate angiotensin-converting enzyme II (ACE2) expression in SARS-CoV-2 target tissues;
● Dual TCZ and CBD-loading in lipid nanoparticles may ameliorate lethal inflammatory responses in COVID-19 patients;
● Lipid nanoparticles are suitable for orally-administered TNF-α inhibitors.
2 COVID-19 – Current therapies and promising drugs
Coronavirus disease-2019 (COVID-19) is caused by the severe acute respiratory syndrome (SARS) coronavirus-2 (SARS-CoV-2). It was first reported in Wuhan, China, in November 2019, when the outbreak was dated. Based on scientific reports, this acute infection is related to a cytokine storm, causing symptoms such as fever, cough, and muscle pain. In most severe cases, bilateral interstitial pneumonia with ground-glass opacity and focal chest infiltrates can be observed by using computerized tomography scans (1).
Despite the urgent need for specified therapeutic intervention, there are no effective antiviral drugs or vaccines against SARS-CoV-2. In October 2020, FDA approved remdesivir as the first promising antiviral drug to treat COVID-19 patients (2, 3). Previously, this antiviral drug has been applied to treat hepatitis C and was also used against Ebola. In the EU, remdesivir is now licensed to treat COVID-19 in adults and adolescents with pneumonia requiring supplemental oxygen (4).
Studies demonstrate that hospitalized COVID-19 patients with a lower respiratory tract infection in the remdesivir group recovered faster than patients in the placebo group (5). However, the clinical status of the patients within the 10-day course of remdesivir did not have any statistical improvement compared to standard care at 11 days after initiation of treatment in the case of moderate COVID-19. On the other hand, patients randomized to a 5-day treatment with remdesivir have shown a statistically significant difference compared to standard care. The obtained clinical importance was unreliable (6). To sum up, the first randomized trial indicated that remdesivir has no significant clinical values. In contrast, the numerical reduction in time to clinical improvement points out the need for more research investment (7).
Even though hydroxychloroquine, lopinavir/ritonavir, and interferon were also proposed against SARS-CoV2 (8), research is still ongoing on more effective treatments. Among available therapeutic regimens, the most common drugs are those used for autoimmune diseases, antiviral agents, and antibodies from people who have recovered from COVID-19. It is worth underlining that due to the reproduction of viruses, an efficient antiviral drug should be able to target the specific part of its life cycle necessary. Moreover, antiviral agents must be able to kill viruses without killing human cells.
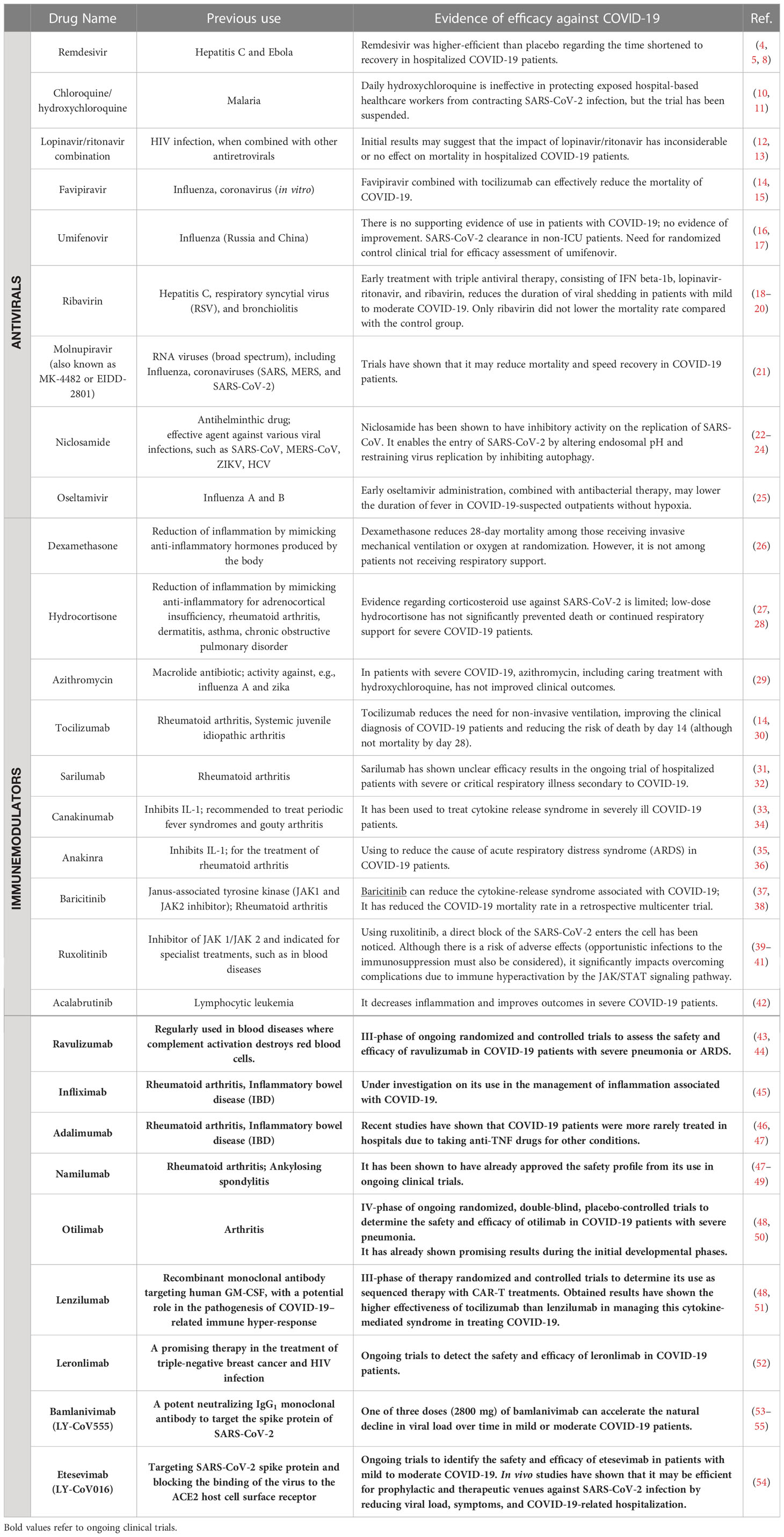
Still, plenty of ongoing clinical trials of COVID-19 treatment are performed worldwide. In June 2020, the European Medicines Agency (EMA) announced negotiations with the developers of 132 potential COVID-19 therapies (9). Different drugs listed in Table 1 have been mentioned among possible medications for treating COVID-19.
The need for drug repurposing has increased to prompt an efficient way to fight against SARS-CoV-2. Various drug repurposing screenings have chosen several potential drug candidates against COVID-19, but no one is fully efficient.
In this review, we discuss the studies that have implemented tocilizumab (TCZ) as an anti-IL-6 receptor antibody in COVID-19 treatment, proposing a new TCZ-coated platform for the targeted delivery of CBD. Many already published results indicate that the combination of dual delivery TCZ and CBD may aid in the recovery of patients with COVID-19 and reduce mortality. A novel approach is therefore discussed here, exploiting opportunities associated with linking nanocarriers loaded with TCZ and its agonist – CBD. Both drugs can inhibit IL-6, a major inflammatory cytokine involved in cytokine release syndrome (CRS) in various inflammatory conditions (56). Therefore, this dual-drug delivery system may have a crucial meaning in the mechanism of SARS-CoV-2 infections.
3 Clinical view of COVID-19
The SARS-CoV-2 virus has already infected millions of people worldwide and has led to numerous deaths. COVID-19 was firstly recognized and described in November 2019 in Wuhan (Hubei Province, China) during a series of cases that initiated the pandemic of this disease (57). The transmission of infection is primarily by droplet infection (58). Still, the SARS-CoV-2 virus is also noted in the stool, suggesting that the gastrointestinal tract is the second - after the respiratory system - target site for initial viral replication (59). The natural course of COVID-19 can be split into three stages of development: early, pulmonary, and hyperinflammatory. Each phase, presented in Figure 1, has slightly different clinical characteristics and minor differences in the proposed treatment algorithms (60). Many infected patients are asymptomatic, augmenting the spread of the virus (61). When symptomatic, cough, fever, fatigue, and dyspnoea are the most frequent manifestations of COVID-19; however, some people develop serious complications resulting in death. People with reduced immunity are at the highest risk of more severe side effects attributed to the infection, such as dysfunction of specific organs or even respiratory failure (61).
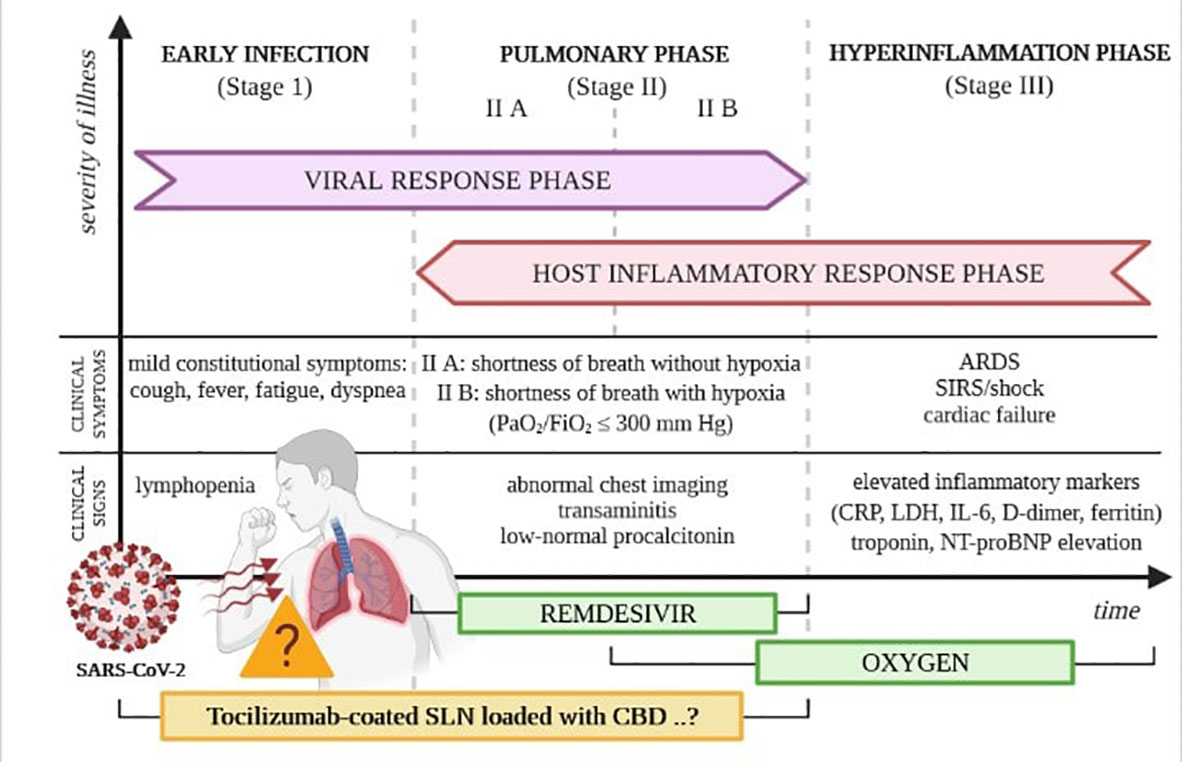
Figure 1 Phases of the clinical course of COVID-19. Own drawing, based on (60).
In many cases, COVID-19 can cause some other less typical clinical manifestations (57). Very often, anxiety is the accompanying symptom. As the expression of specific receptor proteins for the SARS-CoV-2 virus is exceptionally high in intestinal epithelial cells, symptoms at the gastrointestinal level (e.g., diarrhea, abdominal pain, and nausea) are also commonly reported, especially in the early stages of the disease (59, 62). The risk factors for a severe course of the illness with dynamic progression to the hyperinflammatory phase, the clinical manifestation of which is acute respiratory distress syndrome, circulatory failure, and shock, are still not fully understood. The most crucial pathophysiological phenomenon responsible for these processes is the cytokine-associated toxicity resulting from SARS-CoV-2 virus infection (58). One of the critical directions of research into an effective COVID-19 therapy is the search for drugs that can inhibit and prevent the uncontrolled production of pro-inflammatory cytokines, showing significant potential to damage tissues, including respiratory diseases. Cytokine-associated toxicity or cytokine release syndrome (CRS) is mainly associated with pro-inflammatory cytokine IL-6 released in severe COVID-19 infections. Cytokine IL-6 initiates the CRS in the MAPK/NF-κB-IL-6 or JAK-STAT pathway (63), and as an infection trigger, IL-6 has been associated with the symptom progression in this disease (64). The cytokine-associated toxicity has been regarded as the most typical marker of the severity of COVID-19 infection and high mortality risk (65).
As the number of patients infected with the SARS-CoV-2 virus continues to grow worldwide, there is still a need to introduce an effective therapy that can provide effective treatment in all phases of the infection and especially prevent the progression of COVID-19 into clinical forms that constitute a direct threat to the patient’s life.
4 Gene delivery and therapy in SARS-CoV-2 infection
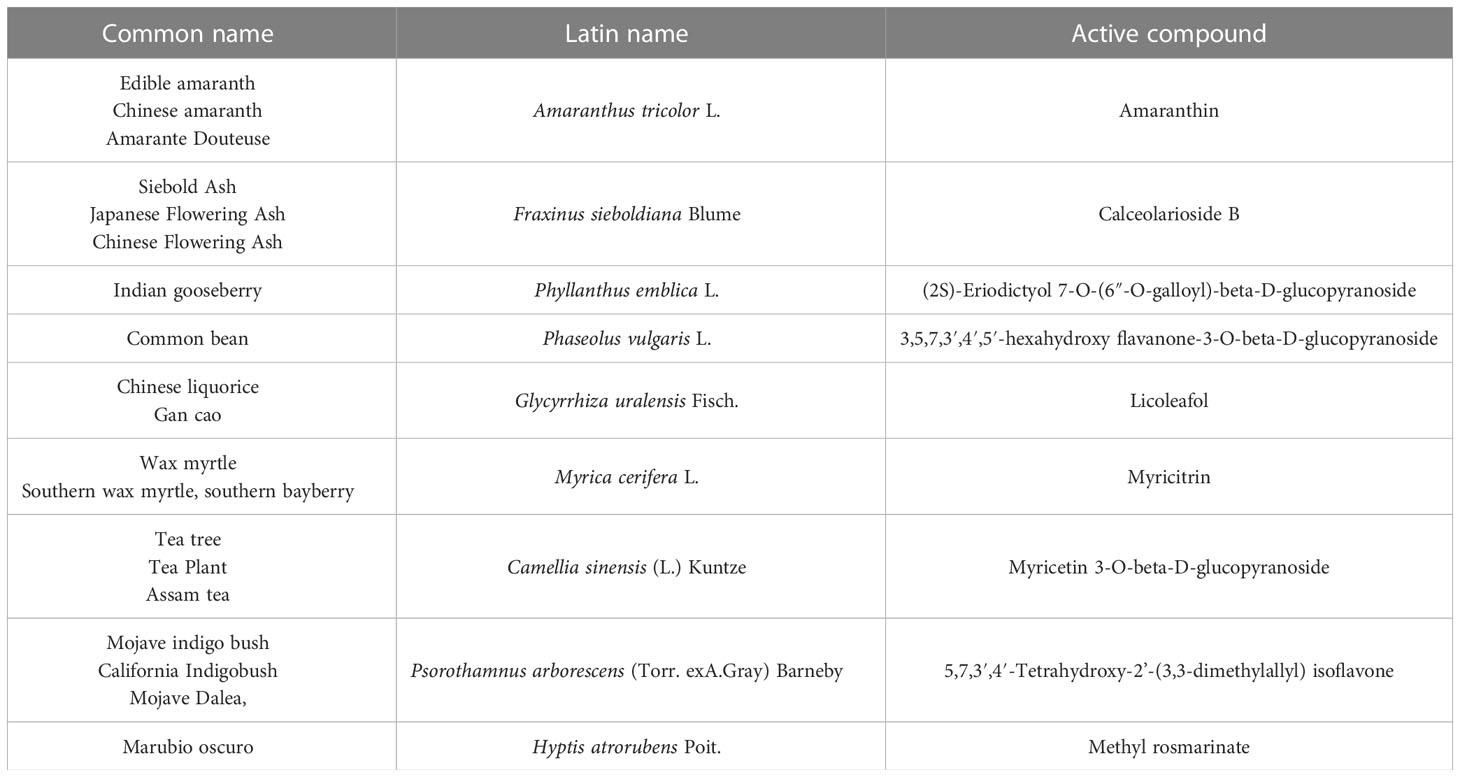
The genome sequence of SARS-CoV-2, shown in Figure 2, shows a very high similarity to SARS-CoV-2. Therefore, 3D homology sequence lines were used to analyze potential antiviral properties based on databases of over 32 000. Recognized medicinal plants and substances used in Chinese medicine are listed in Table 2 (67).
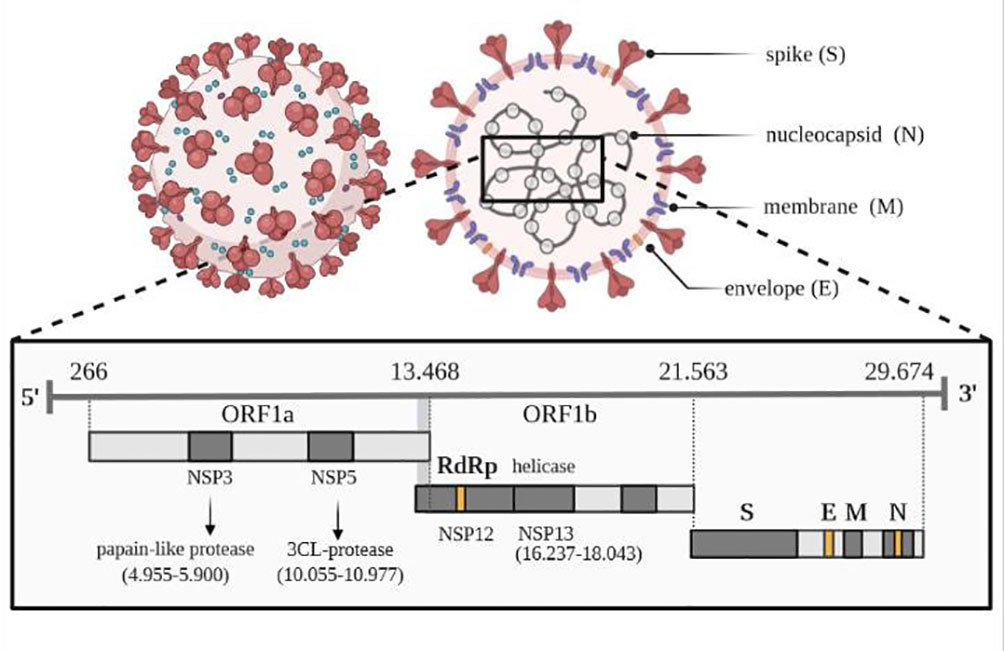
Figure 2 Viral genome of SARS-CoV-2. Own drawing, based on (66).
The genome of the SARS-CoV-2 virus distinguishes genes encoding structural proteins that make up the virus particle, including spike proteins (S protein) essential for infection, as well as envelope proteins (E), nucleocapsid proteins (N) and membrane proteins (M) (66). The SARS-CoV-2 virus also contains sequences encoding non-structural proteins (NSP), which inhibit the host’s innate immune response to infection through the activity of papain-like protease (PLP) encoded by the NSP3 sequence and 3CL protease encoded by the NSP5 sequence, located in the gene designated as ORF1a. The virus has its RNA polymerase (RdRP) that is RNA-dependent (NSP12) and an RNA helicase (NSP13) located within the ORF1b gene. One of the tasks of the 16 non-structural proteins involves the transcription and translation of the genome (RTC replication-transcription complex).
Substances with an antiviral activity that inhibit the main proteases of the SARS-CoV-2 virus (Mpro) can be searched among bioactive compounds from medicinal plants using a molecular docking strategy. Khaerunnis et al. informed that nelfinavir and lopinavir can treat SARS-CoV-2 infection. In addition, the main protease activity is inhibited by apigenin-7-glucoside, curcumin, demethoxycurcumin, catechin, epicatechin-gallate, and oleuropein, luteolin-7-glucoside has been shown, which can be used in therapy (68).
Further work has identified other low-risk drugs that can inhibit the activity of the COVID-19 protease due to its ability to bind to it. They are also characterized by high affinity, suggesting the potential for use in treating viral infection. Among the proposed substances, bilobalide and citral can be distinguished, in addition, to forskolin, ginkgolide A, menthol, or noscapine, salvinorin A and beta selinene and thymoquinone (69). An interesting substance of plant origin is cannabidiol (CDB), which may be used for immunological health support and possible substance protection from infection. The anti-inflammatory effect was found in various immune-mediated disorders, including autoimmune conditions and neurodegeneration. Since cannabidiol can support the body during infection against pathogens, it is assumed that it can play a similar role in COVID-19 disease. Cannabidiol reduces the secretion of cytokines, leading to a decrease in the level of chemokines.
Moreover, the anti-inflammatory effect is associated with limiting cell-mediated immunity with the participation of effector T cells. Similarly, in the central nervous system, it also modulates the activity of microglial cells (70, 71). This information is interesting within the problem of cytokine storm, which strongly influences the odds ratio of survival of affected COVID-19 patients. The main conclusion of many papers is the need for proper analysis of the influence of cannabidiol and tetrahydrocannabinol (THC) as potential therapeutic substances with antiviral properties (71–73).
Patients infected with SARS-CoV2 are treated with various drugs that reduce excessive inflammation associated with the enormous secretion of cytokines. The most commonly used are interleukin inhibitors, Janus kinase inhibitors, corticosteroids, convalescent Plasma, interferons, nitric oxide, statins, and adjunctive nutritional therapies, including zinc and vitamin D (47, 74, 75).
One of the drugs under investigation is tocilizumab (Actemra). This IL-6 inhibitor participates in several phase III clinical trials, all randomized (for participants and investigators) and double-blind. For example, clinical trials in patients with COVID-19 pneumonia, COVACTA, and EMPACTA, included an analysis of tocilizumab and a placebo, and REMDACTA additionally included an analysis of remdesivir. Preliminary results of clinical trials are encouraging (76–78).
An essential part of any therapy is the successful delivery of a drug or new gene construct, for example, based on genome editing, to the destination. Still, the big challenge is how to deliver the therapeutic protein to the specific compartment of the target cell (79). Nanocarriers based on lipids, polymers, graphene, or gold have been proposed. It is assumed that using nanoparticles based on lipids and polymers does not stimulate the immune system’s response and does not cause additional problems for cells or tissues (80).
After administration via systemic injection or by oral route, numerous obstacles must be overcome before reaching specific cell types or cellular compartments. One possibility is to put a drug, nucleic acids, or proteins into a nanoparticle shell, preventing aggregation, immune clearance, kidney removal, or premature release. It is assumed that the carrier, e.g., non-viral nanoparticle, and the transferred payload/cargo should be compatible in electrostatic charge. On the surface of the shell, additional targeting ligands may improve delivery precision. The next step will cover crossing the cell membrane of the target cell, which may be overcome by bombardment, using guiding peptides, or even via endocytosis. The developed formulations have to escape endosomes. After that, they may stay in the cytoplasm or journey to the nucleus. At each point, the risk of degradation has to be considered. For the delivery of nucleic acids, viral vectors (e.g., lentiviruses and adeno-associated viruses), are used. Due to problems related to their limited loading capacity, the possibility to increase immune response connected with mutagenesis and carcinogenesis, and issues with up-scaling, other delivery systems are being proposed. Nonviral vectors use lipids and/or polymers as nanocarriers and may deliver large cargo (81, 82). The choice of a virus-based or a non-viral vector will depend on the load we want to transfer to the cell rather than on the vector itself.
For lipid-based nanoparticles, synthetic lipids containing disulfide bonds, which will break after exposition to the reducing environment in the cell and release the nanoparticle contents, have been proposed (81). Another study used a covalently cross-linked thin polymer carrier that can be removed by glutathione (GSH) to deliver a Cas9 ribonucleoprotein complex for in vivo genome editing. The presence on the surface of the additional nanocapsule peptides may guide the whole structure to the destination cells. Inside the cell, cytosolic glutathione may disrupt the shell releasing the cargo (82).
Biocompatible carriers such as lipid and polymeric nanoparticles, peptide/protein and messenger RNA complexes, and other biomaterials have recently been used for in vivo protection and delivery of various loads (83, 84). For the formulation of lipid-like nanoparticles like cationic lipids, such as DOTAP and DOTMA, and ionizable lipid derivatives such as TT3, 5A2-SC8, LP-01, cKK-E12, and A18-Iso5-2 DC18 to improve the efficiency and lowering toxicity in vivo (83).
Polymeric carriers are of great interest due to their ease of uptake by cells, the ability to combine with proteins, and the ability to form complex systems with biologically active biodegradable substances. Carriers often show the ability to bind to the cell surface; such endocytic uptake is due to their functionalization/modification. Perfluorocarbon nanoemulsions (PFCs) more and more often are used in medicine, enabling the response to emerging stimuli, taking part in the active prevention and control of hemorrhage, actively participating in the transport of oxygen as synthetic artificial blood or in tissue ischemia (79). Such substances are sensitive to ultrasound, which allows them to be tracked and activate selected proteins at the expected target sites. This strategy may find application in the tightly controlled delivery of antibodies.
Cell and gene therapies aim to replace a defective gene. New medicines can be used in the treatment of a whole range of common diseases of great social importance, often with a complex genetic background (e.g., cancer, heart disease, or diabetes), conditioned by single genes (e.g., cystic fibrosis, hemophilia) and infections caused by viruses (e.g., AIDS). Cell and gene therapies still need to be fully commercialized and may be available only as part of a clinical trial, but already some treatments are marketed. Gene therapy possibility as the replacement of a diseased gene variant was mentioned in 1972. Still, it has taken years since the first gene therapy, Gendicine, for skin cancer was commercialized in China in 2003. FDA approved the first gene therapy in 2017. Since the first gene therapy patient Jesse Gelsinger died in 1999 (the ornithine transcarbamylase gene using a recombinant adenovirus), gene therapy may become a reality due to knowledge of the human genome, availability of precise and efficient tools for genes edition, and using of better delivery methods. Currently, several gene therapy products are commercially available, approved by the relevant agencies (Europe: European Medicines Agency, U.S.: Food and Drug Administration); advanced therapy medicines include Kymriah, Luxturna, Tecartus, Yescarta, as well as Zolgensma. Gene therapy aims to cure diseases caused by defined single-gene mutations mainly. Cell-specific delivery and immunogenicity remain challenges in gene therapy. Gene or drug transfer can be based on two strategies that enable the introduction of permanent changes by incorporating them into the genome or obtaining a transient effect; the first often use modified viruses, and the second can use lipid nanoparticles. After using adenovirus as a delivery vector, nowadays mainly for in vivo gene therapy, adeno-associated viruses (AAVs) are used and in vitro modification lentiviruses, for example, in chimeric antigen receptor (CAR) T cells (85, 86). Scientists also focused on decreasing the toxicity of viral vectors by limiting viral dosage by using capsids with increased efficiency of cell penetration, or the possibility of modifying virus expression specific for target tissues, or increasing the purity of the virus injected.
Gene therapy is not only strictly focused on gene delivery; there are attempts to cure polygenic disorders by delivering proteins for disease treatment (87). Another approach involves using a modifier gene platform and providing a functional copy of the gene encoding the retina-specific nuclear receptor NR2E3 using the Adeno-Associated Virus AAV platform for gene therapy instead of correcting the damaged gene (88), changing disease phenotype.
It is also interesting to use specific receptors activated only by dedicated active substances (DREADD) for non-invasive and longitudinal tracking of neuron activity (89). Gene therapy may establish resistance to infectious diseases, and fragments of the SARS-CoV-2 sequence may be delivered within an AAV capsid (vaccine AAVCOVID). Production of antibodies against the adeno-associated virus may be prevented by endopeptidase imlifidase (IdeS) expression without disrupting B lymphocytes (90). The availability of therapy is limited in price.
Gene therapies are available on the market offer insertion of correct genes: Gendicine (China), Glybera (EU), and Imlygic (China, US, and EU); delivery of the DNA for drug production: Holoclar (EU), Kymriah (US and EU), Luxturna (US and EU), Strimvelis (EU), Yescarta (US, EU, in China under clinical trials), Zolgensma (US), Zynteglo (EU); gene interference: Defitelio (US and EU), Exondys 51 (US), Kynamro (US), Macugen (US) and Spinraza (US).
Gene therapy strategies include replacing entire genes with normal genes, repairing a mutated gene fragment, or making abnormal cells more recognizable by the immune system so they can be effectively removed from the body. Problematic is the delivery of genes using a carrier, usually a viral vector, because viruses can recognize specific cells and introduce genetic material into the cell. Another vehicle may include using of stem cells or liposomes. The risk of gene therapy may be associated with the occurrence of an inappropriate reaction of the immune system, the impact on non-target cells, the appearance of infection with the virus used as a carrier, and even the development of cancer. Liposomal carriers are now very often analyzed. Due to COVID-19, many clinical trials are delayed, but new challenging options are concentrated on producing vaccines and finding new drugs against SARS-CoV-2 using an available portfolio of viral vectors. Usually, adeno-associated virus (AAV) may be chosen to deliver spike protein fragments. Using nanolipids as delivery vectors is also auspicious, not only to transport vaccines but also to deliver specific drugs.
WHO reports 42 candidate vaccines under clinical evaluation: 13 working with protein subunit, 10 using Non-Replicating Viral Vectors, 7 Inactivated, 6 RNA, 4 DNA, and two virus-like particles (VLP). Other 151 candidate vaccines are in preclinical evaluation and use mainly protein subunits (54), Non-Replicating Viral Vectors (18), RNA (18), Replicating Viral Vectors (18), virus-like particles VLP (14), DNA (13), Inactivated (11), Live weakened viruses (3), Replication-competent bacterial vector (1) and based on the use of T cells (1). Only 10 developers/manufacturers of the COVID-19 vaccine have passed phase 3 clinical trials. Among them, the following centers can be distinguished: ii) Sinovac, ii) Wuhan Institute of Biological Products in cooperation with Sinopharm, iii) Beijing Institute of Biological Products in cooperation with Sinopharm as well as iv) CanSino Biological Inc. together with the Beijing Institute of Biotechnology, the following centers can be distinguished: v) the University of Oxford in cooperation with AstraZeneca, vi) Gamaleya Research Institute, vii) Janssen Pharmaceutical Companies, viii) Novavax, ix) Moderna with the National Institute of Allergy and Infectious Diseases (NIAID), and v) BioNTech in cooperation with the Chinese company Fosun Pharma and Pfizer.
The SARS-CoV-2 coronavirus can infect cells with angiotensin-converting enzyme 2 (ACE2) receptors on their surface. It also uses type II transmembrane serine protease TMPRSS2, penetrating, among others, lung epithelial and lung endothelial cells, macrophages, or monocytes. Additionally, coronavirus may use antibody-dependent enhancement (ADE) for infection of cells with a lower level of ACE2 and TMPRSS2 receptors (91). Such a possibility may be connected with problems finding a good strategy for producing vaccines against coronavirus (92). The main aim should be focused on the preparation of specific therapies against SARS-CoV2, including RNA interference (RNAi) (93, 94), small interfering RNA (siRNAs) (95), RNA aptamers, Ribozymes, antisense RNA (ASOs), and oligonucleotide therapeutics (96–99). The ideal vaccine has to be immunogenic with minimal side effects. Production of the vaccine should be efficient and affordable, with the possibility of easy scale-up in full compliance with the principles of good manufacturing practice (GMP). Moreover, the vaccine must not lead to adverse post-vaccination reactions, including antibody-dependent aggravation of infection (100). Conventional methods of obtaining vaccines allow for their effective production. Inactivated vaccines can be used based on attenuated viruses and those using immunogenic subunits. Still, they are associated with the possibility of problems involving strain specificity, risks of viral interference, cross-immunity, allergenicity, or triggering only partially of the immune response. Using genetic vaccines (naked DNA or RNA) like replication-defective recombinant adenoviruses may overcome limitations, be more safely, cost-effective, and quicker, and induce an innate and adaptive immune response, including activation of T cells and antibodies. There is also no problem with the derivation of nucleic acids-based vaccine using antigens sequence and choose fragment, generating better immunological response (88, 100, 101). Genetic vaccines based on DNA and RNA still have some limitations, such as lower immunogenicity. Still, high reproducibility, low costs, and relatively short production time are promising (101, 102).
The nucleic acid-based molecules/drugs may influence viral infection by regulating transcription or post-transcriptional processes, leading to the overexpression of protective genes and silencing damaged genes (103, 104). The main types of nucleic acid-based vaccines are DNA and RNA vaccines outlining in detail the mode of action, evidence supporting a therapeutic strategy based on nucleic acids, the use of new research and development solutions, emerging patents, vaccines for SARS-CoV-2 based on the use of DNA and RNA; clinical trials, expenditures related to the development and production of the vaccine and the pros and cons of vaccines based on mRNA and DNA against SARS-CoV-2were presented in the paper prepared by Piyush et al. (2020) (103).
5 Chemical structure, properties, and medical application of tocilizumab
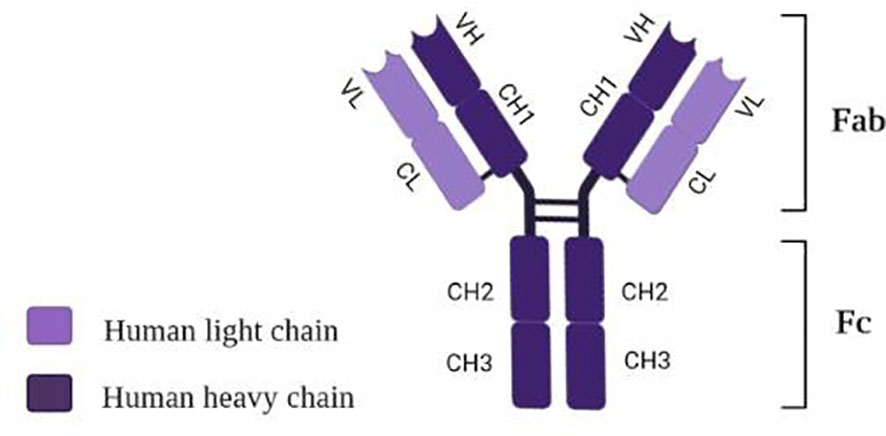
Tocilizumab (TCZ), also known as atlizumab (RoActemra®), is known as a humanized IgG1 monoclonal antibody targeting interleukin-6 (IL-6) receptor (105). The structure of TCZ is schematically shown in Figure 3. The antibody consists of two heavy chains (dark violet) containing a variable VH domain and constant domains CH1, CH2, and CH3. In comparison, two light chains (light violet) consist of a variable VL domain and constant CL. Moreover, an antigen-binding fragment (Fab) and a fragment responsible for antibody effectors’ functions (Fc) are distinguished in the structure of this immunosuppressive drug (106).
Tocilizumab binds with high affinity with both soluble receptors for IL-6 (SIL-6R) and membrane-bound IL-6 receptor (mIL-6R), as well as it inhibits JAK-STAT or MAPK/NF-κB-IL-6 signaling pathway (107, 108). In addition, TCZ can block cytokine storm syndrome (63) and inhibit intracellular signaling in cells expressing soluble gp130 protein (sgp130, Figure 4) (109). Currently, this FDA-approved drug commonly used so far in treating rheumatoid arthritis and juvenile idiopathic arthritis is administered intravenously (107, 110) only in hospital conditions, which is a great difficulty in its use during a pandemic.
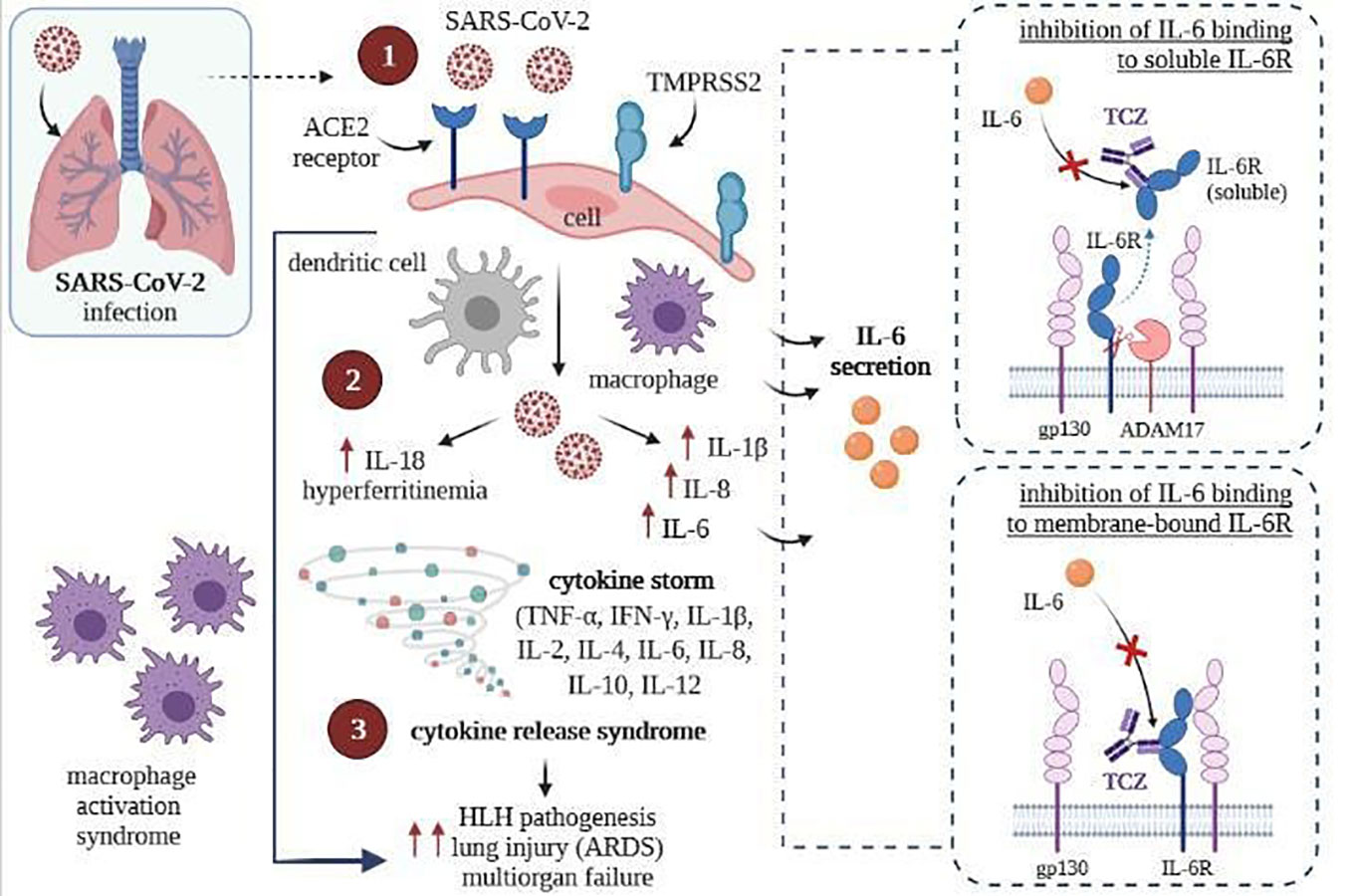
Figure 4 SARS-CoV-2 infection (left) and inhibition of intracellular signaling in cells by TCZ, resulting express of gp130 (right) [own drawing]. (1) Virus entry and infection of pneumocytes expressing the ACE2 receptor, recruiting antigen-presenting cells (dendritic cells and macrophages) into the lungs; (2) Activation of the NLRC4 inflammasome, which causes the overproduction of IL1β and IL 18, and causes the secretion of IL6 and ferritin by macrophages; (3) Upregulation resulting in cytokine release syndrome and macrophage recruitment to the lungs, contributing to ARDS.
5.1 Tocilizumab in the treatment of cytokine-associated toxicity in COVID-19 patients
Several attempts have been made to use tocilizumab in the active hyperinflammatory phase of COVID-19 (63, 111, 112). Exaggerated immune response to infection with the SARS-CoV-2 virus contributes to respiratory distress and multi-organ failure (113). It is caused by the elicitation of the so-called cytokine storm (114) (Figure 4). The results of recent scientific reports have shown that TCZ therapy in COVID-19 can drive a significant reduction in the inflammatory process. It can be explained by the induction of apoptosis of immunocompetent cells in affected tissues and by inhibiting proinflammatory cytokine release (115–117). Although the results are promising, previous studies of cytokine storm associated with other coronavirus and influenza virus infections and CAR (chimeric antigen receptor)-T cell therapy have also proven high levels of interleukin (IL)-6 and other cytokines (113, 114).
The study by Guo et al. (2020) has shown that immunosuppressive drugs targeting the IL-6 receptor, such as TCZ, can ameliorate lethal inflammatory responses in COVID-19 patients (118). The research has proven that the inflammatory cascade caused by excessive immune responses correlated with the death rate of COVID-19 (83, 119). As a result of SARS-CoV-2 infection, an increase in plasma concentrations of several inflammatory cytokines was observed, besides tumor necrosis factor α (TNF-α), interleukins (IL-2,-6,-7,-10), granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF) (118).
Since tocilizumab has been considered effective for treating severe cytokine-release syndrome (120), this immunosuppressive drug has also been applied to treat selected COVID-19 patients (121). Other results have shown that pathogenic T cells and peripheral inflammatory monocytes may induce cytokine-associated toxicity in patients infected with SARS-CoV-2. However, the administration of tocilizumab decreased the patient’s body temperature within 24 hours, and a visible reduction of oxygen inhalation in COVID-19 patients within less than a week of treatment (122). Although it was shown that TCZ might efficiently attenuate the cytokine cascade in COVID-19 patients, no single-cell-level analysis explaining these phenomena has been performed so far. This study would help to disclose the tocilizumab mode of action in the context of a characteristic COVID-19-induced activation of an inflammatory storm (118).
Xu et al. (2020) conducted an uncontrolled study using TCZ as IL- 6 blocker in 21 COVID-19 patients with the most common symptoms. All patients required supplemental oxygen (2 were on ventilators), had worsening ground-glass opacities on chest computed tomography, and showed deterioration of other clinical and laboratory measures (122). It is worth underlining that within 24 hours of TCZ therapy beginning, fevers and increased C-reactive protein levels significantly resolved, while all pro-inflammatory cytokines (especially IL-6) declined. Furthermore, there was no urgent need to use oxygen in 15 patients. In all patients, oxygen saturation levels were improved (122).
One of the most extensive studies regarding the use of tocilizumab in COVID-19 patients was conducted in Northern Italy and reported by Guaraldi et al. (2020) (105). SARS-CoV-2-infected patients were administered 8 mg/kg (up to 800 mg) of TCZ intravenously or 162 mg subcutaneously in two simultaneous doses (81 mg per thigh). Both amounts were based on pharmacokinetic data and were intended to mimic peak plasma concentration (111). Patients who received tocilizumab were compared with a control group with the same inclusion and exclusion criteria. However, the main limitation of this study was that patients and controls were not randomly chosen, thus making it impossible to compare the obtained results and draw reliable conclusions.
Another study has identified the outcomes among SARS-CoV-2-infected patients treated with tocilizumab to target cytokine storms (112). The results helped set specific criteria to define the cytokine storm in all SARS-CoV-2-RNA-positive patients. Moreover, it has been shown that early identification and inhibition of cytokine storms before intubation is much more significant than any anti-inflammatory treatment. Cytokine storm duration should be included, while randomized controlled trials based on targeted anti-cytokine and corticosteroids may also be considered (109, 112, 123).
6 The impact of the endocannabinoid system on COVID-19
Emerging reports on the production of endocannabinoids in the respiratory system and cannabinoid-induced bronchial dilatation allow conclusions about the potential therapeutic use of cannabinoids in treating respiratory diseases, including acute respiratory failure syndrome in severe COVID-19 patients (124). Despite the identification of the first strains of human coronavirus in the 1960s and the molecular similarity of SARS-CoV to SARS-CoV-2, no studies have been conducted to prove the effect of cannabinoids on this family of single-stranded RNA viruses so far. There was also no objective evidence of the therapeutic, anti-inflammatory effects of cannabidiol (CBD) and delta-9-tetrahydrocannabinol (Δ9-THC), the two main cannabinoids would contribute to promote or prevent the application of cannabinoids as compounds to the fight against the virus. Nonetheless, it seems that THC, CBD, or other cannabinoids can act as immune modulators, which could be helpful in the treatment of viral infections, especially those where we have a pathogenic host-inflammatory response, as with SARS-CoV-2 (125).
Infection caused by SARS-CoV-2 leads, for reasons not fully explained, to the overproduction of inflammatory cytokines (mainly from immune cells) with a wide range of biological activity, which is caused by various infections and loss of unfavorable influence on the immune system. On the other hand, these cytokines positively influence different immune cells, guiding them to the inflammation sites, which causes an exponential increase in inflammation, leading to continuous extreme activation of the autoimmune system. This mechanism is called a cytokine storm, a significant cause of acute respiratory distress syndrome, systemic inflammatory response, and multi-organ failure (126). It is suggested that cannabinoids could be part of two schemes to treat these acute inflammatory reactions. The first, with non-steroidal anti-inflammatory drugs (NSAIDs) targeting the immune system, which hurt antiviral therapies due to NSAIDs interactions and weakening the immune response to acute viral infections leading to disease progression, while the second, with drugs specifically binding to pro-inflammatory cytokine receptors, such as tocilizumab that inhibits the transmission of the signals through IL-6 receptors leading to a weakening of IL-6 activity.
7 Cannabinoids and their influence on the treatment of COVID-19
Cannabidiol (CBD; C21H30O2) is a phytocannabinoid without psychoactive activities produced by Cannabis Sativa L and has a structural similarity to Δ9- tetrahydrocannabinol (THC; C21H30O2), the primary psychotropic congener of this cannabis plant. Both cannabinoids are lipophilic compounds characterized by long half-life, bioaccumulation, and shared common metabolic pathways within the cytochrome family, drug carriers, and plasma protein binding substrates (127). Both of them also have anti-inflammatory activity and possible antiviral potential. Nonetheless, the majority of studies indicate the immunosuppressive and anti-inflammatory effects of cannabidiol in various immunological reactions and inflammations (70). CBD, unlike THC, is a non-toxic compound with a high safety margin and drug tolerance, even at doses up to 1500 mg/day. Recently, it was demonstrated that cannabidiol has anti-inflammatory effects in chronic inflammatory diseases preclinical models, apoptotic effects on the mammalian cells (70), or effects that contribute to the host of the viral infection response (128, 129).
Interleukin-6 is effectively suppressed by cannabidiol in numerous models of inflammation, including diabetes, asthma, pancreatitis, and hepatitis. In vivo, cannabidiol use resulted in an IL-6 decrease in ex vivo lipopolysaccharide-stimulated peritoneal macrophages in acute pancreatitis and bronchial-alveolar lavage fluid in lipopolysaccharide-induced pneumonia. Also, in mice with the induced asthma-like disease, IL-4, IL-5, and IL-13 cytokines and chemokines caused in the lungs of mice were shown to be suppressed by CBD (70). Cannabidiol decreased lung inflammation in asthma and acute pneumonia mouse models by inhibiting the production of pro-inflammatory cytokines by immune cells and suppressing the exuberant immune response (130, 131). Nichols and Kaplan (2020) (70) have shown that CBD inhibits the production of pro-inflammatory cytokines such as interleukin IL-1α and β, IL -2, IL-6, Il-17A interferon-γ inducible protein 10, monocyte chemoattractant protein-1, tumor necrosis factor -α and macrophage inflammatory protein-1α, which are associated with the occurrence of polyorgan inflammation and high mortality caused by SARS-CoV-2 (70). The current research results point out that CBD’s immunotherapeutic and anti-inflammatory properties may limit the cytokine storm and reduce the effects of exaggerated inflammation in patients with severe respiratory tract viral infections and ARDS often associated with COVID-19 (124).
To date, no studies about interactions between drugs used to treat SARS-CoV-2 infection and CBD have been published, but considering the inhibition of interleukin 6 receptor by Tocilizumab and the anti-inflammatory role of cannabidiol in the treatment of severe respiratory viral infections, a synergic, significant effect of these compounds on the reduction of inflammation in the acute course of COVID-19 can be expected (Figure 5).
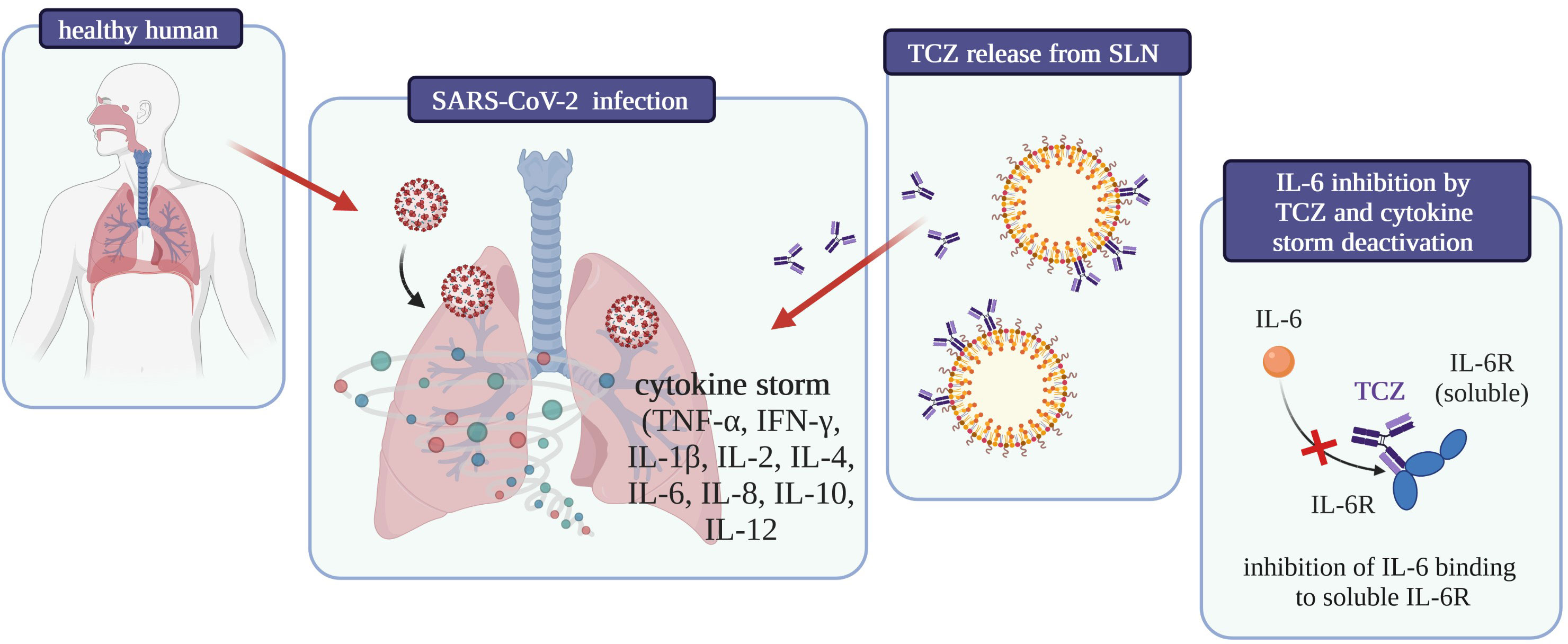
Figure 5 Graphical summary of the influence on COVID-19 treatment of tocilizumab-coated solid lipid nanoparticles [own drawing].
The constant mutations of SARS-CoV-2 make it imperative to identify an effective medication for patients suffering from COVID -19. The possibility of using cannabinoids to treat severe cases of this disease is increasingly being discussed.
The novel coronavirus binds to cellular receptors via angiotensin-converting enzyme 2 (ACE2), characteristic of pulmonary tissue, oral and nasal mucosa, kidneys, IG, and testicles. Furthermore, smokers and patients with chronic obstructive pulmonary disease have been reported to be more susceptible to COVID-19 and develop a severe form of the illness, as they present a high level of ACE2 expression. Therefore, it is believed that ACE2 expression in the oral, respiratory, and intestinal epithelium may provide SARS-CoV-2 with a vital entry point into the host cells, and ACE2 modulation in these tissues may limit SARS-CoV-2 binding to ACE2 receptors and thus reduce susceptibility to COVID-19 (57, 132–134). A study by Wang et al. on artificial human 3D models of the tissues mentioned above allowed these authors to identify 13 extracts from Cannabis sativa with a significant amount of CBD, which affects the expression of the ACE2 gene and the level of the ACE2 protein. Preliminary experiments also demonstrated that the extracts studied may decrease the concentration of the transmembrane protease serine 2 (TMPRSS2), a protein essential in the process of the viruses entering into cells (57).
The cannabinoid system consists of two cannabinoid receptors: CB1, present in the central nervous system (CNS), and CB2, in the immune system. Various ligands activate cannabinoid receptors: endogenous (anandamide, AEA; 2-arachidonoylglycerol, 2-AG), exogenous (e.g., phytocannabinoids from Cannabis sativa L.), or synthetic (135). The most critical phytocannabinoids include psychoactive delta-9-tetrahydrocannabinol (THC) and non-psychoactive cannabidiol (CBD). In contrast to THC, a partial antagonist of both CB1 and CB2 receptors, CBD is a partial antagonist of CB2 and only a weak antagonist of CB1 (136–138). Other cannabinoids are found in dried cannabis in much lower amounts. Cannabigerol and cannabichromene inhibit AEA re-uptake (139). The MOA of numerous other phytocannabinoids, such as cannabidivarin, cannabidiol, or cannabielsoin, has not been fully explored yet (138). When developing an adequate preparation based on cannabis flower extracts, it must be remembered that cannabis contains over 100 identified cannabinoids, Δ9-THC and CBD being the best-known ones, and other compounds like terpenes (140, 141). Terpenes and cannabinoids may interact with each other, affecting a given extract’s overall therapeutic effect. It is assumed that a quote is more potent than a single compound; therefore, it is essential to study the impact of a whole extract obtained from a plant rather than a single compound (142, 143). It has been known that anandamide (AEA) endocannabinoid, an endogenous antagonist with a high affinity to CB1, decreases IL-6 production. In contrast, THC, a partial antagonist of CB1 and CB2, inhibits IL-12 and IFN-γ release (144–148). Another phytocannabinoid (E)-β-caryophyllene [(E)-BCP], as a functional antagonist of CB2, inhibits both the production of pro-inflammatory cytokines in the peripheral blood induced by lipopolysaccharides (LPS) and the LPS-incited phosphorylation of Erk1/2 and JNK1/2 in monocytes (149–151). CBD impedes the expression of Il-6, IL-8, and TNF-α in in vitro models of allergic contact dermatitis and bone and joint inflammation. On the other hand, delta-9-tetrahydrocannabinol reduces the release of TNF-α, IL-1β, IL-6, and IL-8 in MG63 cells incited with LPS, which points to an essential role of the CB2 receptor in the anti-inflammatory response (152, 153). COVID-19 patients show macrophages, monocytes, and low levels of lymphocytes accountable for acute lung injury and leading to acute respiratory distress syndrome or even death. There are two groups of macrophages involved in the inflammatory response, cytokine production, phagocytosis, cell proliferation, and tissue repair: classically activated macrophages (M1) and alternatively activated macrophages (M2) (154–157). CB2 receptors are known as macrophage polarization regulators in inflammation. The use of an antagonist reduces the proliferation of inflammation-stimulating macrophages (M1) and increases the commonness of the second type of macrophages, which have an opposite effect (M2) (148, 158).
In SARS-CoV2 infection, there is a change in cytokine production, very similar to a cytokine storm, accompanied by excessive release of immune cells. Mesenchymal stromal cells (MSCs) have an anti-inflammatory effect. Their use may decrease the production of inflammatory-inducing compounds, which could improve the condition of the lungs previously damaged by, e.g., the flu virus (64, 159–161). MSCs raise the level of peripheral lymphocytes, simultaneously lowering the number of immune cells producing cytokines. Furthermore, MSCs produce leukemia inhibitory factor (LIF), which helps counteract the cytokine storm in viral pneumonia and stimulates CB2 receptors (162–164). The proposed MSC treatment for COVID-19 patients and proper stimulation of CB2 receptors will allow for the repair of damaged stem cells and immune response stimulation. It has been observed that MSCs do not stimulate the synthesis of ACE2 and TMPRSS2 proteins, which are associated with SARS-CoV-2 infection (165–167).
It was demonstrated that, in comparison to women, men are more prone to SARS-CoV-2 infection, which lower estrogen concentrations could explain. This beneficial property of estrogens and the immunosuppressive effect, which reduces excessive inflammation, may be associated with the CB2 cannabinoid receptor, a well-known immune response modulator (168–172).
Selective stimulation of CB2 may limit inflammation in COVID-19 patients through inflammatory cascade control in several checkpoints by reducing cytokine production, limiting immune cell proliferation, or producing antibodies, thus eliminating acute immune response (148, 173, 174). Currently, there are no CB2 antagonists approved for human use. Therefore, while searching for a commercially available CB2 receptor inhibitor is ongoing, treatment with CBD may be an alternative solution for COVID-19 patients (148).
Numerous experimental studies on rodents have shown that CB1 activation is essential for an effective immune response in bacterial infections, whereas CB2 activation prevents further damage caused by inflammation in sepsis due to an immunosuppressive effect (175). El Biali et al. (2020) (175) report a few human studies identifying potential relationships between the endocannabinoid system and the immune response. For instance, it was found that a genetic polymorphism in CB2 (CBQ63R), which reduces CB2 responses, can be linked with a higher probability of hospitalization in small children infected with RSV (n = 83), with the risk of developing severe ARTI being two times higher in allele Q carriers (OR = 2.148; 95% CI:1.09–4.22), and three times higher in the carriers of the QQ genotype (OR = 3.28; 95% CI: 1.22–8.71) (175, 176).
The FDA approved two forms of delta-9-tetrahydrocannabinol (dronabinol and nabilone) for treating some of the adverse effects of chemotherapy (i.e., nausea and vomiting) and for recovery of the appetite in wasting diseases like AIDS. In 2018, CBD was permitted for treating two types of pediatric epilepsy: Dravet syndrome and Lennox-Gastaut syndrome. Apart from these four indications, the most solid evidence of using cannabinoids with desired therapeutic outcomes is observed in chronic pain (including neuropathic pain) and MS-related muscle spasticity (125). Numerous in vitro and in vivo studies on animal and human cells suggest that CBD has an immunosuppressive and anti-inflammatory effect through direct inhibition of microglial cells and T cells, induction of apoptosis in regulatory T cells, or through myeloid-derived regulatory T cell induction (70). In vitro studies in animals suggest that Cannabis sativa extracts have an anti-bacterial and limited anti-fungal properties (177). A study conducted among healthy volunteers (n = 10) demonstrated that a dose of 30 mg of water-soluble or fat-soluble CBD significantly decreased the TNF level in peripheral blood mononuclear cells stimulated with bacterial lipopolysaccharide (178).
To date, there have been no studies investigating the effect of cannabinoids on SARS-CoV-2 infection. Furthermore, there is no epidemiological data on COVID-19 incidence in people using cannabinoids for medical or recreational purposes. A paper by Esposito lists four features of CBD that warrant its use, namely: 1) Cannabis sativa extracts have been proven to regulate the expression of two receptors that are of crucial importance for SARS-CoV-2 in a cellular model, 2) it has been demonstrated that CBD has an extensive range of immunomodulatory and anti-inflammatory effects, which may reduce the over-production of that leads to acute lung injury, 3) as a PPARγ antagonist, CBD may present a direct anti-viral effect, 4) as a PPARγ antagonist, CBD may inhibit the process of pulmonary fibrosis (179).
The characteristic feature of severe SARS-CoV-2 infection is the uncontrolled release of cytokines IL-1β, IL-6, and CCL2, and pro-inflammatory molecules, together with a decrease in the number of NK (natural killer) cells that may cause the cytokine storm. There is much to suggest that the severe course of infection does not stem from the viremia per se but depends on the degree of immune dysregulation. To limit mortality in severe cases of COVID-19, it is necessary to develop new therapeutic options to mitigate the cytokine storm (180). Esposito et al. (2020) indicate that due to the rapid spread of the pandemic, the ideal drug candidate should already be used in treating other diseases, have a good safety profile, and act to mitigate the cytokine storm through immunomodulation rather than immunosuppression (179). Recently, it was shown that Cannabis sativa extracts with a high concentration of CBD down-regulate the activity of ACE2 and TMPRSS2 enzymes, which are vital for SARS-CoV-2 to enter the human body (181). In recent years, CBD has been the subject of much research due to its broad spectrum of therapeutic effects, including anti-seizure, calming, sleep-inducing, anti-psychotic, anti-cancer, anti-inflammatory, and neuroprotective effects (182). What has been emphasized is the lack of adverse psychotropic effects of cannabidiol and its favorable safety profile in humans (182). The pharmacological activity of CBD was tested in patients suffering from various conditions, including respiratory diseases characterized by acute lung injury (179). CBD effect on adenosine A2A receptors limited leukocyte migration to the lungs, which was accompanied by a significant decrease in pro-inflammatory cytokine (TNF-α and IL-6) and chemokine (MCP-1/MIP-2/CXCL2) release, thus clearly improving the compromised lung function (131, 183).
CBD has also been studied as a molecule with a potential anti-viral effect. CBD acts by interacting with nuclear hormone receptors PPAR. It was shown that down-regulation of PPARγ expression by alveolar macrophages significantly reduces lung inflammation and enhances regeneration after viral respiratory tract infections (184). Preventive and therapeutic administration of PPARγ antagonists decreased morbidity and mortality related to influenza A virus infection (185). However, the use of full PPARγ agonists has several adverse effects, including the risk of cardiovascular complications, cardiac insufficiency, and stroke. It needs to be verified whether CBD, as a weak antagonist of PPARγ, could be used without causing such adverse effects (179). There is no direct proof of the anti-viral activity of cannabinoids in viral infections. Such products have been described in vitro studies. Lowe et al. demonstrated an anti-viral effect of CBD against the hepatitis C virus (HCV), but not against HBV, in cell lines for producing these viruses (128). Anti-viral activity of CBD was also confirmed against Kaposi’s sarcoma-associated herpesvirus (KSHV) in a model of KSHV-infected human dermal microvascular endothelial cells (HMVECs) (129). In yet another study, CBD mitigated the effects of neuroinflammation induced by Theiler’s murine encephalomyelitis virus (TMEV) (186). Respiratory syncytial virus (RSV) used in the mouse model has indicated that CB2 activation reduced infection symptoms, and CB1 antagonist administration alleviated pulmonary complications (176).
Research has shown that CBD is a reasonably safe molecule (187). In the case of COVID-19 patients, it is essential to establish the toxicity profile of CBD when administered concomitantly with other drugs used in the current anti-COVID-19 protocols.
First, pharmacokinetic (PK) and pharmacodynamic (PD) interactions between cannabinoids and experimental COVID-19 drugs must be determined. Both Δ9-THC and CBD are lipophilic, highly protein-bound molecules. They have a long half-life, undergo bioaccumulation, and share metabolic pathways with cytochrome P450, drug transporters (e.g., breast cancer resistance protein), and substrates that bind to plasma proteins (127). Furthermore, PK (e.g., warfarin and clobazam) and PD (e.g., valproic acid) interactions were described for THC and CBD (188). Land et al. (2020) (127) compiled a table with 16 compounds examined for their potential use in COVID-19 treatment and their possible pharmacokinetic or pharmacodynamic interactions with cannabinoids.
They found that most candidate drugs could interact with THC and CBD. The authors propose that COVID-19 patients should be asked about their use of substances containing cannabinoids, as these may significantly affect their reaction to the selected treatment method (127).
Esposito points to the possibility of testing the therapeutic potential of CBD in COVID-19 patients at the beginning of the disease to suppress the cytokine storm, prevent the danger of respiratory failure, or assess the effect of CBD on pulmonary fibrosis. The central aspect of being clarified is dosing. In the case of HIV and post-Ebola syndrome, CBD was used as an agent controlling immune activation at doses of 10–20 mg · kg−1 · day−1 and 1.7–10 mg · kg−1 · day−1 (100 mg · day−1 titrating up to 600 mg · day−1) (189, 190).
The results of pre-clinical studies are encouraging. However, evidence is needed to approve cannabidiol as a supportive drug in COVID-19 treatment.
8 Lipid nanoparticles as carriers for proteins and monoclonal antibodies
Lipid nanoparticles (LNPs) are composed of biodegradable and biocompatible lipids (191, 192), and they can be successfully proposed to encapsulate proteins (193–198). The literature describes two classical types of lipid nanoparticles, namely:
● solid lipid nanoparticles (SLN), so-called “1st generation”,
● nanostructured lipid carriers (NLC), so-called “2nd generation”.
SLN and NLC are known to increase the bioavailability of loaded drugs administered by different routes (199, 200). In contrast to SLN, the lipid matrix of NLC consists of a mixture of solid and liquid lipids (that melt above 40°C). The matrix originates a less-ordered matrix with the capacity to load a higher drug amount than SLN, preventing its leakage during storage and allowing a more flexible drug release modulation (201). SLN contains only solid lipids in its matrix, offering the capacity to modulate the release profile of loaded drugs. SLN and NLC require surfactants (e.g., poloxamers, tweens) to stabilize the lipid matrices in aqueous dispersion (202, 203). Acylglycerols, waxes, fatty acids, and hard fats are the most commonly used lipids that should be approved by the Food and Drug Administration (191, 204).
8.1 Drug release form of SLN
There are three basic types of SLN, defined by the location of the drug in the lipid matrix (205, 206). Loaded drugs can be placed between fatty acid chains or between lipid layers. The final drug location affects its release mechanism from the lipid matrices (198).
The SLN type I is defined as the homogeneous matrix model, in which the drug is molecularly dispersed in the lipid core or amorphous clusters. This model is obtained when applying the hot, high-pressure homogenization (HPH) in an optimized drug and lipid ratio or when using the cold HPH. Due to their structure, SLN type I can show controlled release properties.
The SLN type II, or drug-enriched shell model, is obtained when applying the hot HPH technique and the low drug concentration in the melted lipid. During the cooling of the homogenized nanoemulsion, the lipid molecules precipitate first. Then, they lead to a steadily increasing drug concentration in the remaining lipid melt with an increased fraction of solidified lipid. A drug-free (or drug-reduced) lipid core is formed; when the drug reaches its saturation solubility in the remaining melt, an outer shell will solidify, containing both drug and lipid. This model is not suitable for prolonged drug release.
Nonetheless, it may be used to obtain a burst release of medicine and the occlusive properties of the lipid core. The SLN type III, or drug-enriched core model, is formed when the drug concentration is relatively close to or at its saturation solubility in the lipid melt. Under the nanoemulsion cooling, the drug’s solubility will decrease when the saturation solubility is exceeded. This model is also helpful for prolonged-release purposes (204, 207–209). SLN work as an absorption enhancer when orally administered (206, 210, 211). Types of SLN that can be obtained have been shown schematically in Figure 6.
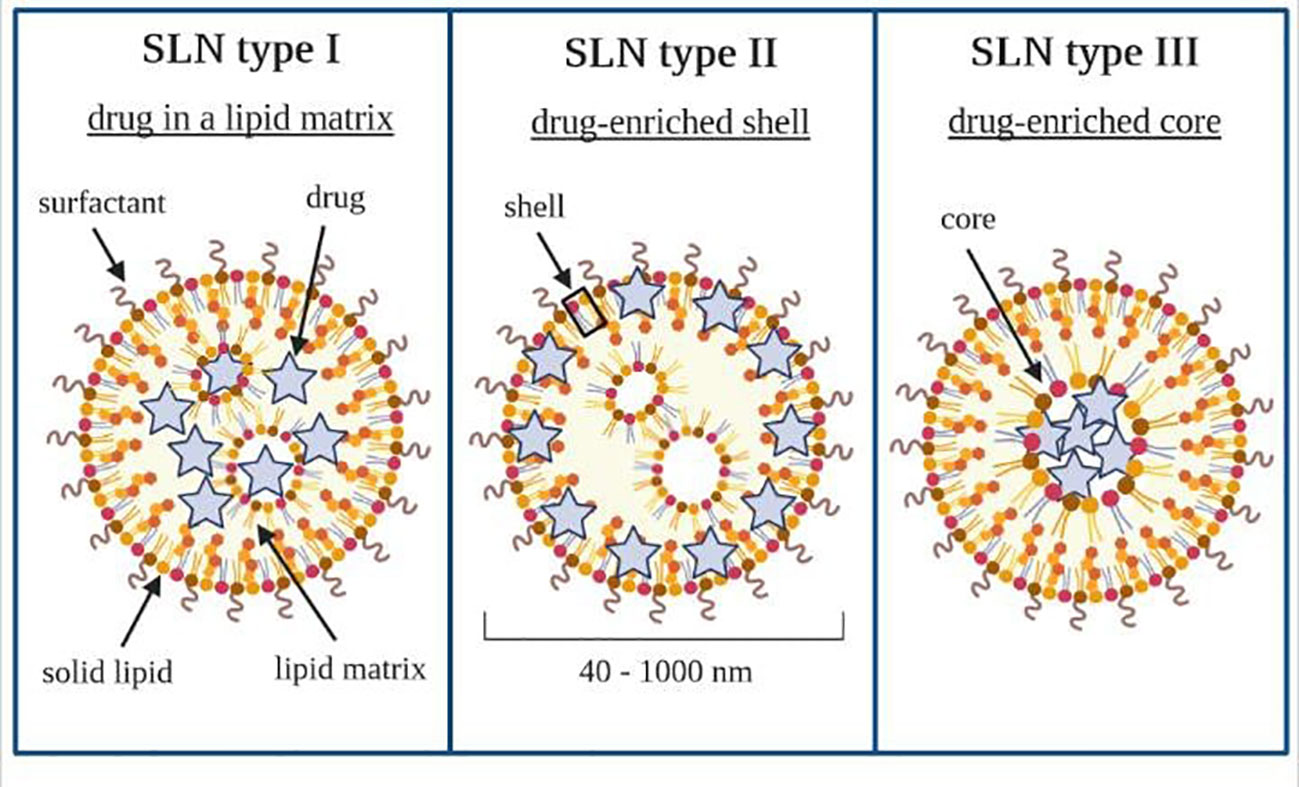
Figure 6 Types of solid lipid nanoparticles. Own drawing based on (206).
8.2 TCZ-coated SLN loaded with CBD
The use of CBD-based SLN as potential TCZ carriers for oral administration is mainly associated with the safety of LNPs and their ability to faster enteral administration with the increased bioavailability of both hydrophilic and lipophilic active substances. On the other hand, understanding the impact of the size and shape of LNPs on their distribution in the intestine can be used in developing improved drug delivery systems to treat COVID-19. The biodegradable lipid matrix of SLN undergoes enzymatic decomposition into components naturally occurring in the human body (191). The novel approach proposed by the authors is the oral form administration of TCZ and CBD simultaneously, which can significantly improve the comfort of patients who have previously used regular intravenous injections of mAb. Moreover, the obtained SLN may be an ideal carrier for TCZ/CBD because of the ability of LNPs to modify drug release, increase bioavailability and thus regulate pharmacological activity (212). The selection of the type of lipids to be used for the production of SLN is governed by the solubility of CBD in the solid lipid. We have recently developed a stabilized SLN formulation based on glycerol behenate for the loading of CBD. The surface tailoring with the mAb is usually carried out by biotinylating as described by Souto et al. (2019) (213). Due to the potential of SLN to delay drug release, there is a high probability of optimizing these nanocarriers for drug release in the colon, thereby protecting the gastrointestinal tract against the destructive influence of COVID-19 at the initial phase. It is worth underlining that a final enteric formulation would be developed for the delayed release of the actives into the colon by encapsulating drugs-loaded LNPs in gastro-resistant capsules to prevent earlier degradation of nanoparticles in the stomach (214).
The development of surface-modified SLN formulations for targeted delivery to the colon requires the production of gastro-resistant capsules in which the TCZ-coated CBD-loaded SLN dispersions are loaded. Enteric coatings for colonic administration exploit the pH differences along the gastrointestinal tract to release the drug only when reaching pH 6.0-7.0 in the colon. Polymethacrylates (e.g., Eudragit® brands) are typical polymers that coat tablets and capsules to protect the drugs from gastric and small intestinal pathways. They are commonly found in commercially available pharmaceutical formulations for ulcerative colitis and Crohn’s disease (215).
The combination of SLN and electroporation has been proposed to enhance drug transport to the colon. Cyanine–type IR 780 and the flavonoid derivative baicalein were co-loaded into SLN for both imaging and therapy of colorectal carcinoma (216). The authors reported that the presence of flavonoids contributed to reducing the dose and, thus, cytotoxicity in chemotherapy. Electroporation generates external electric field pulses and increases cell membrane permeability, which offers the opportunity for intracellular trafficking of the cargo (217). The p53 and manganese superoxide dismutase expression was significantly increased by electroporation, with substantially higher cytotoxicity.
9 Conclusion
In this review, scientific evidence is given about the added value of using cannabidiol (CBD) for the management SARS-CoV-2 infection. CBD was found to down-regulate the activity of ACE2 and TMPRSS2 enzymes, both governing the entry of SARS-CoV-2 in the human body. Besides reducing the secretion of cytokines, cannabidiol also promotes body protection against several pathogenic infections and is also expected to can play a similar role against COVID-19. On the other hand, solid lipid nanoparticles (SLN) are ideal carriers for the oral administration of drugs, provided that lipids are known to be absorption enhancers in the gastrointestinal tract. Given its lipophilic character, CBD is an appropriate candidate to be loaded into SLN. A synergistic effect is expected with the combination of tocilizumab (TCZ), as this monoclonal antibody is an anti-IL-6 receptor antibody in COVID-19 treatment. Indeed, cytokine-associated toxicity is linked to pro-inflammatory cytokine IL-6 released in severe COVID-19 infections. An innovative approach is thus proposed by exploiting the advantages of coating SLN with mAb for site specific targeting. To obtain a suitable pharmaceutical dosage form for the oral administration of TCZ-coated CBD-loaded SLN, further studies are suggested towards the optimization of gastro-resistant gelatin capsules in which the lipid nanoparticle formulations can be encapsulated.
Author contributions
AZ, PE, JK, and EBS were responsible for conceptualizing the manuscript. AZ, PE, JK, MaS, SH, KW, MiS, and EBS were responsible for writing and editing the paper. AD, AGA, RS, and ES were responsible for reviewing, while AZ was responsible for the visualization of the manuscript. All authors have made substantial contributions to the conception and design of the paper, drafting the article and revising it critically for important intellectual content, and final approval of the version to be submitted. All authors contributed to the article and approved the submitted version.
Funding
This research was also supported by the National Centre for Research and Development (grant number INNOMED/I/11/NCBR/2014) from the Innovative Economy Operational Programme funds within the framework of the European Regional Development Fund.
Acknowledgments
EBS acknowledged the national funds from FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences—UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1147991/full#supplementary-material
References
1. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (Covid-19) outbreak. J Autoimmun (2020) 109:102433. doi: 10.1016/j.jaut.2020.102433
2. Agrawal M, Saraf S, Saraf S, Murty US, Kurundkar SB, Roy D, et al. In-line treatments and clinical initiatives to fight against covid-19 outbreak. Respir Med (2020) 106192.
3. Malin JJ, Suárez I, Priesner V, Fätkenheuer G, Rybniker J. Remdesivir against covid-19 and other viral diseases. Clin Microbiol Rev (2020) 34(1):e00162–20. doi: 10.1128/CMR.00162-20
4. Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, et al. Remdesivir: A review of its discovery and development leading to emergency use authorization for treatment of covid-19. ACS Cent Sci (2020). doi: 10.20944/preprints202004.0299.v1
5. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of covid-19. New Engl J Med (2020). doi: 10.1056/NEJMoa2007764
6. Spinner CD, Gottlieb RL, Criner GJ, López JRA, Cattelan AM, Viladomiu AS, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate covid-19: A randomized clinical trial. Jama (2020) 324(11):1048–57. doi: 10.1001/jama.2020.16349
7. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe covid-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet (2020).
8. Organization WH. Solidarity” clinical trial for covid-19 treatments, in: World health organization (WHO) situation reports (2020). Geneva: WHO (Accessed 5 Apr 2020).
9. Aditya NP, Vathsala PG, Vieira V, Murthy RS, Souto EB. Advances in nanomedicines for malaria treatment. Adv Colloid Interface Sci (2013) 201-202:1–17. doi: 10.1016/j.cis.2013.10.014
10. Abella BS, Jolkovsky EL, Biney BT, Uspal JE, Hyman MC, Frank I, et al. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure sars-Cov-2 prophylaxis among health care workers: A randomized clinical trial. JAMA Internal Med (2020). doi: 10.1001/jamainternmed.2020.6319
11. Axfors C, Schmitt AM, Janiaud P, van’t Hooft J, Abd-Elsalam S, Abdo EF, et al. Mortality outcomes with hydroxychloroquine and chloroquine in covid-19: An international collaborative meta-analysis of randomized trials. Nat Commun (2021) 12(1):2349. doi: 10.1038/s41467-021-22446-z
12. Horby PW, Mafham M, Bell JL, Linsell L, Staplin N, Emberson J, et al. Lopinavir–ritonavir in patients admitted to hospital with covid-19 (Recovery): A randomised, controlled, open-label, platform trial. Lancet (2020) 396(10259):1345–52. doi: 10.1016/S0140-6736(20)32013-4
13. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. New Engl J Med (2020) 382(19):1787–99. doi: 10.1056/NEJMoa2001282
14. Zhao H, Zhu Q, Zhang C, Li J, Wei M, Qin Y, et al. Tocilizumab combined with favipiravir in the treatment of covid-19: A multicenter trial in a small sample size. Biomed Pharmacother (2020) 133:110825. doi: 10.1016/j.biopha.2020.110825
15. Drożdżal S, Rosik J, Lechowicz K, Machaj F, Kotfis K, Ghavami S, et al. Fda approved drugs with pharmacotherapeutic potential for sars-Cov-2 (Covid-19) therapy. Drug Resist Updates (2020) 100719.
16. Huang D, Yu H, Wang T, Yang H, Yao R, Liang Z. Efficacy and safety of umifenovir for coronavirus disease 2019 (Covid-19): A systematic review and meta-analysis. J Med Virol (2020). doi: 10.1002/jmv.26256
17. Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: A retrospective study. Clin Microbiol Infect (2020).
18. Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with covid-19: An open-label, randomised, phase 2 trial. Lancet (2020) 395(10238):1695–704. doi: 10.1016/S0140-6736(20)31042-4
19. Tong S, Su Y, Yu Y, Wu C, Chen J, Wang S, et al. Ribavirin therapy for severe covid-19: A retrospective cohort study. Int J Antimicrob Agents (2020) 56(3):106114.
20. Khalili JS, Zhu H, Mak NSA, Yan Y, Zhu Y. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning covid-19. J Med Virol (2020).
22. Chang Y-W, Yeh T-K, Lin K-T, Chen W-C, Yao H-T. Pharmacokinetics of anti-Sars-Cov agent niclosamide and its analogs in rats. J Food Drug Anal (2006) 14(4). doi: 10.38212/2224-6614.2464
23. Wu C-J, Jan J-T, Chen C-M, Hsieh H-P, Hwang D-R, Liu H-W, et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrobial Agents Chemother (2004) 48(7):2693–6. doi: 10.1128/AAC.48.7.2693-2696.2004
24. Pindiprolu SKS, Pindiprolu SH. Plausible mechanisms of niclosamide as an antiviral agent against covid-19. Med Hypotheses (2020) 109765. doi: 10.1016/j.mehy.2020.109765
26. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with covid-19-Preliminary report. New Engl J Med (2020). doi: 10.1101/2020.06.22.20137273
27. Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe covid-19: The remap-cap covid-19 corticosteroid domain randomized clinical trial. Jama (2020) 324(13):1317–29.
28. Dequin P-F, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with covid-19: A randomized clinical trial. Jama (2020) 324(13):1298–306. doi: 10.1001/jama.2020.16761
29. Furtado RH, Berwanger O, Fonseca HA, Corrêa TD, Ferraz LR, Lapa MG, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe covid-19 in Brazil (Coalition ii): A randomised clinical trial. Lancet (2020) 396(10256):959–67. doi: 10.1016/S0140-6736(20)31862-6
30. Hermine O, Mariette X, Tharaux P-L, Resche-Rigon M, Porcher R, Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with covid-19 and moderate or severe pneumonia: A randomized clinical trial. JAMA Internal Med (2020). doi: 10.1001/jamainternmed.2021.2209
31. Alvi MM, Sivasankaran S, Singh M. Pharmacological and non-pharmacological efforts at prevention, mitigation, and treatment for covid-19. J Drug Target (2020) 28(7-8):742–54. doi: 10.1080/1061186X.2020.1793990
32. Chakraborty C, Sharma AR, Bhattacharya M, Sharma G, Lee SS, Agoramoorthy G. Covid-19: Consider Il6 receptor antagonist for the therapy of cytokine storm syndrome in sars-Cov-2 infected patients. J Med Virol (2020). doi: 10.1002/jmv.26078
33. Caracciolo M, Macheda S, Labate D, Tescione M, La Scala S, Vadalà E, et al. Case report: Canakinumab for the treatment of a patient with covid-19 acute respiratory distress syndrome. Front Immunol (2020) 11:1942. doi: 10.3389/fimmu.2020.01942
34. Sheng CC, Sahoo D, Dugar S, Prada RA, Wang TKM, Abou Hassan OK, et al. Canakinumab to reduce deterioration of cardiac and respiratory function in sars-Cov-2 associated myocardial injury with heightened inflammation (Canakinumab in covid-19 cardiac injury: The three c study). Clin Cardiol (2020) 43(10):1055–63. doi: 10.1002/clc.23451
35. Navarro-Millán I, Sattui SE, Lakhanpal A, Zisa D, Siegel CH, Crow MK. Use of anakinra to prevent mechanical ventilation in severe covid-19: A case series. Arthritis Rheumatol (2020). doi: 10.1002/art.41422
36. Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with covid-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol (2020). doi: 10.1016/S2665-9913(20)30127-2
37. Cantini F, Niccoli L, Nannini C, Matarrese D, Di Natale ME, Lotti P, et al. Beneficial impact of baricitinib in covid-19 moderate pneumonia; multicentre study. J Infect (2020) 81(4):647–79. doi: 10.1016/j.jinf.2020.06.052
38. Favalli EG, Biggioggero M, Maioli G, Caporali R. Baricitinib for covid-19: A suitable treatment? Lancet Infect Dis (2020).
39. Yeleswaram S, Smith P, Burn T, Covington M, Juvekar A, Li Y, et al. Inhibition of cytokine signaling by ruxolitinib and implications for covid-19 treatment. Clin Immunol (2020), 108517.
40. Bagca BG, Avci CB. The potential of Jak/Stat pathway inhibition by ruxolitinib in the treatment of covid-19. Cytokine Growth Factor Rev (2020) 54:51.
41. Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (Covid-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol (2020).
42. Roschewski M, Lionakis MS, Sharman JP, Roswarski J, Goy A, Monticelli MA, et al. Inhibition of bruton tyrosine kinase in patients with severe covid-19. Sci Immunol 2020):5(48).
43. Jodele S, Köhl J. Tackling covid-19 infection through complement-targeted immunotherapy. Br J Pharmacol (2020). doi: 10.1111/bph.15187
44. Smith K, Pace A, Ortiz S, Kazani S, Rottinghaus S. A phase 3 open-label, randomized, controlled study to evaluate the efficacy and safety of intravenously administered ravulizumab compared with best supportive care in patients with covid-19 severe pneumonia, acute lung injury, or acute respiratory distress syndrome: A structured summary of a study protocol for a randomised controlled trial. Trials (2020) 21(1):639. doi: 10.1186/s13063-020-04548-z
45. Bezzio C, Manes G, Bini F, Pellegrini L, Saibeni S. Infliximab for severe ulcerative colitis and subsequent sars-Cov-2 pneumonia: A stone for two birds. Gut (2020). doi: 10.1136/gutjnl-2020-321760
46. Tursi A, Angarano G, Monno L, Saracino A, Signorile F, Ricciardi A, et al. Covid-19 infection in crohn’s disease under treatment with adalimumab. Gut (2020) 69(7):1364–5. doi: 10.1136/gutjnl-2020-321240
47. Rizk JG, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Forthal DN. Pharmaco-immunomodulatory therapy in covid-19. Drugs (2020) 80(13):1267–92. doi: 10.1007/s40265-020-01367-z
48. J Gómez-Rial M-T. A strategy targeting monocyte-macrophage differentiation to avoid pulmonary complications in sars-Cov2 infection. In: Clinical immunology. Orlando, Fla (2020).
49. Bonaventura A, Vecchié A, Wang TS, Lee E, Cremer PC, Carey B, et al. Targeting gm-csf in covid-19 pneumonia: Rationale and strategies. Front Immunol (2020) 11:1625. doi: 10.3389/fimmu.2020.01625
50. Mehta P, Porter JC, Manson JJ, Isaacs JD, Openshaw PJM, McInnes IB, et al. Therapeutic blockade of granulocyte macrophage colony-stimulating factor in covid-19-Associated hyperinflammation: Challenges and opportunities. Lancet Respir Med (2020) 8(8):822–30. doi: 10.1016/s2213-2600(20)30267-8
51. Melody M, Nelson J, Hastings J, Propst J, Smerina M, Mendez J, et al. Case report: Use of lenzilumab and tocilizumab for the treatment of coronavirus disease 2019. Immunotherapy (2020) 12(15):1121–6. doi: 10.2217/imt-2020-0136
52. Yang B, Fulcher JA, Ahn J, Berro M, Goodman-Meza D, Dhody K, et al. Clinical characteristics and outcomes of covid-19 patients receiving compassionate use leronlimab. Clin Infect Dis An Off Publ Infect Dis Soc America (2020). doi: 10.1093/cid/ciaa1583
53. Mahase E. Covid-19: Fda authorises neutralising antibody bamlanivimab for non-admitted patients. BMJ (2020) 371:m4362. doi: 10.1136/bmj.m4362
54. Mahase E. Covid-19: Experts advise cautious optimism for neutralising antibodies after early results. BMJ (2020) 371:m3937. doi: 10.1136/bmj.m3937
55. Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. Sars-Cov-2 neutralizing antibody ly-Cov555 in outpatients with covid-19. N Engl J Med (2020). doi: 10.1056/NEJMoa2029849
56. Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. The cytokine release syndrome (Crs) of severe covid-19 and interleukin-6 receptor (Il-6r) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents (2020) 105954.
57. Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. Clinical characteristics of refractory covid-19 pneumonia in wuhan, China. Clin Infect Dis (2020) 73(11):e4208–e4213. doi: 10.1093/cid/ciaa270
58. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The covid-19 cytokine storm; what we know so far. Front Immunol (2020) 11:1446. doi: 10.3389/fimmu.2020.01446
59. Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med (2020) 382(18):1708–20.
60. Siddiqi HK, Mehra MR. Covid-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant (2020) 39(5):405.
61. Ali A, Kamjani MH, Kesselman MM. The role of tocilizumab in cytokine storm and improving outcomes in covid-19. Recent Pat Antiinfect Drug Discov (2020).
62. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of covid-19 in new York city. New Engl J Med (2020).
63. Saha A, Sharma AR, Bhattacharya M, Sharma G, Lee S-S, Chakraborty C. Tocilizumab: A therapeutic option for the treatment of cytokine storm syndrome in covid-19. Arch Med Res (2020).
64. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. Covid-19: Consider cytokine storm syndromes and immunosuppression. Lancet (London England) (2020) 395(10229):1033. doi: 10.1016/S0140-6736(20)30628-0
65. Narain S, Stefanov DG, Chau AS, Weber AG, Marder G, Kaplan B, et al. Comparative survival analysis of immunomodulatory therapy for coronavirus disease 2019 cytokine storm. Chest (2020). doi: 10.2139/ssrn.3627337
66. Alanagreh L, Alzoughool F, Atoum M. The human coronavirus disease covid-19: Its origin, characteristics, and insights into potential drugs and its mechanisms. Pathogens (2020) 9(5):331. doi: 10.3390/pathogens9050331
67. Ul Qamar TM, Alqahtani SM, Alamri MA, Chen LL. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal (2020) 10(4):313–9. doi: 10.1016/j.jpha.2020.03.009
68. Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S. Potential inhibitor of covid-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints (2020) 20944:1–14. doi: 10.20944/preprints202003.0226.v1
69. Shaghaghi N. Molecular docking study of novel covid-19 protease with low risk terpenoides compounds of plants. ChemRxiv (2020) 10:1–9. doi: 10.26434/chemrxiv.11935722.v1
70. Nichols JM, Kaplan BL. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res (2020) 5(1):12–31. doi: 10.1089/can.2018.0073
71. Brown JD. Cannabidiol as prophylaxis for sars-Cov-2 and covid-19? unfounded claims versus potential risks of medications during the pandemic. Res Soc Administrative Pharmacy: RSAP (2021) 17(1):2053. doi: 10.1016/j.sapharm.2020.03.020
72. Nelson KM, Bisson J, Singh G, Graham JG, Chen S-N, Friesen JB, et al. The essential medicinal chemistry of cannabidiol (Cbd). J Med Chem (2020) 63(21):12137–55. doi: 10.1021/acs.jmedchem.0c00724
73. Costiniuk CT, Jenabian M-A. Acute inflammation and pathogenesis of sars-Cov-2 infection: Cannabidiol as a potential anti-inflammatory treatment? Cytokine Growth Factor Rev (2020) 53:63–65. doi: 10.1016/j.cytogfr.2020.05.008
74. Ingraham NE, Lotfi-Emran S, Thielen BK, Techar K, Morris RS, Holtan SG, et al. Immunomodulation in covid-19. Lancet Respir Med (2020) 8(6):544–6. doi: 10.1016/S2213-2600(20)30226-5
75. Cennimo D. Coronavirus disease 2019 (Covid-19) treatment & management: Approach considerations, medical care, prevention. Medscape Online (2020). Available at: https://emedicine.medscape.com/article/2500114-treatment?reg=1.
76. Rosas IO, Bräu N, Waters M, Go RC, Malhotra A, Hunter BD, et al. Tocilizumab in patients hospitalised with COVID-19 pneumonia: Efficacy, safety, viral clearance, and antibody response from a randomised controlled trial (COVACTA). EClinicalMedicine (2022) 47:101409. doi: 10.1016/j.eclinm.2022.101409
77. Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med (2021) 384(1):20–30. doi: 10.1056/NEJMoa2030340
78. Price CC, Altice FL, Shyr Y, Koff A, Pischel L, Goshua G, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized patients with coronavirus disease 2019: Survival and clinical outcomes. Chest (2020) 158(4):1397–408. doi: 10.1016/j.chest.2020.06.006
79. Sloand JN, Nguyen TT, Zinck SA, Cook EC, Zimudzi TJ, Showalter SA, et al. Ultrasound-guided cytosolic protein delivery Via transient fluorous masks. ACS Nano (2020) 14(4):4061–73. doi: 10.1021/acsnano.9b08745
80. Mayer K. Nonviral crispr delivery vehicles lay the smart siege: Lipid-and polymer-based nanoparticles trust in guile more than force, so they're less likely to arouse immune resistance or wreak collateral damage. Genet Eng Biotechnol News (2020) 40(S4):S15–S9. doi: 10.1089/gen.40.S4.05
81. Liu J, Chang J, Jiang Y, Meng X, Sun T, Mao L, et al. Fast and efficient Crispr/Cas9 genome editing in vivo enabled by bioreducible lipid and messenger rna nanoparticles. Adv Mater (2019) 31(33):1902575. doi: 10.1002/adma.201902575
82. Chen G, Abdeen AA, Wang Y, Shahi PK, Robertson S, Xie R, et al. A biodegradable nanocapsule delivers a Cas9 ribonucleoprotein complex for in vivo genome editing. Nat Nanotechnol (2019) 14(10):974–80. doi: 10.1038/s41565-019-0539-2
83. Zhang X, Zhao W, Nguyen GN, Zhang C, Zeng C, Yan J, et al. Functionalized lipid-like nanoparticles for in vivo mrna delivery and base editing. Sci Adv (2020) 6(34):eabc2315. doi: 10.1126/sciadv.abc2315
84. Souto EB, Souto SB, Zielinska A, Durazzo A, Lucarini M, Santini A, et al. Perillaldehyde 1,2-epoxide loaded sln-tailored mab: Production, physicochemical characterization and in vitro cytotoxicity profile in mcf-7 cell lines. Pharmaceutics (2020) 12(2):161. doi: 10.3390/pharmaceutics12020161
85. Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of Raav1-aat gene therapy. Proc Natl Acad Sci (2009) 106(38):16363–8. doi: 10.1073/pnas.0904514106
86. Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of luxturna (and zolgensma and glybera): Where are we, and how did we get here? Annu Rev Virol (2019) 6:601–21. doi: 10.1146/annurev-virology-092818-015530
87. Samanta A, Aziz AA, Jhingan M, Singh SR, Khanani A, Chhablani J. Emerging therapies in neovascular age-related macular degeneration in 2020. Asia-Pac J Ophthalmol (2020) 9(3):250–9. doi: 10.1097/APO.0000000000000291
88. Li S, Datta S, Brabbit E, Love Z, Woytowicz V, Flattery K, et al. Nr2e3 is a genetic modifier that rescues retinal degeneration and promotes homeostasis in multiple models of retinitis pigmentosa. Gene Ther (2021) 28(5):223–41. doi: 10.1038/s41434-020-0134-z
89. Bonaventura J, Eldridge MA, Hu F, Gomez JL, Sanchez-Soto M, Abramyan AM, et al. High-potency ligands for dreadd imaging and activation in rodents and monkeys. Nat Commun (2019) 10(1):1–12. doi: 10.1038/s41467-019-12236-z
90. Leborgne C, Barbon E, Alexander JM, Hanby H, Delignat S, Cohen DM, et al. Igg-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-aav neutralizing antibodies. Nat Med (2020) 26(7):1096–101. doi: 10.1038/s41591-020-0911-7
91. Pedersen NC. An update on feline infectious peritonitis: Virology and immunopathogenesis. Vet J (2014) 201(2):123–32. doi: 10.1016/j.tvjl.2014.04.017
92. Morris KV. The improbability of the rapid development of a vaccine for sars-Cov-2. Mol Ther (2020) 28(7):1548–9. doi: 10.1016/j.ymthe.2020.06.005
93. Tang Q, Li B, Woodle M, Lu PY. Application of sirna against sars in the rhesus macaque model. Rnai (2008) 442:139–58. doi: 10.1007/978-1-59745-191-8_11
94. Casucci M, Falcone L, Camisa B, Norelli M, Porcellini S, Stornaiuolo A, et al. Extracellular ngfr spacers allow efficient tracking and enrichment of fully functional car-T cells Co-expressing a suicide gene. Front Immunol (2018) 9:507. doi: 10.3389/fimmu.2018.00507
95. Wu C-J, Huang H-W, Liu C-Y, Hong C-F, Chan Y-L. Inhibition of sars-cov replication by sirna. Antiviral Res (2005) 65(1):45–8. doi: 10.1016/j.antiviral.2004.09.005
96. Ahn D-G, Lee W, Choi J-K, Kim S-J, Plant EP, Almazán F, et al. Interference of ribosomal frameshifting by antisense peptide nucleic acids suppresses sars coronavirus replication. Antiviral Res (2011) 91(1):1–10. doi: 10.1016/j.antiviral.2011.04.009
97. Shi Y, Luo H, Jia J, Xiong J, Yang D, Huang B, et al. Antisense downregulation of sars-cov gene expression in vero E6 cells. J Gene Med (2005) 7(1):97–107. doi: 10.1002/jgm.640
98. Peeples L. News feature: Avoiding pitfalls in the pursuit of a covid-19 vaccine. Proc Natl Acad Sci (2020) 117(15):8218–21. doi: 10.1073/pnas.2005456117
99. Donia A, Bokhari H. Rna interference as a promising treatment against sars-Cov-2. Int Microbiol (2021) 24(1):123–4. doi: 10.1007/s10123-020-00146-w
100. Conforti A, Marra E, Roscilli G, Palombo F, Ciliberto G, Aurisicchio L. Are genetic vaccines the right weapon against covid-19? Mol Ther (2020) 28(7):1555–6. doi: 10.1016/j.ymthe.2020.06.007
101. Liu MA. A comparison of plasmid DNA and mrna as vaccine technologies. Vaccines (2019) 7(2):37. doi: 10.3390/vaccines7020037
102. Lurie N, Saville M, Hatchett R, Halton J. Developing covid-19 vaccines at pandemic speed. New Engl J Med (2020) 382(21):1969–73. doi: 10.1056/NEJMp2005630
103. Piyush R, Rajarshi K, Chatterjee A, Khan R, Ray S. Nucleic acid-based therapy for coronavirus disease 2019. Heliyon (2020) 6(9):e05007. doi: 10.1016/j.heliyon.2020.e05007
104. Chen J, Tang Y, Liu Y, Dou Y. Nucleic acid-based therapeutics for pulmonary diseases. AAPS PharmSciTech (2018) 19(8):3670–80. doi: 10.1208/s12249-018-1183-0
105. Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe covid-19: A retrospective cohort study. Lancet Rheumatol (2020) 2(8):e474–e84. doi: 10.1016/S2665-9913(20)30173-9
106. Łodyga M, Eder P, Bartnik W, Gonciarz M, Kłopocka M, Linke K, et al. New pharmaceuticals in inflammatory bowel disease. Przeglad Gastroenterol (2015) 10(2):57.
107. Hennigan S, Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manage (2008) 4(4):767.
108. Consortium I-RMRA. The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet (2012) 379(9822):1214–24.
109. Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, et al. Cytokine storm in Covid-19–immunopathological mechanisms, clinical considerations, and therapeutic approaches: The reprogram consortium position paper. Front Immunol (2020) 11:1648. doi: 10.3389/fimmu.2020.01648
110. Scott LJ. Tocilizumab: A review in rheumatoid arthritis. Drugs (2017) 77(17):1865–79. doi: 10.1007/s40265-017-0829-7
111. Schulert GS. Can tocilizumab calm the cytokine storm of covid-19? Lancet Rheumatol (2020) 2(8):e449–e51. doi: 10.1016/S2665-9913(20)30210-1
112. Langer-Gould A, Smith JB, Gonzales EG, Castillo RD, Figueroa JG, Ramanathan A, et al. Early identification of covid-19 cytokine storm and treatment with anakinra or tocilizumab. Int J Infect Dis (2020) 99:291–7. doi: 10.1016/j.ijid.2020.07.081
113. Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med (2008) 26(6):711–5. doi: 10.1016/j.ajem.2007.10.031
114. Hirano T, Murakami M. Covid-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity (2020). doi: 10.1016/j.immuni.2020.04.003
115. Afif W, Loftus EV Jr., Faubion WA, Kane SV, Bruining DH, Hanson KA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol (2010) 105(5):1133. doi: 10.1038/ajg.2010.9
116. Roblin X, Marotte H, Leclerc M, Del Tedesco E, Phelip J, Peyrin-Biroulet L, et al. Combination of c-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohn's Colitis (2015) 9(7):525–31. doi: 10.1093/ecco-jcc/jjv061
117. Jürgens M, John JMM, Cleynen I, Schnitzler F, Fidder H, van Moerkercke W, et al. Levels of c-reactive protein are associated with response to infliximab therapy in patients with crohn's disease. Clin Gastroenterol Hepatol (2011) 9(5):421–7.e1. doi: 10.1016/j.cgh.2011.02.008
118. Guo C, Li B, Ma H, Wang X, Cai P, Yu Q, et al. Single-cell analysis of two severe covid-19 patients reveals a monocyte-associated and tocilizumab-responding cytokine storm. Nat Commun (2020) 11(1):1–11. doi: 10.1038/s41467-020-17834-w
119. Wang C, Xie J, Zhao L, Fei X, Zhang H, Tan Y, et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe covid-19 patients. EBioMedicine (2020) 57:102833. doi: 10.1016/j.ebiom.2020.102833
120. Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol (2019) 15(8):813–22. doi: 10.1080/1744666X.2019.1629904
121. Moore JB, June CH. Cytokine release syndrome in severe covid-19. Science (2020) 368(6490):473–4. doi: 10.1126/science.abb8925
122. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe covid-19 patients with tocilizumab. Proc Natl Acad Sci (2020) 117(20):10970–5.
123. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in covid-19: The current evidence and treatment strategies. Front Immunol (2020) 11:1708.
124. Khodadadi H, Salles ÉL, Jarrahi A, Chibane F, Costigliola V, Yu JC, et al. Cannabidiol modulates cytokine storm in acute respiratory distress syndrome induced by simulated viral infection using synthetic rna. Cannabis Cannabinoid Res (2020) 5(3):197–201. doi: 10.1089/can.2020.0043
125. Hill KP. Cannabinoids and the coronavirus. Cannabis Cannabinoid Res (2020). doi: 10.1089/can.2020.0035
126. Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by sars-Cov-2. Clin Chim Acta (2020). doi: 10.1016/j.cca.2020.06.017
127. Land MH, MacNair L, Thomas BF, Peters EN, Bonn-Miller MO. Letter to the Editor: Possible drug–drug interactions between cannabinoids and candidate covid-19 drugs. Cannabis Cannabinoid Res (2020) 5(4):340–3. doi: 10.1089/can.2020.0054
128. Lowe HI, Toyang NJ, McLaughlin W. Potential of cannabidiol for the treatment of viral hepatitis. Pharmacogn Res (2017) 9(1):116. doi: 10.4103/0974-8490.199780
129. Maor Y, Yu J, Kuzontkoski PM, Dezube BJ, Zhang X, Groopman JE. Cannabidiol inhibits growth and induces programmed cell death in kaposi sarcoma–associated herpesvirus-infected endothelium. Genes Cancer (2012) 3(7-8):512–20. doi: 10.1177/1947601912466556
130. Vuolo F, Abreu SC, Michels M, Xisto DG, Blanco NG, Hallak JE, et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur J Pharmacol (2019) 843:251–9. doi: 10.1016/j.ejphar.2018.11.029
131. Ribeiro A, Almeida V, Costola-de-Souza C, Ferraz-de-Paula V, Pinheiro M, Vitoretti L, et al. Cannabidiol improves lung function and inflammation in mice submitted to lps-induced acute lung injury. Immunopharmacol Immunotoxicol (2015) 37(1):35–41. doi: 10.3109/08923973.2014.976794
132. Turner AJ, Hiscox JA, Hooper NM. Ace2: From vasopeptidase to sars virus receptor. Trends Pharmacol Sci (2004) 25(6):291–4. doi: 10.1016/j.tips.2004.04.001
133. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell rna-seq data analysis on the receptor Ace2 expression reveals the potential risk of different human organs vulnerable to 2019-ncov infection. Front Med (2020), 1–8. doi: 10.1007/s11684-020-0754-0
134. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of Ace2 receptor of 2019-ncov on the epithelial cells of oral mucosa. Int J Oral Sci (2020) 12(1):1–5. doi: 10.1038/s41368-020-0074-x
135. Hernández-Cervantes R, Méndez-Díaz M, Prospéro-García Ó, Morales-Montor J. Immunoregulatory role of cannabinoids during infectious disease. Neuroimmunomodulation (2017) 24(4-5):183–99. doi: 10.1159/000481824
136. Pertwee RG. Cannabinoids [Handbook of experimental pharmacology 168]. Berlin Heidelberg: Springer-Verlag (2005).
137. Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther (2017) 175:133–50. doi: 10.1016/j.pharmthera.2017.02.041
138. Morales P, Hurst DP, Reggio PH. Molecular targets of the phytocannabinoids: A complex picture. In: Kinghorn A., Falk H., Gibbons S., Kobayashi J.(eds) Phytocannabinoids. Progress in the Chemistry of Organic Natural Products. Cham: Springer (2017) 103:103–31. doi: 10.1007/978-3-319-45541-9_4
139. De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on trp channels and endocannabinoid metabolic enzymes. Br J Pharmacol (2011) 163(7):1479–94. doi: 10.1111/j.1476-5381.2010.01166.x
140. Aizpurua-Olaizola O, Soydaner U, Oüztürk E, Schibano D, Simsir Y, Navarro P, et al. Evolution of the cannabinoid and terpene content during the growth of cannabis sativa plants from different chemotypes. J Nat Prod (2016) 79(2):324–31. doi: 10.1021/acs.jnatprod.5b00949
141. Russo EB. Taming thc: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol (2011) 163(7):1344–64. doi: 10.1111/j.1476-5381.2011.01238.x
142. Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther (2006) 318(3):1375–87. doi: 10.1124/jpet.106.105247
143. De Petrocellis L, Ligresti A, Schiano Moriello A, Iappelli M, Verde R, Stott CG, et al. Non-thc cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: Pro-apoptotic effects and underlying mechanisms. Br J Pharmacol (2013) 168(1):79–102. doi: 10.1111/j.1476-5381.2012.02027.x
144. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to covid-19 based on an analysis of data of 150 patients from wuhan, China. Intensive Care Med (2020) 46(5):846–8. doi: 10.1007/s00134-020-05991-x
145. Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of the macrophage activation syndrome: Re-analysis of a prior phase iii trial. Crit Care Med (2016) 44(2):275. doi: 10.1097/CCM.0000000000001402
146. Chang YH, Lee ST, Lin WW. Effects of cannabinoids on lps-stimulated inflammatory mediator release from macrophages: Involvement of eicosanoids. J Cell Biochem (2001) 81(4):715–23. doi: 10.1002/jcb.1103
147. Klein TW, Cabral GA. Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. J Neuroimmune Pharmacol (2006) 1(1):50. doi: 10.1007/s11481-005-9007-x
148. Rossi F, Tortora C, Argenziano M, Di Paola A, Punzo F. Cannabinoid receptor type 2: A possible target in sars-Cov-2 (Cov-19) infection? Int J Mol Sci (2020) 21(11):3809. doi: 10.3390/ijms21113809
149. Sardinha J, Kelly M, Zhou J, Lehmann C. Experimental cannabinoid 2 receptor-mediated immune modulation in sepsis. Mediators Inflamm (2014) 2014:978678. doi: 10.1155/2014/978678
150. Youssef DA, El-Fayoumi HM, Mahmoud MF. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of Cb2 and ppar-Γ receptors. Chemico-Biol Interact (2019) 297:16–24. doi: 10.1016/j.cbi.2018.10.010
151. Gertsch J, Leonti M, Raduner S, Racz I, Chen J-Z, Xie X-Q, et al. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci (2008) 105(26):9099–104. doi: 10.1073/pnas.0803601105
152. Petrosino S, Verde R, Vaia M, Allarà M, Iuvone T, Di Marzo V. Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J Pharmacol Exp Ther (2018) 365(3):652–63. doi: 10.1124/jpet.117.244368
153. Yang L, Li F-F, Han Y-C, Jia B, Ding Y. Cannabinoid receptor Cb2 is involved in tetrahydrocannabinol-induced anti-inflammation against lipopolysaccharide in mg-63 cells. Mediators Inflamm (2015) 2015:362126. doi: 10.1155/2015/362126
154. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin Immunopathol (2017) 39(5):529–39. doi: 10.1007/s00281-017-0629-x
155. Snyder RJ, Lantis J, Kirsner RS, Shah V, Molyneaux M, Carter MJ. Macrophages: A review of their role in wound healing and their therapeutic use. Wound Repair Regen (2016) 24(4):613–29. doi: 10.1111/wrr.12444
156. Punzo F, Bellini G, Tortora C, Di Pinto D, Argenziano M, Pota E, et al. Mifamurtide and Tam-like macrophages: Effect on proliferation, migration and differentiation of osteosarcoma cells. Oncotarget (2020) 11(7):687. doi: 10.18632/oncotarget.27479
157. Du Y, Ren P, Wang Q, Jiang S-K, Zhang M, Li J-Y, et al. Cannabinoid 2 receptor attenuates inflammation during skin wound healing by inhibiting M1 macrophages rather than activating M2 macrophages. J Inflamm (2018) 15(1):1–12. doi: 10.1186/s12950-018-0201-z
158. Braun M, Khan ZT, Khan MB, Kumar M, Ward A, Achyut BR, et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury Via alternative macrophage polarization. Brain Behav Immun (2018) 68:224–37. doi: 10.1016/j.bbi.2017.10.021
159. Starc N, Ingo D, Conforti A, Rossella V, Tomao L, Pitisci A, et al. Biological and functional characterization of bone marrow-derived mesenchymal stromal cells from patients affected by primary immunodeficiency. Sci Rep (2017) 7(1):1–13. doi: 10.1038/s41598-017-08550-5
160. Gao F, Chiu S, Motan D, Zhang Z, Chen L, Ji H, et al. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis (2016) 7(1):e2062–e. doi: 10.1038/cddis.2015.327
161. Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, et al. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic influenza a (H7n9) infection, a hint for covid-19 treatment. Engineering (2020) 6(10):1153–61. doi: 10.1016/j.eng.2020.02.006
162. Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of Ace2 (-) mesenchymal stem cells improves the outcome of patients with covid-19 pneumonia. Aging Dis (2020) 11:216–28. doi: 10.14336/AD.2020.0228
163. Shetty AK. Mesenchymal stem cell infusion shows promise for combating coronavirus (Covid-19)-Induced pneumonia. Aging Dis (2020) 11(2):462. doi: 10.14336/AD.2020.0301
164. Foronjy RF, Dabo AJ, Cummins N, Geraghty P. Leukemia inhibitory factor protects the lung during respiratory syncytial viral infection. BMC Immunol (2014) 15(1):41. doi: 10.1186/s12865-014-0041-4
165. Golchin A, Farahany TZ, Khojasteh A, Soleimanifar F, Ardeshirylajimi A. The clinical trials of mesenchymal stem cell therapy in skin diseases: An update and concise review. Curr Stem Cell Res Ther (2019) 14(1):22–33. doi: 10.2174/1574888X13666180913123424
166. Chen L, Qu J, Xiang C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther (2019) 10(1):1–10. doi: 10.1186/s13287-018-1105-9
167. Metcalfe SM. Mesenchymal stem cells and management of covid-19 pneumonia. Med Drug Discov (2020) 5:100019. doi: 10.1016/j.medidd.2020.100019
168. Franks LN, Ford BM, Prather PL. Selective estrogen receptor modulators: Cannabinoid receptor inverse agonists with differential Cb1 and Cb2 selectivity. Front Pharmacol (2016) 7:503. doi: 10.3389/fphar.2016.00503
169. Kumar P, Song Z-H. Identification of raloxifene as a novel Cb2 inverse agonist. Biochem Biophys Res Commun (2013) 435(1):76–81. doi: 10.1016/j.bbrc.2013.04.040
170. Dobovišek L, Hojnik M, Ferk P. Overlapping molecular pathways between cannabinoid receptors type 1 and 2 and Estrogens/Androgens on the periphery and their involvement in the pathogenesis of common diseases. Int J Mol Med (2016) 38(6):1642–51. doi: 10.3892/ijmm.2016.2779
171. Peretz J, Pekosz A, Lane AP, Klein SL. Estrogenic compounds reduce influenza a virus replication in primary human nasal epithelial cells derived from female, but not Male, donors. Am J Physiology-Lung Cell Mol Physiol (2016) 310(5):L415–L25. doi: 10.1152/ajplung.00398.2015
172. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol (2017) 198(10):4046–53. doi: 10.4049/jimmunol.1601896
173. Rockwell CE, Raman P, Kaplan BL, Kaminski NE. A cox-2 metabolite of the endogenous cannabinoid, 2-arachidonyl glycerol, mediates suppression of il-2 secretion in activated jurkat T cells. Biochem Pharmacol (2008) 76(3):353–61. doi: 10.1016/j.bcp.2008.05.005
174. Carayon P, Marchand J, Dussossoy D, Derocq J-M, Jbilo O, Bord A, et al. Modulation and functional involvement of Cb2 peripheral cannabinoid receptors during b-cell differentiation. Blood J Am Soc Hematol (1998) 92(10):3605–15. doi: 10.1182/blood.V92.10.3605
175. El Biali M, Broers B, Besson M, Demeules J. Cannabinoids and covid-19. Med Cannabis Cannabinoids (2020) 3(2):111–5. doi: 10.1159/000510799
176. Tahamtan A, Samieipoor Y, Nayeri FS, Rahbarimanesh AA, Izadi A, Rashidi-Nezhad A, et al. Effects of cannabinoid receptor type 2 in respiratory syncytial virus infection in human subjects and mice. Virulence (2018) 9(1):217–30. doi: 10.1080/21505594.2017.1389369
177. Farha MA, El-Halfawy OM, Gale RT, MacNair CR, Carfrae LA, Zhang X, et al. Uncovering the hidden antibiotic potential of cannabis. ACS Infect Dis (2020) 6(3):338–46. doi: 10.1021/acsinfecdis.9b00419
178. Hobbs JM, Vazquez AR, Remijan ND, Trotter RE, McMillan TV, Freedman KE, et al. Evaluation of pharmacokinetics and acute anti-inflammatory potential of two oral cannabidiol preparations in healthy adults. Phytother Res (2020) 34(7):1696–1703. doi: 10.1002/ptr.6651
179. Esposito G, Pesce M, Seguella L, Sanseverino W, Lu J, Corpetti C, et al. The potential of cannabidiol in the covid-19 pandemic: A hypothesis letter. Br J Pharmacol (2020) 177(21):4967–70. doi: 10.22541/au.158894349.98427987
180. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
181. Wang B, Kovalchuk A, Li D, Ilnytskyy Y, Kovalchuk I, Kovalchuk O. In search of preventative strategies: Novel anti-inflammatory high-cbd cannabis sativa extracts modulate Ace2 expression in covid-19 gateway tissues. Aging (Albany NY) (2020) 12(22):22425–44. doi: 10.20944/preprints202004.0315.v1
182. Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res (2017) 2(1):139–54. doi: 10.1089/can.2016.0034
183. Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Vitoretti LB, Mariano-Souza DP, Quinteiro-Filho WM, et al. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A2a receptor. Eur J Pharmacol (2012) 678(1-3):78–85. doi: 10.1016/j.ejphar.2011.12.043
184. Huang S, Goplen NP, Zhu B, Cheon IS, Son Y, Wang Z, et al. Macrophage ppar-Γ suppresses long-term lung fibrotic sequelae following acute influenza infection. PloS One (2019) 14(10):e0223430. doi: 10.1371/journal.pone.0223430
185. Bassaganya-Riera J, Song R, Roberts PC, Hontecillas R. Ppar-Γ activation as an anti-inflammatory therapy for respiratory virus infections. Viral Immunol (2010) 23(4):343–52. doi: 10.1089/vim.2010.0016
186. Mecha M, Feliú A, Iñigo P, Mestre L, Carrillo-Salinas F, Guaza C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2a receptors. Neurobiol Dis (2013) 59:141–50. doi: 10.1016/j.nbd.2013.06.016
187. Millar SA, Stone N, Bellman Z, Yates A, England T, O'Sullivan S. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol (2019) 85(9):1888–900. doi: 10.1111/bcp.14038
188. Foster BC, Abramovici H, Harris CS. Cannabis and cannabinoids: Kinetics and interactions. Am J Med (2019) 132(11):1266–70. doi: 10.1016/j.amjmed.2019.05.017
189. Costiniuk CT, Saneei Z, Routy J-P, Margolese S, Mandarino E, Singer J, et al. Oral cannabinoids in people living with hiv on effective antiretroviral therapy: Ctn Pt028–study protocol for a pilot randomised trial to assess safety, tolerability and effect on immune activation. BMJ Open (2019) 9(1):e024793. doi: 10.1136/bmjopen-2018-024793
190. Reznik SE, Gardner EL, Ashby CR Jr. Cannabidiol: A potential treatment for post Ebola syndrome? Int J Infect Dis (2016) 52:74–6. doi: 10.1016/j.ijid.2016.09.020
191. Doktorovová S, Kovačević AB, Garcia ML, Souto EB. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur J Pharmaceut Biopharmaceut (2016) 108:235–52. doi: 10.1016/j.ejpb.2016.08.001
192. Doktorovova S, Souto EB, Silva AM. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers–a systematic review of in vitro data. Eur J Pharmaceut Biopharmaceut (2014) 87(1):1–18. doi: 10.1016/j.ejpb.2014.02.005
193. Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev (2007) 59(6):478–90. doi: 10.1016/j.addr.2007.04.007
194. Martins S, Costa-Lima S, Carneiro T, Cordeiro-da-Silva A, Souto E, Ferreira D. Solid lipid nanoparticles as intracellular drug transporters: An investigation of the uptake mechanism and pathway. Int J Pharmaceut (2012) 430(1-2):216–27. doi: 10.1016/j.ijpharm.2012.03.032
195. Martins S, Sarmento B, Ferreira DC, Souto EB. Lipid-based colloidal carriers for peptide and protein delivery–liposomes versus lipid nanoparticles. Int J nanomed (2007) 2(4):595.
196. Martins S, Silva A, Ferreira D, Souto E. Improving oral absorption of samon calcitonin by trimyristin lipid nanoparticles. J Biomed Nanotechnol (2009) 5(1):76–83. doi: 10.1166/jbn.2009.443
197. Fangueiro J, Gonzalez-Mira E, Martins-Lopes P, Egea M, Garcia M, Souto S, et al. A novel lipid nanocarrier for insulin delivery: Production, characterization and toxicity testing. Pharm Dev Technol (2013) 18(3):545–9. doi: 10.3109/10837450.2011.591804
198. Patel M, Souto EB, Singh KK. Advances in brain drug targeting and delivery: Limitations and challenges of solid lipid nanoparticles. Expert Opin Drug Deliv (2013) 10(7):889–905. doi: 10.1517/17425247.2013.784742
199. Souto E, Müller R. Cosmetic features and applications of lipid nanoparticles (Sln®, nlc®). Int J Cosmetic Sci (2008) 30(3):157–65. doi: 10.1111/j.1468-2494.2008.00433.x
200. Souto EB, Müller RH. Lipid nanoparticles: Effect on bioavailability and pharmacokinetic changes. In: Drug delivery. Springer (2010). p. 115–41.
201. Souto E, Wissing S, Barbosa C, Müller R. Development of a controlled release formulation based on sln and nlc for topical clotrimazole delivery. Int J Pharmaceut (2004) 278(1):71–7. doi: 10.1016/j.ijpharm.2004.02.032
202. Saupe A, Wissing SA, Lenk A, Schmidt C, Müller RH. Solid lipid nanoparticles (Sln) and nanostructured lipid carriers (Nlc)–structural investigations on two different carrier systems. Bio-medical Mater Eng (2005) 15(5):393–402.
203. Teeranachaideekul V, Müller RH, Junyaprasert VB. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (Nlc)–effects of formulation parameters on physicochemical stability. Int J Pharmaceut (2007) 340(1):198–206. doi: 10.1016/j.ijpharm.2007.03.022
204. Souto EB, Fangueiro JF, Müller RH. Solid lipid nanoparticles (Sln™). In: Fundamentals of pharmaceutical nanoscience. Springer (2013). p. 91–116.
205. Souto E, Almeida A, Müller R. Lipid nanoparticles (Sln®, nlc®) for cutaneous drug delivery: Structure, protection and skin effects. J Biomed Nanotechnol (2007) 3(4):317–31. doi: 10.1166/jbn.2007.049
206. Zielińska A, Nowak I. Solid lipid nanoparticles and nanostructured lipid carriers as novel carriers for cosmetic ingredients. In: Nanobiomaterials in galenic formulations and cosmetics. Elsevier (2016). p. 231–55.
207. Zielińska A, Ferreira NR, Durazzo A, Lucarini M, Cicero N, Mamouni SE, et al. Development and optimization of alpha-Pinene-Loaded solid lipid nanoparticles (Sln) using experimental factorial design and dispersion analysis. Molecules (2019) 24(15):2683.
208. Souto EB, Doktorovova S, Zielinska A, Silva AM. Key production parameters for the development of solid lipid nanoparticles by high shear homogenization. Pharm Dev Technol (2019) 24(9):1181–5. doi: 10.1080/10837450.2019.1647235
209. Souto EB, Zielinska A, Souto SB, Durazzo A, Lucarini M, Santini A, et al. (+)-limonene 1, 2-Epoxide-Loaded slns: Evaluation of drug release, antioxidant activity, and cytotoxicity in an hacat cell line. Int J Mol Sci (2020) 21(4):1449. doi: 10.3390/ijms21041449
210. Singh KK, Vingkar SK. Formulation, antimalarial activity and biodistribution of oral lipid nanoemulsion of primaquine. Int J Pharmaceut (2008) 347(1-2):136–43. doi: 10.1016/j.ijpharm.2007.06.035
211. Patil HG, Tiwari RV, Repka MA, Singh KK. Formulation and development of orodispersible sustained release tablet of domperidone. Drug Dev Ind Pharm (2016) 42(6):906–15. doi: 10.3109/03639045.2015.1088864
212. Joshi M, Patravale V. Nanostructured lipid carrier (Nlc) based gel of celecoxib. Int J Pharmaceut (2008) 346(1):124–32. doi: 10.1016/j.ijpharm.2007.05.060
213. Souto EB, Doktorovova S, Campos JR, Martins-Lopes P, Silva AM. Surface-tailored anti-Her2/Neu-Solid lipid nanoparticles for site-specific targeting mcf-7 and bt-474 breast cancer cells. Eur J Pharm Sci (2019) 128:27–35. doi: 10.1016/j.ejps.2018.11.022
214. Eder P, Zielińska A, Karczewski J, Dobrowolska A, Słomski R, Souto EB. How could nanobiotechnology improve treatment outcomes of anti-Tnf-A therapy in inflammatory bowel disease? current knowledge, future directions. J Nanobiotechnol (2021) 19(1):346. doi: 10.1186/s12951-021-01090-1
215. Maroni A, Moutaharrik S, Zema L, Gazzaniga A. Enteric coatings for colonic drug delivery: State of the art. Expert Opin Drug Deliv (2017) 14(9):1027–9. doi: 10.1080/17425247.2017.1360864
216. Kulbacka J, Pucek A, Kotulska M, Dubińska-Magiera M, Rossowska J, Rols M-P, et al. Electroporation and lipid nanoparticles with cyanine ir-780 and flavonoids as efficient vectors to enhanced drug delivery in colon cancer. Bioelectrochemistry (2016) 110:19–31. doi: 10.1016/j.bioelechem.2016.02.013
Keywords: COVID-19, solid lipid nanoparticles (SLN), tocilizumab (TCZ), cannabidiol (CBD), cytokine storm, oral drug therapy
Citation: Zielińska A, Eder P, Karczewski J, Szalata M, Hryhorowicz S, Wielgus K, Szalata M, Dobrowolska A, Atanasov AG, Słomski R and Souto EB (2023) Tocilizumab-coated solid lipid nanoparticles loaded with cannabidiol as a novel drug delivery strategy for treating COVID-19: A review. Front. Immunol. 14:1147991. doi: 10.3389/fimmu.2023.1147991
Received: 19 January 2023; Accepted: 06 March 2023;
Published: 22 March 2023.
Edited by:
Alfonso J. Rodriguez-Morales, Fundacion Universitaria Autónoma de las Américas, ColombiaReviewed by:
Fahad S. Alshehri, Umm Al Qura University, Saudi ArabiaRajashri R. Naik, Al-Ahliyya Amman University, Jordan
Engy Elekhnawy, Tanta University, Egypt
Copyright © 2023 Zielińska, Eder, Karczewski, Szalata, Hryhorowicz, Wielgus, Szalata, Dobrowolska, Atanasov, Słomski and Souto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aleksandra Zielińska, YWxla3NhbmRyYS56aWVsaW5za2FAaWdjei5wb3puYW4ucGw=; Piotr Eder, cGlvdHJlZGVyQHVtcC5lZHUucGw=; Eliana B. Souto, ZWJzb3V0b0BmZi51cC5wdA==
†These authors have contributed equally to this work
‡ORCID: Aleksandra Zielińska, orcid.org/0000-0003-2603-1377
Piotr Eder, orcid.org/0000-0001-9306-5038
Jacek Karczewski, orcid.org/0000-0002-8322-4186
Marlena Szalata, orcid.org/0000-0002-0153-4317
Szymon Hryhorowicz, orcid.org/0000-0003-4388-286X
Karolina Wielgus, orcid.org/0000-0003-0629-2991
Milena Szalata, orcid.org/0000-0003-1500-8251
Agnieszka Dobrowolska, orcid.org/0000-0002-3647-5070
Atanas G. Atanasov, orcid.org/0000-0003-2545-0967
Ryszard Słomski, orcid.org/0000-0001-5601-7002
Eliana B. Souto, orcid.org/0000-0002-9737-6017
 Aleksandra Zielińska
Aleksandra Zielińska Piotr Eder
Piotr Eder Jacek Karczewski
Jacek Karczewski Marlena Szalata
Marlena Szalata Szymon Hryhorowicz
Szymon Hryhorowicz Karolina Wielgus
Karolina Wielgus Milena Szalata
Milena Szalata Agnieszka Dobrowolska2‡
Agnieszka Dobrowolska2‡ Atanas G. Atanasov
Atanas G. Atanasov Eliana B. Souto
Eliana B. Souto