- 1Department of Otorhinolaryngology and Head and Neck Surgery, First Affiliated Hospital, Guangxi Medical University, Nanning, China
- 2Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor (Guangxi Medical University), Ministry of Education, Nanning, China
- 3Guangxi Medical University, Nanning, China
- 4Life Science Institute, Guangxi Medical University, Nanning, China
- 5Department of Radiation Oncology, First Affiliated Hospital of Guangxi Medical University, Nanning, China
Substantial improvement in prognosis among metastatic renal cell carcinoma (mRCC) patients has been achieved, owing to the rapid development and utilization of immunotherapy. In particular, immune checkpoint inhibitors (ICIs) have been considered the backbone of systemic therapy for patients with mRCC alongside multi-targeted tyrosine kinase inhibitors (TKIs) in the latest clinical practice guidelines. However, controversies and challenges in optimal individualized treatment regarding immunotherapy remains still About 2/3 of the patients presented non-response or acquired resistance to ICIs. Besides, immune-related toxicities, namely immune-related adverse events, are still elusive and life-threatening. Thus, reliable biomarkers to predict immunotherapeutic outcomes for mRCC patients are needed urgently. Tumor microenvironment (TME), consisting of immune cells, vasculature, signaling molecules, and extracellular matrix and regulates tumor immune surveillance and immunological evasion through complex interplay, plays a critical role in tumor immune escape and consequently manipulates the efficacy of immunotherapy. Various studied have identified the different TME components are significantly associated with the outcome of mRCC patients receiving immunotherapy, making them potential valuable biomarkers in therapeutic guidance. The present review aims to summarize the latest evidence on the associations between the components of TME including immune cells, cytokines and extracellular matrix, and the therapeutic responses among mRCC patients with ICI-based treatment. We further discuss the feasibility and limitation of these components as biomarkers.
1 Introduction
Around 430,000 newly diagnosed renal cell carcinoma (RCC) are reported yearly worldwide (1). One-fourth of these patients experience metastatic disease (mRCC) at diagnosis and another 30% develop distant metastasis after curative nephrectomy, whose estimated 5-year survival rate is only about 10-18% (2, 3). Fortunately, substantial improvement in prognosis among mRCC patients has been achieved, owing to the rapid development and revolutionized utilization of immunotherapy over the past decade (4–6). In particular, immune checkpoint inhibitors (ICIs) have been considered the backbone of systemic therapy for patients with mRCC alongside multi-targeted tyrosine kinase inhibitors (TKIs) in the latest clinical practice guidelines from the American Society of Clinical Oncology and the European Association of Urology (7, 8). However, there remain controversies and challenges in optimal individualized treatment regarding immunotherapy. Although about 1/3 of the patients experienced objective and durable responses, the majority of the patients did not benefit, presenting non-response or acquired resistance to ICIs (9). Additionally, immune-related toxicities, namely immune-related adverse events, are still elusive and can be life-threatening (10). Around 60% of the patients administrated ICIs experienced grade 3-4 treatment-related adverse events, leading to 7%-33% treatment discontinuation (11–14).
Therefore, seeking reliable biomarkers is crucial for monitoring the therapeutic efficacy and predicting the responses of immunotherapy among mRCC patients, which may be a solution to optimize the outcomes of immunotherapy-based treatment.
Recently, various studies indicate that tumor microenvironment (TME) plays a critical role in anti-tumor immunity and consequently affects the sensitivity to immunotherapy (15–18). TME consists of immune cells, vasculature, signaling molecules, and extracellular matrix and regulates tumor immune surveillance and immunological evasion through complex interplay (Figure 1) (19). Several investigations have revealed the potential of TME components of TME as biomarkers for immunotherapy in various solid tumors, including RCC (11, 20–22). For instance, the expression of Programmed cell death-ligand 1 (PD-L1) in mRCC has been extensively studied as a potential biomarker for immunotherapy (23–25). Besides, researches on cytokines (such as IL-8 and IL-6) and extracellular matrices have shown their potential as biomarkers for mRCC (26–29). Although intriguing progress has been made, no consensus on a reliable and feasible biomarker for immunotherapy among mRCC patients to date.
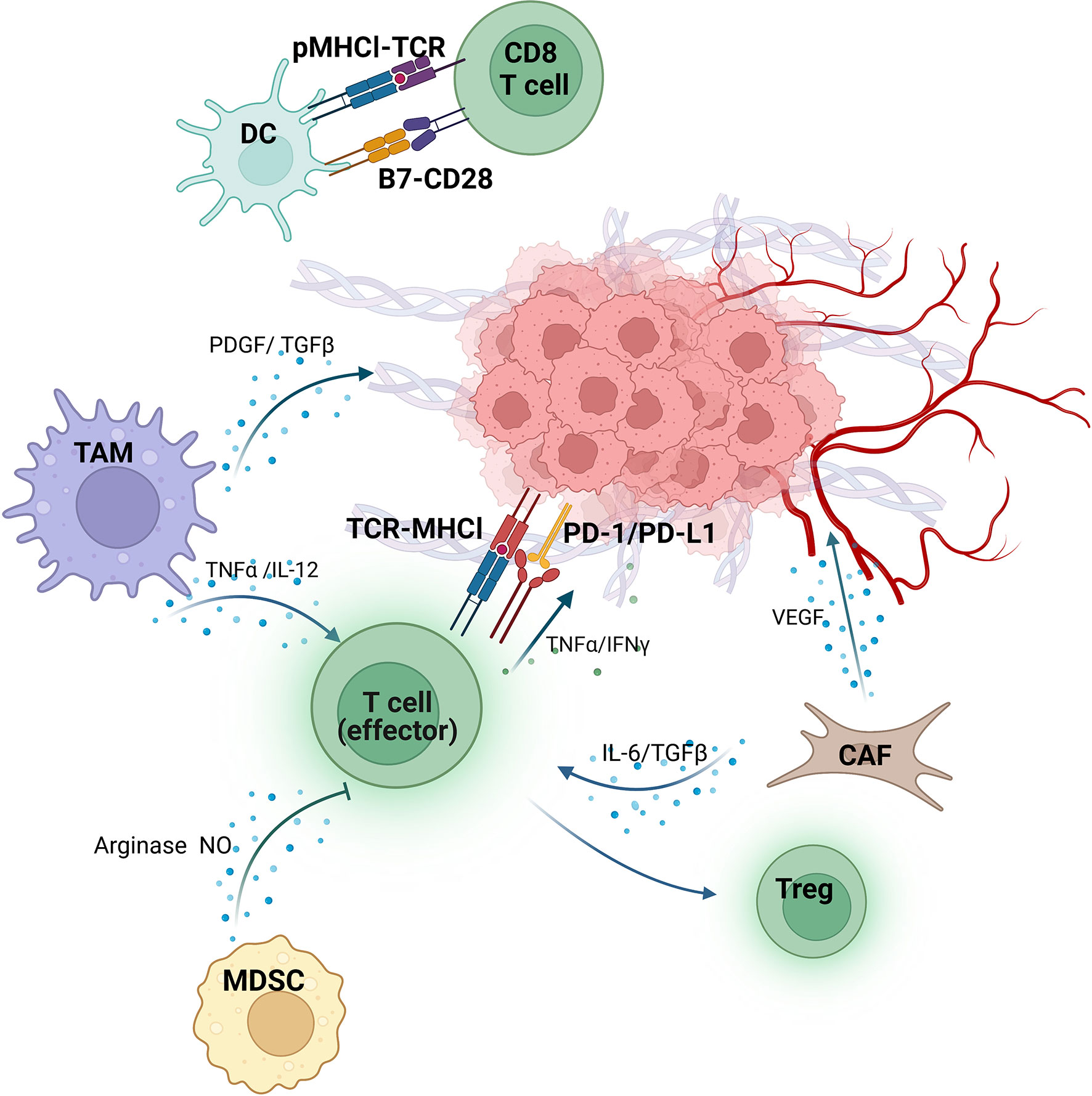
Figure 1 Tumor immunity/antitumor immunity in the TME of RCC. Dendritic cells (DC) present peptide-MHCI (pMHCI) complex to CD8+T cells. B7, a costimulatory molecule expressed on the surface of DC, binds to CD28 and subsequently activates CD8+T cells. The tumor-associated macrophages (TAM) secrete either platelet-derived growth factor (PDGF) and transforming growth factor β (TGF-β) to promote tumor angiogenesis, or tumor necrosis factor α (TNF-α) and IL-12 to enhance the killing function of effector T cells (T-eff). On the other hand, the function of T-eff is inhibited by Myeloid-derived suppressor cells (MDSC) via producing arginase and nitric oxide (NO). The IL-6 and TGF-β from Cancer-associated fibroblasts (CAF) lead to the transformation of T-eff into regulatory T cells (Treg), and release vascular endothelial growth factor (VEGF) to promote extracellular matrix deformation and angiogenesis as well. T-eff recognizes and kills tumor cells by binding the TCR on their surface to MHC-I on the surface of tumor cells. However, PD-L1 expressed by tumors interacts with PD-1 on T-eff significantly block their activity. Despite this, T-eff still plays an antitumor role by secreting TNF-α and interferon-γ (INFγ). (Figure was created with BioRender.com).
The present review aims to summarize the latest evidence on the association between key components of TME (including PD-L1 and other immune checkpoints, tumor-infiltrating T cells and T cell receptors, cytokines, and extracellular matrix) and the therapeutic responses among mRCC patients with ICI-based treatment and discuss the potential and feasibility of these components as a biomarker for mRCC patients receiving immunotherapy.
2 Immunotherapy for metastatic renal cell carcinoma
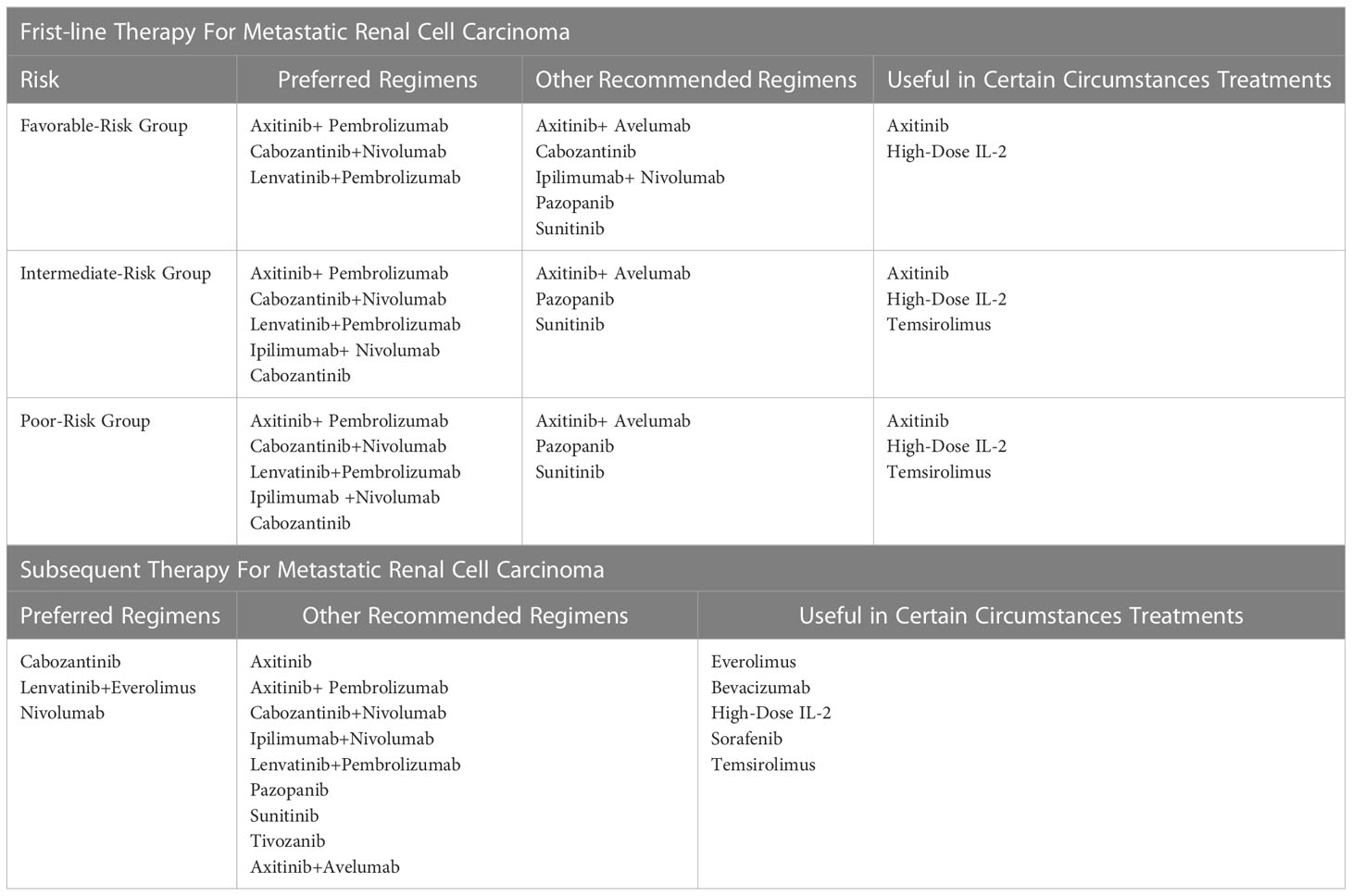
Along with the growing understanding of the immune responses ofRCC and considerable results achieved in numerous solid tumors by applying ICIs, medicines targeting the programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) axis and cytotoxic T-lymphocyte associated antigen (CTLA-4) pathways have been introduced to mRCC patients since 2014 (30, 31) and received the United States Food and Drug Administration approval in 2018 (32). Furthermore, after encouraging evidence reported by a series of milestone clinical trials (9, 12, 14, 33–36), the combination of TKI and ICI was formally recommended for mRCC patients as first-line protocols by the U.S. National Comprehensive Cancer Network clinical guidelines for kidney cancer, since version -3.2022 (37–39). A brief summary of clinical practices for mRCC patients based on NCCN guideline and the results of selected crucial trials are described in Table 1 and Supplementary Table 1. Although an ameliorative advancement has been yielded, a substantial amount of mRCC patients did not benefit from ICI-based therapy (40). Therefore, identifying reliable biomarkers is believed to facilitate further improvement in prolonged survival and minimize toxicities for mRCC patients (41–43).
3 TMEs as a biomarker for immunotherapy in mRCC
TME is the cellular niche surrounding tumor cells, which consists of immune cells, stromal cells, endothelial cells, extracellular matrix (ECM), neuroendocrine cells, adipose cells, and structural molecules, forming a spatially organized, dynamic, and functional network (15, 44, 45). Recent advances suggest that TME may be one of the pivotal players in modulating tumor immune surveillance and immunological evasion, as well as regulating the response to immunotherapy (15, 41, 46). Besides, the majority of RCC, especially clear-cell RCC (ccRCC) accounting for > 70% of tumors, is regarded as immunogenic cancer, featured by infiltration of leukocytes (natural killer cells, CD8+ T cells, and CD4+ T cells) and myeloid cells (macrophages and neutrophils) as surrounding microenvironment (41, 45). Furthermore, transcriptomic studies based on The Cancer Genome Atlas (TCGA) database addressed that ccRCC were highly CD8+ T cell infiltrated (only 27% of the tumor showed non-infiltrated features) and presented the highest scores on both the immune infiltration and T cell infiltration among 19 cancer types (23, 24). Thus, several key components of TME has been considered a candidate for the biomarker for immunotherapy in mRCC patients (28, 47–52).
3.1 Programmed cell death-ligand 1
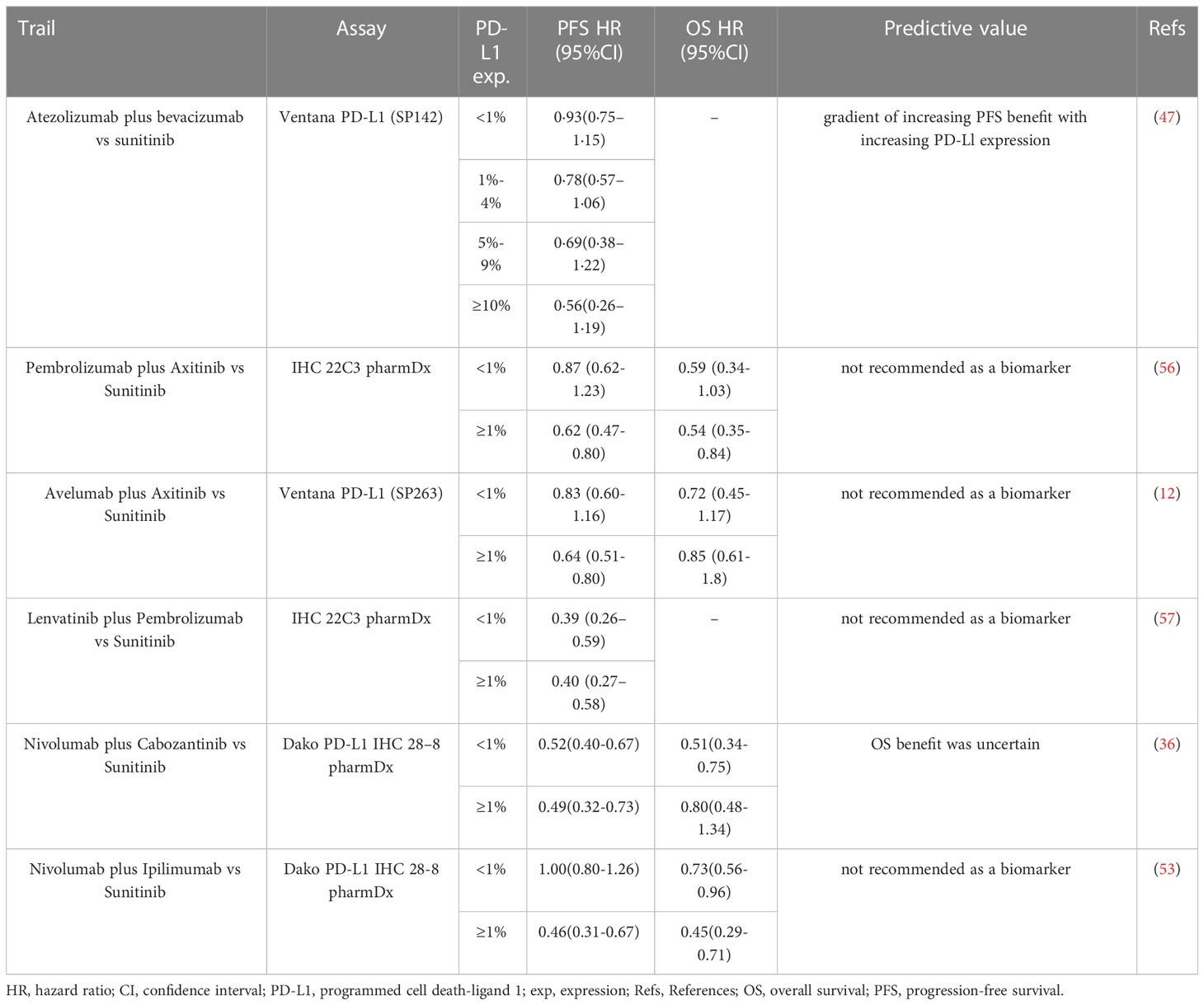
Recent clinical trials administrating ICIs-based immunotherapy in mRCC patients have reported that higher PD-L1 expression was associated with higher objective response rates (ORRs) (47, 53–55). In the PD-L1 positive population (expression ≥1%), mRCC patients treated with ICIs presented improvement in progression-free survival (PFS) or overall survival (OS) compared with those with sunitinib monotherapy (47, 53). Notably, conflict results were addressed in other trials comparing ICIs and sunitinib, where PD-L1 expression showed no predictive value in mRCC patients (12, 36, 56) (Table 2).
Currently, several studies have considered different measurements for PD-L1-based evaluation among mRCC patients with ICI-based treatment. Chen et al. reported that patients with PD-L1+ circulating tumor cells (CTCs) experienced higher disease control rates than others (73%, 11/15 vs. 20%, 1/5). After treatment, 82% of patients with controlled diseases (9/11) presented a decrease in the expression of PD-L1+ CTCs, whereas all the patients with progressive diseases (100%, 4/4) showed an increased or stable expression of PD-L1+ CTCs (58). In addition, Incorvaia and colleagues demonstrated that patients with higher baseline plasma soluble PD-L1 concentrations (> 0.66 ng/ml) might associate with longer PFS after nivolumab treatment (p < 0.0001) (59).Moreover, the combination of PD-L1 expression with other biomarkers is under investigation and expected to improve the predictability of immunotherapy. Among mRCC patients treated with nivolumab, those with high tumor cells PD-L1 expression and a high percentage of CD8+ PD-1+ TIM-3- LAG-3- tumor-infiltrating cells experienced longer immune-related PFS and better immune-related ORR (60). Chouaib and coworkers reported that the expression status of AXL (receptor tyrosine kinases) was closely associated with PD-L1 status, especially in the population with tumor-suppressor gene von Hippel-Lindau (VHL) inactivation (61). They further revealed that patients with accompanied high AXL expression and PD-L1 expression had worst survival than others (61).
However, these evidences mentioned above are insufficient to support if the expression of PD-L1 in tumor tissue alone served as a reliable biomarker. Firstly, lack of a standardized detection method, consistent thresholds of PD-L1 positivity, and consensus on how to score the expression level of PD-L1 in tumor cells only or including immune cells in TME in these studies (62). Secondly, conducting and interpreting the immunohistochemical results is usually subject to standardized guidelines and pathologists’ experience (63). Different immunohistochemical protocols were applied to measure the PD-L1 expression, leading to a difficulty in comparing the results and result interpretation directly. Thirdly, the different histopathological features of RCC and TME may affect the association between PD-L1 expression and treatment outcomes. For example, sarcomatoid and rhabdoid features increased CD8+ T-cell infiltration and up-regulated PD-L1 expression, showing higher response rate ICIs (64). Fourthly, PD-L1 expression was detected in various portions of study subjects, and its association with treatment outcome was analyzed in a subgroup with limited power, leading to failed comparisons among studies. Therefore, researches who had aware those difficulties suggested that the detection of circulating PD-L1 levels might complement the IHC-based measurement of PD-L1 expression in tissue based on experimental but encouraging results (partially presented above). In addition, the single biomarker might not be enough, but establishing a predictive model, a combination with other biomarkers would be a better strategy to improve the accuracy.
3.2 Other immune checkpoints
Beyond PD-1/PD-L1, the expression of cytotoxic T-lymphocyte-associated Protein 4 (CTLA-4) in RCC tumor-infiltrating monocytes was identified as an independent prognostic factor in RCC patients and significantly associated with worse outcome (65). The overexpression of CTLA4 due to promoter hypomethylation in RCC patients treated with ICI might be an independent factor for favorable outcomes (PFS: HR 1.94 (95% CI 1.09 to 3.44), p = 0.024; OS: HR 2.14 (95% CI 1.01 to 4.57), p = 0.048) (49). Besides, Lymphocyte activation gene-3 (LAG-3) and T cell immunoglobulin and mucin-containing molecule 3 (TIM-3) have been found to be associated with poor prognosis among mRCC patients (66, 67). Notably, the evidence on the roles of the immune checkpoints other than PD-1/PD-L1 in mRCC patients with immunotherapy (targeting PD-1/PD-L1 axis) is limited. Their roles in other immunotherapy are under investigation and related results are awaited.
3.3 Tumor-infiltrating T cells and T cell receptors
The abundance and composition of TITCs are valuable for prognostic prediction of cancer patients (68). Increased immune infiltration has been observed in RCC patients with nivolumab administration (69). A positive associations was found between the improved therapeutic response of nivolumab in ccRCC patients and the abundance of T-cell subsets in biopsies collected at baseline (p = 0.03) and day 28 (p < 0.01) of treatment (50). Detailed study of the T cell population may deepen the understanding of TITCs’ role in the immunotherapy of mRCC. Scientists found that patients with raised tumor-infiltrating CD8+ T cells and TCF-1+stem cell-like CD8+ T cells at the time of surgery tend to experienced robust immune responses and improved survival after subsequent immunotherapy (51). Those with larger expansions of HLA-DR+/CD38+/CD8+ T cells in peripheral blood after one cycle of immunotherapy had more significant tumor shrinkage (p < 0.05) and longer PFS (p = 0.006). Similarly, a higher TNFRSF9 CD8+ T cells infiltration was addressed to be associated with greater reduction of tumor size (p = 0.003) and better PFS (p = 0.012) in ccRCC patients receiving nivolumab (70). However, some non-responders can also present high T-cell infiltration (50). In addition, infiltration of some subsets of CD8 T cells such as PD-1+TIM-3+CD8+, CXCL13+CD8+, and CD39+CD8+ were associated with poor prognosis in RCC without immunotherapy (71–73). It is notable that CD8+T cells are activated and eventually differentiate into a phenotypically depleted terminal state in responders, according to single-cell transcriptome analysis of advanced RCC before and after ICI treatment (74). The expression of checkpoint molecules and anti-inflammatory signals was increased. It should be recognized that antitumor immunity is a dynamic process, and the composition and potential function of TITCs in different stages of immunotherapy will change accordingly.
On the other hand, TCR repertoire has been considered as a candidate biomarker for therapeutic monitoring and prognostic evaluation in cancer patients (75, 76). In the analysis of the peripheral TCR of HLA-DR+ CD38+ CD8 T cells, newer additional TCR clonotypes emerge in patients with a clinical benefit after one cycle of treatment (51). In a phase II study of nivolumab-treated mRCC patients, researchers conducted TCR analysis and found a higher pre-treatment expanded TCR clonality in ICI responders than non-responders (p = 0.042) (25). After treatment, expanded TCR clones in ICI responders were more likely to maintain than in non-responders whose TCR clones were usually replaced (p = 0.024). Maintaining similar TCR clones in tumor tissue after treatment was correlated with therapeutic response, but no correlation was found in peripheral TCR clones (25). Similarly, Kato et al. observed that expanded TCR clones preexisted in responders’ circulation before immunotherapy and maintained a long-term antitumor immune response after treatment (77). However, contradicting the former study, they found that patients with increased peripheral TCR clones after treatment had better OS and PFS than those with decreased peripheral TCR clones (p = 0.044 and p = 0.028, respectively) (77).
The presented studies yielded inconsistent associations between TITCs/TCR repertoire and ICI-based therapeutic outcomes. We are aware that our understanding of the diversity of the T cell population and the complexity of their functions is, to date, the tip of the proverbial iceberg.
3.4 Cytokines
Cytokines are involved in the physiological and pathological processes of mRCC patients, as well as the efficacy of treatment. mRCC patients undergoing ICI-based treatment with lower baseline of IL-8 has higher ORR (p = 0.047) (26). Elevated IL-8 expression might induce epithelial-mesenchymal transformation (EMT) and promote distant metastasis (78). Two comprehensive studies based on clinical trials underlined that circulating IL-8 might be a potential prognostic biomarker for patients administrating ICIs. Schalper and colleagues analyzed the data from four trials of patients with various cancers (advanced RCC, melanoma, and non-small-cell lung cancer) (52). They revealed that higher levels of pretreatment serum IL-8 were associated with shorter OS [HR = 2.56 (95% CI 1.89-3.54)] and lower PFS [HR = 1.36 (95% CI 1.07-1.72)] (52). Additionally, higher serum IL-8 before treatment was associated with poorer survival across cancer types, regardless of treatment strategies. In another study looking at ICIs in managing patients with advanced RCC and urothelial carcinoma, a significantly negative association between baseline plasma IL-8 levels and treatment outcomes is demonstrated, which is similar to the former finding (79). Except for IL-8, an analysis of studies in patients with metastatic renal cell carcinoma treated with IFN-α and Bevacizumab showed that IL-6 and hepatocyte growth factor (HGF) were associated with shorter OS [IL-6: HR = 1.27, 95% CI (1.11-1.42); HGF: HR = 1.19; 95% CI (1.00-1.33)] (29). Sang et al. found that among mRCC patients receive treatment with Pembrolizumab plus Axitinib, the shorter PFS [HR = 3.51, 95% CI (1.54-7.98), p = 0.003] and worse OS [HR = 7.18, 95% CI (2.26-22.82), p = 0.001] were found in those patient with higher IL-6, patients with high IL-6 had worse OS than those with low IL-6 (80). Although further validation is required, these promising results highlight the possibility of some cytokines as reliable and easily measurable predictive biomarkers for RCC and other solid tumor upon ICI treatment (81).However, limited information can be captured by a single measurement of serum cytokine at a single time point (82, 83). Besides, cytokines could be affected by numerous diseases beyond tumors (84–86). Therefore, the specificity of cytokines is relatively low, which obstacles its application as a biomarker.
3.5 Extracellular matrix
Substantial alterations of ECM around solid tumor contribute tremendously in the invasion of tumor cells, thereby initiating metastasis (87, 88). A series of investigations have focused on the components of ECM in RCC patients with metastasis and their treatment responses (27, 28, 89–91). For example, higher levels of transmembrane collagen COL23A1, a ligan of integrin α2β1 (92) [HR = 3.024, 95% CI (1.22-7.49)] and hyaluronan, a high molecular weight unbranched polysaccharide [HR = 1.4; 95% CI (1.02-2.0)] were associated with shorter survival CD248, being identified to localize to the stromal compartment in cancers, serves a key role in myofibroblast generation and accumulation (93). mRCC patients with CD248 overexpression and cancer-associated fibroblasts (CAF) infiltration were experienced poorer 5-year OS (58.3%) comparing those with low infiltration (27); Feng et al. reported a higher level of SPARC-related modular calcium-binding (SMOC2), which promotes matrix assembly and stimulate angiogenic activity was associated with worse 5-year survival rates than patients with lower SMOC2 (64% and 79%, respectively) (28). These findings have not yet been validated in prospective studies with sufficient subjects. More data is awaited to qualify the availability of ECM as a candidate biomarker for immunotherapy. On the other hand, evaluating the expression levels of particular components in ECM has been a practically challenge due to all of these components are expressed and function normally among adjacent healthy epithelial cells as well (94).
4 Future perspectives
Over the past decade, the utility of ICI-based systemic therapy has been attributed to the substantial improvement in prognosis among mRCC patients. To further improve clinical efficacy, and early identification of response and non-response in mRCC patients receiving immunotherapy to reduce the cost of treatment and avoid the damage of immune-related adverse reactions, seeking reliable biomarkers is crucial to predict the outcome and monitor therapeutic management.
As the most extensively studied component in TME, PD-L1 expression has not yet been available in monitoring treatment strategy and predicting outcomes due to inconsistent results across cancer types and ICI combinations. Developing a standardized, interchangeable detection assay and defining a uniform threshold of positivity of PD-L1 expression might improve the comparability across studies and practicality in clinical management. In addition, complementary circulating PD-L1 measurements and a combination of PD-L1 and other biomarkers might accelerate the application of PD-L1 to the bedside. Moreover, fully elucidating how distinct histopathological features, TME signatures, and patient characteristics affect the expression of PD-L1 and its relation with ICI-based treatment outcomes could facilitate the progress of seeking reliable biomarkers. Last but not least, any promising results that have been announced should be further validated in well-designed prospective studies with robust power.
In addition, encouraging but inconsistent results of TITCs and TCR as predictive markers for ICI-based treatment among mRCC patients were yielded from previous research. A more particular knowledge of the dynamics of the T cell population in response to immunotherapy among RCC patients is warranted to monitor therapeutic decisions better (95, 96). Comprehensive characterization of immune cell phenotypes and their interrelationships in the tumor microenvironment can promote immune cells such as T cells to become biomarkers and drug targets. Using mass spectrometry, a method that can be used to analyze large numbers of cells, Chevrier et al. identified 17 tumor-associated macrophage phenotypes, 22 T-cell phenotypes, and a unique immune composition associated with progression-free survival in ccRCC (97). The development of single-cell transcriptome analysis and mass spectrometry have revealed the complex network of immune cells in the tumor microenvironment, which is expected to help us better understand the therapeutic response in the context of immunotherapy. TCR repertoire analysis based on deep sequencing provides a reliable assessment to reveal the clonal richness and diversity of the T cell population, to chase the longitudinal changes of T cell clones with repeated sampling on both tissue and blood, and to measure the expansion of T cell clones. Thus, introducing TCR repertoire analysis into future investigations on immunotherapy treatment outcomes among patients with RCC and other solid tumors should facilitate the progression in clinical management.
Serum cytokine IL-8 has been suggested as a feasible biomarker for prognosis prediction of ICI-based treatment. Next step, further validation trials are warranted to confirm its capability and test its specificity and sensitivity as a biomarker. Moreover, the multi-measurement of correlated cytokines which function in a synergistic network (such as IL-8, IL-6, and TNF) from one sample might raise the value of the prediction of outcomes (98), and identification of cytokine composite signatures associated with prognosis may be an important approach to improve specificity and accuracy (99) Recent advances in ECM detection suggested that ECM derivatives measured in blood might be an attractive supplement to ECM detection (100).
5 Conclusions
In summary, accumulating data prompt that components of TMEs, such as PD-L1, CTLA-4, LAG-3, TIM-3, infiltrating T cell and T cell receptors, cytokines, and ECM, might be candidates of predictor for ICI-based treatment outcome among mRCC patients. However, they have yet to be served as reliable and practical biomarkers applying to the clinic immediately. Thus, future investigations in developing standardized measurement, expanding knowledge on the functional network of TMEs, and validations are warranted to overcome these issues and facilitate a continuous improvement of the clinical benefit of ICI-based treatment among mRCC patients. In addition, we believe that a comprehensive prediction model for mRCC patients by incorporating both classical prediction models (such as International Metastatic Renal-Cell Carcinoma Database Consortium criteria) and various TME components would have the potential to improve the accuracy of predictions. Furthermore, novel testing and analyzing techniques and approaches, such as TCR repertoire analysis, single-cell multi-omic analysis, multiplex label-based immunoassays, and mass spectrometry, are encouraged to apply in future studies, both in mechanism exploration and clinical settings.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by grants from the National Natural Science Foundation of China (U22A20322, to ZZ), the National Natural Science Youth Foundation of China (82202939, to TH), the National Scientific Foundation of China (32260170, to XZ) and China Postdoctoral Science Foundation (2022MD723765, to TH).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1146738/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Sharma R, Kadife E, Myers M, Kannourakis G, Prithviraj P, Ahmed N. Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma. J Exp Clin Cancer Res (2021) 40(1):186. doi: 10.1186/s13046-021-01961-3
3. Lavacchi D, Pellegrini E, Palmieri VE, Doni L, Mela MM, Di Maida F, et al. Immune checkpoint inhibitors in the treatment of renal cancer: current state and future perspective. Int J Mol Sci (2020) 21(13):4691. doi: 10.3390/ijms21134691
4. Albiges L, Powles T, Staehler M, Bensalah K, Giles RH, Hora M, et al. Updated European association of urology guidelines on renal cell carcinoma: immune checkpoint inhibition is the new backbone in first-line treatment of metastatic clear-cell renal cell carcinoma. Eur Urol (2019) 76(2):151–6. doi: 10.1016/j.eururo.2019.05.022
5. Sepe P, Mennitto A, Corti F, Procopio G. Immunotherapeutic targets and therapy for renal cell carcinoma. Immunotargets Ther (2020) 9:273–88. doi: 10.2147/ITT.S240889
6. Horodyska J, Hamill RM, Reyer H, Trakooljul N, Lawlor PG, McCormack UM, et al. RNA-Seq of liver from pigs divergent in feed efficiency highlights shifts in macronutrient metabolism, hepatic growth and immune response. Front Genet (2019) 10:117. doi: 10.3389/fgene.2019.00117
7. Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol (2022) 82(4):399–410. doi: 10.1016/j.eururo.2022.03.006
8. Rathmell WK, Rumble RB, Van Veldhuizen PJ, Al-Ahmadie H, Emamekhoo H, Hauke RJ, et al. Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol (2022) 40(25):2957–95. doi: 10.1200/JCO.22.00868
9. Motzer RJ, McDermott DF, Escudier B, Burotto M, Choueiri TK, Hammers HJ, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer (2022) 128(11):2085–97. doi: 10.1002/cncr.34180
10. Cozma A, Sporis ND, Lazar AL, Buruiana A, Ganea AM, Malinescu TV, et al. Cardiac toxicity associated with immune checkpoint inhibitors: a systematic review. Int J Mol Sci (2022) 23(18):10948. doi: 10.3390/ijms231810948
11. Motzer RJ, Powles T, Burotto M, Escudier B, Bourlon MT, Shah AY, et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol (2022) 23(7):888–98. doi: 10.1016/S1470-2045(22)00290-X
12. Choueiri TK, Motzer RJ, Rini BI, Haanen J, Campbell MT, Venugopal B, et al. Updated efficacy results from the JAVELIN renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol (2020) 31(8):1030–9. doi: 10.1016/j.annonc.2020.04.010
13. Motzer RJ, Rini BI, McDermott DF, Aren Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol (2019) 20(10):1370–85. doi: 10.1016/S1470-2045(19)30413-9
14. Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer (2020) 126(18):4156–67. doi: 10.1002/cncr.33033
15. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med (2018) 24(5):541–50. doi: 10.1038/s41591-018-0014-x
16. Zhang Y, Narayanan SP, Mannan R, Raskind G, Wang X, Vats P, et al. Single-cell analyses of renal cell cancers reveal insights into tumor microenvironment, cell of origin, and therapy response. Proc Natl Acad Sci USA (2021) 118(24):e2103240118. doi: 10.1073/pnas.2103240118
17. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet (2019) 51(2):202–6. doi: 10.1038/s41588-018-0312-8
18. Peng YL, Xiong LB, Zhou ZH, Ning K, Li Z, Wu ZS, et al. Single-cell transcriptomics reveals a low CD8(+) T cell infiltrating state mediated by fibroblasts in recurrent renal cell carcinoma. J Immunother Cancer (2022) 10(2):e004206. doi: 10.1136/jitc-2021-004206
19. Khalaf K, Hana D, Chou JT, Singh C, Mackiewicz A, Kaczmarek M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front Immunol (2021) 12:656364. doi: 10.3389/fimmu.2021.656364
20. He J, Xiong X, Yang H, Li D, Liu X, Li S, et al. Defined tumor antigen-specific T cells potentiate personalized TCR-T cell therapy and prediction of immunotherapy response. Cell Res (2022) 32(6):530–42. doi: 10.1038/s41422-022-00627-9
21. Motzer RJ, Choueiri TK, McDermott DF, Powles T, Vano YA, Gupta S, et al. Biomarker analysis from CheckMate 214: nivolumab plus ipilimumab versus sunitinib in renal cell carcinoma. J Immunother Cancer (2022) 10(3):e004316. doi: 10.1136/jitc-2021-004316
22. Phillips D, Matusiak M, Gutierrez BR, Bhate SS, Barlow GL, Jiang S, et al. Immune cell topography predicts response to PD-1 blockade in cutaneous T cell lymphoma. Nat Commun (2021) 12(1):6726. doi: 10.1038/s41467-021-26974-6
23. Senbabaoglu Y, Gejman RS, Winer AG, Liu M, Van Allen EM, de Velasco G, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol (2016) 17(1):231. doi: 10.1186/s13059-016-1092-z
24. Braun DA, Hou Y, Bakouny Z, Ficial M, Sant’ Angelo M, Forman J, et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med (2020) 26(6):909–18. doi: 10.1038/s41591-020-0839-y
25. Au L, Hatipoglu E, Robert de Massy M, Litchfield K, Beattie G, Rowan A, et al. Determinants of anti-PD-1 response and resistance in clear cell renal cell carcinoma. Cancer Cell (2021) 39(11):1497–518 e11. doi: 10.1016/j.ccell.2021.10.001
26. Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-Neblett KL, Martin AM, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol (2012) 13(8):827–37. doi: 10.1016/S1470-2045(12)70241-3
27. Xu C, Zhang K, Yang F, Zhou X, Liu S, Li Y, et al. CD248(+) cancer-associated fibroblasts: a novel prognostic and therapeutic target for renal cell carcinoma. Front Oncol (2021) 11:773063. doi: 10.3389/fonc.2021.773063
28. Feng D, Gao P, Henley N, Dubuissez M, Chen N, Laurin LP, et al. SMOC2 promotes an epithelial-mesenchymal transition and a pro-metastatic phenotype in epithelial cells of renal cell carcinoma origin. Cell Death Dis (2022) 13(7):639. doi: 10.1038/s41419-022-05059-2
29. Nixon AB, Halabi S, Liu Y, Starr MD, Brady JC, Shterev I, et al. Predictive biomarkers of overall survival in patients with metastatic renal cell carcinoma treated with IFNalpha +/- bevacizumab: results from CALGB 90206 (Alliance). Clin Cancer Res (2022) 28(13):2771–8. doi: 10.1158/1078-0432.CCR-21-2386
30. Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol (2015) 33(13):1430–7. doi: 10.1200/JCO.2014.59.0703
31. Khetani VV, Portal DE, Shah MR, Mayer T, Singer EA. Combination drug regimens for metastatic clear cell renal cell carcinoma. World J Clin Oncol (2020) 11(8):541–62. doi: 10.5306/wjco.v11.i8.541
32. Escudier B. Combination therapy as first-line treatment in metastatic renal-cell carcinoma. N Engl J Med (2019) 380(12):1176–8. doi: 10.1056/NEJMe1900887
33. McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med (2018) 24(6):749–57. doi: 10.1038/s41591-018-0053-3
34. Tamada S, Kondoh C, Matsubara N, Mizuno R, Kimura G, Anai S, et al. Pembrolizumab plus axitinib versus sunitinib in metastatic renal cell carcinoma: outcomes of Japanese patients enrolled in the randomized, phase III, open-label KEYNOTE-426 study. Int J Clin Oncol (2022) 27(1):154–64. doi: 10.1007/s10147-021-02014-7
35. Grunwald V, Powles T, Choueiri TK, Hutson TE, Porta C, Eto M, et al. Lenvatinib plus everolimus or pembrolizumab versus sunitinib in advanced renal cell carcinoma: study design and rationale. Future Oncol (2019) 15(9):929–41. doi: 10.2217/fon-2018-0745
36. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med (2021) 384(9):829–41. doi: 10.1056/NEJMoa2026982
37. Motzer RJ, Jonasch E, Boyle S, Carlo MI, Manley B, Agarwal N, et al. NCCN guidelines insights: kidney cancer, version 1.2021. J Natl Compr Canc Netw (2020) 18(9):1160–70. doi: 10.6004/jnccn.2020.0043
38. Navani V, Heng DYC. Treatment selection in first-line metastatic renal cell carcinoma-the contemporary treatment paradigm in the age of combination therapy: a review. JAMA Oncol (2022) 8(2):292–9. doi: 10.1001/jamaoncol.2021.4337
39. Kumar SK, Callander NS, Adekola K, Anderson LD, Baljevic M, Campagnaro E, et al. Kidney cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(1):67–81. doi: 10.6004/jnccn.2022.0001
40. Vento JA, Rini BI. Treatment of refractory metastatic renal cell carcinoma. Cancers (Basel) (2022) 14(20):5005. doi: 10.3390/cancers14205005
41. Diaz-Montero CM, Rini BI, Finke JH. The immunology of renal cell carcinoma. Nat Rev Nephrol (2020) 16(12):721–35. doi: 10.1038/s41581-020-0316-3
42. Conroy M, Naidoo J. Immune-related adverse events and the balancing act of immunotherapy. Nat Commun (2022) 13(1):392. doi: 10.1038/s41467-022-27960-2
43. Rosellini M, Marchetti A, Mollica V, Rizzo A, Santoni M, Massari F. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat Rev Urol (2023) 20(3):133–57. doi: 10.1038/s41585-022-00676-0
44. Heidegger I, Pircher A, Pichler R. Targeting the tumor microenvironment in renal cell cancer biology and therapy. Front Oncol (2019) 9:490. doi: 10.3389/fonc.2019.00490
45. Anker J, Miller J, Taylor N, Kyprianou N, Tsao CK. From bench to bedside: how the tumor microenvironment is impacting the future of immunotherapy for renal cell carcinoma. Cells (2021) 10(11):3231. doi: 10.3390/cells10113231
46. Tang T, Huang X, Zhang G, Hong Z, Bai X, Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther (2021) 6(1):72. doi: 10.1038/s41392-020-00449-4
47. Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet (2019) 393(10189):2404–15. doi: 10.1016/S0140-6736(19)30723-8
48. Rosellini M, Marchetti A, Mollica V, Rizzo A, Santoni M, Massari F. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat Rev Urol (2023) 20(3):133–57. doi: 10.1038/s41585-022-00676-0
49. Klumper N, Ralser DJ, Zarbl R, Schlack K, Schrader AJ, Rehlinghaus M, et al. CTLA4 promoter hypomethylation is a negative prognostic biomarker at initial diagnosis but predicts response and favorable outcome to anti-PD-1 based immunotherapy in clear cell renal cell carcinoma. J Immunother Cancer (2021) 9(8):e002949. doi: 10.1136/jitc-2021-002949
50. Ross-Macdonald P, Walsh AM, Chasalow SD, Ammar R, Papillon-Cavanagh S, Szabo PM, et al. Molecular correlates of response to nivolumab at baseline and on treatment in patients with RCC. J Immunother Cancer (2021) 9(3):e001506. doi: 10.1136/jitc-2020-001506
51. Carlisle JW, Jansen CS, Cardenas MA, Sobierajska E, Reyes AM, Greenwald R, et al. Clinical outcome following checkpoint therapy in renal cell carcinoma is associated with a burst of activated CD8 T cells in blood. J Immunother Cancer (2022) 10(7):e004803. doi: 10.1136/jitc-2022-004803
52. Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med (2020) 26(5):688–92. doi: 10.1038/s41591-020-0856-x
53. Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med (2018) 378(14):1277–90. doi: 10.1056/NEJMoa1712126
54. Choueiri TK, Larkin J, Oya M, Thistlethwaite F, Martignoni M, Nathan P, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol (2018) 19(4):451–60. doi: 10.1016/S1470-2045(18)30107-4
55. Atkins MB, Plimack ER, Puzanov I, Fishman MN, McDermott DF, Cho DC, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol (2018) 19(3):405–15. doi: 10.1016/S1470-2045(18)30081-0
56. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med (2019) 380(12):1116–27. doi: 10.1056/NEJMoa1816714
57. Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med (2021) 384(14):1289–300. doi: 10.1056/NEJMoa2035716
58. Chen BH, Kao CC, Xu T, Yang YN, Cha TL, Tsai YT, et al. Determining programmed cell death ligand 1 expression in circulating tumor cells of patients with clear cell renal cell carcinoma and its correlation with response to programmed cell death protein 1 inhibitors. Int J Urol (2022) 29(9):947–54. doi: 10.1111/iju.14812
59. Incorvaia L, Fanale D, Badalamenti G, Porta C, Olive D, De Luca I, et al. Baseline plasma levels of soluble PD-1, PD-L1, and BTN3A1 predict response to nivolumab treatment in patients with metastatic renal cell carcinoma: a step toward a biomarker for therapeutic decisions. Oncoimmunology (2020) 9(1):1832348. doi: 10.1080/2162402X.2020.1832348
60. Pignon JC, Jegede O, Shukla SA, Braun DA, Horak CE, Wind-Rotolo M, et al. irRECIST for the evaluation of candidate biomarkers of response to nivolumab in metastatic clear cell renal cell carcinoma: analysis of a phase II prospective clinical trial. Clin Cancer Res (2019) 25(7):2174–84. doi: 10.1158/1078-0432.CCR-18-3206
61. Terry S, Dalban C, Rioux-Leclercq N, Adam J, Meylan M, Buart S, et al. Association of AXL and PD-L1 expression with clinical outcomes in patients with advanced renal cell carcinoma treated with PD-1 blockade. Clin Cancer Res (2021) 27(24):6749–60. doi: 10.1158/1078-0432.CCR-21-0972
62. Xu W, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapy in kidney cancer. Nat Rev Urol (2020) 17(3):137–50. doi: 10.1038/s41585-020-0282-3
63. Paver EC, Cooper WA, Colebatch AJ, Ferguson PM, Hill SK, Lum T, et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: a guide to immunohistochemistry implementation and interpretation. Pathology (2021) 53(2):141–56. doi: 10.1016/j.pathol.2020.10.007
64. Bakouny Z, Braun DA, Shukla SA, Pan W, Gao X, Hou Y, et al. Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun (2021) 12(1):808. doi: 10.1038/s41467-021-21068-9
65. Kahlmeyer A, Stohr CG, Hartmann A, Goebell PJ, Wullich B, Wach S, et al. Expression of PD-1 and CTLA-4 are negative prognostic markers in renal cell carcinoma. J Clin Med (2019) 8(5):743. doi: 10.3390/jcm8050743
66. Takamatsu K, Tanaka N, Hakozaki K, Takahashi R, Teranishi Y, Murakami T, et al. Profiling the inhibitory receptors LAG-3, TIM-3, and TIGIT in renal cell carcinoma reveals malignancy. Nat Commun (2021) 12(1):5547. doi: 10.1038/s41467-021-25865-0
67. Yuan J, Jiang B, Zhao H, Huang Q. Prognostic implication of TIM-3 in clear cell renal cell carcinoma. Neoplasma (2014) 61(1):35–40. doi: 10.4149/neo_2014_006
68. Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer (2012) 12(4):298–306. doi: 10.1038/nrc3245
69. Choueiri TK, Fishman MN, Escudier B, McDermott DF, Drake CG, Kluger H, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res (2016) 22(22):5461–71. doi: 10.1158/1078-0432.CCR-15-2839
70. Li Y, Wang Z, Jiang W, Zeng H, Liu Z, Lin Z, et al. Tumor-infiltrating TNFRSF9(+) CD8(+) T cells define different subsets of clear cell renal cell carcinoma with prognosis and immunotherapeutic response. Oncoimmunology (2020) 9(1):1838141. doi: 10.1080/2162402X.2020.1838141
71. Kawashima A, Kanazawa T, Kidani Y, Yoshida T, Hirata M, Nishida K, et al. Tumour grade significantly correlates with total dysfunction of tumour tissue-infiltrating lymphocytes in renal cell carcinoma. Sci Rep (2020) 10(1):6220. doi: 10.1038/s41598-020-63060-1
72. Dai S, Zeng H, Liu Z, Jin K, Jiang W, Wang Z, et al. Intratumoral CXCL13(+)CD8(+)T cell infiltration determines poor clinical outcomes and immunoevasive contexture in patients with clear cell renal cell carcinoma. J Immunother Cancer (2021) 9(2):e001823. doi: 10.1136/jitc-2020-001823
73. Qi Y, Xia Y, Lin Z, Qu Y, Qi Y, Chen Y, et al. Tumor-infiltrating CD39(+)CD8(+) T cells determine poor prognosis and immune evasion in clear cell renal cell carcinoma patients. Cancer Immunol Immunother (2020) 69(8):1565–76. doi: 10.1007/s00262-020-02563-2
74. Bi K, He MX, Bakouny Z, Kanodia A, Napolitano S, Wu J, et al. Tumor and immune reprogramming during immunotherapy in advanced renal cell carcinoma. Cancer Cell (2021) 39(5):649–61 e5. doi: 10.1016/j.ccell.2021.02.015
75. Hopkins AC, Yarchoan M, Durham JN, Yusko EC, Rytlewski JA, Robins HS, et al. T Cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI Insight (2018) 3(13):e122092. doi: 10.1172/jci.insight.122092
76. Liu YY, Yang QF, Yang JS, Cao RB, Liang JY, Liu YT, et al. Characteristics and prognostic significance of profiling the peripheral blood T-cell receptor repertoire in patients with advanced lung cancer. Int J Cancer (2019) 145(5):1423–31. doi: 10.1002/ijc.32145
77. Kato T, Kiyotani K, Tomiyama E, Koh Y, Matsushita M, Hayashi Y, et al. Peripheral T cell receptor repertoire features predict durable responses to anti-PD-1 inhibitor monotherapy in advanced renal cell carcinoma. Oncoimmunology (2021) 10(1):1862948. doi: 10.1080/2162402X.2020.1862948
78. Zhou N, Lu F, Liu C, Xu K, Huang J, Yu D, et al. IL-8 induces the epithelial-mesenchymal transition of renal cell carcinoma cells through the activation of AKT signaling. Oncol Lett (2016) 12(3):1915–20. doi: 10.3892/ol.2016.4900
79. Yuen KC, Liu LF, Gupta V, Madireddi S, Keerthivasan S, Li C, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med (2020) 26(5):693–8. doi: 10.1038/s41591-020-0860-1
80. Sang YB, Yang H, Lee WS, Lee SJ, Kim SG, Cheon J, et al. High serum levels of IL-6 predict poor responses in patients treated with pembrolizumab plus axitinib for advanced renal cell carcinoma. Cancers (Basel) (2022) 14(23):5985. doi: 10.3390/cancers14235985
81. Bakouny Z, Choueiri TK. IL-8 and cancer prognosis on immunotherapy. Nat Med (2020) 26(5):650–1. doi: 10.1038/s41591-020-0873-9
82. Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol (2017) 28(8):1988–95. doi: 10.1093/annonc/mdx190
83. Ozawa Y, Amano Y, Kanata K, Hasegwa H, Matsui T, Kakutani T, et al. Impact of early inflammatory cytokine elevation after commencement of PD-1 inhibitors to predict efficacy in patients with non-small cell lung cancer. Med Oncol (2019) 36(4):33. doi: 10.1007/s12032-019-1255-3
84. Shen W, Bian L, Ma Y, Yin X. Serum IL-6 as a marker of disease progression in interstitial nephritis. Am J Transl Res (2022) 14(5):3189–97.
85. Abdel Galil SM, Ezzeldin N, El-Boshy ME. The role of serum IL-17 and IL-6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine (2015) 76(2):280–7. doi: 10.1016/j.cyto.2015.05.007
86. Yuan L, Wang Q, Zhang S, Zhang L. Correlation between serum inflammatory factors TNF-alpha, IL-8, IL-10 and henoch-schonlein purpura with renal function impairment. Exp Ther Med (2018) 15(4):3924–8. doi: 10.3892/etm.2018.5876
87. Hashmi F, Mollapour M, Bratslavsky G, Bourboulia D. MMPs, tyrosine kinase signaling and extracellular matrix proteolysis in kidney cancer. Urol Oncol (2021) 39(6):316–21. doi: 10.1016/j.urolonc.2020.04.034
88. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov (2022) 12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059
89. Xu F, Chang K, Ma J, Qu Y, Xie H, Dai B, et al. The oncogenic role of COL23A1 in clear cell renal cell carcinoma. Sci Rep (2017) 7(1):9846. doi: 10.1038/s41598-017-10134-2
90. Jokelainen O, Pasonen-Seppanen S, Tammi M, Mannermaa A, Aaltomaa S, Sironen R, et al. Cellular hyaluronan is associated with a poor prognosis in renal cell carcinoma. Urol Oncol (2020) 38(8):686 e11– e22. doi: 10.1016/j.urolonc.2020.03.029
91. Chen J, Ye Z, Liu L, Xuan B. Assessment of the prognostic value of SPOCK1 in clear cell renal cell carcinoma: a bioinformatics analysis. Transl Androl Urol. (2022) 11(4):509–18. doi: 10.21037/tau-22-161
92. Veit G, Zwolanek D, Eckes B, Niland S, Kapyla J, Zweers MC, et al. Novel ligand for integrin alpha2beta1 in the epidermis. J Biol Chem (2011) 286(31):27804–13. doi: 10.1074/jbc.M111.220046
93. Di Benedetto P, Ruscitti P, Liakouli V, Del Galdo F, Giacomelli R, Cipriani P. Linking myofibroblast generation and microvascular alteration: the role of CD248 from pathogenesis to therapeutic target (Review). Mol Med Rep (2019) 20(2):1488–98. doi: 10.3892/mmr.2019.10429
94. Oxburgh L. The extracellular matrix environment of clear cell renal cell carcinoma. Cancers (Basel) (2022) 14(17):4072. doi: 10.3390/cancers14174072
95. Cowell LG. The diagnostic, prognostic, and therapeutic potential of adaptive immune receptor repertoire profiling in cancer. Cancer Res (2020) 80(4):643–54. doi: 10.1158/0008-5472.CAN-19-1457
96. Porciello N, Franzese O, D’Ambrosio L, Palermo B, Nistico P. T-Cell repertoire diversity: friend or foe for protective antitumor response? J Exp Clin Cancer Res (2022) 41(1):356. doi: 10.1186/s13046-022-02566-0
97. Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, et al. An immune atlas of clear cell renal cell carcinoma. Cell (2017) 169(4):736–49 e18. doi: 10.1016/j.cell.2017.04.016
98. Kartikasari AER, Huertas CS, Mitchell A, Plebanski M. Tumor-induced inflammatory cytokines and the emerging diagnostic devices for cancer detection and prognosis. Front Oncol (2021) 11:692142. doi: 10.3389/fonc.2021.692142
99. Wang R, Zheng J, Shao X, Ishii Y, Roy A, Bello A, et al. Development of a prognostic composite cytokine signature based on the correlation with nivolumab clearance: translational PK/PD analysis in patients with renal cell carcinoma. J Immunother Cancer (2019) 7(1):348. doi: 10.1186/s40425-019-0819-2
Keywords: metastatic renal cell carcinoma, immunotherapy, tumor microenvironment, biomarker, PD-L1
Citation: Su J, Zhou L, Zhang Z, Xiao X, Qin Y, Zhou X and Huang T (2023) The components of tumor microenvironment as biomarker for immunotherapy in metastatic renal cell carcinoma. Front. Immunol. 14:1146738. doi: 10.3389/fimmu.2023.1146738
Received: 17 January 2023; Accepted: 26 May 2023;
Published: 07 June 2023.
Edited by:
Jianzhong Ai, Sichuan University, ChinaReviewed by:
Giuseppe Schepisi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyJoanna Bogusławska, Medical Centre for Postgraduate Education, Poland
Copyright © 2023 Su, Zhou, Zhang, Xiao, Qin, Zhou and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingting Huang, dGluZ3RpbmdodWFuZzE5ODZAZ21haWwuY29t; Xiaoying Zhou, emhvdXhpYW95aW5nMTk4MkBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
 Jiaming Su
Jiaming Su Lu Zhou
Lu Zhou Zhe Zhang
Zhe Zhang Xue Xiao
Xue Xiao Yanning Qin3
Yanning Qin3 Xiaoying Zhou
Xiaoying Zhou Tingting Huang
Tingting Huang