- 1National Clinical Research Centre for Haematologic Diseases, Jiangsu Institute of Haematology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Institute of Blood and Marrow Transplantation, Collaborative Innovation Centre of Haematology, Soochow University, Suzhou, China
- 3Key Laboratory of Thrombosis and Haemostasis of Ministry of Health, Suzhou, China
- 4State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou, China
Purpose: Appropriate pre-transplant strategies in patients with myelodysplastic syndromes (MDS) remain challenging. We sought to assess the effect of different pre-transplant therapies and transplantation interval times on patient prognosis.
Methods: We retrospectively analysed clinical data for 371 consecutive MDS patients after myeloablative transplantation between 2007 and 2019.
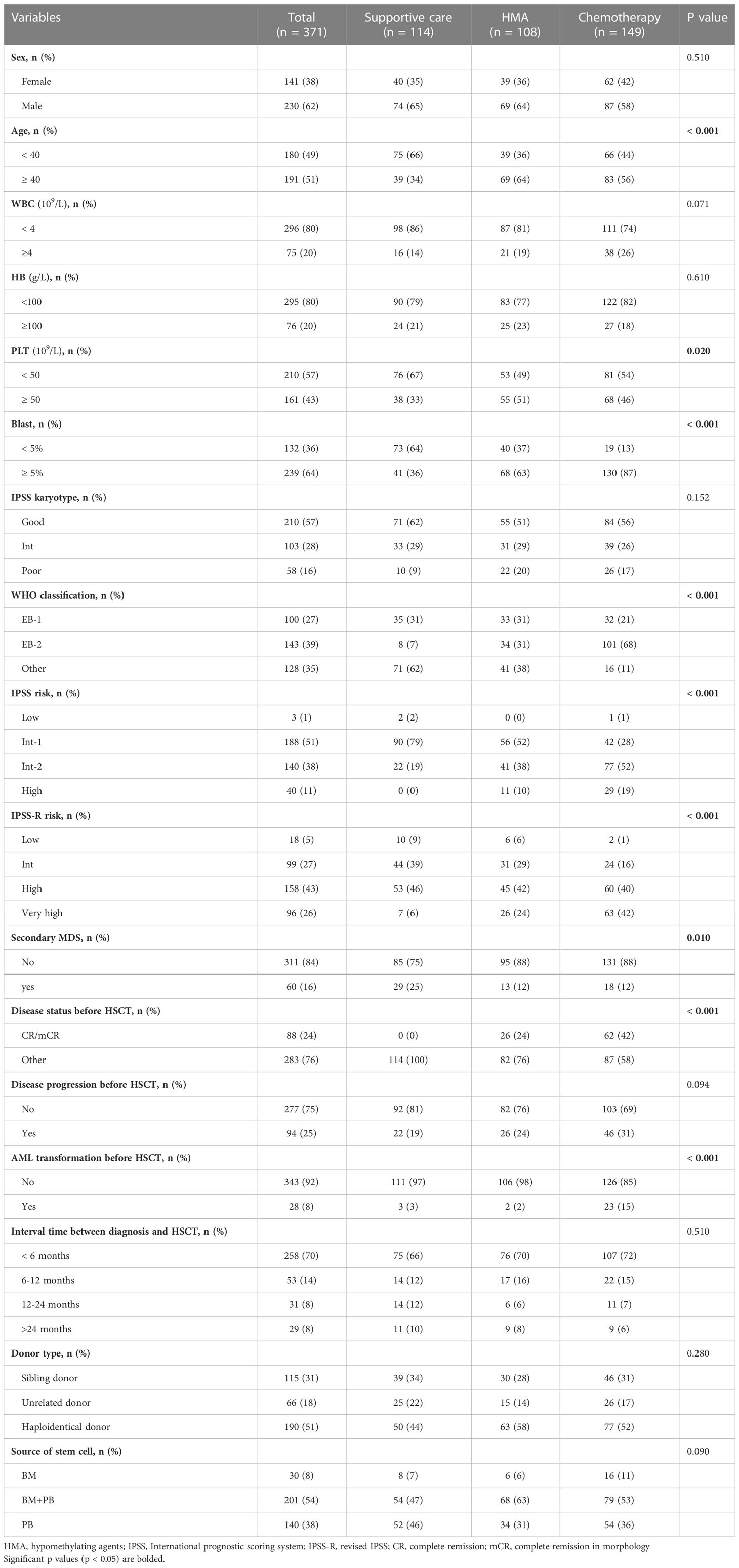
Results: The median age of the patients was 38 years (range, 12–64 years). A total of 114 patients (31%) received supportive care (SC), 108 (29%) hypomethylating agents (HMAs), and 149 (40%) chemotherapy-based therapy before transplantation. In patients who received HMA or SC, there was no significant difference in overall survival (OS; P=0.151) or relapse-free survival (RFS; P=0.330), except that HMA-treated patients had a lower rate of non-relapse mortality (5-year NRM: 18% vs. 32%, P=0.035). However, compared with patients who received HMA, those who received chemotherapy-based therapy had a lower 5-year OS rate (56% vs. 69%, P=0.020) and a slightly higher 5-year NRM rate (28% vs. 18%, P=0.067). Compared to the delayed transplant group (transplant interval ≥6 months), the early transplant group (transplant interval <6 months) had a superior 5-year OS (66% vs. 51%, P=0.001) and a lower 5-year cumulative incidence of NRM (22% vs. 36%, P=0.001).
Conclusion: The findings of the study indicate that receiving an appropriate pre-transplant strategy (SC/HMA + <6 months) significantly improves OS and decreases NRM in MDS patients after myeloablative transplantation.
Introduction
Myelodysplastic syndromes (MDS) are a group of clonal haematopoietic stem cell disorders characterized by cytopaenia, ineffective haematopoiesis, and increased risk of evolution to acute myeloblastic leukaemia (AML) (1). With the growing understanding of the pathophysiology of MDS, epigenetic therapy with hypomethylating agents (HMAs), including azacitidine (AZA) and decitabine (DAC), has become the standard treatment. Despite their low toxicity and ability to induce a haematological response and survival improvement (2–4), HMAs are not curative therapies for MDS.
Allogeneic haematopoietic stem cell transplantation (HSCT) is the only potentially curative therapeutic option; it significantly improves overall survival (OS) and disease-free survival, depending on prognostic features (5–7). The role of different pre-transplant therapies in patients who are transplant candidates has been investigated. In a series of small retrospective analyses, HMAs were found to confer no clear benefit when given pre-HSCT (8–12). Additionally, one study comparing outcomes after chemotherapy, HMAs, or best supportive care (SC) pre-allo-HSCT reported no prognostic advantage with any treatment (13). However, sequential treatment with AZA followed or preceded by chemotherapy was found to adversely influence both OS and event-free survival (9).
In addition to pre-transplant therapies, optimal timing of allo-HSCT for a given patient with MDS is important. The most cited Markov decision model compares life expectancy after myeloablative conditioning (MAC) transplantation for younger patients, showing that early transplantation is associated with maximal life expectancy in patients with International Prognostic Scoring System (IPSS) intermediate-2 and high-risk disease stages (14). Koreth et al. employed the Markov decision model and found similar results for older patients who received HSCT in the reduced intensity conditioning (RIC) setting (15). As different pre-transplant therapies result in different interval times between diagnosis and transplantation, the decision of an appropriate pre-transplantation strategy presents many challenges when considering these two factors.
The aim of this study was to assess the effect of different pre-transplant therapies (HMA, chemotherapy and SC) and transplantation interval times on patient prognosis. Based on the results, we propose an appropriate pre-transplant strategy for MDS patients who received allo-HSCT after MAC.
Patients and methods
Patient selection
Between December 2007 and September 2019, 415 consecutive MDS patients underwent allo-HSCT at the First Affiliated Hospital of Soochow University. To exclude the impact of different conditioning approaches, 44 patients who received the RIC conditioning regimen were excluded from our analysis. Thus, 371 patients who received allo-HSCT after the MAC conditioning regimen were included in the final analysis. Informed consent was obtained from all patients prior to data collection. This study was approved by the Committee for the Ethical Review of Research at The First Affiliated Hospital of Soochow University and conducted in accordance with institutional guidelines and the Declaration of Helsinki.
Diagnosis of MDS was based on the 2016 World Health Organization (WHO) classification (16). IPSS and revised IPSS (IPSS-R) scores were used for risk evaluation (17, 18). Patients were divided into three groups according to treatment received prior to transplantation, as follows: SC, HMA alone and chemotherapy-based treatment (chemotherapy). SC includes growth factors (erythropoietin, granulocyte colony-stimulating factor), hormones, blood transfusion, immunosuppressive treatment and antibiotics. HMA includes DAC (20 mg/m2 on Days 1–5, for each cycle) or AZA (75 mg/m2 on Days 1–7, for each cycle). Chemotherapy-based treatment includes induction chemotherapy alone and HMA plus chemotherapy. Induction chemotherapy includes low-dose CAG or revised CAG regimens (IAG and HAG). The CAG regimen consisted of cytarabine at 10 mg/m2, q12 h on Days 1–7; aclarubicin at 7 mg/m2, qd on Days 1–4; and G-CSF at 200 μg/m2, qd on Days 1–7. The IAG regimen consisted of idarubicin at 8 mg/m2, qd on Days 1–3; cytarabine at 10 mg/m2, q12 h on Days 1–7; and G-CSF at 200 μg/m2, qd on Days 1–7. HAG consisted of homoharringtonine at 2 mg/m², qd, on Days 1–3; cytarabine at 10 mg/m2, q12 h on Days 1–7; and G-CSF at 200 μg/m2, qd on Days 1–7. For HMA plus chemotherapy, hypomethylating agents were administered before CAG or revised CAG regimens.
Transplantation modalities
A total of 115 patients (31%) received HLA-matched related donor transplantation (MRDT), 66 patients (18%) received HLA-matched unrelated donor transplantation (MUDT), and 190 patients (51%) received haploidentical stem cell transplantation (haplo-SCT). All patients were followed until death or June 2021. The patient characteristics are shown in Table 1.
All patients received MAC regimens. For MRDT, the MAC regimens comprised semustine (250 mg/m2, day −10), cytarabine (2 g/m2/d, days −9 to −8), busulfan (3.2 mg/kg/d, days −7 to −5), and cyclophosphamide (1.8 g/m2/d, days −4 to −3). For MUDT and haplo-SCT, patients received a MAC regimen identical to that for MRDT but with higher doses of cytarabine (4 g/m2/d, days −9 to −8). In addition, patients receiving MUDT received hydroxycarbamide (80 mg/kg, day −10). Rabbit anti-thymocyte globulin (ATG; Genzyme Polyclonals S.A.S, Lyon, France), ATG-F (Fresenius Biotech GmbH, Munich, Germany) or porcine antilymphocyte globulin (ALG; Wuhan Institute of Biological Products Co., Ltd., Wuhan, Hubei, China) was administered to patients receiving MUDT and haplo-SCT for graft-versus-host disease (GVHD) prophylaxis. The regimens were as follows: ATG 2.5 mg/kg/day for four days; ATG-F 5 mg/kg/day for four days; and ALG 15 mg/kg/day for four days. For a small number of patients who received MSDT, a lower dose of ATG (2.5 mg/kg/day, for two days) or ATG-F (5 mg/kg/day, for two days) was used.
Patients who underwent MRDT received GVHD prophylaxis consisting of cyclosporine and methotrexate. GVHD prophylaxis in patients who underwent MUDT or haplo-SCT consisted of cyclosporine, mycophenolate mofetil, and methotrexate.
Study endpoints, definitions, and statistical analysis
Marrow CR (mCR) indicates a clinical CR with a morphologically normal marrow but persistent cytopenia (19, 20). Overall survival (OS) was defined from the time of allo-HSCT to death, regardless of cause, or the last follow-up. Relapse-free survival (RFS) was defined as the time from allo-HSCT to treatment failure (death or relapse). NRM was defined as death from any cause in the first 28 days after allo-HSCT or death without evidence of disease recurrence beyond Day 28, with relapse as a competing event. Relapse was defined as disease morphological recurrence and/or reappearance of the underlying disease. Relapse incidence was estimated by considering relapse as the event of interest and death without relapse as a competing event.
OS and RFS were computed using the Kaplan–Meier method, and the log-rank test was used for univariate comparisons. Prognostic factors with values of P ≤ 0.1 in univariate analyses were entered into a Cox proportional hazards model to determine their effects on survival. The cumulative incidence method was applied to compute the incidence of NRM and relapse in a competing risk setting, with the Gray test applied for comparisons of different groups (21). For risk factors for cumulative incidence of relapse (CIR) and NRM, factors with values of P ≤ 0.1 in univariate analyses and those with clinical significance were chosen for further evaluation in the multivariate regression analysis proposed by Fine and Gray (22). All analyses were performed using the R software package (R version 4.0.3; The R Foundation for Statistical Computing, www.r-project.org).
Results
A total of 371 MDS patients received allo-HSCT after MAC conditioning. Of those, 114 patients (31%) received SC, 108 received HMA (29%, DAC in 104 cases and AZA in four cases), and 149 (40%) received low-dose chemotherapy, including 20 cases of chemotherapy alone and 129 DAC plus chemotherapy. The median age of the patients was 38 years (range, 12–64 years). Baseline characteristics were well balanced between the three groups, except as expected for investigator-preselected subgroups, namely, more patients with EB-2, higher blast percentage, or higher IPSS-R risk at diagnosis received chemotherapy (Table 1). After transplantation, 126 patients (34%) developed grade 2 to 4 acute GVHD (aGVHD), 144 (39%) developed chronic GVHD (cGVHD), and 36 (10%) had extensive cGVHD. For the whole patient population, the 3-year OS, 3-year RFS, 3-year CIR, and 3-year cumulative incidence of NRM were 66%, 86%, 10%, and 25%, respectively.
Outcome according to prior-to-transplant treatment
OS, RFS, CIR, and NRM based on different prior-to-transplant therapies are shown in Supplemental Figure 1. Among patients who received HMA or SC, there were no significant differences in OS (P=0.151) or RFS (P=0.330); however, patients in the HMA group had a lower rate of NRM (5-year NRM: 18% vs. 32%, P=0.035). When compared with patients who received HMA, those who received chemotherapy had lower 5-year OS rates (56% vs. 69%, P=0.020) and slightly higher 5-year NRM rates (28% vs. 18%, P=0.067). Based on these findings, we combined patients who received SC and those who received HMA into a non-chemotherapy group and found that the patients in this group had superior OS (P=0.061) and RFS (P=0.010) and a lower rate of relapse (P=0.010), with no significant difference in NRM in comparison to those in the chemotherapy group (Figure 1).
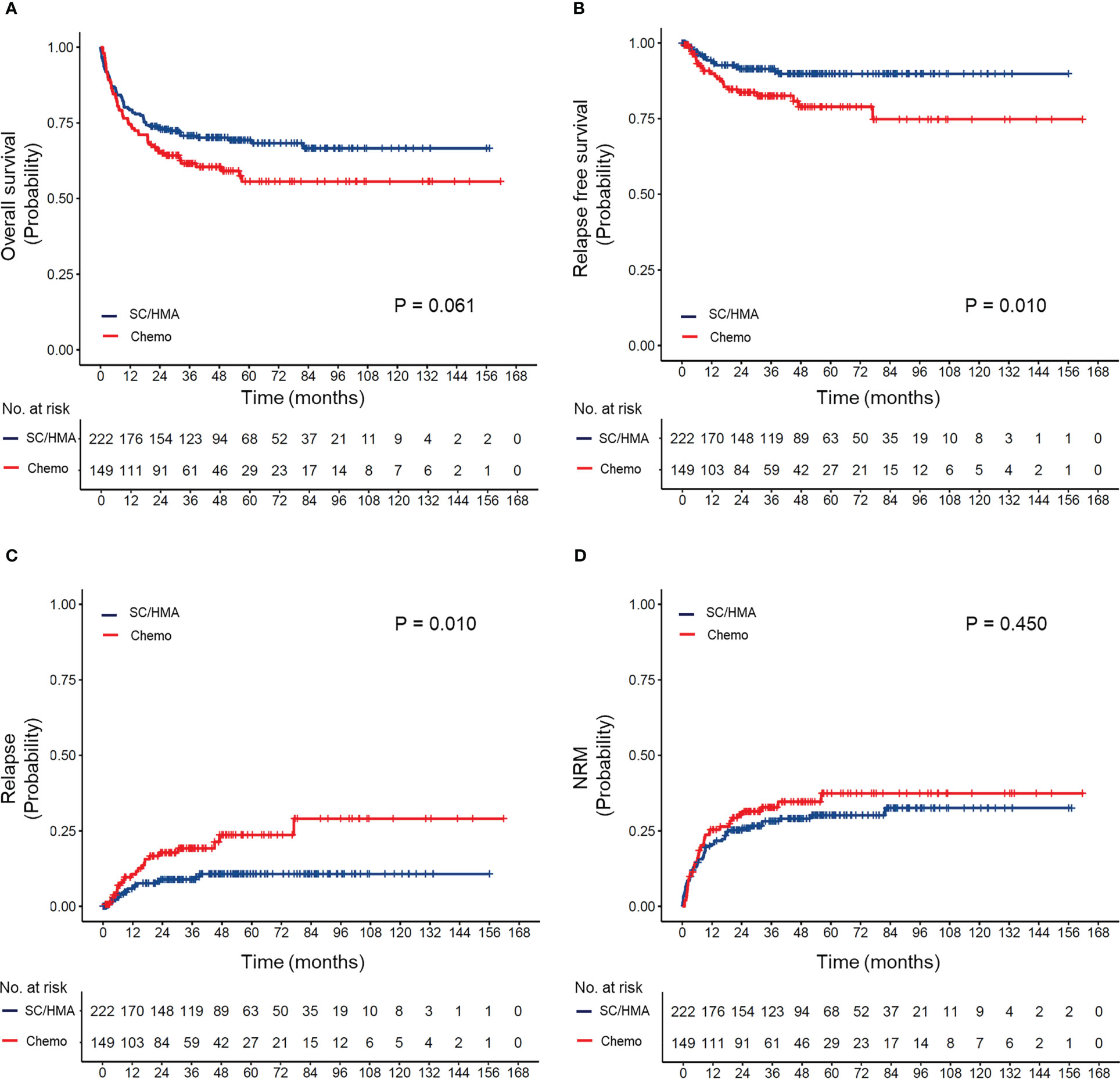
Figure 1 (A) Overall survival, (B) relapse-free survival, (C) cumulative incidence of relapse, and (D) cumulative incidence of non-relapse mortality (NRM) according to prior transplantation treatment received. SC, supportive care; HMA, hypomethylating agents (decitabine and azacitidine); Chemo, chemotherapy.
To a certain extent, HSCT was able to overcome the adverse prognostic impact of disease progression before transplantation, with no significant difference in terms of OS, RFS, CIR, and NRM between patients who experienced progression and those who did not. However, worse RFS and higher CIR were observed when the disease progressed to AML. The proportion of patients who achieved complete remission (CR) or marrow CR (mCR) was significantly higher in the chemotherapy group than in the HMA group (42% vs. 24%, P < 0.001). No patients in the supportive care group achieved CR or mCR. Notably, a higher response rate did not translate to a favourable prognosis in the chemotherapy group. Patients who achieved CR or mCR did not show any significant advantage in OS, RFS, CIR, or NRM (Table 2). Moreover, the type of treatment before HSCT had no significant effect on the incidence or severity of aGVHD or cGVHD. In addition, cytomegalovirus (CMV)/Epstein–Barr virus (EBV) reactivation did not differ among patients who received SC, HMA or chemotherapy pre-transplantation (Table 1).
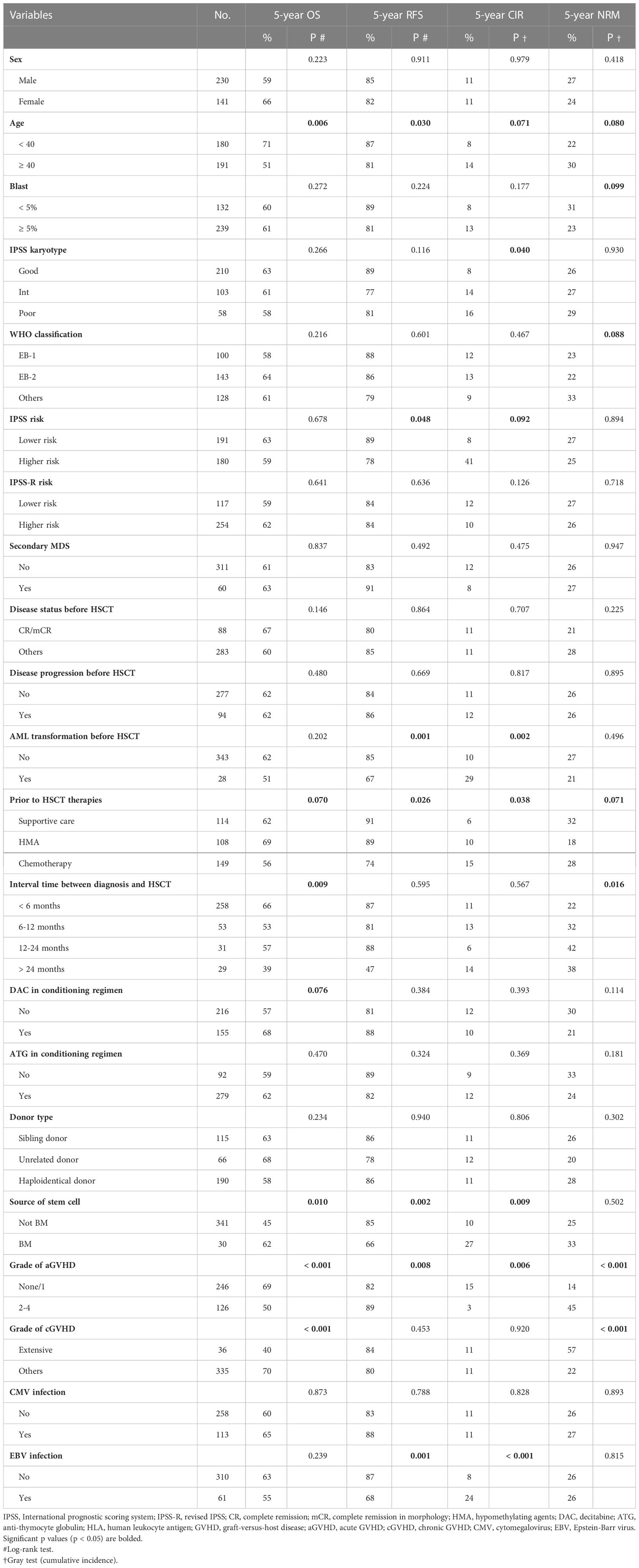
Table 2 Univariate analysis of prognostic factors for OS, RFS, cumulative incidence of relapse, and cumulative incidence of NRM.
Timing of transplantation
To evaluate the effect of differences in the timing of transplantation on transplant outcomes, patients were divided into four groups according to the transplant interval: less than 6 months, 6–12 months, 12–24 months, and more than 24 months. The OS, RFS, CIR, and NRM of the cohort based on different transplant interval times are shown in Supplemental Figure 2. Compared to the delayed transplant group (transplant interval ≥6 months), the early transplant group (transplant interval <6 months) had a superior 5-year OS (66% vs. 51%, P=0.001) and a lower 5-year cumulative incidence of NRM (22% vs. 36%, P=0.001) (Figure 2).
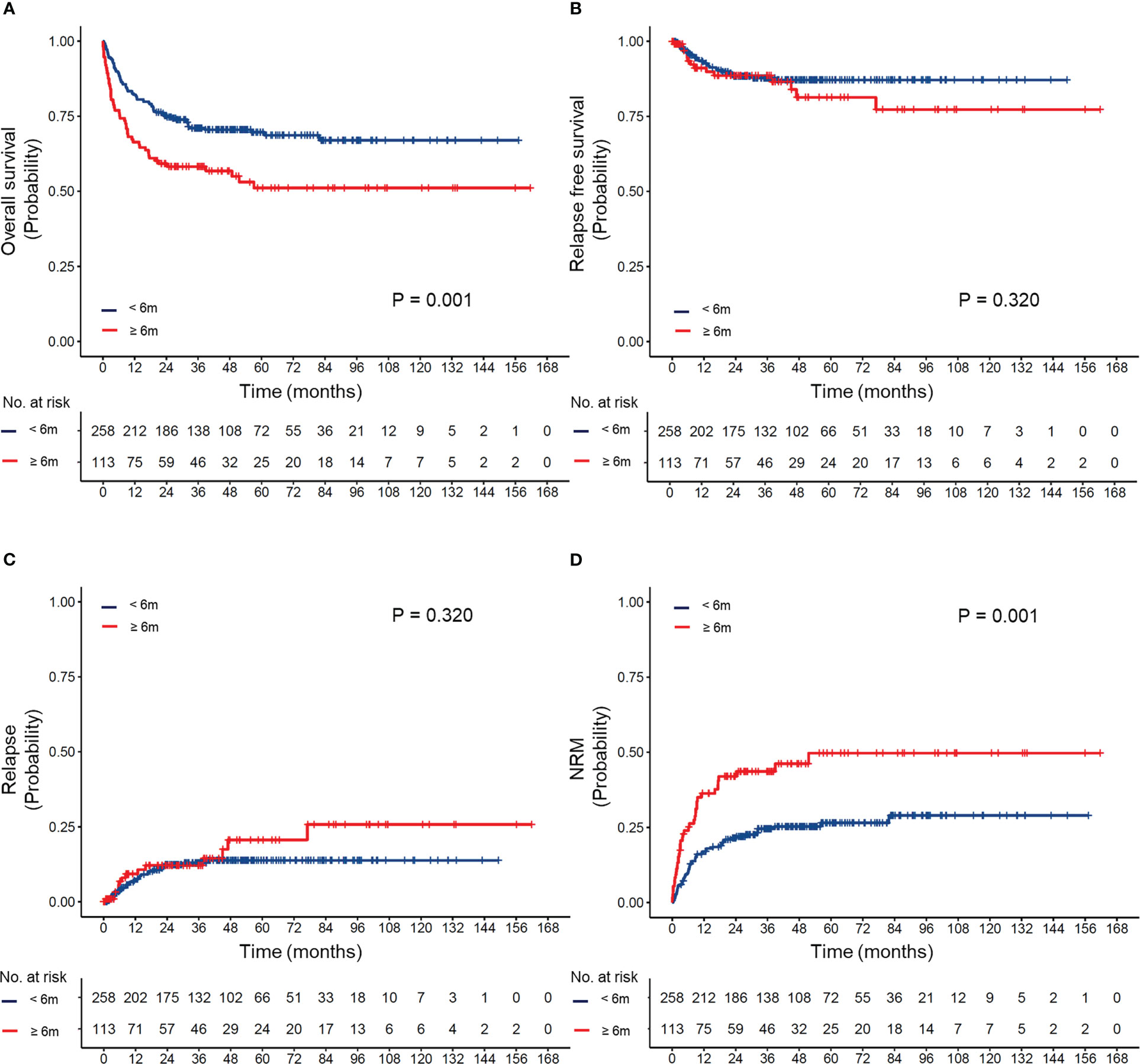
Figure 2 (A) Overall survival, (B) relapse-free survival, (C) cumulative incidence of relapse, and (D) cumulative incidence of non-relapse mortality (NRM) according to different intervals between diagnosis and transplantation.
The effect of differences in the transplant interval in patients with different IPSS-R risks is provided in Supplemental Figure 3 and Figure 3. Our results suggested that early transplantation conferred better OS and lower NRM after HSCT in both lower and higher-risk patients.
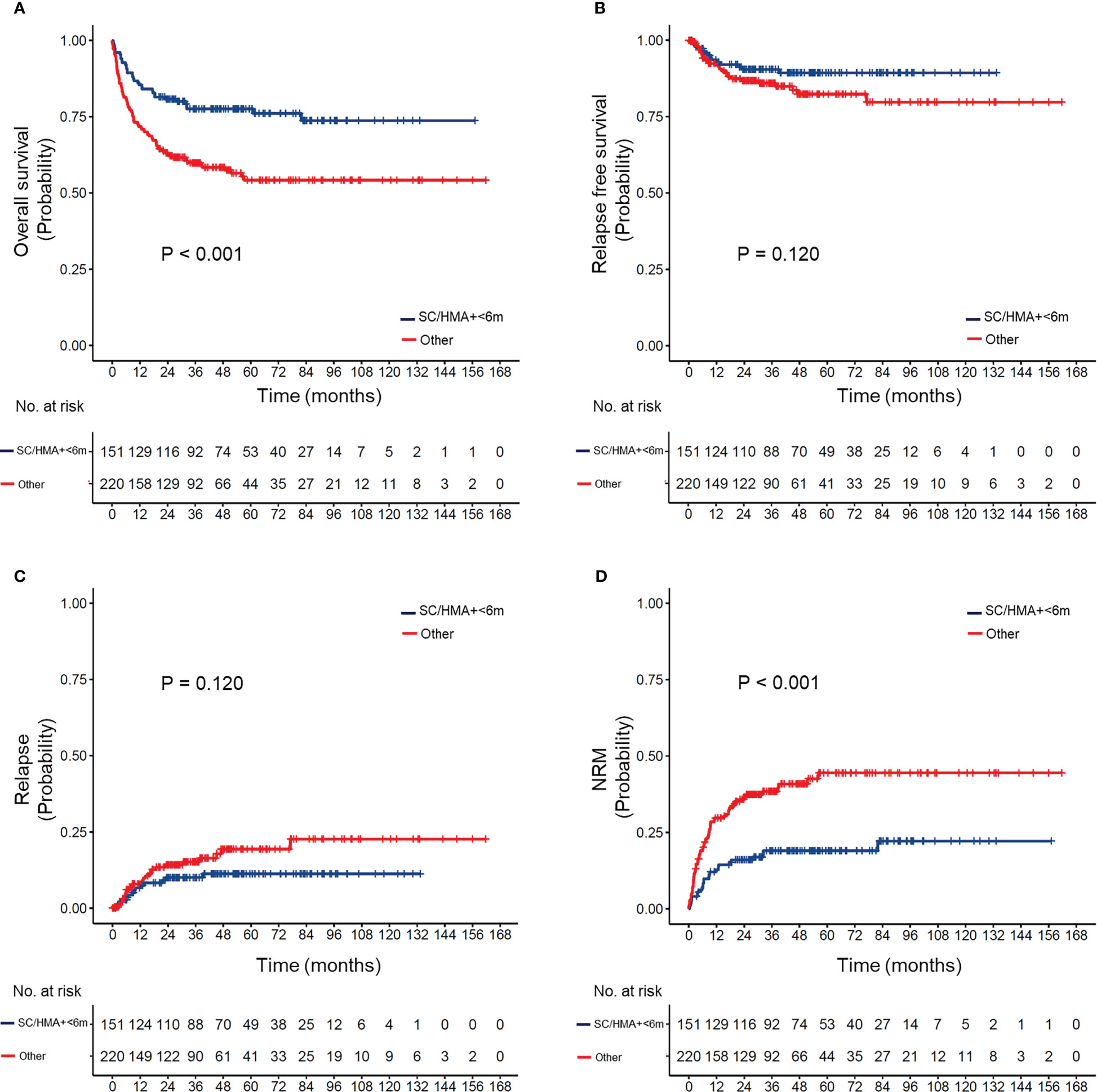
Figure 3 (A) Overall survival, (B) relapse-free survival, (C) cumulative incidence of relapse, and (D) non-relapse mortality (NRM) in MDS patients according to pre-transplant strategy (appropriate strategy vs. others). Appropriate strategy: MDS patients who received hypomethylating agents or supportive care and underwent HSCT within 6 months after diagnosis.
Appropriate pre-transplant treatment strategy
Different pre-transplant therapies may result in different intervals between diagnosis and transplantation. Therefore, we reclassified our patients according to the type of pre-transplant therapy and timing of HSCT. According to our results, patients in the SC/HMA + <6 months group had a superior OS and a lower rate of NRM than those in the other three groups after pairwise comparison (Supplemental Figure 4). No significant differences in OS were observed between the SC/HMA + ≥6 months, Chemo + <6 months, and Chemo + ≥6 months groups, though patients in the Chemo + ≥6 months group showed worse RFS after transplantation (Supplemental Figure 5). Therefore, receiving non-chemotherapy and transplantation within 6 months after diagnosis (SC/HMA + <6 months) was regarded as the most appropriate pre-transplant strategy in MDS patients.
Univariate analysis
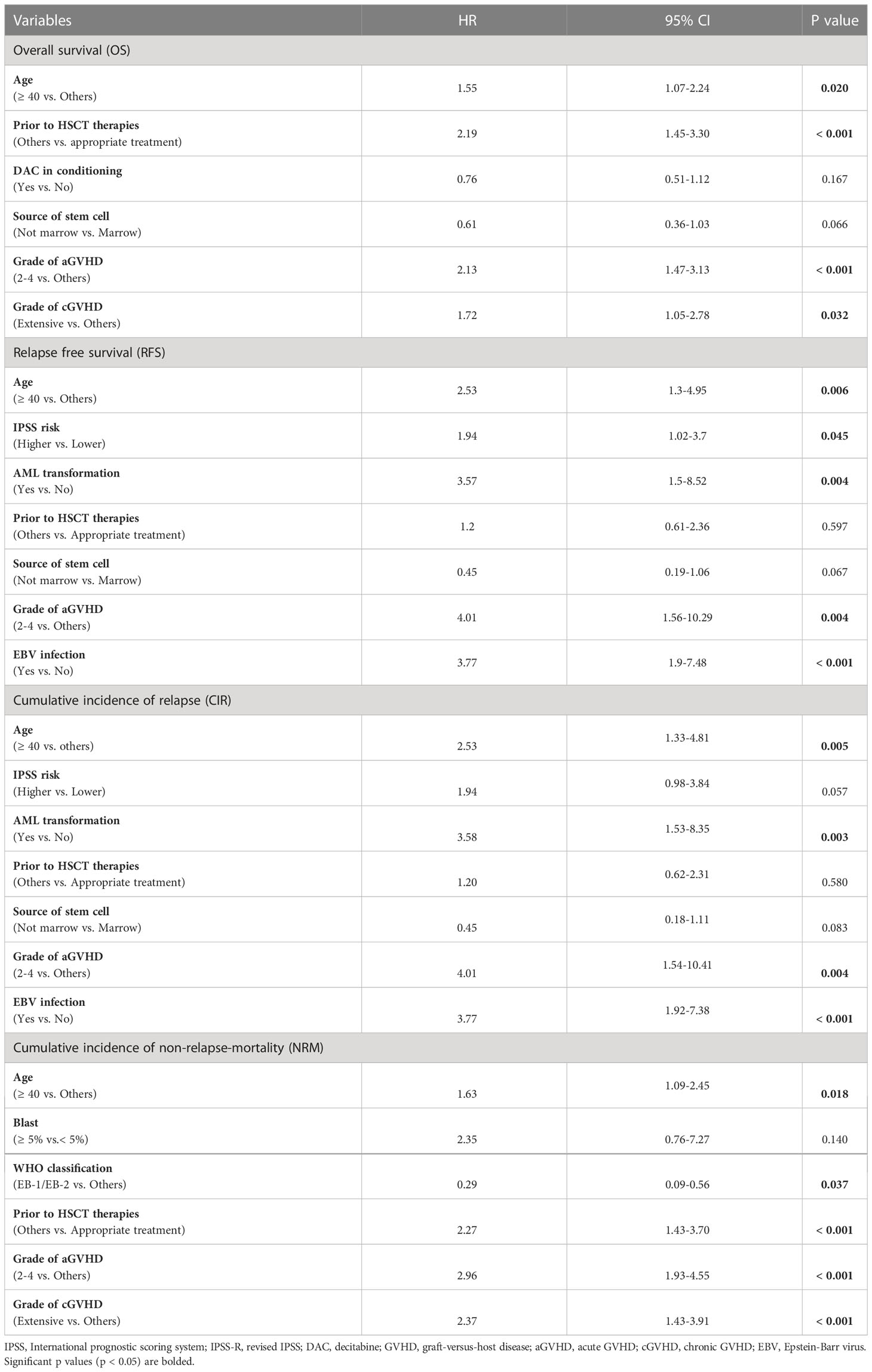
Among all at-diagnosis and/or at-transplantation characteristics studied, MDS patients who aged ≥ 40 years or received marrow-derived stem cells or experienced grade 2 to 4 aGVHD had inferior OS and RFS. Appropriate treatment strategy before HSCT, grade 2 to 4 aGVHD, and extensive cGVHD were found to correlate with OS and NRM (Table 2). Furthermore, AML transformation before HSCT and EBV infection after HSCT were found to be associated with RFS and CIR.
Multivariate analysis
The results of multivariate analysis of OS, RFS, CIR, and NRM are shown in Table 3. Older age (≥ 40 years) was an independent risk factor for OS (HR=1.55, P=0.020), RFS (HR=2.53, P=0.006), CIR (HR=2.53, P=0.005) and NRM (HR=1.63, P=0.018). Additionally, AML transformation before HSCT and EBV infection after HSCT adversely influenced RFS and CIR (Table 3), and grade 2 to 4 aGVHD adversely affected OS (HR=2.13, P<0.001), RFS (HR=4.01, P=0.004), CIR (HR=4.01, P=0.004), and NRM (HR=2.96, P<0.001). Moreover, the prognostic effect of an appropriate treatment strategy before HSCT on OS and NRM was confirmed in multivariate analysis (Table 3).
Discussion
To the best of our knowledge, this is the largest study to evaluate the use of HMAs (especially DAC), chemotherapy, and SC before transplantation in consecutive patients with MDS after long-term follow-up. An appropriate pre-transplant strategy (SC/HMA + <6 months) prolonged OS and decreased NRM in MDS patients receiving myeloablative allo-HSCT.
Disease status at the time of transplantation is one of the most important factors that influences outcomes after allo-HSCT (23), and the presence of more than 5% blasts at the time of transplantation is associated with poor prognosis (24). In our analysis, 24% of the HMA group and 42% of the chemotherapy group achieved CR at the time of HSCT (Supplemental Table 1). A similar result was observed in another clinical trial, with a higher haematological response to chemotherapy than AZA (25). We also assessed the impact of marrow blast on OS or RFS. The results were shown in Supplemental Figure 6. Overall, different blast levels did not impact OS (sFigure 6A, P=0.264). For RFS, patients with blast level of ≥15% showed a trend of worse RFS (sFigure 6B, P=0.056). Although chemotherapy was superior to HMA before transplantation, providing a better and more rapid reduction in the marrow blast percentage, it did not translate to a survival benefit, and the value of reducing the blast percentage before transplantation in MDS remains unclear (26). A recent study by Potter et al. using data from a large registry suggested that the post-transplant outcomes of patients with MDS who were not in CR were not significantly worse than those who were in CR (27). This is in line with our results that the marrow response was not an indepenW1dent predictor of a favourable prognosis for OS and RFS after transplantation. Furthermore, pre-transplant chemotherapy is associated with substantial morbidity and mortality, which may ultimately result in fewer patients undergoing HSCT. These findings suggest that achieving CR or mCR prior to HSCT is not a requisite for prolonged survival (27, 28). Taking the MAC conditioning into account, we prefer lower intensive regimes such as IAG or HAG instead of a 3 + 7 induction regimen for patients with higher-risk disease. In total, although the optimum strategy has not been determined, lower intensive regimes are commonly selected for higher-risk MDS patients with a transplant intention in our centre.
In our study, and in accordance with the report of Fenaux et al., treatment with HMA before transplantation prolonged OS and lowered the risk of relapse compared with treatment with conventional care in MDS (25). The mechanisms of AZA and DAC activity in MDS have been explored. HMA therapy may play a role in stabilizing the disease and may offer benefits with respect to relapse reduction via epigenetic modulation. Damaj et al. reported that 15% of AZA group patients experienced disease progression before transplantation compared with 51% of chemotherapy group patients (9). Similarly, 2% of patients in our study who received HMA experienced progression to AML before transplantation, which was significantly lower than the 15% of those who received chemotherapy. It has been reported that AZA can promote induction of CD8+ T-cell responses in AML (29). Administration of AZA post-transplantation increases the number of Tregs and induces a CD8+ T-cell response to a number of tumour antigens, including MAGE, BAGE, and Wilms’ tumour antigen 1 (29). Addition of DAC to conditioning therapy also induces tumour-associated antigen-specific T-cell responses, augmenting a greater graft-versus-leukaemia response after HSCT (30).
Although it is the only curative treatment available for patients with MDS, allo-HSCT is associated with a significant risk of early morbidity and mortality and therefore is not used as first-line treatment in MDS. For patients who are candidates for HSCT, choosing the optimal time to perform HSCT remains a major challenge in clinical practice. According to Markov model decision analyses, for patients with advanced IPSS risk (intermediate-2 and high), the strategy that maximizes discounted life expectancy is transplantation at the time of diagnosis (14). Similar to the results from decision analyses, our findings support that proceeding to HSCT for patients with higher IPSS-R risk within 6 months after diagnosis is associated with superior OS and RFS (Supplemental Figure 4). For those with lower IPSS risk (low and intermediate-1), watchful waiting and supportive care is appropriate, and delayed transplantation prior to the development of AML is the strategy that maximizes overall discounted life years (14). However, disease progression for lower risk patients is not always gradual and may be abrupt. Our results showed that early transplantation (within 6 months of diagnosis) may offer a survival advantage even in lower risk patients (Supplemental Figure 3). Considering that patients who did not undergo transplant were not included in our study, the conclusions should be treated with caution.
There are some limitations to this study. Next-generation sequencing (NGS) continues to identify specific mutations associated with disease progression. In general, transplant decision-making may be influenced by genetic changes identified using NGS, such as TP53, RUNX1, ASXL1, RAS and JAK2 mutations (31, 32). Due to the limited data regarding NGS, we did not incorporate these data in our analysis. Most importantly, our single-centre study was retrospective, and therefore, the results need to be validated in multicentre clinical trials.
In conclusion, our study indicates that using an appropriate pre-transplant strategy (SC/HMA + <6 months) significantly prolongs OS and decreases NRM in MDS patients receiving myeloablative allo-HSCT. In the absence of randomized data, these results may help in clinical transplant decision-making for MDS patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Soochow University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
HW, QW, JQ, XL, TC: contribution of patients, acquisition of data, analysis and interpretation of data. YH, DW and HW: study design, acquisition of funding, contribution of patients, interpretation of data, supervision of the study, and revision of the manuscript. HQ, CF, XT and CR: contribution of patients and revision of the manuscript. HW, QW and JQ wrote the paper. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81100342, 81873432 and 82070143), Grants from the Jiangsu Province of China (BE2021645), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1146619/full#supplementary-material
References
1. Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med (2009) 361(19):1872–85. doi: 10.1056/NEJMra0902908
2. Santini V. Novel therapeutic strategies: hypomethylating agents and beyond. Hematol Am Soc Hematol Educ Program (2012) 2012:65–73. doi: 10.1182/asheducation-2012.1.65
3. Craddock C, Quek L, Goardon N, Freeman S, Siddique S, Raghavan M, et al. Azacitidine fails to eradicate leukemic stem/progenitor cell populations in patients with acute myeloid leukemia and myelodysplasia. Leukemia (2013) 27(5):1028–36. doi: 10.1038/leu.2012.312
4. Garcia-Manero G, Fenaux P. Hypomethylating agents and other novel strategies in myelodysplastic syndromes. J Clin Oncol (2011) 29(5):516–23. doi: 10.1200/JCO.2010.31.0854
5. de Witte T, Pikkemaat F, Hermans J, van Biezen A, Mackinnan S, Cornelissen J, et al. Genotypically nonidentical related donors for transplantation of patients with myelodysplastic syndromes: comparison with unrelated donor transplantation and autologous stem cell transplantation. Leukemia (2001) 15(12):1878–84. doi: 10.1038/sj.leu.2402296
6. Castro-Malaspina H, Harris RE, Gajewski J, Ramsay N, Collins R, Dharan B, et al. Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the national marrow donor program. Blood (2002) 99(6):1943–51. doi: 10.1182/blood.v99.6.1943
7. Sierra J, Perez WS, Rozman C, Carreras E, Klein JP, Rizzo JD, et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood (2002) 100(6):1997–2004.
8. Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL. Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biol Blood Marrow Transplant (2012) 18(8):1211–8. doi: 10.1016/j.bbmt.2012.01.009
9. Damaj G, Duhamel A, Robin M, Beguin Y, Michallet M, Mohty M, et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the societe francaise de greffe de moelle et de therapie-cellulaire and the groupe-francophone des myelodysplasies. J Clin Oncol (2012) 30(36):4533–40. doi: 10.1200/JCO.2012.44.3499
10. Kim Y, Kim IH, Kim HJ, Park S, Lee KH, Kim SJ, et al. Multicenter study evaluating the impact of hypomethylating agents as bridging therapy to hematopoietic stem cell transplantation in myelodysplastic syndromes. Int J Hematol (2014) 99(5):635–43. doi: 10.1007/s12185-014-1549-3
11. Jabbour E, Mathisen MS, Garcia-Manero G, Champlin R, Popat U, Khouri I, et al. Allogeneic hematopoietic stem cell transplantation versus hypomethylating agents in patients with myelodysplastic syndrome: a retrospective case-control study. Am J Hematol (2013) 88(3):198–200. doi: 10.1002/ajh.23371
12. Field T, Perkins J, Huang Y, Kharfan-Dabaja MA, Alsina M, Ayala E, et al. 5-azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant (2010) 45(2):255–60. doi: 10.1038/bmt.2009.134
13. Oran B, Kongtim P, Popat U, de Lima M, Jabbour E, Lu X, et al. Cytogenetics, donor type, and use of hypomethylating agents in myelodysplastic syndrome with allogeneic stem cell transplantation. Biol Blood Marrow Transplant (2014) 20(10):1618–25. doi: 10.1016/j.bbmt.2014.06.022
14. Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Perez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood (2004) 104(2):579–85. doi: 10.1182/blood-2004-01-0338
15. Koreth J, Pidala J, Perez WS, Deeg HJ, Garcia-Manero G, Malcovati L, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol (2013) 31(21):2662–70. doi: 10.1200/JCO.2012.46.8652
16. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood (2016) 127(20):2391–405. doi: 10.1182/blood-2016-03-643544
17. Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood (2012) 120(12):2454–65. doi: 10.1182/blood-2012-03-420489
18. Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood (1997) 89(6):2079–88. doi: 10.1182/blood.V89.6.2079
19. Kenealy M, Hertzberg M, Benson W, Taylor K, Cunningham I, Stevenson W, et al. Azacitidine with or without lenalidomide in higher risk myelodysplastic syndrome & low blast acute myeloid leukemia. Haematologica (2019) 104(4):700–9. doi: 10.3324/haematol.2018.201152
20. Steensma DP, Baer MR, Slack JL, Buckstein R, Godley LA, Garcia-Manero G, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol (2009) 27(23):3842–8. doi: 10.1200/jco.2008.19.6550
21. Scrucca L, Santucci A, Aversa F. Competing risk analysis using r: an easy guide for clinicians. Bone Marrow Transplant (2007) 40(4):381–7. doi: 10.1038/sj.bmt.1705727
22. Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using r: an in depth guide for clinicians. Bone Marrow Transplant (2010) 45(9):1388–95. doi: 10.1038/bmt.2009.359
23. Lim Z, Brand R, Martino R, van Biezen A, Finke J, Bacigalupo A, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol (2010) 28(3):405–11. doi: 10.1200/JCO.2009.21.8073
24. Warlick ED, Cioc A, Defor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant (2009) 15(1):30–8. doi: 10.1016/j.bbmt.2008.10.012
25. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol (2009) 10(3):223–32. doi: 10.1016/S1470-2045(09)70003-8
26. de Witte T, Suciu S, Verhoef G, Labar B, Archimbaud E, Aul C, et al. Intensive chemotherapy followed by allogeneic or autologous stem cell transplantation for patients with myelodysplastic syndromes (MDSs) and acute myeloid leukemia following MDS. Blood (2001) 98(8):2326–31. doi: 10.1182/blood.v98.8.2326
27. Potter VT, Iacobelli S, van Biezen A, Maertens J, Bourhis JH, Passweg JR, et al. Comparison of intensive chemotherapy and hypomethylating agents before allogeneic stem cell transplantation for advanced myelodysplastic syndromes: A study of the myelodysplastic syndrome subcommittee of the chronic malignancies working party of the European society for blood and marrow transplant research. Biol Blood Marrow Transplant (2016) 22(9):1615–20. doi: 10.1016/j.bbmt.2016.05.026
28. Yahng SA, Kim M, Kim TM, Jeon YW, Yoon JH, Shin SH, et al. Better transplant outcome with pre-transplant marrow response after hypomethylating treatment in higher-risk MDS with excess blasts. Oncotarget (2017) 8(7):12342–54. doi: 10.18632/oncotarget.12511
29. Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood (2012) 119(14):3361–9. doi: 10.1182/blood-2011-09-377044
30. Cruijsen M, Hobo W, van der Velden W, Bremmers MEJ, Woestenenk R, Bar B, et al. Addition of 10-day decitabine to Fludarabine/Total body irradiation conditioning is feasible and induces tumor-associated antigen-specific T cell responses. Biol Blood Marrow Transplant (2016) 22(6):1000–8. doi: 10.1016/j.bbmt.2016.02.003
31. Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med (2011) 364(26):2496–506. doi: 10.1056/NEJMoa1013343
Keywords: myelodysplastic syndrome, pre-transplant strategy, myeloablative conditioning, haematopoietic stem cell transplantation, prognosis
Citation: Wang H, Wang Q, Qi J, Li X, Chu T, Qiu H, Fu C, Tang X, Ruan C, Wu D and Han Y (2023) Appropriate pre-transplant strategy for patients with myelodysplastic syndromes receiving allogeneic haematopoietic stem cell transplantation after myeloablative conditioning. Front. Immunol. 14:1146619. doi: 10.3389/fimmu.2023.1146619
Received: 17 January 2023; Accepted: 16 February 2023;
Published: 28 February 2023.
Edited by:
Eva Maria Weissinger, Hannover Medical School, GermanyReviewed by:
Zhao Xiaosu, Peking University People’s Hospital, ChinaPhilippe Lewalle, Jules Bordet Institute, Belgium
Copyright © 2023 Wang, Wang, Qi, Li, Chu, Qiu, Fu, Tang, Ruan, Wu and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Han, aGFueXVlQHN1ZGEuZWR1LmNu; Depei Wu, d3VkZXBlaXN6QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Hong Wang1,2,3,4†
Hong Wang1,2,3,4† Qingyuan Wang
Qingyuan Wang Jiaqian Qi
Jiaqian Qi Yue Han
Yue Han