- 1The First Clinical Medical College of Lanzhou University, Lanzhou, China
- 2The First Clinical Medical College of Gansu University of Chinese Medicine (Gansu Provincial Hospital), Lanzhou, China
- 3Department of Urology, Gansu Provincial Hospital, Lanzhou, China
Urolithiasis is a common and frequent disease in urology. Percutaneous nephrolithotomy (PCNL) is preferred for the treatment of upper urinary tract stones and complicated renal stones >2 cm in diameter, but it has a higher rate of postoperative complications, especially infection, compared with other minimally invasive treatments for urinary stones. Complications associated with infection after percutaneous nephrolithotomy include transient fever, systemic inflammatory response syndrome (SIRS), and sepsis, which is considered one of the most common causes of perioperative death after percutaneous nephrolithotomy. In contrast, SIRS serves as a sentinel for sepsis, so early intervention of SIRS by biomarker identification can reduce the incidence of postoperative sepsis, which in turn reduces the length of stay and hospital costs for patients. In this paper, we summarize traditional inflammatory indicators, novel inflammatory indicators, composite inflammatory indicators and other biomarkers for early identification of systemic inflammatory response syndrome after percutaneous nephrolithotomy.
Introduction
Urolithiasis is a common and frequent disease in urology, and with the change of diet and lifestyle, the incidence of stones has been increasing in the past three decades at home and abroad (1, 2), and the incidence of stones is 8.8% in the United States and about 5.8% in China (3, 4). Urinary stones often lead to colic, infection, decreased kidney function, and even kidney failure, which greatly reduces patients’ quality of life and increases their financial burden. The treatment of urinary stones has evolved from traditional open surgery to minimally invasive endoluminal urological procedures, of which percutaneous nephrolithotomy (PCNL) has become the treatment of choice for patients with upper urinary tract stones >2 cm and complicated renal stones (5). Compared with traditional open surgery, percutaneous nephrolithoscopy has the advantages of minimal invasiveness, short operative time, short hospital stay, and high stone removal rate (6), but the complexity of its own operation and long learning curve make its postoperative complication rate higher, such as postoperative hemorrhage and postoperative infection (7, 8). Complications related to infection after percutaneous nephrolithotomy can be classified as transient fever, systemic inflammatory response syndrome (SIRS), and sepsis, depending on the severity.
Systemic inflammatory response syndrome is an uncontrolled, self-destructive, and self-sustained amplified systemic inflammatory response caused by severe injury, infection, trauma, surgery, ischemia, and other factors (9), and is also one of the common complications after percutaneous nephrolithotomy, with a high incidence even with preoperative antibiotic prophylaxis. The incidence of systemic inflammatory response syndrome after percutaneous nephrolithotomy has been reported to be approximately 9.8%-43% (10). In addition, the presence of SIRS during hospitalization is strongly associated with poor patient prognosis and can increase the risk of death by 82% (11). Sepsis is considered one of the most common causes of perioperative death after percutaneous nephrolithotomy (12), with a mortality rate of 20-42% (13), and SIRS is the first step in the sepsis cascade and is closely associated with the development of sepsis (14), so systemic inflammatory response syndrome can be used as a sentinel for sepsis. Therefore, early recognition and timely intervention of systemic inflammatory response syndrome is the key to reduce the incidence of sepsis and patient mortality after PCNL. In recent years, there have been numerous studies on biomarkers to predict the occurrence of SIRS after PCNL, so this paper summarizes these biomarkers to predict the occurrence of systemic inflammatory response syndrome early after percutaneous nephrolithotomy.
Procalcitonin
Procalcitonin is a protein consisting of 116 amino acids and is the peptide precursor of calcitonin (15). In normal physiological conditions, PCT is synthesized mainly in thyroid C cells and to a lesser extent by neuroendocrine tissues in other organs such as the lung and gastrointestinal tract (16). In response to stimulation induced by glucocorticoids, calcitonin gene-related peptides, glucagon, gastrin or β-adrenergic signals, PCT is converted to calcitonin before entering the circulatory system and therefore exhibits very low serum PCT levels (< 0.02 ng/mL) under normal physiological conditions (17). In contrast, during inflammation, PCT is mainly produced by two alternative mechanisms: a direct pathway induced by lipopolysaccharide (LPS) or other toxic metabolites from microorganisms and an indirect pathway induced by inflammatory mediators such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) (18). Due to the lack of conversion of PCT to calcitonin under both alternative mechanisms, PCT enters the circulatory system directly, resulting in elevated PCT concentrations in the peripheral circulation.
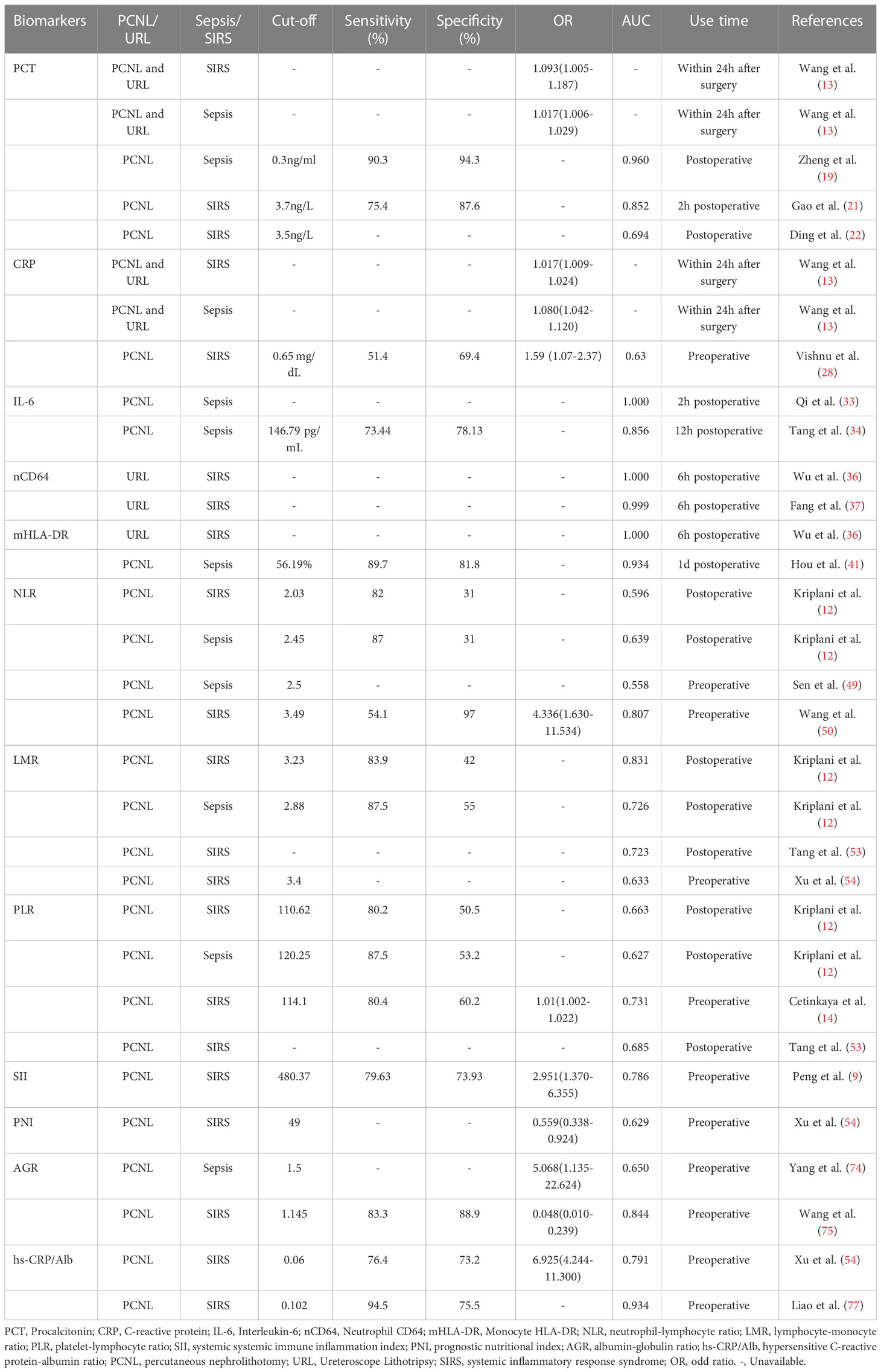
PCT, as a biomarker most widely used in sepsis, is a good predictor of the occurrence, treatment outcome and prognosis of sepsis after PCNL (19, 20). Zheng et al. (19) found that when PCT > 0.3ng/ml its sensitivity to predict post-PCNL sepsis reached 90.3% and its specificity 94.3%. As similarly, the study (13) showed that PCT and C-reactive protein (CRP) were independent risk factors for SIRS after PCNL with good predictive effect and that 88.2% of patients with SIRS occurred within 24 hours after surgery. Gao et al. (21) showed that PCT at 2 hours postoperatively was more effective than CRP and white blood cell in predicting SIRS after PCNL, with a specificity of 87.6% and sensitivity of 75.4% for the diagnosis of SIRS when PCT > 3.7 ng/L at 2 hours postoperatively. This is analogous to the findings of Ding et al. (22). Therefore, PCT at 2 hours postoperatively is a biomarker to predict the occurrence of SIRS after PCNL. However, the sensitivity of PCT as a single indicator to predict postoperative SIRS is not high, and it needs to be combined with other indicators such as CRP to improve the sensitivity (13) for more accurate and sensitive identification of postoperative SIRS. In addition, the severity of postoperative infection can be well evaluated by dynamic monitoring of PCT (22) to guide treatment and thus prevent the misuse of antibiotics leading to antibiotic resistance.
C-reactive protein
C-reactive protein is a biomarker of inflammation in the acute response phase stimulated by inflammatory factors such as interleukin-1 (IL-1), IL-6 and tumor necrosis factor (TNF) produced in the liver (23). CRP is currently widely used mainly in cardiovascular diseases (24), autoimmune diseases (25), oncology (26), and systemic infections (27), while fewer studies have been performed to predict the occurrence of SIRS after PCNL. Vishnu et al. (28) found that preoperative CRP was an independent risk factor for the occurrence of SIRS after PCNL based on a multifactorial logistic analysis, and by constructing a receiver operating characteristic curve (ROC) curve, they found that the preoperative CRP predicted the occurrence of SIRS after PCNL The best cut-off value for predicting SIRS after PCNL was 0.65 mg/dL, with a specificity of 69.4% and sensitivity of 51.4%, which was consistent with the study of Wang et al. (13). In addition, Wang et al. (13) showed that CRP can also predict the development of postoperative sepsis. However, it is not good in predicting SIRS or sepsis after PCNL compared to PCT (21), mainly because CRP is more susceptible to rheumatic diseases, malignancies and drug reactions compared to PCT (29). To address the limited role of CRP in predicting infection, a study found that the CRP-related index C reactive protein velocity (CRPv) (the difference between two CRP measurements before admission divided by the time between the two tests) can better distinguish sepsis from non-sepsis and has a better predictive value (23). However, there are no studies in which CRPv predicted the occurrence of SIRS after PCNL, so future randomized controlled studies with large samples should be conducted to verify the role of CRPv in predicting SIRS after PCNL.
Interleukin-6
Interleukin-6 is a cytokine with multiple biological activities with pro- and anti-inflammatory activities, depending on the immune response environment. IL-6 is mainly produced by monocytes, neutrophils, T lymphocytes, B lymphocytes and NK cells and is involved in the development of systemic infections, autoimmune diseases and tumors through immune regulation (30, 31). In patients with kidney stones, bacteria are present not only in the urine but also in the stones, especially infected stones, and these bacteria and endotoxins are often released during PCNL lithotripsy. In addition, the high pelvic pressure caused by the flushing fluid leads to the entry of bacteria and endotoxins into the circulatory system through the damaged pelvic mucosa (32), which eventually leads to postoperative systemic severe reaction syndrome and even urogenic sepsis. Qi et al. (33) showed that compared to PCT, IL-6 at 2 hours postoperatively was the earliest and most valuable inflammatory biomarker for the diagnosis of urogenic sepsis occurring after PCNL, with an area under the ROC curve of 1.0. Unfortunately, however, their study did not provide an optimal cut-off value for IL-6 at 2 hours postoperatively to further guide the clinic. Similarly, Tang et al. (34) found that with compared to PCT and CRP, IL-6 at 12 hours postoperatively was the best for diagnosing urogenic sepsis after PCNL with a best cut-off value of 146.79 pg/mL, a specificity of 78.13% and a sensitivity of 73.44%. Therefore, IL-6 at 2 hours postoperatively is the earliest and most valuable biomarker of inflammation, but the diagnostic value of either PCT, CRP or IL-6 as a single indicator to predict the occurrence of infection after PCNL is limited and not as accurate as the combined diagnosis of the three indicators.
Neutrophil CD64
CD64 is present on the surface of neutrophils and is a high-affinity receptor for the Fc portion of IgG. Under normal conditions CD64 is expressed at low levels on the surface of peripheral blood neutrophils. However, when the organism is in an infected state, the body produces large amounts of cytokines such as interferon-γ, IL-6, TNF-α, and granulocyte colony-stimulating factor, and these cytokines stimulate neutrophils to express CD64 in large amounts, and their expression peaks within 4 to 6 hours and remains stable for a certain period of time until 7 days after these cytokines return to normal and return to basal expression (35). Cong et al. (17) compared the value of CD64, PCT and IL-6 in the diagnosis of sepsis by Meta-analysis and found that CD64 had the highest diagnostic value for sepsis with a specificity of 88%, a sensitivity of 88% and an area under the ROC curve of 0.94. Given its stability and high diagnostic value for sepsis, CD64 can be used as a biomarker for predicting infection. There are not many studies of CD64 in predicting the emergence of SIRS after lithotripsy, focusing almost exclusively on the emergence of SIRS after ureteroscopic lithotripsy. A retrospective study based on 407 patients found that CD64 had the highest diagnostic value for the development of SIRS after ureteroscopy holmium laser lithotripsy compared to PCT versus CRP, with an area under the ROC curve of 1.0 at 2 hours, 6 hours, and 1 day postoperatively (36), similar to the findings of Fang et al. (37). Consequently, CD64 at 2 and 6 hours postoperatively is an effective biomarker for early prediction of SIRS after lithotripsy, but its validity in early prediction of SIRS after PCNL still needs further validation. In addition, there is no uniform unit of CD64 flow cytometry, and most of the different measurement units are not interconvertible, making it more difficult to apply the best cutoff value of CD64 for predicting the development of SIRS or sepsis.
Monocyte HLA-DR
HLA-DR is a class II antigen and also a glycosylated transmembrane protein expressed on antigen-presenting cells. HLA-DR expressed on monocytes presents ingested pathogenic microbial peptides to CD4 or CD8-positive T cells, thereby initiating a specific immune response to eliminate the underlying pathogen. More studies have shown that reduced HLA-DR expression is a reliable diagnostic and prognostic biomarker for immunosuppression or sepsis in critically ill patients (38, 39). Patients with HLA-DR expression below 30% have a lower survival rate and a 30-fold increased risk of death compared to normal levels (40). Wu et al. (36) found that HLA-DR at 2 hours postoperative and 6 hours postoperative was a good predictor of SIRS after ureteroscopy holmium laser lithotripsy compared with the traditional inflammatory index PCT, but was less effective than CD64, which had significant areas of 0.993 and 0.983 for the 2-hour and 6-hour ROC curves, respectively. Considering the different surgical procedures, the resulting postoperative reaction stress is not the same. However, Hou et al. (41) found that HLA-DR could also be used to predict the development of sepsis after PCNL with an optimal cut-off value of 56.19%, specificity of 81.8% and sensitivity of 89.7%. Nevertheless, although SIRS and sepsis are different stages of development of the same disease, there are differences between them, mainly in organ dysfunction. Monocyte HLA-DR expression was found to be significantly lower in sepsis patients who developed acute renal failure compared to sepsis patients who did not (42). Therefore, the findings of Hou et al. (41) are not well applied in predicting the occurrence of SIRS after PCNL, and future clinical trials with large samples are still needed to validate the role of HLA-DR in predicting the occurrence of SIRS after PCNL.
Complex inflammatory indicators
Blood tests are the most commonly used tests in clinical practice and are quick, economical and easy to perform, so they are often used to make preliminary judgments about inflammation in the body. For a long time, however, clinicians often choose white blood cell count, neutrophil count and neutrophil percentage to determine systemic inflammation, and rarely use other indicators of blood routine to judge, such as monocytes and lymphocytes. It was found that leukocytes are not as valuable in predicting or diagnosing infections compared to other indicators such as PCT and neutrophil-lymphocyte ratio (NLR) (19, 43), probably because some infections do not cause elevated or decreased leukocytes (44). For this reason, more and more studies are being conducted to improve the diagnostic value of systemic reactive syndrome or sepsis by combining the indicators from routine blood and liver function tests. Current composite inflammatory indicators used to predict or diagnose the development of SIRS after PCNL include neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio (LMR), platelet-lymphocyte ratio (PLR), systemic systemic immune inflammation index (SII), prognostic nutritional index (PNI), albumin-globulin ratio (AGR), and hypersensitive C-reactive protein-albumin ratio (hs-CRP/Alb).
Neutrophil-lymphocyte ratio
The neutrophil-lymphocyte ratio is now a common composite inflammatory index, originally described by Goodman et al. (45) in a 1995 study that evaluated its role in the diagnosis of appendicitis. Subsequently, as the relationship between tumors and inflammation was uncovered, more and more studies found that NLR was closely associated with the diagnosis and prognosis of urologic tumors such as prostate cancer (46), renal cell carcinoma (47), and adrenal cortical carcinoma (48). And similarly, NLR was strongly associated with predicting the occurrence of infection after minimally invasive treatment of urinary stones. The presence of kidney stones leads to the release of inflammatory mediators such as IL-6, IL-7, IL-8 and TNF-α, which in turn leads to an increase in neutrophil counts, while the inflammatory response suppresses the immune response by decreasing the cytolytic activity of lymphocytes, T cells and natural killer cells (12). Therefore, increased NLR may indicate a persistent inflammatory response. Sen et al. (49) found that NLR better predicted the development of sepsis after PCNL compared to white blood cell count with an optimal cut-off value of 2.5. This is in agreement with the findings of Kriplani et al. (12), in addition they also predicted the development of SIRS after PCNL by NLR with an optimal cut-off value of 2.03. However, NLR has a higher sensitivity and better value in predicting sepsis compared to predicting SIRS. Wang et al. (50) revealed that NLR was more effective in predicting the occurrence of SIRS after PCNL compared with the traditional index PCT, and its area under the ROC curve was higher than that of PCT with a specificity of 97%. In conclusion, NLR is a simple and easily available marker for predicting the occurrence of SIRS or sepsis after PCNL. In addition, NLR is a simple and easily available marker to predict the development of SIRS or sepsis after PCNL compared to more expensive tests such as PCT and IL-6, but the lack of consensus on the optimal cut-off value for NLR limits its use in clinical practice, and therefore prospective multicenter studies with large samples are needed to improve evidence and standardization.
Lymphocyte-monocyte ratio
As with NLR, the lymphocyte-monocyte ratio is a commonly used indicator of compound inflammation. Several studies have shown the value of LMR in the diagnosis and prognostic assessment of various diseases such as malignancies and SIRS (51, 52). Winkler et al. (38) observed an increase in the number of monocytes during sepsis, while SIRS or sepsis decreased the circulating blood lymphocyte count. Thus, a lower LMR may reflect the inflammatory state. LMR predicted the occurrence of SIRS after PCNL was originally described in a study by Tang et al. (53), but its predictive power was not as good as NLR. This is in agreement with the findings of Kriplani et al. (12), who calculated the best cut-off value of 3.23, sensitivity of 83.9%, specificity of 42% and area under the curve of 0.649 for LMR prediction of SIRS by ROC curve, but they found that LMR predicted postoperative sepsis better than NLR with a best cut-off value of 2.88, sensitivity of 87.5%, specificity of 55%, and area under the curve of 0.726. It is evident that LMR is a better predictor of severe infection, and they found that LMR is an independent risk factor for the development of SIRS after PCNL by multifactorial logistic regression analysis. This is in agreement with the study of Xu et al. (54), whose optimal cut-off value was 3.4. On the flipside, the best cut-off values from a large number of relevant studies were smaller than the mean value of LMR in a healthy Chinese population (male: 5.14; female: 5.50) (12, 53, 55). Therefore, LMR is a biomarker that can predict SIRS or sepsis. Therefore, LMR is a biomarker that can predict SIRS or sepsis, but its specificity is low and needs to be combined with other indicators to further improve the diagnostic efficacy which means reducing the misdiagnosis rate.
Platelet-lymphocyte ratio
The platelet-lymphocyte ratio is a novel composite inflammatory marker in recent years and has been shown to be predictive of a variety of diseases, such as diabetic complications and cancer (56, 57). Numerous current studies have shown that platelets are involved in the pathophysiological processes of sepsis and play a key role in organ dysfunction (58); while lymphopenia is a common marker of sepsis-induced immunosuppression (59). Therefore, PLR may be a biomarker for predicting systemic infection. Kriplani et al. (12) conducted a retrospective analysis of 517 post-PCNL patients and found that preoperative PLR was an independent risk factor for the development of SIRS after PCNL, with a specificity of 50.5% and sensitivity of 80.2% for predicting SIRS when the preoperative PLR was >110.62, which was less sensitive than NLR and LMR. This is similar to the findings of Cetinkaya et al. (14), who concluded that patients’ vital signs should be closely monitored and alerted for the development of postoperative SIRS when the preoperative PLR is >114.1. However, Tang et al. (53) found through a retrospective study that although PLR was statistically different between the non-SIRS and SIRS groups, PLR was not an independent risk factor for predicting the occurrence of SIRS after PCNL by multifactorial logistic analysis, which may be caused by the inherent limitations of the type of retrospective study, so a prospective study with a large sample should be conducted in the future to further verify its validity.
Systemic immune inflammatory index
The systemic immune inflammatory index was first proposed by Hu et al. as a novel index of inflammation obtained by platelet * NLR calculation (60). Currently, SII is mainly applied in the prognosis of cardiovascular diseases and tumors (61, 62), and there are fewer studies in predicting postoperative complications, especially in SIRS or sepsis. There is only one national and international study on SII predicting the occurrence of SIRS after PCNL (9). Their retrospective analysis of 365 patients revealed that SII was an independent risk factor for the development of SIRS after PCNL and had a greater predictive value compared to NLR, LMR and PLR with a sensitivity of 79.63% and a specificity of 73.93%. This is probably because these predictors become unstable when only one or two parameters are involved and are usually susceptible to other confounding factors (63). In contrast, SII contains three parameters that are more stable and more objective in reflecting the balance between host inflammation and immune status (64). Therefore, SII is expected to be a biological indicator to predict the occurrence of SIRS after PCNL surgery. However, their study lacked the optimal cut-off value to directly guide the clinic, so the specific application of SII in the clinic, such as predicting diagnosis and guiding treatment, should be further investigated in the future.
Prognostic nutritional index
The prognostic nutritional index was first proposed by Onodera et al. in 1984 and was used to assess the risk of surgery for gastrointestinal tumors to guide treatment and prognosis with good results (65). Studies after this have focused on tumor prognosis, such as Li et al. who found that PNI >50.2 had a more favorable prognosis for prostate cancer (66). PNI is calculated based on serum albumin level and peripheral lymphocyte count, i.e. serum albumin concentration (g/L) + 5 * total peripheral circulating lymphocyte count (109/L), which can reflect the nutritional, inflammatory and immune status of the patient in a simpler and more comprehensive way. Nutrition affects all physiological processes, including those related to the development and function of our immune system (67), so malnutrition often leads to immune dysfunction (68), which in turn leads to a greater susceptibility of patients to postoperative infections. There are few studies involving PNI to predict the occurrence of SIRS after PCNL. Xu et al. (54) found that PNI was an independent influencing factor for the occurrence of SIRS after PCNL through a retrospective analysis of 556 patients, and the probability of SIRS could be reduced when PNI was >49. They also found that PNI predictive value was greater than PLR and comparable to NLR and LMR. In addition, PNI is significantly more effective than NLR and PLR in predicting all-cause mortality in sepsis (69), so early nutritional support for patients with sepsis may reduce mortality in patients (70). Consequently, PNI may be a potential inflammatory marker for predicting the occurrence of SIRS after PCNL, but future clinical trials with large samples as well as exploring the relationship between it and SIRS should still be conducted to provide a basis for the future application of PNI in the clinic.
Albumin-globulin ratio
Albumin and globulin are important components of serum proteins and play a potential role in the systemic inflammatory response. Low clear albumin concentrations not only reflect malnutrition but also predict infectious complications after oncologic surgery and orthopedic surgery (71, 72). Globulins are acute phase proteins during the host immune response and their concentrations increase shortly after the invasion of pathogens and toxins into the body, and high concentrations indicate a state of systemic inflammation and accumulation of various inflammatory cytokines (73). Therefore, the albumin-globulin ratio, like the PNI, provides a simpler and more comprehensive picture of the patient’s nutritional, inflammatory and immune status. Xun et al. (74) showed that AGR was an independent risk factor for postoperative sepsis in PCNL by multifactorial logistic analysis, and they found that the incidence of sepsis decreased with increasing AGR, and the incidence of postoperative sepsis was 0.78% when AGR was ≥1.5; 5.93% when AGR was between 1 and 1.5; 11.59% when AGR <1.0, the incidence of postoperative sepsis was 11.59%. Therefore, preoperative AGR should be kept above 1.5 to greatly reduce the incidence of sepsis. Wang et al. (75) showed that AGR was also an independent risk factor for SIRS after PCNL, and they found that AGR had the best predictive efficacy compared to traditional inflammatory indicators such as neutrophils, total leukocytes, C-reactive protein, or a single indicator of albumin and globulin, with an optimal cut-off value of 1.145, at which point the sensitivity was 83.3% and the specificity was 88.9%. Therefore, it is recommended that patients with AGR <1.145 be carefully evaluated and treated before undergoing PCNL to reduce the occurrence of systemic inflammatory response syndrome after surgery. However, future multicenter clinical studies with large samples are still needed to determine the optimal cut-off value and influencing factors of AGR for better application in the clinic.
Ultrasensitive C-reactive protein-albumin ratio
Ultrasensitive C-reactive protein-albumin is a novel inflammatory marker, in which ultrasensitive C-reactive protein is the same protein as C-reactive protein, which is produced by mainly hepatocytes and regulated by cytokines such as IL-1β, IL-6, and TNF, the only difference is that ultrasensitive C-reactive protein is detected in a more sensitive manner and can detect very low concentrations of CRP in plasma (76), so it can detect inflammatory responses earlier than CRP. Liao et al. (77) showed that when hs-CRP/Alb > 0.102, it has a good predictive value for the development of SIRS after PCNL with a sensitivity of 94.8% and a specificity of 75.5%. This is consistent with the study of Xu et al. (54), who showed by ROC curve analysis that hs-CRP/Alb has better predictive value than NLR, PLR, LMR and PNI for postoperative SIRS with an optimal cut-off value of 0.06, sensitivity of 76.4% and specificity of 73.2%. They found that an elevated hs-CRP/Alb ratio was associated with female gender, preoperative urine culture, hs-CRP, albumin, hemoglobin, and creatinine. Therefore, the influence of these factors on hs-CRP/Alb should be further explored in the future when hs-CRP/Alb is used in actual clinical practice.
Conclusions
Sepsis is the most serious complication after percutaneous nephrolithotomy and is one of the leading causes of death after PCNL, of which more than half progress from systemic inflammatory response syndrome, so SIRS can be used as a sentinel for sepsis. There is growing evidence that biomarkers such as traditional inflammatory markers (PCT, CRP and IL-6), novel inflammatory markers (neutrophil CD64 and monocyte HLA-DR), and composite inflammatory markers (NLR, LMR, PLR, SII, PNI, AGR and hs-CRP/Alb) play an important role in early prediction of postoperative development of SIRS (Table 1). However, it is worth noting that the novel inflammatory indicators are currently focused on studies of the development of SIRS after ureteroscopic lithotripsy, so their effectiveness in early prediction of the development of SIRS after PCNL will remain to be further validated in the future. In addition, the cut-off value of each biomarker varies somewhat in different studies, which limits its application in clinical practice. Therefore, in the future, it is still necessary to determine the optimal cut-off value of these biomarkers through prospective studies or Meta-analysis with large samples and multiple centers; secondly, the role of a single indicator is limited, and in the future, these indicators should be combined to improve the sensitivity and specificity of prediction in order to better guide timely clinical detection of postoperative SIRS and early intervention to reduce the incidence of postoperative sepsis and patient mortality.
Author contributions
WW: Conceptualization, literature search, and writing the article. DZ: Writing the article. TJ, TL and FZ: Reviewing the article. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kittanamongkolchai W, Vaughan LE, Enders FT, Dhondup T, Mehta RA, Krambeck AE, et al. The changing incidence and presentation of urinary stones over 3 decades. Mayo Clin Proc (2018) 93(3):291–9. doi: 10.1016/j.mayocp.2017.11.018
2. Wang W, Fan J, Huang G, Li J, Zhu X, Tian Y, et al. Prevalence of kidney stones in mainland China: A systematic review. Sci Rep (2017) 7:41630. doi: 10.1038/srep41630
3. Scales CD Jr, Smith AC, Hanley JM, Saigal CS. Urologic diseases in America project. prevalence of kidney stones in the united states. Eur Urol (2012) 62(1):160–5. doi: 10.1016/j.eururo.2012.03.052
4. Zeng G, Mai Z, Xia S, Wang Z, Zhang K, Wang L, et al. Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int (2017) 120(1):109–16. doi: 10.1111/bju.13828
5. Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol (2016) 69(3):468–74. doi: 10.1016/j.eururo.2015.07.040
6. Chung DY, Kang DH, Cho KS, Jeong WS, Jung HD, Kwon JK, et al. Comparison of stone-free rates following shock wave lithotripsy, percutaneous nephrolithotomy, and retrograde intrarenal surgery for treatment of renal stones: A systematic review and network meta-analysis. PloS One (2019) 14(2):e0211316. doi: 10.1371/journal.pone.0211316
7. Seitz C, Desai M, Häcker A, Hakenberg OW, Liatsikos E, Nagele U, et al. Incidence, prevention, and management of complications following percutaneous nephrolitholapaxy. Eur Urol (2012) 61(1):146–58. doi: 10.1016/j.eururo.2011.09.016
8. de la Rosette J, Assimos D, Desai M, Gutierrez J, Lingeman J, Scarpa R, et al. The clinical research office of the endourological society percutaneous nephrolithotomy global study: indications, complications, and outcomes in 5803 patients. J Endourol (2011) 25(1):11–7. doi: 10.1089/end.2010.0424
9. Peng C, Li J, Xu G, Jin J, Chen J, Pan S. Significance of preoperative systemic immune-inflammation (SII) in predicting postoperative systemic inflammatory response syndrome after percutaneous nephrolithotomy. Urolithiasis (2021) 49(6):513–9. doi: 10.1007/s00240-021-01266-2
10. Tan F, Gan X, Deng Y, Li X, Guo N, Hei Z, et al. Intraoperative dexmedetomidine attenuates postoperative systemic inflammatory response syndrome in patients who underwent percutaneous nephrolithotomy: a retrospective cohort study. Ther Clin Risk Manage (2018) 14:287–93. doi: 10.2147/TCRM.S157320
11. Taniguchi LU, Pires EMC, Vieira JM Jr, Azevedo LCP. Systemic inflammatory response syndrome criteria and the prediction of hospital mortality in critically ill patients: a retrospective cohort study. Rev Bras Ter Intensiva (2017) 29(3):317–24. doi: 10.5935/0103-507X.20170047
12. Kriplani A, Pandit S, Chawla A, de la Rosette JJMCH, Laguna P, Jayadeva Reddy S, et al. Neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and lymphocyte-monocyte ratio (LMR) in predicting systemic inflammatory response syndrome (SIRS) and sepsis after percutaneous nephrolithotomy (PNL). Urolithiasis (2022) 50(3):341–8. doi: 10.1007/s00240-022-01319-0
13. Wang C, Xu R, Zhang Y, Wu Y, Zhang T, Dong X, et al. Nomograms for predicting the risk of SIRS and urosepsis after uroscopic minimally invasive lithotripsy. BioMed Res Int (2022) 2022:6808239. doi: 10.1155/2022/6808239
14. Cetinkaya M, Buldu I, Kurt O, Inan R. Platelet-to-Lymphocyte ratio: A new factor for predicting systemic inflammatory response syndrome after percutaneous nephrolithotomy. Urol J (2017) 14(5):4089–93.
16. Sartelli M, Ansaloni L, Bartoletti M, Catena F, Cardi M, Cortese F, et al. The role of procalcitonin in reducing antibiotics across the surgical pathway. World J Emerg Surg (2021) 16(1):15. doi: 10.1186/s13017-021-00357-0
17. Cong S, Ma T, Di X, Tian C, Zhao M, Wang K. Diagnostic value of neutrophil CD64, procalcitonin, and interleukin-6 in sepsis: a meta-analysis. BMC Infect Dis (2021) 21(1):384. doi: 10.1186/s12879-021-06064-0
18. Vijayan AL, Vanimaya, Ravindran S, Saikant R, Lakshmi S, Kartik R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care (2017) 5:51. doi: 10.1186/s40560-017-0246-8
19. Zheng J, Li Q, Fu W, Ren J, Song S, Deng G, et al. Procalcitonin as an early diagnostic and monitoring tool in urosepsis following percutaneous nephrolithotomy. Urolithiasis (2015) 43(1):41–7. doi: 10.1007/s00240-014-0716-6
20. Tan M, Lu Y, Jiang H, Zhang L. The diagnostic accuracy of procalcitonin and c-reactive protein for sepsis: A systematic review and meta-analysis. J Cell Biochem (2019) 120(4):5852–9. doi: 10.1002/jcb.27870
21. Gao XL, Wu ZS, He YH, Yu J. Value of serum procalcitonin in the early diagnosis of systemic inflammatory response syndrome after percutaneous nephrolithotomy. J Clin Urol (China (2020) 35(01):14–6. doi: 10.13201/j.issn.1001-1420.2020.01.004
22. Ding H, Wang XM, Yang T, Hao XD, Chen Y, Yang K, et al. Predictive value of inflammatory markers for SIRS after percutaneous nephrolithotomy. J Tianjin Med Univ (2017) 23(02):108–12.
23. Levinson T, Wasserman A. C-reactive protein velocity (CRPv) as a new biomarker for the early detection of acute Infection/Inflammation. Int J Mol Sci (2022) 23(15):8100. doi: 10.3390/ijms23158100
24. Boncler M, Wu Y, Watala C. The multiple faces of c-reactive protein-physiological and pathophysiological implications in cardiovascular disease. Molecules (2019) 24(11):2062. doi: 10.3390/molecules24112062
25. Enocsson H, Gullstrand B, Eloranta ML, Wetterö J, Leonard D, Rönnblom L, et al. C-reactive protein levels in systemic lupus erythematosus are modulated by the interferon gene signature and CRP gene polymorphism rs1205. Front Immunol (2021) 11:622326. doi: 10.3389/fimmu.2020.622326
26. Zhu M, Ma Z, Zhang X, Hang D, Yin R, Feng J, et al. C-reactive protein and cancer risk: A pan-cancer study of prospective cohort and mendelian randomization analysis. BMC Med (2022) 20(1):301. doi: 10.1186/s12916-022-02506-x
27. Rao H, Dutta S, Menon P, Attri S, Sachdeva N, Malik M. Procalcitonin and c-reactive protein for diagnosing post-operative sepsis in neonates. J Paediatr Child Health (2022) 58(4):593–9. doi: 10.1111/jpc.15774
28. Ganesan V, Brown RD, Jiménez JA, De S, Monga M. C-reactive protein and erythrocyte sedimentation rate predict systemic inflammatory response syndrome after percutaneous nephrolithotomy. J Endourol (2017) 31(7):638–44. doi: 10.1089/end.2016.0884
29. Tang Y, Fung E, Xu A, Lan HY. C-reactive protein and ageing. Clin Exp Pharmacol Physiol (2017) 44 Suppl 1:9–14. doi: 10.1111/1440-1681.12758
30. Smok B, Domagalski K, Pawłowska M. Diagnostic and prognostic value of IL-6 and sTREM-1 in SIRS and sepsis in children. Mediators Inflamm (2020) 2020:8201585. doi: 10.1155/2020/8201585
31. Aliyu M, Zohora FT, Anka AU, Ali K, Maleknia S, Saffarioun M, et al. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int Immunopharmacol (2022) 111:109130. doi: 10.1016/j.intimp.2022.109130
32. Omar M, Noble M, Sivalingam S, El Mahdy A, Gamal A, Farag M, et al. Systemic inflammatory response syndrome after percutaneous nephrolithotomy: A randomized single-blind clinical trial evaluating the impact of irrigation pressure. J Urol (2016) 196(1):109–14. doi: 10.1016/j.juro.2016.01.104
33. Qi T, Lai C, Li Y, Chen X, Jin X. The predictive and diagnostic ability of IL-6 for postoperative urosepsis in patients undergoing percutaneous nephrolithotomy. Urolithiasis (2021) 49(4):367–75. doi: 10.1007/s00240-020-01237-z
34. Tang YC, Fu H, Guo T, Duan B, Wang XX, Chen XC. The significance of serum IL-6 combined with PCT and CRP in the diagnosis of urinary sepsis after percutaneous nephrolithotripsy. J Prac Med (2018) 34(13):2198–203.
35. Patnaik R, Azim A, Agarwal V. Neutrophil CD64 a diagnostic and prognostic marker of sepsis in adult critically ill patients: A brief review. Indian J Crit Care Med (2020) 24(12):1242–50. doi: 10.5005/jp-journals-10071-23558
36. Wu HL, Zhang LQ, Liu Q, Huang YP, Zhang CT, Dai GP, et al. Diagnostic value of neutrophil CD64, monocyte HLA-DR, and serum procalcitonin in the early monitoring of systemic inflammatory response syndrome after holmium laser lithotripsy with ureteroscopy. Chin J Exp Surg (2020) 37(08):1449–51.
37. Fang YQ, Chen ZQ, Mao YF. Changes in peripheral blood neutrophil CD64 levels in holmium laser lithotripsy SIRS patient. ZH J J Traumatic (2021) 26(04):630–2.
38. Winkler MS, Rissiek A, Priefler M, Schwedhelm E, Robbe L, Bauer A, et al. Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFα response: A diagnostic tool for immunosuppression? PloS One (2017) 12(8):e0182427. doi: 10.1371/journal.pone.0182427
39. Xu J, Li J, Xiao K, Zou S, Yan P, Xie X, et al. Dynamic changes in human HLA-DRA gene expression and Th cell subsets in sepsis: Indications of immunosuppression and associated outcomes. Scand J Immunol (2020) 91(1):e12813. doi: 10.1111/sji.12813
40. Zhuang Y, Peng H, Chen Y, Zhou S, Chen Y. Dynamic monitoring of monocyte HLA-DR expression for the diagnosis, prognosis, and prediction of sepsis. Front Biosci (Landmark Ed) (2017) 22(8):1344–54. doi: 10.2741/4547
41. Hou HF, Liu Y, Zhang X, Han Z, Chen T. The value of postoperative HLA-DR expression and high mobility group box 1 level in predictive diagnosis of sepsis in percutaneous nephrolithotomy surgery. Ren Fail (2022) 44(1):1338–44. doi: 10.1080/0886022X.2022.2107541
42. Wu HP, Chuang LP, Liu PH, Chu CM, Yu CC, Lin SW, et al. Decreased monocyte HLA-DR expression in patients with sepsis and acute kidney injury. Medicina (Kaunas) (2022) 58(9):1198. doi: 10.3390/medicina58091198
43. Marik PE, Stephenson E. The ability of procalcitonin, lactate, white blood cell count and neutrophil-lymphocyte count ratio to predict blood stream infection. analysis of a large database. J Crit Care (2020) 60:135–9. doi: 10.1016/j.jcrc.2020.07.026
44. Seigel TA, Cocchi MN, Salciccioli J, Shapiro NI, Howell M, Tang A, et al. Inadequacy of temperature and white blood cell count in predicting bacteremia in patients with suspected infection. J Emerg Med (2012) 42(3):254–9. doi: 10.1016/j.jemermed.2010.05.038
45. Goodman DA, Goodman CB, Monk JS. Use of the neutrophil:lymphocyte ratio in the diagnosis of appendicitis. Am Surg (1995) 61(3):257–9.
46. Chong W, Zhang Z, Luo R, Gu J, Lin J, Wei Q, et al. Integration of circulating tumor cell and neutrophil-lymphocyte ratio to identify high-risk metastatic castration-resistant prostate cancer patients. BMC Cancer (2021) 21(1):655. doi: 10.1186/s12885-021-08405-3
47. Rebuzzi SE, Signori A, Stellato M, Santini D, Maruzzo M, De Giorgi U, et al. The prognostic value of baseline and early variations of peripheral blood inflammatory ratios and their cellular components in patients with metastatic renal cell carcinoma treated with nivolumab: The Δ-Meet-URO analysis. Front Oncol (2022) 12:955501. doi: 10.3389/fonc.2022.955501
48. Solak M, Kraljević I, Zibar Tomšić K, Kaštelan M, Kakarigi L, Kaštelan D, et al. Neutrophil-lymphocyte ratio as a prognostic marker in adrenocortical carcinoma. Endocr Res (2021) 46(2):74–9. doi: 10.1080/07435800.2020.1870234
49. Sen V, Bozkurt IH, Aydogdu O, Yonguc T, Yarimoglu S, Sen P, et al. Significance of preoperative neutrophil-lymphocyte count ratio on predicting postoperative sepsis after percutaneous nephrolithotomy. Kaohsiung J Med Sci (2016) 32(10):507–13. doi: 10.1016/j.kjms.2016.08.008
50. Wang L, Wang Y, Li L, Wang CS. Predictive value of CHR versus NLR for systemic inflammatory response syndrome associated with percutaneous nephrolithotomy. Anhui Med J (2021) 42(02):202–6.
51. Ugel S, Canè S, De Sanctis F, Bronte V. Monocytes in the tumor microenvironment. Annu Rev Pathol (2021) 16:93–122. doi: 10.1146/annurev-pathmechdis-012418-013058
52. Brodska H, Valenta J, Pelinkova K, Stach Z, Sachl R, Balik M, et al. Diagnostic and prognostic value of presepsin vs. established biomarkers in critically ill patients with sepsis or systemic inflammatory response syndrome. Clin Chem Lab Med (2018) 56(4):658–68. doi: 10.1515/cclm-2017-0839
53. Tang K, Liu H, Jiang K, Ye T, Yan L, Liu P, et al. Predictive value of preoperative inflammatory response biomarkers for metabolic syndrome and post-PCNL systemic inflammatory response syndrome in patients with nephrolithiasis. Oncotarget (2017) 8(49):85612–27. doi: 10.18632/oncotarget.20344
54. Xu H, Hu L, Wei X, Niu J, Gao Y, He J, et al. The predictive value of preoperative high-sensitive c-reactive Protein/Albumin ratio in systemic inflammatory response syndrome after percutaneous nephrolithotomy. J Endourol (2019) 33(1):1–8. doi: 10.1089/end.2018.0632
55. Wang J, Zhang F, Jiang F, Hu L, Chen J, Wang Y. Distribution and reference interval establishment of neutral-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) in Chinese healthy adults. J Clin Lab Anal (2021) 35(9):e23935. doi: 10.1002/jcla.23935
56. Liu Y, Wang X, Wang L, Chen W, Liu W, Ye T, et al. Platelet-to-Lymphocyte ratio predicts the presence of diabetic neurogenic bladder. Diabetes Metab Syndr Obes (2022) 15:7–13. doi: 10.2147/DMSO.S335957
57. Merhe A, Labban M, Hout M, Bustros G, Abou Heidar N, El-Asmar JM, et al. Development of a novel nomogram incorporating platelet-to-lymphocyte ratio for the prediction of lymph node involvement in prostate carcinoma. Urol Oncol (2020) 38(12):930. doi: 10.1016/j.urolonc.2020.05.026
58. Chen QS, Wang XY. Research progress of platelets in sepsis. Chin Pediatr Emerg Med (2021) 28(02):145–7.
59. Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock (2014) 42(5):383–91. doi: 10.1097/SHK.0000000000000234
60. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20(23):6212–22. doi: 10.1158/1078-0432.CCR-14-0442
61. Xiang J, He L, Li D, Wei S, Wu Z. Value of the systemic immune-inflammation index in predicting poor postoperative outcomes and the short-term prognosis of heart valve diseases: a retrospective cohort study. BMJ Open (2022) 12(10):e064171. doi: 10.1136/bmjopen-2022-064171
62. Li J, Cao D, Huang Y, Xiong Q, Tan D, Liu L, et al. The prognostic and clinicopathological significance of systemic immune-inflammation index in bladder cancer. Front Immunol (2022) 13:865643. doi: 10.3389/fimmu.2022.865643
63. Bedel C, Korkut M, Armağan HH. NLR, d-NLR and PLR can be affected by many factors. Int Immunopharmacol (2021) 90:107154. doi: 10.1016/j.intimp.2020.107154
64. Zhao R, Shan J, Nie L, Yang X, Yuan Z, Xu H, et al. The predictive value of the ratio of the product of neutrophils and hemoglobin to lymphocytes in non-muscular invasive bladder cancer patients with postoperative recurrence. J Clin Lab Anal (2021) 35(8):e23883. doi: 10.1002/jcla.23883
65. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi (1984) 85(9):1001–5.
66. Li B, Lu Z, Wang S, Hou J, Xia G, Li H, et al. Pretreatment elevated prognostic nutritional index predicts a favorable prognosis in patients with prostate cancer. BMC Cancer (2020) 20(1):361. doi: 10.1186/s12885-020-06879-1
67. Collins N, Belkaid Y. Control of immunity via nutritional interventions. Immunity (2022) 55(2):210–23. doi: 10.1016/j.immuni.2022.01.004
68. Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol (2016) 37(6):386–98. doi: 10.1016/j.it.2016.04.003
69. Wu H, Zhou C, Kong W, Zhang Y, Pan D. Prognostic nutrition index is associated with the all-cause mortality in sepsis patients: A retrospective cohort study. J Clin Lab Anal (2022) 36(4):e24297. doi: 10.1002/jcla.24297
70. Cha JK, Kim HS, Kim EJ, Lee ES, Lee JH, Song IA. Effect of early nutritional support on clinical outcomes of critically ill patients with sepsis and septic shock: A single-center retrospective study. Nutrients (2022) 14(11):2318. doi: 10.3390/nu14112318
71. Tfaily MA, Ghanem P, Farran SH, Dabdoub F, Kanafani ZA. The role of preoperative albumin and white blood cell count in surgical site infections following whipple surgery. Sci Rep (2022) 12(1):19184. doi: 10.1038/s41598-022-21849-2
72. Zhang X, Liu P, You J. Risk factors for surgical site infection following spinal surgery: A meta-analysis. Med (Baltimore) (2022) 101(8):e28836. doi: 10.1097/MD.0000000000028836
73. Busani S, Damiani E, Cavazzuti I, Donati A, Girardis M. Intravenous immunoglobulin in septic shock: review of the mechanisms of action and meta-analysis of the clinical effectiveness. Minerva Anestesiol (2016) 82(5):559–72.
74. Xun Y, Yang Y, Yu X, Li C, Lu J, Wang S. A preoperative nomogram for sepsis in percutaneous nephrolithotomy treating solitary, unilateral and proximal ureteral stones. Peer J (2020) 8:e9435. doi: 10.7717/peerj.9435
75. Wang Q, Jiang K, Chen X, Zeng G, Sun F. The predictive value of preoperative albumin-globulin ratio for systemic inflammatory response syndrome after percutaneous nephrolithotomy. Int J Gen Med (2022), 15:7407–7415. doi: 10.2147/IJGM.S379741
76. Banait T, Wanjari A, Danade V, Banait S, Jain J. Role of high-sensitivity c-reactive protein (Hs-CRP) in non-communicable diseases: A review. Cureus (2022) 14(10):e30225. doi: 10.7759/cureus.30225
Keywords: percutaneous nephrolithotripsy, systemic inflammatory response syndrome, biomarkers, PCNL, SIRS
Citation: Wu W, Zhang D, Jin T, Lu T and Zhou F (2023) Progress in the study of biomarkers for early prediction of systemic inflammatory response syndrome after percutaneous nephrolithotomy. Front. Immunol. 14:1142346. doi: 10.3389/fimmu.2023.1142346
Received: 12 January 2023; Accepted: 22 March 2023;
Published: 30 March 2023.
Edited by:
Dor Golomb, Assuta Ashdod University Hospital, IsraelReviewed by:
Fernanda Gabrigna Berto, Western University, CanadaVladimir M. Pisarev, Federal Research and Clinical Center of Intensive Care Medicine and Rehabilitation, Russia
Copyright © 2023 Wu, Zhang, Jin, Lu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenghai Zhou, WmhvdWZlbmdoQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Wangjian Wu
Wangjian Wu Di Zhang2†
Di Zhang2†