- 1Department of Biotherapy and National Clinical Research Center for Geriatrics, Cancer Center, West China Hospital, Sichuan University, Sichuan, China
- 2Department of Head and Neck Oncology, Department of Radiation Oncology, Cancer Center, and State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Sichuan, China
- 3Research and Development Department Shanghai ETERN Biopharma Co., Ltd., Shanghai, China
- 4West China School of Nursing, West China Hospital, Sichuan University, Sichuan, China
The evidence for chronic hepatitis B virus (HBV) infection and hepatocellular carcinoma (HCC) occurrence is well established. The hepatocyte epithelium carcinogenesis caused by HBV has been investigated and reviewed in depth. Nevertheless, recent findings from preclinical and observational studies suggested that chronic HBV infection is equally important in extrahepatic cancer occurrence and survival, specifically gastrointestinal system-derived cancers. Immune microenvironment changes (immune-suppressive cytokine infiltration), epigenetic modification (N6-methyladenosine), molecular signaling pathways (PI3K–Akt and Wnt), and serum biomarkers such as hepatitis B virus X (HBx) protein are potential underlying mechanisms in chronic HBV infection-induced extrahepatic cancers. This narrative review aimed to comprehensively summarize the most recent advances in evaluating the association between chronic HBV infection and extrahepatic cancer risk and explore the potential underlying molecular mechanisms in the carcinogenesis induction of extrahepatic cancers in chronic HBV conditions.
1 Introduction
Hepatitis B (HBV) infection is one of the leading global public health issues to date. There are approximately 316 million hepatitis B surface antigen (HBsAg)-seropositive carriers, specifically in the developing and poor countries (1, 2). The serum indicators, which include HBsAg, hepatitis B core antigen (HBcAg), hepatitis B e antigen (HBeAg), hepatitis B virus X (HBx) protein, and HBV DNA copies, are crucial in HBV-associated diseases. The correlation between chronic HBV and hepatocellular carcinoma (HCC) has been investigated in depth. The “three steps” of chronic hepatitis, liver cirrhosis, and HCC have been validated (2–4).
Emerging evidence also identified abnormal HBV DNA copies in several extrahepatic tissues, including the stomach, pancreas, and colorectum, which indicated the potential association between HBV infection and extrahepatic cancer risk (5, 6). An epidemiological report evaluating the cancer deaths and cases in China attributable to lifestyle factors and infections revealed that HBV infection accounted for 12% and 7% of cancer-associated mortality in men and women, respectively (7). Recent large-scale population-based studies and meta-analyses have detailed these under-estimated connections (8–12). For example, a Chinese prospective cohort study reported that the HBsAg-seropositive population had a significantly increased stomach cancer, colorectal cancer, oral cancer, pancreatic cancer, and lymphoma risk as compared with the HBsAg-seronegative population (13). Furthermore, US health care organizations reported HBV-induced extrahepatic cancers in the US population in 2006–2018 (14). Therefore, assessments of the clinical connections and underlying mechanisms in HBV infection-induced extrahepatic cancers are equally pivotal as they could aid understanding of the HBV biological characteristics in human tissue carcinogenesis and aid individualized monitoring and treatment modalities in the HBV-infected population. In this review, we summarize the most recent progress in chronic HBV infection and extrahepatic cancers and review the possible underlying mechanisms of these connections (Figure 1).
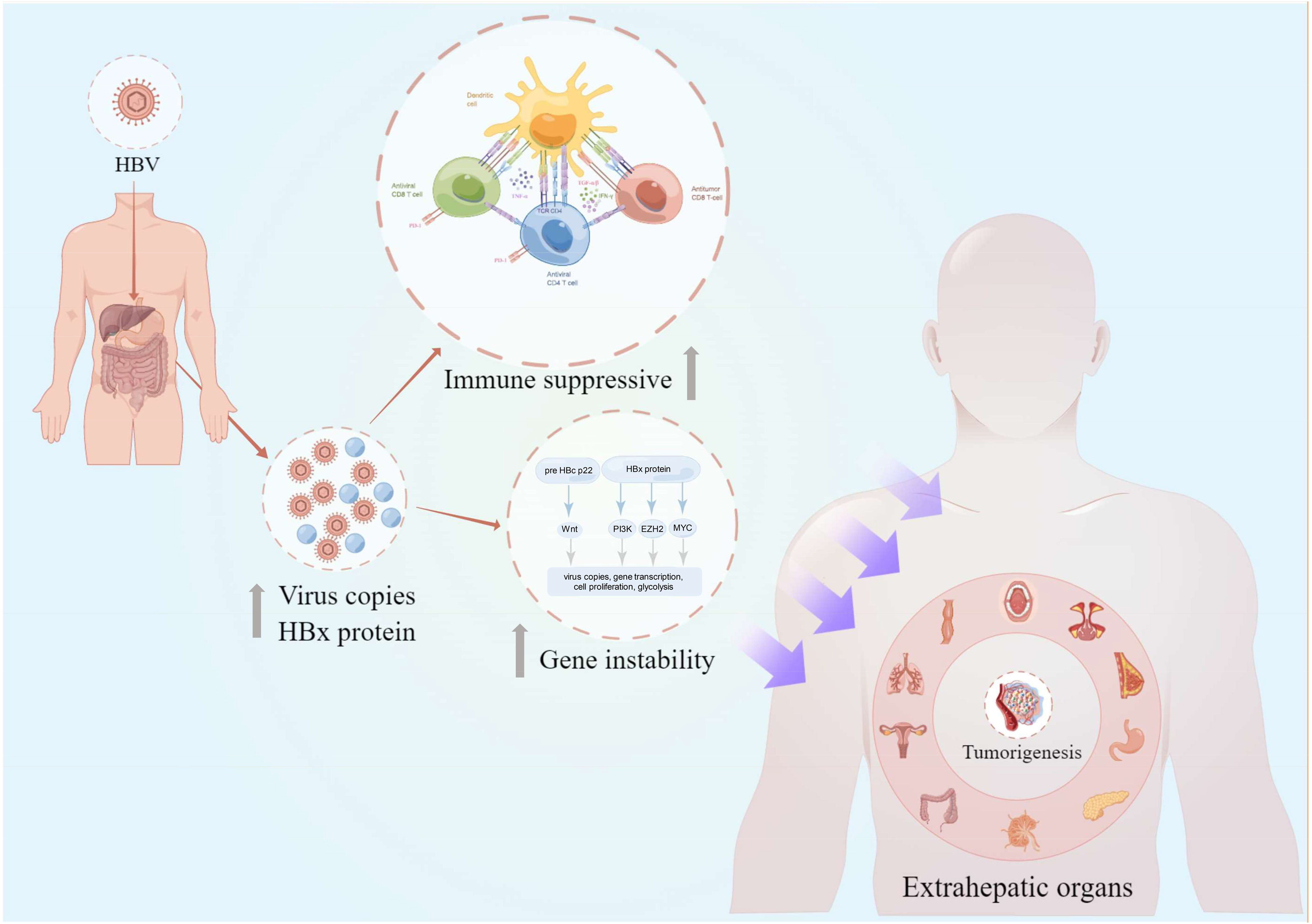
Figure 1 The overview of chronic HBV-mediated extrahepatic cancers in this review. The intrahepatic and extrahepatic HBV copies and HBx protein secretion could lead to the gene instability and host immunosuppressive condition. These adversely effects would further increase the risk of extrahepatic cancer risks in different organs including the oral cavity, nasopharynx, breast, stomach, pancreas, lymph, colorectum, cervix, lung, and esophagus (clockwise description). HBV, hepatitis B virus; pre HBc p22, pre-hepatitis B core protein 22; HBx, hepatitis B virus X; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; IFN-γ, interferon γ; PD-1, programmed cell death protein 1; This figure was drawn with the tool “Figdraw” by Dr. Yu Min and Xiaoyuan Wei. HBV, hepatitis B virus.
2 Epidemiology of chronic hepatitis B
Currently, HBV infection is a major global public health concern (15, 16). Approximately 30% of the global population exhibits serological evidence of current or previous HBV infection (1, 17). Vertical transmission (from mother to neonate) and horizontal transmission (mother to child and child to child) remain the main HBV transmission routes, while the administration of contaminated blood products remains a major transmission mode, particularly in the low- and middle-income countries (18, 19).
Since the 1990s, The World Health Organization (WHO) has called for the formulation of several global public plans (routine HBV vaccine injection, fast virus testing, advanced antiviral therapies) to prevent HBV infection and HBV-related disease progression (1, 20–25). Most recently, the global HBV infection burden was assessed by Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019 collaborators (1) (Figure 2). In detail, the estimated global all-age prevalence of chronic HBV infection was 4.1% (95% uncertainty interval [UI]: 3.7–4.5), which corresponded to approximately 316 million infected people (95%UI: 284–351). With the wide utility of HBV vaccination in different regions, all-age HBV infection prevalence of the whole population has declined by 31.3% (95%UI: 29.0–33.9%) during the past three decades. Furthermore, a more significant decline in HBV infection prevalence was observed in younger children (<5 years) (76.8%, 95%UI: 76.2–77.5%) during the same period (1). However, HBV infection and associated diseases burden and progress across the world were markedly disparate, where the developing and poor countries predominated (1, 16, 21, 23, 26, 27). HBV infection has been endemic in China since the 1980s, where the infant vaccination rate was 99.5% and the timely birth dose vaccination rate was 96% as compared with that in 1992 (28). Subsequently, the national hepatitis B immunization program led to chronic HBV infections decreasing by 33.9% (28). While significantly decreased HBV infection prevalence was reported in most countries and regions owing to HBV vaccination in the general population, the number of remaining HBV-infected patients continues to be substantial and they require early detection, more precise management, and reduction of further transmission.
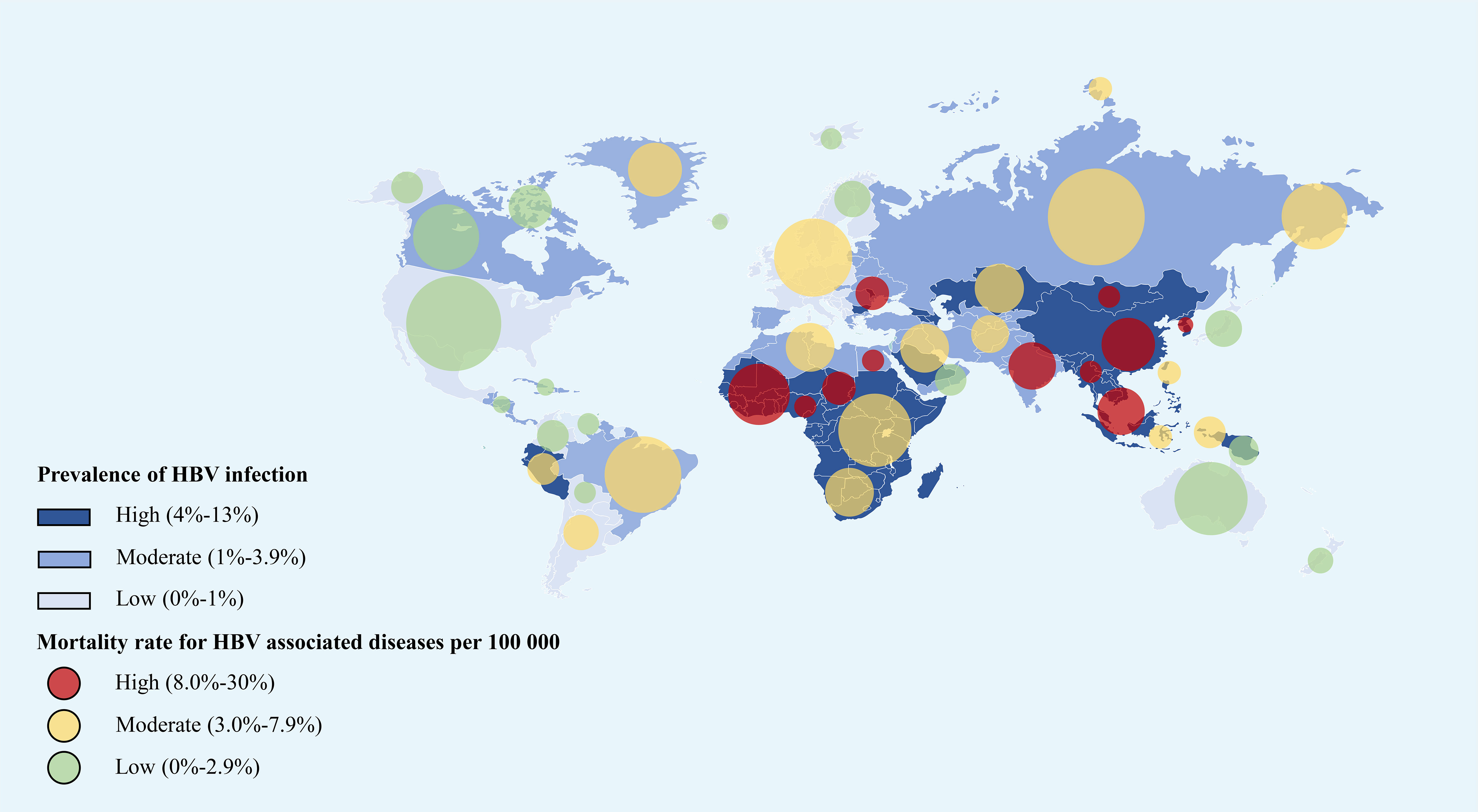
Figure 2 The global prevalence of HBV infection and all-age death rate for HBV associated diseases per 100,000 in 2019 (refers to the Global Burden of Disease Study 2019) (1). The east Asian countries and African countries showed the highest HBV infection rate as well as the mortality rate around the world. This figure was drawn with the tool of “Smart Art” by Dr. Xiaoyuan Wei. HBV, hepatitis B virus.
3 Chronic hepatitis B in extrahepatic cancer risk
The correlation between chronic HBV infection and HCC development has been well investigated (29–32). The integration of virus DNA and the oncoprotein HBx, intrahepatic immune imbalance, and HBV infection-induced cirrhosis are acknowledged as the frequent pivotal factors of hepatocarcinogenesis (3, 33, 34). More recently, epidemiological evidence demonstrated a potential association between HBV infection and extrahepatic cancer risk, which includes gastrointestinal cancers, head and neck cancer, cervical cancer, non-Hodgkin lymphoma, breast cancer, and non-small cell lung cancer (NSCLC) (13, 14, 35–39) (Figure 1). Notably, HBV infection status can not only increase the extrahepatic cancer risk but can also significantly influence subsequent long-term survival patterns (40–45). However, a multi-center study demonstrated that a large proportion of patients with newly diagnosed cancer and concurrent HBV were unaware of their viral infection at the time of cancer diagnosis (46). Therefore, exploring the association between chronic HBV infection and pan-cancer could provide more robust evidence to establish effective public health strategies to guide HBV-infected population surveillance. Table 1 summarizes the recent clinical studies that evaluated the association between chronic HBV infection and extrahepatic cancers.
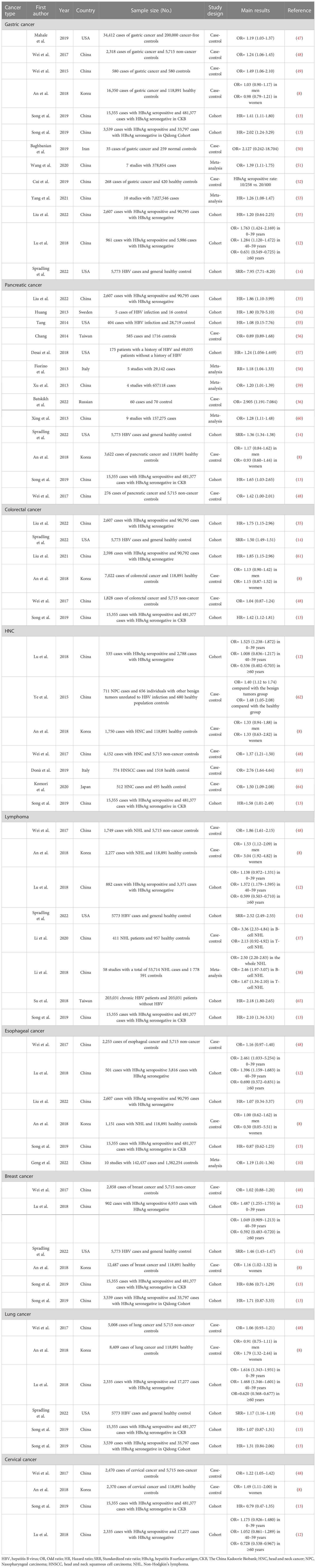
Table 1 The evaluation of associations between chronic HBV infection and extrahepatic cancers in recent studies.
3.1 Gastric cancer
Gastric cancer is one of the specific infection‐associated cancers. Helicobacter pylori (Hp) and Epstein‐Barr virus (EBV) infections are well-known gastric cancer risk factors (66–68). Recent evidence demonstrated a positive association between chronic HBV infections and gastric cancer risk. A Chinese retrospective study was the first to demonstrate this potential correlation (49). The study involved 580 cases and 580 matched controls, and the authors (49) determined that HBsAg seropositivity was significantly associated with increased gastric cancer risk (adjusted odds ratio [adjustedOR] = 1.49, 95% confidence interval [CI]: 1.06–2.10) in the whole group and in patients without a family history of gastric cancer (adjustedOR = 1.49, 95%CI: 1.06–2.11). In a pilot study, the relatively limited evidence of the study design and the lack of records on Hp infection could not resolve the question of whether HBV infection patients required different screening plans or additional surveillance for gastric cancer (39). In the prospective Kailuan Cohort Study (HBsAg seronegative: 93,402 cases; HBsAg seropositive: 2,607 cases), Liu et al. (35) comprehensively evaluated the correlation between HBV infection and newly diagnosed gastrointestinal cancers. However, the subgroup analysis results did not support the hypothesis (adjusted hazard ratio [adjustedHR] = 1.20, 95%CI: 0.64–2.25). Conversely, the results from another Chinese hospital experience (52) revealed no association between HBV infection and gastric cancer risk. Nonetheless, histological examination revealed an increased nuclei‐to‐cytoplasm ratio in the HBV-exposed gastric cells and morphological atypia of the gastric epithelium. Furthermore, the HBV-exposed patients had lower programmed cell death protein 1 (PD-1) expression levels. Notably, information on Hp infection and antivirus treatment records was unavailable during the analysis in the abovementioned studies, which could have led to selection bias and obscured the actual connection between these two diseases. Therefore, there is a lack of robust evidence justifying the further bench-to-bed step for screening and preventing HBV-associated gastric cancer. Interestingly, recent evidence from a cross-sectional study in Iran (50) revealed that HBV infection was only associated with gastritis (OR = 12.46, 95%CI: 3.01–51.61) but not cancer (OR = 2.13, 95%CI: 0.24–18.70). Hp infection was more frequently detected in HBsAg-seropositive patients than in HBsAg-seronegative patients (p = 0.029). While the authors compared the clinicopathological characteristics of HBV infection patients with or without concurrent Hp infection, the study limitations, which included the study design and small sample size, could not be overlooked when concluding on the association between HBV infection and gastric cancer.
Most recently, two meta-analyses based on epidemiological studies reported consistent evidence on this topic. Wang et al. (51) determined that patients with gastric cancer presented a significantly higher HBV infection prevalence (OR = 1.39, 95%CI: 1.11–1.75) after patients with known Hp or EBV infections had been excluded. Yang et al. (53) reported that HBV infection was associated with a remarkably higher gastric cancer risk as compared with the healthy controls (HR = 1.26; 95%CI: 1.08–1.47).
Generally, the evidence supporting the positive relation between HBV infection and gastric cancer risk was mainly based on retrospective case–control and cohort studies. The confounding variables, which included other virus infections and cirrhosis, was not well controlled in many studies. Additionally, the underlying mechanisms of HBV-induced carcinogenesis in gastric cancer remain unknown.
3.2 Pancreatic carcinoma
While pancreatic carcinoma incidence is much lower than that of other gastrointestinal tumors, a worse survival outcome is predicted for patients with pancreatic carcinoma (69). Virus and autoimmune disease-induced inflammation is pivotal in pancreatic carcinoma development (70–72). Nevertheless, the role of HBV infection in pancreatic carcinoma risk remains uncertain. Initially, Hassan et al. (70) confirmed the apparent association between past exposure to HBV infection and pancreatic carcinoma risk in a US population (anti-HBc-seropositive patients: adjustedOR = 2.5; anti-HBc and anti-HBs double-seropositive patients: adjustedOR = 2.3; anti-HBc-seropositive but anti-HBs-seronegative patients: adjustedOR = 4). However, de Gonzalez et al. noted that the study lacked HBsAg testing results, which are considered a sensitive marker of chronic HBV infection. This absence might have led to uncertainties in directly evaluating the potential association between HBV infection and pancreatic cancer risk (73). Notably, de Gonzalez et al. presented evidence derived from a Korean population-based study (74). Interestingly, the findings did not support the strong association suggested by Hassan et al. (70), where HBsAg was considered the basis for diagnosing HBV infection (74). Furthermore, the results reported in the following works are non-concordant. In 2013, a notable meta-analysis that involved nine studies (60) reported more robust clinical evidence supporting the positive association between HBV infection and pancreatic cancer risk (poolOR = 1.28, 95%CI: 1.18–1.48). Other hospital-based studies reported similar results (36, 75). Most recently, a large-scale prospective cohort study also reported a direct association between HBV infection and pancreatic cancer onset (35). By contrast, two retrospective population-based cohort studies and one hospital-based study did not observe this positive correlation (54–56). Notably, each study could have been influenced by confounding factors that included the different pancreatic cancer histological classifications (pancreatic adenocarcinoma, neuroendocrine, or unspecified pancreatic tumors), different ethnic populations, and HBV infection history duration.
A previous study reported that patients with pancreatic cancer had remarkably increased HBV DNA and anti-HBc levels when compared with healthy controls. The results indicated that HBV infection in the pancreatic tissues might be the underlying etiology of pancreatic carcinoma development (76). Similarly, HBV antigens and replicative sequences have been identified in extrahepatic organs, including the pancreas (6). For this reason, some scholars speculated that the existence of HBV in pancreatic tissue could cause chronic pancreatic inflammation such as that in hepatic tissues, with the possibility of progression to malignant transformation (77). Contrastingly, HBV infection patients with pancreatic carcinoma demonstrated different metastatic patterns and survival (44), where HBV infection increased the synchronous liver metastasis rate in the patients. Interestingly, chronic HBV infection patients nevertheless demonstrated better survival when compared with inactive HBsAg carriers (p = 0.039). The authors proposed possible explanations to aid understanding of this phenomenon, where the beneficial role of anti-HBV therapy and subsequent immunological and tumor microenvironment changes could specifically be contributed to these divergence progression patterns. The early detection and diagnosis of pancreatic cancer are challenging (78, 79). Emerging evidence demonstrated a higher rate of early detection of pancreatic cancer in HBV infection patients under HCC surveillance (80). Therefore, whether routine screening for pancreatic conditions can be conducted during the surveillance of HBV-induced HCC is worth investigating.
3.3 Colorectal cancer
To date, compelling evidence supports the premise that HBV, especially chronic HBV infection status, can increase the colorectal cancer risk in populations from different regions. In a report from a large-scale Taiwan cohort, Kamiza et al. (11) determined that HBV infection patients exhibited a higher colon cancer development risk (adjustedHR: 1.36, 95%CI: 1.09–1.70). Several recent population-based epidemiological studies from different regions confirmed this potential correlation. Notably, in the China Kadoorie Biobank prospective cohort study from mainland China (13), HBsAg-seropositive patients had increased colorectal cancer risk (adjustedHR = 1.42, 95%CI: 1.12–1.81). However, this correlation was not validated in another two cohorts due to the limited sample size and cancer types. In line with the positive findings in gastric cancer, the Kailuan Cohort Study (61) also confirmed a positive association between HBV infection and colorectal cancer risk (whole group: adjustedHR = 1.77, 95%CI: 1.11–2.84; after excluding participants in whom HCC occurred within 1 year: adjustedHR = 2.05, 95%CI: 1.28–3.30). Epidemiologic investigations from Western countries presented more robust evidence of this correlation. Among chronic HBV infection patients in the Chronic Hepatitis Cohort Study (CHeCS), the patients with colorectal cancer had a slightly increased standardized rate ratio (SRR) of 1.50 (95%CI: 1.49–1.51) as compared with general health controls (National Program of Cancer Registries and Surveillance, Epidemiology, and End Results program cancer registries, 2006–2018) (14). A Canadian population-based study demonstrated that HBV infection patients had significantly elevated colorectal cancer risk (adjustedHR = 2.47, 95%CI: 1.85–3.28) as compared with uninfected patients (9). In patients with colorectal cancer, chronic HBV infection was observed as a pivotal risk factor of synchronous colorectal cancer liver metastasis and subsequent poor prognosis. By contrast, occult HBV infection instead of HBV DNA serum level decreased metachronous colorectal cancer liver metastasis occurrence with better overall survival (OS) and liver disease-free survival during the 5-year postoperative follow-up (45). Generally, these studies presented robust clinical evidence that chronic HBV infection can not only increase colorectal cancer risk but can also impair short-term survival in different ethnicities and regions. However, the underlying molecular mechanisms in HBV-induced colorectal cancer remain unknown. Furthermore, recent evidence demonstrated that anti-virus treatments in chronic HBV infection patients also increased the colorectal cancer risk (81). Accordingly, future analyses should consider this confounding information.
3.4 Head and neck cancer
HBV plays a role in head and neck cancer development and progression (82–85). Head and neck cancer with HPV-positive status is a specific head and neck cancer that has different tumor-node-metastasis (TNM) stage classifications and warrants varied treatment modalities (86). Recent epidemiological studies investigated the association between HBV infection and head and neck squamous cancer. Notably, Donà et al. (63) identified a positive association between HBV infection and head and neck squamous cancer in their case–control study. Alternatively, head and neck squamous cancer was more likely to coexist with chronic HBV infection (adjustedOR = 2.76; 95%CI: 1.64–4.64). Similar positive results were reported for Asian populations (43, 64). Nevertheless, the retrospective design of these studies meant that the exact causality between these two diseases could not be determined. In contrast, earlier evidence from a large population-based study did not support this correlation (87). Instead of HBV infection, patients with HCV infection presented a 2.28-fold higher oral cavity cancer risk as compared with the non-viral hepatitis group (6.15 vs. 2.69 per 10,000 person-years). Although some studies have examined the association between HBV infection and head and neck cancer, the evidence of the link between HBV and head and neck cancer occurrence is scanty and the mechanism of the high concurrent rate of HBV infection remains unclear.
3.5 Lymphoma
Emerging evidence confirmed that HBV demonstrates a significant capacity to infect peripheral blood mononuclear cells (PBMCs) and replicate in them (88, 89). For this reason, recent clinical studies focused on the association between chronic HBV infection and non-Hodgkin lymphoma, but reported inconsistent results (37, 38, 90–92). Notably, a comprehensive meta-analysis involving 58 studies and a total of 53,714 non-Hodgkin lymphoma cases and 1,778,591 non-cancer controls highlighted a remarkable association between HBV infection and increased B-cell non-Hodgkin lymphoma risk, specifically diffuse large B-cell lymphoma and follicular lymphoma (38). A recent hospital-based study also reported this correlation (37). Notably, the authors detected multiple integrated HBV DNA copies in lymphoma tissue via high-throughput viral integration detection. Correspondingly, the exons of four genes [fat atypical cadherin 2 (FAT2), senataxin (SETX), α10 integrin (ITGA10), lysosome-associated membrane protein-3 (CD63)] were interrupted by the HBV DNA, and the expression levels of seven HBV preferential target genes [ankyrin repeat and sterile alpha motif domain-containing protein 1B (ANKS1B), recombinant histone deacetylase 4 (HDAC4), fibronectin type III and laminin G-like domain-containing protein (EGFLAM), alpha1,2-mannosidase subtype IC (MAN1C1), XK-related 6 genes (XKR6), zinc finger and BTB domain-containing protein 38 (ZBTB38), coiled-coil domain containing 91 (CCDC91)] were significantly altered. In line with other HBV-associated cancers, HBsAg-seropositive diffuse large B-cell lymphoma exhibited unique clinicopathological characteristics that included younger age at diagnosis, more frequent spleen involvement, more advanced disease, and lower myelocytomatosis oncogene (MYC) expression rate when compared with the controls (91). However, HBV infection did not influence the treatment response of lymphoma. Consequently, more attention should be focused on the early screening of peripheral blood component disorders in chronic HBV infection patients. Furthermore, whether HBV infection can impair the survival of patients with non-Hodgkin lymphoma requires further exploration.
3.6 Esophageal cancer
Compelling evidence highlighted the fact that virus infection, particularly human papillomavirus (HPV) infection, could be involved in esophageal cancer development and progress (10, 93–97). Similarly, the role of HBV infection in esophageal cancer has been evaluated. The most recent meta-analysis, which involved 10 studies (142,437 cases and 1,382,254 controls) (10) confirmed that HBV infection patients had significantly increased esophageal cancer risk (adjustedOR = 1.19, 95%CI: 1.01–1.36). However, another hospital-based study (98) determined that HBV infection (referred to as HBsAg-seropositive) was a favorable prognostic factor for OS and disease-free survival (DFS) in patients with operable esophageal squamous cell carcinoma (DFS: adjustedHR = 0.79, 95%CI: 0.66–0.94; OS: adjustedHR = 0.80, 95%CI: 0.65–0.95). Some researchers speculated that HBV-induced immune microenvironment changes could contribute to this cancer progress divergence (99, 100). For example, the possession of specific genetic variants on the interleukin-17 (IL-17) gene might represent the underlying basis of the positive prognostic value of HBV infection on the survival outcomes of patients with esophageal cancer (101). These findings suggested the important but complex role of HBV infection in esophageal cancer. Unlike HPV-induced esophageal cancer, there remains a lack of robust evidence to explain the association between HBV infection and esophageal cancer. The histological examination of HBV-related indicators from esophageal epithelial cells might aid the exploration of detailed associations such as that in other gastrointestinal sites (52, 59, 77).
3.7 Breast cancer
Several studies have investigated the association between nutrition, infection, and environmental exposure with breast cancer risk (102–109); nevertheless, the correlations between HBV infection and breast cancer remain uncertain. Notably, a case–control study in China demonstrated that HBV infection was potentially an independent risk factor regardless of other factors in middle-aged breast cancer development (103), as the positive rate of HBc antibody (HBcAb) in patients with breast cancer (66.4%) was significantly higher than that in the controls (53.7%). Nevertheless, the serum HBsAg levels among the two groups were not markedly different. Another center reported significant associations between breast cancer and HBV infection, which could increase the breast cancer risk by approximately 16% (8). This correlation was validated in a US population-based epidemiological study (14). By contrast, two nationwide cohorts from China did not support the potential risk of HBV infection in inducing breast cancer (13, 107). Su et al. (107) determined that HCV infection but not HBV infection was more pivotal in breast cancer development. On the other hand, HBV infection patients with breast cancer had a higher risk for additional liver metastasis (102). Furthermore, recent studies indicated that chronic HBV infection patients with breast cancer tended to have an earlier tumor stage and higher histological grade (105), but survival outcomes were worse in young adult patients (104). These results supported precision treatment modalities for this subpopulation, and routine screening for HBV infection status could be a promising cost-effective means of preventing HBV reactivation during treatment (106). Further studies are needed to clarify the underlying mechanisms of the association between HBV infection and this highly common endocrine-related cancer.
3.8 Lung cancer
Chronic viral infections are frequent comorbidities in patients with lung cancer. However, whether there are potential connections between virus infection and lung cancer development remains unclear, as there have been few studies on this topic. Based on previous findings, HBV infection has a limited role in lung cancer risk. For example, an epidemiologic study on pan-cancer incidence analysis in HBV patients revealed only a slightly increased SRR on lung cancer risk in HBV conditions when compared with the general population (adjusted incidence: 105.8 vs. 90.4, SRR = 1.17, 95%CI: 1.16–1.18) (14). Similarly, no association was identified with elevated lung cancer risk in an HBsAg-seropositive Chinese population (35).
Recent studies focused on evaluating the prognostic outcomes of chronic HBV infection patients with NSCLC. Chronic HBV infection induces chronic inflammation. Chen et al. revealed that HBV infection patients with NSCLC presented unique survival patterns, which required more individualized clinical management (40, 110, 111). Combining HBV infection status with peripheral blood indicators, clinicians could obtain more precise survival predictions in this subpopulation. Serum HBV DNA levels could also be an independent prognostic factor in patients with NSCLC (41). HBV infection was recently associated with NSCLC immune expression (112, 113). HBV induced a systemic immune response with increased PD1 ligand 1 (PD-L1) expression levels in patients with NSCLC. However, a hospital-based study revealed that HBV infection might not affect the efficacy of immunotherapy (PD-1/PD-L1 inhibitors) in patients with advanced NSCLC. Nevertheless, the potential risk of virus reactivation when receiving treatment remains important, while the mechanism of HBV reactivation induced by immunotherapy therapy remains unclear (114, 115). For this reason, active surveillance before and during immunotherapy for HBV-associated serum indicators is important regardless of the rare incidence of these events.
3.9 Cervical cancer
Generally, HBV and HPV are DNA viruses that almost invariably integrate into the host genome in cancers (116, 117). The infections by both viruses present relatively the same clinical progress and the activated immune response can eradicate the viruses in most infected patients. However, a small sub-group can progress to carcinoma years later. The association between HPV infection and cervical cancer development has been established, where the evidence demonstrated a high concurrent rate of HBV and HPV co-infection (118, 119). Subsequently, recent hospital-based and epidemiological studies investigated the influence of HBV infection on cervical cancer development and prognosis (8, 12, 120–122). However, the influence of HBV infection on cervical cancer risk is debated. A large-scale hospital-based study from Korea (sample = 2,370 cervical cancer cases with an HBsAg-seropositive rate of 4.98%) (8) revealed a similar positive connection between HBV infection and cervical cancer risk as in other extrahepatic cancers (adjustedOR = 1.49). Conversely, this correlation was not reported in another larger-scale study from China (sample = 4,106 cervical cancer cases with an HBsAg-seropositive rate of 12.1%) (12). Moreover, a relatively lower risk for cervical cancer development was observed in the elderly (age > 60 years) HBV infection subpopulation (adjustedOR = 0.73). Nevertheless, the antivirus treatment rates among HBV infection groups in these two studies were unknown. Therefore, the conclusion for the causal effect between HBV infection and cervical cancer risk requires further evaluation with more comprehensive clinical information. An increasing number of studies revealed that anti-HBs seropositivity and prior HBV infection might be independent favorable prognostic factors for the OS and DFS of patients with cervical cancer (120, 121), whereas HBx protein exerted an adverse effect on cervical cancer progression (122). Accordingly, in-depth explorations of the interaction between HBV and HPV in inducing the carcinogenesis of cervical lesions and the immune microenvironment in HPV infection patients with cervical cancer with prior HBV infection could aid understanding of the role of HBV infection status and anti-HBV treatment in cervical cancer risk and future prognosis.
3.10 Nasopharyngeal carcinoma
A region-specific cancer, nasopharyngeal carcinoma (NPC) is frequently observed in Southern China, which accounts for approximately 50% of patients worldwide (age-standardized incidence rate of NPC in China in 2018: 3.0 per 100,000) (123, 124). NPC development likely involves close interplay between multiple factors, including EBV infection, environmental exposure, and genetics (123). Recent clinical studies assessed whether HBV infection played a role in NPC development. For example, a case–control study revealed a higher prevalence of anti-HBcAg seropositivity in patients with NPC as compared with patients with other benign tumors (47.26% vs. 39.33%) and anti-HBcAg seropositivity was an independent indicator of higher NPC risk (adjustedOR = 1.40). Moreover, patients with anti-HBcAg and EBV double-positivity had the highest NPC development risk (adjustedOR = 141.82) (62). While the role of HBsAg was not associated with NPC risk, another Southern China study revealed the pivotal role of HBsAg seropositivity in the prognosis of patients with NPC. That is, HBsAg-seropositive patients with NPC had worse outcomes, and a higher incidence of distant metastasis, specifically liver metastasis, was more common in such patients (125). Nevertheless, the evidence supporting the prediction value of HBV-associated serum indicators in NPC development was based on limited clinical hospital-based studies, and the underlying mechanisms remain unclear. Recent research evaluated the value of HBV infection on NPC prognosis (126, 127). Although HBsAg seropositivity was a negative prognostic factor in NPC, only patients with a particular tumor stage were significantly affected by this condition. In a Southern China report, Liu et al. (126) demonstrated that HBsAg seropositivity was an independent adverse prognostic predictor for survival and recurrence in patients with locoregionally advanced NPC but not patients with early-stage NPC. Interestingly, another recent study (127) from a similar Southern Chinese region indicated that HBsAg seropositivity markedly impaired the OS, DFS, and metastasis-free survival of patients with early-stage NPC. Furthermore, immune dysfunction conditions were observed in the HBsAg-seropositive subpopulation, which had a lower CD4+ T cell count than the HBsAg-seronegative group. The authors also identified a statistically significant beneficial role of anti-HBV treatment in recurrence-free survival (RFS) and metastasis-free survival of the patients.
During the past few years, immunotherapy (particularly PD-1/PD-L1 inhibitors) has become an important treatment strategy against solid tumors (128–130). Immunotherapy was also pivotal in patients with recurrent and/or metastatic NPC (131–133). While recent studies evaluated immunotherapy safety and efficacy among different cancers with HBV infection, research on this topic in patients with NPC is scarce (134–136). A meta-analysis that involved six studies revealed no significant difference in PD-1/PD-L1 inhibitor efficacy on the objective response rate among patients with HBV+HCC and HBV-HCC. However, the HBV+HCC group had a lower disease control rate (136). The association between adjuvant treatment in cancer and HBV reactivation was recently investigated at Chinese medical centers (137, 138). As expected, the results supported the premise that regular monitoring of the serum HBV indicators in HBV infection patients with NPC during treatment courses is warranted. Nevertheless, future studies are needed to quantitatively analyze the safety, efficacy, and cost-effectiveness of these treatment modalities.
4 Molecular pathway in carcinogenesis
HBV is a DNA virus with partly double-stranded relaxed circular DNA (33, 139). HBV infection is a pivotal pathogenesis in HCC development and the subsequent disease progression, and includes epigenetics and metabolic regulation, immune microenvironment dysfunction, HBV DNA integration, gene mutation, and cancer-promoting signaling pathway activation (3, 33, 116, 136, 140–142). Nevertheless, studies on HBV-associated extrahepatic cancers are relatively scarce. Previous study findings supported the premise that HBV might share similar mechanisms of HCC induction in inducing extrahepatic tissue carcinogenesis (103, 116, 122, 140, 141, 143).
4.1 HBV DNA integration
Genomic instability is a pivotal and frequent hallmark of cancer that stems from DNA repair gene mutations and drives cancer development (144, 145). The dynamic nature and unique features of chronic HBV infection such as viral variants, HBV DNA integration into host chromosomes, and extrahepatic reservoirs can induce genome instability to cause cellular transformation and subsequently tumorigenesis (3, 146, 147). Enhanced HBV molecular data and replication were recently identified in genes associated with oncogenic consequences in PBMCs (148). Although previous epidemiological studies determined that chronic HBV infection was significantly associated with extrahepatic cancer development, the exact causalities among the two diseases were not well documented. Given the remarkable advances in whole-genome, transcriptome, and exome sequencing technologies and genome-wide association studies (GWAS), some researchers preliminarily evaluated the role of HBV infection and cancer risk at the genetic level. Khoury et al. (141) reported that RNA sequencing results from The Cancer Genome Atlas (TCGA) database revealed two pronounced gene mutations [telomerase reverse transcriptase (TERT) and mixed-lineage leukemia 4 (MLL4)] in mediating HBV-associated HCC. Zapatka et al. (116) determined that viral integration was associated with local variations in genomic copy numbers and RNA sequencing demonstrated significant exclusivity between HBV infection and mutations in catenin beta-1 (CTNNB1), tumor protein p53 (TP53), and AT-rich interaction domain 1A (ARID1A). Furthermore, next-generation sequencing analysis identified five frequently mutated protein-coding genes in HBV-infected gastric cancer samples [histone-lysine N-methyltransferase 2B (KMT2B), histone-lysine N-methyltransferase 2D (KMT2D), sex-determining region Y box protein 1 (SOX1), fibroblast growth factor 12 (FGF12), and tubulin beta-2B chain (TUBB2B)] (90).
Mendelian randomization (MR) is an analytical approach that can improve causal inference by using genetic single-nucleotide polymorphisms (SNPs) as instrumental variables to infer the causality of exposure to an outcome. In MR analysis that used millions of SNPs in HBV and extrahepatic cancers, Kamiza et al. (143) confirmed that chronic HBV infection had causal effects on cervical cancer and gastric cancer in populations of Asian descent. By contrast, no association was observed between HBV infection and other extrahepatic cancers such as pancreatic and colorectal cancer in these populations. Notably, the SNP data are updated annually and potential connections might be identified in future analysis (149, 150). A recent genome-wide virus-integration analysis (151) revealed that three most common viruses (HBV, HPV, EBV) shared the same insertional mutagenesis (mainly mediated via a synthesis-dependent end-joining DNA repair mechanism). This finding could aid more in-depth understanding of the role of HBV infection in the development of other virus-associated cancers. So far, limited genetic data support the direct association between chronic HBV infection and extrahepatic cancer risk. Nevertheless, this field is worth exploring, which could enhance clinical observational findings and subsequent cancer prevention.
4.2 Chronic inflammation and the immune microenvironment
The intrahepatic inflammation induced by long-term chronic HBV infection significantly alters the host immune system, which is characterized by more immunosuppressive conditions given the weakening of co-activation signals, enhancement of co-inhibitory signals, functional impairment, decreased numbers of effector T cells such as HBV-specific CD8+ T cells but enrichment of T regulatory (Treg) cells (17, 152–160) (Figure 3). The most recent evidence confirmed that the adaptive immune response plays a major role in inducing both viral clearance and liver disease, whereas little innate immune activation was observed in the HBV condition (154). Therefore, the morbidities in chronic HBV infection patients include liver fibrosis and cirrhosis, where even cancers frequently develop in the subsequent years (4), partially owing to the intrahepatic immune disorders of T cell-induced immunopathology. However, the prior changes in the intrahepatic microenvironment of HBV infection contribute to the hepatic carcinogenic process and progress (160). A multidimensional immune landscape analyses revealed a distinct immune microenvironment in HBV-associated HCC that featured more immunosuppressive and exhausted phenotypes when compared with non-HBV-induced HCC (157). Furthermore, the immunopathological results in HBV mouse models revealed that hepatocellular priming of naïve HBV-specific CD8+ T cells led to their local activation and proliferation, but these cells did not further differentiate into effector cells (161, 162).
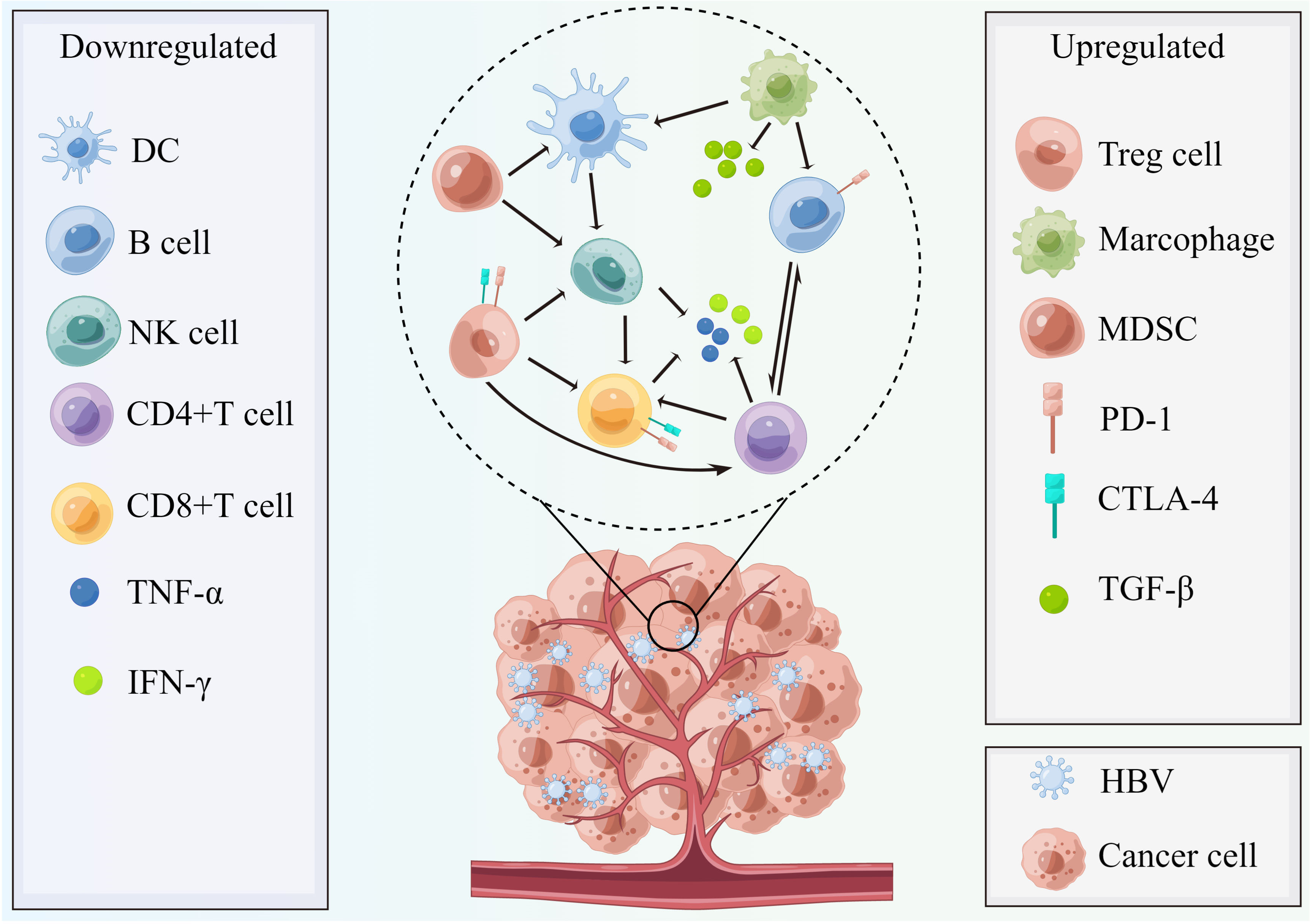
Figure 3 The chronic HBV-mediated tumor microenvironment with characteristics of more immunosuppressive conditions. In HBV infection conditions, the host immune status becomes more immunosuppressive and is characterized by weakening of stimulatory signal (DC, TNF-α, and IFN-γ), enhancement of inhibitory signals (TGF-β), decreased quantity of NK cells, and B cells, as well as effector T cells (CD4+ and CD8+T cell) and enrichment of Tregs cells, myeloid-derived suppressor cells, and M2 macrophage polarization. Therefore, the activated PD-1/PD-L1 pathway plays a suppressive role to help cancer cells’ immune escape and invasion and the CTLA-4 pathways also contribute to this process. This figure was drawn with the tool “Figdraw” by Dr. Yu Min. DC, dendritic cells; MDSC, myeloid-derived suppressor cells; PD-1, programmed cell death protein 1; CTLA-4, cytotoxic T-lymphocyte–associated antigen; TGF-β, transforming growth factor-β; NK cells, nature killer cells; TNF-α, tumor necrosis factor-α; IFN-γ, interferon γ; HBV, hepatitis B virus.
The most recent reviews demonstrated a noteworthy modification of the remaining immune components in the HBV-induced HCC tumor microenvironment (136, 160), with enhanced myeloid-derived suppressor cells (MDSC) cells and macrophages (M2 polarization) but inhibited dendritic cells and natural killer (NK) cells. In HBV-associated HCC, the elevated Treg cell levels aided the maintenance of a tolerogenic environment in the liver and suppressed the proliferation and interferon γ (IFN-γ) production of autologous PBMCs mediated by the HBV condition (163), which were significant immune alterations. Notably, single-cell sequencing results demonstrated that tumor-associated macrophages (TAM) significantly inhibited tumor T-cell infiltration, and T cell immunoreceptor with Ig and ITIM domains–nectin cell adhesion molecule 2 (TIGIT–NECTIN2) interaction regulated the immunosuppressive environment (153). B cells are a major tumor microenvironment component that are predominantly associated with tertiary lymphoid structures. Sufficient evidence demonstrated the double-edged sword of B cells with different phenotypes in cancer occurrence and progress (164). Initially, B cells defend against chronic HBV infection by producing humoral antibodies (136, 165). In chronic HBV infection, B regulatory (Breg) cells and secreted cytokines such as IL-10, IL-35, and transforming growth factor-β (TGF-β) aid the construction of the immune-suppressive landscape in the liver. Therefore, the immune escape caused by the intrahepatic immunosuppressive condition subsequently increases the risk of HBV-induced HCC (155–157). To date, research focused on the tumor-associated immune microenvironment of HBV-induced extrahepatic cancers remains scarce. Nevertheless, immunotherapy safety and efficacy in patients with cancer concurrent with HBV infection were evaluated recently (112, 113). Notably, patients with NSCLC concurrent with HBV infection had a significantly higher percentage of PD-L1 expression when compared with patients without HBV infection. The safety analysis revealed that HBV infection status did not impair the immunotherapy efficacy in patients with NSCLC. Rather, the HBV groups demonstrated more durable clinical benefits and longer progression-free survival and OS than the non-HBV group (113). For this reason, these findings indicate viral mediation of a systemic immune response, which could potentially affect PD-L1 expression-guided immunotherapy in HBV infection patients with extrahepatic cancer. Nevertheless, whether patients with HBV-associated extrahepatic cancers can derive more benefit from immunotherapy requires further exploration. The evaluation of peripheral blood immune cell subsets and single-cell sequencing-related immune infiltration scores aid improved individualized treatment modalities for both HBV-mediated HCC and non-HCC cancer subtypes.
4.3 HBx protein
The detailed biological mechanism linking chronic HBV infection with extrahepatic cancers has not been fully illustrated. Some studies reported elevated HBx protein expression levels and virus DNA copies in several extrahepatic tissues, especially the gastrointestinal system, which suggested that HBV can initiate and promote tumorigenesis outside the liver (5, 6). Currently, it is thought that the transcriptional coactivator HBx protein is crucial in initiating tumorigenesis by modulating key apoptosis regulators, which interferes with the DNA repair pathways and tumor suppressor genes (122, 166–170). Furthermore, HBx protein significantly caused host chromosomal instability (171). Therefore, the target downstream molecules in HBx protein mediate cell carcinogenesis, proliferation, viability, and migration by modulating multiple signaling pathways (167, 170, 172, 173). Specifically, Lei et al. (172) demonstrated that ARRB1 (arrestin β1) was pivotal in HBV-related HCC by modulating autophagy and the CDKN1B–CDK2–CCNE1–E2F1 signaling pathway. However, evaluation studies on HBx protein and extrahepatic cancer occurrence are limited. Notably, Song et al. (13) observed higher HBx protein expression in the cancerous part of the tissue specimen than in the healthy part of the same specimen among several gastrointestinal cancers, which supported the oncogenic role of HBx protein. Moreover, a recent report stated that HBx protein promoted pancreatic cancer development via the PI3K–Akt signaling pathway (166). Based on these non-negligible connections between HBx protein and intrahepatic and extrahepatic cancers, these findings could guide the design and interpretation of subsequent clinical and experimental studies to identify more potential cancer prevention and treatment targets. Furthermore, the low HBx expression levels during HBV infection and the lack of satisfactory detection strategies mean that these findings require verification in models that more closely mimic HBV infection and HBV-associated cancers.
4.4 Cancer-related signaling pathway activation
Over the past few years, compelling evidence indicated that HBV infection induces numerous cancer-related signaling pathways in hepatocellular tissue carcinogenesis (167, 174–178). On the contrary, evaluation data on the underlying molecular mechanisms of chronic HBV infection-associated extrahepatic cancers remain scarce. Nevertheless, some potential pathways described in the literature could aid understanding of the complex mechanisms of HBV infection-mediated extrahepatic cancer occurrence (Figure 4). Notably, Tran et al. (179) used mouse models and determined that HBV pre-core protein p22 alone could activate Wnt signaling. Furthermore, HBV p22 elevated TCF/β-catenin transcription in colon cancer cell lines (SW480 and HCT116) owing to mutations in downstream genes of the Wnt pathway, namely adenomatous polyposis coli (APC) and CTNNB1 (Figure 4A). Similarly, disordered Toll-like receptor 7 and 9 signaling was observed in chronic HBV infection (142). HBx protein activates various signaling pathways in HBV-associated extrahepatic tissue carcinogenesis (166, 168, 169). Specifically, the basic study by Chen et al. (166) demonstrated that HBx increased pancreatic cancer risk by modulating the PI3K–Akt signaling pathway, with elevated expression of human epidermal growth factor receptor 4 (ErbB4) and TGF-α in parallel with HBx protein expression (Figure 4B). PI3K–Akt pathway activation was also observed in gastric cancer samples in vivo and in vitro (168). Furthermore, p53 pathway impairment was observed in gastric cancer samples with HBx protein integration, which promoted glucose metabolic reprogramming in gastric tissue and subsequent cancer development (168). Salerno et al. (170) demonstrated that HBx protein enhanced gene transcription and induced the accumulation of the long non-coding RNA (lncRNA) deleted in lymphocytic leukemia 2 (DLEU2) in infected hepatocytes. The co-recruitment of HBx protein and DLEU2 elevated histone methyltransferase enhancer of zeste homolog 2 (EZH2) expression levels and viral covalently closed circular DNA (cccDNA) levels and enhanced transcription and viral replication in the host (Figure 4C). Moreover, a recent study (169) highlighted that HBx might increase the MYC expression level by inducing the methyltransferase-like protein 3 (METTL3)-mediated N6-methyladenosine (m6A) modification of MYC mRNA, which promoted gastric cancer onset and progression in preclinical analysis (Figure 4D). Generally, while several in vivo and in vitro studies identified the potential different signaling pathways in gastrointestinal cancer occurrence, whether these signaling pathways are also involved in other extrahepatic cancers was not fully investigated. Moreover, the effects of epigenetic modifications in the activation or blocking these signaling pathways of chronic HBV-induced extrahepatic cancers require further investigation.
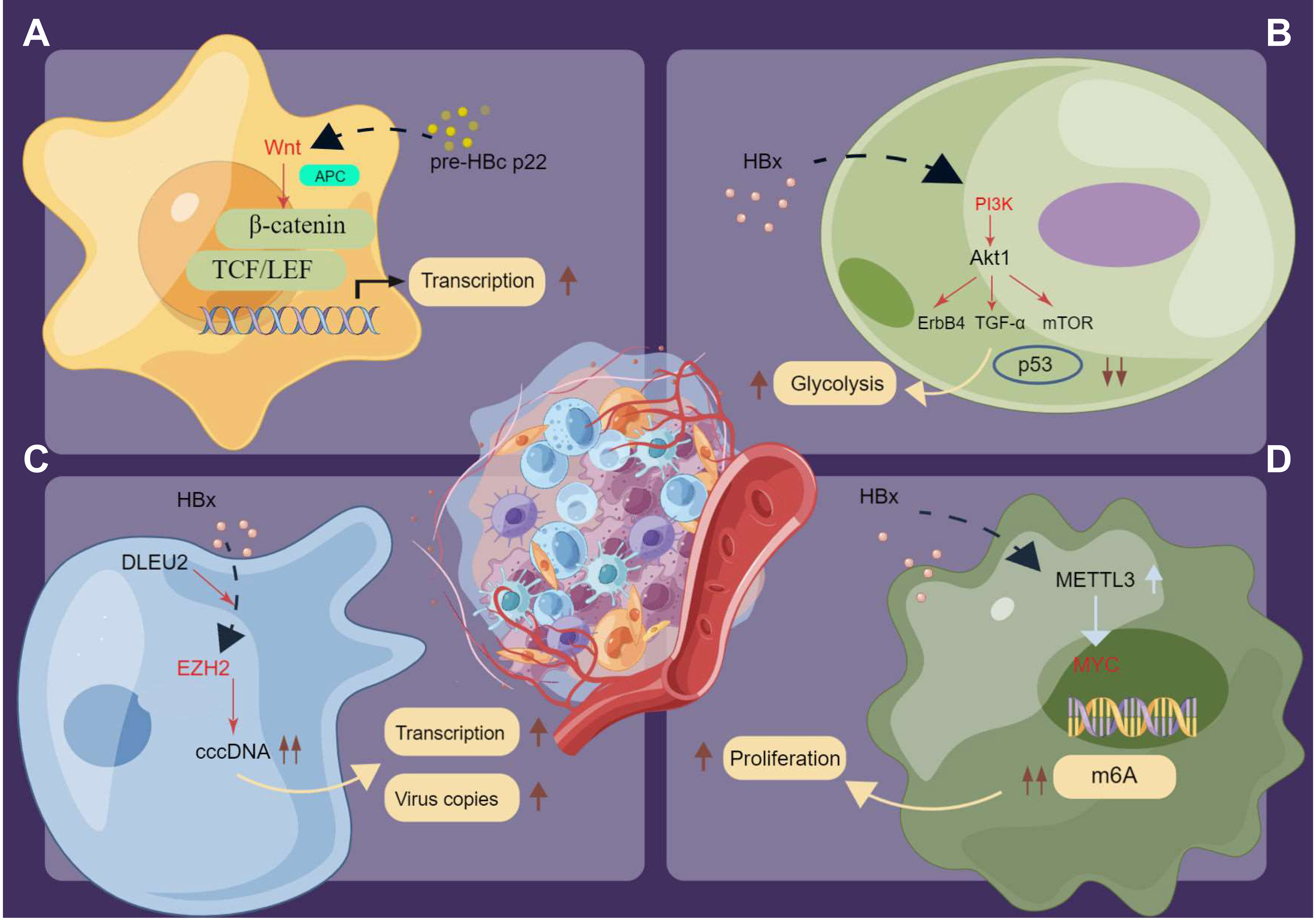
Figure 4 The potential signaling pathways in inducing extrahepatic cancers under the chronic HBV infection. (A) HBV p22 elevates TCF/β-catenin transcription in cancer cells owing to mutations in downstream genes of the Wnt pathway. (B) HBx activates the PI3K/Akt signaling pathway and promotes glucose metabolic reprogramming in extrahepatic cancers. (C) HBx enhances the transcription and induces the accumulation of DLEU2 in infected hepatocytes. The co-recruitment HBx protein and DLEU2 could elevate the expression levels of EZH2 as well as the levels of viral cccDNA and further boost transcription and viral replication in the host. (D) HBx increases the expression level of oncogenes like MYC and the N6-methyladenosine (m6A) modification of MYC mRNA to promote the proliferation of cancer cells. Pre-HBc p22, pre-hepatitis B core protein 22; HBx, Hepatitis B virus X protein; TCF/LEF, T-cell Factor/Lymphoid Enhancing Factor; TGF-α, Transforming Growth Factor α; cccDNA, covalently closed circular DNA; DLEU2, deleted in lymphocytic leukemia 2; EZH2, zeste homolog 2; MYC, myelocytomatosis oncogene; m6A, N6-methyladenosine.
5 Treatment and prognosis
Currently, there remains a lack of specific treatment guidelines for HBV-mediated extrahepatic cancers. Therefore, the established approach is to address the primary malignancy and monitor liver function and the HBV-associated indicators during treatment. Alternatively, clinicians are more interested in assessing the potential virus reactivation risk during novel adjuvant therapy, especially immunotherapy (113–115, 135, 137, 138). Immunotherapy might not significantly increase HBV reactivation risk, but regular monitoring of HBV DNA copies and antiviral prophylaxis could aid the prevention of this potential complication (113, 138). Most studies highlighted that antiviral therapy can reduce cancer metastasis risk and recurrence and subsequently improve long-term survival (42, 127, 180). Additionally, drug repurposing is a recent hot topic in the design of novel anti-cancer treatment modalities (181–183). Notably, Choi et al. (184) demonstrated in the HBV population that statins significantly reduced HBV-associated HCC risk with a dose-dependent effect. Preclinical studies reported that atorvastatin downregulated the HBx protein-induced Akt pathway via the purinergic receptors (P2X) and further decreased hepatocyte proliferation and invasiveness (185). Other studies suggested that anti-platelet therapy contributed to inhibiting HCC development by limiting the intrahepatic accumulation of HBV-specific CD8+ T cells (154, 186). These findings on decreasing the risk of HBV-mediated HCC can also aid exploration of the role of drug repurposing to prevent extrahepatic cancers.
There is no consensus regarding the role of HBV infection in the clinical prognosis of different cancers. Regarding the lymph nodes and distant organ metastasis, a cohort study from an endemic area in China demonstrated that HBV infection decreased the liver metastasis risk in patients with colorectal cancer but did not influence survival (187). Additionally, a pooled meta-analysis involving 11 studies suggested that HBV infection was associated with a lower rate of lymph node metastasis, better DFS, and prolonged OS in patients with intrahepatic cholangiocarcinoma and esophageal cancer (98, 188). By contrast, higher serum titer HBc antibody was recently identified as an adverse factor in increasing liver metastasis risk and poor survival probability in colorectal cancer (189). Similarly, HBsAg seropositivity was a biomarker that predicted worse survival of early-stage NPC (127). It should be noted that most of the existing evidence on this topic was derived from Asian populations (188). Whether these correlations between HBV biomarkers and varied cancer prognosis can be generalized to other ethnicities and regions requires validation. Furthermore, the distinct roles of HBc, HBsAg, and other serum biomarkers in cancer outcomes indicate that further studies are warranted to illustrate the landscapes of altered host immune function and the gene expression profiles under the altered hepatic microenvironment in HBV. Such findings would aid precision medical decision-making to manage these unique cancer populations.
6 Prospects
Currently, there is no guideline to support HBV screening in general patients with cancer. Nevertheless, it may be likely that possible target cancers (determined by their associations with HBV) can be regularly included in the routine surveillance programs of chronic HBV infection patients. Nonetheless, the cost-effectiveness of the proposed policies must be determined before actual clinical application. More importantly, the safety and feasibility of antiviral treatment in the early prevention and survival of HBV infection patients with extrahepatic cancer are also important tasks in future studies. Some key population groups, specifically people living with HIV, have remarkably higher HBV infection prevalence than the general population (27, 118, 190). Notably, much clinical epidemiological data demonstrated significantly increased extrahepatic cancer risk in hepatitis virus and HIV co-infection subpopulations (9, 191). Based on these findings, these key populations with HBV infection may derive more benefits from targeted cancer prevention and diligent clinical monitoring. Furthermore, future studies are warranted to validate the clinical findings and clarify the associated mechanisms, especially for the immunosuppressed population.
7 Conclusions
The clinical epidemiological evidence supports the premise that chronic HBV infection significantly increases the risk of several extrahepatic cancers, especially gastrointestinal cancers. Although compelling studies confirmed the underlying mechanisms in HBV-mediated HCC involving different molecular signaling pathways, there are few explorations of HBV-mediated extrahepatic cancers. Therefore, future studies are warranted to fill these gaps and provide more insightful evidence to aid understanding of the interactions among HBV infection-derived immune microenvironment changes, epigenetic modification, elevated cancer-promoted signaling pathways, serum biomarkers, and the oncogenesis of extrahepatic organs.
Author contributions
XH and XP were responsible for the study’s concept and design. YM and XX did the data and project management. ZL and YM did the literature search and analysis. YM, ZL, RL, ZW and JJ interpreted the data. YM, XW, XX and XP drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the National Key Research and Development Program of China (2021YFE0206600), the National Natural Science Foundation of China (82172842 and 81672386), the Sichuan Province Science and Technology Support grant, and Tianfu Laboratory (2022SYSX0064, 2021YFSY0008, 2020YFS0276), West China Nursing Discipline Development Special Fund Project (HXHL21008). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
YM and XW drew the Figures in this review. The resources of the figures in this review are designed by the online tools of “Figdraw” (https://www.figdraw.com/static/index.html#/) and “Smart Art” on the website (https://smart.servier.com/).
Conflict of interest
Author XX is employed by Shanghai ETERN Biopharma Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. GBD 2019 Hepatitis B Collaborators Global, regional, and national burden of hepatitis b, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol Hepatol (2022) 7(9):796–829. doi: 10.1016/S2468-1253(22)00124-8
3. Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol (2016) 64(1 Suppl):S84–s101. doi: 10.1016/j.jhep.2016.02.021
4. Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, et al. A global scientific strategy to cure hepatitis b. Lancet Gastroenterol Hepatol (2019) 4(7):545–58. doi: 10.1016/S2468-1253(19)30119-0
5. Dejean A, Lugassy C, Zafrani S, Tiollais P, Brechot C. Detection of hepatitis b virus DNA in pancreas, kidney and skin of two human carriers of the virus. J Gen Virol (1984) 65(Pt 3):651–5. doi: 10.1099/0022-1317-65-3-651
6. Mason A, Wick M, White H, Perrillo R. Hepatitis b virus replication in diverse cell types during chronic hepatitis b virus infection. Hepatology (1993) 18(4):781–9. doi: 10.1002/hep.1840180406
7. Islami F, Chen W, Yu XQ, Lortet-Tieulent J, Zheng R, Flanders WD, et al. Cancer deaths and cases attributable to lifestyle factors and infections in China, 2013. Ann Oncol (2017) 28(10):2567–74. doi: 10.1093/annonc/mdx342
8. An J, Kim JW, Shim JH, Han S, Yu CS, Choe J, et al. Chronic hepatitis b infection and non-hepatocellular cancers: A hospital registry-based, case-control study. PloS One (2018) 13(3):e0193232. doi: 10.1371/journal.pone.0193232
9. Darvishian M, Butt ZA, Wong S, Yoshida EM, Khinda J, Otterstatter M, et al. Elevated risk of colorectal, liver, and pancreatic cancers among HCV, HBV and/or HIV (co)infected individuals in a population based cohort in Canada. Ther Adv Med Oncol (2021) 13:1758835921992987. doi: 10.1177/1758835921992987
10. Geng H, Xing Y, Zhang J, Cao K, Ye M, Wang G, et al. Association between viral infection other than human papillomavirus and risk of esophageal carcinoma: a comprehensive meta-analysis of epidemiological studies. Arch Virol (2022) 167(1):1–20. doi: 10.1007/s00705-021-05268-8
11. Kamiza AB, Su FH, Wang WC, Sung FC, Chang SN, Yeh CC. Chronic hepatitis infection is associated with extrahepatic cancer development: A nationwide population-based study in Taiwan. BMC Canc (2016) 16(1):861. doi: 10.1186/s12885-016-2918-5
12. Lu T, Yang Q, Li M, Zhang J, Zou J, Huang L, et al. HBV infection and extra-hepatic cancers in adolescents and 20s: A retrospective study in China. Cancer Epidemiol. (2018) 55:149–55. doi: 10.1016/j.canep.2018.05.012
13. Song C, Lv J, Liu Y, Chen JG, Ge Z, Zhu J, et al. Associations between hepatitis b virus infection and risk of all cancer types. JAMA Netw Open (2019) 2(6):e195718. doi: 10.1001/jamanetworkopen.2019.5718
14. Spradling PR, Xing J, Zhong Y, Rupp LB, Moorman AC, Lu M, et al. Incidence of malignancies among patients with chronic hepatitis b in US health care organizations, 2006-2018. J Infect Dis (2022) 226(5):896–900. doi: 10.1093/infdis/jiac011
15. GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet (2015) 385(9963):117–71. doi: 10.1016/S0140-6736(14)61682-2
16. Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet (2016) 388(10049):1081–8. doi: 10.1016/S0140-6736(16)30579-7
17. Trépo C, Chan HL, Lok A. Hepatitis b virus infection. Lancet (2014) 384(9959):2053–63. doi: 10.1016/S0140-6736(14)60220-8
18. Collaborators PO. Global prevalence, treatment, and prevention of hepatitis b virus infection in 2016: A modelling study. Lancet Gastroenterol Hepatol (2018) 3(6):383–403. doi: 10.1016/S2468-1253(18)30056-6
19. Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis b virus: Advances in prevention, diagnosis, and therapy. Clin Microbiol Rev (2020) 33(2):896–900. doi: 10.1128/CMR.00046-19
20. Scaglione SJ, Lok AS. Effectiveness of hepatitis b treatment in clinical practice. Gastroenterology (2012) 142(6):1360–8.e1. doi: 10.1053/j.gastro.2012.01.044
21. Alaama AS, Khattabi H, Mugisa B, Atta H, Hermez J, Hutin YJ. Progress towards elimination of viral hepatitis by 2030 in the WHO Eastern Mediterranean region, 2019. Lancet Gastroenterol Hepatol (2022) 7(9):862–70. doi: 10.1016/S2468-1253(22)00082-6
22. Wong GL, Hui VW, Yip TC, Liang LY, Zhang X, Tse YK, et al. Universal HBV vaccination dramatically reduces the prevalence of HBV infection and incidence of hepatocellular carcinoma. Aliment Pharmacol Ther (2022) 56(5):869–77. doi: 10.1111/apt.17120
23. Liu Y, Liu L. Changes in the epidemiology of hepatocellular carcinoma in Asia. Cancers (Basel). (2022) 14(18):4473. doi: 10.3390/cancers14184473
24. Hefele L, Vannachone S, Khounvisith V, Nouanthong P, Sayasone S, Kounnavong S, et al. Lasting benefit of infant hepatitis b vaccination in adolescents in the lao people's democratic republic. Int J Infect Dis (2020) 93:217–23. doi: 10.1016/j.ijid.2020.01.055
25. Smith S, Harmanci H, Hutin Y, Hess S, Bulterys M, Peck R, et al. Global progress on the elimination of viral hepatitis as a major public health threat: An analysis of WHO member state responses 2017. JHEP Rep (2019) 1(2):81–9. doi: 10.1016/j.jhepr.2019.04.002
26. Khetsuriani N, Lesi O, Desai S, Armstrong PA, Tohme RA. Progress toward the elimination of mother-to-Child transmission of hepatitis b virus - worldwide, 2016-2021. MMWR Morb Mortal Wkly Rep (2022) 71(30):958–63. doi: 10.15585/mmwr.mm7130a2
27. Leumi S, Bigna JJ, Amougou MA, Ngouo A, Nyaga UF, Noubiap JJ. Global burden of hepatitis b infection in people living with human immunodeficiency virus: A systematic review and meta-analysis. Clin Infect Dis (2020) 71(11):2799–806. doi: 10.1093/cid/ciz1170
28. Liu Z, Li M, Hutton DW, Wagner AL, Yao Y, Zhu W, et al. Impact of the national hepatitis b immunization program in China: A modeling study. Infect Dis Poverty. (2022) 11(1):106. doi: 10.1186/s40249-022-01032-5
29. Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis b (REACH-b): development and validation of a predictive score. Lancet Oncol (2011) 12(6):568–74. doi: 10.1016/S1470-2045(11)70077-8
30. Kim GA, Lim YS, Han S, Choi J, Shim JH, Kim KM, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis b. Gut (2018) 67(5):945–52. doi: 10.1136/gutjnl-2017-314904
31. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology (2012) 142(6):1264–73.e1. doi: 10.1053/j.gastro.2011.12.061
32. McMahon BJ, Nolen LD, Snowball M, Homan C, Negus S, Roik E, et al. HBV genotype: A significant risk factor in determining which patients with chronic HBV infection should undergo surveillance for HCC: The hepatitis b Alaska study. Hepatology (2021) 74(6):2965–73. doi: 10.1002/hep.32065
33. Chen Y, Tian Z. HBV-induced immune imbalance in the development of HCC. Front Immunol (2019) 10:2048. doi: 10.3389/fimmu.2019.02048
34. Quitt O, Luo S, Meyer M, Xie Z, Golsaz-Shirazi F, Loffredo-Verde E, et al. T-Cell engager antibodies enable T cells to control HBV infection and to target HBsAg-positive hepatoma in mice. J Hepatol (2021) 75(5):1058–71. doi: 10.1016/j.jhep.2021.06.022
35. Liu T, Song C, Zhang Y, Siyin ST, Zhang Q, Song M, et al. Hepatitis b virus infection and the risk of gastrointestinal cancers among Chinese population: A prospective cohort study. Int J Canc (2022) 150(6):1018–28. doi: 10.1002/ijc.33891
36. Batskikh S, Morozov S, Dorofeev A, Borunova Z, Kostyushev D, Brezgin S, et al. Previous hepatitis b viral infection-an underestimated cause of pancreatic cancer. World J Gastroenterol (2022) 28(33):4812–22. doi: 10.3748/wjg.v28.i33.4812
37. Li M, Shen Y, Chen Y, Gao H, Zhou J, Wang Q, et al. Characterization of hepatitis b virus infection and viral DNA integration in non-Hodgkin lymphoma. Int J Canc (2020) 147(8):2199–209. doi: 10.1002/ijc.33027
38. Li M, Gan Y, Fan C, Yuan H, Zhang X, Shen Y, et al. Hepatitis b virus and risk of non-Hodgkin lymphoma: An updated meta-analysis of 58 studies. J Viral Hepat. (2018) 25(8):894–903. doi: 10.1111/jvh.12892
39. Lewis R. Possible link between hepatitis b infection and gastric cancer. Lancet Oncol (2015) 16(4):e159. doi: 10.1016/S1470-2045(15)70077-X
40. Chen S, Li X, Lv H, Wen X, Ding Q, Xue N, et al. Prognostic dynamic nomogram integrated with inflammation-based factors for non-small cell lung cancer patients with chronic hepatitis b viral infection. Int J Biol Sci (2018) 14(13):1813–21. doi: 10.7150/ijbs.27260
41. Fu Y, Yang X, Liang H, Wu X. Serum hepatitis b viral (HBV) DNA is a predictive biomarker for survival in non-small cell lung cancer patients with chronic HBV infection. Cancer Manag Res (2019) 11:5091–100. doi: 10.2147/CMAR.S198714
42. Lei Z, Xia Y, Si A, Wang K, Li J, Yan Z, et al. Antiviral therapy improves survival in patients with HBV infection and intrahepatic cholangiocarcinoma undergoing liver resection. J Hepatol (2018) 68(4):655–62. doi: 10.1016/j.jhep.2017.11.015
43. Nayyar SS, Thiagarajan S, Malik A, D'Cruz A, Chaukar D, Patil P, et al. Head and neck squamous cell carcinoma in HIV, HBV and HCV seropositive patients - prognosis and its predictors. J Cancer Res Ther (2020) 16(3):619–23. doi: 10.4103/jcrt.JCRT_166_19
44. Wei XL, Qiu MZ, Chen WW, Jin Y, Ren C, Wang F, et al. The status of HBV infection influences metastatic pattern and survival in Chinese patients with pancreatic cancer. J Transl Med (2013) 11:249. doi: 10.1186/1479-5876-11-249
45. Zhou J, Guo X, Huang P, Tan S, Lin R, Zhan H, et al. HBV infection status indicates different risks of synchronous and metachronous liver metastasis in colorectal cancer: A retrospective study of 3132 patients with a 5-year follow-up. Cancer Manag Res (2022) 14:1581–94. doi: 10.2147/CMAR.S350276
46. Ramsey SD, Unger JM, Baker LH, Little RF, Loomba R, Hwang JP, et al. Prevalence of hepatitis b virus, hepatitis c virus, and HIV infection among patients with newly diagnosed cancer from academic and community oncology practices. JAMA Oncol (2019) 5(4):497–505. doi: 10.1001/jamaoncol.2018.6437
47. Mahale P, Engels EA, Koshiol J. Hepatitis b virus infection and the risk of cancer in the elderly US population. Int J Canc (2019) 144(3):431–9. doi: 10.1002/ijc.31643
48. Wei XL, Luo HY, Li CF, Jin Y, Zeng ZL, Ju HQ, et al. Hepatitis b virus infection is associated with younger median age at diagnosis and death in cancers. Int J Canc (2017) 141(1):152–9. doi: 10.1002/ijc.30719
49. Wei XL, Qiu MZ, Jin Y, Huang YX, Wang RY, Chen WW, et al. Hepatitis b virus infection is associated with gastric cancer in China: An endemic area of both diseases. Br J Canc (2015) 112(7):1283–90. doi: 10.1038/bjc.2014.406
50. Baghbanian M, Hoseini Mousa SA, Doosti M, Moghimi M. Association between gastric pathology and hepatitis b virus infection in patients with or without helicobacter pylori. Asian Pac J Cancer Prev (2019) 20(7):2177–80. doi: 10.31557/APJCP.2019.20.7.2177
51. Wang H, Chen XL, Liu K, Bai D, Zhang WH, Chen XZ, et al. Associations between gastric cancer risk and virus infection other than Epstein-Barr virus: A systematic review and meta-analysis based on epidemiological studies. Clin Transl Gastroenterol (2020) 11(7):e00201. doi: 10.14309/ctg.0000000000000201
52. Cui H, Jin Y, Chen F, Ni H, Hu C, Xu Y, et al. Clinicopathological evidence of hepatitis b virus infection in the development of gastric adenocarcinoma. J Med Virol (2020) 92(1):71–7. doi: 10.1002/jmv.25584
53. Yang Y, Jiang Z, Wu W, Ruan L, Yu C, Xi Y, et al. Chronic hepatitis virus infection are associated with high risk of gastric cancer: A systematic review and cumulative analysis. Front Oncol (2021) 11:703558. doi: 10.3389/fonc.2021.703558
54. Huang J, Magnusson M, Törner A, Ye W, Duberg AS. Risk of pancreatic cancer among individuals with hepatitis c or hepatitis b virus infection: a nationwide study in Sweden. Br J Canc (2013) 109(11):2917–23. doi: 10.1038/bjc.2013.689
55. Tang J, Sharma R, Lamerato L, Sheehan M, Krajenta R, Gordon SC. Is previous exposure to hepatitis b a risk factor for pancreatic cancer or hepatocellular carcinoma? J Clin Gastroenterol (2014) 48(8):729–33. doi: 10.1097/MCG.0000000000000111
56. Chang MC, Chen CH, Liang JD, Tien YW, Hsu C, Wong JM, et al. Hepatitis b and c viruses are not risks for pancreatic adenocarcinoma. World J Gastroenterol (2014) 20(17):5060–5. doi: 10.3748/wjg.v20.i17.5060
57. Desai R, Patel U, Sharma S, Singh S, Doshi S, Shaheen S, et al. Association between hepatitis b infection and pancreatic cancer: A population-based analysis in the united states. Pancreas (2018) 47(7):849–55. doi: 10.1097/MPA.0000000000001095
58. Fiorino S, Chili E, Bacchi-Reggiani L, Masetti M, Deleonardi G, Grondona AG, et al. Association between hepatitis b or hepatitis c virus infection and risk of pancreatic adenocarcinoma development: a systematic review and meta-analysis. Pancreatology (2013) 13(2):147–60. doi: 10.1016/j.pan.2013.01.005
59. Xu JH, Fu JJ, Wang XL, Zhu JY, Ye XH, Chen SD. Hepatitis b or c viral infection and risk of pancreatic cancer: a meta-analysis of observational studies. World J Gastroenterol (2013) 19(26):4234–41. doi: 10.3748/wjg.v19.i26.4234
60. Xing S, Li ZW, Tian YF, Zhang LM, Li MQ, Zhou P. Chronic hepatitis virus infection increases the risk of pancreatic cancer: a meta-analysis. Hepatob Pancreat Dis Int (2013) 12(6):575–83. doi: 10.1016/S1499-3872(13)60091-0
61. Liu T, Li W, Zhang Y, Siyin ST, Zhang Q, Song M, et al. Associations between hepatitis b virus infection and risk of colorectal cancer: a population-based prospective study. BMC Canc (2021) 21(1):1119. doi: 10.1186/s12885-021-08846-w
62. Ye YF, Xiang YQ, Fang F, Gao R, Zhang LF, Xie SH, et al. Hepatitis b virus infection and risk of nasopharyngeal carcinoma in southern China. Cancer Epidemiol Biomarkers Prev (2015) 24(11):1766–73. doi: 10.1158/1055-9965.EPI-15-0344
63. Donà S, Borsetto D, Fussey J, Biscaro V, Vian E, Spinato G, et al. Association between hepatitis c and b viruses and head and neck squamous cell carcinoma. J Clin Virol (2019) 121:104209. doi: 10.1016/j.jcv.2019.104209
64. Komori MF, Kimura T, Kariya S, Onoda T, Takeda S, Mizukawa N, et al. Epidemiological correlations between head and neck cancer and hepatitis b core antibody positivity. Anticancer Res (2020) 40(4):2393–403. doi: 10.21873/anticanres.14209
65. Su TH, Liu CJ, Tseng TC, Chou SW, Liu CH, Yang HC, et al. Chronic hepatitis b is associated with an increased risk of b-cell non-hodgkin's lymphoma and multiple myeloma. Aliment Pharmacol Ther (2019) 49(5):589–98. doi: 10.1111/apt.15132
66. Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, et al. Association between helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology (2016) 150(5):1113–24.e5. doi: 10.1053/j.gastro.2016.01.028
67. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett (2014) 345(2):196–202. doi: 10.1016/j.canlet.2013.08.016
68. Yang J, Liu Z, Zeng B, Hu G, Gan R. Epstein-Barr Virus-associated gastric cancer: A distinct subtype. Cancer Lett (2020) 495:191–9. doi: 10.1016/j.canlet.2020.09.019
69. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol (2016) 22(44):9694–705. doi: 10.3748/wjg.v22.i44.9694
70. Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, Davila M, et al. Association between hepatitis b virus and pancreatic cancer. J Clin Oncol (2008) 26(28):4557–62. doi: 10.1200/JCO.2008.17.3526
71. Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic pancreatitis and pancreatic cancer risk: A systematic review and meta-analysis. Am J Gastroenterol (2017) 112(9):1366–72. doi: 10.1038/ajg.2017.218
72. Everhov ÅH, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Inflammatory bowel disease and pancreatic cancer: a Scandinavian register-based cohort study 1969-2017. Aliment Pharmacol Ther (2020) 52(1):143–54. doi: 10.1111/apt.15785
73. de Gonzalez AB, Jee SH, Engels EA. No association between hepatitis b and pancreatic cancer in a prospective study in Korea. J Clin Oncol (2009) 27(4):648. doi: 10.1200/JCO.2008.20.7514
74. Berrington de Gonzalez A, Yun JE, Lee SY, Klein AP, Jee SH. Pancreatic cancer and factors associated with the insulin resistance syndrome in the Korean cancer prevention study. Cancer Epidemiol Biomarkers Prev (2008) 17(2):359–64. doi: 10.1158/1055-9965.EPI-07-0507
75. Wang DS, Chen DL, Ren C, Wang ZQ, Qiu MZ, Luo HY, et al. ABO blood group, hepatitis b viral infection and risk of pancreatic cancer. Int J Canc (2012) 131(2):461–8. doi: 10.1002/ijc.26376
76. Jin Y, Gao H, Chen H, Wang J, Chen M, Li G, et al. Identification and impact of hepatitis b virus DNA and antigens in pancreatic cancer tissues and adjacent non-cancerous tissues. Cancer Lett (2013) 335(2):447–54. doi: 10.1016/j.canlet.2013.03.001
77. Gheorghe G, Diaconu CC, Ionescu V, Constantinescu G, Bacalbasa N, Bungau S, et al. Risk factors for pancreatic cancer: Emerging role of viral hepatitis. J Pers Med (2022) 12(1):83. doi: 10.3390/jpm12010083
78. Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Screening for pancreatic cancer: US preventive services task force reaffirmation recommendation statement. JAMA (2019) 322(5):438–44. doi: 10.1001/jama.2019.10232
79. Singhi AD, Koay EJ, Chari ST, Maitra A. Early detection of pancreatic cancer: Opportunities and challenges. Gastroenterology (2019) 156(7):2024–40. doi: 10.1053/j.gastro.2019.01.259
80. Kumagi T, Terao T, Yokota T, Azemoto N, Kuroda T, Imamura Y, et al. Early detection of pancreatic cancer in patients with chronic liver disease under hepatocellular carcinoma surveillance. Mayo Clin Proc (2019) 94(10):2004–10. doi: 10.1016/j.mayocp.2018.12.034
81. Lim J, Lee JB, An J, Song GW, Kim KM, Lee HC, et al. Extrahepatic carcinogenicity of oral nucleos(t)ide analogues in chronic hepatitis b carriers: A 35,000-Korean outcome study. J Viral Hepat. (2022) 29(9):756–64. doi: 10.1111/jvh.13721
82. Tewari SR, D'Souza G, Troy T, Wright H, Struijk L, Waterboer T, et al. Association of plasma circulating tumor HPV DNA with HPV-related oropharynx cancer. JAMA Otolaryngol Head Neck Surg (2022) 148(5):488–9. doi: 10.1001/jamaoto.2022.0159
83. Sannigrahi MK, Rajagopalan P, Lai L, Liu X, Sahu V, Nakagawa H, et al. HPV E6 regulates therapy responses in oropharyngeal cancer by repressing the PGC-1α/ERRα axis. JCI Insight (2022) 7(18):e159600. doi: 10.1172/jci.insight.159600
84. Deutsch F, Regina Bullen I, Nguyen K, Tran NH, Elliott M, Tran N. Current state of play for HPV-positive oropharyngeal cancers. Cancer Treat Rev (2022) 110:102439. doi: 10.1016/j.ctrv.2022.102439
85. Sabatini ME, Chiocca S. Human papillomavirus as a driver of head and neck cancers. Br J Canc (2020) 122(3):306–14. doi: 10.1038/s41416-019-0602-7
86. Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and neck cancers-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin (2017) 67(2):122–37. doi: 10.3322/caac.21389
87. Su FH, Chang SN, Chen PC, Sung FC, Huang SF, Chiou HY, et al. Positive association between hepatitis c infection and oral cavity cancer: a nationwide population-based cohort study in Taiwan. PloS One (2012) 7(10):e48109. doi: 10.1371/journal.pone.0048109
88. Shi X, Wang X, Xu X, Feng Y, Li S, Feng S, et al. Impact of HBV replication in peripheral blood mononuclear cell on HBV intrauterine transmission. Front Med (2017) 11(4):548–53. doi: 10.1007/s11684-017-0597-5
89. Pontisso P, Vidalino L, Quarta S, Gatta A. Biological and clinical implications of HBV infection in peripheral blood mononuclear cells. Autoimmun Rev (2008) 8(1):13–7. doi: 10.1016/j.autrev.2008.07.016
90. Li M, Wu S, Luo H, Niu J, Yan Y, Fang Y, et al. Serological and molecular characterization of hepatitis b virus infection in gastric cancer. Front Cell Infect Microbiol (2022) 12:894836. doi: 10.3389/fcimb.2022.894836
91. Chen DG, Chen G, Wang C, Ke LF, Wu H, He HM, et al. Clinicopathological and prognostic features of hepatitis b virus-associated diffuse large b-cell lymphoma: a single-center retrospective study in China. Infect Agent Canc (2021) 16(1):57. doi: 10.1186/s13027-021-00396-x
92. Fwu CW, Chien YC, You SL, Nelson KE, Kirk GD, Kuo HS, et al. Hepatitis b virus infection and risk of intrahepatic cholangiocarcinoma and non-Hodgkin lymphoma: a cohort study of parous women in Taiwan. Hepatology (2011) 53(4):1217–25. doi: 10.1002/hep.24150
93. Yamashina T, Shimatani M, Takeo M, Sasaki K, Orino M, Saito N, et al. Viral infection in esophageal, gastric, and colorectal cancer. Healthcare (Basel) (2022) 10(9):1626. doi: 10.3390/healthcare10091626
94. Baj J, Forma A, Dudek I, Chilimoniuk Z, Dobosz M, Dobrzyński M, et al. The involvement of human papilloma virus in gastrointestinal cancers. Cancers (Basel) (2022) 14(11):2607. doi: 10.3390/cancers14112607
95. Zhang Y, Li Y, Pan E, Zhao C, Zhang H, Liu R, et al. Infection with human papillomavirus 18 promotes alkylating agent-induced malignant transformation in a human esophageal cell line. Chem Res Toxicol (2021) 34(8):1866–78. doi: 10.1021/acs.chemrestox.1c00156
96. Petrelli F, De Santi G, Rampulla V, Ghidini A, Mercurio P, Mariani M, et al. Human papillomavirus (HPV) types 16 and 18 infection and esophageal squamous cell carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol (2021) 147(10):3011–23. doi: 10.1007/s00432-021-03738-9
97. Rajendra S, Pavey D, McKay O, Merrett N, Gautam SD. Human papillomavirus infection in esophageal squamous cell carcinoma and esophageal adenocarcinoma: a concise review. Ann N Y Acad Sci (2020) 1482(1):36–48. doi: 10.1111/nyas.14509
98. Zou J, Chen J, Xie X, Liu Z, Cai X, Liu Q, et al. Hepatitis b virus infection is a prognostic biomarker for better survival in operable esophageal cancer: Analysis of 2,004 patients from an endemic area in China. Cancer Epidemiol Biomarkers Prev (2019) 28(6):1028–35. doi: 10.1158/1055-9965.EPI-18-1095
99. Song E, Chen J, Ou Q, Su F. Rare occurrence of metastatic colorectal cancers in livers with replicative hepatitis b infection. Am J Surg (2001) 181(6):529–33. doi: 10.1016/S0002-9610(01)00634-1
100. Zhao Y, Lin J, Peng J, Deng Y, Zhao R, Sui Q, et al. Hepatitis b virus infection predicts better survival in patients with colorectal liver-only metastases undergoing liver resection. J Canc (2018) 9(9):1560–7. doi: 10.7150/jca.24544
101. Mormile R. Hepatitis b virus infection and susceptibility to esophageal cancer in China: Implications of ethnicity-related interleukin-17 gene polymorphism? J Gastroint Cancer (2020) 51(2):718–9. doi: 10.1007/s12029-019-00271-4
102. Yu P, Liu P, Li N, Xie X, Tang H, Wu J, et al. Hepatitis b virus infection specially increases risk of liver metastasis in breast cancer patients: a propensity-matched analysis. Transl Cancer Res (2020) 9(3):1506–17. doi: 10.21037/tcr.2020.01.63
103. Lu LJ, Adhikari VP, Zhao CX, Wu H, Dai W, Li X, et al. Clinical study on the relationship between hepatitis b virus infection and risk of breast cancer: a large sized case-control and single center study in southwest of China. Oncotarget (2017) 8(42):72044–53. doi: 10.18632/oncotarget.19132
104. Li N, Zhong QQ, Yang XR, Wang QC, Zhang DT, Zheng S, et al. Prognostic value of hepatitis b virus infection in very young patients with curatively resected breast cancer: Analyses from an endemic area in China. Front Oncol (2020) 10:1403. doi: 10.3389/fonc.2020.01403
105. Gao D, Song J, Chen C, Zhu S, Wang Z, Sun S. Relationships of hepatitis b virus infection with clinicopathological features in breast cancer and survival outcomes in central China. Transl Cancer Res (2020) 9(4):2511–7. doi: 10.21037/tcr.2020.03.15
106. Wong WW, Hicks LK, Tu HA, Pritchard KI, Krahn MD, Feld JJ, et al. Hepatitis b virus screening before adjuvant chemotherapy in patients with early-stage breast cancer: a cost-effectiveness analysis. Breast Cancer Res Treat (2015) 151(3):639–52. doi: 10.1007/s10549-015-3382-7
107. Su FH, Chang SN, Chen PC, Sung FC, Su CT, Yeh CC. Association between chronic viral hepatitis infection and breast cancer risk: a nationwide population-based case-control study. BMC Canc (2011) 11:495. doi: 10.1186/1471-2407-11-495
108. Liu Z, Luo Y, Ren J, Yang L, Li J, Wei Z, et al. Association between fish oil supplementation and cancer risk according to fatty fish consumption: A large prospective population-based cohort study using UK biobank. Int J Canc (2022) 150(4):562–71. doi: 10.1002/ijc.33819
109. Li J, Liu Y, Jiang J, Peng X, Hu X. Effect of telehealth interventions on quality of life in cancer survivors: A systematic review and meta-analysis of randomized controlled trials. Int J Nurs Stud (2021) 122:103970. doi: 10.1016/j.ijnurstu.2021.103970
110. Chen S, Huang H, Liu Y, Lai C, Peng S, Zhou L, et al. A multi-parametric prognostic model based on clinical features and serological markers predicts overall survival in non-small cell lung cancer patients with chronic hepatitis b viral infection. Cancer Cell Int (2020) 20(1):555. doi: 10.1186/s12935-020-01635-8
111. Chen S, Lai Y, He Z, Li J, He X, Shen R, et al. Establishment and validation of a predictive nomogram model for non-small cell lung cancer patients with chronic hepatitis b viral infection. J Transl Med (2018) 16(1):116. doi: 10.1186/s12967-018-1496-5
112. Lin G, Zhuang W, Chen XH, Huang C, Lin XD, Huang YJ, et al. Increase of programmed death ligand 1 in non-small-cell lung cancers with chronic hepatitis b. Ann Oncol (2018) 29(2):516–7. doi: 10.1093/annonc/mdx694
113. Zhang X, Tian D, Chen Y, Chen C, He LN, Zhou Y, et al. Association of hepatitis b virus infection status with outcomes of non-small cell lung cancer patients undergoing anti-PD-1/PD-L1 therapy. Transl Lung Cancer Res (2021) 10(7):3191–202. doi: 10.21037/tlcr-21-455
114. Yoo S, Lee D, Shim JH, Kim KM, Lim YS, Lee HC, et al. Risk of hepatitis b virus reactivation in patients treated with immunotherapy for anti-cancer treatment. Clin Gastroenterol Hepatol (2022) 20(4):898–907. doi: 10.1016/j.cgh.2021.06.019
115. Mustafayev K, Torres H. Hepatitis b virus and hepatitis c virus reactivation in cancer patients receiving novel anticancer therapies. Clin Microbiol Infect (2022) 28(10):1321–7. doi: 10.1016/j.cmi.2022.02.042
116. Zapatka M, Borozan I, Brewer DS, Iskar M, Grundhoff A, Alawi M, et al. The landscape of viral associations in human cancers. Nat Genet (2020) 52(3):320–30. doi: 10.1038/s41588-019-0558-9
117. Ferber MJ, Montoya DP, Yu C, Aderca I, McGee A, Thorland EC, et al. Integrations of the hepatitis b virus (HBV) and human papillomavirus (HPV) into the human telomerase reverse transcriptase (hTERT) gene in liver and cervical cancers. Oncogene (2003) 22(24):3813–20. doi: 10.1038/sj.onc.1206528
118. Bomfim-Hyppólito S, Eleuterio J Jr., Nunes GC, Bomfim-Hyppólito E, Franco ES, Neto Rda J. HIV Or human papillomavirus co-infection among Brazilian individuals infected with hepatitis b and/or hepatitis c. Int J Gynaecol Obstet. (2013) 122(3):258–60. doi: 10.1016/j.ijgo.2013.04.012
119. Siu SS, Cheung TH, Chan PK, Lin CK, Lo KW. Patients with malignant or pre-malignant cervical lesion have increased risk of becoming hepatitis b carrier. J Exp Clin Cancer Res (2007) 26(1):77–81.
120. Feng X, Lu H, Wei Y, Guan M, Wang J, Liu C, et al. Prognostic impact of hepatitis b virus infection in patients with primary cervical cancer. Cancer Med (2021) 10(23):8310–9. doi: 10.1002/cam4.4358
121. Wu X, Li L, Li Y, Jiang M, Li K, Li Z, et al. Prognostic value of serological markers of hepatitis b virus infection in squamous cell cervical cancer. J Canc (2021) 12(22):6620–8. doi: 10.7150/jca.61249
122. Li N, Wang Y, Che S, Yang Y, Piao J, Liu S, et al. HBXIP over expression as an independent biomarker for cervical cancer. Exp Mol Pathol (2017) 102(1):133–7. doi: 10.1016/j.yexmp.2017.01.009
123. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet (2019) 394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0
124. Bai R, Sun J, Xu Y, Sun Z, Zhao X. Incidence and mortality trends of nasopharynx cancer from 1990 to 2019 in China: an age-period-cohort analysis. BMC Public Health (2022) 22(1):1351. doi: 10.1186/s12889-022-13688-7
125. Xu T, Huang Z, Deng Y, Wang S, Su B, Wei W, et al. Clinical implications of hepatitis b viral infection in Epstein-Barr virus-associated nasopharyngeal carcinoma. J Clin Virol (2015) 64:64–71. doi: 10.1016/j.jcv.2014.11.024
126. Liu X, Li X, Jiang N, Lei Y, Tang LL, Chen L, et al. Prognostic value of chronic hepatitis b virus infection in patients with nasopharyngeal carcinoma: analysis of 1301 patients from an endemic area in China. Cancer (2014) 120(1):68–76. doi: 10.1002/cncr.28377
127. Weng JJ, Wei JZ, Li M, Lu JL, Qin YD, Jiang H, et al. Effects of hepatitis b virus infection and antiviral therapy on the clinical prognosis of nasopharyngeal carcinoma. Cancer Med (2020) 9(2):541–51. doi: 10.1002/cam4.2715
128. Braun DA, Bakouny Z, Hirsch L, Flippot R, Van Allen EM, Wu CJ, et al. Beyond conventional immune-checkpoint inhibition - novel immunotherapies for renal cell carcinoma. Nat Rev Clin Oncol (2021) 18(4):199–214. doi: 10.1038/s41571-020-00455-z
129. Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol (2017) 14(8):463–82. doi: 10.1038/nrclinonc.2017.43
130. Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-Small-Cell lung cancer. J Clin Oncol (2022) 40(6):586–97. doi: 10.1200/JCO.21.01497
131. Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: An international, multicenter study of the Mayo clinic phase 2 consortium (NCI-9742). J Clin Oncol (2018) 36(14):1412–8. doi: 10.1200/JCO.2017.77.0388
132. Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: A phase II clinical trial (POLARIS-02). J Clin Oncol (2021) 39(7):704–12. doi: 10.1200/JCO.20.02712
133. Jung HA, Park KU, Cho S, Lim J, Lee KW, Hong MH, et al. A phase II study of nivolumab plus gemcitabine in patients with recurrent or metastatic nasopharyngeal carcinoma (KCSG HN17-11). Clin Cancer Res (2022) 28(19):4240–7. doi: 10.1158/1078-0432.CCR-22-1238
134. Cheng YK, Chen P, Chen DW, Lin ZS, Ye SB, Lan P. Comparative safety, efficacy and survival outcome of anti-PD-1 immunotherapy in colorectal cancer patients with vs without hepatitis b virus infection: A multicenter cohort study. Clin Transl Gastroenterol (2022) 13(5):e00475. doi: 10.14309/ctg.0000000000000475
135. Pertejo-Fernandez A, Ricciuti B, Hammond SP, Marty FM, Recondo G, Rangachari D, et al. Safety and efficacy of immune checkpoint inhibitors in patients with non-small cell lung cancer and hepatitis b or hepatitis c infection. Lung Canc (2020) 145:181–5. doi: 10.1016/j.lungcan.2020.02.013
136. Li B, Yan C, Zhu J, Chen X, Fu Q, Zhang H, et al. Anti-PD-1/PD-L1 blockade immunotherapy employed in treating hepatitis b virus infection-related advanced hepatocellular carcinoma: A literature review. Front Immunol (2020) 11:1037. doi: 10.3389/fimmu.2020.01037
137. Lv JW, Chen YP, Huang XD, Zhou GQ, Chen L, Li WF, et al. Hepatitis b virus screening and reactivation and management of patients with nasopharyngeal carcinoma: A large-scale, big-data intelligence platform-based analysis from an endemic area. Cancer (2017) 123(18):3540–9. doi: 10.1002/cncr.30775
138. Zhang X, Zhou Y, Chen C, Fang W, Cai X, Zhang X, et al. Hepatitis b virus reactivation in cancer patients with positive hepatitis b surface antigen undergoing PD-1 inhibition. J Immunother Canc (2019) 7(1):322. doi: 10.1186/s40425-019-0808-5
139. Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, et al. Hepatitis b virus infection. Nat Rev Dis Primers. (2018) 4:18035. doi: 10.1038/nrdp.2018.35
140. Bubie A, Zoulim F, Testoni B, Miles B, Posner M, Villanueva A, et al. Landscape of oncoviral genotype and co-infection via human papilloma and hepatitis b viral tumor in situ profiling. iScience (2021) 24(4):102368. doi: 10.1016/j.isci.2021.102368
141. Khoury JD, Tannir NM, Williams MD, Chen Y, Yao H, Zhang J, et al. Landscape of DNA virus associations across human malignant cancers: Analysis of 3,775 cases using RNA-seq. J Virol (2013) 87(16):8916–26. doi: 10.1128/JVI.00340-13
142. Hirsch I, Caux C, Hasan U, Bendriss-Vermare N, Olive D. Impaired toll-like receptor 7 and 9 signaling: from chronic viral infections to cancer. Trends Immunol (2010) 31(10):391–7. doi: 10.1016/j.it.2010.07.004
143. Kamiza AB, Fatumo S, Singini MG, Yeh CC, Chikowore T. Hepatitis b infection is causally associated with extrahepatic cancers: A mendelian randomization study. EBioMedicine (2022) 79:104003. doi: 10.1016/j.ebiom.2022.104003
144. Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discovery (2022) 12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059
145. Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability–an evolving hallmark of cancer. Nat Rev Mol Cell Biol (2010) 11(3):220–8. doi: 10.1038/nrm2858
146. Zhang D, Guo S, Schrodi SJ. Mechanisms of DNA methylation in virus-host interaction in hepatitis b infection: Pathogenesis and oncogenetic properties. Int J Mol Sci (2021) 22(18):9858. doi: 10.3390/ijms22189858
147. Lau KCK, Burak KW, Coffin CS. Impact of hepatitis b virus genetic variation, integration, and lymphotropism in antiviral treatment and oncogenesis. Microorganisms (2020) 8(10):1470. doi: 10.3390/microorganisms8101470
148. Lau KC, Joshi SS, Gao S, Giles E, Swidinsky K, van Marle G, et al. Oncogenic HBV variants and integration are present in hepatic and lymphoid cells derived from chronic HBV patients. Cancer Lett (2020) 480:39–47. doi: 10.1016/j.canlet.2020.03.022
149. Gazal S, Weissbrod O, Hormozdiari F, Dey KK, Nasser J, Jagadeesh KA, et al. Combining SNP-to-gene linking strategies to identify disease genes and assess disease omnigenicity. Nat Genet (2022) 54(6):827–36. doi: 10.1038/s41588-022-01087-y
150. Wang Z, Emmerich A, Pillon NJ, Moore T, Hemerich D, Cornelis MC, et al. Genome-wide association analyses of physical activity and sedentary behavior provide insights into underlying mechanisms and roles in disease prevention. Nat Genet (2022) 54(9):1332–44. doi: 10.1038/s41588-022-01165-1
151. Tian R, Wang Y, Li W, Cui Z, Pan T, Jin Z, et al. Genome-wide virus-integration analysis reveals a common insertional mechanism of HPV, HBV and EBV. Clin Transl Med (2022) 12(8):e971. doi: 10.1002/ctm2.971
152. Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis b virus infection: towards a cure. Nat Rev Drug Discovery (2019) 18(11):827–44. doi: 10.1038/s41573-019-0037-0
153. Ho DW, Tsui YM, Chan LK, Sze KM, Zhang X, Cheu JW, et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat Commun (2021) 12(1):3684. doi: 10.1038/s41467-021-24010-1
154. Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis b virus infection. Nat Rev Immunol (2022) 22(1):19–32. doi: 10.1038/s41577-021-00549-4
155. Khanam A, Chua JV, Kottilil S. Immunopathology of chronic hepatitis b infection: Role of innate and adaptive immune response in disease progression. Int J Mol Sci (2021) 22(11):5497. doi: 10.3390/ijms22115497
156. Kondo Y, Shimosegawa T. Significant roles of regulatory T cells and myeloid derived suppressor cells in hepatitis b virus persistent infection and hepatitis b virus-related HCCs. Int J Mol Sci (2015) 16(2):3307–22. doi: 10.3390/ijms16023307
157. Lim CJ, Lee YH, Pan L, Lai L, Chua C, Wasser M, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis b virus-related hepatocellular carcinoma. Gut (2019) 68(5):916–27. doi: 10.1136/gutjnl-2018-316510
158. Miyakawa K, Jeremiah SS, Ogawa M, Nishi M, Ohnishi M, Ryo A. Crosstalk between the innate immune system and selective autophagy in hepatitis b virus infection. Autophagy (2022) 18(8):2006–7. doi: 10.1080/15548627.2022.2059747
159. Tout I, Loureiro D, Mansouri A, Soumelis V, Boyer N, Asselah T. Hepatitis b surface antigen seroclearance: Immune mechanisms, clinical impact, importance for drug development. J Hepatol (2020) 73(2):409–22. doi: 10.1016/j.jhep.2020.04.013
160. Yang P, Markowitz GJ, Wang XF. The hepatitis b virus-associated tumor microenvironment in hepatocellular carcinoma. Natl Sci Rev (2014) 1(3):396–412. doi: 10.1093/nsr/nwu038
161. Isogawa M, Chung J, Murata Y, Kakimi K, Chisari FV. CD40 activation rescues antiviral CD8+ T cells from PD-1-mediated exhaustion. PloS Pathog (2013) 9(7):e1003490. doi: 10.1371/journal.ppat.1003490
162. Bénéchet AP, De Simone G, Di Lucia P, Cilenti F, Barbiera G, Le Bert N, et al. Dynamics and genomic landscape of CD8(+) T cells undergoing hepatic priming. Nature (2019) 574(7777):200–5. doi: 10.1038/s41586-019-1620-6
163. Peng G, Li S, Wu W, Sun Z, Chen Y, Chen Z. Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis b infection. Immunology (2008) 123(1):57–65. doi: 10.1111/j.1365-2567.2007.02691.x
164. Fridman WH, Meylan M, Petitprez F, Sun CM, Italiano A, Sautès-Fridman C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol (2022) 19(7):441–57. doi: 10.1038/s41571-022-00619-z
165. Poonia B, Ayithan N, Nandi M, Masur H, Kottilil S. HBV induces inhibitory FcRL receptor on b cells and dysregulates b cell-T follicular helper cell axis. Sci Rep (2018) 8(1):15296. doi: 10.1038/s41598-018-33719-x
166. Chen Y, Bai X, Zhang Q, Wen L, Su W, Fu Q, et al. The hepatitis b virus X protein promotes pancreatic cancer through modulation of the PI3K/AKT signaling pathway. Cancer Lett (2016) 380(1):98–105. doi: 10.1016/j.canlet.2016.06.011
167. Lin X, Li AM, Li YH, Luo RC, Zou YJ, Liu YY, et al. Silencing MYH9 blocks HBx-induced GSK3β ubiquitination and degradation to inhibit tumor stemness in hepatocellular carcinoma. Signal Trans Target Ther (2020) 5(1):13. doi: 10.1038/s41392-020-0111-4
168. Qiu L, Lu F, Zhang L, Wang G, Geng R, Miao Y. HBXIP regulates gastric cancer glucose metabolism and malignancy through PI3K/AKT and p53 signaling. Onco Targets Ther (2020) 13:3359–74. doi: 10.2147/OTT.S243250
169. Yang Z, Jiang X, Li D, Jiang X. HBXIP promotes gastric cancer via METTL3-mediated MYC mRNA m6A modification. Aging (Albany NY). (2020) 12(24):24967–82. doi: 10.18632/aging.103767
170. Salerno D, Chiodo L, Alfano V, Floriot O, Cottone G, Paturel A, et al. Hepatitis b protein HBx binds the DLEU2 lncRNA to sustain cccDNA and host cancer-related gene transcription. Gut (2020) 69(11):2016–24. doi: 10.1136/gutjnl-2019-319637
171. Xie Y. Hepatitis b virus-associated hepatocellular carcinoma. Adv Exp Med Biol (2017) 1018:11–21. doi: 10.1007/978-981-10-5765-6_2
172. Lei Y, Xu X, Liu H, Chen L, Zhou H, Jiang J, et al. HBx induces hepatocellular carcinogenesis through ARRB1-mediated autophagy to drive the G(1)/S cycle. Autophagy (2021) 17(12):4423–41. doi: 10.1080/15548627.2021.1917948
173. Sekiba K, Otsuka M, Funato K, Miyakawa Y, Tanaka E, Seimiya T, et al. HBx-induced degradation of Smc5/6 complex impairs homologous recombination-mediated repair of damaged DNA. J Hepatol (2022) 76(1):53–62. doi: 10.1016/j.jhep.2021.08.010
174. Fang Q, Chen H. The significance of m6A RNA methylation regulators in predicting the prognosis and clinical course of HBV-related hepatocellular carcinoma. Mol Med (2020) 26(1):60. doi: 10.1186/s10020-020-00185-z
175. Wong CM, Tsang FH, Ng IO. Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat Rev Gastroenterol Hepatol (2018) 15(3):137–51. doi: 10.1038/nrgastro.2017.169
176. Yu X, Lan P, Hou X, Han Q, Lu N, Li T, et al. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production. J Hepatol (2017) 66(4):693–702. doi: 10.1016/j.jhep.2016.12.018
177. Zhou C, Liu C, Liu W, Chen W, Yin Y, Li CW, et al. SLFN11 inhibits hepatocellular carcinoma tumorigenesis and metastasis by targeting RPS4X via mTOR pathway. Theranostics (2020) 10(10):4627–43. doi: 10.7150/thno.42869
178. D'Souza S, Lau KC, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol (2020) 26(38):5759–83. doi: 10.3748/wjg.v26.i38.5759
179. Tran BM, Flanagan DJ, Ebert G, Warner N, Tran H, Fifis T, et al. The hepatitis b virus pre-core protein p22 activates wnt signaling. Cancers (Basel) (2020) 12(6):1435. doi: 10.3390/cancers12061435
180. Yu SJ, Kim YJ. Hepatitis b viral load affects prognosis of hepatocellular carcinoma. World J Gastroenterol (2014) 20(34):12039–44. doi: 10.3748/wjg.v20.i34.12039
181. Jiang W, Hu JW, He XR, Jin WL, He XY. Statins: a repurposed drug to fight cancer. J Exp Clin Cancer Res (2021) 40(1):241. doi: 10.1186/s13046-021-02041-2
182. Saka-Herrán C, Jané-Salas E, Mano-Azul A, Torrejón-Moya A, Estrugo-Devesa A, López-López J. Effects of the prior use of statins on head and neck cancer risk: A hospital-based case-control study. Pharm (Basel). (2022) 15(5):579. doi: 10.3390/ph15050579
183. Zhou Q, Zhang Q, Wang K, Huang T, Deng S, Wang Y, et al. Anti-rheumatic drug-induced hepatitis b virus reactivation and preventive strategies for hepatocellular carcinoma. Pharmacol Res (2022) 178:106181. doi: 10.1016/j.phrs.2022.106181
184. Choi WM, Kim HJ, Jo AJ, Choi SH, Han S, Ko MJ, et al. Association of aspirin and statin use with the risk of liver cancer in chronic hepatitis b: A nationwide population-based study. Liver Int (2021) 41(11):2777–85. doi: 10.1111/liv.15011
185. Ghalali A, Martin-Renedo J, Högberg J, Stenius U. Atorvastatin decreases HBx-induced phospho-akt in hepatocytes via P2X receptors. Mol Cancer Res (2017) 15(6):714–22. doi: 10.1158/1541-7786.MCR-16-0373
186. Sitia G, Aiolfi R, Di Lucia P, Mainetti M, Fiocchi A, Mingozzi F, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis b. Proc Natl Acad Sci U S A. (2012) 109(32):E2165–72. doi: 10.1073/pnas.1209182109
187. Qiu HB, Zhang LY, Zeng ZL, Wang ZQ, Luo HY, Keshari RP, et al. HBV infection decreases risk of liver metastasis in patients with colorectal cancer: A cohort study. World J Gastroenterol (2011) 17(6):804–8. doi: 10.3748/wjg.v17.i6.804
188. Wang Z, Sheng YY, Dong QZ, Qin LX. Hepatitis b virus and hepatitis c virus play different prognostic roles in intrahepatic cholangiocarcinoma: A meta-analysis. World J Gastroenterol (2016) 22(10):3038–51. doi: 10.3748/wjg.v22.i10.3038
189. Li Z, Li S, Tao H, Zhan Y, Ni K, Gong J, et al. Higher titer hepatitis b core antibody predicts a higher risk of liver metastases and worse survival in patients with colorectal cancer. World J Surg Oncol (2021) 19(1):251. doi: 10.1186/s12957-021-02369-1
190. Mason LM, Duffell E, Veldhuijzen IK, Petriti U, Bunge EM, Tavoschi L. Hepatitis b and c prevalence and incidence in key population groups with multiple risk factors in the EU/EEA: A systematic review. Euro Surveill. (2019) 24(30):1800614. doi: 10.2807/1560-7917.ES.2019.24.30.1800614
Keywords: hepatitis B virus, extrahepatic cancer, immune microenvironment, risk factor, molecular mechanisms
Citation: Min Y, Wei X, Xia X, Wei Z, Li R, Jin J, Liu Z, Hu X and Peng X (2023) Hepatitis B virus infection: An insight into the clinical connection and molecular interaction between hepatitis B virus and host extrahepatic cancer risk. Front. Immunol. 14:1141956. doi: 10.3389/fimmu.2023.1141956
Received: 11 January 2023; Accepted: 20 February 2023;
Published: 01 March 2023.
Edited by:
Sha Wu, Southern Medical University, ChinaReviewed by:
Azam Roohi, Tehran University of Medical Sciences, IranZhinan Yin, Jinan University, China
Copyright © 2023 Min, Wei, Xia, Wei, Li, Jin, Liu, Hu and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingchen Peng, cHh4MjAxNEAxNjMuY29t; Xiaolin Hu, aHV4aWFvbGluQHdjaHNjdS5jbg==
†These authors have contributed equally in this work
 Yu Min
Yu Min Xiaoyuan Wei
Xiaoyuan Wei Xi Xia3
Xi Xia3 Zhigong Wei
Zhigong Wei Ruidan Li
Ruidan Li Jing Jin
Jing Jin Zheran Liu
Zheran Liu Xiaolin Hu
Xiaolin Hu Xingchen Peng
Xingchen Peng