- 1Department of Clinical Pharmacy, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Medical Oncology, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Zhejiang Provincial Key Laboratory for Drug Evaluation and Clinical Research, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Background: Whether irAEs can predict the efficacy of PD-1 inhibitors in cholangiocarcinoma (CCA) has not been assessed. Therefore, this study aims to investigate the correlation between irAEs and the therapeutic effect of PD-1 inhibitors combination therapy in patients with advanced CCA.
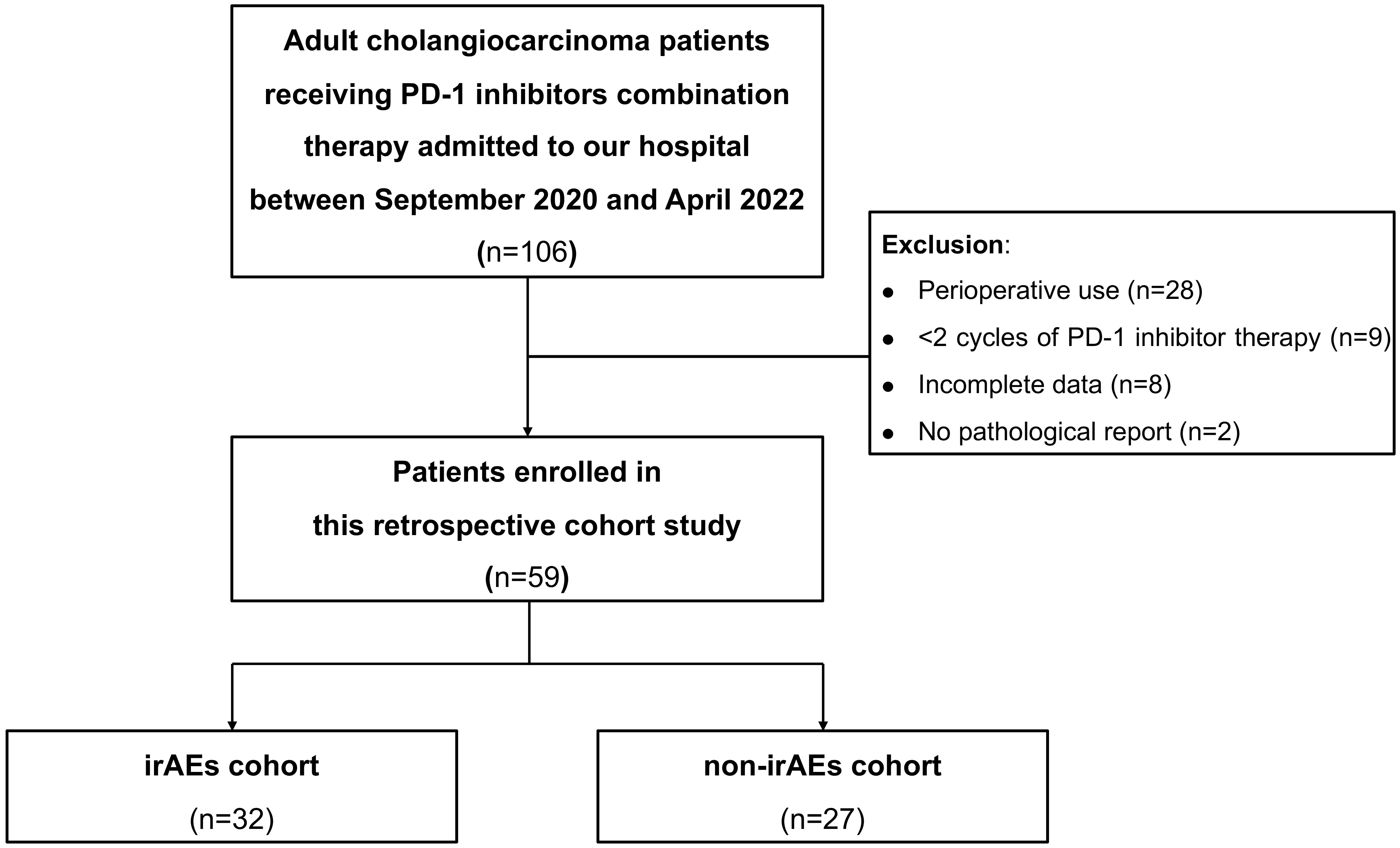
Methods: All patients with CCA who were consecutively admitted to the inpatient unit of our hospital and received PD-1 inhibitors combination therapy between September 2020 and April 2022 were screened. In total, 106 patients with CCA were screened out. We then followed up these patients until October 2022. Due to perioperative use (n=28), less than 2 cycles of PD-1 inhibitor therapy (n=9), incomplete data (n=8) and no pathological report (n=2), 59 patients were included in the final analysis. The patients were divided into the irAEs cohort and the non-irAEs cohort according to whether they experienced irAEs or not. The Log-Rank test was performed to compare the difference in survival time between these two cohorts. We then applied multivariate COX regression analysis to investigate whether irAEs were independent prognostic factors for survival in patients with advanced CCA.
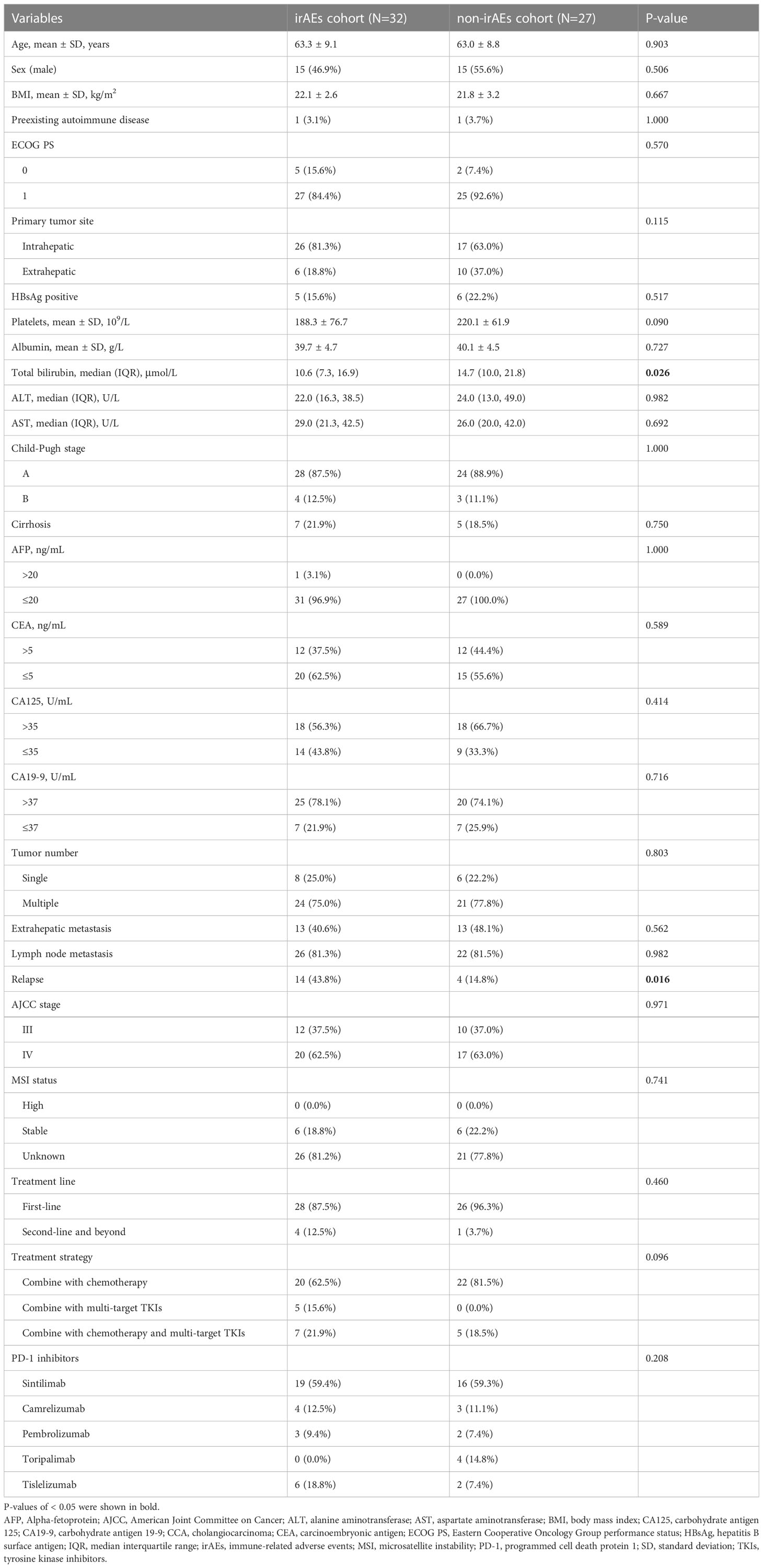
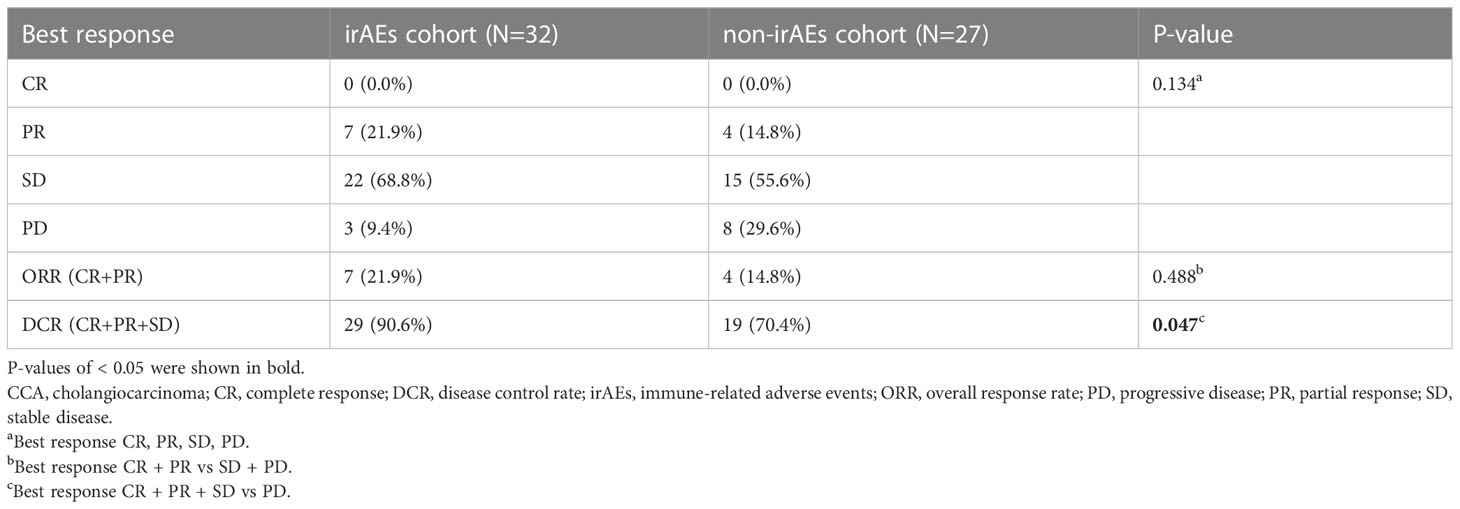
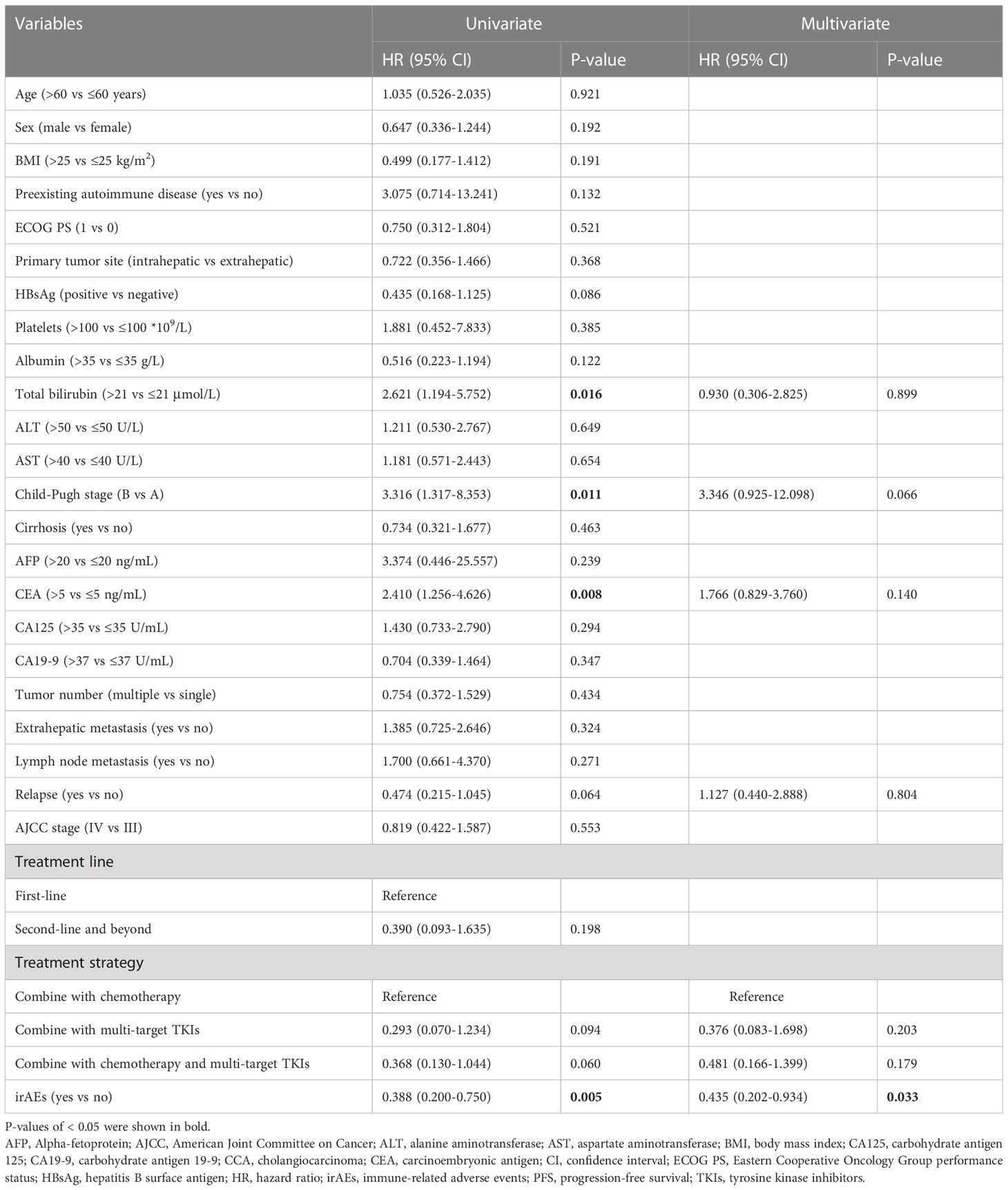
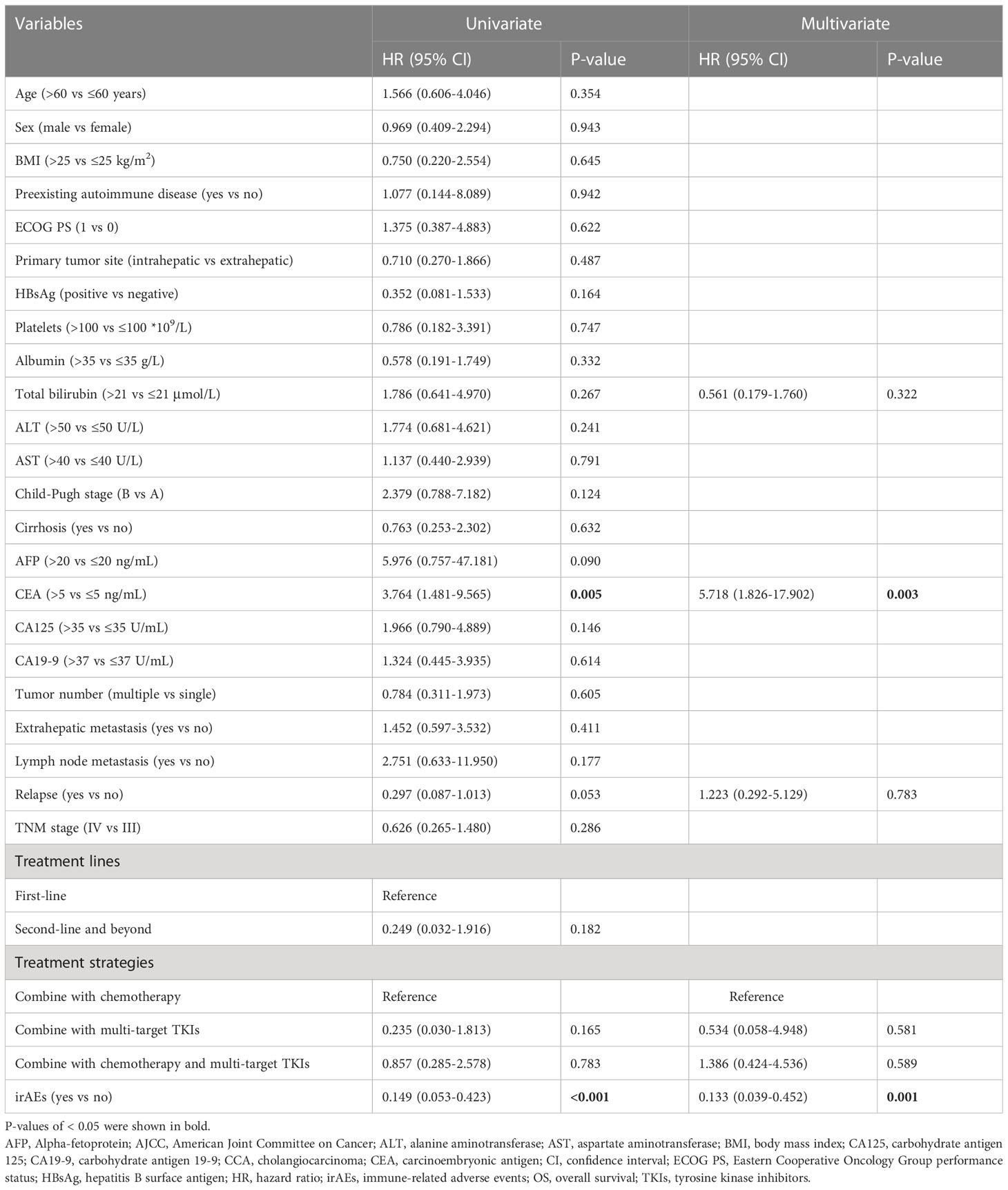
Results: Finally, 32 patients were included in the irAEs cohort and 27 patients in the non-irAEs cohort. A total of 32 patients (54.2%) had any-grade irAEs, of which 4 patients (6.8%) had grade 3-4 irAEs. The most common irAEs were thyroid toxicity (30.5%) and dermatologic toxicity (30.5%). There were no notable differences in demographics and clinical characteristics between the irAEs and non-irAEs cohorts, except for total bilirubin level (P=0.026) and relapse (P=0.016). The disease control rate (DCR) in the irAEs cohort was higher than in the non-irAEs cohort (90.6% vs 70.4%, P=0.047). Median overall survival (OS) and median progression-free survival (PFS) were better in the irAEs cohort than in the non-irAEs cohort (OS: 21.2 vs 10.0 months, P<0.001; PFS: 9.0 vs 4.4 months, P=0.003). Multivariate COX regression analysis showed that irAEs were independent prognostic factors for OS and PFS (OS: HR=0.133, 95% CI: 0.039-0.452, P=0.001; PFS: HR=0.435, 95% CI: 0.202-0.934, P=0.033).
Conclusion: IrAEs correlated with improved DCR, OS, and PFS in advanced CCA patients receiving PD-1 inhibitors combination therapy.
1 Introduction
Cholangiocarcinoma (CCA) is major type of biliary tract cancer (BTC) that originates from the bile duct epithelium (1). It is the second most common hepatobiliary cancer with high malignancy and poor prognosis (1). Depending on its anatomical location, CCA is divided into extrahepatic cholangiocarcinoma (ECC) and intrahepatic cholangiocarcinoma (ICC). CCA has few treatment options and is insensitive to both radiotherapy and chemotherapy. The onset of CCA is latent and is often at a later stage of the tumor when it is diagnosed (2). Surgery is currently the most effective treatment for CCA. However, only about 35% of patients with early-stage CCA can be treated with radical surgery. Even with radical surgery, patients still have a postoperative relapse rate of up to 70-75% (3, 4). Gemcitabine in combination with cisplatin has been the standard first-line treatment for patients with unresectable or metastatic CCA since 2010, based on the phase 3 ABC-02 trial (5). However, the overall survival (OS) of patients with advanced CCA treated with chemotherapy remains unsatisfactory.
With the ongoing development of precision medicine, immunotherapeutic drugs, particularly immune checkpoint inhibitors (ICIs), have made breakthroughs in cancer treatment. Inhibitors of the programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) play an important therapeutic role in a wide range of tumors (6–9). In the phase 3 TOPAZ-1 trial in 685 patients with advanced BTC, durvalumab (a PD-L1 inhibitor) in combination with gemcitabine and cisplatin improved OS compared to placebo plus chemotherapy (median OS: 12.8 months vs 11.5 months, P=0.021) (10). Therefore, durvalumab plus gemcitabine and cisplatin is recommended as one of the preferred first-line regimens for advanced CCA. The efficacy of PD-1 inhibitors in CCA has also been extensively studied, but objective response rates (ORR) vary widely between studies, ranging from 17.5% to 40.9% (11–14). Therefore, there is a need to identify biomarkers that predict response to PD-1 inhibitors in CCA. Currently, deficient mismatch repair/microsatellite instability-high (dMMR/MSI-H), tumor mutation burden-high (TMB-H) and PD-L1 expression have been widely studied as biomarkers related to response to immunotherapy in solid tumors (15–17), but the evidence in CCA is insufficient (11, 12, 14, 18, 19). Recently, several biomarkers have been identified in ICC to predict the efficacy of PD-1inhibitors, including pre-treatment serum lipid levels, inflammation-based scores and immune-related RNA signatures (20–22). However, there is still a lack of clinical markers during treatment to identify patients who may benefit from PD-1 inhibitors in CCA. Interestingly, PD-1 inhibitors not only activated effector T cells, but also reduced regulatory T cells, which may play a key role in maintaining immune tolerance (23). As a result, the immune system can also attack normal cells, leading to immune-related adverse events (irAEs) (24). Several studies have shown that irAEs can serve as an effective clinical biomarker for PD-1 inhibitors in several cancers, such as lung, liver, gastric cancers, and melanoma (24–28). However, there is a lack of studies on whether irAEs may play a similar role in CCA.
Therefore, this retrospective cohort study aims to investigate the correlation between irAEs and the efficacy of PD-1 inhibitors combination therapy in CCA patients. It will provide a theoretical basis for whether irAEs can be considered as a clinical marker for predicting the efficacy of PD-1 inhibitors combination therapy, and provide evidence for the precise screening of CCA patients who may benefit from PD-1 inhibitors combination therapy.
2 Materials and methods
2.1 Participants
All CCA patients consecutively admitted to the inpatient unit of our hospital and received PD-1 inhibitors combination therapy between September 2020 and April 2022 were screened. A total of 106 patients with CCA were screened out. A pathology report was required for each patient. All patients were then followed up until October 2022.
2.2 Inclusion and exclusion criteria
Inclusion criteria; (1) pathologically diagnosed with CCA; (2) ≥18 years of age, no gender restrictions; (3) received PD-1 inhibitors in combination with chemotherapy or targeted therapy [including multi-target tyrosine kinase inhibitors (TKIs)]; (4) received at least two cycles of PD-1 inhibitors combination therapy; (5) Eastern Cooperative Oncology Group (ECOG) performance status (PS) score was 0-1. Exclusion criteria: (1) incomplete medical records; (2) received PD-1 inhibitor combinations therapy for perioperative treatment.
2.3 Measures
We reviewed electronic medical records and extracted demographic information and laboratory parameters of eligible patients, including gender, age, ECOG PS score, TNM stage, total bilirubin, alpha-fetoprotein (AFP), and carcinoembryonic antigen (CEA) before PD-1 inhibitors combination therapy. A fixed dose of 200mg for pembrolizumab, sintilimab, camrelizumab, and tislelizumab and 3 mg/kg or 240 mg for toripalimab was given intravenously every 3 weeks. The choice of PD-1 inhibitor for treatment does not follow strict guidelines, mainly because of the accessibility and price of the drugs. In addition, to date, no studies have shown which PD-1 inhibitor is more effective than others. According to National Comprehensive Cancer Network (NCCN) guidelines, CCA patients who developed irAEs require a decision-making team consisting of oncology, pharmacy, endocrinology, dermatology and pulmonology specialists. This team determined whether the patient needed to discontinue PD-1 inhibitors and received appropriate observation, levothyroxine supplementation, anti-allergy medication, and topical or systemic hormone therapy. Multi-target TKIs combined with PD-1 inhibitors were lenvatinib (8 mg orally per day) or anlotinib (10 mg orally once daily d1-14, every 3 weeks for one cycle), which are recommended by the China Society of Clinical Oncology (CSCO) guidelines for advanced BTC patients. The specific combination regimens for each patient are shown in Supplementary Table 1.
Response to treatment was assessed according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). ORR, defined as the proportion of patients who achieved a partial response (PR) or complete response (CR). The disease control rate (DCR) was defined as the proportion of patients who achieved not only PR and CR but also stable disease (SD). Progression-free survival (PFS) was defined as the time from the first cycle of PD-1 inhibitors combination therapy until death or disease progression, whichever came first. In addition, OS was defined as the time from the first cycle of PD-1 inhibitors combination treatment to death from any cause. The irAEs were assessed and graded for severity according to the Common Terminology Criteria for Adverse Events 5.0 (CTCAE 5.0). Patients were then divided into an irAEs cohort and a non-irAEs cohort based on whether they had irAEs or not.
2.4 Statistical analysis
Data were calculated as mean ± standard deviation (SD) for continuous variables with normal distribution, otherwise as median (interquartile range). Categorical variables were presented as frequencies and percentages. Student’s t-test or Mann-Whitney U test was used to compare differences between the irAEs cohort and the non-irAEs cohort for continuous variables. Fisher’s exact test or Chi-square test was used for categorical variables. Kaplan-Meier survival curves were plotted. Differences in OS and PFS between the irAEs and non-irAEs cohorts were compared using the Log-Rank test. We also performed univariate and multivariate COX regression analyses to investigate whether irAEs were independent prognostic factors for OS and PFS in CCA patients. All statistical analyses were performed using the IBM Statistical Package for Social Sciences (SPSS, version 26). A two-sided P<0.05 was considered a significant difference.
3 Results
3.1 Clinical and demographic characteristics of all included CCA patients
A total of 106 CCA patients receiving PD-1 inhibitors combination therapy at our hospital from September 2020 to April 2022 were screened out. After reviewing the electronic medical records system, 28 patients with perioperative treatments, nine patients with less than two cycles of PD-1 inhibitor treatments, eight patients with incomplete data, and two patients without pathological diagnosis were excluded. Patients were divided into the irAEs cohort and non-irAEs cohort according to whether they had irAEs or not. Finally, 32 patients were included in the irAEs cohort and 27 patients were enrolled in the non-irAEs cohort. The flow chart is shown in Figure 1.
The clinical and demographic characteristics of all CCA patients in the irAEs and non-irAEs cohorts are shown in Table 1. Of these, 35 (59.3%) patients were treated with sintilimab, 7 (11.9%) with camrelizumab, 5 (8.5%) with pembrolizumab, 4 (6.8%) with toripalimab, and 8 (13.6%) with tislelizumab. In two cohorts, the mean age was 63.3 and 63.0 years, respectively. Fifteen patients (46.9%) in the irAEs cohort and 15 patients (55.6%) in the non-irAEs cohort were male. The mean body mass index (BMI) in two cohorts was 22.1 and 21.8 kg/m2, respectively. No pre-existing autoimmune disease (96.9% vs 96.3%), ECOG PS score of 1 (84.4% vs 92.6%), ICC (81.3% vs 63.0%), HBsAg negative (84.4% vs 77.8%), Child-Pugh A (87.5% vs 88.9%), no history of cirrhosis (78.1% vs 81.5%), AFP ≤20 ng/mL (96.9% vs 100.0%), CEA ≤5 ng/mL (62.5% vs 55.6%), CA125 >35 U/mL (56.3% vs 66.7%), CA19-9 >37 U/mL (78.1% vs 74.1%), multiple tumors (75.0% vs 77.8%), lymph node metastasis (81.3% vs 81.5%), AJCC stage IV (62.5% vs 63.0%), PD-1 inhibitors combination therapy as first-line treatment (87.5% vs 96.3%), and PD-1 inhibitors in combination with chemotherapy (62.5% vs 81.5%) were the majority of CCA patients enrolled. There were no statistical differences in most clinical and demographic characteristics between the two cohorts (P>0.05), except for total bilirubin level (P=0.026) and relapse (P=0.016).
3.2 Description of irAEs
In total, 32 (54.2%) patients experienced any- grade irAEs and 4 (6.8%) patients experienced severe (grade 3-4) irAEs. The most common irAEs were thyroid dysfunction (30.5%) and dermatologic adverse events (30.5%), manifesting as hypothyroidism (25.4%), hyperthyroidism (5.1%), rash (22.0%) and pruritus (8.5%). Other irAEs were mainly pneumonia (5.1%), colitis (1.7%), hyperglycaemia (1.7%), pancreatitis (1.7%) and infusion-related reactions (1.7%). The incidence of irAEs is shown in Table 2.
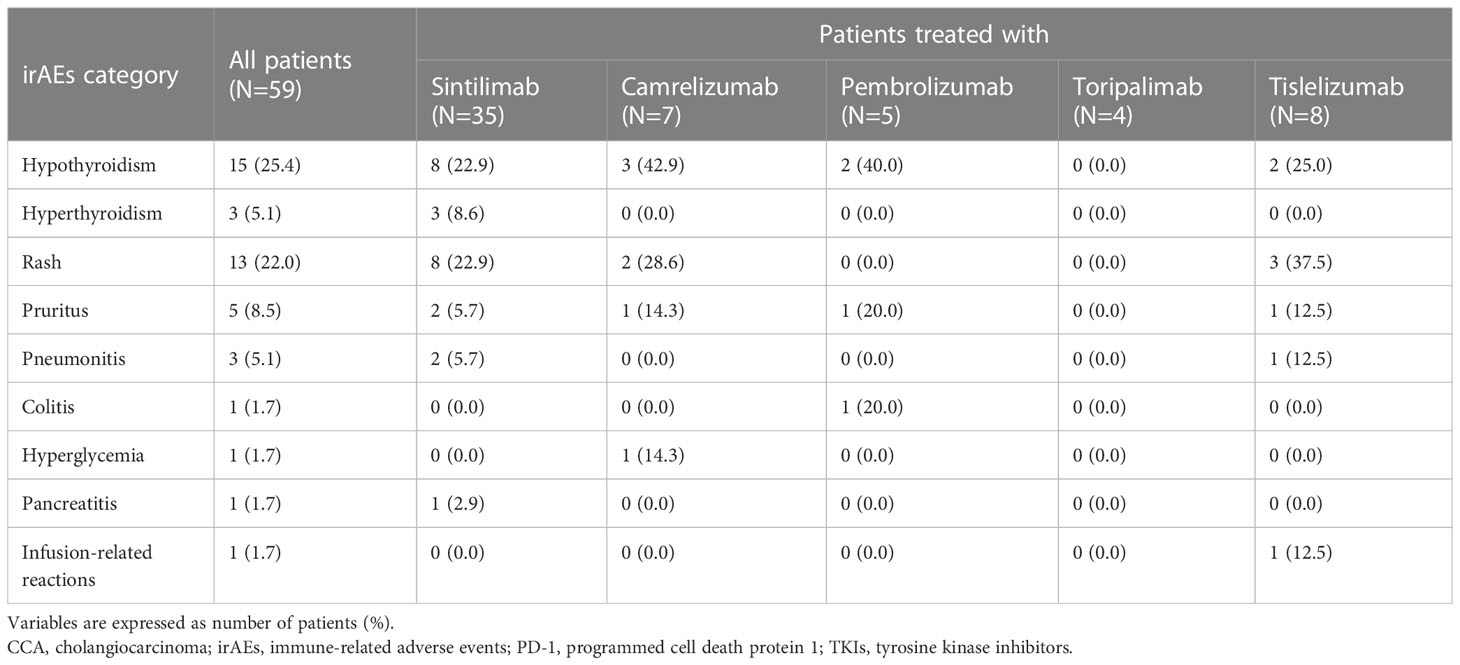
Table 2 Description of irAEs in CCA patients treated with PD-1 inhibitors in combination with chemotherapy and/or multi-target TKIs.
3.3 Comparison of the efficacy between irAEs and non-irAEs cohorts
The median follow-up time was 11.4 (95%CI: 9.1-13.7) months. The ORR was similar in the irAEs and non-irAEs cohorts (21.9% vs 14.8%, P=0.488). The DCR was higher in the irAEs cohort (90.6% vs 70.4%, P=0.047) (Table 3). Median PFS was 9.0 (95% CI: 4.9-13.1) months in the irAEs cohort and 4.4 (95% CI: 3.2-5.6) months in the non-irAEs cohorts. Median OS was 21.2 (95% CI: 10.9-31.5) months and 10.0 (95% CI: 5.2-14.8) months in these two cohorts, respectively. Both PFS and OS were better in the irAEs cohort than in the non-irAEs cohort (P=0.003 and P<0.001, respectively) (Figure 2).
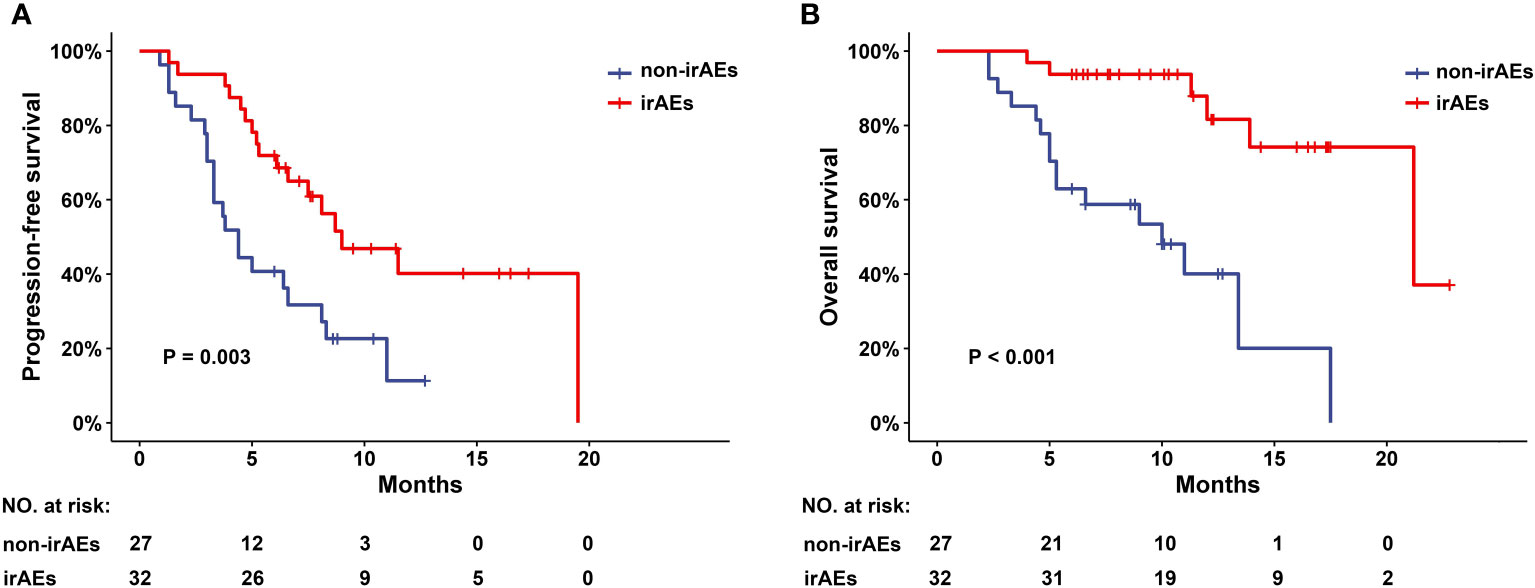
Figure 2 PFS and OS in patients with CCA with or without irAEs.(A) Progression-free survival (PFS) and (B) overall survival (OS) in CCA patients with or without irAEs after receiving programmed cell death protein 1 (PD-1) inhibitors combination therapy. irAEs, immune-related adverse events; CCA, cholangiocarcinoma.
3.4 Correlation of irAEs with PFS and OS
To investigate whether the differences in patients’ clinical and demographic characteristics affected the results of this study, we performed univariate COX regression analysis, the results showed that total bilirubin >21 μmol/L (HR=2.621, 95% CI: 1.194-5.752, P=0.016), Child-Pugh B (HR=3.316, 95% CI: 1.317-8.353, P=0.011), CEA>5 ng/mL (HR=2.410, 95% CI: 1.256-4.626, P=0.008) and irAEs (HR=0.388, 95% CI: 0.200-0.750, P=0.005) were prognostic factors for PFS. In addition, we also constructed a multivariate COX regression model using the above variables as well as relapse and treatment strategy (P=0.060 in univariate analysis), and the results demonstrated that irAEs were an independent protective factor for PFS (HR=0.435, 95% CI: 0.202-0.934, P=0.033) Table 4).
In addition, univariate COX regression analysis indicated that CEA>5 ng/mL (HR=3.764, 95% CI: 1.481-9.565, P=0.005) and irAEs (HR=0.149, 95% CI: 0.053-0.423, P<0.001) were prognostic factors for OS. We constructed a multivariate COX regression model with the above 2 variables as well as total bilirubin, relapse and treatment strategy. The results showed that irAEs were an independent protective factor for OS (HR=0.133, 95% CI: 0.039-0.452, P=0.001), whereas CEA>5 ng/mL was an independent risk factor for OS (HR=5.718, 95% CI: 1.826-17.902, P=0.003) (Table 5).
4 Discussion
PD-1 inhibitors are promising anti-tumor drugs that have led to several breakthroughs in the treatment of tumors such as lung cancer and melanoma. A number of clinical trials of PD-1 inhibitors for the treatment of CCA are ongoing, and several case reports and clinical findings suggest that PD-1 inhibitors have therapeutic effects in CCA (11, 12, 29–31). Data from KEYNOTE-158 (11) showed that among 22 patients with advanced CCA with dMMR/MSI-H who received pembrolizumab monotherapy, the ORR was 40.9% (2 CR and 7 PR), and the median PFS and OS were 4.2 (95% CI: 2.1-NR) months and 24.3 (95% CI: 6.5-NR) months, respectively. In another phase II study in 46 patients with advanced BTC (12), 5 patients (11%) responded to nivolumab, all mismatch repair protein-positive tumors. These studies suggest that dMMR/MSI-H may be associated with improved outcomes in CCA patients treated with PD-1 inhibitors, but the evidence is insufficient. Zhang J (31) et al. reported a patient with metastatic ICC who failed first-line chemotherapy and achieved a CR after three cycles of sintilimab monotherapy. However, this patient had a low TMB and was microsatellite stable (MSS). Therefore, there is still a need to explore new biomarkers to predict the effect of PD-1 inhibitors on CCA.
Interestingly, PD-1 inhibitors have the potential to augment normal immune responses while enhancing antitumor immune responses, leading to dysregulation of immune homeostasis and ultimately to irAEs (23). Weber JS et al (32) were the first to report that irAEs of any grade were associated with higher ORR in patients with advanced melanoma receiving nivolumab. Since then, several studies have shown that the occurrence of irAEs is associated with improved ORR, PFS or OS in patients with non-small cell lung cancer (NSCLC) (25, 33–37), gastric cancer (25, 26, 37), renal cell carcinoma (25, 37), urothelial cell carcinoma (38) and hepatocellular carcinoma (28) treated with immunotherapy. However, whether irAEs have a predictive effect in CCA has not been reported. To our knowledge, this is the first evidence that irAEs are associated with better clinical outcomes in patients with advanced CCA receiving PD-1 inhibitors combination therapy. The results of this study showed that among CCA patients receiving PD-1 inhibitors combination therapy, the irAEs cohort had a higher DCR (90.6% vs 70.4%, P=0.047), longer OS (median OS: 21.2 vs 10.0 months, P<0.001) and PFS (median PFS: 9.0 vs 4.4 months, P=0.003) compared to the non-irAEs cohort.
There were statistically differences in total bilirubin levels and relapse between the irAEs and non-irAEs cohorts in the clinical and demographic characteristics of the CCA patients. To assess whether differences in total bilirubin levels and relapse between these two cohorts would affect outcomes, we further performed multivariate COX regression analysis. The results showed that total bilirubin levels did not affect OS and PFS in CCA patients (P=0.322 and 0.899, respectively). Relapse also had no effect on OS and PFS in CCA patients (P=0.783 and 0.804, respectively). Furthermore, irAEs were an independent prognostic factor for PFS in CCA patients (P=0.033), while irAEs and CEA levels were independent prognostic factors for OS (irAEs: P=0.001; CEA levels: P=0.003). The above results suggest that the occurrence of irAEs may predict better outcomes in patients with advanced CCA receiving PD-1 inhibitors combination therapy. Recently, a multicentre retrospective study (39) showed that a higher grade of initial checkpoint inhibitor-related pneumonitis (CIP) was associated with a higher relapse rate of CIP after rechallenge with ICIs in patients with advanced lung cancer. Therefore, we infer that advanced CCA patients with grade 1-2 irAEs may benefit from rechallenge with PD-1 inhibitors, and appropriate studies are needed to verify this inference.
However, the specific mechanisms to explain why irAEs are positively associated with immunotherapy are limited. Studies have shown that a higher BMI associated with improved PFS and OS and a higher incidence of irAEs in cancer patients receiving PD-1/PD-L1 inhibitors (40–42), suggesting that adipose tissue may play a key role in immunotherapy and irAEs. Taken together, the available evidence suggests that this may be related to an increased inflammatory environment due to obesity (42–46). Furthermore, a prospective study of dermatologic adverse events in NSCLC patients receiving PD-1 inhibitors found that tumor and skin tissues shared nine T-cell antigens, and it has been speculated that T cells targeting cancer cells may also target normal tissues with shared antigens (47), which may partially explain the correlation between irAEs and PD-1/PD-L1 inhibitor efficacy. It has also been shown that intestinal flora can modulate innate and acquired immunity in cancer patients in a variety of ways, further influencing the efficacy of PD-1/PD-L1 inhibitors (48–53). While irAEs may also be associated with intestinal flora, studies to date have been limited to CTLA-4 inhibitors. It was found that OS and PFS were improved in patients with Faecal Bacterium and other Firmicutes, but these patients were more likely to develop colitis (54). The specific roles of different microbial species are not yet known and further relevant prospective clinical trials are needed. Finally, high drug clearance (Clearance, CL) has also been suggested as a possible factor to explain the correlation between immunotherapy efficacy and irAEs. One study indicated that high CL was negatively associated with OS and PFS in cancer patients receiving pembrolizumab (P<0.001 and 0.003, respectively) (55). It is possible that high CL promotes the clearance of PD-1 inhibitors, leading to lower blood levels and possibly reduced efficacy. However, this study found no statistical difference in CL between patients with severe irAEs and those with mild irAEs (P = 0.70) (55). Although the CL of PD-1/PD-L1 inhibitors may help explain the correlation between irAEs and the therapeutic effect of PD-1/PD-L1 inhibitors, further evidence is needed.
This study also has some limitations. First, it is a retrospective cohort study, so there may be an inevitable bias for irAEs. Secondly, due to non-randomization, baseline imbalances in patients resulted in the presence of confounding variables. Although we corrected for this using multivariate analysis, validation is needed in prospective, randomized clinical trials. Thirdly, due to the low prevalence of CCA, the number of advanced CCA patients receiving PD-1 inhibitors combination therapy is limited. Therefore, the sample size in this study is small. Finally, the PD-L1 expression and MSI status of most of the patients enrolled were unknown, so we cannot draw any conclusions on the correlation between MSI status, PD-L1 expression and response/survival in advanced CCA patients. In the future, larger prospective studies are needed to validate this finding and identify molecular biomarkers.
5 Conclusion
Our study suggests for the first time that irAEs may predict higher DCR and longer OS and PFS in patients with advanced CCA receiving PD-1 inhibitors combination therapy. IrAEs may serve as an effective clinical marker to predict the efficacy of PD-1 inhibitors combination therapy in patients with advanced CCA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the First Affiliated Hospital, Zhejiang University School of Medicine (Approval number: IIT20210916A). The patients/participants provided their written informed consent to participate in this study.
Author contributions
YZ and ZY designed this study. XW and YL collected the data of the participants. YZ, YH contributed to the data analysis. YZ, QZ, and ZY participated in the writing and revision of the manuscript. All authors approved the final version of the article and agreed to be responsible for all aspects of this study.
Funding
This study was supported by the Fund of Zhejiang Pharmaceutical Association (Grant number 2022ZYYL01) and the Fund of Zhejiang Medical Doctors Association (Grant number YS2022-3-010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1141148/full#supplementary-material
References
1. Roth GS, Neuzillet C, Sarabi M, Edeline J, Malka D, Lievre A. Cholangiocarcinoma: What are the options in all comers and how has the advent of molecular profiling opened the way to personalised medicine? Eur J Cancer (2023) 179:1–14. doi: 10.1016/j.ejca.2022.11.006
2. Wang M, Chen Z, Guo P, Wang Y, Chen G. Therapy for advanced cholangiocarcinoma: Current knowledge and future potential. J Cell Mol Med (2021) 25(2):618–28. doi: 10.1111/jcmm.16151
3. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol (2013) 31(9):1188–95. doi: 10.1200/JCO.2012.41.5984
4. Goyal L, Zheng H, Yurgelun MB, Abrams TA, Allen JN, Cleary JM, et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer (2017) 123(11):1979–88. doi: 10.1002/cncr.30571
5. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med (2010) 362(14):1273–81. doi: 10.1056/NEJMoa0908721
6. Qin LX. Immunotherapy for hepatobiliary malignancies: Progress and prospective. Hepatobiliary Pancreat Dis Int (2022) 21(5):409–12. doi: 10.1016/j.hbpd.2022.09.002
7. Li Y, Liang X, Li H, Chen X. Comparative efficacy and safety of immune checkpoint inhibitors for unresectable advanced melanoma: A systematic review and network meta-analysis. Int Immunopharmacol (2023) 115:109657. doi: 10.1016/j.intimp.2022.109657
8. Li Q, Han J, Yang Y, Chen Y. Pd-1/Pd-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front Immunol (2022) 13:1070961. doi: 10.3389/fimmu.2022.1070961
9. Pang K, Shi ZD, Wei LY, Dong Y, Ma YY, Wang W, et al. Research progress of therapeutic effects and drug resistance of immunotherapy based on pd-1/Pd-L1 blockade. Drug Resist Update (2022) 66:100907. doi: 10.1016/j.drup.2022.100907
10. Oh D-Y, He AR, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid (2022) 1(8):1–11. doi: 10.1056/EVIDoa2200015
11. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite Instability/Mismatch repair-deficient cancer: Results from the phase ii keynote-158 study. J Clin Oncol (2020) 38(1):1–10. doi: 10.1200/JCO.19.02105
12. Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol (2020) 6(6):888–94. doi: 10.1001/jamaoncol.2020.0930
13. Xie L, Huang J, Wang L, Ren W, Tian H, Hu A, et al. Lenvatinib combined with a pd-1 inhibitor as effective therapy for advanced intrahepatic cholangiocarcinoma. Front Pharmacol (2022) 13:894407. doi: 10.3389/fphar.2022.894407
14. Deng M, Li S, Wang Q, Zhao R, Zou J, Lin W, et al. Real-world outcomes of patients with advanced intrahepatic cholangiocarcinoma treated with programmed cell death protein-1-Targeted immunotherapy. Ann Med (2022) 54(1):803–11. doi: 10.1080/07853890.2022.2048416
15. Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther (2017) 16(11):2598–608. doi: 10.1158/1535-7163.MCT-17-0386
16. Paver EC, Cooper WA, Colebatch AJ, Ferguson PM, Hill SK, Lum T, et al. Programmed death ligand-1 (Pd-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology (2021) 53(2):141–56. doi: 10.1016/j.pathol.2020.10.007
17. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. Pd-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
18. Gou M, Zhang Y, Si H, Dai G. Efficacy and safety of nivolumab for metastatic biliary tract cancer. Onco Targets Ther (2019) 12:861–7. doi: 10.2147/OTT.S195537
19. Zhang W, Shi J, Wang Y, Zhou H, Zhang Z, Han Z, et al. Next-generation sequencing-guided molecular-targeted therapy and immunotherapy for biliary tract cancers. Cancer Immunol Immunother (2021) 70(4):1001–14. doi: 10.1007/s00262-020-02745-y
20. Yang Z, Zhang D, Sima X, Fu Y, Zeng H, Hu Z, et al. Levels of pretreatment serum lipids predict responses to pd-1 inhibitor treatment in advanced intrahepatic cholangiocarcinoma. Int Immunopharmacol (2023) 115:109687. doi: 10.1016/j.intimp.2023.109687
21. Yang Z, Zhang D, Zeng H, Fu Y, Hu Z, Pan Y, et al. Inflammation-based scores predict responses to pd-1 inhibitor treatment in intrahepatic cholangiocarcinoma. J Inflammation Res (2022) 15:5721–31. doi: 10.2147/JIR.S385921
22. Zeng TM, Pan YF, Yuan ZG, Chen DS, Song YJ, Gao Y. Immune-related rna signature predicts outcome of pd-1 inhibitor-combined gemcis therapy in advanced intrahepatic cholangiocarcinoma. Front Immunol (2022) 13:943066. doi: 10.3389/fimmu.2022.943066
23. Cramer P, Bresalier RS. Gastrointestinal and hepatic complications of immune checkpoint inhibitors. Curr Gastroenterol Rep (2017) 19(1):3. doi: 10.1007/s11894-017-0540-6
24. Ye Z, Zheng S, Chen J, Zhang Y, Yang S, Hong Y, et al. Can immune-related adverse events serve as clinical biomarkers of pd-1/Pd-L1 inhibitor efficacy in pan-cancer patients? Int Immunopharmacol (2022) 108:108738. doi: 10.1016/j.intimp.2022.108738
25. Shimozaki K, Sukawa Y, Beppu N, Kurihara I, Suzuki S, Mizuno R, et al. Multiple immune-related adverse events and anti-tumor efficacy: Real-world data on various solid tumors. Cancer Manag Res (2020) 12:4585–93. doi: 10.2147/CMAR.S247554
26. Masuda K, Shoji H, Nagashima K, Yamamoto S, Ishikawa M, Imazeki H, et al. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer (2019) 19(1):974. doi: 10.1186/s12885-019-6150-y
27. Indini A, Di Guardo L, Cimminiello C, Prisciandaro M, Randon G, De Braud F, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-Pd1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol (2019) 145(2):511–21. doi: 10.1007/s00432-018-2819-x
28. Lu L, Xing K, Wei W, Ling Y, Li P, Li S, et al. Immune-related adverse events predict responses to pd-1 blockade immunotherapy in hepatocellular carcinoma. Int J Cancer (2021) e002344. doi: 10.1002/ijc.33609
29. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to pd-1 blockade. Science (2017) 357(6349):409–13. doi: 10.1126/science.aan6733
30. Zhang Z, Zhang W, Wang H, Hu B, Wang Z, Lu S. Successful treatment of advanced intrahepatic cholangiocarcinoma with a high tumor mutational burden and pd-L1 expression by pd-1 blockade combined with tyrosine kinase inhibitors: A case report. Front Immunol (2021) 12:744571. doi: 10.3389/fimmu.2021.744571
31. Zhang J, Wu L, Liu J, Lin M. A metastatic intrahepatic cholangiocarcinoma treated with programmed cell death 1 inhibitor: A case report and literature review. Immunotherapy (2020) 12(8):555–61. doi: 10.2217/imt-2019-0100
32. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol (2017) 35(7):785–92. doi: 10.1200/JCO.2015.66.1389
33. Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-Small-Cell lung cancer. Clin Lung Cancer (2019) 20(3):201–7. doi: 10.1016/j.cllc.2018.10.002
34. Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F, Tiseo M, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-Pd1 immunotherapy in nsclc patients. Clin Lung Cancer (2019) 20(4):237–47.e1. doi: 10.1016/j.cllc.2019.02.006
35. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-Small-Cell lung cancer. JAMA Oncol (2018) 4(3):374–8. doi: 10.1001/jamaoncol.2017.2925
36. Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer (2018) 115:71–4. doi: 10.1016/j.lungcan.2017.11.019
37. Park R, Lopes L, Saeed A. Anti-Pd-1/L1-Associated immune-related adverse events as harbinger of favorable clinical outcome: Systematic review and meta-analysis. Clin Transl Oncol (2021) 23(1):100–9. doi: 10.1007/s12094-020-02397-5
38. Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol (2019) 37(30):2730–7. doi: 10.1200/JCO.19.00318
39. Lin X, Deng H, Chu T, Chen L, Yang Y, Qiu G, et al. Safety and efficacy of immunotherapy rechallenge following checkpoint inhibitor-related pneumonitis in advanced lung cancer patients: A retrospective multi-center cohort study. Transl Lung Cancer Res (2022) 11(11):2289–305. doi: 10.21037/tlcr-22-732
40. McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol (2018) 19(3):310–22. doi: 10.1016/S1470-2045(18)30078-0
41. Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and pd-1 checkpoint blockade. Nat Med (2019) 25(1):141–51. doi: 10.1038/s41591-018-0221-5
42. Cortellini A, Bersanelli M, Santini D, Buti S, Tiseo M, Cannita K, et al. Another side of the association between body mass index (Bmi) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (Pd-1)/Programmed cell death-ligand 1 (Pd-L1) checkpoint inhibitors: A multicentre analysis of immune-related adverse events. Eur J Cancer (2020) 128:17–26. doi: 10.1016/j.ejca.2019.12.031
43. Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, et al. A multicenter study of body mass index in cancer patients treated with anti-Pd-1/Pd-L1 immune checkpoint inhibitors: When overweight becomes favorable. J Immunother Cancer (2019) 7(1):57. doi: 10.1186/s40425-019-0527-y
44. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res (2005) 96(9):939–49. doi: 10.1161/01.RES.0000163635.62927.34
45. Han SJ, Glatman Zaretsky A, Andrade-Oliveira V, Collins N, Dzutsev A, Shaik J, et al. White adipose tissue is a reservoir for memory T cells and promotes protective memory responses to infection. Immunity (2017) 47(6):1154–68.e6. doi: 10.1016/j.immuni.2017.11.009
46. Jordan BF, Gourgue F, Cani PD. Adipose tissue metabolism and cancer progression: Novel insights from gut microbiota? Curr Pathobiol Rep (2017) 5(4):315–22. doi: 10.1007/s40139-017-0154-6
47. Berner F, Bomze D, Diem S, Ali OH, Fassler M, Ring S, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol (2019) 5(7):1043–7. doi: 10.1001/jamaoncol.2019.0402
48. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-Pd-L1 efficacy. Science (2015) 350(6264):1084–9. doi: 10.1126/science.aac4255
49. Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by ctla-4 blockade relies on the gut microbiota. Science (2015) 350(6264):1079–84. doi: 10.1126/science.aad1329
50. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of pd-1-Based immunotherapy against epithelial tumors. Science (2018) 359(6371):91–7. doi: 10.1126/science.aan3706
51. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-Pd-1 efficacy in metastatic melanoma patients. Science (2018) 359(6371):104–8. doi: 10.1126/science.aao3290
52. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-Pd-1 immunotherapy in melanoma patients. Science (2018) 359(6371):97–103. doi: 10.1126/science.aan4236
53. Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol (2017) 17(4):219–32. doi: 10.1038/nri.2017.7
54. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol (2017) 28(6):1368–79. doi: 10.1093/annonc/mdx108
Keywords: advanced cholangiocarcinoma, PD-1 inhibitors combination therapy, immune-related adverse events (irAEs), efficacy prediction, clinical marker
Citation: Zhang Y, Wang X, Li Y, Hong Y, Zhao Q and Ye Z (2023) Immune-related adverse events correlate with the efficacy of PD-1 inhibitors combination therapy in advanced cholangiocarcinoma patients: A retrospective cohort study. Front. Immunol. 14:1141148. doi: 10.3389/fimmu.2023.1141148
Received: 10 January 2023; Accepted: 16 March 2023;
Published: 24 March 2023.
Edited by:
Hongda Liu, Nanjing Medical University, ChinaReviewed by:
Elena Tassi, San Raffaele Hospital (IRCCS), ItalySusanna Ulahannan, University of Oklahoma, United States
Copyright © 2023 Zhang, Wang, Li, Hong, Zhao and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingwei Zhao, cXd6aGFvQHpqdS5lZHUuY24=; Ziqi Ye, emlxaXllQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
 Yanfang Zhang1†
Yanfang Zhang1† Qingwei Zhao
Qingwei Zhao Ziqi Ye
Ziqi Ye