
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 10 March 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1139799
This article is part of the Research Topic Environmental Factors in Autoimmunity View all 14 articles
Background: Evidence from observational studies on the association of folate and vitamin B12 with autoimmune diseases are conflicting.
Objective: We aimed to investigate the relationship of folate and vitamin B12 with autoimmune diseases using Mendelian randomization (MR).
Materials and methods: We selected single-nucleotide polymorphisms associated with folate and vitamin B12 at the genome-wide significance level. Summary-level data for four common autoimmune diseases (vitiligo, inflammatory bowel disease, rheumatoid arthritis, and systemic lupus erythematosus) were obtained from large-scale genome-wide association studies, with a sample size of 44,266, 86,640, 58,284, and 23,210, respectively. MR analyses were conducted using the inverse variance weighted (IVW) approach, and sensitivity analyses were further performed to test the robustness.
Results: We found that a higher genetically determined serum folate level per one standard deviation (SD) was associated with a decreased risk of vitiligo by the IVW method [odds ratios (OR) = 0.47; 95% confidence interval (CI): 0.32–0.69; P = 1.33 × 10-4]. Sensitivity analyses using alternative methods showed similar associations, and no evidence of pleiotropy was detected by MR-Egger regression (P = 0.919). In addition, we observed that vitamin B12 per one SD was positively associated with IBD (IVW: OR = 1.14, 95% CI: 1.03–1.26, P = 0.010; maximum likelihood: OR = 1.14, 95% CI: 1.01–1.29, P = 0.035; MR-PRESSO: OR = 1.14, 95% CI:1.01–1.28, P =0.037), while the association was not significant after Bonferroni correction.
Conclusion: The study provides convincing evidence for an inverse association between serum folate level and risk of vitiligo. Further studies are warranted to elucidate the possible association between vitamin B12 and risk of IBD.
Autoimmune diseases represent a family of illnesses that share a common pathogenesis of an immune-mediated attack on the body’s own organs (1). Accumulating epidemiological studies have shown that autoimmune diseases have caused a serious disease burden on the world—for example, the global prevalence of vitiligo is 0.06%–8.80% according to research published between 1964 and 2017 (2). The Global Burden of Disease study reported that (3) there were 4.90 million prevalent cases of inflammatory bowel disease (IBD) in 2019, resulting in 41,000 deaths and 1.62 million disability adjusted life years (DALYs) in global. Moreover, autoimmune diseases may have serious tissue damage before clinical diagnosis and due to lack of effective treatment (1)—for instance, the hazard of systemic lupus erythematosus (SLE) may involve multiple organs, including the kidneys, skin, joints, and nervous system (4). Rheumatoid arthritis (RA) can cause cartilage and bone damage as well as disability (5). IBD is a chronic inflammatory disorder primarily of, but not restricted to, the gut because extraintestinal manifestations are frequently observed in joints, eyes, hepatobiliary tract, and skin (6).
Folate and vitamin B12 are essential water-soluble vitamins that play a crucial role in the maintenance of one-carbon metabolism. B vitamins control DNA synthesis and methyl donor availability, which are important for the normal function of cells in the immune system (7). Notwithstanding, the association of folate and vitamin B12 with autoimmune diseases is not fully understood. Recently, a meta-analysis involving a total of 804 patients with vitiligo and 518 controls indicated that vitiligo patients had lower vitamin B12 levels [standardized mean difference (SMD) -0.430; 95% CI, -0.738 to -0.121] than controls, while there was no significant difference in the serum folate level between 672 patients and 378 matched controls (8). Similarly, Tsung-Yu Tsai et al. conducted a meta-analysis of eight studies involving 415 patients with SLE and 391 controls which showed that SLE patients had significantly lower vitamin B12 levels than the controls (SMD, -0.359; 95% CI, -0.638 to -0.080), but the serum folate level did not differ markedly between 532 SLE patients and 634 controls (9). For IBD, a meta-analysis including 1,086 IBD patients and 1,484 controls showed that the serum folate concentration in IBD patients was significantly lower than that in control patients (SMD, -0.46; 95% CI, -0.64 to -0.27), whereas the mean serum vitamin B12 concentration did not differ between cases and controls (10). Therefore, the association of folate and vitamin B12 with autoimmune diseases was still debated. Moreover, these associations derived from traditional observational epidemiological studies are susceptible to confounders and reverse causation bias.
Mendelian randomization (MR) is a genetic epidemiological method which uses genetic variants, especially single-nucleotide polymorphisms (SNPs), as instrumental variables (IVs) to infer the potential causality of exposure and outcome (11). The MR approach can overcome confounders and reverse causation bias as the genotype of an individual is determined at conception and cannot be changed. To the best of our knowledge, there are no MR studies inferring the potential causal relationship of folate and B12 with these four autoimmune diseases (vitiligo, IBD, RA, and SLE) to date. Therefore, we applied the two-sample MR method to examine whether the genetically predicted folate and B12 levels were associated with the risk of autoimmune diseases.
The overview of our study design is shown in Figure 1. Since the data that we used were based on published studies and public databases, no additional ethical approval from an institutional review board was required.
We gathered the summary statistics of autoimmune diseases from published genome-wide association studies (GWASs). To be more specific, the sample sizes of datasets for vitiligo (12), IBD (13), RA (14), and SLE (15) are 44,266 (4,680 cases), 86,640 (38,155 cases), 58,284 (14,361 cases), and 23,210 (7,219 cases), respectively. All the participants were of European ancestry. The detailed information of the outcome GWASs is listed in Supplementary Table S1.
Genetic variants for folate and vitamin B12 levels were derived from a GWAS including 37,341 and 45,576 European individuals, respectively (16). The population involved in the GWAS were from Iceland and Denmark. Specifically, the sample size of GWASs for folate and B12 were 28,913 and 37,283 from Iceland and 8,428 and 8,293 from Denmark, respectively. A total of four and 15 SNPs at the genome-wide significance level (P < 5 × 10-8) were selected as IVs for folate and vitamin B12 in primary analysis (Supplementary Table S2). Furthermore, we excluded two SNPs for folate with linkage disequilibrium (LD) (r2 threshold < 0.1) through the Web source (https://snipa.helmholtzmuenchen.de/snipa3/index.php?task=pairwise_ld) in the sensitivity analysis, and no LD was found among the 15 instrumental SNPs for vitamin B12.
Then, we calculated the F-statistics; to quantify the strength of the IVs that we used to assess weak instrumental variable bias, the F-statistic for folate and vitamin B12 ranged from 22.05 to 568.86 (Supplementary Table S3), satisfying the assumption of F >10 for MR analyses (17). The variance explained by the IVs for folate and vitamin B12 with different outcomes was approximately 1.1% and at least 2.7% (Supplementary Table S3). Furthermore, we detected the minimum effect size based on 80% statistical power at an α level of 5% using an online tool (https://shiny.cnsgenomics.com/mRnd/). Based on the sample size of outcome datasets, there was sufficient power to detect significant associations with any autoimmune diseases for folate and vitamin B12 at an effect size (odds ratio, OR) of 1.64 and 1.38 (Supplementary Table S3). Then, we scanned other relevant phenotypes of the selected IVs through the GWAS catalog (https://www.ebi.ac.uk/gwas/, accessed on July 1, 2022) to further certify the impact of horizontal pleiotropy on causality effect (Supplementary Table S4).
We conducted a two-sample Mendelian randomization analysis to estimate the potential causal relationship of folate and vitamin B12 with different autoimmune diseases using the “Mendelian randomization” and “MRPRESSO” packages in R software version 3.6.3. The MR study was based on three core assumptions: (1) there is a robust and strong association between IVs and exposure; (2) the IVs are independent of confounders; and (3) the IVs can only affect the outcome through exposure without other pathways. We mainly used inverse variance weighted (IVW) method for MR analysis, which provided a consistent estimate of the association between exposure and the risk of outcomes when IVs were not pleiotropic (18). Cochran’s Q statistics was performed to assess the heterogeneity across individual SNPs. The fixed-effects model was employed if no substantial heterogeneity (P < 0.05) was observed; otherwise, the random-effects model was utilized (19). We also performed sensitivity analyses to verify the robustness of our results. The weighted median method can obtain a consistent estimate of the overall effect when more than 50% of IVs are valid, which can reduce the bias of the causal effect estimate compared with the IVW method (20). The maximum-likelihood method may provide more reliable estimates when measurement error occurs in the SNP-exposure effects (21). Moreover, the MR-Egger method was used to determine whether the instrumental SNPs are multi-effect. The intercept obtained from the MR-Egger regression was used to measure the directional pleiotropy (22). In addition, we used MR pleiotropy residual sum and outlier test (MR-PRESSO) in order to detect and correct for multi-effects by removing possible outliers (18).
Statistical significance was set at a P-value <6.25 × 10-3 (P = 0.05/(two exposures × four outcomes) corrected for two exposures and four outcomes using the Bonferroni method. A P-value above the Bonferroni-corrected threshold, but lower than 0.05, was considered suggestive evidence for a potential causal association. All statistical analyses were two-sided.
The effect estimates of the MR analyses for the association of folate with four autoimmune diseases are depicted in Figure 2. There was no evidence for heterogeneity (vitiligo: P = 0.382, IBD: P = 0.056; RA: P = 0.480; SLE: P = 0.085) by using Cochran’s Q test, so we chose the fixed-effect IVW model for MR analysis. We observed that a higher genetically predicted serum folate level per one standard deviation (SD) was significantly associated with a decreased risk of vitiligo by the IVW method (OR = 0.47; 95% CI: 0.32–0.69; P =1.33 × 10-4). The sensitivity analysis showed similar results (OR: 0.43, 95% CI: 0.27–0.69, P = 5.00 × 10-4 by weighted median; OR: 0.47, 95% CI: 0.31–0.69, P = 1.76 × 10-4 by maximum-likelihood method; OR: 0.47, 95% CI: 0.32–0.70, P = 1.75 × 10-4 by MR-PRESSO). Moreover, MR-Egger regression did not suggest evidence of potential directional pleiotropy (P for intercept = 0.919). In addition, we found that the direction and effect of the association between folate and vitiligo remained consistent after excluding the two SNPs with LD (Figure 3). The IVW method showed that the genetically predicted serum folate level per one SD increment was associated with a decreased risk of vitiligo (OR = 0.35, 95% CI: 0.21–0.60, P = 1.47×10-4). Moreover, the results were stable by maximum-likelihood method (OR = 0.35, 95% CI: 0.20–0.61, P = 2.12 × 10-4). Here we could not use the MR-Egger method to determine whether the SNPs are horizontally pleiotropic due to the fact that the MR-Egger method required at least three SNPs. Similarly, we cannot detect outlier SNPs by the MR-PRESSO method due to lack of enough SNPs. We further performed a leave-one-out analysis to detect whether the causal estimate was driven by any single SNP, which revealed a consistent inverse association between genetically predicted folate and risk of vitiligo (Supplementary Figure S1).

Figure 2 Mendelian randomization estimates from instrument variants for folate on risk of autoimmune diseases.
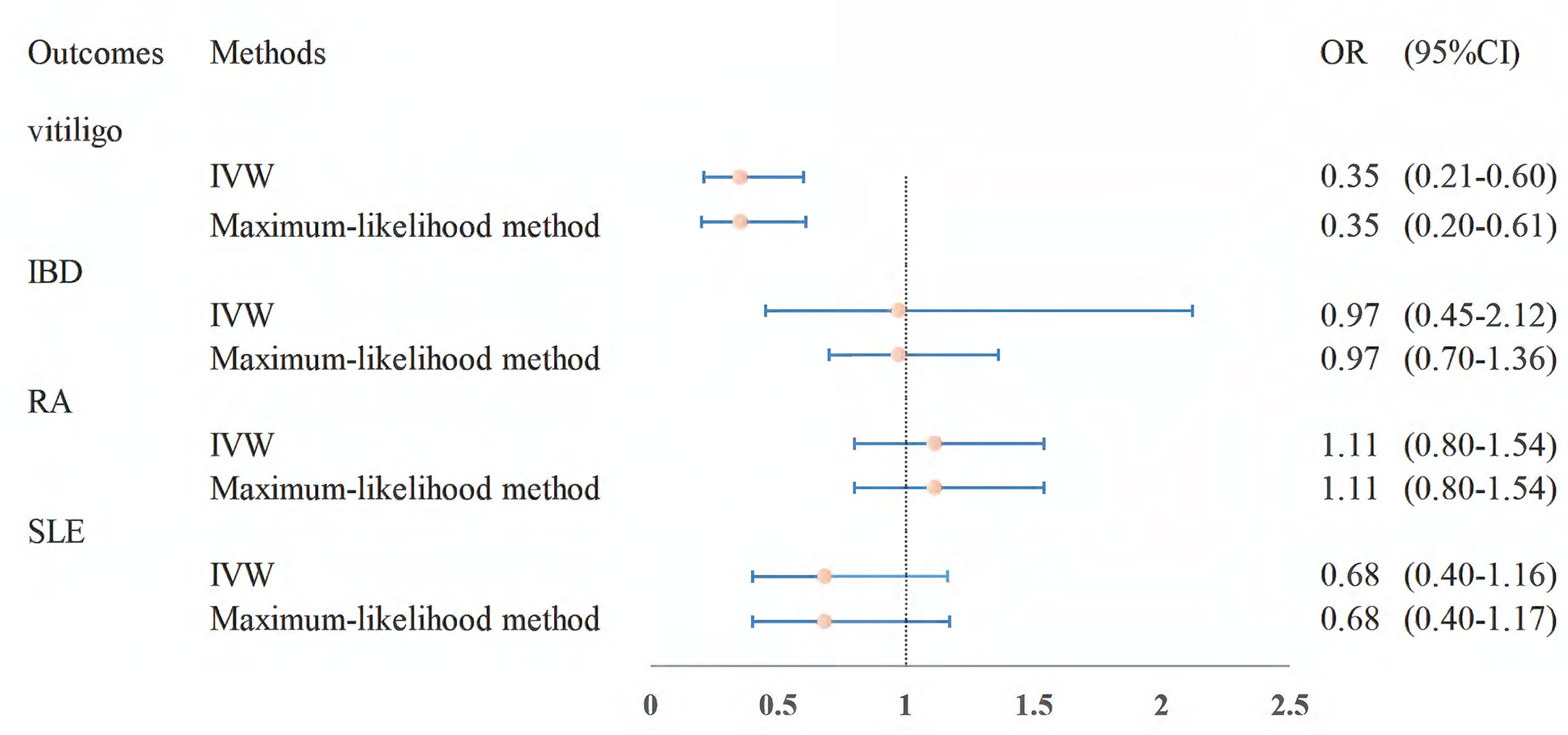
Figure 3 MR estimates from instrument variants for folate on risk of autoimmune diseases after removing SNPs with LD. MR, Mendelian randomization; SNP, single-nucleotide polymorphism; LD, linkage disequilibrium.
However, the results showed no statistically significant associations of folate level with other autoimmune diseases (IBD: OR = 0.87, 95% CI: 0.70–1.10, P = 0.246; RA: OR = 1.16, 95% CI: 0.93–1.43, P = 0.190; SLE: OR = 1.10, 95% CI: 0.75–1.63, P = 0.616; Figure 2). The null findings were supported by the weighted median method, the maximum-likelihood method, and MR-PRESSO. There was also no apparent sign of horizontal pleiotropy as assessed by MR-Egger regression (P-values of the intercept for IBD: P = 0.670; RA: P = 0.469; SLE: P = 0.891). Furthermore, we observed that the results were stable in the sensitivity analysis after excluding the two SNPs with LD (Figure 3).
For vitamin B12, the IVW method showed that the genetically predicted vitamin B12 level per one SD increment may be associated with a 14% increased risk of IBD (95% CI: 3%–26%, P = 0.010), but alternative MR methods showed wider CIs (Table 1). However, all these associations did not meet the statistical test following multiple testing correction (P=6.25×10-3). In the meanwhile, we did not detect any associations of vitamin B12 with other autoimmune diseases (Table 1). Similarly, there was no apparent sign of horizontal pleiotropy as assessed by MR-Egger regression (vitiligo: P = 0.819; IBD: P = 0.196; RA: P = 0.615; SLE: P = 0.914).
Research on preventing autoimmune diseases through a nutritious diet has gradually become attractive because diet is easier to correct than the other modifiable unhealthy lifestyles. However, to the best of our knowledge, randomized controlled trial (RCT) studies on folate and vitamin B12 with autoimmune diseases are rare to date. Our MR study found a significant association of one SD higher genetically predicted folate level with a decreased risk of vitiligo, providing suggestive evidence as well of the potential causal relationship between vitamin B12 and risk of IBD.
Vitiligo is an autoimmune disease which destroys skin melanocytes and gives the appearance of patchy depigmentation (23). The aforementioned meta-analysis (8) including nine previous observational studies showed that folate may be not associated with a decreased risk of vitiligo. However, the studies were heterogeneous due to sample size and ethnic differences among the vitiligo patients. Moreover, the included observational studies were eight case–control studies and one cross-sectional study, which is insufficient to prove the causal association (8). The directionality of the MR association between genetically determined levels of folate and risk of vitiligo is consistent with experimental data showing a preventive role for folate in vitiligo. It is well known that folate plays a role in the transformation of homocysteine (Hcy), which could lower the intracellular Hcy level (24). A recent study published by Chen et al. reported that Hcy could induce melanocyte apoptosis via the PERK-eIF2α-CHOP pathway in vitiligo patients and provide evidence that folate supplementation can reverse the melanin production defects in melanocytes induced by Hcy. Folate can protect melanocytes from Hcy-induced apoptosis and inhibition of melanin synthesis (24). In addition, folate is implicated in the biosynthesis of melanin in human epidermis because it is necessary for the production of tetrahydrobiopterin in melanocytes and keratinocytes (25–27). Tetrahydrobiopterin regulates the tyrosinase activity in melanosome, and the synthesized tetrahydrobiopterin is highly activated in vitiligo (28, 29). Although the explanation of the association between folate and the risk of vitiligo was biologically reasonable, more macro and micro evidence such as related preventive RCTs and animal experimental studies is warranted to elucidate the mechanism.
In a previous MR study on the environmental risk factors for IBD with the outcome dataset including 25,042 IBD cases and 34,915 controls, one SD increase in circulating vitamin B12 was not significantly associated with IBD (OR = 1.07, 95% CI: 1.00–1.15, P = 0.06) (30). Our well-powered MR study with a larger sample size found that there was suggestive evidence of a potential hazard effect of vitamin B12 on the risk of IBD, although the association did not reach statistical significance after correction for multiple comparisons. Vitamin B12 is bound to dietary protein and absorbed after the hydrochloric acid produced in the gastric mucosa separates it from the protein. The released cobalamin then attaches to the R protein and enters into the duodenum, where the R protein is removed and the free cobalamin binds to the intrinsic factor (IF). The IF–cobalamin complex is absorbed by the distal ileum and requires calcium (31). The former studies suggested that the absorption mechanisms of vitamin B12 could reach saturation (32, 33); thus, excessive B12 levels may burden the gut absorption function. A randomized double-blind placebo-controlled trial concluded that surplus treatment with vitamin B12 in IBD patients has no beneficial clinical effect (34). In addition, an animal study reported that vitamin B12 deficiency protects against DSS-induced colitis in mice (35), which confirms that higher B12 levels may increase the risk of IBD.
For RA, there were limited observational studies on the association of folate and vitamin B12 with risk of RA to date. Our study found null associations of folate and vitamin B12 concentrations with RA risk, which suggested that folate and vitamin B12 supplement may not be beneficial for preventing from RA, thus clinicians should use nutritional supplements with caution in practice. Although there was a meta-analysis showing a protective effect of folate on SLE and no marked difference in the folate level of patients with SLE and controls, the including studies were all of case-control studies (9), which are insufficient to prove the causal association due to flaws such as confounders and reverse causation bias in case-control studies. As compared with previous findings, our MR results are more convincing since it avoids confounders and reverse causal association bias to some extent. Consequently, we found that folate and B12 were not significantly associated with SLE.
However, the present study has some limitations. First, the validity of the MR study may be reduced due to the weak IVs. To remedy this problem, we used independent SNPs reaching genome-wide significance level (P < 5 × 10-8), and the F-statistic satisfied the threshold of >10. However, a larger sample size of GWAS is warranted to find more folate-related SNPs in the future. Second, pleiotropy may occur when some instrumental SNPs are associated with other multiple factors. In the present study, we did not observe evidence of pleiotropy for the causal association between folate and risk of vitiligo in any of the above-mentioned MR approaches. Third, since the subjects are limited to Europeans, the results may not be applicable to the entire population, and the research conclusions should be extrapolated cautiously. Fourth, we could not investigate the nonlinear effects of folate and vitamin B12 on these autoimmune diseases due to the utilization of summary-data level in two-sample MR analysis. Nonetheless, our study offers new insights into the relationships between the folate level and vitiligo, providing a better understanding of its etiology.
In summary, genetically determined higher circulating levels of folate were associated with a decreased risk of vitiligo using MR methods. Our research suggests that folate supplementation may be a potential preventive measure for vitiligo, which provides a rationale for using folate supplementation as a promising target for vitiligo prevention. Further studies are needed to determine how folate affects the occurrence and development of vitiligo and confirm the association in large-scale randomized controlled trials and scientific animal experiments.
Datasets used for the MR analyses are public and available under reasonable requests. The authors thank all investigators for sharing these data.
Since the data we used were based on published studies and public databases, no additional ethical approval from an institutional review board was required. The patients/participants provided their written informed consent to participate in this study.
HY conducted the data analysis and drafted the manuscript. JS participated in mining the database and drafting the manuscript. AL and LL searched literatures and analyzed the data. XS took responsibility of the tables and figures of the results. DY and YM designed the study, revised the manuscript, and provided technical support. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (82204843, 81973663 and 82174208), the Natural Science Foundation of Zhejiang Province (LQ21H260001 and LQ20H260008), the Foundation of Zhejiang Chinese Medical University (2020ZG01 and 2020ZG16), and the Research Project of Zhejiang Chinese Medical University (2021JKZKTS004A).
The authors sincerely thank the researchers and the participants of the original GWASs for the collection and management of the large-scale data resources.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1139799/full#supplementary-material
1. Rose NR. Prediction and prevention of autoimmune disease in the 21st century: A review and preview. Am J Epidemiol (2016) 183(5):403–6. doi: 10.1093/aje/kwv292
2. Said-Fernandez SL, Sanchez-Domínguez CN, Salinas-Santander MA, Martinez-Rodriguez HG, Kubelis-Lopez DE, Zapata-Salazar NA, et al. Novel immunological and genetic factors associated with vitiligo: A review. Exp Ther Med (2021) 21(4):312. doi: 10.3892/etm.2021.9743
3. Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9
4. Durcan L, O'Dwyer T, Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet (2019) 393(10188):2332–43. doi: 10.1016/S0140-6736(19)30237-5
5. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet (2016) 388(10055):2023–38. doi: 10.1016/s0140-6736(16)30173-8
6. Greuter T, Vavricka SR. Extraintestinal manifestations in inflammatory bowel disease - epidemiology, genetics, and pathogenesis. Expert Rev Gastroenterol Hepatol (2019) 13(4):307–17. doi: 10.1080/17474124.2019.1574569
7. Lyon P, Strippoli V, Fang B, Cimmino L. B vitamins and one-carbon metabolism: Implications in human health and disease. Nutrients (2020) 12(9):2867. doi: 10.3390/nu12092867
8. Tsai TY, Kuo CY, Huang YC. Serum homocysteine, folate, and vitamin B(12) levels in patients with vitiligo and their potential roles as disease activity biomarkers: A systematic review and meta-analysis. J Am Acad Dermatol (2019) 80(3):646–54.e5. doi: 10.1016/j.jaad.2018.08.029
9. Tsai TY, Lee TH, Wang HH, Yang TH, Chang IJ, Huang YC. Serum homocysteine, folate, and vitamin B(12) levels in patients with systemic lupus erythematosus: A meta-analysis and meta-regression. J Am Coll Nutr (2021) 40(5):443–53. doi: 10.1080/07315724.2020.1788472
10. Pan Y, Liu Y, Guo H, Jabir MS, Liu X, Cui W, et al. Associations between folate and vitamin B12 levels and inflammatory bowel disease: A meta-analysis. Nutrients (2017) 9(4):382. doi: 10.3390/nu9040382
11. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. Jama (2017) 318(19):1925–6. doi: 10.1001/jama.2017.17219
12. Jin Y, Andersen G, Yorgov D, Ferrara TM, Ben S, Brownson KM, et al. Genome-wide association studies of autoimmune vitiligo identify 23 new risk loci and highlight key pathways and regulatory variants. Nat Genet (2016) 48(11):1418–24. doi: 10.1038/ng.3680
13. Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet (2015) 47(9):979–86. doi: 10.1038/ng.3359
14. Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature (2014) 506(7488):376–81. doi: 10.1038/nature12873
15. Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet (2015) 47(12):1457–64. doi: 10.1038/ng.3434
16. Grarup N, Sulem P, Sandholt CH, Thorleifsson G, Ahluwalia TS, Steinthorsdottir V, et al. Genetic architecture of vitamin B12 and folate levels uncovered applying deeply sequenced large datasets. PloS Genet (2013) 9(6):e1003530. doi: 10.1371/journal.pgen.1003530
17. Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res (2012) 21(3):223–42. doi: 10.1177/0962280210394459
18. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol (2013) 37(7):658–65. doi: 10.1002/gepi.21758
19. Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in mendelian randomization studies. Hum Mol Genet (2018) 27(R2):R195–r208. doi: 10.1093/hmg/ddy163
20. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol (2016) 40(4):304–14. doi: 10.1002/gepi.21965
21. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife (2018) 7:e34408. doi: 10.7554/eLife.34408
22. Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet (2016) 48(5):481–7. doi: 10.1038/ng.3538
23. Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol (2017) 77(1):1–13. doi: 10.1016/j.jaad.2016.10.048
24. Chen J, Zhuang T, Chen J, Tian Y, Yi X, Ni Q, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond) (2020) 134(10):1127–41. doi: 10.1042/cs20200218
25. Lerner AB, Fitzpatrick TB. Biochemistry of melanin formation. Physiol Rev (1950) 30(1):91–126. doi: 10.1152/physrev.1950.30.1.91
26. Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem Biophys (2004) 41(3):415–34. doi: 10.1385/CBB:41:3:415
27. Schallreuter KU, Kothari S, Chavan B, Spencer JD. Regulation of melanogenesis–controversies and new concepts. Exp Dermatol (2008) 17(5):395–404. doi: 10.1111/j.1600-0625.2007.00675.x
28. Schallreuter KU. Advances in melanocyte basic science research. Dermatol Clin (2007) 25(3):283–91, vii. doi: 10.1016/j.det.2007.04.010
29. Buglak AA, Telegina TA, Lyudnikova TA, Vechtomova YL, Kritsky MS. Photooxidation of tetrahydrobiopterin under UV irradiation: Possible pathways and mechanisms. Photochem Photobiol (2014) 90(5):1017–26. doi: 10.1111/php.12285
30. Carreras-Torres R, Ibáñez-Sanz G, Obón-Santacana M, Duell EJ, Moreno V. Identifying environmental risk factors for inflammatory bowel diseases: A mendelian randomization study. Sci Rep (2020) 10(1):19273. doi: 10.1038/s41598-020-76361-2
33. Adams JF, Ross SK, Mervyn L, Boddy K, King P. Absorption of cyanocobalamin, coenzyme b 12 , methylcobalamin, and hydroxocobalamin at different dose levels. Scand J Gastroenterol (1971) 6(3):249–52. doi: 10.3109/00365527109180702
34. Scholten AM, Vermeulen E, Dhonukshe-Rutten RAM, Verhagen T, Visscher A, Olivier A, et al. Surplus vitamin B(12) use does not reduce fatigue in patients with irritable bowel syndrome or inflammatory bowel disease: A randomized double-blind placebo-controlled trial. Clin Nutr ESPEN (2018) 23:48–53. doi: 10.1016/j.clnesp.2017.10.004
Keywords: folate, vitamin B12, autoimmune diseases, Mendelian randomization, single nucleotide polymorphism
Citation: Yang H, Song J, Li A, Lv L, Sun X, Mao Y and Ye D (2023) Genetically predicted levels of folate, vitamin B12, and risk of autoimmune diseases: A Mendelian randomization study. Front. Immunol. 14:1139799. doi: 10.3389/fimmu.2023.1139799
Received: 07 January 2023; Accepted: 24 February 2023;
Published: 10 March 2023.
Edited by:
Shannon E. Dunn, St Michael’s Hospital, CanadaCopyright © 2023 Yang, Song, Li, Lv, Sun, Mao and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ding Ye, eWVkaW5nQHpjbXUuZWR1LmNu; Yingying Mao, bXl5QHpjbXUuZWR1LmNu
†These authors have contributed equally to this work
‡ORCID: Yingying Mao, orcid.org/0000-0003-3644-9160
Ding Ye, orcid.org/0000-0001-6654-7832
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.