
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 29 March 2023
Sec. Viral Immunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1139031
This article is part of the Research Topic COVID and Emerging Infectious Diseases View all 47 articles
 Abdullah Reda1*†
Abdullah Reda1*† Basant Ismail Lashin2†
Basant Ismail Lashin2† Mustafa Mohammad Alaaraj3,4
Mustafa Mohammad Alaaraj3,4 Moustafa Abouelkheir5
Moustafa Abouelkheir5 Mahmoud Ibrahim Ahmed6
Mahmoud Ibrahim Ahmed6 Jaffer Shah7*
Jaffer Shah7* Amr Ehab El-Qushayri8†
Amr Ehab El-Qushayri8†Background: The impact of chronic rhinosinusitis (CRS) and subsequent steroid therapy on acquiring COVID-19 and severe outcomes remains controversial. Therefore, we conducted this systematic review and meta-analysis to provide cumulative evidence regarding the risk of COVID-19 and the impact of steroid therapy, length of hospital stay, mechanical ventilation, and mortality among CRC patients.
Methods: We conducted a comprehensive electronic search strategy using the relevant keywords. The outcomes and risk factors of COVID-19 in CRS patients was estimated and compared to a healthy control group when applicable.
Results: A total of seven studies were included, with an estimated prevalence of 6.5% (95% confidence interval (CI): 2.5-15.7) for COVID-19 in the CRS group. COVID-19 prevalence did not differ between CRS and controls (odds ratio (OR): 0.92; 95%CI: 0.84-1.01; p = 0.08). Moreover, using steroid/immunosuppressive therapy did not significantly increase the risk of acquiring COVID-19 in CRS patients compared to the control group (OR: 3.31; 95%CI: 0.72-15.26; p = 0.12). Length of hospital stay, mechanical ventilation, and mortality rates were comparable between the two groups. Furthermore, we found that male sex, cardiovascular morbidity, renal diseases, and hypertension were inversely associated with COVID-19 infection (p < 0.01).
Conclusion: CRS had a neutral effect on acquiring COVID-19 and developing severe outcomes. However, further studies are needed.
Chronic rhinosinusitis (CRS) is an inflammatory condition affecting the nasal cavity and related sinuses. Affected patients are characterized by various symptoms, including nasal discharge with and without facial pain, stuffy nose, and sense of smell-related conditions. Epidemiological data indicate the high prevalence of CRS in the general population, up to 12.5% (1). Evidence shows that nasal cavity-related pathological conditions can significantly lead to serious lower respiratory diseases, the main entry of different pathogens into the respiratory system (2, 3). Furthermore, established evidence shows that oral and nasal cavities are mainly responsible for introducing viral illnesses to the respiratory system, including the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (4). This might be due to specific antibody deficiency, exaggerated immune response, bacterial colonization, and epithelial barrier dysfunction (5, 6).
Since SARS-CoV-2 emerged and caused the coronavirus disease 2019 (COVID-19), different reports have indicated the global burden affection of the different aspects of the communities and their populations by the pandemic (7, 8). In addition, the disease has been associated with not only the development of acute respiratory distress syndromes but other conditions and complications that might even be life-threatening. Further, studies demonstrated that patients with underlying comorbidities are at risk of infection and developing severe COVID-19. These comorbidities include hypertension, diabetes mellitus (DM), obesity, and chronic obstructive pulmonary disease. Similarly, it has been shown that allergic rhinitis and asthma might be associated with an increased risk of COVID-19 and severe disease (9–11). This might be due to the influence of host immune functions on the infectivity and/or severity of COVID-19, based on the different affinities and expression levels of viral entry factors (12).
The exact role of CRS in COVID-19 is still unknown. Some studies were published to investigate this association. However, no cumulative evidence was found in these studies. For instance, some studies reported that CRS is not a significant risk factor for developing COVID-19 and severe disease. Moreover, it has been suggested that the condition might have a protective role against COVID-19 (12–16). On the other hand, other studies demonstrated that CRS increases the risk of hospitalization in COVID-19 patients (17, 18). Increased susceptibility to COVID-19 and increased risk of severe disease with CRS might be attributed to the high expression levels of viral entry genes, epithelial barrier dysfunction, and induced upper airway inflammatory condition. The main aim of this study is to provide cumulative evidence regarding the impact of CRS on COVID-19 infection and outcomes.
The steps of this systematic review were conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (19). We performed a comprehensive search of five electronic databases: PubMed, Scopus, ISI, google scholar, and VHL, for relevant studies published in the literature until July 10, 2022. We conducted another search on January 1, 2023 to check whether further studies were published. The search keywords and corresponding Medical Subject Headings (Mesh) were as follows (“COVID-19” OR “COVID 19” OR “novel coronavirus” OR “SARS-CoV-2”) AND (“chronic sinusitis” OR “chronic rhinosinusitis”). Moreover, we followed a manual search strategy as members looked up the references of relevant studies and reviews to find any relevant articles that might have been missed during the electronic search strategy.
The current systematic review aims to identify the impact of CRS on acquiring COVID-19 and related severe outcomes of previously affected patients. Accordingly, we will mainly assess the prevalence of COVID-19 in CRS patients and compare it to the control group of non-CRS individuals. The intervention group will include patients with CRS, while the control one will include healthy individuals with no CRS or another control group. We will also estimate the rates of hospitalization, intensive care unit (ICU) admissions, mechanical ventilation, and mortality, together with the length of hospital stay. We will also assess the impact of steroids/immunosuppressive therapy used by CRS patients on acquiring COVID-19. These outcomes will be compared to the control group whenever possible.
Other secondary endpoints of the current study will include the risk factors for developing COVID-19 and the difference in symptomatology and COVID-19 positive and negative patients with CRS.
We drafted our criteria based on our intended outcomes. Accordingly, we included articles that 1) were original, including cross-sectional and cohort studies 2) included human populations and 3) investigated the prevalence of COVID-19 (PCR-confirmed) or any related outcome in CRS patients or compared the prevalence between CRS and non-CRS (control) individuals. On the other hand, we excluded articles that 1) were not original (like different types of reviews, abstract-only articles, editorials, thesis, protocols, and conference papers), or 2) included limited sample populations, like case reports and case series, 3) were not human studies (like in vivo and in vitro studies), 4) did not include CRS patients, 5) did not report any COVID-19 related outcomes among these patients, or 6) reported other outcomes related to laboratory data or genetic information.
After we finished the search strategy, all citations from all databases were grouped into a single Endnote library to help us remove all potential duplicates among these databases. Following this step, we used an Excel sheet and gave each citation an ID to help identify and facilitate the screening of the different citations. The screening process was divided into two main steps, including title/abstract followed by full-text screening. Both of these steps were conducted by at least two study members. To prevent bias in selection, each member’s screening results were blinded from the other. However, after they were finished, the senior member grouped and compared the results of each member and highlighted the differences to be discussed to reach a final decision about whether to include or exclude the article according to the inclusion and exclusion criteria.
A pilot extraction sheet was drafted based on the intended outcomes. It was then tested and modified whenever needed throughout the extraction process to enhance the quality of reporting and facilitate the extraction process. The sheet mainly consisted of three parts. The first part was made for the baseline characteristics (including study reference, study design, sample size, age, and gender (male %) of each group, and method of COVID-19 diagnosis. The second part was developed for the study outcomes, including the prevalence of COVID-19, hospitalization, length of hospital stay, mechanical ventilation, ICU admissions, and mortality rates in the CRS and control groups. This part also included the symptoms of each group and the risk factors between COVID-19 positive and negative CRS patients. The third part included the domains of the Newcastle–Ottawa scale (NOS) for assessing the quality of non-randomized studies (Cohorts and Case-controls). The tool mainly assessed the quality of each included study by three domains, including selection, compatibility, and outcomes, which were divided into eight items. The highest score for any study was nine, and studies were stratified based on their totally estimated scores as follows: 1) 0-3 very high risk of bias, 2) 4-6 moderate risk (fair quality), and 3) 7-9 high quality. We also used the modified the NOS for cross-sectional studies, which was also composed of three main domains, divided into seven items with a maximum score of ten. The extraction process was also conducted in a blinded approach to reduce the risk of bias in extraction. A discussion was also made whenever there was a disagreement to reach a final decision.
Data analysis was conducted using the Comprehensive Meta-analysis (CMA, Version 3) software. Random or fixed models were chosen based on the heterogeneity of included studies. We identified heterogeneity as P-value < 0.1, or I (2) ≥ 50%. We expressed data in rates (%) for data presented in events/totals and measuring the prevalence of outcomes. We also compared the outcomes between the two study groups, and data was represented by odds ratio (OR) and 95% confidence interval (CI). A P-value < 0.05 was considered statistically significant.
A total of 690 studies were imported for a title and abstract screening, and after removing duplicates, 578 studies were retained. These articles were screened by title/abstract screening, and as a result, only 21 studies were deemed eligible for full-text review. From the full-text review, seven papers met our criteria and were extracted. The rest of these articles were excluded because they were not original, did not include any of our reported outcomes, or was duplicated with another included article. We detailed this process in the PRISMA flow chart in Figure 1.
A total of seven studies, including five cross-sectional (13, 15–17, 20) and two cohort studies (18, 21), were finally retrieved. The included studies’ sample size remarkably varied, ranging from 52 to 12323 in the CRS group and 942 to 207636 in the control group. Similarly, the age of included participants was remarkably variable among included studies. Besides, the proportion of women was comparable among all CRS and control groups. All COVID-19 patients were diagnosed by reverse transcription-polymerase chain reaction. All baseline characteristics are presented in Table 1.
Results of the quality assessment of each study and domains of the NOS tool are presented in Supplementary Tables 1, 2.
The prevalence of COVID-19 in CRS patients was 6.5% (95%CI: 2.5-15.7) (Figure 2A). Furthermore, COVID-19 prevalence did not differ between CRS and controls (OR: 0.92; 95%CI: 0.84-1.01; p = 0.08) (Figure 2B).
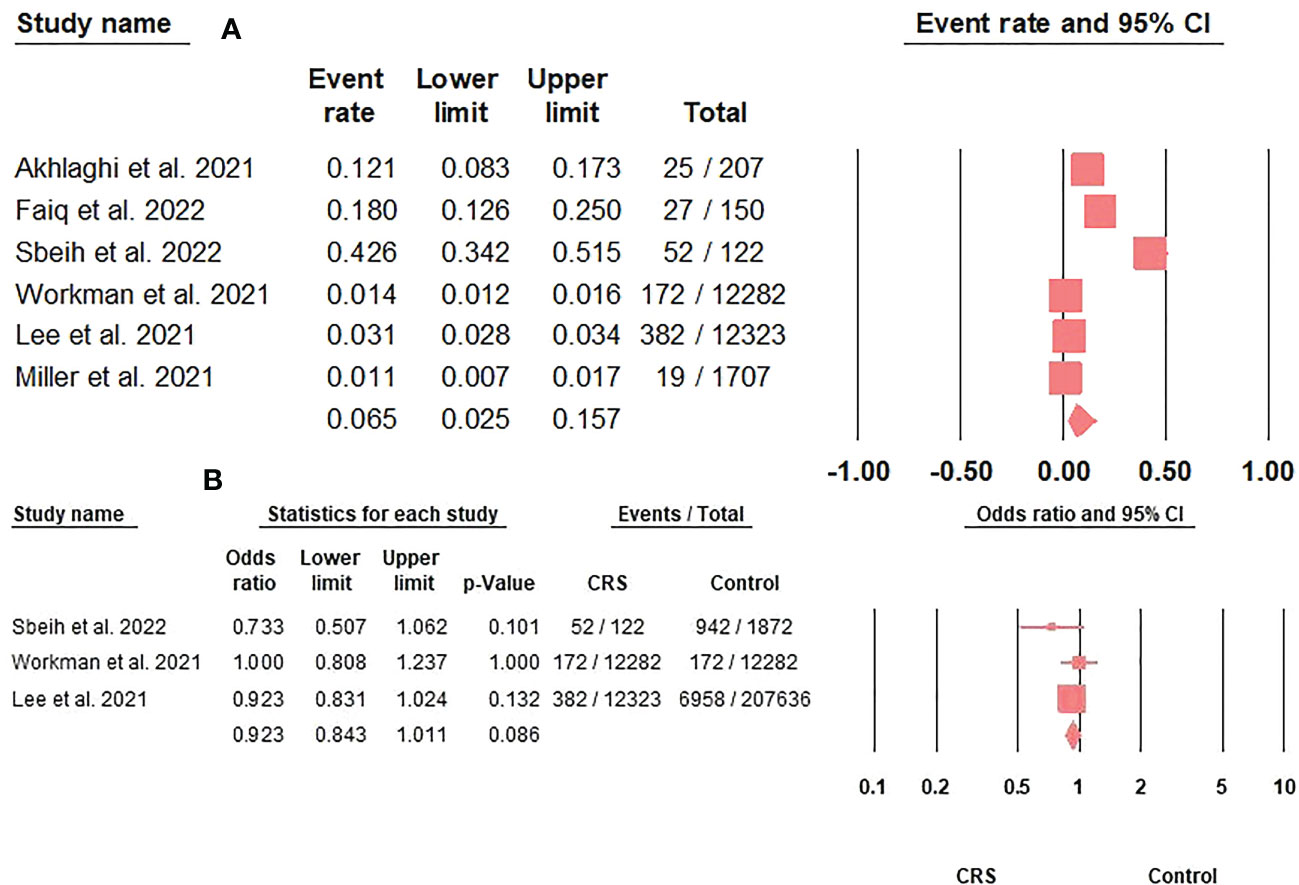
Figure 2 (A). The COVID-19 prevalence rate in CRS patients represented by the event rate and 95% confidence interval. Figure 2 (B). The association between COVID-19 prevalence and CRS represented by the odds ratio and 95% confidence interval.
Around one-fifth (20.8%; 95%CI: 3.7-84) of CRS patients with COVID-19 required hospitalization (Table 2). Moreover, COVID-19 hospitalization rates were significantly higher in CRS patients than in controls (38.46% versus 25.82%; p = 0.03) (17).
The length of hospital stay in COVID-19 patients with CRS (n= 72) was similar to the controls(n=1100) [Median: 18 (IQR: 15 – 23) versus 18 (12 – 21.5) days, respectively] (13).
Only 5.4% (95%CI: 2.5-11.6) of CRS patients with COVID-19 needed mechanical ventilation (Table 2). However, comparing mechanical ventilation rates against controls did not reveal any significant differences (OR: 1.1; 95%CI: 0.47-2.57; p = 0.8) (Figure 3).
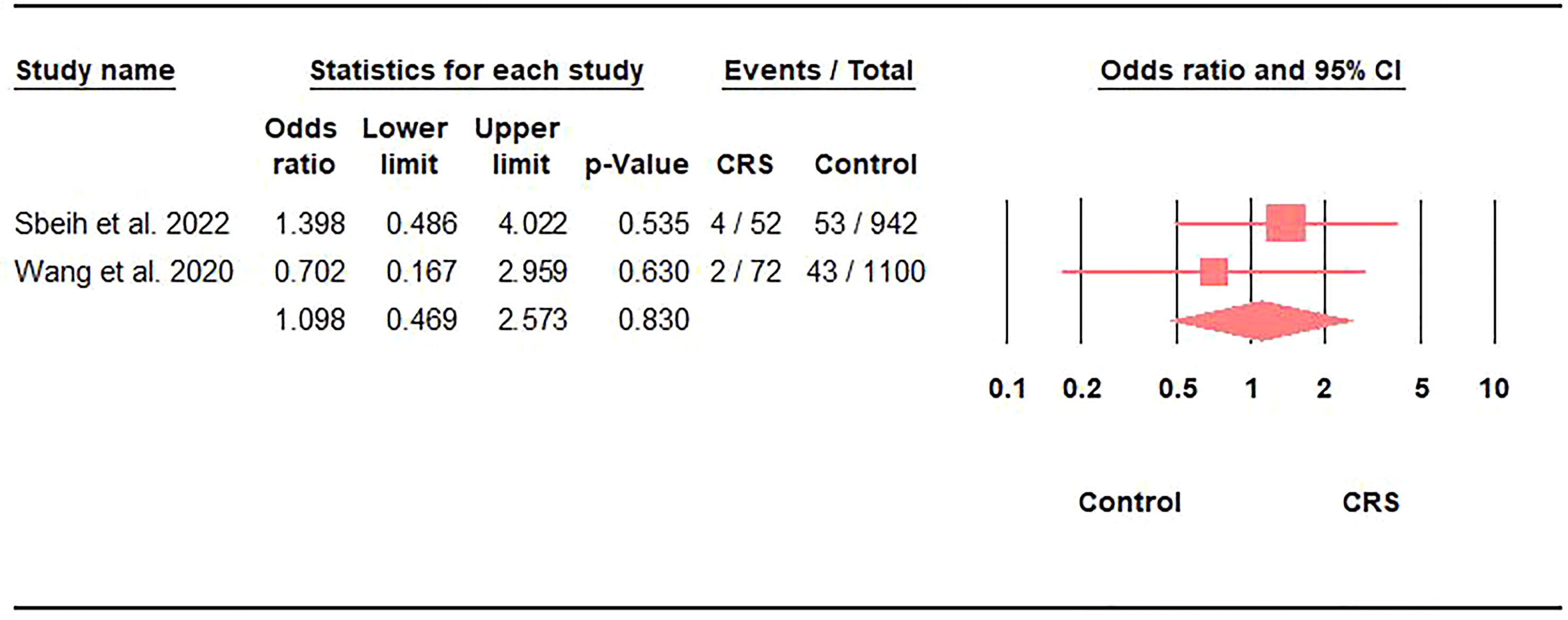
Figure 3 Association between the prevalence of mechanical ventilation in COVID-19 and CRS represented by the odds ratio and 95% confidence interval.
The prevalence of CRS with COVID-19 infection admitted to the ICU was 6.7% (95%CI: 2.8-15) (Table 2).
COVID-19 mortality in CRS patients (n=52) was lower, but not statistically significant than controls (n=942) (2.9% vs. 4.6%, P-value > 0.05) (17).
The prevalence of steroid/immunosuppressive therapy administration in CRS patients with COVID-19 was 20.8% (95%CI: 17.5-24.5) (Figure 4A). Moreover, using steroid/immunosuppressive therapy did not significantly increase the risk of acquiring COVID-19 in CRS patients compared to the control group (OR: 3.31; 95%CI: 0.72-15.26; p = 0.12) (Figure 4B). Finally, CRS patients treated with inhaled corticosteroids had a greater risk of developing severe COVID-19 outcomes than non-CRS patients (25.7% versus 13.3%; adjusted OR: 2.24; 95%CI: 1.16-4.20) (18).
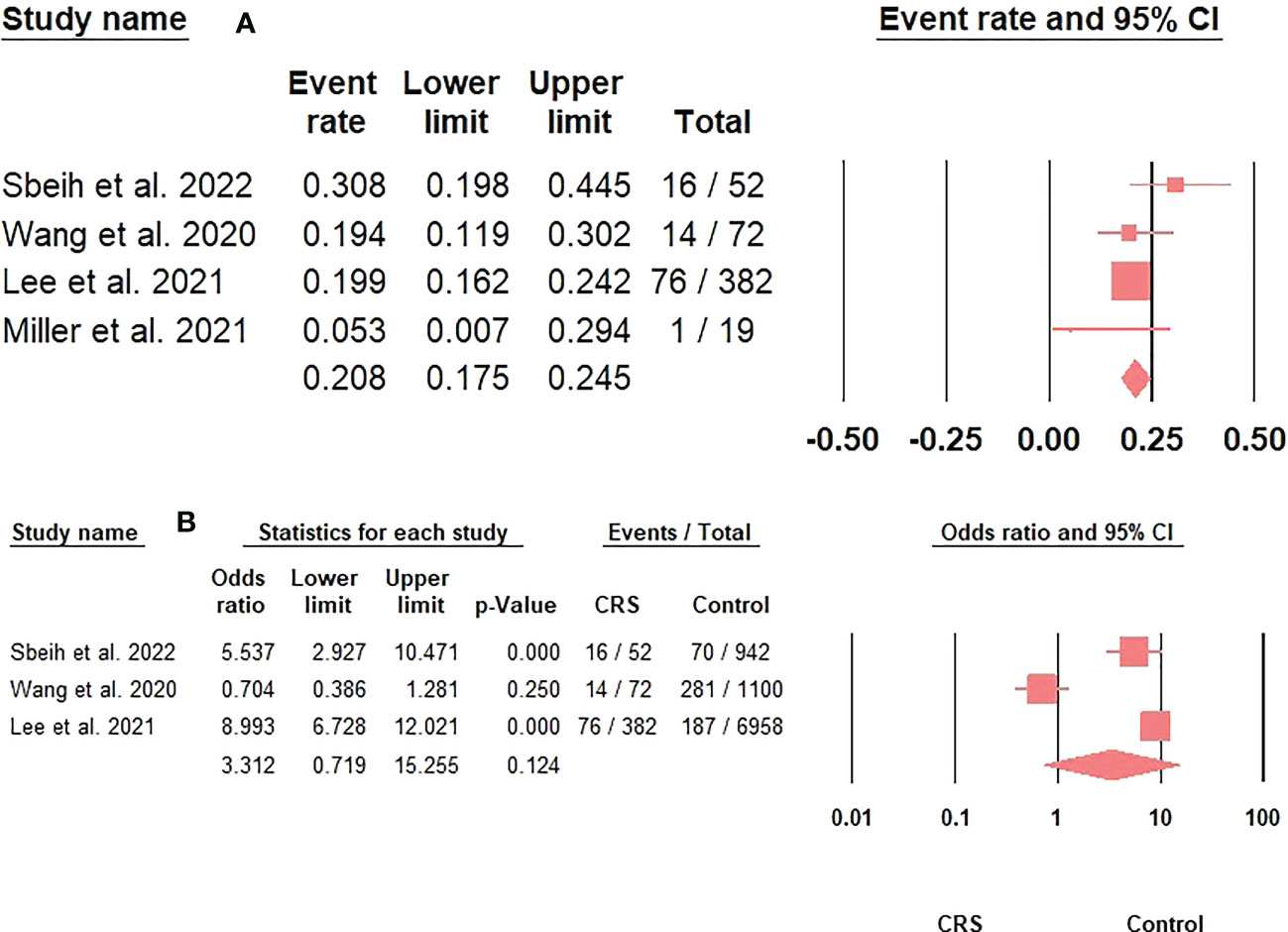
Figure 4 (A). The prevalence of steroids/immunosuppressive therapy use in CRS patients with COVID-19 represented by the event rate and 95% confidence interval. Figure 4 (B). The association between steroids/immunosuppressive therapy use in CRS patients and acquiring COVID-19 represented by the odds ratio and 95% confidence interval.
We found that male sex, cardiovascular morbidity, renal diseases, and hypertension were inversely associated with COVID-19 infection (p < 0.01). However, nasal polyps, cerebrovascular disorders, DM, and steroids/immunosuppressive therapy use were comparable between COVID-19 positive and negative patients (p > 0.05) (Table 3).
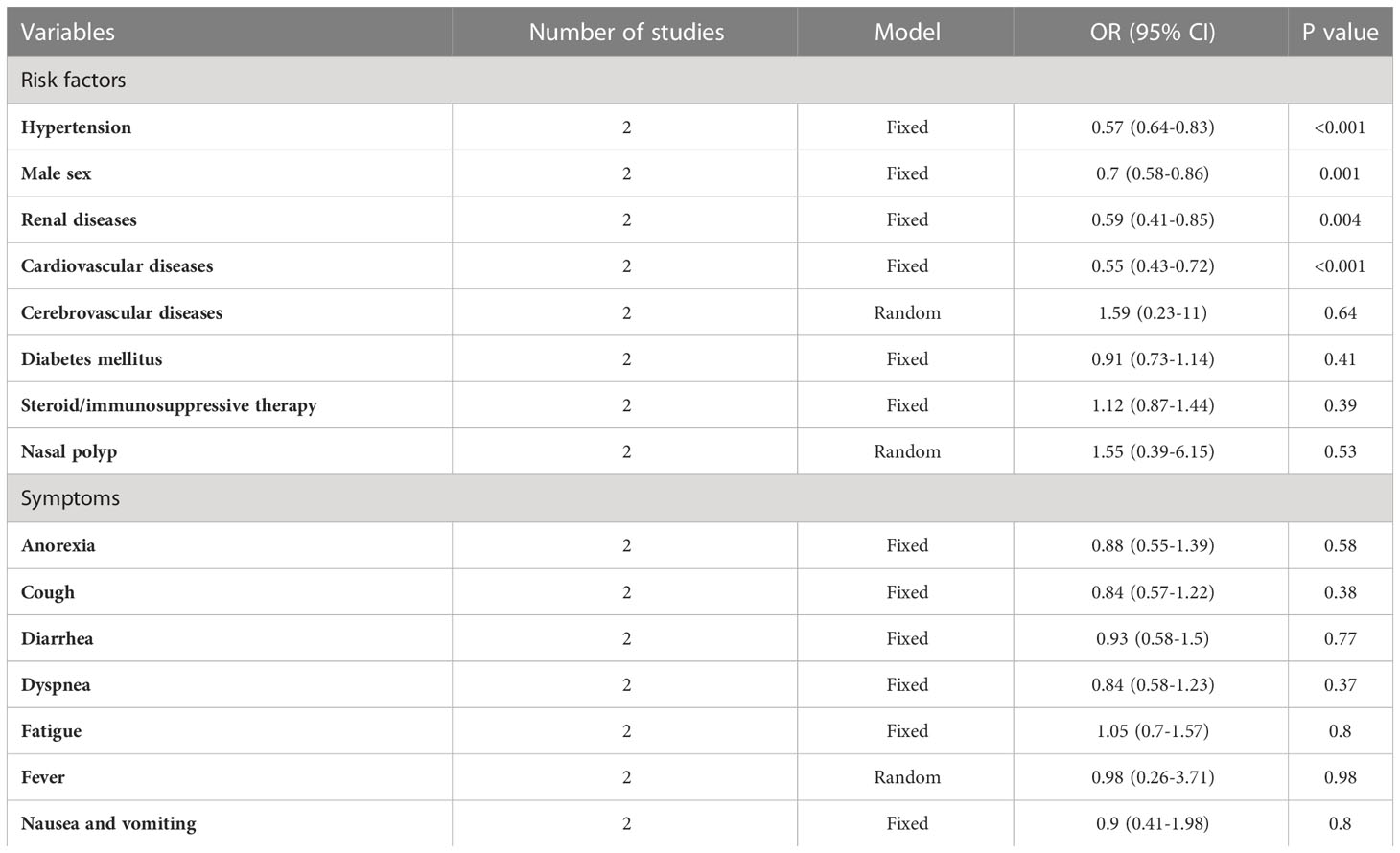
Table 3 Risk factors and symptoms of COVID-19 with chronic rhinosinusitis compared to the control group.
We found that fever, cough, and anosmia were the common COVID-19 symptoms in CRS COVID-19 patients 67%, 55%, and 49.5% (Table 2). On further comparison of COVID-19 symptoms between COVID-19 patients in the CRS and control population, we did not find any significant differences between the two groups regarding fever, fatigue, anorexia, cough, diarrhea, dyspnea, and nausea and vomiting (p > 0.05) (Table 3).
The main aim of this study was to investigate the impact of CRS on susceptibility to COVID-19 and related severity. Our findings indicate that the rates of COVID-19 were comparable in the CRS and non-CRS groups, indicating that CRS does not impact the susceptibility to SARS-CoV-2 infection. Moreover, we found that the prevalence of COVID-19 cases among patients with CRS was 6.5%. This rate is not considered high, especially during times of the pandemic, which might furtherly indicates the neutral impact of CRS on the development of COVID-19. However, it is hard to compare prevalence rates because of different inclusion criteria and trends of COVID-19 per country.
CRS is characterized by type 2 inflammation and is associated with increased levels of interleukin (IL)-4, IL-5, and IL-13 cytokines, together with marked eosinophilia (22). It is difficult to compare our findings to the existing literature since this is the first study to provide cumulative evidence about the impact of CRS on COVID-19. However, asthma is frequently found in CRS patients and is usually associated with type 2 inflammation (23). Previous studies investigated the impact of asthma on developing COVID-19 and compared affected patients’ outcomes between patients with and without asthma. For instance, a previous meta-analysis by Sunjaya et al. (24) reported that the risk of SARS-CoV-2 infection was significantly reduced among patients with asthma, with an estimated pooled prevalence of COVID-19 of 8.08% among these patients, which is slightly higher than ours. A more updated meta-analysis furtherly demonstrated that the risk of infection was significantly lower among patients with asthma than others (25). Accordingly, it can be concluded that CRS, similar to asthma, is not associated with higher rates of SARS-CoV-2 infections.
Regarding COVID-19 severe outcomes, CRS was significantly associated with increased hospitalization rates. On the other hand, mechanical ventilation and mortality rates, and length of hospital stay were comparable between the two groups. Similarly, previous meta-analyses also demonstrated the neutral effects that asthma plays among patients with COVID-19. These studies showed that the hospitalization, MV, ICU admissions, and mortality rates were similar between COVID-19 patients with and without asthma (24, 25). Studies that reported the protective effects of these respiratory diseases proposed some factors for this phenomenon. For instance, it has been suggested that the protective effect of CRS might be related to the expression of the angiotensin-converting enzyme (ACE)-2 receptor in CRS patients. This has been indicated in the analysis by Wang et al. (26), which demonstrated that ACE-2 receptor expression is significantly reduced among patients with eosinophilic CRS with nasal polyps (ECRSwNP). Therefore, although increased expression of ACE-2 receptors was not noticed in the non-eosinophilic CRSwNP type, this evidence indicates that some CRS patients might have a protective effect against COVID-19. This can partially explain the current results. However, a definite explanation could not be reached because we could not conduct a proper analysis that might explain the impact of different CRS subtypes. Moreover, even if we assumed that most patients included in the current analysis have ECRS [which usually presents with more severity and worsened outcomes (27–31)], our results would still show that patients with CRS have comparable outcomes to those without it, excluding the negative impact of CRS on COVID-19 outcomes.
Another important factor to consider is the impact of steroid administration on COVID-19 outcomes among CRS patients. Our findings indicate that the administration of steroid/immunosuppressive therapy did not significantly impact the risk of acquiring COVID-19 in CRS patients. Many concerns have arisen among these patients because of their prolonged administration of steroids, as reports showed that it might impact their immune functions and induce the severity of COVID-19 (32, 33). On the other hand, other investigations demonstrated that administering corticosteroids in patients with respiratory allergic diseases associated with type 2 inflammation is associated with the downregulation of nasal cavity ACE-2 receptors, leading to a reduced risk of SARS-CoV-2 infection (32, 34, 35). This might explain why COVID-19 cases are not high and do not suffer from more severe outcomes in the CRS compared to the control group. Similar conclusions were also reported in studies investigating the impact of asthma (34). Therefore, the administration of steroid therapy in CRS patients should not be avoided, especially to reduce the risk of sneezing and spreading the infection (36). However, it should be noted that these findings were more remarkable in patients with eosinophilia. Moreover, one included study reported that the administration of steroid/immunosuppressive therapy was associated with more severe COVID-19 in CRS patients. However, the authors demonstrated that this finding is not clinically significant and needs further confirmation (18). Although biologics might be used in CRS patients with favorable outcomes, evidence regarding its impact on SARS-CoV-2 infection and severity of outcomes in these patients is limited and needs further investigations (37). However, studies recruiting patients with atopic dermatitis showed that the drug has no effect on acquiring COVID-19 and does not induce severe outcomes (38, 39). However, using these modalities is still controversial and needs further validation (40).
A third factor to be considered is the presence of comorbidities and the administration of treatment regimens that can be protective against COVID-19. However, we found that male sex, cardiovascular morbidity, renal diseases, and hypertension were inversely associated with COVID-19, which contradicts the findings reported by the previous COVID-19 investigation (41–43). The exact reason for this contradiction is unknown. However, it might be due to the potential differences in baseline characteristics of included patients and different management approaches that might reduce the impact and intensity of these comorbidities. The current analysis could not adjust confounders due to data shortage and improper study designs. Accordingly, further future investigations with proper designs and better adjustment of baseline characteristics of their populations are urgently needed.
There are some limitations to be considered in the current analysis. First, many of the included studies did not have proper designs and sample sizes, which might not be sufficient to conduct an ideal analysis that identifies the association between CRS and COVID-19 without the impact of certain confounders, like demographics, treatment regimens, and associated morbidities. Randomized controlled trials are encouraged in this context. Moreover, limitations within included studies should also be considered. For instance, Wang et al. (13) reported that the risk of developing severe COVID-19 was not associated with CRS. However, they also mentioned that deceased patients with CRS were excluded from the study. These could have been patients with severe COVID-19. Therefore, their data might not have been adequately representative.
The impact of CRS on COVID-19 seems neutral. Neither CRS nor related steroid/immunosuppressive therapy increases the risk of acquiring COVID-19. Besides, although CRS increases the risk of hospitalization in COVID-19 patients, it does not induce severe outcomes, including length of hospital stay, ICU admissions, mechanical ventilation, and mortality.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
AR was responsible for the idea and the study design. All authors shared in the data extraction. AR analyzed the data and interpreted it. All authors shared in the writing of the full text and approval of final version before submission. All steps of this study were supervised by AE-Q. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1139031/full#supplementary-material
1. Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol (2011) 128(4):693–707. doi: 10.1016/j.jaci.2011.08.004
2. Beule AG. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr Top otorhinolaryngol Head Neck Surg. (2010) 9:Doc07. doi: 10.3205/cto000071
3. Hakansson AP, Orihuela CJ, Bogaert D. Bacterial-host interactions: Physiology and pathophysiology of respiratory infection. Physiol Rev (2018) 98(2):781–811. doi: 10.1152/physrev.00040.2016
4. Wu Y-C, Chen C-S, Chan Y-J. The outbreak of COVID-19: An overview. J Chin Med Assoc (2020) 83(3):217–20. doi: 10.1097/jcma.0000000000000270
5. Cho SH, Hamilos DL, Han DH, Laidlaw TM. Phenotypes of chronic rhinosinusitis. J Allergy Clin Immunol In Pract (2020) 8(5):1505–11. doi: 10.1016/j.jaip.2019.12.021
6. Keswani A, Dunn NM, Manzur A, Kashani S, Bossuyt X, Grammer LC, et al. The clinical significance of specific antibody deficiency (SAD) severity in chronic rhinosinusitis (CRS). J Allergy Clin Immunol In Pract. (2017) 5(4):1105–11. doi: 10.1016/j.jaip.2016.11.033
7. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature (2020) 579(7798):270–3. doi: 10.1038/s41586-020-2012-7
8. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol (2020) 5(4):536–44. doi: 10.1038/s41564-020-0695-z
9. Yang JM, Koh HY, Moon SY, Yoo IK, Ha EK, You S, et al. Allergic disorders and susceptibility to and severity of COVID-19: A nationwide cohort study. J Allergy Clin Immunol (2020) 146(4):790–8. doi: 10.1016/j.jaci.2020.08.008
10. Guan WJ, Liang WH, Shi Y, Gan LX, Wang HB, He JX, et al. Chronic respiratory diseases and the outcomes of COVID-19: A nationwide retrospective cohort study of 39,420 cases. J Allergy Clin Immunol In Pract. (2021) 9(7):2645–55.e14. doi: 10.1016/j.jaip.2021.02.041
11. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur Respir J (2020) 55(5):2000547. doi: 10.1183/13993003.00547-2020
12. Marin C, Hummel T, Liu Z, Mullol J. Chronic rhinosinusitis and COVID-19. J Allergy Clin Immunol In Pract (2022) 10(6):1423–32. doi: 10.1016/j.jaip.2022.03.003
13. Wang H, Song J, Pan L, Yao Y, Deng YK, Wang ZC, et al. The characterization of chronic rhinosinusitis in hospitalized patients with COVID-19. J Allergy Clin Immunol In Pract (2020) 8(10):3597–3599.e2. doi: 10.1016/j.jaip.2020.09.013
14. Wang M, Wang C, Zhang L. Inflammatory endotypes of CRSwNP and responses to COVID-19. Curr Opin Allergy Clin Immunol (2021) 21(1):8–15. doi: 10.1097/aci.0000000000000700
15. Workman AD, Bhattacharyya N. Do patients with chronic rhinosinusitis exhibit elevated rates of covid-19 infection? Laryngoscope (2022) 132(2):257–8. doi: 10.1002/lary.29961
16. Akhlaghi A, Darabi A, Mahmoodi M, Movahed A, Kaboodkhani R, Mohammadi Z, et al. The frequency and clinical assessment of COVID-19 in patients with chronic rhinosinusitis. Ear Nose Throat J (2021), 1455613211038070. doi: 10.1177/01455613211038070
17. Sbeih F, Gutierrez J, Saieed G, Chaaban MR. Chronic rhinosinusitis is associated with increased risk of COVID-19 hospitalization. Am J Otolaryngol (2022) 43(4):103469. doi: 10.1016/j.amjoto.2022.103469
18. Lee SW, Kim SY, Moon SY, Yang JM, Ha EK, Jee HM, et al. Estimating COVID-19 infection and severity risks in patients with chronic rhinosinusitis: A Korean nationwide cohort study. J Allergy Clin Immunol In Pract (2021) 9(6):2262–2271.e2. doi: 10.1016/j.jaip.2021.03.044
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clinical Res ed) (2021) 372:n71. doi: 10.1136/bmj.n71
20. Faiq TN, Ghareeb OA. Association of chronic rhinosinusitis with risk of COVID-19 infection. J Res Med Dental Sci. (2022) 10(1):407–10.
21. Miller LE, Bhattacharyya N. Risk of COVID-19 infection among chronic rhinosinusitis patients receiving oral corticosteroids. Otolaryngology–head Neck Surg (2022) 166(1):183–5. doi: 10.1177/01945998211006931
22. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol (2008) 122(5):961–8. doi: 10.1016/j.jaci.2008.07.008
23. Massoth L, Anderson C, McKinney KA. Asthma and chronic rhinosinusitis: Diagnosis and medical management. Med Sci (Basel Switzerland). (2019) 7(4):53. doi: 10.3390/medsci7040053
24. Sunjaya AP, Allida SM, Di Tanna GL, Jenkins CR. Asthma and COVID-19 risk: A systematic review and meta-analysis. Eur Respir J (2022) 59(3):2101209. doi: 10.1183/13993003.01209-2021
25. Sunjaya AP, Allida SM, Di Tanna GL, Jenkins C. Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: Systematic review and meta-analysis. J Asthma (2022) 59(5):866–79. doi: 10.1080/02770903.2021.1888116
26. Wang M, Bu X, Fang G, Luan G, Huang Y, Akdis CA, et al. Distinct expression of SARS-CoV-2 receptor ACE2 correlates with endotypes of chronic rhinosinusitis with nasal polyps. Allergy (2021) 76(3):789–803. doi: 10.1111/all.14665
27. Soler ZM, Sauer DA, Mace J, Smith TL. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngology–head Neck Surg (2009) 141(4):454–61. doi: 10.1016/j.otohns.2009.06.085
28. Ferguson BJ. Categorization of eosinophilic chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg (2004) 12(3):237–42. doi: 10.1097/01.moo.0000124938.46948.c7
29. Kountakis SE, Arango P, Bradley D, Wade ZK, Borish L. Molecular and cellular staging for the severity of chronic rhinosinusitis. Laryngoscope (2004) 114(11):1895–905. doi: 10.1097/01.mlg.0000147917.43615.c0
30. Snidvongs K, Chin D, Sacks R, Earls P, Harvey RJ. Eosinophilic rhinosinusitis is not a disease of ostiomeatal occlusion. Laryngoscope (2013) 123(5):1070–4. doi: 10.1002/lary.23721
31. Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngology–head Neck Surg (2010) 142(1):64–71. doi: 10.1016/j.otohns.2009.10.005
32. Klimek L, Jutel M, Bousquet J, Agache I, Akdis CA, Hox V, et al. Management of patients with chronic rhinosinusitis during the COVID-19 pandemic-an EAACI position paper. Allergy (2021) 76(3):677–88. doi: 10.1111/all.14629
33. Wei CC, Adappa ND, Cohen NA. Use of topical nasal therapies in the management of chronic rhinosinusitis. Laryngoscope (2013) 123(10):2347–59. doi: 10.1002/lary.24066
34. Ferastraoaru D, Hudes G, Jerschow E, Jariwala S, Karagic M, de Vos G, et al. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clin Immunol In Pract (2021) 9(3):1152–1162.e3. doi: 10.1016/j.jaip.2020.12.045
35. Jackson DJ, Busse WW, Bacharier LB, Kattan M, O'Connor GT, Wood RA, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol (2020) 146(1):203–206.e3. doi: 10.1016/j.jaci.2020.04.009
36. Bousquet J, Akdis CA, Jutel M, Bachert C, Klimek L, Agache I, et al. Intranasal corticosteroids in allergic rhinitis in COVID-19 infected patients: An ARIA-EAACI statement. Allergy (2020) 75(10):2440–4. doi: 10.1111/all.14302
37. Förster-Ruhrmann U, Szczepek AJ, Bachert C, Olze H. COVID-19 in a patient with severe chronic rhinosinusitis with nasal polyps during therapy with dupilumab. J Allergy Clin Immunol (2020) 146(1):218–220.e2. doi: 10.1016/j.jaci.2020.05.005
38. Carugno A, Raponi F, Locatelli AG, Vezzoli P, Gambini DM, Di Mercurio M, et al. No evidence of increased risk for coronavirus disease 2019 (COVID-19) in patients treated with dupilumab for atopic dermatitis in a high-epidemic area - bergamo, Lombardy, Italy. J Eur Acad Dermatol Venereol: JEADV (2020) 34(9):e433–4. doi: 10.1111/jdv.16552
39. Ferrucci S, Romagnuolo M, Angileri L, Berti E, Tavecchio S. Safety of dupilumab in severe atopic dermatitis and infection of covid-19: Two case reports. J Eur Acad Dermatol Venereol: JEADV (2020) 34(7):e303–4. doi: 10.1111/jdv.16527
40. Roland LT, Pinto JM, Naclerio RM. The treatment paradigm of chronic rhinosinusitis with nasal polyps in the COVD-19 era. J Allergy Clin Immunol: In Pract. (2020) 8(8):2492–4. doi: 10.1016/j.jaip.2020.06.029
41. Rashedi J, Mahdavi Poor B, Asgharzadeh V, Pourostadi M, Samadi Kafil H, Vegari A, et al. Risk factors for COVID-19. Le infezioni medicina (2020) 28(4):469–74.
42. Ko JY, Danielson ML, Town M, Derado G, Greenlund KJ, Kirley PD, et al. Risk factors for coronavirus disease 2019 (COVID-19)-Associated hospitalization: COVID-19-Associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect diseases: an Off Publ Infect Dis Soc America (2021) 72(11):e695–703. doi: 10.1093/cid/ciaa1419
Keywords: chronic rhinosinusitis, steroids, COVID-19, coronavirus, prevalence, epidemiology
Citation: Reda A, Lashin BI, Alaaraj MM, Abouelkheir M, Ahmed MI, Shah J and El-Qushayri AE (2023) The impact of chronic rhinosinusitis on COVID-19 risk and outcomes: A systematic review and meta-analysis. Front. Immunol. 14:1139031. doi: 10.3389/fimmu.2023.1139031
Received: 06 January 2023; Accepted: 20 March 2023;
Published: 29 March 2023.
Edited by:
Pei-Hui Wang, Shandong University, ChinaReviewed by:
Egidia Miftode, Grigore T. Popa University of Medicine and Pharmacy, RomaniaCopyright © 2023 Reda, Lashin, Alaaraj, Abouelkheir, Ahmed, Shah and El-Qushayri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaffer Shah, SmFmZmVyLnNoYWhAa2F0ZWIuZWR1LmFm; Abdullah Reda, QWJkdWxsYWhyZWRhNzdAYXpoYXIuZWR1LmVn
†ORCID: Abdullah Reda, orcid.org/0000-0002-2180-0029
Basant Ismail Lashin, orcid.org/0000-0002-8979-7524
Amr Ehab El-Qushayri, orcid.org/0000-0002-0967-797X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.