
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 07 June 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1136955
This article is part of the Research Topic Epidemiology, Diagnosis, Prognosis and Treatment of Rare Immune-mediated Diseases of the Central Nervous System View all 18 articles
 Wanwan Zhang1†
Wanwan Zhang1† Yinyin Xie1†
Yinyin Xie1† Yali Wang2†
Yali Wang2† Fengxia Liu1
Fengxia Liu1 Li Wang2
Li Wang2 Yajun Lian1
Yajun Lian1 Hongbo Liu1
Hongbo Liu1 Cui Wang3
Cui Wang3 Nanchang Xie1*
Nanchang Xie1*Background: Autoimmune glial fibrillary acidic protein astrocytopathy (GFAP-A) is a recently discovered inflammatory central nervous system (CNS) disease, whose clinical characteristics and prognostic factors for short-term outcomes have not been defined yet. We aimed to assess the symptoms, laboratory tests, imaging findings, treatment, and short-term prognosis of GFAP-A.
Methods: A double-center retrospective cohort study was performed between May 2018 and July 2022. The clinical characteristics and prognostic factors for short-term outcomes were determined.
Results: We enrolled 33 patients with a median age of 28 years (range: 2–68 years), 15 of whom were children (<18 years). The clinical spectrum is dominated by meningoencephalomyelitis. Besides, we also found nausea, vomiting, poor appetite, and neuropathic pain in some GFAP-A patients, which were not mentioned in previous reports. And adults were more prone to limb numbness than children. Magnetic resonance imaging revealed lesions involving the brain parenchyma, meninges, and spinal cord, exhibiting patchy, linear, punctate, and strip T2 hyperintensities. First-line immunotherapy, including corticosteroid and gamma globulin, was effective in most patients in the acute phase (P = 0.02). However, patients with overlapping AQP4 antibodies did not respond well to first-line immunotherapy and coexisting neural autoantibodies were more common in women. Additionally, the short-term prognosis was significantly better in children than in adults (P = 0.04). Positive non-neural autoantibodies and proven viral infection were independent factors associated with poor outcomes (P = 0.03, 0.02, respectively).
Conclusion: We expanded the spectrum of clinical symptoms of autoimmune GFAP-A. The clinical symptoms and short-term prognosis differed between children and adults. Positive non-neural autoantibodies and proven viral infection at admission suggest a poor short-term prognosis.
Glial fibrillary acidic protein (GFAP) is an intermediate filament protein that is mainly found in the astrocytic cytoplasm, and is involved in numerous astrocyte functions. The size of GFAP lies between that of microfilaments and microtubules (1). Tissue-based assays and cell-based assays (CBA) can be used to identify the immunoglobulin G (IgG) reactive with GFAP in the cerebrospinal fluid (CSF) or serum of patients with autoimmune GFAP astrocytopathy (GFAP-A), which is a novel inflammatory central nervous system (CNS) disease reported in 2016 (2, 3). Patients usually present with meningitis (headache and neck stiffness), encephalitis (psychiatric symptoms, seizures, tremor, or delirium), myelitis (weakness and sensory symptoms), optic neuritis (blurred vision), or a combination of the above (4). The characteristic imaging feature is perivascular radial enhancement perpendicular to the ventricles, which resolves with immunotherapy (5). Coexisting neural autoantibodies are common in autoimmune GFAP-A, which makes diagnosis difficult (6). Most patients respond well to first-line immunotherapy, including corticosteroids, intravenous immunoglobulin, and plasma exchange, alone or in combination, but some are prone to relapse or death (7).
Since autoimmune GFAP-A is a recent discovery, the complete range of clinical and imaging phenotypes is still unknown. Although several GFAP-A case series have been reported, to our knowledge, no study has identified the prognostic factors for short-term outcomes in GFAP-A. Therefore, we included 33 GFAP-A patients from two hospitals in China, and retrospectively analyzed the clinical manifestations, magnetic resonance imaging (MRI) findings, laboratory examination results, treatment, and short-term prognosis. This study aims to provide new insights and improve the clinicians’ understanding of autoimmune GFAP-A.
In this double-center retrospective observational cohort study, we enrolled patients who presented with meningitis, encephalitis, and myelitis, and tested positive for GFAP antibodies in the CSF between May 1, 2018 and April 1, 2022 at the First Affiliated Hospital of Zhengzhou University and Henan Children’s Hospital. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (number 2022-KY-0053) and all patients provided their informed consent.
Demographic data, clinical manifestations, CSF examination, serological tests, imaging findings, intensive care unit admission, mechanical ventilation, treatment, and outcomes were recorded. CSF examination included white cell counts, protein level, glucose level, virus antibodies detection, and oligoclonal antibodies (IgG), which were assessed in all patients with GFAP-A. Neutrophilic granulocyte, monocyte, lymphocyte, blood sodium, non-neural autoantibodies, tumor markers, and virus antibodies detection were comprised in the serological tests. Tumor markers comprised ferritin, neuron-specific enolase, alpha-fetoprotein, carcino-embryonic antigen, tumor associated antigen 125, 19-9, 15-3, and 72-4, and non-small cell lung cancer antigen 21-1. Non-neural autoantibodies contained antinuclear, anti-endothelial cell, anti-cardiolipin, anti-neutrophil cytoplasmic, anti-double-stranded DNA, anti-RA33, rheumatoid factor, anti-PM-Scl antibody, anti-SSA, and anti-Ro52 antibodies. Virus antibodies detection in CSF and serum included Epstein-Barr virus, cytomegalovirus, coxsackie virus, measles virus, herpes simplex virus I and II, human parvovirus B-19, influenza b virus, parainfluenza virus, adenovirus, rubella virus, herpes zoster virus, and echovirus. All patients underwent the above serological tests except for one patient who did not undergo virus screenings in serum. Patients exhibiting symptoms such as fever, cough, fatigue, nausea, or vomiting that cannot be explained by other causes and who were positive for the virus antibodies (immunoglobulin M) in serum, were defined as proven viral infection. If they only showed symptoms associated with viral infection without laboratory evidence, patients were defined as suspected viral infection. First-line immunotherapy included intravenous methylprednisolone (IVMP), intravenous immunoglobulin (IVIG), or plasma exchange (PE). Second-line immunotherapy included rituximab (RIT), tacrolimus (TAC), and mycophenolate mofetil (MMF). The Modified Rankin Scale (mRS) was used to evaluate the neurological status at admission, 4 weeks after the initiation of immunotherapy, and last follow-up. A favorable outcome was defined as an mRS score of <3, while a poor outcome was defined as an mRS score of ≥3. If the patient died, the mRS score was recorded as 6. All patients were followed up by telephone or in the outpatient clinic, and the last date for follow-up was July 1, 2022. We defined patients younger than 18 years as children. Relapse was defined as hospital readmission for meningoencephalomyelitis.
The CSF and serum samples of the patients were simultaneously obtained before treatment and sent to the Neurology Laboratory of the First Affiliated Hospital of Zhengzhou University or Zhengzhou Jinyu Clinical Laboratory Center. Both institutes used fixed CBA for confirmation, with 100% agreement among positive results.
The CBA method used human embryonic kidney 293 cells transfected with plasmids (pc DNA3.1) encoding GFAP homo sapiens transcript variant (NM_002055) (Shanghai Genechem Co.,Ltd) using Lipofectamine 2000. 36 hours after transfection, cells were fixed with 4% paraformaldehyde for 20 min and permeabilized with PBS containing 0.25% Triton-100 for 30 min at room temperature. Cells were incubated for 30 min at room temperature (serum diluted at 1:10, and CSF 1:1). The fluid in the wells was removed and washed 3 times with PBS afterward. AlexaFluor 546 anti-human IgG (1:500; Thermo Scientific) was used as the secondary antibody to label autoantibodies for 1 h at room temperature. Images were obtained using a Zeiss Axiovert A1 fluorescence microscope (Figure 1).
Figure 1 IgG in serum of patient (no.5, see Table 1). Those stained in red alone are GFAP antibodies. Images were obtained using a Zeiss Axiovert A1 fluorescence microscope.
In addition, autoantibodies to aquaporin-4 (AQP-4), myelin oligodendrocyte glycoprotein (MOG), myelin basic protein, N-methyl-D-aspartate receptor (NMDAR), glycine receptor, glutamic acid decarboxylase 65, γ−amino butyric acid type A receptor and B receptor, α-amino-3-hydroxy-5-methyl-4-isoxazol-propionic acid receptors 1 and 2, contactin-associated protein 2, leucine-rich glioma-inactivated protein 1, Purkinje cell type 1 (Yo), antineuronal nuclear antibodies type 1 and 2, were detected with fixed CBA to examine possible coexisting autoimmunity.
Statistical analysis was performed using SPSS IBM 25.0 (SPSS Inc., Chicago, IL, USA). We used the fisher exact test to analyze the difference of short-term prognosis and abnormal spine cord MRIs between children and adults. The rank sum test was used for the effectiveness of first-line immunotherapy. The correlation analysis of the disease severity on admission or short-term prognosis with GFAP antibody titers and the number of symptoms was examined by Spearman’s rank correlation. Univariable binary logistic regression models were used to assess the factors affecting the outcome, and factors associated with a poor outcome (P < 0.1) were included in the multivariate binary logistic regression model. All statistical tests were two-sided, and P < 0.05 was considered statistically significant in the multivariate binary logistic regression analysis.
Overall, we identified 33 patients with positive GFAP antibodies in the CSF from May 2018 to April 2022. Of these, 25 patients were from the First Affiliated Hospital of Zhengzhou University, and 8 pediatric patients were from Henan Children’s Hospital. The demographics and clinical characteristics of the patients with autoimmune GFAP-A are summarized in Table 1.
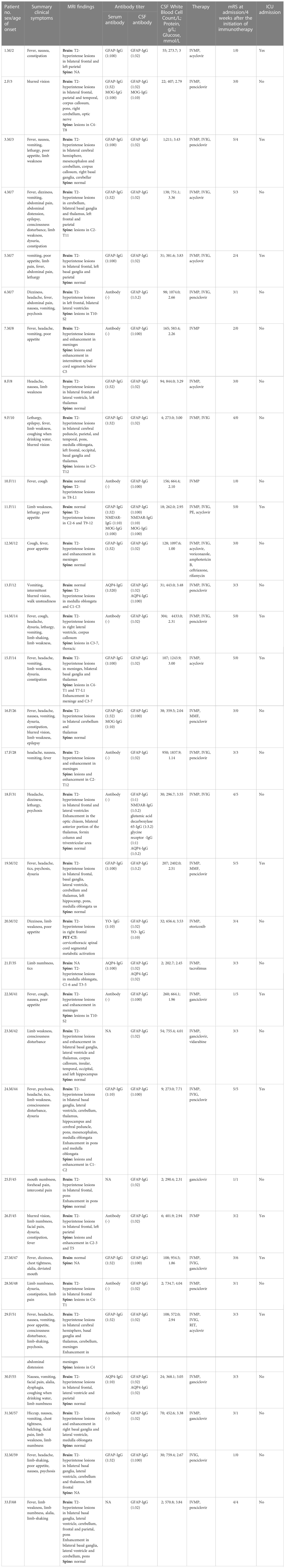
Table 1 Clinical features, auxiliary examinations, treatment strategies, and short-term prognosis in patients positive for GFAP-IgG.
The median age at disease onset was 28 years (range: 2–68 years), and 15 patients were children with a median age of 8 years (range: 2–14 years). There were 17 males and 16 females in the cohort. Of these, 16 cases were positive for GFAP-IgG in both the CSF and serum, while 17 cases were positive for GFAP-IgG only in the CSF. GFAP antibody titers in the serum and CSF were not significantly associated with the disease severity on admission or short-term prognosis (P = 0.17, rs = 0.26; P = 0.60, rs = -0.10; P = 0.20, rs = -0.23; P = 0.15, rs = -0.26; respectively).
A total of 29 patients had three or more symptoms at admission. However, there was no statistical correlation between the number of symptoms and disease severity on admission or short-term prognosis (P = 0.05, rs = 0.34; P = 0.45, rs = 0.14 respectively). Seventeen of the thirty-three patients had prodromal symptoms, including fever (n = 15), vomiting (n = 2). The main clinical symptoms were fever (21 cases); limb weakness (13 cases); vomiting (13 cases); headache (12 cases); nausea (11 cases); disturbance of consciousness (10 cases); dysuria and constipation (9 cases); poor appetite (9 cases); involuntary movements, including tics and limb-shaking (7 cases); psychosis (6 cases); limb numbness (6 cases); neuropathic pain involving the face, limbs, and intercostal region (6 cases). Thirteen patients had hyponatremia (<135 mmol/L); four patients presented with blurred vision; and three patients had focal epilepsy. Ten patients showed neck stiffness on physical examination. Patient #27 died of acute brainstem failure during hospitalization, and patient #29 died of severe pneumonia 3 months after discharge. Figure 2A compared the symptoms in children and adults. And only adult patients presented with limb numbness in our cohort.
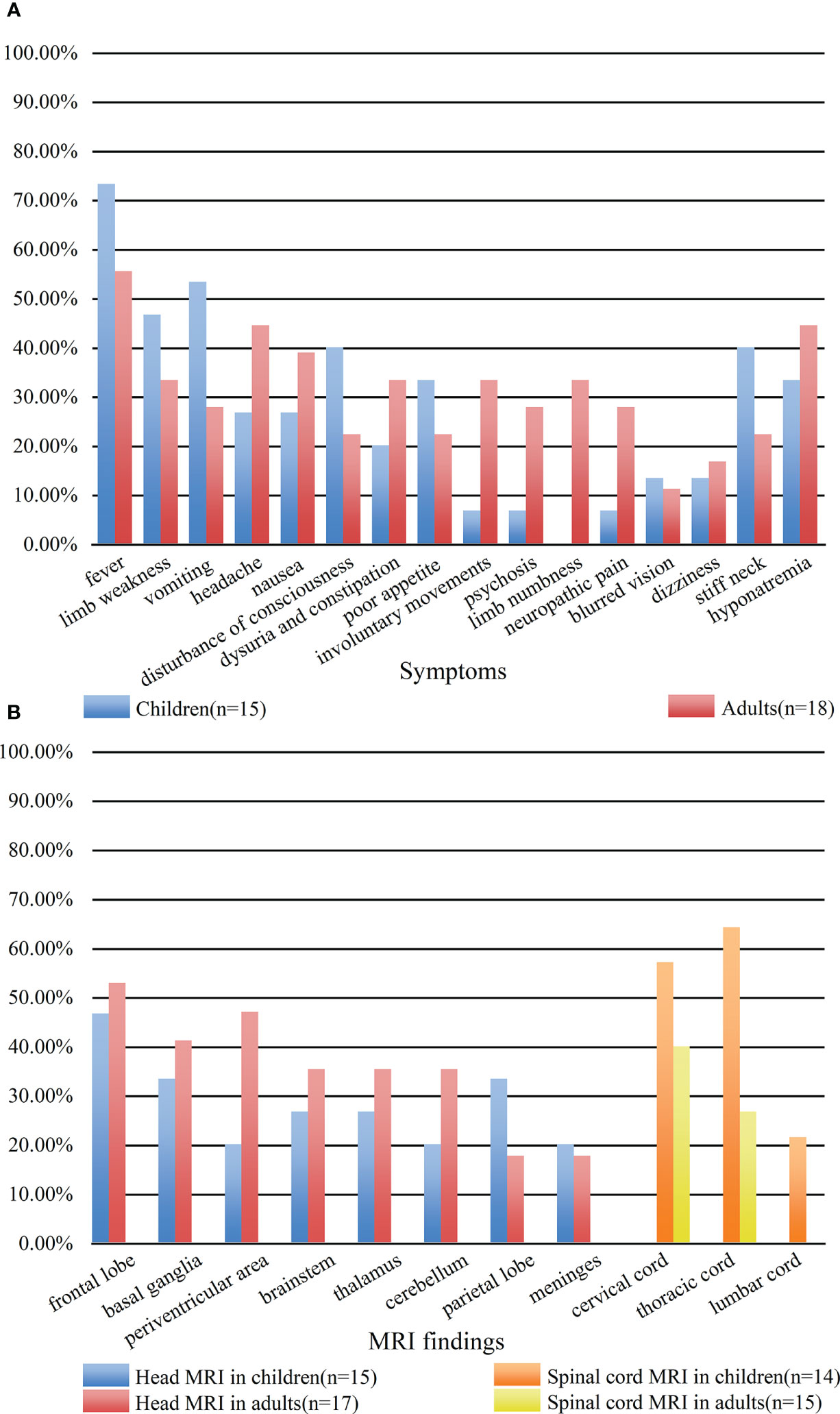
Figure 2 Comparison of clinical symptoms (A) and MRI findings (B) between children and adults. (A) The blue bar represented the clinical symptoms of children. The red bar represented the clinical symptoms of adults. (B) The blue bar represented the head MRI findings of children. The red bar represented the head MRI findings of adults. The orange bar represented the spinal MRI findings of children. The yellow bar represented the spinal MRI findings of adults.
Thirteen patients had abnormal tumor markers in the serum, mainly ferritin (5/13) and neuron-specific enolase (5/13). However, none of the patients were diagnosed with a tumor as of July 2022. Viral antibodies (immunoglobulin M) were detected in the serum of 19 patients, which primarily comprised antibodies to Epstein-Barr virus (12/19), cytomegalovirus (11/19), and coxsackie virus (7/19). Ten patients had non-neural autoantibodies in the serum, including anti-SSA antibody (6/10), antinuclear antibody (5/10), rheumatoid factor (1/10), and anti-PM-Scl antibody (1/10) (Table 2).
CSF abnormalities were found in 30 patients. Pleocytosis was found in 27 patients (mainly lymphocytes), with the highest number being 950 × 106/L (reference range, 0–5 × 106/L). There were 19 cases with elevated proteins up to 4433 mg/L (reference range, 150–450 mg/L). Furthermore, 10 patients showed hypoglycorrhachia, with the minimum value being 1 mmol/L (reference range, 2.5–4.5 mmol/L). Viruses were detected in the CSF of five patients, namely Epstein-Barr virus (3/5), enterovirus (1/5), and herpes simplex virus I (1/5). Aspergillus fumigatus was detected in the CSF of patient #12. Oligoclonal antibodies (IgG) were identified in the CSF of four patients (Table 3).
Of the 33 patients, eight patients had one or more overlapping neural antibodies in the CSF or serum, including seven females and one male. AQP-4 was the most common overlapping antibody in the CSF of GFAP-A patients, followed by MOG and NMDAR. Furthermore, all patients with overlapping AQP4 antibodies responded poorly to first-line immunotherapy (IVMP, IVIG) in the acute phase, and two of them presented with longitudinally extensive transverse myelitis. Table 4 listed the clinical manifestation and MRI characteristics of patients with coexisting neural antibodies.
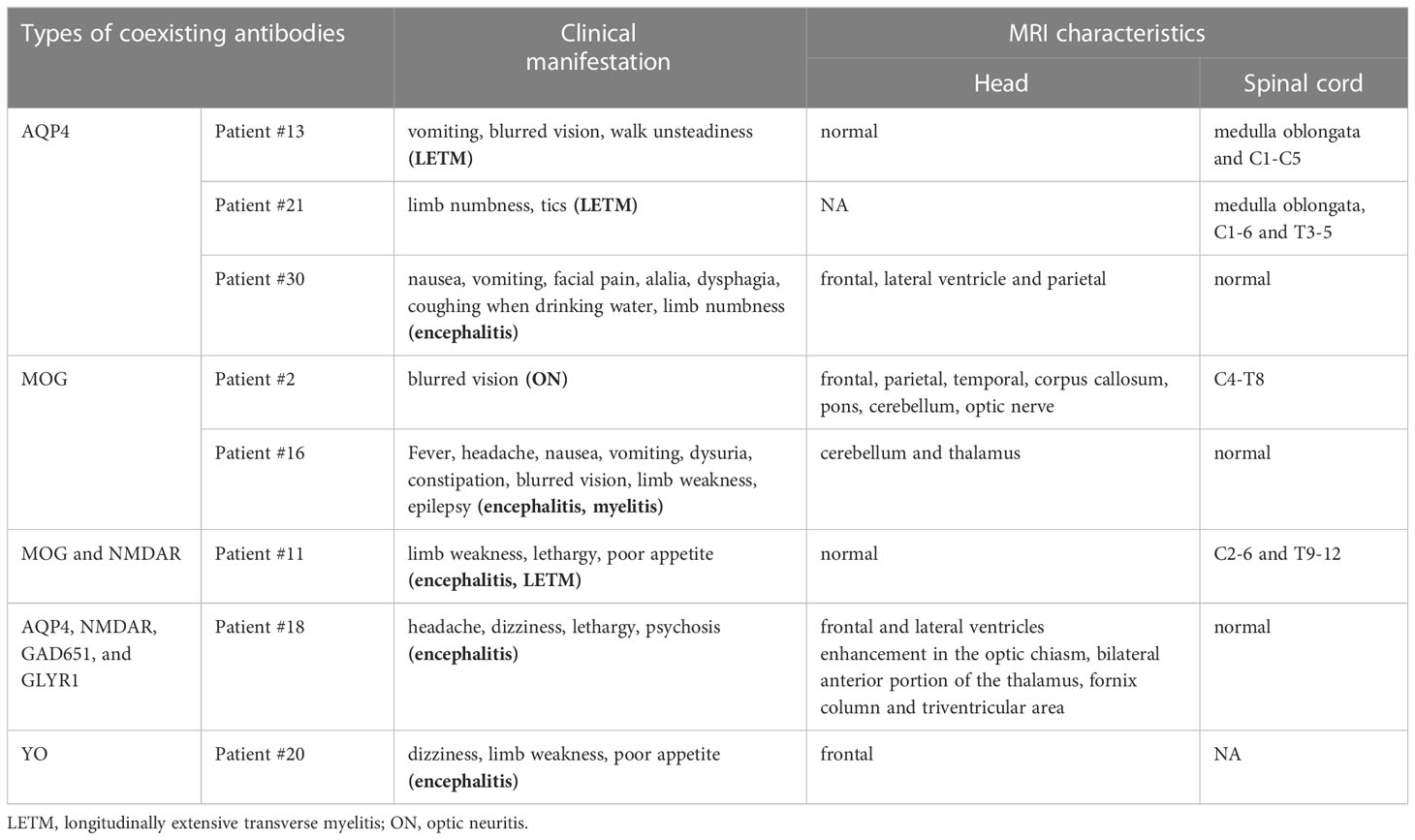
Table 4 Clinical manifestation and MRI characteristics of patients with coexisting neural antibodies.
All 33 cases underwent MRI examinations, including 32 head MRIs and 29 spinal cord MRIs. MRI abnormalities were mainly T2 sequence high signal lesions, which appeared as patchy, linear, punctate, and stripe patterns. Results from head MRIs were varied: four patients had normal imaging, 28 patients demonstrated abnormal T2 hyperintensities, and 12 of 20 patients showed abnormal contrast enhancement. The lesions were mainly located in the frontal lobe (16/32), basal ganglia (12/32), periventricular area (11/32), brainstem (10/32), cerebellum (9/32), thalamus (9/32), parietal lobe (8/32), and meninges (6/32); enhancement patterns were observed in the meninges (n = 6), periventricular area (n = 3), and basal ganglia (n = 3) (Figures 3A, B). Regarding spinal cord MRI, T2 hyperintensities were observed in the cervical cord (14 cases), followed by the thoracic cord (13 cases). Five of nine patients who underwent contrast-enhanced spinal MRI showed abnormal enhancement. The lesion of patient #17 involved up to 18 spinal cord segments (Figures 3C, D). Additionally, there was no statistical difference in abnormal spine cord MRIs between children and adults (P = 0.264). Figure 2B compared the MRI characteristics between adults and children. Besides, the MRI results revealed that the lesions in GFAP-A patients generally reduced in size or disappeared after immunotherapy. Patient #3 presented with multiple lesions in the brain parenchyma on admission MRI (Figure 3E). Re-examination of MRI at 1 and 2 months after treatment with IVMP and IVIG showed that the lesions gradually reduced (Figures 3F, G). Four months after the treatment, his MRI lesions were significantly reduced in size (Figure 3H). Moreover, his symptoms were fully resolved 6 months after the onset.
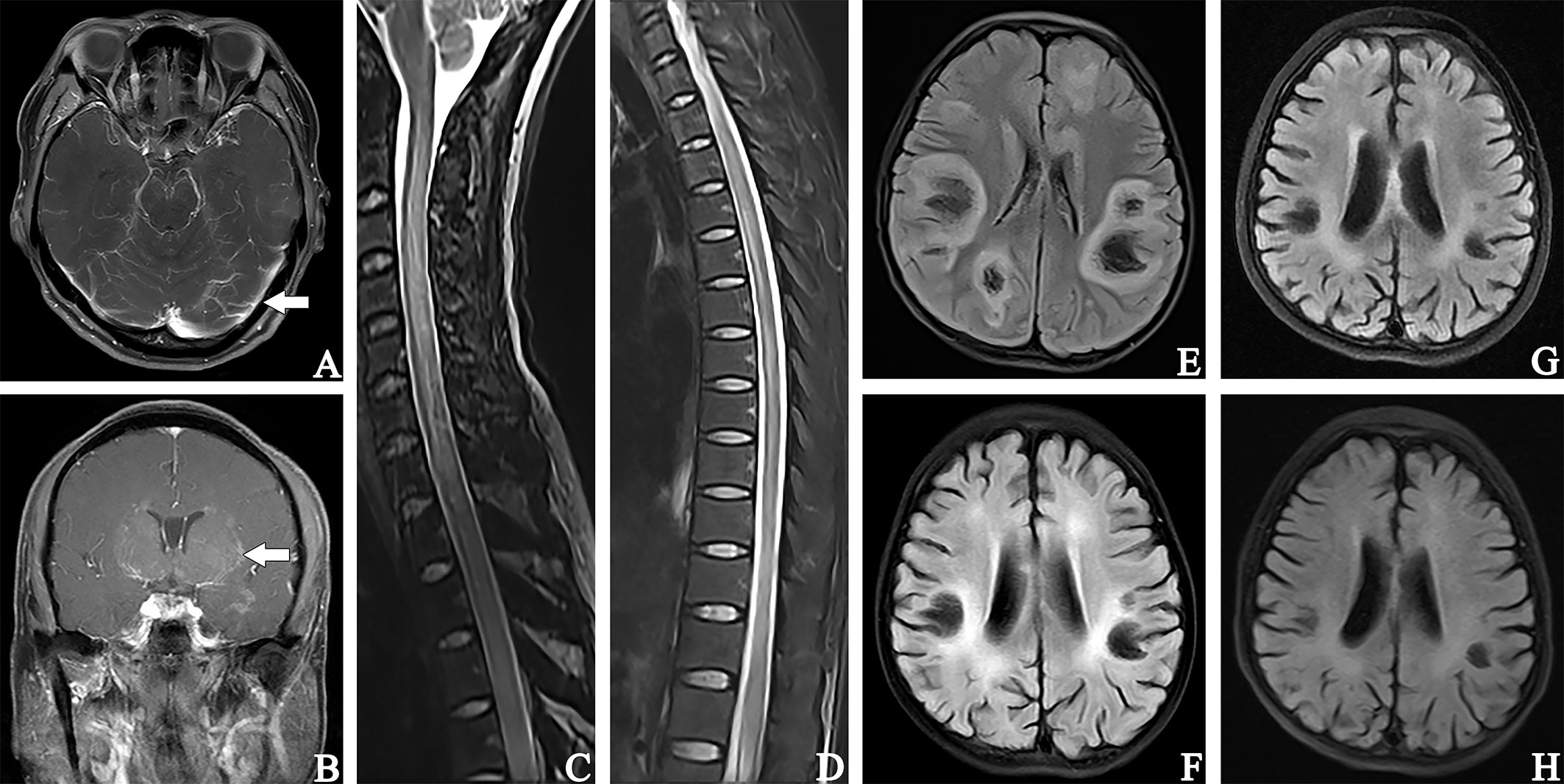
Figure 3 Contrast-enhanced T1-weighted magnetic resonance image (A, B). (A) patient #18, showed soft meningeal linear enhancement (arrow). (B) patient #23, MR image suggested patchy enhancement around the lateral ventricles (arrow). (C, D): patient #18, T2-hyperintense lesions in C2-T12. Fluid attenuated inversion recovery (E–H). MR images of patient #3. (E) MR image showed multiple lesions of bilateral brain parenchyma at admission. (F) (1 month after treatment with IVMP and IVIG) and (G) (2 months after treatment) showed that the extent of lesions gradually decreased and brain atrophy began to appear. (H) 4 months after treatment, a significant reduction in the lesion range and high-signal lesions in the bilateral lateral periventricular. The widening and deepening of the cerebral sulcus and fissure were evident.
Treatment responses and short-term outcomes are summarized in Figure 4. Most patients experienced a significant improvement in their symptoms after first-line immunotherapy (P = 0.02). Among the 33 patients, 17 were treated with IVMP only; 14 underwent IVMP plus IVIG therapy; 1 patient had only IVIG treatment. And 1 patient received PE for poor outcome after receiving IVMP plus IVIG. Nineteen patients underwent oral tapering of steroids in the maintenance period. Three patients responded poorly to treatment and subsequently received immunosuppressive therapy (MMF, RIT, and TAC). Moreover, five patients were mechanically ventilated because of respiratory failure. 19 proven viral infection and 7 suspected viral infection were treated with antiviral drugs in the early stage of admission.
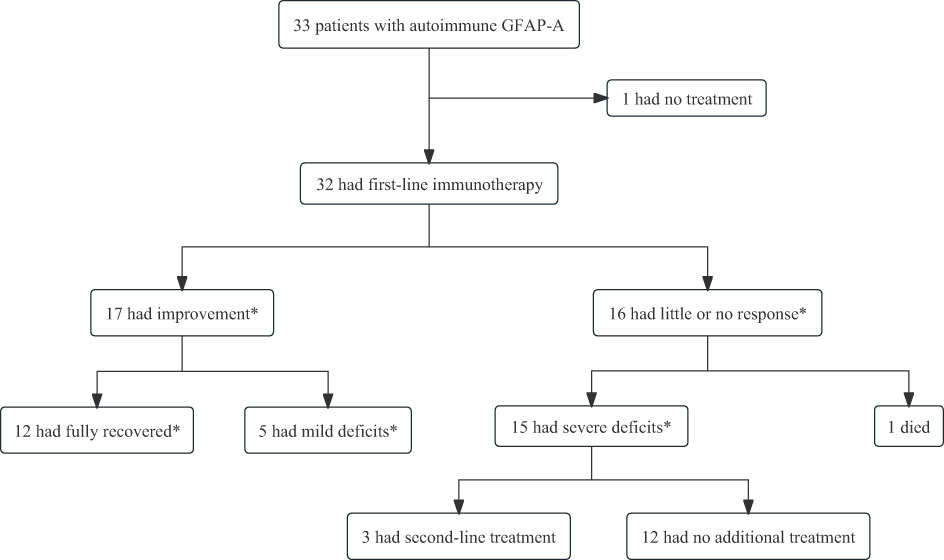
Figure 4 The treatment response and short-term outcomes of patients with autoimmune GFAP astrocytopathy. First-line immunotherapy included intravenous methylprednisolone, intravenous immunoglobulin, and plasma exchange, alone or in combination. Second-line immunotherapy included rituximab, tacrolimus, or mycophenolate mofetil. *The occurrence of improvement or absence of improvement was assessed at 4 weeks after the initiation of immunotherapy. Recovered was defined as mRS=0; mild deficits were defined as mRS= 1,2; and severe deficits were defined as mRS= 3,4.
The median mRS score at admission was 3 and that at 4 weeks after the initiation of immunotherapy was 2. Short-term prognosis was significantly better in children than in adults (P = 0.04). In the univariate binary logistic model, the factors associated with poor outcomes included positive non-neural autoantibodies and proven viral infection (P = 0.03, 0.03, respectively). Multivariate binary logistic regression model identified positive non-neural autoantibodies and proven viral infection as the independent factors associated with a poor outcome (P = 0.03, 0.02, respectively) (Table 5).
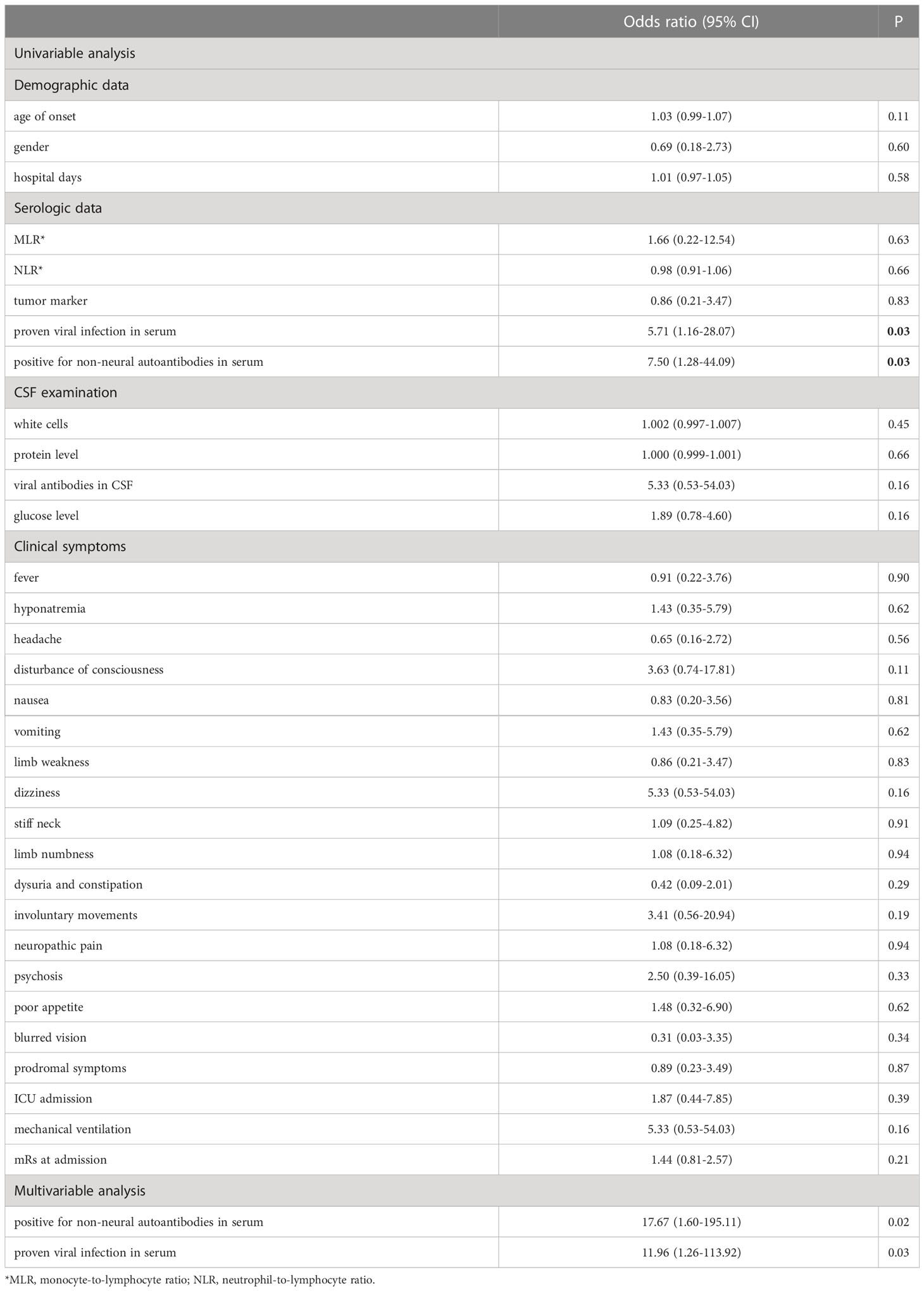
Table 5 Factors associated with poor outcomes at 4 weeks after the initiation of immunotherapy (mRS ≥3).
At the last follow-up, seven patients had poor outcomes and 26 patients had good outcomes, with the median mRS score being 0. The average follow-up duration was 12 months, ranging from 3 to 47 months. 8 of 33 patients were hospitalized more than two times, including two patients who were hospitalized seven times (Patients #13 and #21). In addition, four patients relapsed during oral tapering of steroids: two of them had two relapses; one patient had three relapses; and one patient had five relapses. Three patients experienced worsening or recurrence of previous symptoms, and one patient had new symptoms which were significantly alleviated after immunotherapy.
GFAP-A is a relatively rare autoimmune inflammatory CNS disorder. Despite several studies (2, 3, 7–13), there is limited understanding of its short-term prognosis, which motivated us to conduct this study. Our findings expanded the spectrum of symptoms, which comprise nausea, vomiting, poor appetite, and neuropathic pain. Importantly, patients with positive non-neural autoantibodies and proven viral infections in the serum at admission were found to have a poor short-term prognosis, which was rarely reported before. Additionally, our study results differ from those of previous studies in that adult patients were more likely to have sensory symptoms such as limb numbness and poor short-term prognosis. Hence, the present study provides a relatively comprehensive description of the clinical characteristics and short-term prognosis of GFAP-A.
The median age of disease onset in GFAP-A patients was 28 years (range: 2–68 years) in our study, while it was 40–50 years (range: 11 months to 103 years) in previous studies (8, 10). We think that the difference may be because eight patients in our study were enrolled from children’s hospitals. Our study confirmed that 29 patients with autoimmune GFAP-A had three or more clinical symptoms, which were diverse and non-specific. We also found other clinical presentations, including nausea and vomiting, which were rarely mentioned previously. The underlying mechanisms were heterogeneous, and included area postrema syndrome, hyponatremia, meningitis, and encephalitis. Poor appetite was not a rare symptom in our study, owing to GFAP expression by the enteric glial cells. It is an important component of the enteric nervous system, which regulates enteric neural reflexes and maintains intestinal homeostasis (14, 15). We also found that limb numbness was more likely to occur in adults, which suggests that adults are more prone to sensory disturbance in autoimmune GFAP-A. In contrast to the findings of Zhuang et al. (8), myelitis in children was also relatively common in our cohort, which was supported by no statistical difference in abnormal spinal cord MRIs between children and adults. As already noted by Flanagan et al., most patients had infectious prodromal symptoms, which indicates that autoimmune GFAP-A may be triggered by infection (2, 9). Additionally, hiccups were the main symptom of area postrema syndrome in autoimmune GFAP-A, supported by MRI findings of T2-hyperintense lesions in the dorsal medulla oblongata (16). Interestingly, frequent neuropathic pain was reported in six cases, and patient #13 presented with left-sided peripheral facial nerve palsy at the first relapse. It is worth noting that GFAP is also expressed by Schwann cells and satellite glial cells of peripheral nerves (17).
Thirteen patients with GFAP-A had serum tumor markers detected in our study. Therefore, clinicians should give high priority to tumor screening in autoimmune GFAP-A patients, especially within 2 years of the onset (4). Additionally, we found that viral antibodies are frequently detected in the serum of autoimmune GFAP-A patients. More importantly, proven viral infection in the serum on admission suggests a poor short-term prognosis. Of note, viral infections are related to CNS autoimmune disorders (18). Although a close relationship between GFAP-A and viral infection has been previously demonstrated, all previous studies detected the virus in the CSF (11, 19–21). We speculate that serum viral antibodies enter the CNS through the blood-brain barrier and then participate in the pathophysiological process of autoimmune GFAP-A. The underlying mechanism still needs to be confirmed by further animal experiments. The other factor associated with poor short-term prognosis was overlapping non-neural autoantibodies, which were also encountered in 75% of the first Chinese cohort (11). Even though these antibodies are not specific per se, it suggests that clinicians should be alert for co-morbid autoimmune diseases other than CNS involvement in patients with GFAP-A, which may exacerbate the patient’s condition. Iorio et al. (13) also reported that GFAP-A in combination with other autoimmune diseases was common. In our study, we noted that eight patients had coexisting neural autoantibodies, with the most common being AQP-4 antibodies, which tend to occur in women. Similar to the findings of Xiao et al. (10), patients with overlapping AQP-4 antibodies responded poorly to immunotherapy in the acute phase, but the exact mechanism is unclear.
Our results provide statistical evidence that first-line immunotherapy is effective in most patients with autoimmune GFAP-A in the acute phase, which is more convincing than the results of previous observational studies. As recently described (9), relapse may occur during oral tapering of steroids in most patients. The recurrence rate was 12.1% in our cohort, which was lower than that reported in previous studies (18%) (7, 12). Relapses usually involved the worsening of previous symptoms, although a few patients developed new symptoms. In contrast to the findings of Xiao et al. (10), we noticed that one patient treated with TAC still had frequent relapses during follow-up, questioning the effectiveness of TAC for preventing a relapse. Intriguingly, we found children had a better short-term prognosis, which was reported to be poor previously.
Our study findings provide novel insights into the clinical characteristics and short-term prognosis of GFAP-A patients. However, there are some limitations of our study. Firstly, this was not a randomized and prospective study, but a precursor to future trials to explore the prognostic factors. Secondly, we only evaluated 33 patients including 15 children, which is a small sample size and the presence of population heterogeneity. In the future, more studies are needed in a larger population. Lastly, patients with Alzheimer’s disease and cancer have been shown to have serum GFAP antibodies (22). Therefore, the specificity of serum GFAP antibodies remains uncertain. We only included patients with positive CSF GFAP antibodies, which may have excluded some patients.
In conclusion, our study not only expands the known spectrum of clinical characteristics of GFAP-A, but also statistically confirms the effectiveness of first-line immunotherapy in the acute phase. Furthermore, we identified the prognostic factors associated with the short-term outcomes of GFAP-A as well as significant differences between children and adults with GFAP-A.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
NX and WZ contributed to conception and design of the study. YX and YW organized the database. FL performed the statistical analysis. WZ wrote the first draft of the manuscript. LW, YL, HL, and CW wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (No.81971214) and the Outstanding Young Talent Cultivation Project of Henan Science and Technology Innovation Talents (YXKC2022037).
The authors are grateful to the patients and their families for the support and cooperation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GFAP-A, glial fibrillary acidic protein astrocytopathy; CBA, cell-based assay; CNS, central nervous system; MRI, Magnetic resonance imaging; GFAP, glial fibrillary acidic protein; IgG, immunoglobulin G; CSF, cerebrospinal fluid; IVMP, intravenous methylprednisolone; IVIG, intravenous immunoglobulin; PE, plasma exchange; RIT, rituximab; TAC, tacrolimus; MMF, mycophenolate mofetil; mRS, Modified Rankin Scale; NMDAR, N-methyl-D-aspartate receptor; AQP-4, aquaporin-4; MOG, myelin oligodendrocyte glycoprotein; Yo, Purkinje cell type 1.
1. Li D, Liu X, Liu T, Liu H, Tong L, Jia S, et al. Neurochemical regulation of the expression and function of glial fibrillary acidic protein in astrocytes. Glia (2020) 68(5):878–97. doi: 10.1002/glia.23734
2. Flanagan EP, Hinson SR, Lennon VA, Fang B, Aksamit AJ, Morris PP, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: analysis of 102 patients. Ann Neurol (2017) 81(2):298–309. doi: 10.1002/ana.24881
3. Fang B, McKeon A, Hinson SR, Kryzer TJ, Pittock SJ, Aksamit AJ, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol (2016) 73(11):1297–307. doi: 10.1001/jamaneurol.2016.2549
4. Kunchok A, Zekeridou A, McKeon A. Autoimmune glial fibrillary acidic protein astrocytopathy. Curr Opin Neurol (2019) 32(3):452–8. doi: 10.1097/WCO.0000000000000676
5. Yang X, Liang J, Huang Q, Xu H, Gao C, Long Y, et al. Treatment of autoimmune glial fibrillary acidic protein astrocytopathy: follow-up in 7 cases. Neuroimmunomodulation (2017) 24(2):113–9. doi: 10.1159/000479948
6. Yang X, Xu H, Ding M, Huang Q, Chen B, Yang H, et al. Overlapping autoimmune syndromes in patients with glial fibrillary acidic protein antibodies. Front Neurol (2018) 9:251. doi: 10.3389/fneur.2018.00251
7. Dubey D, Hinson SR, Jolliffe EA, Zekeridou A, Flanagan EP, Pittock SJ, et al. Autoimmune gfap astrocytopathy: prospective evaluation of 90 patients in 1 Year. J Neuroimmunol (2018) 321:157–63. doi: 10.1016/j.jneuroim.2018.04.016
8. Zhuang X, Jin K, Li X, Li J. Autoimmune glial fibrillary acidic protein astrocytopathy in children: a retrospective study. Eur J Med Res (2022) 27(1):11. doi: 10.1186/s40001-022-00641-y
9. Gravier-Dumonceau A, Ameli R, Rogemond V, Ruiz A, Joubert B, Muñiz-Castrillo S, et al. Glial fibrillary acidic protein autoimmunity: a French cohort study. Neurology (2022) 98(6):e653–e68. doi: 10.1212/WNL.0000000000013087
10. Xiao J, Chen X, Shang K, Tang Y, Chen M, Deng G, et al. Clinical, neuroradiological, diagnostic and prognostic profile of autoimmune glial fibrillary acidic protein astrocytopathy: a pooled analysis of 324 cases from published data and a single-center retrospective study. J Neuroimmunol (2021) 360:577718. doi: 10.1016/j.jneuroim.2021.577718
11. Long Y, Liang J, Xu H, Huang Q, Yang J, Gao C, et al. Autoimmune glial fibrillary acidic protein astrocytopathy in Chinese patients: a retrospective study. Eur J Neurol (2018) 25(3):477–83. doi: 10.1111/ene.13531
12. Fang H, Hu W, Jiang Z, Yang H, Liao H, Yang L, et al. Autoimmune glial fibrillary acidic protein astrocytopathy in children: a retrospective analysis of 35 cases. Front Immunol (2021) 12:761354. doi: 10.3389/fimmu.2021.761354
13. Iorio R, Damato V, Evoli A, Gessi M, Gaudino S, Di Lazzaro V, et al. Clinical and immunological characteristics of the spectrum of gfap autoimmunity: a case series of 22 patients. J Neurol Neurosurg Psychiatry (2018) 89(2):138–46. doi: 10.1136/jnnp-2017-316583
14. Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature (1980) 286(5774):736–7. doi: 10.1038/286736a0
15. Seguella L, Gulbransen BD. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol (2021) 18(8):571–87. doi: 10.1038/s41575-021-00423-7
16. Deng B, Wang J, Yu H, Jin L, Qiu Y, Liu X, et al. Area postrema syndrome in autoimmune glial fibrillary acidic protein astrocytopathy: a case series and literature review. Neurol Neuroimmunol Neuroinflamm (2022) 9(6):e200029. doi: 10.1212/NXI.0000000000200029
17. Yang Z, Wang KKW. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci (2015) 38(6):364–74. doi: 10.1016/j.tins.2015.04.003
18. Getts DR, Chastain EML, Terry RL, Miller SD. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev (2013) 255(1):197–209. doi: 10.1111/imr.12091
19. Handoko M, Hong W, Espineli E, Saxena K, Muscal E, Risen S. Autoimmune glial fibrillary acidic protein astrocytopathy following herpes simplex virus encephalitis in a pediatric patient. Pediatr Neurol (2019) 98:85–6. doi: 10.1016/j.pediatrneurol.2019.05.010
20. Issa N, Martin C, Dulau C, Camou F. Severe anti-gfap meningo-encephalomyelitis following viral infection. Mult Scler Relat Disord (2020) 45:102448. doi: 10.1016/j.msard.2020.102448
21. Li J, Xu Y, Ren H, Zhu Y, Peng B, Cui L. Autoimmune gfap astrocytopathy after viral encephalitis: a case report. Mult Scler Relat Disord (2018) 21:84–7. doi: 10.1016/j.msard.2018.02.020
22. Wang KKW, Yang Z, Yue JK, Zhang Z, Winkler EA, Puccio AM, et al. Plasma anti-glial fibrillary acidic protein autoantibody levels during the acute and chronic phases of traumatic brain injury: a transforming research and clinical knowledge in traumatic brain injury pilot study. J Neurotrauma (2016) 33(13):1270–7. doi: 10.1089/neu.2015.3881
Keywords: glial fibrillary acidic protein, autoimmune, inflammatory CNS disease, Short-term prognosis, clinical characteristics
Citation: Zhang W, Xie Y, Wang Y, Liu F, Wang L, Lian Y, Liu H, Wang C and Xie N (2023) Clinical characteristics and prognostic factors for short-term outcomes of autoimmune glial fibrillary acidic protein astrocytopathy: a retrospective analysis of 33 patients. Front. Immunol. 14:1136955. doi: 10.3389/fimmu.2023.1136955
Received: 03 January 2023; Accepted: 23 May 2023;
Published: 07 June 2023.
Edited by:
Oivind Torkildsen, University of Bergen, NorwayReviewed by:
Maria Eleftheria Evangelopoulos, National and Kapodistrian University of Athens, GreeceCopyright © 2023 Zhang, Xie, Wang, Liu, Wang, Lian, Liu, Wang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nanchang Xie, eGllbmFuY2hhbmcyMDAxQDE2My5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.