
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 18 May 2023
Sec. Alloimmunity and Transplantation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1136343
This article is part of the Research TopicEmerging Talents in Alloimmunity and Transplantation: 2022View all 7 articles
Objective: Whether fecal microbiota transplantation (FMT) in patients with irritable bowel syndrome (IBS) is effective in improving outcomes remains controversial. We assessed the safety and efficacy of FMT for patients with IBS.
Methods: In this systematic review and meta-analysis, we searched PubMed, Embase, Web of Science, the Cochrane Library, the clinicaltrials.gov and International Clinical Trials Registry Platform (ICTRP) up to February 25, 2022, updated to March 28, 2023. Randomized controlled trials (RCTs) compared the stool and capsule FMT with placebo in patients with IBS were included. Two authors independently assessed study eligibility, extracted the data, and assessed risk of bias. We did meta-analysis with RevMan, and the Stata software was used for sensitivity analysis and meta-regression. The GRADE system was used to assess the quality of evidences. Mean difference (MD) or standardized Mean difference (SMD) with 95% CI for continuous data, and risk ratios (RR) with 95% CI for dichotomous data were used with random-effects models. The primary outcomes included the clinical response rate and IBS-SSS score. This study is registered with PROSPERO: CRD42022328377.
Results: Nineteen reports from nine RCTs were included finally. Compared with the placebo, a single stool FMT could significantly decrease the IBS-SSS score at 1 month (MD=-65.75, 95%CI [-129.37, -2.13]), 3 months (MD=-102.11, 95% CI [-141.98, -62.24]), 6 months (MD=-84.38, 95%CI [-158.79, -9.97]), 24 months (MD=-110.41, 95%CI [-145.37, -75.46]), and 36 months (MD=-104.71, 95%CI [-137.78, -71.64]). It also could improve the clinical response rate at 3 months (RR=1.91, 95% [1.12, 3.25]), 24 months (RR=2.97, 95% [1.94, 4.54]), and 36 months (RR=2.48, 95% [1.65, 3.72]), and increase the IBS-QoL score at 3 months, 24 months, and 36 months. FMT did not increase the serious adverse event. The risk of bias was low, and the quality of evidence based on GRADE system was moderate in the stool FMT group. However, we did not find positive effect of capsule FMT on patients with IBS based on the current available data.
Conclusion: A single stool FMT is effective and safe for patients with IBS. However, some factors may affect the effectiveness of FMT, and the relationship between the gut microbiome and the effect of FMT for IBS is still unclear.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022328377.
Irritable bowel syndrome (IBS) is one of the most common functional gastrointestinal disorders (FGIDs) which now called disorders of gut-brain interaction (1). The prevalence of IBS appears to vary widely between different countries all over the world, according to the latest research, the average varies between 9.2%-10.1% and 3.8%-4.1% used the Rome III criteria and the Rome IV criteria, respectively (1, 2). IBS is characterized by symptoms including recurrent abdominal pain associated with a change in stool form or frequency, it has resulted in significant global health care costs and impaired health-related quality of life (3–6).
IBS is difficult to treat and conventional therapies are often ineffective at controlling symptoms and restoring function (7). The pathophysiology of IBS is complex and incompletely understood up to now, it may associate with the altered gut-brain axis, stress, disordered gastrointestinal motility, abnormal intestinal secretion, visceral hypersensitivity, immunomodulation, and intestinal permeability, and all of these can be affected by the gut microbial community (3, 8). More and more researches show that gut microbiota dysbiosis plays an important role in IBS pathogenesis (9–11).
Fecal microbiota transplantation (FMT) is a non-conventional therapy in which fecal material from healthy donors is given to patients attempt to cure disease or relieve symptoms (7). It is an efficient way of modulating the gut microbiota and aims to introduce a balanced conglomerate of microorganisms (12). It has shown definite efficacy for the treatment of recurrent Clostridioides difficile infection (13, 14). In addition, it has also been used for some gastrointestinal diseases such as inflammatory bowel disease (15). FMT is being explored as a therapeutic option for the patients of IBS, positive effects on IBS symptoms in various degrees were obtained in some randomized controlled trials (RCTs), while there was no effect in the others, so the results from these RCTs are inconsistent (16).
So far, some meta-analyses have evaluated the efficacy of FMT in the treatment of IBS, and they unanimously concluded that FMT is ineffective for IBS (17–19). Unfortunately, some recently published RCTs (20–22) were not included in these analyses, so the conclusions may not represent the real results very well. We therefore conduct an updated meta-analysis and systematic review of RCTs to re-estimate the efficacy and safety of FMT for the treatment of IBS.
The systematic review and meta-analysis was performed in accordance with the Cochrane Handbook for Systematic Reviews of Intervention (23) and the PRISMA statement (24). The study was registered in PROSPERO (CRD42022328377).
The PICOS (patients, intervention, comparison, outcomes, and study design) tool was used to specify eligibility criteria for the systematic review and meta-analysis (25, 26). Patients with moderate to severe IBS diagnosed according to the Rome III or IV criteria, aged ≥ 18 years, the subtypes of IBS were not restricted; allogenic FMT was used as the intervention, the routes, frequency and does were not restricted; autologous FMT or placebo capsules were used as comparison measures for patients in the placebo group; the main outcomes included clinical response rate, IBS-SSS score, IBS-QoL score, abdominal pain, frequency of stool, side effects and the change of microbiome profiles; only randomized controlled trials were included.
We systematically searched the electronic databases PubMed, Embase, Web of Science, the Cochrane Library, the clinicaltrials.gov and International Clinical Trials Registry Platform up to February 25, 2022, updated to March 28, 2023. This search was performed using both free text and Mesh terms. Search terms included fecal, faecal, feces, faeces, stool, microbiota, microbiome, microflora, bacteria, transplantation, transplant, transfer, irritable bowel syndrome, IBS. The full search syntaxes were supplied in Supplementary Table 1.
The study screening and selection was performed in accordance with the PRISMA 2020 flow diagram (24) by a three-step process. In the first step, all database citations got by preliminary searching were imported and de-duplicated in EndNote (27). In the second step, titles, abstracts, and keywords of citations were screened separately by two authors (MCW and XFX) to identify potentially eligible studies. In the third step, the full texts of these potentially eligible studies were examined to identify the studies that ultimately met the eligibility criteria above. If consensus could not be reached, a third co-author (YCZ) provided input. In the case of multiple papers from the same RCTs, relevant data were extracted from all papers, they were included as a single study in the analysis and identified uniformly by the only register number.
Two reviewers (MCW, XFX) independently extracted data from all full-text articles that met eligibility criteria in a prespecified Microsoft Excel spreadsheet, and any disagreement was resolved by discussion with a third co-author (YCZ). The means and standard deviations were collected for continuous variables, if they were not reported in the text, the data would be extracted from their plots, images, and maps using a web-based tool WebPlotDigitizer 4.5 (28, 29). When these data were not available and whenever possible, the 95% CIs and P values were used to calculate means and standard deviations using the RevMan Calculator which was provided in the Review Manager 5 (Version 5.4). Where sample size, median, range and/or interquartile range were reported, they were converted to means and standard deviations according to the conversion formulas of Wan et al. (30) and Shi et al. (31), which will often give an advantage over the omission of trials with missing means or standard deviations from a meta-analysis (32). Where insufficient data were available to calculate or extract the means and standard deviations, the study was excluded from quantitative analysis.
Two authors (MCW, XFX) independently assessed the quality of the systematic review and meta-analysis, disagreements were resolved by discussing with a third co-author (YCZ). The risk of bias of each included studies was assessed with the Cochrane Collaboration’s tool in the Cochrane Handbook for Systematic Reviews of Interventions (RevMan software, Version 5.4) (23). The quality of evidence for each outcome was assessed with the GRADE system (GRADEpro software, Version 3.6), the quality could be downgraded by one level (serious concern) or two levels (very serious concerns) due to these factors: risk of bias (33), inconsistency (34), indirectness (35), imprecision (36), and publication bias (37), the grade was specified four categories as high, moderate, low, and very low (38, 39).
The primary outcome was the IBS-SSS score at different time points after FMT. The secondary outcomes included the clinical response rate, IBS-QoL score, abdominal pain, frequency of stool, stool consistency, adverse events, and the change of microbiome profiles. The clinical response rate was defined by the relief level of IBS symptoms, and the symptoms were assessed using the IBS-severity scoring system (IBS-SSS) (40), or Gastrointestinal Symptom Rating Scale for IBS (GSRS-IBS) (41), or a daily symptom diary (22).
Data synthesis and analysis was performed using the RevMan software (Version 5.4). we reported data in terms of mean difference (MD) and 95% confidence interval (CI) for continuous data. When different studies used different rating instruments to measure the same outcome, the standardized mean difference (SMD) would be reported (42). For dichotomous data, we reported risk ratios (RR) and 95% CI. We identified heterogeneity from forest plots using the Chi (2) test with a significance level of p= 0.1. The heterogeneity was quantified using the I2 statistic, where I2 ≥ 50% indicated a significant heterogeneity (43). When the I2 ≥ 50%, we would assess the possible sources of heterogeneity using sensitivity analysis. All meta-analysis were performed used random-effects models considering the heterogeneous in terms of interventions, participant characteristics, donor characteristics, and outcome measurements among included studies. Where meta-analysis was not possible or appropriate, we would present results as qualitative synthesis of intervention effects.
Where sufficient data were available, we planned to perform subgroup analyses based on the stool FMT and capsule FMT, meta-regression analysis would also be performed for different routes, dose, frequency of FMT, number of donors, and for different style of stools. When the number of included studies was more than 10 (44), we would assess the publication bias for the outcomes of clinical response rate, IBS-SSS and IBS-QoL using the funnel plot and Egger’s test. The Stata software (Version 12) was used to assess the publication bias, sensitivity analysis and meta-regression analysis.
Nineteen articles (7, 12, 20–22, 45–58) from nine eligible RCTs were included finally with a total sample size of 516. All the RCTs were registered in the clinicaltrials.gov or ICTRP. The flow chart of study selection was shown in the PRISMA 2020 flow diagram (24) (Figure 1). The characteristics of included studies were represented in Table 1. Of the nine included studies, two (7, 54) were conducted in the USA, one (55) in China, and the rest were in European countries. Patients with moderate to severe IBS symptoms were enrolled in the included studies. For the IBS subtypes, IBS-D (diarrhea-predominant IBS) and IBS-M (mixed-diarrhea-and constipation IBS) accounted for about 81.7% of patients in these studies in total. Diagnosis of IBS based on Rome III criteria in 8 RCTs and Rome IV criteria in one RCT (NCT03822299). The follow-up time varied between 10 weeks and 52 weeks. The main outcomes and design of these included studies were represented in Supplementary Table 2.
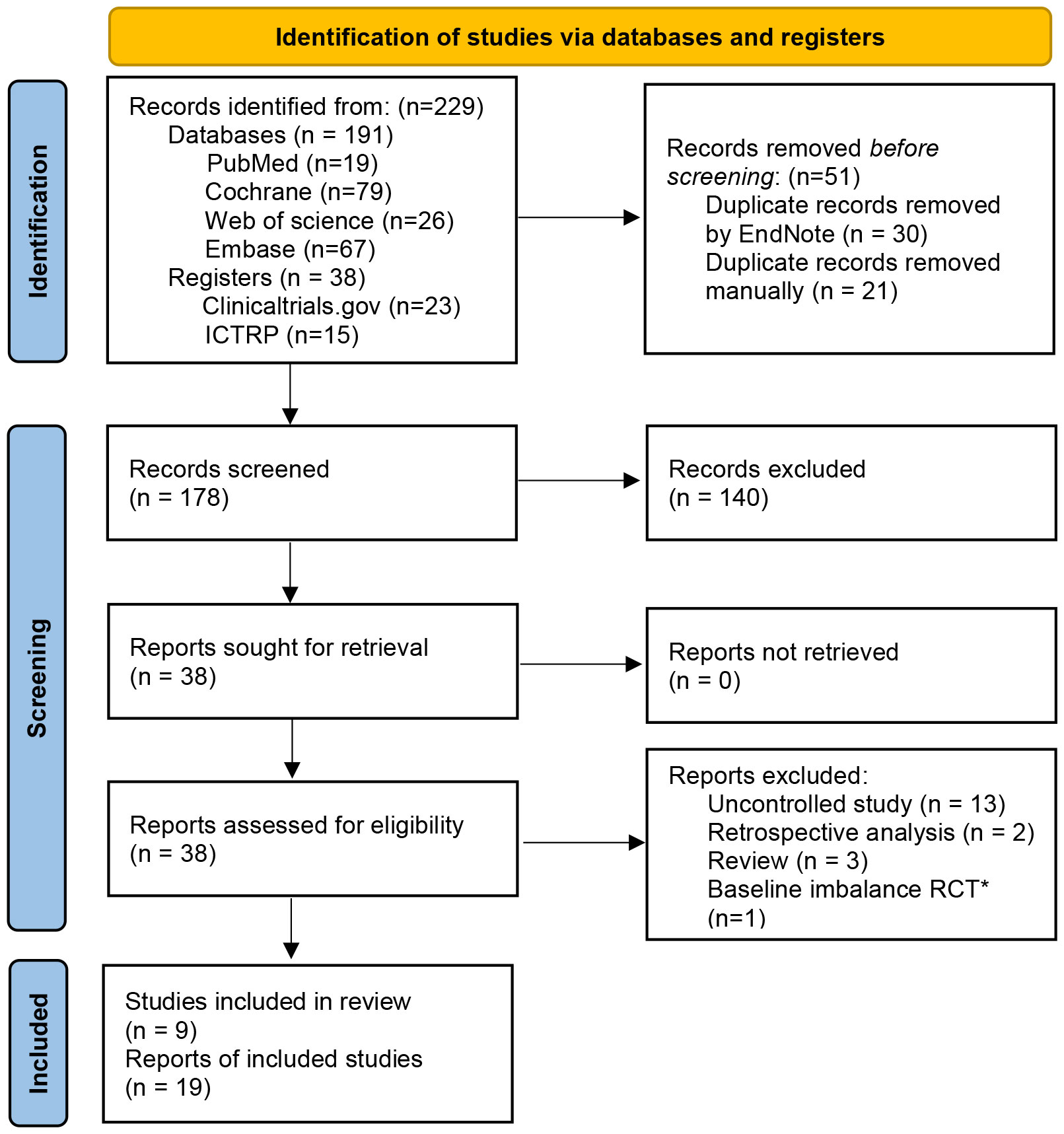
Figure 1 PRISMA 2020 flow diagram ICTRP, International Clinical Trials Registry Platform. *The study did not report the baseline data of patients for different groups in detail, IBS-SSS score in the transplantation day between FMT group and placebo group was significantly statistical difference (352.1±27 vs. 309.8±20).
The characteristics of FMT in these included RCTs were represented in Table 2. The styles of FMT materials included fresh or frozen donor stool and fecal microbiota capsule in the FMT group. In the placebo group, the placebo materials included autologous stool and placebo capsule. The route of FMT administration included nasojejunal probe, gastroscope, colonoscopy and oral capsules. In the stool FMT group, the dose of fresh stool was 30g, 50g, 60g, and 50-80g, and in the capsule FMT group, the dose of fresh stool was 14.25g, 28.5g and 600g. Two RCTs (45, 55) did not reported the dose of stool. All patients were given just a single FMT in the stool FMT group, a second FMT was offered only in one study (45) after the cross-over part in the capsule FMT group.
Risk of bias assessment showed low to moderate risk for all the included studies (Figure 2). Randomized controlled design was performed in all included studies, and the allocation concealment for the random sequences was used in seven RCTs. Meanwhile, study participants and investigators were blinded to treatment allocation. Loss of follow-up was reported in detail in all studies and managed appropriately. The number of lost to follow-up was similar between FMT group and placebo group, there was no significant difference in the meta-analysis (RR=1.30, 95% [0.56, 3.01]).
An important feature of partially included studies (20, 22) was that some of the outcomes in the FMT group changed significantly from baseline at the end of the intervention, which did not occur in the placebo group, but there were no statistically significant differences between the FMT group and placebo group at the end of the intervention. Therefore, in order to comprehensively analyze the effect of FMT on IBS patients, we not only vertically analyzed the differences of these outcomes between the two groups after the end of intervention, but also horizontally analyzed the differences between the baseline and endpoint after intervention in the two groups, separately.
Four RCTs (20, 21, 46, 55) reported the IBS-SSS score at 1 month/4weeks, one (54) reported at 10 weeks, six (7, 20, 21, 46, 50, 55) at 3 months/12 weeks, two (46, 50) at 6 months, one (20) at 52 weeks, one (57) at 24 months, and one (57) at 36 months. Meta-analysis with random-effects models shown that there were statistically significant differences between FMT and placebo groups at 1 month, 3 months, 24months and 36 months (1 month: MD=-55.72, 95%CI [-105.01, -6.43]; 3 months: MD=-69.60, 95%CI [-98.09, -41.12]; 24 months: MD=-110.41, 95%CI [-145.37, -75.46]; 36 months: MD=-104.71, 95%CI [-137.78, -71.64]), but there were not statistically significant differences at other time points (10 weeks: MD=61.10, 95%CI [-30.86, 153.06]; 6 months: MD =-27.87, 95%CI [-138.28, 82.54]; 52 weeks: MD =-12.68, 95%CI [-82.76, 57.40]) (Supplementary Figure 2).
Subgroup analysis based on the stool and capsule FMT shown that, compared with the placebo group, there were statistically significant differences in the stool FMT group at 1 month (MD=-65.75, 95%CI [-129.37, -2.13]), 3 months (MD=-102.11, 95%CI [-141.98, -62.24]), 6 months (MD=-84.38, 95%CI [-158.79, -9.97]), 24 months (MD=-110.41, 95%CI [-145.37, -75.46]), and 36 months (MD=-104.71, 95%CI [-137.78, -71.64]) (Figure 3). Significant heterogeneity existed among these studies at both 1 month and 3 months. After sensitivity analysis, we respectively removed the obviously heterogeneous study Lahtinen et al. (NCT03561519) (20) at 1 month and at 3 months, the results were consistent with that before (Supplementary Figure 3). The differences were not statistically significant in the capsule FMT group compared with the placebo group at 1 month, 10 weeks, 3 months, or 6 months (p>0.05) (Supplementary Figure 4).
Five RCTs (7, 20, 21, 45, 50) reported the clinical response rate at 3 months/12 weeks, one (54) reported at 10 weeks, one (22) at 6 months, one (50) at 12 months, one (57) at 24 months, and one (57) at 36 months. Meta-analysis with random-effects models shown that there were not statistically significant differences between FMT and placebo groups at any time points (10 weeks: RR=0.39, 95% [0.11, 1.41]; 3 months: RR=1.60, 95%CI [0.92, 2.78]; 6 months: RR=4.00, 95% [0.56, 28.40]; 12 months: RR=1.58, 95%CI [0.91, 2.73]) except at 24 months (RR=2.97, 95% [1.94, 4.54]) and 36 months (RR=2.48, 95% [1.65, 3.72]) (Supplementary Figure 1).
Subgroup analysis based on the stool and capsule FMT shown that the clinical response rate in stool FMT group was significantly improved at 3 months/12 weeks compared with the placebo group (four RCTs, RR=1.91, 95% [1.12, 3.25]) (Figure 4). The difference was not statistically significant in the capsule FMT group compared with the placebo group (1 RCT, RR=0.82, 95%CI [0.48, 1.40]) (Figure 4). The average clinical response rate at 3 months with different definition was 70.0% (161/230, 4 RCTs) in the stool FMT group and 32.0% (41/128, 4 RCTs) in the placebo group. However, it should be emphasized that the definition of clinical response rate is not same in different studies (Supplementary Table 3). Significant heterogeneity existed among these studies (Chi2 = 10.30, I2 = 71%). After sensitivity analysis, we removed the obviously heterogeneous study El-Salhy et al. (NCT03822299) (21), the result was consistent with the previous one (Chi2 = 1.56, I2 = 0%; RR=1.48, 95%CI [1.06, 2.08]).
Two RCTs (21, 46) with three pairs of data reported the IBS-QoL score at 1 month/4weeks, one RCT (54) reported at 10 weeks, four RCTs (7, 21, 45, 46) with five pairs of data reported at 3 months/12 weeks, one RCT (57) with two pairs of data reported at 24 months and 36 months. Meta-analysis shown that there were not statistically significant differences between FMT and placebo groups at 1 month/4 weeks (SMD=0.14, 95%CI [-0.11, 0.38]) and 10 weeks (SMD=0.30, 95%CI [-0.53, 1.12]), but there was statistically significant difference at 3 months/12 weeks, 24 months and 36 months (3months: SMD=0.62, 95%CI [0.33, 0.90]; 24 months: SMD=0.85, 95%CI [0.37, 1.33]; 36 months: SMD=1.07, 95%CI [0.67, 1.46]) (Supplementary Figure 5).
Subgroup analysis shown that, compared with the placebo group, there were statistically significant differences in the stool FMT group at 3 months (SMD=0.78, 95%CI [0.53, 1.02]), 24 months (SMD=0.85, 95%CI [0.37, 1.33]), and 36 months (SMD=1.07, 95%CI [0.67, 1.46]) (Figure 5). However, there was no significant difference between capsule FMT group and placebo group at 1 months or 3 months.
The subgroup analysis was performed based on the stool FMT and capsule FMT for the abdominal pain at 3 months. Two RCTs (21, 45) with three pairs of data reported the abdominal pain in the stool FMT group, meta-analysis shown that there was significantly statistical difference compared with the placebo group (Chi2 = 0.05, I2 = 0%, SMD=-0.60, 95%CI [-0.84, -0.35]). One RCT (46) reported the outcome in the capsule group, there was no difference compared with the placebo group (SMD=0.38, 95%CI [-0.17, 0.93]).
The frequency of stool was reported in one RCT each in the stool FMT group (45) and capsule FMT group (46) at 3 months. Compared with the placebo group, there was significantly statistical difference in the stool FMT group (MD=-0.50, 95%CI [-0.93, -0.07]), while there was no difference in the capsule FMT group (MD=0.02, 95%CI [-0.63, 0.67]).
The stool consistency was reported in one RCT each in the stool FMT group (45) and capsule FMT group (46) at 3 months. Compared with the placebo group, there was significantly statistical difference in the stool FMT group (MD=-0.33, 95%CI [-0.61, -0.05]), while there was no difference in the capsule FMT group (MD=0.06, 95%CI [-0.62, 0.44]).
In both the donor stool FMT group (FMT group) and the autologous stool FMT group (placebo group), two RCTs (20, 21) reported IBS-SSS score at baseline and at 1 month and 3 months after FMT, and one RCT (57) reported at 24 months and 36 months. Meta-analysis shown that, compared with its baseline, IBS-SSS score were significantly reduced at 1 month, 3 months, 24 months, and 36 months in the donor stool FMT group (1month: MD=-101.72, 95%CI [-124.49, -78.95]; 3 months: MD=-129.01, 95%CI [-153.05, -104.97]; 24 months: MD=-156.95, 95%CI [-188.41, -125.49]; 36 months: MD=-150.70, 95%CI [-179.91, -121.49]). But there were no statistical differences at 1 month (MD=-23.07, 95%CI [-52.01, 5.87]), 3 months (MD=-18.60, 95%CI [-52.50, 15.29]), 24 months (MD=-28.60, 95%CI [-70.01, 12.81]), or 36 months (MD=-27.70, 95%CI [-68.91, 13.51]) after FMT in the autologous stool FMT group.
In both the fecal microbiota capsule FMT group (FMT group) and the placebo capsule FMT group (placebo group), two RCT (46, 55) reported IBS-SSS score at baseline and at 1 month, three RCTs (7, 46, 55) reported IBS-SSS score at baseline and at 3 months, one RCT (46) reported it at 6 months after FMT. Meta-analysis shown that, compared with its baseline in the fecal microbiota capsule FMT group, IBS-SSS score were significantly reduced at 1 month and 3 months after FMT (MD=-102.66, 95%CI [-158.41, -46.91]; MD=-82.69, 95%CI [-126.74, -38.63]), while there was no difference between baseline and 6 months (MD=-43.95, 95%CI [-107.25, 19.35]). Surprisingly, in the placebo capsule FMT group, IBS-SSS score were also significantly reduced at 3 months and 6 months after FMT in the placebo capsule FMT group when compared with its baseline (MD=-66.92, 95%CI [-117.31, -16.53]; MD=-114.34, 95%CI [-171.73, -56.95]).
In both the donor stool FMT group (FMT group) and the autologous stool FMT group (placebo group), one RCT (21) reported IBS-QoL score at baseline and at 1 month and 3 months after FMT, one RCT (45) reported it at baseline and at 3 months after FMT, and one RCT (57) reported it at 24 months and 36 months. Meta-analysis shown that, compared with its baseline, IBS-QoL score was significantly improved at 1 month, 3 months, 24 months, and 36 months after FMT in the donor stool FMT group (1 month: SMD=0.56, 95%CI [0.29, 0.83]; 3 months: SMD=0.75, 95%CI [0.52, 0.98]; 24 months: MD=27.76, 95%CI [20.79, 34.73]; 36 months: MD=27.61, 95%CI [20.66, 34.56]). However, there were no statistical differences at any time after FMT in the autologous stool FMT group compared with its baseline (1month: SMD=5.10, 95%CI [-3.40, 13.60]; 3 months: SMD=-0.02, 95%CI [-0.52, 0.47]; 24 months: MD=1.80, 95%CI [-8.46, 12.06]; 36 months: MD=-0.60, 95%CI [-9.74, 8.54]).
In both the fecal microbiota capsule FMT group (FMT group) and the placebo capsule FMT group (placebo group), one RCT (46) reported IBS-QoL score at baseline and at 1 month and 3 months after FMT, another RCT (7) reported it at baseline and at 3 months after FMT. Meta-analysis shown that, compared with its baseline, there were no statistical differences at both 1 month and 3 months after FMT in the fecal microbiota capsule FMT group (SMD=-0.49, 95%CI [-1.05, 0.07]; SMD=0.10, 95%CI [-0.30, 0.51]). In the placebo capsule FMT group, the IBS-QoL score was reduced at 1 month after FMT (SMD=-13.73, 95%CI [-22.40, -5.06]), but there was no statistical difference at 3 months after FMT when compared with its baseline (SMD=-0.10, 95%CI [-1.83, 1.63]).
The main adverse events reported in these RCTs included abdominal pain, nausea, diarrhea, constipation, bloating or flatulence, headache, fatigue, fever and others. Only one serious adverse event was reported in one RCT (50). A participant in the FMT group was admitted to hospital for a few hours of observation after the FMT procedure due to transient vertigo and nausea, and the researchers deemed this to be related to the medication and instrumentation used during colonoscopy (50). Meta-analysis with random effects model shown that there were no significant statistical differences for these adverse events between the FMT group and placebo group. When I (2) >50%, we removed studies with significant heterogeneity after sensitivity analysis, meta-analysis with fixed effects model shown that FMT may increase the incidence of abdominal pain, constipation, and diarrhea (Table 3).
Meta-regression analysis was performed for the primary outcome IBS-SSS score at 3 months after FMT, the covariates included the year of study, material of FMT (stool vs. capsule), route of FMT (gastroscope, colonoscopy, and oral capsules), total number of donors for all patients, number of donors for each patient (one to one, or mixed to one), single dose of stool, total dose of stool, and different style of stool (fresh vs. frozen). The results shown that there were significant relations between IBS-SSS score and these covariates of material of FMT (Coef. = 116.63, p=0.03, 95%CI: 15.23, 218.02), route of FMT (Coef. = 78.45, p=0.00, 95%CI: 34.90, 121.99), total number of donors for all patients (Coef. = 40.36, p=0.01, 95%CI: 9.73, 70.99), and number of donors for each patient (Coef. = 46.87, p=0.04, 95%CI: 0.62, 93.13) (Figure 6). However, due to the small number of included RCTs, the statistical reliability of the results above will be significantly reduced, although these four covariates are of great clinical significance.
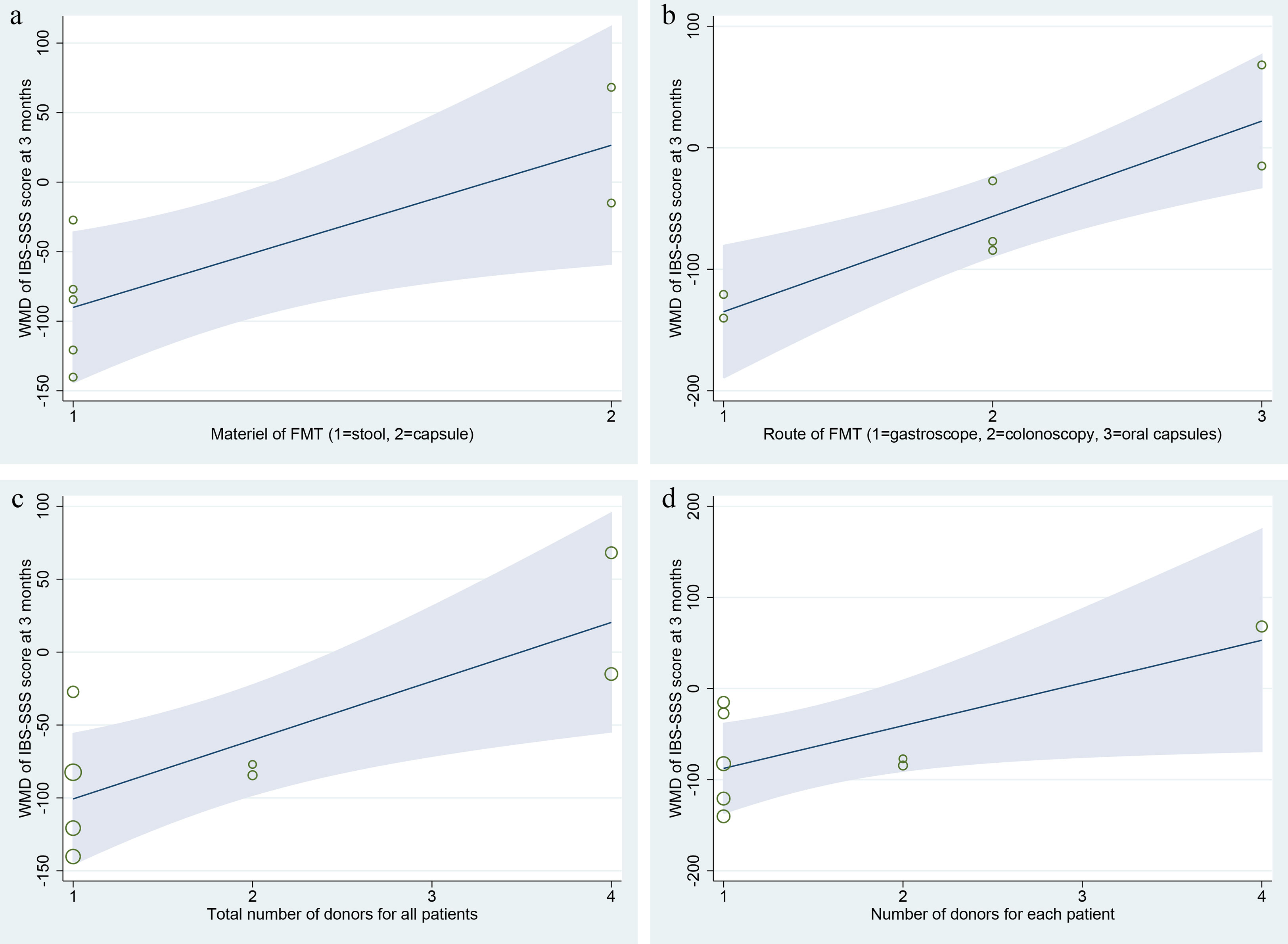
Figure 6 Meta-regression analysis of the primary outcome IBS-SSS score at 3 months after FMT. (A) material of FMT (stool vs. capsule); (B) route of FMT (gastroscope, colonoscopy, and oral capsules); (C) total number of donors for all patients; (D) number of donors for each patient (one to one, or mixed to one).
Because of small number of RCTs included, we did not perform a publication bias analysis. However, the included RCTs were mainly small sample studies, and the possibility of publication bias cannot be ruled out (37).
The summary of findings and the GRADE evidence profile were shown in Table 4 and Supplementary Table 4. In order to comprehensively analyze the effect of FMT on IBS patients, we analyzed the differences between the baseline and endpoint after intervention in different groups, the consistency of different outcomes was shown in Table 5, the best consistency of these different outcomes were shown at 3 months, 24 months, and 36 months after stool FMT, and at 3 months after capsule FMT. The risk of bias of study design was shown in Figure 2. In summary, the quality was moderate to high in terms of the design of the included studies, and the quality of these primary outcomes after pooled was moderate to low.
As is known, IBS is one of the most common disorders of gut-brain interaction worldwide, its effects on the individual in terms of their quality of life, and on health-care delivery and society in terms of economic costs, are considerable (6, 59). As a non-conventional method, FMT is being explored as a therapeutic option for the patients of IBS. So far, twenty RCTs about FMT for IBS have been registered in the clinicaltrials.gov and International Clinical Trials Registry Platform (Supplementary Table 5). Among them, 10 RCTs have been completed, 9 have been included in this study, and the results of one RCT (60) (NCT05088434) was excluded because of imbalanced baseline. Of the 20 registered RCTs, 13 were or would be conducted in European countries, 5 in China, and 2 in the United States, 15 had a sample size of less than 100.
We finally included nine RCTs in this systematic review and meta-analysis, of which five for stool FMT, and four for capsule FMT. Our meta-analysis results shown that the stool FMT could increase the clinical response rate, decrease IBS-SSS score, and improve the quality of life of patients with IBS, without increasing the incidence of serious complications. However, based on the current available data, our study did not confirm the positive effect of capsule FMT on patients with IBS.
The risk of bias of included RCTs was low to moderate. In the stool FMT group, random allocation, allocation concealment and blinding were all performed properly, and the risk of bias was low. In the capsule group, one RCT (54) did not report the specific random allocation scheme, two RCTs (54, 55) did not report whether the allocation concealment for the random sequences was performed, and the risk of bias was moderate (Figure 2). In this study, we used the GRADE system to estimate the quality of evidence for these main outcomes, which based on the five factors of risk of bias, inconsistency, indirectness, imprecision, and publication bias. In the stool FMT group, the qualities of the primary outcomes (included clinical response rate and IBS-SSS score) were moderate, which were downgraded by one level due to publication bias, and the reason for publication bias was that all RCTs included were small sample studies. In the capsule FMT group, the qualities of the same primary outcomes were low, which were downgraded by two levels due to publication bias and imprecision, and the reasons for publication bias and inaccuracy were respectively the small sample size of the included studies and the heterogeneity among different studies (Table 4). Therefore, it can be seen that the smaller sample size of the included RCTs is one of the main reasons for reducing the reliability of the conclusions for the stool FMT. Predictably, this conclusion will be further confirmed with the emergence of larger RCTs in the future.
As well as stool FMT, capsule FMT has also been shown to be effective and safe in the treatment of recurrent Clostridioides difficile infection (13, 61). Halkjær et al. (46) proved that IBS patients in the placebo group experienced greater symptom relief compared with the capsule FMT group after 3 months. Aroniadis et al (7) shown that capsule FMT did not induce symptom relief of IBS patients at 12 weeks compared with placebo. Our study did not also prove that the capsule FMT has a positive therapeutic effect on patients with IBS, but we still refuse to deny the obvious advantages and attractive application prospects of capsule FMT compared with stool FMT. In this systematic review and meta-analysis, the following deficiencies may be the main factors affecting the authenticity of the conclusion that capsule FMT is applied to IBS treatment. First, only four RCTs (7, 46, 54, 55) for capsule FMT were included, the sample size of all included studies was small and the follow-up time was different. As a result, the available data for analysis was insufficient. Second, the heterogeneity among different studies was significant, and the GRADE level of main outcomes was low. Third, in two of the four included studies, the randomization and allocation concealment schemes were unclear, and the risk of bias was moderate. Therefore, it will be very necessary to continue more in-depth and normative research on the application of capsule FMT for patients with IBS in future studies.
Many factors may affect the effectiveness and safety of FMT for patients with IBS (46). These factors were shown in Tables 1–3 in this study. 1) Characteristics of donors. The study by Holvoet et al. (45) shown that higher similarity of microbial community composition between patients and donors at baseline might increase chances of successful FMT in IBS, and the stability of the microbial composition in the donors might be an important predictor of success. Our meta-regression analysis shown that there were significant relations between IBS-SSS score and these covariates of total number of donors for all patients and number of donors for each patient (Figure 6). Most of the trials (5/6) that favored FMT used fecal material from one donor, whereas two thirds of the trials that did not favor FMT used mixed fecal material from multiple donors (Table 2). The standards for donor screening could refer to the recently published consensus statements for FMT (62–64).
2) Material of FMT. The material styles of FMT included stool and capsule. Of the nine included RCTs, all studies using stool FMT were found to be effective in patients with IBS, while only one of the studies using capsule FMT reached the same conclusion (Table 2). Our meta-regression analysis shown that there was significant relation between IBS-SSS score and the material of FMT (Figure 6). All fecal microbiota capsule were stored in a frozen state. In this meta-analysis, two RCTs (45, 50) used fresh stool FMT, and four RCTs (20–22, 50) used frozen FMT. Unfortunately, we were unable to perform a subgroup analysis of these two different stool FMT due to the limited data available for extraction. Although frozen stool FMT has been shown to be non-inferior to fresh stool FMT for patients with recurrent Clostridioides difficile infection (65, 66), this might not be the case for IBS and warrants further study (7).
3) Route of FMT. Several routes of FMT administration are available at the current, such as nasojejunal tube, gastroscope, duodenoscopy, colonoscopy, enema, and oral capsules. Of which nasojejunal tube (45), gastroscope (21), colonoscopy (20, 22, 50) and oral capsules (7, 46, 54, 55) were used in this study. Meta-regression analysis showed that the route of FMT was correlated with IBS-SSS score at 3 months (Figure 6). Similarly, the cure rates of recurrent Clostridioides difficile infection with FMT performed with colonoscopy are superior to enema and nasojejunal tube, while FMT with colonoscopy and capsule are comparable (67).
4) Stool dose and frequency of FMT. In the stool FMT group, the single dose of stool was 30g-80g, and in the capsule FMT group, the single dose of stool was 9.5g-50g (Table 2). Meta-regression analysis showed that neither single stool dose nor total stool dose of FMT were associated with IBS-SSS score (p>0.05). The frequency of FMT has also differed between included RCTs and might account for differences in results (7). Except for Holvoet et al (45), all other RCTs performed a single FMT administration for the stool FMT in this meta-analysis. Our results shown that, ignoring the different definitions of clinical response rate (Supplementary Table 3), the total clinical response rate at 3 months was 70.0% in the stool FMT group and 32.0% in the placebo group. In the study of Holvoet et al (45), the continued response rate was 21% at 1 year after first FMT, and the median time to loss of response was four months (3.5 months-12 months). A second FMT was performed for patients who responded initially to first FMT but lost the effect at 1 year in the study, and it was successful in 67% of patients (45). It suggested that repeated FMT might be a better way to induce a lasting effect in patients with IBS. Previously RCTs that have shown positive results in patients with ulcerative colitis have used an FMT dosing/frequency strategy of enemas once weekly for 6 weeks (68), or 5 days per week for 8 weeks (69, 70).
Four RCTs (7, 46, 54, 55) performed capsule FMT in this systematic review and meta-analysis. The frequencies of oral capsules were respectively 25 capsules per day for 12 days (46), 25 capsules per day for 3 days (7), 19 capsules per day for 1 day (54), and 30 capsule per day for 3 days (55). Three RCTs (7, 46, 54) did not confirm that capsule FMT has a positive effect on patients with IBS, which was consistent with the conclusion of our study. However, considering the effect of dose and frequency of FMT on the results, we suggest that the duration of capsule FMT should be increased to 6-8 weeks in future studies.
5) IBS subtypes. IBS is diagnosed using the Rome criteria, which have volved over the years from the Rome I criteria to the latest Rome IV (71). It is categorized into 4 subtypes based on the predominant stool form or frequency reported by the individual: IBS with constipation (IBS-C); IBS with diarrhea (IBS-D); IBS with mixed bowel habit (IBS-M); or IBS unclassified (IBS-U), where stool form or frequency cannot classify the patient accurately into one of the other 3 subtypes (71). Of the nine RCTs included, eight used Rome III and one used Rome IV, six RCTs included a mixture of patients with differing IBS subtypes, and three RCTs included patients only with IBS-D (Table 1). Compared with Rome III, Rome IV is more restrictive and less stable among both functional bowel disorder groups and IBS subtypes (72, 73), the rate of the subtypes change is respectively 24.5% and 31.7% for Rome III and Rome IV in one year (71). Therefore, we are more inclined to support the Rome III as the diagnostic and classification criteria for studies of patients with IBS, and it may be more preferable to subgrouping IBS patients based on the subtypes in future studies.
6) Gender difference. Holvoet et al (45) shown that there was a clear gender difference in the response to FMT, with female patients responding significantly better to active treatment compared to males. However, another RCT (21) found no effect of gender on FMT. Our meta-analysis based on the IBS subtypes shown that the difference in clinical response rate was statistically significant between male and female for patients with IBS-D and IBS-M (two RCTs, RR=0.58, 95% [0.37, 0.89], p=0.01), but there were no differences in IBS-SSS score and IBS-QoL score for IBS patients of all subtypes (P>0.05). It is worth mentioning that this phenomenon exists not only in the FMT process, but also in other IBS treatment options, such as serotonin antagonist alosetron, ibodutant and adding cognitive behavioral therapy to medical treatment, which in favor of effectiveness towards female in either satisfactory relief of overall IBS symptoms or percentage of pain-free days (74). In addition, studies have confirmed that IBS is more common in females (75, 76), and they are more likely to have severe symptoms and coexistent anxiety or depression (77). Thus, gender is one of the possible factors affecting the effect of FMT, which should be paid attention to in future studies.
The safety of FMT for patients with IBS may still one of the focuses of concern (78–80). In this study, seven RCTs (7, 20–22, 46, 50, 54) reported the adverse events, of which most were mild self-limiting gastrointestinal symptoms (Table 3). Only one serious adverse event was reported in a participant, who was admitted to hospital for a few hours of observation due to transient vertigo and nausea during colonoscopy (50).
It is generally accepted that IBS is characterized by gut microbiome dysbiosis, but a specific microbial pattern that characterizes all patients with IBS has not been identified due to the lack of consistency in results which seems to be related to the heterogeneity of microbiota assessment (81, 82). All of nine included RCTs reported the characteristics and changes of microbiome profiles after FMT for patients with IBS in this systematic review and meta-analysis, the main conclusions were shown in Supplementary Table 6. In all these RCTs, the gut microbiome profiles changed significantly in the groups received FMT. Three RCTs (45, 46, 52) shown that the microbial diversity or richness could be increased after FMT for IBS patients, and five RCTs (7, 20, 22, 46, 52) shown that the microbial composition of the FMT-treated patients shifted towards the donors after the intervention. Holster et al. (53) shown that the microbe-host response was influenced by FMT on the mucosal gene expression level. However, it is a pity that they found none of these changes correlated with clinical improvements. The relationships between the microbiome and the effect of FMT and the etiology of IBS remain unsolved. In addition, microbiota-derived metabolites, such as bile acids, short-chain fatty acids, vitamins, amino acids, serotonin and hypoxanthine, are proposed as possible etiological factors of IBS, and they may provide some new avenues for the diagnosis and treatment of IBS (8, 49).
We believe that this study is the most comprehensive systematic review and meta-analysis so far for use of FMT in patients with IBS. The risk of bias of included RCTs was low in the stool FMT, and was moderate in the capsule FMT. Although there is heterogeneity among different studies, the results of main outcomes obtained after removing the studies with obvious heterogeneity are the same as the former. We used different methods to analyze the quality and reliability of the main outcomes from different perspectives, and the conclusion is reliable (Figure 2; Tables 4, 5).
There are some limitations in this RCTs-based meta-analysis, and we put forward some suggestions for future studies about FMT for IBS patients. First, although nine RCTs were included, they were all small sample studies, the qualities of the primary outcomes were downgraded by one level to moderate. In the clinical practice guidelines for IBS published in recent years, FMT was not recommended as a first-line or even second-line treatment because of the low quality of clinical evidence (9, 83, 84). Thus, RCTs with large sample size is urgently needed, which is of great significance to further improve the qualities of outcomes. Second, most of the included RCTs in this meta-analysis were conducted in European countries, and the epidemiological data shown a wide variation in the prevalence of IBS globally. Considering the influence of an individual’s geographical and cultural context on IBS, researches need to be multicultural in design, encouraging global collaboration (59). Third, most of the RCTs included a mixture of patients with differing IBS subtypes, and the rate of the subtypes change to each other is significant in one year, it may be more preferable to subgrouping IBS patients based on the subtypes in future studies. Fourth, different outcomes were reported in these RCTs, and different criteria were used to define the same outcomes. We suggest that the clinical remission rate, IBS-SSS Score, IBS-QoL score and other outcomes should be reported in future studies. Clinical remission rate should be defined as IBS-SSS score decreased by ≥50 points after FMT (7, 20, 21, 54), IBS-SSS Score and IBS-QoL score should be measured by using the disease-specific questionnaire (40, 85). Fifth, this meta-analysis showed that a single FMT was effective for IBS patients within 3 months. The median time to loss of response is four months (3.5 months-12 months) (45), and repeated FMT may be a better way to induce a lasting effect in the future studies. Sixth, fecal material from one donor may be better than that from multiple donors in the FMT for a single IBS patient. Seventh, capsule FMT needs to be further studied. Eighth, the relationship between the microbiome and the effect of FMT for IBS is still unclear.
In conclusion, a single stool FMT is effective and safe for patients with IBS, and the efficacy of capsule FMT for IBS remains to be studied in the future. Some factors may affect the effect of FMT, and the relationship between the gut microbiome and FMT for IBS is still unclear.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
MW and YZ designed the study. MW, XX and YZ independently assessed studies for possible inclusion and collected the data. XX and SZ analyzed the data. MW, ZW, and XM drafted the manuscript. All authors revised and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (82060800), Gansu Province Youth Science and Technology Fund program (20JR10RA759), Health industry science and technology plan of Gansu Province (GSWSKY2020-30), Natural Science Foundation of Gansu Province (22JR5RA189), Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2021-QN-A01), and the Fundamental Research Funds for the Central Universities (31920200047). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1136343/full#supplementary-material
Supplementary Figure 1 | Clinical response rate at different times between FMT and placebo groups
Supplementary Figure 2 | IBS-SSS score compared FMT and placebo groups at different times
Supplementary Figure 3 | IBS-SSS score in the stool FMT group after removed the obviously heterogeneous study
Supplementary Figure 4 | IBS-SSS score compared the capsule FMT and placebo groups at different times
Supplementary Figure 5 | IBS-QoL score compared FMT and placebo groups at different times
1. Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology (2021) 160:99–114.e3. doi: 10.1053/j.gastro.2020.04.014
2. Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol (2020) 5:908–17. doi: 10.1016/S2468-1253(20)30217-X
3. Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet (2020) 396:1675–88. doi: 10.1016/S0140-6736(20)31548-8
4. Flacco ME, Manzoli L, De Giorgio R, Gasbarrini A, Cicchetti A, Bravi F, et al. Costs of irritable bowel syndrome in European countries with universal healthcare coverage: a meta-analysis. Eur Rev Med Pharmacol Sci (2019) 23:2986–3000. doi: 10.26355/eurrev_201904_17580
5. Zhang F, Xiang W, Li CY, Li SC. Economic burden of irritable bowel syndrome in China. World J Gastroenterol (2016) 22:10450–60. doi: 10.3748/wjg.v22.i47.10450
6. Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the united states: update 2021. Gastroenterology (2022) 162:621–44. doi: 10.1053/j.gastro.2021.10.017
7. Aroniadis OC, Brandt LJ, Oneto C, Feuerstadt P, Sherman A, Wolkoff AW, et al. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: a double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol Hepatol (2019) 4:675–85. doi: 10.1016/S2468-1253(19)30198-0
8. Xiao L, Liu Q, Luo M, Xiong L. Gut microbiota-derived metabolites in irritable bowel syndrome. Front Cell Infect Microbiol (2021) 11:729346. doi: 10.3389/fcimb.2021.729346
9. Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, et al. ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol (2021) 116:17–44. doi: 10.14309/ajg.0000000000001036
10. Jeffery IB, O'Herlihy E, Shanahan F, O' Toole PW. Microbiome alterations in IBS. Gut (2020) 69:2263–4. doi: 10.1136/gutjnl-2020-320919
11. Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: a systematic review and meta-analysis. Dig Liver Dis (2017) 49:331–7. doi: 10.1016/j.dld.2017.01.142
12. Browne PD, Cold F, Petersen AM, Halkjær SI, Christensen AH, Günther S, et al. Engraftment of strictly anaerobic oxygen-sensitive bacteria in irritable bowel syndrome patients following fecal microbiota transplantation does not improve symptoms. Gut Microbes (2021) 13:1–16. doi: 10.1080/19490976.2021.1927635
13. Hui W, Li T, Liu W, Zhou C, Gao F. Fecal microbiota transplantation for treatment of recurrent c. difficile infection: an updated randomized controlled trial meta-analysis. PloS One (2019) 14:e0210016. doi: 10.1371/journal.pone.0210016
14. Hvas CL, Dahl Jørgensen SM, Jørgensen SP, Storgaard M, Lemming L, Hansen MM, et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. Gastroenterology (2019) 156:1324–1332.e3. doi: 10.1053/j.gastro.2018.12.019
15. Caldeira LF, Borba HH, Tonin FS, Wiens A, Fernandez-Llimos F, Pontarolo R, et al. Fecal microbiota transplantation in inflammatory bowel disease patients: a systematic review and meta-analysis. PloS One (2020) 15:e0238910. doi: 10.1371/journal.pone.0238910
16. El-Salhy M, Patcharatrakul T, Gonlachanvit S. Fecal microbiota transplantation for irritable bowel syndrome: an intervention for the 21(st) century. World J Gastroenterol (2021) 27:2921–43. doi: 10.3748/wjg.v27.i22.2921
17. Xu D, Chen VL, Steiner CA, Berinstein JA, Eswaran S, Waljee AK, et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol (2019) 114:1043–50. doi: 10.14309/ajg.0000000000000198
18. Myneedu K, Deoker A, Schmulson MJ, Bashashati M. Fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. United Eur Gastroenterol J (2019) 7:1033–41. doi: 10.1177/2050640619866990
19. Elhusein AM, Fadlalmola HA. Efficacy of fecal microbiota transplantation in irritable bowel syndrome patients: an updated systematic review and meta-analysis. Gastroenterol Nurs (2022) 45:11–20. doi: 10.1097/SGA.0000000000000652
20. Lahtinen P, Jalanka J, Hartikainen A, Mattila E, Hillilä M, Punkkinen J, et al. Randomised clinical trial: faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment Pharmacol Ther (2020) 51:1321–31. doi: 10.1111/apt.15740
21. El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut (2020) 69:859–67. doi: 10.1136/gutjnl-2019-319630
22. Holster S, Lindqvist CM, Repsilber D, Salonen A, de Vos WM, König J, et al. The effect of allogenic versus autologous fecal microbiota transfer on symptoms, visceral perception and fecal and mucosal microbiota in irritable bowel syndrome: a randomized controlled study. Clin Transl Gastroenterol (2019) 10:e00034. doi: 10.14309/ctg.0000000000000034
23. Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions version 6.2 (2021). Available at: www.training.cochrane.org/handbook.
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 372:n71. doi: 10.1136/bmj.n71
25. Amir-Behghadami M, Janati A. Population, intervention, comparison, outcomes and study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J (2020) 37:387. doi: 10.1136/emermed-2020-209567
26. Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res (2014) 14:579. doi: 10.1186/s12913-014-0579-0
27. Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc (2016) 104:240–3. doi: 10.3163/1536-5050.104.3.014
28. Rohatgi A. WebPlotDigitizer 4.5. Pacifica, California, USA (2021). Available at: https://automeris.io/WebPlotDigitizer.
29. Drevon D, Fursa SR, Malcolm AL. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif (2017) 41:323–39. doi: 10.1177/0145445516673998
30. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135
31. Shi J, Luo D, Weng H, Zeng X-T, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods (2020) 11:641–54. doi: 10.1002/jrsm.1429
32. Weir CJ, Butcher I, Assi V, Lewis SC, Murray GD, Langhorne P, et al. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med Res Methodol (2018) 18:25. doi: 10.1186/s12874-018-0483-0
33. Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol (2011) 64:407–15. doi: 10.1016/j.jclinepi.2010.07.017
34. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. rating the quality of evidence–inconsistency. J Clin Epidemiol (2011) 64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017
35. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. rating the quality of evidence–indirectness. J Clin Epidemiol (2011) 64:1303–10. doi: 10.1016/j.jclinepi.2011.04.014
36. Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. rating the quality of evidence–imprecision. J Clin Epidemiol (2011) 64:1283–93. doi: 10.1016/j.jclinepi.2011.01.012
37. Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. rating the quality of evidence–publication bias. J Clin Epidemiol (2011) 64:1277–82. doi: 10.1016/j.jclinepi.2011.01.011
38. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
39. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. Bmj (2004) 328:1490. doi: 10.1136/bmj.328.7454.1490
40. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther (1997) 11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x
41. Wiklund IK, Fullerton S, Hawkey CJ, Jones RH, Longstreth GF, Mayer EA, et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol (2003) 38:947–54. doi: 10.1080/00365520310004209
42. Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry (2020) 81(5):20f13681. doi: 10.4088/JCP.20f13681
43. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
44. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj (2011) 343:d4002. doi: 10.1136/bmj.d4002
45. Holvoet T, Joossens M, Vázquez-Castellanos JF, Christiaens E, Heyerick L, Boelens J, et al. Fecal microbiota transplantation reduces symptoms in some patients with irritable bowel syndrome with predominant abdominal bloating: short- and long-term results from a placebo-controlled randomized trial. Gastroenterology (2021) 160:145–157.e8. doi: 10.1053/j.gastro.2020.07.013
46. Halkjær SI, Christensen AH, Lo BZS, Browne PD, Günther S, Hansen LH, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut (2018) 67:2107–15. doi: 10.1136/gutjnl-2018-316434
47. Madsen AMA, Halkjær SI, Christensen AH, Günther S, Browne PD, Kallemose T, et al. The effect of faecal microbiota transplantation on abdominal pain, stool frequency, and stool form in patients with moderate-to-severe irritable bowel syndrome: results from a randomised, double-blind, placebo-controlled study. Scand J Gastroenterol (2021) 56:761–9. doi: 10.1080/00365521.2021.1915375
48. El-Salhy M, Casen C, Valeur J, Hausken T, Hatlebakk JG. Responses to faecal microbiota transplantation in female and male patients with irritable bowel syndrome. World J Gastroenterol (2021) 27:2219–37. doi: 10.3748/wjg.v27.i18.2219
49. El-Salhy M, Valeur J, Hausken T, Gunnar Hatlebakk J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol Motil (2021) 33:e13983. doi: 10.1111/nmo.13983
50. Johnsen PH, Hilpüsch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol (2018) 3:17–24. doi: 10.1016/S2468-1253(17)30338-2
51. Johnsen PH, Hilpüsch F, Valle PC, Goll R. The effect of fecal microbiota transplantation on IBS related quality of life and fatigue in moderate to severe non-constipated irritable bowel: secondary endpoints of a double blind, randomized, placebo-controlled trial. EBioMedicine (2020) 51:102562. doi: 10.1016/j.ebiom.2019.11.023
52. Goll R, Johnsen PH, Hjerde E, Diab J, Valle PC, Hilpusch F, et al. Effects of fecal microbiota transplantation in subjects with irritable bowel syndrome are mirrored by changes in gut microbiome. Gut Microbes (2020) 12:1794263. doi: 10.1080/19490976.2020.1794263
53. Holster S, Hooiveld GJ, Repsilber D, Vos WM, Brummer RJ, König J. Allogenic faecal microbiota transfer induces immune-related gene sets in the colon mucosa of patients with irritable bowel syndrome. Biomolecules (2019) 9(10):586. doi: 10.3390/biom9100586
54. Singh P, Alm EJ, Kelley JM, Cheng V, Smith M, Kassam Z, et al. Effect of antibiotic pretreatment on bacterial engraftment after fecal microbiota transplant (FMT) in IBS-d. Gut Microbes (2022) 14:2020067. doi: 10.1080/19490976.2021.2020067
55. Lin H, Guo Q, Wen Z, Tan S, Chen J, Lin L, et al. The multiple effects of fecal microbiota transplantation on diarrhea-predominant irritable bowel syndrome (IBS-d) patients with anxiety and depression behaviors. Microb Cell Fact (2021) 20:233. doi: 10.1186/s12934-021-01720-1
56. Mazzawi T, Hausken T, El-Salhy M. Changes in colonic enteroendocrine cells of patients with irritable bowel syndrome following fecal microbiota transplantation. Scand J Gastroenterol (2022) 57:792–6. doi: 10.1080/00365521.2022.2036809
57. El-Salhy M, Winkel R, Casen C, Hausken T, Gilja OH, Hatlebakk JG. Efficacy of fecal microbiota transplantation for patients with irritable bowel syndrome at 3 years after transplantation. Gastroenterology (2022) 163:982–994.e14. doi: 10.1053/j.gastro.2022.06.020
58. El-Salhy M, Mazzawi T, Hausken T, Hatlebakk JG. The fecal microbiota transplantation response differs between patients with severe and moderate irritable bowel symptoms. Scand J Gastroenterol (2022) 57:1036–45. doi: 10.1080/00365521.2022.2064725
59. Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol (2020) 17:473–86. doi: 10.1038/s41575-020-0286-8
60. Mazzawi T, Hausken T, Refsnes PF, Hatlebakk JG, Lied GA. The effect of anaerobically cultivated human intestinal microbiota compared to fecal microbiota transplantation on gut microbiota profile and symptoms of irritable bowel syndrome, a double-blind placebo-controlled study. Microorganisms (2022) 10(9):1819. doi: 10.3390/microorganisms10091819
61. Pomares Bascuñana R, Veses V, Sheth CC. Effectiveness of fecal microbiota transplant for the treatment of clostridioides difficile diarrhea: a systematic review and meta-analysis. Lett Appl Microbiol (2021) 73:149–58. doi: 10.1111/lam.13486
62. Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut (2019) 68:2111–21. doi: 10.1136/gutjnl-2019-319548
63. Haifer C, Kelly CR, Paramsothy S, Andresen D, Papanicolas LE, McKew GL, et al. Australian Consensus statements for the regulation, production and use of faecal microbiota transplantation in clinical practice. Gut (2020) 69:801–10. doi: 10.1136/gutjnl-2019-320260
64. Keller JJ, Ooijevaar RE, Hvas CL, Terveer EM, Lieberknecht SC, Högenauer C, et al. A standardised model for stool banking for faecal microbiota transplantation: a consensus report from a multidisciplinary UEG working group. United Eur Gastroenterol J (2021) 9:229–47. doi: 10.1177/2050640620967898
65. Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent clostridium difficile infection: a randomized clinical trial. Jama (2016) 315:142–9. doi: 10.1001/jama.2015.18098
66. Tang G, Yin W, Liu W. Is frozen fecal microbiota transplantation as effective as fresh fecal microbiota transplantation in patients with recurrent or refractory clostridium difficile infection: a meta-analysis? Diagn Microbiol Infect Dis (2017) 88:322–9. doi: 10.1016/j.diagmicrobio.2017.05.007
67. Ramai D, Zakhia K, Fields PJ, Ofosu A, Patel G, Shahnazarian V, et al. Fecal microbiota transplantation (FMT) with colonoscopy is superior to enema and nasogastric tube while comparable to capsule for the treatment of recurrent clostridioides difficile infection: a systematic review and meta-analysis. Dig Dis Sci (2021) 66:369–80. doi: 10.1007/s10620-020-06185-7
68. Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology (2015) 149:102–109.e6. doi: 10.1053/j.gastro.2015.04.001
69. Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet (2017) 389:1218–28. doi: 10.1016/S0140-6736(17)30182-4
70. Paramsothy S, Nielsen S, Kamm MA, Deshpande NP, Faith JJ, Clemente JC, et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology (2019) 156:1440–1454.e2. doi: 10.1053/j.gastro.2018.12.001
71. Barberio B, Houghton LA, Yiannakou Y, Savarino EV, Black CJ, Ford AC, et al. Symptom stability in Rome IV vs Rome III irritable bowel syndrome. Am J Gastroenterol (2021) 116:362–71. doi: 10.14309/ajg.0000000000000946
72. Black CJ, Ford AC. Assessing the impact of changes to the Rome IV criteria for clinical practice in irritable bowel syndrome. Gastroenterology (2022) 162(2):1758–4.e1. doi: 10.1053/j.gastro.2022.01.021
73. Black CJ, Yiannakou Y, Houghton LA, Ford AC. Epidemiological, clinical, and psychological characteristics of individuals with self-reported irritable bowel syndrome based on the Rome IV vs Rome III criteria. Clin Gastroenterol Hepatol (2020) 18:392–398.e2. doi: 10.1016/j.cgh.2019.05.037
74. van Kessel L, Teunissen D, Lagro-Janssen T. Sex-gender differences in the effectiveness of treatment of irritable bowel syndrome: a systematic review. Int J Gen Med (2021) 14:867–84. doi: 10.2147/IJGM.S291964
75. Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology (2002) 123:1686–701. doi: 10.1053/gast.2002.36603
76. So SY, Savidge TC. Sex-bias in irritable bowel syndrome: linking steroids to the gut-brain axis. Front Endocrinol (Lausanne) (2021) 12:684096. doi: 10.3389/fendo.2021.684096
77. Narayanan SP, Anderson B, Bharucha AE. Sex- and gender-related differences in common functional gastroenterologic disorders. Mayo Clin Proc (2021) 96:1071–89. doi: 10.1016/j.mayocp.2020.10.004
78. Janket SJ, Ackerson LK, Diamandis EP. Drug-resistant bacteremia after fecal microbiota transplant. N Engl J Med (2020) 382:1960. doi: 10.1056/NEJMc2002496
79. DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug-resistant e. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med (2019) 381:2043–50. doi: 10.1056/NEJMoa1910437
80. Blaser MJ. Fecal microbiota transplantation for dysbiosis - predictable risks. N Engl J Med (2019) 381:2064–6. doi: 10.1056/NEJMe1913807
81. Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, et al. Gut microbiota in patients with irritable bowel syndrome-a systematic review. Gastroenterology (2019) 157:97–108. doi: 10.1053/j.gastro.2019.03.049
82. Wang L, Alammar N, Singh R, Nanavati J, Song Y, Chaudhary R, et al. Gut microbial dysbiosis in the irritable bowel syndrome: a systematic review and meta-analysis of case-control studies. J Acad Nutr Diet (2020) 120:565–86. doi: 10.1016/j.jand.2019.05.015
83. Vasant DH, Paine PA, Black CJ, Houghton LA, Everitt HA, Corsetti M, et al. British Society of gastroenterology guidelines on the management of irritable bowel syndrome. Gut (2021) 70:1214–40. doi: 10.1136/gutjnl-2021-324598
84. Smalley W, Falck-Ytter C, Carrasco-Labra A, Wani S, Lytvyn L, Falck-Ytter Y. AGA clinical practice guidelines on the laboratory evaluation of functional diarrhea and diarrhea-predominant irritable bowel syndrome in adults (IBS-d). Gastroenterology (2019) 157:851–4. doi: 10.1053/j.gastro.2019.07.004
Keywords: fecal microbiota transplantation, irritable bowel syndrome, systematic review, meta-analysis, gut microbiome, influence factor
Citation: Wang M, Xie X, Zhao S, Ma X, Wang Z and Zhang Y (2023) Fecal microbiota transplantation for irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Front. Immunol. 14:1136343. doi: 10.3389/fimmu.2023.1136343
Received: 02 January 2023; Accepted: 27 April 2023;
Published: 18 May 2023.
Edited by:
Olaf Penack, Charité University Medicine Berlin, GermanyReviewed by:
Nicolas Benech, Assistance Publique Hopitaux De Paris, FranceCopyright © 2023 Wang, Xie, Zhao, Ma, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youcheng Zhang, emhhbmd5Y2htZEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.