
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 22 February 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1135096
Background/Objectives: Autoimmune pancreatitis (AIP) is a distinct form of pancreatic inflammatory disease that responds well to glucocorticoid therapy. Knowledge on AIP has rapidly evolved over the past two decades. Based on bibliometric analysis, this study aimed to assess the research status of AIP over the past two decades and determine the research focus and emerging topics.
Methods: AIP-related publications published between January 1, 2002, and June 6, 2022, were retrieved from the Web of Science Core Collection. Bibliometric data were analyzed using HisCite, VOSviewer, CiteSpace, and bibliometrix package. Annual output, leading countries/regions, active institutions and authors, core journals and references, and keywords of AIP were evaluated.
Results: Overall, 1,772 publications were retrieved from 501 journals by 6,767 authors from 63 countries/regions. Japan published articles on AIP the most (n=728, 41.1%), followed by the United States (n=336, 19%), Germany (n=147, 8.3%), China (n=127, 7%), and Italy (n=107, 6%). The top three most prolific authors were Terumi Kamisawa from Tokyo Metropolitan Komagome Hospital (n=117), Kazuichi Okazaki from Kansai Medical University (n=103), and Shigeyuki Kawa from Matsumoto Dental University (n=94). Pancreas was the most productive journal regarding AIP research (n=95), followed by the Journal of Gastroenterology (n=67), Internal Medicine (n=66), Pancreatology (n=63), and World Journal of Gastroenterology (n=62). “Diagnosis” was the most mentioned keyword. “Risk,” “malignancy,” “outcome,” “22-gauge needle,” and “fine-needle aspiration” were recognized as emerging topics.
Conclusion: Japan was the leading country in AIP research. Research papers were mainly published in specialized journals. Diagnosis was the research focus. Long-term outcomes and pancreatic tissue acquisition were recognized as research frontiers for AIP.
Autoimmune pancreatitis (AIP) is a distinct form of pancreatic inflammatory disease that responds well to glucocorticoid therapy (1). AIP can be classified into types 1 and 2 based on clinical and pathological findings. Type 1 AIP is the pancreatic manifestation of IgG4-related disease (IgG4-RD), which is characterized by an elevation of serum IgG4 levels and infiltration of IgG4-positive plasmacytes (2, 3), whereas type 2 is more localized in the pancreas, with normal serum IgG4 levels and the presence of neutrophil infiltration (2, 3). As a relatively newly identified disease, the knowledge on the diagnosis, treatment, and clinical outcomes of AIP has rapidly evolved over the past two decades.
Bibliometric analysis enables the qualitative and quantitative profiling of publications (4) and allows researchers to identify not only the productive countries/regions, institutions, and authors but also the research focus and emerging topics within a specific field (5, 6). Additionally, bibliometric analysis has been applied in research on autoimmune digestive diseases, including inflammatory bowel disease and primary biliary cholangitis (7, 8). However, a bibliometric analysis of AIP has not been reported in the literature thus far.
In the current study, bibliometric analysis was utilized to assess the research status of AIP over the past two decades, as well as identify the research focus and emerging topics.
Literature search was performed in the Web of Science Core Collection (WoSCC) on June 6, 2022, at the Ruijin Hospital affiliated to the Shanghai Jiao Tong University School of Medicine. Thesauruses of AIP were identified in the Medical Subject Headings (MeSH) database (https://www.ncbi.nlm.nih.gov/mesh) and added to the search query, as follows: TI = (“autoimmune pancreatitis” OR “IgG4-related pancreatitis” OR “lymphoplasmacytic sclerosing pancreatitis” OR “idiopathic duct centric pancreatitis”) OR AB = (“autoimmune pancreatitis” OR “IgG4-related pancreatitis” OR “lymphoplasmacytic sclerosing pancreatitis” OR “idiopathic duct centric pancreatitis”) OR AK = (“autoimmune pancreatitis” OR “IgG4-related pancreatitis” OR “lymphoplasmacytic sclerosing pancreatitis” OR “idiopathic duct centric pancreatitis”). According to our search query, articles that mentioned AIP or its synonyms in the title, abstract, or keywords were identified. The date of publications was set between January 1, 2002, and June 6, 2022, and the type of publications was restricted to articles and review articles. Documents published earlier than January 1, 2002, were excluded. Moreover, case reports, meeting abstracts, editorial materials, and other documents types were excluded. No restriction on languages was applied.
Information on the literature identified by search query was downloaded from the WoSCC on June 6, 2022. Details of the literature, including author, title, source, sponsors, times cited count, accession number, abstract, address, document type, and cited references, were downloaded in txt and BibTex formats for further analysis. The H-index of the top 10 most productive authors were collected from Web of Science on June 6, 2022. The 2021 impact factor and 2021 Journal Citation Report category quartile of the top 10 core journals in AIP were collected from Web of Science.
Bibliometric data were analyzed using HisCite (version 12.03.17), VOSviewer (version 1.6.18), CiteSpace (version 6.1.R3), and bibliometrix package (version 3.2.1; https://cran.r-project.org/web/packages/bibliometrix/) based on R language (version 4.1.2). HisCite was used to identify the number of publications and the number of citations for productive countries, institutions, and authors. The top 10 publications with the highest number of citations in AIP research were recognized by HisCite. The annual number of publications was also identified by HisCite and visualized by ggplot2 package (version 3.3.6; https://github.com/tidyverse/ggplot2) based on R language. VOSviewer was used to recognize the top 10 keywords with the highest number of occurrences, as well as the clustering of the top 50 keywords. A list of thesauri was employed for better understanding, which included “serum IgG4 concentrations,” represented by “serum IgG4”; “diagnostic criteria,” represented by “diagnosis”; “carcinoma,” represented by “cancer”; “disease,” represented by “IgG4-related disease”; “clinical feature”; and “characteristics,” represented by “features.” CiteSpace was used to construct a dual-map overlay of the journals related to AIP and to perform a keyword burst detection of the top 25 keywords with the strongest emergent strength. CiteSpace was used to measure the collaborative centrality of countries/regions, institutions, and authors. The setting of CiteSpace was as follows: scale factor k=25, the strength of links measured by cosine, the scope of links measured within slices, and pruning with pathfinder and sliced network. The distribution of publications and collaborations between countries/regions and the annual output of the top 10 most productive authors were visualized using bibliometrix package. Clustering of collaboration among countries/regions, institutions, and authors was also visualized by bibliometrix package. The ratios of original and review articles for each year were measured using bibliometrix package.
Overall, 1,772 publications related to AIP published between 2002 and 2022 were found in the WoSCC, including 1,436 original articles and 336 review articles. Figure 1 shows the inclusion and exclusion of publications. Most identified literatures were published in English (n=1,689, 95.3%), followed by German (n=36, 2.0%), Spanish (n=18, 1.0%), French (n=12, 0.7%), Russian (n=4, 0.2%), and other 7 languages. Figure 2 presents the annual number of publications on AIP. According to the annual output, we artificially divided this period into two stages: the growing stage (2002–2009) and the mature stage (2010–2022). The yearly number of publications increased from 16 in 2002 to 113 in 2009, with an average increase of 13.9 publications per year. During the mature stage, the annual output stayed at >80 per year, and the highest output was 127 in 2012. The ratios of original and review articles for each year are displayed in Supplementary Table S1. Notably, the ratio of review articles increased from 6.3% in 2002 to 34.8% in 2021. So far, this collection of articles has been cited 55,504 times, with an average of 27.29 citations per article.
Between 2002 and 2022, 63 countries/regions over 6 continents published articles on AIP, with close collaboration among East Asia, North America, and Western Europe (Figure 3A).

Figure 3 Leading Countries/Regions. (A) Distribution of publications and collaborations between countries/regions and (B) clustering of collaboration among countries/regions.
The top 10 most productive countries are listed in Table 1. Japan was the most productive country with respect to AIP research (n=728, 41.1%), followed by the United States (n=336, 19%), Germany (n=147, 8.3%), China (n=127, 7%), and Italy (n=107, 6%). While articles from Japan received the most total citations (29,705 times), those from the United States showed the highest number of average citations (48.47 times per article). Three clusters of collaboration were identified (Figure 3B). Active collaborations were noted among Japan, the United States, China, and South Korea, whereas Germany had close collaboration with Italy. Collaborative centrality measures the position of a country/institution/author in the network of research collaboration, and a higher level of collaborative centrality reflects a greater number of research connections with partners. The United States showed the highest level of collaborative centrality, followed by Japan and Germany in this study.
A total of 6,767 authors from 1,617 institutions published articles on AIP. The top 10 most productive institutions are listed in Table 2. Tokyo Metropolitan Komagome Hospital (n=112, 6.3%) was the leading institution, followed by Kansai Medical University (n=100, 5.6%), Mayo Clinic (n=95, 5.4%), Shinshu University (n=95, 5.4%), and the University of Ulsan (n=57, 3.2%). Seven out of the ten most productive institutions were located in Japan. Four clusters of collaboration among institutions were identified (Figure 4A). The cluster led by Tokyo Metropolitan Komagome Hospital, Kansai Medical University, Shinshu University, Tohoku University, and Nagoya City University showed the closest cooperation. Mayo Clinic displayed the highest level of collaborative centrality, followed by Tokyo Metropolitan Komagome Hospital and the University of Verona.
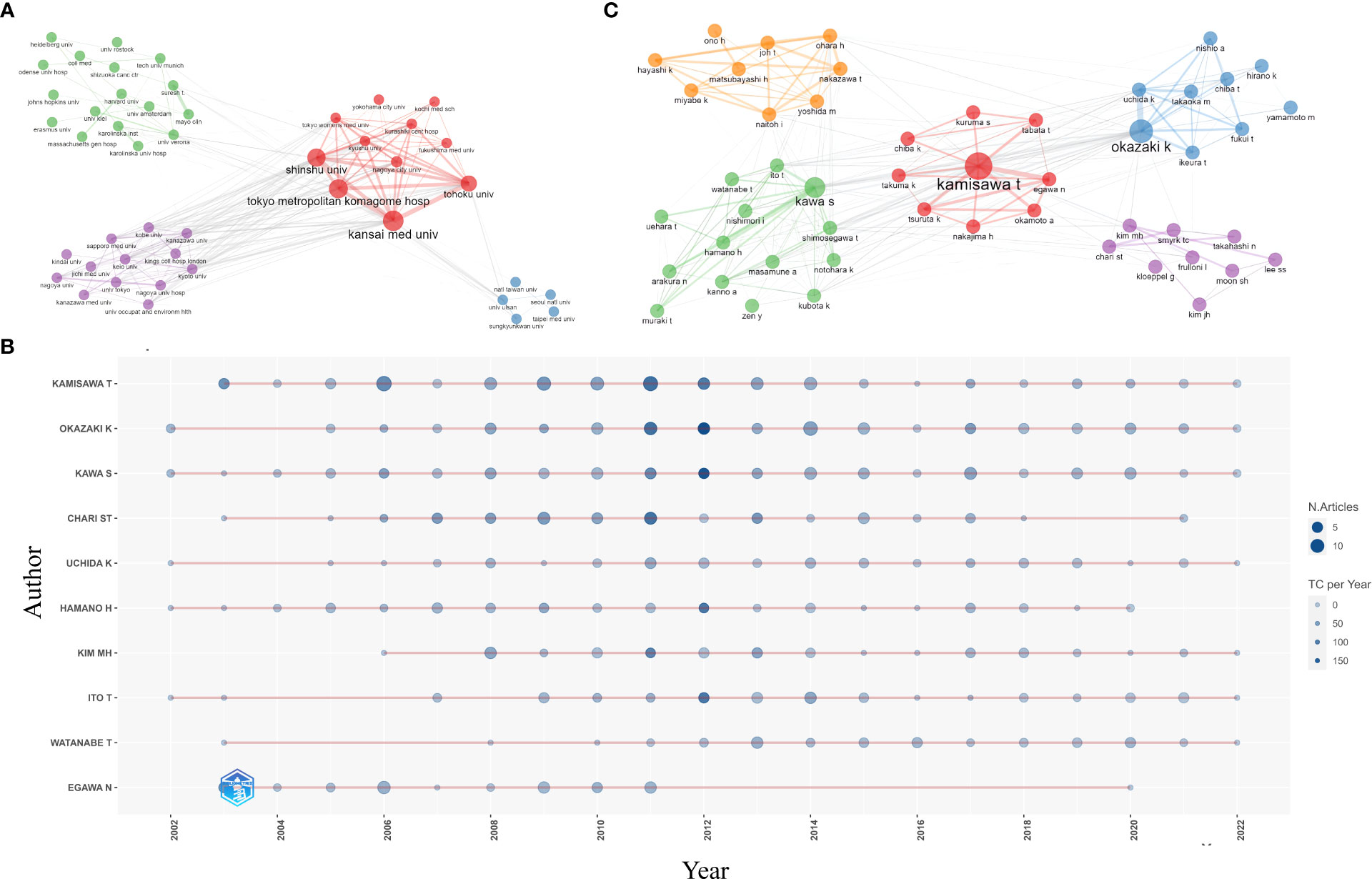
Figure 4 Active institutions and authors. (A) Clustering of collaboration among institutions (B) annual output of the top 10 most productive authors and (C) clustering of collaboration among authors.
The top three most prolific authors were Terumi Kamisawa from Tokyo Metropolitan Komagome Hospital (n=117, 6.6%), Kazuichi Okazaki from Kansai Medical University (n=103, 5.8%), and Shigeyuki Kawa from Matsumoto Dental University (n=94, 5.3%) (Table 3). Eight out of the ten most productive authors came from Japan, one was from Korea, and another was from the United States. Suresh T. Chari of the University of Texas MD Anderson Cancer Center showed the highest H-index of 87. Figure 4B displays the annual output of the top 10 most productive authors. Cooperation among authors was relatively close, with five clusters present (Figure 4C). Terumi Kamisawa showed the highest level of collaborative centrality, followed by Suresh T Chari and Myung-Hwan Kim.
Overall, 501 journals published studies on AIP. The top 10 most productive journals with respect to AIP research are summarized in Table 4. Pancreas was the most productive journal (n=95, 5.4%), followed by the Journal of Gastroenterology (n=67, 3.8%), Internal Medicine (n=66, 3.7%), Pancreatology (n=63, 3.6%), and World Journal of Gastroenterology (n=62, 3.5%). Publications from the American Journal of Gastroenterology had the highest number of average citations (104 times per article). The dual-map overlay revealed multiple inter-domain connections between journals (Figure 5). In Figure 5, the journals on the left are the citing journals, whereas the journals on the right are the cited journals; the lines denote the citation relationship between them (4). Two main citation paths were identified. Publications in the journals of Health/Nursing/Medicine and Molecular/Biology/Genetics were mostly cited by publications in the journals of Medicine/Medical/Clinical.

Figure 5 The dual-map overlay of journals publishing studies on AIP. Citing journals are on the left, cited journals are on the right, and lines represent the citation relationship.
The top 10 references with the highest number of citations are presented in Table 5. In 2003, Kamisawa et al. suggested that AIP could be a pancreatic manifestation of a chronic fibroinflammatory condition currently known as IgG4-RD. In 2009, Kamisawa et al. proposed the standard steroid regimen for AIP. In 2011, Shimosegawa et al. developed the International Consensus Diagnostic Criteria (ICDC) for AIP, which is the diagnostic standard most frequently used in clinical practice and categorizes AIP into types 1 and 2. In summary, the top 10 core references mainly focused on the diagnosis and treatment of AIP.
The top 10 keywords with the highest number of occurrences are listed in Table 6. “Diagnosis” was the most mentioned keyword. Among the top 50 keywords, three clusters were identified according to keyword co-occurrence (i.e., how frequently two keywords appear in the same literature), as shown in Figure 6A. The cluster led by “diagnosis” and “IgG4-related disease” displayed the highest number of occurrences, followed by the cluster led by “cancer” and “features” and then the cluster led by “serum IgG4” and “cholangitis.”
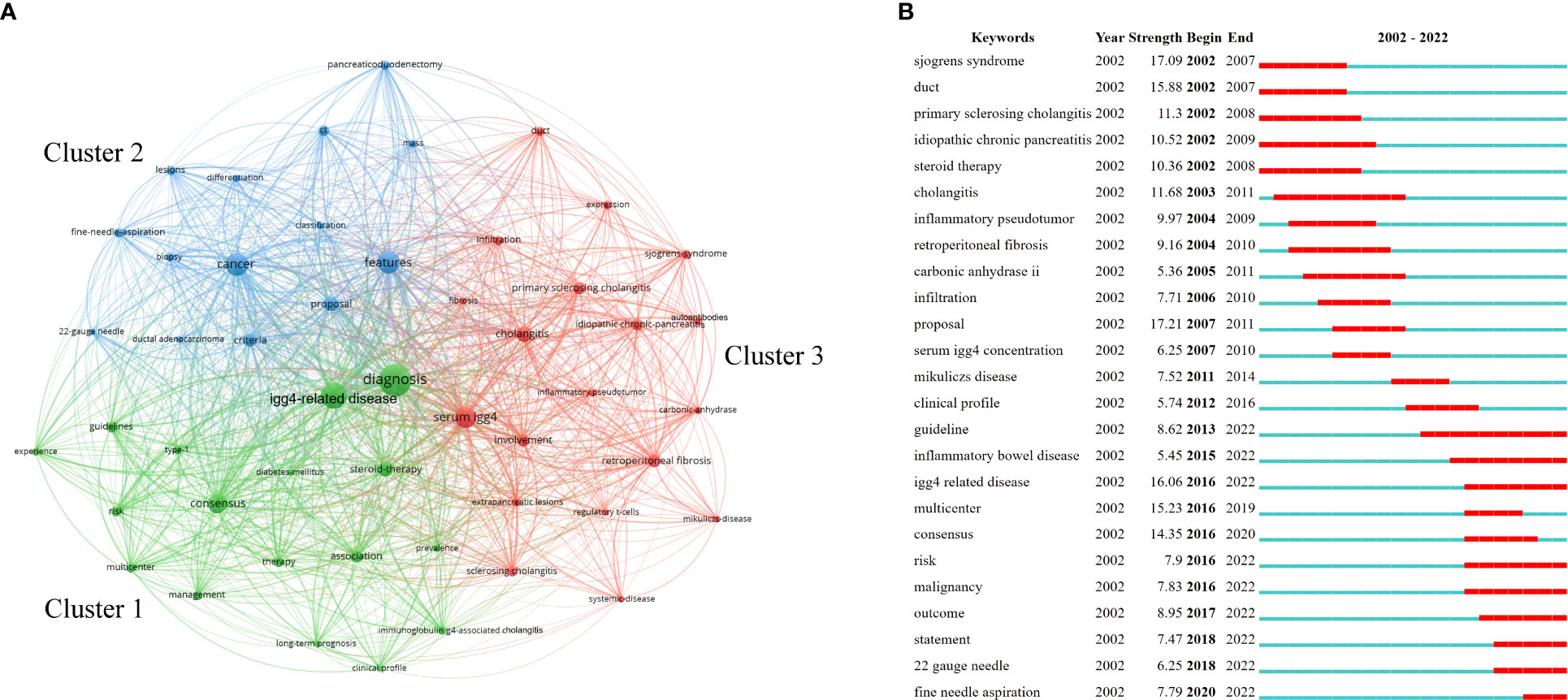
Figure 6 Analysis of keywords related to AIP. (A) Clustering of the top 50 keywords with the highest number of occurrences and (B) keyword burst detection of the top 25 keywords with the strongest emergent strength.
Keyword burst detection is regarded as an indicator of research frontiers or emerging topics in a specific field over time (9, 10). The top 25 keyword terms with the strongest emergent strength are illustrated in Figure 6B. In Figure 6B, “Year” indicates the year in which the keyword first appeared; “Begin” and “End” indicate the starting and ending years of the keyword as a frontier, respectively; and “Strength” indicates the emergent strength. Figure 6B reflects the research frontiers during different time periods. “Proposal” was the focus of research in 2007–2011, with 17.21 being the strongest emergent strength. “Risk,” “malignancy,” “outcome,” “22-gauge needle,” and “fine-needle aspiration” have been the research frontiers in recent years.
Herein, we conducted a bibliometric analysis of AIP-related publications over the last 20 years. The annual number of publications showed an upward trend from 2002 to 2009 and has remained relatively stable since 2010. Leading countries/regions, active institutions and authors, core journals and references, and keywords were evaluated. Some landmark articles were identified (Figure 7). To our knowledge, this is the first bibliometric analysis of AIP reported.
Japan was the leading country with respect to AIP research, contributing over 40% of studies on AIP. Seven out of the ten most productive institutions and eight out of the ten most productive authors were from Japan. Furthermore, six out of the top 10 most cited articles were first authored by Japanese researchers. Collaboration among Tokyo Metropolitan Komagome Hospital, Kansai Medical University, Shinshu University, Tohoku University, and Nagoya City University was relatively close. The Japan Pancreas Society had provided timely updates on the diagnostic criteria for AIP (11–16).
Diagnosis of AIP is currently the research focus. The Japan Pancreas Society proposed the first diagnostic criteria in 2002 (11). Since then, much progress has been made. Kamisawa et al. (17) proposed that AIP might be a pancreatic manifestation of a chronic fibroinflammatory condition currently known as IgG4-RD. Notohara et al. (18) and Zamboni et al. (19) summarized the histopathological findings of AIP and reported two subtypes—namely, lymphoplasmacytic sclerosing pancreatitis and idiopathic duct-centric pancreatitis. In 2006, Chari et al. (20) introduced the HISORt diagnostic criteria, which are based on histology, imaging of the pancreas using computed tomography or magnetic resonance imaging, serum IgG4 levels, other organ involvement, and response to steroid therapy. The integration of histology, radiology, serology, and follow-up formed a pathway for the diagnosis of AIP, which remained in further studies. In 2011, Shimosegawa et al. (2) proposed the ICDC, which are the most widely used diagnostic criteria in clinical practice. According to the ICDC, AIP can be subclassified into types 1 and 2 (2). AIP types 1 and 2 share similar radiological findings such as sausage-like pancreatic enlargement, rim-like enhancement around the lesion, delayed homogenous enhancement in the pancreatic parenchyma, and long or multiple strictures without marked upstream dilatation in the main pancreatic duct (21–23). However, AIP types 1 and 2 differ in terms of serology and histopathology. As the pancreatic manifestation of IgG4-RD, type 1 AIP exhibits elevated serum IgG4 levels (3, 24, 25). Histopathologically, type 1 AIP corresponds to lymphoplasmacytic sclerosing pancreatitis, with abundant IgG4-positive plasma cell infiltration (2). Other organ involvement, including sclerosing cholangitis, retroperitoneal fibrosis, and sclerosing sialadenitis, is common in type 1 AIP (26–29), with IgG4-related sclerosing cholangitis being the most common organ involvement, occurring in up to 80% of type 1 AIP cases (30, 31). In contrast, other organ involvement is less common in type 2 AIP (26). Type 2 AIP accounts for <5% of AIP cases in Eastern countries and is more common in Western countries, accounting for up to 10%–20% of AIP cases in Western countries (32, 33). Approximately 30% of type 2 AIP cases are estimated to be associated with inflammatory bowel disease, particularly ulcerative colitis (2). Type 2 AIP has an earlier onset (in individuals in their 30s) and a lower incidence of disease relapse after initial induction of remission than type 1 AIP (32). Histopathologically, type 2 AIP corresponds to idiopathic duct-centric pancreatitis and usually exhibits infiltration of no or very few IgG4-positive plasma cells (2). Serum IgG4 levels are often within the normal range (3). Owing to the paucity of reliable serum biomarkers, the diagnosis of type 2 AIP heavily relies on pancreatic histopathology (26). Moreover, both types of AIP share a dramatic response to glucocorticoid therapy (32).
Much progress has been made in the treatment of AIP. Glucocorticoids are the first-line therapy. Indications for glucocorticoid therapy are symptoms such as obstructive jaundice, abdominal pain, and other organ involvement (34). As for the induction of remission, Kamisawa et al. (35) recommended an initial dose of 0.6 mg/kg/day of oral prednisolone for 2–4 weeks. Symptoms are anticipated to be relieved within days after commencing the treatment (36). Assessment of the response to initial treatment with biochemical, serological, and radiological work-up at weeks 2–4 is recommended (36). Subsequently, glucocorticoids should be gradually tapered off, usually 5 mg every 1–2 weeks. When glucocorticoid therapy fails to relieve AIP-related symptoms, a reevaluation of diagnosis should be in order (15, 16). Clinicians should be particularly cautious about pancreatic cancer misdiagnoses. There are disputes over glucocorticoid maintenance therapy. In Western countries, glucocorticoid therapy is generally limited to the induction of remission without maintenance (37) because prolonged administration may increase the risk of infections, diabetes, osteoporosis, and cataracts (34). However, Masamune et al. (38) conducted the first AIP-related randomized controlled trial, the results of which favored maintenance therapy. Maintenance therapy with prednisolone at a dose of 5–7.5 mg/day was continued for 3 years. Compared with the cessation group, which had withdrawn at 26 weeks since the initial glucocorticoid therapy, the maintenance group achieved better 3-year relapse-free survival (42.1% vs. 76.7%, p=0.007) (38). Moreover, no major glucocorticoid-related complications requiring treatment cessation were found in the maintenance group (38). Thus, the Japanese consensus guidelines advocate a 3-year maintenance therapy to prevent disease relapse (16). Immunosuppressants such as azathioprine, methotrexate, and mycophenolate mofetil may be beneficial for patients with AIP. A recent meta-analysis had suggested that azathioprine was effective in preventing AIP relapse (39). B-cell depletion therapy has been proposed as a treatment for recurrent type 1 AIP. CD20 is a B-cell surface marker involved in calcium channel activation, cell proliferation, and B-cell differentiation (40). Rituximab is a monoclonal antibody targeting human CD20. Rituximab can induce complement activation and cell-mediated cytotoxicity, leading to B-cell depletion (41). Hart et al. (42) reported rituximab as a treatment for recurrent AIP. A decrease in serum IgG4 concentration and the extinction of pancreatic hypermetabolic signal on positron emission tomography were achieved in type 1 AIP after rituximab treatment (43). Other therapies for type 1 AIP, including rilzabrutinib (Bruton tyrosine kinase inhibitor), belimumab (B-cell activating factor inhibitor), and inebilizumab (anti-CD19 monoclonal antibody), are under investigation (44).
Keyword burst detection is capable of tracing research frontiers (9, 10). Some emerging topics had been recognized, including “risk,” “malignancy,” and “outcome.” Patients with AIP are at a high risk for malignancy (45). A recent meta-analysis of 17 studies involving 2,746 patients revealed that the overall prevalence of malignancy in patients with AIP was 9.6% (46). The top 5 most prevalent malignancies in patients with AIP were gastric, colorectal, bladder, prostate, and pancreatic cancers (46). The majority of pancreatic cancer cases in patients with AIP occurred at no less than 2 years after an AIP diagnosis (47). Other than malignancy, the long-term outcomes of AIP include diabetes mellitus (DM) and pancreatic exocrine insufficiency (PEI) (48, 49). Chronic inflammation in patients with AIP may cause damage to the pancreatic β-cells and acinar cells, leading to DM and PEI (50). By alleviating the inflammation and swelling of pancreatic tissues with glucocorticoid therapy, both endocrine and exocrine functions are supposed to be restored (50, 51). However, DM and PEI are often described at the time of diagnosis and during follow-up. The pooled prevalence of DM and PEI in patients with AIP at the time of diagnosis is 36.5% and 45.2%, respectively (52). Moreover, the pooled prevalence of DM during follow-up is 40.9% (52). The prevalence of PEI during follow-up ranged from 23.8% to 72.7% (49, 53, 54). Studies concerning malignancy, DM, and PEI in patients with AIP are mostly retrospective. More high-quality prospective cohort studies are required to better understand the long-term outcomes of patients with AIP.
In addition to clinical outcomes, pancreatic tissue acquisition is now gaining attention. A “22-gauge needle” and “fine-needle aspiration” have been recognized as research hotspots since the late 2010s by keyword burst analysis. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) have been utilized for pancreatic tissue acquisition in patients with AIP. When pathology specimen collection is necessary for diagnosis or when malignancy is suspected, EUS-FNA or EUS-FNB should be taken into consideration (16). EUS-FNA is designed for the aspiration of cells from the target lesion using a conventional straight needle. Tissue core samples acquired by EUS-FNA are usually limited in size, making the histopathological diagnosis of AIP less than satisfactory. With the help of recently developed core needles, EUS-FNB is capable of obtaining a large amount of tissue core samples with preserved tissue architecture (16, 55). According to the ICDC, the histological findings of AIP can be categorized into levels 1 and 2 (2). A recent meta-analysis demonstrated that EUS-FNB had better diagnostic yield than EUS-FNA (56). The pooled diagnostic yield for level 1 and 2 histological findings was 55.8% for EUS-FNA and 87.2% for EUS-FNB (p=0.03) (56). As for the needle size, a 19-gauge needle exhibited a better pooled diagnostic yield for level 1 and 2 histological findings than a 22-gauge needle (88.9% vs. 60.6%, p=0.023) (56). However, studies investigating the diagnostic yield of EUS-FNA or EUS-FNB had mainly focused on type 1 AIP, rather than type 2 AIP, possibly because these studies were largely conducted in Eastern countries where type 2 AIP is quite rare (<5% of total AIP cases) (32, 33). Moreover, histological diagnosis is more essential for type 2 AIP than for type 1 AIP, as there are no reliable serological markers. Further studies should recruit more patients with type 2 AIP by inviting more European and American centers.
Research cooperation among countries/regions, institutions and authors has been identified by the cooperation network in our study. Active collaborations were noted among Japan, the United States, China, and South Korea. Due to the limited cases of type 2 AIP reported, its genetic predisposition, relationship with inflammatory bowel disease, and long-term outcome have not been characterized in detail (57). This might be because the current knowledge base of AIP is mainly generated from Eastern-population-driven information, especially in Japan, where type 2 AIP is less common. Therefore, further collaborative global research is essential to understand AIP comprehensively.
This study has some limitations. First, the data were retrieved exclusively from the WoSCC, rather than other databases such as Embase and MEDLINE. The WoSCC is the most commonly applied database in bibliometric analysis because it provides timely and comprehensive updates on citation network. Moreover, software that were applied in our bibliometric analysis had difficulties in integrating data from different resources. Second, the increase in the number of publications on AIP from 2002 to 2009 might have resulted from the overall increase in scientific outputs in the medical field over the past two decades. Finally, some new emerging topics related to AIP might not have been identified because of the sensitivity of algorithms applied in the analysis. Although multiple software/packages have been used in our study, the information provided in our study is still constricted by algorithms applied in bibliometric analysis. Further development in the methodology of bibliometric analysis might be helpful in resolving these limitations.
Over the past two decades, Japan was the leading country in AIP research, with more than half of the top 10 most productive institutions and top 10 most productive authors being from Japan. Research papers were mainly published in specialized journals. Diagnosis of AIP was the research focus. Long-term outcomes and pancreatic tissue acquisition are recognized as research frontiers for AIP.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
X-DZ: conceptualization, methodology, software, formal analysis, resources, data curation, visualization, and writing – original draft. YZ: methodology, software, validation, formal analysis, resources, data curation, visualization, and writing – original draft. Y-ZZ: methodology, software, validation, formal analysis, resources, data curation, visualization, and writing – original draft. C-HZ: conceptualization, supervision, funding acquisition, and writing – review and editing. D-WZ: conceptualization, supervision, project administration, and writing – review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Nature Science Foundation of China (grant number: 82270667).
The authors thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1135096/full#supplementary-material
1. Uchida K, Okazaki K. Current status of type 1 (IgG4-related) autoimmune pancreatitis. J Gastroenterol (2022) 57:695–708. doi: 10.1007/s00535-022-01891-7
2. Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the international association of pancreatology. Pancreas (2011) 40:352–8. doi: 10.1097/MPA.0b013e3182142fd2
3. Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology (2015) 149:39–51. doi: 10.1053/j.gastro.2015.03.010
4. Chen C. Science mapping: A systematic review of the literature. J Data Inf Sci (2017) 2:1–40. doi: 10.1515/jdis-2017-0006
5. Chen C, Song M. Visualizing a field of research: A methodology of systematic scientometric reviews. PLoS One (2019) 14:e0223994. doi: 10.1371/journal.pone.0223994
6. Thompson DF, Walker CK. A descriptive and historical review of bibliometrics with applications to medical sciences. Pharmacotherapy (2015) 35:551–9. doi: 10.1002/phar.1586
7. Zhao Y, Yin Z, Du H, Huang K, Zhang F, Chen H. The latest research trends in primary biliary cholangitis: a bibliometric analysis. Clin Exp Med (2022). doi: 10.1007/s10238-022-00825-0
8. Liu C, Yu R, Zhang J, Wei S, Xue F, Guo Y, et al. Research hotspot and trend analysis in the diagnosis of inflammatory bowel disease: A machine learning bibliometric analysis from 2012 to 2021. Front Immunol (2022) 13:972079. doi: 10.3389/fimmu.2022.972079
9. Li D, Dai FM, Xu JJ, Jiang MD. Characterizing hotspots and frontier landscapes of diabetes-specific distress from 2000 to 2018: A bibliometric study. BioMed Res Int (2020) 2020:8691451. doi: 10.1155/2020/8691451
10. Zhou H, Tan W, Qiu Z, Song Y, Gao S. A bibliometric analysis in gene research of myocardial infarction from 2001 to 2015. PeerJ (2018) 6:e4354. doi: 10.7717/peerj.4354
11. Members. Diagnostic criteria for autoimmune pancreatitis by the Japan pancreas society (in Japanese). Suizo (2002) 17:585–7.
12. Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol (2006) 41:626–31. doi: 10.1007/s00535-006-1868-0
13. Shimosegawa T. Working group members of the Japan pancreas society, research committee for intractable pancreatic disease by the ministry of labor, health and welfare of japan. the amendment of the clinical diagnostic criteria in Japan (JPS2011) in response to the proposal of the international consensus of diagnostic criteria (ICDC) for autoimmune pancreatitis. Pancreas (2012) 41:1341–2. doi: 10.1097/MPA.0b013e3182706ed5
14. Okazaki K, Kawa S, Kamisawa T, Ito T, Inui K, Irie H, et al. Amendment of the Japanese consensus guidelines for autoimmune pancreatitis, 2013 i. concept and diagnosis of autoimmune pancreatitis. J Gastroenterol (2014) 49:567–88. doi: 10.1007/s00535-014-0942-2
15. Kawa S, Kamisawa T, Notohara K, Fujinaga Y, Inoue D, Koyama T, et al. Japanese Clinical diagnostic criteria for autoimmune pancreatitis, 2018: revision of Japanese clinical diagnostic criteria for autoimmune pancreatitis, 2011. Pancreas (2020) 49:e13–4. doi: 10.1097/MPA.0000000000001443
16. Okazaki K, Kawa S, Kamisawa T, Ikeura T, Itoi T, Ito T, et al. Amendment of the Japanese consensus guidelines for autoimmune pancreatitis, 2020. J Gastroenterol (2022) 57:225–45. doi: 10.1007/s00535-022-01857-9
17. Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol (2003) 38:982–4. doi: 10.1007/s00535-003-1175-y
18. Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol (2003) 27:1119–27. doi: 10.1097/00000478-200308000-00009
19. Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch (2004) 445:552–63. doi: 10.1007/s00428-004-1140-z
20. Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, et al. Diagnosis of autoimmune pancreatitis: the Mayo clinic experience. Clin Gastroenterol Hepatol (2006) 4:1010–6. doi: 10.1016/j.cgh.2006.05.017
21. Suzuki K, Itoh S, Nagasaka T, Ogawa H, Ota T, Naganawa S. CT findings in autoimmune pancreatitis: assessment using multiphase contrast-enhanced multisection CT. Clin Radiol (2010) 65:735–43. doi: 10.1016/j.crad.2010.06.002
22. Kawai Y, Suzuki K, Itoh S, Takada A, Mori Y, Naganawa S. Autoimmune pancreatitis: assessment of the enhanced duct sign on multiphase contrast-enhanced computed tomography. Eur J Radiol (2012) 81:3055–60. doi: 10.1016/j.ejrad.2012.04.023
23. Tang CSW, Sivarasan N, Griffin N. Abdominal manifestations of IgG4-related disease: a pictorial review. Insights Imaging (2018) 9:437–48. doi: 10.1007/s13244-018-0618-1
24. Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T, et al. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas (2010) 39:549–54. doi: 10.1097/MPA.0b013e3181e4d9e5
25. Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy (2009) 39:469–77. doi: 10.1111/j.1365-2222.2009.03207.x
26. Nagpal SJS, Sharma A, Chari ST. Autoimmune pancreatitis. Am J Gastroenterol (2018) 113:1301. doi: 10.1038/s41395-018-0146-0
27. Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology (2008) 134:706–15. doi: 10.1053/j.gastro.2007.12.009
28. Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol (2012) 22:1–14. doi: 10.1007/s10165-011-0508-6
29. Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol (2012) 22:21–30. doi: 10.1007/s10165-011-0571-z
30. Tanaka A, Tazuma S, Okazaki K, Nakazawa T, Inui K, Chiba T, et al. Clinical features, response to treatment, and outcomes of IgG4-related sclerosing cholangitis. Clin Gastroenterol Hepatol (2017) 15:920–26.e3. doi: 10.1016/j.cgh.2016.12.038
31. Naitoh I, Kamisawa T, Tanaka A, Nakazawa T, Kubota K, Takikawa H, et al. Clinical characteristics of immunoglobulin IgG4-related sclerosing cholangitis: comparison of cases with and without autoimmune pancreatitis in a large cohort. Dig Liver Dis (2021) 53:1308–14. doi: 10.1016/j.dld.2021.02.009
32. de Pretis N, De Marchi G, Frulloni L. Diagnosis and treatment of autoimmune pancreatitis. Curr Opin Gastroenterol (2018) 34:362–6. doi: 10.1097/MOG.0000000000000454
33. Hart PA, Kamisawa T, Brugge WR, Chung JB, Culver EL, Czakó L, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut (2013) 62:1771–6. doi: 10.1136/gutjnl-2012-303617
34. Matsubayashi H, Ishiwatari H, Imai K, Kishida Y, Ito S, Hotta K, et al. Steroid therapy and steroid response in autoimmune pancreatitis. Int J Mol Sci (2019) 21(1):257. doi: 10.3390/ijms21010257
35. Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, et al. Standard steroid treatment for autoimmune pancreatitis. Gut (2009) 58:1504–7. doi: 10.1136/gut.2008.172908
36. Löhr JM, Beuers U, Vujasinovic M, Alvaro D, Frøkjær JB, Buttgereit F, et al. European Guideline on IgG4-related digestive disease - UEG and SGF evidence-based recommendations. U Eur Gastroenterol J (2020) 8:637–66. doi: 10.1177/2050640620934911
37. Ghazale A, Chari ST. Optimising corticosteroid treatment for autoimmune pancreatitis. Gut (2007) 56:1650–2. doi: 10.1136/gut.2007.129833
38. Masamune A, Nishimori I, Kikuta K, Tsuji I, Mizuno N, Iiyama T, et al. Randomised controlled trial of long-term maintenance corticosteroid therapy in patients with autoimmune pancreatitis. Gut (2017) 66:487–94. doi: 10.1136/gutjnl-2016-312049
39. Masaki Y, Nakase H, Tsuji Y, Nojima M, Shimizu K, Mizuno N, et al. The clinical efficacy of azathioprine as maintenance treatment for autoimmune pancreatitis: a systematic review and meta-analysis. J Gastroenterol (2021) 56:869–80. doi: 10.1007/s00535-021-01817-9
40. Pavlasova G, Mraz M. The regulation and function of CD20: an “enigma” of b-cell biology and targeted therapy. Haematologica (2020) 105:1494–506. doi: 10.3324/haematol.2019.243543
41. Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene (2003) 22:7359–68. doi: 10.1038/sj.onc.1206939
42. Hart PA, Topazian MD, Witzig TE, Clain JE, Gleeson FC, Klebig RR, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo clinic experience. Gut (2013) 62:1607–15. doi: 10.1136/gutjnl-2012-302886
43. Le Cosquer G, Ribes D, Faguer S, Jeune M, Alric L, Bournet B, et al. Long-term follow-up and immunomonitoring of relapsing type 1 autoimmune pancreatitis treated with rituximab. Pancreas (2022) 51:452–62. doi: 10.1097/MPA.0000000000002048
44. Löhr JM, Vujasinovic M, Rosendahl J, Stone JH, Beuers U. IgG4-related diseases of the digestive tract. Nat Rev Gastroenterol Hepatol (2022) 19:185–97. doi: 10.1038/s41575-021-00529-y
45. Shiokawa M, Kodama Y, Yoshimura K, Kawanami C, Mimura J, Yamashita Y, et al. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol (2013) 108:610–7. doi: 10.1038/ajg.2012.465
46. Haghbin H, Chuang J, Fatima R, Zakirkhodjaev N, Lee-Smith W, Aziz M. Correlation of autoimmune pancreatitis and malignancy: systematic review and meta-analysis. Dig Dis Sci (2022) 67:3252–64. doi: 10.1007/s10620-021-07179-9
47. Macinga P, Bajer L, Del Chiaro M, Chari ST, Dite P, Frulloni L, et al. Pancreatic cancer in patients with autoimmune pancreatitis: A scoping review. Pancreatology (2021) 21:928–37. doi: 10.1016/j.pan.2021.03.007
48. Vujasinovic M, Valente R, Maier P, von Beckerath V, Haas SL, Arnelo U, et al. Diagnosis, treatment and long-term outcome of autoimmune pancreatitis in Sweden. Pancreatology (2018) 18:900–4. doi: 10.1016/j.pan.2018.09.003
49. Uchida K, Yazumi S, Nishio A, Kusuda T, Koyabu M, Fukata M, et al. Long-term outcome of autoimmune pancreatitis. J Gastroenterol (2009) 44:726–32. doi: 10.1007/s00535-009-0049-3
50. Farris AB 3rd, Lauwers GY, Deshpande V. Autoimmune pancreatitis-related diabetes: quantitative analysis of endocrine islet cells and inflammatory infiltrate. Virchows Arch (2010) 457:329–36. doi: 10.1007/s00428-010-0948-y
51. Frulloni L, Scattolini C, Katsotourchi AM, Amodio A, Gabbrielli A, Zamboni G, et al. Exocrine and endocrine pancreatic function in 21 patients suffering from autoimmune pancreatitis before and after steroid treatment. Pancreatology (2010) 10:129–33. doi: 10.1159/000265945
52. Lanzillotta M, Tacelli M, Falconi M, Arcidiacono PG, Capurso G, Della-Torre E. Incidence of endocrine and exocrine insufficiency in patients with autoimmune pancreatitis at diagnosis and after treatment: a systematic review and meta-analysis. Eur J Intern Med (2022) 100:83–93. doi: 10.1016/j.ejim.2022.03.014
53. Huggett MT, Culver EL, Kumar M, Hurst JM, Rodriguez-Justo M, Chapman MH, et al. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol (2014) 109:1675–83. doi: 10.1038/ajg.2014.223
54. Kuraishi Y, Uehara T, Watanabe T, Ashihara N, Ozawa M, Kanai K, et al. Corticosteroids prevent the progression of autoimmune pancreatitis to chronic pancreatitis. Pancreatology (2020) 20:1062–8. doi: 10.1016/j.pan.2020.07.408
55. de Pretis N, Crinò SF, Frulloni L. The role of EUS-guided FNA and FNB in autoimmune pancreatitis. Diagnostics (Basel) (2021) 11(9):1653. doi: 10.3390/diagnostics11091653
56. Yoon SB, Moon SH, Song TJ, Kim JH, Kim MH. Endoscopic ultrasound-guided fine needle aspiration versus biopsy for diagnosis of autoimmune pancreatitis: systematic review and comparative meta-analysis. Dig Endosc (2021) 33:1024–33. doi: 10.1111/den.13866
Keywords: autoimmune pancreatitis, bibliometrics, CiteSpace, emerging topics, research focus, VOSviewer
Citation: Zhang X-D, Zhang Y, Zhao Y-Z, Zhou C-H and Zou D-W (2023) Autoimmune pancreatitis: A bibliometric analysis from 2002 to 2022. Front. Immunol. 14:1135096. doi: 10.3389/fimmu.2023.1135096
Received: 31 December 2022; Accepted: 09 February 2023;
Published: 22 February 2023.
Edited by:
Stefano Francesco Crinò, University of Verona, ItalyReviewed by:
Tsukasa Ikeura, Kansai Medical University, JapanCopyright © 2023 Zhang, Zhang, Zhao, Zhou and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Hua Zhou, emhvdV9jaHVuaEAxNjMuY29t; Duo-Wu Zou, emR3cmp4aDY2QHNqdHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
‡ORCID: Xian-Da Zhang, orcid.org/0000-0002-9881-4111
Chun-Hua Zhou, orcid.org/0000-0003-2351-578X
Duo-Wu Zou, orcid.org/0000-0002-2461-5304
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.