
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 13 July 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1135061
 Xin Chen†
Xin Chen† Jun Xiao†
Jun Xiao† Luo-Qi Zhou
Luo-Qi Zhou Wen-Xiang Yu
Wen-Xiang Yu Man Chen
Man Chen Yun-Hui Chu
Yun-Hui Chu Ke Shang
Ke Shang Gang Deng
Gang Deng Wen-Hui Song
Wen-Hui Song Chuan Qin*
Chuan Qin* Deng-Ji Pan*
Deng-Ji Pan* Dai-Shi Tian*
Dai-Shi Tian*Neuromyelitis optica spectrum disorders (NMOSD) are demyelinating diseases of the central nervous system, have drawn the attention of many researchers due to the relapsing courses and cumulative disability. A first bibliometric analysis of NMOSD was conducted to identify the research hotspots and emerging trends. Articles relevant to NMOSD published in the core collection of Web of Science were retrieved and analyzed through visualized analysis using CiteSpace and VOSviewer, focusing on annual publication trends, countries, institutions, authors, journals, and keywords. The analysis showed that over the past 30 years, publications related to NMOSD had shown steady growth with slight fluctuations. The United States played an important part in this field, with the highest outputs and the greatest number of citations. Research hotspots of NMOSD had gradually shifted from the definition, biomarkers, and diagnostic criteria to diagnosis and treatment, particularly immunotherapy. This bibliometric analysis provides researchers with a theoretical basis for studying NMOSD and offers guidance for future research directions.
Neuromyelitis optica spectrum disorders (NMOSD) are immune-mediated demyelinating diseases primarily targeting the central nervous system, characterized by severe demyelination and axonal damage (1–3). The prevalence of NMOSD ranges from 0.7 to 10 per 100,000 in different populations worldwide, with the incidence in women significantly higher than in men (4, 5). Relapsing courses and cumulative disability reduce the quality of life in patients and impose great burdens on families and society, which have drawn the attention of many researchers. Bibliometric analyses have been performed in certain professional fields, such as cardiovascular disease (6, 7), infectious diseases (8, 9), and oncology (10–12). In contrast, bibliometric analysis of NMOSD is rare. This bibliometric analysis aims to elucidate the research hotspots and new trends of publications over the past 30 years.
The eligible studies published until 15 October 2022 were downloaded from the core collection of Web of Science. The main search terms were as follows: TS=Neuromyelitis Optica Spectrum disorder* OR Neuromyelitis Optica OR NMOSD OR NMO. The search was restricted to articles and reviews in English. The time span of this analysis was from 1990 to 2022. Two researchers retrieved and screened the raw data independently and then cleared divergences. The detailed screening process is shown in Supplementary Figure 1.
Data analysis and visualization were performed utilizing CiteSpace (version 6.1.R3) and VOSviewer (version 1.6.18). Microsoft Excel summarized the annual number of publications (Np) and plotted the bar chart. CiteSpace and VOSviewer were used to visualize the co-occurrence of authors, countries, journals, institutions, keywords, clusters and burst of keywords, and co-cited authors, co-cited journals, and co-cited references.
Visualization networks were composed of nodes and lines generally. The node size signified the number of publications, and the number of lines among the nodes represented cooperation intensity. In light of burst keywords, a red line segment was used to represent the period when a keyword was calculated with the strongest citation burst (13). The impact factors (IF) of journals were acquired from the latest Journal Citation Reports (JCR) 2021 in Web of Science.
The retrieval identified a total number of 5,974 publications restricted to articles and reviews in this field from 1990 to 2022. The yearly growth of publications reflected the growing trend of research. Figure 1 shows the annual number of publications (Np) on NMOSD. In general, there was an upward trend, despite slight fluctuations. It showed that Np was low from 1990 to 2006, which might result from the lack of understanding of the disease. With the discovery of pathogenic antibodies, the update of diagnostic criteria, and the development of new drugs, an increasing number of scholars concentrated on exploring the etiology, pathogenesis, clinical manifestation, diagnosis, treatment, and prevention of NMOSD. As a result, Np showed a tremendous increase from 2007 to 2021, especially more than 700 in 2021, indicating a rapid development in this field.
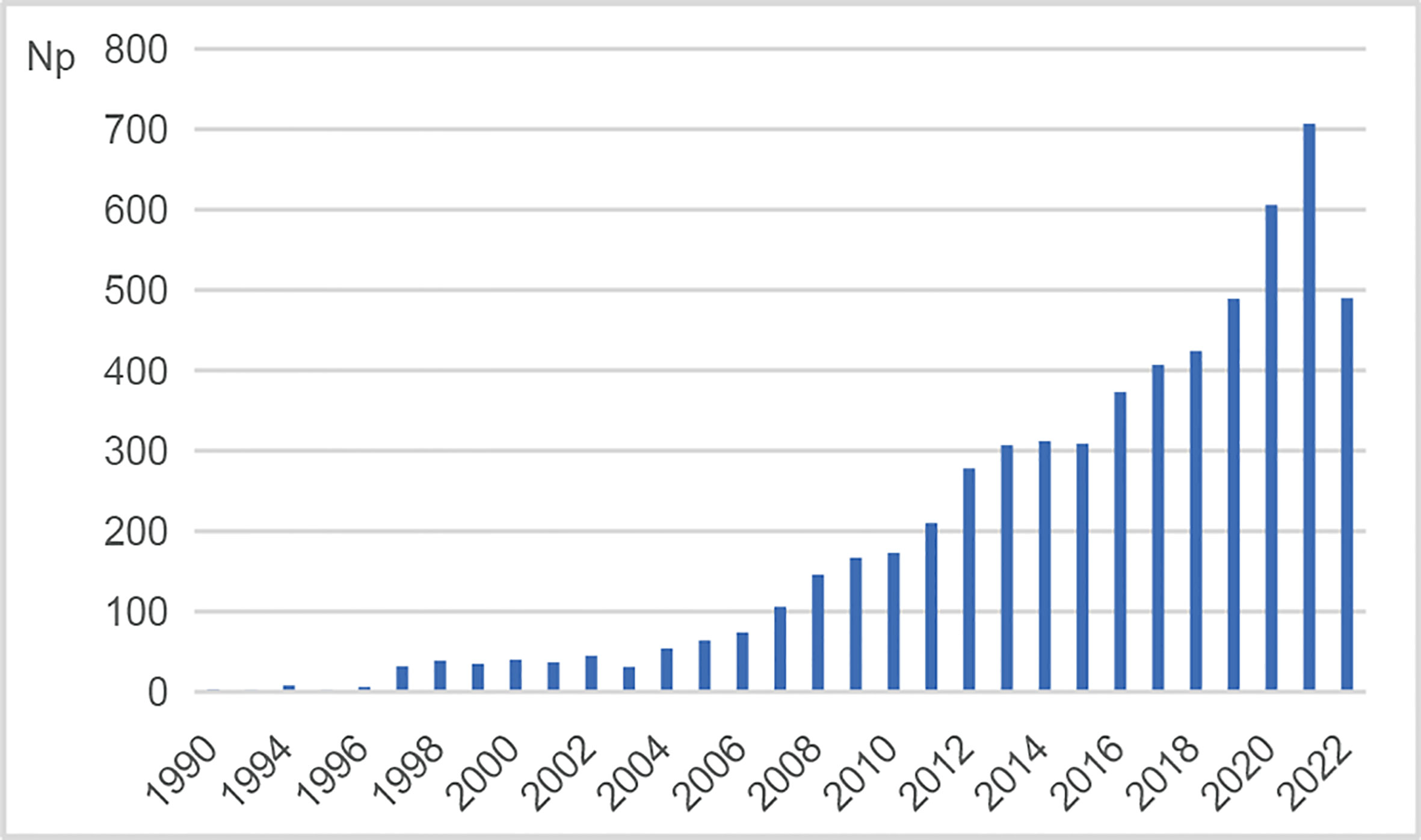
Figure 1 Annual number of publications (Np) on NMOSD research. NMOSD, neuromyelitis optica spectrum disorder.
There were 122 countries involved in publishing articles on NMOSD in the past 30 years. Figure 2 shows the co-occurrence of countries, and Table 1 lists the top 10 countries that delivered the most articles with their number of citations (Nc) and total link strength (TLS). The United States published most of the articles (1,610), followed by China (1,019) and Japan (621). TLS represented cooperation intensity with each other. In terms of TLS, the United States had the highest TLS (1,468), implying the strongest cooperation with others, followed by Germany (1,179) and the UK (1,127).
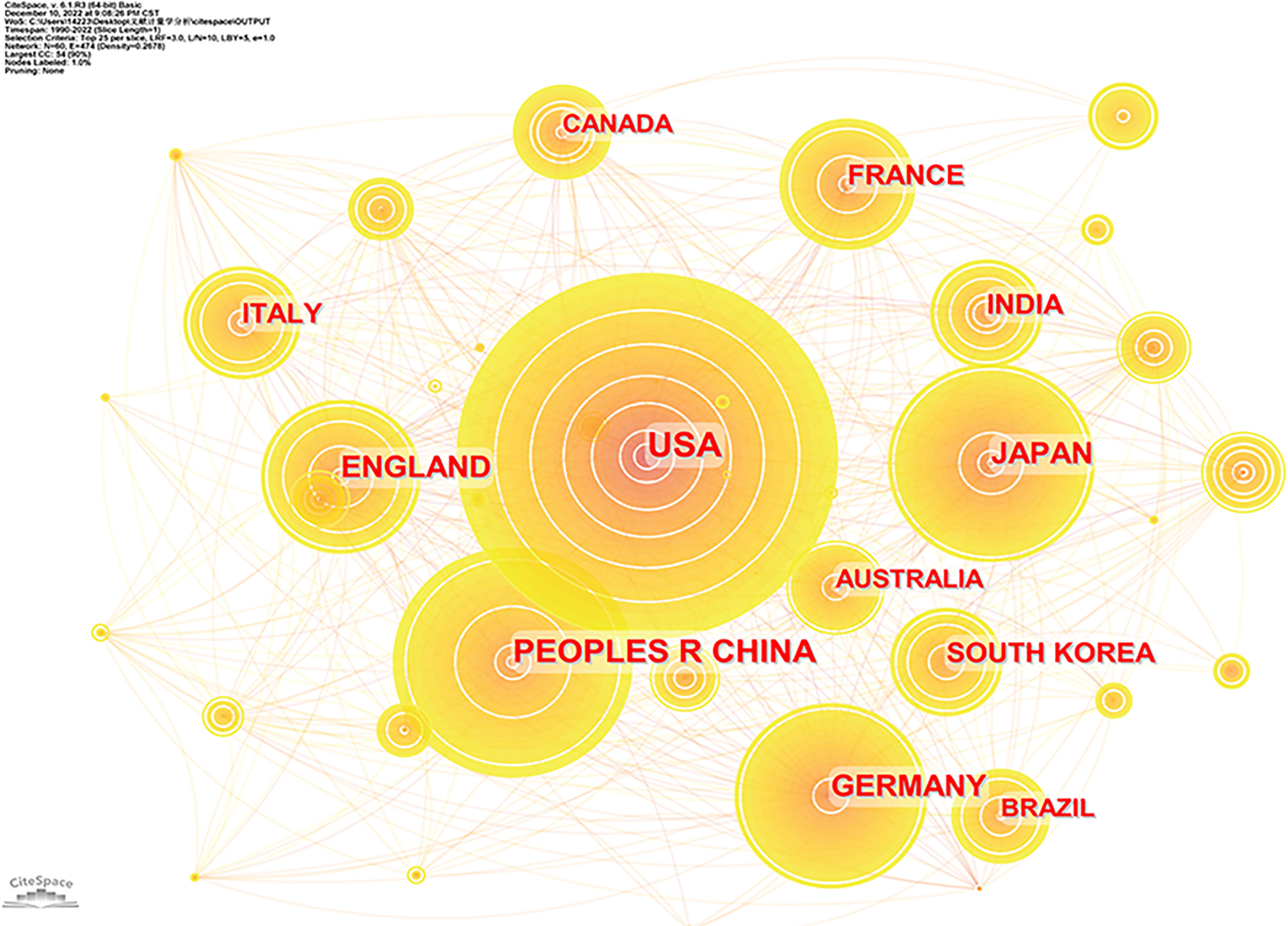
Figure 2 Co-occurrence of countries involved in the study of NMOSD. NMOSD, neuromyelitis optica spectrum disorder.
Supplementary Figure 2 shows the co-occurrence of institutions, and Table 2 lists the top 10 institutions in light of the outputs of NMOSD. These institutions were dispersed in the United States (3), Japan (3), China (2), Germany (1), and Austria (1). Mayo Clinic (247) published the largest number of articles on NMOSD; Tohoku University (202) and the University of California—San Francisco (153) followed closely. The top three institutions regarding TLS were Charité – Universitätsmedizin Berlin (763), Tohoku University (654), and Mayo Clinic (610). As the data showed, there was considerably close cooperation between institutions.
In total, 21,328 authors contributed to the publications related to NMOSD. Supplementary Figure 3 shows the co-occurrence network of authors, and Table 3 lists the top 10 authors who produced the most publications of NMOSD. Fujihara, Kazuo (115), ranking first, had the highest Np with 13,886 citations, followed by Paul, Friedemann (112), and Takahashi, Toshiyuki (108). However, in terms of TLS, Fujihara, Kazuo (770), Paul, Friedemann (756), and Nakashima, Ichiro (576) ranked the top 3 among the top 10, implying that these authors had closer cooperation with others. Notably, the top 10 authors were mainly from the United States and Japan.
Cited authors who appeared concurrently in references were named co-cited authors. Among the 71,271 co-cited authors, 263 were co-cited more than 100 times, and six were co-cited over 1,000 times. The co-occurrence of co-cited authors is visualized in Supplementary Figure 4, and the top 10 co-cited authors are displayed in Supplementary Table 1. Wingerchuk, Dean M. (6,333) was co-cited at most, followed by Jarius, Sven (3,737), and Lennon, Vanda A. (2,808).
In this research, 5,974 articles regarding NMOSD were delivered in 1,188 academic journals, of which the highest IF surpassed 50 (Lancet Neurology). Multiple Sclerosis and Related Disorders ranked first in terms of articles (374), followed by Multiple Sclerosis Journal (295) and Journal of Neuroimmunology (205). Six were in the Q1 JCR partition, and three had an IF over 10 among the top 10 journals (Supplementary Table 2).
The co-citation analysis of journals showed that Neurology (22,933 citations) was cited the most, followed by Multiple Sclerosis Journal (10,386 citations) and Annals of Neurology (8,817 citations) (Table 4 and Supplementary Figure 5). Of the total 13,258 journals, 33 had been co-cited more than 1,000 times, and six journals exceeded 5,000 co-citations. Of the top 10 co-cited journals, Lancet Neurology had the highest IF (59,935), followed by Brain (15.255). All of the top 10 co-cited journals except one (Journal of the Neurological Sciences) were divided in the Q1 JCR partition.
A dual-map overlay of journals on NMOSD was constructed by CiteSpace to explore the subject distributions of scholarly journals (14). There were two main regions on the overlay, with the left areas representing distributions of citing journals and the right representing cited journals. As we could see from Supplementary Figure 6, the citing journals mainly belonged to the areas labeled Medicine, Medical, Clinical and Molecular, Biology, Immunology, and Neurology, Sports, Ophthalmology, whereas the cited journals primarily focused on the fields labeled Molecular, Biology, Genetics, and Health, Nursing, Medicine.
Keywords generalized the main research contents of the articles. Therefore, the keyword co-occurrence network could present research topics intuitively in a certain field (15). In this research, the co-occurrence map of keywords was derived from VOSviewer. With restrictions on keywords that occurred more than 100 times, a total of 68 keywords were selected (Figure 3A). “Neuromyelitis optica” (3,077), “multiple sclerosis” (2,637), “anti-aquaporin 4 antibody” (1,535), “neuromyelitis optica spectrum disorder” (1,007), and “diagnostic criteria” (808) were the top 5 keywords used frequently. Along with the keyword co-occurrence, a density visualization map was also constructed, where keywords were colored according to the occurrence times (Supplementary Figure 7). The larger the number of keywords counted, the closer the color of nodes was to red. On the contrary, the smaller the number of keywords counted, the closer the color of nodes was to blue.
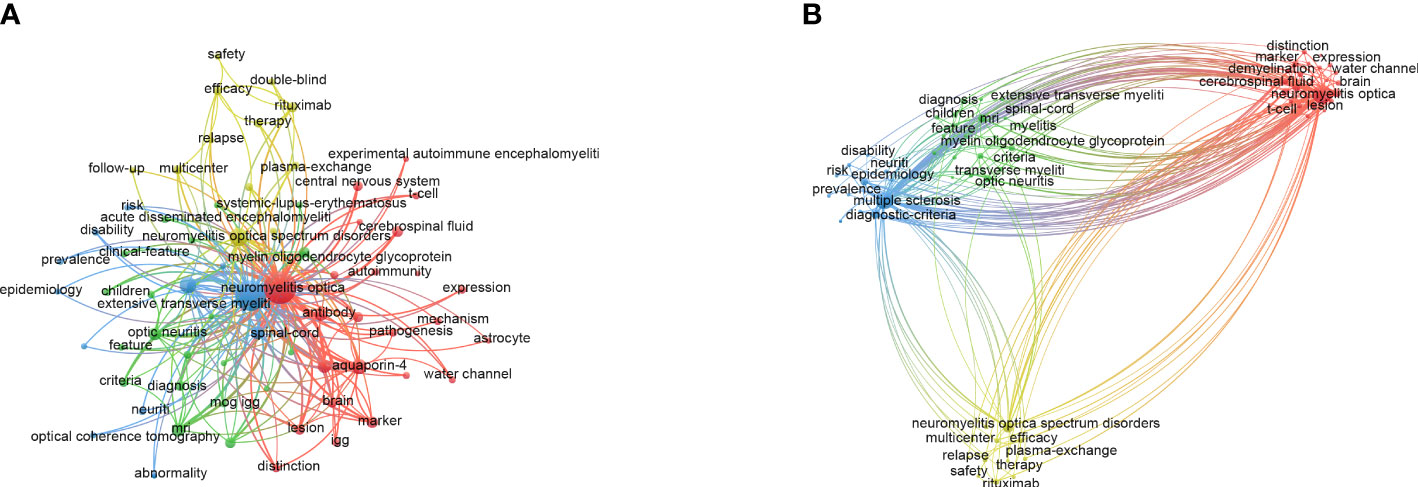
Figure 3 The maps of keywords appearing more than 100 times in the study of NMOSD. (A) Network diagram of keywords. (B) Cluster analysis of co-occurring keywords. NMOSD, neuromyelitis optica spectrum disorder.
Keyword cluster analysis aimed to display the distribution of core contents, which classified keywords based on the similarity degree (16). As shown in Figure 3B, 68 keywords that occurred more than 100 times were grouped into four clusters: cluster 1 (red nodes, 24 items) mainly focused on mechanism and biomarkers such as anti-aquaporin 4 antibody (AQP4-IgG) and cytokines; cluster 2 (green nodes, 20 items) was mainly related to clinical features, other antibodies such as myelin oligodendrocyte glycoprotein antibody, and other demyelination diseases such as acute disseminated encephalomyelitis; cluster 3 (blue nodes, 12 items) concentrated on diagnostic criteria and the manifestation of auxiliary examination such as magnetic resonance imaging and optical coherence tomography; cluster 4 (yellow nodes, 12 items) was mainly related to the treatment of NMOSD, especially in the field of immunotherapy such as immunosuppressants and monoclonal antibodies.
Burst keywords were considered another crucial indicator of research frontiers (13). Burst keywords referred to items that increased rapidly in frequency in a short time, the analysis of which reflected the evolution trend of research hotspots (16). The top 15 burst keywords are displayed after a thorough analysis in Figure 4. Burst keywords of NMOSD mainly focused on the definition and biomarkers in the early years. In recent years, the diagnosis and treatment of NMOSD have become new research hotspots in this field, and the burst is still ongoing.
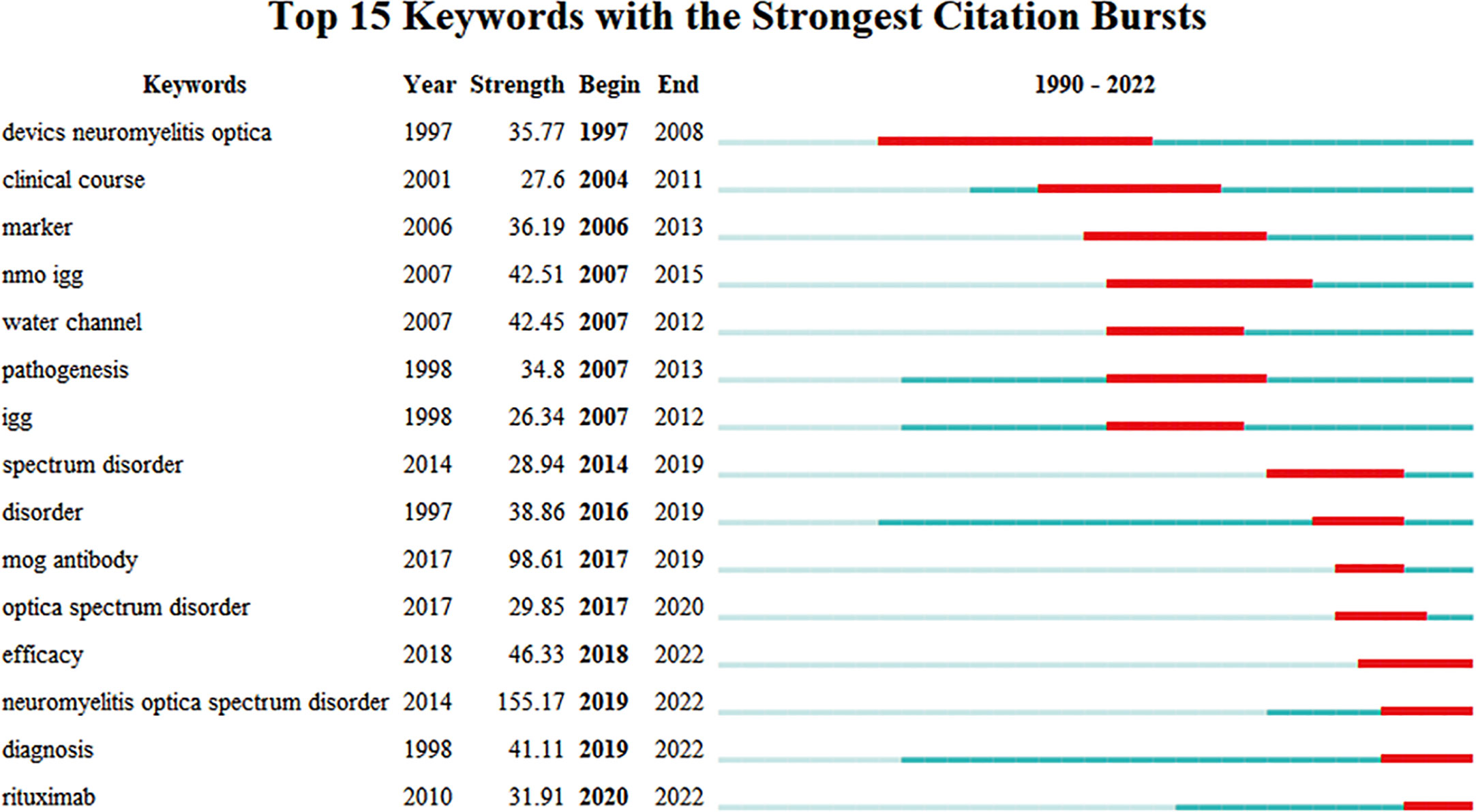
Figure 4 The top 10 keywords with the strongest citation bursts from 1997 to 2022 (generated by CiteSpace).
Co-cited references referred to two articles that were cited simultaneously, and co-citation was confirmed by calculating the frequency of co-cited references (16). Supplementary Figure 8 shows the co-occurrence of co-cited references, and Supplementary Table 3 displays the top 10 co-cited articles whose co-citation appeared 423 times at least. The most co-cited reference on NMOSD was “International consensus diagnostic criteria for neuromyelitis optica spectrum disorders”, authored by Wingerchuk, Dean M. and published in Neurology, followed by an article entitled “A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis”. In general, two of the top 10 co-cited publications were documents about the diagnostic criteria, and three were related to AQP4-IgG, a unique biomarker of NMOSD.
Until now, this is the first comprehensive bibliometric analysis of NMOSD. In this analysis, we chose VOSviewer and CiteSpace to analyze 5,974 documents on NMOSD research filtered from the core collection of Web of Science to discover research hotspots and trends in this field.
As shown in Figure 1, the annual number of publications ranged from 1 to 707 during the period from 1990 to 2021. The number of publications showed an overall upward trend in the past 30 years despite slight fluctuations, implying that this field had gradually entered into a mature phase of development (7).
The top 3 high-yield countries listed were the United States, China, and Japan. The United States, with the strongest TLS, had the highest productivity. Three of the top 10 institutions with the most published research on NMOSD were situated in the United States. In addition, three of the top 10 authors who published the most articles on NMOSD also came from the United States. Thus, it turned out that the United States occupied a leading position in the research area, and there were still differences among nations and regions in the development of this area. Compared to the United States, China had lower Np, Nc, and TLS. It is noteworthy that China started to step into this field in 2005 but ranked second in gross outputs at present, which represented that China had made great contributions to NMOSD research in the past 15 years, which may be triggered by the higher prevalence.
We also explored whether there was a possible relationship between the prevalence of NMOSD and the number of publications. According to previous population-based studies about global epidemiology, the prevalence of NMOSD under 2015 diagnostic criteria ranged from 0.7 to 10/per 100,000 persons in different populations worldwide (5). Africans had the highest prevalence, whereas Whites had the lowest (17). There was also an increased prevalence of NMOSD in Asians and Africans (5). In terms of countries, the top three productive countries were the United States, China, and Japan. The prevalence of NMOSD in East Asia, such as China and Japan, is higher than that in the United States, so it seems that there is no linear correlation between prevalence and the number of publications. The high prevalence of a certain disease in a particular region is bound to attract the attention of researchers and lead to more efforts being devoted to its study. On account of the higher prevalence but lower productivity, countries in Africa and Asia are supposed to devote more energy to NMOSD research and strengthen their international cooperation.
As an indispensable approach to research dissemination, the quality and prestige of journals play a crucial part in transmitting research results (7, 18). In this research, Multiple Sclerosis and Related Disorders, Multiple Sclerosis Journal, and Journal of Neuroimmunology published the most articles over 200. These journals occupied an important position in this field. We manually collected the publication dates of those documents and identified the duration as 2003–2022. During the first decade when the disease emerged, there were no significant publications in the aforementioned journals, possibly due to a lack of awareness regarding these disorders. In terms of co-cited journals, Neurology, Multiple Sclerosis Journal, and Annals of Neurology were cited more than 8800 times. These journals that were all in the Q1 JCR partition might declare consensus and unique insights better.
According to the authors’ analysis, Kazuo, Fujihara, from Japan made the greatest contributions with 115 publications. He was a member of Fukushima Medical University, and his team focused on treatment for NMOSD. His latest research reviewed therapeutic approaches for symptomatic treatment and function restoration in NMOSD, such as remyelinating agents and mesenchymal stem cell transplantation (19), which provided new alternatives for NMOSD restoration treatment. In the co-cited author analysis, the most productive author was Wingerchuk, Dean M., from the United States. He made great contributions to the development of NMOSD, and his articles had been delivered in many authoritative journals such as Lancet and Neurology. His latest network meta-analysis suggested that the complement component C5 inhibitor eculizumab may be an effective approach to decreasing NMOSD relapses (20). Furthermore, the most co-cited reference produced by Wingerchuk, Dean M., was entitled “International consensus diagnostic criteria for neuromyelitis optica spectrum disorders” (2). This record updated the diagnostic criteria detailedly and laid a theoretical foundation for subsequent studies. The second most co-cited article was original research on a highly specific IgG autoantibody (NMO-IgG) (21). This study evaluated the ability of NMO-IgG to differentiate NMOSD from multiple sclerosis (MS). The detection of NMO-IgG allowed for early diagnosis, which in turn facilitated the timely implementation of appropriate immunosuppressive treatment.
The co-occurrence analysis of keywords played a significant role in data visualization. The findings revealed the research focus and evolution of keywords related to NMOSD (13). The results of keyword cluster analysis revealed four significant research orientations in the field of NMOSD: pathogenesis, biomarkers, diagnosis, and treatment. The variation trend of burst keywords indicated that the main research focus had shifted from biomarkers, which were the primary area of obsession in the early stages, to treatment in recent years. In 1894, French neurologists Eugène Devic and Fernand Gault first described published cases of optic neuritis with myelitis and named the disease neuromyelitis optica (NMO) or Devic’s disease (22). Since then, NMO gained the attention of scholars. However, distinguishing NMO from MS was difficult for a long time due to the similarity in clinical manifestations (23, 24). In 2004, the discovery of pathogenic AQP4-IgG, a highly sensitive and specific serum biomarker of NMO, made it possible to distinguish NMO from classic MS (24, 25). In 2007, Wingerchuk D. M. et al. first put forward the concept of NMOSD (26). With the incremental understanding of the disease, the International Panel for NMO Diagnosis (IPND) revised diagnostic criteria and identified the medical term NMOSD in 2015 (2). AQP4-IgG was considered one of the most important criteria for disease diagnosis. Until now, we still use the term NMOSD and the updated diagnostic criteria. In the past decade, the discovery of other antibodies, such as myelin oligodendrocyte glycoprotein antibody (MOG IgG) (27) and glial fibrillary acidic protein antibody (GFAP IgG) (28), has broadened our perception of demyelinating diseases of the central nervous system. Recently, due to the relapse courses, cumulative disability, and poor survival quality of NMOSD patients, scholars have devoted themselves to elucidating the possible pathogenesis (29) and have raised widespread concern regarding treatment, particularly immunotherapy.
It is of great importance to initiate appropriate treatment early in NMOSD management. Acute attacks or relapses are usually treated with intravenous glucocorticoids, which should be started early and aggressively to hasten recovery (1). Plasma exchange or intravenous immunoglobulin can be used as adjunctive or alternative therapies to relieve symptoms for patients who are not sensitive to glucocorticoids (30). The pivotal goals during the remission period are to minimize disability and prevent relapses. Immunotherapy plays a crucial part in these purposes. Currently, there is no drug that can completely prevent a recurrence, but a variety of immunosuppressants and monoclonal antibodies can significantly reduce the relapse frequency of NMOSD. Recently researchers have conducted numerous clinical trials of immunotherapy to verify the efficacy and adverse of new drugs (31–36). As a result, the most commonly used off-label drugs are azathioprine, mycophenolate mofetil, and rituximab. Rituximab has shown the most robust efficacy. With the discovery of AQP4-IgG and astrocytopathy, the era of targeted therapy is burgeoning, which has led to the development of a wealth of monoclonal antibodies targeting B cells, the complement system, and the IL-6 receptor (37). Four randomized placebo-controlled trials, namely, the PREVENT trial (38), the N-MOmentum trial (34), the SAkuraSky study, and the SAkuraStar study (32, 39), have demonstrated the efficacy of three new agents (eculizumab, satralizumab, and inebilizumab) for NMOSD patients. These three monoclonal antibodies have been approved by Food and Drug Administration for NMOSD treatment currently (40). Furthermore, several new agents such as aquaporumab, bortezomib, bevacizumab, ublituximab, and sivelestat are under development (41, 42), and they are expected to be approved in the future. Although all of the agents mentioned are effective in principle, it is difficult to widely recommend them due to factors such as their high cost and adverse effects. The most common adverse effects are infections, cytopenias, and infusion-related reactions (43). Therefore, it is important to continue researching more precise immunotherapy targeting antibody-producing cells in the near future.
More importantly, hematopoietic stem cell transplantation (HSCT) and chimeric antigen receptor T-cell therapy (CAR-T) (36, 44), novel approaches to reconstructing immune tolerance, are arousing great concern among a large number of scholars. Richard et al. reported that patients with relapsed or refractory NMOSD had significant improvements in the Expanded Disability Status Scale (EDSS), neurologic rating scale (NRS), and quality of life after HSCT (45). Recently, our teams, for the first time, have evaluated the safety and efficacy of CAR-T therapy in relapsed or refractory patients with AQP4-IgG-seropositive NMOSD in a phase 1 clinical trial (46). However, treatment evidence is insufficient, and more clinical trials are indispensable to verify the efficacy and safety of these new strategies. Therapeutic exploration relevant to these new methods will emerge as research hotspots in the future. These suggest that treatment has gained a large amount of attention recently and will remain a research priority in the future. However, the complicated pathogenesis but limited treatment lead to partial effectiveness (47). Yang et al. found a potential association between molecular mechanisms underlying NMOSD and proteins encoded by some novel genes (48) and more promising therapies such as gene therapies need to be exploited. However, the immunotherapy mentioned is mainly approved for AQP4-IgG-seropositive NMOSD, and options for seronegative patients are still limited because of obscure pathogenesis. It is not only a conundrum but also a challenge we are supposed to conquer in the future (42).
Furthermore, cumulative disability resulting from relapse requires the prediction of relapse risk, which is essential in guiding the early implementation of rational individualized treatment. Previous studies have reported various relapse predictors, such as female, younger age at onset, European or African descent, coexisting medical conditions, longer lesion length, and serum biomarkers such as GFAP and neurofilament light chain levels (42, 49–51). Wang et al. identified some relapse risk factors and developed an outcome prediction model comprised of gender, AQP4-IgG titer, previous attack under the same therapy, baseline EDSS score, and maintenance therapy to estimate the 1-year and 2-year relapse-free probabilities. The feasibility of this model was confirmed by multicenter cohort external validation (52). However, different predictors have presented inconsistent effects on relapse in various publications. In the future, it is necessary to conduct numerous clinical studies to explore and identify more predictors and establish a convenient and feasible relapse prediction model based on easily obtained clinical indicators. This will enable the assessment of relapse risk earlier, facilitate the generation of an individualized treatment strategy, and help avoid unfavorable outcomes.
There were several limitations to this analysis. First, raw data were only collected from the core collection database of the Web of Science, resulting in the loss of some relevant records. Therefore, in the future, we should retrieve data from additional databases to obtain a more comprehensive picture. Second, we discarded several non-English articles, which might have resulted in missing some critical studies on NMOSD. Finally, as with other bibliometric analyses (7, 13, 16), there may be biases in the analysis results due to the combined utilization of CiteSpace and VOSviewer.
To summarize, this bibliometric analysis provided a comprehensive overview of NMOSD research for the first time. The study included a visualized analysis of NMOSD research by pooling output over the past three decades, covering annual trends of publications, global cooperation, research hotspots, and emerging trends. However, domestic research is still in the early stage of development. Therefore, more attention should be paid to the diagnosis and treatment of NMOSD, and conducting more clinical trials should be considered as the primary research direction and frontier in the future.
D-ST, D-JP, and CQ conducted the conceptualization. D-ST, CQ, and JX identified the methodology. XC, W-XY, and W-HS were responsible for the screening of documents and visualization of the analysis. XC and L-QZ finished the images together. XC wrote the original manuscript. D-ST and CQ revised and retouched the manuscript. All authors contributed to the article and approved the submitted version.
This work was funded by the National Natural Science Foundation of China (Grants 82071380, 81873743, and 82271341), and Tongji Hospital (HUST) Foundation for Excellent Young Scientists (Grant No. 2020YQ06).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1135061/full#supplementary-material
1. Carnero Contentti E, Correale J. Neuromyelitis optica spectrum disorders: from pathophysiology to therapeutic strategies. J Neuroinflamm (2021) 18(1):208. doi: 10.1186/s12974-021-02249-1
2. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology (2015) 85(2):177–89. doi: 10.1212/wnl.0000000000001729
3. Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers (2020) 6(1):85. doi: 10.1038/s41572-020-0214-9
4. Hor JY, Asgari N, Nakashima I, Broadley SA, Leite MI, Kissani N, et al. Epidemiology of neuromyelitis optica spectrum disorder and its prevalence and incidence worldwide. Front Neurol (2020) 11:501. doi: 10.3389/fneur.2020.00501
5. Holroyd KB, Manzano GS, Levy M. Update on neuromyelitis optica spectrum disorder. Curr Opin Ophthalmol (2020) 31(6):462–8. doi: 10.1097/icu.0000000000000703
6. Ding Y, Chen D, Ding X, Wang G, Wan Y, Shen Q. A bibliometric analysis of income and cardiovascular disease: status, hotspots, trends and outlook. Med (Baltimore) (2020) 99(34):e21828. doi: 10.1097/md.0000000000021828
7. Xu X, Wang Y, Li Y, Zhang B, Song Q. The future landscape of macrophage research in cardiovascular disease: a bibliometric analysis. Curr Probl Cardiol (2022) 47(10):101311. doi: 10.1016/j.cpcardiol.2022.101311
8. Phoobane P, Masinde M, Mabhaudhi T. Predicting infectious diseases: a bibliometric review on Africa. Int J Environ Res Public Health (2022) 19(3):1893. doi: 10.3390/ijerph19031893
9. Yang W, Zhang J, Ma R. The prediction of infectious diseases: a bibliometric analysis. Int J Environ Res Public Health (2020) 17(17):6218. doi: 10.3390/ijerph17176218
10. Teles RHG, Yano RS, Villarinho NJ, Yamagata AS, Jaeger RG, Meybohm P, et al. Advances in breast cancer management and extracellular vesicle research, a bibliometric analysis. Curr Oncol (2021) 28(6):4504–20. doi: 10.3390/curroncol28060382
11. Gao Y, Shi S, Ma W, Chen J, Cai Y, Ge L, et al. Bibliometric analysis of global research on pd-1 and pd-L1 in the field of cancer. Int Immunopharmacol (2019) 72:374–84. doi: 10.1016/j.intimp.2019.03.045
12. Darroudi M, Gholami M, Rezayi M, Khazaei M. An overview and bibliometric analysis on the colorectal cancer therapy by magnetic functionalized nanoparticles for the responsive and targeted drug delivery. J Nanobiotechnology (2021) 19(1):399. doi: 10.1186/s12951-021-01150-6
13. He R, Yin T, Pan S, Wang M, Zhang H, Qin R. One hundred most cited article related to pancreaticoduodenectomy surgery: a bibliometric analysis. Int J Surg (2022) 104:106775. doi: 10.1016/j.ijsu.2022.106775
14. Cheng K, Guo Q, Shen Z, Yang W, Wang Y, Sun Z, et al. Bibliometric analysis of global research on cancer photodynamic therapy: focus on nano-related research. Front Pharmacol (2022) 13:927219. doi: 10.3389/fphar.2022.927219
15. Wang H, Shi J, Shi S, Bo R, Zhang X, Hu Y. Bibliometric analysis on the progress of chronic heart failure. Curr Probl Cardiol (2022) 47(9):101213. doi: 10.1016/j.cpcardiol.2022.101213
16. Song L, Zhang J, Ma D, Fan Y, Lai R, Tian W, et al. A bibliometric and knowledge-map analysis of macrophage polarization in atherosclerosis from 2001 to 2021. Front Immunol (2022) 13:910444. doi: 10.3389/fimmu.2022.910444
17. Papp V, Magyari M, Aktas O, Berger T, Broadley SA, Cabre P, et al. Worldwide incidence and prevalence of neuromyelitis optica: a systematic review. Neurology (2021) 96(2):59–77. doi: 10.1212/wnl.0000000000011153
18. Highhouse S, Zickar MJ, Melick SR. Prestige and relevance of the scholarly journals: impressions of siop members. Ind Organizational Psychol (2020) 13(3):273–90. doi: 10.1017/iop.2020.2
19. Abboud H, Salazar-Camelo A, George N, Planchon SM, Matiello M, Mealy MA, et al. Symptomatic and restorative therapies in neuromyelitis optica spectrum disorders. J Neurol (2022) 269(4):1786–801. doi: 10.1007/s00415-021-10783-4
20. Wingerchuk DM, Zhang I, Kielhorn A, Royston M, Levy M, Fujihara K, et al. Network meta-analysis of food and drug administration-approved treatment options for adults with aquaporin-4 immunoglobulin G-positive neuromyelitis optica spectrum disorder. Neurol Ther (2022) 11(1):123–35. doi: 10.1007/s40120-021-00295-8
21. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet (2004) 364(9451):2106–12. doi: 10.1016/s0140-6736(04)17551-x
22. Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol (2012) 11(6):535–44. doi: 10.1016/s1474-4422(12)70133-3
23. Jarius S, Wildemann B. On the contribution of Thomas Clifford allbutt, F.R.S., to the early history of neuromyelitis optica. J Neurol (2013) 260(1):100–4. doi: 10.1007/s00415-012-6594-3
24. Jarius S, Wildemann B. Aquaporin-4 antibodies (Nmo-igg) as a serological marker of neuromyelitis optica: a critical review of the literature. Brain Pathol (2013) 23(6):661–83. doi: 10.1111/bpa.12084
25. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol (2007) 6(9):805–15. doi: 10.1016/s1474-4422(07)70216-8
26. Wingerchuk DM. Neuromyelitis optica: new findings on pathogenesis. Neurobiol Multiple Sclerosis (2007) 79:665–+. doi: 10.1016/s0074-7742(07)79029-3
27. Marignier R, Hacohen Y, Cobo-Calvo A, Pröbstel AK, Aktas O, Alexopoulos H, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol (2021) 20(9):762–72. doi: 10.1016/s1474-4422(21)00218-0
28. Abdelhak A, Foschi M, Abu-Rumeileh S, Yue JK, D'Anna L, Huss A, et al. Blood gfap as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol (2022) 18(3):158–72. doi: 10.1038/s41582-021-00616-3
29. Chihara N, Yamamura T. Immuno-pathogenesis of neuromyelitis optica and emerging therapies. Semin Immunopathol (2022) 44(5):599–610. doi: 10.1007/s00281-022-00941-9
30. Siritho S, Nopsopon T, Pongpirul K. Therapeutic plasma exchange vs conventional treatment with intravenous high dose steroid for neuromyelitis optica spectrum disorders (Nmosd): a systematic review and meta-analysis. J Neurol (2021) 268(12):4549–62. doi: 10.1007/s00415-020-10257-z
31. Wingerchuk DM, Fujihara K, Palace J, Berthele A, Levy M, Kim HJ, et al. Long-term safety and efficacy of eculizumab in aquaporin-4 igg-positive nmosd. Ann Neurol (2021) 89(6):1088–98. doi: 10.1002/ana.26049
32. Traboulsee A, Greenberg BM, Bennett JL, Szczechowski L, Fox E, Shkrobot S, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol (2020) 19(5):402–12. doi: 10.1016/s1474-4422(20)30078-8
33. Tahara M, Oeda T, Okada K, Kiriyama T, Ochi K, Maruyama H, et al. Safety and efficacy of rituximab in neuromyelitis optica spectrum disorders (Rin-1 study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol (2020) 19(4):298–306. doi: 10.1016/s1474-4422(20)30066-1
34. Cree BAC, Bennett JL, Kim HJ, Weinshenker BG, Pittock SJ, Wingerchuk DM, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-momentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet (2019) 394(10206):1352–63. doi: 10.1016/s0140-6736(19)31817-3
35. Zhang C, Zhang M, Qiu W, Ma H, Zhang X, Zhu Z, et al. Safety and efficacy of tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorder (Tango): an open-label, multicentre, randomised, phase 2 trial. Lancet Neurol (2020) 19(5):391–401. doi: 10.1016/s1474-4422(20)30070-3
36. Shi M, Chu F, Jin T, Zhu J. Progress in treatment of neuromyelitis optica spectrum disorders (Nmosd): novel insights into therapeutic possibilities in nmosd. CNS Neurosci Ther (2022) 28(7):981–91. doi: 10.1111/cns.13836
37. Wallach AI, Tremblay M, Kister I. Advances in the treatment of neuromyelitis optica spectrum disorder. Neurol Clin (2021) 39(1):35–49. doi: 10.1016/j.ncl.2020.09.003
38. Pittock SJ, Berthele A, Fujihara K, Kim HJ, Levy M, Palace J, et al. Eculizumab in aquaporin-4-Positive neuromyelitis optica spectrum disorder. N Engl J Med (2019) 381(7):614–25. doi: 10.1056/NEJMoa1900866
39. Yamamura T, Kleiter I, Fujihara K, Palace J, Greenberg B, Zakrzewska-Pniewska B, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med (2019) 381(22):2114–24. doi: 10.1056/NEJMoa1901747
40. Wingerchuk DM, Lucchinetti CF. Neuromyelitis optica spectrum disorder. New Engl J Med (2022) 387(7):631–9. doi: 10.1056/NEJMra1904655
41. Lotan I, Levy M. New treatment perspectives for acute relapses in neuromyelitis optica spectrum disorder. Transfusion Med Rev (2022) 36(4):230–2. doi: 10.1016/j.tmrv.2022.06.008
42. Costello F. Neuromyelitis optica spectrum disorders. Continuum (Minneap Minn) (2022) 28(4):1131–70. doi: 10.1212/con.0000000000001168
43. Giglhuber K, Berthele A. Adverse events in nmosd therapy. Int J Mol Sci (2022) 23(8):4154. doi: 10.3390/ijms23084154
44. Ceglie G, Papetti L, Valeriani M, Merli P. Hematopoietic stem cell transplantation in neuromyelitis optica-spectrum disorders (Nmo-sd): state-of-the-Art and future perspectives. Int J Mol Sci (2020) 21(15):5304. doi: 10.3390/ijms21155304
45. Burt RK, Balabanov R, Han X, Burns C, Gastala J, Jovanovic B, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation for neuromyelitis optica. Neurology (2019) 93(18):e1732–e41. doi: 10.1212/wnl.0000000000008394
46. Qin C, Tian DS, Zhou LQ, Shang K, Huang L, Dong MH, et al. Anti-bcma car T-cell therapy Ct103a in relapsed or refractory Aqp4-igg seropositive neuromyelitis optica spectrum disorders: phase 1 trial interim results. Signal Transduct Target Ther (2023) 8(1):5. doi: 10.1038/s41392-022-01278-3
47. Wu Y, Zhong L, Geng J. Neuromyelitis optica spectrum disorder: pathogenesis, treatment, and experimental models. Mult Scler Relat Disord (2019) 27:412–8. doi: 10.1016/j.msard.2018.12.002
48. Li T, Li H, Li Y, Dong SA, Yi M, Zhang QX, et al. Multi-level analyses of genome-wide association study to reveal significant risk genes and pathways in neuromyelitis optica spectrum disorder. Front Genet (2021) 12:690537. doi: 10.3389/fgene.2021.690537
49. Kimbrough DJ, Mealy MA, Simpson A, Levy M. Predictors of recurrence following an initial episode of transverse myelitis. Neurol Neuroimmunol Neuroinflamm (2014) 1(1):e4. doi: 10.1212/nxi.0000000000000004
50. Kitley J, Leite MI, Nakashima I, Waters P, McNeillis B, Brown R, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the united kingdom and Japan. Brain (2012) 135(Pt 6):1834–49. doi: 10.1093/brain/aws109
51. Palace J, Lin DY, Zeng D, Majed M, Elsone L, Hamid S, et al. Outcome prediction models in Aqp4-igg positive neuromyelitis optica spectrum disorders. Brain (2019) 142(5):1310–23. doi: 10.1093/brain/awz054
Keywords: neuromyelitis optica spectrum disorders, bibliometric analysis, CiteSpace, VOSviewer, research hotspots
Citation: Chen X, Xiao J, Zhou L-Q, Yu W-X, Chen M, Chu Y-H, Shang K, Deng G, Song W-H, Qin C, Pan D-J and Tian D-S (2023) Research hotspots and trends on neuromyelitis optica spectrum disorders: insights from bibliometric analysis. Front. Immunol. 14:1135061. doi: 10.3389/fimmu.2023.1135061
Received: 31 December 2022; Accepted: 19 June 2023;
Published: 13 July 2023.
Edited by:
Giuseppe Murdaca, University of Genoa, ItalyReviewed by:
Abdorreza Naser Moghadasi, Tehran University of Medical Sciences, IranCopyright © 2023 Chen, Xiao, Zhou, Yu, Chen, Chu, Shang, Deng, Song, Qin, Pan and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dai-Shi Tian, dGlhbmRzQHRqaC50am11LmVkdS5jbg==; Deng-Ji Pan, ZGpwYW5AdGpoLnRqbXUuZWR1LmNu; Chuan Qin, Y2h1YW5xaW5AdGpoLnRqbXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.