- 1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing Medical University, Chongqing, China
- 2Department of Cardiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing Medical University, Chongqing, China
Background: To date, many researches have investigated the correlation of folate and asthma occurrence. Nevertheless, few studies have discussed whether folate status is correlated with dis-ease severity, control or progression of asthma. So, we explored the correlation of serum folate and blood eosinophil counts in asthmatic adults to gain the role of folate in the control, progression, and treatment of asthma.
Methods: Data were obtained from the 2011–2018 NHANES, in which serum folate, blood eosinophils, and other covariates were measured among 2332 asthmatic adults. The regression model, XGBoost algorithm model, and generalized linear model were used to explore the potential correlation. Moreover, we conducted stratified analyses to determine certain populations.
Results: Among three models, the multivariate regression analysis demonstrated serum folate levels were negatively correlated with blood eosinophil counts among asthmatic adults with statistical significance. And we observed that blood eosinophil counts decreased by 0.20 (-0.34, -0.06)/uL for each additional unit of serum folate (nmol/L) after adjusting for confounders. Moreover, we used the XGBoost Algorithm model to identify the relative significance of chosen variables correlated with blood eosinophil counts and observed the linear relationship between serum folate levels and blood eosinophil counts by constructing the generalized linear model.
Conclusions: Our study indicated that serum folate levels were inversely associated with blood eosinophil counts in asthmatic adult populations of America, which indicated serum folate might be correlated with the immune status of asthmatic adults in some way. We suggested that serum folate might affect the control, development, and treatment of asthma. Finally, we hope more people will recognize the role of folate in asthma.
1 Introduction
Asthma is a chronic airway disease with various etiologies (1, 2), affecting approximately 358 million people worldwide (3, 4). Epidemiological surveys have reported that about 250000 deaths are directly caused by asthma every year. And asthma was classified as the 23rd leading cause of premature mortality in 2016. With the development of society, the prevalence and socioeconomic burden of asthma are increasing year by year. According to reports, at present, the prevalence of asthma in adults is estimated to be 7.9% in the USA (5). Another study demonstrated that the cost of asthma treatment and mortality in the USA was estimated at $81 billion from 2008 to 2013 (6).
For many years, eosinophils in asthmatic inflammation have been recognized as an essential factor in the pathophysiology of asthma (7). Asthma is a heterogeneous disease comprising several endotypes and clinical phenotypes. Type 2 (T2) inflammation as key immune response in asthma pathobiology, resulting in the broad classification of T2-high and T2-low asthma. And eosinophils are also key inflammatory cells of ‘T2 high’ asthma (8). Quite a few studies reported a significant correlation between blood eosinophil counts and asthma (9–15). For example, eosinophilia has been confirmed to be a risk factor for future asthma exacerbations among asthmatic adults (16–18). And another study indicated increasing blood eosinophil counts might be correlated with rising mortality in patients with asthma (12). Besides, blood eosinophils are also used to guide and regulate treatment with corticosteroids, anti-IL antibodies in asthma, resulting in controlling asthma and reducing exacerbation frequency (19, 20). In short, eosinophils play a vital part in the occurrence, evolution, and therapy of asthma.
Asthma of underlying mechanisms maybe probably multifactorial, which may be affected by environmental, genetic, lifestyle factors, and so on, including dietary intake (21). A noticeable factor in a diet is folate. It takes part in synthesis of amino acid, purine, and pyrimidine, which is also a source of methyl donors for DNA methylation (22). Folate may contribute to the pathogenesis of asthma by affecting DNA methylation, further increasing or decreasing the expression of disease susceptibility genes (23, 24). Previous studies have reported, in animal models, folate plays a potential role in the pathogenesis of asthma (21, 24). In addition, some clinical researches reported that serum folate levels were correlated with the risk of asthma occurrence (21, 25–27).
At present, most studies have focused on exploring the relationship between folate and asthma occurrence. Nevertheless, to date, few researches have discussed whether folate status is correlated with disease severity or progression in asthmatic individuals; whether folate status affects asthma control is also unclear (24, 28). To gain insight into the role of folate in the control, progression, and treatment of asthma, we explored the correlation of serum folate concentration and blood eosinophil counts in asthmatic adults by using the data from the National Health and Nutrition Examination Survey (NHANES) of 2011–2018 cycles.
2 Materials and methods
2.1 Data source
The NHANES, which was conducted by the Centers for Disease Control and Prevention of America, collected information regarding the health and nutritional status of the U.S. population every 2 years. NHANES used a complex, stratified sampling design, which can select representative samples of non-institutionalized civilians. The NHANES database was approved by the NCHS Institutional Review Board in accordance with the revised Helsinki Declaration. The informed consent forms were completed before the data collection procedures and extensive health examinations.
2.2 Study population
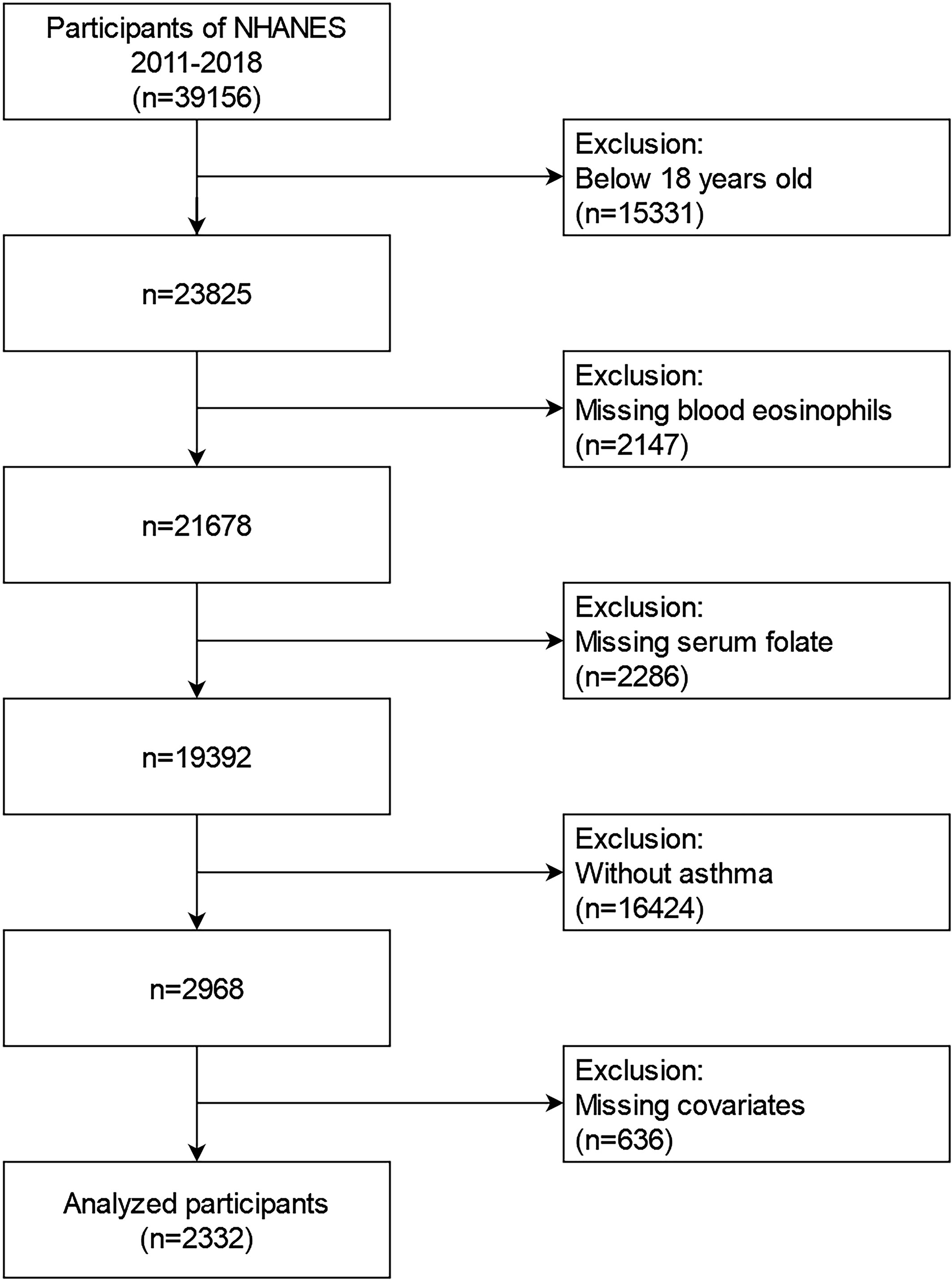
2011-2018 NHANES data were involved into our research. These data contained demographic data, examination data, diet data, laboratory data, and questionnaire data for the second analysis. From 2011 to 2018, a total of 39156 samples were involved in the NHANES. We excluded individuals (1): aged<18 years old (n=15331) (2); missing blood eosinophils data(n=2147) (3); missing serum folate data (n=2286) (4); participants without asthma (n=16424) (5); missing data about covariates at least one of following (n=636): educational status, marital status, the ratio of family income to poverty (PIR), BMI, smoking status, intake of Vitamin A, Vitamin B12, Vitamin D or folate, housing, hypertension history, diabetes history, blood creatinine, and blood cotinine. Finally, a large national representative sample (n=2332) of American adults with asthma was enrolled in our research. The flow chart of the screening process is performed in Figure 1.
2.3 Measurement of serum folate
Folate in the blood contained five forms: 5-methyltetrahydrofolate, folic acid, tetrahydrofolate, 5-formyltetrahydrofolate, 5,10-methenyltetrahydrofolate. Finally, we use the concentration of total folate in serum to represent the overall level of five forms of folate in the blood. Total serum folate concentrations were measured by isotopedilution high performance liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). The assay is performed by combining a specimen (275 μL serum or whole blood hemolysate) with ammonium formate buffer and an internal standard mixture. Values below the lower limit of detection (LOD) for the various folate forms were imputed as LOD/Sqrt (2). Detailed instructions on specimen collection and processing can be found on the NHANES website.
2.4 Blood eosinophil count measurement
Blood differential counts were performed in NHANES 2011-2018 by using the Beckman Coulter HMX (Beckman Coulter, Fullerton, Calif), a quantitative and automated hematologic analyzer and leukocyte differential cell counter for in vitro diagnostic use in clinical laboratories. A detailed description of the laboratory methods can be found on the NHANES website.
2.5 Covariates and asthma assessment
Covariates were selected based on previous studies. Demographic data included gender, age (years old), race (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, others), educational status (less than high school, high school, more than high school), poverty to income ratio (grouped by trisection: low, middle, high; high poverty to income ratio means richer), marital status (married, single, living with a partner). Secondly, we also contained examination data, diet data and personal life history data involving body mass index (kg/m2), smoking status (whether smoked at least 100 cigarettes in life), intake of Vitamin A, Vitamin B12, Vitamin D and folate (average intake from two 24-hours recall data on diet and supplements), housing (owned, rented, other), hypertension history (Yes, No), and diabetes history (Yes, No, Borderline). Finally, variables of laboratory data included blood basophil counts (/uL), blood creatinine (mol/L) and blood cotinine (ng/mL). The assessment of asthma was based on the information from the questionnaire section of the US National Health Interview Survey. In order to assess asthma, participants were asked, “Has a doctor or other health professional ever told you that you have asthma?” If the participant responds “yes,” he or she was regarded as asthma patient. More detailed explanations of all variables could be obtained from the NHANES database website (https://www.cdc.gov/nchs/nhanes/index.htm).
2.6 Statistical analysis
Based on the NHANES database guidelines, we conducted the statistical analyses of serum folate concentrations and blood eosinophil counts. Continuous and categorical variables were presented as the means ± SD and percentage, separately. Firstly, we converted blood eosinophil counts into four quartiles. We utilized the weighted chi-square to calculate the p-value of the basic characteristics of the analyzed individuals’ categorical variables and Kruskal Wallis rank sum test to calculate the p-value of continuous variables. Secondly, we constructed three types of weighted linear regression models which adjusted different variables to identify the association of serum folate concentrations and blood eosinophil counts (Model I, Model II, Model III). Thirdly, we utilized the machine learning of XGBoost algorithm model to discuss the relative significance of chosen variables on the effect of blood eosinophil counts. Next, we found the statistical difference between the serum folate concentrations and blood eosinophil counts. Therefore, we further conducted the stratified analysis to ascertain the stratified association of serum folate concentrations and blood eosinophil counts. Ultimately, according to the penalty spline method, we constructed a smooth curve by the generalized linear model to discuss the potential linear correlation of serum folate concentrations and blood eosinophil counts. All statistical analyses were conducted by R software (Version 4.2.0) using the R package. In our study, the p-value < 0.05 was indicated statistically significant.
3 Results
3.2 Baseline characteristics of the participants
The baseline characteristics, which were weighted distribution, were shown in Table 1, including demographic data, examination data, laboratory data, and questionnaire data of selected individuals from 2011-2018 NHANES survey. In our research, the mean age of selected individuals was 46.7 years old, and non-Hispanic White people were the main population. Then, we converted blood eosinophil counts into quartiles (Q1–Q4). The distribution of gender, age, hypertension history, blood creatinine, Vitamin A intake, Vitamin B12 intake, housing, diabetes history and blood basophil counts showed statistical differences (p values < 0.05), but the distribution of race, educational status, marital status, poverty to income ratio (PIR), housing, smoking status, diabetes history, Vitamin D intake, folate intake, blood cotinine and serum folate wasn’t statistically different (p value > 0.05).
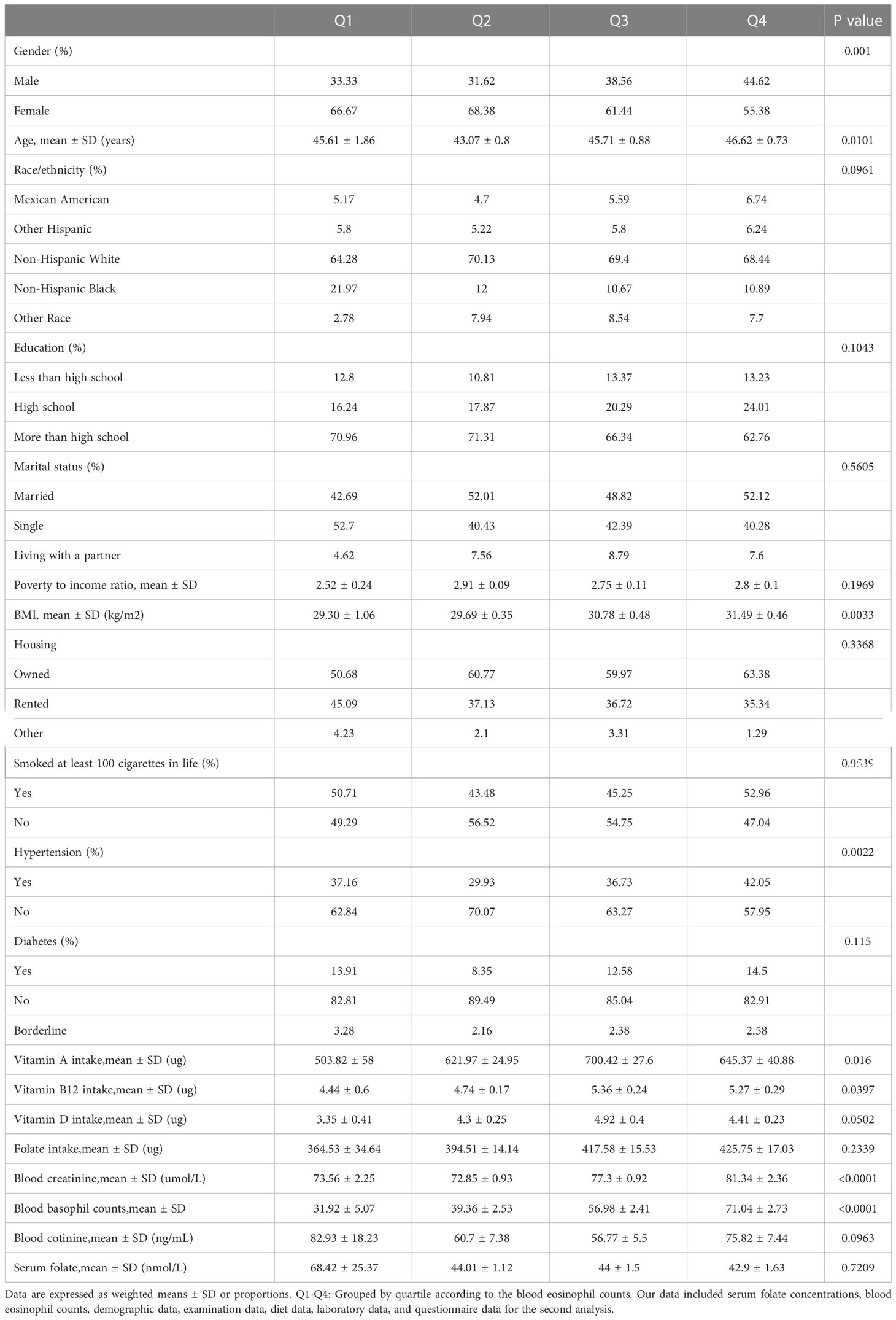
Table 1 Weighted basic characteristics of the analyzed population based on the quartiles of blood eosinophils counts.
3.2 The associations between the serum folate levels and blood eosinophil counts
We used the weighted linear regression models to discuss the correlation of blood folate and blood eosinophil counts in asthmatic adults (Table 2). According to the results, we observed that serum folate levels were inversely correlated with blood eosinophil counts in Model I, Model II and Model III, with statistical significance. In Model I, the blood eosinophil counts decreased by 0.17 (-0.29, -0.06)/uL for each additional unit of serum folate (nmol/L) (p for trend >0.05). In Model II, which adjusted for gender, age, and race, the blood eosinophil counts decreased by 0.18 (-0.30, -0.07)/uL for each additional unit of serum folate (nmol/L) (p for trend <0.05). In Model III, which adjusted for gender, age, race, educational status, marital status, PIR, BMI, smoked status, PIR, BMI, smoked, Vitamin A intake, Vitamin B12 intake, Vitamin D intake, folate intake, housing, hypertension history, diabetes history, blood basophil counts, blood creatinine, and blood cotinine, the blood eosinophil count decreased by 0.20 (-0.34, -0.06)/uL for each additional unit of serum folate (nmol/L) (p for trend >0.05). The above results indicated that serum folate might be correlated with the immune status of asthmatic adults in some way.
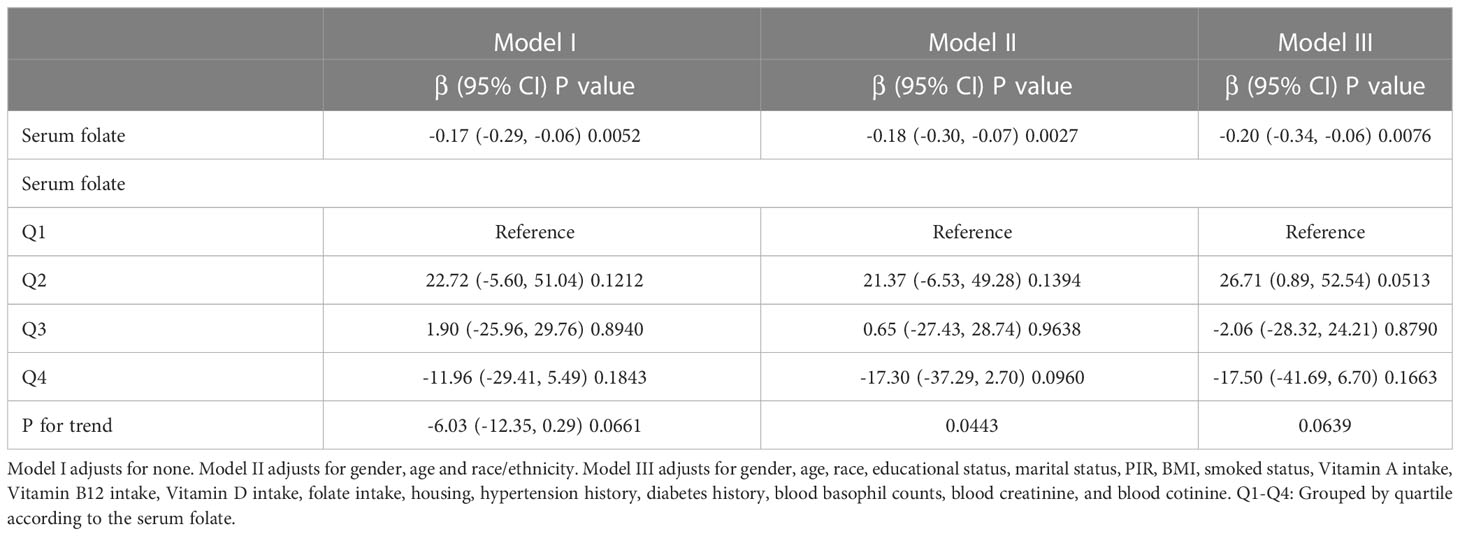
Table 2 Multivariate weighted linear regression model analysis elucidates the correlation between the serum folate and blood eosinophils counts.
3.3 Stratified associations between blood folate levels and blood eosinophil counts
To verify the stability of multivariate regression analysis results, we further analyzed stratified associations of the serum folate and blood eosinophil counts in different subgroups by sex, age, race, educational status, marital status, PIR, BMI, smoke, housing, hypertension history, and diabetes history (Table 3). According to stratified analysis results, it was possible that female, age <40, Non-Hispanic White people, more than high school, single status, high group of PIR, owned housing, BMI≥28, smoke≥100 cigarettes in life, without hypertension, and with diabetes had lower blood eosinophil counts, with increasing serum folate concentrations displaying a significant trend (p<0.05). Besides, we observed statistical differences in the interaction test. Variables of PIR, housing and serum folate may have interaction effects associated with blood eosinophil counts (p for interaction < 0.05).
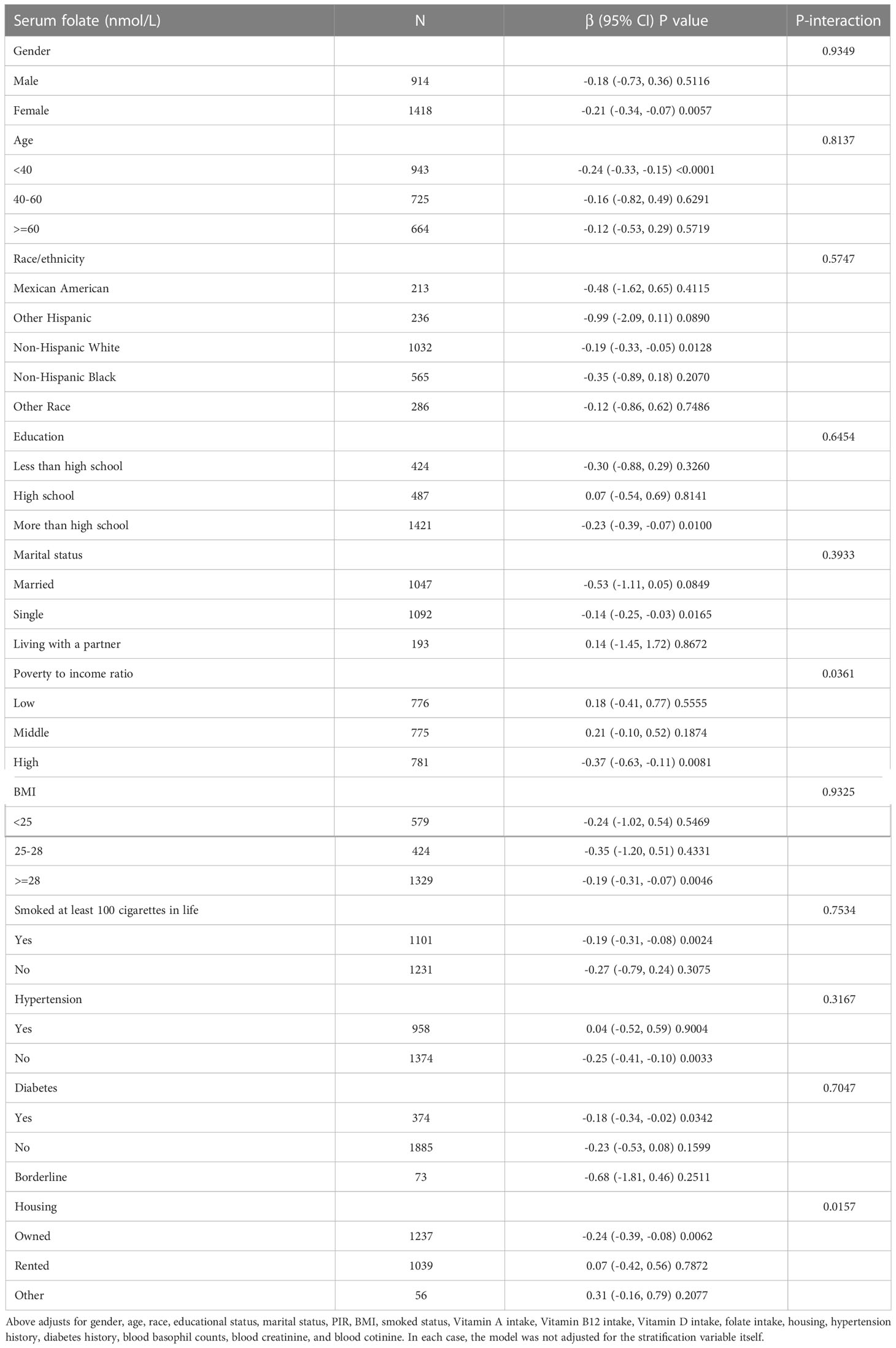
Table 3 Stratified associations of association between the serum folate and blood eosinophils counts.
3.4 Utilizing the XGBoost algorithm model to discuss chosen variables’ relative significance
At the stage of model development and validation, we utilized the XGBoost model of machine learning to identify the relative significance of chosen variables correlated with blood eosinophil counts. Variables contained sex, age, race, educational status, marital status, poverty to income ratio, BMI, smoking status, intake of Vitamin A, intake of Vitamin B12, intake of Vitamin D, intake of folate, housing, hypertension history, diabetes history, blood creatinine, blood cotinine, and serum folate. Based on the results of each variable contribution by the XGBoost model, we observed that folate intake, Vitamin A intake, Vitamin B12 intake, BMI, blood basophil counts, blood creatinine, serum folate, Vitamin D intake, age and blood cotinine, were the ten most important variables in the blood eosinophil counts (Figure 2). Ultimately, serum folate, as the relatively important variable, was further applied to constructing smooth curve models to verify the reliability of regression analyses results in our study.
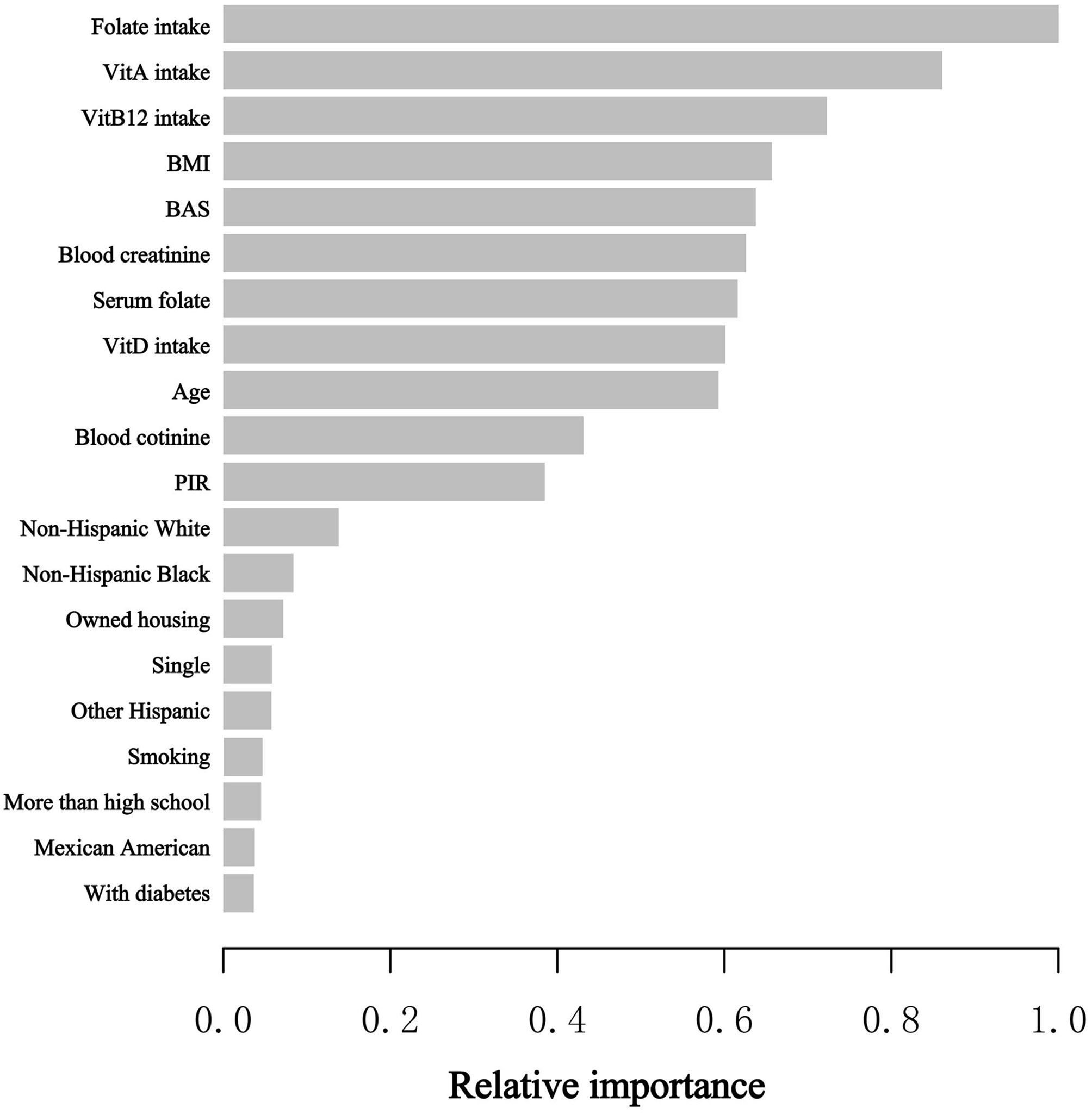
Figure 2 XGBoost model elucidates the relative significance of each variable on blood eosinophil counts and the corresponding variable significance score. The X-axis is the significance score, the relative number of a variable used to distribute the data; the Y-axis is the selected variables. PIR, poverty to income ratio; BAS, blood basophil counts; Vit, Vitamin.
3.5 Exploring dose-response relationships of serum folate levels with blood eosinophil counts by the generalized linear model
The GAM is greatly sensitive to identification of linear or nonlinear correlation. To verify the reliability of regression analyses results, we utilized GAM to discuss the linear or nonlinear correlation of the serum folate and blood eosinophil counts. Based on model 3 (Figure 3), we constructed a smooth fit curve to reflect the probable correlation. We observed the linear relationship between serum folate concentrations with blood eosinophil counts in asthmatic adults after adjusting all variables except for serum folate. All the above results indicated that serum folic acid levels were linearly and inversely associated with blood eosinophil counts.
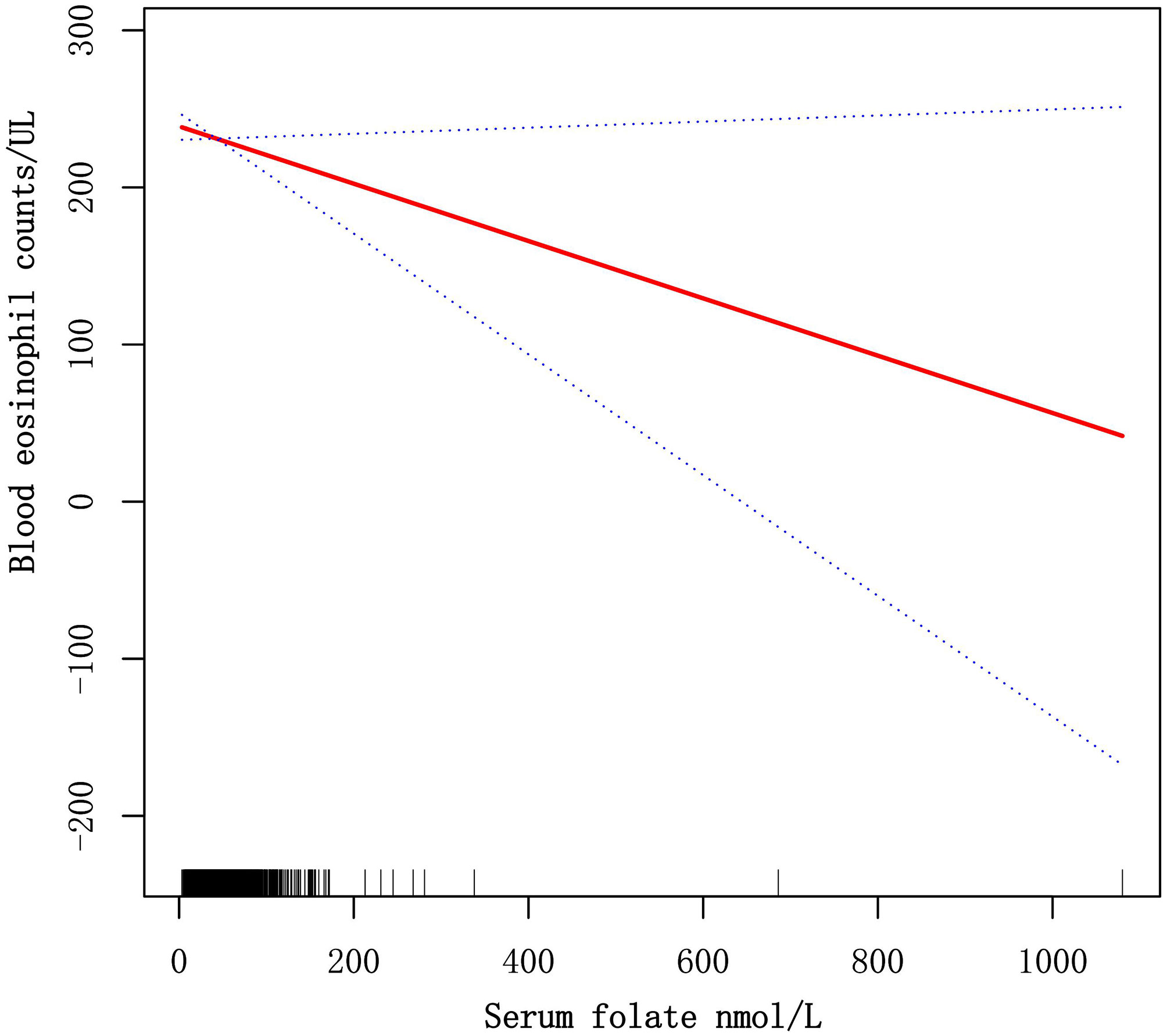
Figure 3 Dose-response relationships of serum folate levels with blood eosinophil counts. The solid red line represents the smooth fitting curve between serum folate levels with blood eosinophil counts, and the blue dotted line represents the 95% CIs of the fitting.
4 Discussion
The prevalence of atopy has risen over recent decades, possibly because of changes in lifestyle and environmental factors. Recently, it was suggested that changes in the intake of certain nutrients might partly explain the asthma epidemic worldwide (29–31). Folic acid, a special nutritional component that has attracted much attention recently, is necessary for protein and DNA protein methylation, and for nucleotide synthesis (24). Folate could result in asthma risk by affecting DNA methylation and, further, genetic expression (23, 32). However, at present, there are few studies on the relationship between folate status with asthma severity or progression in asthmatic individuals. Thus, in our study, we used a largely representative sample of individuals who took part in the 2011-2018 of NHANES to explore the correlation of serum folate concentrations and blood eosinophil counts among asthmatic individuals.
To our knowledge, our investigation is the first research to discuss the correlation of serum folic acid levels and blood eosinophil counts in asthmatic adults and is also one of the most extensive cross-sectional studies. Therefore, we investigated the correlation of serum folic acid levels and blood eosinophil counts among 2332 asthmatic adults who took part in the NHANES survey from 2011 to 2018 in the USA. Three types of weighted multiple linear regression models were used to identify the association between the serum folate concentrations and blood eosinophil counts. Among three models, we all observed that serum folate levels were negatively correlated with blood eosinophil counts with statistical significance. Also, we observed that blood eosinophil counts decreased by 0.20 (-0.34, -0.06)/uL for each additional unit of serum folate (nmol/L) in the Model III, which adjusted for the demographic, laboratory, examination, dietary, and comorbidities data. Next, we constructed the machine learning of XGBoost model to identify the relative importance of chosen variables correlated with blood eosinophils, and we observed that folate intake, Vitamin A intake, Vitamin B12 intake, BMI, blood basophil counts, blood creatinine, serum folate, Vitamin D intake, age and blood cotinine were the ten most important variables in the blood eosinophil counts. Then, we observed that the linear relationship between serum folate concentrations and blood eosinophil counts through constructing the generalized linear model. The above results suggested that serum folate might be correlated with the immune status of asthmatic adults in some way.
DNA methylation, histone modifications and regulation of noncoding RNA, which are primary types of epigenetic regulatory mechanisms in humans, have been confirmed to be correlated with asthma, and might be regarded as biomarkers for asthma (33). DNA methylation, a crucial type of epigenetic regulation, is a mechanism underlying some gene-environment interactions of allergic diseases such as asthma. And DNA methylation plays a vital role in asthma through regulation of a substantial number of genes involved in allergic responses, indicating a strong correlation with the development of asthma and maintenance of inflammation via epigenetic control of IgE, eosinophils and fractional exhaled nitric oxide (FENO), among other mechanisms (34). Folic acid, a water-soluble vitamin, is called vitamin B9, too. It plays a critical role in purine, pyrimidine and amino acid of synthesis, which is also a source of methyl donors for DNA methylation. In a mouse model, one animal experiment demonstrated that a diet rich in folate enhanced allergic humoral responses and lung inflammation. However, in contrast, some studies reported that lower folate levels were correlated with various inflammation-mediated diseases such as rheumatoid arthritis, inflammatory bowel disease (35, 36), and cardiovascular disease (37–39). Thus, it is possible that folic acid may alleviate, rather than promote, allergic diseases.
Several clinical studies have reported the association between folate concentrations and asthma. In Denmark, research including 6784 adults observed that obvious correlations between folic acid of deficiency and physician-diagnosed asthma (40). One case-control study including 1030 adults in the UK observed that intake of folate was negatively correlated with physician-diagnosed asthma (41). But another case-control study including 1469 adults in Australia observed that no correlation between intake of folate and physician-diagnosed asthma (42). And a case-control study involving 754 Peruvian children observed that serum folic acid concentrations were negatively associated with asthma (21). In addition, a Korean study involving 6615 individuals aged >10 years found that serum folic acid concentrations were negatively correlated with physician-diagnosed asthma (26). In contrast, a case-control study in Egypt, which involved 180 adults, found no significant association between serum folate levels and asthma (43). Likewise, in another cross-sectional study including 8083 American participants aged 2–85 years, no significant correlation between serum folate levels with physician-diagnosed asthma was observed (25). Besides, in a prospective cohort study, which mainly included black, urban children and adolescents with asthma, researchers observed that there are significant associations between serum folic acid and FENO and total IgE levels (28). Previous studies demonstrated that increasing folate could serve as a protective factor in certain populations against allergic inflammatory outcomes such as wheeze, atopy, and high total IgE. For example, a cross-sectional study in the USA, which involved 8083 participants aged 2 to 85 years old, observed that serum folic acid concentrations were negatively correlated with wheeze, total IgE, and atopy (25). Likewise, an Egyptian study involving 120 asthmatic adults demonstrated that serum folate was inversely correlated with total IgE, too (43). And lower serum folate concentrations were correlated with a higher risk of uncontrolled asthma in a study of 412 asthmatic children (21). However, above most studies, which explored the correlation of serum folate and asthma, didn’t include accommodation and eating data such as intake of Vitamin A, Vitamin B12, Vitamin D, folate and so on, meanwhile, had small sample populations. In addition, these studies didn’t carry out stratified analyses in various populations to confirm the stability and reliability of analyzed results. Our results were similar to those of the above studies, which demonstrated that serum folate levels were inversely associated with blood eosinophil counts in asthmatic adults. What’s more, we observed that the association between serum folic acid concentrations and blood eosinophil counts had population differences among American asthmatic adults, too. Based on the stratified analysis, we determined possibly protective groups with higher folate levels, including female, age <40, Non-Hispanic White people, more than high school, single status, high group of PIR, owned housing, BMI≥28, smoke≥100 cigarettes in life, without hypertension, which had lower blood eosinophil counts. In a word, serum folate levels may potentially affect the control and progression of asthma through immunomodulation and modulation of inflammation.
Compared with previous investigations, our study has several advantages. Firstly, our research offers a nationally representative, relatively large sample of asthmatic adults, which includes information on a few potential confounders. Second, considering that confounders may affect the results, we identify the possibly protective population with higher folate levels through stratified analysis. Then, we use the machine learning of XGBoost Algorithm model to identify, among all selected variables, serum folate affects blood eosinophil counts most. Finally, we observed the linear relationship between serum folate levels and blood eosinophil counts by constructing the generalized linear model.
Nonetheless, we still acknowledge several limitations in our study. Though our study is national broad, most of the data is based on the American population. And dietary model may be different in different countries on account of unbalance in national development. Secondly, due to the cross-sectional study design limits, we can’t conclude the causal relationship between serum folate and blood eosinophil counts. Finally, we may not take certain potential confounders into consideration. Moreover, in future, information such as lung function, airflow obstruction, atopy and so on will be included into further studies. And more prospective researches will be conducted to get insight into the potential role of folate in the control, progression and treatment of asthma, and to determine potential mechanisms of action.
5 Conclusion
Our study observed that serum folate levels were inversely associated with blood eosinophil counts in asthmatic adult populations of America, which indicated serum folate might be correlated with the immune status of asthmatic adults in some way. We suggested that serum folate might affect the control, development, and treatment of asthma. Finally, we hope more people will recognize the role of folate in asthma.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving human participants were reviewed and approved by the National Centers for Disease Control (CDC) and Prevention National Health Statistics Center. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JW conducted the study design, data extraction, statistical analysis, drafted the manuscript, and revision of the paper. MG conducted data extraction, statistical analysis, and revision of the paper. CW conducted the study design, statistical analysis, drafted the manuscript, and revision of the paper. SG took part in the study design and revision of the paper. All authors contributed to the article and approved the submitted version.
Funding
Chongqing Talents, Teachers and Masters (SG).
Acknowledgments
The NHANES protocol was approved by the NCHS Research Ethics Review Board.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wenzel S. Severe asthma: From characteristics to phenotypes to endotypes. Clin Exp Allergy J Br Soc Allergy Clin Immunol (2012) 42(5):650–8. doi: 10.1111/j.1365-2222.2011.03929.x
2. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J (2014) 43(2):343–73. doi: 10.1183/09031936.00202013
3. Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet Respir Med (2017) 5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X
4. Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the global burden of disease study 2015. Lancet (London England). (2016) 388(10053):1459–544. doi: 10.1016/S0140-6736(16)31012-1
5. Pate CA, Zahran HS, Qin X, Johnson C, Hummelman E, Malilay J. Asthma surveillance - united states, 2006-2018. Morbidity mortality weekly Rep Surveillance Summ. (Washington DC 2002). (2021) 70(5):1–32. doi: 10.15585/mmwr.ss7005a1
6. Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the united states, 2008-2013. Ann Am Thorac Society. (2018) 15(3):348–56. doi: 10.1513/AnnalsATS.201703-259OC
7. Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med (2017) 195(3):302–13. doi: 10.1164/rccm.201602-0419OC
8. Carr TF, Zeki AA, Kraft M. Eosinophilic and noneosinophilic asthma. Am J Respir Crit Care Med (2018) 197(1):22–37. doi: 10.1164/rccm.201611-2232PP
9. Wagener AH, de Nijs SB, Lutter R, Sousa AR, Weersink EJ, Bel EH, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax (2015) 70(2):115–20. doi: 10.1136/thoraxjnl-2014-205634
10. Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol (2013) 132(1):72–80. doi: 10.1016/j.jaci.2013.03.044
11. Fowler SJ, Tavernier G, Niven R. High blood eosinophil counts predict sputum eosinophilia in patients with severe asthma. J Allergy Clin Immunol (2015) 135(3):822–4.e2. doi: 10.1016/j.jaci.2014.09.034
12. Ulrik CS, Frederiksen J. Mortality and markers of risk of asthma death among 1,075 outpatients with asthma. Chest (1995) 108(1):10–5. doi: 10.1378/chest.108.1.10
13. Tran TN, Khatry DB, Ke X, Ward CK, Gossage D. High blood eosinophil count is associated with more frequent asthma attacks in asthma patients. Ann allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol (2014) 113(1):19–24. doi: 10.1016/j.anai.2014.04.011
14. Mallah N, Rodriguez-Segade S, Gonzalez-Barcala FJ, Takkouche B. Blood eosinophil count as predictor of asthma exacerbation. A meta-anal Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol (2021) 32(3):465–78. doi: 10.1111/pai.13403
15. Durham SR, Kay AB. Eosinophils, bronchial hyperreactivity and late-phase asthmatic reactions. Clin Allergy (1985) 15(5):411–8. doi: 10.1111/j.1365-2222.1985.tb02290.x
16. Zeiger RS, Schatz M, Dalal AA, Chen W, Sadikova E, Suruki RY, et al. Blood eosinophil count and outcomes in severe uncontrolled asthma: A prospective study. J Allergy Clin Immunol In practice. (2017) 5(1):144–53.e8. doi: 10.1016/j.jaip.2016.07.015
17. Vedel-Krogh S, Fallgaard Nielsen S, Lange P, Vestbo J, Nordestgaard BG. Association of blood eosinophil and blood neutrophil counts with asthma exacerbations in the Copenhagen general population study. Clin Chem (2017) 63(4):823–32. doi: 10.1373/clinchem.2016.267450
18. Price DB, Rigazio A, Campbell JD, Bleecker ER, Corrigan CJ, Thomas M, et al. Blood eosinophil count and prospective annual asthma disease burden: A UK cohort study. Lancet Respir Med (2015) 3(11):849–58. doi: 10.1016/S2213-2600(15)00367-7
19. Wark PA, McDonald VM, Gibson PG. Adjusting prednisone using blood eosinophils reduces exacerbations and improves asthma control in difficult patients with asthma. Respirol (Carlton Vic). (2015) 20(8):1282–4. doi: 10.1111/resp.12602
20. Kupczyk M, Haque S, Middelveld RJ, Dahlén B, Dahlén SE. Phenotypic predictors of response to oral glucocorticosteroids in severe asthma. Respir Med (2013) 107(10):1521–30. doi: 10.1016/j.rmed.2013.07.014
21. Nicholson A, Pollard SL, Lima JJ, Romero KM, Tarazona-Meza C, Malpartida-Guzmán G, et al. Serum folate concentrations, asthma, atopy, and asthma control in Peruvian children. Respir Med (2017) 133:29–35. doi: 10.1016/j.rmed.2017.10.026
22. Naderi N, House JD. Recent developments in folate nutrition. Adv Food Nutr Res (2018) 83:195–213. doi: 10.1016/bs.afnr.2017.12.006
23. Lovinsky-Desir S, Miller RL. Epigenetics, asthma, and allergic diseases: A review of the latest advancements. Curr Allergy Asthma Rep (2012) 12(3):211–20. doi: 10.1007/s11882-012-0257-4
24. Blatter J, Han YY, Forno E, Brehm J, Bodnar L, Celedón JC. Folate and asthma. Am J Respir Crit Care Med (2013) 188(1):12–7. doi: 10.1164/rccm.201302-0317PP
25. Matsui EC, Matsui W. Higher serum folate levels are associated with a lower risk of atopy and wheeze. J Allergy Clin Immunol (2009) 123(6):1253–9.e2. doi: 10.1016/j.jaci.2009.03.007
26. Kim SR, Park EJ, Cho YH, Lee SY, Choi JI, Lee YI, et al. Association between serum folic acid levels and asthma in the Korean population: A study based on the 2016-2018 Korea national health and nutrition examination survey. Kor. J Family Med (2022) 43(4):241–5. doi: 10.4082/kjfm.21.0143
27. Han YY, Forno E, Rosser F, Celedón JC. Serum folate metabolites, asthma, and lung function in a nationwide US study. J Allergy Clin Immunol (2020) 146(1):220–2.e8. doi: 10.1016/j.jaci.2020.01.034
28. Lin JH, Matsui W, Aloe C, Peng RD, Diette GB, Breysse PN, et al. Relationships between folate and inflammatory features of asthma. J Allergy Clin Immunol (2013) 131(3):918–20. doi: 10.1016/j.jaci.2012.10.046
29. Paul G, Brehm JM, Alcorn JF, Holguín F, Aujla SJ, Celedón JC. Vitamin d and asthma. Am J Respir Crit Care Med (2012) 185(2):124–32. doi: 10.1164/rccm.201108-1502CI
30. Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. Global variation in the prevalence and severity of asthma symptoms: Phase three of the international study of asthma and allergies in childhood (ISAAC). Thorax (2009) 64(6):476–83. doi: 10.1136/thx.2008.106609
31. Allan K, Devereux G. Diet and asthma: Nutrition implications from prevention to treatment. J Am Dietetic Assoc (2011) 111(2):258–68. doi: 10.1016/j.jada.2010.10.048
32. Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J Nutr (2002) 132 (8 Suppl):2333s–5s. doi: 10.1093/jn/132.8.2333S
33. Qi C, Xu CJ, Koppelman GH. The role of epigenetics in the development of childhood asthma. Expert Rev Clin Immunol (2019) 15(12):1287–302. doi: 10.1080/1744666X.2020.1686977
34. Gomez JL. Epigenetics in asthma. Curr Allergy Asthma Rep (2019) 19(12):56. doi: 10.1007/s11882-019-0886-y
35. Schroecksnadel K, Frick B, Kaser S, Wirleitner B, Ledochowski M, Mur E, et al. Moderate hyperhomocysteinaemia and immune activation in patients with rheumatoid arthritis. Clinica chim acta; Int J Clin Chem (2003) 338(1-2):157–64. doi: 10.1016/j.cccn.2003.09.003
36. Kurzawski M, Pawlik A, Safranow K, Herczynska M, Drozdzik M. 677C>T and 1298A>C MTHFR polymorphisms affect methotrexate treatment outcome in rheumatoid arthritis. Pharmacogenomics (2007) 8(11):1551–9. doi: 10.2217/14622416.8.11.1551
37. Moens AL, Claeys MJ, Wuyts FL, Goovaerts I, Van Hertbruggen E, Wendelen LC, et al. Effect of folic acid on endothelial function following acute myocardial infarction. Am J Cardiol (2007) 99(4):476–81. doi: 10.1016/j.amjcard.2006.08.057
38. Forman JP, Rimm EB, Stampfer MJ, Curhan GC. Folate intake and the risk of incident hypertension among US women. Jama (2005) 293(3):320–9. doi: 10.1001/jama.293.3.320
39. Connor SL, Ojeda LS, Sexton G, Weidner G, Connor WE. Diets lower in folic acid and carotenoids are associated with the coronary disease epidemic in central and Eastern Europe. J Am Dietetic Assoc (2004) 104(12):1793–9. doi: 10.1016/j.jada.2004.09.023
40. Thuesen BH, Husemoen LL, Ovesen L, Jørgensen T, Fenger M, Gilderson G, et al. Atopy, asthma, and lung function in relation to folate and vitamin B(12) in adults. Allergy (2010) 65(11):1446–54. doi: 10.1111/j.1398-9995.2010.02378.x
41. Patel BD, Welch AA, Bingham SA, Luben RN, Day NE, Khaw KT, et al. Dietary antioxidants and asthma in adults. Thorax (2006) 61(5):388–93. doi: 10.1136/thx.2004.024935
42. Woods RK, Walters EH, Raven JM, Wolfe R, Ireland PD, Thien FC, et al. Food and nutrient intakes and asthma risk in young adults. Am J Clin Nutr (2003) 78(3):414–21. doi: 10.1093/ajcn/78.3.414
Keywords: folate, eosinophil, asthma, national health and nutrition examination survey, machine learning
Citation: Wen J, Wang C, Giri M and Guo S (2023) Association between serum folate levels and blood eosinophil counts in American adults with asthma: Results from NHANES 2011–2018. Front. Immunol. 14:1134621. doi: 10.3389/fimmu.2023.1134621
Received: 30 December 2022; Accepted: 14 February 2023;
Published: 22 February 2023.
Edited by:
Sara Manti, University of Messina, ItalyReviewed by:
Amruta Naik, University of Pennsylvania, United StatesNathalie Guriec, Centre Hospitalier Regional Universitaire (CHU) de Brest, France
Copyright © 2023 Wen, Wang, Giri and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuliang Guo, Z3Vvc2w5OTlAc2luYS5jb20=
†These authors have contributed equally to this work
 Jun Wen1†
Jun Wen1† Mohan Giri
Mohan Giri Shuliang Guo
Shuliang Guo