- 1Department of Cardiovascular Medicine, The Third People’s Hospital of Kunming, Yunnan Clinical Medicine Center for Infectious Diseases, Kunming, China
- 2Department of Hospice Care, The Third People’s Hospital of Kunming, Yunnan Clinical Medicine Center for Infectious Diseases, Kunming, China
- 3Department of Infectious Diseases, The Third People’s Hospital of Kunming, Yunnan Clinical Medicine Center for Infectious Diseases, Kunming, China
Background: The incidence of hypertension is high in people living with HIV (PLWH). High-sensitivity C-reactive protein (hsCRP), systemic inflammation response index (SIRI), and neutrophil-to-monocyte ratio (NMR) are considered economic and convenient parameters that reflect the levels of inflammation in patients. Our aim was to explore whether indirect inflammation markers are associated with hypertension in PLWH.
Methods: This was a case-control study. The case group (hypertension) comprised PLWH with hypertension, and the control group (non-hypertension) comprised sex- and age-(± 3 years)-matched PLWH without hypertension. Demographic parameters, hsCRP, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune- inflammation index (SII), SIRI, lymphocyte-to-monocyte ratio (LMR), platelet-to-neutrophil ratio (PNR), platelet-to-monocyte ratio (PMR), NMR, time to HIV diagnosis, antiretroviral therapy (ART) duration, recent CD4+ and CD8+ cell counts, recent CD4+/CD8+ ratio, recent HIV viral load (HIV-RNA),and recent ART regimen were obtained from the patients’ electronic medical records. A t-test or Wilcoxon rank-sum test was performed to compare differences between the two groups, and conditional logistic regression was used to analyze the risk factors of hypertension. Correlations between inflammation markers and CD4+ cell counts, CD8+ cell counts, and CD4+/CD8+ ratio were analyzed using Spearman’s correlation.
Results: In the hypertension group, body mass index (BMI), hsCRP, NLR, SII, SIRI, NMR, time to HIV diagnosis, ART duration, CD4+ and CD8+ cell counts, and CD4+/CD8+ ratio, the ratio of HIV-RNA < 100 copies/mL were all higher than those in the non-hypertension group, while the PNR was lower than that in the non-hypertension group. ART duration, CD4+ cell counts, HIV-RNA < 100 copies/mL, hsCRP, SIRI, and NMR were positively associated with hypertensive risk in PLWH. CD8+ cell counts and CD4+/CD8+ ratio was negatively associated with hypertensive risk in PLWH. SIRI was negatively correlated with CD4+ cell counts and CD8+ cell counts, but positively correlated with CD4+/CD8+ ratio.
Conclusions: We identified positive associations between inflammation markers hsCRP, SIRI, NMR and hypertensive risk in PLWH. Alleviating inflammation may help control or delay the occurrence of hypertension in PLWH.
1 Introduction
With the increased use of antiretroviral therapy (ART), the incidence of opportunistic infections associated with human immunodeficiency virus (HIV) have significantly decreased, increasing the life expectancy of people living with HIV (PLWH) (1). However, the incidence of age-related hypertension in PLWH has increased. Although regular ART significantly lowers the level of inflammation in PLWH, it does not completely alleviate inflammation, and patients often maintain a persistent state of low-level inflammation (2, 3) independently of traditional cardiovascular risk factors, such as dyslipidemia, obesity, diabetes, and smoking (4). Chronic inflammation causes major alterations in physiological processes that play important roles in the pathogenesis and progression of cardiovascular diseases (5). PLWH are 2.16 times more likely to develop cardiovascular diseases than individuals without HIV. The proportion of PLWH with cardiovascular diseases has increased from 0.36% in 1990 to 0.92% in 2015 (6). Hypertension, the most common cardiovascular disease, is particularly prevalent in the PLWH population; approximately 1 in 4 PLWH has hypertension, and the prevalence increases with age (7). Therefore, there is an urgent need to explore the risk factors for hypertension in this population, and effective interventions should be implemented at early stages to delay the occurrence of hypertension. Many studies have shown that the risk factors for hypertension in PLWH include unhealthy lifestyle habits, high body mass index (BMI), hyperlipidemia, adverse effects from ART, CD4+ cell counts (8, 9), and CD4+/CD8+ ratio (10). A long-term micro-inflammatory environment may lead to hypertension in PLWH.
Certain parameters can indicate the body’s state of inflammation and affect the progression of some diseases. The neutrophil-to-lymphocyte ratio (NLR) and systemic immune-inflammation index (SII) can predict the occurrence and prognosis of cardiovascular events in non-HIV population (11–16). The NLR, combined with the platelet-to-lymphocyte ratio (PLR), can also predict the prognosis of acute myocardial infarction (17). Interestingly, NLR and PLR are independently associated with all-cause mortality in PLWH (18). However, the role of inflammation markers in predicting hypertension in PLWH remains unknown.
We retrospectively analyzed inflammation marker (high-sensitivity C-reactive protein (hsCRP) and eight inflammation parameters obtained during routine blood tests) levels and clinical factors in patients with and without hypertension who had also been diagnosed with HIV. We explored the associations between clinically accessible markers of inflammation and the risk of developing hypertension in PLWH.
2 Materials and methods
2.1 Study design and setting
This was a case-control study carried out in the Third People’s Hospital of Kunming, the largest PLWH-designated hospital in Kunming, China. The hospital provides HIV testing, treatment for HIV-related comorbidities, ART therapy, and follow-up services. The study was approved by the Ethics Committee of the Third People’s Hospital of Kunming (approval number: 2021120610). All information was collected by professionals and kept confidential.
2.2 Study population
We recruited patients at least 18 years of age who were diagnosed with HIV and hospitalized in the Third People’s Hospital of Kunming from January 1, 2017 to December 31, 2021. The case group included PLWH who were diagnosed with hypertension after being diagnosed with HIV, and the control group included PLWH without hypertension. Patients in both groups were required to have had an acquired immune deficiency syndrome (AIDS) diagnosis for ≥1 year and to have underwent ART for ≥1 year. The electronic medical record (EMR) system search identified 582 patients with hypertension during the study period, after excluding patients who had hypertension or normal blood pressure prior to HIV diagnosis but were taking antihypertensive drugs (n=236), patients with hypertension during pregnancy (n=15), and patients with elevated blood pressure due to other established diseases or causes (n=4). After these exclusions, 327 patients who developed hypertension after being diagnosed with HIV were included. In accordance with the goal of the study, patients with an AIDS diagnosis <1 year (n=36), ART duration <1 year (n=53), or were critically ill (n=10) were also excluded. In the end, 228 PLWH were included in the case group (Figure 1). Similarly, in the study population, 228 PLWH without hypertension were randomly matched 1:1 by sex and age ( ± 3 years) with the control group. The diagnosis of hypertension was made by a consultation’s cardiovascular doctors according to the 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension (19) and the 2020 International Society of Hypertension Global Practice Guidelines for Hypertension (20). The diagnosis of HIV/AIDS was based on the Chinese HIV/AIDS Diagnosis and Treatment Guidelines from the year in which the patient was hospitalized (21–23).
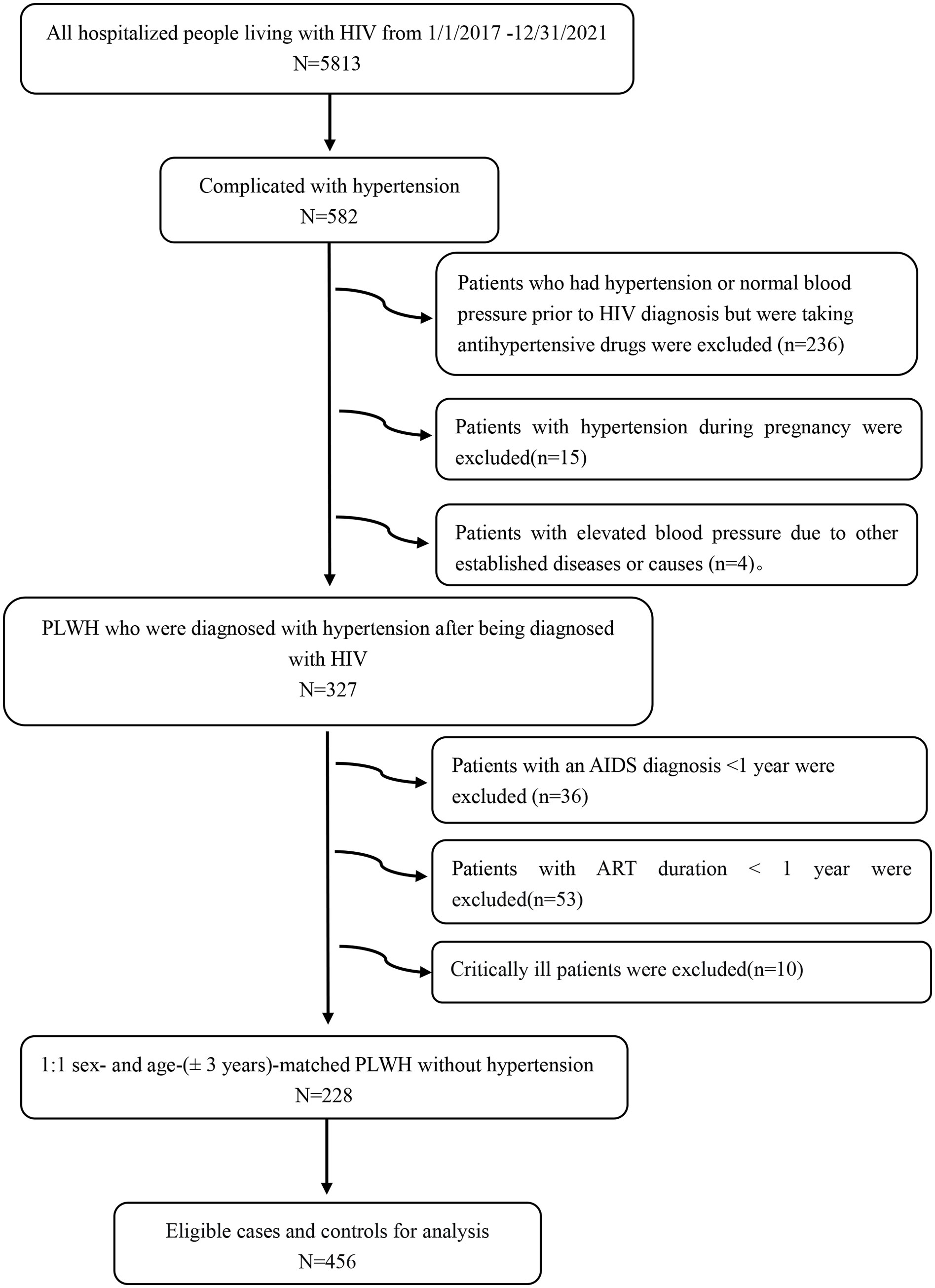
Figure 1 Study population flowchart. HIV, human immunodeficiency virus; AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; PLWH, people living with HIV.
2.3 Study variables
2.3.1 Demographic parameters
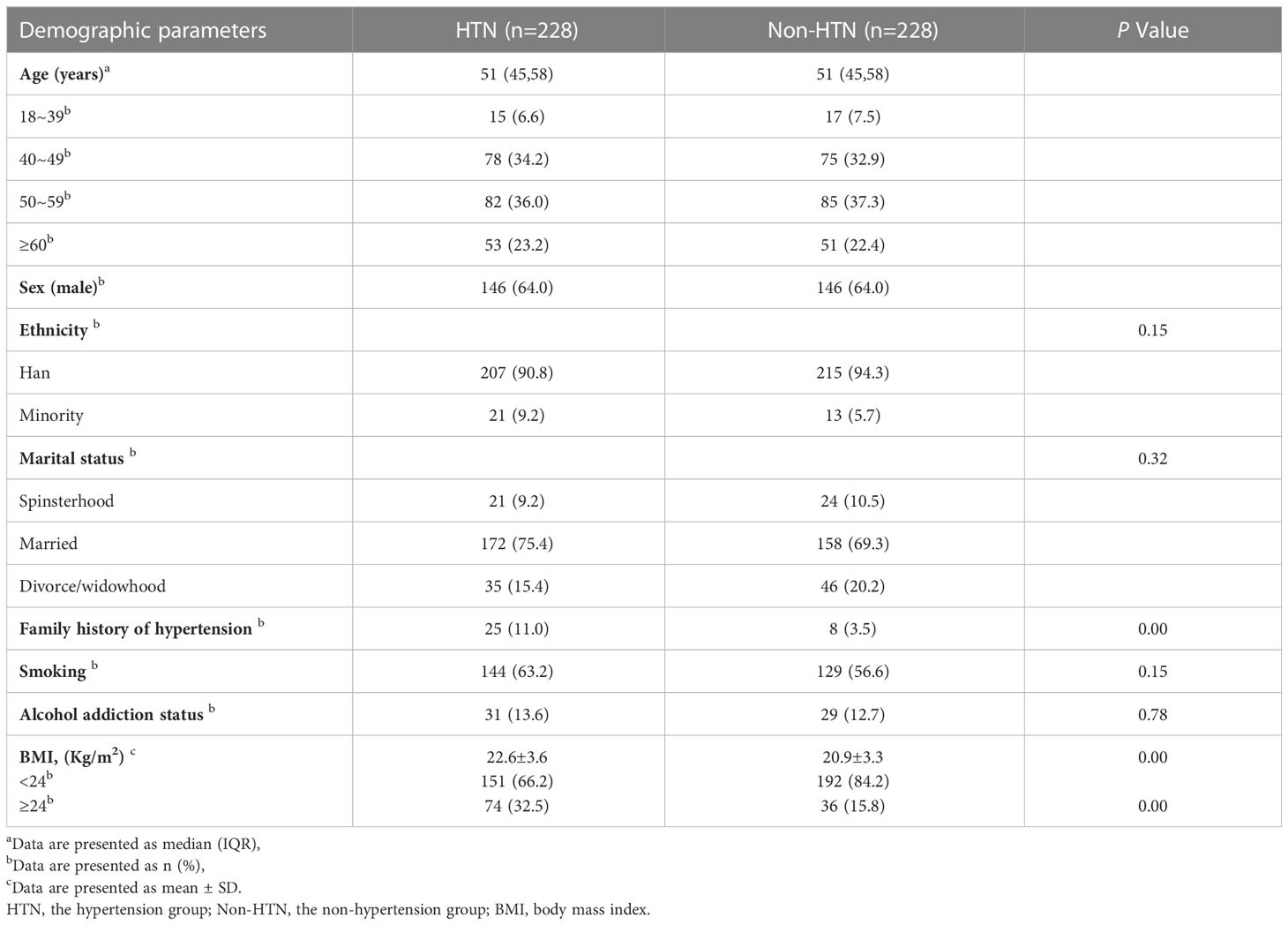
The following research data were collected from the EMR system: age, sex, ethnicity, marital status, smoking, alcohol addiction status, family history of hypertension, BMI, length of HIV diagnosis, ART duration, and recent ART regimen. BMI was calculated as the patient’s weight in kilograms divided by the square of their height in meters, and height and weight measurements were taken when the patients were admitted (barefoot and in light clothing). Based on the criteria for Chinese adults, BMI was classified into the following four categories: underweight <18.5, normal 18.5–23.9, overweight 24–27.9, and obese ≥28.0 kg/m2 (24). However, considering PLWH generally have a relatively low weight compared to the general population, we classified underweight and normal as one category, namely BMI<24 kg/m2, and overweight and obese as another, defined as BMI ≥24 kg/m2. Smoking was defined as smoking ≥1 cigarette/day for at least 6 months consecutively or cumulatively. Alcohol addiction status was defined as drinking ≥80/g once a week for ≥1 year. The length of HIV diagnosis was the difference between the year of the initial HIV diagnosis by the patient as self-reported or laboratory-recorded and the year of admission, and ART duration was the difference between the year of initial ART treatment and the year of admission, as reported by the patient or recorded in the EMR system. ART drug was classified as: nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), and integrase strand transfer inhibitors (INSTIs).
2.3.2 Laboratory indicators
On the first day of admission, blood samples were drawn by professionals from patients who had been fasting for at least 8 hours. Counts of the total neutrophil, monocyte, platelet, and lymphocyte were obtained using a hematology autoanalyzer (Sysmex, XG-550) and expressed as multiples of 109/L. hsCRP was detected with an AU5400 automatic biochemical analyser.CD4+ cell counts, CD8+ cell counts, and CD4+/CD8+ ratio was determined using a flow cytometer (BD Company, New Jersey, USA), and the data were analyzed with Flow Jo software 2.8/3.0 (Tree star, Ashland, OR, USA). HIV primary screening tests were performed using an Enzyme-Linked Immunosorbent Assay (Taiwan Biotechnology, Beijing, China) according to manufacturer instructions, and HIV status was confirmed via western blot analysis. The plasma HIV viral load (HIV-RNA) was quantified by real-time RT- PCR Test (Roche, Mannheim, USA), an HIV-RNA level below 100 copies/mL was classified as undetectable. Calculations of the various parameters were performed as follows: NLR=neutrophil-to-lymphocyte ratio, PLR=platelet-to-lymphocyte ratio, SII=neutrophil/lymphocyte × platelet, systemic inflammation response index (SIRI)=neutrophil × monocyte/lymphocyte, LMR=lymphocyte-to-monocyte ratio, PNR=platelet-to-neutrophil ratio, PMR=platelet-to-monocyte ratio, and NMR=neutrophil-to-monocyte ratio. All test data were from the patients’ most recent hospital admission.
2.4 Statistical analyses
We defined the case group as the hypertensive group and the control group as the non-hypertensive group, conditional logistic regression was used to analyze risk factors for hypertension. Normally distributed measurement data are expressed as the mean ± standard deviation (SD), and non-normally distributed measurement data are presented as median (interquartile range, IQR). Enumeration data are expressed as constituent ratios. A t-test or Wilcoxon rank-sum test was used to compare the measurement data between the two groups, and a chi-squared test was used to compare the enumeration data. Conditional logistic regression analysis was performed on the factors with P<0.1 to analyze the risk factors of hypertension. Nine inflammation markers were treated as continuous variables, and categorical variables included ethnicity (1=Han, 2= minority), marital status(1= spinsterhood, 2=married, 3=divorce/widowhood), family history of hypertension(no=0,yes=1), smoking(no=0, yes=1), alcohol addiction status (no=0, yes=1), BMI (kg/m2)(< 24 = 1, ≥24 = 2), years of HIV diagnosis (1-4 = 1, 5-9 = 2, ≥10 = 3), years of ART duration (1-4 = 1, 5-9 = 2, ≥10 = 3), and number of ART classes (one=1, two=2, three=3), NRTIs (no=0, yes=1), NNRTIs (no=0, yes=1), PIs (no=0, yes=1), and INSTIs (no=0, yes=1). Correlations between hsCRP, SIRI, NMR, and HIV status (CD4+ and CD8+ cell counts and their ratio) were determined using Spearman’s correlation coefficients. All statistical analyses were performed using SPSS software version 20.0 (IBM Corporation, Armonk, New York, USA) and graphs were plotted using GraphPad Prism version 9 (GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was defined as P <0.05.
3 Results
3.1 Participant characteristics
We accessed the medical records of 582 participants. Among them, 327 (56.2%) were PLWH diagnosed with hypertension after their HIV diagnosis. After patient exclusion based on the aforementioned criteria, medical records from 228 PLWH were included in the final analytical set (Figure 1). In addition, 228 PLWH without hypertension were enrolled as controls. Patients in both groups fulfilled the AIDS diagnosis for ≥1 year and ART duration for ≥1year criteria. The median age of the patients in the hypertensive and non-hypertensive groups combined was 51 years old. Patients were mainly between 40–49 and 50–59 years old.146 patients were male, accounting for 64.0% of the study participants. The Han nationality accounted for 90.8% of the hypertension group and 94.3% of the non-hypertension group, respectively. In the marital status, married accounted for 75.4% of the hypertension group and 69.3% of the non-hypertension group, respectively. A family history of hypertension was significantly more common in the hypertensive group than in the non-hypertensive group. Further, compared with the non-hypertensive group, the hypertensive group had a higher rate of smoking and alcohol addiction status. However, these differences were not statistically significant. Compared to the non-hypertensive group, the hypertensive group had a higher BMI value. The hypertensive group had a smaller proportion of underweight and normal weight patients, and a larger proportion of overweight patients. However, overall, underweight and normal weight (<24 kg/m2) patients accounted for the majority of patients in both groups. (Table 1). Regarding ART regimen, the patients in both groups were mainly treated with three-drug regimen, and the most frequent ART regimen was tenofovir disoproxil fumarate plus lamivudine plus efavirenz. Almost all participants received NRTIs, and only a minority received INSTIs (Supplemental Table 1).
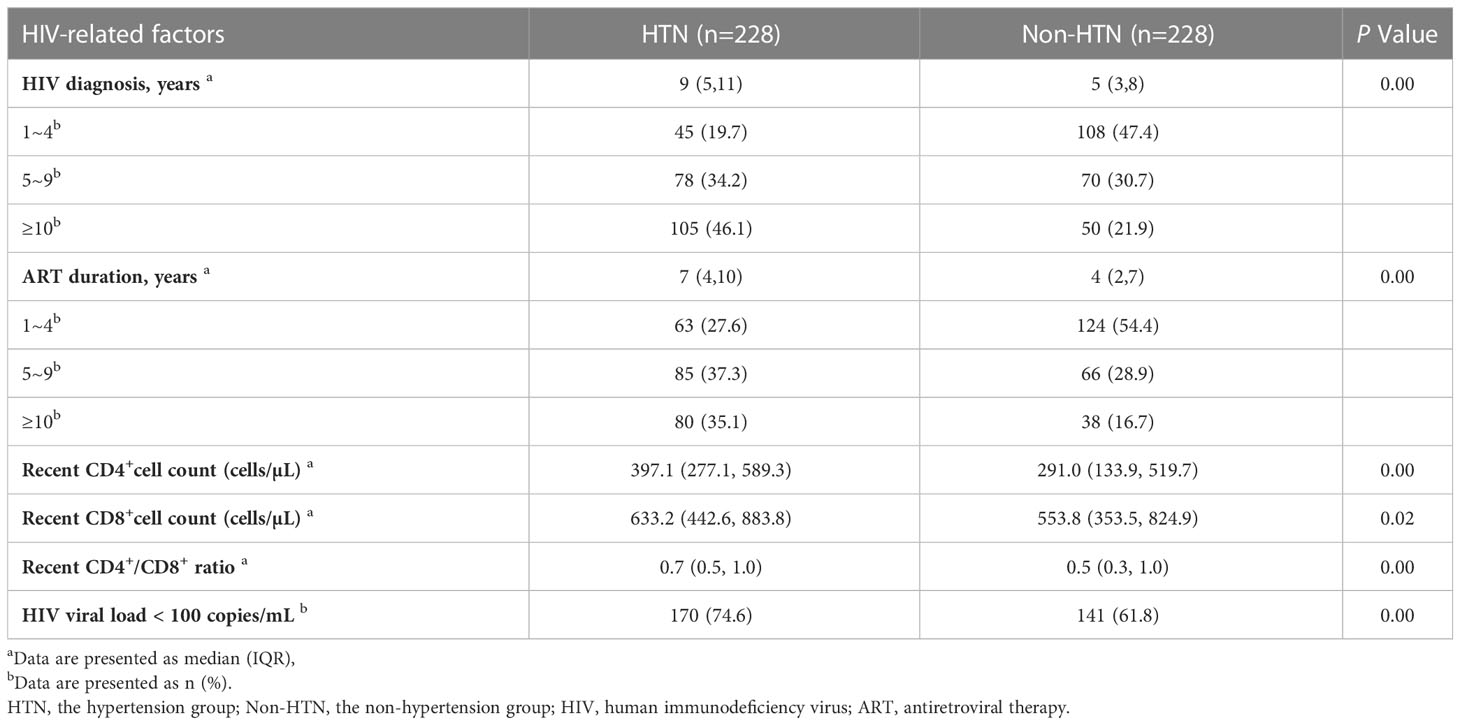
Regarding HIV-related factors, the hypertensive group showed higher CD4+, CD8+ cell counts, CD4+/CD8+ ratio, longer time to HIV diagnosis, and duration of ART. In addition, more patients with undetectable viral loads (<100 copies/mL) were observed in the hypertensive group (Table 2).
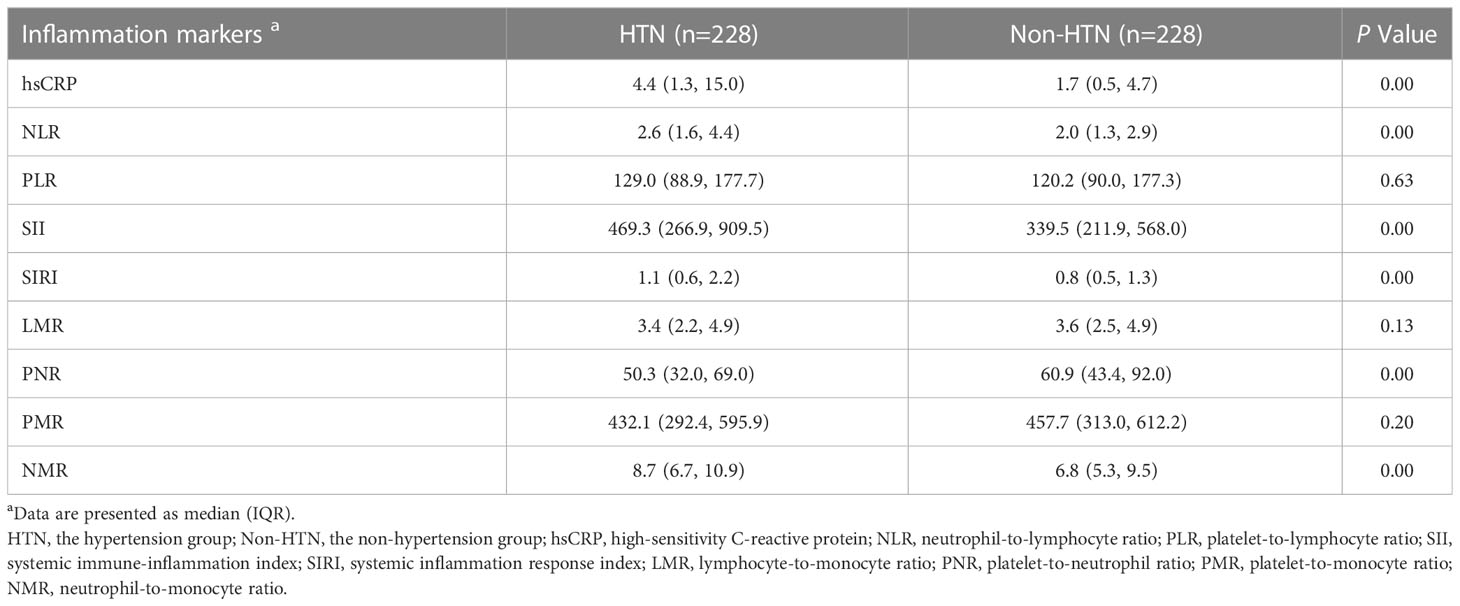
Regarding the inflammation state, we compared the levels of inflammation markers in the two groups: hsCRP, NLR, SII, SIRI and NMR in the hypertension group were significantly higher than those in the non-hypertension group, and PNR was lower than that in the non-hypertension group. The PLR, LMR and PMR levels were no significant differences (Table 3). Among them, hsCRP, NLR, SII, SIRI, PNR, and NMR showed the most obvious differences (Figure 2).
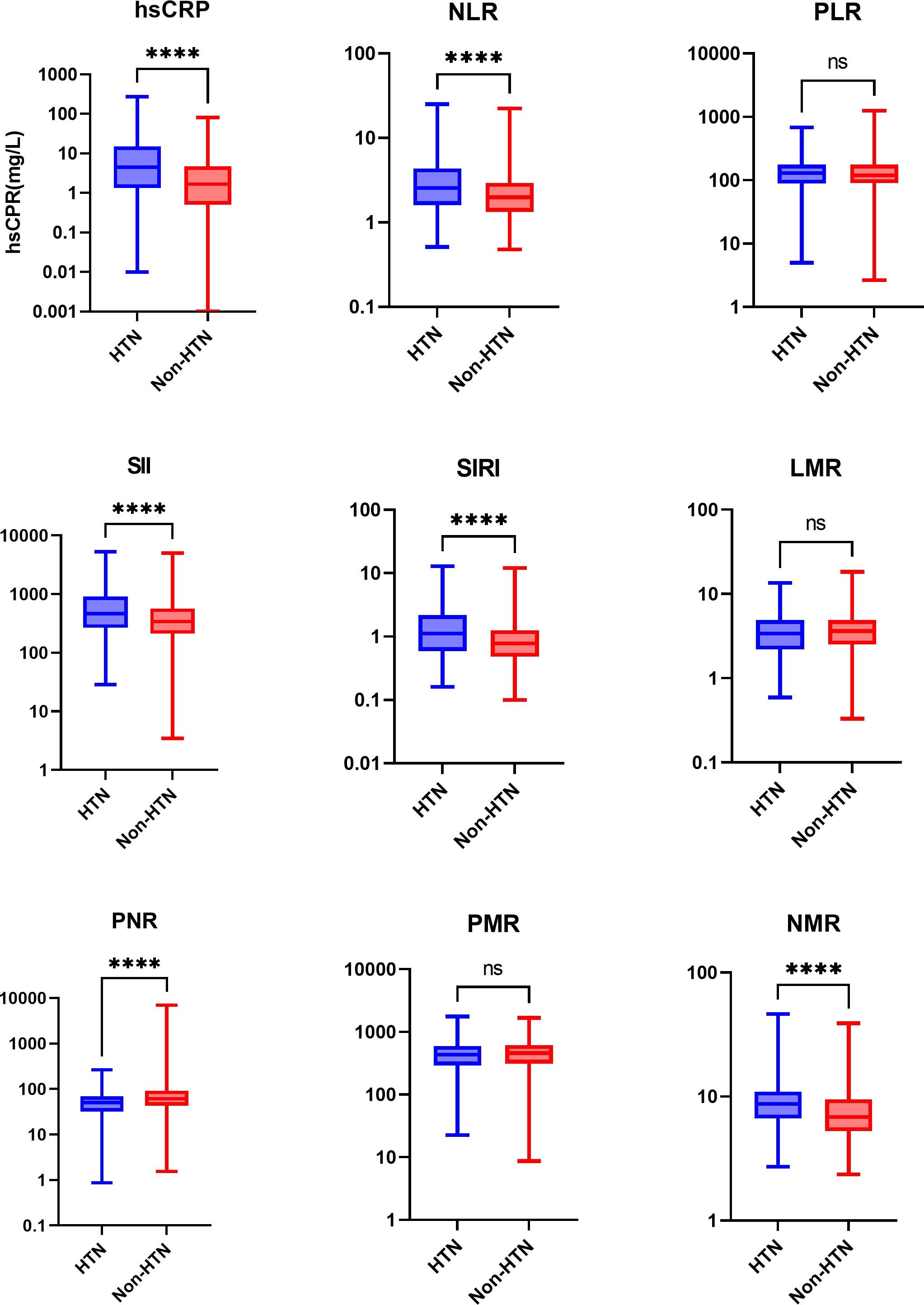
Figure 2 Boxplots comparing the inflammation marker levels of the two groups. Values are presented as median (IQR). log-transformed, ****P<0.0001, and ns, not significant. HTN, the hypertension group; Non-HTN, the non-hypertension group; hsCRP, high-sensitivity C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; LMR, lymphocyte-to-monocyte ratio; PNR, platelet-to-neutrophil ratio; PMR, platelet-to-monocyte ratio; NMR, neutrophil-to-monocyte ratio.
3.2 Risk factors for hypertension
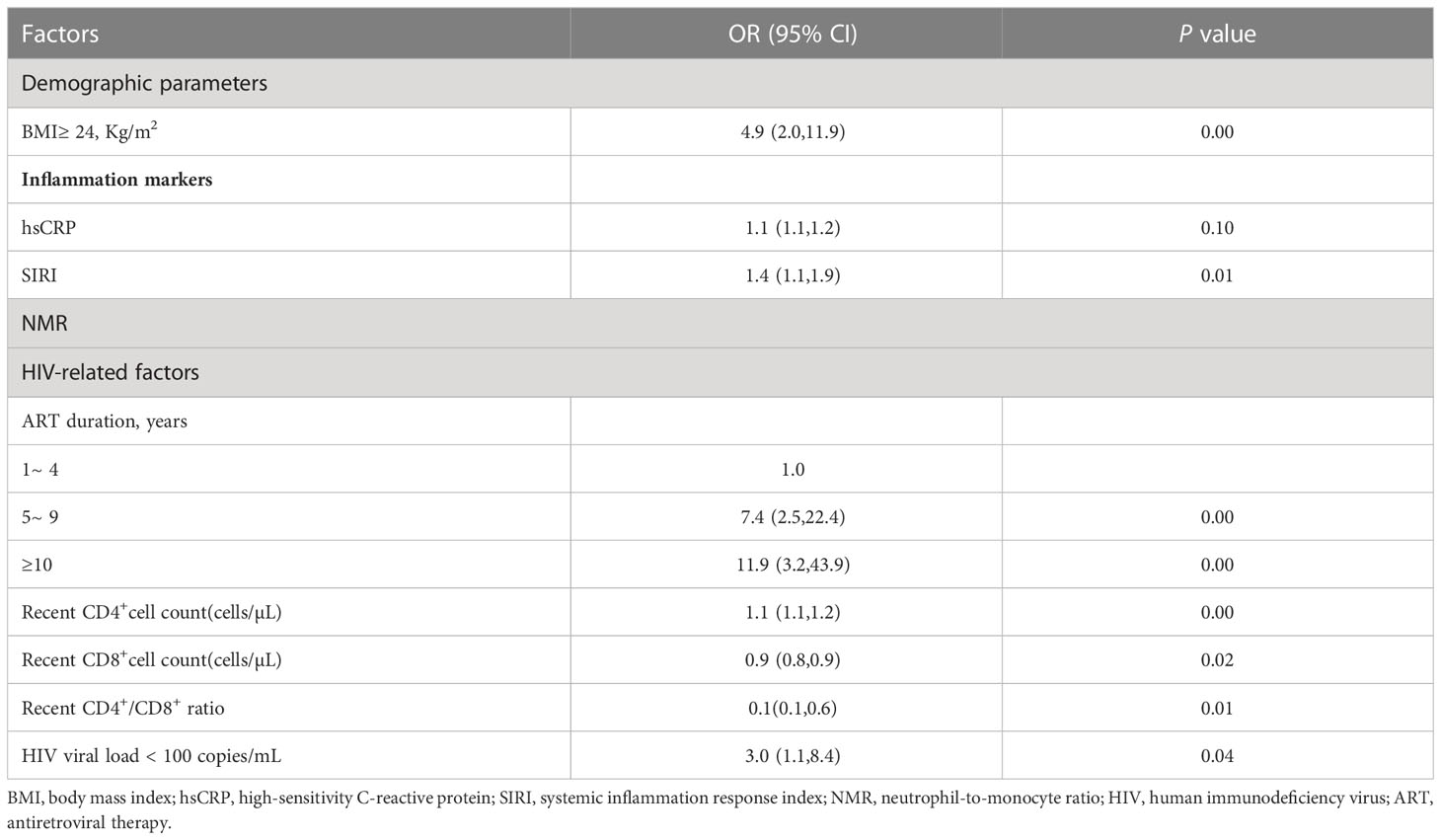
The result showed that higher hsCRP, SIRI, NMR, higher recent CD4+ cell counts and recent HIV-RNA of less than 100 Copies/mL were associated with hypertension. Patients with a BMI more than 24Kg/m2 (compared with BMI<24Kg/m2) had a 5-fold increased risk of developing hypertension. Compared with 1 to 4 years ART duration, ART duration of 5 to 9 years and over 10 years increased risk of hypertension 7-fold and 12-fold, respectively. However, recent CD8+ cell counts and CD4+/CD8+ ratio was negatively associated with risk of hypertension (Table 4).
3.3 Correlation between inflammation markers and HIV-related factors
Spearman’s correlation analysis was performed to explore the relationships between inflammation markers (hsCRP, SIRI, and NMR) and HIV-related parameters (recent CD4+ and CD8+ cell counts and their ratio). As shown in Figure 3, SIRI was negatively correlated with CD4+ cell counts and CD8+ cell counts, but positively correlated with CD4+/CD8+ ratio. Among them, the correlation between SIRI and CD8+ cell counts is the most obvious (Figure 3).
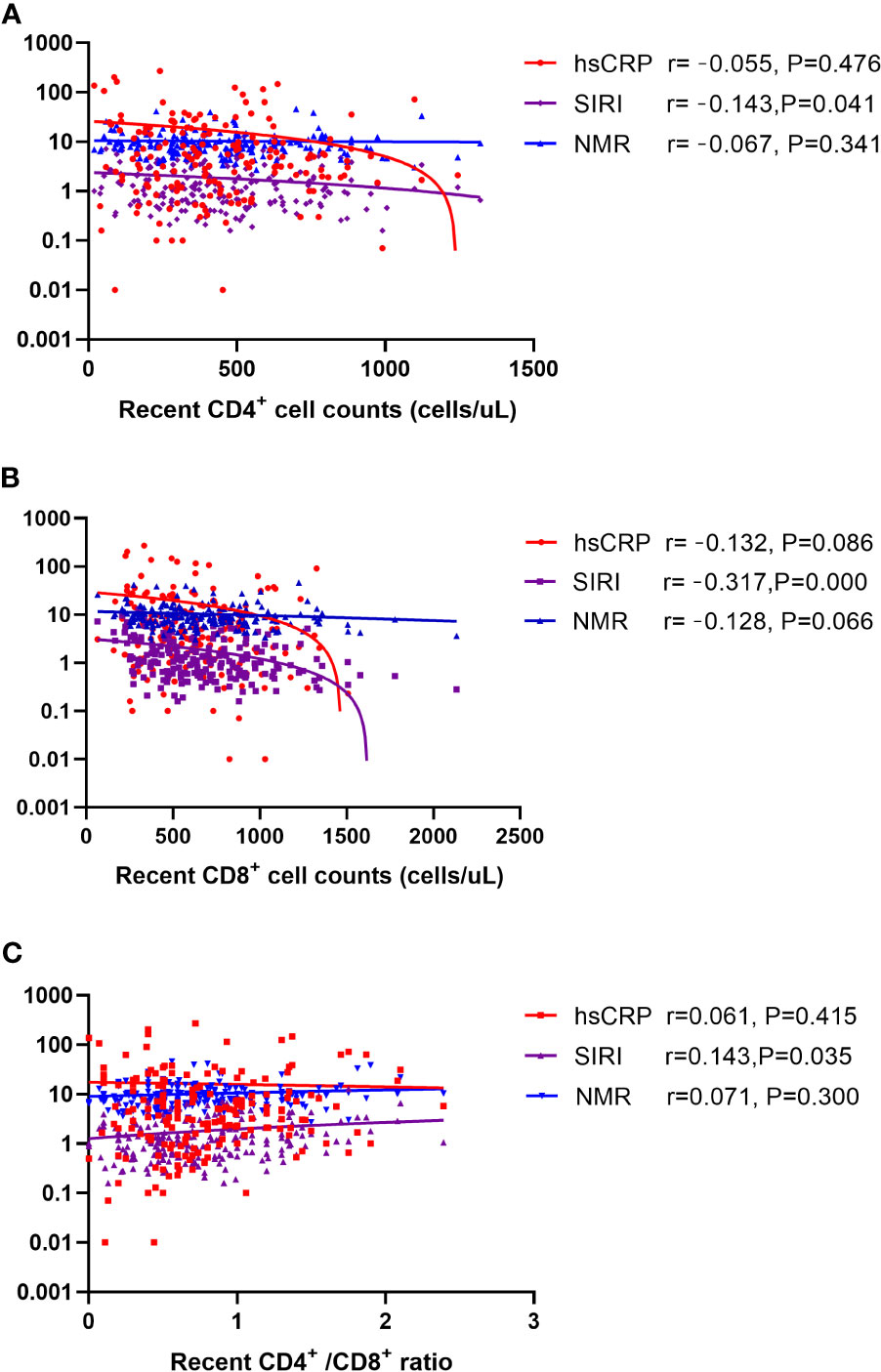
Figure 3 Scatter plot of the correlation between hsCRP, SIRI, and NMR with (A) recent CD4+ cell counts (B) recent CD8+ cell counts (C) CD4+/CD8+ ratio. Note: The ordinate is log transformed. Abbreviations: hsCRP, high-sensitivity C-reactive protein; SIRI, systemic inflammation response index; NMR, neutrophil-to-monocyte ratio.
4 Discussion
The main findings of this study are as follows (1): an ART duration of 5 to 9 years or ≥10 years (compared with 1 to 4 years), CD4+ cell counts, HIV-RNA<100 copies/mL, hsCRP, SIRI, and NMR were positively associated with the prevalence of hypertension in PLWH, while CD8+ cell counts and CD4+/CD8+ ratio was negatively associated with the prevalence of hypertension in PLWH (2). high BMI was also associated with hypertension in PLWH. Among these factors, SIRI and NMR were associated with hypertension, independent of traditional risk factors. Previous studies have hypothesized that ethnic differences in the prevalence of hypertension may be related to dietary habits. However, Ruan et al.’ s research did not confirm this conjecture, they speculated that adaptation of different ethnic population to the local environment and dietary habits reduced the discrepancy in the prevalence of hypertension (25). Similar results were found in the PLWH population. Psychopathological factors caused by being single or poor marriage may indirectly lead to hypertension (26), but we found that in PLWH, marital status has no effect on the prevalence of hypertension. we considered that the mental damage caused by HIV is considerable, which weakens the impact of marriage on hypertension. In fact, HIV-specific factors combine with traditional risk factors to contribute to the high likelihood of PLWH developing hypertension (27). Several studies have demonstrated that inflammation biomarkers, such as interleukin (IL)-6 (28), IL-17, and tumor necrosis factor-α receptor 1(TNF-αR1) are associated with hypertension in PLWH (29). However, it is difficult to obtain the levels of these indicators clinically. We first demonstrate our results regarding easily measurable inflammation markers, such as hsCRP, SIRI, and NMR, which are associated with hypertensive risk in PLWH.
Inflammation is a defense mechanism against infection and trauma, which can stimulate the activation of innate and adaptive immune responses to fight pathogens and restore damaged tissues to their normal state. However, in some cases, chronic inflammation does not completely resolve and may persist across the lifespan (5). A prominent characteristic of HIV infection is the expression of proinflammatory cytokines, which regulate HIV replication and T cell apoptosis during the HIV lifecycle (30). Persistent low-level inflammation causes endothelial dysfunction and promotes the development of hypertension by activating inflammatory pathways and inducing oxidative stress (31). In PLWH, HIV replication is significantly inhibited under ART but does not completely disappear, with the residual virus replicating at low levels over long periods of time, which eventually causes long-term micro-inflammation. Impaired immune and activate inflammatory pathways induce oxidative stress, leading to endothelial dysfunction, which is a key factor in the pathogenesis of hypertension (5, 31). We found that hsCRP is a risk factor for hypertension in PLWH. hsCRP is one of the simplest and most direct indicators of inflammation in clinical practice, and achieves high sensitivity at low costs. In PLWH, elevated hsCRP may be due to HIV or other infections, and may resolve with successful treatment (32). Therefore, we can monitor the progress of low-level inflammation to predict the occurrence of hypertension, and implement early intervention to reduce effects.
Blood analysis is a simple and an easily accessible tool in which monocyte, neutrophil, and lymphocyte counts can be evaluated and used as clinical indicators. SIRI is a more comprehensive marker for chronic low-grade inflammation based on monocyte, neutrophil, and lymphocyte counts (33). NMR is the ratio of neutrophils to monocytes. Neutrophils and monocytes belong to the innate immune system, while lymphocytes are representative of the adaptive immune response, all of which participate in the body’s immune response. Lymphocytes are also the main target cells of the HIV virus. Neutrophils mediate immune responses by phagocytosing pathogenic microorganisms, releasing antimicrobial proteins via degranulation, and producing extracellular traps. By interacting with other innate and adaptive immune cells to attenuate the level of inflammation (such as natural killer cells and T cells), neutrophils can also secrete reactive oxygen species, promote endothelial dysfunction, and directly participate in the occurrence of hypertension (34). Moreover, neutrophil counts are independently associated with hypertension (35, 36). Monocytes participate in the body’s immune response by inducing phagocytosis of pathogens, secreting cytokines, and presenting antigens. For PLWH, HIV can reduce the number of monocytes and increase the proportion of intermediate monocytes (37–40). Even long-term regular ART cannot completely restore the disordered monocytes subsets. Intermediate monocytes, also known as inflammatory monocytes, have a strong pro-inflammatory effect and can injure the vascular endothelium (41, 42), which can secrete inflammation factors leading to persistent systemic inflammation (43). Activated lymphocytes can also induce inflammation and damage the vascular endothelium by secreting cytokines, thereby promoting the occurrence of hypertension (31, 44). In PLWH, persistently low levels of inflammation lead to increased neutrophil levels and decreased monocyte and lymphocyte levels, which translates into high SIRI and NMR values. Therefore, SIRI and NMR can serve as economical and convenient parameters for evaluating the inflammation status of PLWH, and can indicate their susceptibility to hypertension.
Regarding HIV-related factors, our study found that recent elevated CD4+ cell counts was associated with an increased risk of hypertension. Similar to our findings, Xu et al. reported that CD4+ cell counts exceeding 350 cells/µL were associated with higher risks for hypertension (45). In the general population, CD4+ cells are critical to the pathophysiology of hypertension and can produce immunosuppressive cytokines to suppress immune response activation (46). After HIV infection, CD4+ cell counts drop dramatically and then rise again when ART is initiated (27). Low CD4+ cell counts have been associated with higher risks for hypertension in PLWH (47–49), which disagrees with our findings. In fact, we found that low CD4+ cell counts occurred shortly after HIV infection. We inferred that after ART, even though the number of CD4+ cells increased, there was still a functional defect and insufficient ability to produce immunosuppressive factors such as low levels of transforming growth factor (TGF-β) and IL-10 (46). Interestingly, we found that CD8+ cell counts were negatively correlated with hypertension. CD8+ cells can protect against atherosclerosis by inducing plaque cell apoptosis and inflammation (50). A number of HIV antigens are recognized by antibodies which are correlated inversely with CD8+ T cell activation and positively with CD4+ cell counts (51). Early research showed that PLWH share some markers of aging with older noninfected patients, such as increased inflammation marker levels, D-dimers, CD4+/CD8+ inversion, and proportions of memory CD8 + T cells (52). The CD4+/CD8+ ratio might represent a marker that would remind clinicians to distinguish, among regular ART patients, those who require more attentive monitoring for possible early onset complications (52).
Compared with 1 to 4 years, ART for 5 to 9 years or over 10 years increased the risk of hypertension in PLWH. As mentioned earlier, hypertension is a typical age-related disease, long ART time represents a longer survival period of participant to a certain extent, with the prolongation of survival period, the probability of hypertension may be increase correspondingly. Furthermore, although ART is highly effective at inhibiting HIV replication, it is not curative, persistent low level HIV replication will result in micro-inflammation, inflammation is a clear risk factor for hypertension. Interestingly, Low levels of HIV-RNA (lower than 100 copies/mL) was positively associated with the prevalence of hypertensive in PLWH. We speculate that low level of HIV also produce inflammation, moreover, it may be related to time. In our study, most participants had undetectable HIV-RNA, the median time of HIV diagnosis and the median ART duration in the case group were longer than those in the control group. This means that inflammation exists for a longer time and has a higher degree of accumulation in the case group. In fact, a similar conclusion was reached in the Data Collection on Adverse Events of Anti-HIV Drugs (D: A:D) Study, which found that HIV-RNA>100,000 copies/mL was associated with a decreased risk of hypertension (47). HIV single-stranded RNA have been recognized by Toll-like Receptors 7, which then destroy T cells and release inflammatory cytokines (53). HIV-mediated transactivation of transcription protein activates Nuclear Factor-Kappa B (NF-kB) and upregulates the expressions of inflammation cytokines and chemokines (53). Immune activation persists in PLWH under ART, and markers of inflammation (i.e.IL-6) predict mortality in atherosclerotic cardiovascular disease (54). BMI, a traditional risk factor for hypertension, also plays a positive role in PLWH. Weight changes in PLWH after ART initiation is very common. Studies have found that high BMI is negatively associated with mortality and positively with cardiovascular events in PLWH (55, 56). Gender and age were independent risk factors associated with high BMI after 48 weeks of ART (57), but BMI was not associated with traditional biomarkers of inflammation, such as hsCRP, soluble tumor necrosis factor (sTNFR)-2, soluble CD14(sCD14), IL-6 (58).
We also analyzed the correlations between CD4+, CD8+, CD4+/CD8+ ratio, and inflammation markers. The results revealed that only SIRI correlated with CD4+ cell counts, CD8+ cell counts, and the CD4+/CD8+ ratio. SIRI combines neutrophils, monocytes, and lymphocytes to represent the balance between inflammation activators and regulators in the host. Composite indicators are more stable than single indicators, and high SIRI status reflects mediated by monocytes and neutrophils and a weak anti-inflammatory response mediated by lymphocyte (59). To the best of our knowledge, this is the first study to report the role of SIRI in hypertension in PLWH and the correlations between SIRI and T cells. Our findings may be useful for evaluating the immune status of a PLWH indirectly, preliminarily from indicators in blood. We also separately analyzed the correlation between BMI and inflammation markers in the case group, there is no correlation between BMI and inflammation factors (Supplemental Figure 1).
The study has some inevitable limitations. This was a single-center study with a small sample size, and multi center research is needed to further verify the validity of our conclusions. Microbial translocation, chronic inflammation, immune reconstitution, and HIV-related renal disease all influence the development and progression of hypertension. In addition, the use of INSTIs has been reported to affect hypertension (60), due to the limited number of patients using INSTIs, we have not found the effect of drugs on hypertension for the time being. Indeed, the effect of drugs on blood pressure may require longer observation. Due to the limitations of a retrospective study, we were unable to match all the traditional risk factors for hypertension. Although we tried to adjust for confounding factors, the results should be interpreted with caution in clinical practice. In addition, we cannot completely eliminate the influence of unknown opportunistic infections on the results. We tried to select homogeneous cases when manually matching the control group, and the selected control group cases all underwent regular ART. These steps were performed in an attempt to eliminate the influence of confounding factors.
While some limitations exist, we believe that our findings have important clinical significance. To our knowledge, inflammation markers in this study are based on routine blood tests, making all indicators easily available at early stages in hospitals and most communities, and provide a convenient and economical way to monitor inflammation levels. hsCRP, SIRI, and NMR were associated with an increased risk of hypertension in PLWH. Therefore, regular monitoring of inflammation markers is necessary in PLWH to distinguish and identify individuals at high-risk for hypertension. Alleviating inflammation may contribute to controlling or delaying the occurrence of hypertension in PLWH.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Third People’s Hospital of Kunming. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
HO-Y and H-YF wrote the draft of manuscript and contributed equally. YL was responsible for data analysis, and interpretation. H-JL and Y-RD was responsible for the design and provided supervision. Z-YX, RG, J-YD, Y-CM participated in data collection and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Key Laboratory of Drug Dependence and Withdrawal Treatment, National Health Commission of China (NO.2020DAMOP-0013).
Acknowledgments
We would like to acknowledge and thank all the study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1133640/full#supplementary-material
Abbreviations
HTN, the hypertension group; Non-HTN, the non-hypertension group.
References
1. Bigna JJ, Ndoadoumgue AL, Nansseu JR, Tochie JN, Nyaga UF, Nkeck JR, et al. Global burden of hypertension among people living with HIV in the era of increased life expectancy: A systematic review and meta-analysis. J Hypertens (2020) 38(9):1659–68. doi: 10.1097/HJH.0000000000002446
2. Pérez PS, Romaniuk MA, Duette GA, Zhao Z, Huang Y, Martin-Jaular L, et al. Extracellular vesicles and chronic inflammation during HIV infection. J Extracell Vesicles (2019) 8(1):1687275. doi: 10.1080/20013078.2019.1687275
3. Olson A, Coote C, Snyder-Cappione JE, Lin N, Sagar M. HIV-1 transcription but not intact provirus levels are associated with systemic inflammation. J Infect Dis (2021) 223(11):1934–42. doi: 10.1093/infdis/jiaa657
4. Masyuko SJ, Page ST, Polyak SJ, Kinuthia J, Osoti AO, Otieno FC. Et al.Human immunodeficiency virus is associated with higher levels of systemic inflammation among Kenyan adults despite viral suppression. Clin Infect Dis (2021) 73(7):e2034–42. doi: 10.1093/cid/ciaa1650
5. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med (2019) 25(12):1822–32. doi: 10.1038/s41591-019-0675-0
6. Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: Systematic review and meta-analysis. Circulation (2018) 138(11):1100–12. doi: 10.1161/CIRCULATIONAHA.117.033369
7. Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: A systematic review and meta-analysis. J Am Soc Hypertens (2017) 11(8):530–40. doi: 10.1016/j.jash.2017.06.004
8. Sanidas E, Papadopoulos DP, Velliou M, Tsioufis K, Barbetseas J, Papademetriou V. Human immunodeficiency virus infection and hypertension. is there a connection? Am J Hypertens (2018) 31(4):389–93. doi: 10.1093/ajh/hpx208
9. Van Zoest RA, van den Born BH, Reiss P. Hypertension in people living with HIV. Curr Opin HIV AIDS (2017) 12(6):513–22. doi: 10.1097/COH.0000000000000406
10. Fan H, Guo F, Hsieh E, Chen WT, Lv W, Han Y, et al. Incidence of hypertension among persons living with HIV in China: A multicenter cohort study. BMC Public Health (2020) 20(1):834. doi: 10.1186/s12889-020-08586-9
11. Zhao WM, Tao SM, Liu GL. Neutrophil-to-lymphocyte ratio in relation to the risk of all-cause mortality and cardiovascular events in patients with chronic kidney disease: A systematic review and meta-analysis. Ren Fail (2020) 42(1):1059–66. doi: 10.1080/0886022X.2020.1832521
12. Angkananard T, Inthanoo T, Sricholwattana S, Rattanajaruskul N, Wongsoasu A, Roongsangmanoon W. The predictive role of neutrophil-to-Lymphocyte ratio (NLR) and mean platelet volume-to-Lymphocyte ratio (MPVLR) for cardiovascular events in adult patients with acute heart failure. Mediators Inflammation (2021) 2021:6889733. doi: 10.1155/2021/6889733
13. Zhan L, Liu Y, Cheng Y, Guo W, Yang J. Predictive value of Neutrophil/Lymphocyte ratio (NLR) on cardiovascular events in patients with COVID-19. Int J Gen Med (2021) 14:3899–3907. doi: 10.2147/IJGM.S317380
14. Qiao S, Gao W, Guo S. Neutrophil-lymphocyte ratio (NLR) for predicting clinical outcomes in patients with coronary artery disease and type 2 diabetes mellitus: A propensity score matching analysis. Ther Clin Risk Manag (2020) 16:437–43. doi: 10.2147/TCRM.S244623
15. Yang YL, Wu CH, Hsu PF, Chen SC, Huang SS, Chan WL, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest (2020) 50(5):e13230. doi: 10.1111/eci.13230
16. Huang J, Zhang Q, Wang R, Ji H, Chen Y, Quan X, et al. Systemic immune-inflammatory index predicts clinical outcomes for elderly patients with acute myocardial infarction receiving percutaneous coronary intervention. Med Sci Monit (2019) 25:9690–9701. doi: 10.12659/MSM.919802
17. Liu J, Ao W, Zhou J, Luo P, Wang Q, Xiang D. The correlation between PLR-NLR and prognosis in acute myocardial infarction. Am J Transl Res (2021) 13(5):4892–9.
18. Raffetti E, Donato F, Casari S, Castelnuovo F, Sighinolfi L, Bandera A, et al. Systemic inflammation-based scores and mortality for all causes in HIV-infected patients: A MASTER cohort study. BMC Infect Dis (2017) 17(1):193. doi: 10.1186/s12879-017-2280-5
19. Whitworth JA. World health organization, international society of hypertension writing group. 2003 world health organization (WHO)/International society of hypertension (ISH) statement on management of hypertension. J Hypertens (2003) 21(11):1983– 92. doi: 10.1097/00004872-200311000-00002
20. Unger T, Borghi C C, harchar F, NA K, NR P, Prabhakaran D, et al. International society of hypertension global hypertension practice guidelines. J Hypertens(2020) (2020) 38(6):982–1004. doi: 10.1097/HJH.0000000000002453
21. Sun JJ, Lu HZ. [Highlights of the third edition of Chinese guidelines for AIDS diagnosis and treatment (2015)]. Zhejiang Da Xue Xue Bao Yi Xue Ban (2015) 44(6):597–602. doi: 10.3785/j.issn.1008-9292.2015.11.01
22. AIDS and Hepatitis C Professional Group, Society of Infectious Diseases, Chinese Medical Association, Chinese Center for Disease Control and Prevention. [Chinese guidelines for diagnosis and treatment of HIV/AIDS (2018)]. Zhonghua Nei Ke Za Zhi (2018) 57(12):867–84. doi: 10.3760/cma.j.issn.0578-1426.2018.12.002
23. AIDS and Hepatitis C Professional Group, Society of Infectious Diseases, Chinese Medical Association, Chinese Center for Disease Control and Prevention. [Chinese guidelines for diagnosis and treatment of HIV/AIDS (2021 edition)]. Zhonghua Nei Ke Za Zhi (2021) 60(12):1106–28. doi: 10.3760/cma.j.cn112138-20211006-00676
24. Zhou BF. Cooperative meta-analysis group of the working group on obesity in china. predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. BioMed Environ Sci (2002) 15(1):83–96.
25. Ruan Y, Huang Y, Zhang Q, Qin S, Du X, Sun Y. Association between dietary patterns and hypertension among han and multi-ethnic population in southwest China. BMC Public Health (2018) 18(1):1106. doi: 10.1186/s12889-018-6003-7
26. Shen S, Cheng J, Li J, Xie Y, Wang L, Zhou X, et al. Association of marital status with cognitive function in Chinese hypertensive patients: A cross-sectional study. BMC Psychiatry (2022) 22(1):504. doi: 10.1186/s12888-022-04159-9
27. Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV-infected adults: Novel pathophysiologic mechanisms. Hypertension (2018) 72(1):44–55. doi: 10.1161/HYPERTENSIONAHA.118.10893
28. Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis (2014) 210(8):1248– 59. doi: 10.1093/infdis/jiu254
29. Masenga SK, Elijovich F, Hamooya BM, Nzala S, Kwenda G, Heimburger DC, et al. Elevated eosinophils as a feature of inflammation associated with hypertension in virally suppressed people living with HIV. J Am Heart Assoc (2020) 9(4):e011450. doi: 10.1161/JAHA.118.011450
30. Decrion AZ, Dichamp I, Varin A, Herbein G. HIV And inflammation. Curr HIV Res (2005) 3(3):243–59. doi: 10.2174/1570162054368057
31. Barrows IR, Ramezani A, Raj DS. Inflammation, immunity, and oxidative stress in hypertension-partners in crime? Adv Chronic Kidney Dis (2019) 26(2):122–30. doi: 10.1053/j.ackd.2019.03.001
32. Putcharoen O, Pleumkanitkul S, Chutinet A, Vongsayan P, Samajarn J, Sophonphan J, et al. Comparable carotid intima-media thickness among long-term virologically suppressed individuals with HIV and those without HIV in Thailand. J Virus Erad (2019) 5(1):23–7.
33. Zhang Y, Xing Z, Zhou K, Jiang S. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin Interv Aging (2021) 16:1997–2007. doi: 10.2147/CIA.S339221
34. Araos P, Figueroa S, Amador CA. The role of neutrophils in hypertension. Int J Mol Sci (2020) 21(22):8536. doi: 10.3390/ijms21228536
35. Belen E, Sungur A, Sungur MA, Erdoğan G. Increased neutrophil to lymphocyte ratio in patients with resistant hypertension. J Clin Hypertens (Greenwich) (2015) 17(7):532– 7. doi: 10.1111/jch.12533
36. Aydin M, Yuksel M, Yildiz A, Polat N, Bilik MZ, Akil MA, et al. Association between the neutrophil to lymphocyte ratio and prehypertension. Bratisl Lek Listy (2015) 116(8):475– 9. doi: 10.4149/bll_2015_090
37. Knudsen AD, Bouazzi R, Afzal S, Gelpi M, Benfield T, Høgh J, et al. Monocyte count and soluble markers of monocyte activation in people living with HIV and uninfected controls. BMC Infect Dis (2022) 22(1):451. doi: 10.1186/s12879-022-07450-y
38. Huaman MA, Feria MG, Kityo C, Nalukwago S, Nazzinda R, Zidar DA, et al. A sex-stratified analysis of monocyte phenotypes associated with HIV infection in Uganda. Viruses (2021) 13(11):2135. doi: 10.3390/v13112135
39. Prabhu VM, Singh AK, Padwal V, Nagar V, Patil P, Patel V. Monocyte based correlates of immune activation and viremia in HIV-infected long-term non-progressors. Front Immunol (2019) 10:2849. doi: 10.3389/fimmu.2019.02849
40. Chen P, Su B, Zhang T, Zhu X, Xia W, Fu Y, et al. Perturbations of monocyte subsets and their association with T helper cell differentiation in acute and chronic HIV-1-Infected patients. Front Immunol (2017) 8:272. doi: 10.3389/fimmu.2017.00272
41. Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J Leukoc Biol (2007) 81(3):584–92. doi: 10.1189/jlb.0806510
42. Gupta SK, Liu Z, Sims EC, Repass MJ, Haneline LS, Yoder MC. Endothelial colony-forming cell function is reduced during HIV infection. J Infect Dis (2019) 219(7):1076–1083. doi: 10.1093/infdis/jiy550
43. Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol (2002) 168(7):3536– 42. doi: 10.4049/jimmunol.168.7.3536
44. Moro-García MA, Mayo JC, Sainz RM, Alonso-Arias R. Influence of inflammation in the process of T lymphocyte differentiation: Proliferative, metabolic, and oxidative changes. Front Immunol (2018) 9:339. doi: 10.3389/fimmu.2018.00339
45. Xu X, Chen X, Lin H, Zhu B, Shen W, Shi R, et al. General and abdominal obesity and incident hypertension among people living with HIV on antiretroviral therapy. AIDS Care (2021) 33(12):1616–1620. doi: 10.1080/09540121.2020.1852158
46. Agita A, Alsagaff MT. Inflammation, immunity, and hypertension. Acta Med Indones (2017) 49(2):158–65.
47. Hatleberg CI, Ryom L, d'Arminio Monforte A, Fontas E, Reiss P, Kirk O, et al. Association between exposure to antiretroviral drugs and the incidence of hypertension in HIV-positive persons: the data collection on adverse events of anti-HIV drugs (D: A:D) study. HIV Med (2018) 19(9):605–18. doi: 10.1111/hiv.12639
48. Ding Y, Lin H, Liu X, Zhang Y, Wong FY, Sun YV, et al. Hypertension in HIV-infected adults compared with similar but uninfected adults in China: Body mass index-dependent effects of nadir CD4 count. AIDS Res Hum Retroviruses (2017) 33(11):1117–25. doi: 10.1089/AID.2017.0008
49. Oliveira RVC, Shimakura SE, Campos DP, Hökerberg YHM, Victoriano FP, Ribeiro S, et al. Effects of antiretroviral treatment and nadir CD4 count in progression to cardiovascular events and related comorbidities in a HIV Brazilian cohort: a multi-stage approach. AIDS Care (2018) 30(5):551–9. doi: 10.1080/09540121.2017.1391984
50. Cochain C, Zernecke A. Protective and pathogenic roles of CD8+ T cells in atherosclerosis. Basic Res Cardiol (2016) 111(6):71. doi: 10.1007/s00395-016-0589-7
51. Matavele Chissumba R, Magul C, Macamo R, Monteiro V, Enosse M, Macicame I, et al. Helios Expressing regulatory T cells are correlated with decreased IL-2 producing CD8 T cells and antibody diversity in Mozambican individuals living chronically with HIV-1. BMC Immunol (2022) 23(1):12. doi: 10.1186/s12865-022-00487-3
52. Saracino A, Bruno G, Scudeller L, Volpe A, Caricato P, Ladisa N, et al. Chronic inflammation in a long-term cohort of HIV-infected patients according to the normalization of the CD4:CD8 ratio. AIDS Res Hum Retroviruses (2014) 30(12):1178–84. doi: 10.1089/aid.2014.0080
53. Lv T, Cao W, Li T. HIV-Related immune activation and inflammation: Current understanding and strategies. J Immunol Res (2021) 2021:7316456. doi: 10.1155/2021/7316456
54. Dirajlal-Fargo S, Funderburg N. HIV And cardiovascular disease: The role of inflammation. Curr Opin HIV AIDS (2022) 17(5):286–292. doi: 10.1097/COH.0000000000000755
55. Kim DJ, Westfall AO, Chamot E, Willig AL, Mugavero MJ, Ritchie C, et al. Multimorbidity patterns in HIV-infected patients: The role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr (2012) 61(5):600–5. doi: 10.1097/QAI.0b013e31827303d5
56. James WP, Rigby N, Leach R. Obesity and the metabolic syndrome: the stress on society. Ann N Y Acad Sci (2006) 1083:1–10. doi: 10.1196/annals.1367.002
57. Qian Z, Wu H, Wu Y, Liao W, Yu T, Xu X, et al. A model for predicting high BMI of people living with HIV after receiving antiretroviral therapy. Ther Adv Chronic Dis (2022) 13:20406223221102750. doi: 10.1177/20406223221102750
58. Ticona E, Bull ME, Soria J, Tapia K, Legard J, Styrchak SM, et al. Biomarkers of inflammation in HIV-infected Peruvian men and women before and during suppressive antiretroviral therapy. AIDS (2015) 29(13):1617– 22. doi: 10.1097/QAD.0000000000000758
59. Fan W, Wei C, Liu Y, Sun Q, Tian Y, Wang X, et al. The prognostic value of hematologic inflammatory markers in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Clin Appl Thromb Hemost (2022) 28:10760296221146183. doi: 10.1177/10760296221146183
60. Byonanebye DM, Polizzotto MN, Neesgaard B, Sarcletti M, Matulionyte R, Braun DL, et al. Incidence of hypertension in people with HIV who are treated with integrase inhibitors versus other antiretroviral regimens in the RESPOND cohort consortium. HIV Med (2022) 23(8):895–910. doi: 10.1111/hiv.13273
Keywords: HIV, AIDS, hypertension, inflammation markers, high-sensitivity C-reactive protein
Citation: Ou-Yang H, Fu H-Y, Luo Y, Xu Z-Y, Liu J, Gao R, Duan J-Y, Mao Y-C, Li H-J and Du Y-R (2023) Inflammation markers and the risk of hypertension in people living with HIV. Front. Immunol. 14:1133640. doi: 10.3389/fimmu.2023.1133640
Received: 29 December 2022; Accepted: 03 March 2023;
Published: 21 March 2023.
Edited by:
Maria Pini, Alira Health, United StatesReviewed by:
Alex Kayongo, Makerere University, UgandaNicola Squillace, San Gerardo Hospital, Italy
Daniel Edem Kpewou, University for Development Studies, Ghana
Copyright © 2023 Ou-Yang, Fu, Luo, Xu, Liu, Gao, Duan, Mao, Li and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-Rong Du, ZHlyX2ttQDE2My5jb20=; Hong-Juan Li, bGhqX2ttQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Hui Ou-Yang1†
Hui Ou-Yang1† Hai-Yan Fu
Hai-Yan Fu Yu Luo
Yu Luo Ying-Rong Du
Ying-Rong Du