- Department of Urology, The Second Hospital of Lanzhou University, Lanzhou, China
Purpose: The prognostic impact of cytoreductive nephrectomy (CN) for metastatic renal cell carcinoma (mRCC) in the era of immunotherapy is yet to be determined. The aim of our study is to evaluate the correlation between CN and outcomes in the setting of mRCC treated with immunotherapy.
Methods: We conducted a systematic search of the Science, PubMed, Web of Science, and Cochrane Library databases to identify relevant studies published in English up to December 2022. The results were presented as hazard ratio (HR) with 95% confidence intervals (CIs) for overall survival (OS) was extracted to assess their relevance. The study was registered with PROSPERO (CRD42022383026).
Results: A total of 2397 patients were included in eight studies. The CN group was observed to be correlated with superior OS compared to the No CN group (HR = 0.53, 95% CI 0.39–0.71, p < 0.0001). Subgroup analysis according to the type of immunotherapy, sample size, and treatment line of immune checkpoint inhibitor revealed that CN group had a superior OS in all subgroups.
Conclusion: CN is associated with a better outcome in terms of OS benefit in selected patients with mRCC treated by immunotherapy, but further studies are required to verify the conclusions.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022383026.
1 Introduction
In the early twentieth century, the immunogenicity of renal cell carcinoma was discovered, leading to the establishment of interferon-alfa (IFN-alfa) and interleu-kin-2 as first-line therapies for metastatic renal cell carcinoma (mRCC) (1–3). However, the role of cytoreductive nephrectomy (CN) in the treatment of mRCC remains controversial. Two randomized clinical trials (RCTs) conducted by Mickish et al. (4) and Flanigan et al. (5) demonstrated that the combination of CN and IFN-alfa significantly improved overall survival (OS) of patients with mRCC compared to IFN-alfa therapy alone.
Over the past decade, the treatment paradigm for mRCC has significantly evolved, with targeted therapy becoming the new standard of care (6). The introduction of more effective targeted therapies has called into question the role of CN in this context. More recently, the results from a prospective RCT, CARMENA (7), demonstrated that patients with mRCC who received targeted therapy alone had comparable OS to those who received CN followed by targeted therapy. Additionally, another RCT, SURTIME (8), also questioned the value and the optimal timing of CN in relation to the initiation of systemic therapy. However, the universality and availability of both trials have been questioned due to delayed recruitment and unbalanced proportion of patients with poor-risk diseases in CARMENA study population (9). Méjean et al. (10) conducted a study which stratified patients according to the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC), demonstrating that some patients could still benefit from CN. Additionally, a meta-analysis encompassing 14 studies showed that CN can be beneficial for patients receiving targeted therapy (11). More recently, immune checkpoint inhibitors (ICIs) have revolutionized the treatment of mRCC. The outcomes from the CheckMate-025 study have led to the approval of nivolumab as the first ICIs for mRCC patients (12, 13). ICIs therapy, either alone or in combination with targeted therapy, has demonstrated superior efficacy and has been used as a first-line treatment for mRCC (14, 15). Despite the potential benefits of combined therapy of CN and immunotherapy, the impact of CN on patient outcomes remains controversial. Moreover, the small sample sizes of different clinical centers limit the reliability of any conclusions drawn.
This systematic review and meta-analysis aim to integrate the data from comparative studies to evaluate the relationship between CN and outcomes in the setting of mRCC treated with immunotherapy, thereby providing latest evidence for clinical decision-making.
2 Methods
The present study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement 2020 (16, 17), and was registered in PROSPERO (ID: CRD42022383026).
2.1 Literature search strategy, study selection and data collection
We systematically searched the databases such as Science, PubMed, Web of Science, and Cochrane Library to identify published studies till December 2022. The search terms were as follows: ((Renal cell carcinoma OR kidney carcinoma OR renal cell cancer) AND (Metastasis OR advanced) AND (Cytoreductive nephrectomy OR nephrectomy OR radical nephrectomy) AND (Immune checkpoint inhibitor OR immunotherapy OR immune-oncology OR PD-1 inhibitor OR PD-L1 inhibitors OR anti-PD-1 inhibitor)). Furthermore, we manually searched the relevant references and abstracts to avoid any omissions and expand the search scope.
We used the PICOS approach to define the inclusion criteria. P (patients): All the patients were diagnosed with mRCC; I (intervention): patients were undergone CN, either prior to (upfront) or following the initiation of immunotherapy (deferred). The immunotherapy was defined as cytokine-based therapy (IFN-alfa and interleu-kin-2), and ICIs; C (comparator): immunotherapy without CN; O (outcome): survival outcomes; S (study type): randomized controlled trials (RCTs), prospective studies and retrospective studies. Exclusion criteria include (1) duplicate studies and non-comparative studies, (2) the type of letters, comments, meeting abstracts, case reports and reviews, and (3) studies without detailed data for analysis.
Two evaluators (K.L. and S.C.) independently extracted the data from each qualified publication. The following data were extracted: (1) first author, year of publication, center, country, and study period. (2) age, sample size, gender, and follow-up period. (3) IMDC risk score, metastatic sites, number of sites of metastasis, and type of immunotherapy. (4) overall survival (OS). Any discrepancies and disagreements were resolved by discussion with a third evaluators (Y.L.).
In these studies, the risk of bias in non-randomized studies of interventions (ROBINS-I) was used to evaluate the quality of the non-RCTs (18). Furthermore, the Cochrane Collaboration tool was used to evaluate the quality of the RCTs (19). Two independent reviewers access the quality of included literatures, and any discrepancies were settled through discussion.
In the present study, the statistical analysis was processed using Cochrane Collaborative RevMan5.4 software. The hazard ratio (HR) was calculated for all the survival outcomes, and the results were presented with 95% confidence intervals (CIs). Considering the predictable significance between-trial heterogeneity, we used the random-effects model in all analyses. The I2 test was used to evaluate the heterogeneity of each indicator among the studies (20), and statistical significance was considered p < 0.05. Publication bias was evaluated using the Begg’s method funnel plot.
2.2 Subgroup analysis
The subgroup analysis was performed according to the type of immunotherapy, sample size, and treatment line of ICIs.
3 Results
3.1 Baseline characteristics
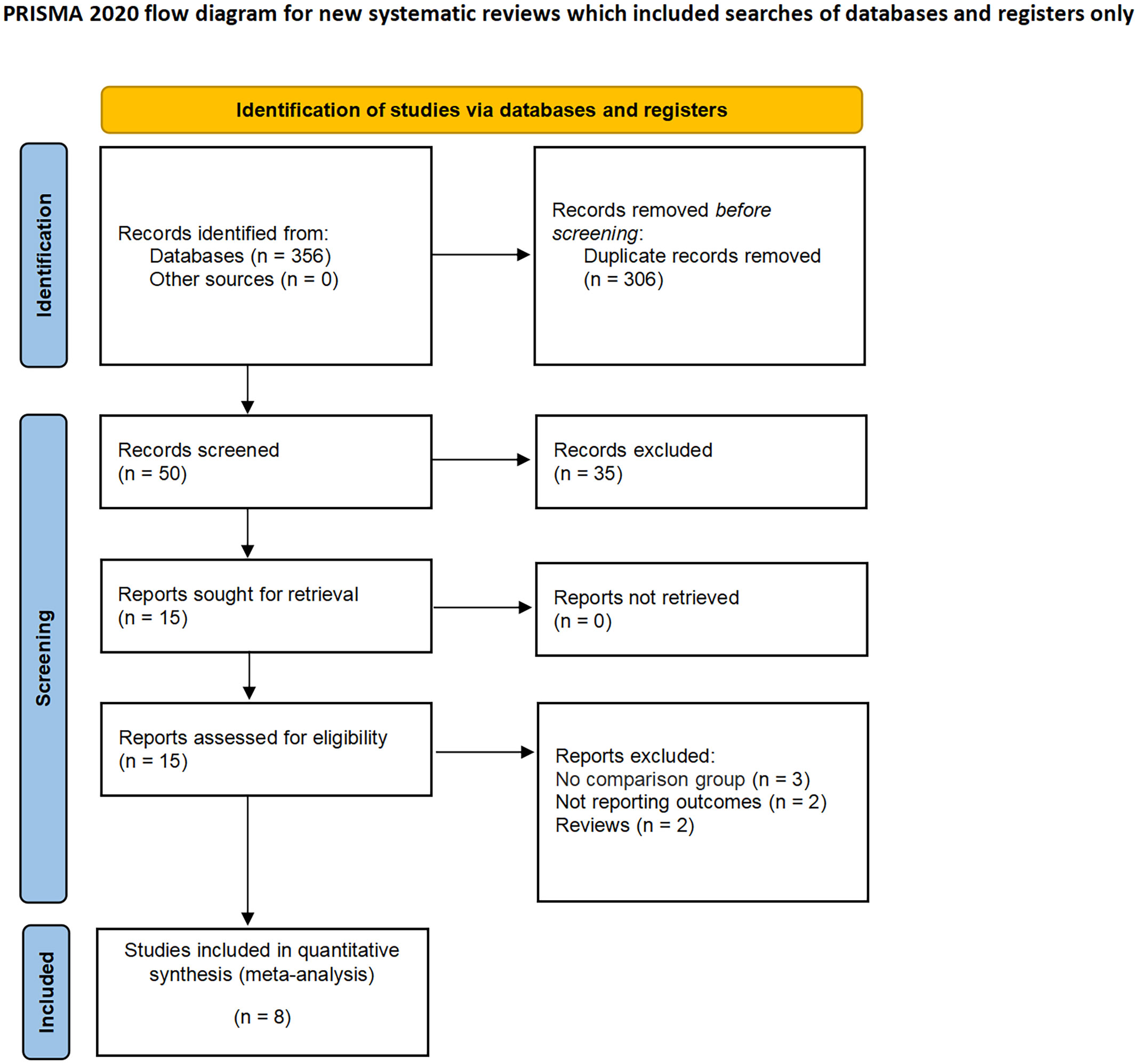
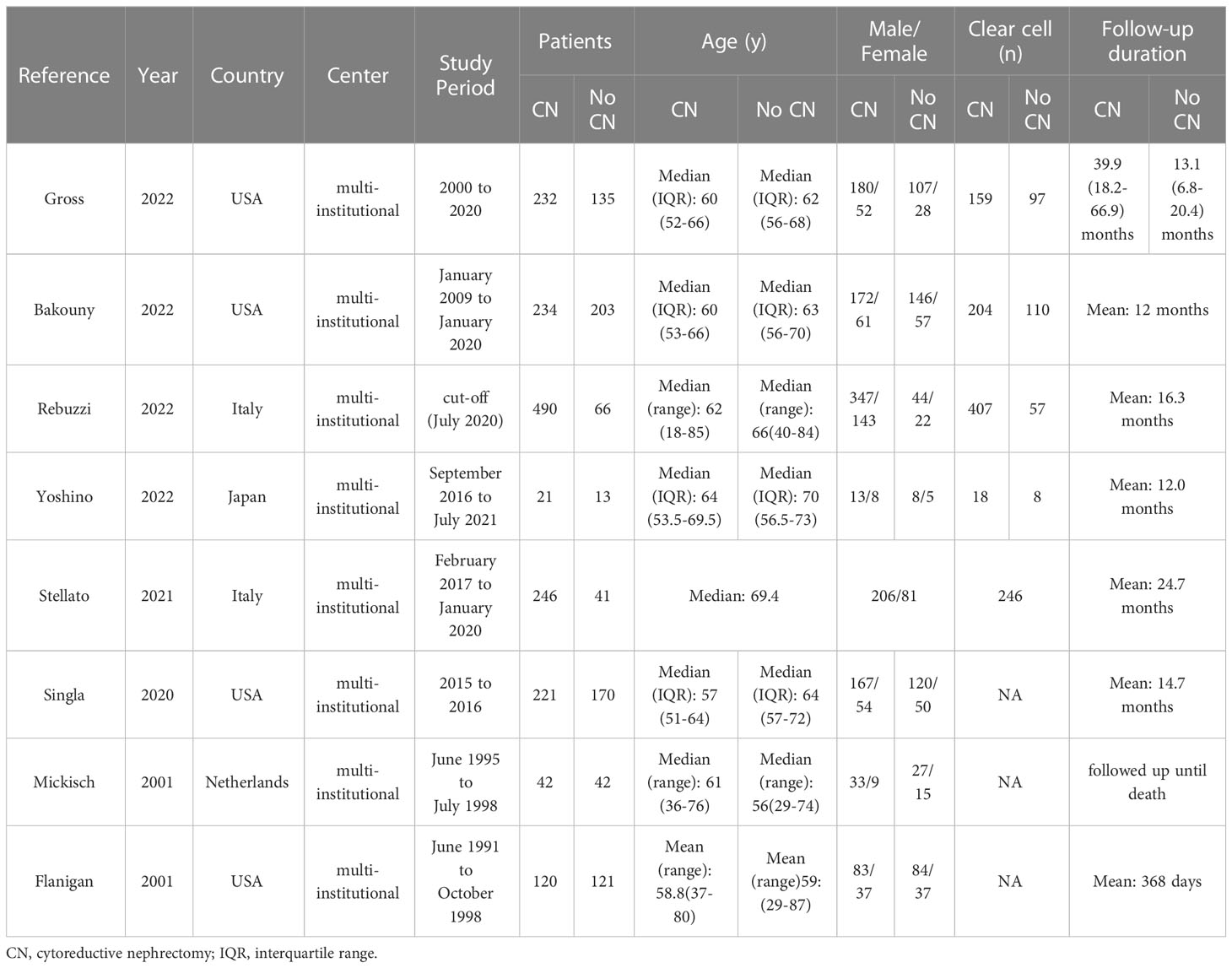
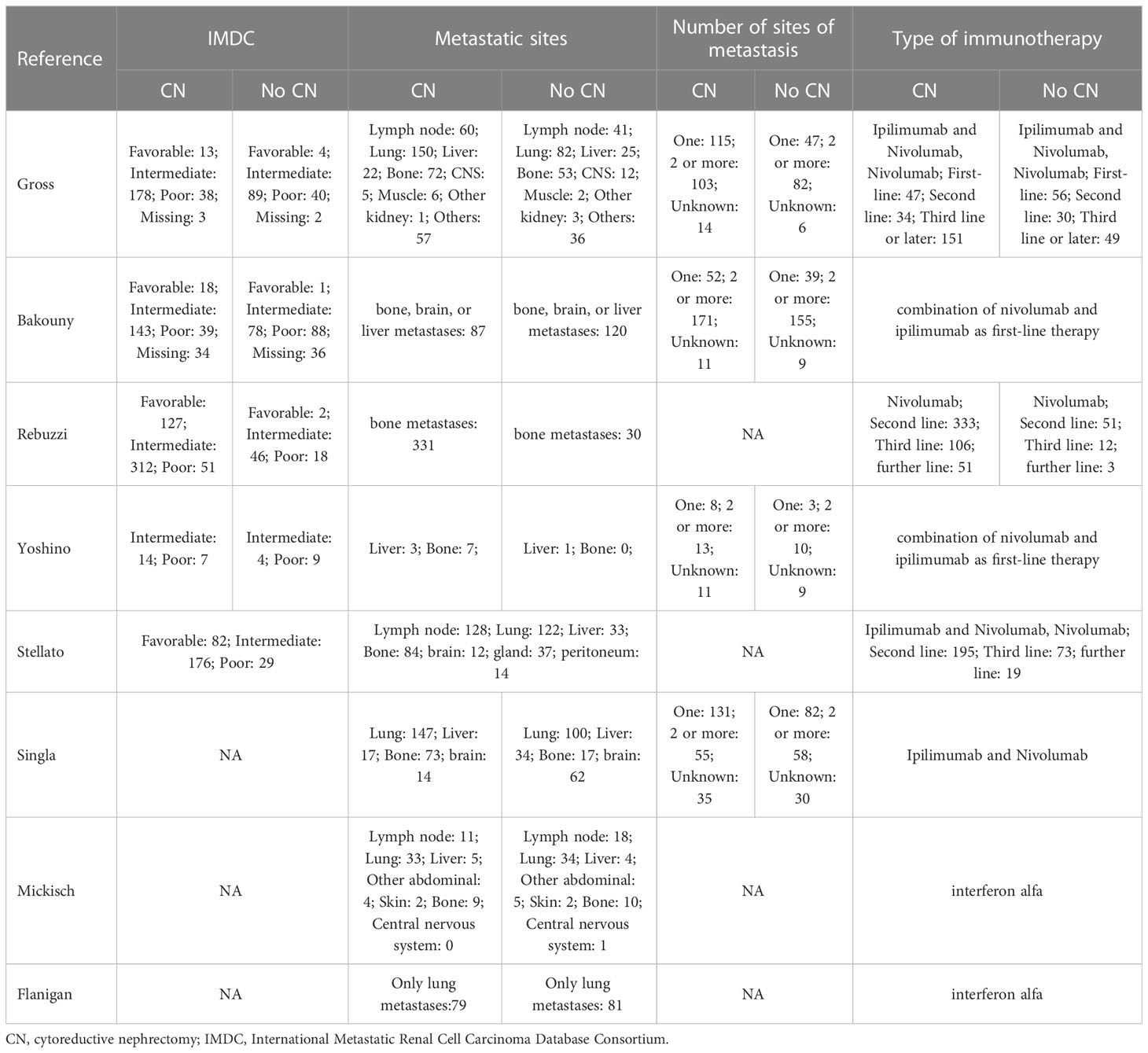
A total of 356 studies were initially identified through electronic search, with 15 remaining after removal of duplicates. After having read and screen the abstracts and full text, eight studies (two RCTs and six non-RCTs) involving 2397 patients were included in the meta-analysis (1606 CN vs. 791 No CN) (Figure 1) (4, 5, 21–26). Six non-RCTs were retrospective comparisons. All the studies were from multi-institutional, with six using ICIs as immunotherapy and the others using IFN-alfa (4, 5). The present studies were conducted in different countries, including the USA, Italy, Japan, and Netherlands, with a follow-up period ranging from 12 to 40 months. Table 1 summarizes the key characteristics of included studies, including their preoperative variables (country, age, sample size, and gender). Table 2 summarize the oncologic outcomes and interventions (IMDC risk score, metastatic sites, number of sites of metastasis, and type of immunotherapy). In the studies included, three studies compared the outcomes of deferred versus upfront CN in patients. Tables S1 summarize the demographic characteristics and oncologic outcomes of the deferred and upfront CN groups (age, gender, race, IMDC risk score, clear cell, metastatic sites, number of sites of metastasis, time from diagnosis to systemic therapy, follow-up duration).
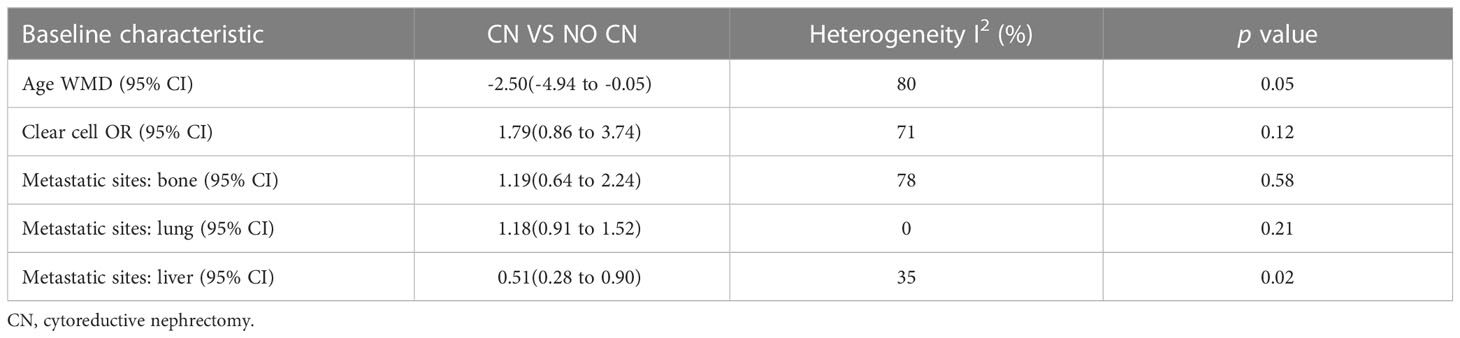
No significant difference was found in age (p = 0.05), clear cell (p = 0.12), bone metastasis (p = 0.58), and lung metastasis (p = 0.21). However, the liver metastasis was significantly less in the CN group compared to the No CN group (p = 0.02) (Table 3).
3.2 Assessment of quality
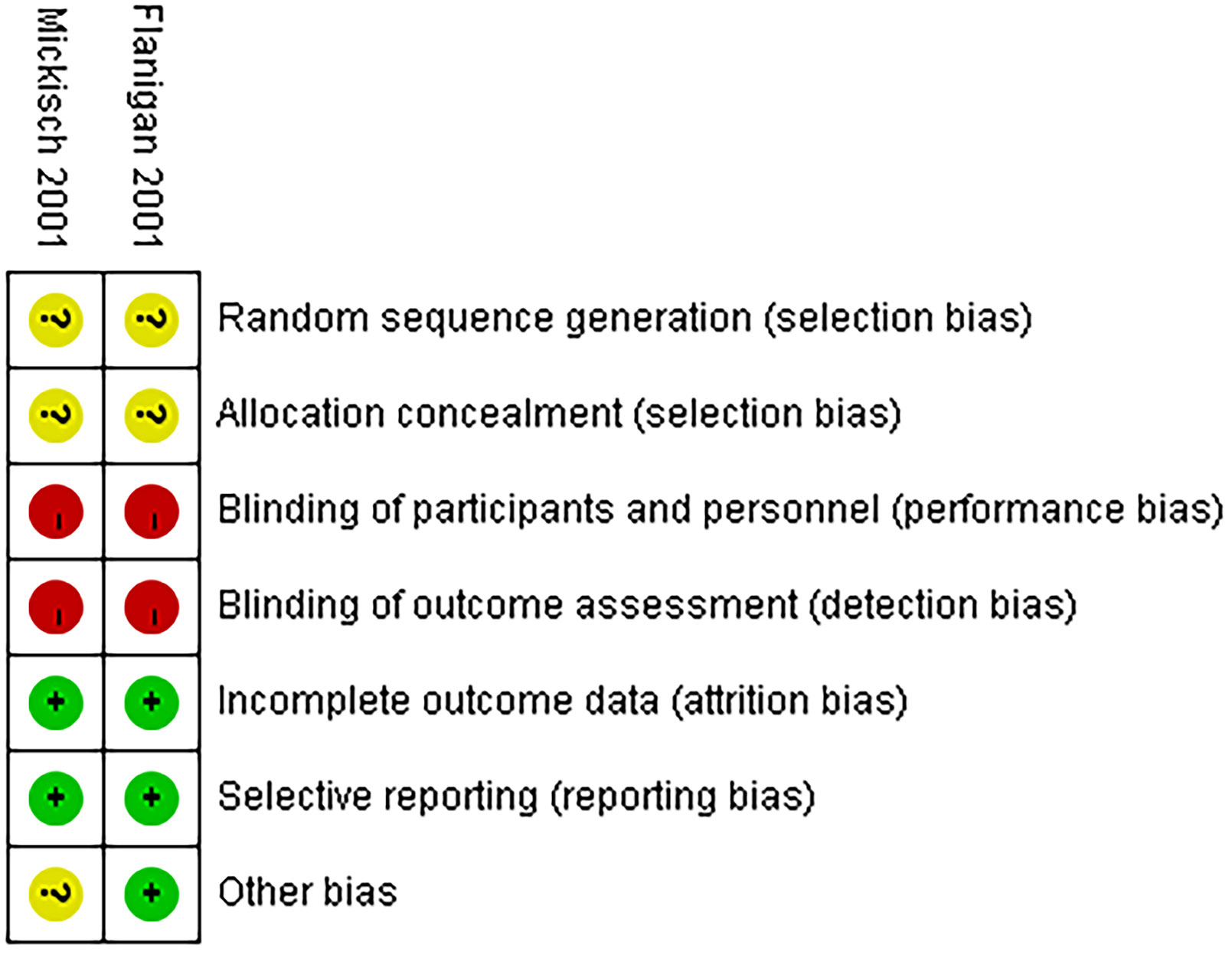
A comparative analysis was performed on all the non-RCTs, of which five studies had a moderate risk of bias (22–26) and one study had a low risk of bias (Table S2). All the non-RCTs were published between 2020 and 2022. Additionally, the two RCTs were not double-blinded, which increased the bias risk, thereby classifying them as high risk (Figure 2) (4, 5).
3.3 Outcome analysis
3.3.1 Overall survival
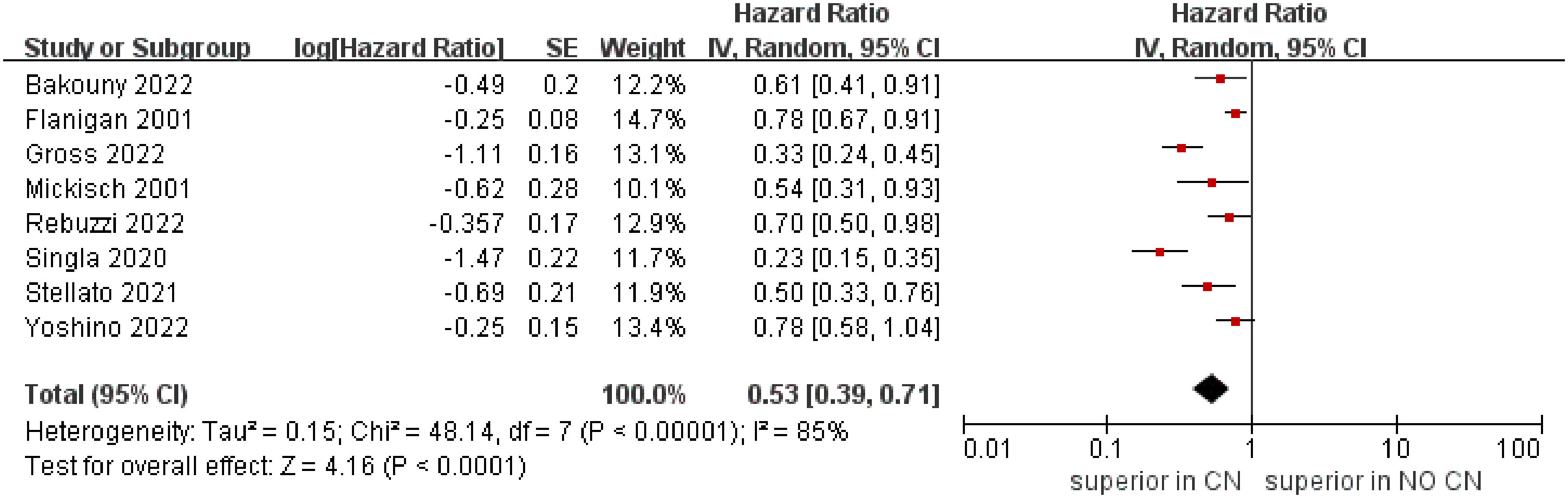
The meta‐analysis included eight studies that reported the OS (4, 5, 21–26). The combined results demonstrated that the CN group was associated with superior OS compared to the No CN group (HR = 0.53, 95% CI 0.39–0.71, p < 0.0001), and with high heterogeneity (I2 = 85%) (Figure 3).
3.3.2 Subgroup analyses
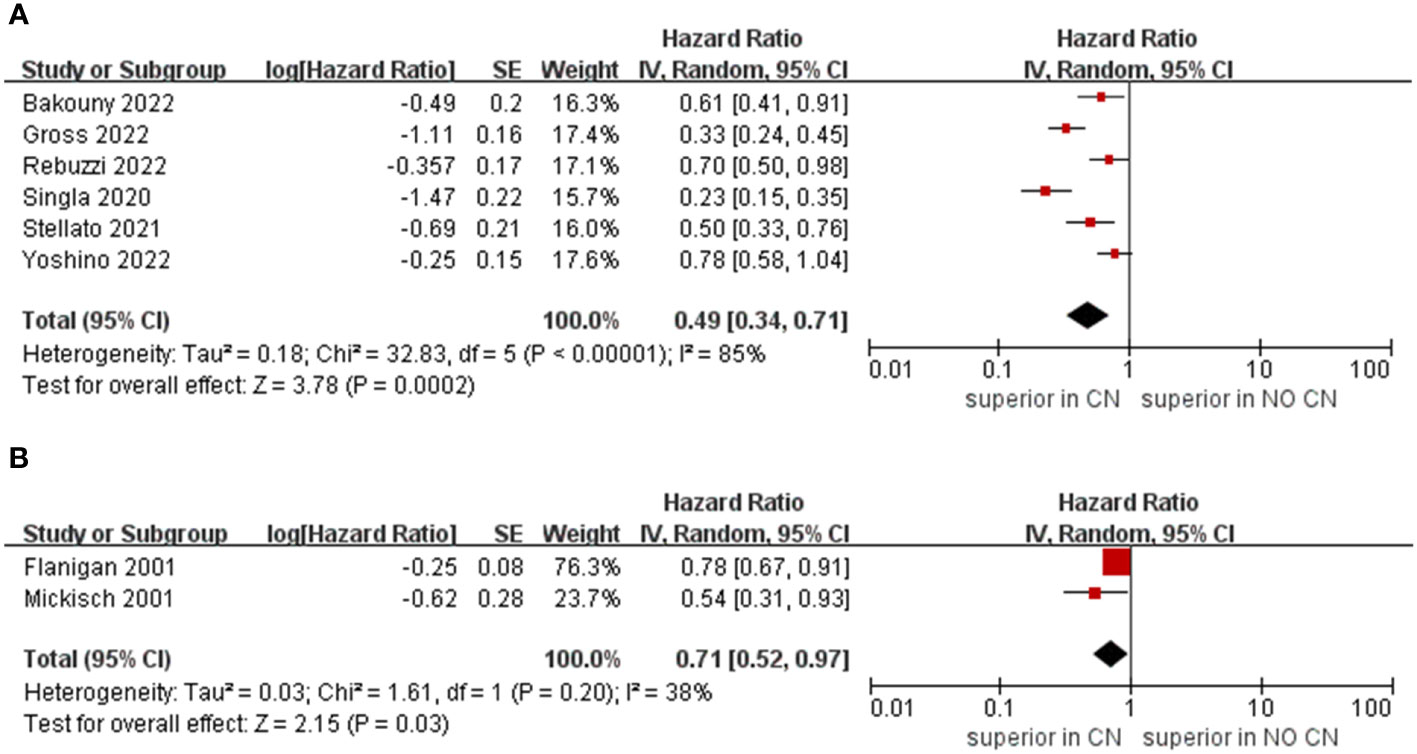
Owing to the insufficient literature included in the meta-analysis, we only preformed subgroup analysis of OS with respect to type of immunotherapy, sample size, and treatment line of ICIs. For studies that include the ICIs, the CN group had significantly lower risk of death compared to the No CN group (HR = 0.49, 95% CI 0.34–0.71, p = 0.0002, I2 = 85%) (21–26). In IFN-alfa subgroup, the CN group was also observed to be correlated with superior OS than for the No CN group (HR = 0.71, 95% CI 0.52–0.97, p = 0.03, I2 = 38%) (Figure 4) (4, 5). In the sample size > 400 subgroup, the CN group had significantly lower risk of death compared to the No CN group (HR = 0.52, 95% CI 0.32–0.84, p = 0.007, I2 = 83%) (21–23). Additionally, for the subgroup with a sample size ≤ 400, the CN group was correlated with better OS than for the No CN group (HR = 0.53, 95% CI 0.35–0.80, p = 0.002, I2 = 87%) (Figure 5) (4, 5, 24–26). In the subgroup analysis of the ICIs as first-line therapy, the CN group had significantly lower risk of death compared to the No CN group (HR = 0.50, 95% CI 0.27–0.92, p = 0.03, I2 = 83%) (21, 22, 24). Similarly, the subgroup analysis revealed that both ICIs as second, third line therapy and first, second and third therapy were associated with superior OS compared to the No CN group (HR = 0.61, 95% CI 0.44–0.84, p = 0.002, I2 = 34%; HR = 0.28, 95% CI 0.20–0.40, p < 0.00001, I2 = 43%) (Figure 6) (21, 23) (25, 26).
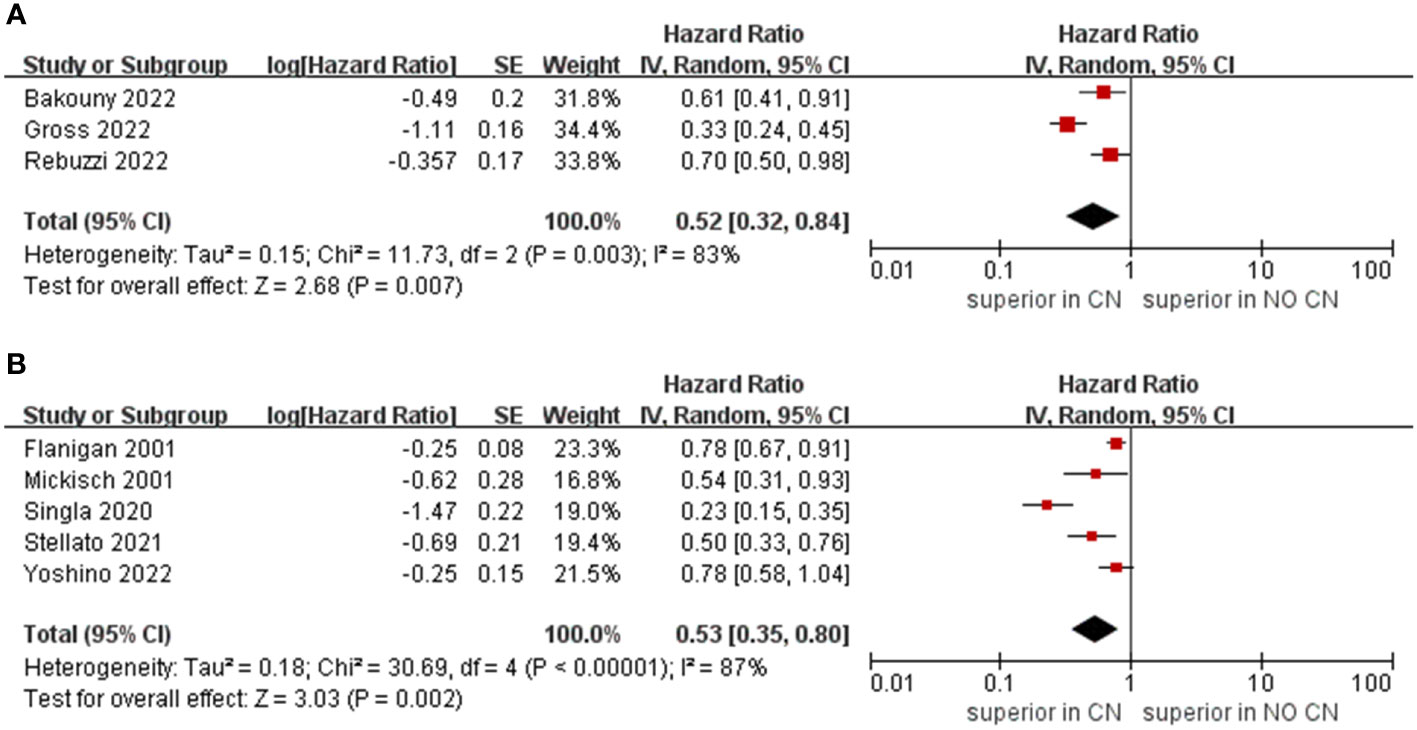
Figure 5 Forest plots of overall survival in subgroup analysis. (A) sample size > 400, (B) sample size ≤ 400.
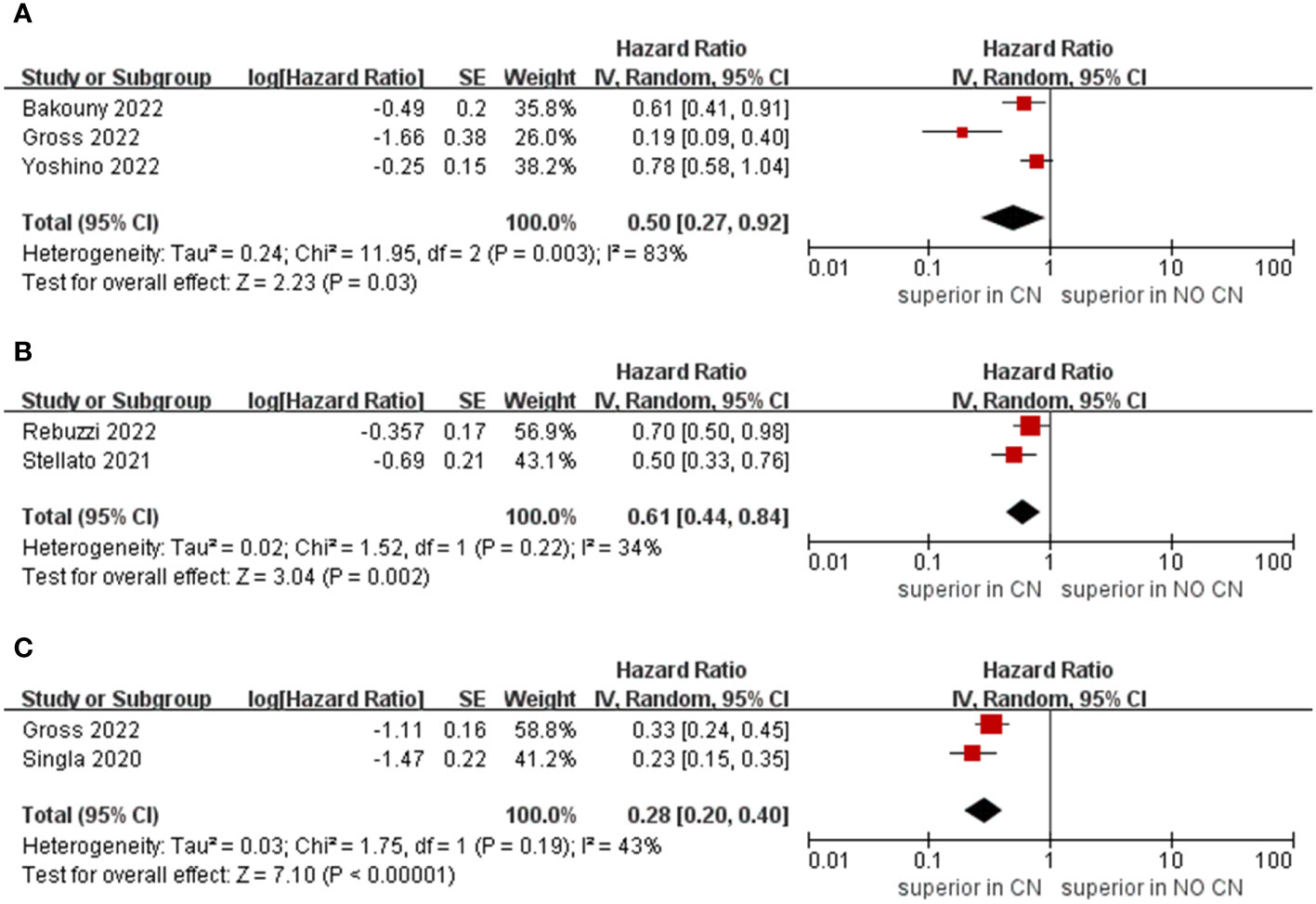
Figure 6 Forest plots of overall survival in subgroup analysis. (A) ICIs as first line therapy, (B) second and third line therapy, (C) first, second and third line or later therapy).
3.4 Sensitivity analysis
We conducted leave-one-out tests to identify the source of heterogeneity and to evaluate the robustness of the results. Ultimately, no substantial change in heterogeneity and pooled HR was found among the studies, regardless of which study was excluded, implying that the source of heterogeneity and the outcomes were stable and reliable. The heterogeneity observed in the outcomes of the studies could be attributed to a variety of factors, including follow-up period, IMDC risk score, metastatic sites, number of sites of metastasis, and type of immunotherapy. Additionally, caution should be taken when interpreting the results of the analyses, as the I2 statistic has been observed to be substantially biased in studies with small sample sizes (27).
3.5 Publication bias
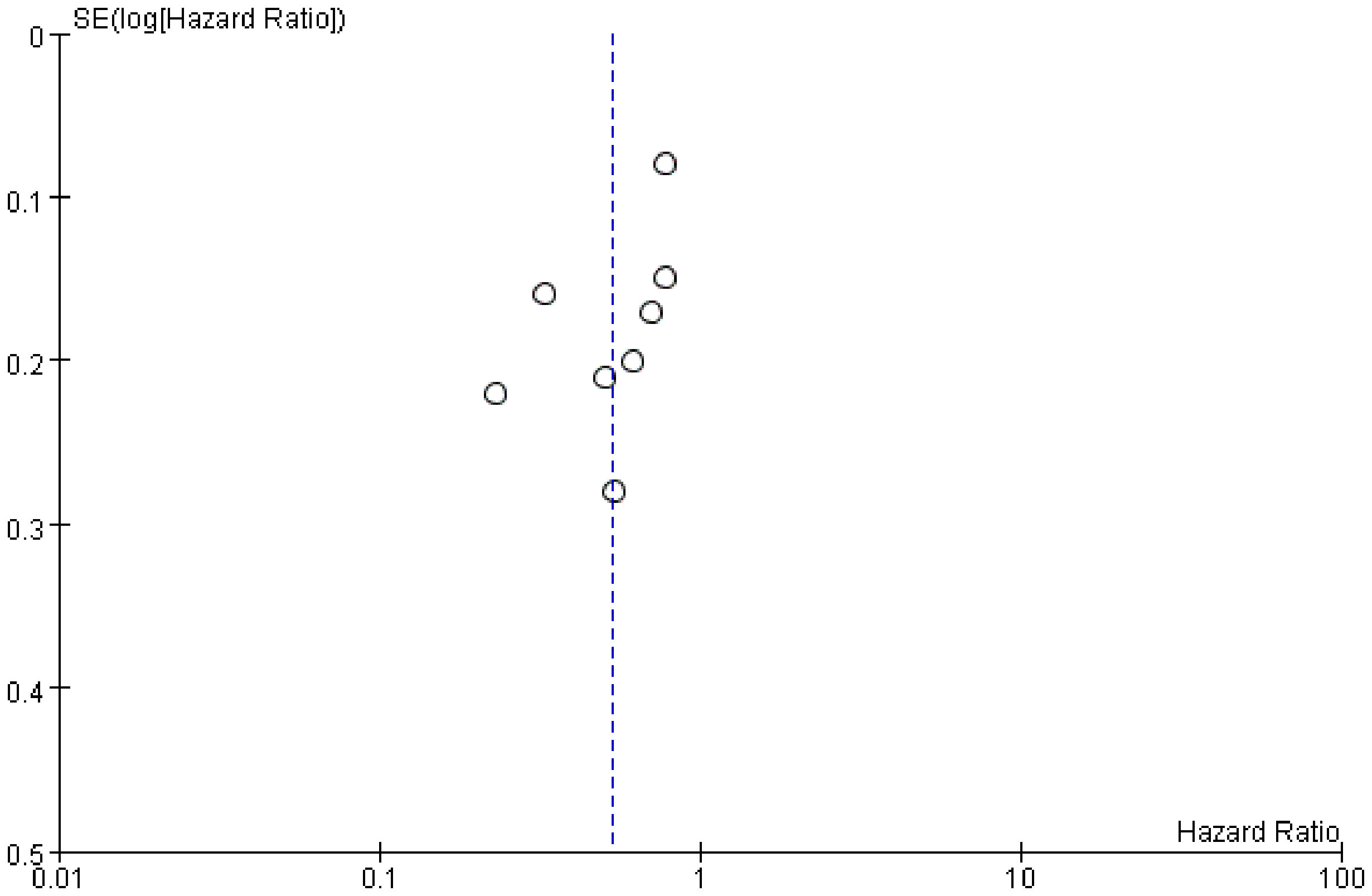
We examined publication bias by the funnel plot. The findings revealed that the distribution of included studies was almost tapered, but there is still some publication bias (Figure 7).
4 Discussion
This is the first systematic review and meta-analysis to evaluate the prognostic impact of CN for mRCC in the era of immunotherapy. Furthermore, some significant findings from this analysis need further discussion.
In the early twentieth century, the cytokine-based therapy was the standard of care for mRCC, whereas surgical management was still the treatment option for patients with mRCC. As more effective targeted therapy for mRCC have been developed, however, the role of CN has been called into question. There are still some inconsistent statements about the benefits of CN for patients with mRCC in the targeted therapy era. The CARMENA trial (7) showed that patients who received CN did not get adequate benefit compared to those who received targeted therapy alone. It is worth mentioning that the proportion of patients with poor-risk disease is higher in the CARMENA study population. Moreover, the patients included in CARMENA trial received therapy immediately after CN. Nevertheless, Roussel et al. (28) demonstrated that patients receive CN after upfront systemic therapy may get better survival outcomes. Recently, Janisch et al. (29) reported that treatment with CN and tyrosine kinase inhibitors was associated with superior survival compared to those without CN for specific patient. Ghatalia et al. (30) conducted a retrospective study to evaluate the role of CN in patients with mRCC, including those receiving ICIs and targeted therapy, and demonstrated that CN had a beneficial effect on select patients with mRCC. Taken together, accumulating evidence suggests that the combination of CN and systemic therapy may provide better outcomes in mRCC.
Although CN has been the important treatment for mRCC, the underlying mechanism of its survival benefits remains unknown. Over the years, various hypotheses have been put forward. First, the immune hypothesis was proposed in the 1990s (31), which was supported by Fujikawa et al.’s (32) research results in that patients who did not receive CN had lower levels of response for interleukin-2 than those who did. This was further corroborated by two RCTs (4, 5), and our meta-analysis also demonstrated that CN group was associated with superior OS than the No CN group in IFN-alfa subgroup. Second, primary tumors are associated with promoting inflammation and suppressing the release of cytokines from T cells, which could impede the systemic anti-tumor immune response (33, 34). Marcus et al. (31) conducted a case report to show that spontaneous regression of mRCC lesions upon CN, which further demonstrated the outcomes. Hence, resection of the primary tumor may enhance the immune response of mRCC. Third, the straightforward explanation is that CN can reduce the overall tumor burden and thus extend the duration of time before tumors reach lethal levels (35). Additionally, the efficacy of CN combined with immunotherapy has been verified in other types of metastatic tumors, such as lung cancer and melanoma, providing further evidence for its application in mRCC (36, 37).
The optimal timing of CN in relation to the initiation of systemic therapy is a critical factor that may influence outcomes. Bhindi et al. (38) conducted a study using real-world data and concluded that deferred CN could significantly improve OS compared to upfront CN. However, Bruijn et al. (39) conducted a comparative study to assess the outcomes of patients receiving targeted therapy followed by CN (deferred CN) against those receiving CN followed by targeted therapy (upfront CN), and the results showed that there was no significant difference in OS between the two groups. In the studies included, three studies compared the outcomes of deferred versus upfront CN in selected patients. Two studies reported that deferred CN did not lead to a superior OS than upfront CN in patients (21, 26), while one study suggested that OS rate tended to be higher with deferred CN in comparison to upfront CN (24). Ghatalia et al. (30) also demonstrated that no statistically significant difference in OS was observed between the upfront and deferred groups. Nevertheless, the insufficient literature barred us from conducting analysis to compare outcomes between the two approaches. Furthermore, the small sample size of the included studies renders it difficult to draw a reliable conclusion. The SURTIME trial revealed that deferred CN did not improve 28-week progression-free rate, while the deferred CN could be associated with improved OS compared to immediate CN (8). Ghanem et al. (40) reported that immediate CN resulted in a lower rate of successful systemic therapy and disease control compared to deferred CN. Due to the dearth of existing research, it is not possible to draw reliable conclusions as to which of the two methods could bring OS advantage for patients with mRCC. Therefore, further research is needed to verify the efficacy of each approach.
Patient selection is also a crucial consideration when evaluating the benefits of CN (41). As an invasive procedure for patients with high disease burden, CN carries a higher mortality risk than standard nephrectomy for T1 or T2 renal tumors (42). Furthermore, the survival benefit for some patients with poor risk score is marginal, and CN might bring postoperative complications that could negatively affect quality of life, prompting further scrutiny of its role (43). Therefore, patient selection for CN may have potential bias. In the included studies, four studies utilized the IMDC risk score to access the baseline risk score of the two groups. However, only two studies revealed that the rates of poor IMDC risk score in the CN group was lower than that of the No CN group. Furthermore, although liver metastases were found to be significantly less in the CN group compared to the non-CN group, no significant difference was found in bone metastasis and lung metastasis. Bakouny et al. (22) proposed that patients without adverse (bone, liver or lung) metastases, favorable IMDC risk score, and good physical condition may gain adequate benefit from CN. Going forward, newer scales should be created during the ICIs and targeted therapy era to evaluate which patient may benefit from CN. Additionally, we also need more studies to assess the outcomes.
Recently, ICIs therapy has shown superior efficacy and has been adopted as front-line therapy for mRCC. Cytokine-based therapy has gradually given way to ICIs therapy. In our meta-analysis, we have included the latest evidence on CN for mRCC in the era of ICIs therapy. Moreover, some ongoing studies should also be taken into account. The PROBE trial (NCT04510597) is recruiting patients with intermediate or poor risk according to the IMDC risk score who are receiving deferred CN following the combination of nivolumab and ipilimumab, and compared to the No CN group. The SWOG-1931 trial (NCT04510597) is assessing the impact of CN on patients receiving the combination therapy with avelumab and axitinib, or pembrolizumab and axitinib. Further research is needed to confirm these findings with larger sample sizes and higher-quality studies.
However, the limitations of this study should be noted. First, all the included studies were from large centers and the patients enrolled were not necessarily representative of the general population. Second, most the studies included in the analysis were non-RCTs, which undoubtedly had potential distribution and blindness bias. Third, the lack of data in the studies did not allow for a pooled analysis to compare other survival outcomes, such as progression free survival. Fourth, significant differences were observed between the CN and No CN groups in terms of the prevalence of liver metastases. Additionally, two studies have demonstrated that rates of poor IMDC risk score in the CN group were lower than those of the No CN group, resulting in certain heterogeneity. Lastly, due to the limited literature available, a subgroup analysis regarding the timing of CN relative to immunotherapy could not be conducted, which may lead to subtle differences.
5 Conclusions
The combination of CN and immunotherapy for mRCC is associated with a better outcome in terms of OS benefit in selected patients compared to immunotherapy alone. Nevertheless, further research is needed to verify these conclusions, such as larger sample sizes, increased follow-up periods and RCTs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
K-PL: Protocol development, data collection and management, data analysis and manuscript writing. S-YC: Protocol development, data collection and management, data analysis and manuscript writing. C-YW: Protocol development, data management, data analysis and manuscript writing. X-RL: Data management, data analysis and manuscript writing. LY: Data management, data analysis and manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82160146); Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (Grant numbers CY2021-MS-A12 and CY2020- MS08); Natural Science Foundation of Gansu Province of China (Grant numbers 21JR1RA151); Second Hospital of Lanzhou University “Cuiying Science and Technology Innovation” project (CY2021-QN-A20); Natural Science Foundation of Gansu Province of China (Grant numbers 22YF7FA090).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1132466/full#supplementary-material
References
1. Biles MJ, Patel HD, Allaf ME. Cytoreductive nephrectomy in the era of tyrosine kinase and immuno-oncology checkpoint inhibitors. Urol Clin North Am (2020) 47(3):359–70. doi: 10.1016/j.ucl.2020.04.009
2. Adashek JJ, Genovese G, Tannir NM, Msaouel P. Recent advancements in the treatment of metastatic clear cell renal cell carcinoma: A review of the evidence using second-generation p-values. Cancer Treat Res Commun (2020) 23:100166. doi: 10.1016/j.ctarc.2020.100166
3. Ravaud A, Dilhuydy MS. Interferon alpha for the treatment of advanced renal cancer. Expert Opin Biol Ther (2005) 5(6):749–62. doi: 10.1517/14712598.5.6.749
4. Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet (2001) 358(9286):966–70. doi: 10.1016/s0140-6736(01)06103-7
5. Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med (2001) 345(23):1655–9. doi: 10.1056/NEJMoa003013
6. Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol (2019) 30(5):706–20. doi: 10.1093/annonc/mdz056
7. Méjean A, Ravaud A, Thezenas S, Colas S, Beauval JB, Bensalah K, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med (2018) 379(5):417–27. doi: 10.1056/NEJMoa1803675
8. Bex A, Mulders P, Jewett M, Wagstaff J, van Thienen JV, Blank CU, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: The SURTIME randomized clinical trial. JAMA Oncol (2019) 5(2):164–70. doi: 10.1001/jamaoncol.2018.5543
9. Psutka SP, Chang SL, Cahn D, Uzzo RG, McGregor BA. Reassessing the role of cytoreductive nephrectomy for metastatic renal cell carcinoma in 2019. Am Soc Clin Oncol Educ Book (2019) 39:276–83. doi: 10.1200/edbk_237453
10. Méjean A, Ravaud A, Thezenas S, Chevreau C, Bensalah K, Geoffrois L, et al. Sunitinib alone or after nephrectomy for patients with metastatic renal cell carcinoma: Is there still a role for cytoreductive nephrectomy? Eur Urol (2021) 80(4):417–24. doi: 10.1016/j.eururo.2021.06.009
11. Esagian SM, Ziogas IA, Kosmidis D, Hossain MD, Tannir NM, Msaouel P. Long-term survival outcomes of cytoreductive nephrectomy combined with targeted therapy for metastatic renal cell carcinoma: A systematic review and individual patient data meta-analysis. Cancers (Basel) (2021) 13(4):695. doi: 10.3390/cancers13040695
12. Brown LC, Desai K, Zhang T, Ornstein MC. The immunotherapy landscape in renal cell carcinoma. BioDrugs (2020) 34(6):733–48. doi: 10.1007/s40259-020-00449-4
13. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med (2015) 373(19):1803–13. doi: 10.1056/NEJMoa1510665
14. Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol (2019) 20(10):1370–85. doi: 10.1016/s1470-2045(19)30413-9
15. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med (2018) 378(14):1277–90. doi: 10.1056/NEJMoa1712126
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 372:n71. doi: 10.1136/bmj.n71
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
18. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj (2016) 355:i4919. doi: 10.1136/bmj.i4919
19. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. Bmj (2011) 343:d5928. doi: 10.1136/bmj.d5928
20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
21. Gross EE, Li M, Yin M, Orcutt D, Hussey D, Trott E, et al. A multicenter study assessing survival in patients with metastatic renal cell carcinoma receiving immune checkpoint inhibitor therapy with and without cytoreductive nephrectomy. Urol Oncol (2023) 41(1):51.e25–31. doi: 10.1016/j.urolonc.2022.08.013
22. Bakouny Z, El Zarif T, Dudani S, Connor Wells J, Gan CL, Donskov F, et al. Upfront cytoreductive nephrectomy for metastatic renal cell carcinoma treated with immune checkpoint inhibitors or targeted therapy: An observational study from the international metastatic renal cell carcinoma database consortium. Eur Urol (2022) 83(2):145–51. doi: 10.1016/j.eururo.2022.10.004
23. Rebuzzi SE, Signori A, Banna GL, Gandini A, Fornarini G, Damassi A, et al. The prognostic value of the previous nephrectomy in pretreated metastatic renal cell carcinoma receiving immunotherapy: A sub-analysis of the meet-URO 15 study. J Transl Med (2022) 20(1):435. doi: 10.1186/s12967-022-03601-6
24. Yoshino M, Ishihara H, Nemoto Y, Nakamura K, Nishimura K, Tachibana H, et al. Therapeutic role of deferred cytoreductive nephrectomy in patients with metastatic renal cell carcinoma treated with nivolumab plus ipilimumab. Jpn J Clin Oncol (2022) 52(10):1208–14. doi: 10.1093/jjco/hyac099
25. Stellato M, Santini D, Verzoni E, De Giorgi U, Pantano F, Casadei C, et al. Impact of previous nephrectomy on clinical outcome of metastatic renal carcinoma treated with immune-oncology: A real-world study on behalf of meet-URO group (MeetUro-7b). Front Oncol (2021) 11:682449. doi: 10.3389/fonc.2021.682449
26. Singla N, Hutchinson RC, Ghandour RA, Freifeld Y, Fang D, Sagalowsky AI, et al. Improved survival after cytoreductive nephrectomy for metastatic renal cell carcinoma in the contemporary immunotherapy era: An analysis of the national cancer database. Urol Oncol (2020) 38(6):604.e9–604.e17. doi: 10.1016/j.urolonc.2020.02.029
27. von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol (2015) 15:35. doi: 10.1186/s12874-015-0024-z
28. Roussel E, Verbiest A, Milenkovic U, Van Cleynenbreugel B, Van Poppel H, Joniau S, et al. Too good for CARMENA: Criteria associated with long systemic therapy free intervals post cytoreductive nephrectomy for metastatic clear cell renal cell carcinoma. Scand J Urol (2020) 54(6):493–9. doi: 10.1080/21681805.2020.1814858
29. Janisch F, Hillemacher T, Fuehner C, D'Andrea D, Meyer CP, Klotzbücher T, et al. The impact of cytoreductive nephrectomy on survival outcomes in patients treated with tyrosine kinase inhibitors for metastatic renal cell carcinoma in a real-world cohort. Urol Oncol (2020) 38(9):739.e9–739.e15. doi: 10.1016/j.urolonc.2020.04.033
30. Ghatalia P, Handorf EA, Geynisman DM, Deng M, Zibelman MR, Abbosh P, et al. The role of cytoreductive nephrectomy in metastatic renal cell carcinoma: A real-world multi-institutional analysis. J Urol (2022) 208(1):71–9. doi: 10.1097/ju.0000000000002495
31. Marcus SG, Choyke PL, Reiter R, Jaffe GS, Alexander RB, Linehan WM, et al. Regression of metastatic renal cell carcinoma after cytoreductive nephrectomy. J Urol (1993) 150(2 Pt 1):463–6. doi: 10.1016/s0022-5347(17)35514-3
32. Fujikawa K, Matsui Y, Oka H, Fukuzawa S, Takeuchi H. Serum c-reactive protein level and the impact of cytoreductive surgery in patients with metastatic renal cell carcinoma. J Urol (1999) 162(6):1934–7. doi: 10.1016/s0022-5347(05)68072-x
33. Flanigan RC. Debulking nephrectomy in metastatic renal cancer. Clin Cancer Res (2004) 10(18 Pt 2):6335s–41s. doi: 10.1158/1078-0432.CCR-sup-040026
34. Lahn M, Fisch P, Köhler G, Kunzmann R, Hentrich I, Jesuiter H, et al. Pro-inflammatory and T cell inhibitory cytokines are secreted at high levels in tumor cell cultures of human renal cell carcinoma. Eur Urol (1999) 35(1):70–80. doi: 10.1159/000019821
35. Choueiri TK, Xie W, Kollmannsberger C, North S, Knox JJ, Lampard JG, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol (2011) 185(1):60–6. doi: 10.1016/j.juro.2010.09.012
36. Huang AC, Orlowski RJ, Xu X, Mick R, George SM, Yan PK, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med (2019) 25(3):454–61. doi: 10.1038/s41591-019-0357-y
37. Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med (2018) 24(11):1649–54. doi: 10.1038/s41591-018-0197-1
38. Bhindi B, Graham J, Wells JC, Bakouny Z, Donskov F, Fraccon A, et al. Deferred cytoreductive nephrectomy in patients with newly diagnosed metastatic renal cell carcinoma. Eur Urol (2020) 78(4):615–23. doi: 10.1016/j.eururo.2020.04.038
39. de Bruijn R, Wimalasingham A, Szabados B, Stewart GD, Welsh SJ, Kuusk T, et al. Deferred cytoreductive nephrectomy following presurgical vascular endothelial growth factor receptor-targeted therapy in patients with primary metastatic clear cell renal cell carcinoma: A pooled analysis of prospective trial data. Eur Urol Oncol (2020) 3(2):168–73. doi: 10.1016/j.euo.2019.12.004
40. Abu-Ghanem Y, van Thienen JV, Blank C, Aarts MJB, Jewett M, de Jong IJ, et al. Cytoreductive nephrectomy and exposure to sunitinib - a post hoc analysis of the immediate surgery or surgery after sunitinib malate in treating patients with metastatic kidney cancer (SURTIME) trial. BJU Int (2022) 130(1):68–75. doi: 10.1111/bju.15625
41. Westerman ME, Shapiro DD, Tannir NM, Campbell MT, Matin SF, Karam JA, et al. Survival following cytoreductive nephrectomy: a comparison of existing prognostic models. BJU Int (2020) 126(6):745–53. doi: 10.1111/bju.15160
42. Abdollah F, Sun M, Thuret R, Schmitges J, Shariat SF, Perrotte P, et al. Mortality and morbidity after cytoreductive nephrectomy for metastatic renal cell carcinoma: a population-based study. Ann Surg Oncol (2011) 18(10):2988–96. doi: 10.1245/s10434-011-1715-2
Keywords: metastatic renal cell carcinoma, cytoreductive nephrectomy, immunotherapy, overall survival, outcomes
Citation: Li K-p, Chen S-y, Wang C-y, Li X-r and Yang L (2023) The impact of cytoreductive nephrectomy on survival outcomes in patients with metastatic renal cell carcinoma receiving immunotherapy: An evidence-based analysis of comparative outcomes. Front. Immunol. 14:1132466. doi: 10.3389/fimmu.2023.1132466
Received: 27 December 2022; Accepted: 01 March 2023;
Published: 14 March 2023.
Edited by:
Hui Dai, Peking University, ChinaReviewed by:
Siming Li, Beijing Cancer Hospital, Peking University, ChinaPietro Pepe, Cannizzaro Hospital, Italy
Copyright © 2023 Li, Chen, Wang, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-ran Li, bGl4aWFvcmFuMDVAMTYzLmNvbQ==; Li Yang, ZXJ5X3lhbmdsaUBsenUuZWR1LmNu
†These authors have contributed equally to this work
 Kun-peng Li
Kun-peng Li Si-yu Chen†
Si-yu Chen† Chen-yang Wang
Chen-yang Wang Li Yang
Li Yang