
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 21 March 2023
Sec. Viral Immunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1132250
This article is part of the Research TopicImmunological Aspects of Emerging and Re-emerging ZoonosesView all 10 articles
 Liyan Niu1,2†
Liyan Niu1,2† Dingfa Liang1,3†
Dingfa Liang1,3† Qin Ling1,4†
Qin Ling1,4† Jing Zhang3
Jing Zhang3 Ziwen Li4
Ziwen Li4 Deju Zhang5
Deju Zhang5 Panpan Xia5
Panpan Xia5 Zicheng Zhu5
Zicheng Zhu5 Jitao Lin5
Jitao Lin5 Ao Shi6,7
Ao Shi6,7 Jianyong Ma6
Jianyong Ma6 Peng Yu5*
Peng Yu5* Xiao Liu8*
Xiao Liu8*On 23rd July 2022, the World Health Organization (WHO) recognized the ongoing monkeypox outbreak as a public medical crisis. Monkeypox virus (MPV), the etiological agent of monkeypox, is a zoonotic, linear, double-stranded DNA virus. In 1970, the Democratic Republic of the Congo reported the first case of MPV infection. Human-to-human transmission can happen through sexual contact, inhaled droplets, or skin-to-skin contact. Once inoculated, the viruses multiply rapidly and spread into the bloodstream to cause viremia, which then affect multiple organs, including the skin, gastrointestinal tract, genitals, lungs, and liver. By September 9, 2022, more than 57,000 cases had been reported in 103 locations, especially in Europe and the United States. Infected patients are characterized by physical symptoms such as red rash, fatigue, backache, muscle aches, headache, and fever. A variety of medical strategies are available for orthopoxviruses, including monkeypox. Monkeypox prevention following the smallpox vaccine has shown up to 85% efficacy, and several antiviral drugs, such as Cidofovir and Brincidofovir, may slow the viral spread. In this article, we review the origin, pathophysiology, global epidemiology, clinical manifestation, and possible treatments of MPV to prevent the propagation of the virus and provide cues to generate specific drugs.
Threats from the pandemic coronavirus disease 2019 (COVID-19) have not been eliminated, and worries about the possibility of another viral pandemic reached a crescendo (1). The monkeypox virus (MPV), a zoonotic DNA orthopoxvirus (OPV) causes the zoonotic disease monkeypox marked by fever and a red rash which is related to smallpox virus and vaccinia virus (VACV) (2, 3). In 1970, the Democratic Republic of Congo (DRC) reported the first human case of infection, accounting for the majority of early cases (4). Recently, more than 57,000 total cases have been found at more than 100 total locations since May 2022 (5). According to the Centers for Disease Control (CDC), there are no specific medications available, and smallpox vaccines, vaccinia immune globulin, and antivirals were used to regulate small-scale outbreaks (6, 7). Such unexpected, unprecedented, and unusually high-frequency transmission outbreaks worldwide have spurred scientific, political, and media attention. Investigation of pathophysiological features, routes of transmission, clinical features and preliminary diagnosis is urgently needed and has the potential to help improve prevention and early intervention and promote the development of specific drugs.
In 1958, MPV was first identified and isolated from cynomolgus (Macaca cynomolgus) monkeys as laboratory animals, which were kept by a research facility after shipment from Singapore to Denmark (8). MPV is a member of the OPV of the Chordopoxvirinae subfamily, Poxviridae family, the same genus as other viruses such as smallpox and cowpox (9). There are some similarities between monkeypox and smallpox, such as structures, clinical manifestations, and responses to some antiviral drugs (10, 11). The MPV of human cases originated in the tropical rainforests of western and central Africa, and there are two clades in the phylogenetic analysis: the West African clade and the Congo clade, with an average mortality rate of up to 11% (12). The first isolates from cynomolgus (Macaca cynomolgus) monkeys belonged to the West African clade while the Congo clade contributed to the first human case in 1970 (13). In addition, monkeypox is a zoonotic disease similar to smallpox, but it is still unclear which is the reservoir host of MPV (14). Rodents from Africa are thought to be the largest animal reservoirs involved in the spread of the virus, and it can be transmitted from some rodents (prairie dogs) to various monkeys and apes, such as anthropoid apes. By coming into contact with the infected animals’ respiratory droplets, skin sores, or body fluids, MPV can transmit from one animal to another. The virus enters a healthy person via the respiratory system, mucous membranes (such as the nose, mouth, or eyes), or skin wounds (15). Simultaneously, it can be transmitted from animals, such as rodents or monkeys, to humans through bushmeat, bites, scratches, and direct or indirect exposure to body substances or fluids from the lesion (16) (Figure 1). In 1970, the first reported case of MPV was a 9-month-old child (A. I.) infected with a smallpox-like disease in the village of Bokenda, Basankusu Province, the Democratic Republic of the Congo (4). Transmission from humans to animals has not been documented. While transmission from person to person is a major problem, it mostly occurs through the respiratory tract by sneezing, coughing, large respiratory droplets, and other similar actions. Direct contact with infected lesions and bodily fluids or indirect contact with contaminated items like patient-used garments or linens (17). In addition to the placenta (congenital monkeypox), intimate contact during labor and after delivery can also result in mother-to-child transmission (18). Currently, with an increasing number of transmissions of Health-Care Associated Infections (HCAI) reported, infection of healthcare workers has recently garnered attention. Prolonged exposure to patients increases the risk of infection among hospital staff and family members (2). According to the European Centre for Disease Prevention and Control, the 2021 incident in the UK was the first chain of transmission to be reported in Europe without epidemiological links with western and central Africa. It was also the first instance documented among males who had sex with men (MSM) (19). Infection preferentially occurs in gays, bisexuals, the MSM community, and other populations more susceptible to other sexually transmitted diseases, such as human immunodeficiency virus/acquired immunodeficiency syndrome (18, 20).
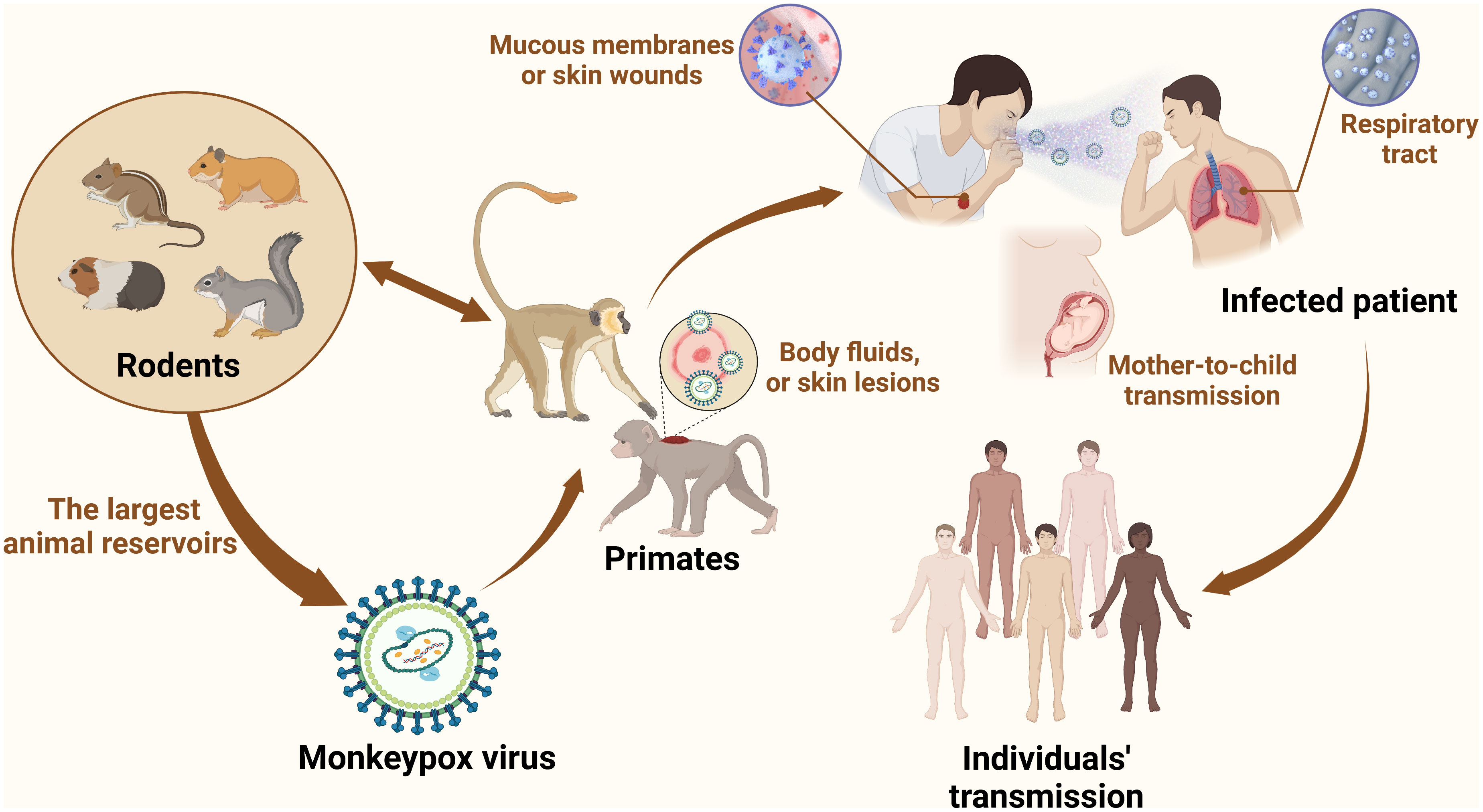
Figure 1 MPV transmission. Zoonotic dissemination of MPV. Animal hosts mainly include rodents (African rope squirrels, prairie dogs, hamsters, and rats) and primates (cynomolgus, rhesus, and gorillas). MPV is an enveloped dsDNA virus belonging to the Poxviridae family, and smallpox is also one of the most prevalent viruses in this family. Wildlife trafficking, habitat degradation, and climate change contribute to the transmission of MPV from new species to other species and increase the bond between people and animals. In the case of wildlife trafficking and illegal hunting, animals are caught, trapped, transported, and sold as food, medicine, and pets. Wildlife trade markets promote disease dissemination, making it possible for viruses from many neighboring species to jump the species barrier. Animal-to-human transmission is mediated by bites, scratches, and slaughtering. Human-to-human transmission occurs through close contact with infected people, such as through respiratory droplets and skin contact, especially MSM. The virus can remain on fomites, such as bedding, linen, and clothes.
MPV can infect the human body through intradermal, mucosal, oropharyngeal, and nasopharyngeal routes after contact with susceptible people. After replication in the inoculated site for an appropriate 6-13 days, they spread to regional lymph nodes and then entered the circulation system (also called viremia) (21). The viruses in the blood infect host cells to spread to multiple sites and exhibit immunomodulation ability to escape immunosurveillance caused by horizontal gene transfer (22, 23). Although viral tropism in human tissue has not been well established, numerous animal models provide important clues regarding this scientific question. Osorio et al. detected MPV antigens in the lung, liver, heart, brain, kidney, ovarian, and pancreatic tissues of severely immunodeficient mice through immunohistochemical and histopathological tests (24). Moreover, the histopathological results of infected cynomolgus monkeys (Macca fasicularis) exhibited viral accumulation in the salivary epithelium, sebaceous and follicular tissue of the lip, especially in lymphoid tissues (25). Numerous animal reservoirs (e.g., dormice, prairie dogs, giant pouched rats) and various infected tissues reveal that MPV has a wide spectrum tropism, making it difficult to identify specific tissues as a site of infection (26).
MPV has structural and functional similarities to other OPVs. An exterior lipoprotein membrane with a geometric pattern of corrugations surrounds the virus’s ovoid or brick-like particle (8). The outer membrane wraps around and protects a tight core and membrane bonds that contain linear double-stranded DNA (197 kb), transcription factors, and enzymes from external stress (27). The genes essential to housekeeping activities are situated in the middle area and are highly conserved, while the genes necessary for virus-host interactions (required for virulence) are found in the terminal area and are less conserved among OPVs (28–31). Although MPV is a DNA virus, its replication, assembly, and maturation are all completed in the cytoplasm of host cells (Figure 2). Furthermore, most of the characteristics of the life cycle of VACV are likely to be common to MPV even though the life cycle of MPV is not well understood (30). In VACV (and probably most MPV), there are two types of infectious virions: intracellular mature virus (IMV) and extracellular-enveloped virus (EEV) generated from infected host cells (32, 33). IMVs enter the host cell through activation of macropinocytosis, while EEVs through membranous fusion (34). When compared to EEVs, which are hypothesized to facilitate viral dissemination within an infected host, IMVs are the more common infective type and mediate host-to-host transmission. For the EEVs, they continuously released from infected cells and are believed to spread locally from tissue to tissue through bodily fluids (35). Although the fact that the aforementioned characteristics are for VACV, they likely apply to all OPVs. Conversely, different agents exist among OPV species. Most OPVs like the cowpox virus evade immune function through downregulation of MHC expression of antigen-presenting cells such as monocytes. While MPV demonstrated the ability to inhibit T-cell responses against other viruses through anti-CD3 stimulation (36).
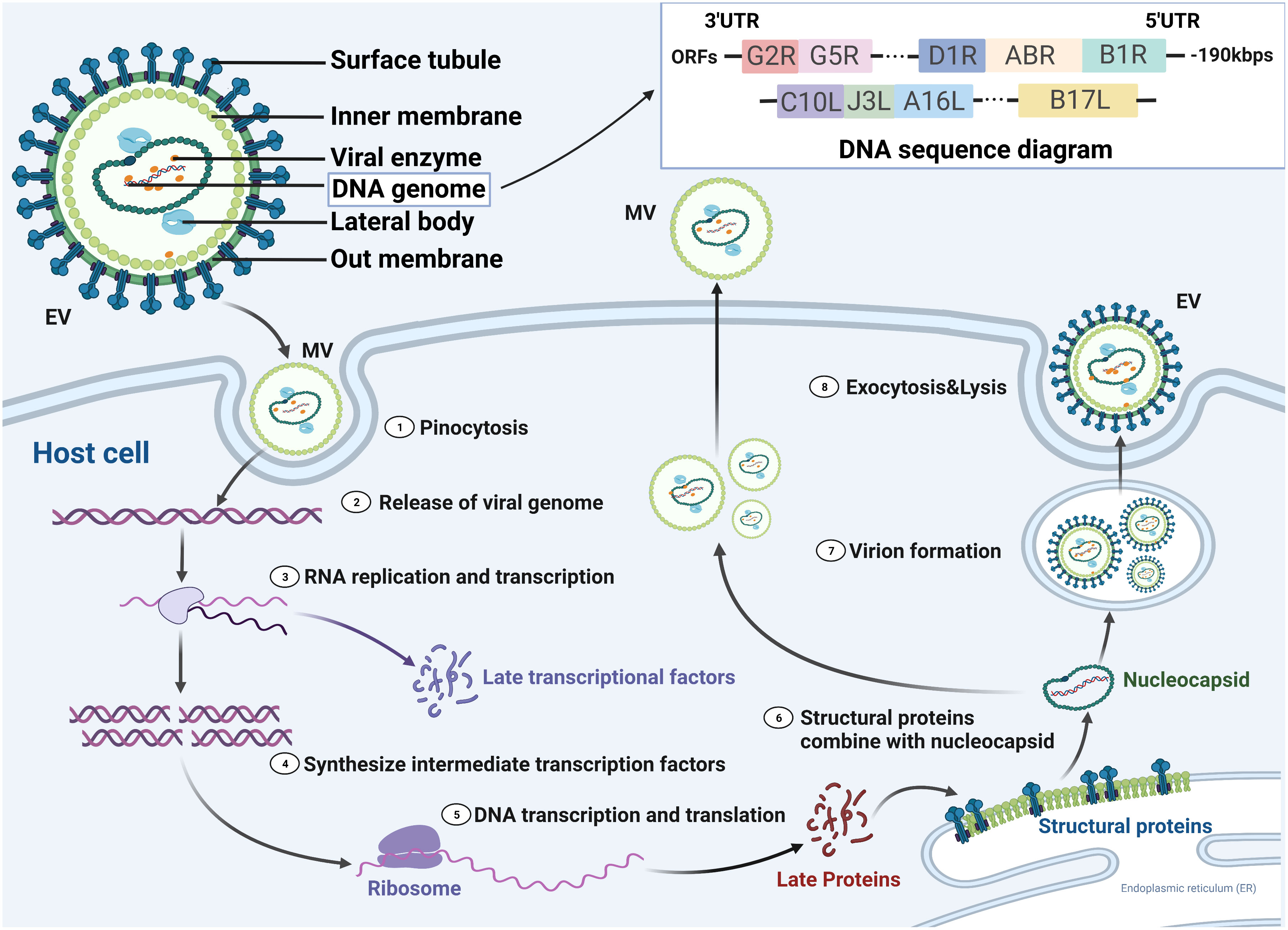
Figure 2 MPV life cycle in susceptible host cells. There are two forms of the virus, enveloped virion (EV) and mature virion (MV), which enter the host cell by fusion and micropinocytosis, respectively. MPV genomic structure and compositions are linear double-stranded DNA with nearly 190 kilobase pairs and contained more than 190 open reading frames (ORFs). 5′- and 3′- ends of the genome were inverted terminal repetitions, which formed hairpin-like structures. Double-stranded DNA is released and partially translated into early proteins that are processed into polymerases and immune modulators, while the others undergo replication in the cytoplasm. The resultant DNA partially transcripts into RNA, and the other part translates into intermediate proteins that are transformed into late transcription factors. Viral proteins translated from cytoplasmic RNA together with replicated DNA are assembled into nucleocapsid proteins and then processed into MVs and EVs, which are transported to the cell membrane for exocytosis. UTR, untranslated region.
Monkeypox is not a novel event and has been known as a zoonotic virus for more than 50 years. As mentioned earlier, in 1958, MPV was first found in an incidence of pox-like symptoms in monkeys at a shipment, which gives the name “monkeypox” (8). But now, it is inappropriate since MPV can infect various species and the majority of animal reservoirs are rodents, including giant pouched rats and squirrels. After the introduction of the virus by an imported animal trading company, there were at least 14 different kinds of rodents infected (37). Correspondingly, in 1970 a 9-month-old boy in the Bukenda village of the Zaire Equatorial region (now DRC) was documented to have the first human case (4). Since then, sporadic, intermittent cases of monkeypox limited in the African continent have been transmitted from local wild animals to humans. The accumulated cases are shown in Figure 3. From 1970 to 1979, 48 confirmed cases were detected in 6 African nations, including DRC (n=38), Cameroon (n = 1), Cote ˆ d’Ivoire (n = 1), Liberia (n = 4), Nigeria (n = 3), and Sierra Leone (n = 1) (18). In the 1980s, over 400 patients were documented, with an appropriate 10% fatality and a nine-fold increase in confirmed and probable cases recorded, and 14 infected patients were found in four additional African nations (38, 39). During the 1990s, 511 infected patients were found in DRC alone, and small outbreaks emerged across equatorial West and Central Africa (39). In 2003, 47 probable and confirmed patients of MPV infection were documented in six states of the USA who might have been exposed to prairie dogs kept as pets. The infected prairie dogs were kept in the same enclosure as Ghanaian small mammals. This was the first time that MPV occurred outside of Africa (40). From 2010 to 2019, increasing reports were documented in seven African nations (CAR, DRC, Cameroon, Nigeria, Liberia, Sierra Leone, and Republic of the Congo), the United Kingdom, Israel, and Singapore compared to past decades (39). On July 15, 2021, a patient with MPV traveling from Nigeria to the United States was found and controlled by the Texas Department of State Health Services and the CDC. More than 200 people contacted the patient and had the potential to be infected by this disease. Fortunately, there was no additional case in early September, and the contacted people were proven to be without MPV (18). However, in May 2022, a new monkeypox outbreak expanded in several countries on almost every continent. As of 14 July 2022, 1,856 laboratory-confirmed patients were documented in the UK. Among them, 12 were from Northern Ireland, 20 were from Wales, 46 were from Scotland, and 1,778 were from England (41). Given that the number of cases in the past few decades has already surpassed the whole number of infections in the first 40 years following the detection of monkeypox, it is apparent that the transmission rate of the disease is rapidly rising. The transmission between humans is increasing exponentially due to adoption by gene loss, instead of progressive mutation such as COVID-19 (42) (Table 1). This phenomenon is a reminder of the outbreak, indicating that prevention of monkeypox transmission and urgent treatment are essential.
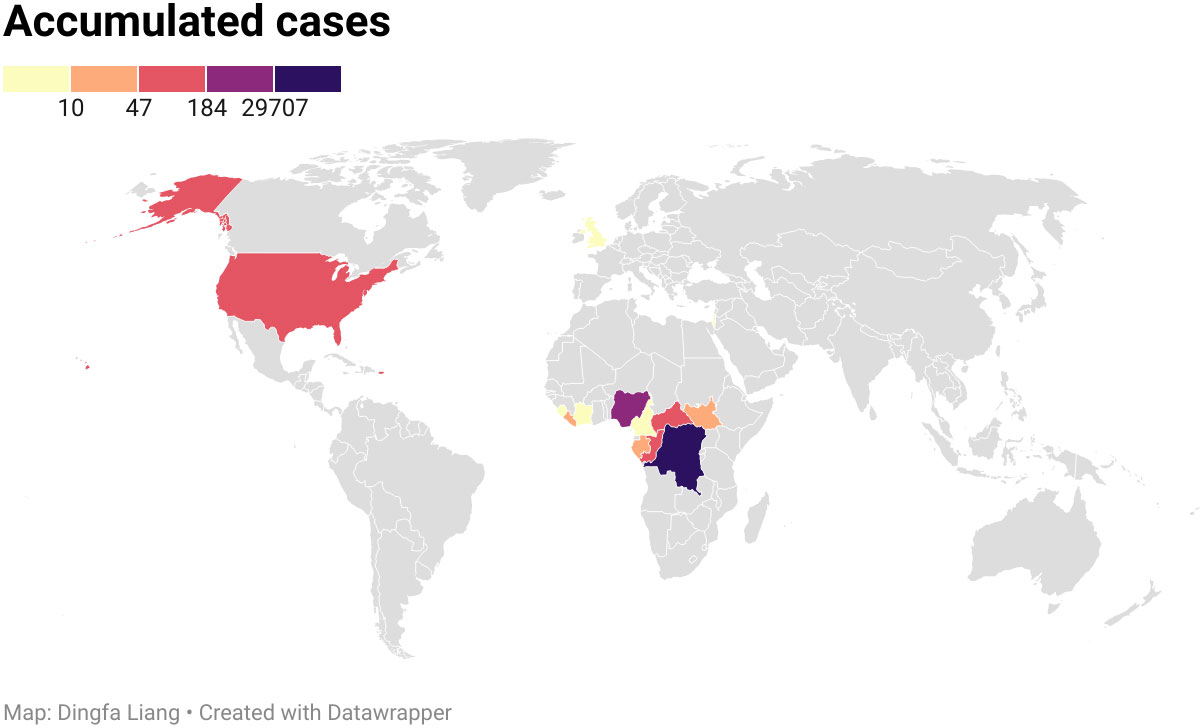
Figure 3 Accumulated cases of MPV from 1970 to 2020. The disease spread within 15 countries, with the Democratic Republic of the Congo being the worst hit area by the epidemic, followed by Nigeria.
MPV infection in humans is currently a growing public health concern as more than 85,000 clinical infections of MPV infection have been published in more than 100 different countries around the world, compared to only 48 cases in the 1970s (18). And the continuing monkeypox outbreak was regarded as a general populace crisis of international concern by the WHO on July 23, 2022 (56). As opposed to other outbreaks connected to travel from endemic nations or contact with animals transported in from the affected area, the infection source has not been identified as of yet (23). It was reported that patients with monkeypox in the current outbreak generally had close, prolonged physical contact with other monkeypox patients. During investigation, many reports documented that approximately 98% of these patients are gays, bisexuals, or other MSM (57–59). According to CDC data published on February 1, 2023, 84,243 cases were located in 103 distinct places that had never before recorded monkeypox, such as Spain (7,528 cases) and Germany (3,692 cases) (60) (Figure 4). A total of 1,293 human patients were documented in 7 places where monkeypox was historically reported, such as Nigeria (775 cases) and the DRC (348 cases). The total number of MPV cases reached 85,536 on February 1 in 2023, with the most of cases accumulating in Europe, the USA, and South America (41) (Table 2).
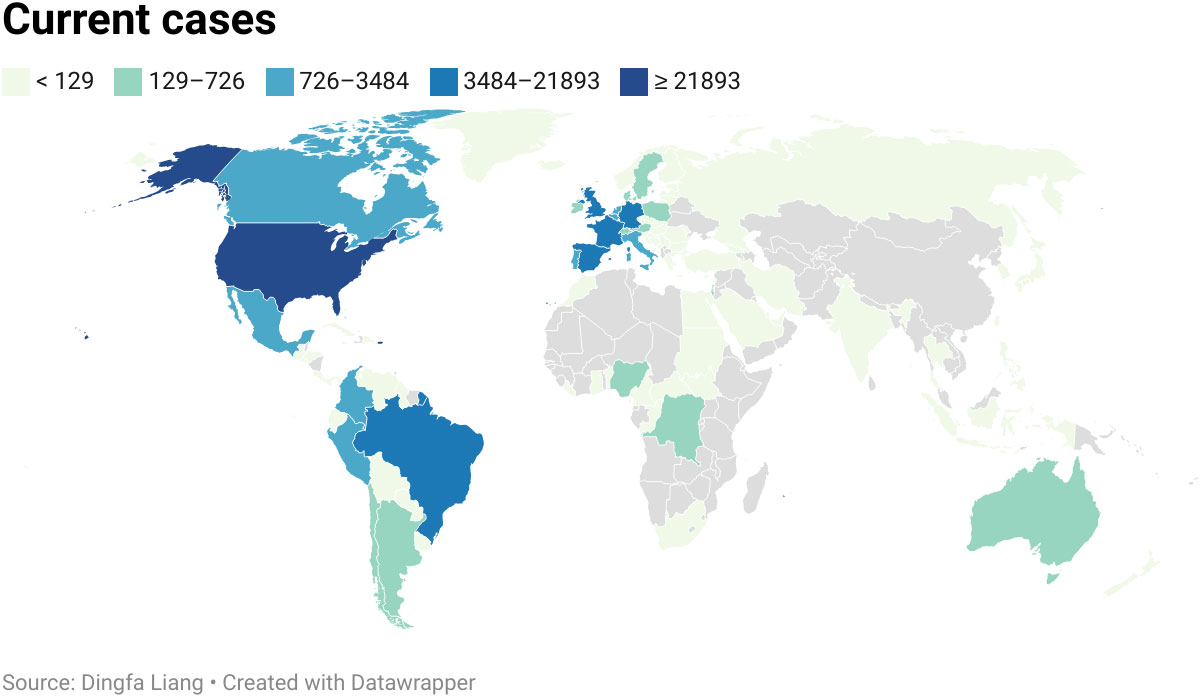
Figure 4 Current cases of MPVX from 1 Jan. to 9 Sep. 2022. More than 57,000 human cases spread globally in more than 100 locations. The United States of America was the worst-hit area, with 2,1893 cases.
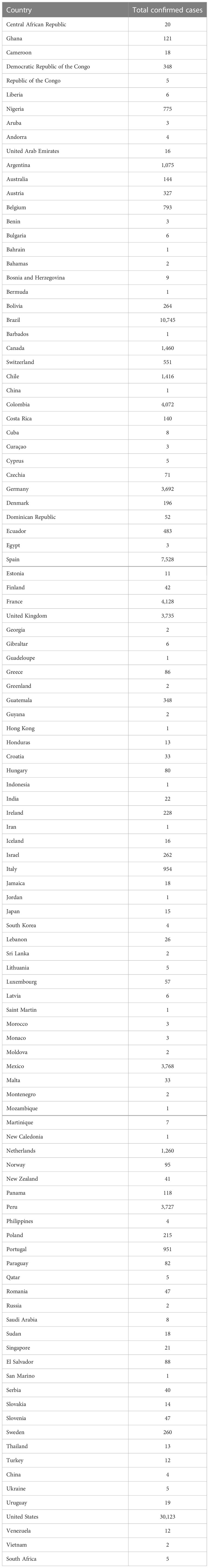
Table 2 The current cases of MPV until 1 Feb. 2023 (41).
From infection to the beginning of clinical manifestations, the incubation period of MPV was estimated to be 12 days and may extend to 21 days in some cases (40). The first signs or symptoms of patients include rash, fever, cough, headache, nausea and/or vomiting, confusion, wheezing, chills, sweats, runny nose, red eyes, stiff neck, lymphadenopathy, shortness of breath, sore throat, joint pain, back pain, chest pain, abdominal pain, myalgia, and conjunctivitis (38) (Table 3). It was reported that patients initially presented with signs or symptoms, such as rash (97%), fever (85%), adenopathy (71%), and chills (71%), which constituted the initial syndrome of MPV infection. The fever lasted for an average of 8 days (range: 2-13 days), and the rash continued for an average of 12 days (range: 7-24 days). The median time interval between the start of the fever and the appearance of the rash was 2 days (range: 0-12 days). Other signs and symptoms were evident between 0 and 14 days after the onset of fever or rash (62) (Figure 5).
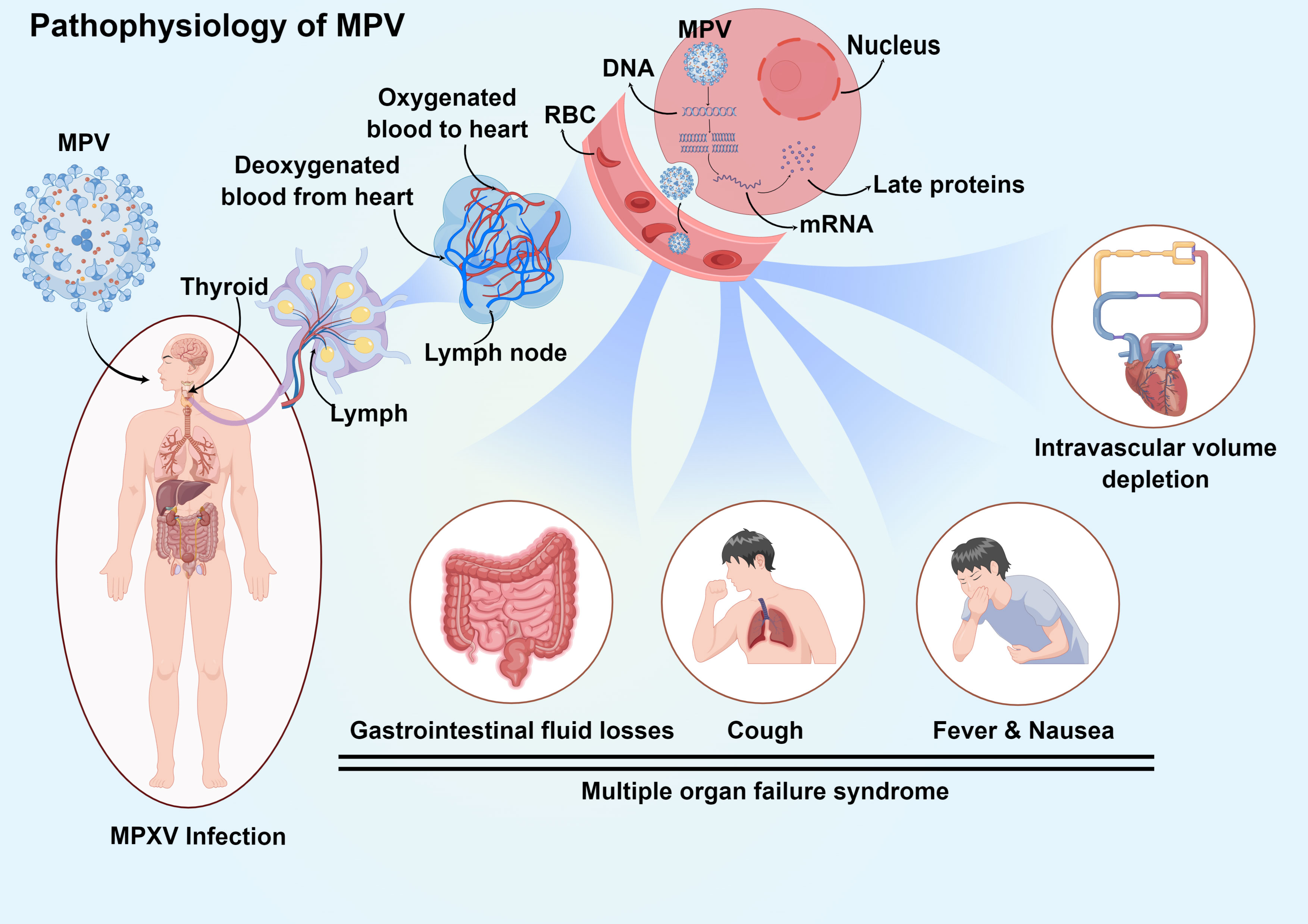
Figure 5 Pathophysiology and clinical manifestation of systemic MPV infection. Infection initiates in the upper respiratory tract and progresses to lymph, and then the virus enters the bloodstream through lymphocytes. The MPV in the blood spreads through the circulatory system to all parts of the body, enters the cell through endocytosis, releases DNA, and uses the substances in the cell to transcribe proteins, finally affecting the normal physiological function of the cell. MPV causes lymphocytosis and leukocytosis, thrombocytopenia, elevated aminotransferase levels, and decreased blood urea nitrogen levels. At the same time, symptoms such as multiorgan inflammation and cough and fever also occur.
The rashes with various sizes appear within 1 to 5 days following the commencement of the fever, initially on the face and then spreading to include the hands, feet, and legs (69). The rash progresses through several stages, from spots and papules to blisters (fluid-filled blisters) and pustules, before gradually going away as crusts and scabs wear off over time (38). Sixty-eight percent of patients had monomorphic lesions, and 48% of patients had centrifugally distributed lesions. Ulcerative or necrotic lesions are reported in 25% of patients, with a very small number presenting as hemorrhagic pustules (62). Different stages of the rash may appear simultaneously, and areas of skin erythema or hyperpigmentation are usually found as discrete lesions (70). Once the prodromal symptoms or rash appear, patients are considered infectious until the lesion crusts and the crusts fall off (71).
Recent studies have shown that in addition to lymphocytopenia and thrombocytopenia observed in over one-third of evaluable patients, leukocytosis, low blood urea nitrogen levels, high transaminase levels, and hypoalbuminemia are common symptoms during the disease (72, 73). Only 2 MPV individuals had hemorrhagic pustular lesions, according to a review of 34 verified patients in the USA. Disseminated intravascular coagulation has not been reported by any individuals, and the thrombocytopenia was typically minor (40).
Despite the symptoms and lesions of monkeypox being difficult to distinguish from those of smallpox, the sign and symptoms of MPV infection are less severe. Up to 90% of MPV patients suffered from lymphadenopathy, which is thought to be a clinical characteristic that separates human monkeypox from smallpox (74). The appearance of swollen lymph nodes, especially in the inguinal, submental, submandibular, and cervical nodes, distinguishes monkeypox from smallpox and chickenpox (75). Meanwhile, the lymphatogenous spread of MPV caused by viremia affects the skin, spleen, thymus, oral mucosa, reproductive system, and gastrointestinal tract in monkeys that have been experimentally infected with aerosolized MPV (26).
According to case studies, MPV-infected individuals also suffer from gastrointestinal disorders, including vomiting, diarrhea, and dehydration (40). Infected individuals with mucosal and gastrointestinal clinical manifestations may require volume replenishment due to gastrointestinal losses caused by poor nutritional balance or negative protein. Gastrointestinal fluid losses and hypoalbuminemia are associated with the fluid transfer from intravascular to extravascular compartments that takes place in systemic illness (76).
A rash normally begins on the face but can extend to other tissues, including the genitalia (19). According to the European Centre for Disease Prevention and Control, many cases in the ongoing outbreak are associated with sexual contact, particularly with men who were identified as gay, bisexual, or males who have sex with other men (58). Furthermore, the transmission of viruses can be achieved via sharing clothing and bedding as well as being directly exposed to infectious ulcers, scar tissue, or bodily fluids (19). Although less severe, the symptoms of monkeypox are similar to those of smallpox and consist of a characteristic rash that is preceded by mild prodromal manifestations (including lymphadenopathy, fever, and flu-like symptoms) (7). The cases in the present outbreak are characterized by a distinct rash that begins in the vaginal and perianal regions and extends to other parts of the human body with or without it (40).
A series of complications reported that 5 cases were identified as severely ill, and 9 cases were hospitalized as inpatients in the United States. Among those cases hospitalized as inpatients, a 6 years old child with encephalitis required intubation and mechanical breathing, while a 10-year-old girl with severe cervical lymphadenopathy and a retropharyngeal abscess had a constructed tracheal airway (77, 78). The intensive care units were allowed for these two patients. In addition, there was a patient with comorbidity (hepatitis C) who had a serious illness and was kept in the hospital as an inpatient. The individual is fully rehabilitated without experiencing any negative consequences. One adult with infection-related issues was found to have bacterial superinfection (unknown microorganism) (79), while another had keratitis and corneal erosion, which eventually necessitated a corneal transplant (40). There was no underlying medical condition in any of these patients. These cases show that monkeypox can cause a range of complications in addition to direct disease. This further illustrates the dangers of monkeypox and the need for prevention and control and provides another way of thinking about reducing the dangers of monkeypox, namely, the prevention of complications.
Tests for diagnosing diseases are of vital importance for the confirmation of MPV infection. Medical history, clinical symptoms, and laboratory tests all contribute to the diagnosis of MPV infection. The enlargement of the lymph nodes is the most typical symptom that separates monkeypox from diseases such as smallpox and chickenpox, which also present with rash symptoms, while confirmation of monkeypox also requires laboratory test assistance (80). Swabs are used to collect crusts or exudates from the infective site to isolate viral nucleic acids for diagnostic purposes. This is followed by an MPV genome-specific real-time polymerase chain reaction (RT-PCR) assay to detect viral DNA (71). Moreover, western blot (WB) analysis using MPV proteins can also be used to confirm MPV infection. WB has lower requirements for detection equipment or laboratories and the detection results are more accurate, but there are some limitations compared with RT-PCR. Such as infection cannot be detected until 7 days after the infection; Blood collection is required and the operation is relatively difficult; not suitable for application to the large-scale collection, etc. (81, 82). So according to the WHO, the preferred test for identifying MPV during acute infection is the RT-PCR test (83). If the mother is infected by MPV, the fetus requires ultrasound surveillance to determine if he/she is infected with MPV by examining the existence of ultrasound anomalies such as fetal hepatomegaly or hydrops (84).
Most people recover from the disease without treatment and the symptoms of monkeypox are typically mild (54). Despite the lack of specific therapies for monkeypox, studies have shown that the smallpox vaccine has an 85% success rate in preventing monkeypox. Furthermore, several antiviral drugs may also be effective in treating monkeypox infections (63) (Table 4).
The US CDC has approved the use of ACAM2000 in emergencies, and it can offer 85% cross-protective resistance against monkeypox (54). Patients must be informed of the abnormal risk of fetal vaccinia by ACAM2000 if high-risk monkeypox exposure occurs during pregnancy. Fetal vaccinia can cause stillbirth, neonatal death, premature delivery, and possibly unfavorable maternal reactions (94). The third-generation MVA-BN (Modified Vaccinia Ankara-Bavarian Nordic) smallpox vaccine, which has received universal approval within the EU, Canada, and the USA, may be gentler because it comprises the virus that cannot replicate and has not been proven to cause problems during pregnancy (95).
Tecovirimat (TPOXX/ST-246), an FDA-approved antiviral medicine, is used to alleviate both pediatric and adult patients with human smallpox illnesses. It acts as a suppressor of the OPV VP37 envelope protein and can be administered as a pill or injection (51). The capsule can be opened and the drug mixed with semisolid food for kids under the weight of 12.9 kilograms (96). Although studies on numerous animal species have shown that tecovirimat is beneficial in treating diseases brought on by OPVs, it is still unclear if tecovirimat is effective in treating human monkeypox infections. One case study was an adult patient who received tecovirimat and made a full recovery after spending a month in the hospital (97), while in another case study, a patient underwent oral tecovirimat (200 mg twice a day for two weeks) treatment without experiencing any negative side effects (67). The CDC has an enhanced access protocol, also known as a “compassionate use” approach, that allows the application of tecovirimat for the mitigation of monkeypox when an outbreak occurs (98, 99).
The FDA has authorized the use of VIGIV, a hyperimmune globulin, to treat the side effects of vaccinia-related conditions such as progressive vaccinia, severe generalized vaccinia, dermatitis vaccinatum, vaccinia infections in persons with skin issues, and abnormal infections brought on by VACV (except in cases of isolated keratitis). The application of VIGIV for the therapy of OPVs outbreak such as monkeypox is permitted by the CDC’s expanded access protocol (100). Although it is a potential strategy, there is little data on how VIGIV acts against smallpox and monkeypox, and the utilization of VIGIV for smallpox or monkeypox has not been tested in humans. However, in extreme circumstances, medical professionals might consider their use incurring monkeypox. Notably, individuals with severely compromised T-cell functionality should not be vaccinated against the MPV. Instead, VIGIV may be administered to them if they have a history of exposure (101).
The antiviral medicine Cidofovir (Vistide), which blocks viral DNA polymerase from functioning, has been proven to be effective against poxviruses in vitro and in preclinical studies (52). It is an antiviral medication that the FDA has approved for use in treating cytomegalovirus (CMV) retinitis in AIDS patients. The effectiveness of Cidofovir in treating monkeypox in people is not well understood. However, investigations on animals and in vitro have shown that it is effective against OPVs (102). To mitigate OPV (including monkeypox) in an outbreak, the CDC has an enhanced access protocol that permits the utilization of stocked cidofovir. Cidofovir treatment may be implemented in cases with severe monkeypox infection, although it is unknown whether such a patient will benefit from it. Cidofovir may be less safe than Brincidofovir since Cidofovir can cause serious renal damage or other side effects (90). Currently, the CDC is working on an Expanded Access Investigational New Drugs to enable the drugs to be used for the treatment of monkeypox. They could pick the CMX-001 medication, a modified version of cidofovir, which has demonstrated antiviral activity against OPV species but lacks the level of nephrotoxicity typically associated with cidofovir (103).
On June 4, 2021, FDA licensed Brincidofovir (Tembexa) as an antiviral drug to alleviate human smallpox in newborns, children, and adults. Although the small numbers involved make it difficult to extrapolate the effectiveness of Brincidofovir in the therapy of monkeypox infection, their efficacy against OPV has been illustrated in vitro and animal models (53, 104). However, a review of patients enrolled since the launch of the HCID (airborne) network between August 15, 2018, and September 10, 2021, revealed that all 3 adult patients receiving oral Brincidofovir (200 mg once a week) had high hepatic enzymes, which led to the termination of therapy (67). The CDC is now working on an EA-IND (Expanded Access Investigational New Drug) to make it easier to utilize Brincidofovir as a monkeypox treatment.
Monkeypox is no longer “a viral zoonotic disease that occurs mainly in remote portions of Central and West Africa, near tropical rainforests”, as the expansion of the disease over the past several years and the ongoing outbreak (105). Although most MPV infections cause self-limited and mild diseases, with supportive treatment being relatively sufficient, there is no specific therapy available for it to date, and it can be transmitted in a variety of ways, such as droplets, contact, and sexual contact. Its possibility for additional regional and worldwide spread is therefore still a serious concern. The majority of information on the illness is gathered from individual cases or outbreak reports, as well as from passive sporadic monitoring, none of which provides a complete overview. To effectively lead data collection, prevention, preparedness, response and surveillance efforts for monkeypox and other emerging or re-emerging diseases with pandemic possibility, there is still an urgent need to improve surveillance and public health skills.
Numerous antiviral drugs that may be beneficial in the mitigation of monkeypox were authorized for the treatment of smallpox based on model studies, such as Tecovirimat, VIGIV, Cidofovir, and Brincidofovir, but the efficacy of these agents has not been completely characterized; thus, more research on these therapies in humans is needed. In addition, there are several variables can affect the prognosis of monkeypox, such as prior vaccination records, baseline health state, and co-occurring illnesses or comorbidities. Therefore, the most sensible course of action is to design treatments specifically for each patient in accordance with their likelihood of contracting a severe illness.
Conceptualization and design: XL and PY; data acquisition and writing-original draft preparation, LN and DL; data analysis and interpretation: JZ and ZL; revision for intellectual content: all authors. All authors agree to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
This work was supported by the Natural Science Foundation of Jiangxi Province [grant numbers 20192ACBL21037, 202004BCJL23049 and 202002BAB216022], the National Natural Science Foundation of China [grant number 82160371, 82100869, 21866019, and 82100347], the China Postdoctoral Science Foundation [grant number 2021M703724], the Natural Science Foundation of Guangdong Province [grant number 2022A1515010582], and the Science and Technology Projects in Guangzhou [grant number 202102010007]. The graphical abstracts were created with BioRender, Figdraw and Datawrapper software (biorender.com, Figdraw.com and datawrapper.de).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
MPV, Monkeypox virus; CDC, Centers for Disease Control; WHO, World Health Organization; COVID-19, coronavirus disease 2019; OPV, orthopoxvirus; RT-PCR, real-time polymerase chain reaction; MSM, males who have sex with other men; VACV, vaccinia virus; EEV, extracellular-enveloped virus; IMV, intracellular mature virus; MV, mature virion; EV, enveloped virion; DRC, Democratic Republic of Congo; VIGIV, Vaccinia Immune Globulin Intravenous; CMV, cytomegalovirus.
1. Lai CC, Hsu CK, Yen MY, Lee PI, Ko WC, Hsueh PR. Monkeypox: An emerging global threat during the COVID-19 pandemic. J Microbiol Immunol Infect (2022) 55(5):787–94. doi: 10.1016/j.jmii.2022.07.004
2. Nolen LD, Osadebe L, Katomba J, Likofata J, Mukadi D, Monroe B, et al. Extended human-to-Human transmission during a monkeypox outbreak in the democratic republic of the Congo. Emerg Infect Dis (2016) 22(6):1014–21. doi: 10.3201/eid2206.150579
3. Falendysz EA, Lopera JG, Lorenzsonn F, Salzer JS, Hutson CL, Doty J, et al. Further assessment of monkeypox virus infection in Gambian pouched rats (Cricetomys gambianus) using In vivo bioluminescent imaging. PloS Negl Trop Dis (2015) 9(10):e0004130. doi: 10.1371/journal.pntd.0004130
4. Marennikova SS, Seluhina EM, Mal'ceva NN, Cimiskjan KL, Macevic GR. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Organ (1972) 46(5):599–611.
5. 2022 monkeypox outbreak global map. Available at: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html2022.
6. Huang YA, Howard-Jones AR, Durrani S, Wang Z, Williams PC. Monkeypox: A clinical update for paediatricians. J Paediatr Child Health (2022) 58(9):1532–8. doi: 10.1111/jpc.16171
7. Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and treatment of monkeypox. Drugs (2022) 82(9):957–63. doi: 10.1007/s40265-022-01742-y
8. Cho CT, Wenner HA. Monkeypox virus. Bacteriol Rev (1973) 37(1):1–18. doi: 10.1128/br.37.1.1-18.1973
9. Luo Q, Han J. Preparedness for a monkeypox outbreak. Infect Med (2022) 1(2):124–34. doi: 10.1016/j.imj.2022.07.001
10. Marennikova SS, Shelukhina EM. Whitepox virus isolated from hamsters inoculated with monkeypox virus. Nature (1978) 276(5685):291–2. doi: 10.1038/276291a0
11. Marennikova SS, Shelukhina EM, Maltseva NN, Matsevich GR. Monkeypox virus as a source of whitepox viruses. Intervirology (1979) 11(6):333–40. doi: 10.1159/000149055
12. Nakazawa Y, Mauldin MR, Emerson GL, Reynolds MG, Lash RR, Gao J, et al. A phylogeographic investigation of African monkeypox. Viruses (2015) 7(4):2168–84. doi: 10.3390/v7042168
13. Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol (2013) 8(2):129–57. doi: 10.2217/fvl.12.130
14. Thakur S, Kelkar D, Garg S, Raina SK, Lateef F, Gilada I, et al. Why should RNA viruses have all the fun - monkeypox, a close relative of smallpox and a DNA virus. J Glob Infect Dis (2022) 14(2):47–9. doi: 10.4103/jgid.jgid_104_22
15. Angelo KM, Petersen BW, Hamer DH, Schwartz E, Brunette G. Monkeypox transmission among international travellers-serious monkey business? J Travel Med (2019) 26(5):taz002. doi: 10.1093/jtm/taz002
16. Grant R, Nguyen LL, Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ (2020) 98(9):638–40. doi: 10.2471/BLT.19.242347
17. Lansiaux E, Jain N, Laivacuma S, Reinis A. The virology of human monkeypox virus (hMPXV): A brief overview. Virus Res (2022) 322:198932. doi: 10.1016/j.virusres.2022.198932
18. Kumar N, Acharya A, Gendelman HE, Byrareddy SN. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun (2022) 102855. doi: 10.1016/j.jaut.2022.102855
19. Mahase E. Monkeypox: What do we know about the outbreaks in Europe and north America? BMJ (2022) 377:o1274. doi: 10.1136/bmj.o1274
20. Inigo Martinez J, Gil Montalban E, Jimenez Bueno S, Martin Martinez F, Nieto Julia A, Sanchez Diaz J, et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Euro Surveill (2022) 27(27):2200471. doi: 10.2807/1560-7917.ES.2022.27.27.2200471
21. Zhu F, Li L, Che D. Monkeypox virus under COVID-19: Caution for sexual transmission - correspondence. Int J Surg (2022) 104:106768. doi: 10.1016/j.ijsu.2022.106768
22. McFadden JWBaG. Origin and evolution of poxviruses. Origin and Evolution of Viruses (Second Edition) Chapter 19. (2008) 431–46. doi: 10.1016/B978-0-12-374153-0.00019-9
23. Velavan TP, Meyer CG. Monkeypox 2022 outbreak: An update. Trop Med Int Health (2022) 27(7):604–5. doi: 10.1111/tmi.13785
24. Thomassen HA, Fuller T, Asefi-Najafabady S, Shiplacoff JA, Mulembakani PM, Blumberg S, et al. Pathogen-host associations and predicted range shifts of human monkeypox in response to climate change in central Africa. PloS One (2013) 8(7):e66071. doi: 10.1371/journal.pone.0066071
25. Osorio JE, Iams KP, Meteyer CU, Rocke TE. Comparison of monkeypox viruses pathogenesis in mice by in vivo imaging. PloS One (2009) 4(8):e6592. doi: 10.1371/journal.pone.0006592
26. Zaucha GM, Jahrling PB, Geisbert TW, Swearengen JR, Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis). Lab Invest (2001) 81(12):1581–600. doi: 10.1038/labinvest.3780373
27. Kugelman JR, Johnston SC, Mulembakani PM, Kisalu N, Lee MS, Koroleva G, et al. Genomic variability of monkeypox virus among humans, democratic republic of the Congo. Emerg Infect Dis (2014) 20(2):232–9. doi: 10.3201/eid2002.130118
28. Esposito JJ, Knight JC. Orthopoxvirus DNA: a comparison of restriction profiles and maps. Virology (1985) 143(1):230–51. doi: 10.1016/0042-6822(85)90111-4
29. Takemura M. Poxviruses and the origin of the eukaryotic nucleus. J Mol Evol (2001) 52(5):419–25. doi: 10.1007/s002390010171
30. Remichkova M. Poxviruses: Smallpox vaccine, its complications and chemotherapy. Virus Adaptat. Treat (2010) 2:41–6. doi: 10.2147/VAAT.S8563
31. Moss B. The molecular biology of poxviruses. Mol Bas. Viral Replicat (1987) 136:499–516. doi: 10.1007/978-1-4684-5350-8_21
32. Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox virus in Nigeria: Infection biology, epidemiology, and evolution. Viruses (2020) 12(11):1257–87. doi: 10.3390/v12111257
33. Schmidt FI, Bleck CK, Mercer J. Poxvirus host cell entry. Curr Opin Virol (2012) 2(1):20–7. doi: 10.1016/j.coviro.2011.11.007
34. Schmelz M, Sodeik B, Ericsson M, Wolffe EJ, Shida H, Hiller G, et al. Assembly of vaccinia virus: The second wrapping cisterna is derived from the trans golgi network. J Virol (1994) 68(1):130–47. doi: 10.1128/jvi.68.1.130-147.1994
35. Smith GL, Murphy BJ, Law M. Vaccinia virus motility. Annu Rev Microbiol (2003) 57:323–42. doi: 10.1146/annurev.micro.57.030502.091037
36. Hammarlund E, Dasgupta A, Pinilla C, Norori P, Fruh K, Slifka MK. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc Natl Acad Sci U S A (2008) 105(38):14567–72. doi: 10.1073/pnas.0800589105
37. Hutson CL, Davidson W, Regnery RL, Reynolds MG, Li YU, Damon IK, et al. Monkeypox zoonotic associations: Insights from laboratory evaluation of animals associated with the multi-state us outbreak. Am J Trop Med Hygiene (2007) 76(4):757–68. doi: 10.4269/ajtmh.2007.76.757
38. Meyer H, Perrichot M, Stemmler M, Emmerich P, Schmitz H, Varaine F, et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the democratic republic of Congo in 2001. J Clin Microbiol (2002) 40(8):2919–21. doi: 10.1128/JCM.40.8.2919-2921.2002
39. Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, et al. The changing epidemiology of human monkeypox-a potential threat? A systematic review. PloS Negl Trop Dis (2022) 16(2):e0010141. doi: 10.1371/journal.pntd.0010141
40. Huhn GD, Bauer AM, Yorita K, Graham MB, Sejvar J, Likos A, et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis (2005) 41(12):1742–51. doi: 10.1086/498115
41. 2022 monkeypox outbreak global map. Available at: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html2022.
42. Hendrickson RC, Wang C, Hatcher EL, Lefkowitz EJ. Orthopoxvirus genome evolution: the role of gene loss. Viruses (2010) 2(9):1933–67. doi: 10.3390/v2091933
43. Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmune Pharmacol (2020) 15(3):359–86. doi: 10.1007/s11481-020-09944-5
44. Anfinrud P, Stadnytskyi V, Bax CE, Bax A. Visualizing speech-generated oral fluid droplets with laser light scattering. N Engl J Med (2020) 382(21):2061–3. doi: 10.1056/NEJMc2007800
45. Guarner J, Johnson BJ, Paddock CD, Shieh WJ, Goldsmith CS, Reynolds MG, et al. Monkeypox transmission and pathogenesis in prairie dogs. Emerg Infect Dis (2004) 10(3):426–31. doi: 10.3201/eid1003.030878
46. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol (2020) 215:108427. doi: 10.1016/j.clim.2020.108427
47. Amoutzias GD, Nikolaidis M, Tryfonopoulou E, Chlichlia K, Markoulatos P, Oliver SG. The remarkable evolutionary plasticity of coronaviruses by mutation and recombination: Insights for the COVID-19 pandemic and the future evolutionary paths of SARS-CoV-2. Viruses (2022) 14(1):78–102. doi: 10.3390/v14010078
48. Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from wuhan, China, 20-28 January 2020. Euro Surveill (2020) 25(5):2000062. doi: 10.2807/1560-7917.ES.2020.25.5.2000062
49. Martinez-Rojas MA, Vega-Vega O, Bobadilla NA. Is the kidney a target of SARS-CoV-2? Am J Physiol Renal Physiol (2020) 318(6):F1454–F62. doi: 10.1152/ajprenal.00160.2020
50. WHO. Weekly epidemiological update on COVID-19 - 1 February 2023 (2023). Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—1-february-2023.
51. Russo AT, Grosenbach DW, Chinsangaram J, Honeychurch KM, Long PG, Lovejoy C, et al. An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications. Expert Rev Anti Infect Ther (2021) 19(3):331–44. doi: 10.1080/14787210.2020.1819791
52. Lanier R, Trost L, Tippin T, Lampert B, Robertson A, Foster S, et al. Development of CMX001 for the treatment of poxvirus infections. Viruses (2010) 2(12):2740–62. doi: 10.3390/v2122740
53. Smee DF. Progress in the discovery of compounds inhibiting orthopoxviruses in animal models. Antivir Chem Chemother (2008) 19(3):115–24. doi: 10.1177/095632020801900302
54. Reynolds MG, McCollum AM, Nguete B, Shongo Lushima R, Petersen BW. Improving the care and treatment of monkeypox patients in low-resource settings: Applying evidence from contemporary biomedical and smallpox biodefense research. Viruses (2017) 9(12):380–94. doi: 10.3390/v9120380
55. Atmar RL, Finch N. New perspectives on antimicrobial agents: Molnupiravir and Nirmatrelvir/Ritonavir for treatment of COVID-19. Antimicrob Agents Chemother (2022) 66(8):e0240421. doi: 10.1128/aac.02404-21
56. See KC. Vaccination for monkeypox virus infection in humans: A review of key considerations. Vaccines (Basel) (2022) 10(8):1342–54. doi: 10.3390/vaccines10081342
57. Control ECfDPa. epidemiological update: Monkeypox outbreak. Available at: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-monkeypox-outbreak2022.
58. Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox virus infection in humans across 16 countries - April-June 2022. N Engl J Med (2022) 387(8):679–91. doi: 10.1056/NEJMoa2207323
59. Philpott D, Hughes CM, Alroy KA, Kerins JL, Pavlick J, Asbel L, et al. Epidemiologic and clinical characteristics of monkeypox cases - united states, may 17-July 22, 2022. MMWR Morb Mortal Wkly Rep (2022) 71(32):1018–22. doi: 10.15585/mmwr.mm7132e3
60. CDC. 2022 monkeypox outbreak global map. Available at: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html2022.
61. Adnan N, Haq ZU, Malik A, Mehmood A, Ishaq U, Faraz M, et al. Human monkeypox virus: An updated review. Med (Baltimore) (2022) 101(35):e30406. doi: 10.1097/MD.0000000000030406
62. Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis (1987) 156(2):293–8. doi: 10.1093/infdis/156.2.293
63. Kmiec D, Kirchhoff F. Monkeypox: A new threat? Int J Mol Sci (2022) 23(14):7866. doi: 10.3390/ijms23147866
64. de Sousa D, Patrocínio J, Frade J, Brazão C, Mancha D, Correia C, et al. Monkeypox diagnosis by cutaneous and mucosal findings. Infect Dis Rep (2022) 14(5):759–64. doi: 10.3390/idr14050077
65. Lapa D, Carletti F, Mazzotta V, Matusali G, Pinnetti C, Meschi S, et al. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect Dis (2022) 22(9):1267–9. doi: 10.1016/S1473-3099(22)00513-8
66. Shafaati M, Zandi M. Monkeypox virus neurological manifestations in comparison to other orthopoxviruses. Travel Med Infect Dis (2022) 49:102414. doi: 10.1016/j.tmaid.2022.102414
67. Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, et al. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect Dis (2022) 22(8):1153–62. doi: 10.1016/S1473-3099(22)00228-6
68. Abdelaal A, Serhan HA, Mahmoud MA, Rodriguez-Morales AJ, Sah R. Ophthalmic manifestations of monkeypox virus. Eye (Lond) (2022) 37(3):383–5. doi: 10.1038/s41433-022-02195-z
69. Petersen E, Kantele A, Koopmans M, Asogun D, Yinka-Ogunleye A, Ihekweazu C, et al. Human monkeypox: Epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am (2019) 33(4):1027–43. doi: 10.1016/j.idc.2019.03.001
70. Singhal T, Kabra SK, Lodha R. Monkeypox: A review. Indian J Pediatr (2022) 89(10):955–60. doi: 10.1007/s12098-022-04348-0
71. Pastula DM, Tyler KL. An overview of monkeypox virus and its neuroinvasive potential. Ann Neurol (2022) 92(4):527–31. doi: 10.1002/ana.26473
72. Haviland JW. Purpura variolosa; its manifestations in skin and blood. Yale J Biol Med (1952) 24(6):518–24.
73. McKenzie PJ, Githens JH, Harwood ME, Roberts JF, Rao AR, Kempe CH. Haemorrhagic smallpox. 2. specific bleeding and coagulation studies. Bull World Health Organ (1965) 33(6):773–82.
74. Gordon SN, Cecchinato V, Andresen V, Heraud JM, Hryniewicz A, Parks RW, et al. Smallpox vaccine safety is dependent on T cells and not b cells. J Infect Dis (2011) 203(8):1043–53. doi: 10.1093/infdis/jiq162
75. Osadebe L, Hughes CM, Shongo Lushima R, Kabamba J, Nguete B, Malekani J, et al. Enhancing case definitions for surveillance of human monkeypox in the democratic republic of Congo. PloS Negl Trop Dis (2017) 11(9):e0005857. doi: 10.1371/journal.pntd.0005857
76. Bone RC. The pathogenesis of sepsis. Ann Intern Med (1991) 115(6):457–69. doi: 10.7326/0003-4819-115-6-457
77. Sejvar JJ, Chowdary Y, Schomogyi M, Stevens J, Patel J, Karem K, et al. Human monkeypox infection: A family cluster in the midwestern united states. J Infect Dis (2004) 190(10):1833–40. doi: 10.1086/425039
78. Anderson MG, Frenkel LD, Homann S, Guffey J. A case of severe monkeypox virus disease in an American child: Emerging infections and changing professional values. Pediatr Infect Dis J (2003) 22(12):1093–6. discussion 6-8. doi: 10.1097/01.inf.0000101821.61387.a5
79. Mailhe M, Beaumont AL, Thy M, Le Pluart D, Perrineau S, Houhou-Fidouh N, et al. Clinical characteristics of ambulatory and hospitalised patients with monkeypox virus infection: An observational cohort study. Clin Microbiol Infect (2022) 29(2):233–9. doi: 10.1016/j.cmi.2022.08.012
80. Macneil A, Reynolds MG, Braden Z, Carroll DS, Bostik V, Karem K, et al. Transmission of atypical varicella-zoster virus infections involving palm and sole manifestations in an area with monkeypox endemicity. Clin Infect Dis (2009) 48(1):e6–8. doi: 10.1086/595552
81. Adalja A, Inglesby T. A novel international monkeypox outbreak. Ann Internal Med (2022) 175(8):1175–6. doi: 10.7326/M22-1581
82. Huggett JF, French D, O'Sullivan DM, Moran-Gilad J, Zumla A. Monkeypox: Another test for PCR. Euro Surveill (2022) 27(32):2200497. doi: 10.2807/1560-7917.ES.2022.27.32.2200497
83. Pal M, Singh R, Gutama KP, Savalia CV, Thakur R. Human monkeypox: An emerging and re-emerging infectious viral disease. Acta Sci Microbiol (2022) 5:146–50. doi: 10.31080/ASMI.2022.05.1045
84. Dashraath P, Nielsen-Saines K, Mattar C, Musso D, Tambyah P, Baud D. Guidelines for pregnant individuals with monkeypox virus exposure. Lancet (2022) 400(10345):21–2. doi: 10.1016/S0140-6736(22)01063-7
85. O’Shea J FT, Morris SB, Weiser J, Petersen B, Brooks JT. Interim guidance for prevention and treatment of monkeypox in persons with HIV infection - United States, August 2022. MMWR Morb Mortal Wkly Rep (2022) 71(32):1023–28. doi: 10.15585/mmwr.mm7132e4
86. Dashraath P, Nielsen-Saines K, Rimoin A, Mattar CNZ, Panchaud A, Baud D. Monkeypox in pregnancy: Virology, clinical presentation, and obstetric management. Am J Obstet Gynecol (2022) 227(6):849–61. doi: 10.1016/j.ajog.2022.08.017
87. Siegrist EA, Sassine J. Antivirals with activity against monkeypox: A clinically oriented review. Clin Infect Dis (2022) 76(1):155–64. doi: 10.1093/cid/ciac622
88. Akazawa D, Ohashi H, Hishiki T, Morita T, Iwanami S, Kim KS, et al. Potential anti-monkeypox virus activity of atovaquone, mefloquine, and molnupiravir, and their potential use as treatments. bioRxiv (2022). doi: 10.1101/2022.08.02.502485
89. Matias WR, Koshy JM, Nagami EH, Kovac V, Moeng LR, Shenoy ES, et al. Tecovirimat for the treatment of human monkeypox: An initial series from Massachusetts, united states. Open Forum Infect Dis (2022) 9(8):ofac377. doi: 10.1093/ofid/ofac377
90. Chittick G, Morrison M, Brundage T, Nichols WG. Short-term clinical safety profile of brincidofovir: A favorable benefit-risk proposition in the treatment of smallpox. Antiviral Res (2017) 143:269–77. doi: 10.1016/j.antiviral.2017.01.009
91. Andrei G, Snoeck R. Cidofovir activity against poxvirus infections. Viruses (2010) 2(12):2803–30. doi: 10.3390/v2122803
92. Islam MR, Hossain MJ, Roy A, Hasan A, Rahman MA, Shahriar M, et al. Repositioning potentials of smallpox vaccines and antiviral agents in monkeypox outbreak: A rapid review on comparative benefits and risks. Health Sci Rep (2022) 5(5):e798. doi: 10.1002/hsr2.798
93. Chan-Tack K, Harrington P, Bensman T, Choi SY, Donaldson E, O'Rear J, et al. Benefit-risk assessment for brincidofovir for the treatment of smallpox: U.S. food and drug administration's evaluation. Antiviral Res (2021) 195:105182. doi: 10.1016/j.antiviral.2021.105182
94. Mbala PK, Huggins JW, Riu-Rovira T, Ahuka SM, Mulembakani P, Rimoin AW, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the democratic republic of Congo. J Infect Dis (2017) 216(7):824–8. doi: 10.1093/infdis/jix260
95. Khalil A, Samara A, O'Brien P, Morris E, Draycott T, Lees C, et al. Monkeypox vaccines in pregnancy: Lessons must be learned from COVID-19. Lancet Global Health (2022) 10(9):e1230–e1. doi: 10.1016/S2214-109X(22)00284-4
96. Gountia IA, Mati P. Monkey pox: A ambient review of transmission, improving care and treatments. Int Res J Modern. Eng Technol Sci (2022) 04(Issue:07):July–2022.
97. Rao AK, Schulte J, Chen TH, Hughes CM, Davidson W, Neff JM, et al. Monkeypox in a traveler returning from Nigeria - Dallas, Texas, July 2021. MMWR Morb Mortal Wkly Rep (2022) 71(14):509–16. doi: 10.15585/mmwr.mm7114a1
98. Laudisoit A, Tepage F, Colebunders R. Oral tecovirimat for the treatment of smallpox. N Engl J Med (2018) 379(21):2084–5. doi: 10.1056/NEJMc1811044
99. Quenelle DC, Buller RM, Parker S, Keith KA, Hruby DE, Jordan R, et al. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob Agents Chemother (2007) 51(2):689–95. doi: 10.1128/AAC.00879-06
100. Wittek R. Vaccinia immune globulin: Current policies, preparedness, and product safety and efficacy. Int J Infect Dis (2006) 10(3):193–201. doi: 10.1016/j.ijid.2005.12.001
101. Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis (2005) 41(12):1765–71. doi: 10.1086/498155
102. Rice AD, Adams MM, Wallace G, Burrage AM, Lindsey SF, Smith AJ, et al. Efficacy of CMX001 as a post exposure antiviral in new Zealand white rabbits infected with rabbitpox virus, a model for orthopoxvirus infections of humans. Viruses (2011) 3(1):47–62. doi: 10.3390/v3010047
103. Parker S, Handley L, Buller RM. Therapeutic and prophylactic drugs to treat orthopoxvirus infections. Future Virol (2008) 3(6):595–612. doi: 10.2217/17460794.3.6.595
104. Baker RO, Bray M, Huggins JW. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res (2003) 57(1-2):13–23. doi: 10.1016/S0166-3542(02)00196-1
Keywords: monkeypox virus (MPV), origin, pathophysiology, global prevalence, clinical manifestation, treatment
Citation: Niu L, Liang D, Ling Q, Zhang J, Li Z, Zhang D, Xia P, Zhu Z, Lin J, Shi A, Ma J, Yu P and Liu X (2023) Insights into monkeypox pathophysiology, global prevalence, clinical manifestation and treatments. Front. Immunol. 14:1132250. doi: 10.3389/fimmu.2023.1132250
Received: 27 December 2022; Accepted: 02 March 2023;
Published: 21 March 2023.
Edited by:
Wei Wang, Jiangsu Institute of Parasitic Diseases (JIPD), ChinaReviewed by:
Celestine Wanjalla, Vanderbilt University Medical Center, United StatesCopyright © 2023 Niu, Liang, Ling, Zhang, Li, Zhang, Xia, Zhu, Lin, Shi, Ma, Yu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Liu, bGl1eDU4N0BtYWlsLnN5c3UuZWR1LmNu; Peng Yu, eXU4MjIwMTgyQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.