- 1Department of Medicine and Infectious Diseases, Stanford University, Palo Alto, CA, United States
- 2Department of Medicine and Infectious Diseases, University of California, San Francisco, San Francisco, CA, United States
- 3Center for Innovations in Brain Science, University of Arizona, Tucson, AZ, United States
- 4Department of Medicine, Infectious Diseases, Icahn School of Medicine at Mount Sinai, Chief, Division of Infectious Disease, New York, NY, United States
- 5Department of Medicine, Infectious Diseases, The University of Texas Health Science Center at San Antonio, San Antonio, TX, United States
- 6Division of Intramural Research, National Institute of Health, Bethesda, MD, United States
- 7National Heart Lung and Blood Institute, Division of Lung Diseases, National Institutes of Health, Bethesda, MD, United States
- 8Department of Medicine, Organ Transplant and Liver Center, Swedish Medical Center, Seattle, WA, United States
- 9Division of Allergy and Infectious Diseases, University of Washington, Seattle, WA, United States
- 10Department of Medicine, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, GA, United States
- 11Department of Clinical Pharmacy, University of California, San Francisco, San Francisco, CA, United States
- 12Department of Rehabilitation Medicine at New York University (NYU) Grossman School of Medicine, Physical Medicine and Rehabilitation Service, New York University (NYU), New York University Medical Center, New York, NY, United States
- 13Applied Clinical Informatics Branch, National Library of Medicine, National Institutes of Health, Bethesda, MD, United States
- 14Department of Medicine at Harvard Medical School, Division of Infectious Disease, Boston, MA, United States
- 15Research Triangle Institute (RTI), International, Durham, NC, United States
- 16Department of Obstetrics and Gynecology, The Ohio State University, Columbus, OH, United States
- 17Long COVID Families, Houston, TX, United States
- 18Utah Covid-19 Long Haulers, Salt Lake City, UT, United States
- 19Department of Medicine, University of Illinois at Chicago, Chicago, IL, United States
- 20Department of Medicine, Albert Einstein College of Medicine, New York, NY, United States
- 21Department of Pediatrics, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States
- 22Department of Medicine, University of Michigan, Ann Arbor, MI, United States
- 23Department of Pediatrics and Medicine, Case Western Reserve University, Cleveland, OH, United States
Although most individuals recover from acute SARS-CoV-2 infection, a significant number continue to suffer from Post-Acute Sequelae of SARS-CoV-2 (PASC), including the unexplained symptoms that are frequently referred to as long COVID, which could last for weeks, months, or even years after the acute phase of illness. The National Institutes of Health is currently funding large multi-center research programs as part of its Researching COVID to Enhance Recover (RECOVER) initiative to understand why some individuals do not recover fully from COVID-19. Several ongoing pathobiology studies have provided clues to potential mechanisms contributing to this condition. These include persistence of SARS-CoV-2 antigen and/or genetic material, immune dysregulation, reactivation of other latent viral infections, microvascular dysfunction, and gut dysbiosis, among others. Although our understanding of the causes of long COVID remains incomplete, these early pathophysiologic studies suggest biological pathways that could be targeted in therapeutic trials that aim to ameliorate symptoms. Repurposed medicines and novel therapeutics deserve formal testing in clinical trial settings prior to adoption. While we endorse clinical trials, especially those that prioritize inclusion of the diverse populations most affected by COVID-19 and long COVID, we discourage off-label experimentation in uncontrolled and/or unsupervised settings. Here, we review ongoing, planned, and potential future therapeutic interventions for long COVID based on the current understanding of the pathobiological processes underlying this condition. We focus on clinical, pharmacological, and feasibility data, with the goal of informing future interventional research studies.
Background
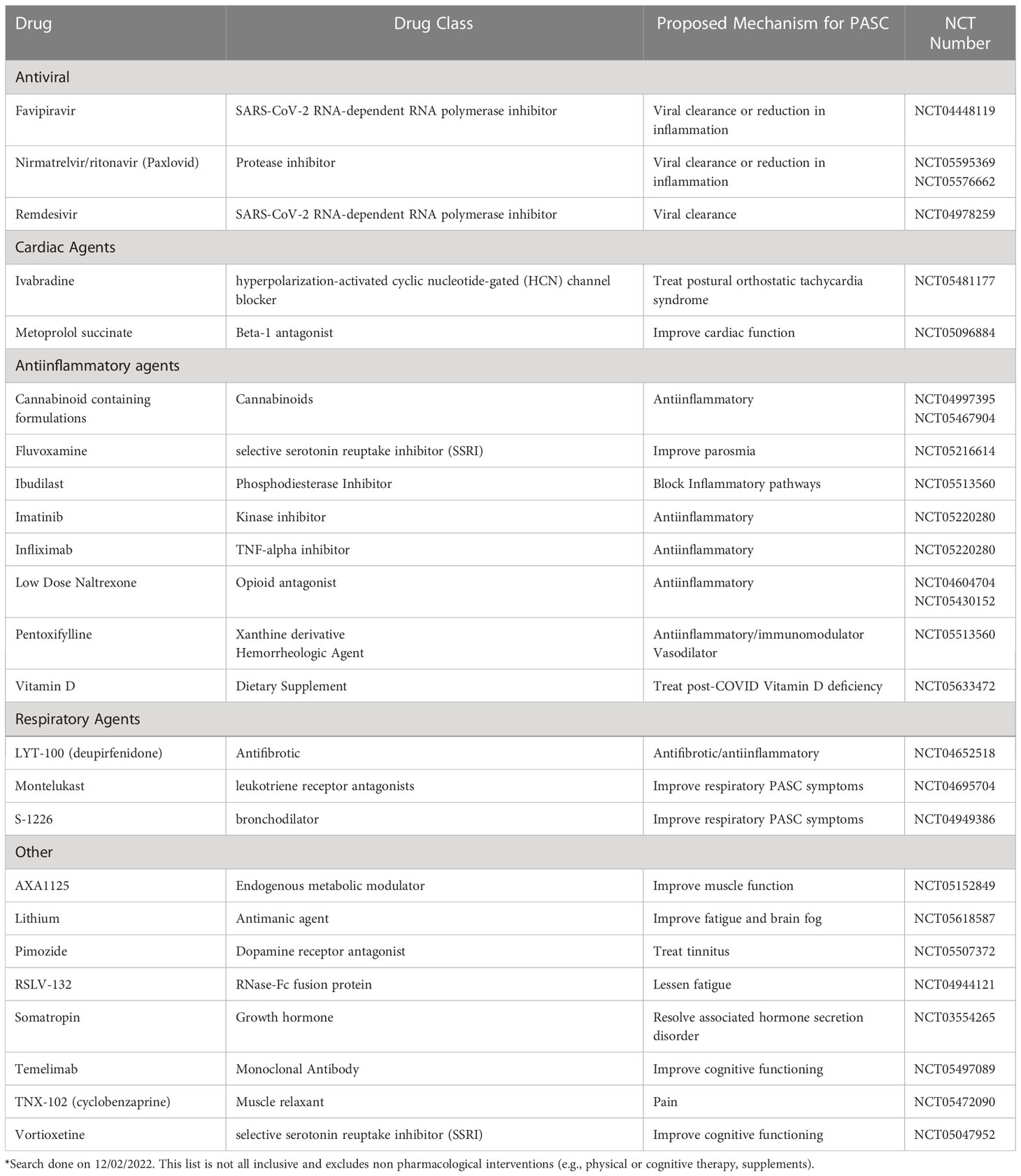
While most people fully recover within weeks of SARS-CoV-2 infection, a subset of individuals experience symptoms that persist well beyond the acute period (1). These symptoms can be debilitating, interfering with return to usual activities and impacting quality of life. While efforts to quantify the scale of the problem are ongoing, there is a growing consensus that post-COVID conditions, collectively known as Post-Acute Sequelae of SARS-CoV-2 infection (PASC), including the unexplained symptoms of long COVID, represent a substantial public health concern. However, there is currently no standard of care for long COVID and no agreed upon treatment. In this article, we review the current state of long COVID management with a view toward therapies with promising early data. Selected studies of pharmaceutical interventions are currently listed in ClinicalTrials.gov* is presented in Table 1. We focus on the biological mechanisms that have been identified as potential drivers of this condition.
Case definitions, epidemiology, and clinical features
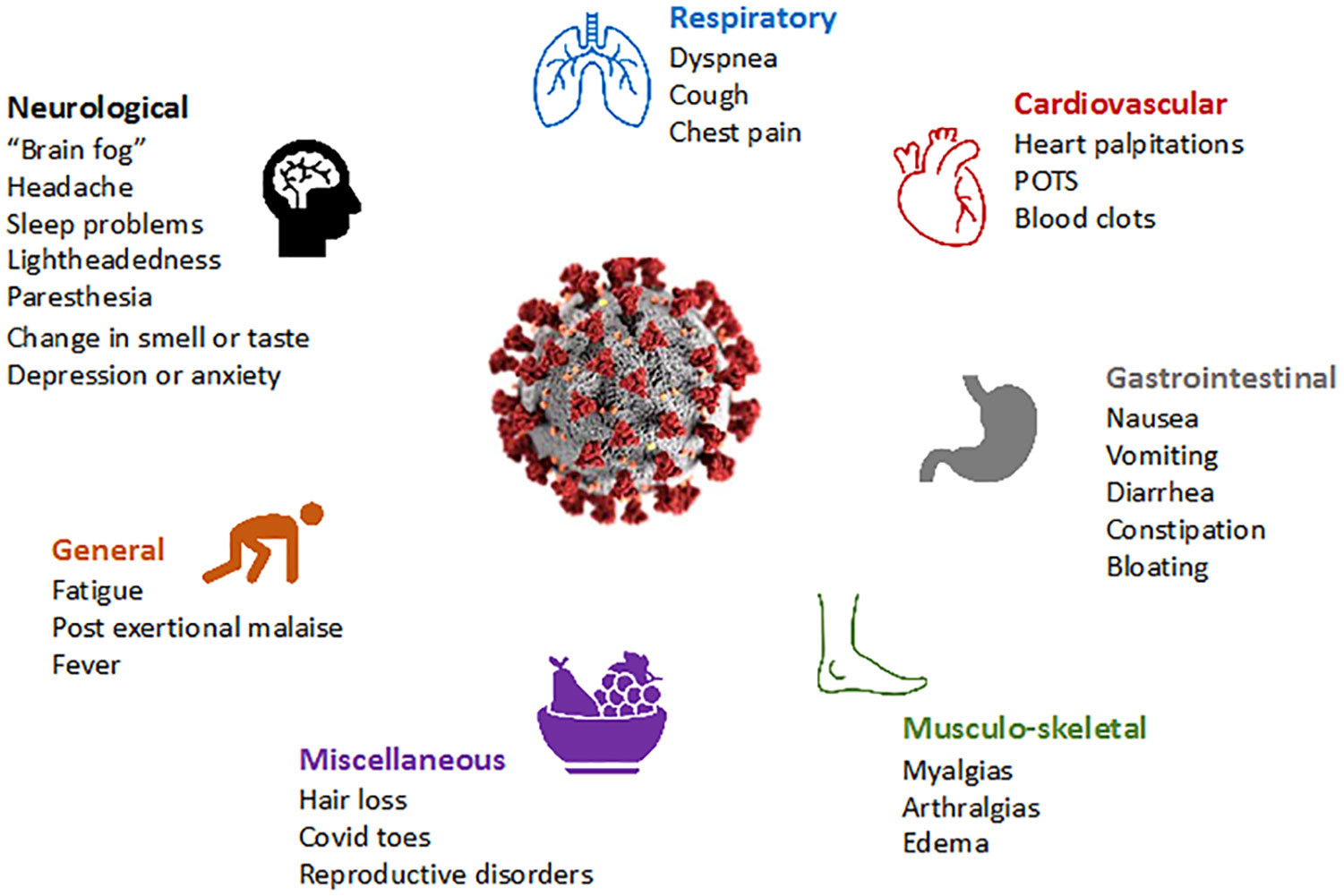
Long COVID is increasingly recognized even in people who experience asymptomatic or mild SARS-CoV-2 infection (2). The U.S. Centers for Disease Control and Prevention (CDC) defines long COVID (which they refer to as “post-COVID conditions”) as symptoms persisting more than 28 days after the initial SARS-CoV-2 infection (3), while the U.K. National Institute for Health and Care Excellence (NICE) (4) and the World Health Organization (WHO) require symptoms to have persisted greater than 12 weeks after the initial infection (5). The American Academy of Physical Medicine and Rehabilitation (AAPM&R) estimates that there are more than 29 million people suffering from long COVID in the US as of December 2022 (6). The overall global prevalence of long COVID is estimated at 43% of the acute cases (hospitalized 54% and non-hospitalized 36%) (7). Recent data from the CDC estimate that 7.5% (over 24 million people) of the adult population has long COVID symptoms. In a meta-analysis which found that fatigue/weakness, myalgia/arthralgia, depression, anxiety, memory loss, concentration difficulties, dyspnea, and insomnia, were the most prevalent symptoms (8, 9) (Figure 1), and that the prevalence of long COVID is three times higher among 50-59 year-olds than over-80-year-olds (10). Even if the prevalence of debilitating symptoms is low, the overall health burden is large given the scale of the pandemic.
While the clinical features of long COVID and epidemiologic risk factors have been described over the last two years, the underlying biological mechanisms remain elusive. Several hypotheses have been proposed. These include persistence of SARS-CoV-2 reservoirs, presence of microthrombi, induction of autoantibodies, hyperreactive immune activation, reactivation of other latent viral coinfections such as Epstein-Barr Virus (EBV), mitochondrial dysfunction, and gut dysfunction/dysbiosis (11). Several of these processes may lead to sustained chronic inflammation driving end-organ disease. Importantly, different clinical phenotypes of long COVID may be driven by different mechanisms, and more than one mechanism may contribute to a particular patient’s condition.
Current state of long COVID management and potential therapeutic approaches
Currently management of long COVID is focused exclusively on symptomatic treatment, and there is no single acceptable approach. While symptomatic management may benefit patients in the short-term, we believe there is an urgent need to identify treatments that target one or more of the aforementioned pathologic mechanisms (12) in order to better understand the biology, alleviate symptoms, reduce morbidity, and return individuals toward their pre-COVID health. In addition, it is important to identify to Identify biomarkers that can help facilitate development and implementation of therapeutic interventions and monitor the effects of an intervention.
The current state of evidence for various interventions is outlined below. We acknowledge that much of this evidence is derived from small and uncontrolled investigations, thus limiting the ability to evaluate changes that might have occurred over time in the absence of an intervention. While we do not endorse any specific strategy, our goal is to summarize the current state of the science as a call for urgent and efficient research to help the millions who continue to suffer from long COVID around the world.
Prevention of long COVID
As of October 2022, two COVID-19 vaccines using novel mRNA technology have been approved by the U.S. Food and Drug Administration (FDA): BNT162b2 (Pfizer/BioNTech), and mRNA-1273 (Moderna) and two have been authorized for emergency use including an adenovirus vector vaccine (Janssen), and a protein subunit vaccine (Novavax) (13). These vaccines are safe and effective at protecting people from severe disease, including hospitalizations and deaths (14–16).
There is mounting evidence suggesting a protective effect of vaccination on the incidence of long COVID. Using the TriNetX Research Network platform including 1,578,719 individuals in the USA with confirmed COVID-19, researchers showed that COVID-19 vaccination prevented the occurrence of PASC (17). At 90 days following COVID-19 diagnosis, the incidence of long COVID was lower in the vaccinated cohort than in a propensity score-matched unvaccinated cohort with relative risks of hypertension 0.33 (95% confidence interval [CI], 0.26-0.42), diabetes 0.28 (95% CI, 0.20-0.38), heart disease 0.35 (95% CI, 0.29-0.44), and death 0.21 (95% CI, 0.16-0.27). However, it is clear that SARS-CoV-2 vaccination does not completely eliminate the incidence of long COVID, as a significant proportion of breakthrough infections remain associated with this condition (18).
Another major question is whether treatment during the acute phase of infection with COVID-19 reduces the risk of PASC. Data not yet peer-reviewed from the Veterans Affairs database shows that nirmatrelvir-ritonavir use during the acute phase is associated with a 26% reduction in the risk of developing PASC (19), suggesting that early antiviral treatment might be of benefit. Notably, the data on remdesivir have been less consistent (20, 21). While the focus of this article is on the management of established long COVID, determining whether treatment during the acute phase of infection impacts the risk of developing long COVID will be important in ongoing efforts
Persistence of viral genetic material and/or antigen
The rationale for the use of antivirals for long COVID treatment is based on evidence suggesting that SARS-CoV-2 may reside in select anatomical reservoirs beyond the initial acute phase, and as such contribute to ongoing inflammation and organ injury. Several studies identified persistence of SARS-CoV-2 genetic material during the post-acute phase. One study described the persistence of SARS-CoV-2 viral RNA shedding for up to 150 days in the upper respiratory tract despite antiviral therapies, with amino acid changes predominantly in the spike gene and the receptor-binding domain leading to viral evolution (22). Another study of 29 patients with long COVID who reported fatigue, muscle pain, dyspnea, inappropriate tachycardia, and low-grade fever found that 13 (45%) had positive plasma RT-PCR results and 51% were positive in at least one RT-PCR sample (plasma, urine, or stool) (23). Another showed that SARS-CoV-2 can be detected in the gut and in the stool for 126 days (24, 25). Other cohorts have identified SARS-CoV-2 proteins during the post-acute phase. For example, a study of individuals with long COVID neuropsychiatric symptoms found both the S1 component of spike and nucleocapsid in neuronal- and astrocytic-derived exosomes (26). In two different cohorts of long COVID patients, SARS-CoV-2 antigenemia was detected in approximately 65% up to 12 months after diagnosis (27, 28). SARS-CoV-2 persistence has also been demonstrated by the presence of genomic RNA, subgenomic RNA, and viral proteins in specific anatomical sites (29, 30) supporting the presence of viral reservoirs in extrapulmonary compartments (24, 30, 31). For example, in one study of autopsy specimens, investigators detected SARS-CoV-2 RNA in multiple anatomical sites including the brain, muscle, gut, and lungs in persons initially diagnosed up to 230 days prior to death (30, 32). These sites were associated with increased inflammatory changes and cytopathological effects. Prolonged gastrointestinal viral shedding for up to seven months, in immunocompetent hosts, and the persistence of SARS-CoV-2 proteins in small and large intestinal mucosal cells correlated with long COVID (24, 25). Although ongoing active viral replication has yet to be confirmed, the growing evidence of persistent antigen and genetic material for months after infection identified in multiple cohorts using multiple mechanisms (27, 28), suggests that the framework that SARS-CoV-2 is a time-limited infection might not be entirely correct.
Although the biological importance of these observations is not yet clear, it remains possible that viral persistence may be an important factor in at least a subset of long COVID cases. An important related question is whether lingering virus directly drives the illness in people experiencing long COVID, or if it induces a dysregulated immune system characterized by heightened release of proinflammatory cytokines that lead to chronic low-grade inflammation and multiorgan symptomatology. This latter mechanism resembles our current understanding of the chronic heightened inflammation seen with chronic HIV infection, which drives several HIV-associated comorbidities (33). For example, people with HIV, even those on suppressive antiretroviral medications, experience ongoing immune activation driven in part by viral persistence in immune privileged sites such as the lymph node (34–36). Over years to decades, this chronic state of immune activation is associated with the acceleration of atherosclerosis, a higher incidence of cardiovascular and pulmonary events, and mild neurocognitive impairment (37, 38). These immunologic complications remain a challenge even in the era of widely available antiretroviral therapy. Similarly, cytomegalovirus infection has broad immunologic effects, including the promotion of immunosenescence and induction of a chronic proinflammatory state (39), and has been associated with cardiovascular disease (40). Other viruses previously thought to be limited to acute illness, including Ebola virus, have been shown to persist in immune privileged sites and cause chronic infection and inflammation (41), providing a framework for persistence that could be evaluated in SARS-CoV-2 infection
While data on the use of antivirals in established long COVID are limited, they support the observation that viral persistence may contribute to some cases of long COVID. For example, there are anecdotal reports of patients whose long COVID symptoms improved following nirmatrelvir-ritonavir administration (42, 43), highlighting the need to study this antiviral agent in controlled and well-designed clinical trials. Two such studies are now underway, evaluating a 15-day course of nirmatrelvir-ritonavir versus placebo in reducing symptom severity in participants with long COVID (44, 45). We believe that studies of antivirals are warranted, and that the sum of the biomarker data, data from treatment during the acute period, and case reports warrant further study of this drug class. This might include currently available antivirals, such as molnupiravir or remdesivir (46, 47), but other agents that have recently been approved in other settings such as ensitrelvir (48) or are under development, e.g. GS441525 (49), could also be considered. Ultimately, the route of administration, side effect profile, and drug-drug interactions will decide which antiviral, if efficacious, is optimal. Monoclonal antibodies may also be worthy of study; however no such agents are currently authorized for treatment of COVID-19 in the United States, due to decreased efficacy in vitro against emerging circulating variants (50).
Therapeutic vaccination for established long COVID
In addition to preventing long COVID, evidence suggests some individuals with established long COVID may benefit from SARS-CoV-2 vaccination. In a meta-analysis of 12 studies evaluating the effects of vaccination on pre-existing long COVID symptoms in 32,726 individuals of whom 8,667 had preexisting signs and symptoms of long COVID. Most of the studies reported improvement in symptoms after one dose, although some reported no benefit or even worsening symptoms (51). Similar observations were noted in other cohorts (52, 53).
In a UK cohort survey of 28,356 participants who previously tested positive for SARS-CoV-2 infection and then received at least one dose of a COVID-19 vaccine, 6629 (23.4%) participants reported long COVID symptoms (presence of symptoms at least 12 weeks after infection) of any severity during follow-up; the first vaccine dose was associated with an initial 12.8% decrease (95% CI −18.6% to −6.6%, P<0.001) in the odds of long COVID, with subsequent data compatible with both increases and decreases in the trajectory (0.3% per week, 95% CI −0.6% to 1.2% per week, P=0.51). A second dose was associated with an initial 8.8% decrease (95% CI −14.1% to −3.1%, P=0.003) in the odds of long COVID, with a subsequent decrease by 0.8% per week (−1.2% to −0.4% per week, P<0.001). While this evidence suggests sustained improvement, the median duration was only 67 days following the second dose suggesting longer follow-up (54).
Postulated hypotheses of the efficacy of the COVID vaccine in established long COVID include the potential correction of dysregulated immune or inflammatory responses or the possible elimination of persisting viruses or viral remnants of SARS-CoV-2, as outlined above (55). Prospective and adequately powered randomized trials are necessary to clarify whether therapeutic immunization will benefit patients with long COVID, and if so, through what mechanism.
Dysregulation of immune system
Acute SARS-CoV-2 infection is characterized by substantial immune dysregulation, particularly among severe cases, and anti-inflammatory therapy is of benefit in individuals who meet criteria for severe disease (56, 57). Multiple studies have demonstrated that SARS-CoV-2 can result in immunologic perturbations that extend beyond the acute phase of infection (58). Early studies of convalescent plasma donors showed persistence of immune activation in people with prior COVID-19 compared with historical donors (59), and early studies of long COVID showed that elevations in certain biomarkers (e.g., IL-6, TNF-α) during early COVID-19 recovery (1-2 months) were associated with the presence of long COVID symptoms at 4 months (60, 61). Additional studies have shown that individuals with long COVID had highly activated innate immune cells, lack naive T and B cells, and showed elevated expression of type-I IFN (IFN-β) and type-III IFN (IFN-λ1) that remained persistently high at 8 months after infection. A classifier based on cytokine profile that measures IFN-β, PTX3, IFN-γ, IFN-λ2/3 and IL-6 predicted long COVID with 78.5–81.6% accuracy (62). Elevations in levels of IL-6, TNF-alpha, and IL-1B have been consistently reported across multiple studies (63–65), suggesting that these might serve as biomarkers for ongoing disease activity in people with long COVID and that the study of agents that could reduce the levels of these cytokines is warranted.
Data in murine models demonstrated that mice, given coronavirus nasally to mimic a mild infection, develop inflammation in the brain, as well as loss of myelin (66). This model could represent a model of immune dysregulation due to SARS-CoV-2 infection and may allow testing of behavioral and pharmacologic interventions. Studies evaluating anti-inflammatory and immunomodulatory drugs to treat acute SARS-Cov-2 infection have shown benefit, however data is limited for in long COVID (67).
The glucocorticoid receptor acts as a transcription regulatory factor and represses the expression of inflammatory cytokines, chemokines, and prostaglandins, suppresses the antigen-stimulated inflammation mediated by macrophages, dendritic cells, and epithelial cells, and impairs cytotoxic immune responses by downregulating interferon-γ production and inhibiting the development of type-1 helper T cells, CD8+ T cells, and natural killer cells. Thus, glucocorticoids regulate the immune balance between antigen response and inflammation in steady-state and stress conditions (68). The RECOVERY Collaborative Group demonstrated that in patients hospitalized with COVID-19, the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone (69). In a case-control observational study, patients who received oral dexamethasone for hospitalized COVID-19 were also less likely to experience persistent symptoms at 8-month follow-up (70). In a small subset of patients with persistent interstitial lung disease post SARS-CoV-2 infection, treatment with prednisolone was associated with improvement of symptoms, radiological abnormalities, and measures on lung spirometry (71). In a study of 24 patients with abnormal chest CT scans as well as resting hypoxia or exertional desaturation, treatment with deflazacort was associated with decline in breathlessness, tachypnea and hypoxia at rest in patients treated in this uncontrolled case-series (72). Decreased levels of cortisol and cortisone have been observed to be associated with PASC (73). Further data also suggest decreased levels of cortisol among individuals with long COVID (74), but this finding remains to be replicated in other cohorts. Long term use of glucocorticoids results in adverse effects on muscle and bone, increase the risk of osteoporosis and sarcopenia, two severe complications of long COVID (75). These therapies should be investigated in randomized controlled studies, especially given the complicated risk-benefit calculation with long-term use.
There are many other anti-inflammatory interventions that will likely be pursued in future monotherapy and combinatory studies, and these anti-inflammatory agents could target general inflammatory pathways or more specific pathways thought to be important in acute SARS-CoV-2 infection, such as JAK inhibitors, IL-6- or TNF- blockers (57, 76). It is important to note that while targeting the IL-6 pathway has shown clinical benefit in acute COVID-19, and this pathway remains aberrant in long COVID, evidence in favor of biological immunomodulatory therapies such as tocilizumab is limited to a case report (77). As for all immunomodulatory agents, the ease of administration and toxicity profile should be carefully considered in the design of future clinical trials. A full review of the scope of immune system dysregulation and all possible targeting immunomodulatory therapies is beyond the scope of this review.
We believe that such studies testing anti-inflammatory and immunomodulatory therapies are justified based on the current understanding of long COVID pathogenesis. In the meantime, already-available agents with potential anti-inflammatory properties are being repurposed for long COVID. In open-label single-site studies, cohorts treated with low dose naltrexone and low dose aripiprazole have demonstrated clinical benefit among patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) (77–79); these medications have subsequently been used off-label for the treatment of long COVID. These agents may represent potential therapies for long COVID due to their anti-inflammatory and immunomodulatory effects, along with a possible impact on amyloid deposition and thrombosis. They are relatively safe, orally active, and of low cost, and therefore, good candidates to be tested as part of therapeutic trials for long COVID.
Low-dose Naltrexone (LDN) is commonly used off-label for people with ME/CFS, a condition characterized by debilitating fatigue and exertional intolerance that has significant clinical overlap with long COVID (78). From a mechanistic perspective, one model of LDN’s efficacy is through suppressive effects on microglia cells of the central nervous system and attenuation of proinflammatory cytokines (80). Microglia are resident macrophages in the brain and spinal cord and normally exist in a resting state but once activated produce proinflammatory factors that interact with neurons to cause hyperalgesia and “sickness response symptoms” such as fatigue, malaise, hypoactivity, sleep changes, etc., which have been demonstrated in animal models (80, 81); there is intense interest in the role that these and other macrophage-derived cells might play in long COVID, particularly in neurocognitive symptoms (82). Additionally, data suggest that the benefits of LDN treatment in ME/CFS are mediated by its regulatory role involving the Transient Receptor Potential Channel Melastatin 3 (83). Other known effects of naltrexone are on Toll-like receptor 4 with downstream effects of re-establishing natural killer cell activation and reducing IL-6, TNF-α and interferon-β levels, pathways that have also been implicated in long COVID (84, 85). Another related mechanistic pathway hypothesized is the possibility of LDN to potentially prevent and treat the immunothrombosis of SARS-CoV-2 and studies are underway to address this question in acute COVID-19 (86). A recently published report evaluated the use of LDN in 38 patients with long COVID, with no control group. In the 36 patients that completed two months of treatment, six of seven evaluated parameters improved over time, with the largest improvements in reduction in pain (79). However, since long COVID symptoms often improve over time, this single-arm study design is insufficient to recommend LDN without further data. A larger study of LDN in 160 participants in a randomized parallel group double-blinded placebo-controlled trial is underway (87).
Aripiprazole is a second-generation antipsychotic agent that was FDA approved in 2002 for use in adult patients with schizophrenia. It has a high affinity for dopamine D2 and D3 receptors as well as for serotonin receptors (88, 89). Besides antipsychotic and antidepressant activities, other pleiotropic properties have been attributed to aripiprazole such as anti-inflammatory actions by decreasing inflammatory and promoting anti-inflammatory cytokines, immunomodulatory effects by a reduction in microglial cell activation and modulation of genes that regulate the immune system, and possible effects on genes implicated in Alzheimer’s disease (90–95). In a retrospective study of 101 patients with ME/CFS, 75 (74%) patients taking low-dose aripiprazole experienced an improvement in one or more symptom categories: fatigue, brain fog, unrefreshing sleep, and frequency of post-exertional malaise (PEM) episodes, or “crashes” (77). These symptoms are frequently reported in patients with long COVID (8, 96, 97), and so further study to assess safety, tolerability, and efficacy in individuals experiencing long COVID are warranted.
Non-pharmacologic approaches are also being pursued for long COVID-associated inflammation. Acupuncture is known to reduce inflammation related to disease and pain, reducing both inflammation and pro-inflammatory cytokines in severe human illnesses such as cancer, multiple sclerosis, and dementia, as well as in allergic respiratory conditions like allergic rhinitis (98). In a preclinical study on the acute phase of burns in rats, acupuncture modulated pain and the inflammatory/proinflammatory cytokine response via the downregulation TLR4 signaling pathway (99, 100). Acupuncture may reduce inflammation and provide immune protection via heme catabolism and upregulation of heme oxygenase-1 (HO-1) gene expression (101, 102). The HO-1-mediated heme breakdown products (biliverdin, bilirubin and carbon monoxide) exhibit both anti-oxidative and anti-inflammatory effects (103). Acupuncture has been recommended as part of a multidisciplinary team approach for long COVID clinics in some countries, such as the UK, but not in the US (98, 99). RCTs should be conducted prior to routinely recommending this intervention.
Microthrombi and hypercoagulability
Some investigators have reported the formation of microthrombi, with an increase in α (2)-antiplasmin (α2AP), various fibrinogen chains, as well as Serum Amyloid A, that were trapped in the microcirculation (104), among people experiencing long COVID. Blockage of the microcirculation leads to decreased blood flow, oxygenation, and impairs nutrition delivery and removal of cellular waste (104). In a study of 845 South African people with long COVID, 70 were identified as having laboratory markers consistent with microthrombi (105). A subset of patients (n=24) were treated with one month of dual antiplatelet therapy (clopidogrel 75mg/aspirin 75mg) once a day, as well as a direct oral anticoagulant (apixiban 5 mg) twice a day. The participants in this small case series (not yet peer-reviewed) reported relief of symptoms, primarily fatigue, and biomarker measurements improved including fibrin amyloid microclots and platelet pathology scores (106). These biomarkers need further clinical validation, and given significant potential for harm, anticoagulation or antiplatelet therapies need to be studied in a RCT. The MICHELLE trial (an open-label, multicenter, randomized, controlled trial) showed the use of rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalization for COVID-19, the thromboprophylaxis with rivaroxaban for 35 days an improvement in clinical outcomes (107). If pursued, an ideal clinical trial would restrict this therapy to individuals who have biomarkers consistent with platelet and/or clotting dysregulation and strict medical oversight would be necessary during treatment to mitigate adverse effects, particularly bleeding and gastric inflammation (108).
Dysbiosis
Changes in the bacterial, fungal, and viral gut microbiome have been reported as a consequence of SARS-CoV-19 infection (109). Patients with prolonged symptoms have demonstrated alterations in the composition of the microbiome with higher levels of Ruminococcus and Bacteroides and lower levels of Faecalibacterium (110). The abundance of genera such as Prevotella and Veillonella are associated with increased inflammation (111). Recently, in a prospective study comparing plant-based fiber or fermented foods in healthy adults, participants consuming a high fermented foods diet had enhanced microbial diversity and a decrease in selected cytokines, chemokines, and other inflammatory serum proteins including IL-6, IL-10, and IL-12b and other inflammatory factors (112). IL-10 is usually considered to be an anti-inflammatory cytokine. However, in some circumstances, IL-10 might be a pro-inflammatory cytokine (113). These results suggest that fermented foods may be powerful modulators of the human microbiome and immune system axis and may provide an alternative to treating post-SARS-CoV-19 associated symptoms. If rigorously studied and demonstrated to be of benefit, dietary interventions could potential be an innovative management approach given the relative ease of implementation. In contrast to the pharmaceutical trial, the dietary clinical trial’s design objective is to measure the impact on health and disease. Some designs include feeding studies, randomized clinical trials, and observational studies. However, nutrition research presents multiple challenges due to the complexity of food environments and the multiple approaches to data collection and analysis, which can influence by the self-reported data, the human nature of the participants, lifestyle, and habits (114, 115).
Reactivation of other latent viral infections
The vast majority of adults (>90%) harbor infection with latent EBV, a ubiquitous human herpesvirus associated with several post-viral conditions (116). EBV is associated with autoimmune conditions (116), and recent studies have demonstrated links to multiple sclerosis (MS), with a close relationship between EBV seroconversion and development of multiple sclerosis (117), and molecular mimicry between anti-EBNA1 antibodies and anti-GlialCAM antibodies prevalent in MS (118). EBV reactivations may also contribute to immune dysregulation.
One early study of long COVID made the observation that two-thirds of individuals experiencing long COVID symptoms demonstrated EBV early antigen-diffuse (EA-D) IgG positivity, suggesting reactivation around the time of SARS-CoV-2 infection (119). More recent efforts demonstrated that the presence of EBV DNA during the acute phase of COVID-19 predicted the presence of symptoms 30-60 days later (73). Another recent larger study of primarily outpatients found that a higher proportion of participants who experienced long COVID had evidence of high-level EBNA IgG levels (47% versus 28%; P<0.05); participants with detectable EBV EA-D IgG responses had a 2.12-fold higher likelihood of post-COVID fatigue, and participants with high levels of EBV nuclear antigen (EBNA) IgG levels (>600 U/mL) had a 2.5-fold higher likelihood of long COVID neurocognitive symptoms (120). These findings are consistent with other post-COVID cohorts (119), and suggest that further investigation of the relationship between EBV-related pathology and long COVID is warranted.
EBV reactivation has also been proposed as a driver of ME/CFS (121–124). One single-arm, uncontrolled case series showed that 9/12 (75%) persons with ME/CFS after treatment with valganciclovir experienced symptomatic improvement allowing return to usual activities; this was sustained off treatment (125). Another retrospective study showed that 52% of patients receiving daily valganciclovir experienced >30% improvement in physical and/or cognitive function (126). In uncontrolled studies such as these, we cannot determine what percent would have improved without treatment. A follow-up randomized study showed improvement in fatigue and cognitive function; those in the intervention group were 7.4 times more likely to respond in this small study (126).
As a result of these observations, there is now growing interest in anti-EBV therapies to modulate disease morbidity and improve symptoms in long COVID. Therapies currently being considered include antivirals like valganciclovir, a guanosine analog with potent anti-human herpesvirus activity, including in vitro and in vivo activity against the lytic phase of EBV that can reduce mucosal shedding of EBV during treatment (127). Other therapies under consideration are considerably more complex, including anti-B cell therapeutics (e.g., rituxmimab) or CAR-T cells. While few clinical trials addressing these potential targets are active as of the time of this writing (128, 129), Bateman Horne Center is running a Phase 2a, open-label trial of Virios Therapeutics’ IMC-2, a combination of valacyclovir and the nonsteroidal anti-inflammatory drug celecoxib, for long COVID symptoms potentially driven by herpesvirus reactivation (130).
Interestingly, despite the associations between latent CMV infection and chronic immune activation, one recent study found that CMV-seropositive individuals had a lower odds of certain types of PASC symptoms, particularly neurocognitive symptoms (131). This pattern was the opposite of what was seen with evidence of recent EBV reactivation. The reason for this observation is unclear, but the authors proposed that it could be related to different immunologic compartmentalization of each of the latent viruses. For example, they suggested that CMV-associated immunoregulatory pathways could decrease inflammation and modulate other potential pathophysiologic mechanisms (e.g., autoantibody formation) (132). In addition, they speculated that CMV seropositivity could be associated with heightened adaptive immune responses as it is with regard to influenza vaccination (133). More work will be needed to determine what role, if any, CMV serostatus plays in the pathogenesis of PASC.
Fibrosis
Residual pulmonary disease is a well described complication of viral pneumonitis which can lead to fibrosis. Myall and colleagues reported that 39% of 837 patients still had respiratory symptoms 4 weeks post discharge following SARS-CoV-2 infection (71). Dyspnea can be secondary to multiple etiologies including alveolar damage with resultant airway fibrosis, pulmonary thrombosis and bronchiolitis (134). Nintendanib and pirfenidone are approved for the treatment of idiopathic pulmonary fibrosis and have both been proposed as treatment for fibrotic lung disease secondary to COVID-19 pneumonia (135). A phase 2 placebo-controlled trial of pirfenidone for post COVID-19 pulmonary fibrosis is ongoing (136). The PINCER trial compares nintendanib with pirfenindone in persons with post COVID-19 pulmonary fibrosis (137). Given that bronchiolitis with air trapping is commonly seen in long COVID (134, 138), clinical trials of an anti-fibrotic targeted to treat constrictive bronchiolitis to prevent fibrosis development should be considered in persons with persistent dyspnea and cough without radiographic evidence of fibrosis. Enrollment of 168 participants in a placebo-controlled trial evaluating deupirfenidone (LYT-100) for treatment of post acute COVID-19 respiratory complications was completed July 2022 with results expected early 2023 (139).
Sleep disturbances and post exertional malaise/fatigue
Long COVID symptoms may include anxiety, depression, brain fog, and sleep disturbances (140, 141). While sleep disturbances are common and more recently have been attributed to societal restrictions during the COVID-19 pandemic, a systematic review and meta-analysis revealed that over 50% of people who had been infected with SARS-CoV-2 suffered with sleep disturbances (142). One small study demonstrated significant sleep impairment four months after having COVID-19 with an increased prevalence among those with obstructive sleep apnea (143). Chronotherapy is an emerging therapeutic in pulmonary and sleep medicine as we understand how the disruption of circadian rhythm affects cells of virally infected organs such as the lung and the immune system (144). Therapies directed at improving sleep and recalibrating circadian rhythm by light therapy, cognitive behavioral therapy for insomnia and melatonin are currently being studied for the sleep disturbances due to the pandemic but have not been initiated yet for long COVID (145).
Post exertional malaise (PEM), also called “crash,” has been clinically defined as an increase in fatigue, pain, cognitive dysfunction, and flu-like illness after physical exertion, mental activities, or stressful events (146, 147). A recent meta-analysis found that PEM is 10.4 times more likely to be associated with ME/CFS (148). Therefore, PEM has been considered the hallmark for diagnosing ME/CFS (146–148). Some patients with long COVID experience similar clinical PEM symptoms. In a pre-print manuscript, investigators described a cohort of 105 patients from the Stanford long COVID clinic with symptoms greater than six months; they found that fatigue, PEM, and brain fog were the predominant and severe symptoms of which 43% were diagnosed with ME/CFS (149). PEM can be mitigated by energy conservation activities known as ‘pacing’ (resting, decreasing stress or overstimulation) (150). A supervised activity program may involve brief walking (~5 minutes), stretching/limb and spine active range of motion, participation in personal or instrumental activities of living, or reading for 5-10 minutes. The duration of these activities is then slowly increased. This method may allow careful introduction of aerobic exercise to prevent deconditioning (cycling, tai chi, yoga or aquatic exercises). For all patients, the goal of this individualized activity program is to regain functional independence, resume participation in life roles, and restore of quality of life (151).
Mitochondrial health
At least one study has identified changes in mitochondrial health to be associated with long COVID (26). AXA1125 is a mixture of six amino acids that can increase fatty acid oxidation, ATP production, ketogenesis, and mitochondrial bioenergetics leading to improved muscle function. Promising Phase 2a results were announced by the company Axcella Health in the third quarter of 2022 for its trial of AXA1125 which targeted fatigue predominant long COVID (152). This small randomized, double-blind, placebo-controlled trial showed significant improvements in fatigue, as measured by the Chalder Fatigue Questionnaire eleven-item scale and in the six-minute walk test endpoint (153). However, the study did not show a significant improvement of mitochondrial function by magnetic resonance spectroscopy. A phase III study is planned and under development. A recently conducted RCT of co-enzyme Q for long COVID did not show an effect (154).
Discussion
Long COVID is now recognized as a major clinical challenge limiting the return to baseline health of a substantial number of people following SARS-CoV-2 infection. While clinical trials were rapidly implemented and conducted to determine the optimal management of acute COVID-19, therapeutic studies for Long COVID have lagged behind. This has occurred for a variety of reasons, including delays in the medical community’s recognition of the clinical significance of the condition as well as the complex and likely multifactorial nature of its pathophysiology, which is still being elucidated. We are grateful that the NIH has made a substantial investment in long COVID and are hopeful that other funders, including industry and philanthropic organizations, will follow suit. It is likely that multiple concurrent and coordinated efforts will be needed to accelerate progress in further defining and understanding this condition.
Although major efforts are now underway to understand the biology of long COVID, the current evidence is limited. At the same time, patients, their clinicians, and their advocates are eager to identify treatments that can provide symptomatic relief and perhaps even reverse the underlying pathophysiology of this condition. The desire to treat and alleviate suffering versus the need to rigorously test such interventions represents a fundamental tension for the field. While we can learn from treatment anecdotes, the case reports, series, and small, uncontrolled studies conducted and reported to date must be interpreted with great caution, as their design makes it impossible to discern whether an intervention was responsible for the clinical changes observed in patients or participants. This is further complicated by the fact that the natural history of long COVID is characterized by within-individual variability in symptoms (which can wax and wane) and by the fact that individuals experiencing long COVID may improve over time even in the absence of an intervention.
We recognize that many individuals experiencing this condition are eager to return to their baseline state of health. Such individuals may choose to work with their care providers to devise a treatment plan that makes use of unapproved or off-label therapies after careful consideration of and counseling regarding the potential risks and benefits. However, it is our position that it is not currently possible to endorse any particular treatment approach aside from seeking clinical care where indicated. Rather, it is our goal to emphasize the urgency for the implementation of well-designed clinical trials that would serve two purposes: to further define the pathophysiology of long COVID and to determine the efficacy and safety of candidate treatments.
There are many important considerations for long COVID clinical trials. Beyond the intervention to be evaluated, those designing such studies must consider whether the study should be focused on one or more long COVID phenotype or enroll more broadly, what phase after SARS-CoV-2 infection is the target for intervention (acute, early post-acute, post-acute), the optimal design and if relevant, randomization scheme, and the optimal outcomes, which in most cases are likely to involve a combination of patient-reported outcomes and biological measurements. While our group has diverse opinions on which types of studies are optimal and which specific agents should be prioritized, we believe that a variety of designs could have a role. For example, a small, proof-of-concept study of an intensive therapy might focus on biological measurements in a few dozen individuals to further define the role of a mechanistic pathway, while a large, randomized trial might seek to determine whether a scalable intervention has an effect on symptom outcomes in a cohort of hundreds or thousands of volunteers. Both approaches, and all those in between, potentially have merit. We believe there is great urgency for these studies to be performed and are encouraged by the recent or planned launch in the near future of several clinical trials, including those through the RECOVER initiative. However, given the complexity of the condition, we believe that ultimately there will need to be ongoing, concerted efforts between people experiencing long COVID, regulators, academic and industry researchers, and funders (including funders outside RECOVER) to get a greater variety of studies into the clinic in order to obtain the answers that patients and clinicians are seeking in a reasonable timeline.
While we sought to be comprehensive, this report has several notable limitations. First, there are numerous mechanisms of long COVID and it is beyond the scope of this review to exhaustively review all hypothesized mechanisms in this article. Second, it is possible that many of the mechanisms we discussed are inter-related and thus a single intervention may in the end address several mechanistic pathways. Third, we do not discuss pathophysiology or interventions in certain subpopulations of interest, including children and pregnant people. While there may be overlap with presentations of long COVID in adults, appropriate management of long COVID in children, including MIS-C, requires consideration of pathophysiology, biology, pharmacology, neurocognitive and behavioral development unique to children (155), which is beyond the scope of this article. Given the paucity of studies evaluating interventions to help children and adolescents with long COVID, clinical trials that leverage approved interventions that pose the sufficient prospect of benefit to justify any associated risks such as antivirals, vaccines, and monoclonal antibodies as well as physical rehabilitation and mental health regimens should be prioritized. Similarly, unique pathophysiology may contribute to long COVID in pregnant people and there are unique risk and benefit considerations during the intra- and post-partum periods. Fourth, we do not consider the costs or complexity of the discussed interventions, although in general we focused on treatments that we believe to be generally practical. Fifth, we do not provide a direct discussion of how these interventions should be prioritized in relation to one another, as that is beyond the scope of our taskforce’s charge. Finally, this is a fast-moving field with new observations and data released on an almost daily basis; such data should be considered in addition to the content of this article for those pursuing clinical trials.
Involving patients, caregivers, and community representatives in intervention research and decision making is crucial. In the instance of Long COVID we are also faced with a novel condition where treatments are still being determined. Patients and caregivers offer the unique and important perspective of living daily with the challenges of Long COVID and can therefore provide key insight into essential topics such as the most common and bothersome symptoms, or which treatment outcomes could offer the best opportunity for improved quality of life. By including patients, caregivers, and community representatives in as many aspects of the process as possible we are creating a collaborative environment which increases the likelihood of better outcomes, strengthens patient trust and acceptance, and broadens our overall understanding of the condition and best intervention approaches.
Enrolling a diverse population of patients can ensure adequate representation and improve access to care for those individuals who are disproportionately impacted by COVID-19 and may be faced with limited availability of vaccines and treatments. This ensures a broader scope of the issue and can even help to uncover hidden disparities or barriers to care which can further guide the most impactful and needed interventions for patients. Engaging diverse populations in research and interventions also expands access to education that can inform both the patients and providers, creating mutually beneficial learning opportunities. Inclusivity also builds trust within the patient and caregiver community, while fostering a sense of belonging that can deepen patient satisfaction and lead to better health outcomes.
Conclusion
As the COVID-19 pandemic continues, substantial numbers of individuals will continue to experience long COVID. Given the vast number of individuals infected by this virus, the magnitude of this health crisis is easily appreciated. Consequently, it is critical that research extend beyond the many important and informative epidemiologic studies describing the extent of the problem to include trials of interventions to prevent, treat or ameliorate the varied symptoms and diverse manifestations associated with long COVID. Although the pathophysiologic basis of long COVID remains incompletely understood and the clinical spectrum of the condition is quite diverse, evidenced-based theories have been proposed, providing potential plausible targets for intervention. This paper describes the early work assessing several potential therapies aimed at preventing and treating long COVID and its various manifestations. It is unknown which, if any of these potential therapies will have an impact alone or in combination with other interventions on the course of long COVID. Moreover, different clinical phenotypes of long COVID may be driven by different mechanisms, and effective therapies may vary from one patient to the next. However, it is established that such therapies are needed and more work in this area is necessary. The current data are limited by the fact that most studies to date are small and uncontrolled, and therefore limited in their ability to evaluate changes over time that might have occurred without intervention. This paper highlights the critical need for timely and efficient research strategies to help the millions suffering from long COVID around the world.
Author contributions
Long COVID is now recognized as a major contributor to post-COVID morbidity and delayed return to health. This article represents the current state of understanding regarding long COVID pathogenesis and therapeutics, with a view toward interventions that the authors believe warrant further evaluation in well-designed clinical trials. It provides an overview of the current state of evidence and proposes a pathway forward for efforts to address this new set of conditions which are anticipated to continue to impact the lives of many individuals recovering from SARS-CoV-2 infection and are already putting a major strain on healthcare systems. All authors contributed to the article and approved the submitted version.
Funding
This study is part of the NIH Researching COVID to Enhance Recover (RECOVER) Initiative, which seeks to understand, treat, and prevent the post-acute sequelae of SARS-CoV-2 infection (PASC). This research was funded by the National Institutes of Health (NIH) Agreement OTA OT2HL161847 as part of the RECOVER research program.
Acknowledgments
We would like to thank the National Community Engagement Group (NCEG), all patients, caregiver and community Representatives, and all the participants enrolled in the RECOVER Initiative. We also acknowledge the RECOVER project for bringing all of these authors together.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AAPM&R, The American Academy of Physical Medicine and Rehabilitation; CDC, U.S. Centers for Disease Control and Prevention; CI, Confidence Interval; EBV, Epstein-Barr Virus; FDA, U.S. Food and Drug Administration; LDN, Low Dose Naltrexone; ME/CFS, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; MIS-C, Multisystem Inflammatory Syndrome in Children; NICE, U.K. National Institute for Health and Care Excellence; PASC, Post-Acute Sequelae of SARS-CoV-2 infection; PEM, Post Exertional Malaise; RECOVER, Researching COVID to Enhance Recover; WHO, World Health Organization; long COVID, PASC.
References
1. Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - united states, march-June 2020. MMWR Morb Mortal Wkly Rep (2020) 69(30):993–8. doi: 10.15585/mmwr.mm6930e1
2. Malkova A, Kudryavtsev I, Starshinova A, Kudlay D, Zinchenko Y, Glushkova A, et al. Post COVID-19 syndrome in patients with Asymptomatic/Mild form. Pathogens (2021) 10(11). doi: 10.3390/pathogens10111408
3. CDC. National center for immunization and respiratory diseases (NCIRD) DoVD. long COVID or post-COVID conditions (2022). Available at: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/.
4. National Institute for Health and Care Excellence (NICE) S, of IGNSaRC, (RCGP) GP. COVID-19 rapid guideline: Managing the long-term effects of COVID-19 (2022). Available at: https://www.nice.org.uk/guidance/ng188.
5. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. Condition WHOCCDWGoP-c-. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis (2022) 22(4):e102–e7. doi: 10.1016/S1473-3099(21)00703-9
6. (AAPM&R) TAAoPMaR. Post-acute sequelae of SARS-CoV-2 infection (PASC) estimates and insights. dashboard (2022). Available at: https://pascdashboard.aapmr.org/.
7. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post COVID-19 condition or long COVID: A meta-analysis and systematic review. J Infect Dis (2022) 226. doi: 10.1093/infdis/jiac136
8. Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: A systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens (2022) 11(2). doi: 10.3390/pathogens11020269
9. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. J Infect Dis (2022) 226(9):1593–607. doi: 10.1093/infdis/jiac136
10. CDC. National center for health statistics. nearly one in five American adults who have had COVID-19 still have “Long COVID” (2022). Available at: https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/20220622.htm.
11. Peluso MJ, Deeks SG. Early clues regarding the pathogenesis of long-COVID. Trends Immunol (2022) 43(4):268–70. doi: 10.1016/j.it.2022.02.008
12. Couzin-Frankel J. Clues to long COVID. Science (2022) 376(6599):1261–5. doi: 10.1126/science.add4297
13. NIH. Understanding COVID-19 vaccines. which COVID-19 vaccines are available in the united states? (2022). Available at: https://covid19.nih.gov/covid-19-vaccines.
14. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med (2021) 384(5):403–16. doi: 10.1056/NEJMoa2035389
15. Dunkle LM, Kotloff KL, Gay CL, Anez G, Adelglass JM, Barrat Hernandez AQ, et al. Efficacy and safety of NVX-CoV2373 in adults in the united states and Mexico. N Engl J Med (2022) 386(6):531–43. doi: 10.1056/NEJMoa2116185
16. CDC. National center for immunization and respiratory diseases (NCIRD), division of viral Diseases Benefits of getting a COVID-19 vaccine (2022). Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html.
17. Zisis SN, Durieux JC, Mouchati C, Perez JA, McComsey GA. The protective effect of coronavirus disease 2019 (COVID-19) vaccination on postacute sequelae of COVID-19: A multicenter study from a Large national health research network. Open Forum Infect Dis (2022) 9(7):ofac228. doi: 10.1093/ofid/ofac228
18. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med (2022) 28(7):1461–7. doi: 10.1038/s41591-022-01840-0
19. Xie Y CT, Al-Aly Z. Nirmatrelvir and the risk of post-acute sequelae of COVID-19. MedRxiv (2022). doi: 10.1101/2022.11.03.22281783v1
20. Nevalainen OPO, Horstia S, Laakkonen S, Rutanen J, Mustonen JMJ, Kalliala IEJ, et al. Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial. Nat Commun (2022) 13(1):6152. doi: 10.1038/s41467-022-33825-5
21. Boglione L, Meli G, Poletti F, Rostagno R, Moglia R, Cantone M, et al. Risk factors and incidence of long-COVID syndrome in hospitalized patients: Does remdesivir have a protective effect? QJM (2022) 114(12):865–71. doi: 10.1093/qjmed/hcab297
22. Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med (2020) 383(23):2291–3. doi: 10.1056/NEJMc2031364
23. Tejerina F, Catalan P, Rodriguez-Grande C, Adan J, Rodriguez-Gonzalez C, Munoz P, et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect Dis (2022) 22(1):211. doi: 10.1186/s12879-022-07153-4
24. Natarajan A, Zlitni S, Brooks EF, Vance SE, Dahlen A, Hedlin H, et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med (NY). (2022) 3(6):371–87 e9. doi: 10.1016/j.medj.2022.04.001
25. Zollner A, Koch R, Jukic A, Pfister A, Meyer M, Rossler A, et al. Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology (2022) 163(2):495–506.e8. doi: 10.1053/j.gastro.2022.04.037
26. Peluso MJ, Deeks SG, Mustapic M, Kapogiannis D, Henrich TJ, Lu S, et al. SARS-CoV-2 and mitochondrial proteins in neural-derived exosomes of COVID-19. Ann Neurol (2022) 91(6):772–81. doi: 10.1002/ana.26350
27. Swank Z, Senussi Y, Manickas-Hill Z, Yu XG, Li JZ, Alter G, et al. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. Clin Infect Dis (2022) 76. doi: 10.1101/2022.06.14.22276401
28. Craddock V MA, Krishnamachary B, Spikes L, Chalise P, Dhillon NK. Persistent presence of spike protein and viral RNA in the circulation of individuals with post-acute sequelae of COVID-19. MedRxiv (2022). doi: 10.1101/2022.08.07.22278520v1
29. Remmelink M, De Mendonca R, D’Haene N, De Clercq S, Verocq C, Lebrun L, et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care (2020) 24(1):495. doi: 10.1186/s13054-020-03218-5
30. Chertow D SS, Ramelli S, Grazioli A, Chung JY, Singh M, Yinda CK, et al. SARS-CoV-2 infection and persistence throughout the human body and brain. Res Square (2021). doi: 10.21203/rs.3.rs-1139035/v1
31. Badran Z, Gaudin A, Struillou X, Amador G, Soueidan A. Periodontal pockets: A potential reservoir for SARS-CoV-2? Med Hypotheses (2020) 143:109907. doi: 10.1016/j.mehy.2020.109907
32. Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CK, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature (2022) 612, 1–6. doi: 10.1038/s41586-022-05542-y
33. Lv T, Cao W, Li T. HIV-Related immune activation and inflammation: Current understanding and strategies. J Immunol Res (2021) 2021:7316456. doi: 10.1155/2021/7316456
34. Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med (2015) 21(2):132–9. doi: 10.1038/nm.3781
35. Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. (2012) 122(9):3271–80. doi: 10.1172/JCI64314
36. Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, et al. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood (2012) 120(20):4172–81. doi: 10.1182/blood-2012-06-437608
37. Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-Associated neurocognitive disorder–pathogenesis and prospects for treatment. Nat Rev Neurol (2016) 12(4):234–48. doi: 10.1038/nrneurol.2016.27
38. Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis (2012) 205 Suppl 3(Suppl 3):S375–82. doi: 10.1093/infdis/jis200
39. van de Berg PJ, Heutinck KM, Raabe R, Minnee RC, Young SL, van Donselaar-van der Pant KA, et al. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J Infect Dis (2010) 202(5):690–9. doi: 10.1086/655472
40. Wang H, Peng G, Bai J, He B, Huang K, Hu X, et al. Cytomegalovirus infection and relative risk of cardiovascular disease (Ischemic heart disease, stroke, and cardiovascular death): A meta-analysis of prospective studies up to 2016. J Am Heart Assoc (2017) 6(7). doi: 10.1161/JAHA.116.005025
41. Forrester JV. Ebola Virus and persistent chronic infection: when does replication cease? Ann Transl Med (2018) 6(Suppl 1):S39. doi: 10.21037/atm.2018.09.60
42. Peluso MJ, Anglin K, Durstenfeld MS, Martin JN, Kelly JD, Hsue PY, et al. Effect of oral nirmatrelvir on long COVID symptoms: 4 cases and rationale for systematic studies. Pathog Immun (2022) 7(1):95–103. doi: 10.20411/pai.v7i1.518
43. Geng LN BH, Shafer RW, Miglis MG, Yang PC. Case report of breakthrough long COVID and the use of nirmatrelvir-ritonavir (2022). Available at: https://www.scienceopen.com/document?vid=e7cc8eb6-98b5-4a69-a1af-09ace722cf4d.
44. University S. Paxlovid for treatment of long covid (STOP-PASC) (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT05576662.
45. Kanecia Obie Zimmerman DU. SARS CoV-2 viral persistence study (PASC) - study of long COVID-19 (PASC) (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT05595369.
46. Aleissa MM, Silverman EA, Paredes Acosta LM, Nutt CT, Richterman A, Marty FM. New perspectives on antimicrobial agents: Remdesivir treatment for COVID-19. Antimicrob Agents Chemother (2020) 65(1). doi: 10.1128/AAC.01814-20
47. Pourkarim F, Pourtaghi-Anvarian S, Rezaee H. Molnupiravir: A new candidate for COVID-19 treatment. Pharmacol Res Perspect (2022) 10(1):e00909. doi: 10.1002/prp2.909
48. Nobori H, Fukao K, Kuroda T, Anan N, Tashima R, Nakashima M, et al. Efficacy of ensitrelvir against SARS-CoV-2 in a delayed-treatment mouse model. J Antimicrob Chemother (2022) 77. doi: 10.1093/jac/dkac257
49. Leegwater E, Moes D, Bosma LBE, Ottens TH, van der Meer IM, van Nieuwkoop C, et al. Population pharmacokinetics of remdesivir and GS-441524 in hospitalized COVID-19 patients. Antimicrob Agents Chemother (2022) 66(6):e0025422. doi: 10.1128/aac.00254-22
50. FDA. FDA Updates on bebtelovimab (2022). Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-bebtelovimab.
51. Mumtaz A, Sheikh AAE, Khan AM, Khalid SN, Khan J, Nasrullah A, et al. COVID-19 vaccine and long COVID: A scoping review. Life (Basel) (2022) 12(7). doi: 10.3390/life12071066
52. Tran V PE, Saldanha J, Pane I, Ravaud P. Efficacy of COVID-19 vaccination on the symptoms of patients with long COVID: A target trial emulation using data from the ComPaRe e-cohort in France (2022). Available at: file:///Users/hectorbonilla/Downloads/SSRN-id3932953-2.pdf.
53. Nehme M, Braillard O, Salamun J, Jacquerioz F, Courvoisier DS, Spechbach H, et al. Symptoms after COVID-19 vaccination in patients with post-acute sequelae of SARS-CoV-2. J Gen Intern Med (2022) 37(6):1585–8. doi: 10.1007/s11606-022-07443-2
54. Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, et al. Trajectory of long covid symptoms after covid-19 vaccination: Community based cohort study. BMJ (2022) 377:e069676. doi: 10.1136/bmj-2021-069676
55. Ledford H. Do vaccines protect against long COVID? what the data say. Nature (2021) 599(7886):546–8. doi: 10.1038/d41586-021-03495-2
56. Investigators R-C, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl J Med (2021) 384(16):1491–502. doi: 10.1056/NEJMoa2100433
57. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med (2021) 384(9):795–807. doi: 10.1056/NEJMoa2031994
58. Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature (2020) 584(7821):463–9. doi: 10.1038/s41586-020-2588-y
59. Bonny TS, Patel EU, Zhu X, Bloch EM, Grabowski MK, Abraham AG, et al. Cytokine and chemokine levels in coronavirus disease 2019 convalescent plasma. Open Forum Infect Dis (2021) 8(2):ofaa574. doi: 10.1093/ofid/ofaa574
60. Peluso MJ, Sans HM, Forman CA, Nylander AN, Ho HE, Lu S, et al. Plasma markers of neurologic injury and inflammation in people with self-reported neurologic postacute sequelae of SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm (2022) 9(5). doi: 10.1212/NXI.0000000000200003
61. Peluso MJ, Lu S, Tang AF, Durstenfeld MS, Ho HE, Goldberg SA, et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis (2021) 224(11):1839–48. doi: 10.1093/infdis/jiab490
62. Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol (2022) 23(2):210–6. doi: 10.1038/s41590-021-01113-x
63. Schultheiss C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes SS, et al. The IL-1beta, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med (2022) 3(6):100663. doi: 10.1016/j.xcrm.2022.100663
64. Queiroz MAF, Neves P, Lima SS, Lopes JDC, Torres M, Vallinoto I, et al. Cytokine profiles associated with acute COVID-19 and long COVID-19 syndrome. Front Cell Infect Microbiol (2022) 12:922422. doi: 10.3389/fcimb.2022.922422
65. Durstenfeld MS, Peluso MJ, Kelly JD, Win S, Swaminathan S, Li D, et al. Role of antibodies, inflammatory markers, and echocardiographic findings in postacute cardiopulmonary symptoms after SARS-CoV-2 infection. JCI Insight (2022) 7(10). doi: 10.1172/jci.insight.157053
66. Fernandez-Castaneda A, Lu P, Geraghty AC, Song E, Lee MH, Wood J, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell (2022) 185(14):2452–68 e16. doi: 10.1016/j.cell.2022.06.008
67. Hertanto DM, Wiratama BS, Sutanto H, Wungu CDK. Immunomodulation as a potent COVID-19 pharmacotherapy: Past, present and future. J Inflammation Res (2021) 14:3419–28. doi: 10.2147/JIR.S322831
68. Shimba A, Ikuta K. Control of immunity by glucocorticoids in health and disease. Semin Immunopathol (2020) 42(6):669–80. doi: 10.1007/s00281-020-00827-8
69. Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med (2021) 384(8):693–704. doi: 10.1056/NEJMoa2021436
70. Milne A MS, Sharp C, Hamilton FW, Arnold DT. Impact of dexamethasone on persistent symptoms of COVID-19: An observational study. medRxiv (2021). doi: 10.1101/2021.11.17.21266392v1
71. Myall KJ, Mukherjee B, Castanheira AM, Lam JL, Benedetti G, Mak SM, et al. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc (2021) 18(5):799–806. doi: 10.1513/AnnalsATS.202008-1002OC
72. Goel N, Goyal N, Nagaraja R, Kumar R. Systemic corticosteroids for management of ‘long-COVID’: An evaluation after 3 months of treatment. Monaldi Arch Chest Dis (2021) 92(2). doi: 10.4081/monaldi.2021.1981
73. Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell (2022) 185(5):881–95 e20. doi: 10.1016/j.cell.2022.01.014
74. Klein J, Wood J, Jaycox J, Lu P, Dhodapkar RM, Gehlhausen JR, et al. Distinguishing features of long COVID identified through immune profiling. medRxiv (2022). doi: 10.1101/2022.08.09.22278592
75. Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. (2016) 15(4):457–65. doi: 10.1517/14740338.2016.1140743
76. Rosas IO, Brau N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe covid-19 pneumonia. N Engl J Med (2021) 384(16):1503–16. doi: 10.1056/NEJMoa2028700
77. Crosby LD, Kalanidhi S, Bonilla A, Subramanian A, Ballon JS, Bonilla H. Off label use of aripiprazole shows promise as a treatment for myalgic Encephalomyelitis/Chronic fatigue syndrome (ME/CFS): A retrospective study of 101 patients treated with a low dose of aripiprazole. J Transl Med (2021) 19(1):50. doi: 10.1186/s12967-021-02721-9
78. Polo O PP, Tuominen E. Low-dose naltrexone in the treatment of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). In: Fatigue: Biomedicine, health & behavior (2019) (Informa UK Limited: Taylor & Francis Group). doi: 10.1080/21641846.2019.1692770
79. O’Kelly B, Vidal L, McHugh T, Woo J, Avramovic G, Lambert JS. Safety and efficacy of low dose naltrexone in a long covid cohort; An interventional pre-post study. Brain Behav Immun Health (2022) 24:100485. doi: 10.1016/j.bbih.2022.100485
80. Kucic N, Racki V, Sverko R, Vidovic T, Grahovac I, Mrsic-Pelcic J. Immunometabolic modulatory role of naltrexone in BV-2 microglia cells. Int J Mol Sci (2021) 22(16). doi: 10.3390/ijms22168429
81. Jin S, Kim JG, Park JW, Koch M, Horvath TL, Lee BJ. Hypothalamic TLR2 triggers sickness behavior via a microglia-neuronal axis. Sci Rep (2016) 6:29424. doi: 10.1038/srep29424
82. Yang AC, Kern F, Losada PM, Agam MR, Maat CA, Schmartz GP, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature (2021) 595(7868):565–71. doi: 10.1038/s41586-021-03710-0
83. Cabanas H, Muraki K, Staines D, Marshall-Gradisnik S. Naltrexone restores impaired transient receptor potential melastatin 3 ion channel function in natural killer cells from myalgic Encephalomyelitis/Chronic fatigue syndrome patients. Front Immunol (2019) 10:2545. doi: 10.3389/fimmu.2019.02545
84. Selfridge BR, Wang X, Zhang Y, Yin H, Grace PM, Watkins LR, et al. Structure-activity relationships of (+)-Naltrexone-Inspired toll-like receptor 4 (TLR4) antagonists. J Med Chem (2015) 58(12):5038–52. doi: 10.1021/acs.jmedchem.5b00426
85. Cant R, Dalgleish AG, Allen RL. Naltrexone inhibits IL-6 and TNFalpha production in human immune cell subsets following stimulation with ligands for intracellular toll-like receptors. Front Immunol (2017) 8:809. doi: 10.3389/fimmu.2017.00809
86. Pitt B, Tate AM, Gluck D, Rosenson RS, Goonewardena SN. Repurposing low-dose naltrexone for the prevention and treatment of immunothrombosis in COVID-19. Eur Heart J Cardiovasc Pharmacother. (2022) 8(4):402–5. doi: 10.1093/ehjcvp/pvac014
87. Nacul L. Low-dose naltrexone for post-COVID fatigue syndrome. Vancouver, Canada: University of British Columbia (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT05430152.
88. de Bartolomeis A, Tomasetti C, Iasevoli F. Update on the mechanism of action of aripiprazole: Translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs (2015) 29(9):773–99. doi: 10.1007/s40263-015-0278-3
89. FDA. Aripipraxole (Abilify). APPROVED AGREED-UPON LABELING (2004). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21436s002lbl.pdf.
90. Zhang Y, Chen Y, Wu J, Manaenko A, Yang P, Tang J, et al. Activation of dopamine D2 receptor suppresses neuroinflammation through alphaB-crystalline by inhibition of NF-kappaB nuclear translocation in experimental ICH mice model. Stroke (2015) 46(9):2637–46. doi: 10.1161/STROKEAHA.115.009792
91. Gil CH, Kim YR, Lee HJ, Jung DH, Shin HK, Choi BT. Aripiprazole exerts a neuroprotective effect in mouse focal cerebral ischemia. Exp Ther Med (2018) 15(1):745–50. doi: 10.3892/etm.2017.5443
92. Sato-Kasai M, Kato TA, Ohgidani M, Mizoguchi Y, Sagata N, Inamine S, et al. Aripiprazole inhibits polyI:C-induced microglial activation possibly via TRPM7. Schizophr Res (2016) 178(1-3):35–43. doi: 10.1016/j.schres.2016.08.022
93. Kato T, Mizoguchi Y, Monji A, Horikawa H, Suzuki SO, Seki Y, et al. Inhibitory effects of aripiprazole on interferon-gamma-induced microglial activation via intracellular Ca2+ regulation in vitro. J Neurochem (2008) 106(2):815–25. doi: 10.1111/j.1471-4159.2008.05435.x
94. Kato TA, Monji A, Yasukawa K, Mizoguchi Y, Horikawa H, Seki Y, et al. Aripiprazole inhibits superoxide generation from phorbol-myristate-acetate (PMA)-stimulated microglia in vitro: Implication for antioxidative psychotropic actions via microglia. Schizophr Res (2011) 129(2-3):172–82. doi: 10.1016/j.schres.2011.03.019
95. Sobis J, Rykaczewska-Czerwinska M, Swietochowska E, Gorczyca P. Therapeutic effect of aripiprazole in chronic schizophrenia is accompanied by anti-inflammatory activity. Pharmacol Rep (2015) 67(2):353–9. doi: 10.1016/j.pharep.2014.09.007
96. Fernandez-de-Las-Penas C, Palacios-Cena D, Gomez-Mayordomo V, Florencio LL, Cuadrado ML, Plaza-Manzano G, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis. Eur J Intern Med (2021) 92:55–70. doi: 10.1016/j.ejim.2021.06.009
97. Bell ML, Catalfamo CJ, Farland LV, Ernst KC, Jacobs ET, Klimentidis YC, et al. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: Results from the Arizona CoVHORT. PloS One (2021) 16(8):e0254347. doi: 10.1371/journal.pone.0254347
98. Lu L, Zhang Y, Tang X, Ge S, Wen H, Zeng J, et al. Evidence on acupuncture therapies is underused in clinical practice and health policy. BMJ (2022) 376:e067475. doi: 10.1136/bmj-2021-067475
99. Nurek M, Rayner C, Freyer A, Taylor S, Jarte L, MacDermott N, et al. Recommendations for the recognition, diagnosis, and management of long COVID: A Delphi study. Br J Gen Pract (2021) 71(712):e815–e25. doi: 10.3399/BJGP.2021.0265
100. Wang L, Yang JW, Lin LT, Huang J, Wang XR, Su XT, et al. Acupuncture attenuates inflammation in microglia of vascular dementia rats by inhibiting miR-93-Mediated TLR4/MyD88/NF-kappaB signaling pathway. Oxid Med Cell Longev (2020) 2020:8253904. doi: 10.1155/2020/8253904
102. Nielsen A, Gua sha Bg. Fascial dysfunction:Traditional East Asian instrument-assisted manual therapies. (2018) (Elsevier Health Sciences).
103. Xia ZW, Zhong WW, Meyrowitz JS, Zhang ZL. The role of heme oxygenase-1 in T cell-mediated immunity: The all encompassing enzyme. Curr Pharm Des (2008) 14(5):454–64. doi: 10.2174/138161208783597326
104. Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp J, et al. Persistent clotting protein pathology in long COVID/Post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol (2021) 20(1):172. doi: 10.1186/s12933-021-01359-7
105. Pretorius E, Venter C, Laubscher GJ, Kotze MJ, Oladejo SO, Watson LR, et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with long COVID/Post-acute sequelae of COVID-19 (PASC). Cardiovasc Diabetol (2022) 21(1):148. doi: 10.1186/s12933-022-01579-5
106. Pretorius E VC, Laubscher GJ, Kotze MJ, Moremi K OS, Watson LR, Rajaratnam K, et al. Combined triple treatment of brin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) can resolve their persistent symptoms. Research Square (2021). doi: 10.21203/rs.3.rs-1205453/v1
107. Ramacciotti E, Barile Agati L, Calderaro D, Aguiar VCR, Spyropoulos AC, de Oliveira CCC, et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet (2022) 399(10319):50–9. doi: 10.1016/S0140-6736(21)02392-8
108. Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: The REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA (2020) 324(13):1317–29. doi: 10.1001/jama.2020.17022
109. Zuo T, Wu X, Wen W, Lan P. Gut microbiome alterations in COVID-19. Genomics Proteomics Bioinf (2021) 19(5):679–88. doi: 10.1016/j.gpb.2021.09.004
110. Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GC, Ng SSS, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut (2022) 71(3):544–52. doi: 10.1136/gutjnl-2021-325989
111. Haran JP, Bradley E, Zeamer AL, Cincotta L, Salive MC, Dutta P, et al. Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID. JCI Insight (2021) 6(20). doi: 10.1172/jci.insight.152346
112. Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell (2021) 184(16):4137–53 e14. doi: 10.1016/j.cell.2021.06.019
113. Islam H, Chamberlain TC, Mui AL, Little JP. Elevated interleukin-10 levels in COVID-19: Potentiation of pro-inflammatory responses or impaired anti-inflammatory action? Front Immunol (2021) 12:677008. doi: 10.3389/fimmu.2021.677008
114. Mirmiran P, Bahadoran Z, Gaeini Z. Common limitations and challenges of dietary clinical trials for translation into clinical practices. Int J Endocrinol Metab (2021) 19(3):e108170. doi: 10.5812/ijem.108170
115. Vitolins MZ, Case TL. What makes nutrition research so difficult to conduct and interpret? Diabetes Spectr (2020) 33(2):113–7. doi: 10.2337/ds19-0077
116. Damania B, Kenney SC, Raab-Traub N. Epstein-Barr Virus: Biology and clinical disease. Cell (2022) 185(20):3652–70. doi: 10.1016/j.cell.2022.08.026
117. Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science (2022) 375(6578):296–301. doi: 10.1126/science.abj8222
118. Lanz TV, Brewer RC, Ho PP, Moon JS, Jude KM, Fernandez D, et al. Clonally expanded b cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature (2022) 603(7900):321–7. doi: 10.1038/s41586-022-04432-7
119. Gold JE, Okyay RA, Licht WE, Hurley DJ. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens (2021) 10(6). doi: 10.3390/pathogens10060763
120. Peluso MJ, Deveau TM, Munter SE, Ryder DM, Buck AM, Beck-Engeser G, et al. Impact of pre-existing chronic viral infection and reactivation on the development of long COVID. J Clin Invest (2022). doi: 10.1172/JCI163669
121. Shikova E, Reshkova V, Kumanova capital AC, Raleva S, Alexandrova D, Capo N, et al. Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic small ie, cyrillicncephalomyelitis/chronic fatigue syndrome. J Med Virol (2020) 92. doi: 10.1002/jmv.25744
122. Kogelnik AM, Loomis K, Hoegh-Petersen M, Rosso F, Hischier C, Montoya JG. Use of valganciclovir in patients with elevated antibody titers against human herpesvirus-6 (HHV-6) and Epstein-Barr virus (EBV) who were experiencing central nervous system dysfunction including long-standing fatigue. J Clin Virol (2006) 37 Suppl 1:S33–8. doi: 10.1016/S1386-6532(06)70009-9
123. Kerr J. Early growth response gene upregulation in Epstein-Barr virus (EBV)-associated myalgic Encephalomyelitis/Chronic fatigue syndrome (ME/CFS). Biomolecules (2020) 10(11). doi: 10.3390/biom10111484
124. Ruiz-Pablos M, Paiva B, Montero-Mateo R, Garcia N, Zabaleta A. Epstein-Barr Virus and the origin of myalgic encephalomyelitis or chronic fatigue syndrome. Front Immunol (2021) 12:656797. doi: 10.3389/fimmu.2021.656797
125. Watt T, Oberfoell S, Balise R, Lunn MR, Kar AK, Merrihew L, et al. Response to valganciclovir in chronic fatigue syndrome patients with human herpesvirus 6 and Epstein-Barr virus IgG antibody titers. J Med Virol (2012) 84(12):1967–74. doi: 10.1002/jmv.23411
126. Montoya JG, Kogelnik AM, Bhangoo M, Lunn MR, Flamand L, Merrihew LE, et al. Randomized clinical trial to evaluate the efficacy and safety of valganciclovir in a subset of patients with chronic fatigue syndrome. J Med Virol (2013) 85(12):2101–9. doi: 10.1002/jmv.23713
127. Yager JE, Magaret AS, Kuntz SR, Selke S, Huang ML, Corey L, et al. Valganciclovir for the suppression of Epstein-Barr virus replication. J Infect Dis (2017) 216(2):198–202. doi: 10.1093/infdis/jix263
128. Coppoletta S, Tedone E, Galano B, Soracco M, Raiola AM, Lamparelli T, et al. Rituximab treatment for Epstein-Barr virus DNAemia after alternative-donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2011) 17(6):901–7. doi: 10.1016/j.bbmt.2010.10.003
129. Slabik C, Kalbarczyk M, Danisch S, Zeidler R, Klawonn F, Volk V, et al. CAR-T cells targeting Epstein-Barr virus gp350 validated in a humanized mouse model of EBV infection and lymphoproliferative disease. Mol Ther Oncolytics. (2020) 18:504–24. doi: 10.1016/j.omto.2020.08.005
130. Virios Therapeutics I. IMC-2 long-COVID treatment trial (2022). Available at: https://www.clinicaltrialsarena.com/news/virios-long-covid-antiviral/.
131. Peluso MJ, Deveau TM, Munter SE, Ryder D, Buck A, Beck-Engeser G, et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J Clin Invest (2023) 133(3). doi: 10.1172/JCI163669
132. Poole E, Neves TC, Oliveira MT, Sinclair J, da Silva MCC. Human cytomegalovirus interleukin 10 homologs: Facing the immune system. Front Cell Infect Microbiol (2020) 10:245. doi: 10.3389/fcimb.2020.00245
133. Furman D, Jojic V, Sharma S, Shen-Orr SS, Angel CJ, Onengut-Gumuscu S, et al. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med (2015) 7(281):281ra43. doi: 10.1126/scitranslmed.aaa2293
134. Cho JL, Villacreses R, Nagpal P, Guo J, Pezzulo AA, Thurman AL, et al. Quantitative chest CT assessment of small airways disease in post-acute SARS-CoV-2 infection. Radiology (2022) 304(1):185–92. doi: 10.1148/radiol.212170
135. Dhooria S. A study of the efficacy and safety of pirfenidone vs. nintedanib in the treatment of fibrotic lung disease after coronavirus disease-19 pneumonia. ClinicalTrialsgov (2021).
136. Maria Molina IdIBdB. Pirfenidone compared to placebo in post-COVID19 pulmonary fibrosis COVID-19 (FIBRO-COVID) (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT04607928.
137. Sahajal Dhooria PIoMEaR. Pirfenidone vs. nintedanib for fibrotic lung disease after coronavirus disease-19 pneumonia (PINCER) (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT04856111.
138. Franquet T, Gimenez A, Ketai L, Mazzini S, Rial A, Pomar V, et al. Air trapping in COVID-19 patients following hospital discharge: retrospective evaluation with paired inspiratory/expiratory thin-section CT. Eur Radiol (2022) 32(7):4427–36. doi: 10.1007/s00330-022-08580-2
139. PureTech. LYT-100 in post-acute COVID-19 respiratory disease (2020). Available at: https://www.clinicaltrials.gov/ct2/show/NCT04652518.
140. Orfei MD, Porcari DE, D’Arcangelo S, Maggi F, Russignaga D, Ricciardi E. A new look on long-COVID effects: The functional brain fog syndrome. J Clin Med (2022) 11(19). doi: 10.3390/jcm11195529
141. Deng J, Zhou F, Hou W, Silver Z, Wong CY, Chang O, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann N Y Acad Sci (2021) 1486(1):90–111. doi: 10.1111/nyas.14506
142. Jahrami HA, Alhaj OA, Humood AM, Alenezi AF, Fekih-Romdhane F, AlRasheed MM, et al. Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Med Rev (2022) 62:101591. doi: 10.1016/j.smrv.2022.101591
143. Henriquez-Beltran M, Labarca G, Cigarroa I, Enos D, Lastra J, Nova-Lamperti E, et al. Sleep health and the circadian rest-activity pattern four months after COVID-19. J Bras Pneumol (2022) 48(3):e20210398. doi: 10.36416/1806-3756/e20210398
144. Giri A, Srinivasan A, Sundar IK. COVID-19: Sleep, circadian rhythms and immunity - repurposing drugs and chronotherapeutics for SARS-CoV-2. Front Neurosci (2021) 15:674204. doi: 10.3389/fnins.2021.674204
145. Wichniak A, Kania A, Sieminski M, Cubala WJ. Melatonin as a potential adjuvant treatment for COVID-19 beyond sleep disorders. Int J Mol Sci (2021) 22(16). doi: 10.3390/ijms22168623
146. Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: International consensus criteria. J Intern Med (2011) 270(4):327–38. doi: 10.1111/j.1365-2796.2011.02428.x
147. McManimen SL, Jason LA. Post-exertional malaise in patients with ME and CFS with comorbid fibromyalgia. SRL Neurol Neurosurg (2017) 3(1):22–7.
148. Brown A, Jason LA. Meta-analysis investigating post-exertional malaise between patients and controls. J Health Psychol (2020) 25(13-14):2053–71. doi: 10.1177/1359105318784161
149. Bonilla H QT, Tiwari A, Bonilla AE, Miglis M, Yang P, Eggert L, et al. Myalgic Encephalomyelitis/Chronic fatigue syndrome (ME/CFS) is common in post-acute sequelae of SARS-CoV-2 infection (PASC): Results from a post-COVID-19 multidisciplinary clinic . medRxiv. Available at: https://wwwmedrxivorg/content/101101/2022080322278363v1fullpdf (Accessed August 4, 2022).
150. Authority PHS. Energy point chart (2021). Available at: http://www.phsa.ca/health-info-site/Documents/post_covid-19-Living_in_your_Energy_Envelope_Tool.pdf.
151. Physio LC. Resources (2022). Available at: https://www.albertahealthservices.ca/assets/info/ppih/if-ppih-covid-19-rehab-allied-health-practice-considerations-post-covid.pdf.
152. Axcella Health I. Efficacy, safety, tolerability of AXA1125 in fatigue after COVID-19 infection (2021). Available at: https://clinicaltrials.gov/ct2/show/NCT05152849.
153. Axcella Health I. Axcella announces highly promising results from phase 2a placebo controlled clinical trial for long COVID (2022). Available at: https://ir.axcellatx.com/news-releases/news-release-details/axcella-announces-highly-promising-results-phase-2a-placebo.
154. Hansen KS, Mogensen TH, Agergaard J, Schiottz-Christensen B, Ostergaard L, Vibholm LK, et al. High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: A randomized, phase 2, crossover trial. Lancet Reg Health Eur (2022) 84, 100539. doi: 10.1016/j.lanepe.2022.100539
Keywords: post-acute sequela of SARS-CoV-2 (PASC), long COVID, SARS- CoV-2, long haulers, treatment, clinical trials, recover
Citation: Bonilla H, Peluso MJ, Rodgers K, Aberg JA, Patterson TF, Tamburro R, Baizer L, Goldman JD, Rouphael N, Deitchman A, Fine J, Fontelo P, Kim AY, Shaw G, Stratford J, Ceger P, Costantine MM, Fisher L, O’Brien L, Maughan C, Quigley JG, Gabbay V, Mohandas S, Williams D and McComsey GA (2023) Therapeutic trials for long COVID-19: A call to action from the interventions taskforce of the RECOVER initiative. Front. Immunol. 14:1129459. doi: 10.3389/fimmu.2023.1129459
Received: 22 December 2022; Accepted: 06 February 2023;
Published: 09 March 2023.
Edited by:
Alan Landay, Rush University, United StatesReviewed by:
James Moy, Rush University, United StatesSara Gianella Weibel, University of California, San Diego, United States
Copyright © 2023 Bonilla, Peluso, Rodgers, Aberg, Patterson, Tamburro, Baizer, Goldman, Rouphael, Deitchman, Fine, Fontelo, Kim, Shaw, Stratford, Ceger, Costantine, Fisher, O’Brien, Maughan, Quigley, Gabbay, Mohandas, Williams and McComsey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hector Bonilla, SGJvbmlsbGFAU3RhbmZvcmQuZWR1
 Hector Bonilla
Hector Bonilla Michael J. Peluso2
Michael J. Peluso2 Judith A. Aberg
Judith A. Aberg Robert Tamburro
Robert Tamburro Jason D. Goldman
Jason D. Goldman Nadine Rouphael
Nadine Rouphael Amelia Deitchman
Amelia Deitchman Jeffrey Fine
Jeffrey Fine Paul Fontelo
Paul Fontelo Patricia Ceger
Patricia Ceger Liza Fisher
Liza Fisher John G. Quigley
John G. Quigley