- 1National Clinical Research Center for Metabolic Diseases, Metabolic Syndrome Research Center, Key Laboratory of Diabetes Immunology, Ministry of Education, and Department of Metabolism and Endocrinology, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China
- 2Department of General Surgery, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China
Background: The overall evidence base of anti-inflammatory therapies in patients with type 2 diabetes mellitus (T2DM) has not been systematically evaluated. The purpose of this study was to assess the effects of anti-inflammatory therapies on glycemic control in patients with T2DM.
Methods: PubMed, Embase, Web of Science, and Cochrane Library were searched up to 21 September 2022 for randomized controlled trials (RCTs) with anti-inflammatory therapies targeting the proinflammatory cytokines, cytokine receptors, and inflammation-associated nuclear transcription factors in the pathogenic processes of diabetes, such as interleukin-1β (IL-1β), interleukin-1β receptor (IL-1βR), tumor necrosis factor-α (TNF-α), and nuclear factor-κB (NF-κB). We synthesized data using mean difference (MD) and 95% confidence interval (CI). Heterogeneity between studies was assessed by I2 tests. Sensitivity and subgroup analyses were also conducted.
Results: We included 16 RCTs comprising 3729 subjects in the meta-analyses. Anti-inflammatory therapies can significantly reduce the level of fasting plasma glucose (FPG) (MD = - 10.04; 95% CI: -17.69, - 2.40; P = 0.01), glycated haemoglobin (HbA1c) (MD = - 0.37; 95% CI: - 0.51, - 0.23; P < 0.00001), and C-reactive protein (CRP) (MD = - 1.05; 95% CI: - 1.50, - 0.60; P < 0.00001) compared with control, and therapies targeting IL-1β in combination with TNF-α have better effects on T2DM than targeting IL-1β or TNF-α alone. Subgroup analyses suggested that patients with short duration of T2DM may benefit more from anti-inflammatory therapies.
Conclusion: Our meta-analyses indicate that anti-inflammatory therapies targeting the pathogenic processes of diabetes can significantly reduce the level of FPG, HbA1c, and CRP in patients with T2DM.
1 Introduction
Obesity and type 2 diabetes mellitus (T2DM) are associated with decreased physical activity and unhealthy high-calorie diets. Obesity is related to insulin resistance and is a crucial risk factor for the development of T2DM (1). Chronic low-grade inflammation plays an important role in the pathogenesis of diabetes and the development of diabetic complications (2, 3). Inflammation has been seen in the pancreatic islets, liver, muscle, adipose tissue, and the sites of diabetic complications (4). Long-term inflammation that occurs in adipose tissue can lead to systemic inflammation and contribute to insulin resistance. In the presence of insulin resistance, β cells secrete more insulin to maintain normal glucose control. Inflammation impairs β cell function and induces β cell apoptosis, and T2DM happens when insulin production fails to reach the insulin needs (5).
Many proinflammatory cytokines and inflammation-associated nuclear transcription factors are related to impaired insulin secretion and contribute to the pathogenesis of T2DM, including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and nuclear factor-κB (NF-κB) etc. (4, 6–8). High concentration glucose can induce IL-1β production and secretion from human pancreatic β cells, and IL-1β was observed in β cells in diabetic patients (9). IL-1β is involved in β cell apoptosis and partially dependent on the activation of NF-κB (10). Obesity can activate the NF-κB signaling pathway, which plays an important role in the development of insulin resistance (8). TNF-α is also involved in β cell apoptosis (11), and more TNF-α expression was found in adipose tissue in obese than lean people, and the plasma level of TNF-α was elevated in patients with T2DM (6, 7).
Anti-inflammatory treatments can improve insulin sensitivity and β cell function in patients with insulin resistance or T2DM (12). Treatments of diabetes focused on inflammation can benefit many inflammatory tissues at the same time, which is less likely to induce hypoglycemia (13). Small molecules or antibody-based molecules targeting inflammatory cytokines, cytokine receptors, or inflammation-associated nuclear transcription factors, such as IL-1β, interleukin-1β receptor (IL-1βR), NF-κB, and TNF-α, can improve metabolism (13, 14). But the effects of anti-inflammatory therapies on glycemic control in patients with T2DM were controversial (15–19). Previous meta-analyses have assessed the effects of anti-IL-1 therapies on T2DM (20, 21). However, the totality of the evidence base of the anti-inflammatory therapies on T2DM has not been systematically assessed. We conducted the meta-analyses to clarify the effects of anti-inflammatory therapies on glycemic control in patients with T2DM.
2 Methods
The meta-analyses were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (22).
2.1 Search strategy
We searched randomized controlled trials (RCTs) from PubMed, Embase, Web of Science and Cochrane Library from database inception up to 21 September 2022. Search terms include Medical Subject Headings (MeSH), keywords and free-text terms related to anti-inflammatory therapies, type 2 diabetes mellitus, T2DM, fasting plasma glucose, FPG, glycated haemoglobin, HbA1c, C-reactive protein, CRP, anakinra, canakinumab, diacerein, gevokizumab, LY2189102, tocilizumab, salsalate, salicylate, etanercept, remicade, infliximab, adalimumab, enbrel, and dapansutrile. The detailed search strategy is available in Table S1. Following the search and removal of duplicates, D Li and J Zhong screened titles and abstracts to identify relevant studies.
2.2 Study selection
Studies were eligible for inclusion if they met the following criteria (1): Participants: patients with T2DM; (2) Interventions: at least one of the following treatments was used, anakinra, canakinumab, diacerein, gevokizumab, LY2189102, tocilizumab, salsalate, salicylate, etanercept, remicade, infliximab, adalimumab, enbrel, or dapansutrile; (3) Controls: placebo with or without approved antidiabetic medications, such as metformin, sulfonylureas, and insulin etc.; (4) Outcomes: at least one of the following outcomes was reported, FPG, HbA1c, or CRP; (5) Studies: RCTs. Trials without accessible data or full text were excluded.
2.3 Data extraction
Data extraction and analyses from included studies were performed by two authors independently, and conflicts were resolved by a third author. The following information was extracted: first author, publication year, agent, dosage and frequency, follow-up duration, number of participants, patient baseline information (mean age, sex distribution, diabetes duration, baseline BMI, and HbA1c) and outcomes of interest (follow-up FPG, HbA1c, and CRP).
2.4 Risk of bias assessment
Risk of bias assessment of the included RCTs was carried out by two authors (D Li and Q Zhang) independently according to the Cochrane Collaboration’s Risk of Bias Tool, which including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias.
2.5 Data analyses
Continuous variables were expressed as mean difference (MD) with 95% confidence interval (CI). When mean and SD were not available, we calculated from SEM, sample size, median, range, or interquartile range (IQR) using methodology from the Cochrane Library Handbook or the article written by Wan et al. (23, 24). Several studies had more than one intervention groups with different dosages, and for these studies, we chose only one comparable dosage as motioned in Table 1. Statistical heterogeneity among studies was assessed with the I2 statistic, considering the I2 value of 50 - 75% was moderate heterogeneity and above 75% was high heterogeneity (25). We performed subgroup analyses based on the targets of interventions, names of the medication, diabetes duration, follow-up duration, and drug administration regimen. Leave-one-out studies were performed for sensitivity analyses to examine the effect of each trial on the overall analyses. Funnel plot and Egger’s test were used to assess the publication bias and tested for statistical significance. All statistical analyses were performed using Review manager 5.3 and Stata 12.0. A value of p ≤ 0.05 was considered statistically significant.
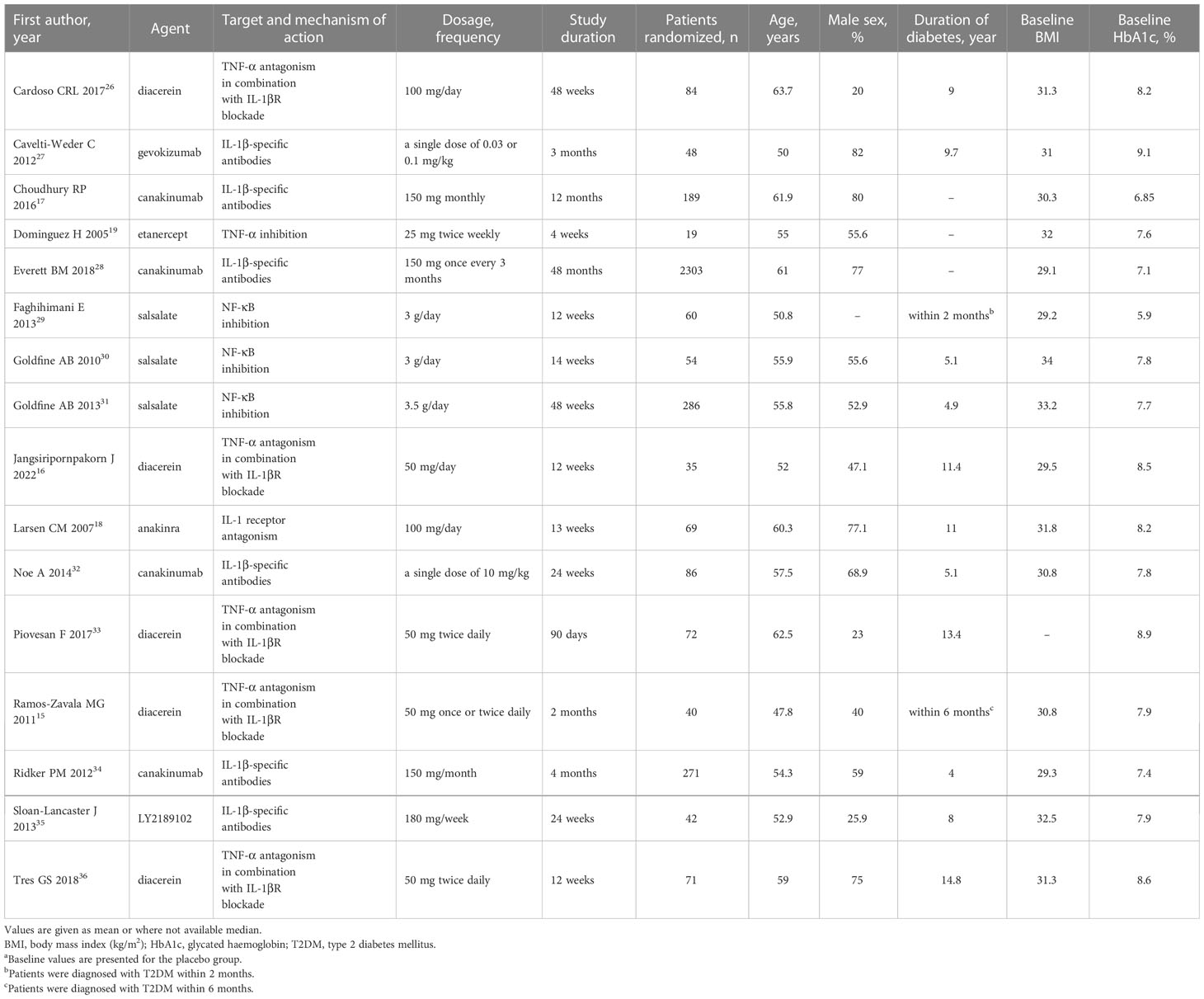
Table 1 Baseline characteristics of included studies a.
3 Results
3.1 Included studies and baseline characteristics
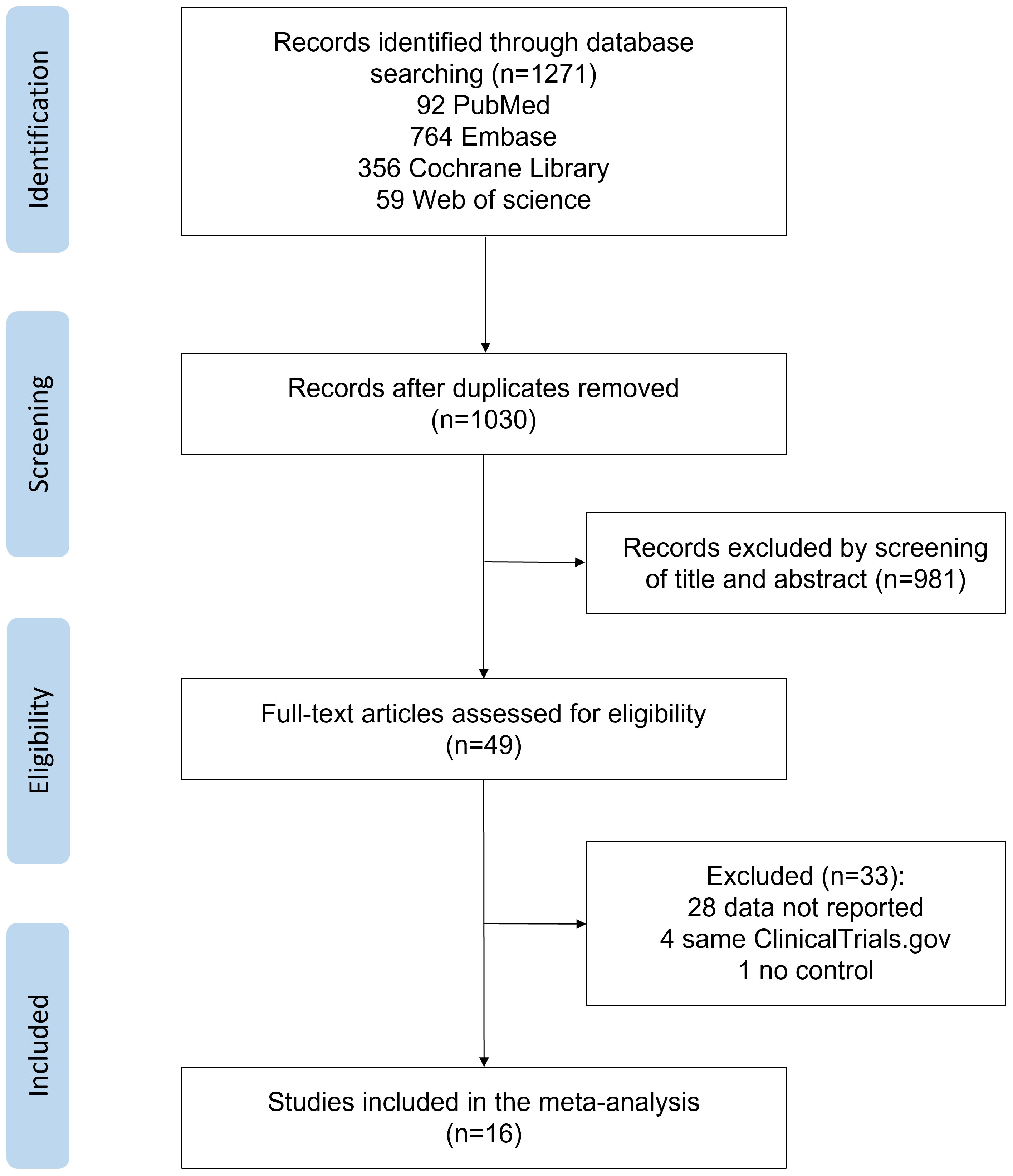
Figure 1 shows the details of the literature search and selection process. Of 1271 reports identified, 241 reports were excluded due to duplication, and 981 were excluded based on titles and abstracts. Of 49 reports reviewed in full, 33 were excluded based on eligibility criteria. A total of 16 reports involving 3729 participants with T2DM were included in the final analyses (15–19, 26–36). Table 1 shows the baseline characteristics of the 16 RCTs. Trials included were published between 2005 and 2022. The follow-up duration was between 1 and 48 months. Trails reported by Everett et al. (28) had the longest follow-up duration (48 months), which was not comparable with all the others, and a more comparable time point (6 months) was used in the subsequent analyses. Among the 16 trails, 4 trails were for canakinumab (17, 28, 32, 34), 5 trails for diacerein (15, 16, 26, 33, 36), 3 trails for salsalate (29–31), and the rest were for anakinra (18), gevokizumab (27), LY2189102 (35), and etanercept (19). The dosage and frequency of the treatments are shown in Table 1.
3.2 Risk of bias of individual studies
The quality of the included trials was assessed according to the criteria of the Cochrane Handbook. A detailed evaluation of the risk of bias for each clinical trial and risk of bias summary are presented in Figure S1. Among the 16 RCTs, only 1 was judged to be at high risk of bias as an open-label randomized trial (19), 6 were judged to be at low risk of bias and 9 as being at unclear risk of bias. Unclear risks were related to selection bias, reporting bias, and other bias.
3.3 Meta-analyses
3.3.1 FPG
Figure 2 shows anti-inflammatory therapies can significantly decrease the level of FPG (n = 12; MD = - 10.04; 95% CI: - 17.69, - 2.40; P =0.01) compared with control, and there was statistically significant heterogeneity among studies (I2 = 77%; P < 0.00001) (Figure 2A). We did a series of subgroup analyses of FPG based on the targets of interventions, diabetes duration, and follow-up duration. Subgroup analyses based on the targets of interventions show that drugs targeting IL-1β plus TNF-α (diacerein) (n = 5; MD = - 13.52; 95% CI: - 23.77, - 3.27; P =0.01) or NF-κB alone (salsalate) (n = 3; MD = - 22.03; 95% CI: - 34.59, - 9.47; P =0.0006) can significantly decrease the level of FPG compared with control, whereas drugs targeting IL-1β (canakinumab) or TNF-α (etanercept) alone had no significant effect on the change of FPG (Figure 2B). Patients with T2DM less than 3 years since diagnosis (n=2, MD = - 20.64; 95% CI: - 32.03, - 9.25; P =0.0004) seem to benefit more from anti-inflammatory therapies than those between 3 and 10 years (n=3, MD = - 14.79; 95% CI: - 28.69, - 0.89; P =0.04), and no significant effect was found in those more than 10 years (n=4, MD = - 7.94; 95% CI: - 20.17, 4.3; P =0.2) (Figure S2A). Anti-inflammatory therapies can decrease the level of FPG in patients whose follow-up duration was less than or equal to 3 months (n=6, MD = - 19.01; 95% CI: - 28.57, -9.45; P < 0.0001), but no significant effect was found in patients with longer follow-up duration (Figure S2B).
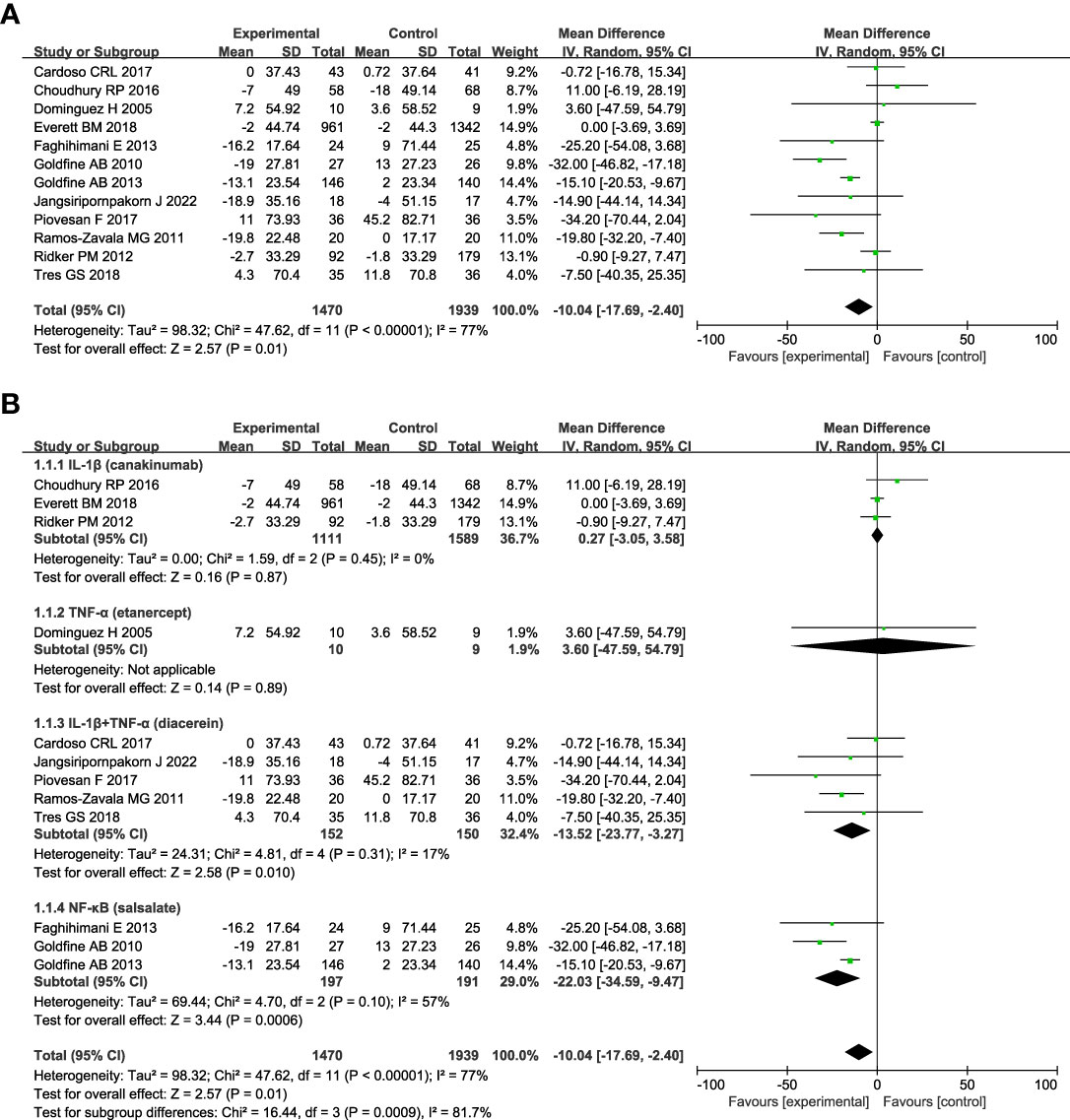
Figure 2 Forest plot of pooled mean difference in change in FPG (mg/dL). (A) Meta-analyses of the effects of anti-inflammatory therapies on FPG in patients with T2DM; (B) The forest plot of FPG in subgroup analyses defined by the targets of interventions. fasting plasma glucose, FPG; CI, confidence interval; IV, inverse variance; SD, standard deviation.
3.3.2 HbA1c
The change in HbA1c was assessed in all studies. Figure 3A shows anti-inflammatory therapies can significantly decrease the level of HbA1c (n = 16; MD = - 0.37; 95% CI: - 0.51, - 0.23; P < 0.00001) with moderate heterogeneity among studies (I2 = 69%; P < 0.0001) (Figure 3A). The sensitivity analyses of HbA1c indicated the stability of the results (Figure S3). Subgroup analyses based on the targets of the interventions show that drugs targeting IL-1β plus TNF-α (diacerein) (n=5; MD = - 0.63; 95% CI: - 1.08, - 0.19; P =0.005) can reduce the level of HbA1c better than targeting IL-1β (gevokizumab, canakinumab, anakinra, or LY2189102) (n=7; MD = - 0.25; 95% CI: - 0.42, - 0.08; P = 0.004) or TNF-α (etanercept) (n=1; MD = 0.00; 95% CI: - 0.88, 0.88; P =1.00) alone (Figure 3B). Anti-inflammatory therapies targeting NF-κB (salsalate) (n = 3; MD = - 0.40; 95% CI: - 0.59, - 0.20; P < 0.0001) can significantly decrease the level of HbA1c compared with control, and there was no heterogeneity among studies (I2 = 27%; P = 0.25). Subgroup analyses according to the name of the medications show in Figure S4A, gevokizumab (n = 1; MD = - 0.85; 95% CI: - 1.60, - 0.10; P =0.03) can reduce the level of HbA1c more than diacerein (n = 5; MD = - 0.63; 95% CI: - 1.08, - 0.19; P =0.005), anakinra (n =1; MD = - 0.46; 95% CI: - 0.61, - 0.31; P < 0.00001), salsalate (n = 3; MD = - 0.40; 95% CI: - 0.59, - 0.20; P < 0.0001), and canakinumab (n = 4; MD = - 0.11; 95% CI: - 0.21, - 0.02; P = 0.02). LY2189102 and etanercept had no significant effect on HbA1c compared with the control. Subgroup analyses based on diabetes duration show that more reduction of HbA1c was seen in patients with T2DM less than 3 years since diagnosis (n = 2, MD = -1.54; 95% CI: - 2.04, - 1.04; P < 0.00001) than those between 3 and 10 years (n = 6, MD = - 0.32; 95% CI: - 0.43, - 0.21; P < 0.00001), and those more han 10 years (n = 5, MD = - 0.44; 95% CI: - 0.56, - 0.31; P < 0.00001) (Figure S4B). Anti-inflammatory therapies were more effective in patients whose follow-up duration was less than or equal to 3 months (n = 7, MD = - 0.71; 95% CI: - 1.16, - 0.26; P = 0.002) (Figure S4C). Repeated drug administration regimen (n = 14, MD = - 0.37; 95% CI: - 0.52, -0.21; P < 0.00001) and single dosing (n = 2, MD = - 0.45; 95% CI: - 1.01, 0.10; P = 0.11) had similar effects on HbA1c (Figure S4D).
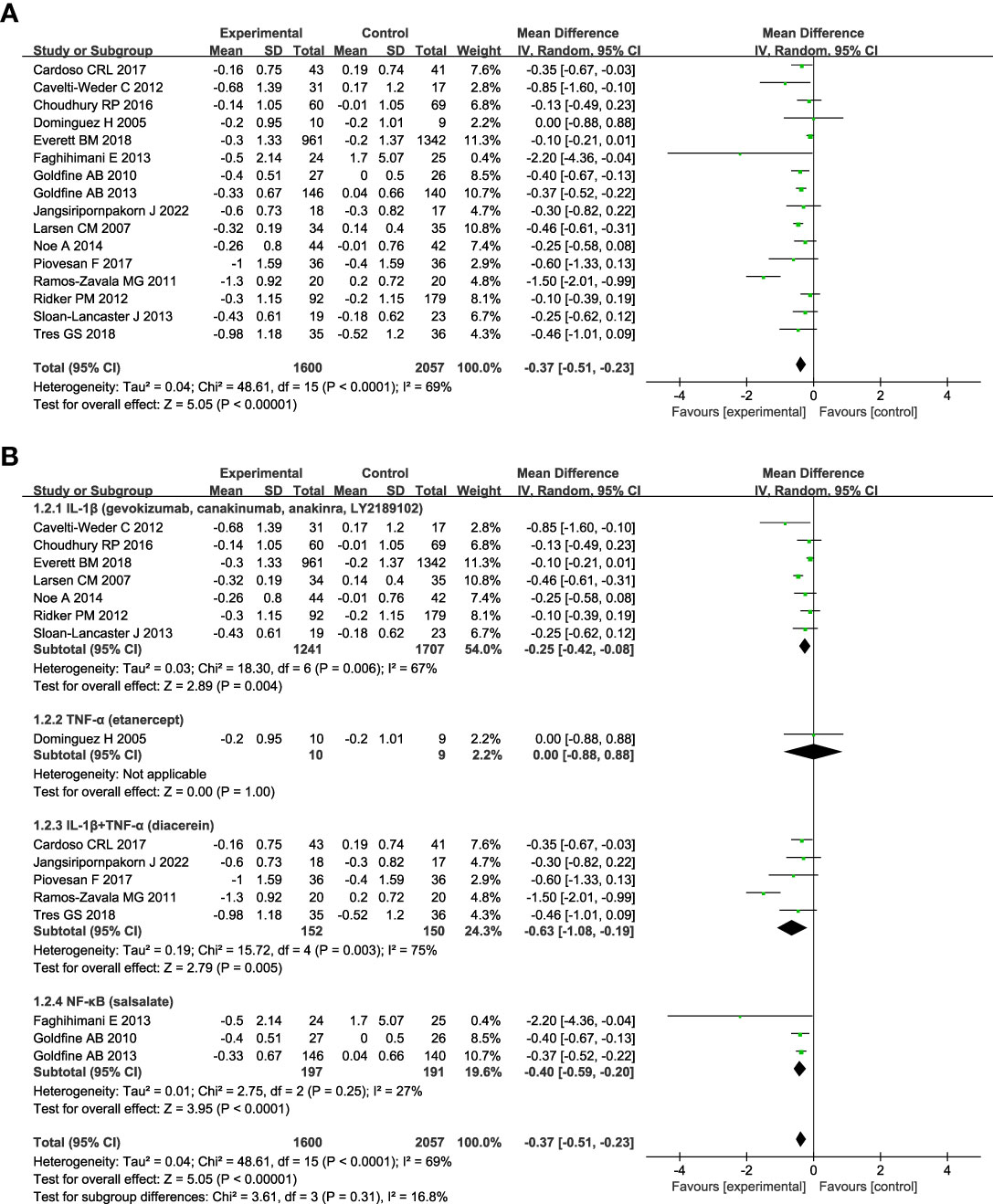
Figure 3 Forest plot of pooled mean difference in change in HbA1c (%). (A) Meta-analyses of the effects of anti-inflammatory therapies on HbA1c in patients with T2DM; (B) The forest plot of HbA1c in subgroup analyses defined by the targets of interventions. glycated haemoglobin, HbA1c; CI, confidence interval; IV, inverse variance; SD, standard deviation.
3.3.3 CRP
Figure 4 shows anti-inflammatory therapies can decrease the level of CRP compared with control (n = 6; MD = - 1.05; 95% CI: - 1.50, - 0.60; P < 0.00001), and there was high heterogeneity among studies (I2 = 77%; P = 0.0007) (Figure 4A). Subgroup analyses based on the targets of interventions show that drugs targeting IL-1β (canakinumab) can significantly reduce the level of CRP (n = 3; MD = - 1.31; 95% CI: - 1.63, - 0.99; P < 0.00001), whereas no significant effect was found in drugs targeting IL-1β plus TNF-α (diacerein) (n = 2; MD = - 1.95; 95% CI: - 4.39, 0.49; P =0.12) or NF-κB (salsalate) (n = 1; MD = - 0.24; 95% CI: - 0.80, 0.32; P =0.40) (Figure 4B).

Figure 4 Forest plot of pooled mean difference in change in CRP (mg/L). (A) Meta-analyses of the effects of anti-inflammatory therapies on CRP in patients with T2DM; (B) The forest plot of CRP in subgroup analyses defined by the targets of interventions. C-reactive protein, CRP; CI, confidence interval; IV, inverse variance; SD, standard deviation.
3.3.4 Publication bias
Egger’s test for HbA1c suggested significant publication bias (p = 0.003) (Figure S5). However, the effect was the same as the original effect after using Duval and Tweedie’s trim and fill, and the result showed that no trimming was performed, and the data stayed unchanged.
4 Discussion
Our meta-analyses of 16 RCTs published between 2005 and 2022 examined the effects of anti-inflammatory therapies on glycemic control in patients with T2DM. Two previous meta-analyses published in 2018 and 2019, concluded that anti-IL-1 therapies can significantly decrease the level of HbA1c and CRP, and have mild hypoglycaemic effect on patients with T2DM (20, 21). However, the effects of anti-inflammatory therapies targeting other inflammatory molecules and the overall effects of anti-inflammatory therapies on T2DM remain to be discovered. Therefore, we performed further analyses of anti-inflammatory therapies based on different inflammatory targets, including IL-1β, IL-1βR, TNF-α, and NF-κB. Our results show that anti-inflammatory therapies, including anti-IL-1 therapies, can significantly decrease the level of FPG, HbA1c and CRP in patients with T2DM. Our findings indicate the clinical efficacy of treating T2DM based on the pathogenesis of diabetes and give suggestions for the future anti-inflammatory clinical trials.
Chronic low-grade inflammation was found in diabetic islets, with increased innate immune cell infiltration and cytokine secretion (37). Immune cell infiltration and cytokine release directly impairs β cell mass and function (38). IL-1β was the first described proinflammatory cytokine in the islets of patients with T2DM (39). IL-1β impairs β cell function and induces the apoptosis of β cells (40). Block IL-1β signaling pathway by antagonists or antibodies had beneficial effects on β cell function and glycemic control in patients with T2DM (41, 42). Anakinra, a recombinant human IL-1βR antagonist, can significantly reduce the level of HbA1c and may improve glycemic control by increasing insulin secretion (18). Canakinumab, gevokizumab and LY2189102 are recombinant human engineered monoclonal antibodies, which can neutralize the activity of IL-1β by forming a complex with circulating IL-1β. Canakinumab can also reduce the blood levels of IL-6 and CRP (17). All the anti-IL-1β therapies mentioned above had significant effect on glucose control as reflected by reductions in HbA1c, which was also reported by previous meta-analyses (20, 21). However, some of the beneficial effects were only detected by certain treatment periods, not the whole follow-up periods (28, 35). As shown in our subgroup analyses, anti-inflammatory therapies may work better in patients with short follow-up duration (less than or equal to 3 months). LY2189102 can improve blood glucose control for 12 weeks, but the effect was attenuated over time and there was no difference at 24 weeks (35). The study reported by Everett BM et al. showed that canakinumab can reduce HbA1c during the first 6 to 9 months of treatment, but no significant effect was found by the end of the follow-up period at 48 months (28). The exact reason for this attenuation is unclear, but the availability of other antidiabetic therapies and lifestyle interventions may contribute to this phenomenon (28).
TNF-α can diminish glucose-dependent insulin secretion and impair the function of β cells both in vitro and in vivo (43, 44). But etanercept, a TNF-α inhibitor, has no significant effect on FPG or HbA1c (19). Etanercept can improve the glucose tolerance of some individuals, but no significant effect was found in the whole group (19). It was difficult to say whether etanercept has a positive effect on β cells since no more than 20 individuals was included in this clinical trial, and studies with a larger number of patients with T2DM are needed to elucidate this issue.
Diacerein is both an IL-1βR blocker and a TNF antagonist. It can inhibit the synthesis and activity of IL-1 and TNF-α by its active metabolite rhein (45). Diacerein can reduce the HbA1c level without affecting the homeostasis model assessment-insulin resistance (HOMA-IR), indicating that it may play a role in insulin secretion (36). And a higher dosage of diacerein (100 mg/day) may be more effective in improving the glycemic outcome (16). Our results show that interventions targeting IL-1β plus TNF-α can reduce the level of HbA1c better than targeting IL-1β or TNFα alone in patients with T2DM. Diacerein had no significant effect on CRP in patients with T2DM, though reduced TNF-α was observed (26, 36). Those studies were carried out in patients with longer duration of diabetes, and most participants were undergoing treatment with metformin, statins, sulfonylureas, or renin-angiotensin system blockers, which have potential roles in anti-inflammation, and might attenuate the anti-inflammatory effect of diacerein (13, 26, 36).
Salsalate, a prodrug form of salicylate, shows anti-inflammatory effects by inhibiting the IKKb/NF-κB and JNK signaling pathways (46, 47). Salsalate can improve glycemic control by affecting cellular kinases nonspecifically and increasing insulin secretion of β cells (48). After 1 year treatment, salsalate still had effects on HbA1c and FPG in patients with T2DM (31). Salsalate can decrease the level of inflammatory mediators, such as leukocytes, neutrophils, and lymphocytes, but had little effect on CRP in patients with T2DM (31). T2DM seems to result from a long-term process of inflammation, even years before diagnosis (35). Greater benefits of salsalate might be seen in patients with newly diagnosed T2DM or longer treatment duration.
Our results show that patients with newly diagnosed T2DM may benefit more from anti-inflammatory therapies. However, Kataria Y et al. reported that the effects of anti-IL-1β therapies depend on the baseline dysmetabolic status, and patients with a more metabolic imbalance at baseline may benefit more after treatment (21). The differences between our studies may come from the different types of medications analyzed, as we included lots of anti-inflammatory medications, not just IL-1β antibodies and IL-1βR antagonists. Since no newly diagnosed T2DM patients were included in the studies of anti-IL-1β therapies, the effects of anti-IL-1β therapies on those patients remain to be seen.
There are some limitations in our study. First, lifestyle modification and antidiabetic medications were allowed in most of the included trials, which may affect or attenuate the efficacy of anti-inflammatory therapies. Second, most of the follow-up duration varied from 1 to 12 months, and longer clinical trials are needed since medication efficacy may change over time. Finally, publication bias exists in the meta-analyses, but the results stay the same after a trim and fill analysis.
5 Conclusions
This study helps us better understand the possibility and efficiency of anti-inflammatory therapies for T2DM based on the pathogenetic processes of the disease. The present analyses demonstrated that targeting cytokines, cytokine receptors, and inflammation-associated nuclear transcription factors, such as IL-1β, IL-1βR, TNF-α, and NF-κB, alone or in combination can significantly reduce the level of FPG, HbA1c, and CRP in patients with T2DM. In addition, patients with a short duration of T2DM may benefit more from anti-inflammatory therapies. Since anti-inflammatory medications can reduce inflammation throughout the body, these medications may be used to treat diseases with similar pathologies, such as cardiovascular disease, chronic kidney disease, and rheumatic arthritis with or without T2DM.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DL and JinZ conceived and designed the study. DL and JiaZ did the scientific literature search and data extraction of the included studies. DL and QZ did the quality assessment and carried out the analyses. DL wrote the first draft of the present manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82070807, 91749118, 81770775, 81730022), Leading Talents Program of Hunan Province (2022RC3078), Natural Science Foundation of Hunan Province, China (2021JJ30976) and National key research and development program (2019YFA0801900, 2018YFC2000100).
Acknowledgments
We are grateful to Central South University Library for the assistance during literature search.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1125116/full#supplementary-material
References
1. Alberti K, Eckel R, Grundy S, Zimmet P, Cleeman J, Donato K, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
2. Pradhan A, Manson J, Rifai N, Buring J, Ridker P. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA (2001) 286:327–34. doi: 10.1001/jama.286.3.327
3. Esser N, Legrand-Poels S, Piette J, Scheen A, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract (2014) 105:141–50. doi: 10.1016/j.diabres.2014.04.006
4. Herder C, Dalmas E, Böni-Schnetzler M, Donath M. The IL-1 pathway in type 2 diabetes and cardiovascular complications. Trends Endocrinol Metab (2015) 26:551–63. doi: 10.1016/j.tem.2015.08.001
5. Kahn S, Cooper M, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet (2014) 383:1068–83. doi: 10.1016/S0140-6736(13)62154-6
6. Winkler G, Salamon F, Harmos G, Salamon D, Speer G, Szekeres O, et al. Elevated serum tumor necrosis factor-alpha concentrations and bioactivity in type 2 diabetics and patients with android type obesity. Diabetes Res Clin Pract (1998) 42:169–74. doi: 10.1016/S0168-8227(98)00109-0
7. Hotamisligil G, Arner P, Caro J, Atkinson R, Spiegelman B. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest (1995) 95:2409–15. doi: 10.1172/JCI117936
8. Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa b axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord (2003) 27 Suppl 3:S49–52. doi: 10.1038/sj.ijo.0802501
9. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka H, Spinas G, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest (2002) 110:851–60. doi: 10.1172/JCI200215318
10. Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care (2008) 31 Suppl 2:S161–164. doi: 10.2337/dc08-s243
11. Donath M, Størling J, Berchtold L, Billestrup N, Mandrup-Poulsen T. Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev (2008) 29:334–50. doi: 10.1210/er.2007-0033
12. Esser N, Paquot N, Scheen A. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs (2015) 24:283–307. doi: 10.1517/13543784.2015.974804
13. Donath M. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discovery (2014) 13:465–76. doi: 10.1038/nrd4275
14. Hotamisligil GS. Inflammation and metabolic disorders. Nature (2006) 444:860–7. doi: 10.1038/nature05485
15. Ramos-Zavala MG, González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, González-López R, Santiago-Hernández NJ. Effect of diacerein on insulin secretion and metabolic control in drug-naive patients with type 2 diabetes: a randomized clinical trial. Diabetes Care (2011) 34:1591–4. doi: 10.2337/dc11-0357
16. Jangsiripornpakorn J, Srisuk S, Chailurkit L, Nimitphong H, Saetung S, Ongphiphadhanakul B. The glucose-lowering effect of low-dose diacerein and its responsiveness metabolic markers in uncontrolled diabetes. BMC Res Notes (2022) 15:91. doi: 10.1186/s13104-022-05974-9
17. Choudhury RP, Birks JS, Mani V, Biasiolli L, Robson MD, L'Allier PL, et al. Arterial effects of canakinumab in patients with atherosclerosis and type 2 diabetes or glucose intolerance. J Am Coll Cardiol (2016) 68:1769–80. doi: 10.1016/j.jacc.2016.07.768
18. Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med (2007) 356:1517–26. doi: 10.1056/NEJMoa065213
19. Dominguez H, Storgaard H, Rask-Madsen C, Steffen Hermann T, Ihlemann N, Baunbjerg Nielsen D, et al. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res (2005) 42:517–25. doi: 10.1159/000088261
20. Huang J, Yang Y, Hu R, Chen L. Anti-interleukin-1 therapy has mild hypoglycaemic effect in type 2 diabetes. Diabetes Obes Metab (2018) 20:1024–8. doi: 10.1111/dom.13140
21. Kataria Y, Ellervik C, Mandrup-Poulsen T. Treatment of type 2 diabetes by targeting interleukin-1: a meta-analysis of 2921 patients. Semin Immunopathol (2019) 41:413–25. doi: 10.1007/s00281-019-00743-6
22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
23. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135
24. Cochrane handbook for systematic reviews of interventions version 6.3. cochrane (2022). Available at: http://www.training.cochrane.org/handbook.
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
26. Cardoso CRL, Leite NC, Carlos FO, Loureiro AA, Viegas BB, Salles GF. Efficacy and safety of diacerein in patients with inadequately controlled type 2 diabetes: A randomized controlled trial. Diabetes Care (2017) 40:1356–63. doi: 10.2337/dc17-0374
27. Cavelti-Weder C, Babians-Brunner A, Keller C, Stahel MA, Kurz-Levin M, Zayed H, et al. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care (2012) 35:1654–62. doi: 10.2337/dc11-2219
28. Everett B, Donath M, Pradhan A, Thuren T, Pais P, Nicolau J, et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol (2018) 71:2392–401. doi: 10.1016/j.jacc.2018.03.002
29. Faghihimani E, Aminorroaya A, Rezvanian H, Adibi P, Ismail-Beigi F, Amini M. Salsalate improves glycemic control in patients with newly diagnosed type 2 diabetes. Acta Diabetol (2013) 50:537–43. doi: 10.1007/s00592-011-0329-2
30. Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med (2010) 152:346–57. doi: 10.7326/0003-4819-152-6-201003160-00004
31. Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, et al. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med (2013) 159:1–12. doi: 10.7326/0003-4819-159-1-201307020-00003
32. Noe A, Howard C, Thuren T, Taylor A, Skerjanec A. Pharmacokinetic and pharmacodynamic characteristics of single-dose canakinumab in patients with type 2 diabetes mellitus. Clin Ther (2014) 36:1625–37. doi: 10.1016/j.clinthera.2014.08.004
33. Piovesan F, Tres GS, Moreira LB, Andrades ME, Lisboa HK, Fuchs SC. Effect of diacerein on renal function and inflammatory cytokines in participants with type 2 diabetes mellitus and chronic kidney disease: A randomized controlled trial. PLoS One (2017) 12:e0186554. doi: 10.1371/journal.pone.0186554
34. Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, c-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation (2012) 126:2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556
35. Sloan-Lancaster J, Abu-Raddad E, Polzer J, Miller JW, Scherer JC, De Gaetano A, et al. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes. Diabetes Care (2013) 36:2239–46. doi: 10.2337/dc12-1835
36. Tres GS, Fuchs SC, Piovesan F, Koehler-Santos P, Pereira FDS, Camey S. Effect of diacerein on metabolic control and inflammatory markers in patients with type 2 diabetes using antidiabetic agents: A randomized controlled trial. J Diabetes Res (2018) 2018:4246521. doi: 10.1155/2018/4246521
37. Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity (2022) 55:31–55. doi: 10.1016/j.immuni.2021.12.013
38. Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes (2007) 56:2356–70. doi: 10.2337/db06-1650
39. Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of langerhans. Science (1986) 232:1545–7. doi: 10.1126/science.3086977
40. Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes (2010) 17:314–21. doi: 10.1097/MED.0b013e32833bf6dc
41. Fève B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol (2009) 5:305–11. doi: 10.1038/nrendo.2009.62
42. Ruscitti P, Masedu F. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK). a multicentre, open-label, randomised controlled trial. PloS Med (2019) 16:e1002901. doi: 10.1371/journal.pmed.1002901
43. Zhang S, Kim K. TNF-alpha inhibits glucose-induced insulin secretion in a pancreatic beta-cell line (INS-1). FEBS Lett (1995) 377:237–9. doi: 10.1016/0014-5793(95)01272-9
44. Kägi D, Ho A, Odermatt B, Zakarian A, Ohashi P, Mak T. TNF receptor 1-dependent beta cell toxicity as an effector pathway in autoimmune diabetes. J Immunol (1999) 162:4598–605. doi: 10.4049/jimmunol.162.8.4598
46. Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of ikkbeta. Science (2001) 293:1673–7. doi: 10.1126/science.1061620
47. Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature (1998) 396:77–80. doi: 10.1038/23948
Keywords: type 2 diabetes, anti-inflammatory therapies, antidiabetic drug, clinical trial, meta-analyses
Citation: Li D, Zhong J, Zhang Q and Zhang J (2023) Effects of anti-inflammatory therapies on glycemic control in type 2 diabetes mellitus. Front. Immunol. 14:1125116. doi: 10.3389/fimmu.2023.1125116
Received: 15 December 2022; Accepted: 15 February 2023;
Published: 01 March 2023.
Edited by:
Pingping Li, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Giovani Marino Favero, Universidade Estadual de Ponta Grossa, BrazilHong-Hui Wang, Hunan University, China
Copyright © 2023 Li, Zhong, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingjing Zhang, ZG9jdG9yemhhbmdqakBjc3UuZWR1LmNu
 Dandan Li
Dandan Li Jiaxin Zhong
Jiaxin Zhong Qirui Zhang
Qirui Zhang Jingjing Zhang
Jingjing Zhang