- Department of Infectious Diseases, Tangdu Hospital, Air Force Medical University, Xi’an, China
Introduction: Heat shock protein (HSPs) are important intracellular factors, which are often involved in the regulation of viral replication including HIV-1 in infected individuals as molecular chaperone proteins. Heat shock proteins 70 (HSP70/HSPA) family play important roles in HIV replication, but this family contain many subtypes, and it is unclear how these subtypes participate in and affect HIV replication.
Methods: To detect the interaction between HSPA14 and HspBP1 by CO-IP. Simulating HIV infection status in vitro to detect the change of intracellular HSPA14 expression after HIV infection in different cells. Constructing HSPA14 overexpression or knockdown cells to detect intracellular HIV replication levels after in vitro infection. Detecting the difference of HSPA expression levels in CD4+ T cells of untreated acute HIV-infected patients with different viral load.
Results: In this study, we found that HIV infection can lead to changes in the transcriptional level of many HSPA subtypes, among which HSPA14 interacts with HIV transcriptional inhibitor HspBP1. The expression of HSPA14 in Jurkat and primary CD4+T cells infected with HIV were inhibited, overexpression of HSPA14 inhibited HIV replication, while knocking down HSPA14 promoted HIV replication. We also found that the expression level of HSPA14 is higher in peripheral blood CD4+T cells of untreated acute HIV infection patients with low viral load.
Conclusion: HSPA14 is a potential HIV replication inhibitor and may restrict HIV replication by regulating the transcriptional inhibitor HspBP1. Further studies are needed to determine the specific mechanism by which HSPA14 regulates viral replication
1 Introduction
After acute HIV infection, there is a major difference in the distribution of HIV RNA and HIV DNA levels in patients, and the high level of virus replication can lead to continuous immune activation, inflammation and progressive damage to the immune function. HIV RNA is a common indicator of the level of virus replication in patients (1). Clinical statistics show that the HIV RNA level in plasma of untreated acute infection is usually 0.25–95.5 × 105 copies/ml (2, 3). The difference in virological level among acute HIV infection patients without treatment is mainly due to two aspects: the characteristics of the virus subtypes and the autoimmune characteristics of the infected individuals. The main virus subtypes in China are the AE recombinant type and BC recombinant type, thus the influence of virus subtypes on HIV replication levels is limited (4), and the level of HIV replication in patients may be mainly regulated by individual host factors (5, 6).
The heat shock protein (HSP) family is a kind of chaperone protein family expressed in many cells, and its role in HIV replication has been widely studied (7–9). Among them, the HSP70 (also called HSPA) subtribe plays an important role in the process of HIV replication. HSPA subtribe exists in various human cells with more than 14 different isoforms, including heat-inducible isoforms (e.g., HSPA1A, HSPA1B, HSPA6, HSPA7, etc.) and non-heat-inducible isoforms (e.g., HSPA13). The subtype-specific roles of HSPAs have been extensively studied. HSPA5 was found to be involved in the folding process of the viral Env protein in the endoplasmic reticulum, while other HSPA isoforms have different interactions with viral proteins such as Tat, Gag, and Vpr (10, 11). Kammula et al. demonstrated the interaction of HSPA9 with the viral Nef protein through a yeast two-hybrid screen, suggesting that HSPA9 could be able to participate in the regulation of HIV replication (12). HSPA14 was first identified in dendritic cells, and it is also widely found in a variety of immune cells such as T lymphocytes and monocytes, playing a role in enhancing Th1-polarizing activity. However, whether HSPA14 can interfere with HIV replication remains unclear. In the current study, we investigated the effect of HSPA14 in regulation of HIV replication. We now report, for the first time, that HSPA14 is an important inhibitory factor for HIV infection in T cells.
2 Materials and methods
2.1 Study subjects
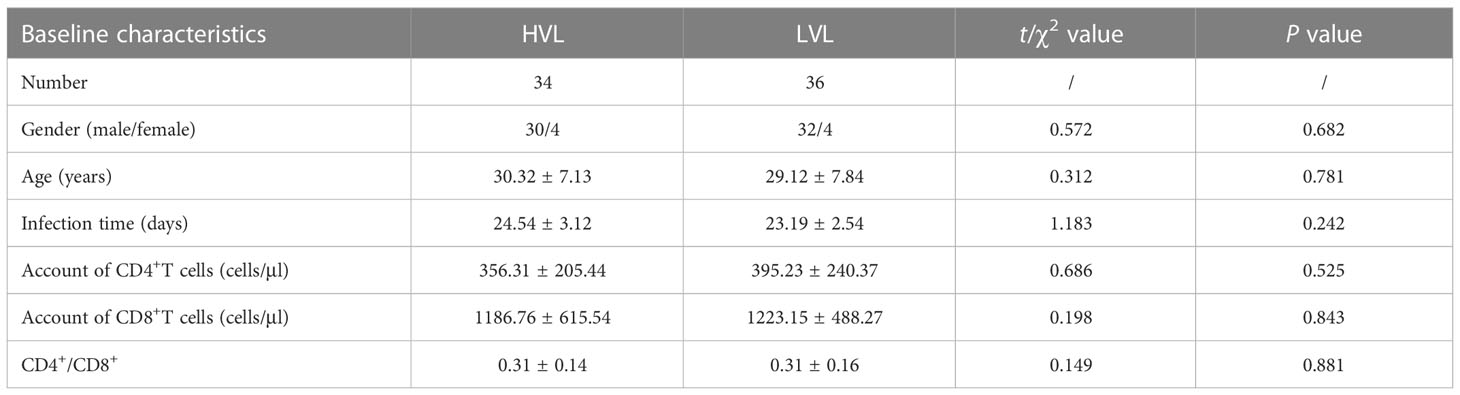
This study was approved by the Medical Ethics Committee of Tangdu Hospital (second affiliated Hospital) of Air Force military Medical University. Untreated acute HIV infection patients came from Department of Infectious Diseases of Tangdu Hospital. The normal seronegative donors came from Department of Blood Transfusion of Tangdu Hospital. This study collects at least 70 cases of untreated acute HIV infection patients and 20 healthy controls, aged from 18 to 60 years old, regardless of gender and excluded trauma, tumor, autoimmune disease or other pathogen infection. Untreated acute HIV infection patients are divided into high viral load group (HVL) and low viral load group (LVL) according to whether HIV RNA level is greater than 1 × 105 IU/ml, and there’s no significant difference in baseline characteristics between two groups (Table 1).
2.2 Pseudovirus preparation
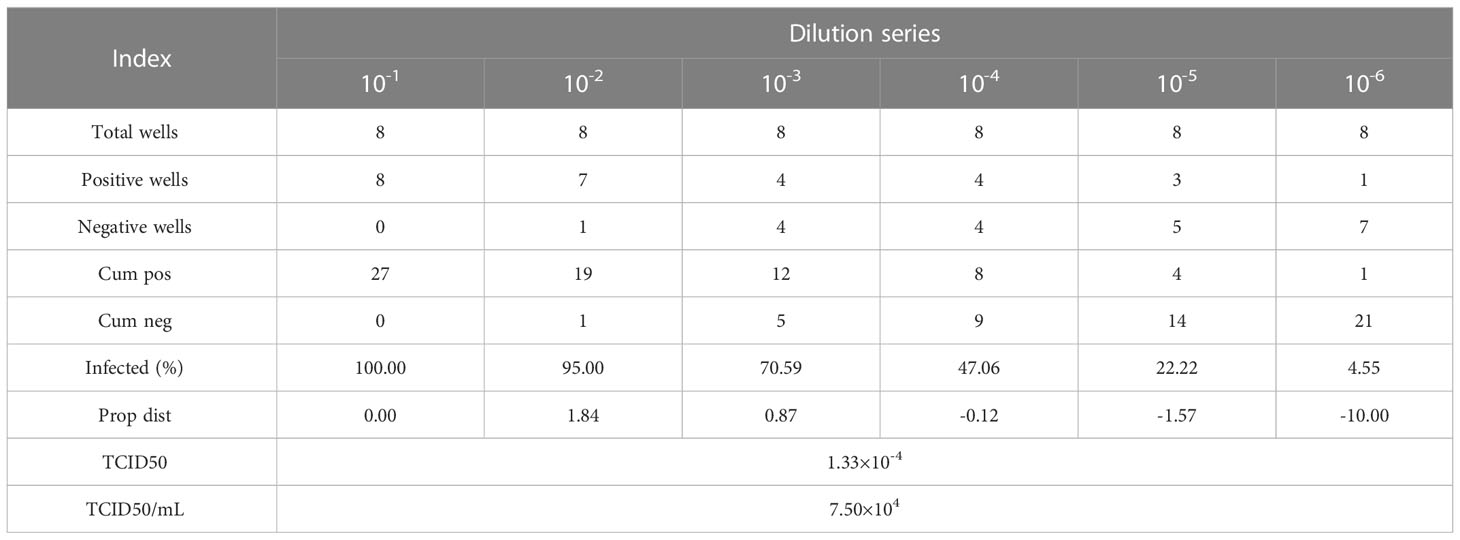
HIV pseudoviral particles were obtained by ultracentrifugation after co-transfection of plasmid pNL-4-3murmurRmure- (From our laboratory) and plasmid pCMV-VSVG (Genechem, China) in HEK293T cells by lipofection (Lipo3000, Invitrogen™, USA), and cultured in a 5% CO2, 37°C cell incubator for 48-72 hours. Collect the cell culture supernatant, centrifuge at 20000×g for 10 minutes at room temperature, and then filter the supernatant with a 0.2 µm filter to obtain the HIV-1 pseudovirus solution. TZM-bl cells were tiled in a 96-well plate and prepared to detect the TCID50 of HIV-1 pseudovirus. Dilute the HIV-1 pseudovirus solution according to a 10-fold gradient, add 100 µL of the solution dropwise to a row of microwells, and continue culturing the 96-well plate after adding HIV-1 pseudovirus solutions of different dilution levels in a 5% CO2, 37°C cell culture incubator for 24 hours. Observe and record the cell state of each microwell in the 96-well plate, and calculate the TCID50 of HIV-1 pseudovirus according to the Reed&Muench method (Table 2).
2.3 Pseudovirus HIV-1 infection of cell lines
CCRF-CEM cell line (Procell, China) and Jurkat cell line (Procell, China) were cultured in RPMI-1640 complete medium [500mlRPMI-1640+50ml fetal bovine serum (FBS) + penicillin streptomycin] at 37°C and 5%CO2. HEK-293T cell line (Procell, China) and TZM-bl cell line (From our laboratory) were cultured in DMEM complete medium [500mlDMEM+50ml fetal bovine serum (FBS) + penicillin streptomycin] at 37°C and 5%CO2. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density gradient centrifugation from blood samples of patients. Commercial CD4+T lymphocyte separation kit (STEMCELL, Canada) was used to isolate CD4+T lymphocytes by negative selection. CD4+T lymphocytes were short-term cultured in RPMI-1640 complete medium [500mlRPMI-1640+50ml fetal bovine serum (FBS) + penicillin streptomycin] at 37°C and 5%CO2. When used in the experiment of infected cells, different cells were inoculated on the culture plate according to the following number of cells: 104/well-96-well plate, 5 × 104/well-24-well plate, 2.5 × 105/well-6-well plate. Using the measured virus titer TCID50/ml, combined with the formula PFU=0.7 × TCID50/ml, MOI=PFU/cell number, when the cell confluence degree reached 60%-70%, the original culture medium was discarded and washed with DPBS for 2 times, and the required amount of virus was added, and the serum-free medium was added. After being cultured in 37°C, CO2 incubator for 2 hours, the complete medium was changed, and then the next experiment was carried out according to different research purposes.
2.4 Overexpression and knockdown of HSPA14
The overexpression plasmids of HSPA14 (GV657 vector) have been provided by Genechem, China. The recombinant product of HSPA14 was transformed into competent bacteria, and the plasmid was obtained by expanding culture and extraction of the correct clone solution. The tool vector plasmid carrying the HSPA14 gene and the virus packaging helper plasmid were co-transfected into HEK293T cells by lipofection (Lipo3000, Invitrogen™, USA). The virus was harvested 48-72 hours after transfection. The lentivirus preservation solution with high titer was obtained by PEG8000 precipitation, concentration and purification, and the lentivirus titer was determined. The obtained lentivirus infection requires cells that overexpress HSPA14, and cells stably expressing HSPA14 can be obtained for follow-up experiments.
According to the nucleotide sequences of HSPA14, the short hairpin RNA (shRNA, GV493 vector) was synthesized by chemical synthesis method from Genechem, China. The target cells in logarithmic growth phase were inoculated with a density of about 50%, and cultured overnight in an incubator with a volume fraction of 5% CO2 at 37°C. The transfection solution was added to cells and cultured in an incubator with a volume fraction of 5% CO2 at 37°C. After 4 hours, the culture medium was discarded and replaced with 10% fetal bovine serum and DMEM culture medium without antibiotics. 48 hours after transfection, the cells were digested with trypsin by hole, and RNA was extracted to detect the interference efficiency after washing with PBS.
For both overexpression and interference with HSPA14, cell lines transfected with vector plasmids (GV657 vector or GV493 vector) were set up as Controls, and cell lines without transfection were used as Blank Controls.
2.5 Immunoblotting and immunoprecipitation assays
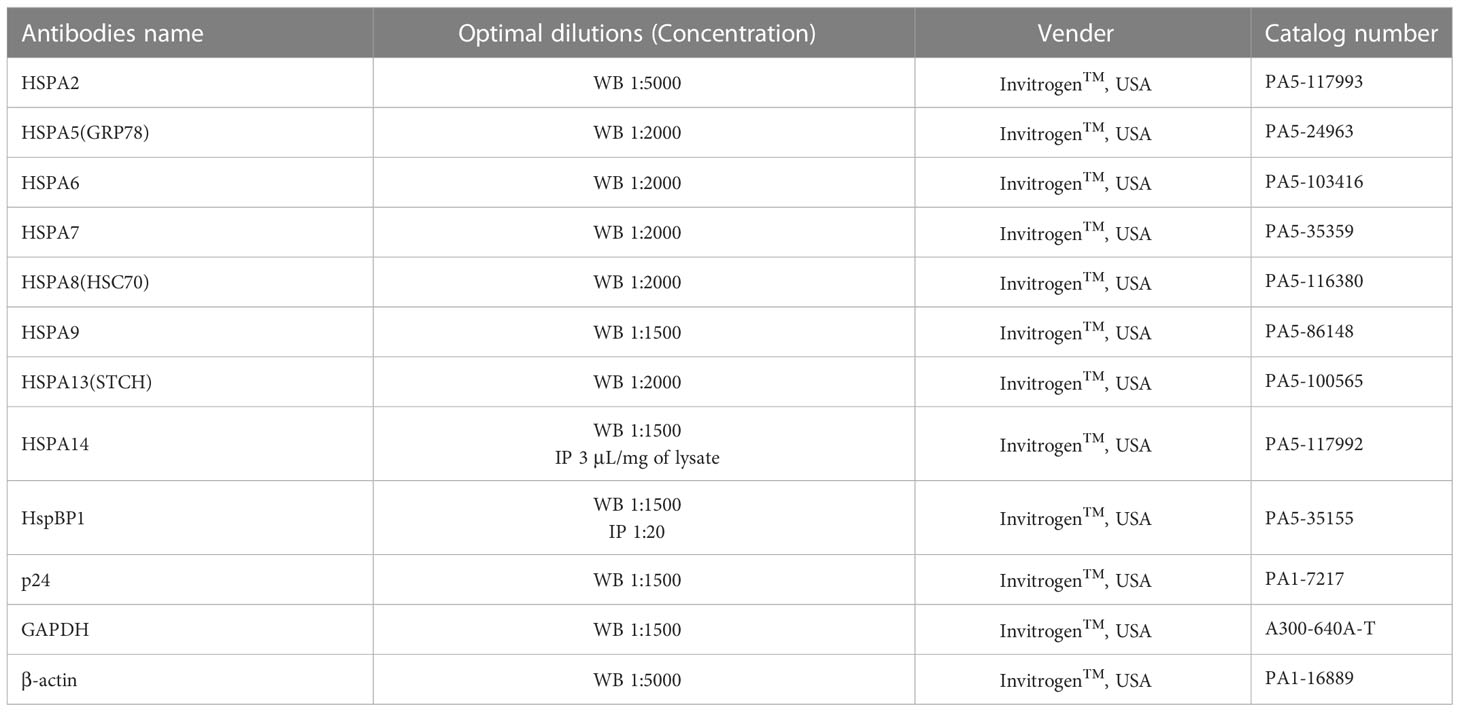
Total cellular proteins were extracted by RIPA lysis buffer (Thermo Scientific™, USA) with protease inhibitor (Thermo Scientific™, USA). The protein concentration was determined by the Rapid Gold BCA Protein Assay Kit (Thermo Scientific™, USA), and the same amount of protein was detected on a NuPAGE 10% Mini Protein Gels (Invitrogen™, USA) by vertical plate electrophoresis tank (Bio-Rad, USA). Then the proteins were transferred from gels to PVDF transfer membranes (Thermo Scientific™, USA) by Trans-Blot SD Semi-Dry transfer tank (Bio-Rad, USA), blocked with 5% skim dried milk or BSA (Thermo Scientific™, USA), and detected with the corresponding antibody (Table 3). Imprinting was performed with the ECL Chemiluminescent Substrate Reagent Kit (Invitrogen™, USA). For coimmunoprecipitation analysis, the total proteins of the corresponding cells were incubated with HSPA14 or HspBP1 antibodies, and the antigen-antibody complex was pulled down by Classic Magnetic IP/Co-IP Kit (Thermo Scientific™, USA) and then identified by the immunoblotting method described above.
2.6 Enzyme-linked immunosorbent assay
The level of HIV p24 in the supernatant of different cell lines infected by HIV pseudovirus was measured by HIV-1 p24 SimpleStep ELISA Kit (Abcam, UK) as follows. (1) Prepare standards and cell culture supernatants according to the protocol. (2) Prepare all regents and add 50µL of all samples and standards to plate wells. (3) Add 50µL of the Antibody Cocktail to each well and incubate for 1 hour on a plate shaker set to 400 rpm (4) Thoroughly wash each well with 1×Wash Buffer PT and remove excess liquid (5) Add 100µL of TMB Development Solution to each well and incubate for 10 minutes in the dark on a plate shaker set to 400 rpm (6) Add 100 µL of Stop Solution to each well and shake plate on a plate shaker for 1 minute to mix. (7) Record the OD of each well at 450 nm and calculate the concentration of HIV p24 in each sample by standard curve.
2.7 Total cQuantitative real-time PCR
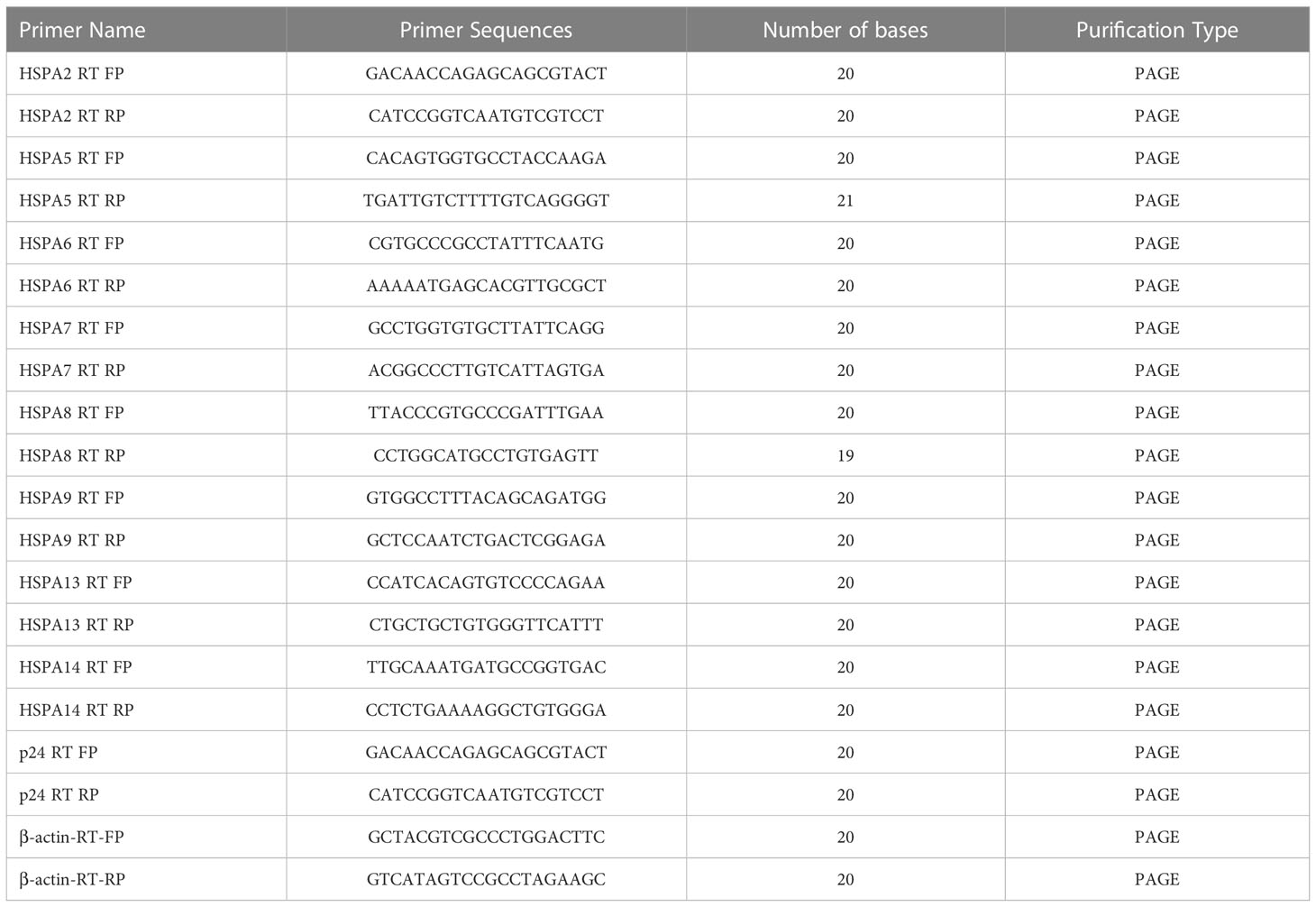
An RNA Easy Fast animal tissue/cell total RNA extraction kit (TIANGEN, China) was used to extract total RNA from cells. cDNA was prepared by a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific™, USA). The expression of HSPAs and p24 was detected by real-time quantitative polymerase chain reaction, and the corresponding forward and reverse primer sequences shown in Table 4. The qRT-PCR procedure is as follows: (1) preincubation: 95°C, ramp 4.4°C/s, 600s; (2) Three-step amplification for 45 cyclers: 95°C, ramp 4.4°C/s, 10s; 60°C, ramp 2.2°C/s, 10s; 72°C, acquisition with ramp 4.4°C/s, 10s; (3) Melting: 95°C, ramp 4.4°C/s, 10s; 65°C, ramp 2.2°C/s, 60s; 97°C, ramp 0.1°C/s, 1s. Finally, the CT value of the corresponding reaction well was obtained, and the 2-Δ(ΔCT) value was calculated by the following formula, which yields the degree of change in the relevant HSPA mRNA level of the corresponding sample.
Fold Change = 2-Δ(ΔCT)
where ΔCT = CT(target) – CT(β-actin)
and Δ(ΔCT) = ΔCT(treated) – ΔCT(control)
2.8 Statistical analysis
SPSS 23.0 was used for statistical analysis, GraphPad Prism 8.0 was used for drawing graphs. All immunoblotting experiments and qRT-PCR experiments were carried out in 3 biological replicates x̄ ± s was used to depict the measurement data, and the measurement data between different groups were compared by Student’s t test. The enumeration data are described by n (%), and the chi-square test was used to compare enumeration data between different groups. When P < 0.05, the difference between groups was considered statistically significant.
3 Result
3.1 Interaction between HspBP1 and HSPA14
HspBP1 is known to play an important role in interfering HIV transcription and interact with some subtypes of HSP70s. Taking these observations into consideration, we investigated whether HSPA14 interacts with HspBP1 in HIV infected cells. To accomplish this, we performed co-immunoprecipitation assays with lysate from CEM cells infected with HIV pseudovirus and CD4+T cells isolated from acute HIV infected patients PBMC. We observed that HSPA14 was pulled down with HspBP1 antibodies. Likewise, HspBP1 was also pulled down with HSPA14 antibody (Figure 1), suggesting that HSPA14 can interact with HspBP1.
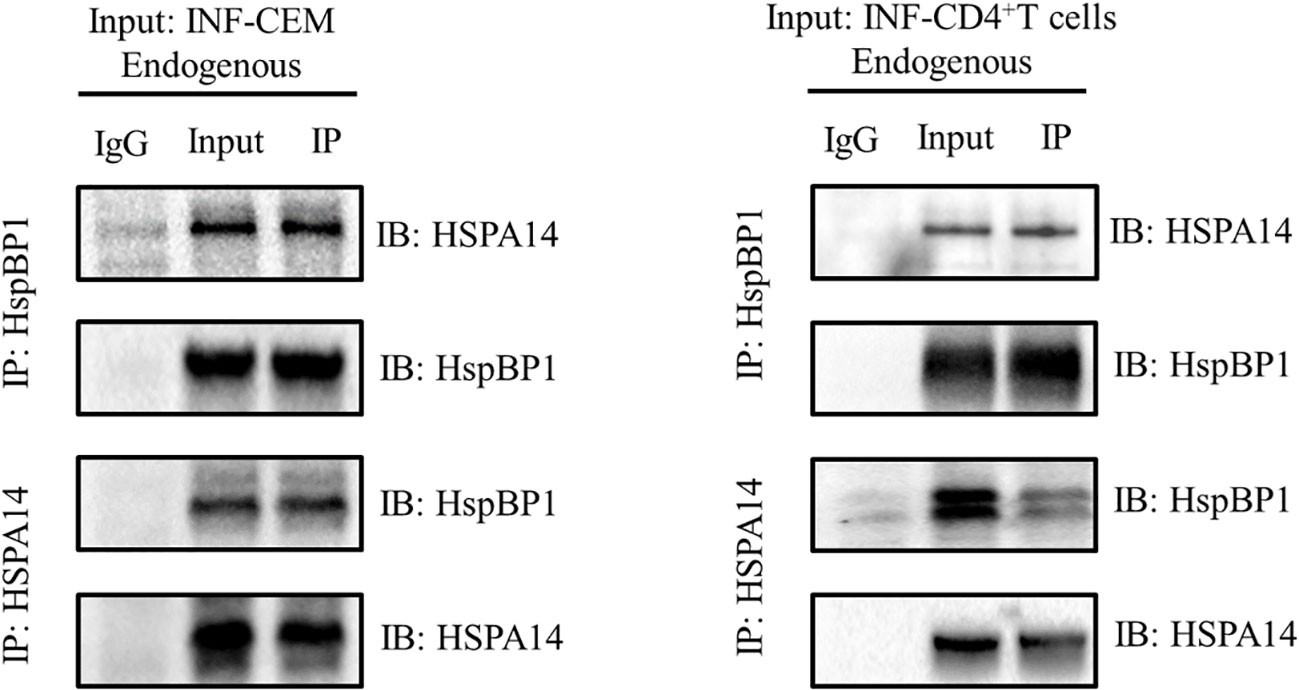
Figure 1 Co-IP to detect the interaction between HspBP1 and HSPA14. Co-immunoprecipitation showing interaction of HspBP1 with HSPA14 at endogenous level in infected CEM cells and CD4+T cells of acute HIV infected patients.
3.2 HSPA14 is down-modulated during HIV-1 infection
We next examined HSPA14 expression levels during HIV-1 infection. Considering the interference of HspBP1 to HIV replication and evidence from Figure 1 that HSPA14 interacts with HspBP1, we were curious to know that what happens to HSPA14 levels during infection. Kinetic expression profile studies for HSPA14 were performed following HIV-1 infection in Jurkat cells, CEM cells and TZM-bl cells. Different cells were infected with HIV pseudovirus and expression of HSPA14 was analyzed at mRNA and protein level at various time points. HIV-1 infection resulted in down-modulation of HSPA14 mRNA and protein expression (Figures 2A–C). This finding was further confirmed by analyzing the expression of HSPA14 in HIV infected human CD4+T cells isolated from the PBMC of three normal seronegative donors (Figures 2D–F). These results clearly indicate that HSPA14 mRNA and protein levels are significantly down-modulated following HIV infection.
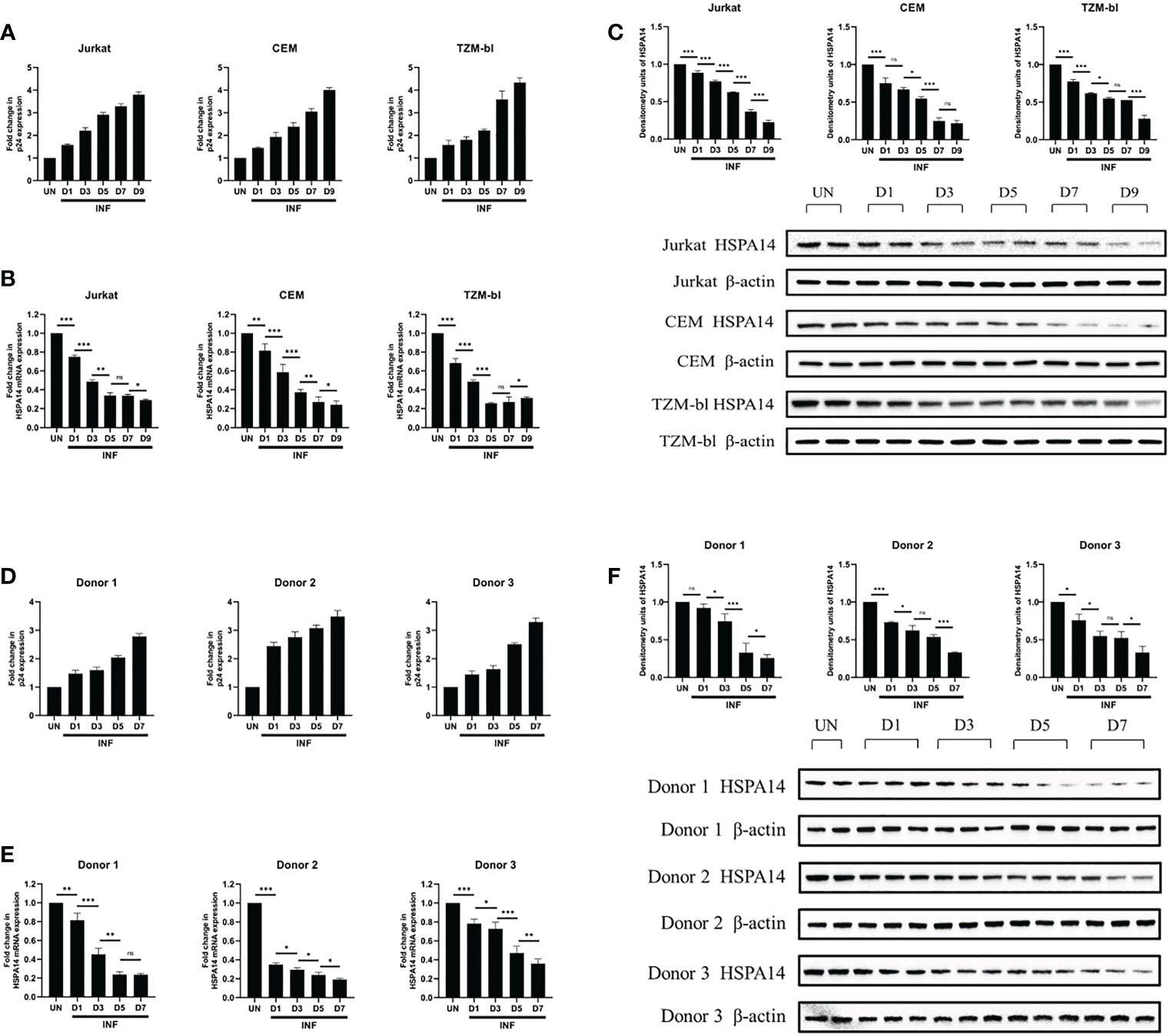
Figure 2 HSPA14 is down modulated during HIV-1 infection. (A, D) The expression of viral capsid protein p24 mRNA was measured to monitor the progression of infection. (B) mRNA expression profiling of HSPA14 during HIV infection. Different cells were infected with HIV pseudovirus and were harvested on day 1 (D1), day 3 (D3), day 5 (D5), day 7 (D7) and day 9 (D9), post infection for quantitative real time PCR analysis. (C) Representative western blot and densitometry analysis showing expression profile of HSPA14 in HIV pseudovirus infected cells at different time points. (E) mRNA expression profiling of HSPA14 during HIV infection. Different cells were infected with HIV pseudovirus and were harvested on day 1 (D1), day 3 (D3), day 5 (D5) and day 7 (D7), post infection for quantitative real time PCR analysis. (F) Representative western blot and densitometry analysis showing expression profile of HSPA14 in HIV pseudovirus infected CD4+T cells at different time points. CD4+T cells were isolated from PBMC of three healthy donors and were infected with HIV pseudovirus. The error bars are presented as the mean ± SD values and significance is defined as *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001. INF: Infected with HIV-1. UN, Uninfected.
3.3 HSPA14 interferes with HIV-1 replication
According to the evidence we observed in Figure 2, we speculate that HSPA14 can also interfere the HIV infection. In order to prove this, we assessed the role of HSPA14 during HIV infection. We examined the effect of HSPA14 on virus production by measuring the expression of viral capsid protein p24. Jurkat cells and CEM cells were transfected with different dose of HSPA14 plasmid or RNAi, followed by HIV pseudovirus infection and different levels assessment of p24. Significant inhibition in a dose-dependent manner of virus production was observed with HSPA14 over-expression in different cells (Figure 3A, B). Moreover, HSPA14 knockdown resulted in increased HIV production in a dose-dependent manner in different cells (Figure 3C, D). These results suggesting that HSP14 suppress viral gene-expression probably both at transcription level and protein level.
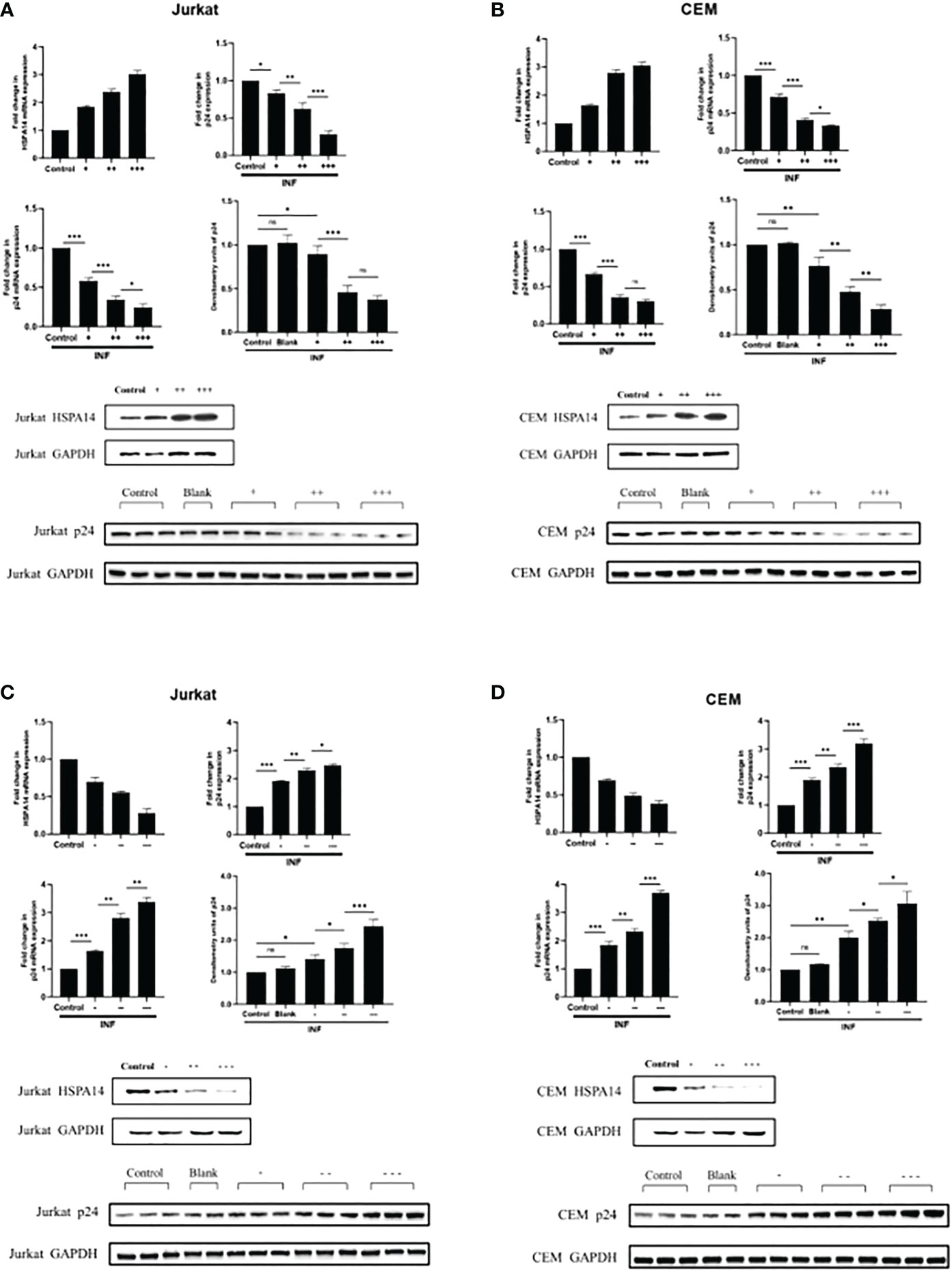
Figure 3 HSPA14 can interfere the HIV-1 replication (A) Jurkat cells were transfected with indicated plasmids. 24 hours post-transfection, cells were infected with HIV pseudovirus. 24 hours post-infection, cells were harvested to isolate total RNA and protein, and culture supernatants were collected. Upper left panel: qRT-PCR analysis showing different dose of HSP14 over-expression in Jurkat cells. Upper right panel: Culture supernatants were examined for amount of virus produced using p24 antigen capture ELISA. Middle left panel: qRT-PCR analysis revealing change of p24 mRNA levels. Middle right panel and lower panel: representative western blot and densitometry analysis revealing change of p24 protein levels. (B) CEM cells were transfected with indicated plasmids as Jurkat cells. Upper left panel: qRT-PCR analysis showing different dose of HSP14 over-expression in CEM cells. Upper right panel: Culture supernatants were examined for amount of virus produced using p24 antigen capture ELISA. Middle left panel: qRT-PCR analysis revealing change of p24 mRNA levels. Middle right panel and lower panel: representative western blot and densitometry analysis revealing change of p24 protein levels. (C) Jurkat cells were transfected with indicated RNAi. 24 hours post-transfection, cells were infected with HIV pseudovirus. 24 hours post-infection, cells were harvested to isolate total RNA and protein, and culture supernatants were collected. Upper left panel: qRT-PCR analysis showing different dose of HSP14 knockdown in Jurkat cells. Upper right panel: Culture supernatants were examined for amount of virus produced using p24 antigen capture ELISA. Middle left panel: qRT-PCR analysis revealing change of p24 mRNA levels. Middle right panel and lower panel: representative western blot and densitometry analysis revealing change of p24 protein levels. (D) CEM cells were transfected with indicated RNAi as Jurkat cells. Upper left panel: qRT-PCR analysis showing different dose of HSP14 knockdown in CEM cells. Upper right panel: Culture supernatants were examined for amount of virus produced using p24 antigen capture ELISA. Middle left panel: qRT-PCR analysis revealing change of p24 mRNA levels. Middle right panel and lower panel: representative western blot and densitometry analysis revealing change of p24 protein levels. Control: cell lines were transfected with vector plasmids as control. Blank: cell lines were not transfected with any plasmids as blank control. +: 500ng/μl of HSPA14 overexpression plasmid, ++: 1μg/μl of HSPA14 overexpression plasmid, +++: 2μg/μl of HSPA14 overexpression plasmid. -: 50nM of HSPA14 shRNA plasmi, –: 100nM of HSPA14 shRNA plasmid, —: 200nM of HSPA14 shRNA plasmid.The error bars are presented as the mean ± SD values and significance is defined as *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001.
3.4 Comparison of HSPAs between acute HIV infected patients with different HIV RNA
We have observed that the HIV infection can suppress the HSPA14 and HSPA14 can interfere the HIV replication in vitro. To further confirm the above findings in vivo, we examined differences in the expression levels of eight HSPAs, including HSPA14, in peripheral blood CD4+ T cells from untreated acute HIV infected patients with similar general statue but different HIV RNA levels. In HVL patients, HSPA2 and HSPA5 were found to have higher transcript and protein expression levels, while HSPA9 and HSPA13 only had higher transcript levels. HSPA14 had lower transcript and protein expression levels, while HSPA6 and HSPA13 only had lower transcript levels (Figure 4). These results are similar to the findings of in vitro studies, which further demonstrates the important inhibitory role of HSPA14 in HIV replication. In the same time, we also identify other HSPA isoforms, such as HSPA2 and HSPA5, that may be involved in and affect HIV replication.
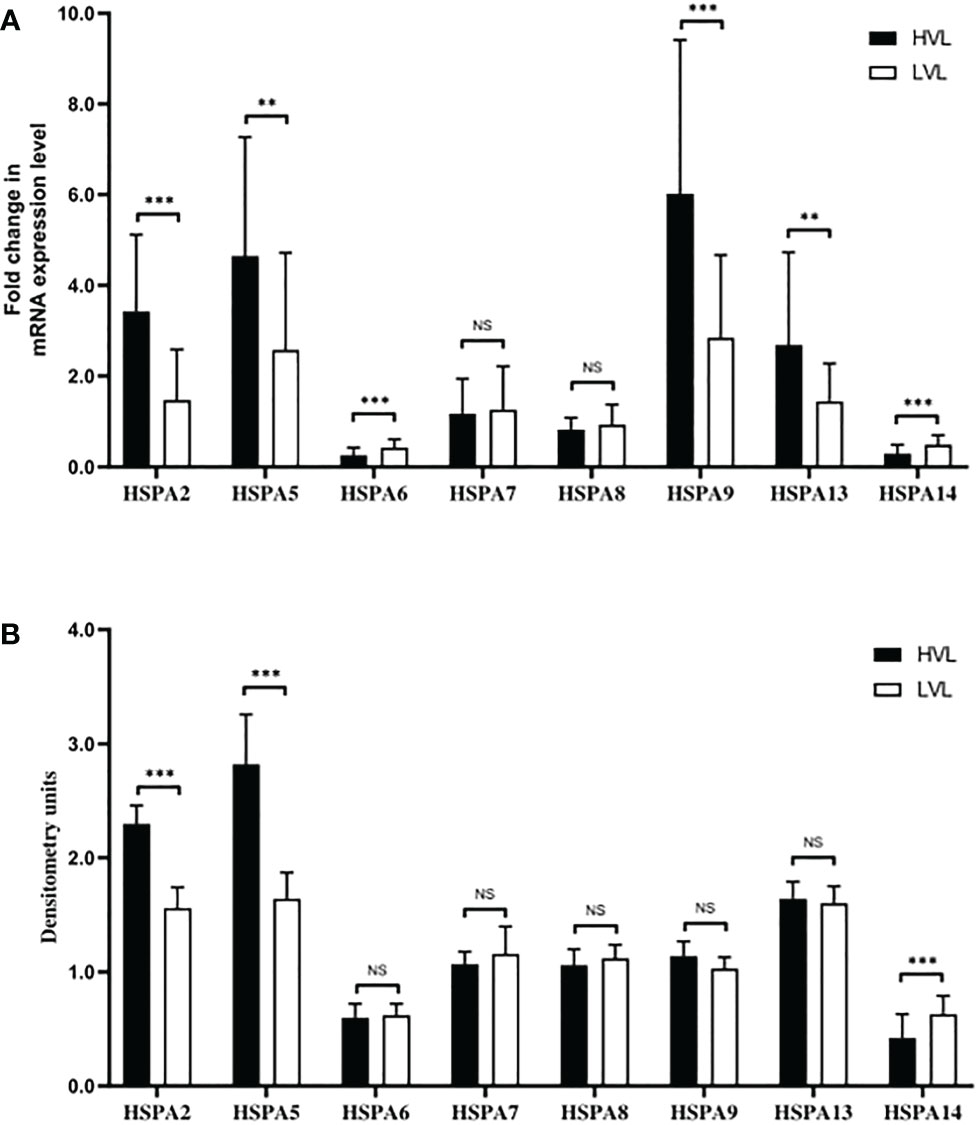
Figure 4 Different expression levels of HSPAs in HVL and LVL patients. CD4+ T cells from PBMC of 70 patients were isolated and harvested for RNA and protein lysate preparation. (A) Analyzing and comparing the different HSPA isoforms (including HSPA2, HSPA5, HSPA6, HSPA7, HSPA8, HSPA9, HSPA13 and HSPA14) transcription levels between the LVL and HVL patients by qRT-PCR. (B) Comparing the different HSPA isoforms protein levels between the LVL and HVL patients by western blot and densitometry analysis. Significance is defined as NS: P>0.05, *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001.
4 Discussion
In the past few decades, several studies have shown that HSPs play important roles in various viral infections. For example, latent infection of herpes simplex virus-2 (HSV-2) increased the HSPs in human neuroblastoma cells, HSP70 mRNA levels increased in mouse L cells after HSV-1 and HSV-2 infection, and the expression level of HSP70 and HSP90 were increased in human B lymphocytes infected with EB virus (13–15). In addition, HSP70 is associated with the capsid precursor P1 of poliovirus type 1 and coxsackievirus B1 (16), the expression levels of HSP70 and HSP40 in lymphocytes of HIV-1 infected patients increased as well (17). A systematic review has summarized the identified “candidate inhibitors” of various HIV-1 infections, and a variety of subtypes of HSP40s, HSP70s and HSP90s are involved in different stages of the HIV-1 life cycle (18). HSP70 contain 13 kinds of subtypes, several reports have demonstrated the significance of HSP70 subtypes in HIV-1 life cycle (19). HSPA5 is involved in the folding of viral Env proteins in the ER, while other HSPA subtypes interact differently with viral proteins such as Tat, Gag, and Vpr (20–22). Compared with HIV-1CladeC gp120 in astrocytoma, HIV-1CladeB gp120 can induce the expression of HSPA5 and other ER stress markers, and HIV-1Tat can also induce HSPA5 and other ER stress markers (23). HSPA9 was proven to be a necessary helper protein for Nef secretion in the exocrine system. Kammula et al. proved that there was an interaction between HSPA9 and Nef by yeast two-hybrid screening (24).
Although the role of HSP70s in HIV-1 infection is more important than that of other HSPs, further research is needed to clarify the specific function of each subtype. For example, although HSPA8 has been shown to play an important role in replication and assembly of other viruses, its role in HIV-1 infection remains unclear (25, 26). HSPA2 and HSPA1L do not show clear changes in other viral infections or HIV-1 infections, because they are enriched in testicular tissue, which may play vital roles in the process of HIV-1 invasion (27, 28). In addition, although HSPA13 has been shown to interact with the Env protein, the effect of HSPA12, HSPA13 and HSPA14 to HIV-1 are unclear. Thus, this study aims to investigate the role of HSPA14 in HIV-1 infection. We found that the mRNA and protein levels of HSPA14 in the Jurkat, CEM and TZM-bl cells decreased gradually with increasing HIV-1 infection time. Further verification with CD4+ T cells isolated from healthy human peripheral blood showed that the mRNA and protein levels of HSPA14 in the CD4+ T cells infected with HIV-1 pseudoviral particles also decreased with increasing infection time. The above results indicate that HIV-1 replication can inhibit the expression of intracellular HSPA14 at the transcriptional and translational levels in vitro infection experiments.
Furthermore, we investigated the effect of HSPA14 on virus replication. The results showed that the mRNA and protein levels of p24 decreased with increasing HSPA14 overexpression in the Jurkat and CEM cell lines overexpressing HSPA14, while the mRNA and protein levels of p24 increased with decreasing HSPA14 expression in the Jurkat and CEM cells infected with HIV-1 pseudovirions. All the above results showed that HSPA14 could inhibit HIV-1 replication. Based on these, we speculate that HSPA14 may act as a transcriptional regulator to inhibit viral replication by directly interacting with cis-acting element binding site on HIV-1 LTR, and may also indirectly inhibit provirus transcription by suppressing transcriptional regulators which promote HIV replication. In addition, HIV-1 might also be developing counteracting strategies to counteract the transcriptional repressive effect caused by HSPA14, such as some viral proteins may reduce intracellular transcription and translation levels of HSPA14 in order to relieve the viral repression by HSPA14.
HspBP1, as a helper protein of HSP70, inhibits its folding activity by binding to the ATPase domain of HSP70 (29). Previous studies have shown that the expression of HspBP1 IgG in the serum of people infected with HIV-1 increases (30). As an intrinsic inhibitor of HIV-1, previous studies have shown that HspBP1 can inhibit HIV-1 replication by competing to inhibit NF-kappa B on the HIV-1LTR promoter. cells (31). In the present study, Co-IP showed that there was a protein-protein interaction between HSPA14 and HspBP1 in both HIV-1 infected Jurkat cells and CEM cells, indicating that the HSPA14 may also participate in regulation of HIV-1 replication by HspBP1.
We further explored the differences in the expression levels of the HSPA subfamily members between HVL patients and LVL patients. The results showed that the transcriptional levels of HSPA2, HSPA5, HSPA9 and HSPA13 were higher and the protein levels of HSPA2 and HSPA5 were also higher in the HVL group. In the LVL group, the transcriptional levels of HSPA6 and HSPA14 were higher and the protein levels of HSPA14 were higher. These results are similar to the findings of previous studies, indicate that besides the HSPA2, HSPA5 and HSPA14 may play important roles in HIV-1 replication, which in ture affects the level of virus replication in vivo (32).
5 Conclusion
Firstly, we found that the HSPA subfamily of HSPs has important roles. Then, by comparing the expression levels of different HSPA subtypes in patients with different virological levels, we found that HSPA14 is most likely to affect the replication level of HIV-1. Finally, through the in vitro cell experiments, we found that the expression level of HSPA14 was inhibited after HIV-1 infection, HSPA14 also inhibited the replication level of the virus and may play a role similar to that of the viral replication inhibitor HspBP1. There are some limitations in this study. First of all, in vivo studies of the effect of HSPA14 on HIV-1 replication are lacking due to the limitations of the study conditions. Secondly, the research on the mechanism of HSPA14 affecting HIV-1 replication is superficial, and the interaction between viral proteins (such as Gag, Pol, Env, Tat, Rev, Nef and Vpr) and HSPA14 has not been explored. At the same time, we only explored the effect of HSPA14 on HIV-1 replication by simulating the infection state with HIV pseudovirus, which may be different from the state of the infection state with real HIV-1 virus.Overall, this study showed that HSPA14 can inhibit HIV-1 replication, which provides a research basis for further exploration of its usage as a target for inhibition of HIV replication.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Tangdu Hospital (Num. 201911-04). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MB, WK, and YS designed the study and collected the blood samples. MB performed the immunoblotting, PCR, and other experiments in vitro. WK and YS provided expert guidance and statistical analysis. MB wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Major Program of National Natural Science Foundation of China (2017ZX10202102-002-001), the Major Program of National Natural Science Foundation of China (2017ZX10202101-004-005) and the Major Program of National Natural Science Foundation of China (2017ZX10202101-003-007).
Acknowledgments
The authors thank the study teams and study participants who provided data and samples for this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Crespo R, Rao S, Mahmoudi T. HibeRNAtion: HIV-1 RNA metabolism and viral latency. Front Cell Infect Microbiol (2022) 12:855092. doi: 10.3389/fcimb.2022.855092
2. Lima VD, Fink V, Yip B, Hogg RS, Harrigan PR, Montaner JS. Association between HIV-1 RNA level and CD4 cell count among untreated HIV-infected individuals. Am J Public Health (2009) 99 Suppl 1(Suppl 1):S193–6. doi: 10.2105/AJPH.2008.137901
3. Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS (2007) 21(13):1723–30. doi: 10.1097/QAD.0b013e3281532c82
4. Liu Y, Margot N, Kitrinos KM, Callebaut C. Development of an HIV-1 integrase genotyping assay for HIV-1 subtype AE. J Med Virol (2019) 91(5):890–3. doi: 10.1002/jmv.25376
5. Ghimire D, Rai M, Gaur R. Novel host restriction factors implicated in HIV-1 replication. J Gen Virol (2018) 99(4):435–46. doi: 10.1099/jgv.0.001026
6. Friedrich BM, Dziuba N, Li G, Endsley MA, Murray JL, Ferguson MR. Host factors mediating HIV-1 replication. Virus Res (2011) 161(2):101–14. doi: 10.1016/j.virusres.2011.08.001
7. Anderson I, Low JS, Weston S, et al. Heat shock protein 90 controls HIV-1 reactivation from latency. Proc Natl Acad Sci U S A (2014) 111(15):E1528–37. doi: 10.1073/pnas.1320178111
8. Li G, Bukrinsky M, Zhao RY. HIV-1 (Vpr) and its interactions with host cell. Curr HIV Res (2009) 7(2):178–83. doi: 10.2174/157016209787581436
9. Bolhassani A, Agi E. Heat shock proteins in infection. Clin Chim Acta (2019) 498:90–100. doi: 10.1016/j.cca.2019.08.015
10. Guerra S, González JM, Climent N, et al. Selective induction of host genes by MVA-b, a candidate vaccine against HIV/AIDS. J Virol (2010) 84(16):8141–52. doi: 10.1128/JVI.00749-10
11. Gomez-Sucerquia LJ, Blas-Garcia A, Marti-Cabrera M, Esplugues JV, Apostolova N. Profile of stress and toxicity gene expression in human hepatic cells treated with efavirenz. Antiviral Res (2012) 94(3):232–41. doi: 10.1016/j.antiviral.2012.04.003
12. Flachbartová Z, Kovacech B. Mortalin - a multipotent chaperone regulating cellular processes ranging from viral infection to neurodegeneration. Acta Virol (2013) 57(1):3–15. doi: 10.4149/av_2013_01_3
13. Pich D, Mrozek-Gorska P, Bouvet M, et al. First days in the life of naive human b lymphocytes infected with Epstein-Barr virus. mBio (2019) 10(5):e01723–19. doi: 10.1128/mBio.01723-19
14. Wong A, Rawson RV, Templeton DJ. Herpes simplex virus-2 (HSV-2) pseudotumour as the initial presentation of human immunodeficiency virus infection. Intern Med J (2021) 51(6):1001–2. doi: 10.1111/imj.15364
15. Oliveira MCPV, Pereira EM, Sereia MJ, et al. Expression of MRJP3 and HSP70 mRNA levels in apis mellifera l. workers after dietary supplementation with proteins, prebiotics, and probiotics. Insects (2022) 13(7):571. doi: 10.3390/insects13070571
16. Macejak DG, Sarnow P. Association of heat shock protein 70 with enterovirus capsid precursor P1 in infected human cells. J Virol (1992) 66(3):1520–7. doi: 10.1128/JVI.66.3.1520-1527.1992
17. Chand K, Iyer K, Mitra D. Comparative analysis of differential gene expression of HSP40 and HSP70 family isoforms during heat stress and HIV-1 infection in T-cells. Cell Stress Chaperones (2021) 26(2):403–16. doi: 10.1007/s12192-020-01185-y
18. Gélinas JF, Gill DR, Hyde SC. Multiple inhibitory factors act in the late phase of HIV-1 replication: a systematic review of the literature. Microbiol Mol Biol Rev (2018) 82(1):e00051–17. doi: 10.1128/MMBR.00051-17
19. Iyer K, Chand K, Mitra A, Trivedi J, Mitra D. Diversity in heat shock protein families: functional implications in virus infection with a comprehensive insight of their role in the HIV-1 life cycle. Cell Stress Chaperones (2021) 26(5):743–68. doi: 10.1007/s12192-021-01223-3
20. Wheeler DS, Dunsmore KE, Wong HR. Intracellular delivery of HSP70 using HIV-1 tat protein transduction domain. Biochem Biophys Res Commun (2003) 301(1):54–9. doi: 10.1016/s0006-291x(02)02986-8
21. SenGupta D, Norris PJ, Suscovich TJ, et al. Heat shock protein-mediated cross-presentation of exogenous HIV antigen on HLA class I and class II. J Immunol (2004) 173(3):1987–93. doi: 10.4049/jimmunol.173.3.1987
22. Iordanskiy S, Zhao Y, DiMarzio P, Agostini I, Dubrovsky L, Bukrinsky M. Heat-shock protein 70 exerts opposing effects on vpr-dependent and vpr-independent HIV-1 replication in macrophages. Blood (2004) 104(6):1867–72. doi: 10.1182/blood-2004-01-0081
23. Sun L, Ishihara M, Middleton DR, Tiemeyer M, Avci FY. Metabolic labeling of HIV-1 envelope glycoprotein gp120 to elucidate the effect of gp120 glycosylation on antigen uptake. J Biol Chem (2018) 293(39):15178–94. doi: 10.1074/jbc.RA118.004798
24. Kammula EC, Mötter J, Gorgels A, Jonas E, Hoffmann S, Willbold D. Brain transcriptome-wide screen for HIV-1 nef protein interaction partners reveals various membrane-associated proteins. PloS One (2012) 7(12):e51578. doi: 10.1371/journal.pone.0051578
25. Zhu P, Lv C, Fang C, et al. Heat shock protein member 8 is an attachment factor for infectious bronchitis virus. Front Microbiol (2020) 11:1630. doi: 10.3389/fmicb.2020.01630
26. Sztuba-Solinska J, Diaz L, Kumar MR, et al. A small stem-loop structure of the Ebola virus trailer is essential for replication and interacts with heat-shock protein A8. Nucleic Acids Res (2016) 44(20):9831–46. doi: 10.1093/nar/gkw825
27. Khachatoorian R, Cohn W, Buzzanco A, et al. HSP70 copurifies with zika virus particles. Virology (2018) 522:228–33. doi: 10.1016/j.virol.2018.07.009
28. Muhammad JS, Saheb Sharif-Askari N, Cui ZG, Hamad M, Halwani R. SARS-CoV-2 infection-induced promoter hypomethylation as an epigenetic modulator of heat shock protein A1L (HSPA1L) gene. Front Genet (2021) 12:622271. doi: 10.3389/fgene.2021.622271
29. McLellan CA, Raynes DA, Guerriero V. HspBP1, an Hsp70 cochaperone, has two structural domains and is capable of altering the conformation of the Hsp70 ATPase domain. J Biol Chem (2003) 278(21):19017–22. doi: 10.1074/jbc.M301109200
30. Ceccin ADF, Souza APD, Hilário GT, Muller DM, Romão PRT, Rodrigues Junior LC. HspBP1 and anti-HspBP1 levels in the serum of HIV-infected individuals are associated to the disease progression. J Appl Microbiol (2019) 127(2):576–85. doi: 10.1111/jam.14230
31. Chaudhary P, Khan SZ, Rawat P, et al. HSP70 binding protein 1 (HspBP1) suppresses HIV-1 replication by inhibiting NF-κB mediated activation of viral gene expression. Nucleic Acids Res (2016) 44(4):1613–29. doi: 10.1093/nar/gkv1151
Keywords: HIV, replication, HSPA14, HspBP1, HIV RNA
Citation: Bi M, Kang W and Sun Y (2023) Expression of HSPA14 in patients with acute HIV-1 infection and its effect on HIV-1 replication. Front. Immunol. 14:1123600. doi: 10.3389/fimmu.2023.1123600
Received: 14 December 2022; Accepted: 30 January 2023;
Published: 09 February 2023.
Edited by:
Tomozumi Imamichi, Frederick National Laboratory for Cancer Research (NIH), United StatesReviewed by:
Tesfaye Gelanew, Armauer Hansen Research Institute, EthiopiaJay Trivedi, Rhode Island Hospital, United States
Copyright © 2023 Bi, Kang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongtao Sun, MTM1NzI5NzI2MTlAMTM5LmNvbQ==; Wen Kang, ODIzOTk1MjZAcXEuY29t
 Mingyuan Bi
Mingyuan Bi Wen Kang*
Wen Kang*