- 1School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Oncology, Nanchong Central Hospital, the Second Clinical Medical College, North Sichuan Medical College, Nanchong, China
- 3Department of Interventional Radiology, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China
Background: Programmed cell death ligand 1 (PD-L1) is highly expressed in intrahepatic cholangiocarcinoma (ICC) tissues. But there is still a dispute over the prognostic value of PD-L1 in patients with ICC. This study aimed to evaluate the prognostic value of PD-L1 expression in patients with ICC.
Methods: We performed a meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Guidelines. We searched the literature from PubMed, Embase, Web of Science, and the Cochrane Library up to December 5, 2022. Hazard ratios (HR) and their 95% confidence intervals (95% CI) were calculated to analyze the overall survival (OS), recurrence-free survival (RFS), and time to relapse. The quality of the studies was assessed using the Newcastle-Ottawa scale. Publication bias was assessed using a funnel plot and Egger’s test.
Results: Ten trials with 1944 cases were included in this meta-analysis. The results showed that the low-PD-L1 group had a statistically significant advantage in OS (HR, 1.57; 95% CI, 1.38–1.79, P <0.00001), RFS (HR, 1.62; 95% CI, 1.34–1.97, P <0.00001), and time to relapse (HR, 1.60; 95% CI, 1.25–2.05, P = 0.0002) compared with the high-PD-L1 group. High programmed cell death (PD1)levels, on the other hand, were correlated with poorer OS (HR, 1.96; 95% CI, 1.43–2.70; P <0.0001) and RFS (HR, 1.87; 95% CI, 1.21–2.91; P = 0.005). Multivariate analysis showed that PD-L1 could act as an independent predictor for OS (HR, 1.48; 95% CI, 1.14–1.91; P = 0.003) and RFS (HR, 1.74; 95% CI, 1.22–2.47; P = 0.002), and PD1 acted as an independent predictor for OS (HR, 1.66; 95% CI, 1.15–2.38; P = 0.006).
Conclusion: This meta-analysis demonstrated that high PD-L1/PD1 expression is associated with poor survival in ICC. PD-L1/PD1 may be a valuable prognostic and predictive biomarker and potential therapeutic target in ICC.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022380093.
1 Introduction
Intrahepatic cholangiocarcinoma (ICC) accounts for over 20% of primary liver cancers and is the second most common primary liver tumor after hepatocellular carcinoma (1). ICC is a highly lethal neoplasm because it is always misdiagnosed and there is no effective therapeutic regimen to control it, which is not sensitive to chemotherapy and radiotherapy (2). Therefore, an effective therapeutic target has recently been focused on ICC worldwide.
The programmed cell death (PD) ligand 1 (PD1)/PD-L1 axis is one of the most important immune checkpoints and a valuable therapeutic target because it plays a critical role in facilitating immune evasion (3). Tumor cells express PD-L1, which binds to PD1 to evade antitumor responses (4). PD-L1 also plays an important role in subsequent tumor progression by binding to PD1 and activating proliferative and survival signaling pathways (5). Furthermore, PD-L1 expression can be modulated by various tumorigenesis-related signaling pathways, including the phosphoinositide 3-kinase–protein kinase B/Akt (PI3K/AKT) (6–8), mitogen-activated protein kinase (MAPK) (9), and Wingless/Integrated (WNT) pathways (10). An increasing number of studies have clearly demonstrated that PD-L1 is overexpressed in various malignant tumors, such as nasopharyngeal carcinoma, lung cancer, and hepatocellular carcinoma (11–16). Additionally, PD-L1 expression is significantly associated with prognosis and clinicopathological features; for example, PD-L1 overexpression contributes to poor prognosis in gastric, colorectal, breast, and pancreatic cancers, and renal cell and hepatocellular carcinomas (17–21). Furthermore, many studies have found that PD-L1 is highly expressed in ICC and is markedly related to the prognosis of patients with ICC (22, 23). However, few studies have reported the efficacy of anti-PD1/PD-L1 treatment for ICC (24, 25), and PD-L1 as a therapeutic target for ICC is still in demand, which requires further evaluation.
Therefore, we performed this meta-analysis to assess the effect of PD-L1 expression on the prognosis of ICC. The purpose of this study was to evaluate the correlation between PD-L1 overexpression and survival in ICC, thereby shedding more light on the development of PD-L1 targeted therapy and prognostic prediction.
2 Materials and methods
This study has been conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Guidelines (26, 27) (Supplementary Table 1).
2.1 Search strategy
Two investigators independently searched the online databases PubMed, Embase, Cochrane Library, and Web of Science for interrelated studies up to December 5, 2022. The search terms were “intrahepatic cholangiocarcinoma” AND “programmed cell death ligand 1” OR “PD-L1” OR “B7-H1” (Supplementary Table 2). Reference lists from published studies were also searched.
2.2 Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) patients who were diagnosed with ICC; (2) articles that were published in English with full texts, involving humans as study subjects; (3) the correlation between PD-L1 and survival (overall survival [OS], recurrence-free survival [RFS], and time to relapse [TTR]) was detected; (4) the hazard ratio (HR) and 95% confidence intervals (CI) for survival times were computed by included articles that provided sufficient data; and (5) PD-L1 expression levels were gauged in clinical ICC tissues. The exclusion criteria were as follows: (1) patients with ICC and other cancers; (2) case reports, abstracts, and reviews; (3) duplicated articles; and (4) articles with incomplete data.
2.3 Data extraction
Two reviewers independently extracted the data. Any disagreements regarding the information were resolved by consensus or by the judgment of a third reviewer. The following data were extracted from each study: authors of the article, year of publication, detection method, region, cutoff value, follow-up time, and HRs with 95% CI (28). The main outcomes were PD-L1 expression-related OS, RFS, and TTR. The following data were also extracted if the study contains: PD1 expression-related survival data. While the original survival data were hardly accessed, the extracted data from the Kaplan–Meier curves were obtained using the software Engauge Digitizer version12.1.
2.4 Quality assessment
Two reviewers independently evaluated the quality of the included literature using a 9-score system of the Newcastle-Ottawa Scale (NOS) (29). The NOS comprises eight items, with a score >6 indicating high-quality research.
2.5 Statistical analysis
All data analyses were performed using Review Manager version 5.3 and Stata Software version 14.0. We applied pooled HRs with their 95% CIs to evaluate the association between prognostic value and the expression levels of PD-L1 in ICC. Cochran’s Q and I2 statistics were used to assess the heterogeneity among these articles (30). Heterogeneity was considered insignificant when P >0.10 or I2 <50%, and a fixed-effects model was used to pool the effects; otherwise, a random-effects model was used. Funnel plot and Egger’s test were used to estimate publication bias, and a P value of <0.05 implied statistical significance.
3 Results
3.1 Selected studies
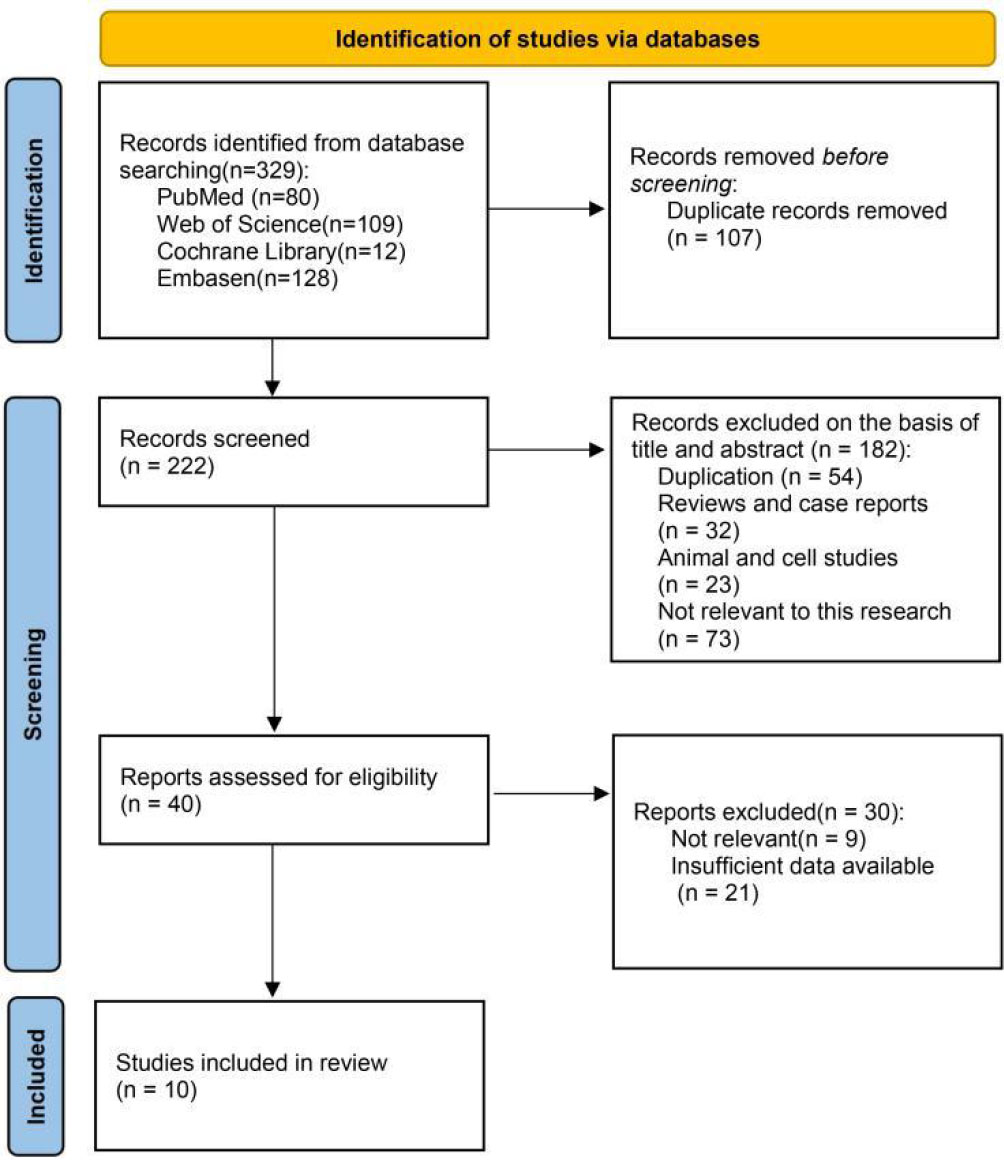
We searched for 329 trials in four electronic databases. Subsequently, we excluded 107 duplicates, 182 records after screening the titles and abstracts, and 30 records due to irrelevant or incomplete data. Eventually, ten trials (22, 31–39) and 1944 cases were included according to the inclusion and exclusion criteria (Figure 1).
3.2 Study characteristics and quality assessment
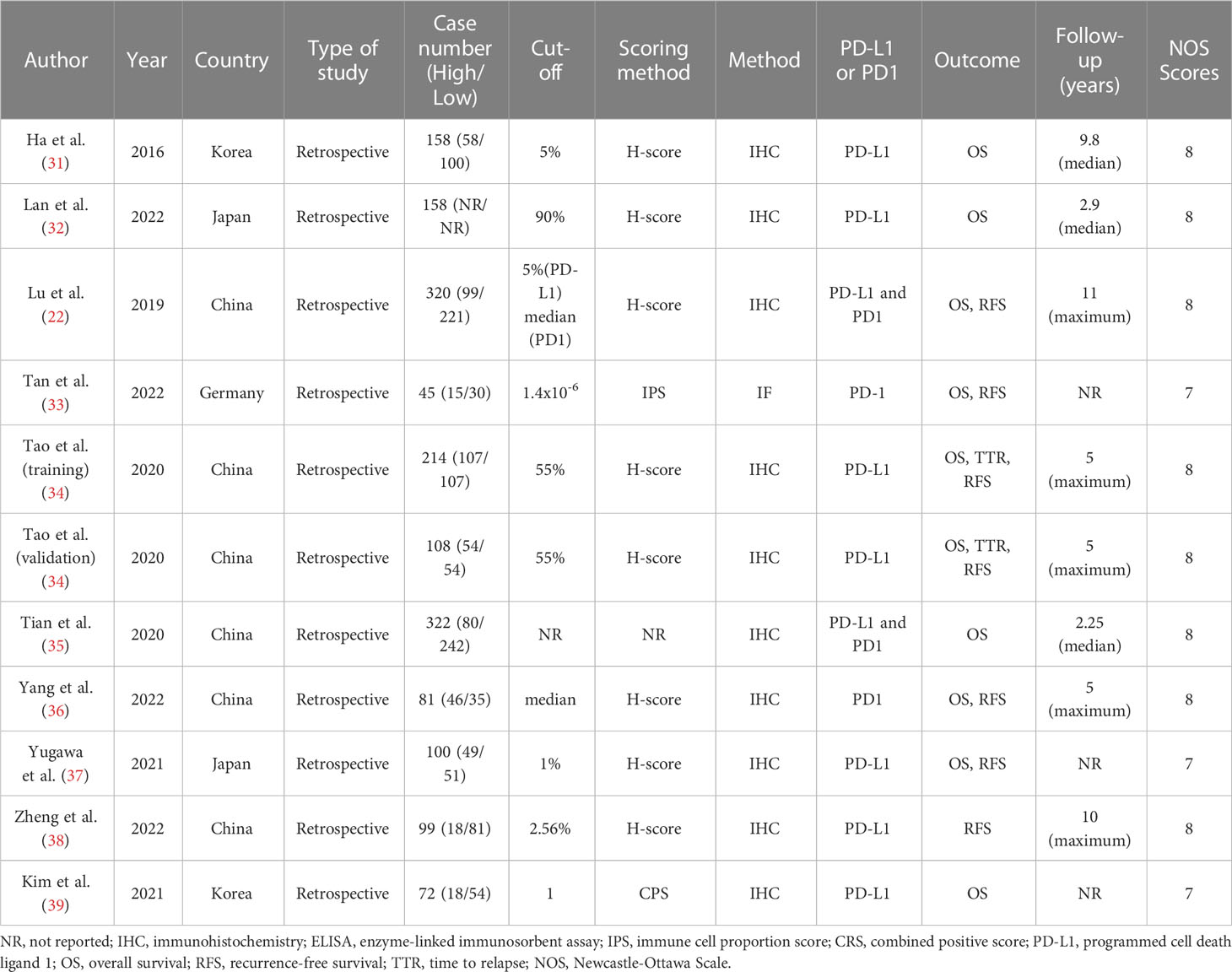
Nine studies were included from Asia (China, Korea, and Japan), and one was from Germany. All of the included studies used either immunohistochemistry or immunofluorescence to detect PD-L1 expression. Moreover, we extracted two sets of survival data from one trial with the training and validation cohorts. Four studies reported the effect of PD1 on the prognosis of ICC. The quality of the included trials was assessed by NOS, and all included studies were high-quality studies. The NOS scores and characteristics of the included studies are summarized in Table 1, the quality evaluations of all included articles are in Supplementary Table 3.
3.3 OS
Regarding the effect of PD-L1 expression on OS, the low-PD-L1 group had a significantly better OS (HR, 1.57; 95% CI, 1.38–1.79; P <0.00001; I2, 26%; P = 0.22) than the high-PD-L1 group (Figure 2A). Furthermore, multivariate analysis showed that PD-L1 was also a significant independent prognostic factor (HR, 1.48; 95% CI, 1.14–1.91; P = 0.003; I2, 14%; P = 0.31) (Figure 2B). While concerning the effect of PD1 expression on OS, a statistically significant difference in OS (HR, 1.96; 95% CI, 1.43–2.70; P <0.0001) was found between the PD1 low and high groups (Figure 2C). Multivariate analysis showed that PD1 was also a significant independent prognostic factor (HR, 1.66; 95% CI, 1.15–2.38; P = 0.006) (Figure 2D). Namely, both high PD-L1 and PD1 expression were associated with poor OS of patients with ICC.
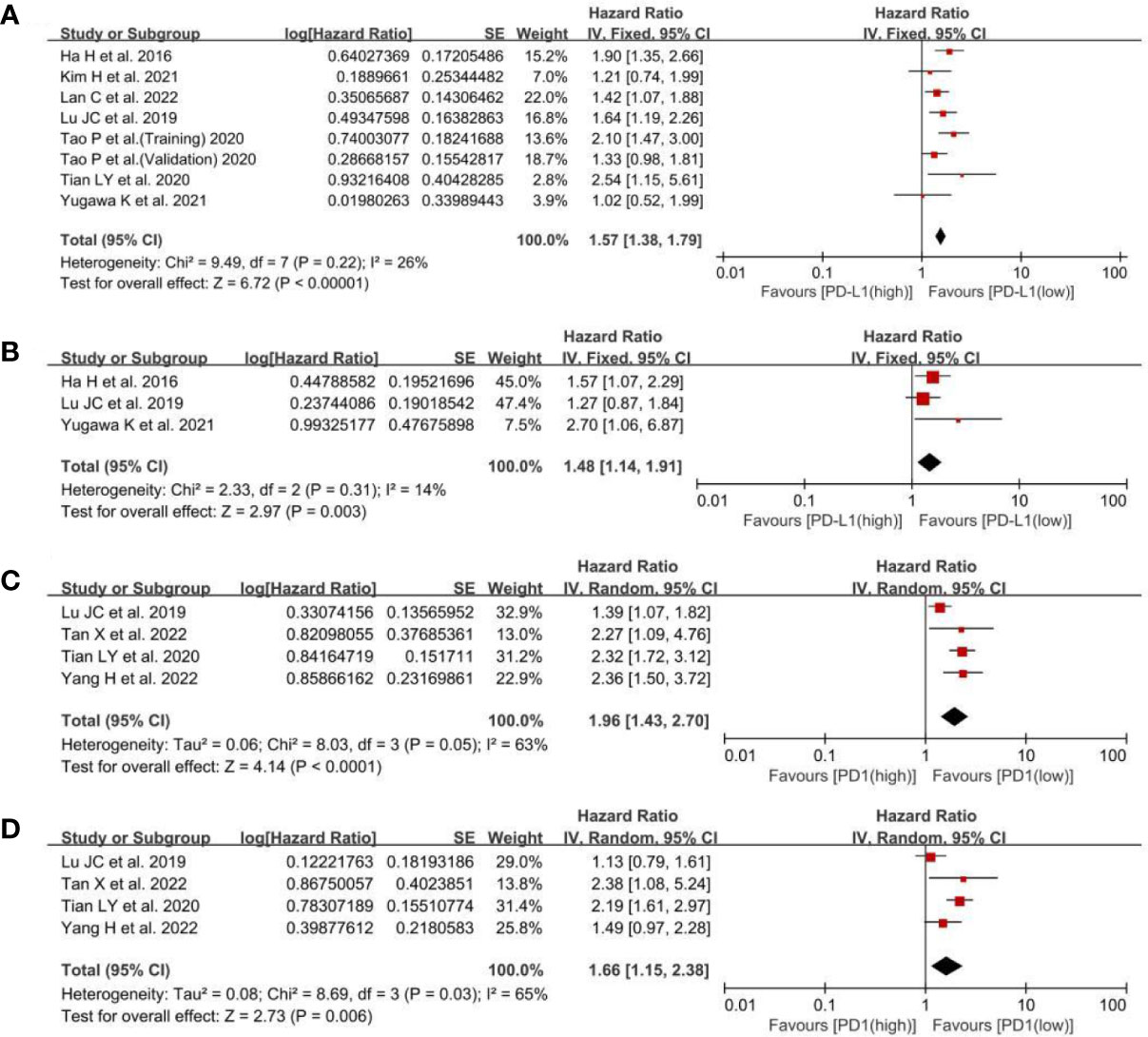
Figure 2 A forest plot of OS between low- and high-PD-L1 expression: (A) pooled OS by univariate analysis; (B) pooled OS by multivariate analysis. A forest plot of OS between low- and high-PD1 expression: (C) pooled OS by univariate analysis; (D) pooled OS by multivariate analysis.PD-L1, programmed cell death ligand 1; OS, overall survival; SE, standard error.
3.4 RFS
Regarding the effect of PD-L1 expression on RFS, the low-PD-L1 group had a statistically significant advantage in RFS (HR, 1.62; 95% CI, 1.34–1.97; P <0.00001) in univariate analysis and (HR, 1.74; 95% CI, 1.22–2.47; P = 0.002) (Figures 3A, B), means that the high level of PD-L1 was relevant to poor RFS and may be used to predict the recurrence time after antitumor therapy. For the effect of PD1 expression on RFS, there was a statistically significant difference in RFS (HR, 1.87; 95% CI, 1.21–2.91; P = 0.005) (Figure 3C), whereas multivariate analysis did not show any statistically significant difference in RFS (HR, 1.31; 95% CI, 0.85–2.04; P = 0.23; Figure 3D). The HRs of the RFS for PD1 expression were pooled using a random-effects model because there was slight heterogeneity among the three studies (I2, 62%; P = 0.07). Based on the above, the PD1 overexpression could be related to poor RFS, but there were not any correlations between PD1 levels and RFS in multivariate analysis.
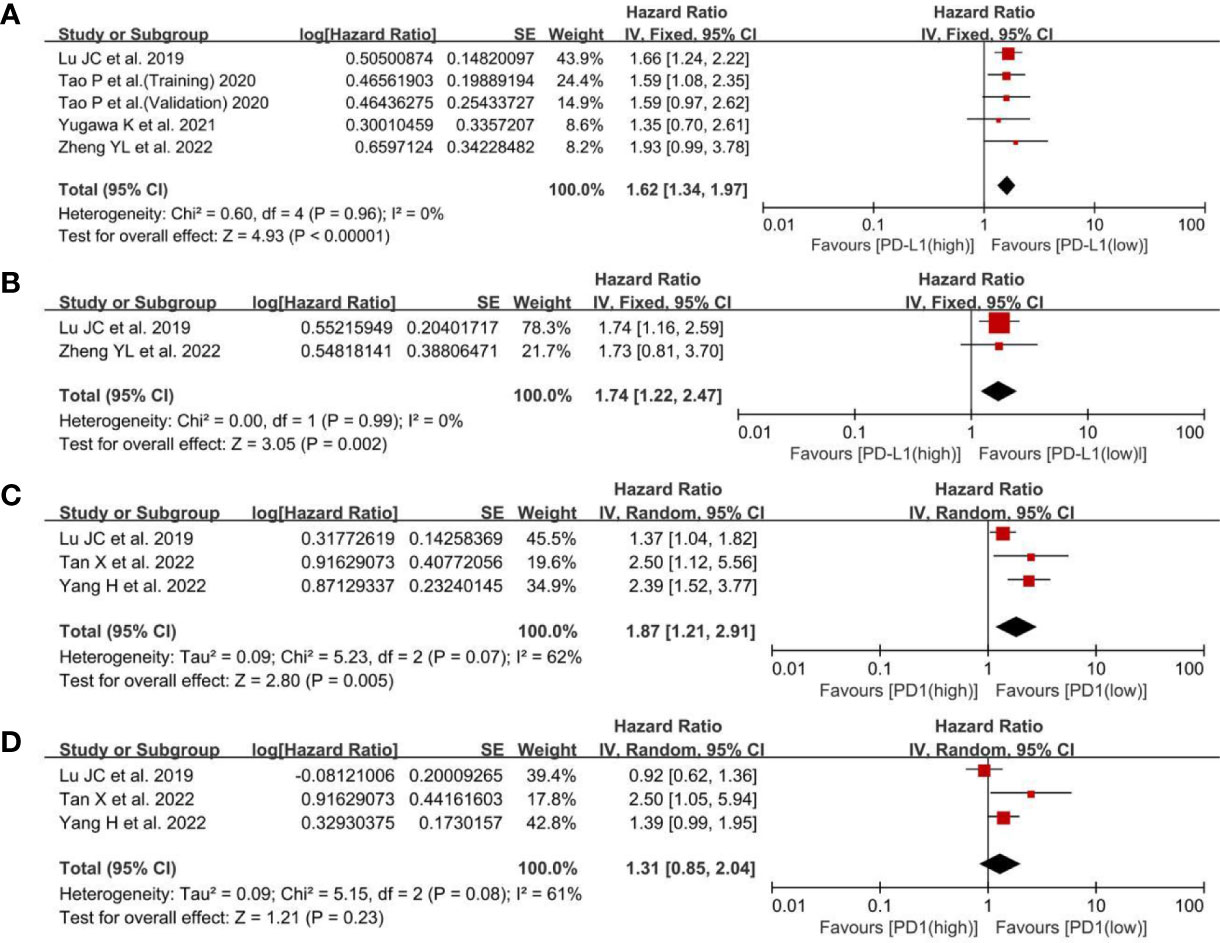
Figure 3 A forest plot of RFS between low- and high-PD-L1 expression: (A) pooled RFS in univariate analysis; (B) pooled RFS in multivariate analysis. A forest plot of RFS between low- and high-PD1 expression: (C) pooled RFS in univariate analysis; (D) pooled RFS in multivariate analysis. RFS, recurrence-free survival; PD-L1, programmed cell death ligand 1; SE, standard error.
3.5 TTR
Two cohorts in one trial reported the TTR of patients with PD-L1 expression. The results showed that the low-PD-L1 group had a statistically significant advantage in TTR (HR, 1.60; 95% CI, 1.25–2.05; P = 0.0002; I2, 0%; P = 0.49) (Figure 4). So, the low level of PD-L1 was beneficial to well TTR of ICC patients.
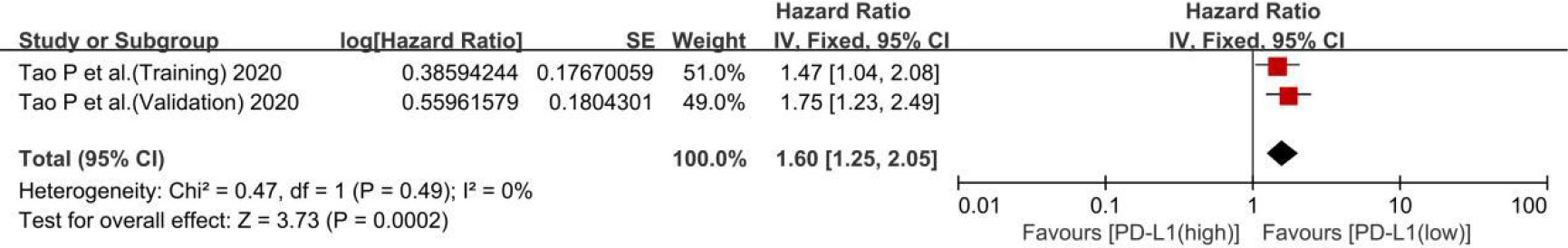
Figure 4 A forest plot of time to relapse between low- and high-PD-L1 expression. PD-L1, programmed cell death ligand 1; SE, standard error.
3.6 Subgroup analysis for PD-L1 expression
Considering that cut-offs for PD-L1 positivity, the scoring method may be important sources of heterogeneity, we conducted the subgroup analyses based on the cut-offs and scoring methods. The subgroups were divided by cut-offs (≤50%, >50% and not reported) and scoring methods (IHC, CPS and not reported). In OS, the results showed there were not any differences among the cut-off groups (I2, 0%; P = 0.42) (Figure 5A), and the scoring method groups (I2, 40.9%; P = 0.18) (Figure 5B). Besides, the cut-off groups also had no differences (I2, 0%; P = 0.87) (Figure 5C). Therefore, the cut-offs and scoring methods may not be the important sources of heterogeneity, means the different cut-offs and scoring method could not affect the pooled HRs.
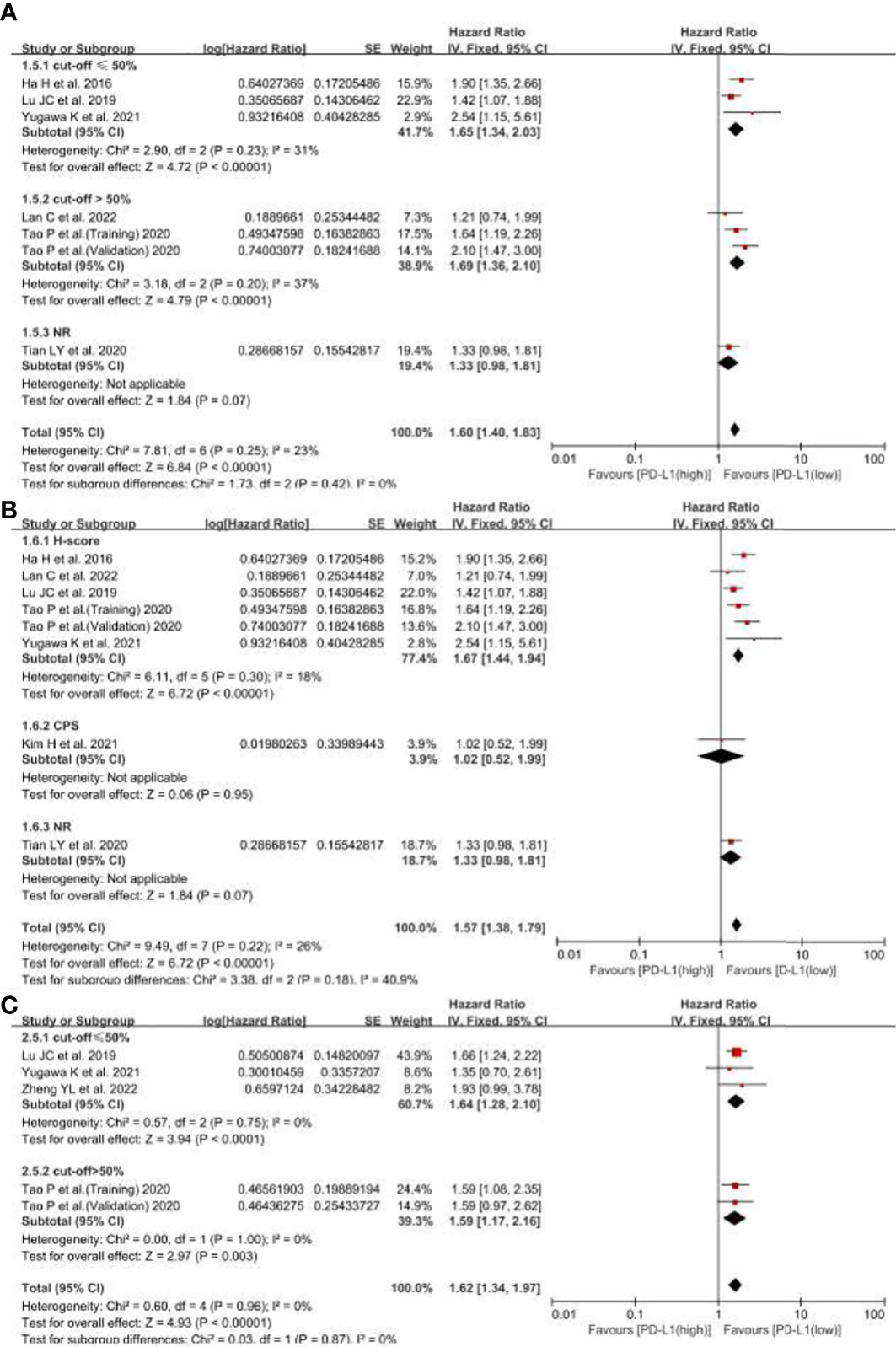
Figure 5 The subgroup analyses for PD-L1 expression related OS and RFS. (A) the subgroup analyses for PD-L1 expression related OS based on different cut-offs, (B) the subgroup analyses for PD-L1 expression related OS based on different scoring methods, (C) the subgroup analyses for PD-L1 expression related RFS based on cut-offs. NR, not reported.
3.7 Sensitivity analysis
We conducted a sensitivity analysis for OS (Figure 6A), RFS (Figure 6B), and TTR (Figure 6C) for PD-L1 expression and OS (Figure 6D) and RFS (Figure 6E) for PD1 expression in ICC by separately removing each study and then merging the effect quality. After excluding each study from the main analysis, the overall results showed no significant changes, which suggests that our combined results are reliable.

Figure 6 Sensitivity analysis of the meta-analysis: (A) OS in PD-L1 expression; (B) RFS in PD-L1 expression; (C) TTR in PD-L1 expression; (D) OS in PD1 expression; (E) RFS in PD1 expression. PD-L1, programmed cell death ligand 1; OS, overall survival; RFS, recurrence-free survival.
3.8 Publication bias
The funnel plots and Egger’s test were used to test for potential publication bias. The funnel plot showed that the left and right sides were symmetrical (Figures 7A–E). Egger’s test showed that all P >0.1, indicating a low probability of publication bias.
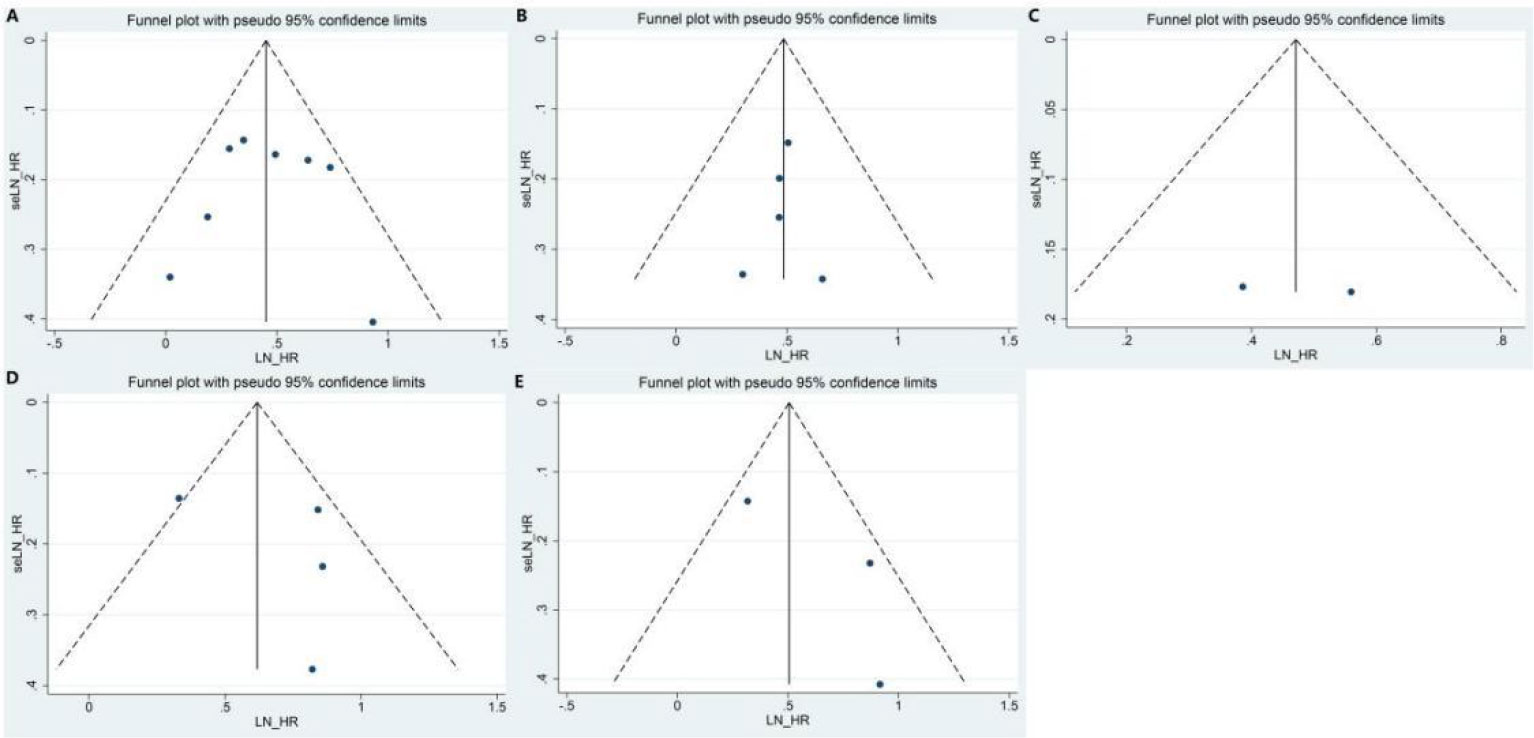
Figure 7 Funnel plot of the meta-analysis: (A) OS in PD-L1 expression; (B) RFS in PD-L1 expression; (C) TTR in PD-L1 expression; (D) OS in PD1 expression; (E) RFS in PD1 expression. PD-L1, programmed cell death ligand 1; OS, overall survival; RFS, recurrence-free survival; TTR, time to relapse.
4 Discussion
The high recurrence rate after surgical removal and low disease control response to treatment for ICC have become a problem for doctors and patients. This study is the first meta-analysis of the prognostic value of PD-L1 expression in patients with ICC. The results showed the prognostic value of PD-L1/PD1 staining in ICC.
In our study, the OS of the low-PD-L1 group was 1.57 times longer than that of the high group. This finding indicates that the prognosis of patients with high PD-L1 levels is worse due to the ability of PD-L1 to facilitate immune evasion (3). Interferon-γ can induce protein kinase D isoform, which can cause PD-L1 upregulation in various solid cancers, and inhibiting its activity could suppress PD-L1 expression and promote an obvious antitumor immune response (40). Moreover, the multivariate analysis also showed that the OS of the low-PD-L1 group was significantly different (HR, 1.96; P<0.0001), indicating that PD-L1 expression was an independent indicator of prognosis in patients with ICC. This finding is consistent with the feature of PD-L1 as a pro-tumorigenic factor (5).
The low-PD-L1 group also had statistically significant advantages in RFS and TTR compared to the high-PD-L1 group (HR, 1.74; P <0.00001 vs. HR, 1.60; P = 0.0002, respectively). These findings confirmed that patients with high PD-L1 expression had significantly higher recurrence rates than those with low PD-L1, especially after curative resection. Multivariate analysis showed that the RFS of the low-PD-L1 group was 1.74 times longer than that of the high-PD-L1 group. This indicates that PD-L1 levels could act as independent predictors of RFS and elevated recurrence. These results may be correlated with the fact that PD-L1 is active and maintains the proliferation of tumor cells (5). For example, the intrinsic pathway of PD-L1 promotes renal cell carcinoma progression by inducing stem cell-like phenotypes in renal cancer cells (41).
Furthermore, we analyzed the effect of PD1 expression on the prognosis of ICC. The results showed that the OS and RFS of PD1 low levels were statistically significantly longer than those of high levels (HR, 1.66; P = 0.006 vs. HR, 1.87; P = 0.005, respectively), which illustrated that PD1 has potential prognostic value for ICC. Multivariate analysis showed that PD1 also acted as an independent predictor for the OS of ICC (HR, 1.87; P = 0.005). PD1 is mainly expressed in activated immune cells as an inhibitor of innate immune responses. PD1 regulates the immune microenvironment by binding to PD-L1; therefore, in theory, PD1 has a similar predictive effect to PD-L1 (42). However, no statistically significant difference in RFS was observed in the multivariate analysis (HR, 1.31; P = 0.23), indicating that PD1 was not an independent predictor of RFS. Which is different from the relationship between PD1 and RFS in other cancers, such as PD1 positivity was an independent predictor of RFS in renal cell carcinoma (43). The number of infiltrated immune cells and the limited number of trials that were included could change the results. The correlation between PD1 expression and the RFS of ICC need to be further explored in future studies.
Therefore, PD-L1/PD1 inhibitors can be used as effective target medications for patients with ICC who exhibit PD-L1/PD1 overexpression. Zhu et al. reported that stage IIIB ICC could be treated with neoadjuvant therapy with a PD1 inhibitor (44). PD-L1/PD1 inhibitors combined with targeted therapy, chemotherapy, and radiotherapy had a synergistic effect on ICC, which improved disease control responses (24, 45, 46). PD-L1/PD1 inhibitors have achieved good results in the treatment of advanced or neoadjuvant ICC. We expect that this regimen could be utilized for patients with ICC as a first-line treatment rather than a salvage treatment.
In order to exclude other factors, we conducted subgroup analyses based on cut-offs and scoring methods. The results showed that both them did not change the pooled results, which is similar to some studies (47). For example, good overall concordance on analytic performance was observed for two assays (Dako 22C3 and Ventana SP263) with both scoring algorithms: the combined positive score (CPS) and tumor infiltrating immune cells (IC), but the SP 142 showed lower positivity rates, especially using the CPS algorithm (48). So different scoring methods could still affect the results. Besides, there were a similar correlation between PD-L1 levels and survival in papillary thyroid carcinoma, PD-L1 expression was significantly associated with a reduced disease-free survival (49). However, no association was found with the OS (49), which is opposite to the results in ICC. Which may be related to the different intensity of promoting cancer progression of PD-L1 in different cancers. Based on the above, the influence of PD-L1 expression on tumor prognosis may vary with different IHC assays, scoring methods and tumor types.
Our study has several limitations. First, due to a lack of data, further subgroup analysis according to clinicopathological features was not conducted. Second, because the majority of patients in our study were from Asia, the results could not fully represent other groups worldwide. Finally, the sample sizes of the included studies were insufficient to conduct a good pooled analysis, especially for PD1. Therefore, we expect that more valuable related trials will be reported in the future.
5 Conclusion
The meta-analysis suggested that high-PD-L1 expression levels predicted poorer OS, RFS, and TTR in patients with ICC. PD1 expression levels are also related to the prognosis of patients with ICC. Moreover, both PD-L1 and PD1 were independent predictors of OS, and PD-L1 was an independent predictor of RFS. Our results indicate that PD-L1/PD1 has great potential as an effective prognostic biomarker and therapeutic target in ICC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
FX and GX designed the study. FX and DR screened the studies and extracted data. The quality of the evidence was assessed using FX and JB. FX and DR analyzed and interpreted the data. FX and DR prepared figures and drafted the manuscript. JB and GX contributed to reviewing and editing the manuscript. All authors have approved the final version of the article, including the authorship list. All authors contributed to the article.
Funding
This study was funded by the Natural Science Foundation of Sichuan (No. 23ZDYF1517). Science and Technology Project of Nanchong (No. 22SXQT0066).
Acknowledgments
We want to thank Jing Wu from North Sichuan Medical College in Nanchong, China, for providing guidance on statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1119168/full#supplementary-material
References
1. Mejia JC, Pasko J. Primary liver cancers: intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Surg Clin North Am (2020) 100(3):535–49. doi: 10.1016/j.suc.2020.02.013
2. Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol (2020) 72(2):353–63. doi: 10.1016/j.jhep.2019.10.009
3. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity (2018) 48(3):434–52. doi: 10.1016/j.immuni.2018.03.014
4. Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med (2015) 21(1):24–33. doi: 10.1016/j.molmed.2014.10.009
5. Dong P, Xiong Y, Yue J, Hanley SJB, Watari H. Tumor-intrinsic PD-L1 signaling in cancer initiation, development and treatment: beyond immune evasion. Front In Oncol (2018) 8:386. doi: 10.3389/fonc.2018.00386
6. Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol (2016) 27(3):409–16. doi: 10.1093/annonc/mdv615
7. Wei F, Zhang T, Deng S-C, Wei J-C, Yang P, Wang Q, et al. PD-L1 promotes colorectal cancer stem cell expansion by activating HMGA1-dependent signaling pathways. Cancer Lett (2019) 450:1–13. doi: 10.1016/j.canlet.2019.02.022
8. Zhao R, Song Y, Wang Y, Huang Y, Li Z, Cui Y, et al. PD-1/PD-L1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Prolif (2019) 52(3):e12571. doi: 10.1111/cpr.12571
9. Stutvoet TS, Kol A, de Vries EG, de Bruyn M, Fehrmann RS. Terwisscha van scheltinga AG, et al. MAPK pathway activity plays a key role in PD-L1 expression of lung adenocarcinoma cells. J Pathol (2019) 249(1):52–64. doi: 10.1002/path.5280
10. Galluzzi L, Spranger S, Fuchs E, López-Soto A. WNT signaling in cancer immunosurveillance. Trends Cell Biol (2019) 29(1):44–65. doi: 10.1016/j.tcb.2018.08.005
11. Cao Y, Chan KI, Xiao G, Chen Y, Qiu X, Hao H, et al. Expression and clinical significance of PD-L1 and BRAF expression in nasopharyngeal carcinoma. BMC Cancer (2019) 19(1):1022. doi: 10.1186/s12885-019-6276-y
12. Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 expression in lung cancer. J Thorac Oncol (2016) 11(7):964–75. doi: 10.1016/j.jtho.2016.04.014
13. Wang W, Zhang T. Expression and analysis of PD-L1 in peripheral blood circulating tumor cells of lung cancer. Future Oncol (2021) 17(13):1625–35. doi: 10.2217/fon-2020-0683
14. Huang C-Y, Wang Y, Luo G-Y, Han F, Li Y-Q, Zhou Z-G, et al. Relationship between PD-L1 expression and CD8+ T-cell immune responses in hepatocellular carcinoma. J Immunother (2017) 40(9):323–33. doi: 10.1097/CJI.0000000000000187
15. Jung HI, Jeong D, Ji S, Ahn TS, Bae SH, Chin S, et al. Overexpression of PD-L1 and PD-L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res Treat (2017) 49(1):246–54. doi: 10.4143/crt.2016.066
16. Ma L-J, Feng F-L, Dong L-Q, Zhang Z, Duan M, Liu L-Z, et al. Clinical significance of PD-1/PD-Ls gene amplification and overexpression in patients with hepatocellular carcinoma. Theranostics (2018) 8(20):5690–702. doi: 10.7150/thno.28742
17. Chen L, Huang X, Zhang W, Liu Y, Chen B, Xiang Y, et al. Correlation of PD-L1 and SOCS3 Co-expression with the prognosis of hepatocellular carcinoma patients. J Cancer (2020) 11(18):5440–8. doi: 10.7150/jca.46158
18. Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PloS One (2017) 12(8):e0182692. doi: 10.1371/journal.pone.0182692
19. Peng Q-H, Wang C-H, Chen H-M, Zhang R-X, Pan Z-Z, Lu Z-H, et al. CMTM6 and PD-L1 coexpression is associated with an active immune microenvironment and a favorable prognosis in colorectal cancer. J For Immunotherapy Cancer (2021) 9(2):e001638. doi: 10.1136/jitc-2020-001638
20. Yin X, Wang Z, Wang J, Xu Y, Kong W, Zhang J. Development of a novel gene signature to predict prognosis and response to PD-1 blockade in clear cell renal cell carcinoma. Oncoimmunology (2021) 10(1):1933332. doi: 10.1080/2162402X.2021.1933332
21. Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, et al. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget (2017) 8(19):31347–54. doi: 10.18632/oncotarget.15532
22. Lu J-C, Zeng H-Y, Sun Q-M, Meng Q-N, Huang X-Y, Zhang P-F, et al. Distinct PD-L1/PD1 profiles and clinical implications in intrahepatic cholangiocarcinoma patients with different risk factors. Theranostics (2019) 9(16):4678–87. doi: 10.7150/thno.36276
23. Wu H, Wei Y, Jian M, Lu H, Song Q, Hao L, et al. Clinicopathological and prognostic significance of immunoscore and PD-L1 in intrahepatic cholangiocarcinoma. OncoTargets Ther (2021) 14:39–51. doi: 10.2147/OTT.S288982
24. Wang Z, Zeng T, Li Y, Zhang D, Yuan Z, Huang M, et al. PD-1 inhibitors plus capecitabine as maintenance therapy for advanced intrahepatic cholangiocarcinoma: a case report and review of literature. Front In Immunol (2021) 12:799822. doi: 10.3389/fimmu.2021.799822
25. Xiong F, Gong J, Wang Q. Olaparib and pembrolizumab treatment for -mutated and PD-L1-Positive intrahepatic cholangiocarcinoma recurrence and metastasis: a case report. OncoTargets Ther (2020) 13:6385–91. doi: 10.2147/OTT.S250454
26. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
28. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
29. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
30. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
31. Ha H, Nam AR, Bang JH, Park JE, Kim TY, Lee KH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget (2016) 7(47):76604–12. doi: 10.18632/oncotarget.12810
32. Lan C, Kitano Y, Yamashita Y, Yamao T, Kajiyama K, Yoshizumi T, et al. Cancer-associated fibroblast senescence and its relation with tumour-infiltrating lymphocytes and PD-L1 expressions in intrahepatic cholangiocarcinoma. Br J Cancer (2022) 126(2):219–27. doi: 10.1038/s41416-021-01569-6
33. Tan XX, Bednarsch J, Rosin M, Appinger S, Liu D, Wiltberger G, et al. PD-1+T-Cells correlate with nerve fiber density as a prognostic biomarker in patients with resected perihilar cholangiocarcinoma. Cancers (2022) 14(9):2190. doi: 10.3390/cancers14092190
34. Tao P, Ma L, Xue R, Wang H, Zhang S. Clinicopathological and prognostic implications of vessels encapsulate tumor clusters with PD-L1 in intrahepatic cholangiocarcinoma patients. Trans Cancer Res (2020) 9(5):3550–63. doi: 10.21037/tcr.2020.04.11
35. Tian L, Ma J, Ma L, Zheng B, Liu L, Song D, et al. PD-1/PD-L1 expression profiles within intrahepatic cholangiocarcinoma predict clinical outcome. World J Surg Oncol (2020) 18(1):303. doi: 10.1186/s12957-020-02082-5
36. Yang H, Yan M, Li W, Xu L. SIRPα and PD1 expression on tumor-associated macrophage predict prognosis of intrahepatic cholangiocarcinoma. J Trans Med (2022) 20(1):140. doi: 10.1186/s12967-022-03342-6
37. Yugawa K, Itoh S, Yoshizumi T, Iseda N, Tomiyama T, Toshima T, et al. Prognostic impact of tumor microvessels in intrahepatic cholangiocarcinoma: association with tumor-infiltrating lymphocytes. Modern Pathol (2021) 34(4):798–807. doi: 10.1038/s41379-020-00702-9
38. Zheng Y, Huang N, Kuang S, Zhang J, Zhao H, Wu J, et al. The clinicopathological significance and relapse predictive role of tumor microenvironment of intrahepatic cholangiocarcinoma after radical surgery. Cancer (2022) 129(3):393–404. doi: 10.1002/cncr.34552
39. Kim H, Kim J, Byeon S, Jang K-T, Hong JY, Lee J, et al. Programmed death ligand 1 expression as a prognostic marker in patients with advanced biliary tract cancer. Oncology (2021) 99(6):365–72. doi: 10.1159/000514404
40. Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer (2015) 112(9):1501–9. doi: 10.1038/bjc.2015.101
41. Nunes-Xavier CE, Angulo JC, Pulido R, López JI. A critical insight into the clinical translation of PD-1/PD-L1 blockade therapy in clear cell renal cell carcinoma. Curr Urol Rep (2019) 20(1):1. doi: 10.1007/s11934-019-0866-8
42. Salmaninejad A, Khoramshahi V, Azani A, Soltaninejad E, Aslani S, Zamani MR, et al. PD-1 and cancer: molecular mechanisms and polymorphisms. Immunogenetics (2018) 70(2):73–86. doi: 10.1007/s00251-017-1015-5
43. Kang MJ, Kim KM, Bae JS, Park HS, Lee H, Chung MJ, et al. Tumor-infiltrating PD1-positive lymphocytes and FoxP3-positive regulatory T cells predict distant metastatic relapse and survival of clear cell renal cell carcinoma. Trans Oncol (2013) 6(3):282–9. doi: 10.1593/tlo.13256
44. Zhu S-G, Li H-B, Dai T-X, Li H, Wang G-Y. Successful treatment of stage IIIB intrahepatic cholangiocarcinoma using neoadjuvant therapy with the PD-1 inhibitor camrelizumab: a case report. World J Clin cases (2022) 10(27):9743–9. doi: 10.12998/wjcc.v10.i27.9743
45. Xie L, Huang J, Wang L, Ren W, Tian H, Hu A, et al. Lenvatinib combined with a PD-1 inhibitor as effective therapy for advanced intrahepatic cholangiocarcinoma. Front Pharmacol (2022) 13:894407. doi: 10.3389/fphar.2022.894407
46. Liu Z-L, Liu X, Peng H, Peng Z-W, Long J-T, Tang D, et al. Anti-PD-1 immunotherapy and radiotherapy for stage IV intrahepatic cholangiocarcinoma: a case report. Front In Med (2020) 7:368. doi: 10.3389/fmed.2020.00368
47. Marletta S, Fusco N, Munari E, Luchini C, Cimadamore A, Brunelli M, et al. Atlas of PD-L1 for pathologists: indications, scores, diagnostic platforms and reporting systems. J Pers Med (2022) 12(7):1073. doi: 10.3390/jpm12071073
48. Munari E, Querzoli G, Brunelli M, Marconi M, Sommaggio M, Cocchi MA, et al. Comparison of three validated PD-L1 immunohistochemical assays in urothelial carcinoma of the bladder: interchangeability and issues related to patient selection. Front In Immunol (2022) 13:954910. doi: 10.3389/fimmu.2022.954910
49. Girolami I, Pantanowitz L, Mete O, Brunelli M, Marletta S, Colato C, et al. Programmed death-ligand 1 (PD-L1) is a potential biomarker of disease-free survival in papillary thyroid carcinoma: a systematic review and meta-analysis of PD-L1 immunoexpression in follicular epithelial derived thyroid carcinoma. Endocr Pathol (2020) 31(3):291–300. doi: 10.1007/s12022-020-09630-5
Keywords: intrahepatic carcinoma, prognostic value, meta-analysis, programmed cell death ligand 1 (PDL1), programmed cell death 1 (PD-1)
Citation: Xian F, Ren D, Bie J and Xu G (2023) Prognostic value of programmed cell death ligand 1 expression in patients with intrahepatic cholangiocarcinoma: a meta-analysis. Front. Immunol. 14:1119168. doi: 10.3389/fimmu.2023.1119168
Received: 08 December 2022; Accepted: 03 April 2023;
Published: 17 April 2023.
Edited by:
Hongda Liu, Nanjing Medical University, ChinaReviewed by:
Albino Eccher, Integrated University Hospital Verona, ItalyBing Feng, Pennington Biomedical Research Center, United States
Jian Zhou, Chinese Academy of Sciences (CAS), China
Copyright © 2023 Xian, Ren, Bie and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohui Xu, eGZ5aXh1ZUBzaW5hLmNvbQ==
 Feng Xian
Feng Xian Dacheng Ren2
Dacheng Ren2 Guohui Xu
Guohui Xu