- 1Molecular and Genetic Epidemiology Laboratory, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
- 2Master’s Program in Medical Sciences, Graduate School of Comprehensive Human Sciences, University of Tsukuba, Tsukuba, Japan
- 3Department of Nephrology, Rheumatology, Endocrinology and Metabolism, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
- 4Department of Clinical Epidemiology, Kochi Medical School, Kochi University, Nankoku, Japan
- 5Department of Rheumatology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan
- 6Department of Lifetime Clinical Immunology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan
- 7Department of Internal Medicine, Juntendo University Koshigaya Hospital, Saitama, Japan
- 8Department of Rheumatology, Ome Municipal General Hospital, Ome, Japan
- 9Department of Rheumatology, Saga University, Saga, Japan
- 10Department of General Medicine, Nara Medical University, Kashihara, Japan
- 11Department of Internal Medicine and Rheumatology, Juntendo University Faculty of Medicine, Tokyo, Japan
- 12Division of Hematology and Rheumatology, Department of Internal Medicine, National Defense Medical College, Tokorozawa, Japan
- 13Department of Internal Medicine, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
- 14Department of Nephrology, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
- 15Juntendo University School of Medicine, Tokyo, Japan
- 16Okayama University, Okayama, Japan
- 17Department of Nephrology and Rheumatology, Kyorin University School of Medicine, Mitaka, Japan
- 18Department of Internal Medicine, Kichijoji Asahi Hospital, Musashino, Japan
- 19Division of Rheumatology, Department of Internal Medicine, Tokyo Women’s Medical University School of Medicine, Tokyo, Japan
Background: Disease relapse remains a major problem in the management of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV). In European populations, HLA-DPB1*04:01 is associated with both susceptibility and relapse risk in proteinase 3-ANCA positive AAV. In a Japanese population, we previously reported an association between HLA-DRB1*09:01 and DQB1*03:03 with susceptibility to, and DRB1*13:02 with protection from, myeloperoxidase-ANCA positive AAV (MPO-AAV). Subsequently, the association of DQA1*03:02, which is in strong linkage disequilibrium with DRB1*09:01 and DQB1*03:03, with MPO-AAV susceptibility was reported in a Chinese population. However, an association between these alleles and risk of relapse has not yet been reported. Here, we examined whether HLA-class II is associated with the risk of relapse in MPO-AAV.
Methods: First, the association of HLA-DQA1*03:02 with susceptibility to MPO-AAV and microscopic polyangiitis (MPA) and its relationship with previously reported DRB1*09:01 and DQB1*03:03 were examined in 440 Japanese patients and 779 healthy controls. Next, the association with risk of relapse was analyzed in 199 MPO-ANCA positive, PR3-ANCA negative patients enrolled in previously reported cohort studies on remission induction therapy. Uncorrected P values (Puncorr) were corrected for multiple comparisons in each analysis using the false discovery rate method.
Results: The association of DQA1*03:02 with susceptibility to MPO-AAV and MPA was confirmed in a Japanese population (MPO-AAV: Puncorr=5.8x10-7, odds ratio [OR] 1.74, 95% confidence interval [CI] 1.40–2.16, MPA: Puncorr=1.1x10-5, OR 1.71, 95%CI 1.34–2.17). DQA1*03:02 was in strong linkage disequilibrium with DRB1*09:01 and DQB1*03:03, and the causal allele could not be determined using conditional logistic regression analysis. Relapse-free survival was shorter with nominal significance in carriers of DRB1*09:01 (Puncorr=0.049, Q=0.42, hazard ratio [HR]:1.87), DQA1*03:02 (Puncorr=0.020, Q=0.22, HR:2.11) and DQB1*03:03 (Puncorr=0.043, Q=0.48, HR:1.91) than in non-carriers in the log-rank test. Conversely, serine carriers at position 13 of HLA-DRβ1 (HLA-DRβ1_13S), including DRB1*13:02 carriers, showed longer relapse-free survival with nominal significance (Puncorr=0.010, Q=0.42, HR:0.31). By combining DQA1*03:02 and HLA-DRβ1_13S, a significant difference was detected between groups with the highest and lowest risk for relapse (Puncorr=0.0055, Q=0.033, HR:4.02).
Conclusion: HLA-class II is associated not only with susceptibility to MPO-AAV but also with risk of relapse in the Japanese population.
Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of necrotizing small vessel vasculitides characterized by ANCA production, mainly against proteinase 3 (PR3) or myeloperoxidase (MPO). AAV is classified as microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), or eosinophilic granulomatosis with polyangiitis (EGPA) according to the European Medicines Agency (EMA) algorithm (1). In AAV, epidemiological differences between European and Asian populations are well known. In European populations, GPA and PR3-ANCA positive AAV (PR3-AAV) are predominant, whereas in the Japanese population, MPA and MPO-ANCA-positive AAV (MPO-AAV) account for the majority of AAV cases (2). Such ethnic differences in epidemiology imply that the genetic background of AAV may play a role in its development.
Although patients with AAV achieve remission with immunosuppressive therapy (3), a substantial proportion experience relapse. In European populations, relapse occurs in approximately half of the patients with GPA within 5 years after achieving complete remission (4). Among AAV patients, the risk of relapse has been shown to be higher in those with GPA and PR3-AAV compared with MPA and MPO-AAV (5). In Japanese observational studies conducted to identify risk factors for AAV relapse, the dosage and tapering speed of prednisolone were associated with relapse (6, 7).
With respect to susceptibility to MPA and GPA, three genome-wide association studies (GWAS) in European populations have been reported thus far (8–10). In GPA and PR3-AAV, the most striking association was identified in the HLA-DP region, which is consistent with a previously reported association of GPA with HLA-DPB1*04:01 in a German population (11). Additionally, PRTN3 and SERPINA1 genes, encoding PR3 and α1-antitrypsin, respectively, were identified as susceptibility genes (8, 10). With respect to MPA and MPO-AAV, the HLA-DQ region has been associated with susceptibility in the GWAS (8, 10). In agreement with this, the HLA-DRB1*09:01-DQB1*03:03 haplotype was found to be associated with susceptibility to MPA and MPO-AAV in the Japanese population (12–15). In addition, the DQA1*03:02-DQB1*03:03 haplotype was recently reported to be associated with MPO-AAV in a Chinese population (16). The HLA-DRB1*09:01-DQA1*03:02-DQB1*03:03 haplotype is common in general East Asian populations, but rare in European populations. Additionally, DRB1*13:02 has been found to be associated with protection from MPA and MPO-AAV in the Japanese population (14).
Although several genes are associated with AAV susceptibility, those associated with relapse have not been well-characterized. In European populations, HLA-DPB1*04:01, the susceptibility allele for GPA, has been shown to be associated with a higher risk of AAV (17) and PR3-AAV relapse (18). However, similar studies have not been conducted in East Asian populations, in which MPA and MPO-AAV account for the majority of AAV cases.
In this study, we focused on MPO-AAV in the Japanese population, and examined whether HLA-DRB1, DQA1, DQB1 and DPB1 alleles are also associated with relapse in MPO-AAV, based on clinical data from Japanese nationwide cohort studies on remission induction therapy in AAV. Data were obtained from “Remission Induction Therapy in Japanese Patients with ANCA-associated Vasculitides” (RemIT-JAV), registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000001648) (19) and “Remission Induction Therapy in Japanese Patients with ANCA-associated Vasculitides and Rapidly Progressive Glomerulonephritis” (RemIT-JAV-RPGN) (UMIN000005136) (20) carried out under the initiatives of Japan Research Committee of the Ministry of Health, Labour, and Welfare for Intractable Vasculitis (JPVAS) and Research Committee of Intractable Renal Disease of the Ministry of Health, Labour, and Welfare of Japan.
Materials and methods
Patients and controls
We first examined whether HLA-DQA1*03:02, which has recently been reported in a Chinese population (16), is also associated with susceptibility to MPO-AAV and MPA in the Japanese population, and investigated its relationship with DRB1*09:01 and DQB1*03:03 (12–15), in 440 patients with AAV (male: 168, female: 272) and 779 controls (male: 306, female: 473) The breakdown is shown in Table 1. Among these subjects, 362 with MPO-AAV, 273 with MPA patients and 514 controls were included in HLA-DRB1 and DPB1 analyses in our previous study (14).
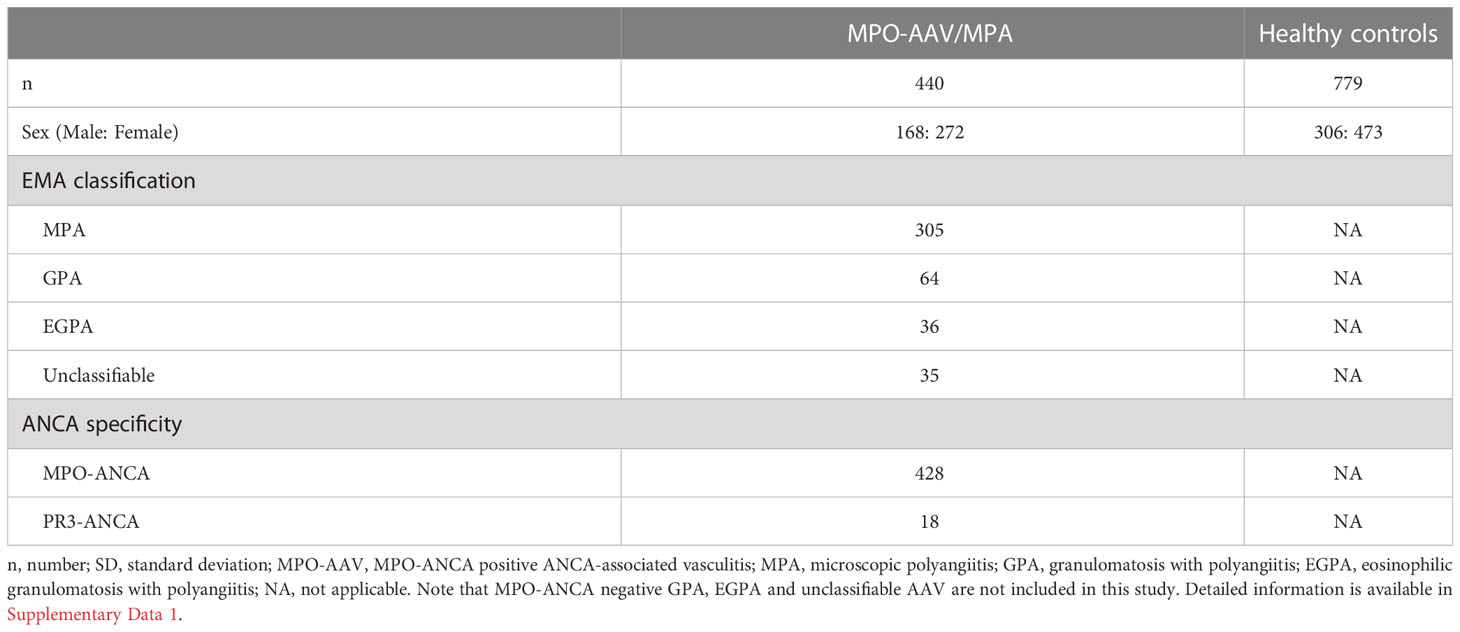
Table 1 Characteristics of the patients and healthy controls examined for the association of HLA-DRB1*09:01 and DQA1*03:02 with susceptibility to MPO-AAV and MPA.
Genomic DNA samples were obtained from institutes participating in Japan Research Committee of the Ministry of Health, Labour, and Welfare for Intractable Vasculitis (JPVAS) and the Research Committee of Progressive Renal Disease, both organized by the Ministry of Health, Labour, and Welfare of Japan, as well as from research groups organized by Tokyo Medical and Dental University and University of Tsukuba. A total of 264 control samples were obtained from the Health Science Research Resources Bank (Osaka, Japan).
Among patients with MPO-AAV, 88 enrolled in RemIT-JAV (19) and 176 enrolled in RemIT-JAV-RPGN (20) were analyzed for relapse-free survival. The characteristics of these cohorts have been previously described (19, 20). Briefly, the enrollment period was from April 2009 to December 2010 (RemIT-JAV) and from April 2011 to March 2014 (RemIT-JAV-RPGN). The enrollment criteria were as follows: 1) receiving a diagnosis of AAV by site investigators, 2) fulfilling the criteria for primary systemic vasculitis as proposed by the EMA algorithm, and 3) starting immunosuppressive treatment based on the discretion of the site investigators. Among these patients, 214 were MPO-ANCA positive and PR3-ANCA negative (thereafter, “MPO-ANCA single-positive”). To examine the rate of relapse after remission, 199 patients with MPO-ANCA single-positive AAV who achieved remission during the observation period were analyzed in this study. The observation period was 730 d after treatment initiation. Of the 199 patients included in this study, 8 died during the observation period (3 after relapse, and 5 without relapse). The causes of death in patients who died without relapse were infection (n=3), heart failure (n=1) and malignancy (n=1). These five patients were censored on the date of death. Remission was defined based on the Birmingham vasculitis activity score 2003 (21) of zero on two consecutive occasions at least one month apart (22). Relapse was defined as the recurrence or new onset of clinical signs and symptoms attributable to active vasculitis (6, 23).
Detailed information on the subjects is provided in Supplementary Data 1 (https://doi.org/10.6084/m9.figshare.21876159.v3).
Genotyping
In all patients and controls, HLA-DRB1 alleles were determined at the four-digit level using a WAKFlow HLA typing kit (Wakunaga Pharmaceutical Co., Ltd., Osaka, Japan) based on polymerase chain reaction-sequence-specific oligonucleotide probes (PCR-SSOP). HLA-DQA1, DQB1 and DPB1 alleles were genotyped using this system for the199 patients with MPO-AAV examined for relapse-free survival,
In susceptibility analysis, rs11545686C was used as a proxy for HLA-DQA1*03:02. Genotyping of rs11545686 was conducted by Sanger sequencing using 3130xl Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA). For amplification of HLA-DQA1 region surrounding rs11545686, forward (5’-TTTGGTTTGGGTGTCTTCAGATT-3’) and reverse (5’-AAAGTTGTTCAGGGAAATTTGAGAATG-3’) primers (Primer ID: Hs00412887_CE, Thermo Fisher Scientific) were used and cycle sequencing reaction was conducted using the forward primer and the BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific). Representative Sanger sequencing chromatograms for each genotype are shown in Supplementary Figure S1.
Statistical analysis
The characteristics of MPO-AAV patients with and without relapse were compared using Fisher’s exact test in two-by-two tables, excluding age, which was compared using the Mann–Whitney U test.
Relapse-free survival curves were generated using the Kaplan-Meier method and were compared among patients who were classified into GPA, MPA, EGPA and unclassifiable (UC) based on the EMA classification, among the patients treated with glucocorticoid (GC) alone, GC plus immunosuppressants, and immunosuppressants alone, and between patients with and without each HLA class II allele (HLA-DRB1, DQA1, DQB1 and DPB1), and each amino acid encoded by these HLA alleles, using log-rank test. Unadjusted hazard ratios (HRs) and adjusted HRs for EMA classification, treatment (GC alone, GC plus immunosuppressants or immunosuppressants alone), age and sex were calculated using Cox proportional hazard model.
The association of HLA-DQA1*03:02 and DRB1*09:01 with susceptibility to MPO-AAV and MPA was tested using logistic regression analysis with an additive model.
Statistical analyses were performed using the R software version 3.5.2. Correction for multiple testing in risk of relapse analysis at each HLA locus and amino acid position was performed by controlling the false discovery rate. Q<0.1 was considered significant. There were 17 DRB1 alleles, 11 DQA1 alleles, 11DQB1 alleles, 8 DPB1 alleles, 79 DRβ1 amino acids, 47 DQα1 amino acids, 71 DQβ1 amino acids, and 26 DPβ1 amino acids. The log-rank test for the pairwise comparison between the combination of presence/absence of DQA1*03:02 and DRβ1_13S was corrected for six comparisons, namely, the combination of 2 out of 4 (4C2 = 6).
Ethics
This study was reviewed and approved by the Faculty of Medicine Ethics Committee of University of Tsukuba (Approval ID: 122, 123, 180, 227, 268).
This study was also approved by the Ethics Committees of the following institutes that participated in the collaboration and/or recruitment of subjects: Aichi Medical University, Asahikawa Medical University, Ehime University, Fukuoka University, Hamamatsu University, Hokkaido University, Iwate Prefectural Central Hospital, Juntendo University, Kagawa University. Kanazawa University, Kitano Hospital, Kyorin University, Kyoto University, Kyushu University, Nagasaki University, Nagoya City University, Nagoya University, Nara Medical University, National Defense Medical College, Okayama University, Okayama Saiseikai General Hospital, Saga University, Saitama Medical Center Hospital, Shimane University, The University of Miyazaki, The University of Tokyo, Toho University, Tokyo Medical and Dental University, Tokyo Medical University Hachioji Medical Center, Tokyo Metropolitan Geriatric Hospital, and Institute of Gerontology, and Tokyo Women’s Medical University.
This study was conducted in accordance with the principles of the Declaration of Helsinki and Ethical Guidelines for Human Genome/Gene Analysis Research implemented by the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labour and Welfare, and the Ministry of Economy, Trade and Industry, of Japan. Written informed consent was obtained from all the participants.
Results
Association of HLA-DRB1*09:01-DQA1*03:02-DQB1*03:03 haplotype with susceptibility to MPO-AAV and MPA in the Japanese population
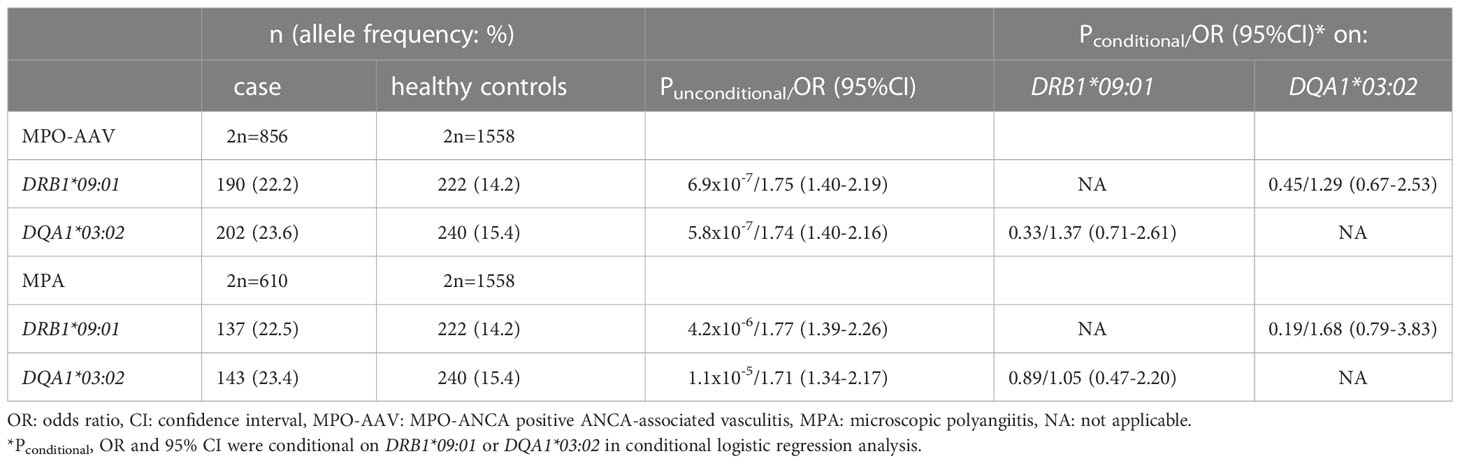
We previously reported that DRB1*09:01 and DQB1*03:03 are associated with susceptibility to, and DRB1*13:02 is associated with protection from, MPO-AAV and MPA in a Japanese population (12–15). Recently, HLA-DQA1*03:02, which is in strong linkage disequilibrium with DRB1*09:01 and DQB1*03:03, was reported to be associated with MPO-AAV in a Chinese population (16). Because the association of DQA1*03:02 with MPO-AAV and its relationship with the DRB1*09:01-DQB1*03:03 haplotype have not been previously analyzed in a Japanese population, we addressed this issue.
To genotype HLA-DQA1*03:02, we used rs11545686 as a tag single nucleotide variant (tagSNV). The rs11545686 encodes a p.Met18Thr substitution within the signal peptide of the HLA-DQ alpha chain. The rs11545686C allele is carried by DQA1*03:02, *03:07, *03:13 and *03:18 based on the IPD-IMGT/HLA Database (Release 3.47.0, https://www.ebi.ac.uk/ipd/imgt/hla/). Except for DQA1*03:02, these alleles are extremely rare in the Japanese population. In fact, rs12722040, which was previously reported to tag DQA1*03:02 (24), is identical to rs11545686. We confirmed that the DQA1*03:02 genotyping system based on rs11545686 was concordant with the results of high-resolution allele typing using PCR-SSOP in 199 samples, except for one sample in which the DQA1 genotype could not be determined using PCR-SSOP.
Using this genotyping system, we examined the association between DQA1*03:02, MPO-AAV, and MPA. DQA1*03:02 was significantly associated with susceptibility to MPO-AAV (Punconditional=5.8x10-7) and MPA (Punconditional=1.1x10-5) (Table 2), which confirmed the recent report on a Chinese population (16).
Strong linkage disequilibrium was observed among DRB1*09:01, DQA1*03:02 and DQB1*03:03 (r2: 0.92-0.97). When the associations were conditioned, none remained significant (Table 2). Therefore, we could not determine the single causative allele among DRB1*09:01, DQA1*03:02 and DQB1*03:03.
Association of HLA class II alleles with relapse-free survival in MPO-AAV
Figure 1 shows a flow chart of the MPO-AAV patients enrolled in the relapse-free survival analysis. Among the 264 AAV patients who enrolled in the cohort studies RemIT-JAV (19) and RemIT-JAV-RPGN (20), more than 80% of patients were MPO-ANCA single-positive. As the frequency of relapse was reported to be higher in AAV patients with PR3-ANCA than in those without it (5), this study focused on 199 MPO-ANCA single-positive patients who achieved remission during the observation period. Patient characteristics are shown in Table 3. During the first 3 months after treatment initiation, 84 (42.2%) patients received GC alone, 112 (56.3%) were treated with GC plus immunosuppressants, and three were treated with immunosuppressants alone (1.5%). In most patients, cyclophosphamide was administered as an immunosuppressant.
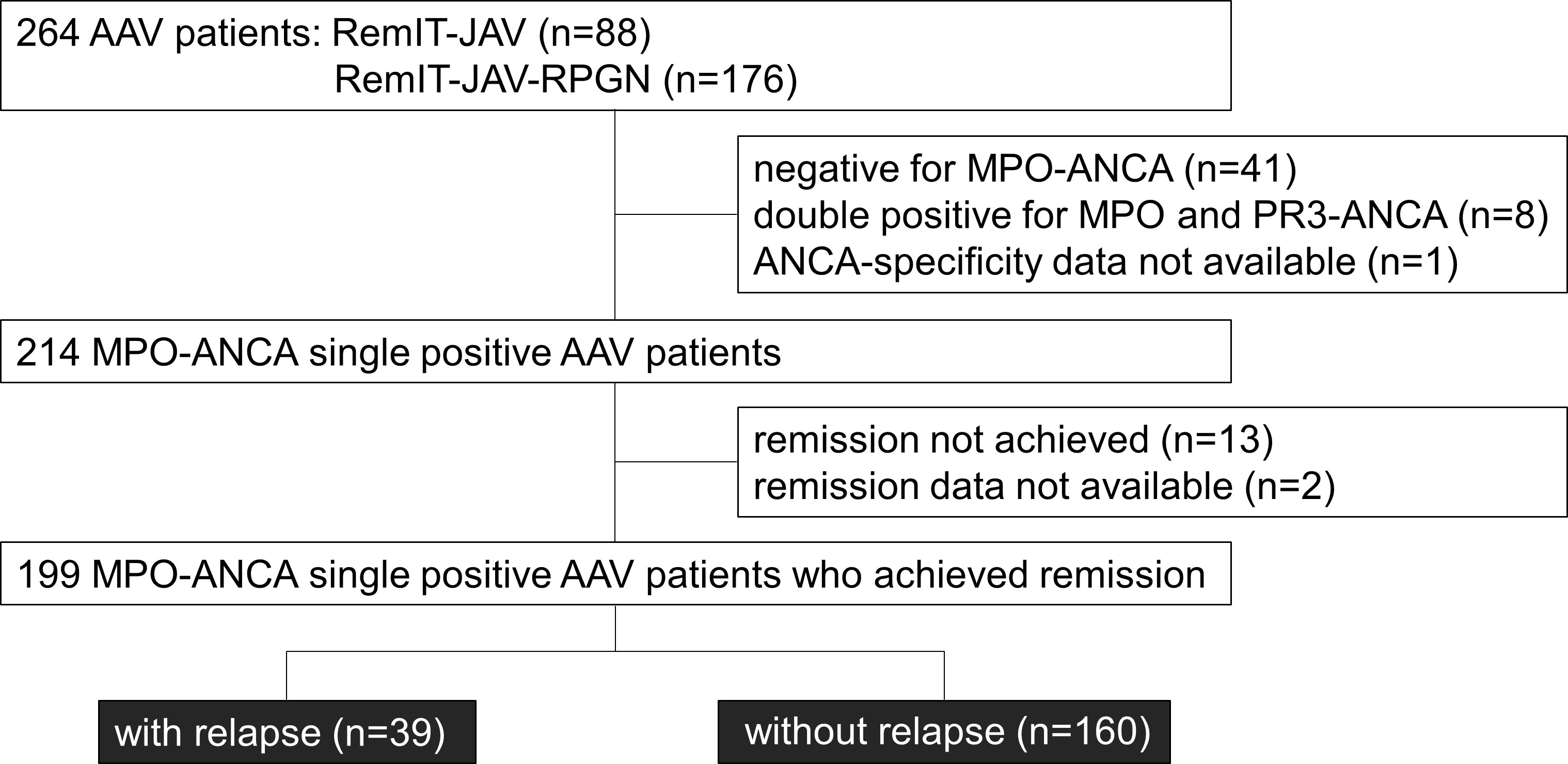
Figure 1 Flow chart of the MPO-AAV patients in the relapse-free survival analysis. Among the patients who entered the cohort studies of remission induction therapy (RemIT-JAV and RemIT-JAV-RPGN), 199 MPO-ANCA positive and PR3-ANCA negative (“MPO-ANCA single-positive”) patients who achieved remission were studied in the relapse-free survival analysis.
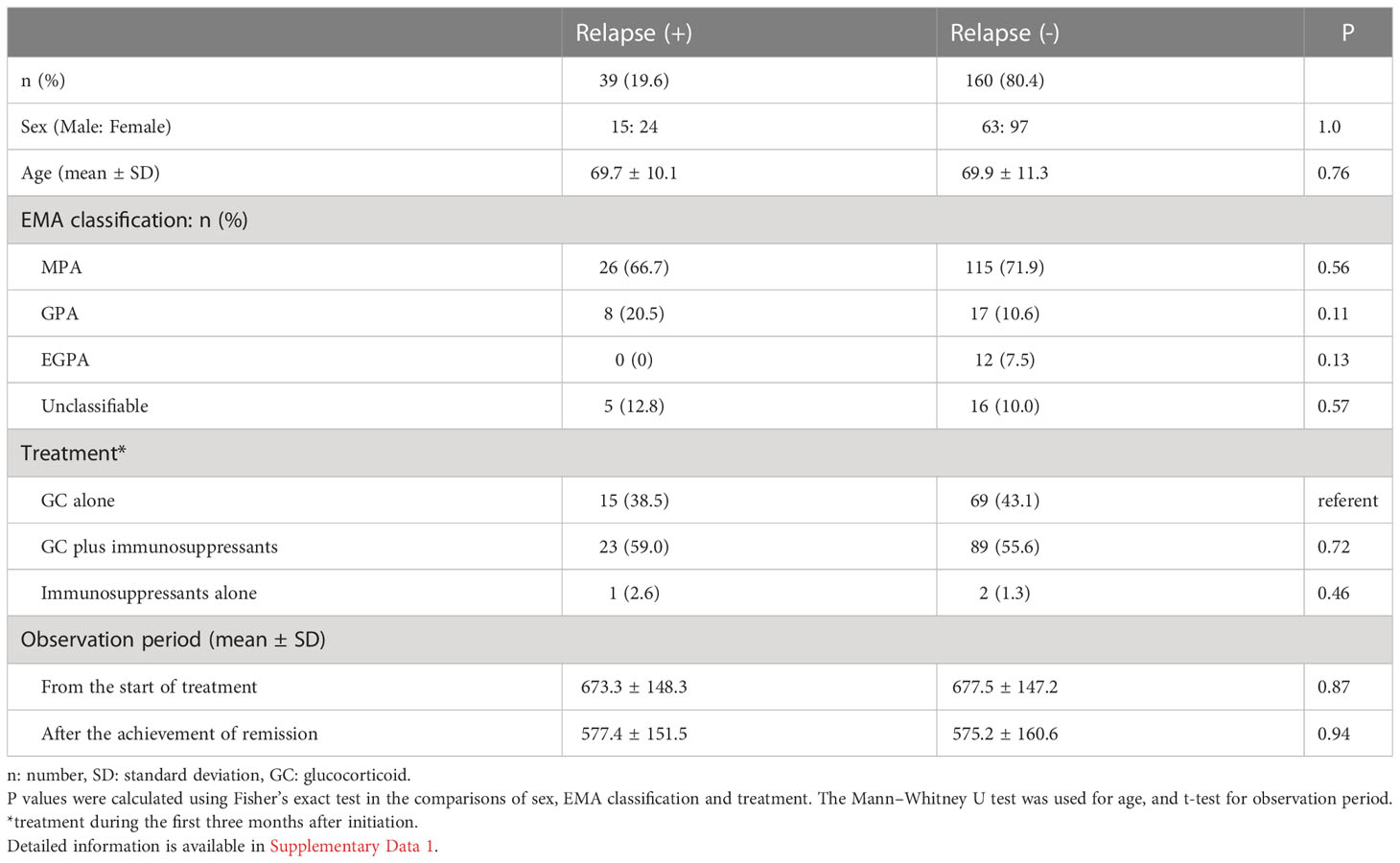
The mean observation period from the start of treatment was 676.7 (SD: 147.0) days. Among the 199 MPO-ANCA single positive AAV patients, 39 patients experienced relapse (19.6%). The mean time to relapse after remission was 228.1 (SD: 173.5) days. No significant difference in sex ratio, age, EMA clinical classification, the treatment received, or observation period were observed between patients with and without relapse (Table 3).
Next, we tested whether the EMA classification and the treatment modalities were associated with the risk of relapse using the Kaplan-Meier method for relapse-free survival. GPA patients have previously been shown to be associated with high occurrence of relapse (5). No significant difference in relapse-free survival was observed among MPO-ANCA single positive AAV patients classified by the EMA algorithm (log-rank test uncorrected P [Puncorr]=0.097) (Figure 2A) or treatment modality (log-rank test Puncorr=0.78)(Figure 2B).
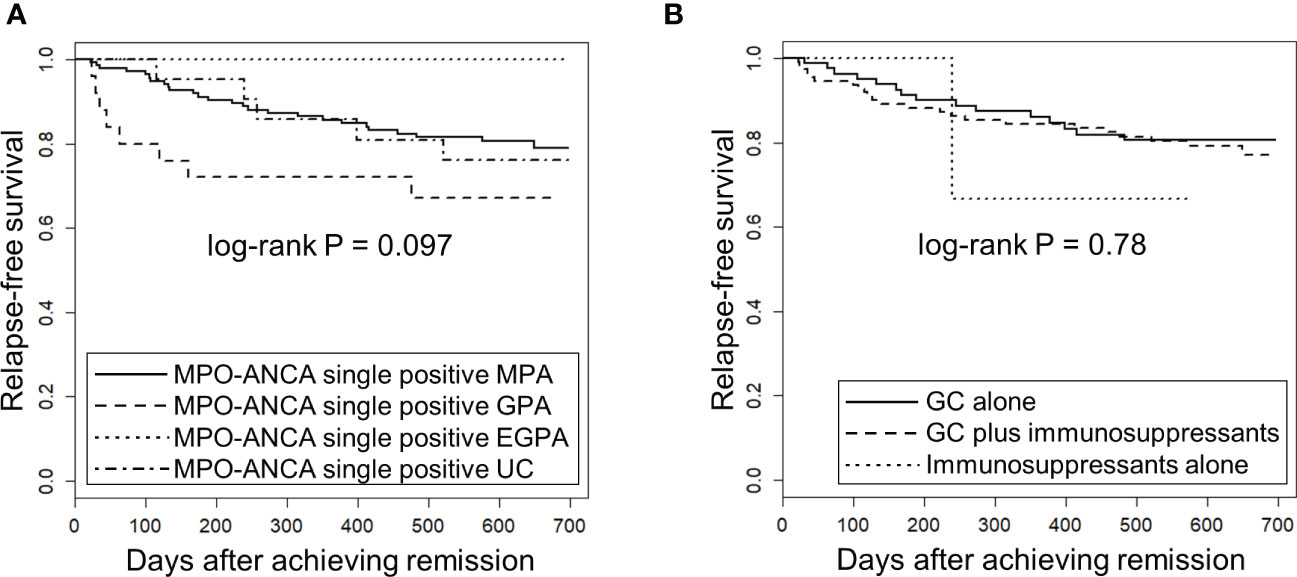
Figure 2 Relapse-free survival in MPO-ANCA single-positive AAV patients according to the EMA classification and treatment modality. The longitudinal and horizontal axes represent the probability of relapse-free survival and days after achievement of remission, respectively. P value was calculated by log-rank test. (A) Relapse-free survival was compared among MPO-ANCA single-positive, MPA (n=141), GPA (n=25), EGPA (n=12) and unclassifiable AAV (UC) (n=21). Note that PR3-ANCA positive patients are not included in any group. (B) Relapse-free survival was compared among MPO-ANCA single-positive AAV patients treated with glucocorticoid (GC) alone (n=84), GC plus immunosuppressants (n=112) and immunosuppressants alone (n=3).
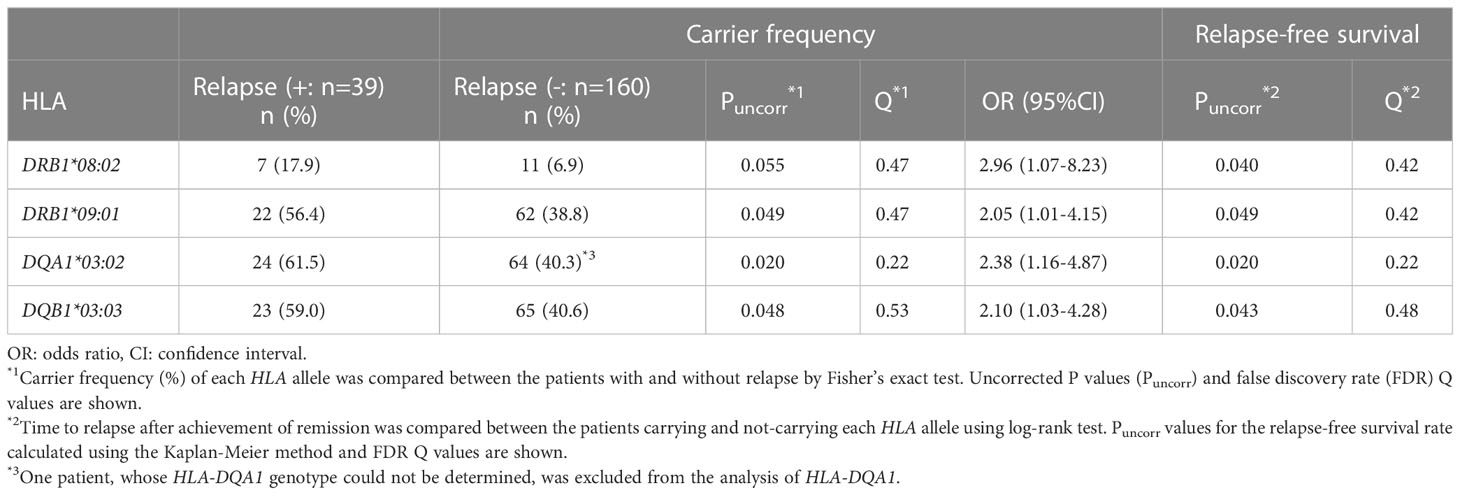
The carrier frequencies of the HLA-DRB1, DQA1, DQB1 and DPB1 alleles in MPO-AAV patients with and without relapse are shown in Supplementary Tables S1-S4. Carrier frequencies of DRB1*09:01 (Puncorr=0.049, Q=0.47, odds ratio [OR]: 2.05, 95% confidence interval [CI]: 1.01-4.15), DQA1*03:02 (Puncorr=0.020, Q=0.22, OR: 2.38, 95%CI: 1.16-4.87) and DQB1*03:03 (Puncorr=0.048, Q=0.53, OR: 2.10, 95%CI: 1.03-4.28) were nominally increased in patients with relapse (Table 4). With respect to DPB1 alleles, no trend toward association was observed among patients with MPO-ANCA single-positive AAV.
Next, we used the Kaplan-Meier method to compare relapse-free survival between the carriers and non-carriers of each HLA allele. As shown in Supplementary Tables S1-S4, a nominal association was observed in DQA1*03:02 (Puncorr=0.020, Q=0.22), DRB1*09:01 (Puncorr=0.049, Q=0.42) and DQB1*03:03 (Puncorr=0.043, Q=0.48) which were in linkage disequilibrium with DQA1*03:02. In addition, DRB1*08:02 (Puncorr=0.040, Q=0.42) was nominally associated with relapse.
Relapse-free survival curves and rates at the end of the observation period for nominally associated HLA alleles are shown in Figures 3A-D and Table 5. The HRs for relapse were 2.11 (95% CI: 1.11-4.02) in DQA1*03:02, 1.87 (0.99-3.52) in DRB1*09:01, 1.91 (1.01-3.61) in DQB1*03:03 and 2.30 (1.01-5.20) in DRB1*08:02. No violation of the proportional hazards assumption was observed for these alleles (P>0.05).
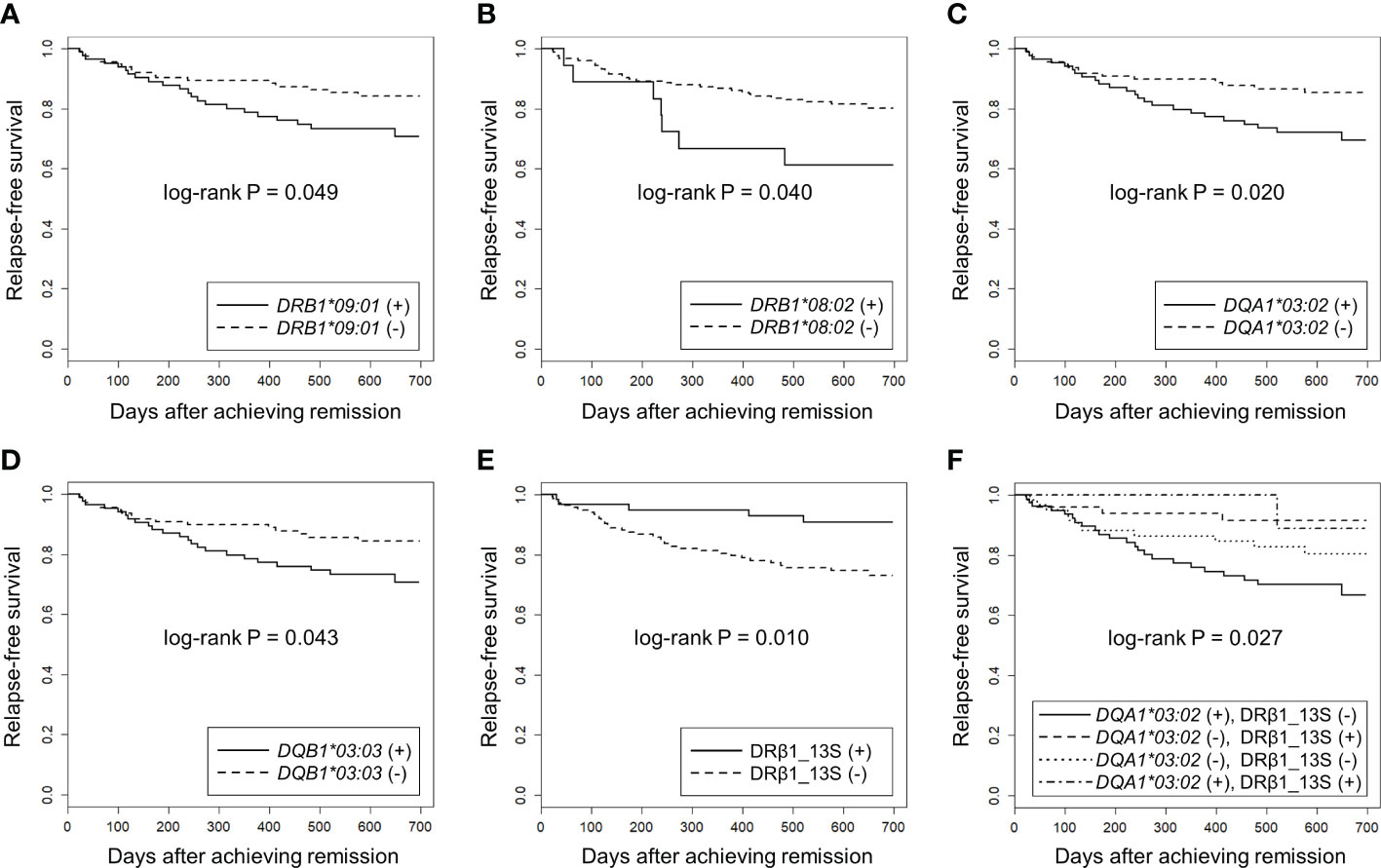
Figure 3 Association of HLA alleles and DRβ1 position 13 serine with relapse-free survival in MPO-ANCA single positive AAV patients using Kaplan-Meier method. The longitudinal and horizontal axes represent the probability of relapse-free survival and days after achievement of remission, respectively. Uncorrected P values calculated by log-rank test are shown. Q values corrected for multiple comparisons for each locus are shown in the text, Table 4 and Table 5. Relapse-free survival was compared between MPO-ANCA single-positive AAV patients carrying and not carrying (A) HLA-DRB1*09:01, (B) HLA-DRB1*08:02, (C) HLA-DQA1*03:02, (D) HLA-DQB1*03:03 and (E) HLA-DRβ1 position 13 serine (HLA-DRβ1_13S). (F) the patients were divided into four groups according to the carriage of HLA-DQA1*03:02 and HLA-DRβ1_13S. Relapse-free survival was compared among DQA1*03:02 (+) and DRβ1_13S (-) (n=79), DQA1*03:02 (-) and DRβ1_13S (+) (n=49), DQA1*03:02 (-) and DRβ1_13S (-) (n=61) and DQA1*03:02 (+) and DRβ1_13S (+) (n=9) groups.
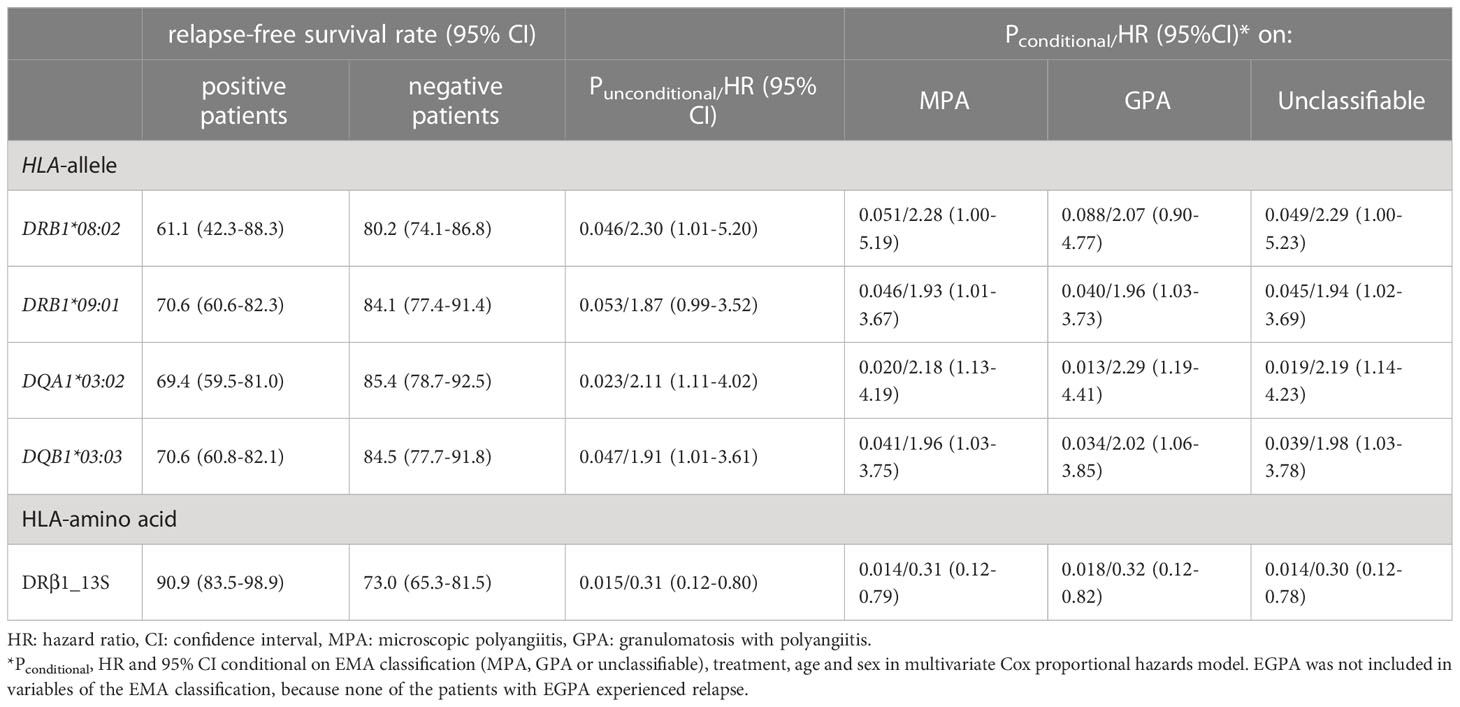
Table 5 Association of HLA class II alleles and DRβ1 position 13 serine with risk of relapse in multivariate Cox proportional hazards model.
When HRs for the associated HLA alleles were conditioned on the EMA classification (MPA, GPA or UC), treatment modality during the initial three months (GC alone, immunosuppressants alone and GC plus immunosuppressants), age and sex using a multivariate Cox proportional hazards model, the associations of DRB1*09:01, DQA1*03:02 and DQB1*03:03 were not affected, whereas the association of DRB1*08:02 was attenuated after conditioning (Table 5). In this analysis, EGPA was not included in the variables of the EMA classification, because no patients with EGPA experienced relapse.
Next, we examined whether specific amino acids in the HLA molecules were associated with relapse. The results of the log-rank test for each amino acid are presented in Supplementary Data 2. The strongest association trend was observed in serine residue at position 13 of HLA-DRβ1 (HLA-DRβ1_13S: log-rank Puncorr=0.010, Q=0.42). The relapse-free survival period was longer in patients carrying HLA-DRβ1_13S (HR: 0.31, 95% CI: 0.12-0.80) (Figure 3E, Table 5).
When the associations of the HLA alleles nominally associated with the risk of relapse and HLA-DRβ1_13S were conditioned on each other, a tendency toward association remained in DRβ1_13S (Pconditional = 0.057 [conditional on DQA1*03:02] and 0.040 [conditional on DQB1*03:03]) (Table 6).
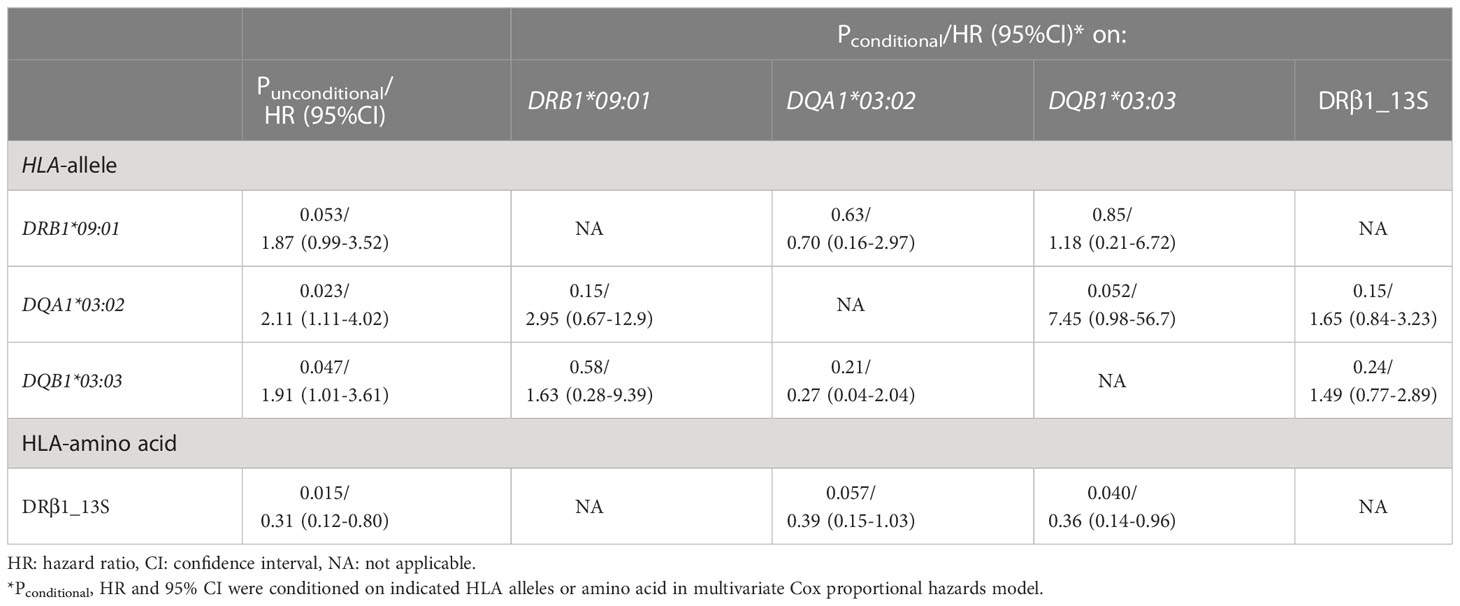
Table 6 Conditional survival analysis of HLA alleles and DRβ1 position 13 serine in multivariate Cox proportional hazards model.
Finally, the patients were divided into four groups according to the carriage a f DQA1*03:02 (as a representative of DRB1*09:01-DQA1*03:02-DQB1*03:03 relapse-risk haplotype) and DRβ1_13S (relapse-protective), and relapse-free survival was compared. As shown in Figure 3F, a significant difference in the time to relapse was detected among the four groups (log-rank P=0.027). In the pairwise comparisons, significant difference was observed between the patients with highest risk combination (DQA1*03:02 positive and DRβ1_13S negative) and with lowest risk for relapse combination (DQA1*03:02 negative and DRβ1_13S positive), even after correction for multiple testing (HR: 4.02, 95% CI: 1.39-11.6, Puncorr=0.0055, Q=0.033).
Discussion
In this study, we examined whether HLA class II alleles and HLA amino acids were associated with relapse in MPO-AAV, using data from two Japanese nationwide cohort studies on remission induction therapy: RemIT-JAV (19) and RemIT-JAV-RPGN (20). We detected that MPO-ANCA single positive AAV patients carrying HLA-DRB1*09:01, DQA1*03:02 or DQB1*03:03 had nominally higher risk of relapse as compared with those without these alleles, while AAV patients carrying serine residue at position 13 of HLA-DRβ1 had a trend toward lower risk of relapse. By combining these two factors, significant differences were observed in relapse-free survival among the patient subgroups. To the best of our knowledge, this is the first study to demonstrate the association between gene and amino acid variations and AAV relapse in an East Asian population.
We previously identified an association between HLA-DRB1*09:01 and DQB1*03:03 and susceptibility to MPA/MPO-AAV in a Japanese population (12–15). In the present study, we confirmed the association of DQA1*03:02 with susceptibility to MPO-AAV in a Japanese population, which was recently reported in a Chinese population (16). Owing to the tight linkage disequilibrium among these three HLA class II alleles, we could not determine which of the three alleles played a primary causative role.
Currently, the molecular mechanisms underlying the association between HLA class II alleles and MPA/MPO-AAV remain unclear. Among the components of the risk haplotype DRB1*09:01-DQA1*03:02-DQB1*03:03, the polymorphic amino acids encoded by DRB1*09:01 and DQB1*03:03 directly affect antigenic peptide specificity. Because MPO-ANCA has been strongly implicated in MPO-AAV pathogenesis (25), it is possible that specific antigenic peptides derived from human MPO (26) or microbial peptides mimicking human MPO peptides (27) might be preferentially presented by HLA-DRB1*09:01 and/or DQB1*03:03 products, although to our knowledge such peptides have not been identified from the patients for DRB1*09:01 or DQB1*03:03 products, Conversely, the specific amino acid encoded by DQA1*03:02, Asp160, is located in the α2 domain of HLA-DQ molecule, and may be involved in stabilizing HLA class II homodimers, thereby affecting antigenic peptide specificity (16). Thus, it is possible that multiple molecular mechanisms associated with MHC-peptide complex formation and T cell receptor signaling are involved in the genetic association between the risk haplotype and susceptibility to MPO-AAV.
A previous report showed that HLA-DPB1*04:01, the susceptibility allele for GPA, was also associated with an increased risk of AAV relapse in the Dutch and German patients (17). In this study, GPA and PR3-AAV were predominant. In contrast, this association was not observed in a Danish population (28). Although the reason for this discrepancy is unclear, differences in DPB1*04:01 carrier frequency among patients between these studies (77% (17) and 94% (28), respectively) may play a role. A recent study from the United States reported an association between HLA-DPB1*04:01 and relapse in PR3-AAV, but not in MPO-AAV (18).
With respect to MPO-AAV, a small-scale study in a European population did not detect a significant association between relapse and HLA-A, -B, or -DR antigens (29). In our study, HLA-DRB1*09:01, -DQA1*03:02 and -DQB1*03:03, the susceptibility alleles for MPO-AAV in East Asian populations (12–16) showed a trend toward a higher risk of relapse of MPO-AAV in a Japanese population, possibly suggesting that the susceptibility alleles for AAV may also be associated with relapse in each ethnic group.
Unlike a European study (17), this study did not detect an association between DPB1*04:01 and the risk of AAV relapse. This discrepancy likely occurred because this study focused on MPO-ANCA positive AAV, whereas in the previous European study, 51.7% of the subjects were positive for PR3-ANCA (17). In our MPO-AAV data, no significant association was detected between DPB1*04:01 and relapse (Supplementary Table S4, https://doi.org/10.6084/m9.figshare.21876159.v3), which is consistent with a report from the United States (18). This difference may not be explained merely by the low carrier frequency of DPB1*04:01 in the Japanese population, because DPB1*04:01 showed a slight tendency toward decrease in patients with relapse (P=0.70, OR: 0.39). In the Japanese population, the protective allele for MPO-AAV susceptibility, DRB1*13:02, is in linkage disequilibrium with DPB1*04:01 (r2 =0.44), and the allele frequency of DPB1*04:01 was also decreased in MPO-AAV (P=2.1x10-4, OR: 0.40) due to linkage disequilibrium with DRB1*13:02 (14). Thus, to evaluate the effect of DPB1*04:01 in the Japanese population, PR3-ANCA positive AAV patients with sufficient sample sizes should be investigated in the future.
In contrast to the HLA-DRB1*09:01-DQA1*03:02-DQB1*03:03 haplotype, alleles carrying HLA-DRβ1_13S were found to show a trend toward protection from relapse. HLA-DRβ1_13S is encoded by DR3, DR11, DR13 and DR14. HLA-DRB1 position 13 amino acid is associated with multiple immune system disorders, and was recently shown to be most strongly associated with individual differences in the T cell receptor complementarity determining region 3 (CDR3) repertoire (30). HRs (95% CI) and log-rank P values for relapse-free survival of alleles encoding HLA-DRβ1_13S with minor allele frequency >0.01 in the Japanese population are shown in Supplementary Table S5. These alleles showed a trend toward protection from relapse, although the differences did not reach statistical significance.
As mentioned above, we previously reported that DRB1*13:02, encoding HLA-DRβ1_13S, exhibited a protective effect against the MPO-AAV development (14). The data on susceptibility to MPO-AAV with HLA-DRβ1_13S, as well as with each allele encoding 13S, are also presented in Supplementary Table S5. HLA-DRβ1_13S was protective against the development of MPO-AAV (Puncorr=0.0029, OR 0.71, 95%CI 0.56-.89). Among the HLA-DRβ1_13S encoding alleles, the protective effect of DRB1*13:02 (Puncorr=6.9x10-5, OR 0.45, 95%CI 0.30-0.66) appeared to be more striking as compared with other HLA-DRβ1_13S encoding alleles. On the other hand, in the relapse analysis, HR was not much different between DRB1*13:02 and other DRβ1_13S encoding alleles. This could suggest a possibility that the protective effect against relapse may be shared by HLA-DRβ1_13S alleles, while protection from disease occurrence may be mainly ascribed to DRB1*13:02. Further studies with larger sample sizes are needed to validate this hypothesis.
When the combined effect of DQA1*03:02 and DRβ1_13S was examined, time to relapse was significantly shorter in the highest risk combination (DQA1*03:02 positive and DRβ1_13S negative) when compared with the lowest risk combination (DQA1*03:02 negative and DRβ1_13S positive), even after correction for multiple testing (Q=0.033). The HR was 4.02, which was higher than that for HLA-DPB1*04:01 in Dutch and American studies (17, 18).
In view of our results, it could be hypothesized that susceptibility alleles other than HLA may also be associated with the risk of MPO-AAV relapse. To date, no gene other than HLA has been established as a susceptibility gene for MPO-AAV in Asian populations. In a European GWAS, PTPN22 rs2476601 was reported to be associated with GPA/PR3-AAV and MPA/MPO-AAV (10); however, the risk allele of PTPN22 is almost absent in Asian populations. Recently, the association of a BACH2 variant was identified using target sequencing (31). According to the Genome Aggregation Database (gnomAD) (https://gnomad.broadinstitute.org/) (32), the risk allele in the BACH2 gene is not detected in East Asian populations. In a European population, it was reported that -463 A allele (rs2333227) in the MPO gene, which is associated with lower expression of MPO, was also associated with the risk of relapse in patients with MPO-AAV (33), which needs to be replicated in independent studies.
This study had several limitations. AAV is a rare disease, with an annual incidence of 22.6/million people in Japan (2). Because of the limited sample size and lack of independent replication cohort, it should be emphasized that this study is of an exploratory nature. For example, the attenuation of a trend toward relapse in DRB1*08:02 after conditioning (Table 5) could possibly be caused by insufficient detection power (0.589) due to the lower carrier frequencies of DRB1*08:02 compared to other alleles (Table 4). In fact, the HR of DRB1*08:02 remained >2.0 even after conditioning (Table 5). Further studies are required to draw a definitive conclusions.
Additionally, because this study was based on observational cohort studies conducted between 2009 and 2016 (19, 20), the potential effects of currently available treatments such as rituximab and avacopan could not be addressed. In this study, relapse was defined as the recurrence or new onset of clinical signs and symptoms attributable to active vasculitis, similar to previous studies on Japanese patients (6, 23). Although some previous studies employed strict definition, including a requirement for treatment escalation (17), we did not use this definition, because we thought that whether treatment is reinforced or not partly depends on each physician’s decision, and might possibly cause a bias. Finally, the classification of AAV is based on the EMA algorithm (1), and the compatibility of our findings with the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria (34–36) needs to be validated. These limitations should be addressed in future studies.
Conclusion
In the present study, we demonstrated that DRB1*09:01, DQA1*03:02 and DQB1*03:03, susceptibility alleles for MPO-AAV, are also nominally associated with the risk of relapse in MPO-AAV in the Japanese population. In addition, we found that the carriers of HLA-DRβ1 allele with serine residue at position 13 are nominally associated with lower risk of relapse. By combining these two factors, a statistically significant difference in the time to relapse was observed. These findings may be relevant in detecting individuals at high risk for AAV relapse of after remission, and adjusting treatment.
Data availability statement
The datasets presented in this study can be found in Figshare: https://doi.org/10.6084/m9.figshare.21876159.v1.
Ethics statement
The studies involving human participants were reviewed and approved by The Faculty of Medicine ethics committee, University of Tsukuba, as well as by all the institutes involved in the recruitment of subjects, as listed in the Materials and Methods. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AK and NTs designed the study, interpreted the data and wrote the manuscript. K-ES, KY, HM, YA and MH coordinated the cohorts. AK, PAK performed genotyping and statistical analysis. K-ES, FH. SK, KN, TS, NO, TF, MK, NTa, YK, KI and TS recruited the participants and collected clinical data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the grants from the Japan Agency for Medical Research and Development “The Study Group for Strategic Exploration of Drug Seeds for ANCA Associated Vasculitis and Construction of Clinical Evidence [grant number 17ek0109104h0003]”, “The Strategic Study Group to Establish the Evidence for Intractable Vasculitis Guideline [grant number 17ek0109121h0003]”, and “Multitiered study to address clinical questions for management of intractable vasculitides [grant number 20ek0109360h003]”, Ministry of Health, Labour and Welfare [grant number JPMH20FC1044], Japan Society for the Promotion of Science KAKENHI [grant number JP17K09967, JP21K08435], research grants from Bristol-Myers Squibb K.K., Ichiro Kanehara Foundation, Takeda Science Foundation, the Uehara Memorial Foundation, collaborative research fund from H.U. Group Research Institute G.K., and award grants from Japan College of Rheumatology and Japan Rheumatism Foundation. The funders had no role in the design, analysis, interpretation and paper writing of this study.
Acknowledgments
The authors are grateful to all the patients for participating in this study, and to the clinical staff associated with Japan Research Committee of the Ministry of Health, Labour, and Welfare for Intractable Vasculitis (JPVAS) and Research Committee of Intractable Renal Disease of the Ministry of Health, Labour, and Welfare of Japan for recruiting the patients and collecting clinical information. We would like to thank Editage (www.editage.com) for English language editing. A preprint of the manuscript is available at the medRxiv preprint server (https://www.medrxiv.org/content/10.1101/2022.12.28.22283983v2) (37).
Conflict of interest
AK has received research grants from Ichiro Kanehara Foundation, Takeda Science Foundation, and Japan College of Rheumatology, and honoraria for lectures from Chugai Pharmaceutical Co. Ltd. K-ES has received a research grant from Pfizer Inc., and honoraria for lectures from Glaxo SmithKline K.K. FH has received honoraria for lectures from Janssen Pharmaceuticals, Ono Pharmaceuticals, and Mitsubishi Tanabe Pharma. SK has received honoraria for the lectures from Novartis Pharma K.K, Eli Lilly Japan K.K., Chugai Pharma, Asahi Kasei Pharma, Gilead Sciences and Janssen Pharma K.K. KN has received speaking fee from Chugai Pharmaceutical Co. Ltd. TS has received grants from AsahiKASEI Co., Ltd., Daiichi Sankyo, Chugai Pharmaceutical Co. and Ono Pharmaceutical, consulting fees from AsahiKASEI Co., Ltd., and honoraria for the lectures from Abbvie Japan Co., Ltd. AsahiKASEI Co., Ltd. Astellas Pharma Inc., Ayumi Pharmaceutical, Bristol Myers Squibb K.K., Chugai Pharmaceutical Co., Ltd, Eli Lilly Japan K.K., Mitsubishi-Tanabe Pharma Co., Ono Pharmaceutical, Pfizer Japan Inc., Takeda Pharmaceutical Co. Ltd., and UCB Japan Co. Ltd. NTa has received grants from Astellas, Ayumi, Asahi Kasei Pharma, Asahi Kasei Medical, AbbVie, Eisai, Nippon Boehringer Ingelheim, Novartis Pharma, Bayer Yakuhin, Tanabe Mitsubishi, Taisho, and Chugai. NTa has received speaker fees and/or consulting fees from AbbVie, Eli Lilly Japan, Eisai, GlaxoSmithKline, Novartis, Bristol Myers Squibb, TanabeMitsubishi, Chugai and Janssen. KI and YK have received grants from Asahi Kasei Pharma, Eizai, Teijin Pharma, and Chugai Pharmaceutical. KI has received honoraria for lectures from Asahi Kasei Pharma and Abbvie. HM has served on advisory boards for Boehringer Ingelheim and Travere Therapeutics. MH has received grants from AbbVie Japan GK, Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., and Teijin Pharma Ltd. MH has received consulting fee from Kissei Pharmaceutical Co., Ltd., honoraria for lectures from AbbVie Japan GK, Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Kissei Pharmaceutical Co., Ltd. Mitsubishi Tanabe Pharma Co., and Teijin Pharma Ltd., participation for Advisory Board for Kissei Pharmaceutical Co., Ltd. NTs has received grants from Bristol-Myers Squibb K.K., the Naito Foundation, the Uehara Memorial Foundation, and collaborative research fund from H.U. Group Research Institute G.K. NTs has received award grants from Japan College of Rheumatology and Japan Rheumatism Foundation, and honoraria for lectures from Teijin Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://doi.org/10.6084/m9.figshare.21876159.v3
Supplementary Figure 1 | Sanger sequencing chromatograms around the HLA-DQA1*03:02 tagSNV rs11545686. Representative Sanger sequencing chromatograms corresponding to each of the three genotypes are shown. The rs11545686C allele tags HLA-DQA1*03:02. HLA-DQA1 genotype determined by PCR-SSOP was DQA1*03:02/03*02 (relapse ID 36), DQA1*03:02/05:07 (relapse ID 156) and DQA1* 01:01/01:02 (relapse ID 164), as shown in Supplementary Data 1.
References
1. Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis (2007) 66:222–7. doi: 10.1136/ard.2006.054593
2. Fujimoto S, Watts RA, Kobayashi S, Suzuki K, Jayne DR, Scott DG, et al. Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the U.K. Rheumatology (2011) 50:1916–20. doi: 10.1093/rheumatology/ker205
3. Novikov PI, Smitienko I, Moiseev SV. Duration of maintenance therapy for ANCA-associated vasculitis: More questions than answers. Ann Rheum Dis (2018) 77:e29. doi: 10.1136/annrheumdis-2017-211972
4. Pierrot-Deseilligny Despujol C, Pouchot J, Pagnoux C, Pagnoux C, Coste J, Guillevin L, et al. Predictors at diagnosis of a first wegener’s granulomatosis relapse after obtaining complete remission. Rheumatology (2010) 49:2181–90. doi: 10.1093/rheumatology/keq244
5. Terrier B, Pagnoux C, Perrodeau É, Karras A, Khouatra C, Aumaître O, et al. Long-term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann Rheum Dis (2018) 77:1151–7. doi: 10.1136/annrheumdis-2017-212768
6. Wada T, Hara A, Arimura Y, Sada KE, Makino H. Risk factors associated with relapse in Japanese patients with microscopic polyangiitis. J Rheumatol (2012) 39:545–51. doi: 10.3899/jrheum.110705
7. Hara A, Wada T, Sada KE, Amano K, Dobashi H, Harigai M, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis in Japan: A nationwide, prospective cohort study. J Rheumatol (2018) 45:521–8. doi: 10.3899/jrheum.170508
8. Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med (2012) 367:214–23. doi: 10.1056/NEJMoa1108735
9. Xie G, Roshandel D, Sherva R, Monach PA, Lu EY, Kung T, et al. Association of granulomatosis with polyangiitis (Wegener’s) with HLA-DPB1*04 and SEMA6A gene variants: evidence from genome-wide analysis. Arthritis Rheum (2013) 65:2457–68. doi: 10.1002/art.38036
10. Merkel PA, Xie G, Monach PA, Ji X, Ciavatta DJ, Byun J, et al. Identification of functional and expression polymorphisms associated with risk for antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol (2017) 69:1054–66. doi: 10.1002/art.40034
11. Heckmann M, Holle JU, Arning L, Knaup S, Hellmich B, Nothnagel M, et al. The wegener’s granulomatosis quantitative trait locus on chromosome 6p21.3 as characterised by tagSNP genotyping. Ann Rheum Dis (2008) 67:972–9. doi: 10.1136/ard.2007.077693
12. Tsuchiya N, Kobayashi S, Kawasaki A, Kyogoku C, Arimura Y, Yoshida M, et al. Genetic background of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis: association of HLA-DRB1*0901 with microscopic polyangiitis. J Rheumatol (2003) 30:1534–40.
13. Tsuchiya N, Kobayashi S, Hashimoto H, Ozaki S, Tokunaga K. Association of HLA-DRB1*0901-DQB1*0303 haplotype with microscopic polyangiitis in Japanese. Genes Immun (2006) 7:81–4. doi: 10.1038/sj.gene.6364262
14. Kawasaki A, Hasebe N, Hidaka M, Hirano F, Sada KE, Kobayashi S, et al. Protective role of HLA-DRB1*13:02 against microscopic polyangiitis and MPO-ANCA-positive vasculitides in a Japanese population: a case-control study. PloS One (2016) 11:e0154393. doi: 10.1371/journal.pone.0154393
15. Kawasaki A, Tsuchiya N. Advances in the genomics of ANCA-associated vasculitis-a view from East Asia. Genes Immun (2021) 22:1–11. doi: 10.1038/s41435-021-00123-x
16. Wang HY, Cui Z, Pei ZY, Fang SB, Chen SF, Zhu L, et al. Risk HLA class II alleles and amino acid residues in myeloperoxidase-ANCA-associated vasculitis. Kidney Int (2019) 96:1010–9. doi: 10.1016/j.kint.2019.06.015
17. Hilhorst M, Arndt F, Kemna MJ, Wieczorek S, Donner Y, Wilde B, et al. HLA-DPB1 as a risk factor for relapse in antineutrophil cytoplasmic antibody-associated vasculitis: a cohort study. Arthritis Rheumatol (2016) 68:1721–30. doi: 10.1002/art.39620
18. Chen DP, McInnis EA, Wu EY, Stember KG, Hogan SL, Hu Y, et al. Immunological interaction of HLA-DPB1 and proteinase 3 in ANCA vasculitis is associated with clinical disease activity. J Am Soc Nephrol (2022) 33:1517–27. doi: 10.1681/ASN.2021081142
19. Sada KE, Yamamura M, Harigai M, Fujii T, Dobashi H, Takasaki Y, et al. Classification and characteristics of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis in a nationwide, prospective, inception cohort study. Arthritis Res Ther (2014) 16:R101. doi: 10.1186/ar4550
20. Sada KE, Harigai M, Amano K, Atsumi T, Fujimoto S, Yuzawa Y, et al. Comparison of severity classification in Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis in a nationwide, prospective, inception cohort study. Mod Rheumatol (2016) 26:730–7. doi: 10.3109/14397595.2016.1140274
21. Flossmann O, Bacon P, de Groot K, Jayne D, Rasmussen N, Seo P, et al. Development of comprehensive disease assessment in systemic vasculitis. Ann Rheum Dis (2007) 66:283–92. doi: 10.1136/ard.2005.051078
22. Hellmich B, Flossmann O, Gross WL, Bacon P, Cohen-Tervaert JW, Guillevin L, et al. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis (2007) 66:605–17. doi: 10.1136/ard.2006.062711
23. Watanabe H, Sada KE, Matsumoto Y, Harigai M, Amano K, Dobashi H, et al. Association between reappearance of myeloperoxidase-antineutrophil cytoplasmic antibody and relapse in antineutrophil cytoplasmic antibody-associated vasculitis: Subgroup analysis of nationwide prospective cohort studies. Arthritis Rheumatol (2018) 70:1626–33. doi: 10.1002/art.40538
24. Zhou F, Cao H, Zuo X, Zhang T, Zhang X, Liu X, et al. Deep sequencing of the MHC region in the Chinese population contributes to studies of complex disease. Nat Genet (2016) 48:740–6. doi: 10.1038/ng.3576
25. Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, et al. ANCA-associated vasculitis. Nat Rev Dis Primers (2020) 6:71. doi: 10.1038/s41572-020-0204-y
26. Free ME, Stember KG, Hess JJ, McInnis EA, Lardinois O, Hogan SL, et al. Restricted myeloperoxidase epitopes drive the adaptive immune response in MPO-ANCA vasculitis. J Autoimmun (2020) 106:102306. doi: 10.1016/j.jaut.2019.102306
27. Ooi JD, Jiang JH, Eggenhuizen PJ, Chua LL, van Timmeren M, Loh KL, et al. A plasmid-encoded peptide from staphylococcus aureus induces anti-myeloperoxidase nephritogenic autoimmunity. Nat Commun (2019) 10:3392. doi: 10.1038/s41467-019-11255-0
28. Gregersen JW, Erikstrup C, Ivarsen P, Glerup R, Krarup E, Keller KK, et al. PR3-ANCA-associated vasculitis is associated with a specific motif in the peptide-binding cleft of HLA-DP molecules. Rheumatology (2019) 58:1942–9. doi: 10.1093/rheumatology/kez111
29. Stassen PM, Cohen-Tervaert JW, Lems SP, Hepkema BG, Kallenberg CGM, Stegeman CA, et al. HLA-DR4, DR13(6) and the ancestral haplotype A1B8DR3 are associated with ANCA-associated vasculitis and wegener’s granulomatosis. Rheumatology (2009) 48:622–5. doi: 10.1093/rheumatology/kep057
30. Ishigaki K, Lagattuta KA, Luo Y, James EA, Buckner JH, Raychaudhuri S. HLA autoimmune risk alleles restrict the hypervariable region of T cell receptors. Nat Genet (2022) 54:393–402. doi: 10.1038/s41588-022-01032-z
31. Dahlqvist J, Ekman D, Sennblad B, Kozyrev SV, Nordin J, Karlsson A, et al. Identification and functional characterization of a novel susceptibility locus for small vessel vasculitis with MPO-ANCA. Rheumatology (2022) 61:3461–70. doi: 10.1093/rheumatology/keab912
32. Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature (2020) 581:434–43. doi: 10.1038/s41586-020-2308-7
33. Reynolds WF, Stegeman CA, Tervaert JW. -463 G/A myeloperoxidase promoter polymorphism is associated with clinical manifestations and the course of disease in MPO-ANCA-associated vasculitis. Clin Immunol (2002) 103:154–60. doi: 10.1006/clim.2002.5206
34. Grayson PC, Ponte C, Suppiah R, Robson JC, Craven A, Judge A, et al. American College of Rheumatology/European alliance of associations for rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis (2022) 81:309–14. doi: 10.1136/annrheumdis-2021-221794
35. Robson JC, Grayson PC, Ponte C, Suppiah R, Craven A, Judge A, et al. American College of Rheumatology/European alliance of associations for rheumatology classification criteria for granulomatosis with polyangiitis. Ann Rheum Dis (2022) 81:315–20. doi: 10.1136/annrheumdis-2021-221795
36. Suppiah R, Robson JC, Grayson PC, Ponte C, Craven A, Khalid S, et al. American College of Rheumatology/European alliance of associations for rheumatology classification criteria for microscopic polyangiitis. Ann Rheum Dis (2022) 81:321–6. doi: 10.1136/annrheumdis-2021-221796
Keywords: ANCA-associated vasculitis (AAV), MPO-ANCA, HLA-class II, relapse, genetics, polymorphism, microscopic polyangiitis (MPA)
Citation: Kawasaki A, Sada K-e, Kusumawati PA, Hirano F, Kobayashi S, Nagasaka K, Sugihara T, Ono N, Fujimoto T, Kusaoi M, Tamura N, Kusanagi Y, Itoh K, Sumida T, Yamagata K, Hashimoto H, Makino H, Arimura Y, Harigai M and Tsuchiya N (2023) Association of HLA-class II alleles with risk of relapse in myeloperoxidase-antineutrophil cytoplasmic antibody positive vasculitis in the Japanese population. Front. Immunol. 14:1119064. doi: 10.3389/fimmu.2023.1119064
Received: 08 December 2022; Accepted: 23 February 2023;
Published: 08 March 2023.
Edited by:
Soheil Tavakolpour, Dana–Farber Cancer Institute, United StatesReviewed by:
Gianluca Moroncini, Marche Polytechnic University, ItalyKouyuki Hirayasu, Kanazawa University, Japan
Copyright © 2023 Kawasaki, Sada, Kusumawati, Hirano, Kobayashi, Nagasaka, Sugihara, Ono, Fujimoto, Kusaoi, Tamura, Kusanagi, Itoh, Sumida, Yamagata, Hashimoto, Makino, Arimura, Harigai and Tsuchiya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aya Kawasaki, YS1rYXdhc2FraUBtZC50c3VrdWJhLmFjLmpw; Naoyuki Tsuchiya, dHN1Y2hpeWEubnlrQGdtYWlsLmNvbQ==
 Aya Kawasaki
Aya Kawasaki Ken-ei Sada3,4
Ken-ei Sada3,4 Nobuyuki Ono
Nobuyuki Ono Takashi Fujimoto
Takashi Fujimoto Naoto Tamura
Naoto Tamura Kenji Itoh
Kenji Itoh Kunihiro Yamagata
Kunihiro Yamagata Masayoshi Harigai
Masayoshi Harigai Naoyuki Tsuchiya
Naoyuki Tsuchiya